Abstract
Arrhythmogenic cardiomyopathy (ACM) is a heart muscle disorder that cannot be explained by ischemic, hypertensive, or valvular heart disease and often results in sudden cardiac death. Arrhythmogenic right ventricular cardiomyopathy (ARVC) is the best-characterized ACM and can be diagnosed using the revised task force criteria. In contrast, there are no accepted clinical diagnostic criteria for arrhythmogenic left ventricular cardiomyopathy (ALVC), another subtype of ACM. Cardiac MRI aids in ARVC diagnosis by delineating biventricular structural and functional abnormalities and can be instrumental in diagnosing ALVC. This report presents a pediatric case of desmoplakin cardiomyopathy, a distinct subtype of ALVC, with findings overlapping myocarditis and LV noncompaction.
Keywords: Pediatrics, Heart, Cardiomyopathies
Supplemental material is available for this article.
© RSNA, 2023
Keywords: Pediatrics, Heart, Cardiomyopathies
Key Points
■ The subtypes of arrhythmogenic cardiomyopathy (ACM) can be difficult to diagnose and differentiate from one another and may have overlapping features with other diseases, such as myocarditis. The task force criteria are insufficient for diagnosing certain subtypes of ACM, particularly those that predominantly involve the left ventricle.
■ Desmoplakin (DSP) cardiomyopathy is a distinct subtype of ACM that predominantly involves the left ventricle and may manifest with a clinical picture that mimics myocarditis.
■ Cardiac MRI can be helpful in the identification and prognostication of DSP cardiomyopathy by delineating biventricular function and the pattern of myocardial fibrosis and edema, but findings may be confused with acute myocarditis.
Introduction
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is the best-characterized arrhythmogenic cardiomyopathy (ACM). It is a genetically determined disease with autosomal dominant inheritance that is characterized by fibrofatty replacement of the myocardium and often results in sudden cardiac death (1,2). Identification of gene mutations linked to ARVC, as well as advancements in cardiac MRI with late gadolinium enhancement (LGE), have aided in detecting disease in probands and screening at-risk family members (3) and have provided insight into the underlying genetic abnormalities and mechanisms of ARVC (4–8). Consequently, the revised task force criteria, which incorporate structural, functional, electrophysiologic, and genetic findings (3), are very useful in diagnosing ARVC (9). In contrast, there are no accepted diagnostic criteria for arrhythmogenic left ventricular cardiomyopathy (ALVC), with diagnosis aided by documenting arrhythmia of isolated or predominantly LV origin in patients with cardiomyopathy not caused by ischemic, valvular, or hypertensive heart disease. Cardiac MRI can also be a useful tool in diagnosing ALVC, particularly in the subtype caused by mutations in the desmoplakin (DSP) gene.
We report a distinct case of ALVC that manifested in fibrotic and inflammatory form, mimicking myocarditis, and that showed phenotypic overlap with LV noncompaction (LVNC).
Case Report
A 13-year-old female patient who was admitted to the psychiatric unit had right axis deviation and T-wave inversion in the lateral leads on an electrocardiogram obtained prior to stimulant therapy. Prior medical history revealed intermittent history of chest pain and an isolated episode of syncope 1 month prior to presentation. The echocardiogram showed LV hypertrabeculation and moderate LV systolic dysfunction. Cardiac biomarkers were abnormal, including elevated N-terminal pro–B-type natriuretic peptide (NT-proBNP) level of 1740 pg/mL and troponin I peak level of 0.14 ng/mL.
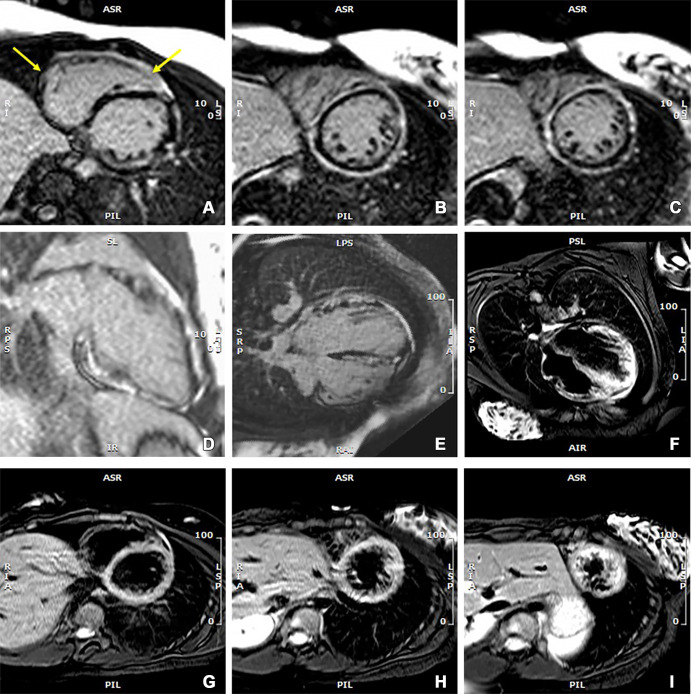
At cardiac MRI, cine imaging in multiple planes using a steady-state free precession sequence (Movie) showed a dilated LV with global hypokinesis, which was most pronounced at the apical half of the ventricle where myocardial noncompaction was also present (noncompacted-to-compacted ratio, 4.2). LV systolic function was moderately impaired (LV ejection fraction [LVEF], 43%). T2-weighted imaging demonstrated patchy edema in the LV myocardium (Figure).
Images from cardiac MRI that were obtained at initial patient presentation when there was a spike in troponin I and NT-proBNP levels. Late gadolinium enhancement (LGE) images in (A–C) short-axis, (D) vertical long-axis, and (E) horizontal long-axis views show diffuse and circumferential LGE in the subepicardial myocardium of the left ventricle and patchy enhancement in the right ventricular myocardium (arrows, A). The images also show patchy and scattered LGE extending into the midmyocardium, which is less intense than the subepicardial enhancement. (F) Horizontal long-axis T2-weighted image and (G–I) short-axis images at three ventricular levels with suppression of blood and fat signals show edema in biventricular myocardium and pericardium that is most visible in the left ventricle. About 55% of the left ventricular myocardium showed LGE in reference to a signal intensity threshold of 5 SDs above the mean in normal myocardium. NT-proBNP = N-terminal pro–B-type natriuretic peptide.
Movie 1a:
(A, B) Short-axis (B, near apex), (C) horizontal long-axis, and (D) vertical long-axis cine MRI sections obtained using a steady-state free precession sequence at initial presentation show dilatation and systolic dysfunction of the left ventricle (LV), along with hypertrabeculation that was consistent with LV noncompaction. LV ejection fraction was 43%, and LV end-diastolic volume index was 113 mL/m2. The right ventricle (RV) was normal in size and had low normal systolic function (RV ejection fraction: 49%).
Movie 1b:
(A, B) Short-axis (B, near apex), (C) horizontal long-axis, and (D) vertical long-axis cine MRI sections obtained using a steady-state free precession sequence at initial presentation show dilatation and systolic dysfunction of the left ventricle (LV), along with hypertrabeculation that was consistent with LV noncompaction. LV ejection fraction was 43%, and LV end-diastolic volume index was 113 mL/m2. The right ventricle (RV) was normal in size and had low normal systolic function (RV ejection fraction: 49%).
Movie 1c:
(A, B) Short-axis (B, near apex), (C) horizontal long-axis, and (D) vertical long-axis cine MRI sections obtained using a steady-state free precession sequence at initial presentation show dilatation and systolic dysfunction of the left ventricle (LV), along with hypertrabeculation that was consistent with LV noncompaction. LV ejection fraction was 43%, and LV end-diastolic volume index was 113 mL/m2. The right ventricle (RV) was normal in size and had low normal systolic function (RV ejection fraction: 49%).
Movie 1d:
(A, B) Short-axis (B, near apex), (C) horizontal long-axis, and (D) vertical long-axis cine MRI sections obtained using a steady-state free precession sequence at initial presentation show dilatation and systolic dysfunction of the left ventricle (LV), along with hypertrabeculation that was consistent with LV noncompaction. LV ejection fraction was 43%, and LV end-diastolic volume index was 113 mL/m2. The right ventricle (RV) was normal in size and had low normal systolic function (RV ejection fraction: 49%).
LGE imaging with a T1-weighted inversion-recovery gradient-echo sequence after intravenous administration of 0.1 mmol of gadobenate dimeglumine per kilogram of body weight showed diffuse circumferential subepicardial LGE in the LV, as well as scattered patchy enhancement in the midmyocardial wall without subendocardial involvement. Patchy edema and LGE were also present in the free wall and interventricular septum of the RV (Figure). Patchy enhancement of the pericardium was also observed.
A working diagnosis of acute myocarditis superimposed on cardiomyopathy was established, and treatment was initiated. Myocarditis workup was inconclusive for a viral cause and was negative for enterovirus, coxsackievirus A, influenza A and B, parvovirus, and SARS-CoV-2 infections. Cytomegalovirus and Epstein-Barr virus antibody levels were suggestive of recovered infection. Workup for bacterial infection was negative, and blood cultures did not reveal any microorganism growth.
During the hospital stay, the patient experienced intermittent substernal chest pain and developed frequent multifocal premature ventricular contractions (PVCs) and an episode of nonsustained ventricular tachycardia (NSVT), requiring β-blocker treatment. After discharge from the hospital, symptoms persisted, and Holter monitoring showed PVCs and NSVT.
Paternal family history was not available, but the mother had cardiac disease as a teenager, without medical follow-up. Additionally, there was a recent death of a second-degree relative who had a history of intravenous drug use and possible cardiomyopathy. Genetic workup of our patient revealed a heterozygous pathogenic variant in DSP due to deletion of the entire coding sequence, which is associated with autosomal dominant ACM. To our knowledge, no other family members have undergone genetic testing.
A few months later, the patient had another episode of chest pain accompanied by a transient increase in troponin level.
Discussion
We presented a case with clinical and imaging findings of acute myocarditis and suspected ALVC. Myocarditis may be a feature of ALVC and may even represent the first manifestation (10,11), which likely has a genetic basis (12). Some subtypes are associated with mutations in genes encoding desmosomal proteins (7). Mutations in the gene encoding the DSP protein, for example, are associated with DSP cardiomyopathy, a subtype of ALVC. DSP is a protein that links desmosomes and intermediate filaments and plays an essential role in maintenance of biomechanical and functional integrity of the myocardium (13,14). Impaired desmosomal integrity may expose cardiomyocytes to loss of cell-cell adhesions and consequent cell death, causing repair and remodeling with fatty and fibrofatty replacement (7). Patients with DSP gene mutations may develop a distinctive phenotype with heightened susceptibility to ventricular arrhythmias and often present with episodes of chest pain and acute myocarditis, ALVC, systolic dysfunction, subepicardial LV fibrosis, and arrhythmias (5,6,13,15–17). About 65% of patients with DSP cardiomyopathy develop acute myocardial injury that can be recurrent (18). Similar to our case, ALVC can initially manifest as acute myocarditis (11), with evidence of ongoing myocardial injury, and manifests at cardiac MRI as myocardial edema, systolic dysfunction, and patchy or diffuse myocardial LGE, often within the subepicardium, with concomitant pericardial involvement (19).
Half of all patients with DSP cardiomyopathy show LGE in the LV, which is most commonly subepicardial (72%) but can also be midmyocardial and, rarely, subendocardial or patchy in distribution, with a predilection for the inferior, lateral, and septal walls and basilar portions of LV (13,18,20). LGE is most commonly observed in individuals with myocardial injury and in those with LVEF less than 50% (18). Circumferential subepicardial LGE may also be present, as in our case (13). Intramyocardial fat can be observed in DSP cardiomyopathy, but LGE and decreased LVEF are the most sensitive indicators of LV involvement in cases of ARVC (5,8,13,21).
Congruent with the common phenotype of DSP cardiomyopathy, cardiac MRI did not demonstrate any findings meeting any major or minor diagnostic criteria for ARVC (Movie). However, it showed an advanced left-predominant phenotype (ie, ALVC), a subtype of ACM (12,22,23). While the task force criteria have limited sensitivity in diagnosing the LV-predominant type (13), patients with DSP cardiomyopathy who meet the criteria, as in our case, experience a higher incidence of sustained ventricular tachycardia and heart failure (18). A subset of patients (14%) with DSP mutation may have phenotypic expression as RV-predominant cardiomyopathy (13,18).
In DSP cardiomyopathy, LVEF less than 35% and RV dysfunction, which can be reliably assessed with cardiac MRI, are independently associated with risk of VT; however, LVEF less than 35% is insufficient in capturing all individuals at risk for severe ventricular arrhythmia (13), as arrhythmia may also develop with LVEFs ranging from 35% to 55% and even with normal LVEF. LV fibrosis may independently contribute to risk prediction in DSP cardiomyopathy (13). Both ALVC and dilated cardiomyopathy may have midwall LGE, but subepicardial distribution favors ALVC (12). The degree of morphologic abnormality and systolic dysfunction in ALVC causes a disproportionately large predisposition to ventricular arrhythmia (12).
LVNC is also an inherited genetic cardiomyopathy, which may coexist with ACM, dilated cardiomyopathy, and hypertrophic cardiomyopathy (24–26). Mutations in several genes, including the gene encoding the intermediate filament protein desmin, are associated with LVNC (27,28). It is uncertain if LVNC in our patient is an overlapping phenotype due to mutations in the DSP gene and other gene(s), or a sequela of malfunctioning DSP protein.
In conclusion, DSP cardiomyopathy is a distinct subtype of ACM that most commonly manifests as the ALVC phenotype, which currently lacks adequate diagnostic and prognostic criteria. This subtype should be distinguished from other types of ACM and dilated cardiomyopathy. Patients with DSP cardiomyopathy may present with clinical, laboratory, and cardiac MRI findings that may be indistinguishable from acute myocarditis. Diffuse or segmental subepicardial LGE pattern is often present at cardiac MRI, with additional patchy midwall foci that may demonstrate temporal inhomogeneity. Molecular genetic evaluation is essential in establishing the diagnosis of DSP cardiomyopathy. Recent research suggests that these patients may be at high risk for severe future adverse events, which may affect not only patients with severe LV dysfunction but also those with mildly impaired and even normal LV systolic function.
Footnotes
Authors declared no funding for this work.
Disclosures of conflicts of interest: G.M. No relevant relationships. S.V. No relevant relationships. R.A. No relevant relationships.
References
- 1. Poloni G , De Bortoli M , Calore M , Rampazzo A , Lorenzon A . Arrhythmogenic right-ventricular cardiomyopathy: molecular genetics into clinical practice in the era of next generation sequencing . J Cardiovasc Med (Hagerstown) 2016. ; 17 ( 6 ): 399 – 407 . [DOI] [PubMed] [Google Scholar]
- 2. Basso C , Corrado D , Marcus FI , Nava A , Thiene G . Arrhythmogenic right ventricular cardiomyopathy . Lancet 2009. ; 373 ( 9671 ): 1289 – 1300 . [DOI] [PubMed] [Google Scholar]
- 3. Marcus FI , McKenna WJ , Sherrill D , et al . Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria . Eur Heart J 2010. ; 31 ( 7 ): 806 – 814 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smith EA , Fuchs E . Defining the interactions between intermediate filaments and desmosomes . J Cell Biol 1998. ; 141 ( 5 ): 1229 – 1241 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Norman M , Simpson M , Mogensen J , et al . Novel mutation in desmoplakin causes arrhythmogenic left ventricular cardiomyopathy . Circulation 2005. ; 112 ( 5 ): 636 – 642 . [DOI] [PubMed] [Google Scholar]
- 6. Sen-Chowdhry S , Syrris P , Prasad SK , et al . Left-dominant arrhythmogenic cardiomyopathy: an under-recognized clinical entity . J Am Coll Cardiol 2008. ; 52 ( 25 ): 2175 – 2187 . [DOI] [PubMed] [Google Scholar]
- 7. Vimalanathan AK , Ehler E , Gehmlich K . Genetics of and pathogenic mechanisms in arrhythmogenic right ventricular cardiomyopathy . Biophys Rev 2018. ; 10 ( 4 ): 973 – 982 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen L , Song J , Chen X , et al . A novel genotype-based clinicopathology classification of arrhythmogenic cardiomyopathy provides novel insights into disease progression . Eur Heart J 2019. ; 40 ( 21 ): 1690 – 1703 . [DOI] [PubMed] [Google Scholar]
- 9. Towbin JA , McKenna WJ , Abrams DJ , et al . 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy . Heart Rhythm 2019. ; 16 ( 11 ): e301 – e372 . [DOI] [PubMed] [Google Scholar]
- 10. Shen MT , Yang ZG , Diao KY , et al . Left ventricular involvement in arrhythmogenic right ventricular dysplasia/cardiomyopathy predicts adverse clinical outcomes: a cardiovascular magnetic resonance feature tracking study . Sci Rep 2019. ; 9 ( 1 ): 14235 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. He J , Xu J , Li G , et al . Arrhythmogenic left ventricular cardiomyopathy: a clinical and CMR Study . Sci Rep 2020. ; 10 ( 1 ): 533 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yelgec NS , Dymarkowski S , Ganame J , Bogaert J . Value of MRI in patients with a clinical suspicion of acute myocarditis . Eur Radiol 2007. ; 17 ( 9 ): 2211 – 2217 . [DOI] [PubMed] [Google Scholar]
- 13. Smith ED , Lakdawala NK , Papoutsidakis N , et al . Desmoplakin cardiomyopathy, a fibrotic and inflammatory form of cardiomyopathy distinct from typical dilated or arrhythmogenic right ventricular cardiomyopathy . Circulation 2020. ; 141 ( 23 ): 1872 – 1884 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mueller H , Franke WW . Biochemical and immunological characterization of desmoplakins I and II, the major polypeptides of the desmosomal plaque . J Mol Biol 1983. ; 163 ( 4 ): 647 – 671 . [DOI] [PubMed] [Google Scholar]
- 15. López-Ayala JM , Gómez-Milanés I , Sánchez Muñoz JJ , et al . Desmoplakin truncations and arrhythmogenic left ventricular cardiomyopathy: characterizing a phenotype . Europace 2014. ; 16 ( 12 ): 1838 – 1846 . [DOI] [PubMed] [Google Scholar]
- 16. Gigli M , Merlo M , Graw SL , et al . Genetic risk of arrhythmic phenotypes in patients with dilated cardiomyopathy . J Am Coll Cardiol 2019. ; 74 ( 11 ): 1480 – 1490 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ghawanmeh M , Simon Frances B , Kerai A , Patel P , Du J , Kumar P . Management of recurrent myocarditis due to desmoplakin cardiomyopathy: diagnostic and therapeutic challenges . JACC Case Rep 2022. ; 4 ( 1 ): 59 – 62 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang W , Murray B , Tichnell C , et al . Clinical characteristics and risk stratification of desmoplakin cardiomyopathy . Europace 2022. ; 24 ( 2 ): 268 – 277 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kohler L , Puertollano R , Raben N . Pompe Disease: from basic science to therapy . Neurotherapeutics 2018. ; 15 ( 4 ): 928 – 942 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Georgiopoulos G , Zampieri M , Molaro S , et al . Cardiac magnetic resonance in patients with ARVC and family members: the potential role of native T1 mapping . Int J Cardiovasc Imaging 2021. ; 37 ( 6 ): 2037 – 2047 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rastegar N , Burt JR , Corona-Villalobos CP , et al . Cardiac MR findings and potential diagnostic pitfalls in patients evaluated for arrhythmogenic right ventricular cardiomyopathy . RadioGraphics 2014. ; 34 ( 6 ): 1553 – 1570 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suzuki H , Sumiyoshi M , Kawai S , et al . Arrhythmogenic right ventricular cardiomyopathy with an initial manifestation of severe left ventricular impairment and normal contraction of the right ventricle . Jpn Circ J 2000. ; 64 ( 3 ): 209 – 213 . [DOI] [PubMed] [Google Scholar]
- 23. Bauce B , Basso C , Rampazzo A , et al . Clinical profile of four families with arrhythmogenic right ventricular cardiomyopathy caused by dominant desmoplakin mutations . Eur Heart J 2005. ; 26 ( 16 ): 1666 – 1675 . [DOI] [PubMed] [Google Scholar]
- 24. Arbustini E , Favalli V , Narula N , Serio A , Grasso M . Left ventricular noncompaction: a distinct genetic cardiomyopathy? . J Am Coll Cardiol 2016. ; 68 ( 9 ): 949 – 966 . [Published correction appears in J Am Coll Cardiol 2016;68(16):1821.] [DOI] [PubMed] [Google Scholar]
- 25. Ayesha B , Ahmed R , Gomceli U , Manrique C , Nicu M , Chilimuri S . A case of isolated left ventricular non-compaction cardiomyopathy in a hiv patient presenting with acute heart failure . Cardiol Res 2019. ; 10 ( 4 ): 236 – 240 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ross SB , Singer ES , Driscoll E , et al . Genetic architecture of left ventricular noncompaction in adults . Hum Genome Var 2020. ; 7 ( 1 ): 33 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Waning JI , Moesker J , Heijsman D , Boersma E , Majoor-Krakauer D . Systematic review of genotype-phenotype correlations in noncompaction cardiomyopathy . J Am Heart Assoc 2019. ; 8 ( 23 ): e012993 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kulikova O , Brodehl A , Kiseleva A , et al . The Desmin (DES) mutation p.A337P is associated with left-ventricular non-compaction cardiomyopathy . Genes (Basel) 2021. ; 12 ( 1 ): 121 . [DOI] [PMC free article] [PubMed] [Google Scholar]