Abstract
Fish losses from infectious diseases are a significant problem in aquaculture worldwide. Therefore, we investigated the ability of cationic antimicrobial peptides to protect against infection caused by the fish pathogen Vibrio anguillarum. To identify effective peptides for fish, the MICs of certain antimicrobial peptides against fish pathogens were determined in vitro. Two of the most effective antimicrobial peptides, CEME, a cecropin-melittin hybrid peptide, and pleurocidin amide, a C-terminally amidated form of the natural flounder peptide, were selected for in vivo studies. A single intraperitoneal injection of CEME did not affect mortality rates in juvenile coho salmon infected with V. anguillarum, the causative agent of vibriosis. Therefore, the peptides were delivered continuously using miniosmotic pumps placed in the peritoneal cavity. Twelve days after pump implantation, the fish received intraperitoneal injections of V. anguillarum at a dose that would kill 50 to 90% of the population. Fish receiving 200 μg of CEME per day survived longer and had significantly lower accumulated mortalities (13%) than the control groups (50 to 58%). Fish receiving pleurocidin amide at 250 μg per day also survived longer and had significantly lower accumulated mortalities (5%) than the control groups (67 to 75%). This clearly shows the potential for antimicrobial peptides to protect fish against infections and indicates that the strategy of overexpressing the peptides in transgenic fish may provide a method of decreasing bacterial disease problems.
During the last decades, gene-encoded cationic antimicrobial peptides have been identified in virtually all species of life, including bacteria, plants, vertebrates, invertebrates, and mammals. Many of these peptides have been shown to play roles in host defenses, providing local nonspecific protection against infectious microbes. Such a role is assisted by the broad spectrum of activity against bacteria, fungi, and/or enveloped viruses and the rapid action of these cationic peptides. Well-known examples of cationic antimicrobial peptides are the cecropins, melittins, magainin, and defensins (8).
Protection of fish against infectious diseases is a major challenge in aquaculture worldwide, and losses due to infectious diseases limit profitability. The use of antibiotics and vaccination has partially alleviated this problem. However antibiotic use has raised concerns of antibiotic resistance development and antibiotic residues in fish. Vaccines are not available for all of the fish pathogens, and vaccinations can involve stressful handling of the animals. One alternative strategy would be to develop disease-resistant fish strains. Considerable evidence has shown that the ectopic expression of genes encoding peptides with in vitro antimicrobial activity can result in increased resistance to fungal and bacterial pathogens in transgenic plants (2, 9, 12, 20) and mice (18). Those peptides could prove to be useful tools for the genetic engineering of disease resistance in transgenic fish. Kelly et al. (10) showed that LSB-37 and Shiva-1, two synthetic derivatives of insect α-helical peptides derived from native cecropin B, were effective against a wide variety of gram-negative fish pathogens in vitro. Furthermore, LSB-37 was able to control Edwardsiella ictaluri infections in channel catfish (11).
Relatively few natural antimicrobial peptides have been discovered in fish. Pardaxin, originally identified as a shark-repellent peptide from Moses sole fish, is a polypeptide toxin that has antimicrobial activity (16). However, that peptide does not possess an amphipathic structure with multiple positive charges like other antimicrobial cationic peptides and is cytotoxic to mammalian cells. Most recently, misgurin (17) and pleurocidin (3) were found in loach (Misgurnus anguillicaudatus) and flounder (Pleuronectes americanus), respectively. Misgurin is a 21-amino-acid peptide with in vitro antimicrobial activity against a broad spectrum of microorganisms and has no significant hemolytic activity (17). Pleurocidin is a 25-residue linear antimicrobial peptide found in the skin mucous secretions of the winter flounder. Pleurocidin has been shown to exert broad-spectrum activity against a wide range of gram-positive and gram-negative bacteria (3). It has high amino acid sequence homology with two other antimicrobial peptides, dermaseptin from the skin of the arboreal frog (15) and ceratotoxin from the Mediterranean fruit fly (14). All three of these peptides have been proposed to form amphipathic α-helices (3, 13, 15).
We investigated the antimicrobial activities of a variety of peptides to identify those that are active against fish pathogens. Two of the more-effective peptides, CEME and amidated pleurocidin, were demonstrated to have the ability to protect coho salmon (Oncorhynchus kisutch) against Vibrio anguillarum infection in vivo.
MATERIALS AND METHODS
Bacterial strains and growth media.
The following bacterial strains were used for testing antimicrobial activity. Field isolates of salmonid pathogens Aeromonas salmonicida and V. anguillarum were satisfactorily identified and kindly provided by Julian Thornton, Microtek International Inc., Victoria, British Columbia, Canada. Pseudomonas aeruginosa strains K799 (parent strain) and Z61 (antibiotic supersusceptible) were described by Angus et al. (1). The parent strain (14028s) and a defensin-supersusceptible strain (MS7953s) of Salmonella enterica serovar Typhimurium were described by Fields et al. (6). Staphylococcus epidermidis C621 was a human clinical isolate obtained from A. Chow, University of British Columbia (UBC). All strains of P. aeruginosa, S. enterica serovar Typhimurium, and S. epidermidis were maintained at 37°C in Mueller-Hinton broth (Difco Laboratories, Detroit, Mich.), while the fish bacteria were maintained at 16°C in tryptic soy broth (Difco; 5 g of NaCl/liter) or tryptic soy broth supplemented with NaCl to a total concentration of 10 g/liter. All strains were stored at −70°C until they were thawed for use and subcultured daily.
Synthesis of peptides.
All peptides were produced using 9-fluorenylmethoxy chemistry by the Nucleic Acid and Protein Services, UBC, Vancouver, Canada. Sequences of the new peptides presented here are included in Table 1. The remaining peptide sequences were as described by Wu et al. (22).
TABLE 1.
Peptide sequences
| Peptide | Sequence |
|---|---|
| Pleurocidin | GWGSFFKKAAHVGKHVGKAALTHYL |
| Pleurocidin amide | GWGSFFKKAAHVGKHVGKAALTHYL-NH2 |
| P-1 | KGWGSFFKKAAHVGKHVGKAALTHYL |
| P-1 amide | KGWGSFFKKAAHVGKHVGKAALTHYL-NH2 |
| P-DER | ALWKTMLKKAAHVGKHVGKAALTHYLN |
| P-CER | SIGSAFKKAAHVGKHVGKAALTHYLN |
| Misgurin | RQRVEELSKFSKKGAAARRRK |
| P-M | GWGSFFKKAAHVGKHVGKAALGAAARRRK |
| DER-M | ALWKTMLKKAAHVGKHVGKAALGAAARRRK |
| CER-M | SIGSAFKKAAHVGKHVGKAALGAAARRRK |
| Dermaseptin | ALWKTMLKKLGTMALHAGKAALGAAADTISQTQ |
| Ceratotoxin | SIGSAFKKALPVAKKIGKAALPIAKAALP |
In vitro activity.
The antimicrobial activities of selected antimicrobial peptides were determined as MICs using the modified microtiter broth dilution method (22). Bacteria were grown overnight to mid-logarithmic phase as described above and diluted to give a final inoculum size of 106 CFU/ml. A suspension of 10 μl of the bacteria was added to each well of a 96-well plate and incubated overnight at the appropriate temperature. Inhibition was defined as growth less than or equal to one-half of the growth observed in control wells, where no peptide was added.
Animals.
Coho salmon were obtained from the Chehalis River of British Columbia and were maintained at 10°C in city water dechlorinated with sodium thiosulfate (free flowing at 1 liter/min in the winter and 2 liters/min in the summer), unless otherwise stated. The fish were kept in a 70-liter tank and fed ad libitum by hand twice a day with a commercial diet.
In vivo experiments.
For single-injection studies, V. anguillarum was grown overnight at room temperature on tryptic soy agar (supplemented with 15 g of NaCl/liter) plates. On the day of the experiment, several colonies were collected and suspended in sterile peptone-saline containing 1 g of Bacto Peptone (Sigma Chemical Co., St. Louis, Mo.) and 8.5 g of NaCl/liter. Juvenile coho salmon (approximately 16 to 18 g) were anesthetized with 0.01% ethyl m-aminobenzoate methanesulfonate (MS 222) in 0.02% sodium bicarbonate and received either peptone-saline alone, CEME (200 μl of a 5-mg/ml concentration in peptone-saline) alone, bacteria (100 μl of a suspension of 106/ml) alone, or a combination of peptides and bacteria, separately injected intraperitoneally. Fish were maintained in a 70-liter tank at approximately 10°C. Mortality was recorded daily.
To mimic physiological conditions, miniosmotic pumps (model 1007D; Alzet Corporation, Palo Alto, Calif.) were used to continuously deliver test agents at a controlled rate into fish. To increase its solubility, before being transferred into the osmotic pumps, CEME was dissolved in 0.129 mM citric acid (2.45 mg/100 μl/pump for the 80-μg/day group and 6.14 mg/100 μl/pump for the 200-μg/day group). Alternatively, pleurocidin amide was dissolved in fish saline (0.85% NaCl) and loaded into the osmotic pumps (7.67 mg/100 μl/pump, 250 μg/day). Juvenile coho salmon (approximately 20 g) were divided into three treatment groups: bacterial injection alone (12 fish); installation of osmotic pumps containing saline, followed by bacterial injection (12 fish); and a combination of installation of osmotic pumps containing CEME followed by bacterial injection (18 fish). The fish were anesthetized as described above, and miniosmotic pumps with a pumping rate of 0.136 μl/h were implanted into the peritoneal cavity. Briefly, the fish were anesthetized and put on a wet pad. A small midline incision of the lower abdomen was made to allow the insertion of the pump. The cut was sutured with one stitch and covered with tissue glue. Heaters were placed in the fish tanks to maintain water temperatures between 11 and 12°C. Pumps were filled with concentrated peptide as described above to deliver approximately 80 μg of peptide/day to fish over a 30-day period. Twelve days after pump implantation, the fish received intraperitoneal injections of V. anguillarum (10,000 CFU/fish). Mortalities were recorded daily. For CEME, the experiment was also repeated at a higher peptide dose of 200 μg/day (12 fish for each control group and 15 for the peptide group). The same procedure was used in the pleurocidin experiment (250 μg/day), but with 12 fish for each control group and 19 fish for the peptide group. In all experiments, fish were treated in a humane fashion under the guidelines of the Canadian Council for Animal Care.
Statistics.
The accumulated mortalities are presented as percentages. Differences between the mortalities were analyzed using Fisher's exact test.
RESULTS
In vitro activities of cationic antimicrobial peptides.
Fish pathogens A. salmonicida and V. anguillarum were selected since they are common causes of infections in coho salmon and rainbow trout reared on fish farms. As shown in Table 2, several α-helical peptides related to CEME showed excellent activity against both A. salmonicida and V. anguillarum (MIC = 1 to 2 μg/ml), but not CP26, which was less effective against V. anguillarum. The extended 13-amino-acid peptide indolicidin and its variant CP11-CN, as well as the cyclic 12-amino-acid peptide bactenecin, had less activity against both pathogens than CEME. The cyclic 10-amino-acid β-structured peptide gramicidin S exhibited moderate activity against the fish pathogens.
TABLE 2.
Activity of antimicrobial peptides against fish pathogens and other gram-negative bacteria and S. epidermidis
| Peptide | MIC (μg/ml) against:
|
||||||
|---|---|---|---|---|---|---|---|
| V. anguillarum | A. salmonicida |
S. enterica serovar Typhimurium strain:
|
P. aeruginosa strain:
|
S. epidermidis strain C621 | |||
| 14028s | MS7953s | K799 | Z61 | ||||
| CEME | 2 | 2 | 2 | 1 | 4 | 2 | 2 |
| CEMA | 2 | 4 | 3a | 2a | 3a | 2a | 4a |
| CP26 | 1 | 32 | 3a | 1a | 4a | 3a | 16a |
| CP29 | 1 | 2 | 2a | 1a | 6a | 3a | 8a |
| Indolicidin | 16 | 32 | 64a | 8a | 64a | 4a | 4a |
| CP11-CN | 8 | 32 | 8a | 1a | 8a | 2a | 1a |
| Bactenecin | 64 | 32 | 8a | 8a | 8a | 0.5a | >64a |
| Gramicidin S | 8 | 8 | 12.5a | 12.5a | 25a | 16a | 3a |
| Pleurocidin | 16 | 2 | 32 | <0.5 | 64 | 16 | 64 |
| Pleurocidin amide | 2 | 1 | 4 | <0.5 | 4 | 4 | 4 |
| P-1 | 16 | 2 | 16 | <0.5 | 64 | 8 | 64 |
| P-1 amide | 2 | 1 | 2 | <0.5 | 2 | 2 | 2 |
| Misgurin | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| P-DER | 4 | 1 | 4 | 0.5 | 4 | 8 | 4 |
| P-CER | 64 | 8 | >64 | 2 | >64 | >64 | >64 |
| P-M | 32 | 1 | 16 | 0.5 | 16 | 16 | 16 |
| DER-M | >64 | 2 | 32 | 0.5 | 32 | 8 | 32 |
| CER-M | >64 | 32 | >64 | 8 | >64 | 64 | >64 |
| Polymyxin B | 16 | <0.5 | <0.5 | <0.5 | 16 | <0.5 | 16 |
| Gentamicin | 1 | <0.5 | <0.5 | <0.5 | 16 | <0.5 | 16 |
Eight derivatives of the two fish peptides pleurocidin and misgurin and sequence homologues of pleurocidin, dermaseptin, and ceratotoxin were constructed. The primary structures of these variants and their parent molecules are depicted in Table 1. Antimicrobial activities of all the peptides against a range of bacteria, including the fish pathogens, were tested and are shown in Table 2. The addition of lysine to the N terminus of pleurocidin (peptide P-1) did not significantly change the antimicrobial activity. However, the amidation of both pleurocidin and P-1 substantially enhanced their antimicrobial activities to a level essentially comparable to that of unamidated CEME. A hybrid of pleurocidin and dermaseptin (P-DER) had more antibacterial activity than unamidated pleurocidin, whereas a hybrid of pleurocidin and ceratotoxin (P-CER) had reduced activity. Misgurin alone was inactive against the bacteria tested. When misgurin was combined as a hybrid with ceratotoxin, the resulting peptide (CER-M) remained quite inactive. However, hybrids of pleurocidin (P-M) or dermaseptin (DER-M) with misgurin showed antibacterial activities approaching those of pleurocidin. These activities were compared to those of cationic antibiotic polymyxin B (a lipopeptide) and gentamicin, which exhibited excellent activity against some bacteria but less activity than CEME and pleurocidin amide against other bacteria. The MICs for the PhoP and -Q S. enterica serovar Typhimurium mutant MS7953s (defensin supersusceptible) were 8- to 64-fold lower than those for its parent strain, whereas MICs of only a few peptides for outer-membrane-altered P. aeruginosa strain Z61 were lower than those for its parent strain (Table 2).
Protection against fish infections.
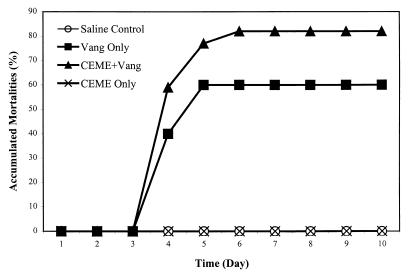
As described above, the antimicrobial peptides CEME and pleurocidin amide were among the most effective antimicrobial peptides tested against the fish pathogens V. anguillarum and A. salmonicida in vitro. Therefore, these two peptides were chosen for in vivo protection studies. In the first experiment, the in vivo effect of CEME was tested by single injections of 1 mg per fish. No fish died during the 10-day trial in either the peptone-saline or the peptide control group (Fig. 1), suggesting that CEME did not have a toxic effect on fish. However, fish injected with only V. anguillarum had 60% mortality, while 82% of fish which received CEME after the bacterial infection died. Although slightly higher mortality in the group receiving peptide combined with bacteria than in the group receiving bacteria alone was observed, the difference was not significant (P = 0.3).
FIG. 1.
Effect of a single CEME injection on V. anguillarum-infected salmon. CEME and V. anguillarum were injected concurrently (CEME+Vang). The control groups were injected with the bacteria alone (Vang only), the peptide alone (CEME only), or peptone-saline alone (saline control). Each infected fish received approximately 10,000 CFU of V. anguillarum. The CEME injection was 1 mg/fish.
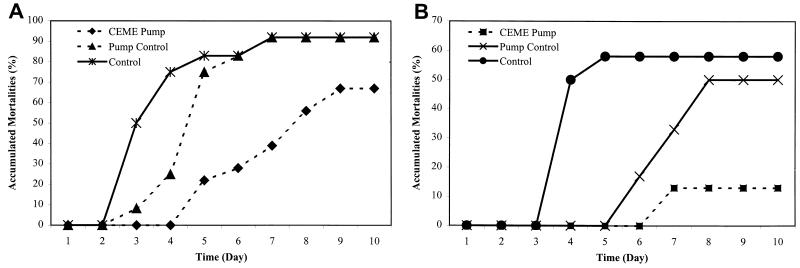
Since a single injection of CEME into coho salmon did not protect fish from V. anguillarum infection, we utilized constant delivery of peptides with miniosmotic pumps. In the studies involving treatment with a low dosage of CEME (80 μg/day), mortalities were first noticed on day 3 for the groups injected with bacteria without peptide treatment and for the group which received saline osmotic pumps as well as bacteria (Fig. 2A). There was no difference in accumulated mortality between the control groups receiving bacterial injection alone and the group receiving saline via the osmotic pump plus bacterial injection (92% for both of them; P = 0.5). However, mortalities were delayed for the group receiving a lower dose of CEME via the osmotic pump (first noticed on day 5), and this group had a relative longer survival time and lower mortalities than the other two groups (67%) although the difference was not significant (P = 0.1). For the high-dosage experiment (200 μg of CEME/day), accumulated mortalities were not significantly different between the control group injected with bacteria alone and the group receiving bacterial injection and saline via the pump (58 versus 50%, respectively; P = 1) (Fig. 2B). However for the group treated with CEME, the accumulated mortality after bacterial injection was significantly reduced (13%; P = 0.007). Some variation in the kinetics of killing in control animals was observed, possibly due to differences in the actual inocula and/or the age of the fish in these experiments. This, however, would not affect the integrity of these experiments since each set of controls was injected with a bacterial inoculum identical to that used in the respective CEME-treated group.
FIG. 2.
Protection of V. anguillarum-infected salmon by CEME delivered via osmotic pump. CEME was constantly administered at the level of 80 μg/day (A) and 200 μg/day (B) into coho salmon though the use of implanted miniosmotic pumps with a pumping rate of 0.136 μl/h (CEME pump). The control groups (control) were injected with bacteria alone. The pump control groups (pump control) were implanted with osmotic pumps containing saline only. The temperature of the tank water was controlled to maintain the constant pumping rate. Twelve days after pump implantation, each fish was challenged with approximately 10,000 CFU of V. anguillarum.
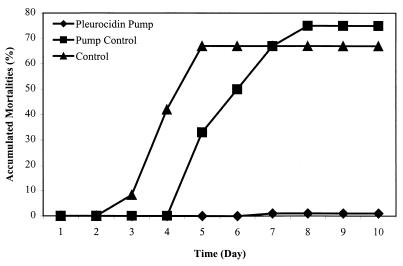
The above study was also performed using pleurocidin amide. Mortalities were first noticed on day 3 for the group injected with bacteria alone and on day 5 for the group which received saline via osmotic pumps as well as bacterial injections (Fig. 3). However, there was no significant difference in mortality between the group which did not have implanted osmotic pumps and the group with the saline osmotic pump (67 versus 75%, respectively; P = 0.6). Only 1 out of 19 fish died (on day 6) in the group receiving 250 μg of pleurocidin amide/day via osmotic pump over the 30 days of this experiment. The accumulated mortality was thus dramatically and significantly (P = 0.0002) reduced to 5% compared with 75% for the saline pump control group.
FIG. 3.
The ability of pleurocidin amide to protect coho salmon from a lethal challenge of V. anguillarum. The peptide was continuously delivered into fish by a miniosmotic pump, implanted intraperitoneally, at a pumping rate of 0.136 μl/h (pleurocidin pump). In the peptide group each fish received pleurocidin amide at approximately 250 μg/day. The control group received bacterial injections only. Fish in the pump control group were implanted with osmotic pumps containing saline only. The water temperature of the tank was controlled to maintain the pumping rate. Twelve days of equilibrating time elapsed before the fish were challenged with approximately 10,000 CFU of V. anguillarum. The accumulated mortalities were determined over 30 days.
DISCUSSION
This study has led to the encouraging finding of in vivo efficacy of certain antimicrobial peptides against bacterial infections. It was previously shown that two synthetic antimicrobial peptide derivatives of the naturally occurring insect peptide cecropin B had significant in vitro activity against bacterial pathogens of fish (10). Due to differences in methodology between the study of Kelly et al. and the present study, it is not possible to perform exact comparisons. However, in general agreement with Kelly et al., we found that CEME, a hybrid of two insect antimicrobial peptides which has both in vitro and in vivo activity against animal pathogens (21), was also very effective in killing two common pathogens of coho salmon, V. anguillarum and A. salmonicida. These findings raised the possibility of utilizing these ectopic peptides through production in transgenic animals, as has been previously done in plants and mice, to enhance disease resistance in aquaculture. Although some α-helical peptides related to CEME also had excellent activity, other natural peptides (including cattle neutrophil indolicidin and bactenecin and bacterial gramicidin S) had a lower potency against fish pathogens. For this reason, and to probe the potential advantages offered by fish-derived peptides, we turned to the flounder-derived peptide pleurocidin (3).
Pleurocidin was found in winter flounder skin mucus and was shown to be active against both gram-negative and gram-positive bacteria in fish (3). Pleurocidin amide, a C-terminally amidated form, was designed in our laboratory and was found to be more active than the native peptide against different groups of bacteria, including the two fish pathogens V. anguillarum and A. salmonicida. We also studied other peptides, including the fish peptide misgurin (which in our hands was inactive), an N-terminally extended version of pleurocidin (P-1), and some hybrids with misgurin, as well as insect and frog peptides related to pleurocidin.
In an attempt to improve the antimicrobial activity of pleurocidin and its related peptides, an effort to design and synthesize a series of different peptide constructs was also made. All three cationic peptide constructs with improved antimicrobial activities (P-DER, P-CN, and P-1-CN) were C-terminally amidated derivatives of pleurocidin. C-terminal amidation has been previously shown to improve the antimicrobial activity of many peptides (5, 16). The improved activities may relate to better lipopolysaccharide binding leading to improved self-promoted uptake across the outer membrane, as demonstrated for the indolicidins (5). Perhaps most surprising was the finding that in our studies the chemically synthesized misgurin exhibited no significant antimicrobial activity and that C-terminal misgurin hybrids showed reduced activities compared to their pleurocidin equivalents. Also, it appeared that the substitution of the N terminus of pleurocidin with the N terminus of dermaseptin had no effect on antimicrobial activity, while the substitution with the N terminus of ceratotoxin decreased this activity. While certain constructs offered some activity against fish and other pathogens, particularly P-DER and P-1 amide, none had sufficient advantages over CEME and pleurocidin amide to merit follow-up in vivo studies.
We chose CEME and pleurocidin amide to investigate the potential efficacy of peptides in vivo. Kelly et al. (11) previously found that a single intraperitoneal injection of their peptide LSB-37 did not have a significant effect on survival but that when the peptide was administered over a longer period using osmotic pumps it significantly reduced mortality from E. ictaluri infections of channel catfish (11). Similarly, in our hands, a single injection of CEME did not have any effect, but constant delivery of the peptide via an implanted miniosmotic pump significantly delayed and reduced mortality in V. anguillarum-infected fish. It appears that better protection was obtained when a higher level of peptide was used in protection studies, suggesting that protection was dose dependent. Constant administration of pleurocidin amide also protected coho salmon from a lethal challenge with V. anguillarum, with the accumulated mortality being dramatically reduced from 75 to 5%. These data are consistent with an important role for fish peptides as a part of the fish nonspecific immune response to infections, as inferred for other animals, including insects, and plants. These results indicate that antimicrobial peptides are not particularly effective in single treatments and that constant administration over a certain period may be necessary to make this therapeutic approach successful. While osmotic pumps are clearly not a feasible option in terms of protection of fish from the threats of disease associated with aquaculture, they do mimic the physiological conditions in the bodies of transgenic animals engineered to overexpress peptides. In this regard, the finding that fish peptides are significantly protective is very encouraging because it has been demonstrated that genes derived from as close a species as possible to the host are most efficiently expressed in transgenic fish (4). The best choice would then be to utilize genes encoding fish antimicrobial peptides to produce disease-resistant fish.
Overall, these studies demonstrate that CEME and pleurocidin amide are potent antimicrobial agents with in vivo activity against the pathogen V. anguillarum in coho salmon. Therefore, great potential exists for the development of disease-resistant transgenic fish using peptide genes. These agents may be powerful tools for preventing disease outbreaks in aquaculture and enhancement-oriented production systems.
ACKNOWLEDGMENTS
This work was funded by separate grants from the Canadian Bacterial Diseases Network to R.E.W.H. and G.K.I. X.J. was a BC Science Council industrial postdoctoral fellow, sponsored by Microtech International Inc., Saanichton, British Columbia, Canada. R.E.W.H. was a recipient of the Medical Research Council of Canada Distinguished Scientist Award. This study was also supported by a grant from Fisheries and Oceans of Canada National Biotechnology Strategy to R.H.D.
The technical assistance of Ellen Teng is gratefully acknowledged.
REFERENCES
- 1.Angus B L, Carey A M, Caron D A, Kropinski A M, Hancock R E. Outer membrane permeability in Pseudomonas aeruginosa: comparison of a wild type with an antibiotic-supersusceptible mutant. Antimicrob Agents Chemother. 1982;21:299–309. doi: 10.1128/aac.21.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broglie K, Chet I, Holliday M, Cressman R, Biddle P, Knowlton C, Mauvai C J, Broglie R. Transgenic plants with enhanced resistance to the fungal pathogen Rhizoctonia solani. Science. 1991;254:1194–1197. doi: 10.1126/science.254.5035.1194. [DOI] [PubMed] [Google Scholar]
- 3.Cole A M, Weis P, Diamond G. Isolation and characterization of pleurocidin, an antimicrobial peptide in the skin secretions of winter flounder. J Biol Chem. 1997;272:12008–12013. doi: 10.1074/jbc.272.18.12008. [DOI] [PubMed] [Google Scholar]
- 4.Devlin R H. Transgenic animals: generation and use. Amsterdam, The Netherlands: Harwood Academic Publishers; 1997. [Google Scholar]
- 5.Falla T J, Karunaratne D N, Hancock R E W. Mode of action of the antimicrobial peptide indolicidin. J Biol Chem. 1996;271:19298–19303. doi: 10.1074/jbc.271.32.19298. [DOI] [PubMed] [Google Scholar]
- 6.Fields P I, Groisman E A, Heffron F. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science. 1989;243:1059–1062. doi: 10.1126/science.2646710. [DOI] [PubMed] [Google Scholar]
- 7.Friedrich C, Scott M G, Karunaratne N, Yan H, Hancock R E. Salt-resistant alpha-helical cationic antimicrobial peptides. Antimicrob Agents Chemother. 1999;43:1542–1548. doi: 10.1128/aac.43.7.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hancock R E, Lehrer R. Cationic peptides: a new source of antibiotics. Trends Biotechnol. 1998;16:82–88. doi: 10.1016/s0167-7799(97)01156-6. [DOI] [PubMed] [Google Scholar]
- 9.Jach G, Gornhardt B, Mundy J, Logemann J, Pinsdorf E, Leah R, Schell J, Maas C. Enhanced quantitative resistance against fungal disease by combinatorial expression of different barley antifungal proteins in transgenic tobacco. Plant J. 1995;8:97–109. doi: 10.1046/j.1365-313x.1995.08010097.x. [DOI] [PubMed] [Google Scholar]
- 10.Kelly D, Wolters W R, Jaynes J M. Effect of lytic peptides on selected fish bacterial pathogens. J Fish Dis. 1990;13:317–321. [Google Scholar]
- 11.Kelly D G, Wolters W R, Jaynes J M, Newton J C. Enhanced disease resistance to enteric septicemia in channel catfish, Ictalurus punctatus, administered lytic peptide. J Appl Aquacult. 1993;3:25–34. [Google Scholar]
- 12.Liu D, Raghothama K G, Hasegawa P M, Bressan R A. Osmotin overexpression in potato delays development of disease symptoms. Proc Natl Acad Sci USA. 1994;91:1888–1892. doi: 10.1073/pnas.91.5.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchini D, Giordano P C, Amons R, Bernini L F, Dallai R. Purification and primary structure of ceratotoxin A and B, two antibacterial peptides from the female reproductive accessory glands of the medfly Ceratitis capitata (Insecta:Diptera) Insect Biochem Mol Biol. 1993;23:591–598. doi: 10.1016/0965-1748(93)90032-n. [DOI] [PubMed] [Google Scholar]
- 14.Marchini D, Manetti A G, Rosetto M, Bernini L F, Telford J L, Baldari C T, Dallai R. cDNA sequence and expression of the ceratotoxin gene encoding an antibacterial sex-specific peptide from the medfly Ceratitis capitata (diptera) J Biol Chem. 1995;270:6199–6204. doi: 10.1074/jbc.270.11.6199. [DOI] [PubMed] [Google Scholar]
- 15.Mor A, Nguyen V H, Delfour A, Migliore-Samour D, Nicolas P. Isolation, amino acid sequence, and synthesis of dermaseptin, a novel antimicrobial peptide of amphibian skin. Biochemistry. 1991;30:8824–8830. doi: 10.1021/bi00100a014. [DOI] [PubMed] [Google Scholar]
- 16.Oren Z, Shai Y. A class of highly potent antibacterial peptides derived from pardaxin, a pore-forming peptide isolated from Moses sole fish Pardachirus marmoratus. Eur J Biochem. 1996;237:303–310. doi: 10.1111/j.1432-1033.1996.0303n.x. [DOI] [PubMed] [Google Scholar]
- 17.Park C B, Lee J H, Park I Y, Kim M S, Kim S C. A novel antimicrobial peptide from the loach, Misgurnus anguillicaudatus. FEBS Lett. 1997;411:173–178. doi: 10.1016/s0014-5793(97)00684-4. [DOI] [PubMed] [Google Scholar]
- 18.Reed W A, Elzer P H, Enright F M, Jaynes J M, Morrey J D, White K L. Interleukin 2 promoter/enhancer controlled expression of a synthetic cecropin-class lytic peptide in transgenic mice and subsequent resistance to Brucella abortus. Transgenic Res. 1997;6:337–347. doi: 10.1023/a:1018423015014. [DOI] [PubMed] [Google Scholar]
- 19.Scott M G, Yan H, Hancock R E. Biological properties of structurally related alpha-helical cationic antimicrobial peptides. Infect Immun. 1999;67:2005–2009. doi: 10.1128/iai.67.4.2005-2009.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terras F R, Eggermont K, Kovaleva V, Raikhel N V, Osborn R W, Kester A, Rees S B, Torrekens S, Van Leuven F, Vanderleyden J, et al. Small cysteine-rich antifungal proteins from radish: their role in host defense. Plant Cell. 1995;7:573–588. doi: 10.1105/tpc.7.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wade D, Boman A, Wahlin B, Drain C M, Andreu D, Boman H G, Merrifield R B. All-D amino acid-containing channel-forming antibiotic peptides. Proc Natl Acad Sci USA. 1990;87:4761–4765. doi: 10.1073/pnas.87.12.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu M, Hancock R E. Improved derivatives of bactenecin, a cyclic dodecameric antimicrobial cationic peptide. Antimicrob Agents Chemother. 1999;43:1274–1276. doi: 10.1128/aac.43.5.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]