Abstract
Objective: To evaluate the potential factors for predicting seroconversion due to the coronavirus disease 2019 (COVID-19) vaccine in people living with HIV (PLWH). Method: We searched the PubMed, Embase and Cochrane databases for eligible studies published from inception to 13th September 2022 on the predictors of serologic response to the COVID-19 vaccine among PLWH. This meta-analysis was registered with PROSPERO (CRD42022359603). Results: A total of 23 studies comprising 4428 PLWH were included in the meta-analysis. Pooled data demonstrated that seroconversion was about 4.6 times in patients with high CD4 T-cell counts (odds ratio (OR) = 4.64, 95% CI 2.63 to 8.19) compared with those with low CD4 T-cell counts. Seroconversion was about 17.5 times in patients receiving mRNA COVID-19 vaccines (OR = 17.48, 95% CI 6.16 to 49.55) compared with those receiving other types of COVID-19 vaccines. There were no differences in seroconversion among patients with different ages, gender, HIV viral load, comorbidities, days after complete vaccination, and mRNA type. Subgroup analyses further validated our findings about the predictive value of CD4 T-cell counts for seroconversion due to COVID-19 vaccines in PLWH (OR range, 2.30 to 9.59). Conclusions: The CD4 T-cell counts were associated with seroconversion in COVID-19 vaccinated PLWH. Precautions should be emphasized in these patients with low CD4 T-cell counts, even after a complete course of vaccination.
Keywords: COVID-19, vaccines, HIV, CD4 T-cell
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic ravaged the globe. Vaccination has become the paramount method for the prevention of worse outcomes, including severe COVID-19 and death during or in SARS-CoV-2 infection [1]. People living with HIV(PLWH) are at higher risk of these worse outcomes due to immunosuppression and its comorbidities [2,3,4,5]. Therefore, PLWH are given priority for COVID-19 vaccination. Accumulating studies demonstrated that PLWH had a lower efficacy of COVID-19 vaccines than the general population [6,7,8]. With the implementation of supplemental vaccination among PLWH [9], it is important to identify the potential predictors for seroconversion due to the COVID-19 vaccine in PLWH. However, the potential factors for predicting seroconversion after the COVID-19 vaccine in PLWH were not completely understood.
Increasing individual studies have attempted to identify the factors associated with the effectiveness of vaccination against COVID-19 [10,11,12]. For example, Anais et al. [13] found that the seroconversion rate was slightly lower among PLWH with CD4 T-cell counts of <350 cell/mm3 and dramatically reduced among those with CD4 T-cell counts of <200 cell/mm3. Moreover, the type of vaccine, the presence of comorbidities, and HIV status might preclude the development of a protective immunological response. However, the lack of studies with large sample sizes highlights the importance of a comprehensive analysis of the potential predictors for seroconversion due to the COVID-19 vaccine in PLWH.
Thus, this systematic review and meta-analysis was to provide a review of the potential factors associated with seroconversion to COVID-19 vaccines among PLWH. We extracted data from observational studies, and the factors included age, gender, CD4 T-cell count, HIV viral load, comorbidities, days after complete vaccination, and vaccine type and were evaluated with respect to the seroconversion rate in PLWH after COVID-19 vaccination. Our findings will help to plan better prevention strategies for this frail population.
2. Methods
We conducted a systematic review and meta-analysis following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRIMSA) guidelines [14]. The protocol of our meta-analysis has been submitted to the International Prospective Register of Systematic Reviews (PROSPERO). The registration number of PROSPERO is CRD42022359603.
2.1. Search Strategy
The PubMed, Embase and Cochrane Library databases were searched for relevant studies from the databases’ inception to 13th September 2022 using the following search terms: (corona[ti] OR covid*[ti] OR sars[ti] OR severe acute respiratory syndrome[ti] OR ncov*[ti] OR “severe acute respiratory syndrome coronavirus 2” [Supplementary Concept] OR “COVID-19” [Supplementary Concept] OR (wuhan[tiab] AND coronavirus[tiab]) OR (wuhan[tiab] AND pneumonia virus[tiab]) OR COVID19[tiab] OR COVID-19[tiab] OR coronavirus 2019[tiab] OR SARS-CoV-2[tiab] OR SARS2[tiab] OR SARS-2[tiab] OR “severe acute respiratory syndrome 2”[tiab] OR 2019-nCoV[tiab] OR (novel coronavirus[tiab] AND 2019[tiab]) NOT (animals[mesh] NOT humans[mesh])) AND (“Vaccines”[MeSH] OR “vaccination”[MeSH] OR vaccine[All Fields] OR vaccination[All Fields] OR vaccin*[All Fields]) AND (“HIV Infections” [MeSH] OR “HIV”[MeSH] OR “hiv”[tw] OR hiv infect*[tw] OR “human immunodeficiency virus”[tw] OR “human immunedeficiency virus”[tw] OR “human immuno-deficiency virus”[tw] OR “human immune-deficiency virus”[tw] OR ((human immun*) AND (“deficiency virus”[tw])) OR “acquired immunodeficiency syndrome”[tw] OR “acquired immunedeficiency syndrome”[tw] OR “acquired immuno-deficiency syndrome”[tw] OR “acquired immune-deficiency syndrome”[tw] OR ((acquired immun*) AND (“deficiency syndrome”[tw]))). No language restrictions were imposed. The full details of the search strategies can be found in Table S1.
2.2. Inclusion and Exclusion Criteria
The study selection was conducted in three steps: removing the initial deduplication, screening titles and abstracts, and reviewing the full text of the potentially eligible articles. Two reviewers (Q.Z. and Y.L.) independently evaluated eligibility, and the discrepancies were solved by a third investigator (G.D.). Articles were included for analysis if they met the following criteria: (1) cohort studies or randomized controlled trials; (2) patients living with HIV; (3) the odds ratio (OR) of potential predictors of seroconversion due to COVID-19 vaccine was reported, or the OR could be calculated according to the data from the studies. There were no restrictions regarding age, sex or duration of the study. The cohort studies were defined as those that sampled participants based on exposure, followed-up participants over time, and ascertained the outcomes [15]. Case reports, case series, and studies with data inaccessible from the corresponding authors were excluded.
2.3. Data Extraction and Quality Assessment
Two investigators (Q.Z. and Y.L.) independently extracted data based on a predetermined proforma in Microsoft Excel. The following information was recorded for the studies: the first author, publication year, country, study type, data source, patient number, vaccine type, vaccine dose, potential predictors for seroconversion due to the COVID-19 vaccine, multivariable analysis, COVID-19 history, and adjustment parameters. We used the Cochrane Risk of Bias 2 tool to assess the risk of bias for the randomized controlled studies [16], while the Risk of Bias in Nonrandomized Studies of Interventions (ROBINS-I) tool was used for the comparative cohort studies [17]. For the Cochrane Risk of Bias 2 tool, the risk of bias judgment per study is noted as low risk when all the domains are judged as being a low risk of bias, noted with some concerns when one or more domains are judged as some concerns, or high risk when at least one domain is judged as being at a high risk of bias, or when multiple domains are judged as some concerns. During our search, 17 eligible comparative cohort studies were included. For the ROBINS-I tool, the risk of bias judgment per study is noted as low risk when all domains are judged as being at low risk of bias, moderate risk when one domain is judged as a moderate risk of bias, serious risk when one domain is judged as serious risk of bias, or critical risk of bias when one domain is judged as being at critical risk of bias. Only one randomized study was founded in our study. The risk of bias for non-comparative cohort studies was regarded as a high risk of bias. We rated the quality of evidence according to the grading of recommendations, assessment, development and evaluation (GRADE) approach to assess the certainty of the evidence obtained from the present meta-analysis of potential risk factors of seroconversion among COVID-19 vaccinated PLWH.
2.4. Definitions of Vaccines
The inactivated vaccines included BBIBP-CorV, Corona Vac or Sinopharm; the mRNA vaccines comprised the BNT162b2 or mRNA-1273; the adenovirus vaccines comprised the ChA-dOx1 nCoV-19 or Ad.26.COV2.S; and mixed vaccines mean more than one type of vaccine.
2.5. Statistical Analysis
The primary outcome was the odds ratio and its corresponding 95% CI of potential predictors for seroconversion to the COVID-19 vaccine in PLWH. If the outcomes were presented as RRs, data were converted to the ORs for analysis by using the formula OR = RR(1-pRef)/(1-RR×pRef), where pRef is the prevalence of the outcome in the control [18]. The p-value by χ2 test < 0.1 or the I2 statistic was ≥ 50% and was considered to indicate significant heterogeneity among the included studies. In this case, the pooled odds ratios were estimated by the fixed-effects model; otherwise, the random-effects model was preferentially performed. Subgroup analyses were conducted to evaluate the predictive value of the CD4 T-cell counts for seroconversion due to the COVID-19 vaccine according to the study location (Europe vs. America vs. Asia), study design (retrospective vs. prospective), source of data (multi-center vs. single-center), sample size (< 100 vs. ≥ 100), cut off of CD4 T-cell counts (200 cell/mm3 vs. 500 cell/mm3 vs. others), vaccine type (inactivated vaccine vs. mRNA vaccine vs. mix), and multivariable analysis (YES vs. NO). Meta-regression analyses were further performed to explore the potential effect of these parameters on the outcomes. The regression coefficient was calculated to describe the change in outcomes with explanatory variables (potential effect modifiers). The potential publication bias was evaluated by Egger’s test, and funnel plots were drawn if the studies were above 10. Trim-and-fill analyses were performed to adjust for publication bias (Egger’s test p < 0.05). Sensitivity analyses were conducted where the outcomes were recalculated by omitting one study at a time. All calculations and graphs were performed and visualized with R statistic software (3.6.3).
3. Results
3.1. Study Selection, Characteristics and Quality Assessment
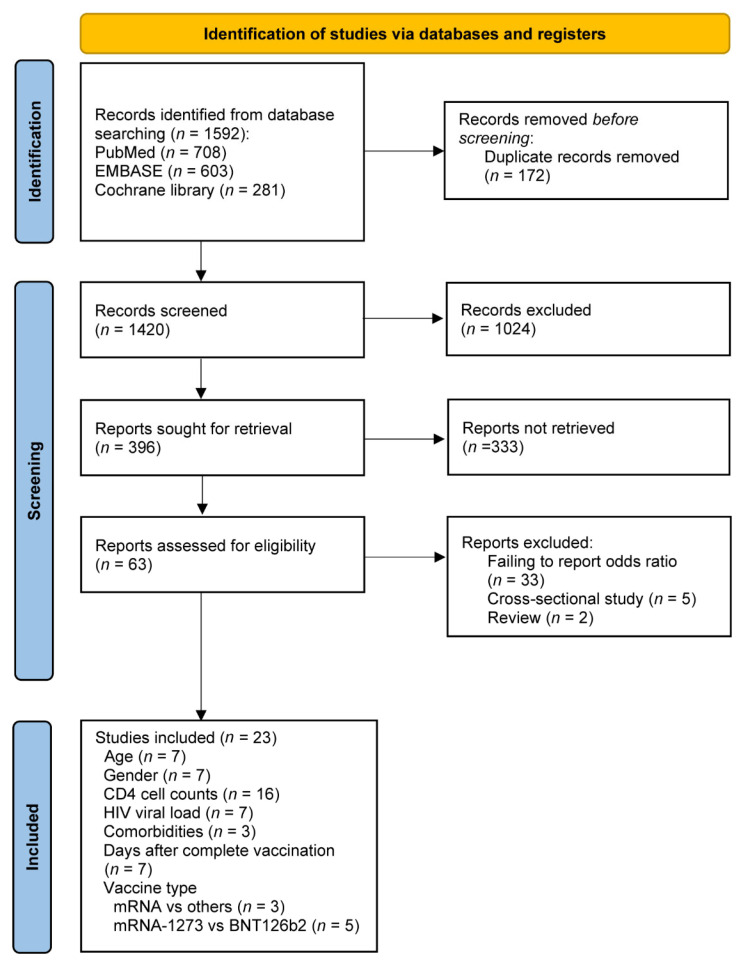
The study selection is shown in Figure 1. A total of 1592 potentially relevant studies were identified through the literature search. After screening the initial titles and abstracts, the full text of 63 studies was further considered for eligibility. After the removal of another 40 studies, including 33 studies that failed to report the odds ratios, 5 cross-sectional studies, and 2 reviews (File S1), 23 studies [13,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40] that included 4428 patients living with HIV were finally included in the meta-analysis (Figure 1).
Figure 1.
Flowcharts illustrating the article selection process.
The main characteristics and clinical outcomes of the studies for quantitative analysis are summarized in Table 1. Of these studies, 11 were from Europe, 5 were from Asia, 5 were from North America, 1 was from South Africa, and 1 was from South America. The studies comprised 14 prospective studies and 9 retrospective studies. A total of 7 studies were multi-center, and 16 were single-center. The number of PLWH in 14 studies was above 100; 6 studies had adjusted for potential confounders; 7 studies were analyzed using multivariable analysis; all PLWH in the 19 studies were not infected with COVID-19 prior to vaccination. In terms of vaccination type, the mRNA vaccines were used in 11 studies; adenovirus vaccines were used in 1 study; inactivated vaccines were used in 6 studies; and another 5 studies involved two or more vaccines or other types of vaccines. PLWH, in 2 studies, received an incomplete dose of vaccines; 20 studies received a complete dose of vaccines; only 1 study received the booster dose of vaccines. Supplementary Table S2 shows the detailed risk of bias for each study, and most of the studies were regarded as critical or at a high risk of bias.
Table 1.
Characteristics of included studies.
| Source | Country | Design | Data Source | Cases | Vaccine Type | Vaccine Dose | COVID-19 History | Outcomes | Multivariable Analysis | Impact Factors | Adjust |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anais 2022 [13] | Spain | Pro | Three university hospitals in Southern Spain | 420 | BNT162b2, mRNA-1273, ChAdOx1 nCoV-19, or Ad26.COV2.S | Complete | NO | anti-S IgG | YES | Age, gender, HIV infection way, CDC clinical category nadir CD4 T-cell counts, Charlson index, cirrhosis, chronic kidney disease, immunosuppressive therapy, CD4 T-cell counts (cutoff = 200 cell/mm3), HIV viral load, vaccine |
- |
| Antinori 2022 [19] |
Italy | Pro | National Institute for Infectious Diseases Lazzaro Spallanzani | 153 | BNT162b2 or mRNA-1273 | Complete | NO | nAbs | NO | CD4 T-cell counts (cutoff = 200 cell/mm3) | - |
| Ao 2022 [20] |
China | Pro | People’s Hospital of Tongliang District | 139 | BBIBP-CorV or Corona Vac | Complete | NO | anti-RBD IgG | YES | Age, gender, days after 2nd vaccination, CD4 T-cell counts (cutoff = 500 cell/mm3), HIV viral load, white blood cell count, lymphocyte count, platelet count, alanine aminotransferase, aspartate aminotransferase, B cells, RBD-specific B cells, RBD-specific MBCs, RBD+ rMBCs, RBD+ actMBCs, RBD+ atyMBCs, RBD+ intMBCs | - |
| Bergman 2021 [21] |
Sweden | Pro | Karolinska University Hospital | 79 | BNT162b2 | Complete | NO | anti-RBD IgG | NO | CD4 T-cell counts (cutoff = 300 cell/mm3) | Age (partially) |
| Brumme 2022 [22] |
Canada | Retro | Three HIV care clinics in | 100 | BNT162b2, mRNA-1273 or ChAdOx1 | Complete | YES | anti-RBD IgG | NO | Days after 2nd vaccination | Age, chronic health conditions |
| Gianserra 2022 [23] |
Italy | Pro | HIV/AIDS Unit of the San Gallicano Dermatological Institute | 42 | BNT162b2 | Complete | NO | SARS-CoV-2 S1/S2 IgG | NO | Days after second vaccination | - |
| Haidar 2022 [24] |
USA | Pro | Unive University of Pittsburgh Medical Center Health System | 94 | BNT162b2, mRNA-1273 or Adenovirus | Complete | NO | anti-RBD IgG | NO, except for days after 2nd dose | Age, gender, race, vaccine, days after second dose | - |
| Han 2022 [25] |
China | Retro | Beijing Ditan Hospital | 47 | CoronaVac or Sinopharm | Complete | NO | nAbs | NO | CD4 T-cell counts (cutoff = 350 cell/mm3) | Age, sex, and interval length |
| Hassold 2022 [26] |
France | Retro | Department of Infectious Diseases of Hospital Avicenne | 105 | BNT162b2, mRNA-1273 or ChAdOx1-nCoV-19 | Complete | NO | Anti-spike IgG | NO | CD4 T-cell counts (cutoff = 200 cell/mm3) | - |
| Hensley 2022 [27] |
Netherlands | Pro | 22 HIV treatment centers | 1154 | BNT162b2, mRNA-1273, ChAdOx1-S or Ad26.COV2.S | Complete | NO | Anti-spike IgG | YES, except for vaccine type | Vaccine type, age, gender, HIV viral load, CD4 T-cell counts (cutoff = 250 cell/mm3), CD4 nadir cell counts | - |
| Khan 2022 [28] |
South African | Pro | Biomedical Research of the University of KwaZulu–Natal | 26 | Ad26.CoV2.S | Complete | YES | Neutralization capacity | NO | HIV viral load | - |
| Milano 2022 [29] |
Italy | Pro | University of Bari | 578 | BNT162b2 | Complete | NO | Anti-RBD IgG | NO | Days after complete vaccination | - |
| Nault 2022 [30] | Canada | Retro | HIV clinics in Montreal | 106 | mRNA-1273 | Uncomplete | YES | Anti-RBD IgG | NO | CD4 T-cell counts (cutoff = 250 cell/mm3) | - |
| Netto 2022 [31] |
Brazil | Pro | University of Sao Paulo HIV/AIDS outpatient clinic | 215 | CoronaVac | Complete | NO | nAbs | NO | CD4 T-cell counts (cutoff = 500 cell/mm3) | - |
| Polvere 2022 [32] |
Italy | Retro | Azienda Ospedaliera Universitaria Senese | 84 | BNT162b2 or mRNA-1273 | Complete | NO | nAbs | NO | Age, gender, vaccine type, BMI, IDU, years from HIV infection, CDC stage, HBV or HCV coinfection, zenith HIV-RNA, CD4 T-cell counts at nadir, years from first ART, type of ART, HIV viral load, time from last HIV-RNA >50 copies/mL, CD4 T-cell counts at baseline (cutoff = 350 cell/mm3), CD4%, CD4/CD8 ratio |
- |
| Speich 2022 [33] |
Switzerland | RCT | University Hospital Basel, University Hospital Bern and University Hospital Zurich | 341 | BNT162b2 or mRNA-1273 | Complete | YES | nAbs | NO | Vaccine type | RCT |
| Spinelli 2022 [34] | USA | Retro | A large outpatient HIV clinic | 100 | BNT162b2 or mRNA-1273 | Complete | NO | nAbs | YES | CD4 T-cell counts (cutoff = NA), HIV viral load, vaccine type | Care for chronic medical conditions on days since completion of second vaccination (minimum 10), sex, age and mRNA vaccine type |
| Tuan 2022 [35] |
USA | Retro | Two HIV clinics of the Yale New Haven Health System | 78 | BNT162b2 | Uncomplete | NO | IgG |
NO, except for CD4 T-cell counts | Age, gender, days after second vaccination, BMI, self-reported substance use, time since HIV diagnosis, HIV ART regimen, CD4 T-cell counts (cutoff = 500 cell/mm3), HIV viral load, comorbidities |
- |
| Vergori 2022 [36] |
Italy | Retro | Infectious Diseases Lazzaro Spallanzani in Rome | 106 | BNT162b2 or Mrna-1273 | Booster | NO | nAbs | NO | CD4 T-cell counts (cutoff = 200 cell/mm3), CD4 T-cell counts at nadir | - |
| Wong 2022 [37] |
China | Pro | The Integrated Treatment Centre or Princess Margaret Hospital HIV Service | 213 | CoronaVac or Comirnaty | Complete | NO | nAbs | NO | Vaccine type | age, sex, CD4 T-cell counts, and suppressed viral load (SVL) at the time point nearest to vaccination. |
| Xu 2022 [38] |
Sweden | Pro | Karolinska University Hospital | 79 | BNT162b2 | Complete | NO | anti-spike-IgG | NO | CD4 T-cell counts (cutoff = 200 cell/mm3) | - |
| Zeng 2022 [39] |
China | Retro | The Third People’s Hospital of Shenzhen | 126 | BBIBP-CorV or CoronaVac | Complete | NO | anti-RBD IgG | NO |
CD4 T-cell counts (cutoff = 350 cell/mm3), days after complete vaccination, vaccine type | - |
| Zou 2022 [40] |
China | Pro | Wuchang district of Wuhan city | 46 | Sinopharm WIBP-CorV | Complete | NO | nAbs and IgG |
YES, except for days after 2nd dose | Age, gender, CD4 T-cell counts (cutoff = NA), days after second dose | - |
Abbreviations: COVID-19, coronavirus disease 2019; Pro, prospective study; Retro, retrospective study; RCT, randomized controlled trial; RBD, receptor binding domain; Ig, immunoglobulin; S, spike; nAbs, neutralizing antibodies; BMI, body mass index; MBC, memory B cell; BV, hepatitis B virus; HCV, hepatitis C virus; IDU, injecting drug users; NA, not available.
3.2. Risk Factors for Seroconversion Rate in PLWH
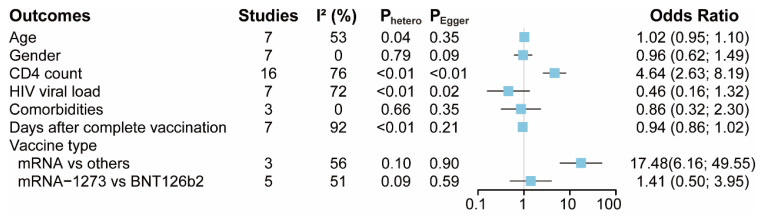
A summary of the findings from the included studies is shown in Figure 2. A total of 7, 7, 16, 7, 3, and 7 studies provided data for the age, gender, CD4 T-cell counts, HIV viral load, comorbidities and days after complete vaccination, respectively (Figures S1A, S2A, S3A, S4A, S5A and S6A). The pooled data showed that there were no statistical differences in seroconversion among patients with different ages, gender, HIV viral load, comorbidities and days after their complete vaccination. Notably, seroconversion was about 4.6 times in patients with higher CD4 T-cell counts (OR = 4.64, 95% CI 2.63 to 8.19) compared with those with lower CD4 T-cell counts. Quantitative synthesis was also accessible for different vaccine types, including mRNA vaccines vs. other vaccines and mRNA-1273 vs. BNT126b2 vaccines (another mRNA vaccine) (Figures S7A and S8A). The pooled data demonstrated that there was no difference in seroconversion between patients receiving the mRNA-1273 vaccines and those receiving the BNT126b2 vaccines. It is worth noting that seroconversion was about 17.5 times in patients receiving mRNA COVID-19 vaccines (OR = 17.48, 95% CI 6.16 to 49.55), compared with those receiving other types of COVID-19 vaccines.
Figure 2.
The pooled odds ratio of potential factors associated with seroconversion rate in people living with HIV(PLWH). Comorbidities mean PLWH with cirrhosis, HBV or HCV coinfections.
3.3. Publication Bias
Egger’s test detected the existence of publication bias in potential predictors for seroconversion due to COVID-19 vaccines, including the CD4 T-cell counts (p < 0.01) and HIV viral load (p = 0.02). The funnel plot also showed the relative asymmetry in CD4 T-cell counts (Figure S3B). After eight studies were filled, the funnel plot showed relative symmetry (Figure S3C), and Egger’s test showed no evidence of significant publication bias (p = 0.60). Patients with high CD4 T-cell counts still had higher seroconversions than those with low CD4 T-cell counts (OR = 1.85, 95% CI 1.05 to 3.28). As for the HIV viral load, after three studies were filled, Egger’s test showed no evidence of significant publication bias (p = 0.78) (Figure S4C) with still no statistical difference in the seroconversions among patients with different HIV viral loads (OR = 1.30, 95% CI 0.40 to 4.21).
3.4. Meta-Regression and Subgroup Analysis
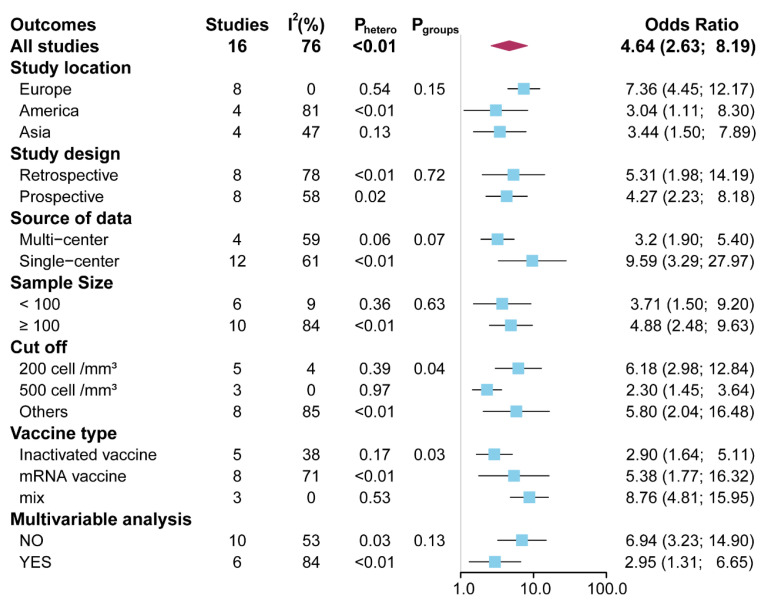
Considering the potential predictive value of the CD4 T-cell counts in seroconversion due to the COVID-19 vaccines in PLWH, univariate meta-regression and subgroup analyses were further carried out to explore the source of heterogeneity. The univariate meta-regression found no significant moderators of heterogeneity (Table S3). All the subgroup analyses arrived at a consistent conclusion (OR range, 2.30 to 9.59) (Figure 3 and Figures S9–S15). Interestingly, a subgroup analysis, conducted according to the cutoff of CD4 T-cell counts, demonstrated that the odds ratio was highest in the cutoff for 200 cell/mm3 (OR = 6.18, 95% CI 2.98 to 12.84), followed by the cutoff for others (OR = 5.80, 95% CI 2.04 to 16.48), and a cutoff for 500 cell/mm3 (OR = 2.30, 95% CI 1.45 to 3.64) (p = 0.04) (Figure 3 and Figure S13). Subgroup analysis, stratified by vaccine type, showed that the odds ratio was lowest when patients received the inactivated vaccine (OR = 2.90, 95% CI 1.64 to 5.11), followed by the mRNA vaccine (OR = 5.38, 95% CI 1.77 to 16.32), and mixed vaccines-type (OR = 8.76, 95% CI 4.81 to 15.95) (p = 0.03) (Figure 3 and Figure S14). No significant heterogeneity was observed in the other subgroup comparisons (all p > 0.05).
Figure 3.
The pooled odds ratio of CD4 T-cell counts in subgroup analyses.
3.5. Sensitivity Analysis
The sensitivity analysis, performed by using the “leave-one-out”, did not markedly change our results in these comparisons (Figures S1–S8).
3.6. Grading the Quality of Evidence
According to the GRADE approach, the quality of evidence was very low for age, gender, CD4 T-cell counts, HIV viral load, comorbidities, days after complete vaccination, and vaccine-type impact factors (Table S4).
4. Discussion
In this meta-analysis, 23 studies with a total of 4428 patients living with HIV were included. We evaluated the potential factors for predicting seroconversion due to the COVID-19 vaccine in PLWH. We demonstrated that the CD4 T-cell counts and mRNA vaccines are associated with seroconversion due to COVID-19 vaccination. Compared with the PLWH with lower CD4 T-cell counts, the seroconversion rate was about 4.6 times in patients with higher CD4 T-cell counts. The mRNA COVID-19-vaccinated PLWH showed about 17.5 times the seroconversion compared with those receiving other types of COVID-19 vaccines, such as the inactivated vaccines. Advanced age, gender, HIV viral load, comorbidities, days after complete vaccination, and different mRNA vaccine types showed no association with seroconversion. The subgroup analysis further validated our finding about the predictive value of CD4 T-cell counts for seroconversion in PLWH.
An effective COVID-19 vaccination strategy becomes the main measure of reducing the risk and mortality of COVID-19 [1]. Immunocompromised patients are of particular interest because of their attenuated responses to various vaccines [41,42,43]. There are several meta-analyses reporting seroconversion due to the COVID-19 vaccine in immunocompromised patients, including PLWH. For example, Mehrabi Nejad et al., demonstrated that immunocompromised patients had a lower overall crude prevalence of seroconversion. Notably, transplant patients were less likely to develop seroconversion after both the first and second dose compared with patients with malignancy or autoimmune disease [44]. However, this study did not include PLWH and also failed to evaluate the risk factors for seroconversion. Yin et al., mainly focused on the PLWH and reported that a second dose could consistently improve the seroconversion, although the seroconversion is still lower in PLWH than in healthy individuals [45]. Conversely, Kang et al., considered the immunogenicity and safety of the COVID-19 vaccine in PLWH to be acceptable because there were no significant differences in the seroconversion rates and incidence rates of adverse events of COVID-19 vaccines between PLWH and healthy controls [46]. However, these two studies still failed to evaluate the risk factors for seroconversion in PLWH. In our study, we comprehensively evaluated the potential risk factors for seroconversion due to the COVID-19 vaccine in PLWH, including age, gender, CD4 T-cell counts, HIV viral load, comorbidities, days after complete days, and vaccine type. We finally found that the CD4 T-cell counts and vaccine type are associated with seroconversion due to COVID-19 vaccination. Our findings were completely different from previous meta-analyses and filled a gap in the risk factors for seroconversion due to the COVID-19 vaccine. PLWH are characterized by impaired immunity with reduced CD4 T-cell counts. For the generation of antibody response to vaccination, an essential step is the interaction of antigen-primed B cells and CD4 T-cells in the germinal center reaction, where CD4 T-cells provide critical helper function for the B cells to undergo proliferation, isotype switching and somatic hypermutation [47,48]. Despite antiretroviral therapy, the immune dysfunction may not be completely reversed [13]. Therefore, PLWH could have decreased response to vaccination due to defects of CD4 T-cells’ help [49,50]. Previous studies showed considerably weaker responses among PLWH with CD4 T-cell counts of < 300 cells/mm3 compared with HIV-negative individuals [19,26,30,51]. Some studies found similar humoral immune responses in PLWH with CD4 T-cell counts of > 500 cells/mm3 compared to the health controls [19,31]. Here, we found that the CD4 T-cell counts are associated with seroconversion in COVID-19-vaccinated PLWH. More importantly, we found that the odds ratio was higher in the cutoff for 200 cell/mm3 than in the cutoff for 500 cell/mm3. These results suggested that lower CD4 T-cell counts predict lower seroconversion in COVID-19-vaccinated PLWH.
It is also worth noting that the mRNA vaccine developed the highest serologic response in PLWH than the inactivated and adenovirus vaccines, which suggested PLWH are given priority for the mRNA vaccine. This finding was consistent with the results from general patients [52,53]. For example, previous studies demonstrated that compared with the mRNA vaccine, the antibody level of inactivated CoronaVac-vaccinees wanes quickly, and patients after the vaccine face a higher risk of breakthrough infection [54,55]. Further, the geometric NAbTs of inactivated vaccinees were 19-fold lower than that of the BNT162b2-vaccines [56,57]. The mRNA vaccines also showed superior cellular immune responses when compared to other vaccines against SARS-CoV-2 [58]. The median levels of CD4 responses following the mRNA vaccine were higher than the adenoviral vaccine, followed by the inactivated vaccine [58]. In a recent UK population-based study, the mRNA BNT162b2 vaccine showed to be more efficacious than the adenovirus ChA-dOx1 nCoV-19 vaccine against SARS-CoV-2 infection and hospital admissions for COVID-19 [59]. Our findings suggest that, just like the general population, PLWH respond better to the mRNA vaccines than other vaccines.
This systematic review and meta-analysis had some limitations. First, some of the studies included were observational studies, which might cause a risk of unbalanced groups for comparison with a high risk of bias. Second, significant heterogeneity and publication bias were found in some analyses, while the outcomes of trim-and-fill analyses, sensitivity analyses, and subgroup analyses were consistent. Third, ART is fundamental to the clinical care of PLWH, while the association between ART and seroconversion in PLWH was not evaluated due to a lack of data. Fourth, we did not evaluate the predictive value of the nadir CD4 counts for the seroconversion in PLWH due to data unavailability. Finally, the subgroup analysis was only performed for the CD4 T-cell counts but not other potential predictors due to fewer than 10 studies. Numerous studies are needed to pool the potential predictors for seroconversion due to the COVID-19 vaccine in PLWH.
5. Conclusions
CD4 T-cell counts are associated with seroconversion in COVID-19-vaccinated PLWH. Precautions should be emphasized in these patients with low CD4 T-cell counts, even after a complete vaccination course. Moreover, the mRNA vaccines might be a priority for PLWH with COVID-19.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines11040789/s1, Figures S1–S8. The forest plot, sensitivity analysis, and publication bias analysis for the pooled odds ratio of age, gender, CD4 T-cell counts, HIV viral load, comorbidities, days after complete vaccination, vaccine type, and mRNA vaccine type associated with serologic response in PLWH after COVID-19 vaccination respectively; Figures S9–S15. Subgroup analysis according to study location, study design, source of data, sample size, CD4 T-cell counts strata, vaccine type, and logistic regression analysis type respectively for the pooled odds ratio of CD4 T-cell counts associated with serologic response in PLWH after COVID-19 vaccination; Table S1. Search strategy; Table S2. Risk of bias of all included studies; Table S3. Meta-regression for odds ratio for the seroconversion in COVID-19 vaccinated PLWH with high and low CD4 T cells; Table S4. Certainty of evidence and summary effect estimates assessed by GRADE; File S1. List of excluded studies.
Author Contributions
Conceptualization, H.L. and G.D.; methodology, Q.Z.; software, Q.Z.; validation, F.Z., Y.M. and G.D.; formal analysis, Q.Z.; investigation, Q.Z. and Y.L.; resources, Q.Z. and Y.L.; data curation, Q.Z.; writing—original draft preparation, Q.Z.; writing—review and editing, Y.L., F.Z., Y.M. and G.D.; visualization, Q.Z.; supervision, H.L. and G.D.; project administration, Q.Z.; funding acquisition, F.Z. and G.D. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting this study’s findings are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research is supported by the National Natural Science Foundation of China (no. 82102803, 82103183, 82272849) and Natural Science Foundation of Hunan province (no. 2021JJ40976, 2022JJ40767).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geretti A.M., Stockdale A.J., Kelly S.H., Cevik M., Collins S., Waters L., Villa G., Docherty A., Harrison E.M., Turtle L., et al. Outcomes of Coronavirus Disease 2019 (COVID-19) Related Hospitalization Among People With Human Immunodeficiency Virus (HIV) in the ISARIC World Health Organization (WHO) Clinical Characterization Protocol (UK): A Prospective Observational Study. Clin. Infect. Dis. 2021;73:e2095–e2106. doi: 10.1093/cid/ciaa1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Western Cape Department of Health in collaboration with the National Institute for Communicable Diseases, South Africa Risk Factors for Coronavirus Disease 2019 (COVID-19) Death in a Population Cohort Study from the Western Cape Province, South Africa. Clin. Infect. Dis. 2021;73:e2005–e2015. doi: 10.1093/cid/ciaa1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhaskaran K., Rentsch C.T., MacKenna B., Schultze A., Mehrkar A., Bates C.J., Eggo R.M., Morton C.E., Bacon S.C.J., Inglesby P., et al. HIV infection and COVID-19 death: A population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV. 2021;8:e24–e32. doi: 10.1016/S2352-3018(20)30305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tesoriero J.M., Swain C.-A.E., Pierce J.L., Zamboni L., Wu M., Holtgrave D.R., Gonzalez C.J., Udo T., Morne J.E., Hart-Malloy R., et al. COVID-19 Outcomes Among Persons Living With or Without Diagnosed HIV Infection in New York State. JAMA Netw. Open. 2021;4:e2037069. doi: 10.1001/jamanetworkopen.2020.37069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balcells M.E., Le Corre N., Durán J., Ceballos M.E., Vizcaya C., Mondaca S., Dib M., Rabagliati R., Sarmiento M., Burgos P.I., et al. Reduced Immune Response to Inactivated Severe Acute Respiratory Syndrome Coronavirus 2 Vaccine in a Cohort of Immunocompromised Patients in Chile. Clin. Infect. Dis. 2022;75:e594–e602. doi: 10.1093/cid/ciac167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang X., Yan Y., Su B., Xiao D., Yu M., Jin X., Duan J., Zhang X., Zheng S., Fang Y., et al. Comparing Immune Responses to Inactivated Vaccines against SARS-CoV-2 between People Living with HIV and HIV-Negative Individuals: A Cross-Sectional Study in China. Viruses. 2022;14:277. doi: 10.3390/v14020277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan Y., Davgadorj C., Lyu C., Zhang S., Qiu Y. Immunogenicity of a third dose of inactivated COVID-19 vaccine in people living with HIV-1, HBV, and tuberculosis during the Omicron variant epidemic: A cross-sectional study. J. Infect. 2022;85:e109–e111. doi: 10.1016/j.jinf.2022.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan D.P.C., Wong N.S., Wong B.C., Chan J.M., Lee S.S. Three-Dose Primary Series of Inactivated COVID-19 Vaccine for Persons Living with HIV, Hong Kong. Emerg. Infect. Dis. 2022;28:2130–2132. doi: 10.3201/eid2810.220691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hafiz I., Illian D.N., Meila O., Utomo A.R.H., Susilowati A., Susetya I.E., Desrita D., Siregar G.A., Basyuni M. Effectiveness and Efficacy of Vaccine on Mutated SARS-CoV-2 Virus and Post Vaccination Surveillance: A Narrative Review. Vaccines. 2022;10:82. doi: 10.3390/vaccines10010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasan T., Beardsley J., Marais B., Nguyen T., Fox G. The Implementation of Mass-Vaccination against SARS-CoV-2: A Systematic Review of Existing Strategies and Guidelines. Vaccines. 2021;9:326. doi: 10.3390/vaccines9040326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petráš M., Máčalík R., Janovská D., Čelko A.M., Dáňová J., Selinger E., Doleček J., Neradová S., Franklová M., Dlouhý P., et al. Risk factors affecting COVID-19 vaccine effectiveness identified from 290 cross-country observational studies until February 2022: A meta-analysis and meta-regression. BMC Med. 2022;20:461. doi: 10.1186/s12916-022-02663-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corma-Gómez A., Fernández-Fuertes M., García E., Fuentes-López A., Gómez-Ayerbe C., Rivero-Juárez A., Domínguez C., Santos M., Viñuela L., Palacios R., et al. Severe immunosuppression is related to poorer immunogenicity to SARS-CoV-2 vaccines among people living with HIV. Clin. Microbiol. Infect. 2022;28:1492–1498. doi: 10.1016/j.cmi.2022.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dekkers O.M., Egger M., Altman D.G., Vandenbroucke J.P. Distinguishing Case Series From Cohort Studies. Ann. Intern. Med. 2012;156:37–40. doi: 10.7326/0003-4819-156-1-201201030-00006. [DOI] [PubMed] [Google Scholar]
- 16.Higgins J.P.T., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., Savović J., Schulz K.F., Weeks L., Sterne J.A.C., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterne J.A.C., Hernán M.A., Reeves B.C., Savović J., Berkman N.D., Viswanathan M., Henry D., Altman D.G., Ansari M.T., Boutron I., et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ziff M., Lane D.A., Samra M., Griffith M., Kirchhof P., Lip G.Y.H., Steeds R., Townend J., Kotecha D. Safety and efficacy of digoxin: Systematic review and meta-analysis of observational and controlled trial data. BMJ. 2015;351:h4451. doi: 10.1136/bmj.h4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antinori A., Cicalini S., Meschi S., Bordoni V., Lorenzini P., Vergori A., Lanini S., De Pascale L., Matusali G., Mariotti D., et al. Humoral and Cellular Immune Response Elicited by mRNA Vaccination Against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in People Living With Human Immunodeficiency Virus Receiving Antiretroviral Therapy Based on Current CD4 T-Lymphocyte Count. Clin. Infect. Dis. 2022;75:e552–e563. doi: 10.1093/cid/ciac238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ao L., Lu T., Cao Y., Chen Z., Wang Y., Li Z., Ren X., Xu P., Peng M., Chen M., et al. Safety and immunogenicity of inactivated SARS-CoV-2 vaccines in people living with HIV. Emerg. Microbes Infect. 2022;11:1126–1134. doi: 10.1080/22221751.2022.2059401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergman P., Blennow O., Hansson L., Mielke S., Nowak P., Chen P., Söderdahl G., Österborg A., Smith C.I.E., Wullimann D., et al. Safety and efficacy of the mRNA BNT162b2 vaccine against SARS-CoV-2 in five groups of immunocompromised patients and healthy controls in a prospective open-label clinical trial. Ebiomedicine. 2021;74:103705. doi: 10.1016/j.ebiom.2021.103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brumme Z.L., Mwimanzi F., Lapointe H.R., Cheung P.K., Sang Y., Duncan M.C., Yaseen F., Agafitei O., Ennis S., Ng K., et al. Humoral immune responses to COVID-19 vaccination in people living with HIV receiving suppressive antiretroviral therapy. Npj Vaccines. 2022;7:28. doi: 10.1038/s41541-022-00452-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gianserra L., Donà M.G., Giuliani E., Stingone C., Pontone M., Buonomini A.R., Giuliani M., Pimpinelli F., Morrone A., Latini A. Immunogenicity and Safety of BNT162b2 Homologous Booster Vaccination in People Living with HIV under Effective cART. Vaccines. 2022;10:1243. doi: 10.3390/vaccines10081243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haidar G., Agha M., Bilderback A., Lukanski A., Linstrum K., Troyan R., Rothenberger S., McMahon D.K., Crandall M.D., Sobolewksi M.D., et al. Prospective Evaluation of Coronavirus Disease 2019 (COVID-19) Vaccine Responses Across a Broad Spectrum of Immunocompromising Conditions: The COVID-19 Vaccination in the Immunocompromised Study (COVICS) Clin. Infect. Dis. 2022;75:e630–e644. doi: 10.1093/cid/ciac103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han X., Yu X., Han Y., Fang Q., Shen C., Liu H., Wang P., Wang Y., Li X. Safety and Immunogenicity of Inactivated COVID-19 Vaccines Among People Living with HIV in China. Infect. Drug Resist. 2022;15:2091–2100. doi: 10.2147/IDR.S353127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hassold N., Brichler S., Ouedraogo E., Leclerc D., Carroue S., Gater Y., Alloui C., Carbonnelle E., Bouchaud O., Mechai F., et al. Impaired antibody response to COVID-19 vaccination in advanced HIV infection. AIDS. 2022;36:F1–F5. doi: 10.1097/QAD.0000000000003166. [DOI] [PubMed] [Google Scholar]
- 27.Hensley K.S., Jongkees M.J., Geers D., GeurtsvanKessel C.H., Mueller Y.M., Dalm V.A.S.H., Papageorgiou G., Steggink H., Gorska A., Bogers S., et al. Immunogenicity and reactogenicity of SARS-CoV-2 vaccines in people living with HIV in The Netherlands: A nationwide prospective cohort study. PLoS Med. 2023;20:e1004159. doi: 10.1371/journal.pmed.1004159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan K., Lustig G., Bernstein M., Archary D., Cele S., Karim F., Smith M., Ganga Y., Jule Z., Reedoy K., et al. Immunogenicity of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection and Ad26.CoV2.S Vaccination in People Living With Human Immunodeficiency Virus (HIV) Clin. Infect. Dis. 2022;75:e857–e864. doi: 10.1093/cid/ciab1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milano E., Ricciardi A., Casciaro R., Pallara E., De Vita E., Bavaro D.F., Larocca A.M.V., Stefanizzi P., Tafuri S., Saracino A. Immunogenicity and safety of the BNT162b2 COVID-19 mRNA vaccine in PLWH: A monocentric study in Bari, Italy. J. Med. Virol. 2022;94:2230–2236. doi: 10.1002/jmv.27629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nault L., Marchitto L., Goyette G., Tremblay-Sher D., Fortin C., Martel-Laferrière V., Trottier B., Richard J., Durand M., Kaufmann D., et al. Covid-19 vaccine immunogenicity in people living with HIV-1. Vaccine. 2022;40:3633–3637. doi: 10.1016/j.vaccine.2022.04.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Netto L.C., Ibrahim K.Y., Picone C.M., Alves A.P.P.S., Aniceto E.V., Santiago M.R., Parmejani P.S.S., Aikawa N.E., Medeiros-Ribeiro A.C., Pasoto S.G., et al. Safety and immunogenicity of CoronaVac in people living with HIV: A prospective cohort study. Lancet HIV. 2022;9:e323–e331. doi: 10.1016/S2352-3018(22)00033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polvere J., Fabbiani M., Pastore G., Rancan I., Rossetti B., Durante M., Zirpoli S., Morelli E., Pettini E., Lucchesi S., et al. B cell response six months after SARS-CoV-2 mRNA vaccination in people living with HIV. Commun. Med. 2022;3:13. doi: 10.1038/s43856-023-00245-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Speich B., Chammartin F., Abela I.A., Amico P., Stoeckle M.P., Eichenberger A.L., Hasse B., Braun D.L., Schuurmans M.M., Müller T.F., et al. Antibody Response in Immunocompromised Patients After the Administration of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccine BNT162b2 or mRNA-1273: A Randomized Controlled Trial. Clin. Infect. Dis. 2022;75:e585–e593. doi: 10.1093/cid/ciac169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spinelli M.A., Peluso M.J., Lynch K.L., Yun C., Glidden D.V., Henrich T.J., Deeks S.G., Gandhi M. Differences in Post-mRNA Vaccination Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Immunoglobulin G (IgG) Concentrations and Surrogate Virus Neutralization Test Response by Human Immunodeficiency Virus (HIV) Status and Type of Vaccine: A Matched Case-Control Observational Study. Clin. Infect. Dis. 2022;75:e916–e919. doi: 10.1093/cid/ciab1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tuan J.J., Zapata H., Critch-Gilfillan T., Ryall L., Turcotte B., Mutic S., Andrews L., Roh M.E., Friedland G., Barakat L., et al. Qualitative assessment of anti-SARS-CoV-2 spike protein immunogenicity (QUASI) after COVID-19 vaccination in older people living with HIV. HIV Med. 2022;23:178–185. doi: 10.1111/hiv.13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vergori A., Cozzi-Lepri A., Matusali G., Colavita F., Cicalini S., Gallì P., Garbuglia A.R., Fusto M., Puro V., Maggi F., et al. SARS-CoV-2 Omicron Variant Neutralization after Third Dose Vaccination in PLWH. Viruses. 2022;14:1710. doi: 10.3390/v14081710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong N.S., Wong B.C.K., Chan J.M.C., Wong K.H., Tsang O.T.Y., Mok C.K.P., Hui D.S.C., Lee S.S., Chan D.P.C. Surrogate neutralization responses following severe acute respiratory syndrome coronavirus 2 vaccination in people with HIV: Comparison between inactivated and mRNA vaccine. AIDS. 2022;36:1255–1264. doi: 10.1097/QAD.0000000000003237. [DOI] [PubMed] [Google Scholar]
- 38.Xu X., Vesterbacka J., Aleman S., Nowak P. High seroconversion rate after vaccination with mRNA BNT162b2 vaccine against SARS-CoV-2 among people with HIV-but HIV viremia matters? AIDS. 2022;36:479–481. doi: 10.1097/QAD.0000000000003135. [DOI] [PubMed] [Google Scholar]
- 39.Zeng G., Xu L., Feng S., Tang J., Wang X., Li G., Gan Y., Zheng C., Zhao J., Yang Z. IgG Antibody Responses and Immune Persistence of Two Doses of BBIBP-CorV Vaccine or CoronaVac Vaccine in People Living with HIV (PLWH) in Shenzhen, China. Vaccines. 2022;10:880. doi: 10.3390/vaccines10060880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zou S., Wu M., Ming F., Wu S., Guo W., Marley G., Xing Z., Zhang Z., Zeng M., Sun C., et al. Immune response and safety to inactivated COVID-19 vaccine: A comparison between people living with HIV and HIV-naive individuals. AIDS Res. Ther. 2022;19:33. doi: 10.1186/s12981-022-00459-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Catherine F.-X., Piroth L. Hepatitis B virus vaccination in HIV-infected people: A review. Hum. Vaccines Immunother. 2017;13:1304–1313. doi: 10.1080/21645515.2016.1277844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pallikkuth S., De Armas L.R., Pahwa R., Rinaldi S., George V.K., Sanchez C.M., Pan L., Dickinson G., Rodriguez A., Fischl M., et al. Impact of aging and HIV infection on serologic response to seasonal influenza vaccination. AIDS. 2018;32:1085–1094. doi: 10.1097/QAD.0000000000001774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grohskopf L.A., Alyanak E., Broder K.R., Walter E.B., Fry A.M., Jernigan D.B. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2019–2020 Influenza Season. MMWR. Recomm. Rep. 2019;68:1–21. doi: 10.15585/mmwr.rr6803a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nejad M.-M.M., Shobeiri P., Dehghanbanadaki H., Tabary M., Aryannejad A., Ghadery A.H., Shabani M., Moosaie F., SeyedAlinaghi S., Rezaei N. Seroconversion following the first, second, and third dose of SARS-CoV-2 vaccines in immunocompromised population: A systematic review and meta-analysis. Virol. J. 2022;19:132. doi: 10.1186/s12985-022-01858-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yin J., Chen Y., Li Y., Wang C., Zhang X. Immunogenicity and efficacy of COVID-19 vaccines in people living with HIV: A systematic review and meta-analysis. Int. J. Infect. Dis. 2022;124:212–223. doi: 10.1016/j.ijid.2022.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang L., Shang W., Gao P., Wang Y., Liu J., Liu M. Immunogenicity and Safety of COVID-19 Vaccines among People Living with HIV: A Systematic Review and Meta-Analysis. Vaccines. 2022;10:1569. doi: 10.3390/vaccines10091569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zotos D., Coquet J.M., Zhang Y., Light A., D’Costa K., Kallies A., Corcoran L.M., Godfrey D.I., Toellner K.-M., Smyth M.J., et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell–intrinsic mechanism. J. Exp. Med. 2010;207:365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruddy J.A., Boyarsky B.J., Werbel W.A., Bailey J.R., Karaba A.H., Garonzik-Wang J.M., Segev D.L., Durand C.M. Safety and antibody response to the first dose of severe acute respiratory syndrome coronavirus 2 messenger RNA vaccine in persons with HIV. AIDS. 2021;35:1872–1874. doi: 10.1097/QAD.0000000000002945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Avelino-Silva V.I., Miyaji K.T., Hunt P.W., Huang Y., Simoes M., Lima S.B., Freire M.S., Caiaffa-Filho H.H., Hong M.A., Costa D.A., et al. CD4/CD8 Ratio and KT Ratio Predict Yellow Fever Vaccine Immunogenicity in HIV-Infected Patients. PLoS Negl. Trop. Dis. 2016;10:e0005219. doi: 10.1371/journal.pntd.0005219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parmigiani A., Alcaide M.L., Freguja R., Pallikkuth S., Frasca D., Fischl M.A., Pahwa S. Impaired Antibody Response to Influenza Vaccine in HIV-Infected and Uninfected Aging Women Is Associated with Immune Activation and Inflammation. PLoS ONE. 2013;8:e79816. doi: 10.1371/journal.pone.0079816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heftdal L.D., Knudsen A.D., Hamm S.R., Hansen C.B., Møller D.L., Pries-Heje M., Fogh K., Hasselbalch R.B., Jarlhelt I., Pérez-Alós L., et al. Humoral response to two doses of BNT162b2 vaccination in people with HIV. J. Intern. Med. 2022;291:513–518. doi: 10.1111/joim.13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fedele G., Trentini F., Schiavoni I., Abrignani S., Antonelli G., Baldo V., Baldovin T., Bandera A., Bonura F., Clerici P., et al. Evaluation of humoral and cellular response to four vaccines against COVID-19 in different age groups: A longitudinal study. Front. Immunol. 2022;13:1021396. doi: 10.3389/fimmu.2022.1021396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maringer Y., Nelde A., Schroeder S.M., Schuhmacher J., Hörber S., Peter A., Karbach J., Jäger E., Walz J.S. Durable spike-specific T cell responses after different COVID-19 vaccination regimens are not further enhanced by booster vaccination. Sci. Immunol. 2022;7:78. doi: 10.1126/sciimmunol.add3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kwok S.L., Cheng S.M., Leung J.N., Leung K., Lee C.-K., Peiris J.M., Wu J.T. Waning antibody levels after COVID-19 vaccination with mRNA Comirnaty and inactivated CoronaVac vaccines in blood donors, Hong Kong, April 2020 to October 2021. Eurosurveillance. 2022;27:2101197. doi: 10.2807/1560-7917.ES.2022.27.2.2101197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peng Q., Zhou R., Wang Y., Zhao M., Liu N., Li S., Huang H., Yang D., Au K.-K., Wang H., et al. Waning immune responses against SARS-CoV-2 variants of concern among vaccinees in Hong Kong. Ebiomedicine. 2022;77:103904. doi: 10.1016/j.ebiom.2022.103904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lim W.W., Mak L., Leung G.M., Cowling B.J., Peiris M. Comparative immunogenicity of mRNA and inactivated vaccines against COVID-19. Lancet Microbe. 2021;2:e423. doi: 10.1016/S2666-5247(21)00177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Y., Shen H., Huang R., Tong X., Wu C. Serum neutralising activity against SARS-CoV-2 variants elicited by CoronaVac. Lancet Infect. Dis. 2021;21:1071–1072. doi: 10.1016/S1473-3099(21)00287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ben Ahmed M., Bellali H., Gdoura M., Zamali I., Kallala O., Ben Hmid A., Hamdi W., Ayari H., Fares H., Mechri K., et al. Humoral and Cellular Immunogenicity of Six Different Vaccines against SARS-CoV-2 in Adults: A Comparative Study in Tunisia (North Africa) Vaccines. 2022;10:1189. doi: 10.3390/vaccines10081189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wei J., Zhang W., Doherty M., Wallace Z.S., Sparks J.A., Lu N., Li X., Zeng C., Lei G. Comparative effectiveness of BNT162b2 and ChAdOx1 nCoV-19 vaccines against COVID-19. BMC Med. 2023;21:78. doi: 10.1186/s12916-023-02795-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this study’s findings are available from the corresponding author upon reasonable request.