Abstract
Glucansucrases of oral streptococci and Leuconostoc mesenteroides are enzymes of medical and biotechnological interest that synthesize α-glucans. They can also synthesize oligosaccharides in the presence of a sugar acceptor. Previous reports have identified an amino acid residue that may affect the structure of the glucan product; therefore, random mutagenesis of the corresponding Asp-569 of Streptococcus downei glucosyltransferase I (GTF-I) was used to further understanding of its involvement in the catalytic mechanism and to evaluate how different amino acids can modulate glucan and oligosaccharide synthesis. GTF-I variants were obtained where Asp-569 was replaced by each of the different possible classes of amino acids. These were expressed in Escherichia coli and purified by means of a His6 tag. The results showed that the amino acid in position 569 influences the structure of the glucan and the size of the oligosaccharides produced by GTF-I. The results suggest that the amino acid occupying this position is more likely to interact with the acceptor molecules (oligosaccharides or elongating glucan chain) than to be directly involved in glucosyl transfer from sucrose. Engineering of the equivalent position in glucansucrases thus appears to be a good target to expand the range of oligosaccharides synthesized.
The glucansucrases (EC 2.4.1.5; commonly called glucosyltransferases [GTFs]) from oral streptococci are enzymes belonging to glycosidase family 70 that catalyze the transfer of glucosyl units from the cleavage of sucrose to a growing α-glucan chain (6). Depending on the enzyme, different sizes and structures of glucan can be produced, and the nature of the linkages between glucosyl units determines the water solubility and properties of the glucan. A higher content of α(1-3) linkages is associated with greater insolubility (13, 20). Glucans are of central importance in adhesive interactions in plaque, where they mediate attachment of bacteria to the tooth surface and to other bacteria, thus stabilizing the plaque biofilm, serve as energy stores aiding the survival of plaque bacteria, and modulate the permeability of plaque and hence the level of acid at the enamel surface (1). In addition to synthesis of glucan (with the release of fructose), GTF can hydrolyze sucrose to glucose and fructose and also transfer glucose to fructose, in which case leucrose [5-O-(α-d-glucopyranosyl)-d-fructopyranose] is formed. If an acceptor molecule such as maltose is present, it is extended to form a series of glucooligosaccharides (GOS).
Oligosaccharides are of potential industrial interest for incorporation in foodstuffs or as prebiotics (14), and we have previously reported that site-directed mutagenesis of GTF can alter the relative balance of the three reaction pathways of synthesis of glucan, hydrolysis, or synthesis of oligosaccharides (12). Further information on the influence on reaction specificity of various amino acid residues in GTF is needed for protein engineering to achieve desired properties as well as providing insights into enzymatic mechanisms and aiding rational design of inhibitors that could have application in dental care products (5, 16).
All glucansucrases possess a common pattern of organization (1, 13). They are of high molecular mass (around 160 kDa) and have a signal sequence followed by a variable stretch of about 200 amino acids, a highly conserved core region of about 900 amino acids including the catalytic domain, and a C-terminal glucan-binding domain covering about 400 amino acids. Sequence alignment and secondary structure prediction showed that the GTF catalytic domain can be related to the α-amylase superfamily (glycosidase family 13), members of which contain a catalytic (β/α)8 barrel domain (9). GTFs are predicted to contain alternating β sheets and α helices, though the homologous elements appear to be circularly permuted with respect to those in amylases (9) and amino acids important in catalysis lie outside the main barrel region (12). Amino acids contributing to the active site have been recognized, and invariant Asp and Glu residues, homologous at those present at the C termini of the β4, β5, and β7 strands of α-amylases and involved in the catalytic triad, have been found to be essential for GTFs (3, 8, 19). In α-amylases, some β-α loops may play an important role in substrate specificity (10), and a critical Asp residue in a region corresponding to the β7 strand of α-amylase has been shown to clearly influence the structure of the glucan produced by Streptococcus mutans GTF-B (18). Shimamura et al. compared sequences of GTF-I, GTF-S, GTF-B, GTF-C, and GTF-D and identified positions where amino acid residues are conserved for the GTFs producing water-insoluble α(1-3)-linked glucan but differ from the residues present in GTFs producing a soluble α(1-6)-linked glucan (18). In addition, site-directed mutagenesis experiments confirmed that in GTF-B and GTF-D, conversion of an Asp to a Thr residue influenced the structure of the glucan produced (18).
In view of the evidence for the importance of an Asp or Thr residue in this position, further analysis of this site should throw light on this phenomenon; we describe mutagenesis of a GTF from Streptococcus downei, one of the mutans group of oral streptococci (4, 17, 21) that produces a water-insoluble glucan containing α(1-3) glucosyl linkages. We have reported the genetic manipulation of the gtfI gene to facilitate purification of the catalytic core (GTF-Ic) and shown that it retains the properties of the intact enzyme (11).
Random mutagenesis of Asp-569 was used to better understand the involvement of this position in the catalytic mechanism and to evaluate how different amino acids can modulate glucan and oligosaccharide synthesis.
MATERIALS AND METHODS
Mutations at position 569 of GTF-I.
Plasmid pGTFIc carrying the gene coding for the 905-amino-acid conserved core region of GTF-I (GTF-Ic) fused with a stretch of six histidine residues (11) was used as the template for mutagenesis. This corresponds to amino acids 148 to 1053 of GTF-I from S. downei MFe28 (4, 21). Random and biased random mutagenesis were performed using two sets of primers (set 1, 5′-GATAGCGAAGTACAANNNCTGATTCGTGACATC and 5′-GATGTCACGAATCAGNNNTTGTACTTCGCTATC; set 2, 5′-GATAGCGAAGTACAAWNSCTGATTCGTGACATC and 5′-GATGTCACGAATCAGSNWTTGTACTTCGCTATC) with a Quick-Change site-directed mutagenesis kit (Stratagene). After transformation into Escherichia coli XL1-Blue and selection on Luria-Bertani medium plates containing ampicillin, a two-step screening was undertaken. Recombinant colonies were first patched on 2xYT (16 g of tryptone, 10 g of yeast extract, and 5 g of NaCl liter−1) containing ampicillin (2xYT+Amp) supplemented with 2% sucrose (wt/vol) and 5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at 37°C. Under these conditions, appearance of a white halo around colonies could be observed, the size of which corresponded with GTF activity. For the second screening step, mutants selected on the basis of halo size were propagated overnight at 37°C in 10 ml of 2xYT+Amp with 2% glucose (wt/vol) and 5 mM IPTG. After protein extraction by sonication, activity was checked by the dinitrosalicylic acid method (11), and the level of expression was monitored by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and staining with Coomassie blue. The selected mutants were then sequenced with an Applied Biosystems 377 DNA sequencer (Molecular Biology Facility, University of Newcastle).
Expression and purification of GTF-Ic mutants.
Selected mutant or wild-type GTF-Ic enzymes were expressed by culturing E. coli XL1-Blue carrying the relevant plasmids overnight at 37°C in 15 ml of 2xYT+Amp supplemented with 2% (wt/vol) glucose and 5 mM IPTG. Cells were harvested and resuspended in 0.5 ml of 50 mM sodium phosphate buffer (pH 8.0)–300 mM NaCl and treated with lysozyme (0.1 mg ml−1) before sonication. Purification of GTF-Ic variants was achieved with an Ni-nitrilotriacetic acid spin column kit (Qiagen) as recommended by the manufacturer. Proteins were eluted in 50 mM sodium phosphate buffer (pH 8.0) containing 300 mM NaCl and 250 mM imidazole and dialyzed overnight at 4°C against 50 mM Tris-HCl buffer (pH 7.0). Protein purity was assayed by SDS-PAGE, and protein concentration was determined by UV absorption at 280 nm using calculated extinction coefficients.
Glucan synthesis activity.
Specific activity at 37°C was assayed by measuring fructose release over a 30-min period in the presence of 50 g of sucrose liter−1 in 50 mM Tris-HCl buffer (pH 7.0) by the dinitrosalicylic acid method (11). One unit is defined as the amount of enzyme that catalyzed the formation of 1 μmol of fructose min−1. After complete depletion of sucrose, concentrations of fructose, glucose, and leucrose released in the reaction medium were assayed by enzymatic methods and high-performance liquid chromatography (HPLC) as previously described (11). Glucan synthesis yield was calculated by subtracting the free glucose concentration from the fructose concentration, since this corresponds to the glucose residues coming from sucrose cleavage and incorporated into glucan. The structure of glucans produced by mutant T569D as well as GTF-Ic were analyzed by 13C nuclear magnetic resonance (NMR) spectroscopy as previously described (12). The peak assignments were made according to Colson et al. (2).
Oligosaccharide synthesis reaction.
Oligosaccharide synthesis was undertaken in the presence of sucrose and maltose as acceptors, using a sucrose/maltose molar ratio of 1:5. The sucrose concentration was 50 g liter−1, and the reaction was allowed to continue until all of the sucrose was depleted. Synthesized oligosaccharides were analyzed by HPLC using a C18 column and water as eluant at a flow rate of 0.7 ml min−1. They were detected using a differential refractometer.
RESULTS
Mutagenesis of GTF-I Asp-569.
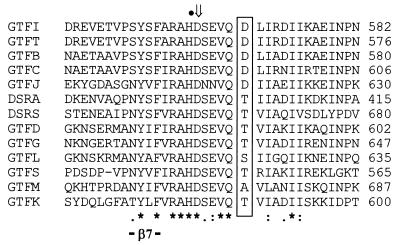
Among streptococcal GTFs and related dextransucrases from Leuconostoc mesenteroides, an Asp residue is always present in sequences of enzymes synthesizing a water-insoluble glucan (Fig. 1). Except for GTF-L and GTF-M, where Ser and Ala residues, respectively, are present, a Thr residue is associated with enzymes synthesizing water-soluble glucan (Fig. 1). Insolubility is thought to be associated with a high content of α(1-3) linkages, though the structures of the glucans made by all GTFs are not yet known. To investigate how the nature of the amino acid at this position modulates both glucan and oligosaccharide synthesis, replacement of Asp-569 from GTF-Ic with different residues was performed by random and biased random mutagenesis. A double-screening procedure after random mutagenesis allowed isolation, from about 100 colonies, of six different GTF-Ic variants where Asp-569 was replaced by Arg, Val, Leu, His, Glu, or Ala. To cover all different possible classes of amino acids, a similar double-screening procedure following biased random mutagenesis of Asp-569 allowed isolation of further GTF-Ic variants where Asp-569 was replaced by Tyr, Ile, Thr, Ser, or Gln.
FIG. 1.
Aligned amino acid sequences of β7 regions of GTFs and related L. mesenteroides dextransucrases. GTF-B, -C, and -D are from S. mutans; GTF-G is from S. gordonii; GTF-S and -I are from S. downei; GTF-J through -M are from S. salivarius. DSR-A and -S are produced by L. mesenteroides (12). Amino acids aligned with Asp-569 from GTF-I are boxed. *, identical residues; :, conserved substitution; ., semiconserved substitution; ⇓, invariant catalytic residue; •, essential His stabilizing transition state in α-amylases (3, 8, 17).
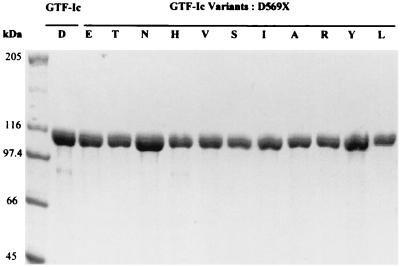
Wild-type GTF-Ic and the 11 variants were expressed in E. coli XL1-Blue and purified by means of the His6 tag at their N-terminal end, using an Ni-nitrilotriacetic acid spin column kit as shown in Fig. 2. No major influence on the level of enzyme production in E. coli by the mutations could be detected.
FIG. 2.
SDS-PAGE analysis followed by Coomassie blue staining of wild-type GTF-Ic and mutant enzymes purified by affinity chromatography. Single letters indicate amino acids substituted for D in GTF-Ic.
Effect of mutations on activity.
Activity assays carried out with purified GTF-Ic and variants showed that all mutations affected the specific activity (Table 1). In the case of D569E and D569T, there was only slight loss of activity, but changing Asp to residues such as Asn, His, Val, Ser, Ile, and Ala resulted in a specific activity less than half that of the wild-type enzyme (mean ± standard deviation, 4.6 ± 0.8 U mg−1 [Table 1]). Change to Tyr, Arg, or Leu had the greatest effect on enzyme activity.
TABLE 1.
Effects of mutation of Asp-569 on glucan and oligosaccharide synthesis
| Amino acid | Sp act (U mg−1)a | Distribution of products from sucrose (%)
|
|||
|---|---|---|---|---|---|
| Glucan synthesisb | Leucrose synthesisb | Hydrolysisb | Yield of oligosaccharidesc | ||
| D (wild type) | 12.07 | 60.5 | 23.9 | 16.7 | 35.0 |
| E | 9.75 | 71.5 | 13.7 | 16.7 | 40.1 |
| T | 8.74 | 77.7 | 10.3 | 13.6 | 41.3 |
| N | 5.84 | 71.4 | 12.9 | 16.5 | 31.5 |
| H | 5.15 | 71.8 | 12.3 | 15.3 | 36.7 |
| V | 5.09 | 76.2 | 11.5 | 14.3 | 33.0 |
| S | 4.39 | 73.6 | 11.9 | 15.1 | 35.9 |
| I | 3.79 | 73.4 | 11.5 | 15.4 | 25.3 |
| A | 3.54 | 74.3 | 11.0 | 15.1 | 37.4 |
| R | 1.96 | 72.2 | 10.6 | 17.7 | 28.8 |
| Y | 1.71 | 72.9 | 11.2 | 16.4 | 38.5 |
| L | 1.47 | 73.4 | 10.5 | 17.0 | 29.0 |
| Avgd | 73.5 ± 2.7 | 11.6 ± 1.1 | 15.7 ± 1.2 | 36.4 ± 5.0 | |
Initial velocity determined in the presence of 50 g of sucrose liter−1. One unit is defined as the amount of enzyme that catalyzed the formation of 1 μmol of fructose min−1.
One liter of the glucan synthesis reaction mixture contained 50 g of sucrose.
One liter of the oligosaccharide synthesis reaction mixture contained 50 g of sucrose and 10 g of maltose (molecular ratio of 5:1).
All experiments were performed in duplicate. Duplicate results never differed by more than ±5%.
Effects of mutations on glucan synthesis.
To investigate the influence of the mutations on the distribution of glucosyl residues deriving from sucrose cleavage during the glucan synthesis reaction, we analyzed the reaction products (Table 1). The rate of sucrose hydrolysis was not significantly modified by any of the mutations, hydrolysis accounting for 16.7% of the sucrose utilization by wild-type GTF-Ic and an average value of 15.7% for the different variants. However, the ratio between the transfer of glucosyl residues to growing glucan chain and to free fructose residues (resulting in leucrose synthesis) was modified by the mutations (Table 1). With GTF-Ic, yields of glucan and leucrose synthesis were 60.8 and 23.9%, respectively. The change of Asp-569 to any other amino acid diverted the transfer of glucosyl residues from fructose toward glucan synthesis. The yield of leucrose synthesis thus decreased to an average value of 11.6% and the yield of glucan synthesis increased to an average value of 73.5%.
Effect of mutation on glucan structure.
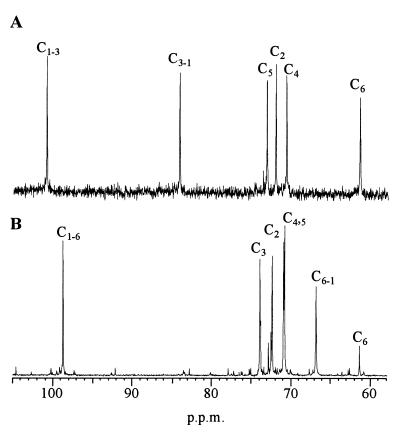
To explore the influence of position 569 on the structure of the polymer produced by GTF-Ic, glucan synthesized by mutant T569D was analyzed. Contrary to GTF-Ic, which produces an insoluble α(1-3)-linked glucan (11, 17), mutant T569D synthesized both insoluble and soluble polymer. The 13C NMR spectrum of the insoluble glucan presented only signals characteristic of glucosyl residues linked through α(1-3) linkages. In addition to signals corresponding to carbon 5 (C-5), C-4, and C-2 in the 68- to 76-ppm area, signals arising at 100.5, 84, and 61 ppm corresponded to C-1 involved in α(1-3) glucosyl linkage, C-3 involved in α(1-3) glucosyl linkage, and nonlinked C-6, respectively (Fig. 3A). The 13C NMR spectrum of the soluble glucan presented only signals characteristic of glucosyl residues linked through α(1-6) linkages (Fig. 3B). In addition to signals corresponding to C-5, C-4, C-3, and C-2 in the 68- to 76-ppm area, signals arising at 98.5, 67, and 61 ppm corresponded to C-1 involved in α(1-6) linkage, C-6 involved in α(1-6) linkage, and nonlinked C-6, respectively.
FIG. 3.
13C NMR analyses of the insoluble (A) and the soluble (B) glucan produced by mutant D569T. Peaks were assigned according to the method of Colson et al. (2).
Effects of mutations on oligosaccharide synthesis in the presence of maltose.
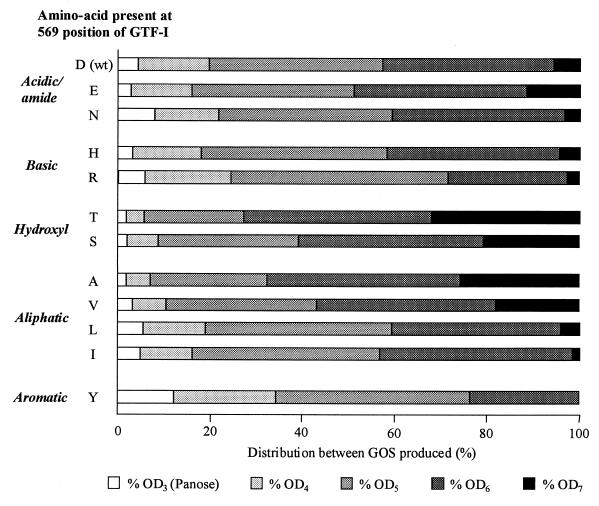
In the presence of maltose as an acceptor, oligosaccharides produced by GTF-I were mainly linear GOS of various degrees of polymerization (DP), according to their retention time during HPLC separation on C18 in comparison with known standard oligosaccharides produced by L. mesenteroides NRRL B-512F dextransucrase (13, 15). GOS of DPn were oligodextrans composed of (n − 2) glucosyl residues linked through α(1-6) bonds and a maltose residue at the reducing end. With a sucrose/maltose molecular ratio of 5:1, GOS ranging from DP3 (panose) to DP7 were produced (Fig. 4). When GOS synthesis was performed with the different GTF-Ic variants, no additional peaks corresponding to the synthesis of new oligosaccharides were observed during HPLC analysis. The total yield of GOS produced by the variants was very close to that obtained with wild-type enzyme (Table 1). Most of the mutations resulted in a change of less than 5% from the yield obtained with GTF-Ic, and only the D569I variant decreased this yield by 10%. On the contrary, the distribution between the different lengths of GOS and especially the yield of oligodextran of DP7 (OD7) was strongly influenced by mutations (Fig. 4), these differences being dependent on the nature of the amino acid change. The replacement of Asp-569 by hydroxyl amino acids (Thr and Ser) or by an aliphatic amino acid with a short lateral chain (Ala) increased the yield of OD7 synthesis 3.5- to 5-fold (Fig. 4). However, an increase in the size of the lateral chain of aliphatic amino acids resulted in a reduction of the OD7 synthesis yield, from 26% of overall GOS with D569A, 18% with D569V, 4% with D569L, and only 1.5% with D569I. The D569Y change virtually abolished the synthesis of OD7. Changes to basic residues such as His and Arg had the same small effect as the change to Asn (Fig. 4).
FIG. 4.
Effects of mutations on the sizes of oligosaccharides produced in the presence of sucrose and maltose as acceptors. One liter of the oligosaccharide synthesis reaction mixture contained 50 g of sucrose and 10 g of maltose. For each reaction, the total amount of linear GOS was defined as 100%.
DISCUSSION
Site-directed mutagenesis of the position corresponding to that studied here in GTF-B and GTF-D of S. mutans has previously been shown to influence the nature of the glucan produced (18). Sequence alignment of GTFs and related L. mesenteroides dextransucrases seemed to indicate that the presence of an Asp residue resulted in the synthesis of insoluble glucan whereas the presence of a Thr residue led to the synthesis of soluble glucan. This suggested that other changes to different amino acids might enlarge GTF specificity. The aim of this study was therefore to find out how this position modulates the activities of glucan and oligosaccharide synthesis by replacing the equivalent Asp-569 of the core region of S. downei GTF-I with different amino acids by random and biased random mutagenesis.
As for GTF-B or GTF-D, this position influences the structure of the glucan produced by GTF-I. In addition to the insoluble α(1-3)-linked glucan produced by GTF-Ic (11), the variant carrying the D569T mutation also produces a soluble α(1-6)-linked glucan, showing that the mutation has affected the orientation of the growing glucan chain mechanism or the transfer of glucosyl residues coming from sucrose cleavage. It was therefore of interest to investigate whether mutation also affected transfer to other acceptors. However, in the presence of maltose as an acceptor, no GOS additional to those produced by GTF-Ic were synthesized by the different variants.
The various replacements of Asp-569 of GTF-Ic resulted in a decrease in activity, the magnitude depending on the nature of the substituent residue. Because Shimamura et al. (18) showed that mutating this position did not significantly modify the sucrose binding of GTF, substitutions of Asp-569 are likely to affect the turnover of the reaction. Local structural disturbances that mutations might cause may explain some small variations, but general features can also be noticed. The change of Asp to Thr does not result in a significant loss of activity, 72% of the initial activity being retained. However, the location of the hydroxyl group itself appears to be essential, as the change to Ser resulted in a twofold decrease of activity. The lateral location of the methyl group in Leu is also critical for activity, as D569L reduced the activity 2.6-fold in comparison to D569I, D569V, and D569A variants, which were all similar.
In the presence of sucrose, the different mutations had no significant effect on the rate of sucrose hydrolysis. In the presence of sucrose and maltose, the total yield of GOS is also largely unchanged. Position 569 is therefore unlikely to be involved directly in glucosyl transfer from sucrose to acceptors. However, the destination of the glucosyl was affected by the mutations. All mutations of Asp-569 had the same effect in increasing glucan yield at the expense of the leucrose yield. As all mutations of Asp-569 have the same effect, this suggests that only interactions promoted by Asp-569 allow fructose molecules to be bound in a way that results in a high yield of leucrose. Distribution between the different lengths of GOS was also clearly affected by the mutations, indicating the influence of Asp-569 on binding of growing oligosaccharides or glucan. In addition, distribution between GOS was dependent on the nature of the residue replacing Asp-569, suggesting that certain amino acids are able to promote specific interactions with the products. The change to a hydroxyl amino acid increased the yield of longer GOS; the difference between the D569T and D569S mutations may illustrate the importance of its orientation. The size of the aliphatic chain seems also to be important, as D569A and D569V mutations increased the yield of longer GOS while D569L and D569I mutations increased the yield of shorter GOS.
Random mutagenesis of Asp-569 from GTF-I has thus shown that the amino acid in this position is more likely to interact with the acceptor molecules (oligosaccharides or elongating glucan) than to be directly involved into the glucosyl transfer from sucrose. Secondary structure predictions locate Asp-569 in a region equivalent to the seventh β-α loop in α-amylase (9), just after the conserved catalytic Asp-567 essential for GTF activity (3). In α-amylases, besides loop 7, residues in the fourth and fifth loops are involved in enzyme specificity (7, 10). We have previously shown by mutation of His-355 of GTF-I that it may play a role in a subsite involved in product binding (12). Other residues may also be directly involved in determining the structure of products (18), and this may explain why a single change at Asp-569 is insufficient to drastically modify enzyme specificity. Nevertheless, this study shows that while engineering of this position does not influence the structure of oligosaccharides, it may be an important target in tailoring the size of the oligosaccharides produced. Investigation of a wider range of reaction conditions may make it possible to enhance this effect. Furthermore, modification of the corresponding residue in GTFs that make predominantly other types of linkage [α(1-6) and α(1-2)] has the potential to expand the range of GOS that can conveniently be synthesized.
ACKNOWLEDGMENTS
This work was supported by Wellcome Trust grant 049554 and the European project BIOTECH CT98-0022.
REFERENCES
- 1.Colby S M, Russell R R B. Sugar metabolism by mutans streptococci. Soc J Appl Microbiol Symp Suppl. 1997;83:80S–88S. doi: 10.1046/j.1365-2672.83.s1.9.x. [DOI] [PubMed] [Google Scholar]
- 2.Colson P, Jennings H J, Smith I C. Composition, sequence, and conformation of polymers and oligomers of glucose as revealed by carbon-13 nuclear magnetic resonance. J Am Chem Soc. 1974;96:8081–8087. doi: 10.1021/ja00833a038. [DOI] [PubMed] [Google Scholar]
- 3.Devulapalle K S, Goodman S D, Gao Q, Hemsley A, Mooser G. Knowledge-based model of a glucosyltransferase from the oral bacterial group of mutans streptococci. Protein Sci. 1997;6:2489–2493. doi: 10.1002/pro.5560061201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferretti J J, Gilpin M L, Russell R R B. Nucleotide sequence of a glucosyltransferase gene from Streptococcus sobrinus MFe28. J Bacteriol. 1987;169:4271–4278. doi: 10.1128/jb.169.9.4271-4278.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hajishengallis G, Michalek S M. Current status of a mucosal vaccine against dental caries. Oral Microbiol Immunol. 1999;14:1–20. doi: 10.1034/j.1399-302x.1999.140101.x. [DOI] [PubMed] [Google Scholar]
- 6.Henrissat B. Glycosidase families. Biochem Soc Trans. 1998;26:153–156. doi: 10.1042/bst0260153. [DOI] [PubMed] [Google Scholar]
- 7.Jespersen H M, MacGregor E A, Henrissat B, Sierks M R, Svensson B. Starch- and glycogen-debranching and branching enzymes: prediction of structural features of the catalytic (β/α)8-barrel domain and evolutionary relationship to other amylolytic enzymes. J Protein Chem. 1993;12:791–805. doi: 10.1007/BF01024938. [DOI] [PubMed] [Google Scholar]
- 8.Kato C, Nakano Y, Lis M, Kuramitsu H K. Molecular genetic analysis of the catalytic site of Streptococcus mutans glucosyltransferases. Biochem Biophys Res Commun. 1992;189:1184–1188. doi: 10.1016/0006-291x(92)92329-v. [DOI] [PubMed] [Google Scholar]
- 9.MacGregor E A, Jespersen H M, Svensson B. A circularly permuted α-amylase-type (β/α)8-barrel structure in glucan-synthesizing glucosyltransferases. FEBS Lett. 1996;378:263–266. doi: 10.1016/0014-5793(95)01428-4. [DOI] [PubMed] [Google Scholar]
- 10.Matsui I, Svensson B. Improved activity and modulated action pattern obtained by random mutagenesis at the fourth beta-alpha loop involved in substrate binding to the catalytic (β/α)8-barrel domain of barley α-amylase 1. J Biol Chem. 1997;272:22456–22463. doi: 10.1074/jbc.272.36.22456. [DOI] [PubMed] [Google Scholar]
- 11.Monchois V, Arguello-Morales M, Russell R R B. Isolation of an active catalytic core of Streptococcus downei MFe28 GTF-I glucosyltransferase. J Bacteriol. 1999;181:2290–2292. doi: 10.1128/jb.181.7.2290-2292.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monchois V, Vignon M, Russell R R B. Isolation of key amino acid residues at the N-terminal end of the core region Streptococcus downei glucansucrase, GTF-I. Appl Microbiol Biotechnol. 1999;52:660–665. doi: 10.1007/s002530051575. [DOI] [PubMed] [Google Scholar]
- 13.Monchois V, Willemot R M, Monsan P. Glucansucrases: mechanism of action and structure-function relationships. FEMS Microbiol Rev. 1999;23:131–151. doi: 10.1111/j.1574-6976.1999.tb00394.x. [DOI] [PubMed] [Google Scholar]
- 14.Monsan P, Paul F. Enzymatic synthesis of oligosaccharides. FEMS Microbiol Rev. 1995;16:187–192. [Google Scholar]
- 15.Robyt J F, Eklund S H. Relative, quantitative effects of acceptors in the reaction of Leuconostoc mesenteroides B-512F dextransucrase. Carbohydr Res. 1983;121:279–286. doi: 10.1016/0008-6215(83)84024-5. [DOI] [PubMed] [Google Scholar]
- 16.Russell R R B. Control of specific plaque bacteria. Adv Dent Res. 1994;8:285–290. doi: 10.1177/08959374940080022301. [DOI] [PubMed] [Google Scholar]
- 17.Russell R R B, Gilpin M L, Mukasa H, Dougan G. Characterization of glucosyltransferase expressed from a Streptococcus sobrinus gene cloned in Escherichia coli. J Gen Microbiol. 1987;133:935–944. doi: 10.1099/00221287-133-4-935. [DOI] [PubMed] [Google Scholar]
- 18.Shimamura A, Nakano Y J, Mukasa H, Kuramitsu H K. Identification of amino acid residues in Streptococcus mutans glucosyltransferases influencing the structure of the glucan product. J Bacteriol. 1994;176:4845–4850. doi: 10.1128/jb.176.16.4845-4850.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsumori H, Minami T, Kuramitsu H K. Identification of essential amino acids in the Streptococcus mutans glucosyltransferases. J Bacteriol. 1997;179:3391–3396. doi: 10.1128/jb.179.11.3391-3396.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker G J. Dextrans. Int Rev Biochem. 1978;16:75–126. [Google Scholar]
- 21.Whiley R A, Russell R R B, Hardie J M, Beighton D. Streptococcus downei sp. nov. of strains previously described as Streptococcus mutans serotype h. Int J Syst Bacteriol. 1988;38:25–29. [Google Scholar]