Abstract
The chromosomal arsenic resistance genes of the acidophilic, chemolithoautotrophic, biomining bacterium Thiobacillus ferrooxidans were cloned and sequenced. Homologues of four arsenic resistance genes, arsB, arsC, arsH, and a putative arsR gene, were identified. The T. ferrooxidans arsB (arsenite export) and arsC (arsenate reductase) gene products were functional when they were cloned in an Escherichia coli ars deletion mutant and conferred increased resistance to arsenite, arsenate, and antimony. Therefore, despite the fact that the ars genes originated from an obligately acidophilic bacterium, they were functional in E. coli. Although T. ferrooxidans is gram negative, its ArsC was more closely related to the ArsC molecules of gram-positive bacteria. Furthermore, a functional trxA (thioredoxin) gene was required for ArsC-mediated arsenate resistance in E. coli; this finding confirmed the gram-positive ArsC-like status of this resistance and indicated that the division of ArsC molecules based on Gram staining results is artificial. Although arsH was expressed in an E. coli-derived in vitro transcription-translation system, ArsH was not required for and did not enhance arsenic resistance in E. coli. The T. ferrooxidans ars genes were arranged in an unusual manner, and the putative arsR and arsC genes and the arsBH genes were translated in opposite directions. This divergent orientation was conserved in the four T. ferrooxidans strains investigated.
Thiobacillus ferrooxidans is an acidophilic (optimum pH, 1.8 to 2.5), obligately chemolithotrophic bacterium that obtains its energy through oxidation of ferrous iron to ferric iron or oxidation of reduced inorganic sulfur compounds to sulfuric acid. It is a member of a consortium of bacteria (which includes Thiobacillus caldus and Leptospirillum ferrooxidans) that is used in commercial biooxidation processes to recover gold from arsenopyrite ores (22). Although recent analysis of microbial populations in continuous-flow biooxidation tanks has revealed that T. ferrooxidans may not be as dominant as was once thought, this organism is nevertheless usually present in such tanks (21). Total arsenic levels greater than 13 g liter−1 may be present in arsenopyrite biooxidation tanks, and therefore the microorganisms present must have a mechanism of resistance to arsenic (8).
Plasmid-associated arsenic efflux resistance mechanisms have been known for many years and have been extensively reviewed (5, 23, 30–32, 35). Although the number of components of these systems varies, in the case of Escherichia coli plasmids R773 and R46, as well as Acidiphilium multivorum plasmid pKW301 (34), as many as five genes (arsRDABC) are present. In the case of R773, the genes are transcribed in a single operon. The arsR and arsD genes encode repressors that control the basal and upper levels of ars operon expression, while the arsABC genes encode the structural components of the arsenic resistance mechanism. ArsA is an ATPase which forms a complex with ArsB, the transmembrane arsenite efflux pump. ArsC is a small, cytoplasmically located arsenate reductase which reduces arsenate to arsenite, which can then be pumped out of the cell. The ArsB protein is capable of exporting arsenite even in the absence of ArsA (9).
The arsenic resistance systems of Staphylococcus plasmids pSX267 and pI258, as well as the chromosomally located arsenic resistance systems of E. coli (4) and Pseudomonas aeruginosa (3), consist of only three genes, arsRBC. Nevertheless, the ars operons are capable of exporting arsenate, arsenite, and antimony oxyanions in the absence of arsA by using membrane potential rather than ATP as an energy source. Recently, an arsenic resistance system which consists of arsRBC and a fourth open reading frame (ORF) of unknown function was discovered in the skin element of Bacillus subtilis (28). An arsenic resistance system has been discovered in Tn2502 located on plasmid pYV of Yersinia enterocolitica, and this system consists of arsRBC, as well as a divergently transcribed gene, arsH (17). The function of arsH is not known, but the presence of this gene either in cis or in trans is essential for arsenic resistance in Y. enterocolitica.
Here we describe isolation and analysis of the evolutionary relationships of the arsenic resistance genes of T. ferrooxidans. We found that these genes are functional in E. coli and have an unusual divergent arsCRBH operon structure, which appeared to be conserved in all of the T. ferrooxidans strains which we examined.
MATERIALS AND METHODS
Bacterial strains, plasmids, primers, and media.
The strains, plasmids, and primers used in this study are shown in Table 1. E. coli strains were grown on Luria-Bertani medium (25). T. ferrooxidans strains were grown in tetrathionate medium or iron sulfate medium (19) at 30°C. Ampicillin (100 μg/ml), chloramphenicol (20 μg/ml), and tetracycline (20 μg/ml) were used as required.
TABLE 1.
Strains, plasmids, and primers used
| Strain, plasmid, or primer | Description | Reference or sourcea |
|---|---|---|
| Strains | ||
| Escherichia coli strains | ||
| W3110 | K-12 F− IN(rrnD-rrnE) | Rosen |
| AW3110 | W3110 Δars::cam | 4 |
| MC1061 | K-12 F−araD139? galU galK hsdR rpsL | Persson |
| BH5262 | K-12 araD139? galU galK hsdR rpsL argH1 trxA7004 ilvC::Tn10 gshA | 14 |
| BH2012 | K-12 araD139? galU galK hsdR rpsL metA46 argH1 trxA7004 ilvC::Tn5 | 14 |
| Thiobacillus ferrooxidans strains | ||
| ATCC 33020 | Wild type | ATCC |
| ATCC 23270 | Wild type | ATCC |
| ATCC 19859 | Wild type | ATCC |
| ATCC 13598 | Wild type | ATCC |
| Thiobacillus caldus MNG | Wild type | This laboratory |
| Thiobacillus thiooxidans ATCC 19377 | Wild type | ATCC |
| Leptospirillum ferrooxidans DSM 2705 | Wild type | DSM |
| Plasmids | ||
| pBluescript(SK) | AmprlacZ′, ColE1 replicon, cloning vector | Stratagene |
| pUCBM21 | AmprlacZ′, ColE1 replicon, cloning vector | Stratagene |
| pACYC184 | Tcr Cmr, p15A replicon, cloning vector | 6 |
| pTfars1a | Apr (from T. ferrooxidans plasmid library, 7-kb Sau3A1 fragment cloned into pEcoR251 digested with BglII) | Library of R. S. Ramesar |
| pTfars1b | Apr (6.7-kb HindIII-BglII fragment of pTfars1a cloned into pBluescript) | This study |
| pTfarsCRBH | Apr (5.3-kb HindIII-StuI fragment of pTfars1b cloned into pBluescript cut with EcoRV and HindIII) | This study |
| pTfarsCRB | Apr (EcoRV-XbaI fragment of pTfars1b, which contained the 5′ end of arsB and all of arsH, was replaced with the EcoRV-XbaI fragment of pBB08, which contained only the 5′ end of arsH) | This study |
| pTfarsBH | Apr (KpnI deletion of pTfarsCRBH) | This study |
| pTfarsB | Apr (PstI deletion of pTfarsBH) | This study |
| pTfarsH | Apr (PCR product obtained with primers ARSHF and ARSHR, cloned into pBluescript) | This study |
| pTfarsC | Apr (PCR product obtained with primers ARSCF and ARSR, cloned into pBluescript) | This study |
| pTfarsR | Apr (0.55-kb SphI fragment of pTfarsCRBH, cloned into pUCBM21) | This study |
| pBB08 | Apr (0.8-kb EcoRV-PstI fragment of pTfars1b, cloned into pBluescript) | This study |
| pTfarsCRBH-Cm | Cmr (6.7-kb HindIII-BglII fragment of pTfars1a, cloned into the tet gene of pACYC184) | This study |
| pTrx6 | Apr (contains T. ferrooxidans ATCC 33020 trxA gene, cloned into pBluescript) | 19 |
| Primers | ||
| BBARSB | (BamHI) 5′-GCGGATCCAGGGTGACGAGAAATATGGC-3′ | This study |
| BBARSC | (BamHI) 5′-GCGGATCCGGGGTTTTCATCACTGG-3′ | This study |
| ARSHF | (EcoRI) 5′-GCGAATTCTGGTGGCTGCCGCTGGCTTG-3′ | This study |
| ARSHR | (HindIII) 5′-GAAAGCTTGCGTACCCCCAACCTCATGCC3′ | This study |
| BBARSCF | (HindIII) 5′-GCAAGCTTCGGTGAAACCCGCTCTCCCT-3′ | This study |
| BBARSCR | (EcoRI) 5′-GCGAATTCTGTGCGCGCCTTGATGGGTGGC-3′ | This study |
Rosen, B. Rosen, Wayne State University, Detroit, Mich.; Persson, Britt Persson, University of Umea, Umea, Sweden; ATCC, American Type Culture Collection, Rockville, Md.; DSM, Deutsche Sammlung von Mikroorganismen, Braunschweig, Germany; Stratagene, Stratagene, La Jolla, Calif.
DNA techniques, sequencing, and analysis.
A T. ferrooxidans ATCC 33020 gene bank consisting of 4- to 9-kb fragments obtained from a partial Sau3A digest cloned into the BglII site of the suicide vector pEcoR251 (20) was transformed into E. coli ars deletion mutant AW3110, which was made competent by the simple and efficient method (10). Plasmid preparation, restriction endonuclease digestion, gel electrophoresis, ligation, and Southern blot hybridization were carried out by using standard methods (20). Pulsed-field gel electrophoresis was carried out by using a Beckman Geneline transverse alternating field electrophoresis apparatus. Labeling of probes, hybridization, and detection were performed by using a dioxigenin-dUTP nonradioactive DNA labeling and detection kit (Roche). Sequences were determined by the dideoxy chain termination method (27) by using a thermosequenase fluorescently labeled primer cycle sequencing kit (Amersham Pharmacia Biotech UK Ltd.). Sequencing reactions were performed with an ALFexpress automated sequencer (Pharmacia Biotech, Uppsala, Sweden). Results were analyzed by using the VAX-based Genetics Computer Group Inc. sequence analysis package (version 7.1) and its associated programs and the PC-based DNAMAN software (version 4.1) from Lynnon BioSoft. Multiple sequence alignments were shaded by using the Genedoc Multiple Sequence Alignment Editor and Shading Utility (version 2.5.000). Comparison searches were performed by using the gapped-BLAST program of the National Center for Biotechnology Information (NCBI) (1). Homology trees were constructed by using the Multiple Sequence Alignment tool in DNAMAN.
Requirement for thioredoxin for arsenate resistance.
A 7.1-kb HindIII-BglII fragment of pTfars1a, which contained the ars genes of T. ferrooxidans, was cloned into pACYC digested with HindIII and BamHI. The resulting clone, pTfarsCRBH-Cm, was used to test pBluescriptSK-based plasmids in trans. E. coli BH2012, BH5262, and MC1061 were made competent with CaCl2 and were transformed with pTfarsCRBH-Cm. E. coli BH5262 was also transformed with pTrx6, pTTn1, and pTT150 (Table 1). All of the strains mentioned above were streaked onto Luria agar plates (24) containing 0, 2, 5, 7, 10, and 15 mM sodium arsenate and were incubated at 37°C overnight.
PCR.
A PCR was performed with the primers described in Table 1, which were synthesized at the Synthetic DNA Laboratory, Department of Biochemistry, University of Cape Town. The reaction was performed with a Biometra Personal cycler by using Redhot Taq DNA polymerase (Advanced Biotechnologies). After an initial denaturation step consisting of 60 s at 94°C, 25 cycles consisting of 30 s at 94°C, 30 s at 57°C (for primers BBARSB and BBARSC) or 63°C (for primers ARSHF and ARSHR), and 90 s at 72°C were performed. A final extension step consisting of 120 s at 72°C before cooling to 25°C completed the reaction.
Arsenic and antimony resistance assays.
Constructs were transformed into competent E. coli AW3110 cells. Assays to determine resistance to arsenite and antimonite were carried out in Luria broth (LB). In assays to determine resistance to arsenate, cells were grown in low-phosphate medium (18) supplemented with 2 mM K2HPO4. Overnight cultures were diluted 100-fold into fresh medium containing the appropriate antibiotics and different concentrations of sodium arsenite, potassium antimonite, or sodium arsenate. The cultures were incubated at 37°C for 5 h in the case of LB or for 12 h in the case of low-phosphate medium, and the absorbance at 600 nm was determined. The incubation times used corresponded to the end of the log phase of growth of controls under the same conditions.
In vitro transcription-translation analysis.
A prokaryotic DNA-directed, E. coli S30 extract-based, in vitro transcription-translation kit for circular DNA (Promega) was used to analyze the polypeptides synthesized from clones that conferred arsenic resistance and subclones. The [35S]methionine-labeled translation products were separated on a sodium dodecyl sulfate-polyacrylamide electrophoresis gel and detected by autoradiography.
Nucleotide sequence accession number.
The nucleotide sequence determined in this study has been deposited in the GenBank database under accession no. AF173880.
RESULTS
Cloning of the ars genes of T. ferrooxidans.
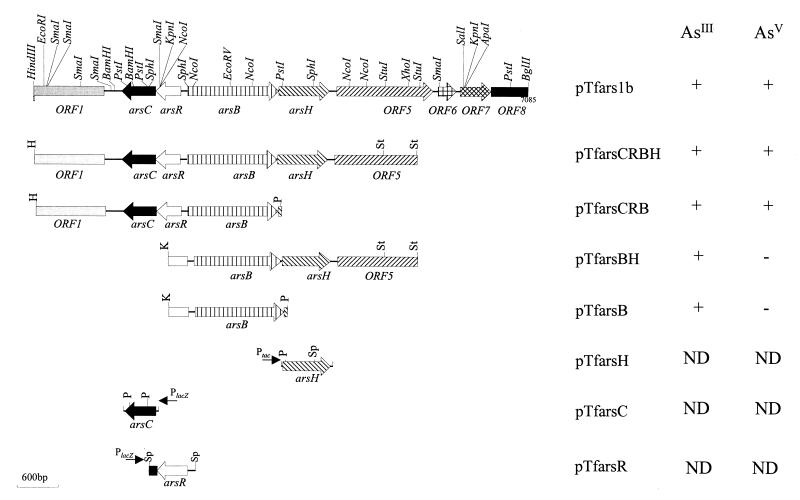
The E. coli ars operon deletion mutant (AW3110) was transformed with a partial Sau3A plasmid bank, and colonies were selected on the basis of their ability to complement the mutant on LB plates containing 0.5 mM sodium arsenite. One plasmid (pTfars1a), which contained a 7.4-kb insert and which retransformed E. coli AW3110 to arsenite resistance at a high frequency, was selected for further study. The plasmid was mapped to determine the positions of restriction endonuclease sites, as shown in Fig. 1, and a 7.1-kb HindIII-BglII fragment was cloned into pBluescriptSK (pTfars1b). The source of the insert DNA was confirmed by Southern hybridization. A 5.3-kb internal HindIII-StuI fragment was labeled and used to probe the chromosomal DNA of T. ferrooxidans ATCC 33020 and pTfars1b digested with PstI. The sizes of PstI fragments that were 3.5 and 2.0 kb long and were inside the insert of pTfars1b corresponded exactly to the sizes of PstI fragments of T. ferrooxidans ATCC 33020 chromosomal DNA (data not shown). This indicated that the insert DNA originated from T. ferrooxidans ATCC 33020, that a single copy was present, and that no rearrangements in the region which included the two PstI fragments occurred during cloning. Chromosomal DNA was also digested with two rarely cutting 8-bp recognition sequence restriction enzymes, PacI and SwaI, as well as with a combination of both of these enzymes. Restriction fragments were separated by using pulsed-field gel electrophoresis and were hybridized to a T. ferrooxidans arsBH probe. Signals of hybridization to PacI, SwaI, and PacI-SwaI fragments that were approximately 450, 620, and 280 kb long, respectively, were obtained (data not shown). During chromosomal mapping experiments (unpublished data), DNA fragments that were the same sizes hybridized to a T. ferrooxidans ntrBC chromosomal gene probe. This indicated that the two sets of genes are located within 280 kb of each other and that the T. ferrooxidans ars genes are located on the chromosome.
FIG. 1.
pTfars1b and deletion clones constructed in this study. The diagram shows the genes, ORFs, restriction endonuclease map, and whether the clones were resistant to 0.5 mM arsenite in Luria agar (AsIII) or 0.2 mM arsenate in low-phosphate medium (AsV). ND, not determined. The directions and types of vector promoters are indicated by labeled arrows.
Sequence analysis of pTfars1b.
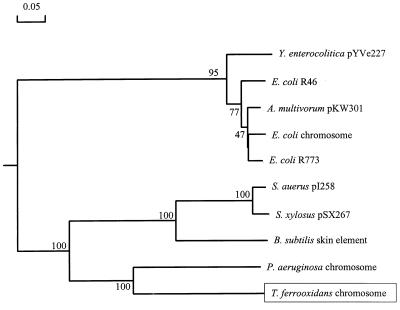
The entire insert DNA was sequenced in both directions, and nine ORFs or partial ORFs were identified (Fig. 1). The characteristics of the predicted products of the nine ORFs are shown in Table 2. ORF 2 and ORF 3 were homologues of the arsB and arsC genes of other bacteria, but unlike other systems, in which the arsC gene is downstream of the arsB gene, the T. ferrooxidans arsC gene was upstream of arsB and the genes were divergently transcribed (Fig. 1). A fourth ORF was found between the arsC and arsB genes, and this ORF was also divergent with respect to arsB. This ORF exhibited weak but clear homology (30 to 40% identity) to many transcriptional regulators, including some members of the ArsR family. Although the putative ArsR of T. ferrooxidans contains two possible helix-turn-helix motifs, the positions of these motifs do not correspond to the position of the helix-turn-helix DNA binding domain identified in other ArsR proteins that have been studied. The putative ArsR of T. ferrooxidans also does not contain the conserved motif that includes two cysteine residues (ELCVCDL), which has been shown to be required for binding of the arsenite inducer (29). Downstream of the arsB gene was a homologue of a gene previously identified as arsH. This gene was first discovered in Y. enterocolitica (17) and has been reported to be essential for arsenic resistance, although its function is not known. By using a BLASTN search of the nonredundant GenBank-EMBL-DDBJ-PDB database at the NCBI, we found a third homologue of ArsH on the chromosome of Synechocystis sp. The predicted amino acid sequence of the T. ferrooxidans ArsH-like homologue was 68% identical to the predicted amino acid sequence of the Y. enterocolitica molecule and 65% identical to the amino acid sequence of the hypothetical protein of Synechocystis sp. Alignment and phylogenetic analysis of all of the ArsC proteins in the NCBI nonredundant database showed that ArsC of T. ferrooxidans is most closely related to ArsC of the P. aeruginosa chromosomal ars operon (Fig. 2). Both T. ferrooxidans and P. aeruginosa are unusual in that although their ArsB proteins cluster with the ArsB proteins of other gram-negative organisms (data not shown), their ArsC proteins are more closely related to the ArsC proteins of gram-positive bacteria. The ArsC arsenate reductases of gram-positive bacteria have four conserved cysteine residues (of which two are essential [13]), whereas two cysteines (of which one is essential [15]) are present in the ArsC proteins of gram-negative bacteria. The putative ArsC protein of T. ferrooxidans contains four cysteine residues with spacing similar to the spacing in the ArsC proteins of gram-positive bacteria (data not shown).
TABLE 2.
Locations and sizes of ORFs in pTfars1b
| ORF | Position | No. of amino acids | Size (kDa) | Closest relationship to a known proteina | Product detectedb |
|---|---|---|---|---|---|
| ORF 1 | 1014-NDc | 338 | ND | 41% identity and 57% similarity to putative membrane protein of E. coli (accession no. ACC73882) | Yes |
| arsC | 1752-1261 | 163 | 18.1 | 53% identity and 65% similarity to P. aeruginosa ArsC (accession no. AAC69644) | Yes |
| arsR | 1752-2108 | 118 | 13.1 | 35% identity and 47% similarity to Mycobacterium tuberculosis hypothetical protein Rv2640c (accession no. CAB02346) | Yes |
| arsB | 2202-3512 | 436 | 48.4 | 57% identity and 70% similarity to ArsB from E. coli plasmid R773 (accession no. P08691) | Yes |
| arsH | 3518-4240 | 240 | 26.7 | 68% identity and 81% similarity to Y. enterocolitica ArsH (accession no. AAB42206) | Yes |
| ORF 5 | 4348-5724 | 458 | 44.7 | 58% identity and 72% similarity to signal recognition particle protein (fifty-four homologue) (P48) of E. coli (accession no. BAA16495) | Yes |
| ORF 6 | 5795-6055 | 86 | 9.56 | 66% identity and 79% similarity to 30S ribosomal protein S16 of Haemophilus influenzae Rd KW20 (accession no. P44382) | No |
| ORF 7 | 6111-6554 | 147 | 12.6 | 44% identity and 58% similarity to 16S rRNA-processing protein RIMM of Haemophilus influenzae Rd KW20 (accession no. P44568) | No |
| ORF 8 | 6554-ND | 94 | ND | 34% identity and 52% similarity to tRNA (guanine-N1)-methyltransferase of Salmonella typhimurium (accession no. P36245) | No |
The identity and similarity values are based on amino acid sequence data. The accession numbers are GenBank-EMBL database accession numbers.
Protein products were detected by using an in vitro E. coli transcription-translation system.
ND, not determined. The ORF was incomplete, and the molecular mass was not calculated; however, the number of amino acids is indicated.
FIG. 2.
Phylogenetic tree based on ArsC proteins. Bootstrap values (expressed as percentages) based on 100 attempts are indicated at the branch points. The accession numbers for the protein sequences are as follows: Y. enterocolitica pYVe227, AAD16858; E. coli R46, AAB09628; A. multivorum pKW301, BAA24824; E. coli chromosome, AAC76528; E. coli R773, AAA21096; S. aureus pI258, AAA25638; Staphylococcus xylosus pSX267, AAA27589; B. subtilis skin element, BAA06970; P. aeruginosa chromosome, AAC69644.
Thioredoxin is required for arsenate reduction by T. ferrooxidans ArsC.
A major difference between the ArsC proteins of gram-positive bacteria and the ArsC proteins of gram-negative bacteria is the source of the reducing power used for reduction of arsenate. It has been shown that reduction of arsenate by a gram-positive ArsC is coupled to thioredoxin (11) and that reduction by a gram-negative ArsC is coupled to glutathione (18). Since ArsC of T. ferrooxidans was clearly like the ArsC molecules of gram-positive bacteria, thioredoxin and not glutathione should have been required for arsenate reduction. To investigate this, E. coli strains with mutations in the thioredoxin gene (trxA) or both the thioredoxin gene and the γ-glutamylcysteinyl synthetase gene (gshA; responsible for the synthesis of glutathione) were examined to determine their resistance to arsenate. The trxA mutant strain was resistant to arsenate and was able to grow in the presence of 15 mM sodium arsenate, while the double mutant (trxA gshA) was sensitive to arsenate and was not able to grow in the presence of 2 mM sodium arsenate. This indicated that the glutathione-requiring E. coli chromosomal ars genes were able to confer resistance to arsenate in the absence of thioredoxin but not in the absence of glutathione. When the E. coli double mutant strain was transformed with the T. ferrooxidans ars genes (pTfarsCRBH-Cm), a similar result was obtained because neither the E. coli ars genes nor the T. ferrooxidans ars genes were functional in the double mutant. However, when a plasmid containing the thioredoxin gene from T. ferrooxidans, pTrx6, was added in trans together with the T. ferrooxidans ars genes to the E. coli double mutant (trxA gshA), resistance to arsenate was restored. If the double mutant was transformed with only pTrx6, the cells remained sensitive to arsenate. This result provided genetic evidence that reduction of arsenate by ArsC of T. ferrooxidans is coupled to thioredoxin.
Conservation of the unusual ars operon structure in other T. ferrooxidans strains.
To determine whether the divergent arrangement of the arsBH and putative arsR and arsC genes found in T. ferrooxidans ATCC 33020 was unique to this strain, primers BBARSB and BBARSC (Table 1) were designed within the 5′ ends of the arsB and arsC genes, which allowed amplification of the 450-bp putative arsR-promoter region (Fig. 3A). If other strains of T. ferrooxidans also have divergent arsBH and putative arsR and arsC genes, the primers would be orientated towards each other, and amplification of a 450-bp fragment would occur. If the genes were not divergently arranged, the primers would face in the same direction, and no amplification would be detected. A PCR product of the predicted size was obtained from chromosomal preparations of T. ferrooxidans ATCC 33020, ATCC 23270, ATCC 19859, and ATCC 13598 but not from chromosomal preparations of other acidophilic bacteria, such as L. ferrooxidans DSM 2705, Thiobacillus thiooxidans ATCC 19377, and T. caldus MNG, or from E. coli DH5α (Fig. 3B). PCR experiments performed with primers which amplified the arsH gene (primers ARSHF and ARSHR [Table 1]) yielded a product of the predicted size for all of the T. ferrooxidans strains tested but not for the other bacteria (data not shown). This indicated that other strains of T. ferrooxidans also possessed the arsH gene.
FIG. 3.
(A) Locations of primers used to determine the divergent arrangement of the putative arsR and arsC genes and the arsBH genes in different strains of T. ferrooxidans and other biomining bacteria. Primers located within the arsB and arsC genes were used to amplify the 450-bp region between these two genes. (B) Ethidium bromide-stained agarose gel of the PCR amplification products, prepared by using chromosomal DNA from different biomining bacteria. Abbreviations: T.f., T. ferrooxidans; L.f., L. ferrooxidans; T.t., T. thiooxidans; T.c., T. caldus; E.c., E. coli. +ve, positive; -ve, negative.
Expression of T. ferrooxidans ars gene products by using an E. coli in vitro transcription-translation system.
Before investigating which of the predicted ORFs described in Table 2 contributed to arsenic resistance in E. coli, we examined which polypeptides were expressed in an E. coli in vitro transcription-translation system. Compared with the vector (Fig. 4, lanes 5 and 10), the pTfars1b clone yielded additional polypeptides at approximately 45, 41, 35, 27, 25, 18, 14, and 12 kDa (Fig. 4, lane 6). The 18-kDa protein was clearly identified as ArsC. The size of this protein was consistent with the size predicted from the sequence (18.2 kDa); this protein was present only when arsC was included in a test construct and was the only polypeptide produced by pTfarsC (data not shown). The 27-kDa protein was identified as ArsH; its size was close to the predicted size (26.7 kDa), it was synthesized only when an arsH gene was present, and it was the only polypeptide synthesized by pTfarsH (Fig. 4B, lane 9). A broad band at about 35 kDa was the only polypeptide band produced by pTfarsB and was always present when arsB was present. This protein appeared to be smaller than the 48.5-kDa protein predicted, but membrane-located proteins often migrate anomalously (2, 12, 24, 26) and ArsB must have been synthesized since all of the cells containing arsB constructs were resistant to arsenite. The 14-kDa band corresponded to the putative ArsR protein as it was the only additional band (compared to the vector) produced by pTfarsR (Fig. 4, lane 7). Based on the polypeptides produced by pTfars1b and the predicted sizes of the ORFs, the 41- and 45-kDa polypeptides were products of ORFs 1 and 5, respectively. We presumed that the 25- and 12-kDa proteins present in pTFars1b but not pTfarsCRBH were synthesized from genes downstream of ORF 5.
FIG. 4.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of the proteins expressed from pTfars1a and subclones by using an E. coli-derived in vitro transcription-translation system.
Ability of the cloned T. ferrooxidans ars gene products to confer increased resistance to arsenic compounds and antimonite in E. coli AW3110.
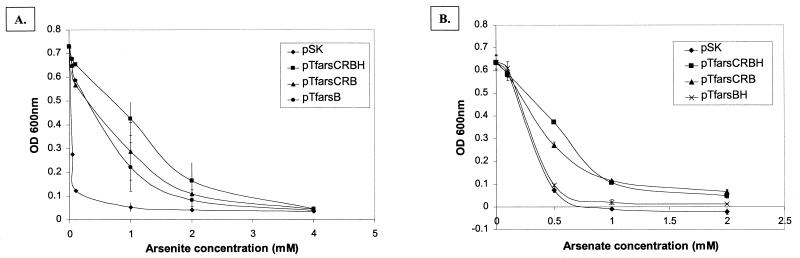
Constructs pTfars1b and pTfarsCRBH conferred equivalent levels of resistance to arsenite [As(III)] and arsenate [As(V)] in E. coli AW3110 (data not shown). The abilities of pTfarsCRBH and subclones to confer resistance to arsenite in E. coli AW3110 were tested further, as shown in Fig. 5A. A construct containing only the arsB gene (pTfarsB) conferred levels of resistance to arsenite similar to the levels of resistance conferred by pTfarsCRBH or pTfarsCRB. This experiment was repeated four times, and although the results of the experiments varied, we obtained no clear evidence that arsenite resistance in E. coli was improved by the presence of arsC or arsH. Therefore, only arsB was required for resistance to arsenite in E. coli. The cloned T. ferrooxidans arsB gene was also required to enhance the resistance of E. coli to antimony, and this resistance was not enhanced further by the presence of arsC or arsH (data not shown). As expected, the arsC and arsB genes were essential for increased resistance of E. coli to arsenate, and this resistance was not increased by the presence of arsH (Fig. 5B).
FIG. 5.
Growth of E. coli AW3110 containing pSK, pTfarsCRBH, pTfarsCRB, pTfarsBH, and pTfarsB in the presence of arsenite (A) and arsenate (B). Each data point represents the results of three assays of three independent experiments. The error bars indicate standard deviations. OD 600 nm, optical density at 600 nm.
DISCUSSION
During biooxidation of arsenopyrite ores and concentrates large quantities of arsenic are released into the surrounding solution. Since T. ferrooxidans is a member of the consortium of bacteria involved in arsenopyrite biooxidation, we expected that T. ferrooxidans would possess arsenic resistance genes. However, the unusual arrangement of the T. ferrooxidans ars operon was not expected. No arsD gene or arsA-like gene (ATPase subunit) was found in the immediate vicinity of the arsenic resistance genes, and only arsC, arsB, and arsH-like genes were identified based on initial sequence comparisons. More careful analysis resulted in identification of a putative regulator between the arsB and arsC genes. However, the predicted protein exhibited only relatively weak homology to the ArsR proteins produced by previously described ars operons. This protein also lacked the conserved metal-binding box to which the arsenite inducer binds, which causes a conformational change in the helix-turn-helix domain and results in dissociation of the repressor from the DNA (29). Recently, the regulator of the chromosomal zinc resistance operon of Staphylococcus aureus, ZntR, which appears to belong to the ArsR family of transcriptional regulators, was also found to lack the metal-binding motif (33). The binding reaction of the ZntR protein and the znt promoter was, however, still inhibited by the presence of 25 μM Zn2+ or Co2+. There is, therefore, some indication that there may be other unknown interactions involved in induction of the operon. The orientation of the putative arsR and arsC genes and the arsBH genes indicated that the genes must be divergently transcribed in a manner that has not been observed before. Furthermore, the divergent gene arrangement was conserved in T. ferrooxidans strains that originated from Canada, the United States, and Japan but was not found in L. ferrooxidans, T. thiooxidans, or T. caldus. In such a divergent arrangement, it is possible that transcription of arsC and transcription of arsB are regulated separately. It has been suggested that TCAT-N7-TTTG may represent a consensus binding site for the E. coli chromosomal ArsR and R773 ArsR repressors (36), but no corresponding sequences were detected in the region between the T. ferrooxidans arsC and arsB genes (data not shown). Work is in progress to investigate regulation of the T. ferrooxidans ars operon. In addition to Northern blotting and transcript mapping, this work will involve construction of arsR-, arsC-, and arsB-lacZ promoter-fusion reporter genes, a ptac-arsR IPTG (isopropyl-β-d-thiogalactopyranoside)-controlled expression construct, and an E. coli lac-Δars mutant strain. This study should reveal how the divergent operon is regulated and whether the activity of the putative ArsR protein of T. ferrooxidans is affected by arsenic.
An ORF with homology to the arsH gene was located immediately downstream of the arsB gene. The arsH gene was first identified in Y. enterocolitica, in which it is divergently transcribed from the arsRBC genes (17). Although it has been reported that the arsH gene is necessary for arsenic resistance, the function of the gene is unknown, and Neyt et al. (17) hypothesized that it might act as some type of regulator. When BLAST, Prosite (http://www.expasy.ch/prosite), and pfam (http://pfam.wustl.edu/pfam) were used, ArsH proteins exhibited no clearly discernable overall or motif similarity to other proteins. The finding that the T. ferrooxidans arsH-like gene was expressed in an E. coli in vitro transcription-translation system but was not required for resistance to arsenite, arsenate, or antimony in E. coli is in apparent contrast to data obtained for the arsenic resistance genes present on the Y. enterocolitica pYV virulence plasmid. However, the effect of pYV arsH was studied in Y. enterocolitica, while the effect of the T. ferrooxidans arsH was studied in a heterologous E. coli host. It is possible that arsH has an effect on arsenic resistance in T. ferrooxidans that is not observed when arsH is cloned in E. coli. The presence of an arsH-like gene appears to be a feature of T. ferrooxidans, as such a gene was detected in all four strains examined. Whether ArsH plays a role in ars regulation will be examined in another study.
As an obligately chemolithotrophic acidophilic bacterium, T. ferrooxidans comes from an environment which could be predicted to be genetically more isolated than the environments of most other bacteria in which ars genes have been studied. Therefore, it was interesting to compare the predicted amino acid sequences of the T. ferrooxidans ArsB and ArsC proteins with the amino acid sequences of proteins of other bacteria. T. ferrooxidans ArsB clearly grouped with the ArsB proteins of other gram-negative bacteria. The predicted amino acid sequence of T. ferrooxidans ArsC was most closely related to the amino acid sequence of ArsC of P. aeruginosa. However, unlike P. aeruginosa ArsC, which did not reduce arsenate when arsC was cloned in E. coli (3), cloned arsC of T. ferrooxidans was expressed and functional in the heterologous host. In contrast to the ArsB proteins, the ArsC proteins of T. ferrooxidans and P. aeruginosa were more similar to ArsC proteins of gram-positive bacteria. Genetic evidence that ArsC of T. ferrooxidans is biochemically like the thioredoxin-requiring gram-positive type of ArsC proteins supports this grouping. Therefore, it is clear that there are at least two groups of ArsC proteins, a four-conserved-cysteine thioredoxin-requiring ArsC group and a two-conserved-cysteine glutathione-requiring ArsC group (31). Since T. ferrooxidans is the second gram-negative bacterium found to have a four-conserved-cysteine thioredoxin-requiring ArsC, correlation of this type of ArsC with gram-positive bacteria, which appeared to be valid based on the initial data for several of the arsenic resistance genes examined, appears to be an oversimplification.
Like other workers, we found that the ArsB protein is difficult to detect unequivocally in an E. coli in vitro system (34). Nevertheless, the arsB and arsC genes of T. ferrooxidans were clearly expressed and functional in E. coli. As found with other ars operons, the T. ferrooxidans arsB gene on its own was able to confer resistance to arsenite and antimony, but arsC was required in addition to arsB for arsenate resistance (Fig. 5). The ability of the T. ferrooxidans ars system to function in E. coli is noteworthy when how arsenic resistance systems may be energized is considered. It is thought that in arsenic resistance systems which lack the ArsA (ATPase) membrane potential rather than ATP, hydrolysis is the energy source (9, 12, 35). The obligately acidophilic organism T. ferrooxidans has an internal pH of about 6.5, and when cells are growing on Fe2+ at pH 2.0, they maintain a ΔpH of 4.5 U (7). Unlike other bacteria, acidophilic bacteria with a large ΔpH have a positive inside membrane potential rather than a negative inside membrane potential, which subtracts from the H+ gradient instead of augmenting it (16). Nevertheless, in spite of the fact that it originated from a bacterium that has a positive inside membrane potential, the T. ferrooxidans ArsB arsenite efflux pump membrane was functional in E. coli.
This initial investigation of the T. ferrooxidans arsenic resistance system was performed with E. coli. In future work we will have to include studies of arsenic resistance and gene expression in T. ferrooxidans, which, because of the rudimentary genetic system available, the lack of mutants, and the difficulty of readily obtaining large quantities of cells, will present a considerable challenge.
ACKNOWLEDGMENTS
We thank Barry Rosen for providing E. coli W3110 and AW3110 and much useful advice, especially advice concerning low-phosphate media. We also thank Simon Silver for his interest and advice.
This work was supported by grants from the National Research Foundation (Pretoria, South Africa) and Billton Process Research Laboratories (Randburg, South Africa).
REFERENCES
- 1.Altshul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein data base search programs. Nucleic Acids Res. 1997;25:3308–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai J, Dubow M S. Expression of the Escherichia coli ars operon. Can J Microbiol. 1996;42:662–671. doi: 10.1139/m96-091. [DOI] [PubMed] [Google Scholar]
- 3.Cai J, Salmon K, Dubow M S. A chromosomal ars operon homologue of Pseudomonas aeruginosa confers increased resistance to arsenic and antimony in Escherichia coli. Microbiology. 1998;144:2705–2713. doi: 10.1099/00221287-144-10-2705. [DOI] [PubMed] [Google Scholar]
- 4.Carlin A, Shi W, Dey S, Rosen B P. The ars operon of Escherichia coli confers arsenical and antimonal resistance. J Bacteriol. 1995;177:981–986. doi: 10.1128/jb.177.4.981-986.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cervantes C, Ji G, Ramirez J L, Silver S. Resistance to arsenic compounds in microorganisms. FEMS Microbiol Rev. 1994;15:355–367. doi: 10.1111/j.1574-6976.1994.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 6.Chang A C Y, Cohen S N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the p15A cryptic miniplasmid. J Bacteriol. 1978;134:1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox J C, Nicholls D G, Ingledew W J. Transmembrane electrical potential and transmembrane pH gradient in acidophile Thiobacillus ferrooxidans. Biochem J. 1979;178:195–200. doi: 10.1042/bj1780195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dew D W, Lawson E N, Broadhurst J L. The Biox® process for biooxidation of gold-bearing ores or concentrates. In: Rawlings D E, editor. Biomining: theory, microbes and industrial processes. Berlin, Germany: Springer-Verlag; 1997. pp. 45–80. [Google Scholar]
- 9.Dey S, Rosen B P. Dual mode of energy coupling by the oxyanion-translocating ArsB protein. J Bacteriol. 1995;177:385–389. doi: 10.1128/jb.177.2.385-389.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoue H, Nojima H, Okayama H. High efficiency transformation of Escherichia coli with plasmids. Gene. 1990;96:23–28. doi: 10.1016/0378-1119(90)90336-p. [DOI] [PubMed] [Google Scholar]
- 11.Ji G, Silver S. Reduction of arsenate to arsenite by the ArsC protein of the arsenic resistance operon of Staphylococcus aureus plasmid pI258. Proc Natl Acad Sci USA. 1992;89:7974–7978. doi: 10.1073/pnas.89.20.9474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji G, Silver S. Regulation and expression of the arsenic resistance operon from Staphylococcus aureus plasmid pI258. J Bacteriol. 1992;174:3684–3694. doi: 10.1128/jb.174.11.3684-3694.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji G, Garber E A, Ames L G, Chen C-M, Fuchs J A, Silver S. Arsenate reductase of Staphylococcus aureus plasmid pI258. Biochemistry. 1994;33:7294–7299. doi: 10.1021/bi00189a034. [DOI] [PubMed] [Google Scholar]
- 14.Lim C-J, Gleason F K, Fuchs J A. Cloning, expression and characterization of the Anabaena thioredoxin gene in Escherichia coli. J Bacteriol. 1986;168:1258–1264. doi: 10.1128/jb.168.3.1258-1264.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lui J, Gladysheva T B, Lee L, Rosen B P. Identification of an essential cysteinyl residue in the arsenate reductase of plasmid R773. Biochemistry. 1995;34:13472–13476. doi: 10.1021/bi00041a026. [DOI] [PubMed] [Google Scholar]
- 16.Matin A. Keeping a neutral cytoplasm: the bioenergetics of obligate acidophiles. FEMS Microbiol Rev. 1990;75:307–318. [Google Scholar]
- 17.Neyt C, Iriarte M, Ha Thi V, Cornelis G R. Virulence and arsenic resistance in yersiniae. J Bacteriol. 1997;179:612–619. doi: 10.1128/jb.179.3.612-619.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oden K L, Gladysheva T B, Rosen B P. Arsenate reduction mediated by the plasmid-encoded ArsC protein is coupled to glutathione. Mol Microbiol. 1994;12:301–306. doi: 10.1111/j.1365-2958.1994.tb01018.x. [DOI] [PubMed] [Google Scholar]
- 19.Powles R E, Deane S M, Rawlings D E. Molecular genetic analysis of a thioredoxin gene from Thiobacillus ferrooxidans. Microbiology. 1995;141:2175–2181. doi: 10.1099/13500872-141-9-2175. [DOI] [PubMed] [Google Scholar]
- 20.Ramesar R S. Developmental studies on Thiobacillus ferrooxidans. Ph.D. thesis. Cape Town, South Africa: University of Cape Town; 1988. [Google Scholar]
- 21.Rawlings D E, Tributsch H, Hansford G S. Reasons why ‘Leptospirillum’-like species rather than Thiobacillus ferrooxidans are the dominant iron-oxidizing bacteria in many commercial processes for the biooxidation of pyrite and related ores. Microbiology. 1999;145:5–13. doi: 10.1099/13500872-145-1-5. [DOI] [PubMed] [Google Scholar]
- 22.Rawlings D E, Silver S. Mining with microbes. Bio/Technology. 1995;13:773–778. [Google Scholar]
- 23.Rensing C, Ghosh M, Rosen B P. Families of soft-metal-ion-transporting ATPases. J Bacteriol. 1999;181:5891–5897. doi: 10.1128/jb.181.19.5891-5897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenstein R, Peschel A, Wieland B, Gotz F. Expression and regulation of the antimonite, arsenite, and arsenate resistance of Staphylococcus xylosus plasmid pSX267. J Bacteriol. 1992;174:3676–3683. doi: 10.1128/jb.174.11.3676-3683.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 26.San Fransisco M J D, Hope C L, Owolabi J B, Tisa L S, Rosen B P. Identification of the metalloregulatory element of the plasmid-encoded arsenical resistance operon. Nucleic Acids Res. 1990;18:619–624. doi: 10.1093/nar/18.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato T, Kobayashi Y. The ars operon in the skin element of Bacillus subtilis confers resistance to arsenate and arsenite. J Bacteriol. 1998;180:1655–1661. doi: 10.1128/jb.180.7.1655-1661.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi W, Wu J, Rosen B P. Identification of a putative metal binding site in a new family of metalloregulatory proteins. J Biol Chem. 1994;269:19826–19829. [PubMed] [Google Scholar]
- 30.Silver S. Bacterial resistances to toxic metals—a review. Gene. 1996;179:9–19. doi: 10.1016/s0378-1119(96)00323-x. [DOI] [PubMed] [Google Scholar]
- 31.Silver S, Phung L T. Bacterial heavy metal resistance: new surprises. Annu Rev Microbiol. 1996;50:753–789. doi: 10.1146/annurev.micro.50.1.753. [DOI] [PubMed] [Google Scholar]
- 32.Silver S, Walderhaug M. Gene regulation of plasmid- and chromosome-determined inorganic ion transport in bacteria. Microbiol Rev. 1992;56:195–228. doi: 10.1128/mr.56.1.195-228.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh V K, Xiong A, Usgaard T R, Chakrabarti S, Deora R, Misra T K, Jayaswal R K. ZntR is an autoregulatory protein and negatively regulates the chromosomal zinc resistance operon znt of Staphylococcus aureus. Mol Microbiol. 1999;33:200–207. doi: 10.1046/j.1365-2958.1999.01466.x. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki K, Wakao N, Kimura T, Sakka K, Ohmiya K. Expression and regulation of the arsenic resistance operon of Acidiphilium multivorum AIU 301 plasmid pK301 in Escherichia coli. Appl Environ Microbiol. 1998;64:411–418. doi: 10.1128/aem.64.2.411-418.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai K-J, Hsu C-M, Rosen B P. Efflux mechanisms of resistance to cadmium, arsenic and antimony in prokaryotes and eukaryotes. Zool Stud. 1997;36:1–16. [Google Scholar]
- 36.Xu C, Shi W, Rosen B P. The chromosomal arsR gene of Escherichia coli encodes a trans-acting metalloregulatory protein. J Biol Chem. 1996;271:2427–2432. doi: 10.1074/jbc.271.5.2427. [DOI] [PubMed] [Google Scholar]