Abstract:
Purpose: Approaching treatment for elderly patients with atrial fibrillation is difficult. A prospective phase II trial evaluating LINAC-based stereotactic arrhythmia radioablation (STAR) safety in this population started in 2021. Dosimetric and planning data were reported. Materials and Methods: A vac-lock bag was used for immobilization in the supine position and a computed tomography (CT, 1 mm) was performed. The clinical target volume (CTV) was defined as the area around the pulmonary veins. An internal target volume (ITV) was added to the CTV to compensate heart and respiratory movement. The planning target volume (PTV) was defined by adding 0–3 mm to the ITV. STAR was performed during free-breathing with a PTV prescription total dose (Dp) of 25 Gy/1 fraction. Flattening filter-free volumetric-modulated arc therapy plans were generated, optimized, and delivered by TrueBeamTM. Image-guided radiotherapy with cone-beam CT and surface-guided radiotherapy with Align-RT (Vision RT) were employed. Results: From May 2021 to March 2022, 10 elderly patients were treated. Mean CTVs, ITVs, and PTVs were 23.6 cc, 44.32 cc, and 62.9 cc, respectively; the mean prescription isodose level and D2% were 76.5% and 31.2 Gy, respectively. The average heart and left anterior descending artery (LAD) Dmean were 3.9 and 6.3 Gy, respectively; the mean Dmax for LAD, spinal cord, left and right bronchus, and esophagus were 11.2, 7.5, 14.3, 12.4, and 13.6 Gy, respectively. The overall treatment time (OTT) was 3 min. Conclusions: The data showed an optimal target coverage, sparing surrounding tissue, in 3 min of OTT. LINAC-based STAR for AF could represent a valid non-invasive alternative for elderly patients who were excluded from catheter ablation.
Keywords: radiosurgery, atrial fibrillation, stereotactic radiotherapy, arrhythmia
1. Introduction
More than 40 million individuals in the world are affected by atrial fibrillation (AF) and, age being a prominent risk factor, such incidence is expected to grow, considering the increasing average lifespan. The current international guidelines recommend pulmonary vein (PV) isolation with catheter ablation (CA) in symptomatic patients, refractory to antiarrhythmic therapy (AAT) [1].
In elderly patients, which represent the majority of the AF population, the treatment approach is quite complex and invasive: paroxysmal AF is difficult to treat with drugs, since it can cause an alternation between alternate sinus bradycardia and fast-rate AF. As regards the CA approach, the high incidence of complication makes the study of more conservative methods a reasonable consideration [2].
One of the most promising and novel alternative approaches for the management of such patients is stereotactic radiotherapy, a safe and effective discipline that uses a high radiation dose to produce relevant cell damage through multifactorial processes (DNA double-strand breaks, apoptosis, vascular damage, and ischemic cell-death).
In recent years, several stereotactic arrhythmia radioablation (STAR) clinical approaches have been published for ventricular tachycardia, while only studies on animals and three case reports with CyberKnife have been reported for AF [3,4,5].
Animal models for radioablation to block signals at the pulmonary venin antrum showed that scarring effects arise with dosages over 30 Gy [6,7,8]. Recent studies introduced the concept of radio modulation in the STAR treatment, according to which 25 Gy single-fraction radiation does not increase cardiac fibrosis, but it modulates the expression of sodium channels, producing a marked decrease in the arrhythmic burden [9,10].
Based on this background, a prospective phase II trial was designed, intended to evaluate the safety of LINAC-based STAR (ClinicalTrials.gov: NCT04575662) in elderly patients, with the first clinical data of five patients recently published [11].
The aim of this study was to report the dosimetric and planning data of the first 10 enrolled patients, proposing a novel planning strategy for the physical management of this treatment.
2. Methods
The NCT04575662 Phase II study was approved by the local Ethics Committee and all patients signed informed consent forms. As previously reported [11], the following inclusion criteria were considered: age higher than 70 years; symptomatic paroxysmal AF; intolerance or non-response to AAT.
The primary endpoint was the 1-month post-STAR safety check, in terms of complete STAR delivery with no acute treatment-related adverse events above G3, assessed according to the Common Terminology Criteria for Adverse Events (version 5.0).
Secondary endpoints were reductions in AF episodes, reduction in AAT, and overall survival.
The sample size was planned for 20 cases based on 95% success for the primary endpoint, with a significance level of 5% and a statistical power of 90%.
A vac-lock bag was used for patient immobilization in the supine position. Three computed tomography (CT) scans were acquired: (1) free-breathing CT for dose calculation; (2) 4-Dimension CT (4D-CT) for motion evaluation with 10 phases; and (3) CT with contrast for anatomical accuracy [11]. The following organs at risk (OaRs) were delineated referring to the international atlas [12,13,14]: lungs, trachea, main bronchus, esophagus, breasts, aorta, superior and inferior vena cava, pulmonary artery, pulmonary veins, left atrium, right atrium, left ventricle, right ventricle, left anterior descending coronary, circumflex artery, and heart including pericardium.
As regards the esophagus and main bronchus, a 3 mm planning risk volume (PRV) was also considered.
The clinical target volume (CTV) was identified in accordance with radiation oncology and cardiology, considering the area around PVs, based on anatomical CT imaging, and generating 2 separate target volumes: one around the left PVs (CTVleft) and the other on the right PVs (CTVright).
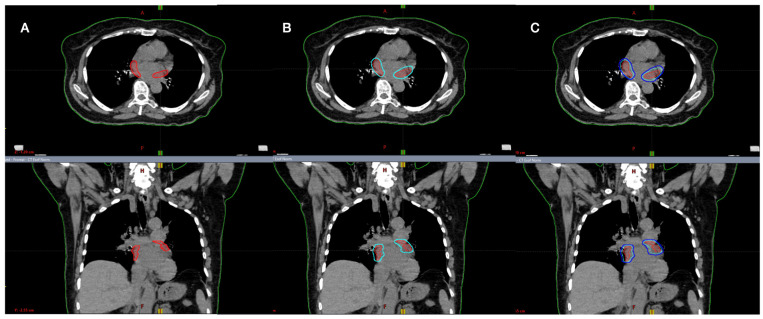
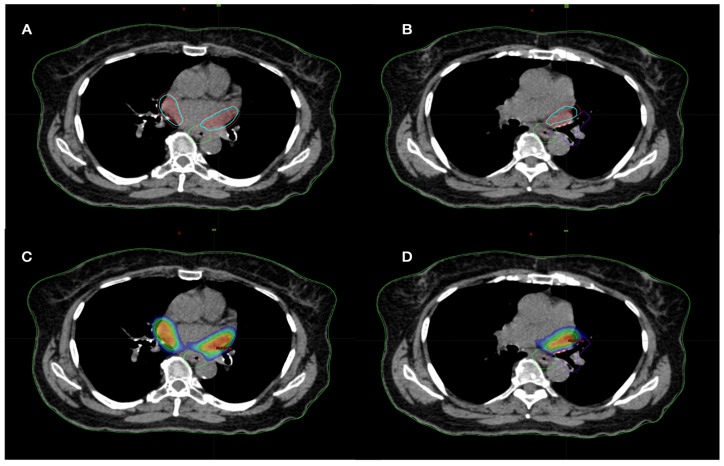
Based on 4D-CT acquisition, an internal target volume (ITV) was added to CTVs in order to compensate for respiratory motion. Although the CT scan was not synchronized to the electrocardiogram (ECG), the magnitude of ITV was adequate to also include heart displacements [15]. Finally, the planning target volume (PTV) was defined by adding 0–2 mm to the ITV, excluding the overlap area with OaRs/PRV, where PTV was cropped (Figure 1 and Figure 2).
Figure 1.
Definition of (A) clinical target volume (CTV); (B) internal target volume (ITV); (C) planning target volume (PTV).
Figure 2.
(A,B) PTV and ITV cropped from OaRs (esophagus and bronchus); (C,D) STAR Planning with simultaneous integrated protection dose.
Medial-lateral (M-L), anterior-posterior (A-P) and superior-inferior (S-I) displacements for the center of mass of CTVright and CTVleft were evaluated during all respiratory phases on 4D-CT. STAR was performed in free-breathing, prescribing 25 Gy in single fraction. A “simultaneous integrated protection” (Figure 2) dose was realized to the interface between PTV-PRV to ensure the tolerability of critical structures [16]. To avoid dose hotspots in the ITV-PTV expansion, it was decided to prescribe the dose to ITV volume, with an additional PTV dose coverage of 95% dose to 95% of the volume.
A flattening filter-free (FFF), volumetric-modulated arc therapy (VMAT) plan was generated, normalizing 100% Dp to 95% of the volume, while large intra-target dose heterogeneity D2% (PTV) < 150%Dp was accepted. The radiation treatment was delivered on a linear accelerator (TrueBeamTM, Varian Medical System, Mountain View, US). Image-guided radiotherapy (IGRT) using cone-beam CT (CBCT) and surface-guided radiotherapy (SGRT) (Align-RT, Vision RT) were used to reduce setup error and monitor patients during treatment.
In addition, a real time ECG was acquired for the entire procedure for each patient.
The plan conformity index (defined as the volume of 100% of the prescription dose to the volume of PTV) and the gradient measures (GM) were evaluated for all plans.
3. Results
From May 2021 to March 2022, 10 elderly patients were treated; the median age was 79.5 years old (range 72–90), and 7 out 10 (70%) patients were female.
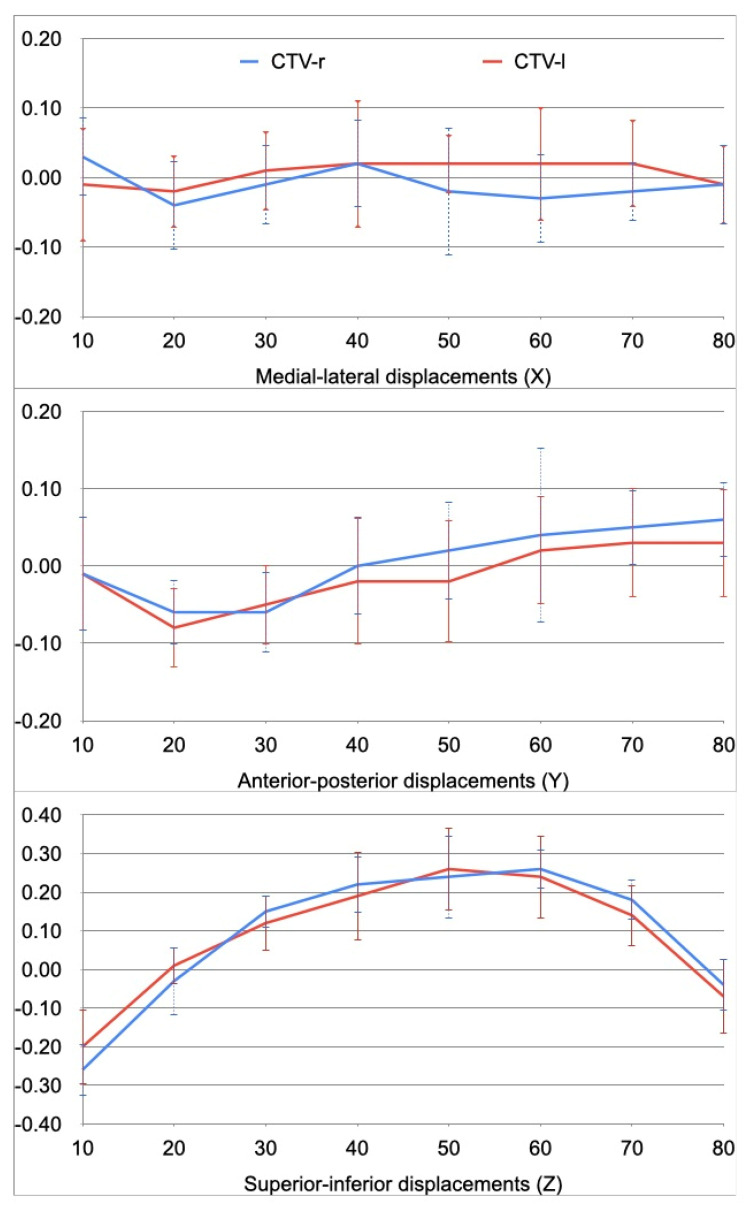
In terms of 4D-CT data, the average Cohort displacements of the CTV during the respiratory phases are shown in Figure 3. The average directions for CTVright and CTVleft were −0.01; 0.01; 0.09; 0.01; −0.01; and 0.09 cm, respectively.
Figure 3.
Displacements of CTVs (right CTV-r and left CTV-l) and standard deviation.
However, only S-I movements reported a motion amplitude of 0.6 cm; for M-L and A-P, an amplitude of 0.1 cm was documented.
The main STAR data are summarized in Table 1: average CTVs (right plus left), ITVs (right plus left), and PTVs (right plus left) were 23.6 cc, 44.32 cc, and 62.9 cc, respectively, while the mean prescription isodose level and D2% were 76.5% and 31.2 Gy, respectively.
Table 1.
Main treatment planning and dosimetric data.
| PT 1 | PT 2 | PT 3 | PT 4 | PT 5 | PT 6 | PT 7 | PT 8 | PT 9 | PT 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) |
72 | 78 | 84 | 90 | 80 | 86 | 75 | 83 | 77 | 79 |
| Sex | F | F | F | M | F | M | F | F | M | F |
| CTV tot (right plus left) (cc) |
25.31 | 12.4 | 16.3 | 15.86 | 25.12 | 15.83 | 28.95 | 18.68 | 48.05 | 29.81 |
| ITV tot (cc) |
59.10 | 37.3 | 38.7 | 44.55 | 50.56 | 32.58 | 36.57 | 31.26 | 68.09 | 44.65 |
| ITV tot cropped (cc) |
54.10 | 35.2 | 36 | 40.22 | 39.7 | 28.21 | 29.19 | 25.58 | 63.9 | 39.13 |
| PTV tot (right plus left) (cc) |
78.24 | 55 | 58.9 | 56.6 | 90.69 | 49 | 48.2 | 43.11 | 90.15 | 59.46 |
| PTV tot cropped (cc) |
69.88 | 47.3 | 53.5 | 51.71 | 71.89 | 42.3 | 43.8 | 38 | 89.6 | 56.8 |
| Dose 2% (Gy) |
31.45 | 30.6 | 30.3 | 30.3 | 30.2 | 31 | 32.6 | 31.4 | 33 | 33 |
| CI | 1.80 | 1.06 | 1.99 | 2.82 | 1.14 | 1.15 | 1.09 | 1.07 | 1.01 | 1.00 |
| GM (cm) |
1.34 | 1.28 | 1.28 | 1.44 | 1.60 | 1.24 | 1.28 | 1.31 | 1.45 | 1.35 |
| OTT (minutes) |
3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
F—female; M—male; Gy—Gray; CTV—clinical target volume; ITV—internal target volume; PTV—planning target volume; tot—total (right plus left); OTT—overall treatment time.
The plan conformity index and the GM were evaluated for all plans (Table 1), reporting a median value of 1.15 (range 1–2.8), and 1.34, respectively.
In terms of beam arrangement, all of the plans were delivered with three coplanar or non-coplanar 10 MV-FFF arcs, as showed in Table 2.
Table 2.
Geometrical characteristics of STAR treatment plans.
| Plan # | Collimator Degree |
Beam Energy (MeV) |
Arcs (Number and Degree) |
Couch Angle (Degree) |
Monitor Units |
|---|---|---|---|---|---|
| #1 | 25–335–105 | 10 × FFF | 3 arcs 240–110 |
0 | 8559 |
| #2 | 75–35–335 | 10 × FFF | 3 arcs 250–110 50–250 320–110 |
0–7–353 | 7622 |
| #3 | 75–35–335 | 10 × FFF | 3 arcs 250–110 50–250 320–110 |
0–7–353 | 7028 |
| #4 | 75–35–335 | 10 × FFF | 3 arcs 250–110 50–250 320–110 |
0–7–353 | 7990 |
| #5 | 75–35–335 | 10 × FFF | 3 arcs 250–110 50–250 320–110 |
0–7–353 | 8014 |
| #6 | 75–35–335 | 10 × FFF | 3 arcs 250–110 50–250 320–110 |
0–7–353 | 8100 |
| #7 | 75–35–335 | 10 × FFF | 3 arcs 250–110 50–250 320–110 |
0–7–353 | 8500 |
| #8 | 75–35–335 | 10 × FFF | 3 arcs 250–110 50–250 320–110 |
0–7–353 | 8040 |
| #9 | 75–35–335 | 10 × FFF | 3 arcs 250–110 50–250 320–110 |
0–7–353 | 6679 |
| #10 | 75–35–335 | 10 × FFF | 3 arcs 180–179 179–330 30–180 |
0–5–355 | 6679 |
MeV—megaelectron volt; FFF—flattening filter-free.
The mean value of monitor units was 7700 (range), while the mean treatment time was 3 min.
Table 3 reports the dose values observed for all of the OaRs case by case. The average mean dose for heart and LAD were 3.9 and 6.3 Gy, respectively; the mean values observed for the maximum dose of LAD, spinal cord, left and right bronchus, and esophagus were 11.2, 7.5, 14.3, 12.4, and 13.6 Gy, respectively.
Table 3.
Dose constraints of Organs at Risk.
| Plan # | Heart Dmean | LAD Dmean | LAD Dmax | Spinal Cord Dmax | Left Bronchus Dmax | Right Bronchus Dmax | Esophagus Dmax | Esophagus D2.5 | Esophagus V12 |
|---|---|---|---|---|---|---|---|---|---|
| #1 | 4.7 Gy | 4.5 Gy | 6.5 Gy | 4 Gy | 10.8 Gy | 4.6 Gy | 11.8 Gy | 9.37 Gy | / |
| #2 | 3.9 Gy | 6.5 Gy | 12 Gy | 8.3 Gy | 8.1 Gy | 18 Gy | 10.7 Gy | 8.59 Gy | / |
| #3 | 3.8 Gy | 7.8 Gy | 13 Gy | 7.1 Gy | 15 Gy | 14 Gy | 12.9 Gy | 10.96 Gy | 0.25 cc |
| #4 | 4 Gy | 6.3 Gy | 9 Gy | 7.1 Gy | 19 Gy | 6.7 Gy | 13.2 Gy | 9.38 Gy | 0.12 cc |
| #5 | 4.2 Gy | 4.1 Gy | 11.2 Gy | 8.9 Gy | 19 Gy | 16.4 Gy | 14.1 Gy | 11.4 Gy | 0.91 cc |
| #6 | 3.7 Gy | 8 Gy | 18 Gy | 7.7 Gy | 10 Gy | 7.5 Gy | 15.4 Gy | 11.48 Gy | 1.38 cc |
| #7 | 3.4 Gy | 8.9 Gy | 14.8 Gy | 7.7 Gy | 15.7 Gy | 16.1 Gy | 14.2 Gy | 11.46 Gy | 0.94 cc |
| #8 | 3.84 Gy | 3.9 Gy | 8.3 Gy | 6 Gy | 19 Gy | 12.6 Gy | 15.2 Gy | 12.07 Gy | 2.76 cc |
| #9 | 3.7 Gy | 8 Gy | 11 Gy | 10.4 Gy | 15 Gy | 15 Gy | 14.6 Gy | 11.03 Gy | 0.91 cc |
| #10 | 4.4 Gy | 5 Gy | 8.6 Gy | 8.4 Gy | 11.2 Gy | 13 Gy | 14.7 Gy | 11.74 Gy | 1.55 cc |
Dmean—mean dose; Dmax—maximum dose; Heart—heart minus PTV (planning target volume); LAD—left anterior descending artery; Gy—Gray; D2.5—dose to 2.5 cc; V12—volume receiving 12 Gy.
Table 4 reports the evaluation of radiation dosage to normal tissue (defined as the total body): Dmean, V5 Gy, and V10 Gy were 0.7 Gy; 3.6%, and 0.8%, respectively.
Table 4.
Dosimetric parameters for normal tissue.
| Plan # | Dmean (Gy) | V5 (%) | V10 (%) |
|---|---|---|---|
| #1 | 0.7 | 3.9 | 0.85 |
| #2 | 0.68 | 4 | 0.9 |
| #3 | 0.58 | 3 | 0.7 |
| #4 | 0.7 | 3.9 | 0.8 |
| #5 | 0.9 | 5.8 | 1.3 |
| #6 | 0.7 | 3.4 | 0.8 |
| #7 | 0.65 | 3 | 0.7 |
| #8 | 0.65 | 2.9 | 0.7 |
| #9 | 0.5 | 2.7 | 0.6 |
| #10 | 0.6 | 3.7 | 0.9 |
Dmean—mean dose; Gy—Gray; V5—volume receiving 5 Gy; V10—volume receiving 10 Gy.
As the first aim of the trial was to prevent grade 3 toxicities, PTVs were cropped to the PRV of OaRs (Figure 2), so in this way, all dose constraints, in terms of maximal dose for OaRs, were respected [15,17].
4. Discussion
In this report, planning and dosimetric analysis on the first 10 patients enrolled in the STAR trial were outlined, with the aim of defining a technical guideline for improving STAR approaches in radiotherapy departments.
In terms of planning parameters, we observed that the majority of patients were planned using three arcs with the combination of 250–110, 50–250, and 320–110 degrees, and 75–35–335 collimator degree. The use of 10FFF was preferred due to the reduction in time of beam exposure, in patients without cardiac devices.
Since there are no other analyses in the literature about the use of LINAC for AF-STAR, we could only compare these results with a few published cases performed with a different technology (Cyberknife) [18,19]. As previously reported, the target definition was not the same. In the Cyberknife cases, the PVs and the left atrial posterior wall were irradiated. The mainstay AF ablation approach is PVs isolation, while appropriate/effective ablation targets, including the atrial wall, remain poorly defined [1,18,19]. Thus, in the present trial, the target was defined as the area around the PVs, defined by radiation oncology and cardiology (Figure 1 and Figure 2). In fact, the present mean CTV was 23 cc, while for Cyberknife, data is roundly 50 cc for all cases.
For all patients enrolled in our phase II trial, a 4D-CT was performed in order to evaluate respiratory movements. CTVLeft and CTVRight displace laterally, A-P and S-I by a few millimeters on average (max amplitude 0.1–0.6 cm). The displacements of CTVLeft and CTVRight were similar during respiratory phases, even if it seems that CTVright was more mobile, with respect to the left, for M-L and A-P displacements (Figure 3).
Based on these results, deep inspiration breath hold (DIBH) could be of limited use for elderly patients with AF (increasing treatment time and difficulty for elderly patients, DIBH could be a trigger of AF), and a free-breathing STAR with 4D-CT should be more efficient.
Based on the targets (2 PTVs for each patient, Figure 1), a 10MV-FFF VMAT plan with three coplanar or non-coplanar arcs is useful in order to obtain better target coverage, sparing OaRs, as shown in Table 2 and Table 3. Surely, considering the “protection of OaRs” and the dose prescription on ITV, the conformity index calculated on PTV was higher than 1, but acceptable (range 1–1.99).
Moreover, Cox et al. showed that, in order to minimize the risk of esophagitis above grade 3, esophagus D2.5 cc and V12 Gy should be less than 14 Gy and 3.78 cc respectively, with a maximum esophagus dose of 22 Gy [20]. For all patients enrolled in the present phase II study, the latter dose constraints for the esophagus were considered and respected.
The OaRs dose differences from LINAC and Cyberknife are negligible: 14 Gy versus 16 Gy for the esophagus, respectively; 4 Gy versus 7.8 Gy for the heart (ventricles) in a Cyberknife case, and 10 Gy (myocardium) in the other two Cyberknife cases [19,21].
The most important advantage of LINAC-based STAR is the delivery time and the MU, and the present report is in line with previous publication reports [3,4,19,21]: treatment time of 3 versus 90 min and MUs of 7700 versus 46,000. However, the shorter time is essential for reducing intrafraction motion, so in the present trial, due to the motion study and IGRT/SGRT monitoring, the introduction of fiducials was not necessary [3,4,5,22].
One limitation that we must mention is, as explained earlier in the text, the lack of synchronization of the CT scan with the ECG; to fill this gap, the magnitude of the ITV was adjusted to effectively include cardiac displacements. Finally, a criticism for STAR in a non-oncological population could be the hypothetical role of low-dose exposure for RT-related carcinogenesis on surrounding healthy tissues. The hypothesis of RT-carcinogenesis derived from atomic bomb survivors, reporting a second tumor probability of 8% at 30 years from primary cancer, with a risk mortality attributable to RT-induced secondary cancer estimated at 1–2% after 10-years [23,24,25,26,27].
Thus, for this reason, the importance of RT-related tumor growth in the elderly population with AF is still negligible. Moreover, in the present analysis, all the conceived dose-volume parameters for the normal tissue structures were low and acceptable (see Table 4: Dmean for all body is 0.7 Gy).
5. Conclusions
LINAC-based STAR can be a promising treatment option for elderly patients affected by AF [28], representing a valid non-invasive alternative for elderly patients who were excluded from CA.
The present dosimetric data showed an optimal target coverage, sparing surrounding tissue, in a reduced treatment time, with comfortable results for elderly AF patients. Considering the large diffusion of LINAC in the world, and the large AF population that could be treated with radiation, the present collected data (regarding target definition, motion evaluation, planning, and dosimetry) are interesting for all radiation oncologists who want to implement LINAC-based STAR for AF in their oncological department.
Author Contributions
Conceptualization: I.B., F.G., A.D.M., M.G. and A.F.; methodology: A.D.M., A.F. and M.G.; software: I.B. and E.P.; validation: R.C. (Roberta Carbonara), A.S., M.P.C. and A.F.; formal analysis: I.B. and F.G.; investigation: F.G., A.D.M. and A.F.; data curation: F.G.; writing—original draft preparation: A.F.; writing—review and editing: D.C., F.T., F.G., A.S., E.L., R.C. (Roberta Carbonara), I.R. and F.C.D.G.; visualization: D.C., F.T., F.G., A.S., E.L., R.C. (Roberto Calbi), I.R., F.C.D.G., G.S., C.D.P., N.V. and F.Q.; supervision: A.F., A.D.M. and M.G.; project administration: M.G. and A.F. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of UOC Advanced Radiation Therapy Miulli.art. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee, and with the 1964 Helsinki declaration and its later amendments, or comparable ethical standards.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The data can be acquired from the corresponding author.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Hindricks G., Potpara T., Dagres N., Arbelo E., Bax J.J., Blomström-Lundqvist C., Boriani G., Castella M., Dan G.-A., Dilaveris P.E., et al. ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021;42:4194. doi: 10.1093/eurheartj/ehab648. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy R., Oral H. Catheter ablation of atrial fibrillation in the elderly: Does the benefit outweigh the risk? Expert. Rev. Cardiovasc. Ther. 2013;11:697–704. doi: 10.1586/erc.13.2. [DOI] [PubMed] [Google Scholar]
- 3.Fiorentino A., Gregucci F., Bonaparte I., Vitulano N., Surgo A., Mazzola R., Di Monaco A., Carbonara R., Alongi F., Langialonga T., et al. Stereotactic Ablative radiation therapy (SABR) for cardiac arrhythmia: A new therapeutic option? Radiol. Med. 2021;126:155–162. doi: 10.1007/s11547-020-01218-7. [DOI] [PubMed] [Google Scholar]
- 4.Bonaparte I., Gregucci F., Surgo A., Di Monaco A., Vitulano N., Ludovico E., Carbonara R., Ciliberti M.P., Quadrini F., Grimaldi M., et al. Linac-based STereotactic Arrhythmia Radioablation (STAR) for ventricular tachycardia: A treatment planning study. Jpn. J. Radiol. 2021;39:1223–1228. doi: 10.1007/s11604-021-01159-9. [DOI] [PubMed] [Google Scholar]
- 5.Fiorentino A., Di Monaco A., Surgo A., Vitulano N., Gregucci F., Ludovico E., Carbonara R., Quadrini F., Rubini G., Bonaparte I., et al. Linac-based STereotactic Arrhythmia Radioablation (STAR) of ventricular tachycardia: Case report and literature review. Clin. Case Rep. 2020;9:362–366. doi: 10.1002/ccr3.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanck O., Bode F., Gebhard M., Hunold P., Brandt S., Bruder R., Grossherr M., Vonthein R., Rades D., Dunst J. Dose-escalation study for cardiac radiosurgery in a porcine model. Int. J. Radiat. Oncol. Biol. Phys. 2014;89:590–598. doi: 10.1016/j.ijrobp.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 7.Bode F., Blanck O., Gebhard M., Hunold P., Grossherr M., Brandt S., Vonthein R., Thiele H., Dunst J., Rades D. Pulmonary vein isolation by radiosurgery: Implications for non-invasive treatment of atrial fibrillation. Europace. 2015;17:1868–1874. doi: 10.1093/europace/euu406. [DOI] [PubMed] [Google Scholar]
- 8.Chang J.H., Cha M.-J., Seo J.-W., Kim H.J., Park S.-Y., Kim B.H., Lee E., Kim M.-K., Yoon H.-S., Oh S. Feasibility study on stereotactic radiotherapy for total pulmonary vein isolation in a canine model. Sci. Rep. 2021;11:12369. doi: 10.1038/s41598-021-91660-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang D.M., Navara R., Yin T., Szymanski J., Goldsztejn U., Kenkel C., Lang A., Mpoy C., Lipovsky C.E., Qiao Y., et al. Cardiac radiotherapy induces electrical conduction reprogramming in the absence of transmural fibrosis. Nat. Commun. 2021;12:5558. doi: 10.1038/s41467-021-25730-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cha M.J., Seo J.W., Kim H.J., Kim M.K., Yoon H.S., Jo S.W., Oh S., Chang J.H. Early Changes in Rat Heart After High-Dose Irradiation: Implications for Antiarrhythmic Effects of Cardiac Radioablation. J. Am. Heart Assoc. 2021;10:e019072. doi: 10.1161/JAHA.120.019072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Monaco A., Gregucci F., Bonaparte I., Troisi F., Surgo A., Di Molfetta D., Vitulano N., Quadrini F., Carbonara R., Martinelli G., et al. Paroxysmal Atrial Fibrillation in Elderly: Worldwide Preliminary Data of LINAC-Based Stereotactic Arrhythmia Radioablation Prospective Phase II Trial. Front. Cardiovasc. Med. 2022;9:832446. doi: 10.3389/fcvm.2022.832446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong F.M., Ritter T., Quint D.J., Senan S., Gaspar L.E., Komaki R.U., Hurkmans C.W., Timmerman R., Bezjak A., Bradley J.D., et al. Consideration of dose limits for organs at risk of thoracic radiotherapy: Atlas for lung, proximal bronchial tree, esophagus, spinal cord, ribs, and brachial plexus. Int. J. Radiat. Oncol. Biol. Phys. 2011;81:1442–1457. doi: 10.1016/j.ijrobp.2010.07.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milo M.L.H., Offersen B.V., Bechmann T., Diederichsen A.C.P., Hansen C.R., Holtved E., Josipovic M., Lörincz T., Maraldo M.V., Nielsen M.H., et al. Delineation of whole heart and substructures in thoracic radiation therapy: National guidelines and contouring atlas by the Danish Multidisciplinary Cancer Groups. Radiother. Oncol. 2020;150:121–127. doi: 10.1016/j.radonc.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 14.Duane F., Aznar M.C., Bartlett F., Cutter D.J., Darby S.C., Jagsi R., Lorenzen E.L., McArdle O., McGale P., Myerson S., et al. A cardiac contouring atlas for radiotherapy. Radiother. Oncol. 2017;122:416–422. doi: 10.1016/j.radonc.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bahig H., De Guise J., Vu T., Chartrand-Lefebvre C., Blais D., Lebeau M., Nguyen N.T., Roberge D. Analysis of Pulmonary Vein Antrums Motion with Cardiac Contraction Using Dual-Source Computed Tomography. Cureus. 2016;8:e712. doi: 10.7759/cureus.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benedict S.H., Yenice K.M., Followill D., Galvin J.M., Hinson W., Kavanagh B., Keall P., Lovelock M., Meeks S., Papiez L., et al. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med. Phys. 2010;37:4078–4101. doi: 10.1118/1.3438081. [DOI] [PubMed] [Google Scholar]
- 17.Boda-Heggemann J., Blanck O., Mehrhof F., Ernst F., Buergy D., Fleckenstein J., Tülümen E., Krug D., Siebert F.-A., Zaman A., et al. Interdisciplinary Clinical Target Volume Generation for Cardiac Radioablation: Multicenter Benchmarking for the RAdiosurgery for VENtricular TAchycardia (RAVENTA) Trial. Int. J. Radiat. Oncol. Biol. Phys. 2021;110:745–756. doi: 10.1016/j.ijrobp.2021.01.028. [DOI] [PubMed] [Google Scholar]
- 18.Franzetti J., Volpe S., Catto V., Conte E., Piccolo C., Pepa M., Piperno G., Camarda A.M., Cattani F., Andreini D., et al. Stereotactic Radiotherapy Ablation and Atrial Fibrillation: Technical Issues and Clinical Expectations Derived From a Systematic Review. Front. Cardiovasc. Med. 2022;9:849201. doi: 10.3389/fcvm.2022.849201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monroy E., Azpiri J., De La Peña C., Cardona C., Hinojosa M., Zamarripa R., Assad J. Late Gadolinium Enhancement Cardiac Magnetic Resonance Imaging Post-robotic Radiosurgical Pulmonary Vein Isolation (RRPVI): First Case in the World. Cureus. 2016;8:e738. doi: 10.7759/cureus.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox B.W., Jackson A., Hunt M., Bilsky M., Yamada Y. Esophageal toxicity from high-dose, single-fraction paraspinal stereotactic radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 2012;83:e661–e667. doi: 10.1016/j.ijrobp.2012.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qian P.C., Azpiri J.R., Assad J., Aceves E.N.G., Ibarra C.E.C., De La Pena C., Bs M.H., Wong D., Fogarty T., Maguire P., et al. Noninvasive stereotactic radioablation for the treatment of atrial fibrillation: First-in-man experience. J. Arrhyth. 2020;36:67–74. doi: 10.1002/joa3.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jumeau R., Ozsahin M., Schwitter J., Elicin O., Reichlin T., Roten L., Andratschke N., Mayinger M., Saguner A.M., Steffel J., et al. Stereotactic Radiotherapy for the Management of Refractory Ventricular Tachycardia: Promise and Future Directions. Front. Cardiovasc. Med. 2020;7:108. doi: 10.3389/fcvm.2020.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoeller U., Borgmann K., Oertel M., Haverkamp U., Budach V., Eich H.T. Late Sequelae of Radiotherapy. Dtsch. Arztebl. Int. 2021;118:205–211. doi: 10.3238/arztebl.m2021.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Gonzalez A.B., Curtis R.E., Kry S.F., Gilbert E., Lamart S., Berg C.D., Stovall M., Ron E. Proportion of second cancers attributable to radiotherapy treatment in adults: A cohort study in the US SEER cancer registries. Lancet Oncol. 2011;12:353–360. doi: 10.1016/S1470-2045(11)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Preston D.L., Ron E., Tokuoka S., Funamoto S., Nishi N., Soda M., Mabuchi K., Kodama K. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat. Res. 2007;168:1–64. doi: 10.1667/RR0763.1. [DOI] [PubMed] [Google Scholar]
- 26.Bhatia S., Robison L.L., Oberlin O., Greenberg M., Bunin G., Fossati-Bellani F., Meadows A.T. Breast cancer and other second neoplasms after childhood Hodgkin’s disease. N. Engl. J. Med. 1996;334:745–751. doi: 10.1056/NEJM199603213341201. [DOI] [PubMed] [Google Scholar]
- 27.Dörr W., Herrmann T. Second primary tumors after radiotherapy for malignancies, Treatment related parameters. Strahlenther. Onkol. 2002;178:357–362. doi: 10.1007/s00066-002-0951-6. [DOI] [PubMed] [Google Scholar]
- 28.Di Monaco A., Gregucci F., Bonaparte I., Troisi F., Surgo A., Di Molfetta D., Vitulano N., Quadrini F., Carbonara R., Ludovico E., et al. First pulmonary vein isolation using LINAC Based STAR. Arryth. Electrophysiol. 2022;15:e010880. doi: 10.1161/CIRCEP.122.010880. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data can be acquired from the corresponding author.