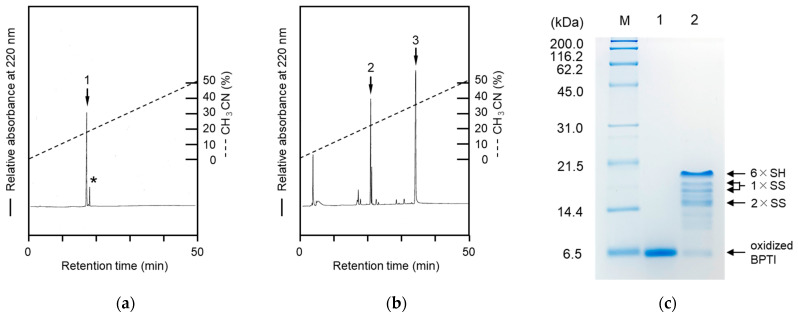
Figure 1.
RP-HPLC profiles of the Arg5-Tyr-NH2 peptide (a) and reaction mixtures (b) of the Arg5-Tyr-NH2 peptide treated with N-succinimidyl 6-maleimidohexanoate. Tricine SDS-PAGE analyses (c) of the refolding solutions of BPTI. Peaks 1 and 2 were assigned to the Arg5-Tyr-NH2 and the Male-Arg5-Tyr-NH2 peptides, respectively, as evidenced by MALDI-TOF/MS. The asterisk and peak 3 represent an impurity and N-succinimidyl 6-maleimidohexanoate, respectively. Lanes 1 and 2 show the refolding solutions (1 h reaction) treated without or with the Male-Arg5-Tyr-NH2 reagent. “M” represents marker proteins (Nacalai Tesque, Kyoto, Japan). RP-HPLC analyses were performed using a Hydrosphere C18 column and the acetonitrile concentration was increased from 1% to 51% for 50 min.