Abstract
Bradyrhizobium japonicum strain 110spc4 was capable of chemolithoautotrophic growth with carbon monoxide (CO) as a sole energy and carbon source under aerobic conditions. The enzyme carbon monoxide dehydrogenase (CODH; EC 1.2.99.2) has been purified 21-fold, with a yield of 16% and a specific activity of 58 nmol of CO oxidized/min/mg of protein, by a procedure that involved differential ultracentrifugation, anion-exchange chromatography, hydrophobic interaction chromatography, and gel filtration. The purified enzyme gave a single protein and activity band on nondenaturing polyacrylamide gel electrophoresis and had a molecular mass of 230,000 Da. The 230-kDa enzyme was composed of large (L; 75-kDa), medium (M; 28.4-kDa), and small (S; 17.2-kDa) subunits occurring in heterohexameric (LMS)2 subunit composition. The 75-kDa polypeptide exhibited immunological cross-reactivity with the large subunit of the CODH of Oligotropha carboxidovorans. The B. japonicum enzyme contained, per mole, 2.29 atoms of Mo, 7.96 atoms of Fe, 7.60 atoms of labile S, and 1.99 mol of flavin. Treatment of the enzyme with iodoacetamide yielded di(carboxamidomethyl)molybdopterin cytosine dinucleotide, identifying molybdopterin cytosine dinucleotide as the organic portion of the B. japonicum CODH molybdenum cofactor. The absorption spectrum of the purified enzyme was characteristic of a molybdenum-containing iron-sulfur flavoprotein.
Carbon monoxide (CO) dehydrogenases (CODHs) are key enzymes in the CO metabolism of physiologically and phylogenetically diverse microbes. Carboxidotrophic bacteria are aerobic chemolithoautotrophs characterized by the utilization of CO as a sole source of carbon and energy. They are taxonomically diverse bacteria, encompassing more than 15 described species in eight genera (5, 27). The CODHs of Oligotropha carboxidovorans (37) and Pseudomonas thermocarboxydovorans (32) have been cloned and sequenced. The CODH from O. carboxidovorans is the best characterized (21–23, 30). The enzyme is an O2-stable, molybdenum-iron-sulfur-flavin hydroxylase that catalyzes the oxidation of CO to CO2 according to the equation CO + H2O → CO2 + 2e− + 2H+. It contains the molybdopterin cytosine dinucleotide-type molybdenum cofactor (29) and [2Fe-2S] centers of type I and type II (2, 10). In anaerobic microorganisms, CODHs are nickel-iron-sulfur proteins, usually O2 labile, that function in a variety of energy-yielding pathways. The CODH from acetogenic Moorella thermoacetica reduces CO2 to acetyl coenzyme A, providing acetate as the major end product (33), and that from acetotrophic Methanosarcina and Methanothrix obtains energy by fermenting acetate to CH4 and CO2 (17). A similar enzyme from phototrophic Rubrivivax gelatinosus and Rhodospirillum rubrum reduces CO and water to CO2 and H2 (40–42). Acetotrophic sulfate reducers also utilize CODH to cleave acetyl coenzyme A, whereas sulfate is reduced to sulfide (12, 36). CO oxidation and the fundamental role of CODHs in aerobic and anaerobic pathways of carbon metabolism have been reviewed previously (5, 28, 30).
Bradyrhizobium japonicum species are gram-negative soil bacteria with the unique ability to establish N2-fixing symbiosis with soybeans (Glycine max). Although all rhizobia have been considered to be aerobic chemoorganotrophs that grow best on complex media (38, 43), some hydrogenase uptake-positive (Hup+) B. japonicum strains have been shown to grow chemolithoautotrophically utilizing H2 and CO2 as sole sources of energy and carbon, respectively (9, 19). With only very few exceptions, carboxidotrophs not only oxidize CO but can use H2 plus CO2 as well, indicating the presence of two different capacities for chemolithoautotrophic growth (25). Given the similarities observed between carboxidotrophic bacteria and hydrogen bacteria, of which B. japonicum is an example, we decided to investigate whether B. japonicum is a carboxidotroph. In this paper, we report on the purification of the CODH enzyme from B. japonicum 110spc4 and on the ability of this bacterium to use CO as a sole source of carbon and energy.
MATERIALS AND METHODS
Bacterial strain and culture conditions.
B. japonicum 110spc4 (35) was used throughout this study. Cells were routinely grown at 28°C on peptone-salts-yeast extract (PSY) medium (35). The medium used for CO-dependent chemolithoautotrophic growth contained the following in 1 liter of distilled water: KH2PO4, 0.3 g; Na2HPO4 · 12H2O, 0.3 g; MgSO4 · 7H2O, 0.1 g; CaCl2 · 2H2O, 5 mg; NH4Cl, 1 g; H3BO3, 10 mg; ZnSO4 · 7H2O, 1 mg; FeCl3 · 6H2O, 0.2 mg; MnCl2 · 4H2O, 0.1 mg; Na2MoO4 · 2H2O, 0.1 mg; and biotin, 100 μg. The pH was adjusted to 7.0 with NaOH prior to autoclaving. Autotrophic cultures of 110spc4 were grown in 250-ml flasks, each containing 50 ml of autotrophic medium supplemented with spectinomycin (100 μg/ml). Inocula for autotrophic growth were 1% of an autotrophically grown culture with an optical density at 600 nm (OD600) of 0.1. Cultures were flushed continuously through sintered glass discs with an atmosphere of 50% (vol/vol) CO–50% (vol/vol) air and incubated at 28°C. Serial dilutions of 1-ml aliquots taken every 48 h were prepared, and 0.1-ml aliquots from the appropriate dilutions were plated in triplicate onto PSY medium solidified with 1.5% of agar. Counts were made after incubation for 5 days at 28°C.
Cell mass of B. japonicum 110spc4 was produced in a 50-liter fermentor containing PSY medium. Fermentors were supplied with a gas mixture of 10% (vol/vol) CO, 5% (vol/vol) CO2, and 85% (vol/vol) air at a flow rate of 2 liters/min, kept at 28°C, and stirred at 200 rpm. The pH was maintained at 7.0. Bacteria were harvested by centrifugation (8,000 × g for 10 min at 4°C), washed with 50 mM potassium phosphate (pH 7.2), and kept frozen at −20°C until use.
Enzyme assays.
CODH activity was determined by following spectrophotometrically the reduction of nitroblue tetrazolium chloride (NBT). Phenazine methosulfate (PMS) served as an electron carrier between CODH and NBT. The reaction mixtures contained (1 ml, final volume) 50 mM Tris-HCl buffer (pH 7.5), 0.05 mM NBT, and 0.1 mM PMS. Serum-stoppered cuvettes (diameter of 1 cm) were flushed with CO for at least 5 min. The reactions were initiated with 100 to 300 μl of enzyme-containing fractions. The formation of red formazan upon the reduction of NBT was followed at 540 nm (ɛ540 = 7.2 mmol−1 cm−1) in a spectrophotometer. Activity staining of CODH on nondenaturing polyacrylamide gel electrophoresis (PAGE) was done by published procedures (24). Gels were immersed in 50 mM Tris-HCl buffer (pH 7.5) containing 0.05 mM NBT and 0.1 mM PMS under an atmosphere of pure CO. After appearance of the bands, the gel was washed several times with water and kept in a 7.5% acetic acid solution. Nitrate reductase was determined at 30°C by measuring the reduction of nitrate to nitrite with dithionite-methyl viologen as the electron donor (4). The reaction was started by addition of the dithionite and terminated after 5 min by vigorous shaking until samples had lost their blue color. Nitrite was determined by a diazotation procedure (31).
Enzyme purification.
Except for hydrophobic interaction chromatography, which was performed at room temperature, all purification steps were carried out at 4°C. Frozen cells (50 g, wet mass) in 55 ml of 50 mM potassium phosphate (pH 7.2) containing 0.2 mM phenylmethylsulfonyl fluoride and a few crystals of DNase were homogenized and disrupted in a high-pressure homogenizer (Rannie AS). Unbroken cells were removed by centrifugation (8,000 × g for 10 min at 4°C). DNA was precipitated with 0.8% protamine sulfate. Cytoplasmic fractions were obtained by ultracentrifugation (100,000 × g for 2 h). Anion-exchange chromatography was performed on Accell QHA resin (Amersham-Pharmacia) equilibrated with 50 mM potassium phosphate (pH 7.2). After washing with 50 mM potassium phosphate, bound proteins were eluted with 200 ml of a linear gradient of 0 to 1 M KCl in potassium phosphate. Fractions of 20 ml containing CODH activity were pooled and adjusted to 50% ammonium sulfate saturation. After gentle stirring for 60 min, precipitated protein was removed by centrifugation (20,000 × g for 60 min). The supernatant was concentrated to about 50 ml in an Amicon Diaflo ultrafiltration cell equipped with a PM-10 membrane and subjected to hydrophobic interaction chromatography on a 15 ISO column (Amersham-Pharmacia) equilibrated with 1.2 M ammonium sulfate in 50 mM potassium phosphate (pH 7.2). After washing with equilibration buffer, bound proteins were eluted with 100 ml of a gradient of 1.2 to 0 M ammonium sulfate in 50 mM potassium phosphate. Fractions with CODH activity were concentrated by ultrafiltration as described above, desalted by gel filtration on Sephadex G-25 (PD10; Amersham-Pharmacia), and subjected to gel filtration through a Sephacryl HR-S300 column (Amersham-Pharmacia) equilibrated with 50 mM HEPES (pH 7.2) containing 150 mM NaCl. After Sephacryl HR-S300 column filtration, the molecular mass of the CODH enzyme was determined using a calibration kit ranging from 67 to 669 kDa (Amersham-Pharmacia).
Electrophoresis and blotting.
Analytical PAGE was carried in a discontinuous system (18). For nondenaturing PAGE, a 5% acrylamide stacking gel and a 7.5% acrylamide running gel were used; molecular mass standards obtained from Amersham-Pharmacia were thyroglobulin (669 kDa), ferritin (440 kDa), catalase (232 kDa), lactate dehydrogenase (142 kDa), and bovine serum albumin (67 kDa). For denaturing PAGE, a 7.5% acrylamide–2.5% (wt/vol) sodium dodecyl sulfate (SDS) stacking gel and a 12% acrylamide–2.5% (wt/vol) SDS running gel were used; the molecular mass standard was the 10-kDa protein ladder system from Gibco-BRL. Protein staining of gels was performed with Coomassie brilliant blue G-250. For Western blot analysis, proteins were transferred electrophoretically from polyacrylamide gels onto polyvinylidene difluoride Immobilon-P membranes (Bio-Rad) as previously described (39). CODH was detected by immunoblotting with a rabbit antiserum (immunoglobulin G [IgG]) raised against the purified CoxL subunit of CODH from O. carboxidovorans OM5 as described elsewhere (26). Staining of blotted proteins was with amido black 10B.
Analytical methods.
Metal contents were estimated by inductively coupled plasma mass spectroscopy (model VG Plasmaquad PQ2 Turbo Plus; Fisons Instruments/VG Elemental). Acid-labile sulfide was determined with p-dimethylaniline by methylene blue formation according to the method of Fogo and Popowsky (6). Purified CODH from O. carboxidovorans was used as a standard. UV-visible spectra were recorded at room temperature on a Kontron double-beam spectrophotometer. The method of Bradford (1) was used for protein determination.
Analysis of flavins and the molybdenum cofactor.
Flavins were analyzed in supernatants of trichloroacetic acid precipitates from CODH prepared as indicated by Meyer (21). The absorption spectrum of the released flavins was recorded before and after reduction with dithionite. A ΔmM at 450 nm of 10,500 for oxidized-minus-reduced flavin was used (44).
Extraction and carboxamidomethylation of pterins were performed according to published procedures (7, 13, 14). High-pressure liquid chromatography (HPLC) analysis involved an ET 250/8/4 Nucleosil 120-7 C18 column (Macherey-Nagel, Düren, Germany) and a photodiode array detector (model 991; Waters) connected to a computer. The mobile phase was 50 mM ammonium acetate (pH 4.5) at a flow rate of 1 ml/min.
RESULTS
CO-dependent growth.
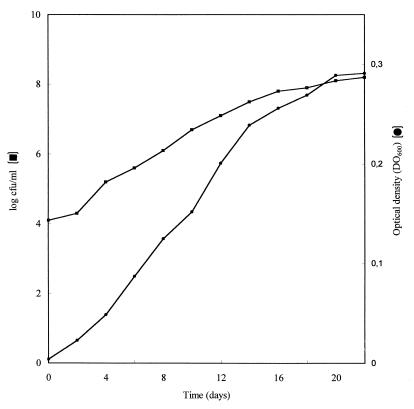
Cells of B. japonicum 110spc4 could utilize CO as a sole source of carbon and energy under aerobic chemolithoautotrophic conditions in a mineral salts medium containing ammonium as the nitrogen source (Fig. 1). During growth under an atmosphere of 50% CO–50% air for a 22-day incubation period, an OD600 of 0.291 was reached (Fig. 1). Plate counts at harvest revealed that the number of cells increased from 1.26 × 104 per ml, which was added as the inoculum, to 1.58 × 108 during the 22-day period (Fig. 1). Growth was not apparent when CO was replaced with an atmosphere of N2. After incubation for 5 days, cultures of 110spc4 grown in PSY medium under an atmosphere of 50% CO–50% air produced as much growth, as measured by OD or number of CFU, as comparable cultures incubated without CO (data not shown). The presence of CO therefore did not affect growth of 110spc4 in PSY medium. Assays of CODH activity based on the CO-dependent reduction of NBT with PMS showed that specific activity in the cytoplasmic fraction from cells grown heterotrophically in PSY medium with CO was 2.8 nmol of CO oxidized/min/mg of protein (Table 1). CO-oxidizing activity was much higher in similar fractions from CO autotrophically grown cells, amounting to 17.37 nmol of CO oxidized/min/mg of protein. Utilization of methylene blue, thionine, 2-(4-iodophenyl)-3-(4-nitrophenyl)-2H-tetrazolium, methyl viologen, benzyl viologen, NAD, NADP, flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN), and ferricyanide as potential electron acceptors gave negative results.
FIG. 1.
CO-dependent growth of B. japonicum 110spc4 in a liquid mineral salts-vitamin medium. Cultures were incubated under an atmosphere of 50% air and 50% CO. Values are averages of the results of three replicate determinations.
TABLE 1.
Purification of CODH from B. japonicum 110spc4
| Step | Protein (mg) | Total activity (nmol of CO oxidized/min) | Sp act (nmol of CO oxidized/min/mg of protein) | Purification (fold) | Yield (%) |
|---|---|---|---|---|---|
| Cytoplasmic fraction | 1,296 | 3,583 | 2.8 | 1 | 100 |
| Anion-exchange chromatography | 280 | 2,423 | 8.6 | 3.1 | 67.6 |
| Hydrophobic interaction chromatography | 24 | 630 | 26 | 9.3 | 17.6 |
| Gel filtration | 9.6 | 554 | 57.7 | 20.6 | 15.5 |
Purification and properties of CODH.
CODH was prepared from cytoplasmic fractions of B. japonicum 110spc4 grown heterotrophically under a CO-containing atmosphere. The enzyme was purified 21-fold with a specific activity of 58 nmol of CO oxidized/min/mg of protein and a yield of 16% (Table 1). In addition to CO-oxidizing activity, the purified enzyme had nitrate reductase activity, with rates of 1.38 nmol of NO2− produced/min/mg of protein. The purified protein had no oxidizing activity with H2, nicotine, N-methylnicotinamide, isonicotinic acid, xanthine, hypoxanthine, purine, quinalic acid, quinaldine, quinoline, and isoquinoline as potential electron donors.
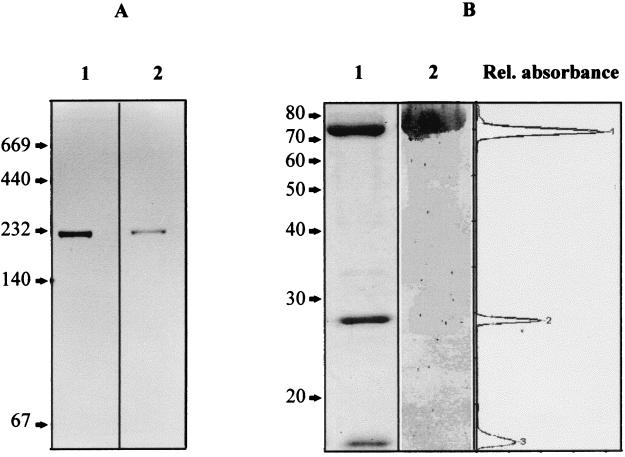
The CODH preparations obtained were homogeneous according to native PAGE, which revealed a single protein band of about 230 kDa (Fig. 2A, lane 1) that corresponded to a single band of CODH activity (Fig. 2A, lane 2). SDS-PAGE revealed three protein bands of 75, 28.4, and 17.2 kDa (Fig. 2B, lane 1). Densitometric analysis of CODH polypeptides in SDS-gels showed 63.4, 20.2, or 16.4% of the protein associated with the 75-kDa (large [L]), 28.4-kDa (medium [M]), or 17.2-kDa (small [S]) polypeptide, respectively (Fig. 2B, right). Assuming that all bands stained equally with Coomassie brilliant blue, these data indicate a 1.13:1.34:1 molar ratio of the enzyme subunits and suggest an (LMS)2 heterohexameric subunit structure as well as a mass of 241 kDa for the native protein. This value agrees with that of 230 kDa found upon native PAGE of the purified enzyme. It also correlated well with that of 235 kDa observed after gel filtration through Sephacryl HR-S300, which is a more precise method than gel electrophoresis.
FIG. 2.
(A) Detection of CODH activity in native polyacrylamide gels. Lane 1, purified CODH enzyme (5 μg) stained with Coomassie brilliant blue; lane 2, purified CODH enzyme (3 μg) stained with PMS-NBT. (B) Subunit composition and immunoblot analysis of CODH. Lane 1, SDS-PAGE of the purified protein (50 μg) stained with Coomassie brilliant blue; lane 2, immunoblot analysis with IgG antibodies raised against the CoxL subunit of O. carboxidovorans. The densitometric scan (right) refers to lane 1. Numbers in panels A and B indicate the molecular masses of reference proteins in kilodaltons. Rel., relative.
After separation on SDS-PAGE and blotting on polyvinylidene difluoride membranes, immunostaining with IgG antibodies raised against the CoxL subunit of O. carboxidovorans revealed a strong cross-reactivity of the band at 75 kDa (Fig. 2B, lane 2).
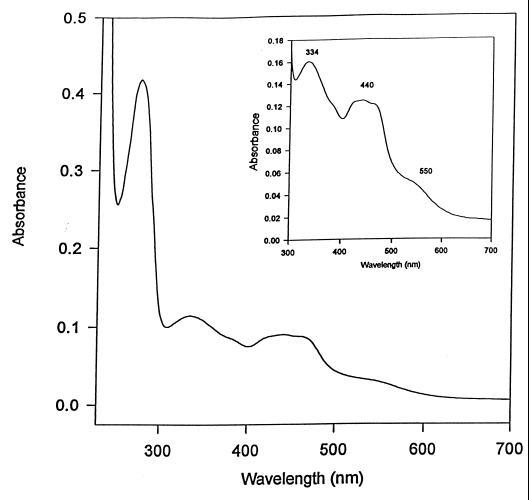
The purified protein was brownish in color and had an air-oxidized absorption spectrum characterized by a protein peak at 280 nm, three broad peaks at 338, 441, and 450 nm, and shoulders in the 390- and 550-nm regions (Fig. 3). The A280/A450 and A450/A550 ratios of different enzyme preparations were 4.6 and 3.03, respectively.
FIG. 3.
UV-visible spectrum of purified CODH as prepared (0.8 mg of protein in 50 mM HEPES, 150 mM NaCl [pH 7.2]) from B. japonicum 110spc4. The inset shows the visible part of the spectrum.
Metal contents and analysis of flavins and carboxamidomethylated pterins.
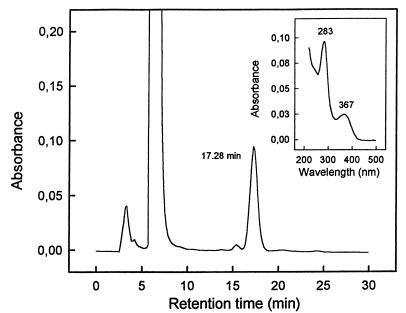
From three separate determinations on two different preparations of CODH, the enzyme revealed (per mole of enzyme) 2.29 ± 0.31 mol of Mo, 7.96 ± 0.76 mol of Fe, 7.60 ± 0.94 mol of S, and 1.99 ± 0.22 mol of flavins at a 1.15:4:3.82:1 molar ratio. Tungsten was not present in the purified CODH. The HPLC elution profile of iodoacetamide-treated protein revealed the presence of a pterin-like compound that eluted as a homogeneous peak at 17.28 min (Fig. 4). This material was identified as di(carboxamidomethyl) molybdopterin cytosine dinucleotide [(MeCONH)2MCD] on the basis of its characteristic UV-visible absorption spectrum with maxima at 283 and 367 nm and an A283/A367 ratio of 2.48 (8, 14).
FIG. 4.
HPLC profile of iodoacetamide-reacted pterin obtained from 0.65 mg of CODH of B. japonicum 110spc4. The inset shows the UV-visible absorption spectrum of the material eluting at 17.28 min.
DISCUSSION
B. japonicum 110spc4 cultured under CO-autotrophic conditions showed convincing increases in OD and the number of CFU (Fig. 1). Nevertheless, under an atmosphere of 50% CO–50% air, bacterial growth was slow, with a doubling time of about 40 h (Fig. 1). During growth on PSY, the doubling time was much shorter and amounted to about 7 h, regardless of the presence or the absence of CO in the gas phase. Experiments designed to further improve autotrophic CO-dependent growth or to define requirements for or characteristics of such a type of growth have not been pursued. Previous work has established that Hup+ strains of B. japonicum not only can oxidize hydrogen (20) but also are hydrogen bacteria that grow as chemolithotrophs utilizing CO2 and H2 as sole sources of carbon and energy, respectively (9, 19). Results in this study extend those findings by showing that B. japonicum 110spc4 is also able to grow with CO as a sole source of carbon and energy.
Specific CODH activity can be readily detected in subcellular fractions prepared from O. carboxidovorans and P. carboxydohydrogena following published procedures that utilize thionine (15), 2-(4-iodophenyl)-3-(4-nitrophenyl)-2H-tetrazolium (16) or methylene blue (23, 24) as a potential electron acceptor. In contrast, CODH in 110spc4 could be detected only when activity was determined by a spectrophotometric assay based on the CO-dependent reduction of NBT with PMS as an electron carrier. Values of CO-oxidizing activity (17.37 nmol of CO oxidized/min/mg of protein) in subcellular fractions from cells grown with CO under autotrophic conditions were lower than those reported for similar fractions from cells of P. carboxydohydrogena (16 μmol of thionine reduced/min/mg of protein) (15) and P. carboxidovorans (131 nmol of CO oxidized/min/mg of protein) (24). Besides CODH activity, the purified enzyme from 110spc4 also carried nitrate reductase activity, with values that were 2.39% of the CO-oxidizing activity. CODHs purified from O. carboxidovorans, P. carboxydoflava, and Streptomyces thermoautotrophicus, as well as the mammalian xanthine oxidase and the chicken liver xanthine oxidase, have been shown to express methyl or benzyl viologen-nitrate reductase activity, with values that did not exceed about 2.5% of the CO-oxidizing activity (reference 28 and references therein).
CODHs from different carboxidotrophic bacteria have been shown to be composed of three polypeptides of 70 to 85 kDa (L), 25 to 33 kDa (M), and 14 to 17 kDa (S) in a stoichiometry of (LMS)2 or LMS with S. thermoautotrophicus (28). On the basis of a molecular mass of 400 kDa, an unusual (LMS)3 subunit structure has been suggested for the P. carboxydohydrogena CODH (15). The estimated molecular mass of 230 kDa for the CODH from 110spc4 falls within the range of molecular masses determined for the (LMS)2-structured enzymes from other carboxidotrophic bacteria. In addition, the 75-kDa polypeptide of 110spc4 CODH showed strong immunoreactivity (Fig. 2B, lane 2) against antibodies specific for the CoxL subunit of the O. carboxidovorans CODH, which indicates that the two enzymes are immunologically related.
The CODH from B. japonicum 110spc4 also resembled the enzymes from O. carboxidovorans, P. carboxydohydrogena, and other molybdenum hydroxylases in UV-visible absorption spectrum and in Mo, S, and flavin content (15, 21, 34). The A280/A450 ratio of different enzyme preparations was 4.6, a value which is close to the 5 used as a purity criterion for xanthine oxidases and related enzymes (3). Furthermore, the A450/A550 ratio of the purified enzyme was 3.03, a value similar to those reported for other molybdenum hydroxylases (3, 28), a value of 3 being indicative of a ratio of 8 FeS/2 FAD (34).
Based on the retention time, its characteristic UV-visible absorption spectrum, and the A283/A367 ratio (8, 14), (MeCONH)2MCD was identified as the material released from CODH after treatment with iodoacetamide. This suggests that MCD is the organic component of the molybdenum cofactor in B. japonicum 110spc4 CODH. The presence of MCD was first demonstrated in CODH from Hydrogenophaga pseudodoflava (14) and subsequently identified in all carboxidotrophic CO dehydrogenases analyzed so far (29), as well as in quinoline oxidoreductase from P. putida and Rhodococcus sp. (11) and xanthine dehydrogenase from Veillonella atypica (8).
Taken together, the results we present here indicate that cells of B. japonicum 110spc4 are able to utilize CO as a sole source of carbon and energy for chemolithoautotrophic growth and that they contain a CODH enzyme closely related to other molybdenum hydroxylases from carboxidotrophic bacteria.
ACKNOWLEDGMENTS
We thank H.-M. Fischer (Mikrobiologisches Institut, Eidgenössiche Technische Hochschule, ETH Zentrum, Zürich, Switzerland) for supplying B. japonicum 110spc4.
Financial support was obtained from the Dirección General de Enseñanza Superior e Investigación Científica, grant PB97-1216; the Deutsche Forschungsgemeinschaft (Bonn, Germany); and the Fonds der Chemischen Industrie (Frankfurt/Main, Germany). M.J.L. also thanks CSIC and DGF for financial support.
REFERENCES
- 1.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 2.Bray R C, George G N, Lange R, Meyer O. Studies by e.p.r. spectroscopy of carbon monoxide oxidase from Pseudomonas carboxydovorans and Pseudomonas carboxyhydrogena. Biochem J. 1983;211:687–694. doi: 10.1042/bj2110687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coughlan M P. Aldehyde oxidase, xanthine oxidase and xanthine dehydrogenase: hydroxylases containing molybdenum, iron-sulfur and flavin. In: Coughlan M P, editor. Molybdenum and molybdenum-containing enzymes. Oxford, United Kingdom: Pergamon Press; 1980. pp. 119–185. [Google Scholar]
- 4.Fernández-López M, Olivares J, Bedmar E J. Two differentially regulated nitrate reductases required for nitrate-dependent microaerobic growth of Bradyrhizobium japonicum. Arch Microbiol. 1994;162:310–315. [Google Scholar]
- 5.Ferry J G. CO dehydrogenase. Annu Rev Microbiol. 1995;49:305–333. doi: 10.1146/annurev.mi.49.100195.001513. [DOI] [PubMed] [Google Scholar]
- 6.Fogo J K, Popowsky M. Spectrophotometric determination of hydrogen sulfide. Anal Chem. 1949;21:732–734. [Google Scholar]
- 7.Frunzke K, Heiss B, Meyer O, Zumft W G. Molybdopterin guanine dinucleotide is the organic moiety of the molybdenum cofactor in respiratory nitrate reductase from Pseudomonas stutzeri. FEMS Microbiol Lett. 1993;113:241–246. [Google Scholar]
- 8.Gremer L, Meyer O. Characterization of xanthine dehydrogenase from the anaerobic bacterium Veillonella atypica and identification of a molybdopterin-cytosine-dinucleotide-containing cofactor. Eur J Biochem. 1996;238:862–866. doi: 10.1111/j.1432-1033.1996.0862w.x. [DOI] [PubMed] [Google Scholar]
- 9.Hanus F J, Maier R J, Evans H J. Autotrophic growth of H2-uptake positive strains of Rhizobium japonicum in an atmosphere supplied with hydrogen gas. Proc Natl Acad Sci USA. 1979;76:1788–1792. doi: 10.1073/pnas.76.4.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hänzelmann P, Meyer O. Effect of molybdate and tungstate on the biosynthesis of CO dehydrogenase and the molybdopterin cytosine-dinucleotide-type of molybdenum cofactors in Hydrogenophaga pseudoflava. Eur J Biochem. 1998;255:755–765. doi: 10.1046/j.1432-1327.1998.2550755.x. [DOI] [PubMed] [Google Scholar]
- 11.Hettrich D, Peschke B, Tshisuata B, Lingens F. Microbial metabolism of quinoline and related compounds: the molybdopterin cofactors of quinoline oxidoreductase from Pseudomonas putida 86 and Rhodococcus spec. B1 and xanthine dehydrogenase from Pseudomonas putida 86. Biol Chem Hoppe-Seyler. 1991;372:513–517. doi: 10.1515/bchm3.1991.372.2.513. [DOI] [PubMed] [Google Scholar]
- 12.Jansen K, Fuchs G, Thauer R K. Autotrophic CO2 fixation by Desulfovibrio baarsii: demonstration of enzyme activities characteristic for the acetyl-CoA pathway. FEMS Microbiol Lett. 1985;28:311–315. [Google Scholar]
- 13.Johnson J L, Bastian N R, Rajagopalan K V. Molybdopterin guanine dinucleotide: a modified form of molybdopterin identified in the molybdenum cofactor of dimethyl sulfoxide reductase from Rhodobacter sphaeroides forma specialis denitrificans. Proc Natl Acad Sci USA. 1990;87:3190–3194. doi: 10.1073/pnas.87.8.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson J L, Rajagopalan K V, Meyer O. Isolation and characterization of a second molybdopterin dinucleotide: molybdopterin cytosine dinucleotide. Arch Biochem Biophys. 1990;283:542–545. doi: 10.1016/0003-9861(90)90681-n. [DOI] [PubMed] [Google Scholar]
- 15.Kim Y M, Hegeman G D. Purification and some properties of carbon monoxide dehydrogenase from Pseudomonas carboxydohydrogena. J Bacteriol. 1981;148:904–911. doi: 10.1128/jb.148.3.904-911.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kraut M, Hugendieck I, Herwig S, Meyer O. Homology and distribution of CO dehydrogenase structural genes in carboxydotrophic bacteria. Arch Microbiol. 1989;152:335–341. doi: 10.1007/BF00425170. [DOI] [PubMed] [Google Scholar]
- 17.Krzycki J A, Lehman L J, Zeikus J G. Acetate catabolism by Methanosarcina barkeri: evidence for involvement of carbon monoxide dehydrogenase, methyl coenzyme M and methylreductase. J Bacteriol. 1985;163:1000–1006. doi: 10.1128/jb.163.3.1000-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laemmli U K. Cleavage of structural proteins during the assembly of the head of the phage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Lepo J E, Hanus F J, Evans H J. Chemoautotrophic growth of hydrogen-uptake-positive strains of Rhizobium japonicum. J Bacteriol. 1980;141:664–670. doi: 10.1128/jb.141.2.664-670.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maier R J, Campbell N E R, Hanus F J, Simpson F B, Russell S A, Evans H J. Expression of hydrogenase activity in free-living Rhizobium japonicum. Proc Natl Acad Sci USA. 1978;75:3558–3562. doi: 10.1073/pnas.75.7.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer O. Chemical and spectral properties of carbon monoxide:methylene blue oxidoreductase. The molybdenum-containing iron-sulfur flavoprotein from Pseudomonas carboxydovorans. J Biol Chem. 1982;257:1333–1341. [PubMed] [Google Scholar]
- 22.Meyer O, Schlegel H G. Reisolation of the carbon monoxide utilizing hydrogen bacterium Pseudomonas carboxydovorans (Kitsner) comb. nov. Arch Microbiol. 1978;118:36–43. doi: 10.1007/BF00406071. [DOI] [PubMed] [Google Scholar]
- 23.Meyer O, Schlegel H G. Oxidation of carbon monoxide in cell extracts of Pseudomonas carboxydovorans. J Bacteriol. 1979;137:811–817. doi: 10.1128/jb.137.2.811-817.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer O, Schlegel H G. Carbon monoxide:methylene blue oxidoreductase from Pseudomonas carboxydovorans. J Bacteriol. 1980;141:74–80. doi: 10.1128/jb.141.1.74-80.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer O, Schlegel H G. Biology of aerobic carbon monoxide-oxidizing bacteria. Annu Rev Microbiol. 1983;37:277–310. doi: 10.1146/annurev.mi.37.100183.001425. [DOI] [PubMed] [Google Scholar]
- 26.Meyer O, Rohde M. Enzymology and bioenergetics of carbon monoxide-oxidizing bacteria. In: Crawford R L, Hanson R S, editors. Microbial growth on C1 compounds. Washington, D.C.: American Society for Microbiology; 1984. pp. 26–33. [Google Scholar]
- 27.Meyer O, Jacobitz S, Krüger B. Biochemistry and physiology of aerobic carbon monoxide-utilizing bacteria. FEMS Microbiol Rev. 1986;39:161–179. [Google Scholar]
- 28.Meyer O, Frunzke K, Mösdorf G. Biochemistry of the aerobic utilization of carbon monoxide. In: Murray J C, Kelly D K, editors. Microbial growth on C1 compounds. Andover, Hampshire, United Kingdom: Intercept, Ltd.; 1993. pp. 433–459. [Google Scholar]
- 29.Meyer O, Frunzke K, Tachil J, Volk M. The bacterial molybdenum cofactor. In: Stiefel E I, Coucouvanis D, Newton W E, editors. Molybdenum enzymes, cofactors and model systems. Washington, D.C.: American Chemical Society; 1993. pp. 50–68. [Google Scholar]
- 30.Mörsdorf G, Frunzke K, Gadkari D, Meyer O. Microbial growth on carbon monoxide. Biodegradation. 1992;3:61–82. [Google Scholar]
- 31.Nicholas D J D, Nason A. Determination of nitrate and nitrite. Methods Enzymol. 1957;3:981–984. [Google Scholar]
- 32.Pearson D M, O'Reilly C, Colby J, Black G W. DNA sequence of the cut A, B and C genes, encoding the molybdenum containing hydroxylase carbon monoxide dehydrogenase, from Pseudomonas thermocarboxydovorans strain C2. Biochim Biophys Acta. 1994;1188:432–438. doi: 10.1016/0005-2728(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 33.Ragsdale S W. Enzymology of the acetyl-CoA pathway of CO2 fixation. Crit Rev Biochem Mol Biol. 1991;26:261–300. doi: 10.3109/10409239109114070. [DOI] [PubMed] [Google Scholar]
- 34.Rajagopalan K V, Handler P. Absorption spectra of Fe-flavoproteins. J Biol Chem. 1964;239:1509–1514. [PubMed] [Google Scholar]
- 35.Regensburger B, Hennecke H. RNA polymerase from Rhizobium japonicum. Arch Microbiol. 1983;135:103–109. doi: 10.1007/BF00408017. [DOI] [PubMed] [Google Scholar]
- 36.Schauder R, Preu B A, Jetten M, Fuchs G. Oxidative and reductive acetyl CoA/carbon monoxide dehydrogenase pathway in Desulfobacterium autotrophicum. 2. Demonstration of the enzymes of the pathway and comparison of CO dehydrogenase. Arch Microbiol. 1989;151:84–89. [Google Scholar]
- 37.Schübel U, Kraut M, Mörsdorf G, Meyer O. Molecular characterization of the gene cluster coxMSL encoding the molybdenum-containing carbon monoxide dehydrogenase of Oligotropha carboxidovorans. J Bacteriol. 1995;177:2197–2203. doi: 10.1128/jb.177.8.2197-2203.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stower M D. Carbon metabolism in Rhizobium species. Annu Rev Microbiol. 1985;39:89–108. doi: 10.1146/annurev.mi.39.100185.000513. [DOI] [PubMed] [Google Scholar]
- 39.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uffen R L. Anaerobic growth of a Rhodopseudomonas species in the dark with carbon monoxide as sole carbon and energy substrate. Proc Natl Acad Sci USA. 1976;73:3298–3302. doi: 10.1073/pnas.73.9.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uffen R L. Metabolism of carbon monoxide. Enzyme Microbiol Technol. 1981;3:197–206. [Google Scholar]
- 42.Uffen R L. Metabolism of carbon monoxide by Rhodopseudomonas gelatinosa: cell growth and properties of the oxidation system. J Bacteriol. 1983;155:956–965. doi: 10.1128/jb.155.3.956-965.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vincent J M. Root-nodule symbioses with Rhizobium. In: Quispel A, editor. The biology of nitrogen fixation. New York, N.Y: American Elsevier Publishing Company Inc; 1974. pp. 265–341. [Google Scholar]
- 44.Waud W R, Brady F O, Wiley R D, Rajagopalan K V. A new purification procedure for bovine milk xanthine oxidase: effect of proteolysis on the subunit structure. Arch Biochem Biophys. 1975;169:695–701. doi: 10.1016/0003-9861(75)90214-3. [DOI] [PubMed] [Google Scholar]