Abstract
Species belonging to the genus Lippia are used worldwide as foods, beverages, and seasonings. Studies have demonstrated that these species have antioxidant, sedative, analgesic, anti-inflammatory, and antipyretic activities. This work aimed to evaluate the antibacterial activity and anxiolytic effect by different pathways of essential oils and ethanolic extracts of three species of Lippia (Lippia alba, Lippia sidoides, and Lippia gracilis). The ethanolic extracts were characterized by HPLC-DAD-ESI-MSn and their phenolics were quantified. The antibacterial activity was evaluated by determining the minimal inhibitory concentration and modulation of antibiotic activity, and toxic and anxiolytic effects were evaluated in the zebrafish model. The extracts showed compositions with a low ratio and shared compounds. L. alba and L. gracilis showed higher amounts of phenols and flavonoids, respectively. All extracts and essential oils presented antibacterial activity, especially those obtained from L. sidoides. On the other hand, L. alba extract presented the most significant antibiotic-enhancing effect. The samples were not toxic after 96 h of exposure, but showed an anxiolytic effect through modulation of the GABAA receptor, while L. alba extract acted via modulation of the 5-HT receptor. This new pharmacological evidence opens horizons for therapeutic approaches targeting anxiolytic and antibacterial therapies and food conservation using these species and their constituents.
Keywords: verbenaceae, essential oil, phenolic compounds, antibacterial, anxiolytic, toxicity, zebrafish
1. Introduction
Anxiety disorders are among the ten major global causes of disabilities. In 2015, Brazil was considered by the World Health Organization (WHO) as the country with the highest rate of anxiety disorders in the Americas, affecting 9.3% of the population [1]. Benzodiazepines (BZD), which act as positive allosteric modulators of the GABAA receptor, have historically been used as the mainstay of therapy against anxiety for decades, also exhibiting amnesiac, anticonvulsant, sedative, and muscle relaxant effects. However, their chronic use is known to induce tolerance and cause considerable side effects [2,3].
In the list of global health issues, antimicrobial resistance stands out for its worrying spread. Several pathogenic bacteria have developed resistance to first-line antibiotics, causing intractable infections, which, therefore, are associated with high morbidity and mortality rates owing to the lack of effective therapies [4,5].
Importantly, developing new treatment options to combat antibiotic resistance is urgently needed before the inexistence of effective prevention measures and the low availability of antibiotics [6]. In addition, like anxiolytic drugs, antibacterial agents are not free from side effects; therefore, searching for a novel, safe, and effective compound remains an important research topic [7].
Medicinal plants are traditionally used for the treatment of a great variety of diseases. Moreover, many species have served as raw materials for the extraction of bioactive compounds whose health-promoting benefits have long been demonstrated [8]. Studies have shown that different plant extracts and natural products have pharmacological potential, including dietary phytochemicals such as terpenes and phenolic compounds, among others [7].
The species of the genus Lippia are used worldwide as food, beverages, and seasonings. Previous research has demonstrated that these species are helpful in treating diseases of the gastrointestinal and respiratory tracts, which is in line with their well-described analgesic, anti-inflammatory, antipyretic [9], antioxidant, antiulcerogenic, antiseptic, and antimicrobial activities. Importantly, studies have demonstrated the effects of Lippia species in the central nervous system, including analgesic, anxiolytic, anticonvulsant, sedative, and antinociceptive [10]. On the other hand, the toxic effects of many species in this genus remain to be better investigated.
Considering the evidence that Lippia species have the potential to be used in drug development, this study aimed to evaluate the antibacterial and anxiolytic effects of three species of the genus Lippia found in the region of Cariri-Ceará, Brazil.
2. Results and Discussion
2.1. Chemical Composition
The three ethanol extracts were essentially rich in phenolic compounds, as shown in Table 1. Lippia alba ethanolic extract (LaEE) showed a greater diversity of compounds, containing 5 iridoids, 1 phenylethanoid glycoside, 1 oxylipin, 1 fatty acid, and 11 flavonoids. While Lippia sidoides ethanolic extract (LsEE) and Lippia gracilis ethanolic extract (LgEE) presented only flavonoids, with 12 and 15 compounds, respectively, some compounds were identified in more than one sample, such as emodin-8-O-glycoside (20) in LaEE and LsEE; luteolin-6-O-glycoside (16), eriodictyol (23), and luteolin (26) in LsEE and LgEE; and the flavonoids naringenin (24) and cirsimaritin (34) were present in all studied extracts.
Table 1.
Compounds identified by HPLC-DAD-ESI-MSn in ethanolic extracts of L. alba, L. sidoides, and L. gracilis.
| N° | R.T. | [M-H]− | Molecular Formula | Error (ppm) | MSn m/z | Assignment | Extract | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | 11.1 | 389.1083 | C16H21O11 | 1.6 | MS2 [389.0]: 227.0, 191.0, 165.0, 147.0 | monotropein | LaEE | [11] |
| 2 | 22.3 | 373.1138 | C16H21O10 | 0.7 | MS2 [373.0]: 211.0, 193.0, 167.0, 149.0, 123.0 | geniposidic acid | LaEE | [12] |
| 3 | 27.4 | 375.1294 | C16H23O10 | 0.8 | MS2 411.0 [M + Cl−H]−: 375.0 MS3 [411.0 → 375.0]: 213.0, 169.0, 151.0 |
loganic acid | LaEE | [13] |
| 4 | 31.4 | 405.1406 | C17H25O11 | 0.9 | MS2 451.0 [M + HCOOH−H]−: 405.0 MS3 [451.0 → 405.0]: 373.0, 243.0 MS4 [405.0 → 243.0]: 225.0, 123.0, 101.0 |
shanzhiside methyl ester | LaEE | [12] |
| 5 | 36.2 | 387.1656 | C18H27O9 | 1.2 | MS2 [387.0]: 207.0, 163.0 | tuberonic acid glucoside | LaEE | [14] |
| 6 | 38.8 | 593.0112 | C27H29O16 | 0.3 | MS2 [593.0]: 575.0, 503.0, 473.0, 383.0, 353.0 | apigenin-6,8-C-diglucoside | LgEE | [15] |
| 7 | 39.5 | 389.1452 | C17H25O10 | 0.4 | MS2 435.0[M + HCOOH−H]−: 389.0 MS3 [435.0 → 389.0]: 227.0, 101.0 |
loganin | LaEE | [16] |
| 8 | 43.2 | 303.0519 | C15H12O7 | 1.6 | MS2 [303.0]: 284.9, 176.9, 124.8 | taxifolin | LsEE LgEE |
[17] |
| 9 | 43.4 | 449.1093 | C21H22O11 | 0.5 | MS2 [449.0]: 286.9 MS3 [449.0 → 286.9]: 150.9 MS4 [449.0 → 286.9 → 150.9]: 106.9 |
eriodictyol-7-O-glicoside | LsEE | [18] |
| 10 | 43.4 | 447.0421 | C21H19O11 | 0.2 | MS2 [447.0]: 392.9, 356.9, 327.0 | orientin | LgEE | [19] |
| 11 | 44.1 | 447.0235 | C21H19O11 | 0.7 | MS2 [447.0]: 429.0, 411.0, 357.0, 327.0 | isoorientin | LgEE | [19] |
| 12 | 44.3 | 623.1989 | C29H35O15 | 1.2 | MS2 [623.0]: 461.0, 315.0 | acteoside | LaEE | [20] |
| 13 | 45.8 | 431.0431 | C21H20O10 | 1.0 | MS2 [431.0]: 310.9, 340.9 MS3 [431.0 → 310.9]: 282.9 |
vitexin | LgEE | [21] |
| 14 | 47.2 | 451.1231 | C21H24O11 | −2.6 | MS2 [451.0]: 288.9 MS3 [451.0 → 288.9]: 270.9, 166.8, 124.9 |
3-hydroxyphlorizin | LsEE | [22] |
| 15 | 48.2 | 431.0169 | C21H20O10 | 0.9 | MS2 [431.0]: 413.0, 395.0; 310.9, 341.0 MS3 [431.0 → 310.9]: 282.9 |
isovitexin | LgEE | [21] |
| 16 | 48.5 | 447.0940 | C21H20O11 | −1.3 | MS2 [447.0]: 284.9 MS3 [447.0 → 284.9]: 240.9, 198.8, 174.9, 150.8, 132.9 |
luteolin-6-O-glicoside | LsEE LgEE |
[18] |
| 17 | 49.5 | 286.9134 | C15H12O6 | 0.4 | MS2 [286.9]: 269.0, 258.9, 243.0, 201.0, 124.9 | dihydrokaempferol | LgEE | [23] |
| 18 | 50.3 | 507.1145 | C23H23O13 | 0.1 | MS2 [507.0]: 345.0, 330.0, 315.0 | quercetagetin-dimethyl-O-hexoside | LaEE | [24] |
| 19 | 51.9 | 435.1296 | C21H24O10 | 0.1 | MS2 [435.0]: 272.9 MS3 [435.0 → 272.9]: 166.8 |
phloridzin | LsEE | [25] |
| 20 | 52.5 | 431.0994 | C21H20O11 | −2.4 | MS2 [431.0]: 269.0 MS3 [431.0 → 269.0]: 225.0 |
emodin-8-O-glicoside | LaEE LsEE |
[26] |
| 21 | 53.2 | 505.1002 | C23H21O13 | 2.8 | MS2 [505.0]: 329.0, 314.0, 299.0 | tricin-7-O-glucuronide | LaEE | [27] |
| 22 | 53.4 | 445.0764 | C21H17O11 | 2.8 | MS2 [445.0]: 269.0, 175.0 MS3 [445.0 → 269.0]: 225.0, 183.0 |
apigenin-7-O-glucuronide | LaEE | [28] |
| 23 | 55.5 | 287.0596 | C15H12O6 | −2.0 | MS2 [287.0]: 268.8, 150.8, 124.9, 106.9 | eriodictyol | LsEE LgEE |
[29] |
| 24 | 60.6 | 271.0619 | C15H11O5 | 2.7 | MS2 [270.9]: 176.8, 150.8, 118.9 | naringenin | LaEE LsEE LgEE |
[29] |
| 25 | 61.4 | 300.9852 | C15H10O7 | 0.1 | MS2 [300.9]: 272.9, 178.8, 150.8 | quercetin | LgEE | [30] |
| 26 | 62.3 | 285.0408 | C15H10O6 | −1.3 | MS2 [284.9]: 266.9, 256.8, 242.9, 240.9, 216.9, 198.9, 174.9, 150.9, 132.9 | luteolin | LsEE LgEE |
[29] |
| 27 | 62.9 | 315.0502 | C16H11O7 | 2.7 | MS2 [315.0]: 300.0 | isorhamnetin | LaEE | [31] |
| 28 | 64.1 | 593.1489 | C27H30O15 | −2.5 | MS2 [593.0]: 446.9, 284.9 MS3 [593.0 → 284.9]: 240.8, 198.7, 174.8, 150.9, 132.9 |
luteolin-7-O-rutinose | LsEE | [32] |
| 29 | 66.7 | 299.0555 | C16H11O6 | 2.1 | MS2 [299.0]: 284.0 MS3 [299.0 → 284.0]: 256.0, 227.0, 212.0 |
chrysoeriol | LaEE | [33] |
| 30 | 66.9 | 268.0459 | C15H10O5 | −1.5 | MS2 [268.9]: 224.8, 226.9, 200.9, 150.9, 148.8 | apigenin | LsEE | [34] |
| 31 | 67.0 | 329.0662 | C17H13O7 | 1.6 | MS2 [329.0]: 314.0 MS3 [329.0 → 314.0]: 299.0, 285.0 |
tricin | LaEE | [33] |
| 32 | 69.1 | 373.0925 | C19H17O8 | 1.1 | MS2 [373.0]: 358.0, 343.0 MS3 [373.0 → 358.0]: 343.0 MS4 [373.0 → 358.0 → 343.0]: 328.0, 300.0 |
dihydroxy-tetramethoxy flavone | LaEE | [24] |
| 33 | 69.5 | 327.2166 | C18H31O5 | 3.4 | MS2 [327.0]: 309.0, 291.0, 229.0, 211.0, 209.0, 171.0 | oxo-dihydroxy-octadecenoic acid | LaEE | [28] |
| 34 | 70.0 | 313.0706 | C17H13O6 | 3.6 | MS2 [313.0]: 297.9, 283.0, 269.0 | cirsimaritin | LaEE LsEE LgEE |
[35] |
| 35 | 71.7 | 284.9821 | C16H14O5 | 0.6 | MS2 [284.9]: 190.8, 164.9, 118.9 | sakuranetin | LgEE | [36] |
| 36 | 71.8 | 343.0823 | C18H17O7 | 0.0 | MS2 [343.0]: 328.0, 313.0 MS3 [343.0 → 328.0]: 313.0, 298.0, 285.0, 270.0 |
dihydroxy-trimethoxyflavone | LaEE | [24] |
| 37 | 72.4 | 254.9103 | C15H12O4 | 0.1 | MS2 [254.9]: 212.9, 186.9, 150.8, 144.8, 135.8, 124.9 | pinocembrin | LgEE | [37] |
R.T.: retention time. LaEE: Lippia alba ethanolic extract; LsEE: Lippia sidoides ethanolic extract; LgEE: Lippia gracilis ethanolic extract.
Iridoid glycosides are secondary metabolites known to be found in species of the Verbenaceae family [38]. In ethanolic and methanolic extracts from L. alba leaves, the presence of geniposidic acid (2) and shanzhiside methyl ester (4) was reported [39,40]. On the other hand, for the compounds monotropein (1), loganic acid (3), and loganin (7), this is the first report for extracts of this species. Commonly, 8-epi-loganin is found in different extracts of L. alba, which is a stereoisomer of loganin [39,40,41]. The difference in composition between individuals of the same species may be linked to the variation in abiotic factors to which they are subjected, such as temperature, humidity, and precipitation, among others [42].
Acteoside (12), also called Verbacoside, is a phenylethanoid glycoside already found in extracts of L. alba [39,43]. It is recognized for its biological activities, such as an anticonvulsant, antiparkinsonian, cytotoxic, hypotensive, anticancer, antioxidant, and so on [44]. Tuberonic acid glycoside (5), on the other hand, was only seen in extracts of L. graveolens Kunth and L. citriodora (Palau) Kunth [45,46], with this being the first report in L. alba.
The chemical compositions of the ethanolic extract of Lippia species showed an abundance of secondary metabolites of the flavonoids class [45], corroborating with the results of this study. The flavonoids apigenin-7-O-glucuronide (22), tricin-7-O-glucuronide (21), isorhamnetin (27), and naringenin appear in different extracts of L. alba [39,43,47], as well as taxifolin (8), luteolin, naringenin, and apigenin (30) in L. sidoides [48] and naringenin, quercetin (25), orientin (10), and isoorientin (11) in L. gracilis [49]. The diglycosylated form of the flavone chrysoeriol (29) has also been reported for L. alba [41].
Among the flavonoids identified, naringenin and cirsimaritin were found in the three extracts studied, which were reported in the species L. salviifolia Cham., L. velutina Schauer, L. balansae Briq., L. lupulina Cham., L. graveolens Kunth, L. citriodora (Palau) Kunth, L. javanica (Burm.f.) Spreng., L. chevalieri Moldenke, and L. lacunosa Mart. & Schauer [9,45,46,48,50,51]. Thus, we suggest that these flavonoids can be considered as chemical markers for the genus. The use of these markers for medicinal plants is essential considering that bioactivities can, in most cases, be related to a specific chemotype [52]. Figure 1 shows the proposed fragmentations for naringenin and cirsimaritin.
Figure 1.
Proposed fragmentation pattern for deprotonated naringenin (A) and cirsimaritin (B).
The results show that LaEE has a significantly higher content of total phenolics of 30.11 ± 1.24 mg GA/g Ext., followed by LgEE and LsEE (Table 2). Studies show a high phenolic content for ethanolic, methanolic, and aqueous extracts of L. alba leaves, with 117.78 ± 2.69, 367.49 ± 38.90, and 505.11 ± 2.55 µg GAE/g dry weight, respectively [53]. Garmus et al. [54], studying the influence of different types of extraction on the phenolic composition of L. siodides, obtained contents ranging between 38.20 ± 0.06 and 230.5 ± 0.1 mg GAE/g extract.
Table 2.
Total content of phenols and flavonoids in the ethanolic extracts of L. alba, L. sidoides, and L. gracilis.
| Samples | Total Phenolics (mg GA/g Ext.) |
Total Flavonoids (mg QE/g Ext.) |
|---|---|---|
| LaEE | 30.11 ± 1.24 a | 6.99 ± 0.28 a |
| LsEE | 9.17 ± 0.26 b | 8.55 ± 0.10 b |
| LgEE | 9.87 ± 0.71 b | 8.76 ± 0.27 b |
These results are expressed as mean ± SD (n = 3). Means followed by different letters differ by Tukey’s test with p < 0.05. LaEE: Lippia alba ethanolic extract; LsEE: Lippia sidoides ethanolic extract; LgEE: Lippia gracilis ethanolic extract.
In terms of total flavonoids, LgEE had the highest rate of these compounds, with 8.76 ± 0.27 mg QE/g Ext., followed by LsEE and LaEE (Table 2). However, these contents were lower than those obtained for different extracts of L. sidoides, with 43.5 ± 0.3 to 262.3 ± 0.4 mg QE/g extract, and of L. alba, with 371.33 ± 4.50 to 463.94 ± 6.49 g QE/gm dry weight [53,54]. Many studies consider the influence of genetic factors, abiotic factors, seasonality, and type of extraction on the composition of a species [55].
The results show that total flavonoid content is equivalent to 93.23%, 88.75%, and 23.21% of the total phenolic values obtained for LsEE, LgEE, and LaEE, respectively. These percentages agree with the compositions identified in the HPLC-DAD-ESI-MSn analysis. Many studies presented in the literature data, with chemical compositions similar to those presented in this manuscript, have reported the significant antioxidant activity of L. alba, L. gracilis, and L. sidoides [43,49,54].
2.2. Antibacterial Activity
The present analysis demonstrated that all Lippia-derived natural products investigated in this study exhibited significant antibacterial activity (Table 3), with LsEO showing the most remarkable effectiveness in inhibiting bacterial growth, as observed in tests with the strain S. aureus Sa 358 (MIC = 53.3 μg/mL). The most potent activity of this extract was observed against most bacterial strains, except for E. coli Ec 27, which showed significant sensitivity to all tested samples, as confirmed through the low MICs of the products. We hypothesize that the effectiveness of LsEO may be related to constituents such as thymol and eucalyptol, whose antimicrobial activity has been consistently demonstrated [56,57,58].
Table 3.
Minimum inhibitory concentrations of essential oils and ethanolic extracts obtained from L. alba, L. sidoides, and L. gracilis.
| Bacterial Strains | MIC (µg/mL) | |||||
|---|---|---|---|---|---|---|
| LaEO | LsEO | LgEO | LaEE | LsEE | LgEE | |
| Staphylococcus aureus Sa 358 | 256 | 53.3 | 512 | 853.3 | 128 | 512 |
| Streptococcus mutans INCQS 00446 | 213.3 | 106.6 | 512 | ≥1024 | 170.6 | 853.3 |
| Escherichia coli Ec 27 | 106.6 | 106.6 | 426.6 | 768 | 74.6 | 256 |
| Pseudomonas aeruginosa ATCC 15442 | 213.3 | 128 | 512 | ≥1024 | 298.6 | 682.6 |
INCQS: National Institute for Quality Control in Health; ATCC: American Type Culture Collection. LaEO: Lippia alba essential oil; LsEO: Lippia sidoides essential oil; LgEO: Lippia gracilis essential oil; LaEE: Lippia alba ethanolic extract; LsEE: Lippia sidoides ethanolic extract; LgEE: Lippia gracilis ethanolic extract.
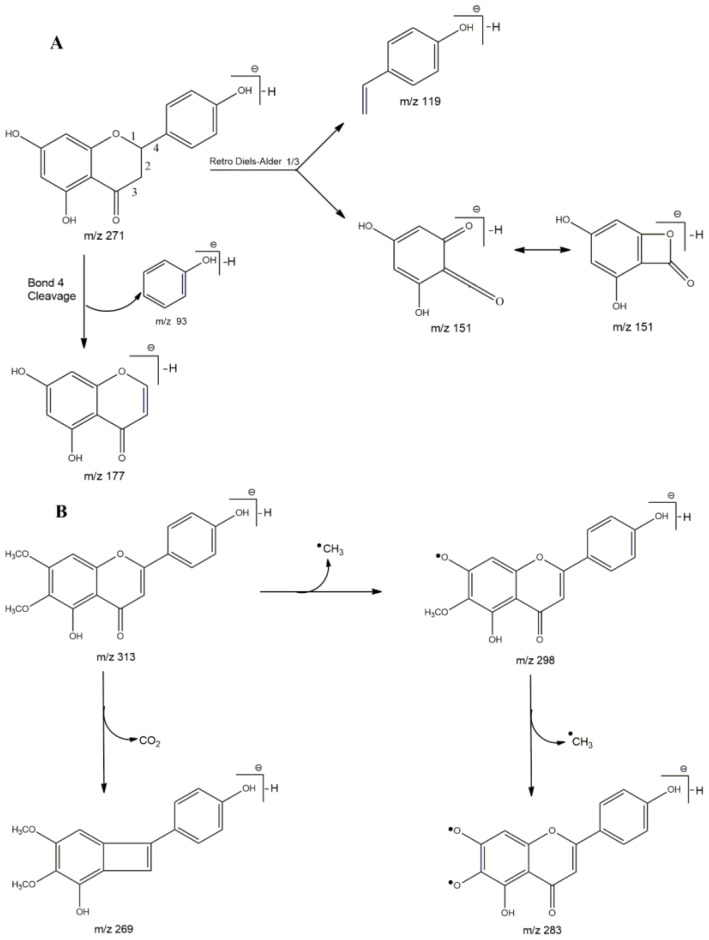
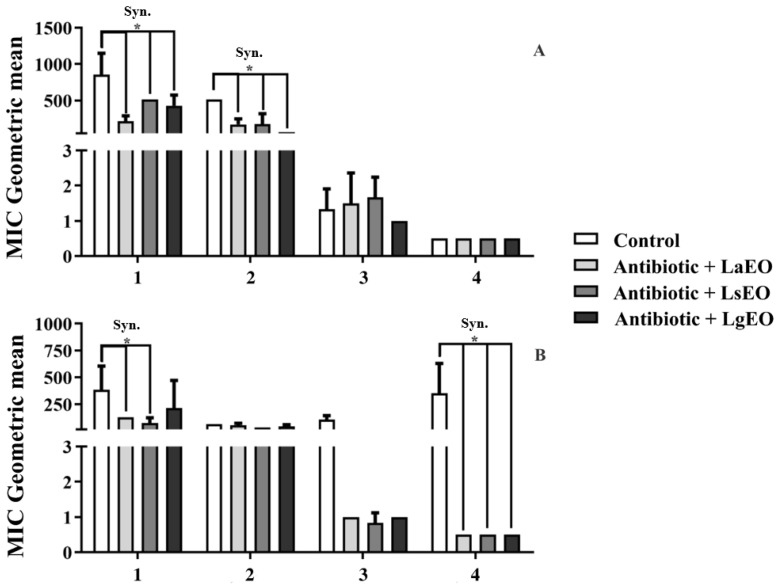
Figure 2 and Figure 3 show the antibacterial activity of conventional antibiotics in association with essential oils and ethanolic extracts of Lippia species against Staphylococcus aureus Sa 358 and Escherichia coli Ec 27. The experiments with the S. aureus strain (Figure 2A) revealed that the combination of amikacin with LaEO resulted in a significant synergistic effect as the MIC of the antibiotic was reduced from 853.3 μg/mL to 213.3 μg/mL. A similar effect was observed when this antibiotic was associated with LsEO and LgEO, which reduced its MIC to 512 μg/mL and 426.6 μg/mL, respectively. When associated with LaEO and LsEO, the MIC of gentamicin was reduced from 512 μg/mL to 170.6 μg/mL and 173.3 μg/mL, respectively, whereas for the combination with LgEO, the antibiotic MIC was reduced to 64 μg/mL, indicating a more remarkable antibiotic-enhancing effect.
Figure 2.
Effects of the essential oils of L. alba (LaEO), L. sidoides (LsEO), and L. gracilis (LgEO) on the antibiotic activity of amikacin (1), gentamicin (2), benzylpenicillin (3), and cephalothin (4) against Staphylococcus aureus Sa 358 (A) and Escherichia coli Ec 27 (B). Syn.: synergism; *: p < 0.05 (ANOVA and Bonferroni’s post-test).
Figure 3.
Effects of the ethanolic extracts of L. alba (LaEE), L. sidoides (LsEE), and L. gracilis (LgEE) on the antibiotic activity of amikacin (1), gentamicin (2), benzylpenicillin (3), and cephalothin (4) against Staphylococcus aureus Sa 358 (A) and Escherichia coli Ec 27 (B). Syn.: synergism; Ant.: antagonism; *: p < 0.05 (ANOVA and Bonferroni’s post-test).
Previous research has demonstrated that the essential oils of Lippia species can potentiate the activity of antibiotics. It was observed that, with the addition of L. alba essential oil (at a concentration of 12%), the diameter of the erythromycin-mediated inhibition zone against S. aureus ATCC 25923 increased by 221.4% [59]. Moreover, the essential oil of L. sidoides significantly reduced the MIC of gentamicin and neomycin against S. aureus ATCC 12624 [60]. In addition, studies have demonstrated the effectiveness of essential oils obtained from these species against food-borne pathogens, indicating that they have potential applications in the food industry [61].
Citral initially affects membrane structure, membrane-associated electron transfer, and cellular respiration, leading to rapid energy depletion and, consequently, bacterial cell death [62]. Thymol, on the other hand, acts through cell membrane disruption, biofilm reduction, motility inhibition, inhibition of membrane-bound ATPases, and inhibition of efflux pumps [57]. These mechanisms may be associated with the action of the tested essential oils.
When combined with beta-lactams (Figure 2A), none of the essential oils significantly improved their antibacterial effects. In this context, the most remarkable effect was obtained with LgEO, which reduced the MIC of benzylpenicillin from 1.3 μg/mL to 1 μg/mL. On the other hand, combining this antibiotic with LaEO and LsEO resulted in antagonistic effects, as the MIC increased to 1.5 μg/mL and 1.6 μg/mL, respectively. Finally, none of these essential oils affected the antibacterial effect of cephalothin. This finding is corroborated by the study of Veras et al. [60]. They observed that the essential oil of L. sidoides failed in modulating the activity of benzylpenicillin and ceftriaxone against Gram-positive and Gram-negative bacteria.
All combinations between essential oils and antibiotics resulted in synergistic effects in experiments with the E. coli strain (Figure 2B). Adding LaEO and LsEO decreased the MIC of amikacin from 384 μg/mL to 128 μg/mL and 74.6 μg/mL, respectively. Interestingly, when combined with cephalothin, each essential oil reduced the antibiotic MIC from 352 μg/mL to 0.5 μg/mL. The combination of LaEO or LgEO decreased the MIC of benzylpenicillin from 106.6 μg/mL to 1 μg/mL. Similarly, an MIC of 0.83 μg/mL resulted from the association of this antibiotic with LsEO. According to the literature, the lipophilic character of essential oils favors their interaction with the lipopolysaccharides of the outer membrane of the cell wall of Gram-negative bacteria, altering its structure and function, which may trigger cell lysis [63].
The ethanolic extracts of the tested species showed significant synergistic effects when combined with aminoglycosides against S. aureus (Figure 3A). In this context, the most significant effect was observed with LaEE, whose combination decreased the MIC of amikacin from 853.3 μg/mL to 32 μg/mL. Similarly, LgEE and LsEE decreased the antibiotic MIC to 53.3 μg/mL and 85.3 μg/mL, respectively. Finally, the MIC of gentamicin decreased from 512 μg/mL to 21.3 μg/mL, 32 μg/mL, and 53.3 μg/mL in the presence of LaEE, LgEE, and LsEE, respectively.
Compounds present in ethanolic extracts, such as quercetin, luteolin, pinocebrin, and apigenin, among others, are known for their antibacterial potential [64,65]. It is reasonable to hypothesize that the antibiotic-enhancing effects of these extracts are associated with the presence of flavonoids in their compositions. These secondary metabolites are known to interfere with bacteria growth through mechanisms that involve inhibition of nucleic acid synthesis, changes in the function and permeability of the cytoplasmic membrane, inhibition of energy metabolism, reduction in cell adhesion and biofilm formation of biofilm, and inhibition of porins in the cell membrane [66].
Unlike the experiments with S. aureus, the results obtained with E. coli cultures (Figure 3B) were quite heterogeneous. While LsEE significantly reduced the MIC of amikacin (from 384 μg/mL to 128 μg/mL), the association with LsEE and LgEE resulted in an antagonistic effect as the antibiotic MIC increased to 512 μg/mL. Moreover, LaEE antagonized the effect of benzylpenicillin, increasing its MIC from 106.6 μg/mL to 426.6 μg/mL. On the other hand, all extracts potentiated the effects of cephalothin. Antagonistic effects from the combination of different antibiotics and natural products are reported in the literature. Evidence indicates that the mechanisms underlying this phenomenon involve competition by the active site and chelation, which frequently results in decreased antibiotic activity [67].
2.3. Effects of Lippia Species in the Zebrafish Experimental Model
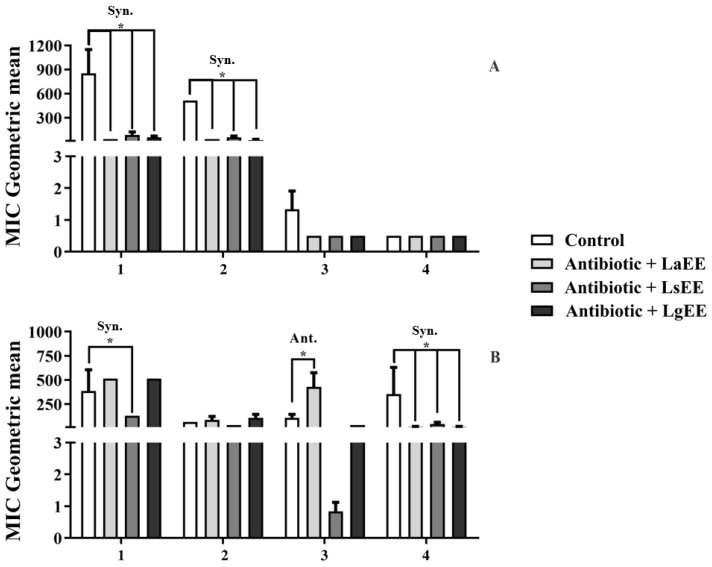
As shown in Table 4, none of the extracts and essential oils induced significant toxicity to adult zebrafish after 96 h (acute toxicity), as there was no death or apparent anatomical alteration in the animals during this period. In the open field test (Figure 4), LgEO and LaEO (at the lowest dose) did not cause motor impairment in the animals. On the other hand, like DZP, the higher doses of LaEO and LsEO altered locomotion. Regarding the ethanolic extracts, LsEE, even at the highest dose, did not affect the locomotion of the animals, different from what was observed following the treatment with LgEE.
Table 4.
Zebrafish mortality following the treatment with L. alba, L. sidoides, and L. gracilis.
| Sample | Mortality | 96 h LD50 (mg/kg)/CI |
|||
|---|---|---|---|---|---|
| NC | D1 | D2 | D3 | ||
| LaEO | 0 | 0 | 0 | 0 | >40 |
| LsEO | 0 | 0 | 0 | 0 | >40 |
| LgEO | 0 | 0 | 0 | 0 | >40 |
| LaEE | 0 | 0 | 0 | 0 | >400 |
| LsEE | 0 | 0 | 0 | 0 | >400 |
| LgEE | 0 | 0 | 0 | 0 | >400 |
NC: negative control group (DMSO 3%); D1: dose 1 (4 mg/kg¹; 40 mg/kg²); D2: dose 2 (20 mg/kg¹; 200 mg/kg²); D3: dose 3 (40 mg/kg¹; 400 mg/kg²). LD50: half-maximal lethal dose; CI: confidence interval; LaEO: Lippia alba essential oil; LsEO: Lippia sidoides essential oil; LgEO: Lippia gracilis essential oil; LaEE: Lippia alba ethanolic extract; LsEE: Lippia sidoides ethanolic extract; LgEE: Lippia gracilis ethanolic extract.
Figure 4.
Effect of essential oils and ethanolic extracts of L. alba, L. sidoides, and L. gracilis on the locomotion of adult zebrafish animals in the open field test (0–5 min). The values represent the mean ± standard error of the mean of six animals/group; ANOVA followed by Tukey test (* p < 0.05, ** p < 0.01, **** p < 0.0001, vs. control; # p < 0.05, ## p < 0.01, ### p < 0.001, #### p < 0.0001 vs. DZP). LaEO: Lippia alba essential oil; LsEO: Lippia sidoides essential oil; LgEO: Lippia gracilis essential oil; LaEE: Lippia alba ethanolic extract; LsEE: Lippia sidoides ethanolic extract; LgEE: Lippia gracilis ethanolic extract; DZP: diazepam.
Anxiolytic drugs such as BZD are known to reduce the locomotion of zebrafish animals, providing a model to analyze the impact of chemical substances on the central nervous system (CNS) [68,69]. This study showed that, although the natural products caused no evident toxicity, some of them affected the locomotion of the animals, indicating CNS action. Ethnobotanical reports have indicated the use of medicinal plants to treat CNS disorders such as depression, epilepsy, anxiety, and pain. However, the effectiveness of most of these species remains to be confirmed experimentally [10].
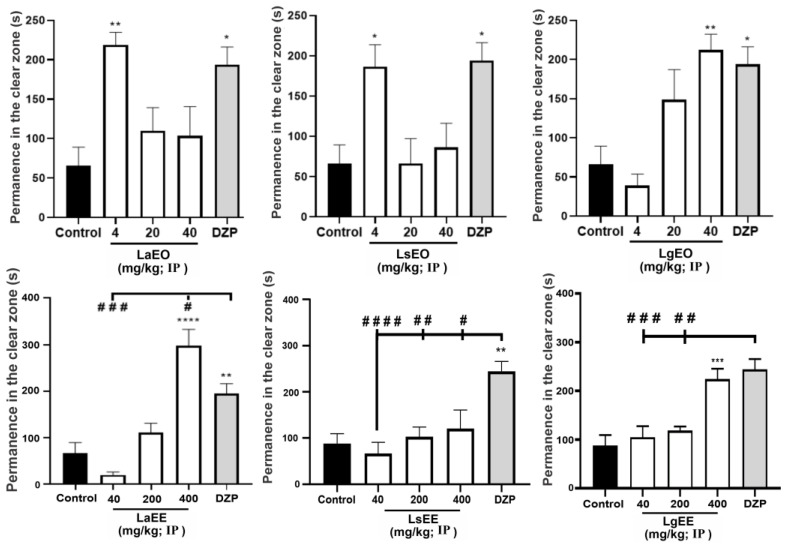
The light/dark test follows the observation that experimental animals escape to the dark compartment under anxiogenic conditions as an adaptive natural antipredatory response. Thus, studies in adult zebrafish animals have shown that anxiolytic compounds reduce their preference for the dark compartment [70]. The treatment with LgEO, LaEE, LaEO, LsEO, and LgEE induced an anxiolytic behavior that increased the permanence of the animals in the light area of the aquarium (Figure 5), similar to the DZP group. However, the treatment with LsEE did not affect the anxiety behavior of the animals. Previous research has demonstrated the sedative, anxiolytic, and anticonvulsant effects of L. alba [10] and L. sidoides [71], probably owing to the presence of constituents such as citral, β-myrcene, limonene, thymol, and carvacrol [72,73]. Moreover, naringenin and quercetin, commonly found in Lippia species, demonstrated anxiolytic activity in the zebrafish model [74,75]. In addition, other species of the Verbenaceae family demonstrated anxiolytic potential in the same model, corroborating the present findings [76].
Figure 5.
The anxiolytic effect of essential oils and ethanolic extracts of L. alba, L. sidoides, and L. gracilis in the light/dark test (0–5 min). The values represent the mean ± standard error of mean of six animals/group; ANOVA followed by Tukey’s test (* p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, vs. control; # p < 0.05, ## p < 0.01, ### p < 0.001, #### p < 0.0001 vs. DZP). LaEO: Lippia alba essential oil; LsEO: Lippia sidoides essential oil; LgEO: Lippia gracilis essential oil; LaEE: Lippia alba ethanolic extract; LsEE: Lippia sidoides ethanolic extract; LgEE: Lippia gracilis ethanolic extract; DZP: diazepam.
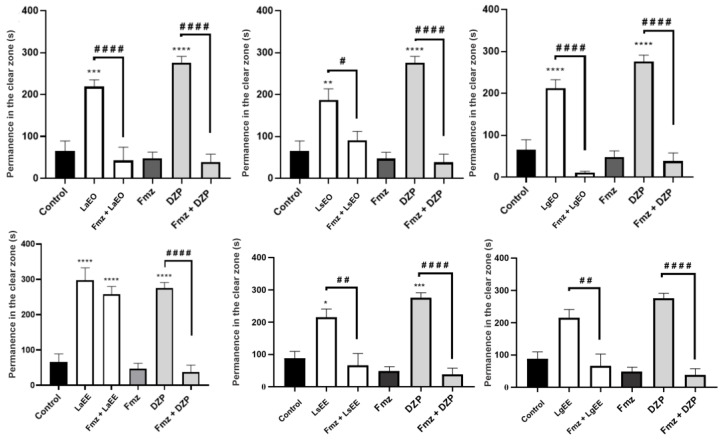
The involvement of GABAergic neurotransmission in the anxiolytic effect of the natural products was evaluated through pre-treatment with the GABAA receptor antagonist flumazenil. It was observed that this treatment blocked the anxiolytic effect of all samples except LaEE (Figure 6), increasing the animals’ permanence in the aquarium’s dark region. This finding suggests that the tested natural products have constituents capable of activating the GABAA receptor in the same region as benzodiazepines, such as DZP [77]. This hypothesis is corroborated by studies demonstrating the effectiveness of L. alba essential oils as anxiolytics and anesthetics through interference with GABAergic neurotransmission in zebrafish and silver catfish models [78,79]. Flavonoids such as quercetin, apigenin, luteolin, vitexin, isovitexin, naringenin, and eriodctyol act as second-order modulators of first-order modulation by BZD and modify the Fmz-insensitive modulation of the GABAA receptor, where incorporation of electronegative groups into C-6 and C-3’ in the flavone backbone increases the affinity for the BZD binding site [80].
Figure 6.
Involvement of GABAergic neurotransmission in the anxiolytic effect of essential oils and ethanolic extracts of L. alba, L. sidoides, and L. gracilis in the light/dark test (0–5 min). The values represent the mean ± standard error of the mean of six animals/group; ANOVA followed by Tukey’s test (* p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, vs. control; # p < 0.05, ## p < 0.01, #### p < 0.0001 vs. DZP or Lippia). LaEO: Lippia alba essential oil; LsEO: Lippia sidoides essential oil; LgEO: Lippia gracilis essential oil; LaEE: Lippia alba ethanolic extract; LsEE: Lippia sidoides ethanolic extract; LgEE: Lippia gracilis ethanolic extract; DZP: diazepam; Fmz: flumazenil.
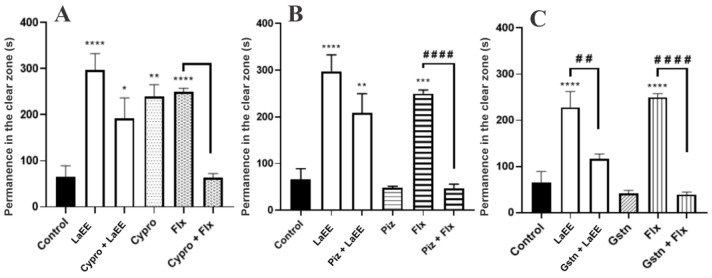
As LaEE treatment showed no interference with GABAergic neurotransmission, we investigated whether this extract could exert its anxiolytic effects through interference with the serotonergic neurotransmission, which was investigated using 5-HT receptor antagonists. Pre-treatment with granisetron blocked the anxiolytic effect of LaEE (Figure 7), while pizotifen and cyproheptadine treatments did not affect the activity of the extract, indicating that LaEE has anxiolytic effects associated with the activation of 5-HT3A/3B receptors.
Figure 7.
Effects of the pre-treatment with cyproheptadine (A), pizotifen (B), and granisetron (C) in the anxiolytic effect of L. alba ethanolic extract through the light/dark test (0–5 min). The values represent the mean ± standard error of the mean of six animals/group; two-way ANOVA followed by Tukey’s test (* p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, vs. control; ## p < 0.01, #### p < 0.0001 vs. Flx or Lippia). LaEE: Lippia alba ethanolic extract; Cypro: cyproheptadine; Piz: pizotifen; Gstn: granisetron; Flx: fluoxetine.
It has been demonstrated that neurotransmitters, such as serotonin (5-HT), dopamine, and gamma-aminobutyric acid (GABA), can regulate the activation of neurons that govern behaviors and emotions [81,82]. At the same time, high levels of 5-HT can induce anxiety-like effects, while low levels of 5-HT cause anxiolytic behavior in the zebrafish model [83]. 5-HTR3 is located in the regions of the brain that regulate mood and emotions; therefore, its activation may be associated with the anxiolytic and antidepressant effects of drugs [84]. Accordingly, the aqueous extract of L. multiflora Moldenke, whose composition is characterized by the high content of flavonoids, showed an anxiolytic effect mediated by the activation of serotonergic receptors such as 5-HT2C/2B, 5-HT1A, and 5-HT2A/2C [85]. In silico and in vivo analyses show the antidepressant and anxiolytic effect of apigenin, a flavonoid present in LaEE, through its effective coupling to serotonin receptors, possibly acting through 5-HT2A inhibition and 5-HT1A agonism [86].
3. Materials and Methods
3.1. Botanical Material and Extract Preparation
The collection of the botanical material as well as the extraction and composition of the essential oils used in this study are described in Nonato et al. [56]. Briefly, the leaves of Lippia alba (Mill.) N.E.Br. ex Britton & P.Wilson and Lippia sidoides Cham. were collected in the botanical garden of the Regional University of Cariri (7°14′20.1″ S 39°24′53.1″ W) in April 2019 and the voucher specimen of each species was registered in the Herbarium Caririense Dárdano de Andrade Lima (HDCAL/URCA) under identification numbers 13,907 and 3038, respectively. The leaves of Lippia gracilis Schauer were collected in the municipality of Crato, Ceará, Brazil (7°13′05.2″ S 39°25′44.9″ W) in April 2019, and its voucher specimen was registered in the Herbarium Prisco Bezerra of the Federal University of Ceará, under registration number 44,456.
The essential oils were extracted by hydrodistillation in a Clevenger-type apparatus. Briefly, fresh leaves were crushed and extracted for 2 h. After extraction, the essential oils of L. alba (LaEO), L. sidoides (LsEO), and L. gracilis (LgEO) were dried with Na2SO4 [56]. In another set of extractions, fresh leaves of these species (500 g of each) were subjected to exhaustive maceration in 99% ethanol for 72 h. The solutions obtained were concentrated in an evaporator at 50 °C under reduced pressure, obtaining a yield of 31.6%, 11.5%, and 1.48% for the ethanolic extracts of L. alba (LaEE), L. sidoides (LsEE), and L. gracilis (LgEE), respectively.
3.2. Drugs and Reagents
The following substances were used in this study: methanol (MeOH), Na2CO3, AlCl3, CH3CO2K, gentamycin, amikacin, benzylpenicillin, cephalothin (Sigma Chemical Corporation, San Luis, Missouri, USA); diazepam (DZP; Neo Química®), flumazenil (Fmz; Sandoz®), dimethylsulfoxide (DMSO; Dynamic®), granisetron chloride (Gstn; Corepharma/Middlesex, NJ, USA), pizotifen maleate (Piz; Central Pharmacy of Manipulation/São Paulo, SP, Brazil), fluoxetine (Flx; Eli Lilly/Indianapolis, IN, USA), and cyproheptadine (Cypro; Evidence Pharmaceutical Solutions/Fortaleza, CE, Brazil).
3.3. Analysis of Non-Volatile Compounds by HPLC-DAD-ESI-MSn
The three extracts were analyzed by Shimadzu HPLC, using a C18 analytical chromatographic column (Kromasil—250 mm × 4.6 mm × 5 μm), coupled to a mass spectrometer (Ion-Trap AmazonX, Bruker or microTOFII, Bruker, Billerica, MA, USA), with ionization by electrospray (ESI). The samples were solubilized in MeOH (1 mg/mL), with subsequent filtration through PVDF (polyvinylidene fluoride) filters, with a mesh size of 0.5 μm. The method used chromatographic-grade MeOH (solvent B) and type I ultrapure H2O (Milli-Q), acidified with formic acid (0.1%, v/v) (solvent A), in a concentration gradient (5 to 100% of B in 95 min). The injection volume was 10 μL and the flow rate was 0.6 mL/min. In the mass spectrometer, the samples were submitted to a sequential fragmentation in MS3. The acquisition parameters in the ion trap and TOF were capillary of 4.5 kV, final plate offset of 500 V, nebulizer gas at 35 psi, dry gas (N2) with flow of 8 mL/min, and a temperature of 300 °C. The sample was analyzed in negative ionization mode and the identification of compounds was based on data (MS/MS) reported in the literature.
3.4. Quantification of Total Phenols
The quantification of total phenols followed the Folin–Ciocalteu oxidation method proposed by Singleton et al. [87]. Here, 25 µL aliquots of extract concentrations (50 to 1000 µg/mL in MeOH) were added to Folin–Ciocalteu reagent (625 µL, 10%) and Na2CO3 (500 µL, 7.5%). Absorbances were measured in a spectrophotometer (Kasuaki DR-200BS, Wuxi Hiwell Diatek Instruments, 209 China) at 765 nm after incubation for 15 min at 45 °C and protected from light. For the calibration curve, gallic acid was used as a standard and, for the blank test, MeOH was used. The analysis was performed in triplicate and the results were expressed as mg equivalent of gallic acid per g of extract (mg GA/g Ext.).
3.5. Quantification of Total Flavonoids
The total flavonoid content was measured by the AlCl3 colorimetric method according to Kosalec et al. [88]. Here, 1160 µL aliquots of extract concentrations (50 to 1000 µg/mL in MeOH) were added to MeOH (760 µL), AlCl3 (40 µL, 10%) and CH3CO2K (40 µL, 0.1 M). Absorbances were measured in a spectrophotometer at 415 nm after incubation for 30 min in the dark. Quercetin was used as a standard for the calibration curve and MeOH was used for the blank test. All analyses were performed in triplicate and the results were expressed as mg quercetin equivalent per g of extract (mg QE/g Ext.).
3.6. Antibacterial Activity Analysis
3.6.1. Minimum Inhibitory Concentration Determination
The antibacterial activity was analyzed through the microdilution method according to the CLSI M100 document [89], using the following bacterial strains: Staphylococcus aureus Sa 358, Streptococcus mutans INCQS 00446, Escherichia coli Ec 27, and Pseudomonas aeruginosa ATCC 15442.
To this end, the samples were previously diluted to 1024 μg/mL in sterile distilled water and DMSO. The wells on a 96-well plate were filled with a bacterial suspension (105 CFU/mL in 10% BHI medium), and then the samples were added to the wells where serial dilutions were performed to reach concentrations of the natural products ranging from 512 to 8 μg/mL. The plate was incubated at 35 ± 2 °C for 24 h. The reading was performed by colorimetry after adding 25 μL of resazurin solution (0.01%) to each well after incubation. The MIC was defined as the lowest concentration of extracts capable of inhibiting the growth of microorganisms. All tests were performed in triplicate.
3.6.2. Evaluation of Antibiotic-Enhancing Activity by Direct Contact
This study followed the methodology proposed by Coutinho et al. [90] to analyze the effectiveness of essential oils and ethanolic extracts as potentiators of the antibacterial activity of aminoglycosides (amikacin and gentamicin) and beta-lactams (benzylpenicillin and cephalothin) against the multidrug-resistant bacterial strains Staphylococcus aureus Sa 358 and Escherichia coli Ec 27 (Table 5). To this end, the antibiotics were tested alone or combined with natural products added to the wells at a concentration equivalent to their MIC/8. The plates were incubated and the antibiotic MIC was determined as previously described.
Table 5.
Bacterial origin and antibiotic resistance profile.
| Bacterial Strain | Origin | Resistance Profile |
|---|---|---|
| Staphylococcus aureus Sa 358 | Surgical Wound | AMK, BTN, CPN, GEN, NEO, NET, OXA, PRM, SISO, TOB. |
| Escherichia coli Ec 27 | Surgical Wound | AMK, AMP, AMX, AZM, CAZ, CEC, CEF, CHL, CIP, CPN, IPM, KAN, SMX, TET, TOB. |
AMK: amikacin; AMP: ampicillin; AMX: amoxicillin; AZM: azithromycin; BTN: butirosin; CAZ: ceftazinidime; CEC: cefaclor; CEF: cephalothin; CHL: chloramphenicol; CIP: ciprofloxacin; CPN: cephalexin; GEN: gentamicin; IPM: imipenem; KAN: kanamycin; NEO: neomycin; NET: netilmicin; OXA: oxacillin; PRM: paramomycin; SISO: sisomicin; SMX: sulfamethoxazole; TET: tetracycline; TOB: tobramycin. Adapted from Sobral et al. [91].
3.7. Zebrafish Experimental Model
3.7.1. Animals
Wild-type adult zebrafish animals (Danio renio) of both sexes aged between 60 and 90 days (0.4 ± 0.1 g) were obtained from a commercial supplier (Fortaleza, CE). The animals were kept in a glass aquarium containing anti-chlorine-treated water (n = 5/L), at a temperature of 25 ± 2 °C, in light/dark cycles for 24 h. The experiments followed the Ethical Principles of Animal Experimentation and were approved by the Ethics Committee for the Use of Animals (CEUA) of the State University of Ceará (04983945/2021). After the experiments, the animals were euthanized by freezing through immersion in cold water (2–4 °C) until the loss of opercular movements (approximately 10 min).
3.7.2. Acute Toxicity Determination
The acute toxicity evaluation in adult zebrafish animals was conducted according to the Organization of Economic Cooperation and Standard Method of Development [92]. The animals (n = 6/group) were treated intraperitoneally (IP) with 20 μL of essential oils (4, 20, or 40 mg/kg), extracts (40; 200, and 400 mg/kg), or vehicle (3% DMSO). After 96 h, the number of dead animals in each group was counted, and the LD50 was determined using the trimmed Spearman–Karber mathematical method with a 95% confidence interval [93].
3.7.3. Evaluation of Locomotor Activity (Open Field Test)
The open field test evaluated changes in the motor system due to sedation or muscle relaxation [94]. The animals (n = 6/group) were treated as previously described, except for a diazepam-treated (4 or 10 mg/kg) group of animals, which was included as a pharmacological control group. After 30 min of the treatments, the animals were placed on water-filled Petri dishes (10 × 15 cm) marked with four quadrants. The locomotor activity was analyzed by counting the line-crossing (LC) frequency.
3.7.4. Anxiolytic Activity Analysis (Light/Dark Test)
The light/dark test can detect the anxiety-like behavior of zebrafish animals because, like rodents, they naturally avoid illuminated areas [84]. A glass aquarium (30 cm × 15 cm × 20 cm), divided into light and dark areas, was filled with up to 3 cm of tap water free of chlorine and heavy metals, which simulated a shallow environment that, unlike the conventional aquarium, is capable of inducing anxiety-like behavior. The animals were treated following the same treatment conditions described in the previous experimental section and, 1 h later, placed individually in the light zone of the aquarium. The anxiolytic effect was measured based on the time spent in the light area of the aquarium within a 5 min time interval of observation [95].
3.7.5. Evaluation of GABAergic Neuromodulation
The animals were pre-treated with Flumazenil (Fmz), a GABAA receptor antagonist, to analyze the interference of extracts and the essential oils on the GABAergic neuromodulation, and thus elucidate the mechanisms underlying the anxiolytic effects of the extracts and essential oils [96]. Briefly, zebrafish (n = 6/group) were pre-treated with Fmz (4 mg/kg) 15 min before the treatment with the natural products. Here, the best dose of each substance was selected according to the light/dark test (4 mg/kg for LaEO and LsEO; 40 mg/kg for LgEO; 400 mg/kg for all extracts). Groups of mice treated with the vehicle (3% DMSO) and DZP (4 mg/kg) were used as negative and pharmacological controls, respectively. Following 30 min of the treatment, the light/dark test was applied as described in the previous section. All treatments were performed intraperitoneally in a volume of 20 μL.
3.7.6. Evaluation of Serotonergic Neuromodulation
The potential participation of the serotoninergic pathway in the anxiolytic effect of LaEE was investigated using the following receptor antagonists: cyproheptadine (5-HTR2A antagonist), pizotifen (5-HTR1/5-HTR2A/5-HTR2C antagonist), and granisetron (5-HTR3A/5-HTR3B antagonist) [96]. Zebrafish animals (n = 6/group) were pretreated orally (p.o) with cyproheptadine (32 mg/kg), pizotifen (32 mg/kg), or granisetron (20 mg/kg). After 15 min, these animals were treated intraperitoneally with LaEE (400 mg/kg), following the anxiolytic effect observed in the light/dark test. The mice treated intraperitoneally with the vehicle (3% DMSO) and fluoxetine (0.05 mg/kg) were used as negative and pharmacological controls, respectively. Following 1 h of the treatment, the light/dark test was applied as described in the previous section.
3.8. Statistical Analysis
The data obtained in the antibacterial activity analysis had the normal distribution evaluated and were then analyzed by one-way ANOVA with Bonferroni’s post-test. For the zebrafish tests, the results were expressed as the mean ± standard error of the mean for each group of 6 animals. After confirming the normality of the distribution and homogeneity of the data, the differences between the groups were analyzed by one-way ANOVA (or two-way ANOVA for GABAergic and serotonergic evaluation), followed by Tukey’s test. GraphPad Prism software version 8.0 (GraphPad Software, San Diego, CA, USA) was used in all analyses, and the results with p < 0.05 were considered statistically significant.
4. Conclusions
According to the results obtained, the ethanolic extracts were essentially rich in flavonoids, with naringenin and cirsimaritin present in the three extracts, indicating the possibility of a chemical marker for this genus. The essential oils and extracts studied showed significant antibacterial and antibiotic effects, demonstrating the ability both to inhibit bacterial growth (especially L. sidoides) and to potentiate the activity of conventional antibiotics (especially L. alba). Furthermore, these natural products caused anxiolytic behavior in zebrafish through interference in GABAergic and serotoninergic pathways. These showed no toxic effect, demonstrating the safe use of these species both for new biological studies and for ethnopharmacological uses.
The volatile and non-volatile compounds identified in these samples are reported in the literature with the studied biological effects, which strengthens the results obtained here. New studies are needed to verify the correlation of the composition with these potentials, as well as their individual mechanisms of action in silico and in vivo. It is noteworthy that this work is the first report of the activity of all extracts and essential oils of L. sidoides and L. gracilis in the zebrafish model. This new pharmacological evidence opens horizons for therapeutic approaches targeting anxiolytic and antibacterial therapies, as well as food preservation using these species and their constituents.
Author Contributions
Conceptualization, C.d.F.A.N. and J.G.M.d.C.; Investigation, C.d.F.A.N., E.V.S.d.M., C.J.C., M.K.A.F., J.E.A.d.M., J.P.R.e.S., J.F.T. and A.W.d.S.; Software, C.d.F.A.N.; Writing—original draft preparation, C.d.F.A.N. and J.G.M.d.C.; Writing—review and editing, J.G.M.d.C., H.S.d.S., I.R.A.d.M., H.D.M.C., J.R.-F. and R.K.; Supervision, G.K. and T.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Ethics Committee for the Use of Animals (CEUA) of the State University of Ceará (04983945/2021).
Informed Consent Statement
Not Applicable.
Data Availability Statement
The data will be available after a reasonable request to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.WHO . Depression and Other Common Mental Disorders: Global Health Estimates. World Health Organization; Geneva, Switzerland: 2017. [Google Scholar]
- 2.Griessner J., Pasieka M., Böhm V., Grössl F., Kaczanowska J., Pliota P., Kargl D., Werner B., Kaouane N., Strobelt S., et al. Central Amygdala Circuit Dynamics Underlying the Benzodiazepine Anxiolytic Effect. Mol. Psych. 2021;26:534–544. doi: 10.1038/s41380-018-0310-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vossen L.E., Cerveny D., Österkrans M., Thörnqvist P.O., Jutfelt F., Fick J., Brodin T., Winberg S. Chronic Exposure to Oxazepam Pollution Produces Tolerance to Anxiolytic Effects in Zebrafish (Danio rerio) Environ. Sci. Technol. 2020;54:1760–1769. doi: 10.1021/acs.est.9b06052. [DOI] [PubMed] [Google Scholar]
- 4.MacLean R.C., Millan A.S. The Evolution of Antibiotic Resistance. Science. 2019;365:1082–1083. doi: 10.1126/science.aax3879. [DOI] [PubMed] [Google Scholar]
- 5.Akova M. Epidemiology of Antimicrobial Resistance in Bloodstream Infections. Virulence. 2016;7:252–266. doi: 10.1080/21505594.2016.1159366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frieri M., Kumar K., Boutin A. Antibiotic Resistance. J. Infect. Public Health. 2017;10:369–378. doi: 10.1016/j.jiph.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Fedotova J., Kubatka P., Büsselberg D., Shleikin A.G., Caprnda M., Dragasek J., Rodrigo L., Pohanka M., Gasparova I., Nosal V., et al. Therapeutical Strategies for Anxiety and Anxiety-like Disorders Using Plant-Derived Natural Compounds and Plant Extracts. Biomed. Pharmacother. 2017;95:437–446. doi: 10.1016/j.biopha.2017.08.107. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Oliveira P., Barral M., Carpena M., Gullón P., Fraga-Corral M., Otero P., Prieto M.A., Simal-Gandara J. Traditional Plants from Asteraceae Family as Potential Candidates for Functional Food Industry. Food Funct. 2021;12:2850–2873. doi: 10.1039/D0FO03433A. [DOI] [PubMed] [Google Scholar]
- 9.Ombito J.O., Salano E.N., Yegon P.K., Ngetich W.K., Mwangi E.M. A Review of the Chemistry of Some Species of Genus Lippia (Verbenaceae Family) J. Sci. Innov. Res. 2014;3:460–466. doi: 10.31254/jsir.2014.3411. [DOI] [Google Scholar]
- 10.Siqueira-Lima P.S., Passos F.R.S., Lucchese A.M., Menezes I.R.A., Coutinho H.D.M., Lima A.A.N., Zengin G., Quintans J.S.S., Quintans-Júnior L.J. Central Nervous System and Analgesic Profiles of Lippia Genus. Rev. Bras. Farmacogn. 2019;29:125–135. doi: 10.1016/j.bjp.2018.11.006. [DOI] [Google Scholar]
- 11.Heffels P., Müller L., Schieber A., Weber F. Profiling of Iridoid Glycosides in Vaccinium Species by UHPLC-MS. Food Res. Int. 2017;100:462–468. doi: 10.1016/j.foodres.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 12.Zhou T., Liu H., Wen J., Fan G., Chai Y., Wu Y. Fragmentation Study of Iridoid Glycosides Including Epimers by Liquid Chromatography-Diode Array Detection/Electrospray Ionization Mass Spectrometry and Its Application in Metabolic Fingerprint Analysis of Gardenia jasminoides Ellis. Rapid Commun. Mass Spectrom. 2010;24:2520–2528. doi: 10.1002/rcm.4643. [DOI] [PubMed] [Google Scholar]
- 13.Li Y., Liu Y., Liu R., Liu S., Zhang X., Wang Z., Zhang J., Lu J. HPLC-LTQ-Orbitrap MSn Profiling Method to Comprehensively Characterize Multiple Chemical Constituents in Xiao-Er-Qing-Jie Granules. Anal. Methods. 2015;7:7511–7526. doi: 10.1039/C5AY00420A. [DOI] [Google Scholar]
- 14.Sánchez-Marzo N., Lozano-Sánchez J., Cádiz-Gurrea M.L., Herranz-López M., Micol V., Segura-Carretero A. Relationships between Chemical Structure and Antioxidant Activity of Isolated Phytocompounds from Lemon Verbena. Antioxidants. 2019;8:324. doi: 10.3390/antiox8080324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El Sayed A.M., Ezzat S.M., El Naggar M.M., El Hawary S.S. In Vivo Diabetic Wound Healing Effect and HPLC–DAD–ESI–MS/MS Profiling of the Methanol Extracts of Eight Aloe Species. Rev. Bras. Farmacogn. 2016;26:352–362. doi: 10.1016/j.bjp.2016.01.009. [DOI] [Google Scholar]
- 16.Tao J., Zhao M., Jiang S., Pu X., Wei X. Comparative Metabolism of Two Major Compounds in Fructus Corni Extracts by Gut Microflora from Normal and Chronic Nephropathy Rats in Vitro by UPLC-Q-TOF/MS. J. Chromatogr. B. 2018;1073:170–176. doi: 10.1016/j.jchromb.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 17.Santos S.A.O., Vilela C., Freire C.S.R., Pascoal-Neto C., Silvestre A.J.D. Ultra-High Performance Liquid Chromatography Coupled to Mass Spectrometry Applied to the Identification of Valuable Phenolic Compounds from Eucalyptus Wood. J. Chromatogr. B. 2013;938:65–74. doi: 10.1016/j.jchromb.2013.08.034. [DOI] [PubMed] [Google Scholar]
- 18.Pereira O.R., Peres A.M., Silva A.M.S., Domingues M.R.M., Cardoso S.M. Simultaneous Characterization and Quantification of Phenolic Compounds in Thymus x citriodorus Using a Validated HPLC-UV and ESI-MS Combined Method. Food Res. Int. 2013;54:1773–1780. doi: 10.1016/j.foodres.2013.09.016. [DOI] [Google Scholar]
- 19.Iswaldi I., Arráez-Román D., Rodríguez-Medina I., Beltrán-Debón R., Joven J., Segura-Carretero A., Fernández-Gutiérrez A. Identification of Phenolic Compounds in Aqueous and Ethanolic Rooibos Extracts (Aspalathus linearis) by HPLC-ESI-MS (TOF/IT) Anal. Bioanal. Chem. 2011;400:3643–3654. doi: 10.1007/s00216-011-4998-z. [DOI] [PubMed] [Google Scholar]
- 20.Tóth G., Barabás C., Tóth A., Kéry Á., Béni S., Boldizsár I., Varga E., Noszál B. Characterization of Antioxidant Phenolics in Syringa vulgaris L. Flowers and Fruits by HPLC-DAD-ESI-MS. Biomed. Chromatogr. 2016;30:923–932. doi: 10.1002/bmc.3630. [DOI] [PubMed] [Google Scholar]
- 21.Spínola V., Pinto J., Castilho P.C. Identification and Quantification of Phenolic Compounds of Selected Fruits from Madeira Island by HPLC-DAD–ESI-MSn and Screening for Their Antioxidant Activity. Food Chem. 2015;173:14–30. doi: 10.1016/j.foodchem.2014.09.163. [DOI] [PubMed] [Google Scholar]
- 22.Petkovska A., Gjamovski V., Stanoeva J.P., Stefova M. Characterization of the Polyphenolic Profiles of Peel, Flesh and Leaves of Malus domestica Cultivars Using UHPLC-DAD-HESI-MSn. Nat. Prod. Commun. 2017;12:35–42. doi: 10.1177/1934578X1701200111. [DOI] [PubMed] [Google Scholar]
- 23.Mbakidi-Ngouaby H., Pinault E., Gloaguen V., Costa G., Sol V., Millot M., Mambu L. Profiling and Seasonal Variation of Chemical Constituents from Pseudotsuga menziesii Wood. Ind. Crops Prod. 2018;117:34–49. doi: 10.1016/j.indcrop.2018.02.069. [DOI] [Google Scholar]
- 24.Olennikov D.N., Chirikova N.K., Kashchenko N.I., Nikolaev V.M., Kim S.W., Vennos C. Bioactive Phenolics of the Genus Artemisia (Asteraceae): HPLC-DAD-ESI-TQ-MS/MS Profile of the Siberian Species and Their Inhibitory Potential against α-Amylase and α-Glucosidase. Front. Pharmacol. 2018;9:756. doi: 10.3389/fphar.2018.00756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mena P., Calani L., Dall’Asta C., Galaverna G., García-Viguera C., Bruni R., Crozier A., Del Rio D. Rapid and Comprehensive Evaluation of (Poly)Phenolic Compounds in Pomegranate (Punica granatum L.) Juice by UHPLC-MSn. Molecules. 2012;17:14821–14840. doi: 10.3390/molecules171214821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao H.-Y., Fan M.-X., Wu X., Wang H.-J., Yang J., Si N., Bian B.-L. Chemical Profiling of the Chinese Herb Formula Xiao-Cheng-Qi Decoction Using Liquid Chromatography Coupled with Electrospray Ionization Mass Spectrometry. J. Chromatogr. Sci. 2013;51:273–285. doi: 10.1093/chromsci/bms138. [DOI] [PubMed] [Google Scholar]
- 27.Marczak Ł., Znajdek-Awizeń P., Bylka W. The Use of Mass Spectrometric Techniques to Differentiate Isobaric and Isomeric Flavonoid Conjugates from Axyris amaranthoides. Molecules. 2016;21:1229. doi: 10.3390/molecules21091229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friščić M., Bucar F., Pilepić K.H. LC-PDA-ESI-MSn Analysis of Phenolic and Iridoid Compounds from Globularia Spp. J. Mass Spectrom. 2016;51:1211–1236. doi: 10.1002/jms.3844. [DOI] [PubMed] [Google Scholar]
- 29.Kang J., Price W.E., Ashton J., Tapsell L.C., Johnson S. Identification and Characterization of Phenolic Compounds in Hydromethanolic Extracts of Sorghum Wholegrains by LC-ESI-MSn. Food Chem. 2016;211:215–226. doi: 10.1016/j.foodchem.2016.05.052. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y., Yu H., Wu H., Pan Y., Wang K., Jin Y., Zhang C. Characterization and Quantification by LC-MS/MS of the Chemical Components of the Heating Products of the Flavonoids Extract in Pollen Typhae for Transformation Rule Exploration. Molecules. 2015;20:18352–18366. doi: 10.3390/molecules201018352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Falcão S.I., Vale N., Gomes P., Domingues M.R.M., Freire C., Cardoso S.M., Vilas-Boas M. Phenolic Profiling of Portuguese Propolis by LC-MS Spectrometry: Uncommon Propolis Rich in Flavonoid Glycosides. Phytochem. Anal. 2012;24:309–318. doi: 10.1002/pca.2412. [DOI] [PubMed] [Google Scholar]
- 32.Brito A., Ramirez J.E., Areche C., Sepúlveda B., Simirgiotis M.J. HPLC-UV-MS Profiles of Phenolic Compounds and Antioxidant Activity of Fruits from Three Citrus Species Consumed in Northern Chile. Molecules. 2014;19:17400–17421. doi: 10.3390/molecules191117400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhong L., Yuan Z., Rong L., Zhang Y., Xiong G., Liu Y., Li C. An Optimized Method for Extraction and Characterization of Phenolic Compounds in Dendranthema indicum Var. aromaticum Flower. Sci. Rep. 2019;9:7745. doi: 10.1038/s41598-019-44102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gouveia S., Castilho P.C. Characterisation of Phenolic Acid Derivatives and Flavonoids from Different Morphological Parts of Helichrysum obconicum by a RP-HPLC-DAD-(-)-ESI-MSn Method. Food Chem. 2011;129:333–344. doi: 10.1016/j.foodchem.2011.04.078. [DOI] [PubMed] [Google Scholar]
- 35.Peter S.R., Peru K.M., Fahlman B., McMartin D.W., Headley J.V. The Application of HPLC ESI MS in the Investigation of the Flavonoids and Flavonoid Glycosides of a Caribbean Lamiaceae Plant with Potential for Bioaccumulation. J. Environ. Sci. Health Part B. 2015;50:819–826. doi: 10.1080/03601234.2015.1058103. [DOI] [PubMed] [Google Scholar]
- 36.Ristivojević P., Trifković J., Gašić U., Andrić F., Nedić N., Tešić Ž., Milojković-Opsenica D. Ultrahigh-Performance Liquid Chromatography and Mass Spectrometry (UHPLC-LTQ/Orbitrap/MS/MS) Study of Phenolic Profile of Serbian Poplar Type Propolis. Phytochem. Anal. 2015;26:127–136. doi: 10.1002/pca.2544. [DOI] [PubMed] [Google Scholar]
- 37.Pellati F., Orlandini G., Pinetti D., Benvenuti S. HPLC-DAD and HPLC-ESI-MS/MS Methods for Metabolite Profiling of Propolis Extracts. J. Pharm. Biomed. Anal. 2011;55:934–948. doi: 10.1016/j.jpba.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 38.Hussain H., Green I.R., Saleem M., Raza M.L., Nazir M. Therapeutic Potential of Iridoid Derivatives: Patent Review. Inventions. 2019;4:29. doi: 10.3390/inventions4020029. [DOI] [Google Scholar]
- 39.Gomes A.F., Almeida M.P., Leite M.F., Schwaiger S., Stuppner H., Halabalaki M., Amaral J.G., David J.M. Seasonal Variation in the Chemical Composition of Two Chemotypes of Lippia alba. Food Chem. 2019;273:186–193. doi: 10.1016/j.foodchem.2017.11.089. [DOI] [PubMed] [Google Scholar]
- 40.Hennebelle T., Sahpaz S., Gressier B., Joseph H., Bailleul F. Antioxidant and Neurosedative Properties of Polyphenols and Iridoids from Lippia alba. Phyther. Res. 2008;22:256–258. doi: 10.1002/ptr.2266. [DOI] [PubMed] [Google Scholar]
- 41.Timóteo P., Karioti A., Leitão S.G., Vincieri F.F., Bilia A.R. A Validated HPLC Method for the Analysis of Herbal Teas from Three Chemotypes of Brazilian Lippia alba. Food Chem. 2015;175:366–373. doi: 10.1016/j.foodchem.2014.11.129. [DOI] [PubMed] [Google Scholar]
- 42.Li Y., Kong D., Fu Y., Sussman M.R., Wu H. The Effect of Developmental and Environmental Factors on Secondary Metabolites in Medicinal Plants. Plant Physiol. Biochem. 2020;148:80–89. doi: 10.1016/j.plaphy.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 43.Trevisan M.T.S., Marques R.A., Silva M.G.V., Scherer D., Haubner R., Ulrich C.M., Owen R.W. Composition of Essential Oils and Ethanol Extracts of the Leaves of Lippia Species: Identification, Quantitation and Antioxidant Capacity. Rec. Nat. Prod. 2016;10:485–496. [Google Scholar]
- 44.Khan R.A., Hossain R., Roy P., Jain D., Saikat A.S.M., Shuvo A.P.R., Akram M., Elbossaty W.F., Khan I.N., Painuli S., et al. Anticancer Effects of Acteoside: Mechanistic Insights and Therapeutic Status. Eur. J. Pharmacol. 2022;916:174699. doi: 10.1016/j.ejphar.2021.174699. [DOI] [PubMed] [Google Scholar]
- 45.Cortés-Chitala M.C., Flores-Martínez H., Orozco-Ávila I., León-Campos C., Suárez-Jacobo Á., Estarrón-Espinosa M., López-Muraira I. Identification and Quantification of Phenolic Compounds from Mexican Oregano (Lippia graveolens HBK) Hydroethanolic Extracts and Evaluation of Its Antioxidant Capacity. Molecules. 2021;26:702. doi: 10.3390/molecules26030702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leyva-Jiménez F.J., Lozano-Sánchez J., Cádiz-Gurrea M.L., Arráez-Román D., Segura-Carretero A. Functional Ingredients Based on Nutritional Phenolics. A Case Study against Inflammation: Lippia Genus. Nutrients. 2019;11:1646. doi: 10.3390/nu11071646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oliveira G.T., Ferreira J.M.S., Lima W.G., Alves L.F., Duarte-Almeida J.M., Lima L.A.R.S. Phytochemical Characterisation and Bioprospection for Antibacterial and Antioxidant Activities of Lippia alba Brown Ex Britton & Wilson (Verbenaceae) Nat. Prod. Res. 2018;32:723–731. doi: 10.1080/14786419.2017.1335727. [DOI] [PubMed] [Google Scholar]
- 48.Funari C.S., Eugster P.J., Martel S., Carrupt P.A., Wolfender J.L., Silva D.H.S. High Resolution Ultra High Pressure Liquid Chromatography-Time-of-Flight Mass Spectrometry Dereplication Strategy for the Metabolite Profiling of Brazilian Lippia Species. J. Chromatogr. A. 2012;1259:167–178. doi: 10.1016/j.chroma.2012.03.069. [DOI] [PubMed] [Google Scholar]
- 49.Moraes V.R.S., Nogueira P.C.L., Costa E.V., Santos L.S., Silva V.R., Bomfim L.M., Bezerra D.P. Phytochemical and biological properties of lippia gracilis. In: Akhtar M.S., Swamy M.K., editors. Anticancer Plants: Properties and Application. Volume 1. Springer; Singapore: 2018. pp. 37–55. [Google Scholar]
- 50.Castellar A., Coelho T.S., Silva P.E.A., Ramos D.F., Lourenço M.C.S., Lage C.L.S., Julião L.S., Barbosa Y.G., Leitão S.G. The Activity of Flavones and Oleanolic Acid from Lippia lacunosa against Susceptible and Resistant Mycobacterium tuberculosis Strains. Rev. Bras. Farmacogn. 2011;21:835–840. doi: 10.1590/S0102-695X2011005000076. [DOI] [Google Scholar]
- 51.Bangou M.J., Abarca N.A., Nâg-Tiero M.R., Mouhibatou Y.Z., Jeanne M.R., Germaine N.O. Lippia chevalieri Moldenke: A Brief Review of Traditional Uses, Phytochemistry and Pharmacology. Int. J. Drug Deliv. 2012;4:289–296. doi: 10.5138/ijdd.v4i3.795. [DOI] [Google Scholar]
- 52.Almeida M.C., Pina E.S., Hernandes C., Zingaretti S.M., Taleb-Contini S.H., Salimena F.R.G., Slavov S.N., Haddad S.K., França S.C., Pereira A.M.S., et al. Genetic Diversity and Chemical Variability of Lippia spp. (Verbenaceae) BMC Res. Notes. 2018;11:725. doi: 10.1186/s13104-018-3839-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dubey S., Ojha K., Chandrakar J., Dehariya R., Vinodia S., Singh A., Dixit A.K. Assessment of Total Phenolic Content and Antioxidant Potentiality of Selected Indian Folk Medicinal Plants by Spectrophotometric Method. Plant Sci. Today. 2020;7:383–390. doi: 10.14719/pst.2020.7.3.765. [DOI] [Google Scholar]
- 54.Garmus T.T., Paviani L.C., Queiroga C.L., Cabral F.A. Extraction of Phenolic Compounds from Pepper-Rosmarin (Lippia sidoides Cham.) Leaves by Sequential Extraction in Fixed Bed Extractor Using Supercritical CO2, Ethanol and Water as Solvents. J. Supercrit. Fluids. 2015;99:68–75. doi: 10.1016/j.supflu.2015.01.016. [DOI] [Google Scholar]
- 55.Shen N., Wang T., Gan Q., Liu S., Wang L., Jin B. Plant Flavonoids: Classification, Distribution, Biosynthesis, and Antioxidant Activity. Food Chem. 2022;383:132531. doi: 10.1016/j.foodchem.2022.132531. [DOI] [PubMed] [Google Scholar]
- 56.Nonato C.F.A., Camilo C.J., Leite D.O.D., Nobrega M.G.L.A., Ribeiro-Filho J., Menezes I.R.A., Tavares J.F., Costa J.G.M. Comparative Analysis of Chemical Profiles and Antioxidant Activities of Essential Oils Obtained from Species of Lippia L. by Chemometrics. Food Chem. 2022;384:132614. doi: 10.1016/j.foodchem.2022.132614. [DOI] [PubMed] [Google Scholar]
- 57.Kachur K., Suntres Z. The Antibacterial Properties of Phenolic Isomers, Carvacrol and Thymol. Crit. Rev. Food Sci. Nutr. 2020;60:3042–3053. doi: 10.1080/10408398.2019.1675585. [DOI] [PubMed] [Google Scholar]
- 58.Saraiva C.R.N., Nonato C.F.A., Camilo C.J., Araújo A.C.J., Rodrigues F.F.G., Coutinho H.D.M., Costa J.G.M. Chemical Profile and Inhibition of MDR Bacteria by the Essential Oil of Laurus nobilis L. and Its Major Compound 1,8-Cineol. Biocatal. Agric. Biotechnol. 2021;36:102148. doi: 10.1016/j.bcab.2021.102148. [DOI] [Google Scholar]
- 59.Veras H.N.H., Campos A.R., Rodrigues F.F.G., Botelho M.A., Coutinho H.D.M., Menezes I.R.A., Costa J.G.M. Enhancement of the Antibiotic Activity of Erythromycin by Volatile Compounds of Lippia alba (Mill.) N.E. Brown against Staphylococcus aureus. Pharmacogn. Mag. 2011;7:334–337. doi: 10.4103/0973-1296.90415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Veras H.N.H., Rodrigues F.F.G., Botelho M.A., Menezes I.R.A., Coutinho H.D.M., Costa J.G.M. Enhancement of Aminoglycosides and β-Lactams Antibiotic Activity by Essential Oil of Lippia sidoides Cham. and the Thymol. Arab. J. Chem. 2017;10((Suppl. 2)):S2790–S2795. doi: 10.1016/j.arabjc.2013.10.030. [DOI] [Google Scholar]
- 61.Costa R.A., Cavalcante T.T.A., Melo C.T.V., Barroso D.L.A., Melo H.M., Carvalho M.G., Catunda-Júnior F.E.A. Antioxidant and Antibacterial Activities of Essential Oil of Lippia sidoides against Drug-Resistant Staphylococcus aureus from Food. Afr. J. Biotechnol. 2018;17:232–238. doi: 10.5897/AJB2017.16355. [DOI] [Google Scholar]
- 62.Thielmann J., Muranyi P. Review on the Chemical Composition of Litsea cubeba Essential Oils and the Bioactivity of Its Major Constituents Citral and Limonene. J. Essent. Oil Res. 2019;31:361–378. doi: 10.1080/10412905.2019.1611671. [DOI] [Google Scholar]
- 63.Souza R.C., Costa M.M., Baldisserotto B., Heinzmann B.M., Schmidt D., Caron B.O., Copatti C.E. Antimicrobial and Synergistic Activity of Essential Oils of Aloysia triphylla and Lippia alba against Aeromonas spp. Microb. Pathog. 2017;113:29–33. doi: 10.1016/j.micpath.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 64.Alizadeh S.R., Ebrahimzadeh M.A. Quercetin Derivatives: Drug Design, Development, and Biological Activities, a Review. Eur. J. Med. Chem. 2022;229:114068. doi: 10.1016/j.ejmech.2021.114068. [DOI] [PubMed] [Google Scholar]
- 65.Wang M., Firrman J., Liu L.S., Yam K. A Review on Flavonoid Apigenin: Dietary Intake, ADME, Antimicrobial Effects, and Interactions with Human Gut Microbiota. Biomed Res. Int. 2019;2019:7010467. doi: 10.1155/2019/7010467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Farhadi F., Khameneh B., Iranshahi M., Iranshahy M. Antibacterial Activity of Flavonoids and Their Structure–Activity Relationship: An Update Review. Phyther. Res. 2019;33:13–40. doi: 10.1002/ptr.6208. [DOI] [PubMed] [Google Scholar]
- 67.Costa M.S., Rocha J.E., Campina F.F., Silva A.R.P., Cruz R.P., Pereira R.L.S., Quintans-Júnior L.J., Menezes I.R.A., Araújo A.A.S., Freitas T.S., et al. Comparative Analysis of the Antibacterial and Drug-Modulatory Effect of d-Limonene Alone and Complexed with β-Cyclodextrin. Eur. J. Pharm. Sci. 2019;128:158–161. doi: 10.1016/j.ejps.2018.11.036. [DOI] [PubMed] [Google Scholar]
- 68.Gupta P., Khobragade S., Rajaram S., Shingatgeri V. Assessment of Locomotion Behavior in Adult Zebrafish after Acute Exposure to Different Pharmacological Reference Compounds. Drug Dev. Ther. 2014;5:127. doi: 10.4103/2394-2002.139626. [DOI] [Google Scholar]
- 69.Ferreira M.K.A., Silva A.W., Santos Moura A.L., Sales K.V.B., Marinho E.M., Cardoso J.N.M., Marinho M.M., Bandeira P.N., Magalhães F.E.A., Marinho E.S., et al. Chalcones Reverse the Anxiety and Convulsive Behavior of Adult Zebrafish. Epilepsy Behav. 2021;117:107881. doi: 10.1016/j.yebeh.2021.107881. [DOI] [PubMed] [Google Scholar]
- 70.Maximino C., Silva A.W.B., Gouveia A., Herculano A.M. Pharmacological Analysis of Zebrafish (Danio rerio) Scototaxis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2011;35:624–635. doi: 10.1016/j.pnpbp.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 71.Parente M.S.R., Custódio F.R., Cardoso N.A., Lima M.J.A., De Melo T.S., Linhares M.I., Siqueira R.M.P., Nascimento A.Á., Catunda Júnior F.E.A., De Melo C.T.V. Antidepressant-Like Effect of Lippia sidoides Cham (Verbenaceae) Essential Oil and Its Major Compound Thymol in Mice. Sci. Pharm. 2018;86:27. doi: 10.3390/scipharm86030027. [DOI] [PubMed] [Google Scholar]
- 72.Bianchini A.E., Garlet Q.I., Da Cunha J.A., Bandeira Junior G., Brusque I.C.M., Salbego J., Heinzmann B.M., Baldisserotto B. Monoterpenoids (Thymol, Carvacrol and S-(+)-Linalool) with Anesthetic Activity in Silver Catfish (Rhamdia quelen): Evaluation of Acetylcholinesterase and GABAergic Activity. Braz. J. Med. Biol. Res. 2017;50:1–8. doi: 10.1590/1414-431x20176346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Castañeda R., Cáceres A., Velásquez D., Rodríguez C., Morales D., Castillo A. Medicinal Plants Used in Traditional Mayan Medicine for the Treatment of Central Nervous System Disorders: An Overview. J. Ethnopharmacol. 2022;283:114746. doi: 10.1016/j.jep.2021.114746. [DOI] [PubMed] [Google Scholar]
- 74.Nachammai V., Jeyabalan S., Muthusamy S. Anxiolytic Effects of Silibinin and Naringenin on Zebrafish Model: A Preclinical Study. Indian J. Pharmacol. 2021;53:457–464. doi: 10.4103/ijp.IJP_18_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang J., Liu M., Cui W., Yang L., Zhang C. Quercetin Affects Shoaling and Anxiety Behaviors in Zebrafish: Involvement of Neuroinflammation and Neuron Apoptosis. Fish Shellfish Immunol. 2020;105:359–368. doi: 10.1016/j.fsi.2020.06.058. [DOI] [PubMed] [Google Scholar]
- 76.Melo N.C., Sánchez-Ortiz B.L., Sampaio T.I.S., Pereira A.C.M., Silva-Neto F.L.P., Silva H.R., Cruz R.A.S., Keita H., Pereira A.M.S., Carvalho J.C.T. Anxiolytic and Antidepressant Effects of the Hydroethanolic Extract from the Leaves of Aloysia polystachya (Griseb.) Moldenke: A Study on Zebrafish (Danio rerio) Pharmaceuticals. 2019;12:106. doi: 10.3390/ph12030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Müller Herde A., Benke D., Ralvenius W.T., Mu L., Schibli R., Zeilhofer H.U., Krämer S.D. GABAA Receptor Subtypes in the Mouse Brain: Regional Mapping and Diazepam Receptor Occupancy by in vivo [18F]Flumazenil PET. Neuroimage. 2017;150:279–291. doi: 10.1016/j.neuroimage.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 78.Heldwein C.G., Silva L.L., Reckziegel P., Barros F.M.C., Bürger M.E., Baldisserotto B., Mallmann C.A., Schmidt D., Caron B.O., Heinzmann B.M. Participation of the GABAergic System in the Anesthetic Effect of Lippia alba (Mill.) N.E. Brown Essential Oil. Brazilian J. Med. Biol. Res. 2012;45:436–443. doi: 10.1590/S0100-879X2012007500052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bandeira-Junior G., Abreu M.S., Rosa J.G.S., Pinheiro C.G., Heinzmann B.M., Baldisserotto B., Barcellos L.J.G. Lippia alba and Aloysia triphylla Essential Oils Are Anxiolytic without Inducing Aversiveness in Fish. Aquaculture. 2018;482:49–56. doi: 10.1016/j.aquaculture.2017.09.023. [DOI] [Google Scholar]
- 80.Ríos J.L., Schinella G.R., Moragrega I. Phenolics as GABAA Receptor Ligands: An Updated Review. Molecules. 2022;27:1770. doi: 10.3390/molecules27061770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Handley S.L. 5-Hydroxytryptamine Pathways in Anxiety and Its Treatment. Pharmacol. Ther. 1995;66:103–148. doi: 10.1016/0163-7258(95)00004-Z. [DOI] [PubMed] [Google Scholar]
- 82.Jia M., Pittman J. Deficits in Striatal Dopamine and Hippocampal Serotonin Following Induction of Anxiety/Depressive-Like Behaviors by Bisphenol A. Arch. Neurosci. 2014;2:e18555. doi: 10.5812/archneurosci.18555. [DOI] [Google Scholar]
- 83.Nowicki M., Tran S., Muraleetharan A., Markovic S., Gerlai R. Serotonin Antagonists Induce Anxiolytic and Anxiogenic-like Behavior in Zebrafish in a Receptor-Subtype Dependent Manner. Pharmacol. Biochem. Behav. 2014;126:170–180. doi: 10.1016/j.pbb.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 84.Gonçalves N.G.G., de Araújo J.I.F., Magalhães F.E.A., Mendes F.R.S., Lobo M.D.P., Moreira A.C.D.O.M., de Azevedo Moreira R. Protein Fraction from Artocarpus altilis Pulp Exhibits Antioxidant Properties and Reverses Anxiety Behavior in Adult Zebrafish via the Serotoninergic System. J. Funct. Foods. 2020;66:103772. doi: 10.1016/j.jff.2019.103772. [DOI] [Google Scholar]
- 85.Ngaibi J., Taiwe G.S., Njapdounke J.S.K., Bigued, Nguezeye Y., Sidiki N., Bum E.N. Potential of an Aqueous Extract of Lippia multiflora Moldenke (Verbenaceae) in the Treatment of Anxiety Disorders: Possible Involvement of Serotoninergic Transmission. GSC Biol. Pharm. Sci. 2021;14:277–289. doi: 10.30574/gscbps.2021.14.3.0079. [DOI] [Google Scholar]
- 86.Amin F., Ibrahim M.A.A., Rizwan-ul-hasan S., Khaliq S., Gabr G.A., Khan A., Sidhom P.A., Tikmani P., Shawky A.M., Ahmad S., et al. Interactions of Apigenin and Safranal with the 5-HT1A and 5-HT2A Receptors and Behavioral Effects in Depression and Anxiety: A Molecular Docking, Lipid-Mediated Molecular Dynamics, and In Vivo Analysis. Molecules. 2022;27:8658. doi: 10.3390/molecules27248658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Singleton V.L., Orthofer R., Lamuela-Raventós R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- 88.Kosalec I., Bakmaz M., Pepeljnjak S., Vladimir-Knezević S. Quantitative Analysis of the Flavonoids in Raw Propolis from Northern Croatia. Acta Pharm. 2004;54:65–72. [PubMed] [Google Scholar]
- 89.CLSI . Performance Standards for Antimicrobial Susceptibility Testing. 28th ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2018. [Google Scholar]
- 90.Coutinho H.D.M., Costa J.G.M., Lima E.O., Falcão-Silva V.S., Siqueira-Júnior J.P. Enhancement of the Antibiotic Activity against a Multiresistant Escherichia coli by Mentha arvensis L. and Chlorpromazine. Chemotherapy. 2008;54:328–330. doi: 10.1159/000151267. [DOI] [PubMed] [Google Scholar]
- 91.Sobral A.M.F., Andreza R.S., Alves E.F., Cruz J.A.F., Talita A., Sousa T.A.L., Oliveira C.D.M., Tintino S.R., Aquino E.A., Lima L.F. Atividade Antibacteriana e Moduladora in vitro de Extrato Metanólico e Hexânico de Beta vulgaris spp. (Linnaeus) Rev. Cuba. Plantas Med. 2016;21:20–30. [Google Scholar]
- 92.OECD . OECD Guidelines for the Testing of Chemicals. OECD Publishing; Paris, France: 2006. Test No. 203: Fish, acute toxicity test. Section 2. [DOI] [Google Scholar]
- 93.Assessment R., Case P., Arellano-aguilar O., Montero-montoya R.D. Use of the Zebrafish Embryo Toxicity Test for Use of the Zebrafish Embryo Toxicity Test for Risk Assessment Purpose: Case Study. J. Fish. Sci. 2015;9:52–62. [Google Scholar]
- 94.Magalhães F.E.A., Sousa C.Á.P.B., Santos S.A.A.R., Menezes R.B., Batista F.L.A., Abreu Â.O., de Oliveira M.V., Moura L.F.W.G., Raposo R.D.S., Campos A.R. Adult Zebrafish (Danio rerio): An Alternative Behavioral Model of Formalin-Induced Nociception. Zebrafish. 2017;14:zeb.2017.1436. doi: 10.1089/zeb.2017.1436. [DOI] [PubMed] [Google Scholar]
- 95.Gebauer D.L., Pagnussat N., Piato Â.L., Schaefer I.C., Bonan C.D., Lara D.R. Effects of Anxiolytics in Zebrafish: Similarities and Differences between Benzodiazepines, Buspirone and Ethanol. Pharmacol. Biochem. Behav. 2011;99:480–486. doi: 10.1016/j.pbb.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 96.Benneh C.K., Biney R.P., Mante P.K., Tandoh A., Adongo D.W., Woode E. Maerua angolensis Stem Bark Extract Reverses Anxiety and Related Behaviours in Zebrafish—Involvement of GABAergic and 5-HT Systems. J. Ethnopharmacol. 2017;207:129–145. doi: 10.1016/j.jep.2017.06.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data will be available after a reasonable request to the corresponding authors.