Abstract
Transgenic potato plants expressing the phage T4 lysozyme gene which are resistant to the plant-pathogenic enterobacterium Erwinia carotovora subsp. carotovora have been constructed. The agricultural growth of these potatoes might have harmful effects on soil microbiota as a result of T4 lysozyme release into the rhizosphere. To assess the bactericidal effect of roots, we have developed a novel method to associate the cells of Bacillus subtilis with hair roots of plants and to quantify the survival of cells directly on the root surface by appropriate staining and fluorescence microscopy. With this technique, we found that the roots of potato plants (Désirée and transgenic control lines) without T4 lysozyme gene display measurable killing activity on root-adsorbed B. subtilis cells. Killing was largely independent of the plant age and growth of plants in greenhouse or field plots. Roots from potato lines expressing the T4 lysozyme gene always showed significantly (1.5- to 3.5-fold) higher killing. It is concluded that T4 lysozyme is released from the root epidermis cells and is active in the fluid film on the root surface. We discuss why strong negative effects of T4 lysozyme-producing potatoes on soil bacteria in field trials may not be observed. We propose that the novel method presented here to study interactions of bacteria with roots can be applied not only to bacterial killing but also to interactions leading to growth-sustaining effects of plants on bacteria.
Plant-microbe interactions often involve plant activities to suppress growth of bacteria in the rhizosphere, e.g., by secretion of bactericidal substances (3, 9, 11). Recently, transgenic plants producing antimicrobial agents have been developed. Particularly plants expressing foreign lysozyme genes, including tobacco (15) and potato (8), were constructed. In potato plants, T4 lysozyme gene expression has been used to protect the plants against the phytopathogenic enterobacterium Erwinia carotovora (8), which causes blackleg and soft rot in Solanum tuberosum (12). T4 lysozyme is an endoacetylmuramidase which degrades the murein of the bacterial cell wall by cleavage of the β(1-4) glycosidic bond between N-acetylmuramic acid and N-acetylglucosamine (17). It is active against gram-negative and gram-positive bacteria (4, 16). For ecological evaluation of the use of lysozyme-producing plants in agriculture, a comparison of the microbial community in the rhizosphere of T4 lysozyme-producing and control plants can yield valuable information. In such a study, no significant differences in the aerobic plate counts and functions of beneficial bacteria including indole-3-acetic acid-producing and antagonistic (against E. carotovora and Verticillium dahliae) organisms was found (10). However, the authors point out that isolates of seven antagonistic species were obtained only from the control plants not producing T4 lysozyme (10). To obtain more direct data on whether T4 lysozyme is released from the plants into the soil, a direct and more specific test on lysozyme leakage would be desirable. Previously, the release of antigenic material from the transgenic plant roots acting with anti-T4 lysozyme antibodies was obtained in aquacultures of transgenic plants with T4 lysozyme gene, but actual lysozyme activity could not be determined (4). Leakage of T4 lysozyme from the rhizodermis would be feasible because the transgene-encoded T4 lysozyme is fused to the α-amylase leader peptide, which leads to the export of the protein into the apoplast (7).
The aim of this study was to explore the bacterial killing effect of potato plant roots and to determine whether T4 lysozyme production of plants contributes to killing.
MATERIALS AND METHODS
Bacterial strain and media.
Bacillus subtilis 168 (Marburg strain, DSM 401) was obtained from the Deutsche Sammlung für Mikroorganismen und Zellkulturen (Braunschweig, Germany). B. subtilis was grown in TBY broth (5) at 25 or 30°C as indicated.
Plant material.
Transgenic potato plants (lines DL10 and DL12) were derived from cv. Désirée and contained the T-DNA of the binary vector pSR8-36 (13). Besides an nptII marker gene, this T-DNA carried the gene coding for T4 lysozyme fused to a barley amylase signal peptide-encoding region for secretion into the apoplast. The chimeric gene is controlled by the cauliflower mosaic virus 35S promoter. A control line (DC1) bearing the T-DNA from the binary vector pSR8-30 (6) contained only the nptII+ marker gene. The potato roots were obtained from field plants grown in a sandy loam soil (Groß Lüsewitz, Germany) or from greenhouse plants grown in the same soil.
T4 lysozyme.
The enzyme was purified by affinity chromatography of hexahistidine-tagged protein on a Ni-nitrilotriacetic acid agarose column by Düring (7). The enzyme was dissolved in phosphate-buffered saline (PBS) (14) and stored at 4°C. A stock solution containing 10 mg/ml was used for all experiments. It maintained its full activity over a period of 1 year.
Photometric lysozyme assay.
The assay was essentially performed as described previously (4) except that the cultures were incubated at 30°C and the cells were harvested at a titer of 4 × 108/ml (late log phase). In the linear range of the optical density (OD) decrease (≈0.6 to 0.3) with time, the negative inclination (ΔOD/Δt) of turbidity is proportional to the lytic activity (4).
Fluorescence lysozyme assay with B. subtilis cells in suspension.
B. subtilis was grown by aeration in TBY at 25°C to a titer of 4 × 108/ml, sedimented by centrifugation, and resuspended in PBS. The temperature of 25°C was chosen, because the number of dead cells in cultures grown at 25°C was consistently lower than the number in cultures grown at 30°C. The cell suspension was diluted to a titer of 108/ml and a buffer concentration of 0.3× PBS. Various concentrations of purified T4 lysozyme were added in volumes of 2 μl per 100 μl of cell suspension. Following incubation for various time periods at 25°C, 50 μl of the cell suspension was mixed with 50 μl of 100% glycerol and stained with 60 μM red (propidium iodide) and 10 μM green (SYTO 9) fluorescent nucleic acid stains (final concentrations; LIVE/DEAD BacLight; Molecular Probes, Leiden, The Netherlands). After 15 min in the dark, the fluorescent red and green cells were counted in a fluorescence microscope (Olympus BH2; excitation filter, 495 nm).
Adsorption of T4 lysozyme to roots.
From freshly harvested potato roots, lateral roots about 0.5 to 1 cm long were cut off with a pair of scissors, handled with a pair of forceps, and washed by gentle swirling for 5 s in a volume of 10 ml of 0.5 mM CaCl2. The roots were incubated in 10 μl of PBS containing purified T4 lysozyme (0.1 to 100 μg) at 23°C for 30 min. After addition of 90 μl of PBS, the roots were removed, washed once for 2 s in 100 μl of PBS, and incubated again for 30 min in 100 μl of PBS. The amount of nonadsorbed T4 lysozyme in all three solutions was quantified by the photometric lysozyme assay in order to estimate the amount of T4 lysozyme associated with the roots.
Adsorption of B. subtilis cells to roots and staining on the roots.
B. subtilis was grown by aeration in TBY at 25°C to a titer of 4 × 108/ml (late log phase). For studies on the adsorption of cells to roots in buffer solution, the cells were sedimented by centrifugation and resuspended in 1/20 volume of 0.3× PBS plus spermidine (250 μM), protamine sulfate (100 ng/ml), CaCl2 (250 μM), and leupeptin (350 μM). The latter was added to block unspecific proteolysis. In the main studies on the killing activity of root surfaces, freshly harvested roots were washed for 5 s in 0.5 mM CaCl2 and directly incubated for 1 h at 23°C in 15 μl of a culture of B. subtilis grown in TBY (4 × 108 cells/ml) supplemented with spermidine, protamine sulfate, leupeptin, and CaCl2 at the final concentrations given above. Then the roots with adsorbed B. subtilis cells were transferred into 100 μl of 50% glycerol and stained as described for the fluorescence lysozyme assay with B. subtilis cells in suspension. The fraction of red fluorescent cells in these cultures was 1.7% ± 0.8%.
RESULTS
Quantification of T4 lysozyme activity by fluorescence microscopy.
As a measure of cell killing by lysozyme action, we established a sensitive fluorescence assay (Materials and Methods) in which the cells were stained with fluorescent nucleic acid stains which give bacteria with intact cell membranes a green fluorescence (SYTO 9) and bacteria with irreversibly damaged membranes a red fluorescence (propidium iodide). The killing of B. subtilis 168 cells in suspension was quantified by determining the proportion of red fluorescent cells in a fluorescence microscope. By a parallel plate count assay of T4 lysozyme-treated cells, it was confirmed that the proportion of red cells is a quantitative measure for the dead cells (not shown).
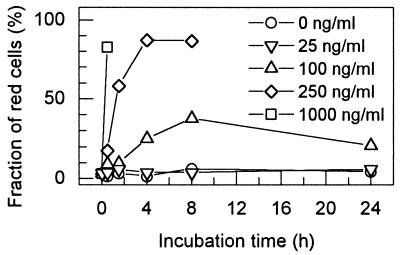
In a time course experiment, the kinetics of killing of B. subtilis by various T4 lysozyme concentrations was determined (Fig. 1). Cell killing was observed at T4 lysozyme concentrations of 100 ng/ml and higher, while at 25 ng/ml killing was not seen even after long incubations. With 250 ng of T4 lysozyme per ml, maximum killing was obtained after 4 h (87% red cells). A concentration of 1,000 ng/ml led to the appearance of 83% red cells within 30 min, after which the killed cells lysed and were no longer detectable (Fig. 1). This occurred similarly at 250 ng/ml after 8 h. The 24-h incubation at 100 ng/ml (38% of cells killed after 8 h) also resulted in lysis of dead cells, and this led to a decline of the fraction of red cells (21%) (Fig. 1). Although Fig. 1 represents the data for a single determination, the killing measurement was found to be very reproducible (see the legend to Fig. 1). These observations indicate that B. subtilis cells are suited as indicator cells to measure T4 lysozyme activity by its killing action in the fluorescence assay. Within a time window of 60 min, concentrations between 100 and 250 ng/ml can be measured (Fig. 1).
FIG. 1.
Killing by T4 lysozyme of B. subtilis 168 cells in 0.3× PBS with 50% glycerol followed by fluorescence microscopy. The suspensions were incubated at various T4 lysozyme concentrations for up to 24 h and stained with the BacLight bacterial viability fluorescence dyes (see Materials and Methods). The fraction of red (dead) cells is given for each time point. Several independent determinations of the fraction of red cells during a 30-min interval with different T4 lysozyme concentrations gave the following data (means with standard deviations): 100 ng/ml, 10.4% ± 5.2% (n = 4); 250 ng/ml, 17.3% ± 6.9% (n = 3); 1,000 ng/ml, 70.5% ± 12.9% (n = 4).
Adsorption of B. subtilis cells to roots.
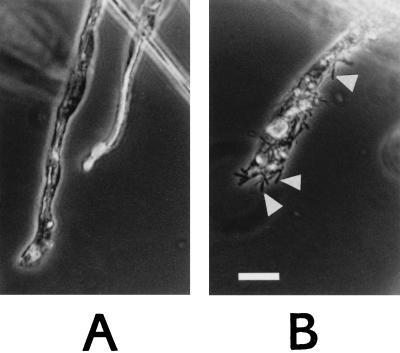
To investigate the bactericidal activity at the surface of potato roots, we developed a protocol to associate B. subtilis cells with the surface of the roots. In PBS, B. subtilis cells did not attach to the roots, possibly because both B. subtilis cells (1) and the roots (2) are negatively charged on their surface. The adsorption of B. subtilis cells in PBS was made possible by the addition of bipolar positively charged molecules (spermidine and protamine sulfate) and CaCl2 to the buffer (see Materials and Methods). Roughly 103 cells per lateral root were bound from the cell suspensions in buffer. Figure 2 shows B. subtilis cells on the surface of root hairs from nontransgenic potato plants. Binding occurred also when roots were incubated directly in supplemented TBY broth culture of B. subtilis. Separate experiments using the photometric assay with T4 lysozyme confirmed that the supplements at the applied concentrations had no effect on the T4 lysozyme sensitivity of the cells (data not shown).
FIG. 2.
Adsorption of B. subtilis 168 cells to roots of greenhouse potato plants. (A) Root after incubation for 60 min in TBY plus supplements; (B) root after incubation for 60 min in B. subtilis culture in TBY broth plus supplements. For details, see Materials and Methods. The scale bar is 25 μm. Bacteria are visible as black rods (arrowheads).
Effect of preadsorbed T4 lysozyme on root-adsorbed B. subtilis cells.
To examine whether T4 lysozyme present on the root surface can cause killing of root-adsorbed B. subtilis cells, we added purified T4 lysozyme to roots of the nontransgenic line Désirée. For this purpose, the roots were incubated in 10 μl of PBS containing 1, 10, or 100 μg of T4 lysozyme for 30 min and washed twice (Materials and Methods). The lysozyme activity in the recovered incubation and wash buffers was quantified by the photometric lysozyme assay. It was found that independently of the added amount of lysozyme, about 25% of the activity (24, 26, or 19% of 1, 10, or 100 μg, respectively) was not recovered from the roots, indicating binding to the roots or inactivation, perhaps by proteolytic cleavage. Subsequently, we adsorbed B. subtilis cells to the washed lysozyme-treated roots and assayed the cell killing activity on the root surface by fluorescence microscopy. Roots to which T4 lysozyme had been added caused increased killing compared to roots without added T4 lysozyme. For example, on a root initially incubated with 10 μg of T4 lysozyme, 81% of the adsorbed cells appeared red, while there were only 30% of red cells on a root without added T4 lysozyme. This suggested that at least a part of the T4 lysozyme which was not recovered from the root supernatants retained its enzymatic activity on the root surface. The data also demonstrated that T4 lysozyme can be active against B. subtilis cells on the root surface.
Bactericidal effect of nontransgenic and transgenic roots of greenhouse plants.
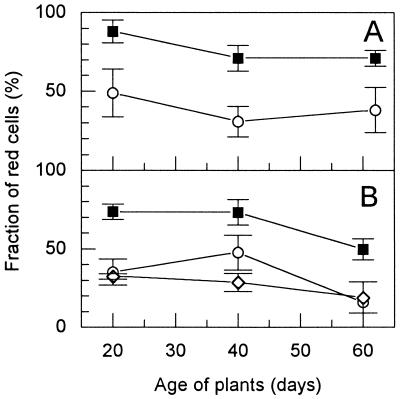
In the following, we measured the bactericidal effect of roots from nontransgenic and transgenic greenhouse plants on root-adsorbed cells of B. subtilis. For each experiment, roots were directly taken from the soil, washed, and incubated in a bacterial culture (see Materials and Methods). Roots were taken at various time intervals after planting of tubers. As shown in Fig. 3A, the nontransgenic line Désirée caused substantial killing of B. subtilis cells 20, 40, and 62 days after planting. However, compared to the Désirée roots, the roots of the transgenic plant DL10 had a significant higher bactericidal activity at each measured time. Similar results were obtained with other plants of Désirée and DL10 which were planted out 3 weeks later (Fig. 3B). A transgenic control line without T4 lysozyme gene (DC1) (Fig. 3B) yielded similar results as Désirée.
FIG. 3.
Killing of B. subtilis 168 cells adsorbed to roots of greenhouse potato plants at various time points after planting out (analyzed by fluorescence microscopy; see Materials and Methods). The tubers were planted out at two different dates (A and B) and were from the lines Désirée (○; nontransgenic), DC1 (◊; transgenic without T4 lysozyme gene), and DL10 (■; transgenic with T4 lysozyme gene). The fraction of red cells corresponds to the fraction of dead cells. The data (with standard deviations) are means of at least four independent experiments. At any time, the values for Désirée and DC1 were not significantly different from each other (t test; P = 0.06 to 0.53) but differed from those for DL10 (P = 10−2 to 4 × 10−6).
When glycerol was omitted during staining of cells on the roots, the killing was apparently lower with both the nontransgenic potato roots (10% red cells instead of 45%) and the transgenic (14% red cells instead of 87%). In control experiments in which nonadsorbed B. subtilis cells were removed from the adsorption mixtures of several transgenic and nontransgenic roots and then stained in the 50% glycerol solution, the fraction of red cells was always at a background level (2.4% ± 0.88% red cells). This showed that the glycerol in the staining solution had no killing effect on cells that were not associated with roots. Thus, after subtraction of the background of dead cells, in the absence of glycerol, cell killing of T4 lysozyme roots was 1.5-fold higher than that of the control roots, while in the presence of glycerol this ratio was 1.9. We concluded that the presence of glycerol during cell staining on roots did not strongly affect the relative killing potential of the roots but increased considerably the sensitivity of the killing assay.
Studies with field plants.
To examine whether the bactericidal effect found with roots of greenhouse plants is also observed with field plants, we assayed the roots of several 40-day-old transgenic and nontransgenic plants from an agricultural plot. The results are shown in Table 1. The roots of the plants without T4 lysozyme gene (nontransgenic line Désirée and transgenic line DC1) had a significantly lower bactericidal activity than the roots of the T4 lysozyme-expressing plants (lines DL10 and DL12). There was no significant difference between the lines DL10 and DL12 and between lines Désirée and DC1, while the differences between lines with and without lysozyme gene were always significant (Table 1). These results are consistent with the results obtained with the greenhouse plants and indicate that expression of the T4 lysozyme gene increases the bactericidal activity of the plant roots 1.5- to 3.5-fold (Fig. 3 and Table 1).
TABLE 1.
Killing of B. subtilis 168 cells on roots from field plot potatoes, harvested 40 days after planting out of tubers
| Line | T4 lysozyme gene | Fraction of red cells (mean % ± SD)a | No. of individual plants tested |
|---|---|---|---|
| DL10 | + | 45.5 ± 3.8 | 5 |
| DL12 | + | 55.5 ± 11.4 | 3 |
| Désirée | − | 15.9 ± 2.9 | 4 |
| DC1 | − | 20.7 ± 8.3 | 6 |
Statistical significance (t test, P values) of pairwise comparisons: DL10-DL12, 0.11; Désirée-DC1, 0.30; DL10-Désirée, 3.9 × 10−6; DL10-DC1, 1.8 × 10−4; DL12-Désirée, 10−3; DL12-DC1, 1.1 × 10−3.
DISCUSSION
In this investigation, we studied the killing effect of potato roots on root-adsorbed B. subtilis cells. With the methods developed for this study, particularly for the adsorption of B. subtilis cells to the roots of potato plants and the fluorescence microscopic survival analysis of the bacteria on the root surface, we demonstrated that the bactericidal effect of roots can be visualized and quantified directly on the root. Interestingly, we observed that the roots of lines without T4 lysozyme gene produced considerable cell killing. This may be the result of bactericidal substances naturally released from the plant roots, which may include toxic components like benzofurans, terpenoids, butyrolactones, and other phytoalexins (2, 11). The fact that the roots of T4 lysozyme gene-expressing plants had a significantly higher bactericidal effect suggests that T4 lysozyme is released from the root cells and that it is active as a bactericide on the root surface, presumably by its muramidase activity. The fusion of the α-amylase leader peptide to the T4 lysozyme results in the presence of the enzyme in the apoplast and probably facilitates the release of enzyme from the root by diffusion. This would be consistent with the previously reported detection of the T4 lysozyme antigen in the root growth solution of transgenic plants by Western blotting (4). The hyperosmotic condition during the fluorescence staining (50% glycerol) perhaps accelerated the release of T4 lysozyme from the apoplast. We assume that the released enzyme is mainly active in a thin liquid film covering the root surface. Considering the fact that T4 lysozyme makes up 0.0005 to 0.0007% of total soluble protein in transgenic potato plants (K. Düring and A. Mahn, personal communication), it is conceivable that T4 lysozyme concentrations of about 0.1 μg/ml and higher can be reached in this film. The in vitro experiments indicated that considerable killing of B. subtilis cells occurs at these concentrations (Fig. 1). Further, it is possible that the background bactericidal effect of plant roots could act synergistically with the T4 lysozyme, so that relatively low T4 lysozyme concentrations would elicit the rather strong killing by DL10 and DL12 roots.
It is known that plant defense against pathogens includes both permanent and inducible defense barriers (9, 11). A somewhat variable but parallel run of the killing curves by T4 lysozyme-producing and -nonproducing plants is apparent in separate experiments (Fig. 3). This suggests that the variations in bactericidal background, i.e., the bactericidal effect of the nontransgenic plants, resulted from variations in the plant culture conditions and may be dependent on complex regulation mechanisms. This may also explain the generally somewhat lower bactericidal activity of the field plant roots than of roots of the greenhouse plants, although the latter were grown in the same soil (Table 1 and Fig. 3). The difference between the background bactericidal effect of the lines Désirée and DC1 (without T4 lysozyme gene) and the bactericidal effect of the T4 lysozyme-producing lines DL10 and DL12 was nearly the same under the different conditions (36.2% ± 6.0% for the greenhouse plants and 32.3% ± 6.4% for the field plants) (Fig. 3 and Table 1). This indicates that the killing effect of the T4 lysozyme is rather constant and independent of the culture conditions. It should be noted that the T4 lysozyme gene in the transgenic plants is controlled by the 35S promoter of cauliflower mosaic virus, which provides constitutive expression (9).
The question whether the T4 lysozyme produced and released by the transgenic plants in the field would produce harmful effects on soil bacteria cannot be answered by our studies, although they demonstrate a negative effect on a single soil bacterial strain in the experimental setup. In a study on the plant-beneficial bacteria in the rhizosphere of T4 lysozyme-producing and control plants, it was found that the apparent abundance and diversity differed in these habitats, but a clear correlation with T4 lysozyme release from the roots was not found (10). It is conceivable that the released enzyme is rapidly inactivated in soil either by chemical conditions not previously detected when the T4 lysozyme activity was measured in aqueous soil extracts (4) or by adsorption to solid surfaces. If this were the case, strong effects of T4 lysozyme release from the potato plants on the soil bacterial community would not be expected although most of the tested soil bacteria turned out to be sensitive to T4 lysozyme in vitro (4).
The demonstration of killing of root-adsorbed B. subtilis cells by potato plant roots directly detects a plant-microbe interaction. Other plant-microbe interactions may also be analyzed by the cell adsorption method, such as the action of other bactericidal substances like phytoalexins produced by wounded tissues. Under the conditions described in this study, the association of B. subtilis cells with roots of other soil-grown plants including Arabidopsis thaliana, Zea mays, Alnus glutinosa, and Nerium oleander has been observed (I. Ahrenholtz, J. de Vries, and W. Wackernagel, unpublished data), suggesting broad applicability of the method. Growth-promoting effects of plant roots on bacteria may also be quantified by a modification of our protocol, which would include removal of the bacteria from the roots after incubation and the subsequent determination of their growth or metabolic activity.
ACKNOWLEDGMENTS
We thank K. Düring for the purified T4 lysozyme.
This work was supported by the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie and the Fonds der Chemischen Industrie.
REFERENCES
- 1.Archibald A R, Hancock I C, Harwood C R. Cell wall structure, synthesis, and turnover. In: Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria. Washington, D.C.: American Society for Microbiology; 1993. pp. 381–410. [Google Scholar]
- 2.Bowen G D, Rovira A D. The rhizosphere. In: Waisel Y, Eshel A, Kafkafi U, editors. Plant roots—the hidden half. New York, N.Y: Marcel Dekker, Inc.; 1991. pp. 641–669. [Google Scholar]
- 3.Clarke H R, Leigh J A, Douglas C J. Molecular signals in the interactions between plants and microbes. Cell. 1992;71:191–199. doi: 10.1016/0092-8674(92)90348-g. [DOI] [PubMed] [Google Scholar]
- 4.de Vries J, Harms K, Broer I, Kriete G, Mahn A, Düring K, Wackernagel W. The bacteriolytic activity in transgenic potatoes expressing a chimeric T4 lysozyme gene and the effect of T4 lysozyme on soil- and phytopathogenic bacteria. Syst Appl Microbiol. 1999;22:280–286. [Google Scholar]
- 5.de Vries J, Wackernagel W. Recombination and UV resistance of Escherichia coli with the cloned recA and recBCD genes of Serratia marcescens and Proteus mirabilis: evidence for an advantage of intraspecies combination of P. mirabilis RecA protein and RecBCD enzyme. J Gen Microbiol. 1992;138:31–38. doi: 10.1099/00221287-138-1-31. [DOI] [PubMed] [Google Scholar]
- 6.Düring K. A plant transformation vector with a minimal T-DNA. Transgenic Res. 1994;3:138–140. doi: 10.1007/BF01974093. [DOI] [PubMed] [Google Scholar]
- 7.Düring K. A tightly regulated system for overproduction of bacteriophage T4-lysozyme in Escherichia coli. Protein Expr Purif. 1993;4:412–416. doi: 10.1006/prep.1993.1054. [DOI] [PubMed] [Google Scholar]
- 8.Düring K, Porsch P, Fladung M, Lörz H. Transgenic potato plants resistant to the phytopathogenic bacterium Erwinia carotovora. Plant J. 1993;3:587–598. [Google Scholar]
- 9.Garcia-Olmedo F, Molina A, Alamillo J M, Rodriguez-Palenzuela P. Plant defense peptides. Biopolymers. 1998;47:479–491. doi: 10.1002/(SICI)1097-0282(1998)47:6<479::AID-BIP6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 10.Lottmann J, Heuer H, Smalla K, Berg G. Influence of transgenic T4-lysozyme-producing potato plants on potentially beneficial plant-associated bacteria. FEMS Microbiol Ecol. 1999;29:365–377. [Google Scholar]
- 11.Mansfield J W. Antimicrobial compounds. In: Callow J A, editor. Biochemical plant pathology. Chichester, United Kingdom: John Wiley & Sons; 1983. pp. 237–265. [Google Scholar]
- 12.Perombelon M C M, Kelman A. Ecology of the soft rot Erwinias. Annu Rev Phytopathol. 1980;18:361–387. [Google Scholar]
- 13.Porsch P, Jahnke A, Düring K. A plant transformation vector with a minimal T-DNA. II. Irregular integration patterns of the T-DNA in the plant genome. Plant Mol Biol. 1998;37:581–585. doi: 10.1023/a:1006035500546. [DOI] [PubMed] [Google Scholar]
- 14.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 15.Trudel J, Potvin C, Asselin A. Expression of active hen egg white lysozyme in transgenic tobacco. Plant Sci. 1992;87:55–67. [Google Scholar]
- 16.Tsugita A. Phage lysozyme and other lytic enzymes. In: Boyer P D, editor. The enzymes. Vol. 5. New York, N.Y: Academic Press; 1971. pp. 343–411. [Google Scholar]
- 17.Tsugita A, Inouye M, Terzaghi E, Streisinger G. Purification of bacteriophage T4 lysozyme. J Biol Chem. 1968;243:391–397. [PubMed] [Google Scholar]