Abstract
Background
AMBRA1 is an intrinsically disordered protein, working as a scaffold molecule to coordinate, by protein-protein interaction, many cellular processes, including autophagy, mitophagy, apoptosis and cell cycle progression. The zebrafish genome contains two ambra1 paralogous genes (a and b), both involved in development and expressed at high levels in the gonads. Characterization of the zebrafish paralogous genes mutant lines generated by CRISPR/Cas9 approach showed that ambra1b knockout leads to an all-male population.
Results
We demonstrated that the silencing of the ambra1b gene determines a reduction of primordial germ cells (PGCs), a condition that, in the zebrafish, leads to the development of all-male progeny. PGC reduction was confirmed by knockdown experiments and rescued by injection of ambra1b and human AMBRA1 mRNAs, but not ambra1a mRNA. Moreover, PGC loss was not rescued by injection with human AMBRA1 mRNA mutated in the CUL4-DDB1 binding region, thus suggesting that interaction with this complex is involved in PGC protection from loss. Results from zebrafish embryos injected with murine Stat3 mRNA and stat3 morpholino suggest that Ambra1b could indirectly regulate this protein through CUL4-DDB1 interaction. According to this, Ambra1+/− mice showed a reduced Stat3 expression in the ovary together with a low number of antral follicles and an increase of atretic follicles, indicating a function of Ambra1 in the ovary of mammals as well. Moreover, in agreement with the high expression of these genes in the testis and ovary, we found significant impairment of the reproductive process and pathological alterations, including tumors, mainly limited to the gonads.
Conclusions
By exploiting ambra1a and ambra1b knockout zebrafish lines, we prove the sub-functionalization between the two paralogous zebrafish genes and uncover a novel function of Ambra1 in the protection from excessive PGC loss, which seems to require binding with the CUL4-DDB1 complex. Both genes seem to play a role in the regulation of reproductive physiology.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40659-023-00430-9.
Keywords: Ambra1, PGCs, Sex differentiation, Reproduction, Zebrafish, Mouse
Background
AMBRA1 (Activating Molecule in Beclin-1-Regulated Autophagy Protein 1) is a multifunctional scaffold protein whose intrinsically disordered structure allows high protein-protein interaction plasticity, resulting in its involvement in a plethora of different and critical cellular pathways including autophagy, mitophagy, apoptosis and cell cycle progression [1–10]. The protein was initially identified by its crucial role in regulating neurogenesis and neural tube closure [3, 11, 12]. While its knockout is embryonically lethal in mice [10, 11], heterozygous Ambra1+/− mice are characterized by pre-diabetic conditions [13], autism-like phenotype limited to female sex [14] and higher cancer susceptibility [5].
The zebrafish genome contains two ambra1 paralogous genes, both involved in the autophagic process as well as in neural, muscular, and cardiac development, as demonstrated by morpholino (MO) knockdown [15–17]. Since data that can be obtained by knockdown approaches are limited to the first developmental stages, we recently generated a mutant zebrafish line for each paralogous gene. ambra1aia35 (called ambra1a−/− hereafter) and ambra1bia36 (called ambra1b−/− hereafter) mutants do not display overt developmental defects, due to the activation of genetic compensation mechanisms with up-regulation of the paralogous gene remaining active [17]. Conversely, the generation of an ambra1a−/− /ambra1b−/− mutant line was not possible as double mutants cannot survive after larval stages [17]. In agreement with the embryonic lethality of Ambra1 inactivation in the mouse models [10, 11], this result suggests that silencing of both paralogous ambra1 genes is incompatible with life. Despite the lack of morphological differences between ambra1a−/− and ambra1b−/− fish lines, ambra1b homozygous mutants develop exclusively as males following the achievement of sexual maturity, whereas heterozygous adult ambra1b+/− and ambra1a mutants do not display sex ratio alterations [17].
Considering the different effects of ambra1a and ambra1b on sexual differentiation, we decided to investigate the role of Ambra1 in this process. Sex determination in Danio rerio domesticated lines is controlled by a polygenic system and environmental factors, such as temperature or social cues [18]. Zebrafish gonads initially develop as a bipotential organ containing germ cells and immature oocytes. At 20–25 dpf (days post fertilization), during sex-specific differentiation, the immature oocytes of presumptive females progress through oogenesis, giving rise to adult females. Conversely, in the presumptive males, the immature oocytes undertake apoptosis, and the gonads develop as testis. Therefore, the number of oocytes and their molecular signalling are considered to play critical roles in sexual development and represent a prerequisite for ovary formation [18].
In addition, experimental reduction of primordial germ cell (PGC) number during the first day of development produces all-male progeny with normal testis, while the total ablation of PGCs generates males with somatic gonad cells organized as testis but devoid of sperm [19]. Hence, as previously demonstrated [20], ovarian development is promoted by abundance of PGCs also at early developmental stages. Moreover, some proteins, such as Dead end and Nanos3, are involved in the control of PGC survival and migration, and their silencing results in all-male development [21].
In this study, we focused our attention on the possible role of Ambra1b on zebrafish sex determination and found that the all-male phenotype of ambra1b mutants relies on PGC reduction during early developmental stages. Remarkably, the PGC loss could be rescued with human AMBRA1 (hAMBRA1) mRNA, but not with the hAMBRA1 mRNA mutated in the CUL4-DDB1 complex binding domain, suggesting a possible interaction with this complex in regulating PGC development. Altogether, the high expression of Ambra1 proteins in zebrafish and mouse ovaries and the impairment of zebrafish reproductive capabilities unveil a new role of Ambra1 in reproductive physiology.
Results
High expression of ambra1a and ambra1b in zebrafish gonads points to a role in reproduction
The maintenance of both ambra1a and ambra1b paralogous genes in zebrafish and the absence of female individuals in the ambra1b knockout (KO) line suggested a sub-functionalization of these paralogs in zebrafish after the ancestral genome duplication [17], and possibly a different tissue-specific expression of the two paralogs. To verify this, we performed RT-qPCR on adult wild-type (WT) fish to assess the expression levels in different tissues (brain, intestine, liver, muscle, ovary, and testis). As reported in Fig. 1A, this analysis confirmed the high and comparable level of expression of both genes in the brain, in agreement with the expression and function of Ambra1 in adult mouse brain [22, 23], whereas expression was very low in intestine and different between the two paralogs in liver and muscle, with a higher level of ambra1a in liver and of ambra1b in muscle. Furthermore, both paralogs displayed high expression levels in ovary and, although in smaller amounts, in testis, suggesting that both genes may play a role in zebrafish reproductive processes (Fig. 1A).
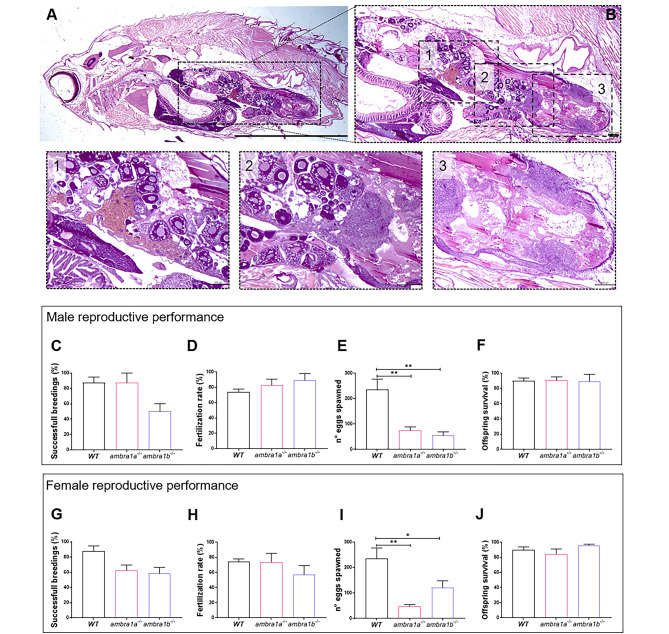
Fig. 1.
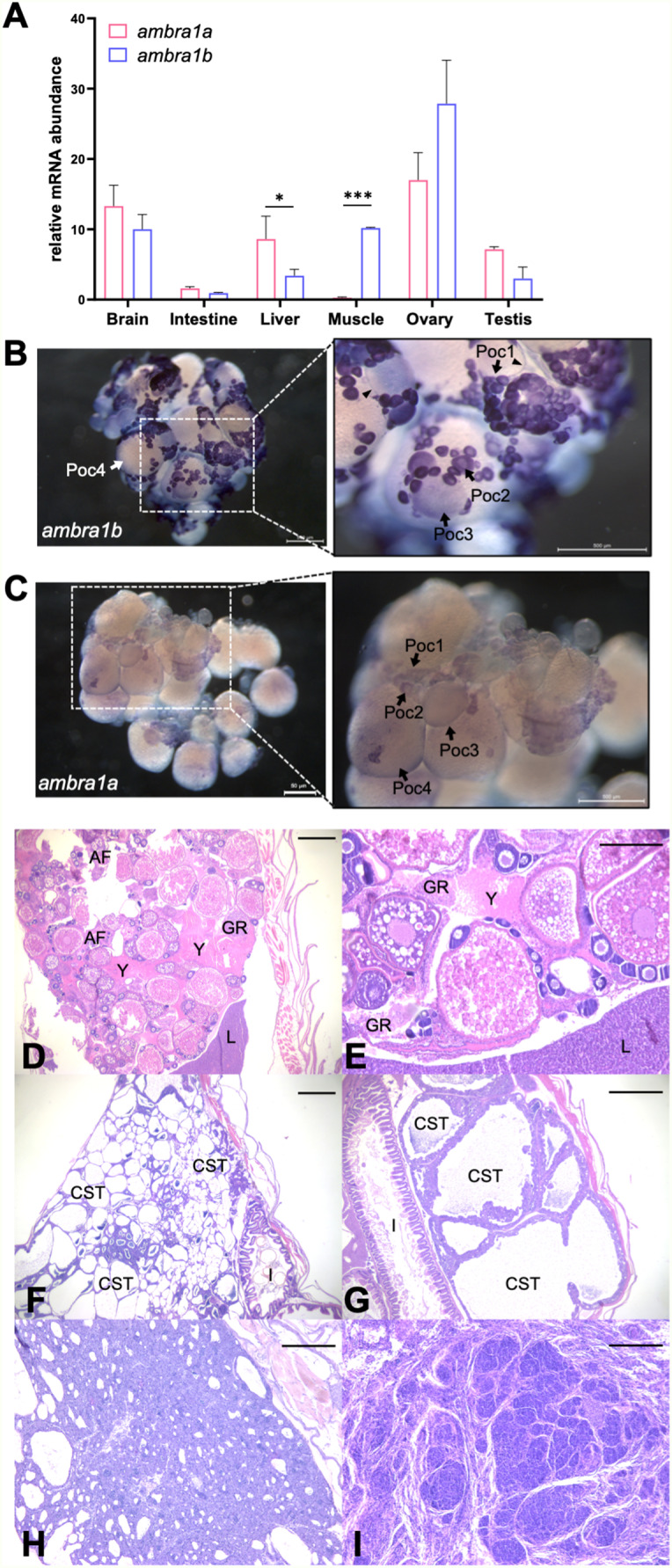
Expression patterns of ambra1 mRNAs and pathological findings in the zebrafish ambra1a and ambra1b KO lines. (A) RT-qPCR analysis of ambra1a and ambra1b mRNA expression in different adult zebrafish tissues. Data were generated from different biological replicates, each consisting of tissue samples from single individual (intestine, liver, ovary n = 4; brain, muscle n = 3; testis n = 2; intestine, liver, brain and muscle were a mix of female and male tissues). qPCR data were analyzed by 2−∆CT method and actb2 was selected as reference gene for normalization. Values represent the mean ± SEM. Statistical analysis was performed using Student’s t-test. * P < 0.05; *** P < 0.001. (B, C) Whole mount in situ hybridization with ambra1b (B) and ambra1a probes (C) in ovaries of 6-month-old WT zebrafish. Arrowheads point at somatic cells surrounding oocytes. Poc1, primary oocytes stage 1; Poc2, primary oocytes stage 2; Poc3, previtellogenic oocytes; Poc4, vitellogenic oocytes. (D) Representative ovary of a 18-mpf ambra1a−/− female showing follicular degeneration and presence of yolk free in the coelomic cavity as a result of degenerated follicles (H&E; scale bar, 200 μm). (E) Higher magnification of panel D, showing a moderate granulomatous reaction towards proteinaceous material (H&E; scale bar, 400 μm). (F) Testis of a 20-mpf ambra1b−/− male, showing diffuse cystic degeneration of the coelomic cavity (H&E; scale bar, 400 μm). (G) Testis of a 15-mpf ambra1b−/− male, showing diffuse ectasia of seminiferous tubules and hyperplasia of spermatogonia lining the tubules (H&E; scale bar, 200 μm). (H) Testis of 18-mpf ambra1a−/− male, displaying seminoma (H&E; scale bar, 200 μm). (I) Testis of a 17-mpf ambra1b−/− male, containing an undifferentiated germ cell tumor (H&E; scale bar, 200 μm). AF, atretic follicle; CST, cystic seminiferous tubule; GR, granulomatous reaction; I, intestine; L, liver; Y, yolk.
As both paralogous genes were highly expressed in the ovary, a whole mount in situ hybridization on adult WT ovaries was also performed to assess whether the two transcripts were differently localized in this organ. The analysis confirmed the RT-qPCR results, showing a higher expression of ambra1b transcripts (Fig. 1B,C). However, while ambra1b mRNA was strongly expressed in stage 1 and stage 2 primary oocytes (Fig. 1B), the ambra1a signal was found at lower levels in these cells but was maintained at more advanced stages of oocyte maturation such as previtellogenic and vitellogenic oocytes (Fig. 1C). ambra1b expression was also displayed by somatic cells surrounding previtellogenic oocytes (Fig. 1B). These data suggested a possible role for Ambra1b at the early stages of ovarian follicle development with respect to Ambra1a, whose expression seems to be maintained, although at low levels, in the maturing oocytes. This latter result is also in agreement with the higher expression of ambra1a mRNA in ovulated oocytes compared to ambra1b, as previously demonstrated [15].
We then assessed the occurrence of morphological alteration due to the genetic ablation of the two ambra1 paralogs and carried out histological analyses on ambra1a−/− (n = 14; 9 males and 5 females) and ambra1b−/− (n = 30, 26 males and 4 females) individuals sacrificed at different ages, from 3 to 20 mpf (months post fertilization). WT (n = 19, 10 males and 9 females) were also analysed for comparative purposes.
The main pathological signs displayed by the different groups of mutants were found in individuals older than 12 mpf and, in agreement with expression data, morphological changes were mainly located in the gonads in both males and females. Pathological findings include a plethora of different changes, ranging from testicular and ovarian degeneration to inflammation and testicular neoplasia. Other phenotypic features, not related to gonads, included mild hepatic degeneration with intracytoplasmic hepatocellular vacuolar changes and skeletal muscle defects, which were constantly present, albeit at different extents, in all the examined groups except for WT subjects. The main phenotypic features of the ovarian tissue were represented by degeneration of follicles, often associated with mineralization and with an occasional inflammatory chronic granulomatous reaction towards follicular components (mainly yolk) released in the coelomic cavity (Fig. 1D,E).
Degenerative cystic changes of the seminiferous tubules were observed in two subjects, in absence of atypia (20-mpf ambra1b−/−, Fig. 1F), or only associated to a moderate dysplasia and hyperplasia of spermatogonia (15-mpf ambra1b−/−, Fig. 1G). Tumors of the germ cells lineage were found, ranging from well-differentiated seminomas (one 18-mpf ambra1b−/− and one ambra1a−/− at the same age, Fig. 1H) to undifferentiated germ cell tumor with infiltration of adjacent coelomic organs (17-mpf ambra1b−/−, Fig. 1I). Altogether, we identified one seminoma in a total of three ambra1a−/− males and four pathological changes of the testis, two of which were cancerous, in 12 ambra1b−/− males over one year of age.
Both ambra1 mutant lines display reduced reproductive capability
Although the main feature of the ambra1b mutant line is the lack of females, one single fish out of more than four hundred ambra1b−/− obtained with the line propagation developed as female (less than 0,25%) and can thus be considered a “mutant escaper”. This individual (generation 1, dead at 20 mpf) displayed the classic female phenotypic features and was able to reproduce with ambra1b−/− males in 2 out of 7 reproductive trials. The two reproductive events resulted in 53 adults, of which only 10 (19%) developed as females (generation 2). One individual of generation 2 was sacrificed at 16 mpf to perform histological analysis, which revealed the presence of all oocytes stages (Fig. 2A,B). Moreover, together with classical asynchronous development, the ovary displayed large areas of tissue degeneration, celomatic egg retention and granulomatous inflammation (Fig. 2A,B). One female from generation 2 was used to perform reproductive trials with other ambra1b−/− males, generating generation 3, in which we found three females up to a total of 14 adult individuals. While we still do not know which compensative process allowed the development of the first escaper, it is clear that ambra1b−/− females produce a sex-imbalanced offspring. Moreover, the ovaries of ambra1b−/− showed clear and consistent morphological alterations, as demonstrated by histological analysis of three more female individuals (data not shown).
Fig. 2.
KO of ambra1a and ambra1b reduces the reproductive capabilities of zebrafish in both sexes. (A) Representative histological analysis of 16-mpf ambra1b−/− female, showing H&E staining of total body sagittal section. Scale bar, 5 mm. (B) Magnification of the dotted area of panel A, showing the ovary region. Scale bar, 400 μm. The lower panels show higher magnifications of the respective dotted areas (1–3), corresponding to different regions of the ovary. Scale bar, 200 μm. (C-J) Quantification of the reproductive performance of WT, ambra1a−/− and ambra1b−/− males (C-F) and females (G-J). Panels C and G show the percentage of spawning success in mutant male and female tests, respectively. Panels D and H show the percentage of fertilized eggs on the total number of eggs. Panels E and I show the mean number of eggs spawned in successful breeding events. Panels F and J show the percentage of survival of the offspring at 5 dpf. Males: ambra1a−/− n = 4; ambra1b−/− n = 4; WT n = 4. Females: ambra1a−/− n = 4; ambra1b−/− n = 3; WT n = 4. Error bars indicate SEM. Statistical analysis was performed using One way ANOVA. * P < 0.05; ** P < 0.01
Considering the high expression level of both ambra1a and ambra1b in ovaries and testes, the morphological alteration in gonads and the availability of ambra1b−/− females (from the female escaper described above), we assessed the reproductive capabilities of ambra1a−/− and ambra1b−/− males and females. Towards this aim, we used four 10-mpf zebrafish from both mutant lines (except for ambra1b−/− females in which only three 10-mpf individuals were available) and compared them to WT zebrafish of the same age. Every animal was bred with WT individuals. The analysis of male reproductive capabilities showed a reduction, although not significant, of ambra1b−/− male reproductive success compared to WT and ambra1a−/− males (Fig. 2C). This could not be ascribed to problems with egg fertilization (Fig. 2D) or offspring survival, since these parameters were not different from WT crosses (Fig. 2F). Moreover, in both mutant lines we observed a significant reduction in the number of eggs released by their WT female partners (Fig. 2E, ambra1a−/−, p = 0,0032, ambra1b−/−, p = 0,0015).
The three ambra1b−/− females of generation 3 obtained as described above were used to assess the reproductive abilities of ambra1b−/− females in parallel with four ambra1a−/− females. Reproductive capabilities were reduced in both mutant lines, although not significantly (Fig. 2G). WT males could fertilize the eggs released by mutant females and the embryos reached 5-dpf stage (Fig. 2H,J). Again, both lines showed a clear reduction in the number of eggs released per reproductive event, compared to WT (Fig. 2I, ambra1a−/−, p = 0,0024, ambra1b−/−, p = 0,0470).
The lack of ambra1b−/− females is not due to female-specific lethality and is associated with delayed gonadal development
As previously shown, ambra1b−/− individuals, obtained from heterozygous ambra1b crosses, appear to be all males [17]. To exclude the possibility of female-specific lethality, we analyzed the Mendelian proportion of a breeding between female ambra1b+/− and male ambra1b−/−. The progeny was raised to adulthood and their sex determined according to zebrafish sexually dimorphic phenotypic traits: body shape, dimorphic colour of the anal fin and abdomen, and appearance of the genital pore with the prominent genital papilla in females [18]. Among the 65 adult zebrafish that resulted from this breeding, 48% were heterozygotes with an equal proportion of male and female (ratio 1:1, female to male), and 52% were homozygotes and all males (ratio 0:2, female to male). This result had strong statistical support (χ2 test with a p-value < 0.001) and excluded the possibility of female lethality, since the ratio predicted in this case should be 1 (ambra1b+/− females) ∶ 1 (ambra1b+/− males) ∶ 0 (ambra1b−/− females) ∶ 1 (ambra1b−/− males), leading to 66% heterozygotes and 33% homozygous mutants.
Knowing that zebrafish has a window of sex determination in which the sex is decided by a system that integrates the expression of different specific genes with environmental factors [24], we performed histological analysis of gonads on 35-dpf juveniles obtained from the crossing of heterozygous female with homozygous male of both ambra1 lines. In zebrafish, juveniles develop a “juvenile or presumptive ovary”, a bipotential gonad that histologically resembles an immature ovary. Depending on multiple signals, this structure becomes a functional ovary in about 50% of individuals. In the remaining half of individuals, the juvenile ovary degenerates, and immature oocytes undergo apoptosis while gonads start developing as testis at around 30 dpf. Then, at the 35-dpf stage, the fate decision toward the male or female phenotype has normally already been taken [25].
We analysed 12 individuals of each of the following genotypes, ambra1a−/−, ambra1b+/− and ambra1b−/−, as well as 6 WT at the same age and classified the gonads of these juveniles as undifferentiated (without any sign of differentiation towards one or the other sex), juvenile ovary, juvenile ovary to testis transition, and ovary (Additional file 1, Fig. A1, A-D). As shown in Table 1, we could find ovaries and juvenile ovaries to testis transition in WT, ambra1b+/− and ambra1a−/−. On the other hand, all the ambra1b−/− individuals presented undifferentiated gonads or juvenile ovaries, but not gonads developing into female structures. These results confirmed the lack of females only in ambra1b−/− and also pointed at a general delay in gonadal differentiation, as demonstrated by the high number of undifferentiated gonads found in heterozygous ambra1b+/− and in homozygous ambra1a−/− larvae. The time window of sex differentiation depends on rearing condition and diet, but since no undifferentiated gonads were found in WT larvae with this analysis, the delay appeared to be correlated with ambra1 KO.
Table 1.
Histological analysis of 35-dpf zebrafish gonads
| Undifferentiated gonad | Juvenile gonad | Juvenile ovary to testis transition | Ovary | |
|---|---|---|---|---|
| ambra1a −/− | 3/12 | 5/12 | 2/12 | 2/12 |
| ambra1b +/− | 5/12 | 3/12 | 2/12 | 2/12 |
| ambra1b −/− | 6/12 | 6/12 | - | - |
| WT | - | 1/6 | 2/6 | 3/6 |
To confirm the all-male ambra1b−/− phenotype, histological analysis was performed on 6 ambra1b−/− individuals at 60 dpf. No ovaries were found, and all individuals presented a well-organized testis (Additional file 1, Fig. A1, E).
Table 1
Summary of the data obtained with the histological analysis of 35-dpf zebrafish sibling of the two ambra1 lines and WT samples. The numbers reported refer to the number of individuals presenting a specific type of gonad out of the total number of fish analysed.
Ambra1b loss causes a reduction of PGCs resulting in the all-males phenotype
In zebrafish, experimental reduction of PGC number at early developmental stages or their complete ablation has been shown to produce adult males with normal or sperm-free testis, respectively [19]. The loss of germ cells in the form of primary oocytes, on the other hand, occurs in juvenile ovaries during sex determination and differentiation. At this stage, the balance between low or high apoptosis levels is supposed to control oocytes survival and the maturation of a female or a male gonad [26]. While the loss of oocytes in juvenile gonads is part of the normal process of sex differentiation to testis, an early decrease of PGCs during the first developmental stages can be due to the loss of fundamental maternal or zygotic instructions, resulting in the formation of all-male adults that can suffer from fertility problems [27] Starting from these data, we sought to assess whether the absence of females in ambra1b−/− was due to a reduction of PGCs by visualizing and counting the PGCs using whole-mount immunohistochemistry against the Vasa protein, a specific marker of germ cells [27]. This analysis was performed with 10-hpf (hours post fertilization) embryos obtained by the mating of ambra1a and ambra1b heterozygous parents. Interestingly, while we found a statistically significant reduction of the number of PGCs in ambra1b−/− embryos compared to ambra1b+/− (27% reduction, p = 0,011) and ambra1b+/+ (30% reduction, p = 0,0237) siblings (Fig. 3B), no reduction in PGCs was detected in the ambra1a−/− embryos (Fig. 3A).
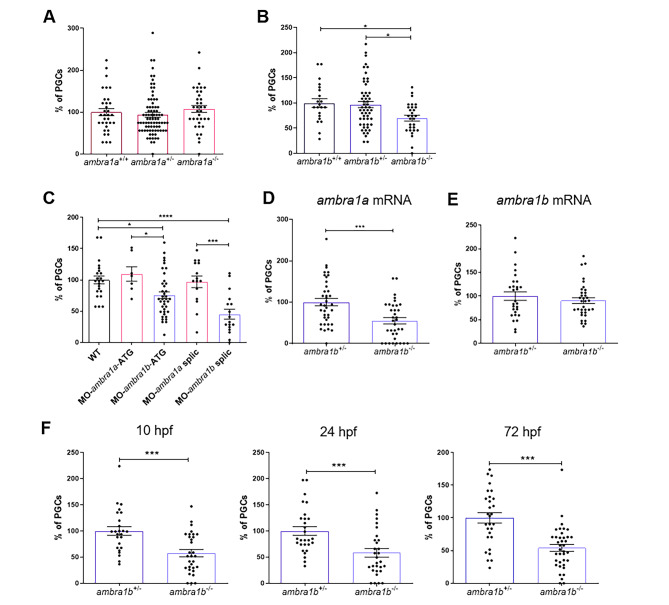
Fig. 3.
KO of ambra1b leads to decreased PGC number. (A, B) Quantification of PGCs in 10-hpf embryos of ambra1a (A), ambra1b (B) genotypes, expressed as percentage of PGCs in heterozygous and homozygous siblings compared to their WT controls. Histograms show data pooled together from three independent experiments. Only ambra1b−/− embryos show significant reduction of PGC number (ambra1a+/+ n = 36, ambra1a+/− n = 78, ambra1a−/− n = 35; ambra1b+/+ n = 21, ambra1b+/− n = 54, ambra1b−/− n = 29). Statistical analysis was performed using One way ANOVA. *, P < 0.05. (C) Percentage of PGCs in WT embryos injected with ambra1a or ambra1b ATG MOs or splicing MOs. Analysis was performed at 10 hpf by immunohistochemistry for Vasa protein. ambra1b knockdown performed with both types of MO results in a significant reduction of PGCs. Histograms show data pooled together from three independent experiments (WT n = 23, MO-ambra1a-ATG n = 14, MO-ambra1b-ATG n = 41, MO-ambra1a-splic n = 20, MO-ambra1b-splic n = 20). Statistical analysis was performed using One way ANOVA. *, P < 0.05; ** P < 0.01; *** P < 0.001; **** P < 0.0001. (D, E) Results of rescue experiments by co-injection of GFP-nos1-3’UTR mRNA with ambra1a or ambra1b mRNA, respectively. PGC number is expressed as percentage with respect to ambra1b+/− animals, in which the PGC number was settled as 100%. Histograms show data pooled together from three independent experiments (D: ambra1b+/− n = 38, ambra1b−/− n = 35; E: ambra1b+/− n = 28, ambra1b−/− n = 34). Statistical analysis was performed using Student’s t-test. *** P < 0.001. (F) Quantification of PGC number in ambra1b+/− and ambra1b−/− embryos at 10, 24,72 hpf after injection with GFP-nos1-3’UTR mRNA. Number of PGCs in controls (ambra1b+/−) was settled as 100%. Histograms show data pooled together from three independent experiments (10 hpf: ambra1b+/− n = 25, ambra1b−/− n = 31; 24 hpf: ambra1b+/− n = 28, ambra1b−/− n = 30; 72 hpf: ambra1b+/− n = 29, ambra1b−/− n = 38). Statistical analysis was performed using Student’s t-test. *** P < 0.001. Error bars indicate SEM.
These results were confirmed by performing the same immunohistochemical analysis in WT embryos after knockdown of the two paralogous genes with MO-ambra1b-ATG and MO-ambra1a-ATG, as well as with the corresponding splicing MOs. The specificity of these MOs has been previously verified [15–17]. As shown in Fig. 3C, silencing of both maternal and zygotic ambra1a transcripts did not affect PGC number. Conversely, we obtained a statistically significant reduction of PGCs with both ambra1b MOs, thus confirming the data obtained with the PGC analysis of ambra1b−/− embryos of Fig. 3B. Precisely, the injection with ambra1b ATG MO determined the loss of the 25% of the PGCs (Fig. 3C, p = 0,0221). Moreover, injection of ambra1b splicing MO determined an even more consistent PGC reduction (55% reduction, p < 0,0001), indicating that the control of PGC number cannot be only ascribed to the contribution of maternal ambra1b transcripts (present in the eggs of the heterozygous female used in the experiment of Fig. 3B). These results suggested that Ambra1b-dependent PGC regulation occurs later, after mid-blastula transition, and requires zygotic transcripts.
Since we did not detect any statistically significant difference in PGC number between ambra1b+/+ and ambra1b+/−, the subsequent studies were carried out, unless otherwise specified, with larvae obtained from breedings between ambra1b heterozygous females and homozygous males.
To better visualize PGCs in living embryos and at later stages of development, we injected GFP-nos1-3’UTR mRNA in the one-cell stage embryos used for the experiments. As previously demonstrated [28], nos-1 mRNA specifically labels PGCs, and its 3’-UTR stabilizes the transcript in PGCs but not in somatic cells. Therefore, with this approach, we could detect and quantify PGCs based on the GFP linked with nos1 3’-UTR in the construct used for mRNA preparation. To confirm that the PGC reduction is elicited only by the ablation of ambra1b paralog, we co-injected GFP-nos1-3’UTR mRNA and ambra1a or ambra1b mRNAs in one-cell stage embryos obtained by breeding of ambra1b heterozygous females and homozygous males. As expected, co-injection with ambra1a mRNA did not rescue PGC numbers in ambra1b−/− embryos, which still displayed a 45% reduction of PGCs when compared to ambra1b+/− control siblings (Fig. 3D, p = 0,0004). On the other hand, when injected with ambra1b mRNA, ambra1b−/− embryos showed an almost complete recovery in the number of PGCs, with a residual loss of only 9% (Fig. 3E).
In addition, this experimental approach allowed to trace PGCs during development up to 72 hpf and revealed that PGC loss in ambra1b−/− embryos is maintained at least until these developmental stages (Fig. 3F, 10 hpf: 43% reduction of PGCs, p = 0,0002; 24 hpf: 42% reduction of PGCs, p = 0,0008; 72 hpf: 46% reduction of PGCs, p = 0,0001). To understand whether Ambra1b is required for the correct development of germ cells only during the early developmental stages or also in later ones, we injected one-cell stage WT embryos with the ambra1b-splicing MO and let them grow to sexual maturity until 90 dpf. The resulting fish were 42% females and 58% males, indicating that knockdown of ambra1b transcripts early in development is not sufficient to prevent ovarian formation. This result suggests that the function of Ambra1b is not exclusively limited to the regulation of PGC number during early developmental stages, but it is also required at later stages.
Human AMBRA1 mRNA can rescue PGC number and involves interaction with the CUL4–DDB1 complex
Injection of hAMBRA1 mRNA has already been proven to be effective in recovering the loss of Ambra1a and Ambra1b functions in previous studies in which MOs injection was used to down-regulate zebrafish ambra1 isoforms [16, 17].
To further validate the rescue experiments performed in the present study, we verified that the hAMBRA1 mRNA injected is indeed translated into protein. Two different transcripts, hAMBRA1 [17] and hAMBRA1-RFP-sspB [8], both containing the entire coding region of hAMBRA1, were injected into one-cell stage WT embryos. Western blotting with antibody against hAMBRA1 using proteins extracted from 24-hpf embryos confirmed the translation of injected RNAs (Additional file 1, Fig. A2).
Hence, we crossed female ambra1b+/− with male ambra1b−/− and injected the one-cell stage embryos thus obtained with both GFP-nos1-3’UTR and hAMBRA1 mRNAs. Then we analyzed the number of PGCs at 10, 24 and 72 hpf (Fig. 4A). hAMBRA1 mRNA effectively rescued the PGC number at 10 and 24 hpf, with no cell loss at 10 hpf and only 5% loss at 24 and 72 hpf (Fig. 4A). Moreover, we injected one-cell stage embryos with hAMBRA1 mRNA and analysed the gonads at 45 dpf. The injected ambra1b−/− fish did not show ovaries, even at 45 dpf, thus confirming that, although there were no significant differences in the PGC number at 3 dpf, a single injection of hAMBRA1 cannot elicit a permanent rescue of the phenotype (Table 2). This result confirmed that Ambra1b is required for a prolonged period to assure the development of a functional ovary and that lack of this protein delays gonadal development.
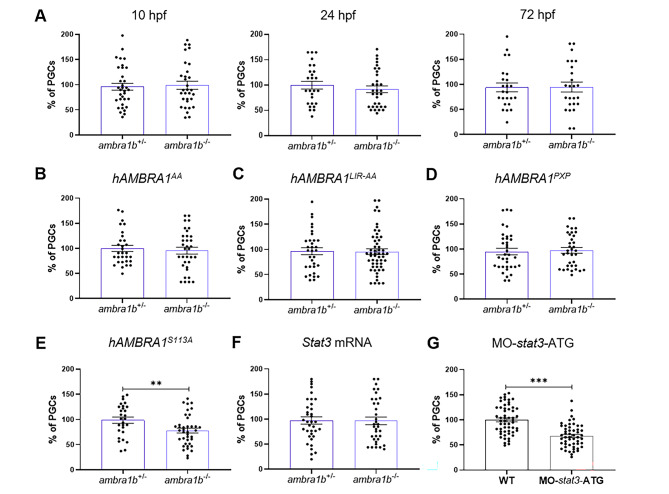
Fig. 4.
Injection of hAMBRA1 mRNA counteracts the loss of PGCs. (A) Quantification of PGCs at 10, 24 and 72 hpf after co-injection of one-cell embryos with GFP-nos1-3’UTR and hAMBRA1 mRNA (10 hpf: ambra1b+/− n = 34, ambra1b−/− n = 31; 24 hpf: ambra1b+/− n = 26, ambra1b−/− n = 32; 72 hpf: ambra1b+/− n = 24, ambra1b−/− n = 25). (B-E) Quantification of PGCs after injection with GFP-nos1-3’UTR mRNA and hAMBRA1 mRNA mutated in TRAF6 (hAMBRA1AA, B), LC3 (hAMBRA1LIR − AA, C), PP2A (hAMBRA1PXP, D) and CUL4–DDB1 (hAMBRA1S113A, E) binding sites (B: ambra1b+/− n = 30, ambra1b−/− n = 34; C: ambra1b+/− n = 33, ambra1b−/− n = 54; D: ambra1b+/− n = 36, ambra1b−/− n = 35; E: ambra1b+/− n = 28, ambra1b−/− n = 39). (F) Injection of one-cell stage ambra1b+/− and ambra1b−/− embryos with murine Stat3 mRNA and quantification of PGCs at 10-hpf (ambra1b+/− n = 29, ambra1b−/− n = 27). (G) Injection of one-cell stage WT embryos with MO-stat3-ATG and quantification of PGCs at 10-hpf (ambra1b+/− n = 55, ambra1b−/− n = 52). In all panels, the PGC number is expressed as percentage with respect to ambra1b+/− animals, in which the PGC number was settled as 100%. The histograms show data pooled together from four (A, F) or three (B, C, D, E, G) independent experiments. Error bars indicate SEM. Statistical analysis was performed using Student’s t-test. ** P < 0.01, *** P < 0.001
Table 2.
Histological analysis of 45-dpf zebrafish gonads after injection of hAMBRA1 mRNA
| Undifferentiated gonad | Juvenile gonad | Juvenile ovary to testis transition | Ovary | |
|---|---|---|---|---|
| ambra1b +/− | - | - | 2/8 | 6/8 |
| ambra1b −/− | 1/8 | 3/8 | 4/8 | - |
Table 2
Summary of the data obtained with the histological analysis of 45-dpf ambra1b+/− and ambra1b−/− zebrafish siblings after injection of hAMBRA1 mRNA. The numbers reported refer to the individuals presenting a specific type of gonad out of the total number of fish analysed.
AMBRA1 is involved in several biological processes through specific interactions with other proteins. The better-described interactors of AMBRA1 are LC3, TRAF6, CUL4–DDB1 and PP2A, which work as regulatory partners of AMBRA1 [1, 2, 5, 29]. To better understand how AMBRA1 acts in the regulation of PGC number in zebrafish, we injected in one-cell stage embryos four mutated forms of hAMBRA1 mRNA which produce an AMBRA1 protein that cannot interact with either LC3 (hAMBRA1LIR − AA; [29]), TRAF6 (hAMBRA1AA; [1]), PP2A (hAMBRA1PXP; [5]) or CUL4–DDB1 (hAMBRA1S113A; [2]). Failure to bind LC3, TRAF6 or PP2A did not affect the hAMBRA1-dependent recovery of PGC number in ambra1b−/− embryos (Fig. 4B-D), whereas hAMBRA1S113A elicited only partial rescue of PGC number (Fig. 4E, 49% reduction of PGCs, p = 0,010), suggesting that AMBRA1-CUL4–DDB1 interaction is required for PGC maintenance.
A recent study has shown a relationship among AMBRA1, CUL4–DDB1 complex, and STAT3 in medulloblastoma (MB) stem cells, demonstrating that AMBRA1, through its direct interaction with the CUL4–DDB1 complex, is involved in the degradation of SOCS3, an inhibitor of STAT3 activity [30]. In this way, up-regulation of AMBRA1 activates STAT3 and leads to enhanced stem potential, whereas knockdown of AMBRA1 reduces MB stem cell growth and migratory potential.
Based on these findings, we assessed the involvement of STAT3 in the regulation of zebrafish PGCs by providing murine Stat3 mRNA to ambra1b KO embryos, as well as by injecting a zebrafish stat3 ATG-MO to block the translation of maternal and zygotic stat3 transcripts in WT embryos. Remarkably, injection of murine Stat3 mRNA was able to completely recover the PGC loss of 10 hpf ambra1b KO embryos (Fig. 4F), whereas injection of zebrafish stat3 MO in WT embryos determined a significantly decreased PGC number when compared to non-injected WT embryos (Fig. 4G, 32% reduction of PGCs, p = 0,001). These results point at the Ambra1b-Cul4-Stat3 molecular pathway in the regulation of PGC number during the first zebrafish developmental stages.
Ambra1 is highly expressed in mouse ovary and its haploinsufficiency affects gene expression and follicle maturation
Since the above results showed the ability of the hAMBRA1 mRNA to recover the number of PGCs in mutant ambra1b−/− zebrafish, we carried out further work aimed at assessing the role of Ambra1 in the differentiation of mice ovaries. Similar to zebrafish, Ambra1 showed high levels of expression in 3-months-old mouse ovaries (Fig. 5A). We then assessed the expression of Ambra1, Stat3, and Myc (one STAT3 target gene) at three and ten months of life, using ovaries from Ambra1+/− and Ambra1+/+ sibling mice (Fig. 5B,C). All heterozygous ovaries showed a significant downregulation of Ambra1 transcripts (Fig. 5B, 3 months, p = 0,0165; Fig. 5C, 10 months, p = 0,0002) and a general reduction of the expression levels of Stat3 and Myc. The first was found to be statistically significant at three months (Fig. 5B, p = 0,0154), pointing at an Ambra1-related function for STAT3 in this organ.
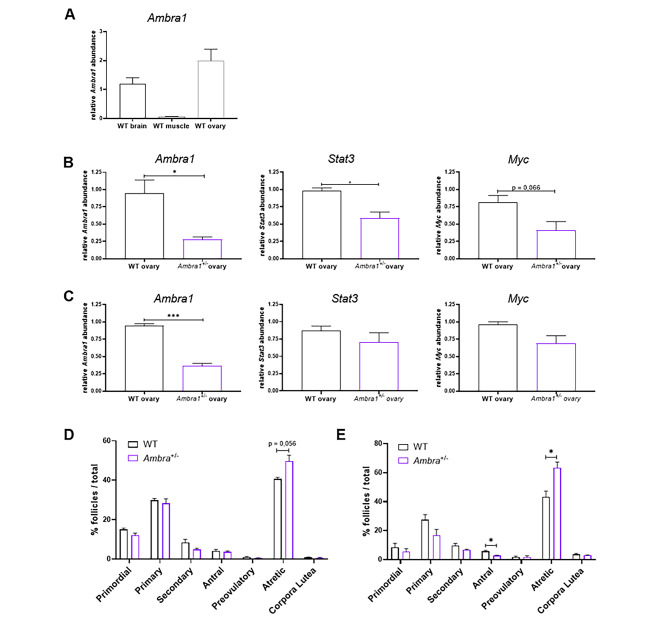
Fig. 5.
Ambra1 deficiency affects ovary physiology in mice. (A) RT-qPCR analysis of Ambra1 mRNA levels in brain, muscle and ovaries of 3-month-old WT mice. (B, C) RT-qPCR analysis of Ambra1, Stat3 and Myc expression in 3-month-old (B) and 10-month-old (C) Ambra1+/+ (WT) and Ambra1+/− sibling mice. Data were generated from three mice of each genotype for each time point. Values represent the mean ± SEM. Data were normalized with the housekeeping gene Gapdh. Statistical analysis was performed using Student’s t-test. *, P < 0.05. (D, E) Comparison of follicle proportion in ovaries of 3-month-old (D) and 10-month-old (E) Ambra1+/+ and Ambra1+/− mice. The proportion of follicles is displayed as the percentage of each follicle type over the total number of follicles (primordial, primary, secondary, antral, preovulatory, atretic and corpora lutea) over the total number of follicles. Values represent the mean ± SEM. Data were generated from three mice of each genotype for each time point, except for 3-month-old Ambra1+/− mice (four ovaries). Statistical analysis was performed using Student’s t-test. *, P < 0.05
The proportion of the different types of follicles in the ovaries of Ambra1+/+ and Ambra1+/− sibling mice did not show significant differences at three months of age (Fig. 5D). However, a significant reduction of antral follicles in parallel with a significant increase in atretic follicles was found at 10 months, thus pointing at a role for Ambra1 in mouse follicolar development regulation (Fig. 5E, antral follicles p = 0,0213; atretic follicles p = 0,0232).
Discussion
AMBRA1 is a well-known protein in the biological landscape, whose biochemical characteristics, presence of specific domains and stretches of intrinsically disordered structure, allow interaction with several proteins, thus regulating a plethora of different functions, as demonstrated in mammalian cell lines and mouse models [1, 2, 4–7, 9, 10].
In zebrafish, Ambra1 is encoded by two paralogous genes, for which we recently generated two knockout zebrafish lines using the CRISPR/Cas9 technology [17]. Unlike the results previously obtained with knockdown MOs approaches, in which silencing of both paralogs led to severe phenotypes, the activation of genetic compensation mechanisms in stable mutant lines led to the lack of overt phenotypes, at least during the developmental stages [17]. Nevertheless, in agreement with the persistence of duplicated genes only after acquisition of new functions, sub-functioning of ancestral ones or tissue and/or temporal-specific expression, we found that the two ambra1 genes show different tissue-specific expression in the adult districts we analysed. Moreover, the lack of ambra1b−/− female individuals, together with the high level of expression of both transcripts in gonads and particularly in the ovary, pointed to a significant role of Ambra1 in gonadal development, physiology and pathology.
Since the number of germ cells represents a critical factor in zebrafish sex determination, we analysed the PGC number in our mutant lines and found that ambra1b KO causes a severe reduction of these stem cells, thus suggesting a critical role in PGC survival. To confirm these data, we adopted different experimental approaches, starting with ATG and splicing MOs, which have already been validated for both paralogous genes [15–17]. These experiments confirmed that the protective function of PGCs is limited to ambra1b, whereas ambra1a silencing does not affect PGC number. In addition, although we cannot rule out the role of maternal ambra1b mRNAs, the results we obtained with splicing MOs suggest that proteins of embryonic origin may have an even more critical role in this process. Rescue experiments added further validations to the exclusive role of Ambra1b, since only ambra1b transient overexpression hampers the loss of PGCs.
Moreover, rescue experiments with the hAMBRA1 transcript led to a complete recovery of the PGC number, suggesting that this function, or the protein interaction on which it is based, is evolutionary conserved. Since AMBRA1 can interact with several proteins involved in different cellular functions, we replicated rescue experiments using hAMBRA1 mRNA mutated in specific binding sites for known AMBRA1 interacting partners. The results showed that only the interaction with the CUL4-DDB1 complex is unable to fully recover PGC number in ambra1b−/− embryos, thus suggesting that the protective function of Ambra1b relies on the interaction with this complex. The CUL4-DDB1 ubiquitin ligase complex can regulate a wide range of cellular processes through interaction with substrate receptors (called DCAFs, DDB1 and CUL4-associated factors) and ubiquitination of specific target proteins [31]. AMBRA1, also known as DCAF3, is one of the WD40 repeat-containing proteins that can act as substrate receptor [6].
Interestingly, Cul4b, a member of the CUL4-DDB1 complex, initially studied in C. elegans where it is involved in the replication of DNA [32], was found to be fundamental for the maintenance of germ cells in the testes of D. melanogaster [33]. Moreover, in mouse, it has both a cell-autonomous and a non-cell-autonomous function in testis physiology, as it is required for male germ cells spermatogenesis, and in somatic cells to maintain the spermatogonial stem cell population in the testis [34].
The functions of this complex in mouse ovary physiology and oocyte survival have been analysed by means of oocyte-specific KO of DDB1, the linker between CUL4 and the DCAF proteins. The silencing of this protein, as well as deletion of DCAF1, determines oocyte loss and ovarian insufficiency [35]. However, other DCAFs are involved in gonadal physiology: DCAF13 was found to regulate ovarian follicle maintenance and oocyte development and growth [36], while DCAF8 and DCAF17 have critical roles in spermatogenesis [37, 38].
AMBRA1 binds CUL4-DDB1 complex and targets D-type cyclins for ubiquitin-mediated degradation, thus acting as a tumor suppressor and assuring normal cell cycle progression [9, 39]. Whereas regulation of this process does not correspond to a protective role from germ cell loss, interaction of AMBRA1 with the CUL4-DDB1 complex allows for degradation of SOCS3, an inhibitor of STAT3 activity [40]. STAT3 is a fundamental transcription factor implicated in the proliferation and migration of stem cells, including those of medulloblastoma [30]. Hence, considering the role of STAT3 in stem cell homeostasis [41, 42], we sought to assess whether Ambra1 role in PGCs is related to STAT3.
Knockdown experiments with a stat3 ATG-morpholino resulted in a significant reduction in PGC number at 10 hpf, suggesting the involvement of Stat3 in the regulation of PGC number in zebrafish. This hypothesis is also supported by the rescue of PGC number after injection with the murine Stat3 mRNA. To validate this interaction, we analysed Ambra1, Stat3 and Myc expression in the ovary of Ambra1+/+ and Ambra1+/− mice. Ambra1 was found highly expressed in WT ovaries and reduced in heterozygous samples. Interestingly, in heterozygous mouse ovaries the expression of Stat3 and Myc, was also reduced, although significantly only at three months of age for Stat3. Moreover, analysis of the different types of follicles showed a significant increase in atretic follicles and a reduction of the antral ones in Ambra1+/− mice at 10 months of age. Sex determination is driven by different mechanisms in mammals and in zebrafish. Indeed, murine sexual differentiation is genetically determined, whereas in zebrafish it is settled by a combination of genetic and environmental conditions. However, an excessive loss of germ cells is associated with reproductive problems in mammals [43] and therefore the results we obtained in mice support a possible role for Ambra1 in the quality control and fertility capabilities of female mice. Future insights into Ambra1 role in gonadal development and physiology may help uncover new aspects of male and female infertility, a pathological problem in which many cases are still considered idiopathic.
The high level of expression of both paralogs in the gonads suggests the involvement of these proteins in other aspects of reproductive physiology. In anticipation of more in-depth studies in this field, we analysed the reproductive performance of both sexes, taking advantage of the ambra1b−/− females derived from the single mutant escaper we found up to now, and found a significant impairment of the reproductive process in all cases. Notably, the administration of the probiotic L. rhamnosus to zebrafish females increases the expression of genes involved in the autophagic process, including ambra1b, and downregulates transcripts related to apoptosis, thus modulating the balance between the two processes and regulating ovarian functions [44].
Finally, in agreement with the AMBRA1 role as a tumor suppressor, among the mutant males we analysed we found two cystic degenerations of the seminiferous tubules, a pre-cancer condition, two seminomas and an undifferentiated germ cell tumor. Although testicular lesions such as seminomas and cystic degeneration of seminiferous tubules with or without hyperplasia of spermatogones are common changes in adult zebrafish [45], seminomas were found to be less than 2% in a survey of nearly 10,000 2-year-old zebrafish [46] and 17% in WT zebrafish of 30–34 months of age [47]. Since we did not detect seminomas or undifferentiated germ cell tumors in any WT, the higher incidence of tumors in the testis of mutants suggests a key role of Ambra1a and Ambra1b as tumor suppressors in this organ.
Conclusion
In conclusion, by exploiting ambra1a and ambra1b KO zebrafish lines, we were able to prove the sub-functionalization between these two genes and uncover a novel function of Ambra1 in the protection from excessive PGC loss, which seems to require binding with the CUL4-DDB1 complex. Since both Ambra1a and Ambra1b proteins contain the binding domain for CUL4-DDB1, but only Ambra1b is involved in PGC maintenance, it can be speculated that this interaction requires an additional functional domain that is present in the human AMBRA1 but not in the zebrafish Ambra1a isoform. Further work in the near future may allow to dissect the molecular basis of this interaction together with a more precise identification of all actors involved, as well as of the roles played by the two Ambra1 isoforms in ovarian and testicular physiology.
Materials and methods
Animal maintenance and handling
Zebrafish embryos, larvae, and adults were maintained according to standard procedures [48]. Embryos were obtained from natural spawning and raised at 28.5 °C in a 12:12 light:dark (LD) cycle in fish water (50X: 25 g Instant Ocean, 39.25 g CaSO4 and 5 g NaHCO3 for 1 L). All husbandry and experimental procedures complied with the Italian and European Legislation for the Protection of Animals used for Scientific Purposes (Directive 2010/63/EU) and were approved by the Animal Ethics Committee of the University of Padua and by the Italian Ministry of Health (Authorization Number 568/2016-PR). The experiments described in this paper were performed with wild-type (WT) zebrafish or with the ambra1a and ambra1b mutant lines whose generation has been previously described [17]. Non-mutant fish indicated as WT and ambra1a+/+/ambra1b+/+ correspond to animals deriving from different or the same batches of the knockouts, respectively. However, the genetic background of the WT fish was the same used for the generation of the ambra1a and ambra1b mutant lines.
Heterozygous whole-body Ambra1 knockout (Ambra1+/–) mice [10] were housed in controlled temperature (23 °C) and light (12:12 light:dark cycle) conditions, with free access to water and food. Animal procedures were approved by the Animal Ethics Committee of the University of Padua and by the Italian Ministry of Health (Authorization Number 581/2017-PR). All animals were in C57BL/6 N genetic background. Ambra1+/– mice and the age-matched littermate controls Ambra1+/+ were sacrificed by cervical dislocation. Ovaries were dissected, isolated and immediately frozen in liquid nitrogen vapours or fixed in Bouin’s solution for further experiments.
DNA extraction and genotyping
Biopsies from the caudal fin of larvae and adult fish were used for fin clips genomic DNA extraction performed with the HotSHOT protocol [49]. For the screening of each genotype, fragments at the target sites were amplified by PCR and the locus-specific primers [17] are reported in in the Additional file 2, Table A1.
Mice genotyping was determined by PCR from digested ear biopsies with specific primers for Ambra1 knockout allele and Ambra1 wild-type allele [10] (Additional file 2, Table A1).
Morphological and histological analyses
Mutant and WT fish, heterozygote, and WT mice ovaries were fixed for 24/48 h in Bouin’s solution at room temperature. Samples were dehydrated through a graded ethanol series, infiltrated with xylene, and embedded in Paraplast plus (Leica Biosystem, 39,602,004). Samples were serially cut into 7–8 μm sections on an LKB microtome. After rehydration, sections were stained with hematoxylin and eosin and mounted with Eukitt (BioOptica, 09–00100) for microscopy examination.
Morpholinos injection
Morpholino (MO) (Gene Tools) treatment was performed with MOs against the ATG translation initiation sites of either ambra1a or ambra1b transcripts (MO-ambra1a-ATG and MO-ambra1b-ATG) and with splice-blocking MOs designed at the exon 3/intron 3 junction sequence of both genes (MO-ambra1a-splice and MO-ambra1b-splice). All MOs were previously described and validated [15–17]. A MO against the ATG translation initiation sites of Stat3 (MO-stat3-ATG) was also used, as previously described and validated [50]. For each ambra1 MO, 8.2 ng were injected in the yolk of one-cell stage embryos whereas 10 ng were injected for the MO-stat3-ATG. Injections were performed under a dissecting microscope using a microinjector (Leica Microsystems).
Vasa immunohistochemistry
The number of PGCs was analysed in WT, knockout (KO), and knockdown (KD) 10-hpf zebrafish embryos by whole-mount immunohistochemistry with an anti-Vasa antibody (1:5000 polyclonal rabbit) [51] kindly provided by Prof. Knaut (New York University School of Medicine, USA). PGCs were manually counted by visualization in brightfield with Leica M165 FC microscope equipped with a Nikon DS-Fi2 digital camera.
GFP-nos1-3’UTR mRNA synthesis and injection
PGCs were visualized with fluorescence microscopy by injection of mRNA produced using the construct GFP-nos1-3’UTR [27], kindly provided by Prof. Raz (University of Münster, Germany). The mRNA was transcribed with the SP6 promoter and the mMessage Machine kit (Ambion) according to manufacturer’s instruction, after plasmid linearization with NotI restriction enzyme (Promega).
For rescue experiments, cDNAs of human AMBRA1 (hAMBRA1), as well as hAMBRA1 mutated in the PP2A, LC3, TRAF6 and CULLIN4 binding sites (hAMBRA1PXP, hAMBRA1LIR−AA, hAMBRA1AA and hAMBRA1S113A) were inserted in pCS2 + plasmids as stated in a previous paper [17] or produced for this study. mRNAs were then transcribed using the T3 promoter and the mMessage Machine kit, after plasmid linearization with HindIII restriction enzyme (Promega). Zebrafish ambra1a1 and ambra1b were inserted in pCS2 + plasmids and transcribed using the SP6 promoter and the mMessage Machine kit, after plasmid linearization with NotI restriction enzyme (Promega). All mRNAs were polyadenylated at the 3’-termini using a Poly(A) Tailing Kit (AM1350; Invitrogen). The mRNAs were injected in one-cell stage embryos at the concentration of 80 pg per embryo for the GFP-nos1-3’UTR mRNA and 40 pg per embryo for the various hAMBRA1 mRNAs. AMBRA1 mutants in LC3, TRAF6, CUL4-DDB1 and PP2A binding sites were generated by using the site-directed mutagenesis kit (Agilent Technologies) respectively. The sequences used are as reported in [1, 2, 5, 29]. The murine Stat3 mRNA (mStat3) was also prepared as described [52] and injected in one-cell stage embryos at the concentration of 50 pg.
Protein extraction and western blotting
Pools of 50 WT embryos (24 hpf) were injected with hAMBRA1 mRNA and hAMBRA1-RFP-sspB mRNA [8, 17], deyolked with Ringer’s solution and frozen in liquid nitrogen. For the protein extraction, samples were added with protease inhibitors (Roche, COEDTAF-RO) and mechanically homogenized in Tissue Extraction Reagent I (Invitrogen, FNN0071). Following brief centrifugation, the supernatant was collected, and the protein content was determined by BCA assay (Thermo-Fisher Scientific, 23,225). 30 µg of total proteins per sample were boiled for 10 min at 90 °C and loaded into 12% polyacrylamide Novex NuPAGE Bis-Tris gels (Thermo- Fisher Scientific, NP0341). Following SDS-PAGE, proteins were transferred onto a PVDF membrane (Millipore, IPVH00010). This was subsequently saturated for 1 h at RT with 5% non-fat dry milk (Bio-Rad, 1,706,404) in 1X Tris-Buffered Saline added with 0.1% Tween20 detergent (Sigma-Aldrich, P9416) and then hybridized O/N at 4 °C with primary antibodies (anti-hAMBRA1 1:1000, Thermo-Fisher Scientific, PA1-16930; anti ACTB/β-actin 1:2000, Sigma-Aldrich, A5316) and for 1 h at RT with secondary antibodies (HRP-conjugated goat anti-rabbit, Bethyl Laboratories Inc.; anti-mouse IgG-Fc fragments, A120-111P and A90-131P). The chemiluminescent signal was revealed with SuperSignal West Pico Chemiluminescent Substrate (Thermo-Fisher Scientific, 340,779). Densitometric quantification was carried out by FIJI software.
RNA extraction, reverse transcription and qPCR
Total RNA was extracted using Quick-RNA Miniprep kit (Zymo Research R1054) from single ovaries of 3- and 10-months-old WT and heterozygote mice, and from organs of 6 mpf WT zebrafish (brain, intestine, liver, muscle, ovary, testis). RNA samples were kept at -80 °C until use. 5 µg of the total RNA obtained from mice ovaries were used for mRNA isolation with Dynabeads mRNA DIRECT Kit (00460456; Invitrogen). All the mRNA obtained with this procedure as well as 1 µg of the total RNA obtained from zebrafish samples was used for cDNA synthesis, employing FIREScript Reverse Transcriptase Kit (06-13-00050; Solis BioDyne) and following the manufacturer’s protocol. Quantitative polymerase chain reaction (qPCR) was performed with SYBR green method on Biorad cfx384 (Biorad). The reaction conditions were as follows: enzyme activation at 95 °C for 15 min followed by 45 cycles of denaturation (30 s at 95 °C), annealing (30 s at 60 °C), and extension (20 s at 72 °C). Fluorescence monitoring occurred at the end of each cycle. Gapdh (mice samples) and actb2 (zebrafish samples) mRNAs were used as normalizers. The primer sequences are reported in the Additional file 2, Table A1.
Zebrafish gonads in situ hybridization
Ovaries and testicles extracted from 6-mpf WT zebrafish were fixed overnight at 4 °C with PFA 4% in PBS added with 0.1% Tween-20 and 0.1% DMSO and then stored in 100% methanol at -20 °C until used. For in situ hybridization, samples were rehydrated through a graded series of methanol in PBT (PBS and 0.1% Tween-20), permeabilized with proteinase K for 30 min at room temperature and fixed again with PFA 4% in PBT for 30 min at room temperature. Fixation was stopped with several brief washes in PBT; then organs were prepared for in situ hybridization as previously described [15]. Briefly, ovaries and testis were prehybridized for 2–5 h at 65 °C and then incubated with specified probes at 65 °C overnight. Hybridized probes for the ambra1a and ambra1b transcripts [15] were detected with sheep anti-digoxigenin-AP Fab fragments (Roche Diagnostics, Mannheim, Germany) and visualized with the chromogen substrate NBT/BCIP Stock solution (Roche Diagnostics, Mannheim, Germany). The chromogenic reaction was stopped by PBT rinsing followed by a final fixation in 4% PFA in PBS overnight at 4 °C. Samples were dehydrated in a graded series of methanol, then cleared in a clearing solution (2:1 benzyl benzoate:benzyl alcohol), and finally rehydrated to PBS. Organs were equilibrated in a graded series of glycerol for image acquisition.
Zebrafish reproductive performance analysis
The reproductive performance of ambra1a−/− and ambra1b−/− males and females was assessed by natural reproductions in spawning tanks under standard aquarium conditions (Westerfield 1995). Four 8-mpf animals were analysed for each sex and genotype. WT partners were randomly chosen each time between the batch of WT males and females (housed separately from the opposite sex). Animals underwent 4 consecutive reproductive rounds, once every 10 days, to ensure we had a realistic mean of the reproductive performances of each individual. We excluded a specific male-female interaction effect by performing repeated reproductive rounds for each animal coupled with different WT partners. At the end of each trial, spawned eggs were collected in a Petri dish with fish water, following the standard husbandry rules [48]. Reproductive performances were quantified as the number of times in which the male was able to induce the female to spawn on the total 4 chances. Considering the successful trials, it was evaluated the mean number of eggs spawned, the mean fertilization rate (fertilised eggs/total eggs) and the mean offspring survival rate at 6 dpf [53].
Mice ovaries follicle count
An equal number of ovaries from WT and AMBRA1+/− heterozygous mice were scored for each age point (3 and 10 months). The scoring system was set up following the methods proposed by previous literature [54, 55].
Briefly, the total follicle number was estimated by counting and classifying all the follicles in every 9th section of the ovary. Only follicles in which the nucleus was visible were included in the count. The total follicle number was then calculated by multiplying the raw counts per 9 [54, 55]. Counts were collected in customized Excel files, and the total and estimated numbers of follicles for each category were calculated. To allow for comparison between ovaries of different sizes we used the percentage of each category instead of the total number. For the accurate estimation and correct classification of ovarian follicles, the recommendations of Myers and collaborators were followed [56]. Representative examples are provided in Additional file 1, Fig. A3.
Imaging
For imaging of PGCs at 10 and 24 hpf, embryos were anaesthetized with 0.04% tricaine and mounted in 2% methylcellulose on a depression slide. Images of PGCs at these embryonic stages were recorded with a Leica DMR using a Nikon DS- Fi2 digital camera. Three dpf embryos were instead embedded in 0.8% low-melting agarose and analysed with the Nikon C2 confocal system provided with the software NIS ELEMENTS.
WMISH-stained organs, as well as immunohistochemistry embryos, were mounted in 80% glycerol in phosphate-buffered saline plus 0.1% Tween 20 (Sigma, P1379), observed under a Leica M165 FC microscope, and photographed with a Nikon DS-Fi2 digital camera. Histological samples were photographed on a Leica DMR using a Nikon DS- Fi2 digital camera.
Statistical analysis
Statistical analysis was performed with Graph Pad Prism V9.0.1. Data are presented as the means ± SEM. Comparisons between WT and KO/KD zebrafish/mice were performed with the tests reported on the figure captions. The p-values are indicated with the following symbols: *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to acknowledge Prof. Erez Raz (University of Münster, Germany) for providing the GFP-nos1-3’UTR construct and Prof. Holger Knaut (New York University School of Medicine, USA) for providing the Vasa antibody. We are grateful to the technical staff of the Zebrafish Facility at the Department of Biology, UniPD.
Abbreviations
- AMBRA1
Activating molecule in beclin-1-regulated autophagy protein 1
- CUL4-DDB1
Cullin4-DNA damage binding protein 1 complex
- DCAF
DDB1- and CUL4-associated factor
- dpf
Days post fertilization
- GFP
Green fluorescent protein
- hAMBRA1
Human AMBRA1
- hpf
Hours post fertilization
- KD
Knockdown
- KO
Knockout
- LC3
Microtubule-associated protein 1 A/1B-light chain 3
- LD
Light:dark
- MB
Medulloblastoma
- MO
Morpholino
- mpf
Months post fertilization
- mStat3
Murine Stat3
- Myc
Myc proto-oncogene protein
- nos1
Nanos1
- PGCs
Primordial germ cells
- PP2A
Protein phosphatase 2 A
- RT-qPCR
Real-time quantitative PCR
- SOCS3
Suppressor of cytokine signaling 3
- STAT3
Signal transducer and activator of transcription 3
- TRAF6
TNF receptor-associated factor 6
- WT
Wild type
Authors’ contributions
LDV designed the study; FCM and MG supervised the study; BP, CO, CF provided conceptual advice and troubleshooting; FCM, TF, FN, MG, DA, GL, ZA, CM carried out the experimental work; FCM, TF, DA and FN analyzed data; BG and VR performed histopathological evaluation; NF generated critical reagents; FCM, DA and LDV wrote the manuscript; LDV, BP, CO, FCM, TF, NF and DA revised the manuscript. BP, CF and LDV provided funding acquisition. All authors read and approved the final manuscript.
Funding
This work was supported by the Fondazione Telethon [GGP14202] and by Università degli Studi di Padova [RFO-ex 60%]. Francesco Cecconi’s laboratory is supported by ‘‘Associazione Italiana per la Ricerca sul Cancro’’ (AIRC IG-2019). Francesca Nazio’s laboratory is supported by grants from the Italian Ministry of Health #GR-2019-2369231 and from CureSearch for Children’s Cancer - Young Investigator Award in Pediatric Oncology Drug Development.
Availability of data and materials
Mutant lines are available upon request. Supplemental information including three figures can be found in Additional file 1. All primers used in this study are listed in Additional file 2, Table A1.
Declarations
Ethics approval
All husbandry and experimental procedures complied with the Italian and European Legislation for the Protection of Animals used for Scientific Purposes (Directive 2010/63/EU) and were approved by the Animal Ethics Committee of the University of Padua and by the Italian Ministry of Health (Authorization Number 568/2016-PR).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nazio F, Strappazzon F, Antonioli M, Bielli P, Cianfanelli V, Bordi M, et al. mTOR inhibits autophagy by controlling ULK1 ubiquitylation, self-association and function through AMBRA1 and TRAF6. Nat Cell Biol. 2013 doi: 10.1038/ncb2708. [DOI] [PubMed] [Google Scholar]
- 2.Antonioli M, Albiero F, Nazio F, Vescovo T, Perdomo AB, Corazzari M, et al. AMBRA1 interplay with cullin E3 ubiquitin ligases regulates autophagy dynamics. Dev Cell. 2014 doi: 10.1016/j.devcel.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Cianfanelli V, Cecconi F. AMBRA1: when autophagy meets cell proliferation. Autophagy. 2015 doi: 10.1080/15548627.2015.1053681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cianfanelli V, De Zio D, Di Bartolomeo S, Nazio F, Strappazzon F, Cecconi F. Ambra1 at a glance. J Cell Sci. 2015 doi: 10.1242/jcs.168153. [DOI] [PubMed] [Google Scholar]
- 5.Cianfanelli V, Fuoco C, Lorente M, Salazar M, Quondamatteo F, Gherardini PF, et al. AMBRA1 links autophagy to cell proliferation and tumorigenesis by promoting c-Myc dephosphorylation and degradation. Nat Cell Biol. 2015 doi: 10.1038/ncb3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cianfanelli V, Nazio F, Cecconi F. Connecting autophagy: AMBRA1 and its network of regulation. Mol Cell Oncol. 2015 doi: 10.4161/23723548.2014.970059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Rita A, Peschiaroli A, Acunzo D, Strobbe P, Hu D, Gruber Z. HUWE1 E3 ligase promotes PINK1/PARKIN-independent mitophagy by regulating AMBRA1 activation via IKKα. Nat Commun. 2018 doi: 10.1038/s41467-018-05722-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Acunzo P, Strappazzon F, Caruana I, Meneghetti G, Di Rita A, Simula L, et al. Reversible induction of mitophagy by an optogenetic bimodular system. Nat Commun. 2019 doi: 10.1038/s41467-019-09487-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maiani E, Milletti G, Nazio F, Holdgaard SG, Bartkova J, Rizza S, et al. AMBRA1 regulates cyclin D to guard S-phase entry and genomic integrity. Nature. 2021 doi: 10.1038/s41586-021-03422-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gambarotto L, Metti S, Chrisam M, Cerqua C, Sabatelli P, Armani A, et al. Ambra1 deficiency impairs mitophagy in skeletal muscle. J Cachexia Sarcopenia Muscle. 2022 doi: 10.1002/jcsm.13010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fimia GM, Stoykova A, Romagnoli A, Giunta L, Di Bartolomeo S, Nardacci R, et al. Ambra1 regulates autophagy and development of the nervous system. Nature. 2007 doi: 10.1038/nature05925. [DOI] [PubMed] [Google Scholar]
- 12.Ye J, Tong Y, Lv J, Peng R, Chen S, Kuang L, et al. Rare mutations in the autophagy-regulating gene AMBRA1 contribute to human neural tube defects. Hum Mutat. 2020 doi: 10.1002/humu.24028. [DOI] [PubMed] [Google Scholar]
- 13.Coccurello R, Nazio F, Rossi C, De Angelis F, Vacca V, Giacovazzo G, et al. Effects of caloric restriction on neuropathic pain, peripheral nerve degeneration and inflammation in normometabolic and autophagy defective prediabetic Ambra1 mice. PLoS ONE. 2018 doi: 10.1371/journal.pone.0208596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dere E, Dahm L, Lu D, Hammerschmidt K, Ju A, Tantra M, et al. Heterozygous ambra1 deficiency in mice: a genetic trait with autism-like behavior restricted to the female gender. Front Behav Neurosci. 2014 doi: 10.3389/fnbeh.2014.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benato F, Skobo T, Gioacchini G, Moro I, Ciccosanti F, Piacentini M, et al. Ambra1 knockdown in zebrafish leads to incomplete development due to severe defects in organogenesis. Autophagy. 2013 doi: 10.4161/auto.23278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skobo T, Benato F, Grumati P, Meneghetti G, Cianfanelli V, Castagnaro S, et al. Zebrafish ambra1a and ambra1b knockdown impairs skeletal muscle development. PLoS ONE. 2014 doi: 10.1371/journal.pone.0099210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meneghetti G, Skobo T, Chrisam M, Fontana CM, Facchinello N, Nazio F, et al. Zebrafish ambra1a and ambra1b silencing affect Heart Development. Zebrafish. 2020 doi: 10.1089/zeb.2020.1860. [DOI] [PubMed] [Google Scholar]
- 18.Kossack ME, Draper BW. Genetic regulation of sex determination and maintenance in zebrafish (Danio rerio) Curr Top Dev Biol. 2019 doi: 10.1016/bs.ctdb.2019.02.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tzung KW, Goto R, Saju JM, Sreenivasan R, Saito T, Arai K, et al. Early depletion of primordial germ cells in zebrafish promotes testis formation. Stem Cell Reports. 2015 doi: 10.1016/j.stemcr.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye D, Zhu L, Zhang Q, Xiong F, Wang H, Wang X, et al. Abundance of early embryonic primordial germ cells promotes zebrafish female differentiation as revealed by lifetime labeling of germline. Mar Biotechnol (NY) 2019 doi: 10.1007/s10126-019-09874-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aharon D, Marlow FL. Sexual determination in zebrafish. Cell Mol Life Sci. 2021 doi: 10.1007/s00018-021-04066-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yazdankhah M, Farioli-Vecchioli S, Tonchev AB, Stoykova A, Cecconi F. The autophagy regulators Ambra1 and beclin 1 are required for adult neurogenesis in the brain subventricular zone. Cell Death Dis. 2014 doi: 10.1038/cddis.2014.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sepe S, Nardacci R, Fanelli F, Rosso P, Bernardi C, Cecconi F, et al. Expression of Ambra1 in mouse brain during physiological and Alzheimer type aging. Neurobiol Aging. 2014 doi: 10.1016/j.neurobiolaging.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Santos D, Luzio A, Coimbra AM. Zebrafish sex differentiation and gonad development: a review on the impact of environmental factors. Aquat Toxicol. 2017 doi: 10.1016/j.aquatox.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Liew WC, Orbán L. Zebrafish sex: a complicated affair. Brief Funct Genomics. 2013 doi: 10.1093/bfgp/elt041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodríguez-Marí A, Cañestro C, Bremiller RA, Nguyen-Johnson A, Asakawa K, Kawakami K, et al. Sex reversal in zebrafish fancl mutants is caused by Tp53-Mediated germ cell apoptosis. PLoS Genet. 2010 doi: 10.1371/journal.pgen.1001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Köprunner M, Thisse C, Thisse B, Raz E. A zebrafish nanos-related gene is essential for the development of primordial germ cells. Genes Dev. 2001 doi: 10.1101/gad.212401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saito T, Fujimoto T, Maegawa S, Inoue K, Tanaka M, Arai K, et al. Visualization of primordial germ cells in vivo using GFP-nos1 3’UTR mRNA. Int J Dev Biol. 2006 doi: 10.1387/ijdb.062143ts. [DOI] [PubMed] [Google Scholar]
- 29.Strappazzon F, Nazio F, Corrado M, Cianfanelli V, Romagnoli A, Fimia GM, et al. AMBRA1 is able to induce mitophagy via LC3 binding, regardless of PARKIN and p62/SQSTM1. Cell Death Differ. 2015 doi: 10.1038/cdd.2014.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nazio F, Po A, Abballe L, Ballabio C, Diomedi Camassei F, Bordi M, et al. Targeting cancer stem cells in medulloblastoma by inhibiting AMBRA1 dual function in autophagy and STAT3 signalling. Acta Neuropathol. 2021 doi: 10.1007/s00401-021-02347-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J, Zhou P. DCAFs, the missing link of the CUL4-DDB1 ubiquitin ligase. Mol Cell. 2007 doi: 10.1016/j.molcel.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 32.Sarikas A, Hartmann T, Pan ZQ. The cullin protein family. Genome Biol. 2011 doi: 10.1186/gb-2011-12-4-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu J, Lan X, Chen X, Yu C, Xu Y, Liu Y, et al. Protein synthesis and degradation are essential to regulate germline stem cell homeostasis in Drosophila testes. Development. 2016 doi: 10.1242/dev.134247. [DOI] [PubMed] [Google Scholar]
- 34.Yin Y, Liu L, Yang C, Lin C, Veith GM, Wang C, et al. Cell Autonomous and nonautonomous function of CUL4B in mouse spermatogenesis. J Biol Chem. 2016 doi: 10.1074/jbc.M115.699660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu C, Zhang YL, Pan WW, Li XM, Wang ZW, Ge ZJ, et al. CRL4 complex regulates mammalian oocyte survival and reprogramming by activation of TET proteins. Science. 2013 doi: 10.1126/science.1244587. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Zhang YL, Zhao LW, Guo JX, Yu JL, Ji SY, et al. Mammalian nucleolar protein DCAF13 is essential for ovarian follicle maintenance and oocyte growth by mediating rRNA processing. Cell Death Differ. 2019 doi: 10.1038/s41418-018-0203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X, Xia Z, Lv X, Li D, Liu M, Zhang R, et al. DDB1- and CUL4-associated factor 8 plays a critical role in spermatogenesis. Front Med. 2021 doi: 10.1007/s11684-021-0851-8. [DOI] [PubMed] [Google Scholar]
- 38.Ali A, Mistry BV, Ahmed HA, Abdulla R, Amer HA, Prince A, et al. Deletion of DDB1- and CUL4- associated factor-17 (Dcaf17) gene causes spermatogenesis defects and male infertility in mice. Sci Rep. 2018 doi: 10.1038/s41598-018-27379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simoneschi D, Rona G, Zhou N, Jeong YT, Jiang S, Milletti G, et al. CRL4AMBRA1 is a master regulator of D-type cyclins. Nature. 2021 doi: 10.1038/s41586-021-03445-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen SH, Jang GM, Hüttenhain R, Gordon DE, Du D, Newton BW, et al. CRL4AMBRA1 targets Elongin C for ubiquitination and degradation to modulate CRL5 signaling. EMBO J. 2018 doi: 10.15252/embj.201797508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martello G, Bertone P, Smith A. Identification of the missing pluripotency mediator downstream of leukaemia inhibitory factor. EMBO J. 2013 doi: 10.1038/emboj.2013.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galoczova M, Coates P, Vojtesek B. STAT3, stem cells, cancer stem cells and p63. Cell Mol Biol Lett. 2018 doi: 10.1186/s11658-018-0078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matzuk MM, Lamb DJ. The biology of infertility: research advances and clinical challenges. Nat Med. 2008 doi: 10.1038/nm.f.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gioacchini G, Dalla Valle L, Benato F, Fimia GM, Nardacci R, Ciccosanti F, et al. Interplay between autophagy and apoptosis in the development of Danio rerio follicles and the effects of a probiotic. Reprod Fertil Dev. 2012 doi: 10.1071/RD12187. [DOI] [PubMed] [Google Scholar]
- 45.Spitsbergen JM, Buhler DR, Peterson TS. Neoplasia and neoplasm-associated lesions in laboratory colonies of zebrafish emphasizing key influences of diet and aquaculture system design. ILAR J. 2012 doi: 10.1093/ilar.53.2.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amsterdam A, Lai K, Komisarczuk AZ, Becker TS, Bronson RT, Hopkins N, et al. Zebrafish hagoromo mutants up-regulate fgf8 postembryonically and develop neuroblastoma. Mol Cancer Res. 2009 doi: 10.1158/1541-7786.MCR-08-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neumann JC, Lillard K, Damoulis V, Amatruda JF. Zebrafish models of germ cell tumor. Methods Cell Biol. 2011 doi: 10.1016/B978-0-12-381320-6.00001-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Westerfield M. (1995) The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio), 3rd Edition. Eugene, OR, University of Oregon Press.
- 49.Meeker ND, Hutchinson SA, Ho L, Trede NS. Method for isolation of PCR-ready genomic DNA from zebrafish tissues. Biotechniques. 2007 doi: 10.2144/000112619. [DOI] [PubMed] [Google Scholar]
- 50.Yamashita S, Miyagi C, Carmany-Rampey A, Shimizu T, Fujii R, et al. Stat3 controls cell movements during zebrafish gastrulation. Dev Cell. 2002 doi: 10.1016/s1534-5807(02)00126-0. [DOI] [PubMed] [Google Scholar]
- 51.Knaut H, Pelegri F, Bohmann K, Schwarz H, Nüsslein-Volhard C. Zebrafish vasa RNA but not its protein is a component of the germ plasm and segregates asymmetrically before germline specification. J Cell Biol. 2000 doi: 10.1083/jcb.149.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peron M, Dinarello A, Meneghetti G, Martorano L, Betto RM, Facchinello N, et al. Y705 and S727 are required for the mitochondrial import and transcriptional activities of STAT3, and for regulation of stem cell proliferation. Development. 2021 doi: 10.1242/dev.199477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fontana CM, Locatello L, Sabatelli P, Facchinello N, Lidron E, Maradonna F, et al. epg5 knockout leads to the impairment of reproductive success and courtship behaviour in a zebrafish model of autophagy-related diseases. Biomed J. 2022 doi: 10.1016/j.bj.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sarma UC, Winship AL, Hutt KJ. Comparison of methods for quantifying primordial follicles in the mouse ovary. J Ovarian Res. 2020 doi: 10.1186/s13048-020-00724-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Winship AL, Sarma UC, Alesi LR, Hutt KJ. Accurate follicle enumeration in adult mouse ovaries. J Vis Exp. 2020 doi: 10.3791/61782. [DOI] [PubMed] [Google Scholar]
- 56.Myers M, Britt KL, Wreford NG, Ebling FJ, Kerr JB. Methods for quantifying follicular numbers within the mouse ovary. Reproduction. 2014 doi: 10.1530/rep.1.00095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Mutant lines are available upon request. Supplemental information including three figures can be found in Additional file 1. All primers used in this study are listed in Additional file 2, Table A1.