Abstract
Lead (Pb2+) poisoning is a public health concern of global dimensions. Although several public health guidelines and workplace safety policies are existing and enforced, lead toxicity cases are drastically increasing. Lead exposure leads to numerous harmful consequences and causes adverse effects on different body organs and systems, mainly via the generation of reactive oxygen species, leading to augmented oxidative stress, competing with metal ions, and binding with the sulfhydryl groups. In several instances, lead poisoning cases remain undiagnosed and untreated or receive only symptomatic treatment. Estimation of blood lead levels reflects only a recent exposure, however, which does not reveal the total body burden. This review summarizes the effects of lead with special reference to hepatotoxicity and some of the potential diagnostic biomarkers. Furthermore, it also focuses on synthetic chelators used in the treatment of lead poisoning and the advantage of using bioactive compounds with an emphasis on the ameliorative effect of garlic.
Keywords: lead toxicity, biomarkers, chelation, antioxidants, bioactive compounds, allicin
Introduction
Lead is an extremely toxic, divalent heavy metal, which occurs naturally in the earth’s crust. It is ubiquitous in the human environment due to relentless anthropogenic activities. Unique properties of lead, such as high malleability, low melting point, softness, high ductility, and corrosion resistance, have led to its extensive use in paint, petrol, ceramics, soldering, automobiles, smelting, plumbing, building material, ammunition, mining, glass manufacturing, pottery, printing, plastic, and pipe manufacturing. Additionally, lead had uncertain primary uses in ancient cosmetics and traditional medicines. Environmental and occupational exposure to lead has become a global concern. Occupational exposure is the major source of lead poisoning; however, nonoccupational lead exposure can occur through various sources and pathways. Lead is nonbiodegradable and is the root cause of its constant presence in the environment. Air, water, dust, and soil are the major pathways through which lead can enter the human body (Fig. 1). Lead does not have any biological role in the human body and affects several biological activities, mainly disturbing the hematopoietic, hepatic, renal, and central nervous systems, creating serious disorders.1 World Health Organization has recognized lead as one of the 10 chemicals of major global public health concern. Lead toxicity can be caused either by acute or chronic exposure. Acute toxicity occurs due to the inhalation or ingestion of high amounts of lead in a single or short period of exposure, and chronic toxicity occurs when the individual gets exposed to lead over a long duration. Lead levels as low as 5 μg/dl in children have shown to cause a reduction in intelligent quotient (IQ), cognitive performance, and academic achievement, and increase the incidences of hyperactivity disorder, behavioral problems, and attention deficit.2 Lead gets into the human body mainly through inhalation (about 70%), ingestion (about 30%), and a negligible amount by dermal contact (<1%) (Fig. 2). Absorbed lead (either ingested or inhaled) is deposited in soft tissues. Autopsy results of lead-exposed individuals specify that among the soft tissues, the liver is the major repository (~33%) of lead followed by the kidney medulla and cortex.3 Lead has a half-life of about a month in blood, 1–1.5 months in soft tissues, and about 25–30 years in bone.4 It can be released at times of hormonal imbalances, stress, pregnancy, and lactation. Estimation of blood lead level is widely used as an indicator of lead exposure; however, it reflects only the recent or acute exposure to lead and not the actual amount of lead in the body.5 X-ray fluorescence technique can noninvasively estimate the bone lead which can reflect the long-term cumulative exposure to lead.6 However, its utilization as a diagnostic marker is restricted due to the constrained availability of the facility to only a few specialized centers. Once lead gets deposited in the soft and hard tissues, it takes a long duration and several courses of chelation therapy to be eliminated from the body.7 The diet and nutritional status of the individuals play a major role in the absorption and deposition of lead in the body. Lead gets eliminated from the body through the glomerular filtrations by the kidney in the urine and biliary secretion by the gastrointestinal tract in the feces. A minor amount of lead is also lost through sweat, hair, nail, and exfoliated skin (Fig. 2).
Fig. 1.
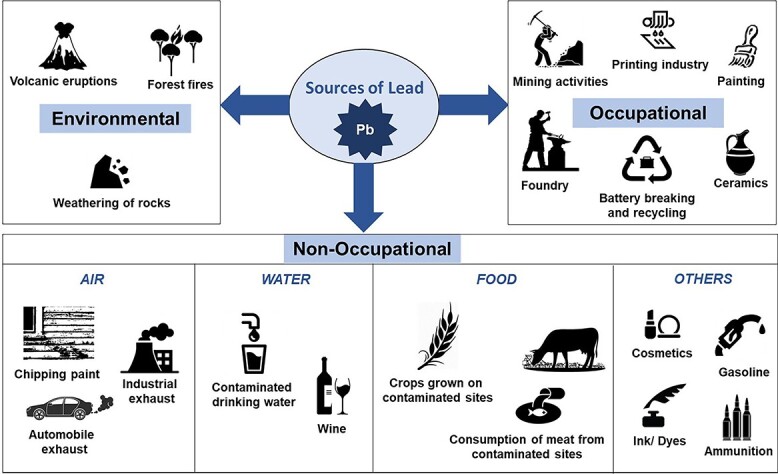
Major sources and pathways of human lead exposure.
Fig. 2.
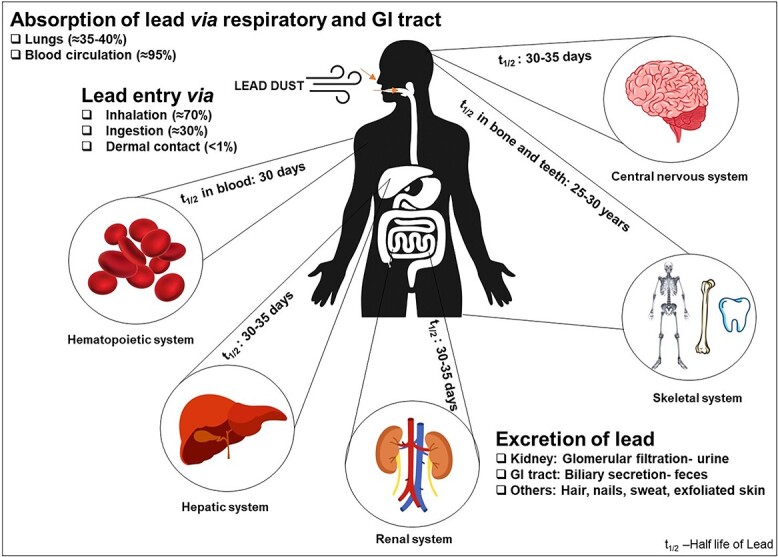
Absorption, distribution, and excretion of lead.
Mechanism of lead toxicity
Free radical damage is caused by lead toxicity through two different pathways, primarily by the generation of reactive oxygen species (ROS) including hydrogen peroxide, singlet oxygen, and secondly by direct antioxidant depletion. ROS enhances membrane lipid peroxidation, and thus, lead causes oxidative damage, which is a detrimental process carried out only by free radicals that in turn affects the role of membrane-bound proteins, such as receptors and enzymes.8 Studies have reported that lead exposure leads to increased levels of lipopolysaccharide-induced tumor necrosis factor-α (TNF-α), which is responsible for inflammation, malignancy, and apoptosis, thus leading to liver injury.9
ROS generation and binding to sulfhydryl (-SH) groups
Oxidative stress signifies an inequality between the ability of a biological system for easy detoxification of the volatile intermediary compounds and restoration of the subsequent impairment and the formation of free radicals. It is considered to play a major role in the development of hepatotoxicity. The impact of lead is notably linked with the formation of oxidative injury which carries in diverse pathways, functioning synchronously; the first one is by the formation of ROS including singlet oxygen (1O2), hydroperoxides (HO2•), and hydrogen peroxide (H2O2); the second one is by the depletion of stored antioxidants.10 The antioxidant defense mechanism is critical in preventing the detrimental effects of ROS produced in the body. The utmost significant antioxidant in cells is glutathione. In the reduced state of glutathione (GSH), the thiol group of cysteinyl residue is a source of one reducing equivalent. The cysteinyl portion of glutathione has a sulfhydryl (SH) group, which is responsible for its strong electron-donating character and is involved in reduction and conjugation reactions. Reduced glutathione (GSH) reacts with ROS and protects cells from oxidative damage by reducing them getting converted to the oxidized form glutathione disulfide (GSSG). Glutathione peroxidase (GPX) enzyme is involved in the detoxification of H2O2 and a range of organic peroxides utilizing reduced glutathione (GSH), which in turn gets oxidized. GSSG is transformed to its reduced form (GSH) due to the presence of the glutathione reductase (GR) enzyme. Conjugation reactions involving glutathione are facilitated by the enzyme glutathione S-transferases (GST) thereby enabling the metabolism of xenobiotics. Lead exposure can result in the depletion of GSH leading to the accumulation of lipid peroxidation products, which eventually alters the activity of GR, GST, and glucose 6-phosphate dehydrogenase (G6PD).11
Lead has a high affinity for the -SH groups and its binding results in the inhibition of functional-SH groups of glutathione, which in turn hinder its replenishment leading to alteration in antioxidant activity and oxidative stress. Other enzymes with -SH groups, such as delta-aminolevulinic acid dehydratase (δ-ALAD), GR, GPX, and GST, are also inhibited by lead. A few other prominent antioxidant enzymes, which are affected by lead, include catalase (CAT) and superoxide dismutase (SOD).
Maximum examined scenarios of ROS are on lipid membranes and lipid peroxidation as a biomarker of oxidative stress. Other than lipid peroxidation, hemoglobin oxidation is also caused by lead exposure leading to red blood cell (RBC) lysis. This happens due to a decrease in the activity of δ-ALAD.12
Ionic mechanism
Lead has the ability to replace divalent cations, such as magnesium, iron, calcium, and monovalent cation such as sodium, and can affect numerous essential biological functions of the body and also responsible for its ionic mechanism of action.13 It disturbs the sodium ion concentration, which is important for various biological actions such as in the excitatory tissues where it is required for the generation of an action potential, intercellular communication, neurotransmitters uptake, functioning of neurons, and osmoregulation.14 The absorption and retention of calcium by synaptosomes is also regulated by sodium. Even at picomolar quantities, lead interferes with sodium ions and can substitute calcium. Lead disrupts intracellular calcium homeostasis by competing with calcium for transport through calcium channels and pumps at the plasma membrane.13 The stored lead can be mobilized during the pathological and physiological states of increased demand for calcium, including osteoporosis, pregnancy, and lactation. About 85–95% of lead is deposited in the bone and the movement of maternal lead deposits during the time of pregnancy and postpartum into the circulation signifies an endogenous basis of exposure that may cause a substantial hazard for the growing fetus and infant.15
Lead toxicity and its effects on the human body
Lead is one of the most extensively studied toxic heavy metals. It does not spare any organ in the human body and affects almost every organ system, including nervous, renal, cardiovascular, hematological, skeletal, reproductive, gastrointestinal, and endocrinal. Lead poisoning also known as saturnism or plumbism is the clinical indication of lead-induced toxicity and has been recognized since early times. Children below the age of 6 years are more vulnerable to the toxic effects due to their brain and nervous system is in a developing stage and lead can interfere with this process.16 Children exhibit numerous symptoms related to lead poisoning, including abdominal pain, nausea, vomiting, constipation, and anemia. The children may also suffer from developmental delay, irritability, hyperactivity, weak motor skills, and particularly low concentration abilities, reaction time, and reduced IQ.17 Chronic exposure to lead leads to structural changes in the liver, increased lipid peroxidation, decreased antioxidant activity, and changes in mitochondrial configuration. Cytotoxicity of lead has an adverse effect on mitochondrial DNA (mtDNA) and repair mechanism through ROS generation due to interaction with sulfhydryl class of proteins or enzymes which are generally a target for heavy metals.
Lead-induced hepatotoxicity
The first organ exposed to xenobiotics and other externally absorbed nutrients is the liver. The liver tissue is metabolically very active and can detoxify the xenobiotics principally by transforming them from lipid-soluble to hydrophilic form in the smooth endoplasmic reticulum of liver cells through phase I and II reactions.
Effect of lead on histopathology of the liver
The liver is mainly involved in detoxification processes. The liver is the largest repository of lead, which accounts for about 33% of soft tissue lead. Hence, the accumulation of lead in the liver can cause the degeneration of liver cells and the congestion of the central vein.
Animal experiments have reported that exposure to lead causes damage to the regular structure and function of the liver leading to metabolic disturbances causing lymphocyte infiltration and cirrhosis of liver cells.18,19 Studies have also demonstrated lead-induced cytoplasmic inclusions in hepatocytes, hyperplasia of Kupffer cells, and mild chronic inflammation of portal triads in lead-exposed Wistar albino rats.20
Metabolic markers of lead-induced hepatotoxicity
Drug metabolism in the liver
The liver is the major site for drug metabolism, where the majority of drugs are metabolized. Enzymes present in the liver either convert prodrugs to an active metabolite or the active form of the drug to an inactive form.
Phase I enzymes
Are a group of cytochrome P450 enzymes involved in metabolizing drugs in the liver. Based on the shared amino acid sequences, almost 50 different human P-450 enzymes were identified and categorized. The phase I reactions mainly consist of oxidation, reduction, and hydrolysis. These reactions are involved in the addition of groups, which assist the drugs to undergo phase II reactions and produce conjugation products.
Phase II enzymes
Phase II reactions are involved in inactivating the drugs or active metabolites formed from phase I reactions. Phase II conjugation reactions, such as glucuronidation, sulphation, acetylation, and methylation, which are involved in the attachment of the ionized groups and the drug, lead to increased water solubility and elimination in the urine and bile.
Biochemical markers of lead-induced hepatotoxicity
Lead is known to cause acute hepatitis, cirrhosis of the liver, and jaundice. Because of the hepatotoxic effects of lead, its exposure leads to variations in the serum levels of enzymes of hepatic origin.
Heme synthetic pathway
Lead inhibits 3 key enzymes responsible for heme synthesis, δ-ALAD, coproporphyrinogen oxidase, and ferrochelatase. The ferrochelatase catalyzes the final reaction of the heme synthesis, involved in the incorporation of Fe2+ into the protoporphyrin IX. As a result, there will be decreased hemoglobin production and heme-containing compounds such as cytochromes. Due to δ-ALAD inhibition, its substrate δ-aminolevulinic acid (δ-ALA), which is a gamma-aminobutyric acid (GABA) analog accumulates and its concentration, increases in the blood. Despite δ-ALA being a GABA agonist, it is not involved in the activation of GABA receptors instead, and it is involved in the reduced production of the second messenger, cyclic adenosine-monophosphate (cAMP), probably by altering the adenylate cyclase activity.21 In lead-induced ALAD inhibition, patients may develop porphyria-like symptoms.
Inhibition of ferrochelatase leads to the formation of erythrocyte protoporphyrin (EPP), and significant protoporphyrin present in the erythrocytes can bind to zinc ions resulting in the formation of zinc protoporphyrin (ZPP).22
Lactate dehydrogenase
Lactate dehydrogenase (LDH) is an intracellular enzyme and is extensively dispersed all over the body. When the patients are exposed to lead, a rise in LDH can imitate injury to many different tissues (liver, kidney, skeletal, or cardiac muscle). Studies have reported a lead-induced increase in LDH activity in various tissues, predominantly in the liver.23 Lead affects metabolic enzyme activities and it is also able to slow down the intermediary metabolism leading to an increase in LDH levels. Besides inducing the production of ROS, lead also inhibit the enzyme pyrimidine 5′ nucleotidase causing hemolysis and RBCs to contain a higher amount of LDH, as well as its lysis, which might lead to increased cytoplasmic LDH level.24
Gamma-glutamyl transferase
Gamma-glutamyl transferase (GGT) is a membrane-bound enzyme found in the kidneys and the liver. It participates in glutathione breakdown and transport of amino acids through the cell. Cellular injury and cholestasis cause its release into the blood. Elevated levels in the blood are also seen in chronic alcoholics. In addition to liver function index and alcohol intake biomarker, it can also be used as a sensitive biomarker of oxidative stress. Higher levels of GGT are reported in individuals exposed to lead.25,26
Transaminases: alanine transaminase and aspartate transaminase
Alanine transaminase (ALT) and aspartate transaminase (AST) are used as markers for liver injury. In the liver, ALT is cytoplasmic, whereas AST is both cytosolic and mitochondrial. Both these enzymes are present in the liver. Besides the liver, AST is also found in the heart and to some extent in the skeletal muscle, kidneys, pancreas, spleen, lungs, and RBCs. The ALT concentrations are low in the kidney, myocardium, pancreas, spleen, lung, skeletal muscle, and RBCs. Any variation in serum levels of ALT is more prone to liver damage and can be used as an ideal biomarker of hepatocellular injury. Studies have reported that lead-induced oxidative stress causes liver damage.27
Lead-induced hepatocellular injury causes the release of ALT into the extracellular fluid (ECF). It is a precise biomarker for mitochondrial and cytoplasmic injuries, where its increased concentration is much more than that of AST.
Alkaline phosphatase
Alkaline phosphatase (ALP) levels can increase because of physiologic reasons or pathologic lesions of the liver.28 Generally, the cells coating the bile ducts in the liver synthesize ALP as the first enzyme during hepatotoxicity, which is seen in patients who are exposed to lead. The liver is the main source of ALP, and it is also synthesized in the bones, intestine, pancreas, and kidney. Animal experiments showed a significant increase in ALP levels in plasma, liver, kidney, and ovary of rats exposed to lead.29
Pyrimidine 5′-nucleotidase (P5′N) activity
P5′N, the enzyme responsible for the maturation of RBCs, is known to be inhibited by lead. As a consequence of this inhibition, in circulating reticulocytes and erythrocytes, aggregates of the ribosomes and fragments of ribosomal RNA or ribonuclear proteins can form and precipitate in the cytoplasm of the erythrocyte leading to hemolysis, anemia, and basophilic stippling of RBCs.30
Serum bilirubin levels
Bilirubin is produced as a result of heme degradation in the body and it has been demonstrated to have antioxidant activity against peroxyl radicals, although its higher concentration leads to a disease condition called hyperbilirubinemia or jaundice.31 Human studies showed no significant relation between bilirubin and chronic lead exposure.25 On the contrary, animal studies have reported elevated levels of bilirubin in rats exposed to lead.32,33 Lead-induced elevation in the serum bilirubin may be because of the induction of isoform of heme oxygenase, which plays a major role in heme catabolism and may also be due to the induction of eryptosis, which causes an increase in the degradation of heme.34,35
Impact of lead exposure on liver in humans
There are limited studies conducted to demonstrate the effect of lead on humans. Studies conducted in the human population exposed to lead at work place reported lead-induced mild elevation in AST and a significant increase in ALT levels.36 Studies conducted in paint factory workers showed a slight elevation in levels of AST, ALT, and ALP compared with nonexposed individuals.37 A study conducted in plastic industry workers showed a significant increase in AST, ALT, ALP, and GGT levels.38 Similarly finding of study conducted in occupationally exposed workers showed a significant increase in ALP, ALT, and AST levels. Recent study conducted in lead-mine workers showed a significant increase in the serum LDH, AST, ALT, ALP, and bilirubin levels compared with the nonexposed individuals.39 All these studies suggest that long-term exposure to lead may induce a deleterious effect on the liver. Individuals working in the lead based industries are at higher risk and the absorption of lead is influence by the individuals dietary and nutritional status.40
Factors associated with inflammation
Lead can cause a negative effect on the immune system. Cytokines include a collection of small bioactive proteins which are synthesized by the cells of the immune system, and responsible for regulating inflammatory responses.41 Lead-induced liver injury leads to the reduction in the activity of cytokines, which prevents the normal functioning of cytokines. Cytokines are divided into growth-promoting factors, interleukins (IL), chemokines, and interferons (IFN). Results of the studies conducted in rats exposed to prenatal and postnatal lead exposure have reported increased brain cytokine levels as well as alterations in the prostaglandin and thromboxane synthetic pathway; furthermore, it also showed higher expression of the nuclear factor Kappa B (NF- κB) mRNA and cyclooxygenase-1 (COX-1) and COX-2 enzymes.42
Studies have reported lead-induced dysregulation of T-helper (TH) cells and alterations in the availability of key cytokines.43 Lead can induce TH2 development affecting the proliferation of TH1 cells, leading to the shift in TH1/TH2 balance.44 Studies have demonstrated suppression of the TH1-type immune responses, delayed-type hypersensitivity and production of interferon (IFN) γ, and promotion of TH2 immune responses, interleukin (IL)-4, and TNF-α production in macrophages of lead-exposed mice.45
MicroRNA (miRNA) markers for lead-induced liver toxicity
miRNAs are single-stranded noncoding RNA molecules, 18–25 nucleotide in length, which are newly documented as ideal components participating in posttranscriptional regulation over many eukaryotic genomes. By binding to complementary sequences, miRNAs are responsible for mediating the gene silencing on the 3′-untranslated regions (UTR) of target mRNAs, leading to mRNA degradation or translational repression.46
Several studies have reported changes in the miRNA expressions associated with lead. A positive association was observed in the expression of miR-146a, miR-10a, miR-190b, and miR-431, and a negative association was observed with the placental expression of miR-651.47
A study conducted by Guo et al. (2019) in 145 individuals exposed to toxic metals, miRNA-21-5p, and miRNA-122-5p was reported to be higher compared with the nonexposed.48,49 Results of the study conducted in animal models by Masoud et al. (2016) showed that early-life exposure to lead has a significant influence on the short- and long-term expression of miRNA, which in turn targets epigenetic mediators and neurotoxic proteins later in life.50
Treatment for lead poisoning-chelation therapy
Chelation therapy is used to treat acute poisonings caused by metals such as lead, arsenic, mercury, and iron. However, most chelators are known to have serious adverse effects (Table 1).
Table 1.
Adverse effects caused by common chelators.
| Chelator | Adverse effects | Reference |
|---|---|---|
| CaNa2EDTA | Tetany, Drop in blood pressure, Heart rhythm changes, Fatigue, Headache, Nasal congestion, Lacrimation, Mucocutaneous lesions, Myalgia, Hepatotoxicity, Hypocalcaemia, Tremors, Arrhythmias, Renal failure. | Knudtson et al. (2002). Flora and Pachauri (2010). |
| DMSA (Succimer) | Diarrhoea, Nausea, Vomiting, Drowsiness, Dizziness, Loss of appetite, Skin rashes, Chills, Transient modest rise in transaminase activity, Urine copper and zinc excretion, Sensorimotor neuropathy, Sleepiness and paraesthesia. | Blanusa et al. (2005), Bradberry and Vale 2009. |
| D-Penicillamine | Anorexia, Nausea, vomiting, Gastrointestinal disturbances, Degenerative dermatoses, Wound healing defects, Gastrointestinal disturbances, Loss of taste, Polymyositis/dermatomyositis, Alopecia and proteinuria. | Iozumi (1997), Khandpur et al. (2015). |
| DMPS | Headache, Fatigue, Nausea, Taste impairment, Pruritus and rash, Gastrointestinal discomfort, Skin reactions, Mild neutropenia, Elevated liver transaminases. | Blanusa et al. (2005), Hoet et al. (2020) |
| Dimercaprol (BAL) | Headache, Nausea, Vomiting, Hypertension, Paraesthesia, Liver damage, Tachycardia, Salivation, Rhinorrhoea, Sweating, Flushing, Restlessness, Nephrotoxicity, Seizures, Sweating, Haemolysis, Lacrimation, Hyperpyrexia, Conjunctivitis, Urticaria, Paraesthesia, Hematomas at the site of injection. Generalized myalgia. | Goldstein et al. (1962), Kosnett (2013). |
In the blood, there is no safe level of lead; levels as low as 3.5 μg/dL in the blood may lead to adverse health effects.51 Environmental factors, habits, nutritional status, and parental occupation contribute to a child’s blood lead levels. In cases of high blood lead levels, environmental intervention and preventing the child from further exposure is of prior importance to bring down the levels. Chelation therapy is not generally prescribed to children with blood lead levels below 45 μg/dl; however, even after the thorough environmental intervention, for persistent blood lead levels within 20–44 μg/dl, chelation therapy can be prescribed in consultation with an expert. Children having blood lead levels above 45 μg/dl need hospitalization and chelation therapy.51,52
Chelating agents are used for treating metal toxicity. The characteristic features of a perfect chelator include: (i) high affinity for the toxic metal elements; (ii) less toxicity; (iii) capacity to enter the cell membrane; (iv) quick excretion of metal from the body; and (v) high solubility in water. Chelation is a classical method to treat lead toxicity. Generally, used lead chelators are calcium disodium ethylenediaminetetraacetate (CaNa2EDTA), D-penicillamine (DPA), Dimercaprol, or British Anti Lewisite (BAL), and succimer or meso 2,3-dimercaptosuccinic acid (DMSA), Unithiol or sodium 2,3-Dimercaptopropanesulfonate (DMPS). Synthetic chelators generally cause adverse effects such as papular rash, cardiac problems due to hypocalcemia, renal toxicity, vomiting, diarrhea, glossitis, pruritis, abdominal pain, stomatitis, alopecia, and skin lesions.53
Calcium disodium ethylenediaminetetraacetic acid (CaNa2EDTA)
It is a derivative of ethylenediaminetetraacetic acid (EDTA) and one of the most commonly used chelating agents. The ability of lead to be chelated in the presence of calcium is the reason for the effective use of CaNa2EDTA in the chelation therapy of lead toxicity. Major drawbacks of CaNa2EDTA are that it can cause the redistribution of lead from other tissues to the brain, and hence, often the administration of CaNa2EDTA is preceded by intramuscular administration of BAL to limit this redistribution into the central nervous system.54
D-Penicillamine (DPA, β-β-dimethylcysteine or 3-mercapto-d-valine)
It is a derivative produced from penicillin and a sulfhydryl comprising amino acid cysteine. It is administered via oral or intravenous routes and can be readily absorbed in the gastrointestinal tract. It is an approved drug for the treatment of Wilson disease, cystinuria, and rheumatoid arthritis; however, few studies have reported the use of DPA in the treatment of lead poisoning.7,55 It is used for patients who are not able to tolerate succimer and CaNa2EDTA.51,52
British antilewisite (2,3-Dimercaptopropanol or dimercaprol)
British antilewisite (BAL) is used for toxic metal poisoning therapy. It is clinically used for the treatment of lead, arsenic, mercury, and copper toxicity. BAL is a simple propanol molecule with two sulfhydryl groups and weak dibasic acid. It needs to be administered parenterally. Due to its lipophilic nature, it is usually administered by dissolving in peanut oil. BAL is unsteady and can easily be oxidized, hence is very hard to store, and needs to be prepared fresh before each use. The antidote efficiency of BAL is most active when given instantly after exposure.53
Succimer or DMSA
A chemical derivative of dimercaprol having two thiols (dithiol) functional groups is an operative chelator of metal toxicity mainly lead, mercury, arsenic, and cadmium. It is less toxic than BAL and can neither cross the blood–brain barrier nor can cause the relocation of metal between various organs. It is an FDA-approved medication for the treatment of elevated blood lead levels in pediatric patients.56
Sodium 2,3 Dimercaptopropane-l-Sulphonate (DMPS)
It is an analog of BAL having dithiol groups, which extensively used in the treatment of lead, mercury, and arsenic poisoning. It is majorly dispersed in the ECF as it is hydrophilic in nature. It can be administered either orally or intravenously. Elimination from the body occurs majorly through the kidney in the urine and also by biliary excretion. It is not able to cross the blood–brain barrier and does not redistribute toxic metals such as lead, arsenic, and mercury to the brain.57
Antioxidants as chelating agents
Both enzymatic antioxidants and nonenzymatic antioxidants are known to counter the consequence of ROS and reactive nitrogen species. These antioxidants decrease the effects of free radicals resulting in a minimum danger of oxidative stress. Antioxidants can chelate the metal ions that can generate ROS as they can act in both membrane and aqueous domains.58
Antioxidant’s role in shielding lead-induced oxidative stress
Antioxidants are constituents that are present in cells in low concentrations and are responsible for reducing or preventing the oxidation of an oxidizable substrate. The antioxidant can be either endogenous, which can be produced in the body, or exogenous, which can be obtained from the diet.
The antioxidant defense system is divided into the (i) enzymatic antioxidants system and (ii) nonenzymatic antioxidants system. The enzymatic antioxidant system includes SOD, CAT, GPX, GR, and G6PD. The nonenzymatic antioxidant system comprises minerals (zinc, selenium), vitamins (Vitamin A, E, K, and C), carotenoids (β-carotene, lycopene, zeaxanthin), organosulfur compounds (allicin, allium, allyl sulfide), and polyphenols (flavonoids, gallic acid).
The enzymatic and nonenzymatic antioxidant systems function synergistically and collectively with one another to guard the cells and organ systems against free radical damage. Generally, antioxidants reverse lead toxicity by one of the following three mechanisms.3,59
(a) By dismissing the radical chain reaction through the deactivation of ROS produced, when it is at a molecular level.
(b) By chelating lead ion that is already present and further avoiding additional production of ROS.
(c) By its inability to reduce molecular oxygen through the chelation of lead and maintaining it in a redox form.
Use of natural antioxidants
Thiamine and pyridoxine
Vitamin B1 (thiamine) and B6 (pyridoxine) are effective in ameliorating lead-induced adverse effects. Studies have shown the therapeutic potential of thiamine in preventing the toxic effects of lead.60
Pyridoxine is known to ameliorate lead-induced lipid peroxidation. Animal studies conducted in rat models have shown the role of pyridoxine in alleviating lead-induced lipid peroxidation by directly reacting with peroxy radicals.61
Ascorbic acid (vitamin C)
It is a water-soluble vitamin and is the most extensively studied potent antioxidant concerning lead-induced oxidative stress. It can directly scavenge free radicals. Thus, has an important role in protecting the cells from oxidative damage and lipid peroxidation and protecting against lead toxicity.62
α-Tocopherol (vitamin E)
It is a fat-soluble vitamin with various biological activities and can counteract the harmful impact of lead by chelating and maintaining it in the redox state and thus reducing the further production of ROS. Vitamin E acts as an antioxidant and can hinder the free radical chain reaction and thus prevents lipid peroxidation.63
Flavonoids
Flavonoids are natural polyphenolic substances widely present in plants having antioxidant activity. Quercetin (3,5,7,3′,4′-pentahydroxyflavon) is a potent flavonoid rich in food such as green leafy vegetables, fruits, and beverages.64 Animal studies have shown that supplementing quercetin at 50-mg/kg body weight was found to have ameliorative effects against lead-induced toxic effects.65
α-Lipoic acid
α-Lipoic acid (α-LA) is an organic compound synthesized in the body which is having antioxidant properties. Studies conducted in male Wistar albino rats have shown that the therapeutic intervention for lead poisoning using α-LA in combination with a thiol chelator is effective in lowering the oxidative stress and brain lead levels.66 Studies have also reported that α-LA can regenerate antioxidants and stimulate antioxidant enzyme activities such as SOD, GPx, GST, and CAT.67
Carotenoids
Carotenoids are present in plants, algae, and photosynthetic bacteria. These are fat-soluble isoprenoid compounds comprising more than 700 compounds. In nature, two important classes of carotenes are present (i) β-carotene, which is a precursor of retinol, having a long chain of hydrocarbons attached to the ring structure at one or both ends and (ii) xanthophylls, which are a diverse group of oxygenated carotenoids.68
The antioxidant function of carotenoids originates basically as a sign of the capability of the conjugated double-bonded structure to move the unpaired electrons. This is the initial reason for the chemical susceptibility of the α-carotene to ROS such as the superoxide radicals (O2•-), hydroxyl (•OH), and peroxyl (ROO•), and for the excellent ability of α-carotene to quench singlet oxygen.69 At adequately high concentrations, carotenoids can prevent cell oxidation and lipids against peroxidative injury.70
Melatonin (N-acetyl-5-methoxy tryptamine)
The pineal gland synthesizes this endogenous neurohormone from amino acid tryptophan. It stimulates antioxidant enzymes and thus acts as a powerful scavenger of free radicals and ROS, preventing lipid peroxidation.71 Experiments conducted in rats exposed to subacute lead treatment have shown melatonin can reduce the blood, brain, and bone lead levels, and increase lead excretion.72
Use of herbal antioxidants as chelators
Spices and herbs have been used by mankind over several years as flavoring agents because of their medicinal and other useful properties. Herbal antioxidants have been reported to act as metal quenchers and play a role in alleviating lead-induced toxic effects.73
Allium sativum (garlic)
Garlic (A. sativum) is a perennial, pungent plant bulb of the onion family. Garlic is known for its beneficial properties such as its ability to act as a hypolipidemic, antioxidant, antidiabetic, antitumor, and immunomodulatory agent. It also has a role in the prevention of endothelial dysfunction. Allicin (diallyl-dithiosulfinate) is the major sulfur-containing compound present. In addition to this, the other major active components present in garlic are diallyl-sulfide (DAS), diallyl-di-sulfide (DADS), diallyl trisulfide (DATS), S-allyl-cysteine sulfoxide (alliin), S-allyl-cysteine (SAC), and E-ajoene and Z-ajoene.74
Allicin is present in fresh garlic extract and it is produced by the action of alliinase on alliin.75 The strong antioxidant properties and sulfhydryl groups of allicin make it a powerful natural chelator and an ideal candidate for the treatment of lead poisoning. It has the ability to reduce the accumulation of lead in bone and soft tissues such as the liver, kidney, and brain.76 The effectiveness of garlic was possible because of the occurrence of these sulfur-possessing amino acids and compounds containing the free amino (-NH2) and carboxyl (-COO) groups in their structures.77 S-allylcystein and S-allylmercaptocystein are known to inhibit the absorption of lead from the gastrointestinal tract.78
Animal studies conducted showed the ameliorative effect of garlic treatment on lead-induced toxicity during pregnancy and lactation and resulted in a reduced concentration of lead in the blood and brain and moderately prevented lead-induced apoptosis of neurons during the hippocampal development.79 Experimental evidence has shown the ameliorative effects of garlic on lead-induced hepatotoxicity in Swiss albino mice exposed to lead treatment.80
Coriandrum sativum (coriander)
Coriander is a significant annual herb in the world having very good antioxidant properties. Animal studies have reported that C. sativum has strong antioxidant and metal-chelating properties, thus able to reduce lead-induced oxidative stress.81 Studies conducted to evaluate the histological changes have reported that treatment with coriander extracts protects the tissues from toxic effects showing lesser damage compared with the control group; hence, extracts showing antioxidant ability are thus considered as a natural chelating agent for lead intoxication.82
Curcuma longa (turmeric)
Curcumin stops the production of ROS, such as nitrile radicals and superoxide anions. Curcumin has numerous pharmaceutical applications owing to its effective anti-inflammatory and antioxidant properties. Studies have shown that curcumin suppresses the lead-induced genotoxicity caused by its interaction with DNA and proteins producing double- and single-strand breaks and cross-linking of DNA protein.83
Tea (black and green)
Catechin is the main green tea polyphenol. Catechin is a free radical scavenger and can scavenge both superoxide and hydroxyl radicals and also peroxyl radicals and lipid-free radicals. Catechins also raise the number of endogenous antioxidants in addition to their ability to shield the effects of oxidative injury, lipid peroxidation, and chelating metal ions.84
Green tea extract has been found to protect against lead-induced liver toxicity. Studies conducted in experimental animals have shown that green tea extract and its components are moderately effective in preventing lead-induced disturbances of the antioxidant defense system in the brain.85
Zingiber officinale (ginger)
Ginger has intense antioxidant, antimicrobial, cytoprotective, and anti-inflammatory properties. It is capable of reducing the lead-induced oxidative stress. Ginger has been shown to reduce the lead-induced harmful effects and rise plasma SOD and catalase levels.86
Phyllanthus emblica Linn/Emblica Officinalis Gaertn (Amla/Indian gooseberry)
It is a medicinal plant having a variety of therapeutic applications. It has antioxidative and immunomodulatory properties. The fruit has various important constituents, such as gallic acid, ellagic acid, tannins, vitamins, minerals, amino acids, and flavonoids such as rutin and quercetin, which can protect against the harmful action of ROS. In addition to its medical value, its easy accessibility makes amla a major basis of protection and treatment for lead-induced toxicity. Studies conducted in 1-day-old male broiler chicks have reported that amla can ameliorate lead-induced toxic effects by reducing free radical production.87
Bacopa monnieri (Brahmi)
It is an ayurvedic indigenous medicinal herb having versatile antioxidant properties. Bacopa monnieri is known to improve cognitive function in humans. It has a strong free radical scavenging property and thus can prevent the formation of lipid peroxides and divalent metals.88 Several phenolics, flavonoids, alkaloids, saponins, and steroids have been identified from the extracts of the B. monnieri. Studies have reported that it can exert its antioxidant effects by breaking oxidative chain reactions, chelating metal ions, scavenging free radicals, and improving the activities of antioxidative defense enzymes.89
Conclusion
Lead is one of the main persistent and common environmental pollutants. Lead is used by human beings because of its feasible properties and wide range of applications. Hence, lead is one of the greatest and most extensively used industrial metals. Several cases of lead poisoning are reported due to occupational lead exposure. Nonoccupational exposure in the general population can occur through various pathways, including contaminated food, water, and air. In numerous instances, the symptoms of lead poisoning are very general or mimic that of other disorders; hence in several instances, lead poisoning cases remain undiagnosed or receive only symptomatic treatment. This review sheds light on various biomarkers which can be used in the diagnosis of lead toxicity. Estimation of blood lead levels reflects only the recent exposure; hence in the case of chronic exposure, along with the estimation of blood lead levels, various biomarkers discussed in this review might be of significance and further help to confirm the diagnosis. Lead deposited over a long period may need several courses of expensive chelation therapy having severe adverse effects. Consuming natural chelators along with ongoing lead exposure may be useful in chronic lead exposure to hasten the excretion and decrease the body burden of lead and thus might prove to be beneficial and avoid the hazards of several courses of expensive chelation therapy.
Acknowledgments
The authors acknowledge FIST-DST Government of India, TIFAC-CORE and Manipal School of Life Sciences, Manipal Academy of Higher Education for the infrastructure and facility.
Contributor Information
Netranandini Lakka, Manipal School of Life Sciences, Manipal Academy of Higher Education, Manipal, Karnataka 576104, India.
Bhagyashree Pai, Manipal School of Life Sciences, Manipal Academy of Higher Education, Manipal, Karnataka 576104, India.
Monica Shirley Mani, Department of Radiation Biology and Toxicology, Manipal School of Life Sciences, Manipal Academy of Higher Education, Manipal, Karnataka 576104, India.
Herman Sunil Dsouza, Department of Radiation Biology and Toxicology, Manipal School of Life Sciences, Manipal Academy of Higher Education, Manipal, Karnataka 576104, India.
Funding
This study did not receive any funding.
Conflict of interest statement: Authors declare that they do not have any conflict of interests with respect to the research, authorship, and publication of this article.
References
- 1. Durgut R, Koc A, Gonenci R, Bal R, Celik S, Guzel M, Altuğ ME, Atesoglu E. Effects of high dose lead toxication on liver, kidneys, heart, brain and blood in rabbits: an experimental study. J Appl Biol Sci. 2008:2:11–18. https://www.jabsonline.org/index.php/jabs/article/view/93. [Google Scholar]
- 2. World Health Organization (WHO) . Global elimination of lead paint: why and how countries should take action: technical brief. 2020 Geneva: World Health Organization. https://apps.who.int/iris/handle/10665/333840.
- 3. Flora G, Gupta D, Tiwari A. Toxicity of lead: a review with recent updates. Interdiscip Toxicol. 2012:5(2):47–58. 10.2478/v10102-012-0009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abadin H, Ashizawa A, Stevens YW, Llados F, Diamond G, Sage G, Citra M, Quinones A, Bosch SJ, Swarts SG. Toxicological profile for lead. Atlanta (GA): Agency for Toxic Substances and Disease Registry (US); 2007. PMID: 24049859. [PubMed] [Google Scholar]
- 5. Wani AL, Ara A, Usmani JA. Lead toxicity: a review. Interdiscip Toxicol. 2015:8(2):55–64. 10.1515/intox-2015-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Specht AJ, Dickerson AS, Weisskopf MG. Comparison of bone lead measured via portable x-ray fluorescence across and within bones. Environ Res. 2019:172:273–278. 10.1016/j.envres.2019.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Menezes G, D'souza H, Venkatesh T. Chronic lead poisoning in an adult battery worker. Occup Med. 2003:53(7):476–478. 10.1093/occmed/kqg091. [DOI] [PubMed] [Google Scholar]
- 8. Sharma A, Sharma V, Kansal L. Amelioration of lead-induced hepatotoxicity by Allium sativum extracts in Swiss albino mice. Libyan J Med. 2010:5(1):4621. 10.3402/ljm.v5i0.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cheng YJ, Chang BC, Liu MY. Lead increases lipopolysaccharide-induced liver injury through tumor necrosis factor-α overexpression by monocytes/macrophages: role of protien kinase C and p42/44 mitogen-activated protein kinase. Environ Health Perspect. 2006:114:507–513. 10.1289/ehp.8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Flora SJS, Pachauri V. Chelation in metal intoxication. Int J Environ Res Public Health. 2010:7(7):2745–2788. 10.3390/ijerph7072745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dobrakowski M, Pawlas N, Hudziec E, Kozłowska A, Mikołajczyk A, Birkner E, Kasperczyk S. Glutathione, glutathione-related enzymes, and oxidative stress in individuals with subacute occupational exposure to lead. Environ Toxicol Pharmacol. 2016:45:235–240. 10.1016/j.etap.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 12. Ahamed M, Siddiqui MKJ. Low level lead exposure and oxidative stress: current opinions. Clin Chim Acta. 2007:383(1–2):57–64. 10.1016/j.cca.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 13. Olufemi AC, Mji A, Mukhola MS. Potential health risks of lead exposure from early life through later life: implications for public health education. Int J Environ Res Public Health. 2022:19(23):16006. 10.3390/ijerph192316006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garza A, Vega R, Soto E. Cellular mechanisms of lead neurotoxicity. Med Sci Monit. 2006:12:57–65. https://pubmed.ncbi.nlm.nih.gov/16501435/. [PubMed] [Google Scholar]
- 15. Ettinger AS, Hu H, Hernandez-Avila M. Dietary calcium supplementation to lower blood lead levels in pregnancy and lactation. J Nutr Biochem. 2007:18(3):172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Health Organization (WHO) . Childhood lead poisoning. Geneva: World Health Organization; 2010. https://apps.who.int/iris/handle/10665/136571. [Google Scholar]
- 17. D’souza HS, Dsouza SA, Menezes G, Venkatesh T. Diagnosis, evaluation, and treatment of lead poisoning in general population. Indian JClin Biochem. 2011:26(2):197–201. 10.1007/s12291-011-0122-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aziz FM. Effects of melatonin, vitamin C and E alone or in combination on lead-induced injury in liver and kidney organs of rats. IOSR J Pharm. 2012:2(5):13–18. 10.9790/3013-25201318. [DOI] [Google Scholar]
- 19. Chang WJ, Joe KT, Park HY, Jeong JD, Lee DH. The relationship of liver function tests to mixed exposure to lead and organic solvents. Ann Occup Environ Med. 2013:25(1):5. 10.1186/2052-4374-25-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jarrar BM, Taib NT. Histological and histochemical alterations in the liver induced by lead chronic toxicity. Saudi J Biol Sci. 2012:19(2):203–210. 10.1016/j.sjbs.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sadiq S, Ghazala Z, Chowdhury A, Busselberg D. Metal toxicity at the synapse: presynaptic, postsynaptic, and long-term effects. J Toxicol. 2012:2012:132671. 10.1155/2012/132671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ortega F, Counter SA, Buchanan LH, Parra AM, Collaguaso MA, Jacobs AB. Tracking blood lead and zinc protoporphyrin levels in Andean adults working in a lead contaminated environment. J Toxicol Environ Health A. 2013:76(19):1111–1120. 10.1080/15287394.2013.840708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baghshani H, Shahsavani D. Effects of lead acetate exposure on metabolic enzyme activities in selected tissues of common carp (Cyprinus carpio). Comp Clin Pathol. 2013:22(5):903–907. 10.1007/s00580-012-1497-3. [DOI] [Google Scholar]
- 24. Al-Johany AM, Haffor AS. Effects of cold temperature on the activities of glutathione peroxidase, lactate dehydrogenase and free radicals production in Uromastyx aegyptius. J Med Sci. 2007:7(3):408–412. 10.3923/jms.2007.408.412. [DOI] [Google Scholar]
- 25. Khan D, Qayyum S, Saleem S, Khan FA. Lead-induced oxidative stress adversely affects health of the occupational workers. Toxicol Ind Health. 2008:24(9):611–618. 10.1177/0748233708098127. [DOI] [PubMed] [Google Scholar]
- 26. Obeng-Gyasi E, Armijos R, Weigel M, Filippelli G, Sayegh M. Hepatobiliary-related outcomes in US adults exposed to lead. Environments. 2018:5(4):46. 10.3390/environments5040046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Long M, Liu Y, Cao Y, Wang N, Dang M, He J. Proanthocyanidins attenuation of chronic lead-induced liver oxidative damage in Kunming mice via the Nrf2/ARE pathway. Nutrients. 2016:8(10):656. 10.3390/nu8100656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fernandez NJ, Kidney BA. Alkaline phosphatase: beyond the liver. Vet Clin Pathol. 2007:36(3):223–233. 10.1111/j.1939-165X.2007.tb00216.x. [DOI] [PubMed] [Google Scholar]
- 29. Dhir V, Dhand P. Toxicological approach in chronic exposure to lead on reproductive functions in female rats (Rattus Norvegicus). Toxicol Int. 2010:17(1):1–7. 10.4103/0971-6580.68340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fakoor M, Akhgari M, Shafaroodi H. Lead poisoning in opium-addicted subjects, its correlation with pyrimidine 5′-nucleotidase activity and liver function tests. Int J Prev Med. 2019:10:36. 10.4103/ijpvm.IJPVM_490_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fuhua P, Xuhui D, Zhiyang Z, Ying J, Yu Y, Feng T, Jia L, Lijia G, Xueqiang H. Antioxidant status of bilirubin and uric acid in patients with myasthenia gravis. Neuroimmunomodulation. 2011:19(1):43–49. 10.1159/000327727. [DOI] [PubMed] [Google Scholar]
- 32. Abdel-Moneim AE, Dkhil MA, Al-Quraishy S. The redox status in rats treated with flaxseed oil and lead-induced hepatotoxicity. Biol Trace Elem Res. 2011:143(1):457–467. 10.1007/s12011-010-8882-z. [DOI] [PubMed] [Google Scholar]
- 33. Annabi Berrahal A, Nehdi A, Hajjaji N, Gharbi N, el-Fazâa S. Antioxidant enzymes activities and bilirubin level in adult rat treated with lead. C R Biol. 2007:330(8):581–588. 10.1016/j.crvi.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 34. Vargas H, Castillo C, Posadas F, Escalante B. Acute lead exposure induces renal haeme oxygenase-1 and decreases urinary Na+ excretion. Hum Exp Toxicol. 2003:22(5):237–244. 10.1191/0960327103ht360oa. [DOI] [PubMed] [Google Scholar]
- 35. Aguilar-Dorado IC, Hernández G, Quintanar-Escorza MA, Maldonado-Vega M, Rosas-Flores M, Calderón-Salinas JV. Eryptosis in lead-exposed workers. Toxicol Appl Pharmacol. 2014:281(2):195–202. 10.1016/j.taap.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 36. Teerasarntipan T, Chaiteerakij R, Prueksapanich P, Werawatganon D. Changes in inflammatory cytokines, antioxidants and liver stiffness after chelation therapy in individuals with chronic lead poisoning. BMC Gastroenterol. 2021:21(1):108. 10.1186/s12876-021-01634-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Orisakwe OE, Nwachukwu E, Osadolor HB, Afonne OJ, Okocha CE. Liver and kidney function tests amongst paint factory workers in Nkpor, Nigeria. Toxicol Ind Health. 2007:23(3):161–165. 10.1177/0748233707081908. [DOI] [PubMed] [Google Scholar]
- 38. Mazumdar I, Goswami K. Chronic exposure to lead: a cause of oxidative stress and altered lver function in plastic industry workers in Kolkata, India. Indian J Clin Biochem. 2014:29(1):89–92. 10.1007/s12291-013-0337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Firoozichahak A, Rahimnejad S, Rahmani A, Parvizimehr A, Aghaei A, Rahimpoor R. Effect of occupational exposure to lead on serum levels of lipid profile and liver enzymes: an occupational cohort study. Toxicol Rep. 2022:9:269–275. 10.1016/j.toxrep.2022.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rădulescu A, Lundgren S. A pharmacokinetic model of lead absorption and calcium competitive dynamics. Sci Rep. 2019:9(1):14225. 10.1038/s41598-019-50654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kany S, Vollrath JT, Relja B. Cytokines in inflammatory disease. Int J Mol Sci. 2019:20(23):6008. 10.3390/ijms20236008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chibowska K, Korbecki J, Gutowska I, Metryka E, Tarnowski M, Goschorska M, Barczak K, Chlubek D, Baranowska-Bosiacka I. Pre- and neonatal exposure to lead (Pb) induces Neuroinflammation in the forebrain cortex, hippocampus and cerebellum of rat pups. Int J Mol Sci. 2020:21(3):1–18. 10.3390/ijms21031083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hsiao CL, Wu KH, Wan KS. Effects of environmental lead exposure on T-helper cell-specific cytokines in children. J Immunotoxicol. 2011:8(4):284–287. 10.3109/1547691X.2011.592162. [DOI] [PubMed] [Google Scholar]
- 44. Heo Y, Mondal TK, Gao D, Kasten-Jolly J, Kishikawa H, Lawrence DA. Posttranscriptional inhibition of interferon-gamma production by lead. Toxicol Sci. 2007:96(1):92–100. 10.1093/toxsci/kfl182. [DOI] [PubMed] [Google Scholar]
- 45. Gao D, Kasten-Jolly J, Lawrence DA. The paradoxical effects of lead in interferon-gamma knockout BALB/c mice. Toxicol Sci. 2006:89(2):444–453. 10.1093/toxsci/kfj043. [DOI] [PubMed] [Google Scholar]
- 46. Stavast CJ, Erkeland SJ. The non-canonical aspects of microRNAs: many roads to gene regulation. Cell. 2019:8(11):1465. 10.3390/cells8111465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li Q, Kappil MA, Li A, Dassanayake PS, Darrah TH, Friedman AE, Friedman M, Lambertini L, Landrigan P, Stodgell CJ, et al. Exploring the associations between microRNA expression profiles and environmental pollutants in human placenta from the National Children’s study (NCS). Epigenetics. 2015:10(9):793–802. 10.1080/15592294.2015.1066960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Guo X, Yang Q, Zhang W, Chen Y, Ren J, Gao A. Associations of blood levels of trace elements and heavy metals with metabolic syndrome in Chinese male adults with microRNA as mediators involved. Environ Pollut. 2019:248:66–73. 10.1016/j.envpol.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 49. Masoud AM, Bihaqi SW, Machan JT, Zawia NH, Renehan WE. Early-life exposure to lead (Pb) alters the expression of micro-RNA that target proteins associated with Alzheimer’s disease. J Alzheimers Dis. 2016:51(4):1257–1264. 10.3233/JAD-151018. [DOI] [PubMed] [Google Scholar]
- 50. Bollati V, Marinelli B, Apostoli P, Bonzini M, Nordio F, Hoxha M, Pegoraro V, Motta V, Tarantini L, Cantone L, et al. Exposure to metal-rich particulate matter modifies the expression of candidate microRNAs in peripheral blood leukocytes. Environ Health Perspect. 2010:118(6):763–768. 10.1289/ehp.0901300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hauptman M, Bruccoleri R, Woolf AD. An update on childhood lead poisoning. Clin Pediatr Emerg Med. 2017:18(3):181–192. 10.1016/j.cpem.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Council on Environmental Health . Prevention of childhood lead toxicity. Pediatrics. 2016:138(1):e20161493. 10.1542/peds.2016-1493. [DOI] [PubMed] [Google Scholar]
- 53. U.S. Department of Health and Human Services . Toxicological profile for Lead (update) Public Health Service Agency for Toxic Substances and Disease Registry. Atlanta (GA): Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profiles. 2007. https://www.atsdr.cdc.gov/toxprofiles/tp13.pdf.
- 54. Flora SJS, Bhattacharya R, Vijayaraghavan R. Combined therapeutic potential of meso-2,3-dimercaptosuccinic acid and calcium disodium edetate on the mobilization and distribution of lead in experimental lead intoxication in rats. Fundam ApplToxicol. 1995:25(2):233–240. 10.1006/faat.1995.1059. [DOI] [PubMed] [Google Scholar]
- 55. Paeezi M, Zamani N, Hassanian-Moghaddam H, Shadnia S, Zamani N, Chaleshi V, Mafi AA. Treatment of adult lead poisoning with D-penicillamine. Drug Metab Pers Ther. 2019:34(2):2019–0003. 10.1515/dmpt-2019-0003. [DOI] [PubMed] [Google Scholar]
- 56. Arnold J, Morgan B. Management of Lead Encephalopathy with DMSA after exposure to lead-contaminated moonshine. J Med Toxicol. 2015:11(4):464–467. 10.1007/s13181-015-0493-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Blaurock-Busch E, Busch YM. Comparison of chelating agents DMPS, DMSA and EDTA for the diagnosis and treatment of chronic metal exposure. J Adv Med Med Res. 2014:4(9):1821–1835. 10.9734/BJMMR/2014/6875. [DOI] [Google Scholar]
- 58. Flora SJS. Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxidative Med Cell Longev. 2009:2(4):191–206. 10.4161/oxim.2.4.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Antonio-García MT, Massó-Gonzalez EL. Toxic effects of perinatal lead exposure on the brain of rats: involvement of oxidative stress and the beneficial role of antioxidants. Food Chem Toxicol. 2008:46(6):2089–2095. 10.1016/j.fct.2008.01.053. [DOI] [PubMed] [Google Scholar]
- 60. Najarnezhad V, Aslani MR, Balali-Mood M. The therapeutic potential of thiamine for treatment of experimentally induced subacute lead poisoning in sheep. Comp Clin Pathol. 2010:19(1):69–73. 10.1007/s00580-009-0897-5. [DOI] [Google Scholar]
- 61. Keles M, al B, Gumustekin K, Demircan B, Ozbey I, Akyuz M, Yilmaz A, Demir E, Uyanik A, Ziypak T, et al. Antioxidative status and lipid peroxidation in kidney tissue of rats fed with vitamin B6-deficient diet. Ren Fail. 2010:32(5):618–622. 10.3109/0886022X.2010.481737. [DOI] [PubMed] [Google Scholar]
- 62. Chang B, Jang B, Son TG, Cho IH, Quan FS, Choe NH, Nahm SS, Lee JH. Ascorbic acid ameliorates oxidative damage induced by maternal low-level lead exposure in the hippocampus of rat pups during gestation and lactation. Food Chem Toxicol. 2012:50(2):104–108. 10.1016/j.fct.2011.09.043. [DOI] [PubMed] [Google Scholar]
- 63. Rendón-Ramirez A, Cerbón-Solórzano J, Maldonado-Vega M, Quintanar-Escorza MA, Calderón-Salinas JV. Vitamin-E reduces the oxidative damage on δ-aminolevulinic dehydratase induced by lead intoxication in rat erythrocytes. Toxicol in Vitro. 2007:21(6):1121–1126. 10.1016/j.tiv.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 64. Spencer JPE. Metabolism of tea flavonoids in the gastrointestinal tract. JNutr. 2003:133(10):S3255–S3261. 10.1093/jn/133.10.3255S. [DOI] [PubMed] [Google Scholar]
- 65. al-Omair MA, Sedky A, Ali A, Elsawy H. Ameliorative potentials of quercetin against lead-induced hematological and testicular alterations in albino rats. Chin J Physiol. 2017:60(1):54–61. 10.4077/CJP.2017.BAF440. [DOI] [PubMed] [Google Scholar]
- 66. Pande M, Flora SJS. Lead induced oxidative damage and its response to combined administration of α-lipoic acid and succimers in rats. Toxicology. 2002:177(2–3):187–196. 10.1016/S0300-483X(02)00223-8. [DOI] [PubMed] [Google Scholar]
- 67. Haleagraha N, Jackie T, Chakravart S, Kulur AB. Protective effect of alpha-lipoic acid against lead acetate-induced oxidative stress in the bone marrow of rats. Int J Pharm. 2011:7(2):217–227. https://scialert.net/abstract/?doi=ijp.2011.217.227. [Google Scholar]
- 68. Botella-Pavia P, Rodriguez-Concepcion M. Carotenoid biotechnology in plants for nutritionally improved foods. Physiol Plant. 2006:126(3):369–381. 10.1111/j.1399-3054.2006.00632.x. [DOI] [Google Scholar]
- 69. Mordi RC, Ademosun OT, Ajanaku CO, Olanrewaju IO, Walton JC. Free radical mediated oxidative degradation of carotenes and xanthophylls. Molecules. 2020:25(5):1–13. 10.3390/molecules25051038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Perez-galvez A, Viera I, Roca M. Carotenoids and chlorophylls as antioxidants. Antioxidants. 2020:9(6):1–39. 10.3390/antiox9060505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bonnefont-Rousselot D, Collin F. Melatonin: action as antioxidant and potential applications in human disease and aging. Toxicology. 2010:278(1):55–67. 10.1016/j.tox.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 72. Hernández-Plata E, Quiroz-Compeán F, Ramírez-Garcia G, Barrientos EY, Rodríguez-Morales NM, Flores A, Wrobel K, Wrobel K, Méndez I, Díaz-Muñoz M, et al. Melatonin reduces lead levels in blood, brain and bone and increases lead excretion in rats subjected to subacute lead treatment. Toxicol Lett. 2015:233(2):78–83. 10.1016/j.toxlet.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 73. Shah FC, Jain NK. Ameliorative action of synthetic and herbal antioxidants on lead induced hepatotoxicity: an in vitro study. Asian J Pharm Clin Res. 2016:9:364–370. [Google Scholar]
- 74. Yoo M, Lee S, Kim S, Hwang JB, Choe J, Shin D. Composition of organosulfur compounds from cool- and warm-type garlic (Allium sativum L.) in Korea. Food Sci Biotechnol. 2014:23(2):337–344. 10.1007/s10068-014-0047-y. [DOI] [Google Scholar]
- 75. Chhabria S, Desai K. Purification and characterisation of alliinase produced by Cupriavidus necator and its application for generation of cytotoxic agent: Allicin. Saudi J Biol Sci. 2018:25(7):1429–1438. 10.1016/j.sjbs.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Aslani MR, Najarnezhad V, Mohri M, Azad M. The effect of allicin on blood and tissue lead content in mice. Comp ClinPathol. 2011:20(2):121–125. 10.1007/s00580-010-0964-y. [DOI] [Google Scholar]
- 77. Miron T, Rabinkov A, Mirelman D, Wilchek M, Weiner L. The mode of action of allicin: its ready permeability through phospholipid membranes may contribute to its biological activity. Biochim Biophys Acta Biomembr. 2000:1463(1):20–30. 10.1016/S0005-2736(99)00174-1. [DOI] [PubMed] [Google Scholar]
- 78. Senapati SK, Dey S, Dwivedi SK, Swarup D. Effect of garlic (Allium sativum L .) extract on tissue lead level in rats. J Ethnopharmacol. 2001:76(3):229–232. 10.1016/s0378-8741(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 79. Ebrahimzadeh-Bideskan AR, Hami J, Alipour F, Haghir H, Fazel AR, Sadeghi A. Protective effects of ascorbic acid and garlic extract against lead-induced apoptosis in developing rat hippocampus. Metab Brain Dis. 2016:31(5):1123–1132. 10.1007/s11011-016-9837-7. [DOI] [PubMed] [Google Scholar]
- 80. Sharma V, Sharma A, Kansal L. The effect of oral administration of Allium sativum extracts on lead nitrate induced toxicity in male mice. Food Chem Toxicol. 2010:48(3):928–936. 10.1016/j.fct.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 81. Velaga MK, Yallapragada PR, Williams D, Rajanna S, Bettaiya R. Hydroalcoholic seed extract of Coriandrum sativum (coriander) alleviates lead-induced oxidative stress in different regions of rat brain. Biol Trace Elem Res. 2014:159(1–3):351–363. 10.1007/s12011-014-9989-4. [DOI] [PubMed] [Google Scholar]
- 82. Tellez-lopez MA, Mora-Tovar G, Ceniceros-Mendez IM, Garcia-Lujan C, Puente-Valenzuela CO, Vega-Menchaca MC, Serrano-Gallardo LB, Garza RG, Moran-Martinez J. Evaluation of the chelating effect of methanolic extract of coriandrum sativum and its fractions on wistar rats poisoned with lead acetate. Afr J Tradit Complement Altern Med. 2017:14(2):92–102. 10.21010/ajtcam.v14i2.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. el-Ashmawy IM, Ashry KM, el-Nahas AF, Salama OM. Protection by turmeric and myrrh against liver oxidative damage and genotoxicity induced by lead acetate in mice. Basic Clin Pharmacol Toxicol. 2006:98(1):32–37. 10.1111/j.1742-7843.2006.pto_228.x. [DOI] [PubMed] [Google Scholar]
- 84. Ostrowska J, Skrzydlewska E. The comparison of effect of catechins and green tea extract on oxidative modification of LDL in vitro. Adv Med Sci. 2006:51:298–303PMID: 17357329. [PubMed] [Google Scholar]
- 85. Khalaf AA, Moselhy WA, Abdel-Hamed MI. The protective effect of green tea extract on lead induced oxidative and DNA damage on rat brain. Neurotoxicology. 2012:33(3):280–289. 10.1016/j.neuro.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 86. Khaki AA. Antioxidant effect of ginger to prevents lead-induced liver tissue apoptosis in rat. J Med Plant Res. 2010:4:1492–1495. 10.5897/JMPR09.397. [DOI] [Google Scholar]
- 87. Poltanov EA, Shikov AN, Dorman HJD, Pozharitskaya ON, Makarov VG, Tikhonov VP, Hiltunen R. Chemical and antioxidant evaluation of Indian gooseberry (Emblica officinalis Gaertn., syn. Phyllanthus emblica L.) supplements. Phytother Res. 2009:23(9):1309–1315. 10.1002/ptr.2775. [DOI] [PubMed] [Google Scholar]
- 88. Brimson JM, Brimson S, Prasanth MI, Thitilertdecha P, Malar DS, Tencomnao T. The effectiveness of Bacopa monnieri (Linn.) Wettst. as a nootropic, neuroprotective, or antidepressant supplement: analysis of the available clinical data. Sci Rep. 2021:11(1):596. 10.1038/s41598-020-80045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Jyoti A, Sharma D. Neuroprotective role of Bacopa monniera extract against aluminium-induced oxidative stress in the hippocampus of rat brain. Neurotoxicology. 2006:27(4):451–457. 10.1016/j.neuro.2005.12.007. [DOI] [PubMed] [Google Scholar]


