Abstract
The sugar molecule N-glycolylneuraminic acid (Neu5Gc) is one of the most common sialic acids discovered in mammals. Cytidine monophospho-N-acetylneuraminic acid hydroxylase (CMAH) catalyses the conversion of N-acetylneuraminic acid (Neu5Ac) to Neu5Gc, and it is encoded by the CMAH gene. On the one hand, food metabolic incorporation of Neu5Gc has been linked to specific human diseases. On the other hand, Neu5Gc has been shown to be highly preferred by some pathogens linked to certain bovine diseases. We used various computational techniques to perform an in silico functional analysis of five non-synonymous single-nucleotide polymorphisms (nsSNPs) of the bovine CMAH (bCMAH) gene identified from the 1000 Bull Genomes sequence data. The c.1271C>T (P424L) nsSNP was predicted to be pathogenic based on the consensus result from different computational tools. The nsSNP was also predicted to be critical based on sequence conservation, stability, and post-translational modification site analysis. According to the molecular dynamic simulation and stability analysis, all variations promoted stability of the bCMAH protein, but mutation A210S significantly promoted CMAH stability. In conclusion, c.1271C>T (P424L) is expected to be the most harmful nsSNP among the five detected nsSNPs based on the overall studies. This research could pave the way for more research associating pathogenic nsSNPs in the bCMAH gene with diseases.
Keywords: sialic acid, Neu5Gc, Neu5Ac, CMAH, pathogenesis, SNPs, virus, bacteria
1. Introduction
Infectious diseases in cattle have an unpredictable economic impact on livestock output, and the effects of pathogens such as viruses and bacteria have been extensively investigated over the past decades. A critical feature of this research is the interaction between pathogens with host cells via sialic acids (Sias). Sias are acidic sugars with a 9-carbon backbone at the terminals of glycan chains in glycoconjugates on the surface of vertebrate cells [1]. They are components of cell surface glycans and are involved in cell communication as well as pathogen–host interactions during infectious processes. Sias are located at the terminal position and serve as the principal interface between pathogens and host cells [2].
The principal Sias in mammalian cells are N-glycolylneuraminic acid (Neu5Gc) and N-acetylneuraminic acid (Neu5Ac). Cytidine monophospho-N-acetylneuraminic acid hydroxylase (CMAH) catalyses the conversion of the Neu5Ac to the Neu5Gc molecule, which is encoded by the CMAH gene. In humans, Neu5Gc is absent due to the inactivation of the CMAH gene by mutation [2,3], and dietary metabolic incorporation of Neu5Gc has been linked to specific diseases and disorders [4].
Additionally, Sias can act as receptors for a variety of influenza viruses, including Influenza A and B [5,6,7]. Sias also regulate receptor binding by modulating transmembrane signalling, fertilisation, and cell differentiation [8]. Certain bacteria and viruses have a strong affinity for Neu5Gc, as demonstrated in different studies [9,10,11]. Schwegmann et al. [11] showed that E. coli K99 induces Neonatal Calf Diarrhoea (NCD) by selectively recognising Neu5Gc glycoconjugates. The findings corroborated prior research indicating that Neu5Gc glycoconjugates act as receptors [12,13]. Additionally, the bovine strain of Nebraska Calf Diarrhoea Virus, a primary pathogenic virus responsible for NCD, shows a high affinity for Neu5Gc glycoconjugates [14,15].
As sequencing technology has advanced in recent years, livestock breeding programs have reaped enormous benefits from genome sequence data [16,17]. Single-nucleotide polymorphisms (SNPs) have been utilised to investigate correlations and genetic links to segments of the genomes associated with various disorders [18,19,20]. A non-synonymous SNP (nsSNP) is an SNP that results in an amino acid substitution in the protein sequence. This mutation may impair the protein’s overall function or be linked to pathogenesis [21,22].
In silico analyses of nsSNPs in the bovine CMAH (bCMAH) gene are limited. Several research studies [23,24] have applied bioinformatics tools in determining the association of diverse nsSNPs with diseases. Additionally, studies have found SNPs in the feline and canine CMAH genes related to alterations in CMAH function [25,26,27]. Considering the importance of CMAH in pathogenesis [28], it is critical to find SNPs within the CMAH gene that may be associated with cattle diseases. Individual animals with a high level of Neu5Gc expression may be prone to certain diseases, and dietary inclusion of such cattle products may also raise the risk of certain diseases in humans [29,30]. The present study aimed to study the disease-causing nsSNP of the CMAH gene and evaluate its impact on the structural stability and functioning of CMAH. The study, coupled with sequencing and bioinformatics tools, facilitated the identification of pathogenic variants. The outcomes of the study facilitated an understanding of the genetic variation influence on CMAH conservation and protein stability.
2. Materials and Methods
2.1. Identification of nsSNPs in bCMAH from the 1000 Bull Genomes Sequence Data
In this study, DNA samples were analysed from a total of 165 individuals belonging to 29 different breeds and 5 unknown breeds. PCR-free fragment libraries with 300–500 bp insert sizes were prepared using the TruSeq DNA PCR-Free protocol [31] and sequenced on Illumina HiSeq3000 lanes with paired-end reads (2 × 150 bp), and the fastq files were created using Casava 1.8. The paired-end reads were then mapped to the cow reference genome UMD3.1/bosTau6 and aligned using the Burrows–Wheeler Aligner (BWA) version 0.5.9-r16, with default settings [32]. The SAM file generated by the BWA was then converted to BAM, and the reads were sorted by chromosome using samtools (http://samtools.sourceforge.net, accessed on 5 April 2023). The PCR duplicates were marked using Picard tools (http://sourceforge.net/projects/picard/, accessed on 5 April 2023). The Genome Analysis Tool Kit (GATK version 2.4.9 [33] was used to carry out local realignment and to produce a cleaned BAM file. Variant calls were then made with the unified genotype module of GATK. The variant data for each sample were obtained in variant call format (.vcf), as were raw calls for all samples and sites flagged using the variant filtration module of GATK. Variant filtration was carried out, following the best practice documentation of GATK version 4. The snpEFF software [34], together with the UMD3.1/bosTau Ensembl annotation, was used to predict the functional effects of the detected variants. Based on the obtained information, DNA sequence data from 2724 individuals were retrieved and analysed from the 1000 Bull Genomes sequence data [35]. For the sequence analysis and identification of different nsSNPs, the exon table of the mRNA transcript variant X6 (XM_024984024.1), comprising 16 exons, of which 14 are coding, was used from the NCBI database (https://www.ncbi.nlm.nih.gov, accessed on 5 April 2023). The total spliced RNA is 3374 bp long and encodes for the protein isoform X2 (XP_024839792.1), which is 577 amino acids (AA) in length. The nsSNPs were retrieved between the genomic positions 32,458,973 bp and 32,755,484 bp of the bovine chromosome 23 (NC_037350.1).
2.2. Prediction, Refinement, and Validation of Tertiary Structure of Bovine CMAH Protein
Due to the absence of the tertiary structure for the bCMAH protein in the protein database and sequence homology between the target and template proteins of less than 30%, we used ab initio structure prediction with all-atom refinement via the Robetta online tool (https://robetta.bakerlab.org/, accessed on 5 April 2023) [36]. The TrRosetta (TR, a method based on deep learning) was utilised as the default. A Monte Carlo minimisation methodology was employed that involves perturbing randomly chosen backbone torsion angles while optimising sidechain rotamer conformations and performing Quasi-Newton minimisation on all backbone and sidechain torsion angles [36]. Protein modelling was performed using the sequence of the bCMAH protein isoform X2 (XP_024839792.1). Further refining of the protein structure was carried out using the GalaxyWEB refiner tool (https://galaxy.seoklab.org/index.html, accessed on 5 April 2023). The server refines loop or terminal areas using ab initio modelling and then uses molecular dynamics simulations to execute repetitive structure perturbation and eventual overall structural relaxation [37]. The predicted bCMAH protein structure was further validated using the PDBsum (http://www.ebi.ac.uk/thornton-srv/databases/pdbsum/Generate.html, accessed on 5 April 2023) [38] and ProSA online tools (https://prosa.services.came.sbg.ac.at/prosa.php, accessed on 5 April 2023) [39]. The domain information of CMAH in bovine was obtained through the UniProt database (https://www.uniprot.org/, accessed on 5 April 2023 [40]) and PROSITE (https://prosite.expasy.org/, accessed on 5 April 2023 [41]). The disordered region on the protein was predicted through the D2P2 webserver tool [42].
2.3. Evaluation of the Functional Impacts of the nsSNPs on the Function and Stability of the Bovine CMAH Protein
To assess the functional effects of nsSNPs and to deduce their potential role in pathogenesis, we used a combination of five different computational online tools based on different algorithms. The primary amino acid sequence of bCMAH was uploaded into various tools. The online computational tools were: PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/, accessed on 5 April 2023) [43], SNPs&GO (https://snps.biofold.org/snps-and-go/, accessed on 5 April 2023) [44], Sorting Intolerant From Tolerant (SIFT; https://sift.bii.a-star.edu.sg/www/SIFT_dbSNP.html, accessed on 5 April 2023) [45], Protein Variation Effect Analyzer (PROVEAN; http://provean.jcvi.org/index.php, accessed on 5 April 2023) [21], and PANTHER (http://www.pantherdb.org/, accessed on 5 April 2023) [46]. The overall criteria for the classification of nsSNP into deleterious and benign classes were taken from [47].
The influence of variations was further evaluated for their effect on the structural stability of the bCMAH protein. The 3D structure of bCMAH was uploaded into DynaMut (http://biosig.unimelb.edu.au/dynamut/, accessed on 5 April 2023), an online computational tool, and SNP information was fed into it [48]. DynaMut investigates the effects of point mutations on the dynamics and stability of proteins due to variations in vibrational entropy. Additionally, it incorporates graph-based signatures and normal-mode dynamics to produce a consensus forecast [48].
2.4. Sequence Conservational Analysis
The web-based program ConSurf (http://consurf.tau.ac.il, accessed on 5 April 2023) was used to analyse sequence conservation. ConSurf analyses the evolutionary trend of functional region amino acids. It classifies the protein’s amino acid residues on a scale of 1 to 9, with 1–3 denoting variable, 4–6 denoting average, and 7–9 denoting conserved or highly conserved sections [49]. Variations that fell into the conserved region were taken as the most deleterious.
2.5. Post-Translational Modification Sites’ Prediction
In order to fully comprehend protein activities and regulation, it is critical to identify and analyse post-translational modification sites (PTMs). By inputting the protein FASTA sequence of the bCMAH, the PTMs online tool MusiteDeep (https://www.musite.net, accessed on 5 April 2023), a deep learning framework, was utilised to predict the PTMs [50]. The tool incorporates numerous ensemble tools that facilitate better prediction of PTM sites.
2.6. Stability Analysis
The predicted tertiary structure of CMAH was analysed using I-Mutant v2 to evaluate the impact of nsSNPs on the structural stability (https://folding.biofold.org/i-mutant/i-mutant2.0.html, accessed on 5 April 2023 [51]). Any nsSNP with a ΔΔG value lower than −0.5 was considered to have a destabilizing effect.
2.7. Active Site Prediction and Molecular Dynamic Simulation
To identify potential binding sites within the tertiary structure of the bCMAH protein, we used SiteMap 3.5, inbuilt within the Schrödinger software package [48], to characterise those binding sites. The SiteMap provides quantitative and graphical information that can help guide efforts to critically assess virtual hits in a lead-discovery application or to modify the ligand structure to enhance the potency or improve physical properties in a lead-optimisation context [52]. Further validation of the predicted active sites was carried out using MetaPocket 2.0 [53].
In this study, the PyMol v4.0.4 mutagenesis wizard tool was employed to introduce point mutations in the predicted CMAH structure [54]. To evaluate the stability of the predicted and mutagenic structures, molecular dynamics (MD) simulations were carried out using the CHARMMS forcefield in GROMACS 2016 [55]. The system was prepared by first subjecting it to solvation, followed by the addition of SPC216 water molecules, and then neutralization with Na+/Cl− ions. An energy minimisation (EM) step was performed using the steepest descent method with a total timestep of 50,000 steps to obtain an optimised system. The modelled system was then subjected to NVT (constant number of particles, volume, and temperature) and NPT (constant number of particles, pressure, and temperature) equilibration for 100 ps. Trajectories were initiated from the same random seed to minimize any biases during the MD simulations.
Following equilibration, MD simulations were carried out for 50 ns, and trajectory coordinates were captured every 10 ps. Structural analysis was performed using GROMACS 2016 built-in programs. Trajectories were constructed using gmx_trjconv, and the root mean square deviations (RMSD) were calculated using gmx_rms. The root mean square fluctuations (RMSF) were computed using gmx_rmsf, the radius of gyration (Rg) using gmx_gyrate, the number of hydrogen bonds using gmx_bond, and the solvent accessibility surface area (SASA) using gmx_sasa. RMSD, RMSF, and Rg calculations were carried out for the backbone. The data were represented through scatter smooth-line plots to visualize any changes in the protein structure. These analyses helped to determine the stability of the predicted and mutagenic structures. Overall, the study aimed to provide a deeper understanding of the effects of the introduced point mutations on the CMAH structure and to identify any potential changes in its stability.
3. Results
3.1. Distribution of nsSNPs in Different Breeds Analysed in 1000 Bull Genomes Sequence Data
The new genomic positions and coding exon variants of the bovine CMAH gene sequenced in 165 individuals were identified in the UMD_3.1.1 and re-mapped on ARS-UCD1.2 assemblies, resulting in the identification of novel variants, as well as the confirmation of previously known variants through their corresponding RefSNP I.D. A total of 2724 DNA sequences were examined and classified into 3 categories: 13 dairy breeds (1349 samples), 9 beef breeds (774 samples), and 9 dual-purpose or crossbred types (601 samples). Within the bCMAH gene, five non-synonymous single-nucleotide polymorphisms (nsSNPs) were discovered, which are predicted to result in missense mutations (Table 1). The frequencies of the identified genotypes in the 1000 Bull Genomes sequence dataset are displayed in Table 2. The nsSNP c.319A>G, located in exon 4, exhibited the highest frequency in both heterozygous and homozygous forms. Conversely, the nsSNP c.1271C>T demonstrated the lowest frequency, appearing solely in a heterozygous state.
Table 1.
nsSNPs detected within bovine CMAH in the 1000 Bull Genomes dataset compared with the reference sequence.
| Coding Exon |
cDNA Variant (XM_024984024.1) |
Protein Variant (XP_024839792.1) | Genomic Position | RefSNP ID (dbSNP) |
|---|---|---|---|---|
| 4 | c.319A>G | K107E | BTA23:g.32,721,570 | rs208635220 |
| 6 | c.628G>T | A210S | BTA23:g.32,727,639 | rs435799892 |
| 6 | c.725A>G | N242S | BTA23:g.32,727,736 | rs109811989 |
| 11 | c.1271C>T | P424L | BTA23:g.32,743,918 | rs518400910 |
| 12 | c.1535T>A | F512Y | BTA23:g.32,745,866 | rs380571713 |
Reference: ARS-UCD1.2.
Table 2.
The frequencies of the bovine CMAH genotypes identified.
| Coding Exon |
cDNA Variant (XM_024984024.1) |
Heterozygous (n) |
Homozygous Alternative Allele (n) | Reference Allele (n) | Null Data (n) |
Total (n) |
|---|---|---|---|---|---|---|
| 4 | c.319A>G | 1091 | 362 | 1140 | 131 | 2724 |
| 6 | c.628G>T | 40 | 4 | 2662 | 18 | 2724 |
| 6 | c.725A>G | 234 | 27 | 2443 | 20 | 2724 |
| 11 | c.1271C>T | 15 | 0 | 2689 | 20 | 2724 |
| 12 | c.1535T>A | 77 | 14 | 2610 | 23 | 2724 |
Null data = no sequence data at the position, n = number of samples.
3.2. Secondary and Tertiary Structure Prediction and Validation
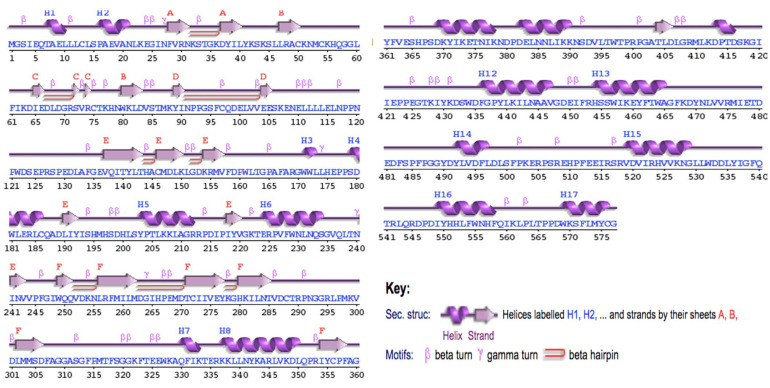
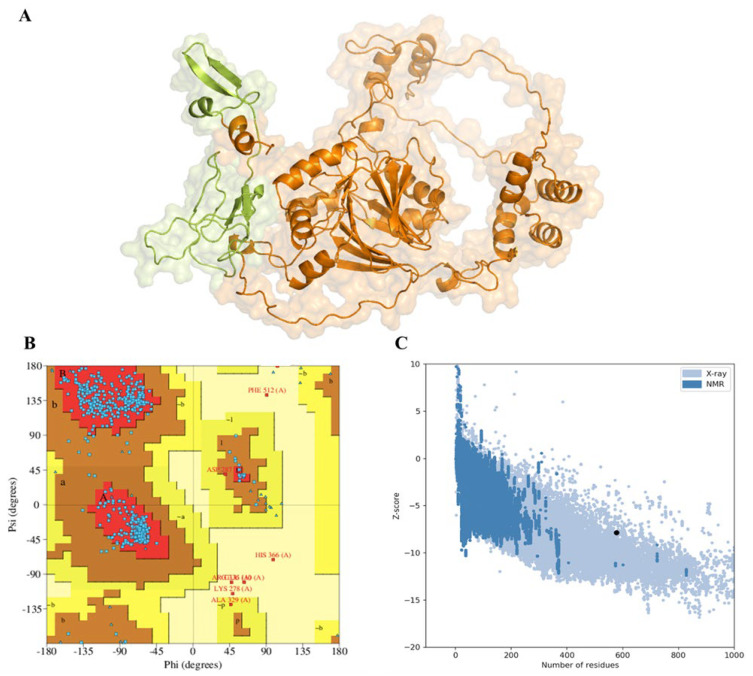
To validate the tertiary structure, the PDBsum’s PROCHECK tool was applied, and the results provided a thorough breakdown of the protein’s composition. According to PDBsum, the protein includes a strand containing 89 residues (15.4%), an alpha helix with 129 residues (22.4%), a helix with 13 residues (2.3%), and other components with 346 residues (60.0%). Additionally, K107 and N242 are predicted to be strand turns, while A210S, P424, and F512 are anticipated to be helix turns (Figure 1). The Ramachandran plot analysis, which assesses the stereochemical quality of the tertiary structure, revealed that an impressive 92.6% of the residues were in the most favoured regions. ProSa, an interactive web service designed for identifying errors in tertiary protein structures [39], demonstrated that the modelled protein achieved a z-score of −7.78. This score is indicative of a high-quality structure. Furthermore, the protein structure was found to be consistent with the standard X-ray crystallography parameters for proteins of a similar size (Figure 2). We also predicted a disordered region in the bCMAH protein that showed a high presence of disorder and provided insight into the presence of loops in the CMAH protein’s structure.
Figure 1.
Secondary structure of bovine CMAH protein.
Figure 2.
The predicted tertiary structure of bovine CMAH protein and its validation. (A) Tertiary structure of CMAH. Lime colour indicates iron-sulphur domain (14–112). (B) Assessment and validation of the protein. Ramachandran plot depicted that 92.6% of the amino acids are located in the most favoured region with a total of 462 residues (A, B, L), 5.8% in the additional allowed region with a total of 29 residues (a, b, l, p), 0.4% in the generously allowed region with two residues (~a, ~b, ~l, ~p), and 1.2% in the disallowed region with six residues. (C) Prosa plot with a z-score of −7.86. The black dot represents the position of the bCMAH structure compared with the standard X-ray crystallography parameters for proteins of a similar size.
3.3. Prediction of Pathogenic and Damaging Amino Acids of Bovine CMAH Protein
Identifying variants associated with pathogenesis is critical, as this might help determine the druggability of such variations. The matching results provided by the five tools (Polyphen-2, SNPs&Go, PROVEAN, SIFT, and PANTHER) independently demonstrated the reliability of the predictions, despite using different algorithms. The K107E, A210S, N242S, and F512Y variants were expected to be neutral, tolerable, or benign, while the P424L variant was anticipated to be probably harmful, deleterious, not tolerated, or disease-causing (Table 3).
Table 3.
Protein variants analysed by PolyPhen-2, SNPs&GO, PROVEAN, SIFT, and PANTHER.
|
Protein Variant (XP_024839792.1) |
PolyPhen-2 | SNPs&GO | PROVEAN | SIFT | PANTHER |
|---|---|---|---|---|---|
| K107E | Benign | Neutral | Neutral | Tolerated | Probably Benign |
| A210S | Benign | Neutral | Neutral | Tolerated | Possibly Damaging |
| N242S | Benign | Neutral | Neutral | Tolerated | Probably Benign |
| P424L | Probably Damaging |
Disease | Deleterious | Deleterious | Probably Damaging |
| F512Y | Benign | Neutral | Neutral | Tolerated | Probably Benign |
Predictions of the impacts of amino acid substitutions as a result of nsSNPs on bovine CMAH using different computational tools.
3.4. Prediction of the Effects of Amino Acid Substitutions on Bovine CMAH Protein Stability
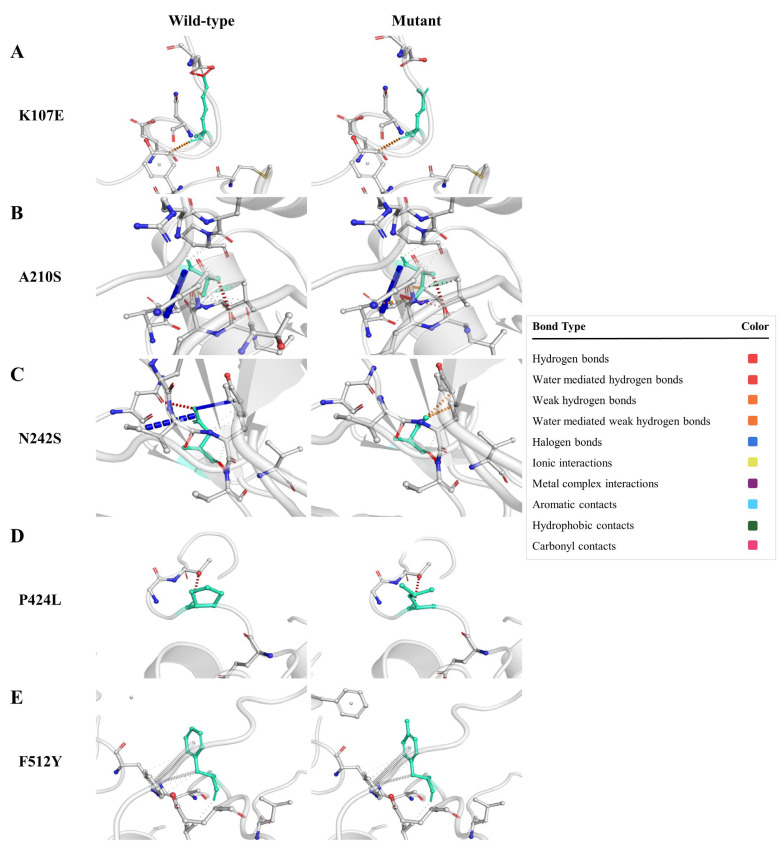
In this study, we used the DynaMut online program to investigate the structural implications of amino acid substitutions in a protein of interest. The program utilizes a computational approach to predict the impact of mutations on protein stability. To carry out this analysis, we uploaded the tertiary structure of our protein in PDB format to the DynaMut webserver. Our analysis revealed that the substitutions K107E, A210S, and N242S (Figure 3A–C) were predicted to stabilize the protein structure. On the other hand, the substitutions P424L and F512Y (Figure 3D,E) were projected to destabilize the protein. These findings are consistent with previous research showing that mutations can have both stabilizing and destabilizing effects on protein structures. Mutations also altered molecular interactions within the protein. Mutation K107E led to the loss of the hydrogen bond, mutation A210S led to the loss of hydrogen bonds as well as hydrophobic interactions, mutation N242S introduced hydrogen bonds, mutation P424L caused the loss of aromatic contact, and mutation F512Y caused the loss of hydrogen bonds.
Figure 3.
Structural impacts of the amino acid substitutions on protein stability, computed by DynaMut. K107E variant (A), A210S variant (B), N242S variant (C), P424L variant (D), and F512Y variant (E), with prediction outcome, ΔΔG, of 0.496, 0.271, 0.118, −0.121, and −0.804 (kcal/mol), respectively. ΔΔG = vibrational entropy change.
To gain a deeper understanding of the interatomic variations between the variants and wildtype residues, we examined the associated changes in vibrational entropy. Our analysis revealed that the substitutions K107E, A210S, and N242S resulted in decreased vibrational entropy, indicating a more ordered protein structure. In contrast, the substitutions P424L and F512Y led to increased vibrational entropy, indicating a more disordered protein structure [43].
3.5. Sequence Conservational Analysis and the Predicted PTMs
Conserved regions of protein sequences and PTMs are vital in analysing disease-causing or structure-altering mutations [56,57]. The FASTA protein sequence was analysed using the ConSurf web tool. ConSurf predicted K107E to be present in a variable region, A210S and N242S in the average region, and the last two variants (P424L and F512Y) in the highly conserved region (Figure 4). Two variants were also predicted to be located in the PTMs by the MusiteDeep webserver tool. While variant K107E was predicted to be associated with ubiquitination, SUMOylation, acetylation, methylation, or hydroxylation, the variant P424L was only associated with hydroxylation (Table 4).
Figure 4.
ConSurf results for residue conservation. The colours show different confidence levels for sequence conservation, with dark green being highly variable and dark red being highly conserved. The five rectangular boxes depict the conversation confidence levels of the five variants (Table 4).
Table 4.
Conservational analysis of the variants and the predicted PTMs.
|
Protein Variant (XP_024839792.1) |
Conservation | PTMs |
|---|---|---|
| K107E | Variable | Ub, Su, Ac, Me, Hy |
| A210S | Average | None |
| N242S | Average | None |
| P424L | Highly Conserved | Hy |
| F512Y | Highly Conserved | None |
ConSurf conservational scale: Using the UniRef90 protein database. PTMs by MusiteDeep with a PTM threshold score of 0.05: Ub (Ubiquitination), Su (SUMOylation), Ac (Acetylation), Me (Methylation), Hy (Hydroxylation).
3.6. Active Sites’ Prediction and Molecular Dynamics Simulations
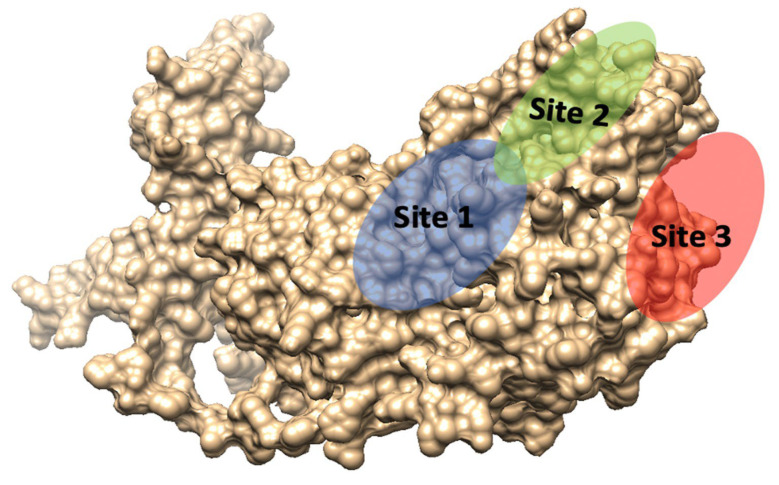
The active site of bCMAH was predicted using Sitemap3.5 [49] and cross-validated with MetaPocket2.0 [53]. This binding pocket was chosen based on the druggability (Dscore) score, which measures a protein’s ability to bind small molecules tightly. Based on the Dscore, tentative active site residues and three locations were predicted, as shown in Table 5 and Figure 5. The results show that none of the amino acid substitutions were located in the predicted active sites.
Table 5.
Highlights of the residues making up the active sites, with a description of the physicochemical properties of each active site.
| Site | Site Score | Size | Volume | DScore | Residues |
|---|---|---|---|---|---|
| 1 | 1.017 | 90 | 184.53 | 0.961 | Ser72, Cys75, Thr76, Asn79, Asp83, Val84, Ser85, Thr86, Met87, Lys88, Pro93, Gly94, Ser95, Phe96, Lys222, Met262, Asp263, Gly264, Ile265, His266, Pro267, Glu268, Asp270 |
| 2 | 1.016 | 214 | 619.11 | 1.057 | Tyr41, Lys42, Ser43, Leu46, Arg48, Lys51, Cys54, Lys55, Leu60, Thr163, Gly164, Pro165, Ala166, Phe167, Ala168, Gly170, Trp171, Trp172, Leu173, Leu174, His175, Pro177, Pro178, Trp181, Met196, His197, Ser198, Leu201, Ser202, Tyr203, Pro204, Lys208, Pro226, Val227, Trp229, Asn230, Leu231, Asn232, Gln233, Glu513, Glu514 |
| 3 | 0.962 | 71 | 154.69 | 1.001 | Pro381, Asp382, Leu384, Asn385, Val394, Thr396, Trp397, Thr398, Lys468, Asp469, Leu530, Leu531, Leu535 |
Figure 5.
Potential active sites predicted by SiteMap and cross-validated by MetaPocket. Site 1 (blue), Site 2 (green) and Site 3 (red).
3.6.1. Bovine CMAH Mutations’ Impact on Its Structural Stability
The impact of bCMAH mutations on its structural stability was also evaluated through I-Mutant. The analysis revealed that all mutations promoted structural stability in bCMAH. The stability impact was predicted through DDG values that indicate the delta-free energy. Table 6 indicates each mutation’s impact on the structure and function of the bCMAH protein.
Table 6.
Impact of mutations on bovine CMAH structural stability.
| Mutation | DDG | Stability |
|---|---|---|
| K107E | −0.17 | Increase |
| A210S | −0.44 | Increase |
| N242S | −0.49 | Increase |
| P424L | −0.32 | Increase |
| F512Y | −0.33 | Increase |
3.6.2. Mutations Elicited Structural Distortion in Bovine CMAH Protein
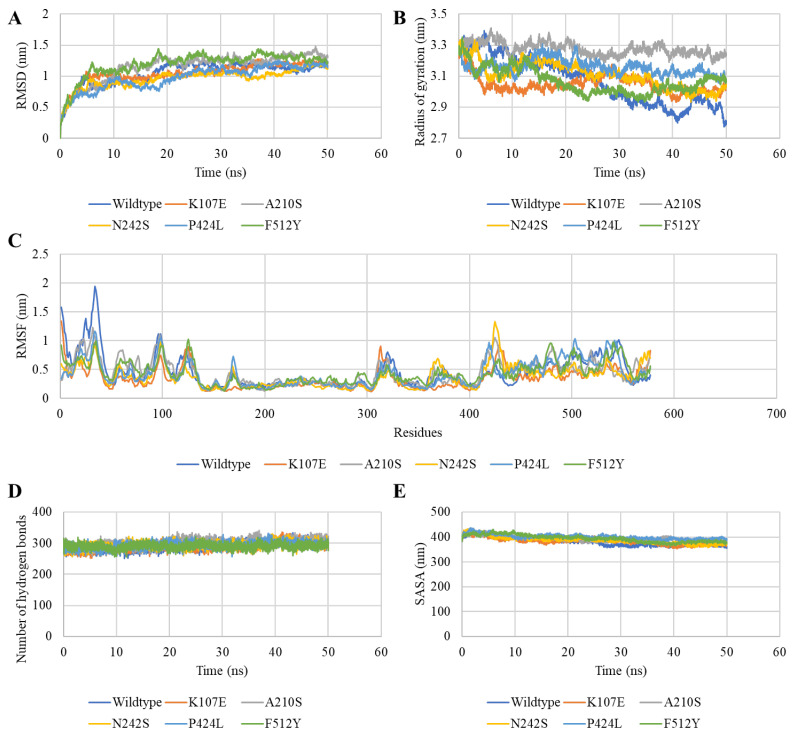
To understand the structural perturbation of bovine CMAH caused by the polymorphisms, we used root mean square deviation (RMSD), the radius of gyration (Rg) and root mean square fluctuation (RMSF) to characterise the structural events in the proteins during the 50 ns simulation. Compared to the wildtype structure, an increase in the RMSD of the mutant F512Y was observed. Mutants K107E, A210S, N242S, and P424L RMSD values were lower than the wildtype (Figure 6A). The wildtype’s Rg decreased after 20 ns, suggesting that the wildtype structure compacts and reaches the point where its activity diminishes. However, all mutations enhanced the Rg of bCMAH, and the values remained in a closer range throughout the simulation (Figure 6B).
Figure 6.
Plots depicting the MD simulation analysis of the impacts of mutations on bCMAH. Wildtype (black), K107E (red), P424L (green), A210S (blue), N242S (lemon), and F512Y (pink) across the MD simulation run. RMSD (A). Rg (B). RMSF (C). Hydrogen bonds analysis (D). SASA analysis (E).
RMSF indicates the influence of mutations on the stability of the domain in which they are located and the nearby regions. High fluctuations in residues 21–39 were observed in the wildtype, and all the mutant structures had a lower fluctuation in these residues. However, mutant K107E had lower fluctuations than the wildtype from residues 92–109. Mutant N242S showed high fluctuations in residues 421–520 and had overall lower fluctuations at its C-terminus (Figure 6C). Among the mutant structures, mutant A210S was found to be the most stable. No significant impact of mutations on the number of hydrogen bonds and solvent accessible surface areas (SASA) was recorded (Figure 6D,E). Overall, compared to the wildtype, all mutations promoted the structural stability of the bCMAH protein, whereas the mutation A210S greatly enhanced bCMAH stability, while the stability of the F512Y mutant was comparatively lower than the other mutants.
4. Discussion
Neu5Gc and Neu5Ac are mammals’ predominant sialic acid sugar molecules. While both Neu5Gc and Neu5Ac are prevalent in bovine species, the Neu5Gc cannot be endogenously synthesised in humans because the CMAH is inactivated [2,3]. On the one hand, the association between Neu5Gc and some bovine diseases is due to the affinity of specific pathogens for Neu5Gc glycoconjugates [14,15]. On the other hand, dietary incorporation of Neu5Gc sugar molecules via red meat has been associated with various human diseases and disorders [4]. As previously noted, cattle expressing a high level of Neu5Gc may be prone to certain diseases, and intake of their meat products may enhance human vulnerability to certain diseases.
SNPs are frequent genetic variants that occur approximately every 500–1000 base pairs and are useful for genome association and pharmaco-genomic investigations [58]. Computational or in silico analysis is becoming more and more popular for mapping SNPs to changes in protein functions or diseases [24,47,59]. Apart from being cost- and time-effective, other studies have demonstrated the efficacy of various computational or in silico analysis tools in precisely identifying SNPs associated with various diseases or changes in protein functions [23,24]. The nsSNPs that lead to changes in the amino acid sequence of a protein may disrupt the protein’s overall tertiary structure, which can result in diseases and disorders [21,45].
This study identified five non-synonymous single-nucleotide polymorphisms (nsSNPs) within the bCMAH gene using data from the 1000 Bull Genomes project [35]. This dataset comprises whole-genome sequences from a diverse range of cattle populations across the globe. The majority of nsSNPs were observed in heterozygous and homozygous states. The c.319A>G variant displayed the highest frequency for both heterozygous and homozygous genotypes, while the c.1271C>T variant showed the lowest frequency as a heterozygous genotype. Previous research has linked CMAH variations to blood subtypes in various cat species [26,60].
Multiple computational tools were employed to assess the potential impact of these nsSNPs, including PolyPhen-2, SNPs&GO, PROVEAN, SIFT, and PANTHER. Despite utilizing different algorithms, all tools reached a consensus based on the provided amino acid sequence. This consensus allowed for a more reliable determination of the potential consequences of these nsSNPs. The c.1271C>T variant was anticipated to be associated with a disease or disorder, harmful, probably detrimental, or intolerable. This could account for its low frequency in the 1000 Bull Genomes sequence data.
For thoroughly evaluating the impact of these variations on the bCMAH protein structure, its structure was predicted using an ab initio approach. The obtained structure was found to be high in disorder. Intrinsically disordered proteins (IDPs) play essential roles in various cellular regulatory processes. Their lack of a well-defined structure in their free state, and sometimes when interacting with physiological partners, is a fundamental aspect of their functionality. Disordered domains often contain numerous sites for post-translational modifications, which serve as crucial elements in cellular metabolic control. Upon binding to a partner, a disordered domain may fold, leading to the formation of a complex that buries a substantially larger surface area compared to interactions between similarly sized folded proteins. This characteristic allows IDPs to maximize specificity while maintaining a relatively small size [61,62]. However, further analysis investigating the functional role of the disorder in CMAH protein interactions will be useful.
Proteins are dynamic in nature, and the effects of amino acid substitutions on their dynamics and stability may affect the protein’s function and may be associated with disease [45,48]. The DynaMut online tool projected the effects of these five mutations on the conformation, flexibility, and stability of proteins due to variations in vibrational entropy. K107E, A210S, and N242S variants were anticipated to stabilise the protein shape, whereas P424L and F512Y variants were predicted to disrupt the protein’s stability.
Additionally, the degree of conservation of the amino acid sequence of proteins strongly correlates with the functional sections of proteins, such as motifs [49]. Generally, variants in highly conserved regions are not tolerated, and those discovered within these areas may affect the protein’s function and contribute to disease [56,57]. According to the ConSurf online tool for analysing evolutionarily conserved regions, both P424L and F512Y are projected to be located in a highly conserved region, K107E in the variable region, and A210S and N242S in the averagely conserved region.
Neu5Gc’s absence or presence in felines is used to identify blood groups. Its absence is linked with type B, which might be caused by variations in the CMAH’s 5′UTR region [25,63]. Furthermore, CMAH variations were reported to have a deleterious influence in cats. Kehl et al. further found the association of the CMAH gene’s deleterious variations with blood group types. They reported that the c.179G>T variant in a Turkish cat breed is linked with blood type B and is reported to be deleterious [60]. Uno et al. identified 15 SNPs (11 intronic and 4 exonic) in 11 dog breeds. The nsSNP, c.554 A>G, is reported to be majorly distributed among canines. However, no loss of function or gain of function mutations with severe consequences is reported in canines [27].
PTMs are also crucial protein locations because they promote proteome diversity, which is required for biological processes such as protein–protein interactions and disease-related signalling cascades. Variants in these locations might be associated with disease [64]. Only K107E and P424L variants were predicted to be related to PTMs using the MusiteDeep online tool. While K107E was anticipated to be linked with a variety of PTMs, including ubiquitination, SUMOylation, acetylation, methylation, and hydroxylation, P424L was projected to be exclusively involved in hydroxylation. Proline hydroxylation is critical for protein stability; for example, it aids in the effective twisting of the collagen helix [65]. Proline hydroxylation is also required to control hypoxia-induced factor-1 alpha (HIF-1), a critical oxygen-dependent transcription factor [66]. Any variant detected in these PTMs may be harmful, implying that variants in the proline hydroxylation region of the bovine CMAH protein may also be associated with diseases or changes in the protein’s function.
Additionally, MD simulations were applied to evaluate the dynamics of the protein following mutation by examining the protein movements and tracking the structural changes of wildtype and mutant proteins over time using GROMACS. The 50 ns MD simulation revealed that all variations enhanced the overall stability of the CMAH protein. As the CMAH enzyme is responsible for Neu5Ac’s catalytic conversion to Neu5Gc, the enhanced stability of CMAH due to mutations might enhance the reaction rate of Neu5Gc production. Such mutations might lead to an increase in the biosynthesis of Neu5Gc, which can also be lethal for humans, causing diseases such as atherosclerosis and cancer, as earlier mentioned. Further investigation describing CMAH mutations’ impact on its interaction with Neu5Ac will further facilitate understanding of the pathogenicity of CMAH in diseases.
5. Conclusions and Recommendations
In silico analysis is a cost-effective and time-efficient method for analysing nsSNPs related to protein structural changes and pathogenicity. The functional and structural consequences of nsSNPs in the bovine CMAH gene were studied utilising a number of computational prediction tools. Five nsSNPs were identified, with c.1271C>T (P424L) having the lowest frequency in the 1000 Bull Genomes sequence data and being expected to be pathogenic or intolerable. Additionally, this P424L variant, similar to F512Y, was predicted to be present in the highly conserved region and destabilise the protein structure due to changes in vibrational entropy. It was predicted that P424L and K107E variants are located in PTMs. Consensus results from all computational techniques indicate that P424L variation may be relevant for confirming the structural disruption of the bovine CMAH protein and its association with pathogenesis. Although the MD simulation revealed that mutation A210S might significantly enhance the CMAH protein stability, based on consensus information, the P424L might be the most harmful mutation. The fundamental limitation of this study is that most computational methods utilised were optimised for the human genome. However, similar computational techniques have been successfully used for in silico analysis of SNPs in non-human species [67,68,69]. In vitro analysis, for example, site-directed mutagenesis, is recommended for future studies to validate the impacts of these nsSNPs, particularly for c.1271C>T (P424L).
Acknowledgments
We want to thank Cord Drögemüller of the Institute of Genetics at the Vetsuisse faculty of the University of Bern, Switzerland, for providing sequence data for the analysis of bovine CMAH variants. We also acknowledge financial support by DFG within the funding programme Open Access Publikationsfonds.
Author Contributions
Conceptualisation, O.J.O., O.S.S. and D.B.; writing and data analysis—original draft preparation, O.J.O. and O.S.S.; writing—review and editing, D.B. and G.T.; visualisation, O.J.O.; supervision, D.B. and G.T. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
O.J.O. is supported by the Federal State Funding at the Kiel University, in accordance with the Landesverordnung über die Förderung des wissenschaftlichen und künstleri-schen Nachwuchses (Stipendiumsverordnung—StpVO).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Varki A. Uniquely human evolution of sialic acid genetics and biology. Proc. Natl. Acad. Sci. USA. 2010;107((Suppl. S2)):8939–8946. doi: 10.1073/pnas.0914634107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angata T., Varki A. Chemical diversity in the sialic acids and related α-keto acids: An evolutionary perspective. Chem. Rev. 2002;102:439–470. doi: 10.1021/cr000407m. [DOI] [PubMed] [Google Scholar]
- 3.Kooner A.S., Yu H., Chen X. Synthesis of N-glycolylneuraminic acid (Neu5Gc) and its glycosides. Front. Immunol. 2019;10:2004. doi: 10.3389/fimmu.2019.02004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhar C., Sasmal A., Varki A. From “serum sickness” to “xenosialitis”: Past, present, and future significance of the non-human sialic acid Neu5Gc. Front. Immunol. 2019;10:807. doi: 10.3389/fimmu.2019.00807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magre S., Takeuchi Y., Bartosch B. Xenotransplantation and pig endogenous retroviruses. Rev. Med. Virol. 2003;13:311–329. doi: 10.1002/rmv.404. [DOI] [PubMed] [Google Scholar]
- 6.Matrosovich M., Herrler G., Klenk H.D. Sialoglyco Chemistry and Biology II: Tools and Techniques to Identify and Capture Sialoglycans. Springer; Cham, Switzerland: 2015. Sialic acid receptors of viruses; pp. 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Payne S. Viruses: From Understanding to Investigation. Elsevier; Amsterdam, The Netherlands: 2022. [Google Scholar]
- 8.Varki A., Schauer R. Essentials of Glycobiology. 2nd ed. Cold Spring Harbor Laboratory Press; Cold Spring, NY, USA: 2009. Sialic acids. [PubMed] [Google Scholar]
- 9.Delorme C., Brüssow H., Sidoti J., Roche N., Karlsson K.-A., Neeser J.-R., Teneberg S. Glycosphingolipid binding specificities of rotavirus: Identification of a sialic acid-binding epitope. J. Virol. 2001;75:2276–2287. doi: 10.1128/JVI.75.5.2276-2287.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kyogashima M., Ginsburg V., Krivan H.C. Escherichia coli K99 binds to N-glycolylsialoparagloboside and N-glycolyl-GM3 found in piglet small intestine. Arch. Biochem. Biophys. 1989;270:391–397. doi: 10.1016/0003-9861(89)90042-8. [DOI] [PubMed] [Google Scholar]
- 11.Schwegmann C., Zimmer G., Yoshino T., Enss M.-L., Herrler G. Comparison of the sialic acid binding activity of transmissible gastroenteritis coronavirus and E. coli K99. Virus Res. 2001;75:69–73. doi: 10.1016/S0168-1702(01)00228-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ono E., Abe K., Nakazawa M., Naiki M. Ganglioside epitope recognized by K99 fimbriae from enterotoxigenic Escherichia coli. Infect. Immun. 1989;57:907–911. doi: 10.1128/iai.57.3.907-911.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teneberg S., Willemsen P., de Graaf F.K., Karlsson K.-A. Receptor-active glycolipids of epithelial cells of the small intestine of young and adult pigs in relation to susceptibility to infection with Escherichia coli K99. FEBS Lett. 1990;263:10–14. doi: 10.1016/0014-5793(90)80693-D. [DOI] [PubMed] [Google Scholar]
- 14.Wasik B.R., Barnard K.N., Parrish C.R. Effects of sialic acid modifications on virus binding and infection. Trends Microbiol. 2016;24:991–1001. doi: 10.1016/j.tim.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu X., Dang V.T., Fleming F.E., von Itzstein M., Coulson B.S., Blanchard H. Structural basis of rotavirus strain preference toward N-acetyl-or N-glycolylneuraminic acid-containing receptors. J. Virol. 2012;86:13456–13466. doi: 10.1128/JVI.06975-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Consortium B.H., Gibbs R.A., Taylor J.F., Van Tassell C.P., Barendse W., Eversole K.A., Gill C.A., Green R.D., Hamernik D.L., Kappes S.M. Genome-wide survey of SNP variation uncovers the genetic structure of cattle breeds. Science. 2009;324:528–532. doi: 10.1126/science.1167936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghosh M., Sharma N., Singh A.K., Gera M., Pulicherla K.K., Jeong D.K. Transformation of animal genomics by next-generation sequencing technologies: A decade of challenges and their impact on genetic architecture. Crit. Rev. Biotechnol. 2018;38:1157–1175. doi: 10.1080/07388551.2018.1451819. [DOI] [PubMed] [Google Scholar]
- 18.Charlier C., Coppieters W., Rollin F., Desmecht D., Agerholm J.S., Cambisano N., Carta E., Dardano S., Dive M., Fasquelle C. Highly effective SNP-based association mapping and management of recessive defects in livestock. Nat. Genet. 2008;40:449–454. doi: 10.1038/ng.96. [DOI] [PubMed] [Google Scholar]
- 19.Schaub M.A., Boyle A.P., Kundaje A., Batzoglou S., Snyder M. Linking disease associations with regulatory information in the human genome. Genome Res. 2012;22:1748–1759. doi: 10.1101/gr.136127.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van der Spek D., Van Arendonk J., Bovenhuis H. Genome-wide association study for claw disorders and trimming status in dairy cattle. J. Dairy Sci. 2015;98:1286–1295. doi: 10.3168/jds.2014-8302. [DOI] [PubMed] [Google Scholar]
- 21.Choi Y., Chan A.P. PROVEAN web server: A tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics. 2015;31:2745–2747. doi: 10.1093/bioinformatics/btv195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ng P.C., Henikoff S. Predicting the effects of amino acid substitutions on protein function. Annu. Rev. Genom. Hum. Genet. 2006;7:61–80. doi: 10.1146/annurev.genom.7.080505.115630. [DOI] [PubMed] [Google Scholar]
- 23.Rafaee A., Kashani-Amin E., Meybodi A.M., Ebrahim-Habibi A., Sabbaghian M. Structural modeling of human AKAP3 protein and in silico analysis of single nucleotide polymorphisms associated with sperm motility. Sci. Rep. 2022;12:3656. doi: 10.1038/s41598-022-07513-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang M., Huang C., Wang Z., Lv H., Li X. In silico analysis of non-synonymous single nucleotide polymorphisms (nsSNPs) in the human GJA3 gene associated with congenital cataract. BMC Mol. Cell Biol. 2020;21:12. doi: 10.1186/s12860-020-00252-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bighignoli B., Niini T., Grahn R.A., Pedersen N.C., Millon L.V., Polli M., Longeri M., Lyons L.A. Cytidine monophospho-N-acetylneuraminic acid hydroxylase (CMAH) mutations associated with the domestic cat AB blood group. BMC Genet. 2007;8:27. doi: 10.1186/1471-2156-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gandolfi B., Grahn R.A., Gustafson N.A., Proverbio D., Spada E., Adhikari B., Cheng J., Andrews G., Lyons L.A., Helps C.R. A novel variant in CMAH is associated with blood type AB in Ragdoll cats. PLoS ONE. 2016;11:e0154973. doi: 10.1371/journal.pone.0154973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uno Y., Kawakami S., Ochiai K., Omi T. Molecular characterization of cytidine monophospho-N-acetylneuraminic acid hydroxylase (CMAH) associated with the erythrocyte antigens in dogs. Canine Genet. Epidemiol. 2019;6:9. doi: 10.1186/s40575-019-0076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spruit C.M., Nemanichvili N., Okamatsu M., Takematsu H., Boons G.-J., de Vries R.P. N-glycolylneuraminic acid in animal models for human influenza A virus. Viruses. 2021;13:815. doi: 10.3390/v13050815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alisson-Silva F., Liu J.Z., Diaz S.L., Deng L., Gareau M.G., Marchelletta R., Chen X., Nizet V., Varki N., Barrett K.E. Human evolutionary loss of epithelial Neu5Gc expression and species-specific susceptibility to cholera. PLoS Pathol. 2018;14:e1007133. doi: 10.1371/journal.ppat.1007133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reuven E.M., Leviatan Ben-Arye S., Marshanski T., Breimer M.E., Yu H., Fellah-Hebia I., Roussel J.C., Costa C., Galiñanes M., Mañez R. Characterization of immunogenic Neu5Gc in bioprosthetic heart valves. Xenotransplantation. 2016;23:381–392. doi: 10.1111/xen.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.TruSeq DNA PCR-Free | Simple Prep for Sequencing Complex Genomes. [(accessed on 5 April 2023)]. Available online: https://www.illumina.com/products/by-type/sequencing-kits/library-prep-kits/truseq-dna-pcr-free.html.
- 32.Li H., Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatic. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKenna A., Hanna M., Banks E., Sivachenko A., Cibulskis K., Kernytsky A., Garimella K., Altshuler D., Gabriel S., Daly M., et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cingolani P., Platts A., Wang L.L., Coon M., Nguyen T., Wang L., Land S.J., Lu X., Ruden D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly. 2012;6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayes B.J., Daetwyler H.D. 1000 bull genomes project to map simple and complex genetic traits in cattle: Applications and outcomes. Annu. Rev. Anim. Biosci. 2019;7:89–102. doi: 10.1146/annurev-animal-020518-115024. [DOI] [PubMed] [Google Scholar]
- 36.Raman S., Vernon R., Thompson J., Tyka M., Sadreyev R., Pei J., Kim D., Kellogg E., DiMaio F., Lange O. Structure prediction for CASP8 with all-atom refinement using Rosetta. Proteins. 2009;77:89–99. doi: 10.1002/prot.22540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ko J., Park H., Heo L., Seok C. GalaxyWEB server for protein structure prediction and refinement. Nucleic Acids Res. 2012;40:W294–W297. doi: 10.1093/nar/gks493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laskowski R.A., Jabłońska J., Pravda L., Vařeková R.S., Thornton J.M. PDBsum: Structural summaries of PDB entries. Protein Sci. 2018;27:129–134. doi: 10.1002/pro.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiederstein M., Sippl M.J. ProSA-web: Interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007;35:W407–W410. doi: 10.1093/nar/gkm290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Consortium U. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019;47:D506–D515. doi: 10.1093/nar/gky1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sigrist C.J., De Castro E., Cerutti L., Cuche B.A., Hulo N., Bridge A., Bougueleret L., Xenarios I. New and continuing developments at PROSITE. Nucleic Acids Res. 2012;41:D344–D347. doi: 10.1093/nar/gks1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oates M.E., Romero P., Ishida T., Ghalwash M., Mizianty M.J., Xue B., Dosztanyi Z., Uversky V.N., Obradovic Z., Kurgan L. D2P2: Database of disordered protein predictions. Nucleic Acids Res. 2012;41:D508–D516. doi: 10.1093/nar/gks1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adzhubei I.A., Schmidt S., Peshkin L., Ramensky V.E., Gerasimova A., Bork P., Kondrashov A.S., Sunyaev S.R. A method and server for predicting damaging missense mutations. Nat. Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Capriotti E., Calabrese R., Fariselli P., Martelli P.L., Altman R.B., Casadio R. WS-SNPs&GO: A web server for predicting the deleterious effect of human protein variants using functional annotation. BMC Genom. 2013;14:S6. doi: 10.1186/1471-2164-14-S3-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ng P.C., Henikoff S. SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003;31:3812–3814. doi: 10.1093/nar/gkg509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mi H., Huang X., Muruganujan A., Tang H., Mills C., Kang D., Thomas P.D. PANTHER version 11: Expanded annotation data from Gene Ontology and Reactome pathways, and data analysis tool enhancements. Nucleic Acids Res. 2017;45:D183–D189. doi: 10.1093/nar/gkw1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khan K., Shah H., Rehman A., Badshah Y., Ashraf N.M., Shabbir M. Influence of PRKCE non-synonymous variants on protein dynamics and functionality. Hum. Mol. Genet. 2022;31:2236–2261. doi: 10.1093/hmg/ddac029. [DOI] [PubMed] [Google Scholar]
- 48.Rodrigues C.H., Pires D.E., Ascher D.B. DynaMut: Predicting the impact of mutations on protein conformation, flexibility and stability. Nucleic Acids Res. 2018;46:W350–W355. doi: 10.1093/nar/gky300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ashkenazy H., Abadi S., Martz E., Chay O., Mayrose I., Pupko T., Ben-Tal N. ConSurf 2016: An improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 2016;44:W344–W350. doi: 10.1093/nar/gkw408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang D., Liu D., Yuchi J., He F., Jiang Y., Cai S., Li J., Xu D. MusiteDeep: A deep-learning based webserver for protein post-translational modification site prediction and visualization. Nucleic Acids Res. 2020;48:W140–W146. doi: 10.1093/nar/gkaa275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Capriotti E., Fariselli P., Casadio R. I-Mutant2.0: Predicting stability changes upon mutation from the protein sequence or structure. Nucleic Acids Res. 2005;33:W306–W310. doi: 10.1093/nar/gki375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Halgren T.A. Identifying and characterizing binding sites and assessing druggability. J. Chem. Inf. Model. 2009;49:377–389. doi: 10.1021/ci800324m. [DOI] [PubMed] [Google Scholar]
- 53.Huang B. MetaPocket: A meta approach to improve protein ligand binding site prediction. OMICS A J. Integr. Biol. 2009;13:325–330. doi: 10.1089/omi.2009.0045. [DOI] [PubMed] [Google Scholar]
- 54.DeLano W.L. Pymol: An open-source molecular graphics tool. CCP4 Newsl. Protein Crystallogr. 2002;40:82–92. [Google Scholar]
- 55.Huang J., Rauscher S., Nawrocki G., Ran T., Feig M., de Groot B.L., Grubmüller H., MacKerell A.D. CHARMM36: An improved force field for folded and intrinsically disordered proteins. Biophys. J. 2017;112:175a–176a. doi: 10.1016/j.bpj.2016.11.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Armenta S., Sánchez-Cuapio Z., Munguia M.E., Pulido N.O., Farrés A., Manoutcharian K., Hernandez-Santoyo A., Moreno-Mendieta S., Sánchez S., Rodríguez-Sanoja R. The role of conserved non-aromatic residues in the Lactobacillus amylovorus α-amylase CBM26-starch interaction. Int. J. Biol. Macromol. 2019;121:829–838. doi: 10.1016/j.ijbiomac.2018.10.061. [DOI] [PubMed] [Google Scholar]
- 57.Parolin Schnekenberg R., Perkins E.M., Miller J.W., Davies W.I., D’Adamo M.C., Pessia M., Fawcett K.A., Sims D., Gillard E., Hudspith K. De novo point mutations in patients diagnosed with ataxic cerebral palsy. Brain. 2015;138:1817–1832. doi: 10.1093/brain/awv117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smigielski E.M., Sirotkin K., Ward M., Sherry S.T. dbSNP: A database of single nucleotide polymorphisms. Nucleic Acids Res. 2000;28:352–355. doi: 10.1093/nar/28.1.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Z., Teng S., Wang L., Schwartz C.E., Alexov E. Computational analysis of missense mutations causing Snyder-Robinson syndrome. Hum. Mutat. 2010;31:1043–1049. doi: 10.1002/humu.21310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kehl A., Heimberger K., Langbein-Detsch I., Boehmer S., Raj K., Mueller E., Giger U. Molecular characterization of blood type A, B, and C (AB) in domestic cats and a CMAH genotyping scheme. PLoS ONE. 2018;13:e0204287. doi: 10.1371/journal.pone.0204287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berlow R.B., Dyson H.J., Wright P.E. Functional advantages of dynamic protein disorder. FEBS Lett. 2015;589:2433–2440. doi: 10.1016/j.febslet.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Babu M.M., Kriwacki R.W., Pappu R.V. Versatility from protein disorder. Science. 2012;337:1460–1461. doi: 10.1126/science.1228775. [DOI] [PubMed] [Google Scholar]
- 63.Omi T., Nakazawa S., Udagawa C., Tada N., Ochiai K., Chong Y.H., Kato Y., Mitsui H., Gin A., Oda H. Molecular characterization of the cytidine monophosphate-N-acetylneuraminic acid hydroxylase (CMAH) gene associated with the feline AB blood group system. PLoS ONE. 2016;11:e0165000. doi: 10.1371/journal.pone.0165000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li S., Iakoucheva L.M., Mooney S.D., Radivojac P. Biocomputing 2010. World Scientific; Singapore: 2010. Loss of post-translational modification sites in disease; pp. 337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kotch F.W., Guzei I.A., Raines R.T. Stabilization of the collagen triple helix by O-methylation of hydroxyproline residues. J. Am. Chem. Soc. 2008;130:2952–2953. doi: 10.1021/ja800225k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee J.-W., Bae S.-H., Jeong J.-W., Kim S.-H., Kim K.-W. Hypoxia-inducible factor (HIF-1) α: Its protein stability and biological functions. Exp. Mol. Med. 2004;36:1–12. doi: 10.1038/emm.2004.1. [DOI] [PubMed] [Google Scholar]
- 67.Jacob K.K., Radhika G., Aravindakshan T. An in silico evaluation of non-synonymous single nucleotide polymorphisms of mastitis resistance genes in cattle. Anim. Biotechnol. 2020;31:25–31. doi: 10.1080/10495398.2018.1524770. [DOI] [PubMed] [Google Scholar]
- 68.Ali A., Rehman M.U., Ahmad S.M., Mehraj T., Hussain I., Nadeem A., Mir M.U.R., Ganie S.A. In Silico Tools for Analysis of Single-Nucleotide Polymorphisms in the Bovine Transferrin Gene. Animals. 2022;12:693. doi: 10.3390/ani12060693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shin D., Oh J.-D., Won K.-H., Song K.-D. In silico approaches to identify the functional and structural effects of non-synonymous SNPs in selective sweeps of the Berkshire pig genome. Asian-Australas. J. Anim. Sci. 2018;31:1150. doi: 10.5713/ajas.17.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.