Abstract
The resistance of cancer and Helicobacter pylori to several drugs reflects a worldwide problem, and it has been the intention of numerous researchers to overcome this problem. Thus, in this study, Acacia nilotica fruits were subjected to HPLC analysis to detect their phenolic compounds and flavonoids. Moreover, A. nilotica‘s anti-H. pylori activity and its inhibitory activity against human hepatocellular carcinoma (HepG-2 cells) were reported. Various compounds with different concentrations, such as ferulic acid (5451.04 µg/mL), chlorogenic acid (4572.26 µg/mL), quercetin (3733.37 µg/mL), rutin (2393.13 µg/mL), gallic acid (2116.77 µg/mL), cinnamic acid (69.72 µg/mL), hesperetin (121.39 µg/mL) and methyl gallate (140.45 µg/mL), were detected. Strong anti-H. pylori activity at 31 mm was reported, compared to the positive control of the 21.67 mm inhibition zone. Moreover, the MIC and MBC were 7.8 µg/mL and 15.62 µg/mL, respectively, while the MIC and MBC of the positive control were 31.25 µg/mL. The concentration of MBC at 25%, 50% and 75% reflected H. pylori’s anti-biofilm activity of 70.38%, 82.29% and 94.22%, respectively. Good antioxidant properties of the A. nilotica flower extract were documented at 15.63, 62.50, 250 and 1000 µg/mL, causing the DPPH scavenging percentages of 42.3%, 52.6%, 65.5% and 80.6%, respectively, with a IC50 of 36.74 µg/mL. HepG-2 cell proliferation was inhibited (91.26%) using 500 µg/mL of flower extract with an IC50 of 176.15 µg/mL, compared to an IC50 of 395.30 µg/mL used against human normal melanocytes. Molecular docking was applied to investigate ferulic acid with the H. pylori (4HI0) crystal structure to determine the best binding mode that interacted most energetically with the binding sites. Molecular docking indicated that ferulic acid was a proper inhibitor for the 4HI0 protein enzyme of H. pylori. A low energy score (−5.58 Kcal/mol) was recorded as a result of the interaction of ferulic acid with the residue’s SER 139 active site caused by the O 29 atom, which was important for its antibacterial activity.
Keywords: Acacia nilotica, flowers, Helicobacter pylori, hepatocellular carcinoma, in silico
1. Introduction
Since the dawn of ancient times, humans have used medicinal plants, and they have continued to play a significant role in the development of safer and more efficient natural drug delivery methods [1,2,3]. The black babul, kikar or the gum arabic tree, often referred to as Acacia nilotica (native to Egypt), is a member of the Fabaceae family, which is extensively distributed throughout several nations. Being a common and significant plant, it is used in a variety of ways from medicine (root, bark, leaves, forbear, gum and pods) to feed (leaves and shoots for animals) and dyeing (leather color) [4]. All parts of A. nilotica, including the seeds, gum, flowers, leaves, bark, roots and pods, are therapeutically important and have been applied in the traditional medicines [5]. It is effective in the treatment of coughs, congestion, cold, nerve stimulation, dysentery, leucorrhea, hemorrhages, and ophthalmia, can offer sclerosis relief and wound healing, and has antiulcer, anti-inflammatory, anthelmintic, diuretic, antihypertensive and antipyretic effects [6]. Pharmacological reports have indicated that A. nilotica extract has antioxidant activity and insulin sensitizing properties, which help with declining obesity rates and minimizing hyperlipidemia [7]. Edible and nutritional characteristics were associated with A. nilotica seed due to the presence of numerous phytochemicals [8].
Using a variety of in vitro experiments, Kaur et al. [4] extracted betulin from the ethyl acetate fraction of the methanol extract of A. nilotica and evaluated its various antioxidant, cytoprotective and anti-inflammatory properties. A. nilotica may be found in many tropical and subtropical areas. It has been used to prevent and treat a variety of illnesses and infectious disorders since it contains many bioactive chemicals. Extracts of A. nilotica have positive antioxidant and antimalarial properties [9]. The plant contains many polyphenolic chemicals such as catechins, which are thought to have anti-inflammatory and antioxidant properties [10]. Hepatitis C virus protease and multidrug resistant bacterial pathogens have both been shown to be inhibited by A. nilotica [11]. Several phenolic constituents with an extensive range of biological activity were found in the plant’s aerial parts [12]. The use of green extraction techniques has developed and increased in recent years to obtain the natural molecules. These techniques, such as enzyme-aided extraction, pressurized liquid extraction, supercritical fluid extraction, ultrasonic/microwave-assisted extraction, maceration and hydrodistillation, have been used to increase the yield of extracted molecules and reduce the negative impact of chemicals on the environment [13,14,15]. The biological effects of flower and leaf extracts from Acacia species, notably, A. saligna, have not been well studied. The antioxidant and antibacterial effects of Egyptian A. saligna flower water extracts were ineffective, while the antifungal effects were strong [16]. In comparison to A. laeta extracts, Egyptian A. nilotica and A. seyal leaf extracts have shown stronger antioxidant activity [17]. Previous studies have demonstrated that leaf extracts of the Egyptian A. saligna may qualitatively include phenolic acids (e.g., gallic acid) and flavonoids (e.g., quercetin, quercitrin, apigenin, apigenin 7-glucoside, astragalin, luteolin, myricetin and kaempferol) [18]. A study conducted in an eastern region of Saudi Arabia on the aerial parts of Acacia species (A. salicina, A. laeta, A. hamulosa and A. tortilis) showed significant cytotoxic activity in A. laeta and A. hamulosa against HepG2 and breast cancer cell lines [19]. Hordeum murinum was resistant to flower extracts from other Acacia species [20]. Another study on the flower’s extracts has documented the presence of phenolic acids (benzoic acid, caffeic acid, o-coumaric acid, p- hydroxybenzoic acid and ellagic acid) and flavonoids (quercetin, naringenin and kaempferol) [16].
It is known that Helicobacter pylori, which thrives and multiplies in acidic environments, is typically responsible for gastric and duodenal ulcers [21,22]. It is well known that, early in life, human stomachs can suffer from the invasion of H. pylori, but the detection of this pathogen may be discovered later. Although 50% of individuals worldwide are infected with H. pylori, as mentioned in several reports from developing nations [20,21], but about 15% are only characterized with the appearance of infection symptoms, such as gastritis, peptic ulcer and gastric adenocarcinoma [23]. H. pylori infection was induced by urease (a nickel-containing enzyme) via an appropriate pH to prolong its survival; thus, the urease activity inhibition should be a strong tool to control the infection of H. pylori. The urease of H. pylori breaks down urea into ammonia, and the released ammonia shields it from the stomach’s acidic environment.
Until now, the management of H. pylori infections is a worldwide problem, so the discovery or development of bioproducts can represent a strategy to overcome this problem. An A. vera gel loaded with chitosan nanoparticles was a promising treatment tool for H. pylori infection [24], as documented in the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the A. nilotica leaf extract against H. pylori of 180 g/mL and 5 mg/mL, respectively. The stronger anti-H. pylori and significant inhibition of urease activity has been reported via the extracts of A. nilotica [25]. In vitro and in vivo tests of the A. nilotica extract revealed high anti-urease activity and significant anti-adhesion properties [26]. Therefore, these studies indicated that A. nilotica could influence the colonization of H. pylori in the stomach, and its potential pharmaceutical use for the eradication of H. pylori could be further investigated. Moreover, these compounds were chosen for molecular docking studies with targeted H. pylori virulent proteins such as cytotoxic-associated gene A (CagA), vacuolating-associated gene (VacA), urease, and gastric cancer-induced signaling proteins such as the epidermal growth factor receptor and the vascular endothelial growth factor receptor. All the compounds of the A. nilotica leaf extract demonstrated good drug-likeness scores. A. nilotica leaf extract could act as a potent anti-H. pylori and a gastric cancer drug, as demonstrated by the validation of the in vitro and in silico results of Sampath et al. [27].
Different species of Acacia were tested to repress the proliferation of several cancer cells. For instance, kidney, breast and liver cells were repressed by the extract of aerial parts of Acacia hamulosa, A. salicina, A. laeta and A. tortilis [19]. In addition, human cancer cell lines, such as those of MCF-7, A549 and THP-1 cells, were influenced by A. catechu and A. nilotica extracts [28,29]. A. nilotica leaf extracts exhibited anticancer activity against Hep-2 (cervical cancer) and MDA-MB-231 (breast cancer) [30]. Numerous scientific texts have almost wholly focused on the biological screening as well as the phytochemical constituents of the leaves, seeds, pod, root and bark of A. nilotica. However, the biological effects of A. nilotica flowers are limited. In this study, therefore, an investigation has been conducted to test the extract of A. nilotica flowers against H. pylori and its anticancer activities.
2. Materials and Methods
2.1. Chemicals
Analytical grade chemicals were obtained from Sigma-Aldrich, Taufkirchen, Germany, including DPPH (2,20 -diphenyl-1-picrylhy drazyl), dimethyl sulfoxide (DMSO), ascorbic acid, trypan blue dye, crystal violet, L-glutamine, 25% Trypsin-EDTA, gentamycin, DMEM, RPMI-1640, fetal bovine serum and bacterial growth medium. Active Fine Chemicals Limited, Dhaka, Bangladesh was the source of solvents and reagents.
2.2. Collection and Extraction of Acacia nilotica Flowers
A. nilotica was grown at the canal banks crossing the Delta, Monufia Governorate, Egypt. Flowers of A. nilotica were collected from three plants of the same species that were identified according to the botanical keys [31]. The plant was identified by Prof. Marei A. Hamed, Prof. of plant taxonomy. The voucher sample of A. nilotica has been placed in the herbal collection in the Botany and Microbiology Department, Faculty of Science, Al-Azhar University, Egypt.
Healthy and fresh flowers were collected and washed with clean water. Then, they were dried at 50 °C until a constant weight was obtained. The ground flowers were extracted using methanol via rotary evaporator. The obtained extract was weighed and stored at 5 °C in a refrigerator until further analysis.
2.3. HPLC Analysis of Acacia nilotica Flower Extract
Once the extraction process finished, 5 μL of the A. nilotica flower extract was inserted into the HPLC apparatus (Agilent 1260 series, Agilent Technologies, Santa Clara, CA, USA). The specifics of the utilized Eclipse C18 column comprised 4.6 mm × 250 mm i.d., 5 μm and 40 °C. Mobile phase included the use of two buffers: A (0.05% trifluoroacetic acid with mili Q water) and B (0.05% trifluoroacetic acid with acetonitrile). The buffers were applied at 0.9 mL/min as a rate of flow. The serial for buffer A, which had a 20 min run for the mobile phase, was 82, 80, 60, 60, 82, 82 and 82% for 0, 0–5, 5–8, 8–12, 12–15, 15–16, 20–20 min. By using an ultraviolet (UV) detector at a wavelength of 280 nm, the contents of phenolic compounds and flavonoids were identified. The quantity of each was documented based on the injected standard compounds [32].
2.4. Assessment of Anti-H. pylori Activity of A. nilotica Flower Extract
H. pylori was obtained from Ain Shams University Hospital, Cairo, Egypt as a tested organism to perform these experiments. The in vitro anti-H. pylori activities were detected using a well agar diffusion technique with the following procedures: A volume of 100 μL suspension of H. pylori (1.0 × 108 colony forming units (CFUs)/mL) was seeded in Mueller Hinton agar amended with 10% blood and blood products and poured in plates. After solidification, a hole with a diameter of 6 mm was punched aseptically with a sterile cork borer. Then, 100 µL of the A. nilotica flower extract was loaded into the well. The well loaded with DMSO was utilized as the negative control, while the well loaded with clarithromycin (0.05 mg/mL) was utilized as the positive control. The inoculated plates were incubated at 37 °C with specific conditions (microaerophilic with humidity) for three days. Then, the diameter of the visualized inhibition zone was detected [33].
2.5. Minimal Inhibitory Concentration (MIC) Experiment
A. nilotica flower extract was subjected to detect its MIC against H. pylori via micro-dilution broth, utilizing Mueller–Hinton broth containing lysed horse blood. Different serial dilutions containing 0.98 to 1000 μg/mL of A. nilotica flower extract were prepared. Each appropriate dilution of A. nilotica flower extract (200 μL) was dispensed per well of sterile in 96-well polystyrene microtitrate plates. The inoculum of H. pylori from fresh culture was prepared in sterile NaCl (0.85%) to equal the standard of 1.0 McFarland turbidity. A total of 2 µL of H. pylori inoculum was added to each well to obtain a final dose of 3.0 × 106 colony forming units)/mL. The plates were incubated under microaerophilic situations (15% CO2) at 35 °C for 3 days. Then, the MIC was evaluated visually, reflecting the whole growth inhibition of H. pylori. H. pylori inoculum without A. nilotica flower extract was used as a positive control, while A. nilotica flower extract without H. pylori inoculum was used as a negative control in each microplate [34].
2.6. Minimal Bactericidal Concentration (MBC) Experiment
MBC was performed via sub-culturing 100 mL of the H. pylori culture from each well that presented thorough inhibition of growth, from the last positive and from the growth control, onto the Mueller–Hinton agar plates (5% horse blood). The plates were incubated under microaerophilic situations (15% CO2) at 35 °C for 3 days. Then, the MBC was evaluated visually, reflecting complete inhibition of H. pylori growth by the lowest concentration of A. nilotica flower extract. To detect either the bactericidal or bacteriostatic agent of the A. nilotica flower extract, the ratios of MBC/MIC were estimated. If the MBC/MIC ratio was no higher than four times that of the MIC, the extract possessed bactericidal efficacy [35].
2.7. Microtiter Plate Test for Biofilm Quantification
The influence of A. nilotica flower extract on the formation of H. pylori biofilm was assessed in 96-well polystyrene flatbottom plates. Fresh trypticase soy yeast broth inoculated with H. pylori (250 μL containing 106 CFU/mL) was transferred to each microplate and was well supported with the different levels of the sub-lethal quantity of MBC (25, 50 and 75%), which was detected in the current investigation. The plates were incubated for 48 h at 37 °C. Then, after finishing this period, the supernatant was removed. The sterile distilled water for each well was then used to remove free-floating cells. Then, the plates were dried for 30 min in the air. The formed H. pylori biofilm was stained by 0.1% aqueous solution of crystal violet for 15 min at 25 °C (room temperature), followed by removal of the excess of crystal violet via sterile distilled water. To completely remove the dye bound to the H. pylori cells, 250 μL of ethanol (95%) was added to each well as a dye-solubilized agent. Then, all wells were incubated for 15 min, and, via a microplate reader, the absorbance was measured at 570 nm [36].
| (1) |
The absorbance of the media is represented with Blank, the absorbance of H. pylori with treatment is represented with sample, and the absorbance of H. pylori without any treatment is represented with control.
2.8. Urease Activity Inhibition Assessment
The examined solution mixture was contained of urea (850 μL), the extract (in the range of 0 to 100 μL) and phosphate buffer (100 mM, pH 7.4) to reach the total value of 985 μL. The enzymatic reactions began with the addition of urease (15 μL). Then, they were measured via detecting the concentration of ammonia after 60 min utilizing 500 μL of solution A (composed of 0.5 g phenol and 2.5 mg of sodium nitroprusside in 50 mL of distilled H2O) and 500 μL of solution B (composed of 250 mg NaOH and 820 μL of NaOCl 5% in 50 mL of distilled H2O) for 30 min at 37 °C. The activity of uninhibited urease was selected as 100% of the control activity. The absorbance of the reaction mixture was assessed at 625 nm, and, by using the following formula, the enzymatic activity was determined:
| (2) |
The absorbance of the flower extract in the existence of urease is represented as T, and the absorbance of the solvent in the existence of urease is represented as C (control) [37].
The concentration that induces an inhibition halfway among the minimum and maximum response of each compound (IC50) was determined to monitor the inhibition influence of different concentrations of flower extract in the assay. Inhibition activity of hydroxyurea was also evaluated as the standard compound.
2.9. Estimation of A. nilotica Flower Extract Antioxidant Activity via DPPH Radical Scavenging Method
A. nilotica flower extract was tested for its capacity to scavenge free radicals using 1,1-diphenyl-2-picryl hydrazyl (DPPH). A total of 0.1 mM DPPH solution in ethanol was prepared; then, 1 mL of this solution was added to 3 mL of A. nilotica flower extract in ethanol at various levels (3.9, 7.8, 15.62, 31.25, 62.5, 125, 250, 500 and 1000 g/mL). Only those extracts that were soluble in ethanol were used in this case, and different concentrations of those extracts were created using the dilution method. The mixture was vigorously shaken before being left to stand at room temperature for 30 min. Then, absorbance was determined at 517 nm (UV-VIS Milton Roy), using spectrophotometer. Ascorbic acid was used as the reference standard compound, and three copies of the experiment were run. Using a log dose inhibition curve, the sample’s IC50 value—the amount of A. nilotica flower extract necessary to inhibit 50% of the DPPH free radical—was determined [24]. The following equation was used to estimate the percentage of the DPPH scavenging outcome:
| DPPH scavenging (%) = A0 − A1/A0 × 100 |
where A0 is the absorbance of the control reaction and A1 is the absorbance in the presence of A. nilotica flower extract or ascorbic acid.
2.10. Viability Assay for the Evaluation of the Cytotoxicity of A. nilotica Flower Extract
Cytotoxic effects of A. nilotica flower extract were tested against human hepatocellular carcinoma cells (HepG-2 cells) that were obtained from VACSERA Tissue Culture Unit, Egypt, and human normal melanocytes (HFB-4 cells) that were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA).
All cells were sub-cultured twice a week and kept at 37 °C in a humid environment with 5% CO2. Cells were seeded in a 96-well plate at a density of 1 × 104 cells per well containing growth medium (100 µL). Different concentrations of A. nilotica flower extract were added to the fresh medium after 24 h of cell seeding. Serial two-fold dilutions of A. nilotica flower extract were added to reach the confluency in 96-well flat-bottomed microtiter plates (Falcon, NJ, USA) utilizing a multichannel pipette. Cells were then incubated at 37 °C in a humid environment with 5% CO2 for a time of 24 h. Control cells were incubated without A. nilotica flower extract and with or without DMSO. Three wells were utilized for each dose of the A. nilotica flower extract. At the end of the incubation period, the cultivated medium was aspirated. Then, 1% of the crystal violet solution (0.5% w/v crystal violet and 50% methanol made up to volume with ddH2O, followed by filtration using Whatmann No.1 filter paper) was added to each well for 30 min. The crystal violet solution was then aspirated. Then, the plates were washed using tap water to remove the excess crystal violet. An amount of 30% glacial acetic acid was added to all wells and mixed carefully. Then, the absorbance of the plates was recorded at 490 nm, and the contents were gently shaken onto the microplate reader (TECAN, Inc.), to detect the yield of viable cells. The optical density was measured with the microplate reader (SunRise, TECAN, Inc., Albany, NY, USA) to determine the viable cells percentage, which was calculated using the following formula:
| [(ODt/ODc)] × 100% |
where ODt is the mean optical density of wells exposed to the A. nilotica flower extract, and ODc iss the mean optical density of control cells [38].
The relation among living cells and A. nilotica flower extract concentration was plotted to find the survival curve of the cancer cell line after being exposed to A. nilotica flower extract. From graphic plots of the dose response curve for each concentration, the 50% inhibitory concentration (IC50), or the concentration required to have toxic effects in 50% of intact cells, was calculated using the Graphpad Prism software (San Diego, CA, USA). All investigations were performed in triplicate [39]. Vinblastine sulfate was applied as a positive control against cancer cells under the same experimental conditions.
2.11. Experimental Docking Study
Computer research has been used to determine the structural and chemical properties of this particular group of molecules that may have an effect on the apoptotic process. In this study, we looked at the positions of the inhibitor ferulic acid in the H. pylori (4HI0) binding site using molecular docking modeling (MOE) 2019.0102 program. The receptor structures were obtained directly from Protein Data Bank (https://www.rcsb.org/, accessed on 19 July 2022). The downloaded structures were then prepared for docking by removing all water molecules and other metal ions or ligands. The primary chain was docked, and the selected chain was then fixed and protonated utilizing the tools for structure preparation. In order to build the dummy sites that served as the binding pocket, the MOE site finder generated the active binding sites. The studied compounds were minimized and optimized for the docking process [40]. The dock scoring in MOE software was calculated using the London dG scoring function and refined using two different methods. The best five conformations that were presented in the crystal structure with a lower RMSD value were predicted by the docking process. Using the visualizing program PyMol, the complexes were examined for interactions and their 3D images were captured. Additionally, the RMSD and RMSD-refine fields were used to compare the results of pose-with-pose in the co-crystal ligand position prior to and after modification, respectively.
2.12. Statistical Study
All statistical studies have been expressed as mean ± standard error (SD) and the outcomes were established at least three times.
3. Results and Discussion
3.1. Phytochemical Constituents
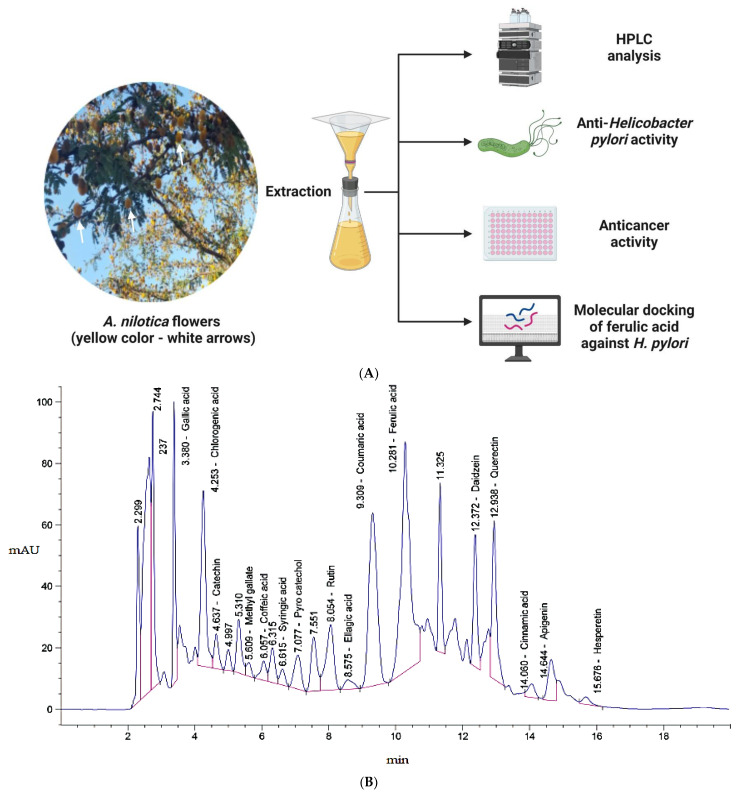
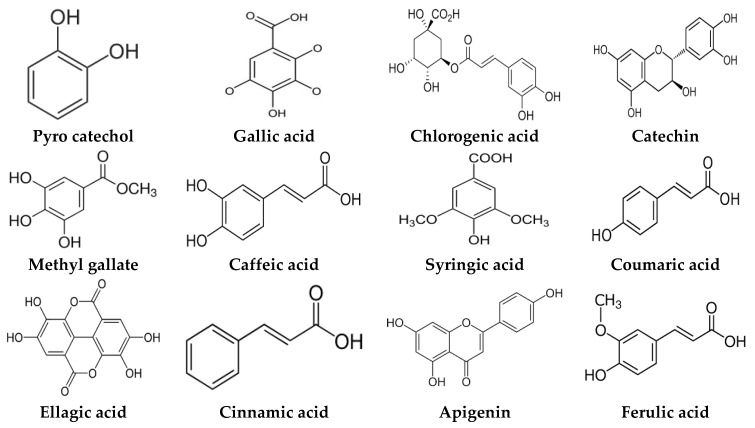
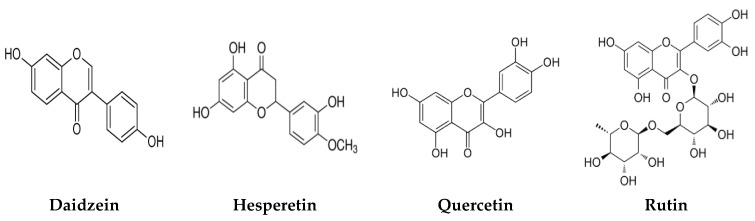
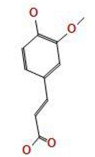
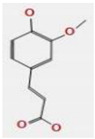
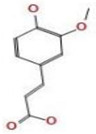
A. nilotica flower (Figure 1A) extract was subjected to HPLC analysis, and the incidence of different phenolic and flavonoid constituents with different retention times, concentrations, area and area % (Figure 1B and Table 1), and chemical formulas was determined (Figure 2). Three compounds were recognized in the extract with great quantity: 5451.04, 4572.26 and 3733.37 µg/mL for ferulic acid, chlorogenic acid and quercetin, respectively. Other detected compounds such as rutin (2393.13 µg/mL) and gallic acid (2116.77 µg/mL) were reported with moderate concentrations. Cinnamic acid (69.72 µg/mL), hesperetin (121.39 µg/mL) and methyl gallate (140.45 µg/mL) were distinguished with the lowest quantity. Moreover, other compounds in the extract, including catechin, caffeic acid, syringic acid, pyro catechol, ellagic acid, apigenin daidzein and coumaric acid were detected. Unfortunately, eight unidentified compounds were present in the extract due to the defect of the standard compounds in the HPLC system. On the other hand, some compounds including vanillin, naringenin and kaempferol were not found in the extract. There are differences in the detected phenolic and flavonoid compounds according to the organ, the geographical location of the cultivation area, the extraction methods and the nutritional and climatic condition of the plant. Sayed et al. [41] recorded the presence of catechin, catechin 7-O-gallate, gallic acid, naringenin 7-O-β-glucopyranoside and quercetin in A. nilotica flower extract. The major detected compounds in A. nilotica ripe fruits were gallic acid methyl ester, followed by catechin and catechin-7-gallate [42]. In another recent study, Kaur et al. [4] studied the phytoconstituents of A. nilotica bark extract via HPLC, showing the existence of numerous phenolic constituents such as ferulic acid, myricetin, kaempferol, rutin, quercetin, gallic acid, betulin, catechin and epicatechin. The extracted A. nilotica flowers with aqueous methanol reflected the presence of gallic acid, catechin, 7-Galloyl catechin, quercetin 3-O-rhanmnopyranosyl (1→6) glucopyranoside, quercetin-3-O-α-L-rhamnopyranoside, quercetin 3-O-β-glucopyranoside and quercetin [29]. Several studies have focused on the biological activities of the natural phenolic and flavonoid molecules in the fields of pharmaceutics and nutrition. Most of the detected compounds in the extract have been shown to have anticancer, antimicrobial, antioxidant, anti-inflammatory and anti-diabetic properties, but these were illustrated in studies that were conducted on other plants. Yersinia enterocolitica was inhibited by chlorogenic acid [43]. Quercetin exhibited therapeutic potential for treating inflammation, Alzheimer, diabetes and cancer, showing some antioxidant activities [44,45]. Rutin was also reported in inflammation and thrombosis treatments [46].
Figure 1.
Image presenting A. nilotica flowers (white color), which experienced a successful extraction method and various relevant assays to detect the phytoconstituents and the anti-H. pylori, anti-biofilm and anticancer activities of the extracted product. This figure was created with BioRender.com, 30 March 2023 (A). Chromatograms of phenolic and flavonoid compounds detected in A. nilotica flower extract via high-performance liquid chromatography (B). White arrows pointed to plant flowers (yellow color).
Table 1.
Flavonoid and phenolic compounds detected in A. nilotica flower extract.
| Compound | Retention Time | Area | Area (%) | Concentration (µg/mL) |
|---|---|---|---|---|
| Unknown | 2.299 | 337.98 | 4.16 | Undetected |
| Unknown | 2.637 | 1081.92 | 13.31 | Undetected |
| Unknown | 2.744 | 488.57 | 6.01 | Undetected |
| Gallic acid | 3.38 | 420.91 | 5.18 | 2116.77 |
| Chlorogenic acid | 4.253 | 574.10 | 7.06 | 4572.26 |
| Catechin | 4.637 | 106.39 | 1.31 | 1524.96 |
| Unknown | 4.997 | 58.61 | 0.72 | Undetected |
| Unknown | 5.310 | 174.94 | 2.15 | Undetected |
| Methyl gallate | 5.609 | 44.12 | 0.54 | 140.45 |
| Caffeic acid | 6.057 | 73.77 | 0.91 | 333.93 |
| Unknown | 6.315 | 121.44 | 1.49 | Undetected |
| Syringic acid | 6.615 | 54.70 | 0.67 | 222.45 |
| Pyro catechol | 7.077 | 158.71 | 1.95 | 1303.73 |
| Unknown | 7.551 | 217.63 | 2.68 | Undetected |
| Rutin | 8.054 | 349.54 | 4.30 | 2393.13 |
| Ellagic acid | 8.575 | 57.27 | 0.70 | 683.49 |
| Coumaric acid | 9.309 | 969.16 | 11.92 | 1715.35 |
| Vanillin | 9.808 | 0.00 | 0.00 | 0.00 |
| Ferulic acid | 10.281 | 1381.53 | 16.99 | 5451.04 |
| Naringenin | 10.494 | 0.00 | 0.00 | 0.00 |
| Unknown | 11.325 | 346.87 | 4.27 | Undetected |
| Daidzein | 12.372 | 333.41 | 4.10 | 1190.40 |
| Quercetin | 12.938 | 491.94 | 6.05 | 3733.37 |
| Cinnamic acid | 14.060 | 65.05 | 0.80 | 69.72 |
| Apigenin | 14.644 | 182.79 | 2.25 | 796.88 |
| Kaempferol | 15.061 | 0.00 | 0.00 | 0.00 |
| Hesperetin | 15.676 | 39.19 | 0.48 | 121.39 |
Figure 2.
Chemical formula detected in A. nilotica flower extract.
3.2. Anti-Helicobacter Pylori Activity of A. nilotica Flower Extract
Inhibitory activity was attributed to A. nilotica flower extract against H. pylori with an inhibition zone of 31 mm compared to the clarithromycin of 21.67 mm as a positive control (Table 2 and Figure 3A,B). Clarithromycin was selected because it is a broad-spectrum antibiotic that is utilized for the treatment of a wide variety of infections caused by bacteria, including H. pylori infection. Clarithromycin inhibits bacteria via binding to the subunit 50S, leading to the inhibition of protein synthesis. Excellent MIC and MBC were recorded in the A. nilotica flower extract with 7.8 µg/mL and 15.62 µg/mL, respectively; however, both the MIC and the MBC of the positive control were 31.25 µg/mL. Via the calculation of the MBC/MIC index, it is clear that the A. nilotica flower extract is determined to be a bactericidal agent due to MBC/MIC values that were less (2.0) than the value of the MIC in the positive control, not exceeding four times that of the positive control MIC (Table 2). Amin et al. [25] reported that the MIC of Helicobacter pylori was found to be 8 μg/mL with the treatment of A. nilotica extract. According to Auwal et al. [47], a pod crude extract of A. nilotica exhibited a killing effect against Staphylococcus aureus (MIC, 200 mg/mL; MBC, 200 mg/mL), Streptococcus pyogenes (MIC, 25 mg/mL; MBC, 100 mg/mL), Bacillus subtilis (MIC, 12.5 mg/mL; MBC, 25 mg/mL), Corynebacterium pyogenes (MIC, 12.5 mg/mL; MBC, 200 mg/mL), Klebsiella pneumoniae (MIC, 12.5 mg/mL; MBC, 25 mg/mL) and Candida albicans (MIC, 25 mg/mL; MBC, 25 mg/mL). This investigation showed the importance of A. nilotica flower extract as an anti-H. pylori agent and its applications in the treatment of H. pylori ulcers. The inhibitory mechanism of phenolic and flavonoid compounds against pathogenic microorganisms has been elucidated in a number of studies [48]. Asha et al. [49] reported that dihydrofolate reductase, DNA gyrase and protein synthesis in H. pylori were inhibited by a flavonoid-rich plant extract. One of the virulence factors, urease of H. pylori, was also inhibited by definite phenolic compounds [50]. Campos et al. [51] studied the antibacterial activity mechanism of ferulic, p-coumaric and caffeic acids and mentioned the capability of these compounds to interfere with bacterial membrane integrity. Flavonoids such as naringenin, taxifolin and eriodictyol can also interact with several enzymes responsible for the creation of bacterial cell membrane precursors and those responsible for the elongation of fatty acid cycle in Enterococcus faecalis [52]. In another study, methicillin-resistant Staphylococcus aureus peptidoglycan and cell wall biosynthesis were damaged as a result of the exposure to sophoraflavanone B [53].
Table 2.
Inhibitory activity, MIC and MBC of A. nilotica flower extract against H. pylori.
| Treatment | Mean Inhibitions Zones (mm) | MIC (µg/mL) | MBC (µg/mL) | MBC/MIC Index |
|---|---|---|---|---|
| Extract | 31.00 ± 1.00 | 7.8 | 15.62 | 2.0 |
| Control | 21.67 ± 1.53 | 31.25 | 31.25 | 1.0 |
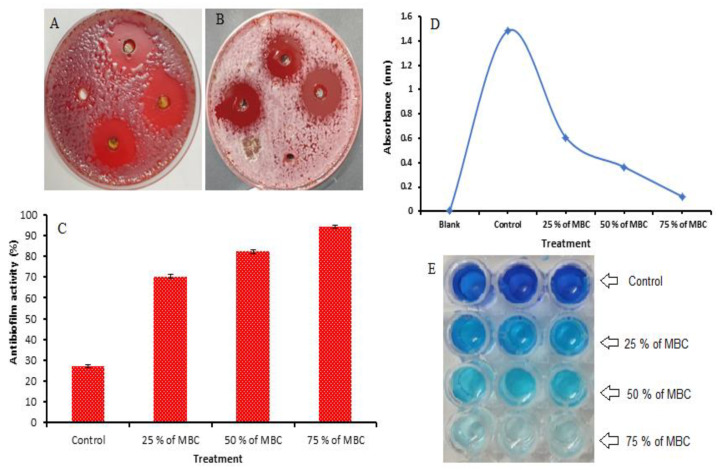
Figure 3.
Inhibitory activity of A. nilotica flower extract (A). Clarithromycin as a positive control against H. pylori. Wells without inhibition zone are considered as negative control (DMSO) (B). Anti-biofilm of A. nilotica flower extract against H. Pylori (C). Biofilm analysis of A. nilotica flower extract against H. Pylori at different concentration of MBC (D). Microtiter plate showing color changes as an indicator of decreased H. Pylori viability at media+ H. Pylori (Control), 25% of MBC of flower extract, 50% of MBC of flower extract and 75% of MBC of flower extract (E).
Moreover, anti-biofilm activity (%) with the increment of the MBC of the A. nilotica flower extract against H. pylori showed 70.38, 82.29 and 94.22% using 25%, 50% and 75% of MBC, respectively. In the control, the anti-biofilm activity was 27.12% (Figure 3C). A plot of the absorbance versus the levels of MBC utilized is visualized (Figure 3D). The color change in the stained biofilm in the microtiter plate was dependent on the viability of the biofilm (Figure 3E). Several bacterial species, such as Bacillus subtilis, B. cereus, B. megaterium, S. aureus, B. aryabhattai, Pseudomonas putida, Serratia marcescens, Escherichia coli and K. pneumoniae, were impacted by A. nilotica leaf extracts [30].
In this study, anti-Helicobacter pylori by a A. nilotica flower extract was assessed and the possible inhibitory influence on its connected urease was also documented. Urease inhibition (%) increased with the increased concentration of the flower extract and reached 76.6% at 1000 µg/mL. The recorded IC50 was 67.4 µg/mL (Table 3). Zhou et al. [54] reported the inhibition of H. pylori urease by almatine from Coptis chinensis, in a dose-dependent manner, with IC50 values of 0.53 ± 0.01 mM, in comparison to acetohydroxamic acid, a well-known urease inhibitor (0.07 ± 0.01 mM). Gastric urease permits the H. pylori to inhibit the acidic stomach and functions as a biosign for the occurrence of H. pylori. One of the virulence factors of H. pylori was associated with urease to create the environment’s alkaline by transforming urea into ammonia and CO2 [55].
Table 3.
Effect of different concentrations of flower extract on urease activity.
| Concentration (µg/mL) | Flower Extract | ||
|---|---|---|---|
| OD Mean | Urease Inhibition % | ±SD | |
| 1000 | 0.172 | 76.6 | 0.003 |
| 500 | 0.236 | 67.9 | 0.004 |
| 250 | 0.276 | 62.4 | 0.006 |
| 125 | 0.338 | 54.0 | 0.004 |
| 62.5 | 0.375 | 48.9 | 0.004 |
| 31.25 | 0.422 | 42.6 | 0.003 |
| 15.625 | 0.458 | 37.6 | 0.002 |
| 7.8125 | 0.512 | 30.3 | 0.004 |
| 3.9 | 0.553 | 24.8 | 0.002 |
| 1.95 | 0.621 | 15.5 | 0.002 |
| 0.0 | 0.735 | 0.0 | 0.026 |
| IC50 | 67.4 µg/mL | ||
3.3. Molecular Docking of Ferulic Acid with 4HI0 Protein of H. pylori
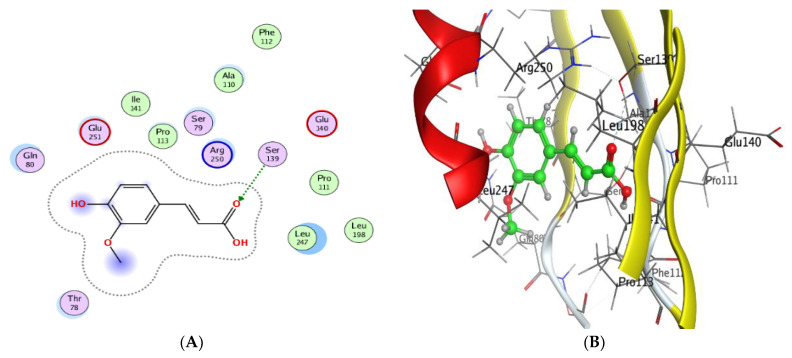
Ferulic acid, which was the highest detected compound in the A. nilotica flower extract, was investigated against 4HI0 protein of H. pylori. The goal of molecular docking, an optimization issue, is to identify the ligand binding mode that has the lowest potential energy. The target binding site’s coordinate space was sampled during docking, and each potential ligand, which had been posed within that site, was scored. The ligand posture with the highest score was then used to forecast the binding mode for that chemical. A 2D molecular structure of ferulic acid has been assigned to the MOE (Molecular Operating Environment), which was designed to visualize the active sites of the complexes (protein–ligand) (Figure 4A–F). The ligand was arranged and rendered using an improved version of the 2D algorithm layout representation, and the protein residues were arranged around it to indicate spatial proximity bonds. The obtained results revealed that ferulic acid was a good inhibitor for the (4HI0) enzyme to slow the progression of H. pylori. Ferulic acid has been found to interact with the residue’s SER 139 active site via the O 29 atom and had a low energy score (−5.58 Kcal/mol), which was important for their biological activity (Table 4 and Table 5). The increased negative score of the free binding energy in the current study confirmed the mechanism of bacterial growth inhibition by ferulic acid. This result was in agreement with recent studies [24,56].
Figure 4.
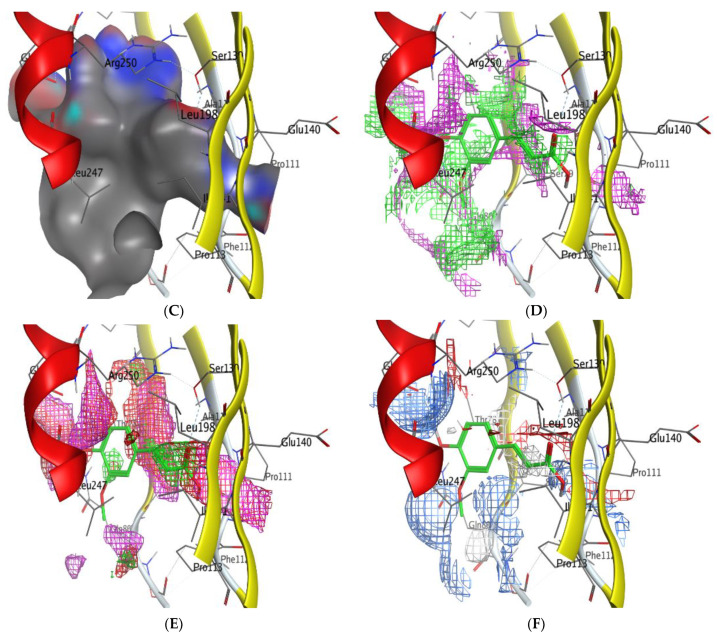
Molecular docking process of ferulic acid with 4HI0. The interaction between ferulic acid and active sites of 4HI0 protein (A). The most likely binding conformation of ferulic acid and the corresponding intermolecular interactions are identified (B). Molecular surface of ferulic acid with 4HI0 (C). The contact preference of ferulic acid with 4HI0 (D). Interaction potential of ferulic acid with 4HI0 (E) and the electrostatic map of ferulic acid with 4HI0 (F).
Table 4.
Docking scores and energies of ferulic acid with crystal structure of H. pylori 4HI0.
| Mol (Five Poses of Ferulic Acid) | rseq | mseq | S | rmsd_refine | E_conf | E_place | E_score1 | E_refine | E_score2 |
|---|---|---|---|---|---|---|---|---|---|
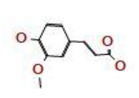
|
1 | 1 | −5.58 | 3.35 | −42.86 | −61.32 | −11.22 | −27.30 | −5.58 |
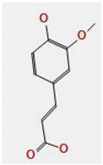
|
1 | 1 | −5.35 | 1.81 | −40.17 | −64.29 | −11.15 | −29.20 | −5.35 |
|
1 | 1 | −5.31 | 2.06 | −41.95 | −63.97 | −9.95 | −28.10 | −5.31 |
|
1 | 1 | −5.29 | 1.73 | −41.47 | −67.91 | −10.93 | −27.96 | −5.29 |
|
1 | 1 | −5.24 | 2.00 | −40.25 | −74.10 | −9.96 | −28.98 | −5.24 |
S, the final score (score of the last stage that was not set to none); rmsd, the root-mean-square deviation of the pose, in Å, from the original ligand; rmsd_refine, the root-mean-square deviation among the pose before refinement and the pose after refinement; E_conf, the energy of the conformer; E_place, the score from the placement stage; E_score 1 and E_score 2, the score from rescoring stages 1 and 2, respectively; E_refine, the score from the refinement stage, which is determined to be the totality of the solvation energies and van der Waals electrostatics, according to the generalized Born solvation model.
Table 5.
Interaction of ferulic acid with crystal structure of H. pylori 4HI0.
| Mol | Ligand | Receptor | Interaction | Distance | E (kcal/mol) |
|---|---|---|---|---|---|
| Ferulic acid | O 20 | OG SER 139 (B) | H-acceptor | 3.02 | −0.7 |
The molecular docking of natural constituents such as chlorogenic acid and pyrocatechol was reported with the H. pylori (4HI0) protein in a recent study [24], which supported the efficacy of these constituents against H. pylori growth. Other investigations also documented the application of the molecular docking of natural ingredient interactions with the E. coli 7C7N protein (value of energy −6.04 kcal mol−1) [40] and Proteus vulgaris [2].
3.4. Antioxidant Activity A. nilotica Flower Extract
The A. nilotica flower extract presented appropriate antioxidant activity depending on the extract concentration. As the concentration increased, the antioxidant properties of the extract increased. For instance, at 15.63, 62.50, 250 and 1000 µg/mL, the DPPH scavenging percentage was 42.3, 52.6, 65.5 and 80.6%, respectively, with an IC50 of 36.74 µg/mL (Table 6). All findings of the antioxidant properties were judged using standard drug ascorbic acid, which reflected an IC50 of 4.08 µg/mL. The promising antioxidant activity was associated with the A. nilotica flower extract content of the phenolic and flavonoids constituents. In the current study, the antioxidant activity of A. nilotica flower extract was better than using other parts of the plant, as shown in comparison to some previous studies. For example, Subhaswaraj et al. [57] studied the antioxidant activity A. nilotica leaf extract and showed an IC50 of 75.157 µg/mL. Polyphenols from the extract of A. nilotica ripe fruits demonstrated an IC50 of 41.91 µg/mL [42]. Another study showed that catechins, a constituent of the A. nilotica flowers, could show antioxidative potential [10].
Table 6.
DPPH scavenging % of A. nilotica flower extract and ascorbic acid.
| Concentration (µg/mL) | Ascorbic Acid | Flower Extract | ||||
|---|---|---|---|---|---|---|
| OD Mean | DPPH Scavenging % | SD | OD Mean | DPPH Scavenging % | SD | |
| 1000 | 0.049 | 97.0 | 0.002 | 0.322 | 80.6 | 0.007 |
| 500 | 0.091 | 94.5 | 0.004 | 0.437 | 73.6 | 0.007 |
| 250 | 0.122 | 92.7 | 0.005 | 0.572 | 65.5 | 0.004 |
| 125 | 0.225 | 86.4 | 0.006 | 0.689 | 58.4 | 0.008 |
| 62.50 | 0.365 | 78.0 | 0.006 | 0.787 | 52.6 | 0.005 |
| 31.25 | 0.478 | 71.2 | 0.004 | 0.866 | 47.8 | 0.009 |
| 15.63 | 0.593 | 64.2 | 0.005 | 0.956 | 42.3 | 0.010 |
| 7.81 | 0.725 | 56.3 | 0.003 | 1.054 | 36.4 | 0.009 |
| 3.90 | 0.898 | 45.8 | 0.002 | 1.130 | 31.8 | 0.004 |
| 1.95 | 0.966 | 41.7 | 0.007 | 1.215 | 26.7 | 0.003 |
| 0 | 1.658 | 0.0 | 0.004 | 1.658 | 0.0 | 0.004 |
| IC50 | 4.08 µg/mL | 36.74 µg/mL | ||||
3.5. Anticancer of A. nilotica Flower Extract
The aerial part extracts of Acacia hamulosa, A. salicina, A. laeta and A. tortilis displayed a moderate effect against cancer proliferation in kidney, breast and liver cells [19]. In the current study, the flower extract of A. nilotica exhibited antitumor potential against HepG-2 cells with different levels dependent on the concentration of the extract. A promising result was obtained at a high concentration, in which the inhibitory activity was 91.26% and 96.73% at 500 µg/mL of the flower extract and Vinblastine sulfate against HepG-2 cells, respectively (Table 7). The IC50 was 176.15 µg/mL against HepG-2 cells, while the IC50 was 395.30 µg/mL against HFB-4 cells, which reflects its safe utilization against cancer cells. However, several studies reported the anticancer properties of natural ingredients, but, after reviewing these studies, it appeared that anticancer potential depended on chemical structure, the type of the ingredients and concentration and genomes of tumor cells [29,58]. These remarks coincided with other reports using different parts of A. nilotica [59]. Different IC50 values of A. catechu fruit extract ranging from 9.7 to 42.8 µg/mL were reported against human cancer cell lines such as those of MCF-7, A549 and THP-1 cells [28]. In a recent study, the anticancer effects of A. nilotica with IC50 values of 59.3 µg/mL and 96.9 µg/mL were observed against A549 and MCF-7 cells, respectively, while less anticancer effects were observed against THP-1 cells with an IC50 of more than 100 µg/mL [29]. The influence of A. nilotica flower extract on normal cells was parallel with Revathi et al. [30], in which the A. nilotica leaf extracts documented the anticancer activity of the extracts against Hep-2 (cervical cancer) and MDA-MB-231 (breast cancer) and showed sparing effects on normal Vero cells. As mentioned from the HPLC analysis, ferulic acid was the main component detected in the A. nilotica flower extract. The mechanisms of ferulic acid cytotoxicity against cancer cells was studied, from which autophagy and apoptosis of HepG2 were observed with the disruption of nucleoli, morphological alterations, cell proliferation decline, mitochondrial membrane potential decrement, and an increased % of cells in the S phase [60]. In a review report [61], cancer cells treated with ferulic acid characterized with DNA fragmentation lowered the rate of protein kinase phosphorylation. Sundarraj et al. [62] reported that the MCF-7 cell cycle at the G2/M phase was blocked as a result of the exposure to a leaf extract of A. nilotica. Another species, namely, Acacia catechu, also arrested the human colon cancer cell cycle at this phase and showed a decline in the potential mitochondrial membrane [63].
Table 7.
Cytotoxic activities of A. nilotica flower extract and vinblastine sulfate.
| Concentration (µg/mL) | Flower Extract | Vinblastine Sulfate | ||||
|---|---|---|---|---|---|---|
| HepG-2 Cells | HFB-4 Cells | HepG-2 Cells | ||||
| Viability % | Inhibitory % | Viability % | Inhibitory % | Viability % | Inhibitory % | |
| 500 | 8.74 | 91.26 ± 0.62 | 32.65 | 67.35 ± 2.31 | 3.27 | 96.73 ± 1.48 |
| 250 | 30.96 | 69.04 ± 1.48 | 74.08 | 25.92 ± 2.14 | 5.89 | 94.11 ± 1.30 |
| 125 | 63.19 | 36.81 ± 2.35 | 91.47 | 8.53 ± 0.69 | 10.92 | 89.08 ± 1.25 |
| 62.5 | 84.23 | 15.77 ± 1.09 | 98.16 | 1.84 ± 0.48 | 14.36 | 85.64 ± 0.31 |
| 31.25 | 97.58 | 2.42 ± 0.64 | 100 | 0.0 | 19.24 | 80.76 ± 0.48 |
| 15.6 | 100 | 0.0 | 100 | 0.0 | 26.85 | 73.15 ± 1.25 |
| 7.8 | 100 | 0.0 | 100 | 0.0 | 34.19 | 65.81 ± 0.50 |
| 3.9 | 100 | 0.0 | 100 | 0.0 | 45.06 | 54.94 ± 1.33 |
| 2 | 100 | 0.0 | 100 | 0.0 | 54.28 | 45.72 ± 1.42 |
| 1 | 100 | 0.0 | 100 | 0.0 | 60.94 | 39.06 ± 0.45 |
| 0 | 100 | 0.0 | 100 | 0.0 | 100 | 0.0 |
| IC50 | 176.15 ± 5.08 µg/mL | 395.30 ± 11.49 µg/mL | 2.93± 0.33 µg/mL | |||
4. Conclusions
This study offered additional scientific support to the utilization of A. nilotica flower extract to treat H. pylori-related gastrointestinal infections. A. nilotica flower extract might be a helpful treatment for peptic and gastritis ulcers induced by H. pylori infection and other urease-related illnesses. The results of this study suggest that the A. nilotica flower extract, due to its considerable antioxidant potential, might play a role in protection via the avoidance of lipid peroxidation generated by free radicals. Furthermore, based on the obtained findings, further studies must be conducted to document the biological effects of A. nilotica flower extract in vivo, and detailed mechanistic studies are required to validate these biological applications.
Acknowledgments
All authors thanks Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R217), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author Contributions
Conceptualization, A.M.H.A.-R. and H.Q.; methodology, A.S.B.; software, T.M.A.; formal analysis, H.Q.; investigation, T.M.A.; resources, T.M.A.; writing—original draft preparation, A.M.H.A.-R.; writing—review and editing, N.K.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R217), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Abdelghany T.M., Reham Y., Bakri M.M., Ganash M., Basma H.A., Qanash H. Effect of Thevetia peruviana Seeds Extract for Microbial Pathogens and Cancer Control. Int. J. Pharmacol. 2021;17:643–655. doi: 10.3923/ijp.2021.643.655. [DOI] [Google Scholar]
- 2.Qanash H., Yahya R., Bakri M.M., Bazaid A.S., Qanash S., Shater A.F., Abdelghany T.M. Anticancer, Antioxidant, Antiviral and Antimicrobial Activities of Kei Apple (Dovyalis caffra) Fruit. Sci. Rep. 2022;12:5914. doi: 10.1038/s41598-022-09993-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qanash H., Bazaid A.S., Aldarhami A., Alharbi B., Almashjary M.N., Hazzazi M.S., Felemban H.R., Abdelghany T.M. Phytochemical Characterization and Efficacy of Artemisia judaica Extract Loaded Chitosan Nanoparticles as Inhibitors of Cancer Proliferation and Microbial Growth. Polymers. 2023;15:391. doi: 10.3390/polym15020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaur P., Arora S., Singh R. Isolation, Characterization and Biological Activities of Betulin from Acacia nilotica Bark. Sci. Rep. 2022;12:9370. doi: 10.1038/s41598-022-13338-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar G., Singh N.K., Srivastava M. Comparative Phytochemical Investigation and Antioxidant Activity in Different Parts of Acacia nilotica Seed. Indian J. Pharm. Sci. 2022;84:552–559. doi: 10.36468/pharmaceutical-sciences.949. [DOI] [Google Scholar]
- 6.Rather L.J., Shahid-ul-Islam, Mohammad F. Acacia nilotica (L.): A review of its Traditional Uses, Phytochemistry, and Pharmacology. Sustain. Chem. Pharm. 2015;2:12–30. doi: 10.1016/j.scp.2015.08.002. [DOI] [Google Scholar]
- 7.Khalaf S.S., Shalaby O.A., Hassan A.R., El-Kherbetawy M.K., Mehanna E.T. Acacia nilotica Stem Bark Extract Ameliorates Obesity, Hyperlipidemia, and Insulin Resistance in a Rat Model of High Fat Diet-induced Obesity. J. Tradit. Complement. Med. 2023. in press . [DOI] [PMC free article] [PubMed]
- 8.Ojo O.A., Oyetayo F.L., Oladipo A.S., Oluwatosin V.O. Polyphenolic Contents, Free Radical Scavenging Properties, and Enzyme Inhibitory Activities of Acacia nilotica (L.) Delile Seed and Pod Extracts. Vegetos. 2023 doi: 10.1007/s42535-023-00599-0. [DOI] [Google Scholar]
- 9.Sadiq M.B., Tharaphan P., Chotivanich K., Tarning J., Anal A.K. In Vitro Antioxidant and Antimalarial Activities of Leaves, Pods and Bark Extracts of Acacia nilotica (L.) Del. BMC Complement. Med. Ther. 2017;17:372. doi: 10.1186/s12906-017-1878-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maldini M., Montoro P., Hamed A.I., Mahalel U.A., Oleszek W., Stochmal A., Piacente S. Strong Antioxidant Phenolics From Acacia nilotica: Profiling by ESI-MS and Qualitative-quantitative Determination by LC-ESI-MS. J. Pharm. Biomed. Anal. 2011;56:228–239. doi: 10.1016/j.jpba.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 11.Hussein G., Miyashiro H., Nakamura N., Hattori M., Kakiuchi N., Shimotohno K. Inhibitory Effects of Sudanese Medicinal Plant Extracts on Hepatitis C Virus (HCV) Protease. Phytother. Res. 2000;14:510–516. doi: 10.1002/1099-1573(200011)14:7<510::AID-PTR646>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 12.Singh R., Singh B., Singh S., Kumar N., Kumar S., Arora S. Anti-free Radical Activities of Kaempferol Isolated From Acacia nilotica (L.) Willd. Ex. Del. Toxicol. In Vitro. 2008;22:1965–1970. doi: 10.1016/j.tiv.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Adeel S., Fazal-Ur R., Abdul H., Habib N., Shumaila K., Khalid M.Z., Zuber M. Sustainable Extraction and Dyeing of Microwave-treated Silk Fabric Using Arjun Bark Colorant. J. Nat. Fibers. 2020;17:745–758. doi: 10.1080/15440478.2018.1534182. [DOI] [Google Scholar]
- 14.Lizárraga-Velázquez C.E., Leyva-López N., Hernández C., Gutiérrez-Grijalva E.P., Salazar-Leyva J.A., Osuna-Ruíz I., Martínez-Montaño E., Arrizon J., Guerrero A., Benitez-Hernández A., et al. Antioxidant Molecules from Plant Waste: Extraction Techniques and Biological Properties. Processes. 2020;8:1566. doi: 10.3390/pr8121566. [DOI] [Google Scholar]
- 15.Zuber M., Adeel S., Fazal-Ur R., Fozia A., Majid M., Muhammad A., Khalid M.Z. Influence of Microwave Radiation on Dyeing of Bio-mordanted Silk Fabric using Neem Bark (Azadirachta indica)-Based Tannin Natural Dye. J. Nat. Fibers. 2020;17:1410–1422. doi: 10.1080/15440478.2019.1576569. [DOI] [Google Scholar]
- 16.Al-Huqail A.A., Behiry S.I., Salem M.Z.M., Ali H.M., Siddiqui M.H., Salem A.Z.M. Antifungal, Antibacterial, and Antioxidant Activities of Acacia saligna (Labill.) H. L. Wendl. Flower Extract: HPLC Analysis of Phenolic and Flavonoid Compounds. Molecules. 2019;24:700. doi: 10.3390/molecules24040700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdel-Farid I.B., Sheded M.G., Mohamed E.A. Metabolomic Profiling and Antioxidant Activity of Some Acacia Species. Saudi Arab. J. Biol. Sci. 2014;21:400–408. doi: 10.1016/j.sjbs.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Toumy S., Salib J., Mohamed W., Morsy F. Phytochemical and Antimicrobial Studies on Acacia saligna Leaves. Egypt J. Chem. 2010;53:705. [Google Scholar]
- 19.Alajmi M.F., Alam P., Alqasoumi S.I., Ali Siddiqui N., Basudan O.A., Hussain A., Mabood Husain F., Ali Khan A. Comparative Anticancer and Antimicrobial Activity of Aerial Parts of Acacia salicina, Acacia laeta, Acacia hamulosa and Acacia tortilis grown in Saudi Arabia. Saudi Arab. Pharm. J. 2017;25:1248–1252. doi: 10.1016/j.jsps.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abd-ElGawad A., El-Amier Y. Allelopathy and Potential Impact of Invasive Acacia saligna (Labill.) Wendl. on Plant Diversity in the Nile Delta Coast of Egypt. Int. J. Environ. Res. 2015;9:923–932. [Google Scholar]
- 21.Dunn B.E., Cohen H., Blaser M.J. Helicobacter pylori. Clin. Microbiol. Rev. 1997;10:720–741. doi: 10.1128/CMR.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amin M., Iqbal M.S., Hughes R.W., Khan S.A., Reynolds P.A., Enne V.I., Sajjad-ur-Rahman, Mirza A.S. Mechanochemical Synthesis and In Vitro Anti-Helicobacter pylori and Uresase Inhibitory Activities of Novel Zinc(II)-famotidine Complex. J. Enzyme Inhib. Med. Chem. 2010;25:383–390. doi: 10.3109/14756360903179518. [DOI] [PubMed] [Google Scholar]
- 23.Bhandari A., Crowe S.E. Helicobacter pylori in Gastric Malignancies. Curr. Gastroenterol. Rep. 2012;14:489–496. doi: 10.1007/s11894-012-0296-y. [DOI] [PubMed] [Google Scholar]
- 24.Yahya R., Al-Rajhi A.M.H., Alzaid S.Z., Al Abboud M.A., Almuhayawi M.S., Al Jaouni S.K., Selim S., Ismail K.S., Abdelghany T.M. Molecular Docking and Efficacy of Aloe vera gel Based on Chitosan Nanoparticles Against Helicobacter pylori and its Antioxidant and Anti-inflammatory Activities. Polymers. 2022;14:2994. doi: 10.3390/polym14152994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amin M., Anwar F., Naz F., Mehmood T., Saari N. Anti-Helicobacter pylori and Urease Inhibition Activities of Some Traditional Medicinal Plants. Molecules. 2013;18:2135–2149. doi: 10.3390/molecules18022135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fowora M., Aina O., Omoregie E.S., Smith S., Osubor C.C. The Effect of Acacia nilotica on Helicobacter pylori Colonization. Int. J. Med. Plants Nat. Prod. 2021;7:1–10. doi: 10.20431/2454-7999.0703001. [DOI] [Google Scholar]
- 27.Sampath G., Douglas J.H., Neelamegam R., Muthukalingan K., Nagarajan K. In Vitro anti-Helicobacter pylori and Anti-gastric Cancer Activities of Acacia nilotica Aqueous Leaf Extract and its Validation Using In Silico Molecular Docking Approach. Mater. Today Proc. 2022;51:1675–1684. doi: 10.1016/j.matpr.2020.09.032. [DOI] [Google Scholar]
- 28.Diab K.A., Guru S.K., Bhushan S., Saxena A.K. In Vitro Anticancer Activities of Anogeissus latifolia, Terminalia bellerica, Acacia catechu and Moringa oleiferna Indian Plants. Asian Pac. J. Cancer Prev. 2015;16:6423–6428. doi: 10.7314/APJCP.2015.16.15.6423. [DOI] [PubMed] [Google Scholar]
- 29.Diab K.A., Fahmy M.A., Hassan E.M., El-Toumy S.A. Evaluation of the Cytotoxic, Anticancer, and Genotoxic Activities of Acacia nilotica Flowers and Their Effects on N-methyl-N-nitrosourea-induced Genotoxicity in Mice. Mol. Biol. Rep. 2022;49:8439–8448. doi: 10.1007/s11033-022-07662-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Revathi S., Govindarajan R.K., Rameshkumar N., Hakkim F.L., Al-Buloshi M., Muthukalingan K., Nagarajan K. Anti-cancer, Anti-microbial and Anti-oxidant Properties of Acacia nilotica and Their Chemical Profiling. Biocatal. Agric. Biotechnol. 2017;11:322–329. doi: 10.1016/j.bcab.2017.08.005. [DOI] [Google Scholar]
- 31.Arber A. Water Plants: A Study of Aquatic Angiosperm. Cambridge University Press; Cambridge, UK: 1972. p. 436. [Google Scholar]
- 32.Al-Rajhi A.M.H., Qanash H., Almuhayawi M.S., Al Jaouni S.K., Bakri M.M., Ganash M., Salama H.M., Selim S., Abdelghany T.M. Molecular Interaction Studies and Phytochemical Characterization of Mentha pulegium L. Constituents with Multiple Biological Utilities as Antioxidant, Antimicrobial, Anticancer and Anti-Hemolytic Agents. Molecules. 2022;27:4824. doi: 10.3390/molecules27154824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castillo-Juarez I., Rivero-Cruz F., Celis H., Romero I. Anti-Helicobacter pylori Activity of Anacardic Acids From Amphipterygium adstringens. J. Ethnopharmacol. 2007;114:72–77. doi: 10.1016/j.jep.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 34.Andrews J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001;48:5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- 35.French G.L. Bactericidal Agents in the Treatment of MRSA Infections—The Potential Role of Daptomycin. J. Antimicrob. Chemother. 2006;58:1107. doi: 10.1093/jac/dkl393. [DOI] [PubMed] [Google Scholar]
- 36.Stepanović S., Vuković D., Hola V., Di Bonaventura G., Djukić S., Cirković I., Ruzicka F. Quantification of Biofilm in Microtiter Plates: Overview of Testing Conditions and Practical Recommendations for Assessment of Biofilm Production by Staphylococci. APMIS. 2007;115:891–899. doi: 10.1111/j.1600-0463.2007.apm_630.x. [DOI] [PubMed] [Google Scholar]
- 37.Kuwahara H., Miyamoto T., Kubota T., Sawa T., Okamoto S., Maeda H. Helicobacter pylori Urease Suppresses Bactericidal Activity of Peroxynitrite Via Carbon Dioxide Production. Infect. Immun. 2000;68:4378–4383. doi: 10.1128/IAI.68.8.4378-4383.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to Proliferation and Cytotoxicity assays. J. Immunol. Method. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 39.Al-Rajhi A.M.H., Yahya R., Abdelghany T.M., Fareid M.A., Mohamed A.M., Amin B.H., Masrahi A.S. Anticancer, Anticoagulant, Antioxidant and Antimicrobial Activities of Thevetia peruviana Latex with Molecular Docking of Antimicrobial and Anticancer Activities. Molecules. 2022;27:3165. doi: 10.3390/molecules27103165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qanash H., Alotaibi K.A.A., Bazaid A.S., Ganash M., Saeedi N.H., Ghany T.A. Effectiveness of Oil-based Nanoemulsions With Molecular Docking of its Antimicrobial Potential. BioResources. 2023;18:1554–1576. doi: 10.15376/biores.18.1.1554-1576. [DOI] [Google Scholar]
- 41.Sayed A., El-Toumy S.A., Mohamed S.M., Hassan E.M., Mossa A.T.H. Phenolic Metabolites from Acacia nilotica Flowers and Evaluation of its Free Radical Scavenging Activity. J. Am. Sci. 2011;7:287–295. [Google Scholar]
- 42.Foyzun T., Mahmud A.A., Ahammed M.S., Manik M.I.N., Hasan M.K., Islam K.M.M., Lopa S.S., Al-Amin M.Y., Biswas K., Afrin M.R., et al. Polyphenolics with Strong Antioxidant Activity from Acacia nilotica Ameliorate Some Biochemical Signs of Arsenic-Induced Neurotoxicity and Oxidative Stress in Mice. Molecules. 2022;27:1037. doi: 10.3390/molecules27031037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen K., Peng C., Chi F., Yu C., Yang Q., Li Z. Antibacterial and Antibiofilm Activities of Chlorogenic Acid Against Yersinia enterocolitica. Front Microbiol. 2022;13:885092. doi: 10.3389/fmicb.2022.885092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salehi B., Machin L., Monzote L., Sharifi-Rad J., Ezzat S.M., Salem M.A., Merghany R.M., El Mahdy N.M., Kılıç C.S., Sytar O., et al. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS Omega. 2020;5:11849–11872. doi: 10.1021/acsomega.0c01818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Asgharian P., Tazekand A.P., Hosseini K., Forouhandeh H., Ghasemnejad T., Ranjbar M., Hasan M., Kumar M., Beirami S.M., Tarhriz V., et al. Potential Mechanisms of Quercetin in Cancer Prevention: Focus on cellular and molecular targets. Cancer Cell Int. 2022;22:257. doi: 10.1186/s12935-022-02677-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen S.-C., Yang C.-S., Chen J.-J. Main Bioactive Components and Their Biological Activities from Natural and Processed Rhizomes of Polygonum sibiricum. Antioxidants. 2022;11:1383. doi: 10.3390/antiox11071383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Auwal M.S., Shuaibu A., Ibrahim A., Mustapha M. Antibacterial Properties of Crude Pod Extract of Acacia nilotica (fabaceae) Haryana Vet. 2015;54:29–32. [Google Scholar]
- 48.Khameneh B., Iranshahy M., Soheili V., Sedigheh B., Bazzaz F. Review on Plant Antimicrobials: A mechanistic Viewpoint. Antimicrob. Resist. Infect Control. 2019;8:118. doi: 10.1186/s13756-019-0559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asha M.K., Debraj D., Prashanth D., Edwin J.R., Srikanth H.S., Muruganantham N., Dethe S.M., Anirban B., Jaya B., Deepak M., et al. In vitro Anti-Helicobacter pylori Activity of a Alavonoid Rich Extract of Glycyrrhiza glabra and its Probable Mechanisms of Action. J. Ethnopharmacol. 2013;145:581–586. doi: 10.1016/j.jep.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 50.Xiao Z.P., Peng Z.Y., Dong J.J., He J., Ouyang H., Feng Y.T., Lu C.L., Lin W.Q., Wang J.X., Xiang Y.P., et al. Synthesis, Structure-Activity Relationship Analysis and Kinetics Study of Reductive Derivatives of Flavonoids as Helicobacter pylori Urease Inhibitors. Eur. J. Med. Chem. 2013;63:685–695. doi: 10.1016/j.ejmech.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 51.Campos F.M., Couto J.A., Figueiredo A.R., Tóth I.V., Rangel A.O., Hogg T.A. Cell Membrane Damage Induced by Phenolic Acids on Wine Lactic Acid Bacteria. Int. J. Food Microbiol. 2009;135:144–151. doi: 10.1016/j.ijfoodmicro.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 52.Jeong K.W., Lee J.Y., Kang D.I., Lee J.U., Shin S.Y., Kim Y. Screening of Flavonoids as Candidate Antibiotics Against Enterococcus faecalis. J. Nat. Prod. 2009;72:719–724. doi: 10.1021/np800698d. [DOI] [PubMed] [Google Scholar]
- 53.Mun S.H., Joung D.K., Kim S.B., Park S.J., Seo Y.S., Gong R., Choi J.G., Shin D.W., Rho J.R., Kang O.H., et al. The Mechanism of Antimicrobial Activity of Sophoraflavanone B Against Methicillin-resistant Staphylococcus aureus. Foodborne Pathog. Dis. 2014;11:234–239. doi: 10.1089/fpd.2013.1627. [DOI] [PubMed] [Google Scholar]
- 54.Zhou J.-T., Li C.-L., Tan L.-H., Xu Y.-F., Liu Y.-H., Mo Z.-Z., Dou Y.-X., Su R., Su Z.-R., Huang P., et al. Inhibition of Helicobacter pylori and Its Associated Urease by Palmatine: Investigation on the Potential Mechanism. PLoS ONE. 2017;12:e0168944. doi: 10.1371/journal.pone.0168944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zahid R., Akram M., Riaz M., Munir N., Shehzad M. Phytotherapeutic Modalities for the Management of Helicobacter pylori Associated Peptic Ulcer. Eur. J. Inflamm. 2020;18:1–16. doi: 10.1177/2058739220968308. [DOI] [Google Scholar]
- 56.Divyashri G., Murthy T.K., Sundareshan S., Kamath P., Murahari M., Saraswathy G.R., Sadanandan B. In Silico Approach Towards the Identification of Potential Inhibitors From Curcuma amada Roxb Against H. pylori: ADMET Screening and Molecular Docking Studies. BioImpacts. 2021;11:119–127. doi: 10.34172/bi.2021.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Subhaswaraj P., Sowmya M., Jobina R., Sudharshan S.J., Dyavaiah M., Siddhardha B. Determination of Antioxidant Potential of Acacia nilotica Leaf Extract in Oxidative Stress Response System of Saccharomyces cerevisiae. J. Sci. Food. Agric. 2017;97:5247–5253. doi: 10.1002/jsfa.8409. [DOI] [PubMed] [Google Scholar]
- 58.Ravishankar D., Rajora A.K., Greco F., Osborn H.M. Flavonoids as Prospective Compounds for Anti-cancer therapy. Int. J. Biochem. Cell Biol. 2013;45:2821–2831. doi: 10.1016/j.biocel.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 59.De Jesús Manríquez-Torres J., Hernández-Lepe M.A., Chávez-Méndez J.R., González-Reyes S., Serafín-Higuera I.R., Rodríguez-Uribe G., Torres-Valencia J.M. Isolation and Cytotoxic Activity of Phyllocladanes From the Roots of Acacia schafneri (Leguminosae) Molecules. 2020;25:3944. doi: 10.3390/molecules25173944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang J., Lai X., Yuan D., Liu Y., Wang J., Liang Y. Effects of Ferulic acid, a Major Component of Rice Bran, on Proliferation, Apoptosis, and Autophagy of HepG2 Cells. Food Res. Int. 2022;161:111816. doi: 10.1016/j.foodres.2022.111816. [DOI] [PubMed] [Google Scholar]
- 61.Singh T.H., Kumar A., Ramniwas S., Coudhary R., Aggarwal D., Kumar M., Sharma U., Chaturvedi Parashar N., Haque S., Sak K. Ferulic Acid: A Natural Phenol That Inhibits Neoplastic Events through Modulation of Oncogenic Signaling. Molecules. 2022;27:7653. doi: 10.3390/molecules27217653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sundarraj S., Thangam R., Sreevani V., Kaveri K., Gunasekaran P., Achiraman S., Kannan S. γ-Sitosterol from Acacia nilotica L. Induces G2/M Cell Cycle Arrest and Apoptosis Through c-Myc Suppression in MCF-7 and A549 cells. J. Ethnopharmacol. 2012;141:803–809. doi: 10.1016/j.jep.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 63.Chiaino E., Micucci M., Durante M., Budriesi R., Gotti R., Marzetti C., Chiarini A., Frosini M. Apoptotic-Induced Effects of Acacia catechu Willd. Extract in Human Colon Cancer Cells. Int. J. Mol. Sci. 2020;21:2102. doi: 10.3390/ijms21062102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.