Abstract
Irisin is an exercise-induced cytokine mainly secreted by myocytes. Circulating level of irisin can increase in response to acute exercise, promoting pleiotropic effects on health. Generally, irisin is evaluated in blood, however, its collection is invasive. Saliva sample would not have any risk associated with blood collection and would represent a less invasive method for irisin detection. Until now, there are only a few studies that have analyzed irisin levels in saliva. In the present research, five healthy male adults performed an incremental exercise until exhaustion on cycle ergometer. Serum and saliva samples were collected before exercise and 15min, 24h and 48h after reaching the exhaustion. Irisin was detected by ELISA assay. Serum and salivary irisin levels increased from baseline to 24h post exercise and reverted to basal levels after 48h of rest. A significant rise of both serum and salivary irisin level at 24h (p≤0.05) compared to baseline levels was found. Furthermore, a significant correlation between irisin percentage change in serum and saliva from baseline to 24h post exercise was detected (r=0.92, p<0.05). Despite the relatively limited sample, this research suggests that collecting saliva samples might represent a valid and less invasive method to detect irisin level changes induced by exercise.
Key Words: Irisin, saliva, serum, muscle, physical exercise
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Ten years ago, a novel myokine called irisin, usuallysecreted by exercising muscles, was discovered.1 Physical exercise induces an increase of the peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) that determines Fibronectin type III domain-containing protein 5 (FNDC5) expression. Irisin is obtained through a proteolytic cleavage of FNDC5 protein and it is released into the bloodstream.1 Primary roles of irisin include browning white adipose tissue, improving liver and systemic glucose metabolism, keeping bone and musculoskeletal homeostasis, and playing a neuroprotective function (Figure 1).2 This myokine modulates muscle cells activity in an autocrine or paracrine manner.3 In vitro studies demonstrated the ability of irisin to stimulate the expression of specific mitochondrial transcription factors, resulting in mitochondrial content and oxygen consumption increase. Moreover, irisin can play a regulatory role in muscle growth and differentiation. Indeed, it stimulates muscle regeneration and hypertrophy through the activation of satellite cells, and promotes the improvement of muscle function, mass and healing.2,4-6
Irisin is generally analyzed in blood,7 the best body fluid for evaluation of different biomarkers.8 However, blood collection exposes to potential risks the individuals, including discomfort of needle, infection at the venipuncture site, and bruising. Moreover, it is also less preferred in research involving children, elderly, as well as patients for whom venous access is not easy. As a result, an alternative method for irisin detection appears desirable. Collection of saliva holds several advantages, such as being a noninvasive, easy, safe, and cost-effective procedure that can be applied to large populations compared to conventional venipuncture procedure and requires no special qualified personnel. In addition, participants’ acceptance of saliva sample collection might be much better when compared to obtaining serum or biopsy samples.
Fig 1.
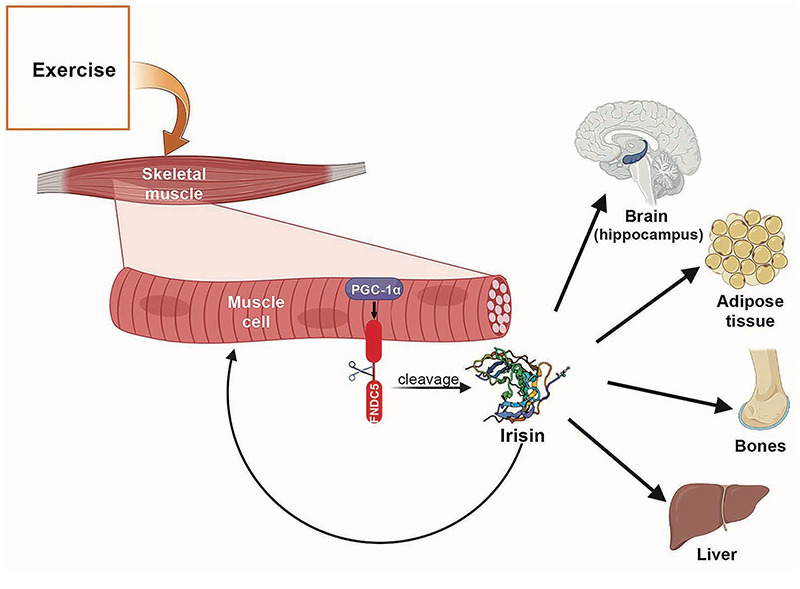
In muscle cells exercise enhances the production of PGC-1α which promotes FNDC5 gene expression. FNDC5 membrane protein is cleaved to generate irisin. This myokine can act locally, in an autocrine/paracrine manner or in a hormone-like way when released into the bloodstream. In muscles, irisin stimulates regeneration and hypertrophy. Moreover, irisin has multi-spectrum functions on various tissues or organs: improves mitochondria function and enhances synaptic plasticity through BDNF signaling in central nervous system; promotes “browning” of mature white adipocytes in response to exercise; promotes osteogenic differentiation; influences the function of pancreas and liver
Saliva is the product of secretion from the salivary glands.9 Moreover, it is known that some molecules enter the saliva from the blood by passing through the intercellular spaces using a passive diffusion process, filtration, or active transport.10 These molecules, such as miRNAs, can be used as diagnostic biomarkers of tumors, autism, Parkinson disease and sleep disorder.11
Only a few studies have investigated the irisin level in saliva.12-17 Findings have shown that this myokine is present in a different concentration over blood. Despite this, saliva sample seems to represent a valid biological specimen for irisin detection. The limited number of studies, measuring irisin in saliva, present many limitations regarding the collection and analysis of samples. Indeed, there are some variables that can significantly influence the results, like the method of collection as well as the degree of stimulation.18 Moreover, as the consistency of saliva may vary within a person and between people,19 this aspect needs to be considered when analyzing irisin and validating a possible method for exercise-induced cytokine detection. Finally, several commercial kits for irisin detection are available, nevertheless, not every kit is extremely sensitive and capable of detecting irisin with high specificity.13
In this study we analyzed the irisin levels in saliva and serum samples obtained from healthy adults before and after an exercise protocol. Moreover, we verified whether there was a correlation between the level changes of salivary and serum irisin in these subjects.
Materials and Methods
Five healthy males ages 20-55 years, mean body mass index (BMI) 22.9±3.7 kg/m2, peak of oxygen consumption 54.0±16.5 mL/kg/min were recruited.
Procedures of present study was approved by the Ethics Commission of the Department of Psychology (CERPS) of the Catholic University of the Sacred Heart of Milan and written informed consent for study participation, permission for personal data treatment and biochemical analysis was obtained from all participants upon enrolment. This study was conducted in accordance with the ethical standards of the responsible committee on human experimentation and the ethical principles established in the Declaration of Helsinki of 1975, as revised in 2013.
Anthropometric parameters included weight and height measurement, following the procedures described by Gordon et al.,20 and the calculation of BMI.
Participants were asked to arrive in the laboratory after 2 h fast and 48 h of rest. All the participants performed an incremental exercise until exhaustion on a cycle ergometer. The exercise took place in a room with a relative humidity less than 60% and a temperature ranging from 18°C to 22°C.
To avoid the influence of circadian variation, assessments took place at the same time of the day, from 9:00 a.m. to 1:00 p.m. Four samples of peripheral venous blood (3 mL each one) and saliva (1.5 mL each one) were drawn simultaneously at each sampling time. First samples were collected at baseline, second samples were obtained 15 min after reaching the exhaustion during the maximal exercise test, third samples were taken approximately 24 h after and the last samples were collected after 48 h of rest. Vacutainers were drawn using a vacutainer system and were immediately centrifuged at 10,000 rpm for 10 min. Soon after, serum was separated and stored at -80°C for subsequent analysis. After the oral rinse with water, participants were asked to drool into a sterile Eppendorf tube. Saliva samples were quickly centrifuged at 10,000 rpm for 2 min and kept at -80°C. In saliva samples, protein concentration was quantified using Bradford Assay (Bio-Rad). Total protein amount was used to normalize the irisin concentration in saliva samples. Serum and saliva irisin levels were detected by ELISA (Irisin kit: Cat. EK-067-29, Phoenix Pharmaceuticals), according to the manufacturer instructions, using the Victor Nivo multimode plate reader (PerkinElmer). Inter-assay variation for irisin was <10%. SPSS software (Version 27) was used for statistical analyses. The effect of exercise on irisin level was analyzed by non parametric test (Wilcoxon signed-rank test). Spearman’s correlation analysis was used to assess the relationships between serum and saliva irisin percentage change. The limit for statistical significance was always considered p<0.05.
Fig 2.
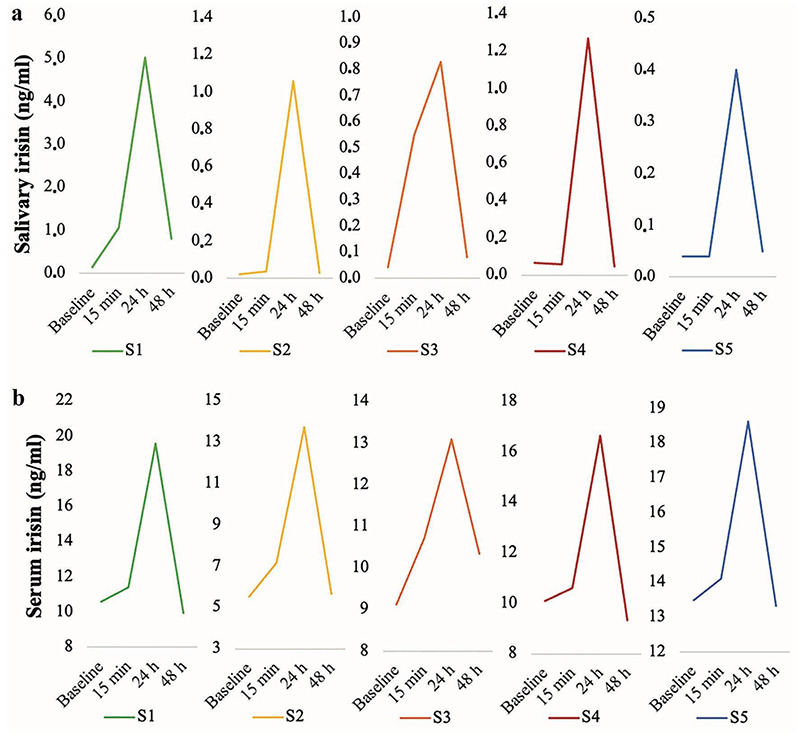
Time course of individual salivary irisin level before and 15 min, 24 h and 48 h post exercise detected in five participants, S1-S5; b) Concentration of serum irisin before and after 15 min, 24 h and 48 h post exercise in five participants.
Results and Discussion
Primary purpose of the current research was to evaluate the effect of an exercise protocol on irisin levels in saliva and serum of healthy adults. We assessed serum and salivary irisin in 5 males before and 15 min, 24 h and 48 h post exercise. Figure 2a and b shows salivary and serum irisin profile of each participant before and after exercise, evidencing that physical activity causes a consistent increase of this myokine. Salivary irisin, nomalized for total protein content, (baseline: 0.06±0.05 ng/ml; 15 min: 0.35±0.45 ng/mL; 24 h: 1.71±1.87 ng/mL) and serum (baseline: 9.77±2.87 ng/mL; 15 min: 10.80±2.48 ng/mL; 24 h: 15.93±2.42 ng/mL) increased from baseline to 24 h post exercise, and then the irisin concentration decreased up to 48 h post exercise (saliva 48 h: 0.20±0.33 ng/mL; serum 48 h: 9.72±2.75 ng/mL). A significant difference of serum irisin levels at 15 min (p≤0.05) and 24 h (p≤0.05), and of salivary irisin levels at 24 h (p≤0.05) compared to baseline levels was detected. Data in Figure 2 also indicates that irisin is more concentrated in serum in comparison with saliva.
Fig 3.
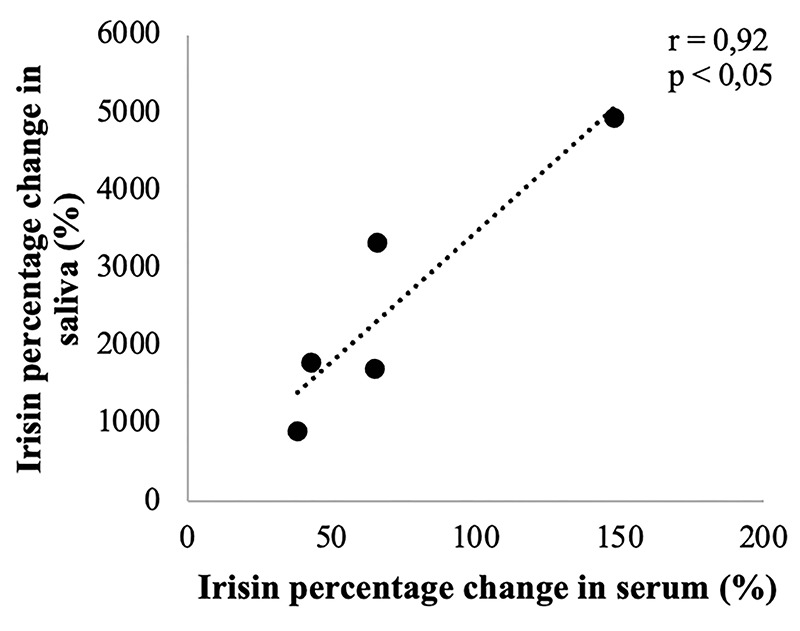
Correlation between irisin percentage change in saliva and serum from baseline to 24 h postexercise in 5 subjects
The second purpose of this study was to ascertain a possible correlation between the expression level of serum and salivary irisin induced by physical exercise. As shown in Figure 3, a significant and positive correlation was found between irisin percentage change in serum and saliva from baseline to 24 h post exercise (r=0.92, p<0.05). Hence, the results reported here show that changes in irisin levels can be detected in both serum and saliva after an incremental exercise. Furthermore, the variations in irisin present the same trend in both biological samples. However, in order to achieve reliable results, we setted up an experimental plan that took into account different variables for the quantification of salivary irisin: i) method of collection; ii) hydration; iii) sensitive and specific method for irisin detection.
It has been demonstrated that the degree of stimulation (active o passive) and the method of collection influence the relative contribution of the different salivary glands to whole saliva and can modify saliva analytes contents.21,22 Ravara et al. pointed out the importance to standardize the methods for collection, processing, and storage of saliva specimens to ameliorate the use of this body fluid in clinical analisys.22 In previous studies researchers indifferently used unstimulated or stimulated methods to obtain whole saliva samples for irisin analysis.12-14,16,17 As recently Jasim et al., by comparing different saliva collection approaches, identified stimulated whole saliva as the simplest and with low variability method for analyses of biomarkers,18 we also collected saliva through a self-stimulated approach.
None of the previous research standardized salivary irisin levels by considering the intra- and interpersonal diversity of total salivary protein amount, like indicator of hydration status. For this reason, contrasting results were reported. Indeed, some studies have revealed that salivary irisin levels were higher than blood levels in healthy adults.12,13 Instead, Bakal and colleagues,15 assessing healthy subjects and patients with acute appendicitis and abdominal pain, have found similar irisin concentration in blood compared to saliva. In another study,14 the total protein concentration in saliva from five Prader Willi Syndrome patients and five healthy controls was previously quantified. However, the amount of proteins has not been considered for standardizing the saliva concentration, despite patients’ saliva was thicker, and total protein concentration was higher compared to that of healthy controls.
Finding from this study revealed that salivary irisin levels in both controls and patients were higher than serum.14 Considering the interpersonal and intrapersonal differences of the saliva consistency, we quantified the total protein concentration in saliva samples, and we related the abundance of the target protein (irisin) to the total protein amount in each sample. This approach takes account of the state of hydration of participants.
There is no consensus for the normal concentration of circulating irisin in humans. This could be due to the use of different ELISA kits, determining enormous variation in irisin quantification. The best method to measure irisin levels is still quantitative mass spectrometry. Nevertheless, it has been shown that some commercial ELISA kits allow to obtain results similar to those of mass spectrometry.23 We have measured irisin levels using an ELISA kit that has been validated by Western blot, which has visualized three bands (15, 20, and 26 kDa), and the amino acid sequence of the molecules isolated from each of the three bands has been verified via mass spectrometry.24
In conclusion, we have focused on the potential role of saliva to detect irisin changes induced by physical exercise. In serum and saliva samples of all assessed participants, increased levels of this myokine 24 h post exercise were observed, which reverted to baseline levels after 48 h of rest. A key strength of the study is the use of a high sensitive and specific kit for irisin detection and the normalization of salivary irisin level with total protein amount within a sample, allowing us to obtain standardized results, not dependent on the different saliva concentration, due to the “personal hydration status“. This study is limited by the relatively small number of subjects involved.
Nevertheless, the finding, while preliminary, suggests that collecting saliva samples might represent a valid method for quantify irisin level changes in response to exercise and can be used especially for frail population or when a time course analysis is required. Future studies conducted on a larger sample size should be conducted to confirm these preliminary observations.
Acknowledgments
The authors are grateful to the partecipants for their kind cooperation and to Alessandro Molinello, Mario Toller and Danny D’Agostini for generous scientific assistance.
List of acronyms
- BMI
body mass index
- CERPS
Ethics Commission of the Department of Psychology
- FNDC5
Fibronectin type III domain - containing protein 5
- PGC1-α
PPARγ-coactivator-1-α
Funding Statement
Funding: This work was supported by Università Cattolica del Sacro Cuore (Italy).
Contributor Information
Sara Missaglia, Email: sara.missaglia@unicatt.it.
Ester Tommasini, Email: ester.tommasini@unicatt.it.
Paola Vago, Email: paola.vago@unicatt.it.
Claudio Pecci, Email: claudio.pecci@mapeisport.it.
Christel Galvani, Email: christel.galvani@unicatt.it.
Andrea Silvestrini, Email: andrea.silvestrini@unicatt.it.
Alvaro Mordente, Email: alvaro.mordente@unicatt.it.
References
- 1.Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Højlund K, Gygi SP, Spiegelman BM. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012. Jan 11;481(7382):463-8. doi: 10.1038/nature10777. PMID: 22237023; PMCID: PMC3522098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu S, Cui F, Ning K, Wang Z, Fu P, Wang D, Xu H. Role of irisin in physiology and pathology. Front Endocrinol (Lausanne). 2022. Sep 26;13:962968. doi: 10.3389/fendo.2022.962968. PMID: 36225200; PMCID: PMC9549367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Momenzadeh S, Jami MS, Jalalvand A, Esfarjani F, Shahabi S, Zamani S. Irisin, A Mediator of Muscle Crosstalk with Other Organs: From Metabolism Regulation to Protective and Regenerative Effects. Curr Protein Pept Sci. 2022;23(2):89-104. doi: 10.2174/1389203723666220217141918. PMID: 35176985. [DOI] [PubMed] [Google Scholar]
- 4.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012. Apr 3;8(8):457-65. doi: 10.1038/nrendo.2012.49. PMID: 22473333. [DOI] [PubMed] [Google Scholar]
- 5.Reza MM, Sim CM, Subramaniyam N, Ge X, Sharma M, Kambadur R, McFarlane C. Irisin treatment improves healing of dystrophic skeletal muscle. Oncotarget. 2017. Oct 6;8(58):98553-98566. doi: 10.18632/oncotarget.21636. PMID: 29228710; PMCID: PMC5716750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reza MM, Subramaniyam N, Sim CM, Ge X, Sathiakumar D, McFarlane C, Sharma M, Kambadur R. Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy. Nat Commun. 2017. Oct 24;8(1):1104. doi: 10.1038/s41467-017-01131-0. PMID: 29062100; PMCID: PMC5653663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox J, Rioux BV, Goulet EDB, Johanssen NM, Swift DL, Bouchard DR, Loewen H, Sénéchal M. Effect of an acute exercise bout on immediate post-exercise irisin concentration in adults: A meta-analysis. Scand J Med Sci Sports. 2018. Jan;28(1):16-28. doi: 10.1111/sms.12904. Epub 2017. May 24. PMID: 28453881. [DOI] [PubMed] [Google Scholar]
- 8.Williamson S, Munro C, Pickler R, Grap MJ, Elswick RK Jr. Comparison of biomarkers in blood and saliva in healthy adults. Nurs Res Pract. 2012;2012:246178. doi: 10.1155/2012/246178. Epub 2012. Apr 30. PMID: 22619709; PMCID: PMC3350846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edgar WM. Saliva: its secretion, composition and functions. Br Dent J. 1992. Apr 25;172(8):305-12. doi: 10.1038/sj.bdj.4807861. PMID: 1591115. [DOI] [PubMed] [Google Scholar]
- 10.Drobitch RK, Svensson CK. Therapeutic drug monitoring in saliva. An update. Clin Pharmacokinet. 1992. Nov;23(5):365-79. doi: 10.2165/00003088-199223050-00003. PMID: 1478004. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida Y, Yajima Y, Fujikura Y, Zhuang H, Higo-Yamamoto S, Toyoda A, Oishi K. Identification of salivary microRNA profiles in male mouse model of chronic sleep disorder. Stress. 2023. Jan;26(1):21-28. doi: 10.1080/10253890.2022.2156783. PMID: 36522611 [DOI] [PubMed] [Google Scholar]
- 12.Aydin S, Aydin S, Kuloglu T, Yilmaz M, Kalayci M, Sahin I, Cicek D. Alterations of irisin concentrations in saliva and serum of obese and normal-weight subjects, before and after 45 min of a Turkish bath or running. Peptides. 2013. Dec;50:13-8. doi: 10.1016/j.peptides.2013.09.011. Epub 2013. Oct 1. PMID: 24096106. [DOI] [PubMed] [Google Scholar]
- 13.Aydin S, Aydin S, Kobat MA, Kalayci M, Eren MN, Yilmaz M, Kuloglu T, Gul E, Secen O, Alatas OD, Baydas A. Decreased saliva/serum irisin concentrations in the acute myocardial infarction promising for being a new candidate biomarker for diagnosis of this pathology. Peptides. 2014. Jun;56:141-5. doi: 10.1016/j.peptides.2014.04.002. Epub 2014. Apr 18. PMID: 24747283. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch HJ, Gross I, Pollak Y, Eldar-Geva T, Gross-Tsur V. Irisin and the Metabolic Phenotype of Adults with Prader-Willi Syndrome. PLoS One. 2015. Sep 3;10(9):e0136864. doi: 10.1371/journal.pone.0136864. PMID: 26334732; PMCID: PMC4559418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bakal U, Aydin S, Sarac M, Kuloglu T, Kalayci M, Artas G, Yardim M, Kazez A. Serum, Saliva, and Urine Irisin with and Without Acute Appendicitis and Abdominal Pain. Biochem Insights. 2016. Jun 15;9:11-7. doi: 10.4137/BCI.S39671. PMID: 27330302; PMCID: PMC4910648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altay DU, Korkmaz M, Ergun S, Korkmaz H, Noyan T. Salivary irisin: potential inflammatory biomarker in recurrent apthous stomatitis patients. Eur Rev Med Pharmacol Sci. 2021. Mar;25(5):2252-2259. doi: 10.26355/eurrev_202103_25257. PMID: 33755963. [DOI] [PubMed] [Google Scholar]
- 17.Khan SU, Ghafoor S, Khaliq S, Syed AR. Salivary Irisin and periodontal clinical parameters in patients of chronic periodontitis and healthy individuals: A novel salivary myokine for periodontal disease. J Pak Med Assoc. 2022. Jan;72(1):27-33. doi: 10.47391/JPMA.01367. PMID: 35099433. [DOI] [PubMed] [Google Scholar]
- 18.Jasim H, Carlsson A, Hedenberg-Magnusson B, Ghafouri B, Ernberg M. Saliva as a medium to detect and measure biomarkers related to pain. Sci Rep. 2018. Feb 19;8(1):3220. doi: 10.1038/s41598-018-21131-4. PMID: 29459715; PMCID: PMC5818517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aro K, Wei F, Wong DT, Tu M. Saliva Liquid Biopsy for Point-of-Care Applications. Front Public Health. 2017. Apr 11;5:77. doi: 10.3389/fpubh.2017.00077. PMID: 28443278; PMCID: PMC5387045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordon CC, Chumlea WC, Roche AF. Stature, recumbent length and weight. In: Lohman TG, Roche AF, Martorell R, eds. Anthropometric standardization reference manual. Champaign, IL: Human Kinetics; 1988. pp 1-5. [Google Scholar]
- 21.Helmerhorst EJ, Oppenheim FG. Saliva: a dynamic proteome. J Dent Res. 2007. Aug;86(8):680-93. doi: 10.1177/154405910708600802. PMID: 17652194. [DOI] [PubMed] [Google Scholar]
- 22.Ravara B, Zampieri S, Kern H, Carraro U. Blood contamination, a problem or a lucky chance to analyze non-invasively Myokines in mouth fluids? Eur J Transl Myol. 2019. Dec 10;29(4):8713. doi: 10.4081/ejtm.2019.8713. PMID: 31908751; PMCID: PMC6926435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polyzos SA, Mantzoros CS. An update on the validity of irisin assays and the link between irisin and hepatic metabolism. Metabolism. 2015. Sep;64(9):937-42. doi: 10.1016/j.metabol.2015.06.005. Epub 2015. Jun 11. PMID: 26130607. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Li R, Meng Y, Li S, Donelan W, Zhao Y, Qi L, Zhang M, Wang X, Cui T, Yang LJ, Tang D. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes. 2014. Feb;63(2):514-25. doi: 10.2337/db13-1106. Epub 2013. Oct 22. PMID: 24150604. [DOI] [PubMed] [Google Scholar]


