Abstract
Photon-counting detector CT (PCD CT) has increasingly garnered interest in cardiothoracic imaging due to its high spatial resolution and ability to perform spectral imaging. CT plays an important role in the planning and postprocedural assessment of transcatheter aortic valve replacement (TAVR). Limitations of current CT technology resulting in blooming and metal artifacts may be addressed with PCD CT. This case series demonstrates the potential advantages of PCD CT in patients prior to and post-TAVR. In TAVR planning, PCD CT allowed for a detailed depiction of the aortic valve, aortic root, coronary arteries, and potential vascular access routes. The high-spatial-resolution reconstructions enabled assessment of hypoattenuating leaflet thickening and periprosthetic leakage for prosthetic valves. This study shows promising initial results, but further research is needed to determine the clinical impact of PCD CT in patients prior to and post-TAVR.
Keywords: Transcatheter Aortic Valve Replacement, Cardiac, Coronary Arteries, Heart, Valves, Photon-counting Detector CT
© RSNA, 2023
An earlier incorrect version appeared online. This article was corrected on October 27, 2023.
Keywords: Transcatheter Aortic Valve Replacement, Cardiac, Coronary Arteries, Heart, Valves, Photon-counting Detector CT
Summary
Due to its high spatial resolution and spectral capabilities, dualsource photon-counting detector CT is a promising technique for cardiovascular imaging and may improve planning and follow-up of transcatheter aortic valve replacement procedures.
Key Points
■ Photon-counting detector CT (PCD CT) is a next-generation CT scanner equipped with a semiconductor detector instead of a scintillation detector.
■ PCD CT is useful in cardiovascular imaging, such as for imaging the aortic valve, aortic root, coronary arteries, and potential vascular access routes, due to its high spatial resolution and spectral capabilities.
■ PCD CT may aid in planning of transcatheter aortic valve replacement and postprocedural assessment of the implanted valve.
Introduction
CT angiography (CTA) plays an essential role in the preprocedural planning of transcatheter aortic valve replacement (TAVR) and is frequently used to assess valve function after implantation (1). Recently, photon-counting detector CT (PCD CT) has become clinically available, which has the potential to address several limitations of current CT technology. PCD CT is a next-generation CT scanner equipped with a semiconductor detector instead of a scintillation detector (2). This detector enables x-ray photons to be directly converted into an electrical signal and be individually measured. The semiconductor detectors are more geometrically efficient than the conventional energy-integrating detectors, allowing for ultra-high-resolution (UHR) imaging with 0.2-mm section thickness. The individually measured photons enable spectral imaging and reduce beam-hardening as well as blooming artifacts (3,4). To date, limited research has been conducted to investigate the advantages of PCD CT in patients prior to and post-TAVR. We present our initial experience with a dual-source PCD CT scanner (NAEOTOM Alpha; Siemens Healthineers) in various TAVR devices and patients prior to and post-TAVR.
Case Presentations
Case 1
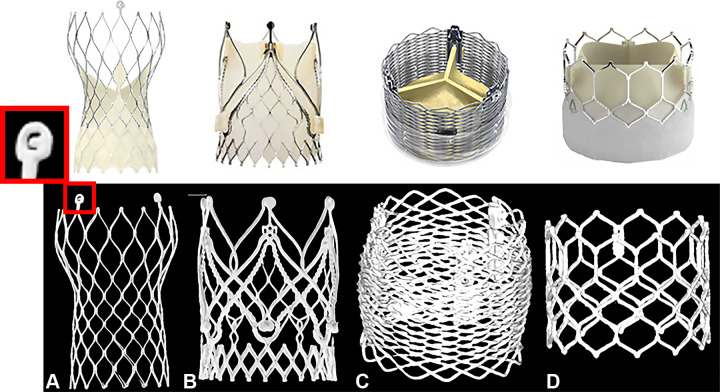
Figure 1 shows examples of ex vivo transcatheter heart valves scanned in UHR mode with PCD CT. The UHR mode enables image acquisition at 0.2-mm section thickness, which is twice as thin as the regular PCD CT acquisition mode (0.4 mm) and approximately three times thinner than most conventional CT scanners with energy-integrating detectors (5). The z-coverage is 144 × 0.4 mm and 120 × 0.2 mm for the regular and UHR modes, respectively. UHR PCD CT imaging allows for a detailed depiction of the stent frame of four valves such as the “C” mark in the CoreValve (Medtronic) stent frame (Fig 1A).
Figure 1:
Transcatheter heart valves scanned ex vivo in ultra-high-resolution (UHR) mode with photon-counting detector CT. Four different ex vivo transcatheter heart valves were scanned in UHR mode with 140 kV and reconstructed with a Qr89/Qr76 kernel at quantum iterative reconstruction strength 4. CT images are displayed as three-dimensional volume-rendered images: (A) CoreValve Evolut R (Medtronic), (B) JenaValve (JenaValve Technology), (C) Lotus Edge (Boston Scientific), (D) SAPIEN 3 Ultra TM transcatheter heart valve (Edwards Lifesciences). (Reprinted, with permission, from Medtronic, JenaValve Technology, ©2023 Boston Scientific Corporation, and Edwards Lifesciences Corporation, respectively. All rights reserved.)
Case 2
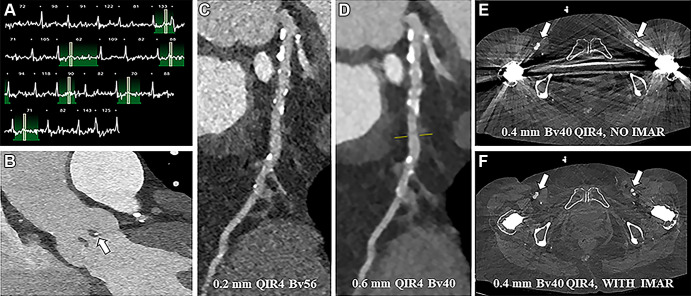
An 81-year-old female patient underwent dual-source PCD CT for TAVR planning (Fig 2). The scan protocol consisted of a non–contrast-enhanced acquisition of the heart, prospectively electrocardiographically triggered UHR CTA at 15%–53% of the R-R interval (120 kVp; image quality level, 43; volume CT dose index [CTDIvol], 26.8 mGy) directly followed by a high-pitch acquisition from the skull base to the groin at 0.4-mm section thickness (120 kVp; image quality level, 41; CTDIvol, 2.45 mGy). The coronary calcium score was 1385. Despite the irregular heart rate at the time of scanning, the aortic annulus and native aortic valve were visualized with sharp delineation of the leaflets and a small calcification in the nodule of Arantius (white arrow in Fig 2B). UHR imaging of the coronary arteries and a sharp reconstruction kernel (Bv56) improved spatial resolution and reduced calcium blooming (Fig 2C) compared with conventional reconstructions at 0.6-mm section thickness with a regular Bv40 kernel (Fig 2D). Assessment of the femoral arteries to determine the most suitable access route in this patient with bilateral hip prostheses is possible by applying iterative metal artifact reduction.
Figure 2:
Photon-counting detector CT scan for pre–transcatheter aortic valve replacement planning in an 81-year-old female patient (with contrast material). (A) Electrocardiogram shows a highly irregular heart rate was present at the time of scanning. (B) Contrast-enhanced visualization of the aortic annulus and native aortic valve in the sagittal plane with sharp delineation of the leaflets and a small calcification in the nodule of Arantius (white arrow). (C) Ultra-high-resolution image shows the coronary arteries (Bv56 kernel, multiplanar reformatted image). (D) Conventional reconstructions at 0.6-mm section thickness (Bv40 kernel) for comparison (multiplanar reformatted image). (E, F) Axial reconstructions at the level of the femoral arteries (arrows) without (E) and with (F) iterative metal artifact reduction (IMAR) reconstruction. QIR = quantitative iterative reconstruction.
Case 3
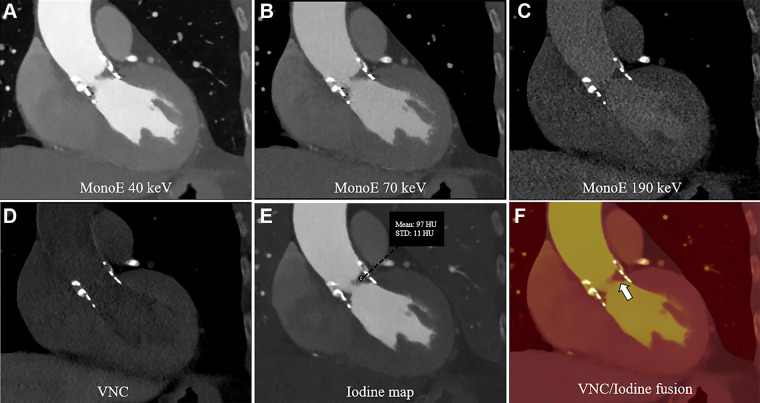
An 82-year-old female patient underwent TAVR with a SAPIEN 3 (Edwards Lifesciences) prosthesis implanted 4 years and 4 months prior to imaging. Follow-up CTA was performed with a PCD CT scanner (120 kV; image quality level, 50; CTDIvol, 15.2 mGy) to assess valve function. The scan depicts hypoattenuating leaflet thickening at the former left coronary cusp and less pronounced thickening of the right coronary cusp (Fig 3). With PCD CT, multiple spectral reconstructions can be computed from CTA. Virtual monoenergetic images (VMIs), reconstructed using preset energy levels without additional scanning (2), were acquired in the patient. The virtual noncontrast algorithm, which can virtually remove iodine from the image to visualize the potential presence of calcification in the native valve, was applied. The iodine map and fused iodine and virtual noncontrast maps showed no iodine uptake in the leaflets (Fig 3E, 3F).
Figure 3:
Contrast-enhanced photon-counting detector CT images of SAPIEN 3 valve with hypoattenuated leaflet thickening in an 82-year-old female patient following transcatheter aortic valve replacement. (A–C) Virtual monoenergetic (mono-E) images at different kiloelectron voltage levels in the sagittal plane. A and B show hypoattenuated leaflet thickening at the former left coronary cusp and less pronounced thickening of the right coronary cusp. (D) Virtual noncontrast (VNC) reconstruction and (E) iodine map. (F) Fused iodine and VNC maps show no iodine uptake in the coronary cusps (white arrow).
Case 4
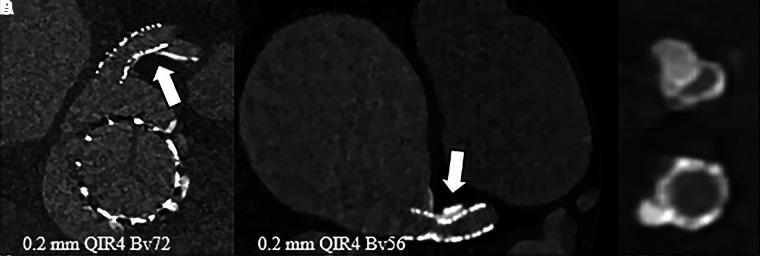
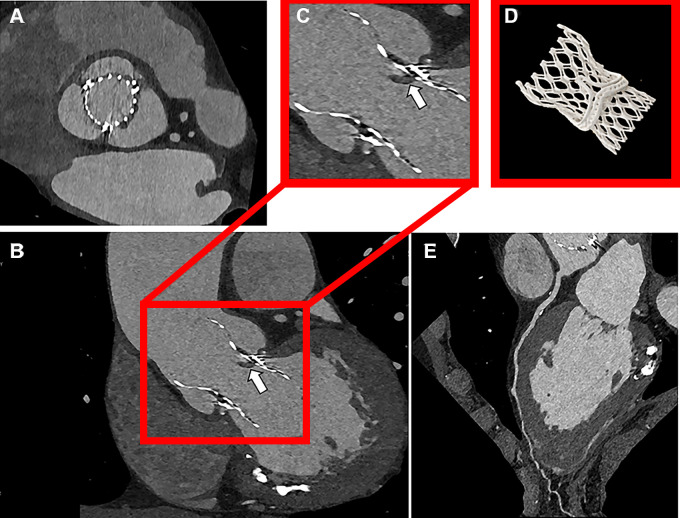
An 85-year-old female patient with a SAPIEN 3 Ultra valve and stent (SYNERGY MEGATRON 5 × 16 mm; Boston Scientific) in the left main coronary artery underwent UHR PCD CT with retrospective electrocardiographic gating (120 kV; image quality level, 60; CTDIvol, 58 mGy). The patient was scanned due to suspected periprosthetic valve leakage; however, no evident leakage was found at PCD CT. UHR imaging visualized both the valve and stent with minimal artifacts (Fig 4A). UHR imaging allowed assessment of the left main stent, demonstrating the absence of in-stent thrombus or intima hyperplasia. With UHR imaging, it was also possible to recognize a large coronary calcification causing incomplete stent expansion, leading to focal narrowing (Fig 4B–4D).
Figure 4:
Contrast-enhanced photon-counting detector CT images in an 85-year-old female patient following transcatheter aortic valve replacement with a stent in the left main coronary artery. (A) Ultra-high-resolution (UHR) image of the valve and stent (axial plane). UHR image shows (B) incomplete stent expansion (arrow) with (C, D) axial reconstructions perpendicular to the stent axis. QIR = quantitative iterative reconstruction.
Case 5
A 72-year-old male patient with a valve-in-valve TAVR was scanned with a dual-source UHR PCD CT scanner with full heart cycle retrospective electrocardiographic gating at 120 kV (image quality level, 65; CTDIvol, 61.5 mGy) to assess valve function (Fig 5). Degeneration of the previous surgically implanted perimount bioprosthesis required subsequent TAVR with a CoreValve Evolut R valve. Images were acquired with UHR mode and reconstructed with a Bv56 kernel with quantum iterative reconstruction strength 4 (Fig 5A). Evident but limited thickening of one valve leaflet was observed, representing hypoattenuating leaflet thickening (white arrow) without a reduction in leaflet motion (Fig 5B). Both the surgical valve and the transcatheter heart valve frames were sharply depicted (Fig 5B, 5C). The three-dimensional rendered image showed the valve-in-valve configuration (Fig 5D). Coronary artery calcifications were well-defined with sharp edges (Fig 5E).
Figure 5:
Ultra-high-resolution (UHR) photon-counting detector CT images show valve-in-valve transcatheter aortic valve replacement with hypoattenuated leaflet thickening in a 72-year-old male patient scanned with contrast medium. (A) Axial UHR image (Bv56 kernel, quantum iterative reconstruction strength 4). (B) CT image shows hypoattenuating leaflet thickening (white arrow). (B, C) Surgical valve and transcatheter heart valve frames are sharply depicted in the sagittal plane. (D) Three-dimensional volume-rendered image. (E) Coronary artery calcifications in left anterior descending artery (multiplanar reformatted images).
Discussion
This case series demonstrates possible applications of dual-source PCD CT in ex vivo valves and four patients prior to and post-TAVR. PCD CT has the potential to be a rapidly emerging modality in cardiovascular imaging, primarily due to its higher spatial resolution and spectral capabilities (2). The dual-source design of the PCD CT scanner used in this case series ensures that a high temporal resolution was maintained (2).
The increased spatial resolution of PCD CT compared with conventional imaging reduces blooming artifacts of calcifications, as demonstrated in cases 2, 4, and 5. The sharp delineation can improve calcium quantification not only of the aortic valve but also of the coronary arteries. Furthermore, the reduced calcium blooming is expected to result in more accurate coronary stenosis grading at CTA. This may be especially useful in the TAVR population, as these older patients often have more extensive calcifications. Reliably ruling out significant coronary stenosis prior to TAVR may eliminate the need for invasive coronary angiography. The more detailed depiction of plaque could also contribute to improved characterization of plaque components (6). These potential benefits of PCD CT need to be fully explored and confirmed in more clinical studies.
An accurate assessment of access routes is crucial for pre-TAVR planning. Case 2 demonstrates poor visibility of the femoral artery, the most common access route for TAVR, because of bilateral hip prostheses (7). The advanced metal artifact reduction capabilities allow for a substantial reduction of the streak artifacts and facilitate accurate TAVR planning for this patient (8,9). Case 5 shows the feasibility of complex prosthetic valve PCD CT imaging after valve-in-valve TAVR. Detailed stent frame imaging without artifacts allows for assessment of valve placement and movement of the valve leaflets. Visualizing stents with conventional CT is troublesome not only because of metal artifacts but also due to blooming artifacts of the surrounding calcifications (10). Case 4 features a stent in the left anterior descending artery with an assessable in-stent lumen, creating opportunities to evaluate in-stent restenosis with PCD CT. Case 3 demonstrates multiple spectral reconstructions with PCD CT and their clinical applications. Spectral maps such as iodine maps and virtual noncontrast reconstructions can detect the absence or presence of contrast agents and calcifications (2). In patients with TAVR, this can be helpful to evaluate periprosthetic valve leakage or to separate the signal of valve calcifications from iodine-infused blood. VMIs can enhance the contrast between different tissues and contrast agents, which can aid in visualization of leaflets, vessel patency, and tissue perfusion. VMIs enable enhanced contrast for iodine at low kiloelectron voltage and seemingly less blooming of the valve frame at high kiloelectron voltage and can be used to administer less contrast agent to patients without compromising image quality (11).
To summarize, dual-source PCD CT may play an important role in the imaging of patients prior to and post-TAVR. This initial case series demonstrates the advantages of high spatial resolution for imaging of the aortic valve, aortic root, coronary arteries, and potential vascular access routes. The visualization of leaflets and stents may be improved by the spectral reconstruction possibilities of PCD CT, such as VMIs and metal artifact reduction. Altogether, PCD CT might improve the planning and follow-up of patients undergoing TAVR, but these potential improvements need to be assessed in larger clinical studies.
J.v.d.B. and S.P.S. contributed equally to this work.
Authors declared no funding for this work.
Abbreviations:
- CTA
- CT angiography
- CTDIvol
- volume CT dose index
- PCD CT
- photon-counting detector CT
- TAVR
- transcatheter aortic valve replacement
- UHR
- ultra high resolution
- VMI
- virtual monoenergetic image
References
- 1. Francone M , Budde RPJ , Bremerich J , et al . CT and MR imaging prior to transcatheter aortic valve implantation: standardisation of scanning protocols, measurements and reporting-a consensus document by the European Society of Cardiovascular Radiology (ESCR) . Eur Radiol 2020. ; 30 ( 5 ): 2627 – 2650 . [Published correction appears in Eur Radiol 2020;30(7):4143-4144.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Willemink MJ , Persson M , Pourmorteza A , Pelc NJ , Fleischmann D . Photon-counting CT: Technical Principles and Clinical Prospects . Radiology 2018. ; 289 ( 2 ): 293 – 312 . [DOI] [PubMed] [Google Scholar]
- 3. Flohr T , Petersilka M , Henning A , Ulzheimer S , Ferda J , Schmidt B . Photon-counting CT review . Phys Med 2020. ; 79 : 126 – 136 . [DOI] [PubMed] [Google Scholar]
- 4. Leng S , Bruesewitz M , Tao S , et al . Photon-counting Detector CT: System Design and Clinical Applications of an Emerging Technology . RadioGraphics 2019. ; 39 ( 3 ): 729 – 743 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rajendran K , Petersilka M , Henning A , et al . Full field-of-view, high-resolution, photon-counting detector CT: technical assessment and initial patient experience . Phys Med Biol 2021. ; 66 ( 20 ): 205019 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mergen V , Eberhard M , Manka R , Euler A , Alkadhi H . First in-human quantitative plaque characterization with ultra-high resolution coronary photon-counting CT angiography . Front Cardiovasc Med 2022. ; 9 : 981012 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Biasco L , Ferrari E , Pedrazzini G , et al . Access Sites for TAVI: Patient Selection Criteria, Technical Aspects, and Outcomes . Front Cardiovasc Med 2018. ; 5 : 88 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou W , Bartlett DJ , Diehn FE , et al . Reduction of Metal Artifacts and Improvement in Dose Efficiency Using Photon-Counting Detector Computed Tomography and Tin Filtration . Invest Radiol 2019. ; 54 ( 4 ): 204 – 211 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Richtsmeier D , O’Connell J , Rodesch PA , Iniewski K , Bazalova-Carter M . Metal artifact correction in photon-counting detector computed tomography: metal trace replacement using high-energy data . Med Phys 2023. ; 50 ( 1 ): 380 – 396 . [DOI] [PubMed] [Google Scholar]
- 10. Boccalini S , Si-Mohamed SA , Lacombe H , et al . First In-Human Results of Computed Tomography Angiography for Coronary Stent Assessment With a Spectral Photon Counting Computed Tomography . Invest Radiol 2022. ; 57 ( 4 ): 212 – 221 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Emrich T , O’Doherty J , Schoepf UJ , et al . Reduced Iodinated Contrast Media Administration in Coronary CT Angiography on a Clinical Photon-Counting Detector CT System: A Phantom Study Using a Dynamic Circulation Model . Invest Radiol 2023. ; 58 ( 2 ): 148 – 155 . [DOI] [PubMed] [Google Scholar]