Abstract
The surfactant linear alkylbenzenesulfonate (LAS; 0.5 mM) or linear monoalkyldiphenyletherdisulfonate (LADPEDS; 0.5 mM) in salts medium was easily degraded in laboratory trickling filters, whereas carbon-limited, aerobic enrichment cultures in suspended culture with the same inocula did not grow. We took portions of the trickling filters which degraded LADPEDS, shook the organisms from the solid support (polyester), and found that growth in suspended culture in LADPEDS-salts medium occurred only in the presence of some solid support (polyester fleece or glass wool), though little biomass was immobilized on the support. The end products in suspended culture were identical with those from the trickling filters. There was low plating efficiency of LADPEDS-grown cultures on complex medium, and no picked colony or mixture of colonies grew in LADPEDS-salts-glass wool medium. However, selective plates containing LADPEDS-salts medium solidified with agarose yielded LADPEDS-dependent, pinpoint colonies which could be picked singly and subcultured in selective liquid medium. Isolate DS-1 was a bacterium which showed 93% sequence homology (16S ribosomal DNA) to its nearest phylogenetic neighbor, an α-proteobacterium. Strain DS-1 grew heterotrophically in LADPEDS-salts-glass wool medium and converted the set of aryl-substituted alkanes to the corresponding aryl-substituted carboxylic acids of shorter chain length. Similarly, strain DS-1 grew heterotrophically with commercial LAS, converting it to a set of sulfophenylcarboxylates. Growth with a single isomer of LAS [3-(4-sulfophenyl)dodecane] was concomitant with excretion of 4-(4-sulfophenyl)hexanoate, which was identified by matrix-assisted laser desorption ionization mass spectrometry. The growth yield (6.4 g of protein/mol of C) indicated mass balance, which, with the specific growth rate (0.05 h−1), indicated a specific utilization rate of LAS of 2.2 mkat/kg of protein.
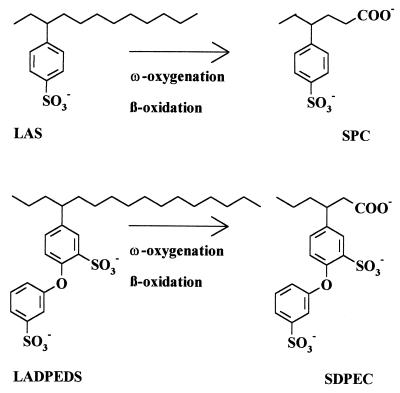
The linear alkylbenzenesulfonate surfactants (LAS; Fig. 1) have been in use for about 40 years. They are well known because they are in household use, and they are the major synthetic surfactants worldwide (40). It is widely accepted that commercial LAS itself is fully degradable (28, 36–38, 42). What is not known is how (4). There are only the generalizations of ω oxygenation and β oxidation (Fig. 1) which were deduced from transient intermediates, sulfophenylcarboxylates (SPC, Fig. 1), excreted during degradation of commercial LAS in mixed cultures (e.g., sewage) (10, 36, 37, 42 [compare reference 2]).
FIG. 1.
A representative LAS congener with its conversion to a defined SPC and a hypothetical LADPEDS congener and with its presumed metabolism to a SDPEC. Commercial LAS always shows the subterminal substitution of the 4-sulfophenyl moiety; the chain length usually ranges from C10 to C13 (23). Commercial C16 LADPEDS always shows the subterminal substitution of the diphenylether. It thus has seven positional isomers on the alkyl chain, each of which has a chiral center. Ten combinations of the alkyl, sulfonate, and ether substituents on the first ring are possible, as are three positional isomers of sulfonation on the second ring. Although substituents are nominally LADPEDS, dialkylated species and monosulfonated ethers also occur (Dowfax surfactant reaction mixture, Dow product information 1510-017A, p. 1, 1997).
The linear monoalkyldiphenyletherdisulfonate surfactants (LADPEDS; Fig. 1) have also been in extensive use for some 40 years in industrial processes ranging from the production of synthetic latex, used in carpet production, paints, and paper coatings (17), to subsurface remediation (34, 35). Despite this widespread usage, LADPEDS seem to be mentioned largely in specialist texts (22, 30), and despite their classification as biodegradable (34) little is known about their metabolism. A company report (Degradation of Dowfax surfactants, Dow product information 1510-014A, p. 1–5, 1996) on work with a typical 14C-labeled LADPEDS congener indicates side chain oxidation to a corresponding sulfodiphenylether carboxylate (SDPEC; Fig. 1), and we presume this to represent ω oxygenation and β oxidation (Fig. 1).
We now report the degradation of LADPEDS and of LAS in pure culture by an α-proteobacterium. The organism requires the presence of a solid support for growth. The products of heterotrophic growth with LADPEDS and LAS have been identified as SDPEC and SPC, respectively.
MATERIALS AND METHODS
Materials.
LADPEDS (Fig. 1), as DOWFAX 8390, was supplied by Dow (Midland, Mich.). This commercial material was an aqueous solution containing 36% active matter as the disodium salt. We were able to confirm the identity of the free acid and the monosodium salt by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) in the negative-ion mode (Table 1). We calculated disodium LADPEDS to have a molecular weight of 598; 1 mM DOWFAX 8390 thus represented about 336 mg of C/liter. Commercial LAS with a mean chain length of 11.4 carbon atoms (19), Marlon A350, was provided by Hüls (Marl, Germany). 3-(4-Sulfophenyl)dodecane (3-C12-LAS) was made available by CONDEA-Vista (Austin, Tex.). The identity of the major component, separated by high-pressure liquid chromatography (HPLC), was confirmed by MALDI-TOF MS in the negative-ion mode (Table 1); 3-C12-LAS was chromatographed and showed the same retention time as 3-C12-LAS in commercial LAS, and some minor components were tentatively identified as 3-C10-LAS, 3-C11-LAS, and 5-C11-LAS (20). 2-(4-Sulfophenyl)butyrate (2-C4-SPC) was available in the laboratory (39).
TABLE 1.
Data from MALDI-TOF MS scans of commercial LADPEDS, 3-C12-LAS, and the product from 3-C12-LAS generated by α-proteobacterium strain DS-1a
| Sample | m/z (a.i.b) | Interpretation |
|---|---|---|
| LADPEDS | 553 (3,400) | [M − H]− |
| 575 (800) | [M − 2H + Na]− | |
| 3-C12-LAS | 325 | [M − H]− |
| Main product from 3-C12-LAS | 271 (7,000) | [M − H]− (C6 SPC) |
| 293 (2,000) | [M − 2H + Na]− | |
| Minor products from 3-C12-LAS | 285 (1,200) | [M − H]− (C7 SPC) |
| 299 (800) | [M − H]− (C8 SPC) |
Control experiments showed that no system peaks interfered in the m/z regions relevant to the data.
a.i., absolute intensity.
Activated sludge as an inoculum for experiments was obtained from the urban sewage treatment plants in Konstanz and Radolfzell, Germany, and from the industrial plant in Ludwigshafen, Germany. The American forest soil inoculum was that used by Dow in earlier work (Dow product information 1510-014A, p. 1–5, 1996). The polyester fleece was described elsewhere (1); it was cut to 1-cm3 cubes when used in suspended culture. The glass wool, sold used as isolation material (Isover, Mönchen-Gladbach, Germany), was homogenized to a powder and washed repeatedly in basal salts medium; the material was thus short fibers of 1 to 5 μm in diameter. Since dry glass fibers are a hazard for eye and lung, stocks were maintained in water. Sinapinic acid (3,5-dimethoxy-4-hydroxycinnamic acid) and α-cyano-4-hydroxycinnamic acid were from Sigma, St. Louis, Mo. The sources of routine chemicals were given elsewhere (18, 24).
Growth medium and enrichment cultures.
Phosphate-buffered minimal-salts medium (31) was used. Enrichment cultures to obtain biodegradation of commercial LAS or commercial LADPEDS as the sole source of carbon and energy for growth were done with a 5% (vol/vol) inoculum in 3-ml cultures in 30-ml screw-cap tubes which were aerated at 30°C in a roller. Cultures were considered positive when both growth and substrate disappearance (HPLC) occurred. A useful spot test for the degradation of these surfactants was whether a sample still foamed after shaking; absence of foam was an indicator of biodegradation.
Anaerobic mineral salts medium, which was suitable for Rhodobium spp. (43), was supplemented with 1.0 mM LAS, 20 mM succinate, and 20 mM acetate, and cultures were incubated at 25°C about 25 cm from a 40-W lamp.
Trickling filter.
The simplest version of Baumann's laboratory trickling filter (1, 18) was used with the original geometry and flow rates, which represent a communal sewage works. Each trickling filter consisted of a vertical glass tube with silicone stoppers pierced by needles through which growth medium and air were pumped in and eluate and gas left the system; the biomass immobilized spontaneously on the strip of inert polyester fleece, which was suspended from the upper stopper.
Growth of organisms from the trickling filter.
Portions (2 cm) of the polyester fleece from a trickling filter were suspended in sterile basal salts solution and vortexed to detach microorganisms. The fleece was removed aseptically, and the biomass was added to 50 ml of sterile carbon-limited medium with or without fleece (1 cm3) in a 500-ml Erlenmeyer flask which was incubated at 30°C on a rotary shaker. Samples were taken at intervals for turbidity and analysis by HPLC.
Isolation of a pure culture.
Enrichment cultures were streaked on selective agarose plates prepared by solidifying 1 mM LADPEDS-salts medium with 1% agarose. Pinpoint colonies developed after 3 weeks at 30°C in the dark. Single colonies were successfully picked into selective liquid medium (1 mM LADPEDS-salts), e.g., 3-ml cultures with 10 mg of glass wool in 30-ml tubes aerated on a roller. The outgrown culture was subjected to two further rounds of plating and picking, and microscopic purity was confirmed by the absence of colonies on nutrient agar within 1 week.
Growth experiments in suspended culture.
Strain DS-1 could be grown up to at least the 1-1 scale. The quantitative growth experiments with 3-C12-LAS were done in 20-ml cultures in 200-ml shaken Erlenmeyer flasks containing about 2 mM LAS-salts-glass wool medium. Samples (1 ml in Eppendorf tubes) were taken at intervals for turbidity and protein measurements and analyses of the substrate and product. Samples for turbidity and protein measurements were pulsed to 12,000 × g to remove the glass wool but leave the bacteria in suspension. The samples for analyses of the substrate and product were centrifuged to remove wool and bacteria, and the supernatant fluid was diluted 1:20 in 0.1 M H3PO4 and stored frozen.
Analytical methods.
Commercial LAS and SPC as well as LADPEDS and the SDPECs were routinely determined by gradient elutions by reversed-phase HPLC with 125- by 3-mm columns of Nucleosil-5-C18. A diode array detector was used (24). The two mobile phases were 0.11 M NaClO4 and acetonitrile in a system adapted from our published method (20) to new column geometry. The column was equilibrated with 100% of the polar solvent at 0.5 ml/min. This condition was maintained for 2 min after injection (20 to 100 μl), when a linear gradient to 70% nonpolar solvent over 15 min was generated. The new condition was maintained for 10 min, when the concentration of acetonitrile was reduced to 0% over 2 min, and the column was equilibrated for 10 min prior to the next injection. This method was satisfactory for LADPEDS and SDPECs, where individual congeners were present at low concentrations. A different gradient was used for LAS. Here the nonpolar solvent was ramped to 40% over 10 min, the column was eluted isocratically for 5 min, and the nonpolar phase was ramped to 70% over 10 min. The initial conditions were regenerated over 2 min and were equilibrated for 5 min. Peak areas were proportional to the amount of LAS congener at only very low levels of analyte (≤5 nmol); the corresponding value for 2-C4-SPC was about 60 nmol. The main product from 3-C12-LAS, 4-C6-SPC, was quantified with the standard curve for 2-C4-SPC. This presented little problem with LADPEDS or commercial LAS, where ≥20 congeners were present (legend to Fig. 1). When 3-C12-LAS was used in growth experiments, however, samples had to be diluted very strongly to quantify the LAS, and we chose to quantify the product(s). Semipreparative HPLC was done with a 250- by 10-mm Ultrasphere-5-C18 column (Beckman) at 3 ml/min using the mobile phases described above. Ion chromatography with suppressed-conductivity detection was done for sulfate, nitrate, or nitrite as described elsewhere (24). Infrared (IR) spectra of material in tetrahydrofuran were measured in the 0.5-cm cuvette of a Bio-Rad FTS-60 spectrometer.
MALDI-TOF MS was done with a Reflex III spectrometer (Brucker, Bremen, Germany). Samples were cocrystallized with a sinapinic acid or an α-cyano-4-hydroxycinnamic acid matrix and desorbed by pulses from a nitrogen laser at 336 nm.
1H nuclear magnetic resonance (NMR) spectra of material in D2O were obtained in a 400-MHz machine (GX 400; JEOL).
Solid-phase extraction was done on Chromabond HR-P (200 mg; Macherey & Nagel, Düren, Germany) (26). Substrate-dependent oxygen uptake rates in whole cells were measured essentially as described elsewhere (see, e.g., reference 16); in this case, glass wool was removed (see above) before the cells were harvested and used in experiments at 10 times the concentration in the culture. The reaction was initiated by the addition of LADPEDS or LAS to 1 mM in the 0.5-ml reaction vessel. Protein in bacterial cells was assayed by a Lowry-type method (5). Optical density at 580 nm (turbidity) was measured in a Novaspec II spectrophotometer (Pharmacia). Dissolved organic carbon (DOC) was measured in a total organic carbon analyzer (9); we found that the apparatus gave 98 to 102% recovery of carbon for p-toluenesulfonate and 2-C4-SPC but only 30 to 50% for commercial LAS, and we attributed the poor recovery to losses of LAS sorbing to plastic tubing in the apparatus. Methods to establish the Gram reaction and to measure catalase and cytochrome c oxidase activities were described elsewhere (8).
A partial 16S ribosomal DNA sequence of strain DS-1 was determined by the German Culture Collection (Braunschweig, Germany), where the sequence data were aligned and compared as described elsewhere (25, 33).
RESULTS
Enrichment cultures.
We experienced ready degradation of commercial LAS (0.5 mM) as the sole source of carbon and energy in oxic laboratory trickling filters inoculated with sewage from all sources, corresponding to earlier reports (20), but we obtained no satisfactory degradation of 0.5 mM LAS, as determined by HPLC and by the continued foaming in enrichment cultures containing only suspended microorganisms. Similarly, enrichment cultures failed to degrade 0.25 or 0.5 mM commercial LADPEDS as the sole source of carbon and energy for growth with inocula from three sewage treatment plants. No growth was observed in 4-week incubations, and no significant disappearance of LADPEDS was determined by HPLC; correspondingly, the foaming due to LADPEDS did not decrease.
Immobilized mixed cultures.
Trickling filter experiments with, initially, 0.1 mM commercial LADPEDS as the carbon and energy source for growth led to eluates which soon ceased to foam in the spot test; this was interpreted as biodegradation. The inoculum was derived from a mixture of activated sludge from an industrial waste treatment plant (Ludwigshafen, Germany) and the American soil shown to attack LADPEDS. This trickling filter was replaced (see below) with another containing an inoculum from the industrial sludge alone, so the soil was not necessary. The concentration of LADPEDS was slowly increased to 1 mM, and the eluate did not foam; at higher concentrations (e.g., 2.5 mM), foaming returned and bacteria were washed from the filter.
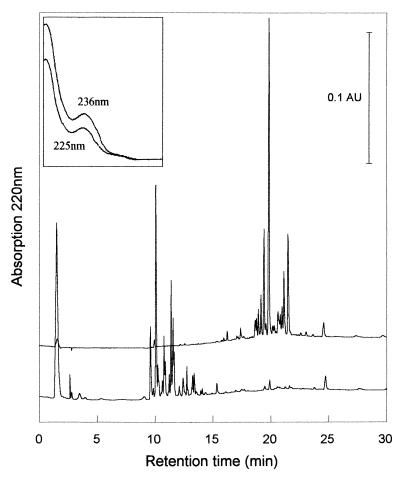
The degradative behavior was examined in detail at 0.33 mM commercial LADPEDS, where, within a week of increasing the influent concentration, reversed-phase HPLC analysis of the eluent showed extensive disappearance (95%) of LADPEDS as well as formation of putative products at a lower retention time (Fig. 2), i.e., of higher polarity. Each product had the same UV spectrum as the substrates (Fig. 2, inset; see also below). We compared the peak areas of the LADPEDS utilized with those of the product peaks (Fig. 2) and found the latter to be 90 to 95% of the former. This implied that the rings were subject to negligible cleavage and desulfonation. We could support this hypothesis when we compared the sulfate concentrations in the inflow to and eluent from the column and determined them to be indistinguishable. The DOC values of the influent and of the effluent were compared. The value(s) for the influent was too low, and we presume that there was loss by sorption to tubing in the total organic carbon apparatus (see Materials and Methods), whereas values for nonpolar standards were accurate. So comparison of the theoretical influent and the observed effluent supports the hypothesis that the C16 LADPEDS (C28 compounds) were converted to C20 compounds, presumably SDPECs with an average side chain of C8.
FIG. 2.
Primary degradation of commercial LADPEDS in a trickling filter as determined by HPLC. LADPEDS-salts medium (0.33 mM; upper chromatogram) was pumped onto the trickling filter, and the eluate in the steady state (lower chromatogram) contained largely peaks of shorter retention time. Inset, UV spectra of representative peaks of substrate (upper scan) and product (lower scan).
The putative SDPECs were subject to solid-phase extraction and analyzed by IR spectroscopy, and the spectra were compared with that of the parent LADPEDS. The products in tetrahydrofuran showed the characteristic band, 1,734 cm−1, for the carbonyl of a carboxylate group (11), which was absent in LADPEDS. We separated out individual products from the putative SDPEC by semipreparative HPLC and examined them by MALDI-TOF MS, but although the apparatus could ionize the parent LADPEDS (Table 1), it did not yield any data that identified the metabolic products.
Requirement for a solid support.
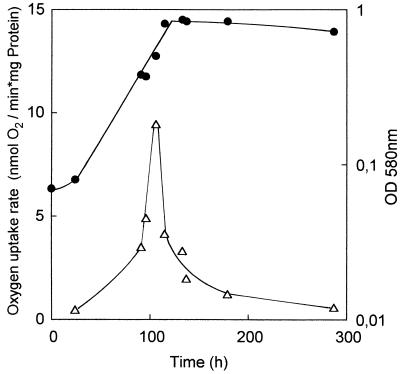
We explored the discrepancy between the enrichment cultures which failed to utilize the commercial LADPEDS (or LAS; see above) and the biotransformation observed in the trickling filters (Fig. 2). Cells which were washed from a portion of the polyester fleece from a trickling filter would only grow in an enrichment culture in the presence of fleece. The fleece, which was sterile at the start of the experiment, was not heavily colonized, and most organisms grew in suspended culture. The products from LADPEDS in these cultures were indistinguishable from those in Fig. 2. We found that glass wool could be used in place of the polyester fleece; the products were unchanged. Here again, the solid phase was not heavily colonized; most of the culture grew in suspension. The change of the solid phase from fleece to glass wool allowed ready harvesting of the degradative organisms, because the glass wool sedimented out of the shaken medium and the supernatant fluid, the growth medium, could be centrifuged to collect the bacteria. It was thus possible to generate growth curves (specific growth rate of 0.02 h−1) with the mixed culture utilizing LADPEDS (Fig. 3) and observe that the specific oxygen consumption rate of the culture was maximal just before the end of growth, after which it fell rapidly (Fig. 3). The culture had a higher specific activity of oxygen consumption with LAS than with LADPEDS, though the patterns were identical (not shown).
FIG. 3.
Growth of the mixed culture in 1 mM LADPEDS-salts-glass wool medium (●) and the specific activity of LADPEDS-dependent oxygen uptake during growth (▵). OD, optical density.
Heterotrophic or specialist organisms?
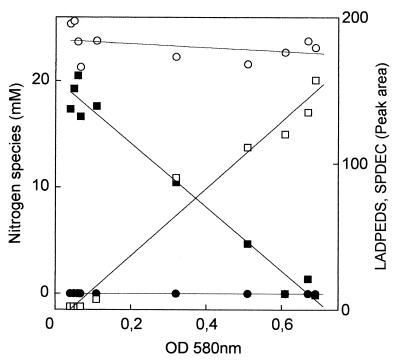
It has been shown that LAS can be degraded after an initial monooxygenation by nonspecific methane monooxygenase (13) and that ammonia monooxygenase could catalyze the same reaction (W. Dong and A. M. Cook, unpublished data); heterotrophic organisms would then catalyze the further reactions. The conditions in the trickling filter did not support methanotrophs but did support ammonia oxidizers (W. Dong and A. M. Cook, unpublished data). We explored the possibility that ammonia oxidizers contributed to the degradation of LADPEDS (Fig. 4). Utilization of LADPEDS and formation of SDPECs were concomitant with growth. No ammonia was consumed during growth, apart from that required for the synthesis of polymers, and neither nitrite nor nitrate was formed (Fig. 4). The culture did not grow when the nitrogen source was changed to nitrate, but we attributed this to an inability to utilize nitrate as the nitrogen source rather than a failure to conserve energy in the absence of the ammonium ion. We concluded that the utilization of LADPEDS in this culture involved solely heterotrophs.
FIG. 4.
Heterotrophic growth of the mixed culture utilizing commercial LADPEDS in suspended culture, including data for LADPEDS (■), SDPEC (□), the ammonium ion (○), and the sum of nitrate and nitrite formed (●). OD, optical density.
Isolation and phylogeny of strain DS-1.
The cultures able to utilize LADPEDS grew with very poor plating efficiency on complex medium, and no single colony or mixture of colonies grew in LADPEDS-salts medium supplemented with glass wool. The experience with ammonia oxidizers (21) led us to try solidifying agents of higher purity and to try selective solid media. Minute colonies were detected in poured LADPEDS-salts medium solidified with agarose; medium without LADPEDS gave negligible growth. Initially we had to pick several colonies to achieve growth in liquid LADPEDS-salts-glass wool medium, but dilution of samples prior to plating and a long incubation period gave pinpoint colonies large enough and well enough separated to be picked individually. Subcultures from one colony gave reproducible growth in selective liquid medium, a homogeneous microscopic picture, and the inability to grow on complex media. We termed this bacterium strain DS-1; it required glass wool or polyester fleece in the medium.
Strain DS-1 growing in LAS-salts-glass wool medium was a short (0.4 to 0.6 μm in length and 0.3 μm in diameter), nonmotile rod, which was gram negative, catalase negative, and oxidase positive. It has been deposited with the German Culture Collection as DSM 13023.
Analysis of a 450-bp fragment of 16S ribosomal DNA indicated that the nearest phylogenetic neighbors are Rhodobium marinum GN-14 (92.7% similarity), R. marinum DSM 2698T (92.4%), and Rhodobium orientis JCM 9337T (90.2%). The Rhodobium spp. are considerably larger (>1 μm) (12) than strain DS-1, and we detected no phototrophic growth. The phylogeny of the isolate will be explored elsewhere.
Growth of α-proteobacterium strain DS-1 with LAS.
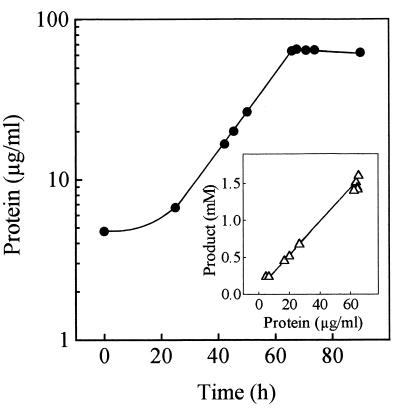
Strain DS-1 in LADPEDS-salts-glass wool medium gave the same products as the mixed culture and the trickling filter (not shown), but the complexity of LADPEDS (Fig. 2 and legend to Fig. 1) meant doing physiological experiments with a range of substrates, so we tested whether strain DS-1 could utilize commercial LAS as well as oxygenate it (see above). We found the turnover of LAS to be essentially 100%, analogous to what is shown in Fig. 2 (not shown), with about nine major products and many minor products formed; each product had the same UV spectrum as the substrate, so we suspected that SPCs were being formed (see Introduction). We then examined the growth of strain DS-1 in 3-C12-LAS medium, where one major product was formed (not shown), as well as several minor products. Analysis of the total products by MALDI-TOF MS yielded an [M − H]− fragment of m/z 271 (Table 1), giving the identity of the major product as a sulfophenylhexanoate, presumably 4-(4-sulfophenyl)hexanoate (4-C6-SPC) (Fig. 1). Correspondingly, an IR spectrum of this product gave a signal at 1,734 cm−1 (carbonyl of a carboxy group [see above]), which was absent in the substrate. The 1H NMR spectrum of the major product in D2O gave four sets of peaks with chemical shifts downfield of tetramethylsilane: 0.89 to 0.93 ppm (t, 3H), CH3 (C-6); 1.74 to 2.44 ppm (5m, 6H), 3 CH2 (C-5, C-3, C-2); 2.75 to 2.8 ppm (m, 1H), CH (C-4); and 7.55 to 7.93 ppm (pq, 4H); the aromatic protons indicated para substitution of the sulfonate group (27). The spectrum supports the identity of the product as 4-C6-SPC. The organism grew with a specific growth rate of 0.05 h−1. Product formation was concomitant with growth and was quantitative (1.6 mM product formed from 1.7 mM 3-C-12-LAS) (Fig. 5). The molar growth yield was about 6.4 g of protein/mol of C (Fig. 5), allowing for six carbon atoms utilized per mole of 3-C12-LAS (Fig. 1).
FIG. 5.
Growth of α-proteobacterium strain DS-1 in 1.7 mM 3-C12-LAS–salts–glass wool medium (●) with concomitant quantitative formation of 4-C6-SPC (inset).
Analysis by MALDI-TOF MS (Table 1) of all material extracted from growth medium by solid-phase extraction showed several other peaks. We inferred that the peak at m/z 293 also represented 4-C6-SPC [M − 2H + Na]−, and we presumed that the peaks at m/z 285 and 299 represented homologs deriving from impurities in the substrate.
The LAS concentration routinely used in laboratory trickling filters was 0.5 mM (20), but experiments were often initiated with 0.1 mM LAS to avoid toxic effects of LAS on the inoculum. Strain DS-1 was tested for growth at different concentrations in suspended culture and was found to grow at the highest concentration tested, in 5 mM LAS-salts-glass wool medium. All these experiments showed LAS to be present in solution; whatever sorption to the solid phase occurred, it did not significantly reduce the concentration of LAS to which the bacteria were exposed.
DISCUSSION
We may not be the first to isolate an organism able to utilize commercial LAS as a sole source of carbon and energy for growth (2, 14, 41), but we are the first to present a pure culture whose mass balance during heterotrophic growth is established (Fig. 5; see below) and whose product is identified (Table 1). In addition, the organism is able to grow at concentrations of LAS which are toxic to many other bacteria. We do not yet know whether strain DS-1 is representative of many LAS degraders. Cometabolic reactions of multicomponent monooxygenases in autotrophs or methanotrophs are suspected or known (W. Dong and A. M. Cook, unpublished data; 13), and the anticipated follow-up product of such a monooxygenation is observed in the field (10). However, the requirement of strain DS-1 for a surface to allow growth in pure culture, coupled to the lack of growth on complex medium, would be a very good explanation for the failure of many attempts to obtain such organisms.
The requirement for a surface to support the growth of organisms utilizing LAS or LADPEDS in an activated sludge plant presents no problem. Indeed, a major aim in the treatment plant is to have the degradative organisms flock out. For the unsuspecting microbiologist, however, this phenomenon can be a serious stumbling block, which we overcame by using a laboratory trickling filter (1, 18) to establish biodegradation in the laboratory before considering the organisms involved. The filter can be set up simply and reproducibly (e.g., after ruining a column by adding excessive concentrations of LADPEDS). These data confirm extensive biotransformation to SDPECs (Fig. 2) (see also below), as indicated previously (Dow product information 1510-014A, p. 1–5, 1996).
The molar growth yield we observe with a single substrate (Fig. 5) is 6.4 g of protein/mol of C. This is the expected value, allowing for 50% incorporation of substrate carbon into cell material and 50% respiration (3), and indicates mass balance for metabolism of the carbon removed from LAS. Quantitative formation of 4-C6-SPC concomitant with growth (Fig. 5) confirms the overall mass balance. A specific degradation rate of LAS of 2.2 mkat/kg of protein can be calculated from the specific growth rate and the growth yield we measured. This is a relatively high value (16) and should allow biochemical work with the LAS oxygenase in cell extracts. Qualitatively similar data were obtained for growth with LADPEDS (Fig. 3) in mixed culture.
Phylogenetically, strain DS-1 belongs to the α-proteobacteria, the closest relationship being to R. marinum (basonym Rhodopseudomonas palustris). We found no support for anaerobic, phototrophic growth and atypically small cells for Rhodobium, so we suspect that strain DS-1 may represent a new species or even a new genus.
We have, allowing for impurities in the substrate, a single product from 3-C12-LAS (Table 1), which we have identified as 4-(4-sulfophenyl)hexanoate. This product is consistent with the theory that metabolism of LAS proceeds via an ω oxygenation to the alcohol, oxidation to the acid, and thioesterification to allow β oxidation, followed by de-esterification and excretion (see, e.g., reference 4). It does not exclude the suggestion that subterminal monooxygenation yields a secondary alcohol and that a Bayer-Villiger monooxygenation generates an ester, which is de-esterified and excreted while the alcohol is utilized for growth; the latter hypothesis would explain why some reviewers (37, 42) note SPC of the wrong length for cycles of β oxidation. We hope to be able to answer the question with strain DS-1. Whatever happens, we observe about half as many products (not shown) from commercial LAS as there are congeners of LAS, which may suggest that there is one attack only on the LAS molecule, that the C10 and C12 homologues give about four products, and that the C11 and C13 homologues give another five products.
The release of the free carboxylate, the SPC (Fig. 1 and 5), from LAS by strain DS-1 corresponds to the degradative intermediates observed in, e.g., sewage works and rivers (see, e.g., references 6 and 7). Our data (UV and IR spectra) on the degradation of LADPEDS indicate that here too the free carboxylate is formed. We have, however, no mass spectrum information to confirm this. But the general similarity of LAS and LADPEDS as arylsulfonate-substituted alkanes and the identification of the 4-C6-SPC from 3-C12-LAS are strong arguments in favor of the same enzymes being involved in the metabolism of both sets of compounds. Further, the identical patterns of oxygenation of LAS and LADPEDS by whole cells from a LADPEDS-grown culture (Fig. 3) support this hypothesis.
The pattern of measurable specific activity of oxygenation of LAS and LADPEDS (Fig. 3) is reminiscent of the pattern observed under analogous conditions with many substrates involving multicomponent oxygenations (15, 16). The effect is apparently due to specific degradation of one component of these oxygenases (29), many examples of which are deduced by Junker et al. (15). We must wait for the biochemical elucidation of the pathway to establish which kind of multicomponent oxygenase (see, e.g., reference 35) is involved in strain DS-1.
Whatever enzymes are involved in the initial attack on commercial LAS, it is now clear that several organisms are needed for its complete degradation. This was, in any case, the generally accepted view (4) before any pure culture was available (14, 41). However, now that we have a pure culture (strain DS-1) with a known product, as well as a pure culture of narrow substrate range, Delftia acidovorans SPB1, able to degrade a defined SPC, 2-C4-SPC (39), it is clear that three tiers of organisms are needed to degrade commercial LAS, (i) organisms of the metabolic type represented by strain DS-1, (ii) organisms which further shorten the side chain, and (iii) the ring cleavage and desulfonation specialists. This corresponds to data from Hrsak and Begonja (13), who observe (i) a methanotroph that oxygenates LAS to the corresponding SPC and (ii) a set of heterotrophs that yield short-chain SPC, which is not degraded in their culture. Given the high tonnage of LAS degraded worldwide, it will be interesting to see how many other tactics are used by other organisms (see, e.g., reference 2).
ACKNOWLEDGMENTS
We are grateful to U. Baumann, EMPA St. Gallen, CH, for advice on the trickling filter and to T. Hitzler, who did the LAS enrichments in suspended culture. The MALDI-TOF MS measurements were made by K. Hollemeyer. The NMR spectra were obtained by E. Krienitz. Bodensee Wasserversorgung, Sipplingen, kindly did the DOC analyses.
D.S. was supported by funds from Dow; W.D. was supported by an exchange studentship from the China-Gesellschaft EV and consumables from ECOSOL (Brussels, Belgium) and the University of Konstanz.
REFERENCES
- 1.Baumann U, Kuhn G, Schefer W. Rasche Bestimmung des Bioabbaus organischer Stoffe in einem Labor-Tropfkörper. Z Wasser-Abwasser-Forsch. 1990;23:129–132. [Google Scholar]
- 2.Campos-García J, Esteve A, Vázquez-Duhalt R, Ramos J L, Soberón-Chávez G. The branched-chain dodecylbenzene sulfonate degradation pathway of Pseudomonas aeruginosa W51D involves a novel route for degradation of the surfactant lateral alkyl chain. Appl Environ Microbiol. 1999;65:3730–3734. doi: 10.1128/aem.65.8.3730-3734.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cook A M. Biodegradation of s-triazine xenobiotics. FEMS Microbiol Rev. 1987;46:93–116. [Google Scholar]
- 4.Cook A M. Sulfonated surfactants and related compounds: facets of their desulfonation by aerobic and anaerobic bacteria. Tenside Surfactants Deterg. 1998;35:52–56. [Google Scholar]
- 5.Cook A M, Hütter R. s-Triazines as nitrogen sources for bacteria. J Agric Food Chem. 1981;29:1135–1143. [Google Scholar]
- 6.di Corcia A, Samperi R, Marcomini A. Monitoring aromatic surfactants and their biodegradation intermediates in raw and treated sewages by solid-phase extraction and liquid chromatography. Environ Sci Technol. 1994;28:850–858. doi: 10.1021/es00054a016. [DOI] [PubMed] [Google Scholar]
- 7.Field J A, Miller D J, Field T M, Hawthorne S B, Giger W. Quantitative determination of sulfonated aliphatic and aromatic surfactants in sewage sludge by ion-pair/supercritical fluid extraction and derivatization gas chromatography/mass spectrometry. Anal Chem. 1992;64:3161–3167. doi: 10.1021/ac00048a013. [DOI] [PubMed] [Google Scholar]
- 8.Gerhardt P, Murray R G E, Wood W A, Krieg N R, editors. Methods for general and molecular bacteriology. Washington, D.C.: American Society for Microbiology; 1994. [Google Scholar]
- 9.Gesellschaft Deutscher Chemiker. German standard methods for the laboratory examination of water, waste water and sludge. Weinheim, Germany: VCH; 1996. [Google Scholar]
- 10.González-Mazo E, Honing M, Barceló D, Gómez-Parra A. Monitoring long-chain intermediate products from the degradation of linear alkylbenzene sulfonates in the marine environment by solid-phase extraction followed by liquid chromatography/ionspray mass spectrometry. Environ Sci Technol. 1997;31:504–510. [Google Scholar]
- 11.Hellmann H. Aluminiumoxid als Ionenaustauscher bei der Analyse von Kationtensiden und Alkylbenzolsulfonaten (LAS) in Klärschlamm. Z Wasser-Abwasser-Forsch. 1989;22:4–12. [Google Scholar]
- 12.Holt J G, Krieg N R, Sneath P H A, Staley J T, Williams S T, editors. Bergey's manual of determinative bacteriology. 9th ed. Baltimore, Md: Williams & Wilkins; 1994. [Google Scholar]
- 13.Hrsak D, Begonja A. Growth characteristics and metabolic activities of the methanotrophic-heterotrophic groundwater community. J Appl Microbiol. 1998;85:448–456. doi: 10.1046/j.1365-2672.1998.853505.x. [DOI] [PubMed] [Google Scholar]
- 14.Jiménez L, Breen A, Thomas N, Federle T W, Sayler G S. Mineralization of linear alkylbenzene sulfonate by a four-member aerobic bacterial consortium. Appl Environ Microbiol. 1991;57:1566–1569. doi: 10.1128/aem.57.5.1566-1569.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Junker F, Field J A, Bangerter F, Ramsteiner K, Kohler H-P, Joannou C L, Mason J R, Leisinger T, Cook A M. Oxygenation and spontaneous deamination of 2-aminobenzenesulphonic acid in Alcaligenes sp. strain O-1 with subsequent meta ring cleavage and spontaneous desulphonation to 2-hydroxymuconic acid. Biochem J. 1994;300:429–436. doi: 10.1042/bj3000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Junker F, Leisinger T, Cook A M. 3-Sulphocatechol 2,3-dioxygenase and other dioxygenases (EC 1.13.11.2 and EC 1.14.12.-) in the degradative pathways of 2-aminobenzenesulphonic, benzenesulphonic and 4-toluenesulphonic acids in Alcaligenes sp. strain O-1. Microbiology (Reading) 1994;140:1713–1722. doi: 10.1099/13500872-140-7-1713. [DOI] [PubMed] [Google Scholar]
- 17.Klein A. Latex technology. In: Grayson M, editor. Kirk-Othmer encyclopedia of chemical technology. 3rd ed. Vol. 14. New York, N.Y: Wiley; 1983. pp. 82–97. [Google Scholar]
- 18.Kölbener P, Baumann U, Cook A M, Leisinger T. 3-Nitrobenzenesulfonic acid and 3-aminobenzenesulfonic acid in a laboratory trickling filter: biodegradability with different activated sludges. Water Res. 1994;28:1855–1860. [Google Scholar]
- 19.Kölbener P, Baumann U, Leisinger T, Cook A M. Linear alkylbenzenesulfonate (LAS) surfactants in a simple test to detect refractory organic carbon (ROC): attribution of recalcitrants to impurities in LAS. Environ Toxicol Chem. 1995;14:571–577. [Google Scholar]
- 20.Kölbener P, Baumann U, Leisinger T, Cook A M. Non-degraded metabolites arising from the biodegradation of commercial linear alkylbenzenesulfonate (LAS) surfactants in a laboratory trickling filter. Environ Toxicol Chem. 1995;14:561–569. [Google Scholar]
- 21.Koops H-P, Möller U C. The lithotrophic ammonia-oxidizing bacteria. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. 2nd ed. Vol. 2. Berlin, Germany: Springer-Verlag; 1992. pp. 2625–2637. [Google Scholar]
- 22.Kosswig K. Herstellung, Eigenschaften und Verwendung von Tensiden. In: Kosswig K, Stache H, editors. Die Tenside. Munich, Germany: Carl Hanser Verlag; 1993. pp. 115–177. [Google Scholar]
- 23.Kosswig K. Surfactants. In: Gerhartz W, Elvers B, editors. Ullmann's encyclopedia of industrial chemistry, 5 ed. A25. Weinheim, Germany: VCH; 1994. pp. 747–817. [Google Scholar]
- 24.Laue H, Field J A, Cook A M. Bacterial desulfonation of the ethanesulfonate metabolite of the chloroacetanilide herbicide metazachlor. Environ Sci Technol. 1996;30:1129–1132. [Google Scholar]
- 25.Maidak B L, Olsen G J, Larsen N, Overbeek R, McCaughey M J, Woese C R. The ribosomal database project (RDP) Nucleic Acids Res. 1996;24:82–85. doi: 10.1093/nar/24.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mampel J, Hitzler T, Ritter A, Cook A M. Desulfonation of biotransformation products from commercial linear alkylbenzenesulfonates (LAS) Environ Toxicol Chem. 1998;17:1960–1963. [Google Scholar]
- 27.Marcomini A, di Corcia A, Samperi R, Capri S. Reversed-phase high-performance liquid chromatographic determination of linear alkylbenzene sulfonates, nonylphenol polyethoxylates and their carboxylic biotransformation products. J Chromatogr. 1993;644:59–71. [Google Scholar]
- 28.Matthijs E, Holt M S, Kiewiet A, Rijs G B J. Fate of surfactants in activated sludge waste water treatment plants: outcome of field studies. Tenside Surfactants Deterg. 1997;34:238–241. [Google Scholar]
- 29.Moodie F D L, Woodland M P, Mason J R. The reductase component of the chromosomally encoded benzoate dioxygenase from Pseudomonas putida C-1 is immunologically homologous with a product of the plasmid encoded xylD gene (toluate dioxygenase) from Pseudomonas putida mt-2. FEMS Microbiol Lett. 1990;71:163–168. [Google Scholar]
- 30.Morse P M. Soaps & detergents. Chem Eng News. 1999;77:35–48. [Google Scholar]
- 31.Organization for Economic Cooperation and Development. Guidelines for testing chemicals. Paris, France: Organization for Economic Cooperation and Development; 1992. [Google Scholar]
- 32.Powlowski J, Shingler V. Genetics and biochemistry of phenol degradation by Pseudomonas sp. CF600. Biodegradation. 1994;5:219–236. doi: 10.1007/BF00696461. [DOI] [PubMed] [Google Scholar]
- 33.Rainey F A, Ward-Rainey N, Kroppenstedt R M, Stackebrandt E. The genus Nocardiopsis represents a phylogenetically coherent taxon and a distinct actinomycete lineage: proposal of Nocardiopsaceae fam. nov. Int J Syst Bacteriol. 1996;46:1088–1092. doi: 10.1099/00207713-46-4-1088. [DOI] [PubMed] [Google Scholar]
- 34.Rouse J D, Sabatini D A, Harwell J H. Minimizing surfactant losses using twin-headed anionic surfactants in subsurface remediation. Environ Sci Technol. 1993;27:2072–2078. [Google Scholar]
- 35.Sabatini D A, Knox R C, Harwell J H, Soerens T, Chen L, Brown R E, West C C. Design of a surfactant remediation field demonstration based on laboratory and modeling studies. Ground Water. 1997;36:954–963. [Google Scholar]
- 36.Schöberl P. Basic principles of LAS biodegradation. Tenside Surfactants Deterg. 1989;26:86–94. [Google Scholar]
- 37.Schöberl P. Biologischer Tensid-Abbau. In: Kosswig K, Stache H, editors. Die Tenside. Munich, Germany: Carl Hanser Verlag; 1993. pp. 407–464. [Google Scholar]
- 38.Schöberl P. Linear alkylbenzenesulphonate (LAS) monitoring in Germany. Tenside Surfactants Deterg. 1997;34:233–237. [Google Scholar]
- 39.Schulz S, Dong W, Groth U, Cook A M. Enantiomeric degradation of 2-(4-sulfophenyl)butyrate via 4-sulfocatechol in Delftia acidovorans SPB1. Appl Environ Microbiol. 2000;66:1905–1910. doi: 10.1128/aem.66.5.1905-1910.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schulze K. Der westeuropäische Tensidmarkt 1994/1995. Tenside Surfactants Deterg. 1996;33:94–95. [Google Scholar]
- 41.Sigoillot J-C, Nguyen M-H. Complete oxidation of linear alkylbenzene sulfonate by bacterial communities selected from coastal seawater. Appl Environ Microbiol. 1992;58:1308–1312. doi: 10.1128/aem.58.4.1308-1312.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swisher R D. Surfactant biodegradation. 2nd ed. New York, N.Y: Marcel Dekker; 1987. [Google Scholar]
- 43.Widdel F, Bak F. Gram-negative mesophilic sulfate-reducing bacteria. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. 2nd ed. Vol. 4. Berlin, Germany: Springer-Verlag; 1992. pp. 3352–3378. [Google Scholar]