Abstract
We collected 111 Agrobacterium isolates from galls of various origins (most of them from France) and analyzed both their plasmid-borne and chromosome-encoded traits. Phenotypic analysis of these strains allowed their classification in three phena which exactly matched the delineation of biovars 1, 2, and 3. A fourth phenon was identified which comprises three atypical strains. The phenotypic analysis has also allowed us to identify 12 additional characteristics which could be used to identify the three biovars of Agrobacterium. Our results also suggest that biovar 1 and 2 represent distinct species. Analysis of plasmid-borne traits confirmed that tartrate utilization is a common feature of biovar 3 strains (now named Agrobacterium vitis) and of Agrobacterium grapevine strains in general. Among pathogenic strains of Agrobacterium, several exhibited unusual opine synthesis and degradation patterns, and one strain of biovar 3 induced tumors containing vitopine and a novel opine-like molecule derived from putrescine. We have named this compound ridéopine.
Agrobacterium sp. is a pathogenic bacterium responsible for two plant diseases: crown gall and hairy root. As these names suggest, the visible symptoms at the infection site are the appearance of tumorous overgrowths and roots for crown gall and hairy root, respectively. Both diseases are examples of natural interkingdom genetic exchange, because the infectious process relies on the transfer of a DNA fragment(s) from the prokaryote Agrobacterium to the eukaryotic plant cells. This transferred DNA, or T-DNA, is borne on extrachromosomal bacterial replicons. These replicons are the Ti (tumor-inducing) plasmid found in bacteria responsible for crown gall disease, and the Ri (root-inducing) plasmid found in bacteria responsible for hairy root disease. Once transferred to the plant, the T-DNA integrates into the nuclear genome of the cell, where T-DNA genes are transcribed. The molecular mechanism underlying the transfer of DNA has been extensively reviewed (e.g., see references 11, 27 and 41).
Genes located on the T-DNA fall into two groups. The first one includes genes responsible for tumor or root formation (for reviews, see references 4 and 18). The second group of T-DNA genes encode enzymes catalyzing the synthesis of the low-molecular-weight compounds specific for the crown gall or hairy root cells. These compounds, termed opines, generally result from the condensation of amino acids and alpha-ketoacids, or aminoacids and sugars; they play a key role in the ecology of the plant-Agrobacterium interaction (for reviews, see references 12 and 13). The combination of opines, the synthesis and the degradation of which are due to genes borne on Ti and Ri plasmids, provides the basis for a simple classification of the pathogenic plasmids of Agrobacterium (4, 13). However, data collected from the analysis of Ti plasmids isolated from grapevine isolates strongly suggest that these plasmids are mosaic plasmids, with conserved and variable regions (30, 31, 52).
It appears that the type of disease induced by Agrobacterium depends on the type of plasmid hosted by the bacteria. In this respect, the former delineation of Agrobacterium species based on the disease symptoms, hence on traits due to plasmid-borne genes, is of little value (for a review, see reference 51). A stronger classification of Agrobacterium species has been performed using numerical taxonomy of phenotypic properties (22, 54), analysis of fatty acid methyl ester profiles (20, 44), or comparison of electrophoregrams of soluble proteins (23). These results indicate clearly that the genus Agrobacterium can be divided into three different clusters which correspond to biovars 1, 2, and 3, as termed by Keane et al. (21). Biovar 3 is now regarded as the Agrobacterium species A. vitis, which includes strains isolated from grapes (29). Similarly, biovars 1 and 2 could define different species of Agrobacterium. Further studies will be crucial to confirm or refute this hypothesis. Such studies may lead to a deep reorganization of the Rhizobium-Agrobacterium clusters within the family Rhizobiaceae, since some Agrobacterium strains have more characteristics in common with Rhizobium than with Agrobacterium (51).
Among commonly infected plants, grapevine is of major commercial importance. In France, grapevine galls have been reported in cold parts of the Rhone Valley, but also in the Bordeaux and Loire Valley regions (39). The spread has resulted from a combination of cold climatic conditions and the poor sanitary status of the cultivated material (3, 7, 8, 17, 25, 26, 28, 33, 40, 46, 48, 50, 55, 56; for a review, see reference 14). A better characterization of the Agrobacterium strains would facilitate their routine identification and subsequent control of plant sanitary conditions. To this end, we have collected 61 isolates from grapevine galls and analyzed their traits due to both plasmid-borne and chromosome-encoded genes with respect to other Agrobacterium strains, including reference strains. The results of this study are reported below.
MATERIALS AND METHODS
Bacterial strains.
Out of 111 Agrobacterium strains used in this study, 88 were isolated in France between 1976 and 1989, and 23 were of various origins and deposited in the French Collection of Phytopathogenic Bacteria (CFBP). Two clinical isolates were obtained from the Pasteur Institute (Paris, France) (Table 1). Agrobacterium isolates were grown on LPGA medium (38) which consisted of yeast extract (Difco Laboratories, Detroit, Mich.), 5 g/liter; Bacto Peptone (Difco), 5 g/liter; glucose, 10 g/liter; and 15 g/liter (pH adjusted to between 7 and 7.2).
TABLE 1.
Origin of strains; results of pathogenicity tests on various plants; and opine production and utilization of biovar 1, 2, and 3 strains
| Biovar | Host plant or source | Yr | Laboratory no. | CFBP strain | Geographical origind | Person who isolated | Pathogenicity ona:
|
Opinec
|
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T | D | Kb | Production | Utilization | |||||||
| 1 | Vitis vinifera | ||||||||||
| Danam | 1982 | 143 | 2407 | F34 | Ridé | − | NTe | +++RE | C, O | C, O | |
| Cabernet sauvignon 41B | 1987 | 279 | 2683 | F33 | Ridé | − | − | −E | NT | NT | |
| Pinot noir 41B | 1987 | 311 | 2732 | F51 | Petit | − | − | −E | C, O | N, C, O | |
| Merlot 3309 | 1987 | 277 | 2682 | F33 | Ridé | − | − | −E | C | N, C, O | |
| Grenache | 360-1 | 2514 | S (Navarra) | Lopez | − | − | −E | NT | NT | ||
| Sultana | LBA 649 | 2883 | GR | Hoekema | NT | NT | + | NT | NT | ||
| Sultana | Ag20 | 1904 | GR | Panagopoulos | + | + | +++Re | N | N | ||
| Cabernet sauvignon | 1985 | 224 | 2642 | F33 | Ridé | − | − | ++rE | C, O | C, O | |
| Cabernet sauvignon R140 | 1987 | 276 | 2655 | F33 | Ridé | − | − | ++RE | C, O | C, O | |
| Cabernet sauvignon R140 | 1987 | 275 | 2654 | F33 | Ridé | + | − | ++RE | C, O | C, O | |
| Prunoideae | |||||||||||
| Prunus × GF677 | 1987 | 302 | 2716 | F84 | Ridé | + | NT | +++ST | NT | M | |
| Prunus × GF677 | 1987 | 299 | 2713 | F84 | Ridé | + | + | +++rS | MA | M | |
| P. cerasus | C58 | 1903 | USA | Dickey | NT | NT | +++rs | N | NT | ||
| Prunus × GF677 | 1987 | 300 | 2714 | F84 | Ridé | + | + | +++rS | MA | M | |
| P. rubiera | 1988 | 317 | 2741 | F13 | Ridé | − | NT | − | NT | N, O | |
| Prunus × GF677 | 1987 | 303 | 2717 | F84 | Ridé | + | NT | +++rs | NT | M | |
| Prunus × GF677 | 1987 | 298 | 2712 | F84 | Ridé | + | − | +++rS | MA | M | |
| P. rubiera | 1988 | 318 | 2879 | F13 | Ridé | + | NT | NT | NT | N | |
| Pomoideae | |||||||||||
| Malus pumila | 1977 | 96 | 1933 | F02 | Lopez | − | − | +++rS | N | NT | |
| Malus sp. | B6 | 2413 | USA | Braun | NT | NT | +++R | O | NT | ||
| Pyrus syriaca | U85 | 2747 | SYR | Abu-Ghorrah | + | + | ++R | NT | NT | ||
| Populus | |||||||||||
| P. tremula × P. alba 712-1-856 | 1988 | 341 | 2795 | F87 | Ridé | + | + | +++Rs | N | NT | |
| P. tremula | 1982 | 146 | 2177 | F45 | Ridé | + | NT | +++rs | N | N | |
| Populus × (Leuce) | 1985 | 10 | 2517 | F45 | Michel | + | + | ++r | N | NT | |
| P. alba | 1988 | 347 | 2885 | F87 | Ridé | + | + | + | N | NT | |
| Chrysanthemum | 1988 | 330 | 2788 | F72 | Ridé | + | + | +++s | N | NT | |
| From hospital | |||||||||||
| Vagina | A65-97 (H8) | 2243 | USA | NT | NT | NT | NT | NT | |||
| Blood | Ag032 (H5) | 2884 | F | Pasteur Institute | NT | NT | NT | NT | NT | ||
| 2 | Vitis vinifera (unknown) | Ag28 | 1905 | GR | Panagopoulos | + | + | +E | N | N | |
| Prunoideae | |||||||||||
| P. persica | 1976 | 76 | 1804 | F24 | Lopez | + | NT | +++rS | N | NT | |
| P. mahaleb | 1977 | 94 | 1962 | F24 | Lopez | + | NT | +++Rs | N | NT | |
| P. avium F12-1 | 1982 | 139 | 2178 | F45 | Ridé | + | NT | +++rS | N | N | |
| P. avium F12-1 | 1982 | 145 | 2719 | F45 | Ridé | + | + | +++Rs | N | NT | |
| P. avium F12-1 | 1982 | 144 | 2718 | F45 | Ridé | + | NT | +++rs | N | NT | |
| P. avium × P. cerasus Colt | 1982 | 149 | 2326 | F34 | Audusseau | + | NT | NT | N | NT | |
| P. avium × P. cerasus Colt | 1984 | 207 | 2417 | F30 | Audusseau | + | NT | NT | N | NT | |
| P. avium × P. cerasus Colt | 1984 | 210 | 2420 | F30 | Audusseau | + | NT | +++Re | N | NT | |
| P. silvestris | 1987 | 264 | 2691 | F30 | Ridé | + | NT | +++rS | NT | NT | |
| Prunus sp. | 1987 | 265 | 2692 | F30 | Ridé | + | NT | +++rS | MA | NT | |
| Prunus sp. | 1987 | 266 | 2693 | F30 | Ridé | + | NT | −E | MA | NT | |
| Prunus sp. | 1987 | 260 | 2687 | F84 | Ridé | + | NT | +++ST | N | NT | |
| Prunus sp. | 1987 | 261 | 2688 | F84 | Ridé | + | NT | +++rST | N | NT | |
| Prunus sp. | 1987 | 262 | 2689 | F84 | Ridé | + | NT | +++rS | N | NT | |
| P. mariana | 1988 | 356 | 2942 | F47 | Ridé | + | NT | + | NT | NT | |
| P. persica | 1988 | 316 | 2740 | F13 | Ridé | + | NT | +E | NT | N | |
| Prunus × GF677 | 1988 | 328 | 2744 | F84 | Nesme | + | + | ++rS | N | NT | |
| Pomoideae | |||||||||||
| Malus M9 | 1977 | M9 | 1931 | F24 | Lopez | − | − | −E | NT | NT | |
| M. pumila | 1987 | 310 | 2728 | F49 | Ridé | − | − | −E | − | − | |
| M. pumila | 1987 | 313 | 2729 | F84 | Ridé | − | − | − | − | − | |
| M. pumila | 1988 | 358 | 2944 | F49 | Ridé | − | NT | − | NT | NT | |
| Pyrus communis | 1988 | 359 | 2945 | F49 | Ridé | − | NT | − | NT | NT | |
| Malus M9 | 1988 | 327 | 2880 | F49 | Ridé | − | − | −E | NT | NT | |
| Populus | |||||||||||
| P. bolleana | 1976 | 74 | 1961 | F78 | Ridé | + | + | +++ | N | NT | |
| P. alba | 1976 | 75 | 1840 | F78 | Ridé | + | + | ++RSE | N | N | |
| P. tremula × P. alba 709-27 | 1988 | 338 | 2881 | F87 | Ridé | + | − | ++R | N | NT | |
| P. tremula × P. alba 712-8 | 1988 | 336 | 2792 | F87 | Ridé | + | + | +++R | N | NT | |
| Others | |||||||||||
| Rosa sp. | 1979 | 115 | 1935 | TAH | Ridé | + | + | +++rSe | N | N | |
| Rosa sp. | 1979 | 116 | 1936 | TAH | Ridé | + | NT | +++rS | N | NT | |
| Actinidia | 1988 | 319 | 2742 | F64 | Ridé | − | NT | − | NT | − | |
| Soil | K84 | 1937 | AUS | Kerr | − | − | −E | NT | N | ||
| 3 | Vitis vinifera | ||||||||||
| Danam | 1982 | 140 | 2179 | F34 | Ridé | + | + | +++RST | N | N | |
| Unknown | 1982 | 339-6 | 2513 | S (Orense) | Lopez | + | + | ++rS | C, O | N, C, O | |
| Ribol | 1984 | 230 | 2607 | F84 | Ridé | + | − | +++RE | C, O | C, O | |
| Cabernet franc | 1985 | 221 | 2641 | F33 | Ridé | + | − | +++Re | C, O | N, O | |
| Cabernet franc | 1985 | 222 | 2608 | F33 | Ridé | + | − | +++Rs | C, O | N, C, O | |
| Cabernet franc | 1985 | 223 | 2609 | F33 | Ridé | + | NT | ++rST | N | N | |
| Cabernet sauvignon | 1985 | 228 | 2610 | F33 | Ridé | + | NT | +++S | N | N | |
| Cabernet sauvignon | 1985 | 229 | 2643 | F33 | Ridé | + | + | ++rSE | N | N | |
| Cabernet franc | 1985 | 226 | 2674 | F33 | Ridé | + | NT | ++Se | N | N | |
| Cabernet franc | 1985 | 225 | 2673 | F33 | Ridé | + | + | ++rS | N | N | |
| Unknown | 1985 | 550-2 | 2515 | S (Portevedro) | Lopez | + | + | ++rse | C, O | C, O | |
| Unknown | 1985 | 565-5 | 2512 | S (Bajoz) | Lopez | + | + | ++S | N | N | |
| Unknown | K305 | 2736 | AUS | Kerr | + | − | +++rs | C, O | NT | ||
| Unknown | K308 | 2737 | AUS | Kerr | + | − | +++rs | C, O | NT | ||
| Unknown | K374 | 2620 | AUS | Kerr | + | NT | +++RST | N | N | ||
| Sultana | 1963 | 63-85 | 2622 | GR (Crete) | Panagopoulos | + | − | +++RE | C, O | C, O | |
| Sultana | Ag82-81 | 2738 | GR | Panagopoulos | + | NT | +++ | C, O | C, O | ||
| Ugni blanc | 1986 | 258 | 2650 | F17 | Ridé | + | − | ++RE | C, O | C, O | |
| Cabernet franc | 1986 | 243 | 2644 | F49 | Ridé | + | − | +++rE | C, O | C, O | |
| Chenin | 1986 | 254 | 2617 | F49 | Ridé | + | − | +++Re | C, O | C, O | |
| Cabernet franc | 1986 | 242 | 2675 | F49 | Ridé | + | − | ++RE | C, O | C, O | |
| Cabernet franc | 1986 | 245 | 2676 | F49 | Ridé | + | + | ++RE | C, O | C, O | |
| Chenin | 1986 | 247 | 2645 | F49 | Ridé | + | − | +++RSE | C, O | N, C, O | |
| Chenin | 1986 | 249 | 2615 | F49 | Ridé | + | + | ++++rS | C, O | N, C, O | |
| Chenin | 1986 | 250 | 2616 | F49 | Ridé | + | − | +++Rse | C, O | C, O | |
| Chenin | 1986 | 251 | 2646 | F49 | Ridé | + | − | +++RS | C, O | N, C, O | |
| Chenin | 1986 | 253 | 2648 | F49 | Ridé | + | − | +++rse | C, O | N, C, O | |
| Cabernet sauvignon | 1986 | 252 | 2647 | F49 | Ridé | + | NT | ++RSe | C, O | N, C, O | |
| Cabernet sauvignon | 1986 | 255 | 2618 | F49 | Ridé | + | + | +++rS | C, O | N, C, O | |
| Cabernet franc (1 yr) | 1986 | 246 | 2613 | F49 | Ridé | + | − | ++RE | C, O | C, O | |
| Cabernet franc (1 yr) | 1986 | 248 | 2615 | F49 | Ridé | + | + | +++RE | C, O | C, O | |
| Chenin | 1987 | 259 | 2651 | F44 | Ridé | + | − | ++RE | C, O | C, O | |
| Melon | 1986 | 256 | 2649 | F44 | Ridé | + | NT | +++RS | C, O | C, O | |
| Melon | 1987 | 257 | 2677 | F44 | Ridé | + | − | ++Re | C, O | C, O | |
| Cabernet sauvignon | 1987 | 273 | 2653 | F33 | Ridé | + | − | +++RE | C, O | C, O | |
| Cabernet sauvignon | 1987 | 280 | 2656 | F33 | Ridé | + | NT | +++RE | C, O | C, O | |
| Grenache | 1987 | 270 | 2679 | F49 | Ridé | + | NT | +++RS | C, O | N, C, O | |
| Grenache | 1987 | 268 | 2652 | F49 | Ridé | + | NT | +++rsE | C, O | C, O | |
| Grenache | 1987 | 269 | 2678 | F49 | Ridé | + | NT | +++RS | C, O | C, O | |
| Cabernet franc | 1987 | 284 | 2657 | F37 | Ridé | + | − | +++RS | C, O | N, C, O | |
| Cabernet sauvignon | 1987 | 285 | 2668 | F37 | Ridé | + | NT | +++RS | C, O | N, C, O | |
| Grenache | 1987 | 272 | 2680 | F49 | Ridé | + | − | +++RSe | C, O | C, O | |
| Sultana | 1970 | 57-81 | 2621 | GR (Crete) | Panagopoulos | + | + | ++RE | C, O | C, O | |
| Unknown | A260 (S4) | 2660 | H | Szegedi | + | + | ++rs | V | NT | ||
| Navanesizu | A258 (NI-1) | 2659 | H | Szegedi | + | + | +++ST | N | N | ||
| Pinot meunier | 1988 | 332 | 2770 | F02 | Ridé | + | + | +++rs | C, O | NT | |
| Cabernet sauvignon | 1987 | 274 | 2681 | F33 | Ridé | + | + | +++RE | V, R | NT | |
| Uncertain | |||||||||||
| Cabernet franc | 1987 | 306 | 2724 | F37 | Ridé | − | − | − | C, O | C, O | |
| Cabernet franc | 1987 | 307 | 2725 | F37 | Ridé | − | − | − | N | C, O | |
| Pinot noir | 1988 | 331 | 2771 | F51 | Ridé | − | − | + | N | NT | |
Pathogenicity tests were performed with on tomato (T), datura (D), and kalanchoe (K) plants. Reactions: +, positive; −, negative.
Reactions observed on the tumors: R, r: roots (many, some); S, s: shoots (many, some); T: teratogenic tumor; E, e: embryo-like organs (many, some).
Opine names are indicated as follows: C, cucumopine; O, octopine; N, nopaline; MA, mannopine; V, vitopine; R, ridéopine.
Abbreviations for French departments are indicated by an F followed by the number of the department: 02 (Aisne), 13 (Bouches du Rhône), 17 (Charente-Maritime), 24 (Dordogne), 30 (Gard), 33 (Gironde), 34 (Hérault), 37 (Indre-et-Loire), 44 (Loire-Atlantique), 45 (Loiret), 47 (Lot-et-Garonne), 49 (Maine-et-Loire), 51 (Marne), 64 (Pyrénées-Atlantiques), 72 (Sarthe), 78 (Yvelines), 84 (Vaucluse), or 87 (Haute-Vienne). Other abbreviations: AUS, Australia; S, Spain; GR, Greece; SYR, Syria; USA, United States; TAH, Tahiti.
NT, not tested.
Biochemical characters for presumptive diagnosis of Agrobacterium.
Gram strain response was determined using the aminopeptidase test from Merck (Darmstadt, Germany). The following conventional biochemical characteristics were assessed according to the method of Popoff et al. (36): presence of esculin-β-glucosidase, urease (in urea-indol medium; Diagnostics Pasteur, Marne-la-Coquette, France), orthonitro-phenyl-β-d-galactopyranoside (ONPG) β-galactosidase, gelatinase, Tween 80 esterase, DNase on DNA agar (Diagnostics Pasteur). 3-Ketolactose production (according to Bernaerts and De Ley [2]) and phenylalanine desaminase (PAD) activity were also assayed. PAD detection was carried out on phenylalanine agar, which was made of dl-phenylalanine, 2 g/liter; yeast extract (Difco), 3 g/liter; NaCl, 5 g/liter; K2HPO4, 1 g/liter; and agar, 12 g/liter. Agrobacterium strains were streaked on this medium to a high density and kept at 26 to 27°C. After 40 to 48 h, the culture was covered with a few drops of FeCl3 (density, 1.26) diluted 1/3 (vol/vol) with distilled water. A positive assay is indicated by an olive-green coloration appearing rapidly and remaining stable for 1 to several hours. Characteristics presumptive for Agrobacterium species were confirmed for all assayed strains using the identification system for Pseudomonas and related bacteria (Diagnostics Pasteur). This system also gave data on nitrate and arginine metabolism.
Nutritive characteristics.
Utilization (with acid formation) of melizitose, dulcitol, erythritol, and ethanol and utilization (with alkali formation) of l-(+)-tartrate and malonate were assayed. These compounds were added at 1% (vol/vol or wt/vol) to the minimal medium, which consisted of NH4H2PO4, 1 g/liter; KCl, 2 g/liter; MgSO4 · 7H2O 0.2 g/liter; yeast extract (Difco), 0.1 g/liter; and bromothymol blue, 0.08 g/liter (pH 7.2) (1). Five milliliters of this medium inoculated with Agrobacterium strains using 48-h precultures performed on LPGA medium (38), and incubated in a shaker (120 rpm) at 27°C. Growth and acid production were generally stopped after 72 h of incubation but for some strains were stopped after 5 days of incubation.
The assimilation of 49 carbohydrates, 49 organic acids, and 49 amino acids was studied using API 50 CH, LRA 50 AO, and LRA 50 AA strip tests (BioMérieux, La Balme Les Grottes, France). The inoculated strips were maintained at 26°C, and growth was assessed after 5 days.
Digital-numerical taxonomy.
A total of 167 characteristics (based on 20 biochemical and physiological tests plus assimilation of carbon sources) were included in the digital-numerical taxonomy analysis. A distance matrix was calculated using the Jaccard coefficient (47). Cluster analysis was done by using the unweighted pair group method of average with arithmetic mean (47).
Pathogenicity assays.
Three plant species were used: Kalanchoe tubiflora, Datura stramonium, and Lycopersicon esculentum (var. Montfavet 63/5). These were kept in a growth chamber at a day temperature of 23°C and a night temperature of 18°C, with a 16-h light, 8-h dark photoperiod and a relative humidity of 80 to 85%. Suspensions of the bacteria to be assayed were made in sterile water and adjusted to ca. 108 CFU/ml. Of these suspensions, 50-μl aliquots were used to inoculate the plants wounded at the second, fifth, and sixth internodes starting from the apex (K. tubiflora) or at the second and fourth internodes (D. stramonium and L. esculentum) at the stage when four leaves had expanded. The reactivity to inoculation was estimated after 40 days to differentiate the various types of reaction, particularly on K. tubiflora. Appearance of tumorous outgrowths was assessed by visual inspection of the inoculated plants.
Opine detection in the tumors and opine utilization by the bacteria.
Detection of opines in tumorous tissues and their utilization by the inducing bacteria were performed by using high-voltage paper electrophoresis, as reviewed by Dessaux et al. (12).
RESULTS AND DISCUSSION
Analysis of phenotypic, chromosome-encoded characteristics. (i) Identification of Agrobacterium strains.
All assayed strains (n = 111) exhibited ONPG-hydrolase (β-galactosidase) and urease activities, and were able to degrade esculin. This confirmed that these strains belonged to the genus Agrobacterium (24). Additionally, the assayed strains were not able to degrade gelatin or to reduce tetrathionate. It is noteworthy that a negative response for the DNase and Tween esterase assays cannot be used as an orientation test for identifying Agrobacterium strains because, out of 111 assayed strains, 12 exhibited DNase activity while 6 produced a Tween esterase.
(ii) Numerical taxonomy.
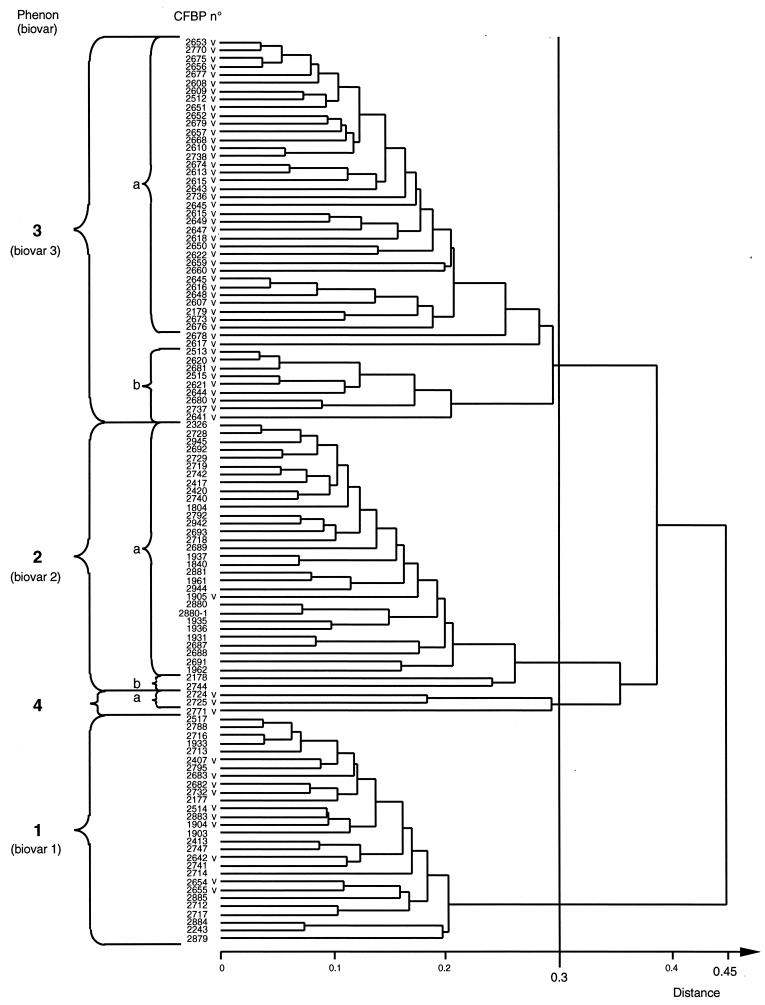
The dendrogram displaying the distance relationships amongst the 111 strains included in this study is shown in Fig. 1. At a phenotypic distance of 0.3, three major and one minor phena were delineated. The major phena 1, 2, and 3 precisely group strains of the three biovars, 1, 2, and 3, respectively. Phenon 4 included three strains, CFBP 2724, 2725, and 2771. Although these strains clustered with biovar 2 strains at a distance of 0.354, they must be regarded as atypical since they exhibit many characteristics which are not common to those of biovar 2 strains (Table 2). Whether the three above-mentioned strains are related to those described by Bouzar et al. (6) remains to determined. At a shorter distance (0.254), phenon 3 divided into two subphena (3a and 3b) which comprised, respectively, 36 and 9 strains, leaving 2 isolated strains (CFBP 2617 and CFBP 2678). At the same distance (0.254), phenon 2 divided into two subphena (2a and 2b) which comprised, respectively, 31 and 2 strains. Strains isolated from grapevines clustered as follows: 10 strains in phenon 1 (which includes 28 strains), 1 strain in phenon 2 (which includes 33 strains), 47 strains in phenon 3 (which includes 47 strains), and 3 strains in phenon 4 (which includes 3 strains). Overall, and except for the three strains CFBP 2724, 2725, and 2771, biovar determination yields clear-cut results. The perfect correspondence between phena 1 and 2 and biovars 1 and 2, respectively, strongly suggests that biovars 1 and 2 could correspond to two distinct species. Bouzar (5) and Sawada et al. (45) previously made this proposal.
FIG. 1.
Phenotypic analysis of 111 Agrobacterium strains. Results are presented as a dendrogram based on phenotypic distance value calculation using the Jaccard coefficient and the unweighted pair group method of average with arithmetic mean method. V, from grapevine.
TABLE 2.
Phenotypic characteristics that differentiate biovars and subphena and strainsa
| Characteristic | Biovar (phenon)
|
Strain
|
||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2
|
3
|
2771 | 2724 | 2725 | |||
| a | b | a | b | |||||
| Dulcitol | + | + | + | − | − | + | + | + |
| β-Methyl-d-xyloside | + | + | − | − | − | + | − | − |
| PAD | − | − | − | + | + | − | + | + |
| Erythritol | − | + | + | − | − | + | + | + |
| Oxydase | + | − | − | + | + | + | + | − |
| Malonate | − | + | + | + | + | − | − | − |
| l-Ornithine | + | − | − | − | − | − | − | − |
| Sarcosine | + | − | − | − | − | − | − | − |
| Arginine dihydrolase | + | − | − | − | − | + | + | − |
| 3-Ketolactose | + | − | − | − | − | − | − | − |
| Melezitose | + | − | − | − | − | + | + | + |
| d-Fucose | + | − | − | − | − | + | + | + |
| Proprionate | + | − | − | − | − | − | − | − |
| l-Arabitol | + | − | − | − | − | − | − | − |
| Citrate | − | + | + | + | + | − | − | − |
| Xylitol | + | + | + | − | − | − | + | + |
| d-Arabinose | + | + | + | − | − | + | + | − |
| Aconitate | + | d | d | + | − | − | − | − |
| α-Methyl-glucoside | + | − | + | + | − | + | − | − |
| l-Rhamnose | + | − | + | − | + | − | − | − |
| l-Arginine | + | − | + | + | − | + | − | − |
| d-Tagatose | + | − | + | + | − | + | − | − |
+, 90 to 100% of the strains are positive; −, 0 to 10% of the strains are positive; d, 11 to 89% of the strains are positive.
(iii) Differential characteristics.
The characteristics that differentiate the three phena and the three strains of phenon 4 are shown in Table 2. Ten assays (3-ketolactose production, presence of oxidase, presence of PAD, and utilization of dulcitol, melezitose, l-rhamnose, malonate, propionate, citrate, and l-ornithine) have been used previously by Kersters and De Ley (24) to differentiate among biovars of Agrobacterium. As shown in Table 2, 12 additional characteristics could be used to identify the biovars and the three isolated strains. Interestingly, our results confirm the validity of using the 3-ketolactose criterion to identify biovar 1 strains, since all these strains produced this lactose derivative (Table 2).
The 47 grapevine strains (clustered in phenon 3), strains CFBP 2724 and 2725 (biovar undetermined, phenon 4), and the two clinical isolates CFBP 2243 and 2884 (biovar 1, from human origin) produce a PAD. This result is in agreement with those of Popof et al. (36), who previously reported on clinical isolates harboring PAD activity. Though it is not an absolute criterion, production of PAD therefore might be a useful orientation assay to identify grapevine strains belonging to the species A. vitis (biovar 3).
Arginine dihydrolase (assayed using the Pasteur gallery of tests) was detected only in biovar 1 strains and in the atypical strains CFBP 2724 and 2771. This characteristic therefore allows the differentiation of biovar 1 strains from strains of the biovars 2 and 3. However, while no arginine dihydrolase was found in strains of biovars 2 and 3, some of them assimilated arginine. These were biovar 2 strains CFBP 1936, 2178, 2688, and 1931 and biovar 3 strains CFBP 2736, 2737, and 2620 (from Australia); CFBP 2621 and 2738 (from Greece); and CFBP 2513 and 2515 from Spain. This feature can be related to the existence of different pathways for assimilation of arginine in this bacterium and to the presence on some Agrobacterium plasmids of genes responsible for arginine degradation (15; for a review, see reference 13).
Among the three biovars, reduction of nitrates is a variable character. Only 10 out of 28 biovar 1 strains reduced nitrate to nitrogen. One biovar 2 strain and 12 of the 47 biovar 3 strains reduced nitrate to nitrite.
Analysis of traits due to plasmid-borne genes. (i) Utilization of l-(+)-tartrate.
Utilization of l-(+)-tartrate yielded positive results for all biovar 2 and 3 strains and only for the biovar 1 strains isolated from grapevine tumors. Though it has been demonstrated that utilization of l-(+)-tartrate is characteristic of many plant-pathogenic bacteria, Szegedi (49) suggested that the degradation of this compound by A. vitis (biovar 3) strains might be due to their adaptation to grapevines. Indeed, tartaric acid is a major chemical component of grapevines (37, 42). Our results are consistent with Szegedi's hypothesis.
Among strains of A. vitis, two independent pathways for tartrate metabolism exist. In the model A. vitis strain AB3, the enzymes defining a first pathway are encoded by genes located on pTrAB3 at the TARI region while enzymes defining a second pathway are encoded by genes located on pTiAB3 at the TARII region (10, 32, 43). Because tartrate utilization in biovar 1 strains is restricted solely to the strains isolated from grapevines, it is tempting to speculate that utilization of l-(+)-tartrate by these strains is due to the in planta transfer of a plasmid bearing the genes encoding utilization of l-(+)-tartrate, possibly from biovar 3 to biovar 1 strains. Moreover, biovar 3 and 1 strains were indeed isolated from the same grapevine plant.
(ii) Pathogenicity assays.
The results of the pathogenicity assays are summarized in Table 1. Only ca. 60% of the biovar 1 strains induced tumors upon inoculation of tomato plants and daturas. Among the strains which were nonpathogenic on tomato plants, seven were isolated from grapevines. Out of these seven strains, three induced overgrowths on daturas, suggesting a possible host range limitation. On the other hand, biovar 1 strains isolated from other host plants (Prunoideae, Pomoideae, Populus sp. and Chrysanthemum sp.) were pathogenic on most if not all test plants, with the exception of strain CFBP 2741 isolated from Prunus rubiera tumors.
Interestingly, biovar 2 strains CFBP 1931, 2728, 2729, 2944, 2945, and 2880 isolated from rootstocks of apple trees and Pyrus communis and strain CFBP 2742 isolated from kiwi plants did not induce tumors on the test plants. The results obtained with the Agrobacterium strains isolated from apple rootstock are reminiscent of those reported by Picard (35). All the other biovar 2 strains, isolated from Prunoideae, Populus sp., and Rosa sp., induced tumor formation on tomato plants daturas or kalanchoes. On kalanchoes, ca. 70% of biovar 2 strains induced large tumors.
Biovar 3 strains always induced tumors on both tomato plants and kalanchoes, but most of them did not induce tumors on daturas. On kalanchoes, ca. 65% of the strains incited large tumors.
In addition to variation affecting the size of tumors, we also observed a wide range of tumor morphologies upon inoculations of kalanchoes. To take into account all these results, we utilized the following traits (Table 1): presence of roots at the lower part of the tumors, presence of shoots at the upper part of the tumors, teratogenic organization defined as tumors covered with fasciated shoots and hypertropic roots, and presence of embryolike organs defined as plantlets growing on leaf edges of the inoculated plants. Six strains incited only tumors: two from each biovar 1 and 2, one from biovar 3, and one from the unidentified biovar. The presence of embryolike organs only (assessed with respect to the uninoculated control plants) was observed on plants inoculated with four biovar 1 grapevine strains, six biovar 2 strains (including one grapevine strain), strains isolated from Prunoidae and Pomoidae, and strain K84 (though this strain is nonpathogenic). The different response patterns described above (also see Table 1) may be attributed to particular phytohormone balances, sensitivity of the transformed gall cells, or production of limited amounts of phytohormones by the bacterium itself (for reviews, see references 9, 18, and 19).
(iii) Production and utilization of opines.
Opines synthesized in the tumors and opines degraded by Agrobacterium strains were analyzed, and results are summarized in Table 1. Four opine groups can be defined from the analysis of tumors induced by biovar 1 strains: octopine, nopaline, mannopine-agropine, and cucumopine-octopine. However, two opine degradation patterns were unusual. Firstly, some cucumopine-octopine grapevine strains degraded both opines, while others degraded cucumopine, octopine, and nopaline. Two of these strains (CFBP 2732 and 2682) remained nonpathogenic on the three test plants. Though not formally demonstrated, their opine degradation capability suggests that they do, however, harbor a Ti plasmid. The second unusual degradation pattern was detected in strains that induced mannopine-agropine-type tumors (CFBP 2712, 2713, and 2714): these degraded only mannopine. If this result is not artifactual, it could be attributed either to a mutation, as reported for mannopinic acid utilization in strain 89.10 (16), or to plasmid dissociation (34) or cointegration (53).
Two pathogenic biovar 2 strains (CFBP 2692 and CFBP 2693) most probably harbor a mannopine-agropine-type Ti plasmid. The other strains, representing over 90% of the pathogenic biovar 2 isolates, harbored a nopaline-type Ti plasmid. Interestingly, the nonpathogenic biovar 2 Malus strains were unable to degrade any assayed opines, suggesting that they do not harbor a Ti plasmid or that they possess a Ti plasmid of an unknown type (35).
The cucumopine-octopine type accounted for ca. 75% of the biovar 3 strains, the remaining strains being either nopaline type (ca. 20%) or vitopine type (ca. 5%) Agrobacterium strains. Among the cucumopine-octopine type strains, some degraded both cucumopine and octopine only while others degraded these two opines plus nopaline, as reported above for the biovar 1 grapevine strains. One strain (CFBP 2641) is of particular interest since it induced tumors synthesizing octopine and cucumopine but degraded cucumopine and nopaline.
An interesting outcome of this study is the identification of a new opine-like molecule in the tumors induced by strain CFBP 2681. Aside from containing vitopine, tumors induced by this strain contained a ninhydrin-positive compound which was specifically degraded by strain CFBP 2681. Examination of the electrophoretic mobilities of this compound and its reaction with ninhydrin (presence of a free NH2 group) indicated that this molecule could result from the condensation of alpha-ketoglutarate and putrescine. Further experiments demonstrated the validity of this hypothesis (Chilton et al., unpublished data). This compound was termed ridéopine and may define a new class of opines (polyamine derivatives).
Though further studies involving DNA-DNA hybridization will be necessary to precisely organize the taxonomy of biovar 1 and 2, the survey of a large collection of original strains belonging to several biovars has proved very useful. It has enabled us to isolate strains of unidentified biovars, to propose new phenotypic properties that can be used to define biovar-discriminating markers, and to identify a novel opine-like molecule.
ACKNOWLEDGMENTS
This work was supported in part by grants from the Bureau des Ressources Génétiques to Y.D.
We thank Rupert Fray (University of Nottinghan) and James Bauley and Phil Oger (CNRS, Gif-sur-Yvette, France) for evaluation and correction of the manuscript.
REFERENCES
- 1.Ayers S H, Rupp P, Johnson W T. A study of alcali-forming bacteria in milk. Bulletin no. 782. U.S. Washington, D.C.: Department of Agriculture; 1919. [Google Scholar]
- 2.Bernaerts M J, De Ley J. A biochemical test for crown-gall bacteria. Nature (London) 1963;197:406–407. [Google Scholar]
- 3.Bien E. Isolierung und Charakterisierung von Agrobacterium tumefaciens Biovar 3, dem Erreger der Mauke an Reben. Ph.D. thesis. Kaiserslautern, Federal Republic of Germany: University of Kaiserslautern; 1988. [Google Scholar]
- 4.Binns A N, Costantino P. The Agrobacterium oncogenes. In: Spaink H P, Kondorosi A, Hooykaas P, editors. The Rhizobiaceae. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 251–266. [Google Scholar]
- 5.Bouzar H. Request for a judicial opinion concerning the type species of Agrobacterium. Int J Syst Bacteriol. 1994;44:373–374. [Google Scholar]
- 6.Bouzar H, Chilton W S, Nesme X, Dessaux Y, Vaudequin V, Petit A, Jones J B, Hodge N C. A new Agrobacterium strain isolated from aerial tumors on Ficus benjamina L. Appl Environ Microbiol. 1995;61:65–73. doi: 10.1128/aem.61.1.65-73.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burr T J, Katz B H. Isolation of Agrobacterium tumefaciens biovar 3 from grapevine galls and saf and from vineyard soil. Phytopathology. 1983;73:163–165. [Google Scholar]
- 8.Burr T S A, Bishop A L, Katz L M, Bazzi C. A root specific decay of grapevine caused by Agrobacterium tumefaciens and Agrobacterium radiobacter biovar 3. Phytopathology. 1987;77:1424–1427. [Google Scholar]
- 9.Cervera M, Lopez M M, Navarro L, Penal L. Virulence and supervirulence of Agrobacterium tumefaciens in woody fruit plants. Phys Mol Plant Pathol. 1998;52:67–78. [Google Scholar]
- 10.Crouzet P, Otten L. Sequence and nutritional analysis of a tartrate utilization operon from Agrobacterium vitis. J Bacteriol. 1995;177:6518–6526. doi: 10.1128/jb.177.22.6518-6526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de la Cruz F, Lanka E. Function of the Ti-plasmid Vir proteins. T-complex formation and transfer to plant cell. In: Spaink H P, Kondorosi A, Hooykaas P, editors. The Rhizobiaceae. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 281–301. [Google Scholar]
- 12.Dessaux Y, Petit A, Tempé J. Opines in Agrobacterium biology. In: Verona D P S, editor. Molecular signals in plant-microbe communications. Boca Raton, Fla: C.R.C. Press, Inc.; 1992. pp. 109–136. [Google Scholar]
- 13.Dessaux Y, Petit A, Farrand S K, Murphy P M. Opine and opine-like molecules involved in plant-Rhizobiaceaea interactions. In: Spaink H P, Kondorosi A, Hooykaas P, editors. The Rhizobiaceae. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 173–197. [Google Scholar]
- 14.El-Fiki F, Giles K L. Agrobacterium tumefaciens in agriculture and research. In: Giles K L, Atherly A G, editors. Biology of the Rhizobiaceae. International Review of Cytology, supplement 13. New York, N.Y: Academic Press; 1981. pp. 47–58. [Google Scholar]
- 15.Ellis G, Kerr A, Tempé J, Petit A. Arginine catabolism: a new function of both octopine and Nopaline Ti plasmids of Agrobacterium. Mol Gen Genet. 1979;173:263–269. doi: 10.1007/BF00268636. [DOI] [PubMed] [Google Scholar]
- 16.Farrand S K, Tempé J, Dessaux Y. Localization and characterization of the region encoding catabolism of manopinic acid from the octopine-type Ti plasmid pTi15955. Mol Plant-Microbe Interact. 1990;3:259–267. doi: 10.1094/mpmi-3-259. [DOI] [PubMed] [Google Scholar]
- 17.Ferreira J H S, Vanzyl F G H. Susceptibility of grapevine root-stocks to strains of Agrobacterium tumefaciens biovar 3. S Afr J Evol Vitic. 1986;7:101–104. [Google Scholar]
- 18.Gaudin V, Vrain T, Jouanin L. Bacterial genes modifying hormone balances in plants. Plant Physiol Biochem. 1994;32:11–29. [Google Scholar]
- 19.Gresshoff P M, Skotnicki M L, Rofle B G. Crown gall teratoma formation is plasmid and plant controlled. J Bacteriol. 1970;137:1020–1021. doi: 10.1128/jb.137.2.1020-1021.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jarvis B W D, Sivakumaran S, Tighe S W, Gillis M. Identification of Agrobacterium and Rhizobium species based on cellular fatty acid composition. Plant Soil. 1996;184:143–153. [Google Scholar]
- 21.Keane P J, Kerr A, New P B. Crown gall of stone fruits. II. Identification and nomenclature of Agrobacterium isolates. Aust J Biol Sci. 1970;23:585–595. [Google Scholar]
- 22.Kersters K, Deley J, Sneath P H A, Sackin M. Numerical taxonomic analysis of Agrobacterium. J Gen Microbiol. 1973;78:227–239. [Google Scholar]
- 23.Kersters K, Deley J. Identification and grouping of bacteria by numerical analysis of their electrophoretic protein patterns. J Gen Microbiol. 1975;87:333–342. doi: 10.1099/00221287-87-2-333. [DOI] [PubMed] [Google Scholar]
- 24.Kersters K, Deley J. Genus III. Agrobacterium Conn 1942. In: Krieg N R, editor. Bergey's manual of systematic bacteriology. Baltimore, Md: Williams and Wilkins Co.; 1984. pp. 244–254. [Google Scholar]
- 25.Lehoczky J. Proceedings of 4th International Conference on Plant Pathogenic Bacteria, 1978. Angers, France: I.N.R.A.; 1978. Root system of the grapevine as a reservoir of Agrobacterium tumefaciens cells; pp. 239–243. [Google Scholar]
- 26.Malenine I. Recherche de l'influence du cancer bactérien sur le cépage Bolgar. Hortic Vitic Sci. 1971;3:101–107. [Google Scholar]
- 27.Nester E W, Gordon M P, Amasino R M, Yanofsky M F. Crown gall: a molecular and physiological analysis. Annu Rev Plant Physiol. 1984;35:387–413. [Google Scholar]
- 28.Ophel K, Burr T J, Magarey P, Kerr A. Detection of Agrobacterium tumefaciens biovar 3 in South Australian grapevine propagation material. Aust Plant Pathol. 1988;17:61–86. [Google Scholar]
- 29.Ophel K, Kerr A. Agrobacterium vitis sp. nov. for strains of Agrobacterium biovar 3 from grapevines. Int J Syst Bacteriol. 1990;40:236–241. [Google Scholar]
- 30.Otten L, Canaday J, Gérard J-C, Fournier P, Crouzet P, Paulus F. Evolution of agrobacteria and their Ti plasmids—a review. Mol Plant-Microbe Interact. 1992;4:279–287. doi: 10.1094/mpmi-5-279. [DOI] [PubMed] [Google Scholar]
- 31.Otten L, De Ruffray P. Agrobacterium vitis nopaline Ti plasmid pTiAB4: relationship to other Ti plasmids and T-DNA structure. Mol Gen Genet. 1994;4:493–505. doi: 10.1007/BF00302262. [DOI] [PubMed] [Google Scholar]
- 32.Otten L, Crouzet P, Salomone J Y, de Ruffray P, Szegedi E. Agrobacterium vitis strain AB3 harbors two independent tartrate utilization systems, one of which is encoded by Ti plasmid. Mol Plant-Microbe Interact. 1995;8:138–146. [Google Scholar]
- 33.Panagopoulos C, Psallidas P G, Alivizatos A S. Proceedings of 4th International Conference on Plant Pathogenic Bacteria, 1978. Angers, France: I.N.R.A.; 1978. Studies on biotype 3 of Agrobacterium radiobacter var. tumefaciens; pp. 221–228. [Google Scholar]
- 34.Petit A, Dahl D C, Ellis G A, Guyon G J, Casse-Delbart F, Tempé J. Further extension of the opine concept: plasmids in Agrobacterium rhizogenes cooperate for opine degradation. Mol Gen Genet. 1983;190:204–214. [Google Scholar]
- 35.Picard C. Caractérisation et détection dans le sol des Agrobacterium du pommier. Ph.D. thesis. Lyon, France: University of Lyon I; 1993. [Google Scholar]
- 36.Popoff M Y, Kersters K, Kiredjian M, Miras I, Coynault C. Position taxonomique de souches de Agrobacterium d'origine hospitalière. Ann Microbiol (Inst Pasteur) 1984;135A:427–442. [PubMed] [Google Scholar]
- 37.Riberau-Gayon J, Peynaud E. Dunod ed. 1971. Sciences et techniques de la vigne. Paris, France. [Google Scholar]
- 38.Ridé M. FAO/Internat. Casale Monferrato, Italy: Poplar Commission; 1963. Our present knowledge of bacterial canker on poplar caused by Aplanobacterium populi; pp. 1–11. [Google Scholar]
- 39.Ridé M. Galle du collet, broussin: une maladie spectaculaire. Vigne. 1991;3:26–27. [Google Scholar]
- 40.Ridé M. Crown gall: vigilance dès la pépinière. Viticulture. 1993;3:47–49. [Google Scholar]
- 41.Rossi L, Tinland B, Hohn B. Role of virulence proteins of Agrobacterium in the plant. In: Spaink H P, Kondorosi A, Hooykaas P, editors. The Rhizobiaceae. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 303–320. [Google Scholar]
- 42.Ruffner H P. Metabolism of tartaric and malic acids in vitis. Vitis. 1982;21:247–259. [Google Scholar]
- 43.Salomone J Y, Crouzet P, de Ruffray P, Otten L. Characterization and distribution of tartrate utilization genes in the grapevine pathogen Agrobacterium vitis. Mol Plant-Microbe Interact. 1996;9:401–408. doi: 10.1094/mpmi-9-0401. [DOI] [PubMed] [Google Scholar]
- 44.Sawada H, Takikawa Y, Ieki H. Fatty acid methyl ester profiles of the genus Agrobacterium. Ann Phytopathol Soc Jpn. 1992;58:46–51. [Google Scholar]
- 45.Sawada H, Ieki H, Oyaizu H, Matsumoto S. Proposal for rejection of Agrobacterium tumefaciens and revised descriptions for the genus Agrobacterium and for Agrobacterium radiobacter and Agrobacterium rhizogenes. Int J Syst Bacteriol. 1993;43:694–702. doi: 10.1099/00207713-43-4-694. [DOI] [PubMed] [Google Scholar]
- 46.Schroth M N, McCain A H, Foott J H, Huisman O C. Reduction in yield and vigor of grapevine caused by crown gall disease. Plant Dis. 1988;72:241–246. [Google Scholar]
- 47.Sneath P H A, Sokal R R. Numerical taxonomy. The principle and practice of numeral classification. San Francisco, Calif: W.H. Freeman and Co.; 1973. [Google Scholar]
- 48.Süle S. Biotypes of Agrobacterium tumefaciens in Hungary. J Appl Bacteriol. 1978;44:207–213. [Google Scholar]
- 49.Szegedi E. Host range and specific L+ tartrate utilization of biotype 3 Agrobacterium tumefaciens. Acta Phytopathol Acad Sci Hung. 1985;20:1–2. , 17–22. [Google Scholar]
- 50.Szegedi E, Korbuly J, Otten L. Types of resistance of grapevine varieties to isolates of Agrobacterium tumefaciens biotype 3. Physiol Mol Plant Pathol. 1989;35:35–45. [Google Scholar]
- 51.van Berkum P, Eardly B D. Molecular evolutionary systematics of the Rhizobiaceae. In: Spaink H P, Kondorosi A, Hooykaas P, editors. The Rhizobiaceae. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 1–24. [Google Scholar]
- 52.Van Nuenen M, de Ruffray P, Otten L. Rapid divergence of Agrobacterium vitis octopine-cucumopine Ti plasmids from a recent common ancestor. Mol Gen Genet. 1993;240:49–57. doi: 10.1007/BF00276883. [DOI] [PubMed] [Google Scholar]
- 53.Vaudequin-Dransart V, Petit A, Chilton W S, Dessaux Y. The cryptic plasmid of Agrobacterium tumefaciens cointegrates with the Ti plasmid and cooperates for opine degradation. Mol Plant-Microbe Interact. 1998;11:583–591. [Google Scholar]
- 54.White L O. The taxonomy of the crown gall organism Agrobacterium tumefaciens and its relationships to rhizobia and other bacteria. J Gen Genet. 1972;72:565–574. [Google Scholar]
- 55.Zinca N, Rajkov E. Tumeurs bactériennes de la vigne (Agrobacterium tumefaciens). Rôle du gel. Influence d'autres facteurs. Méthodes de lutte contre la maladie. Bull Off Int Vigne Vin. 1969;42:1159–1179. [Google Scholar]
- 56.Zoz N N, Avdienko I, Lemanova N B, Sultanova O D, Sakharova M N, Puglazov A B, Khmel R J, Chernin L S. Biochemical and genetic characteristics of Agrobacterium tumefaciens strains isolated on grapevine in Moldavia. Mol Gen Mikrobiol. 1986;2:22–27. [Google Scholar]