Abstract
Background: Structured exercise as part of lifestyle modification plays an important role in the improvement of non-alcoholic fatty liver disease (NAFLD); however, its effectiveness has been shown to vary. This systematic review with meta-analysis investigated the effects of exercise on liver function and insulin resistance markers in patients with NAFLD. Methods: Six electronic databases were searched using terms related to exercise and NAFLD up to March 2022. Data were analyzed using a random-effects model to estimate the standardized mean difference (SMD) and 95% confidence interval. Results: The systematic search identified 2583 articles, of which a total of 26 studies met the inclusion criteria and were eligible. Exercise training had a moderate effect on reducing ALT (SMD: −0.59, p = 0.01) and small effects on reducing AST (SMD: −0.40, p = 0.01) and insulin (SMD: −0.43, p = 0.02). Significant reductions in ALT were found following aerobic training (SMD: −0.63, p < 0.01) and resistance training (SMD: −0.45, p < 0.001). Moreover, reductions in AST were found following resistance training (SMD: −0.54, p = 0.001), but not after aerobic training and combined training. However, reductions in insulin were found following aerobic training (SMD: −0.55, p = 0.03). Exercise interventions for <12 weeks compared to ≥12 weeks were more effective in reducing FBG and HOMA-IR, while interventions for ≥12 weeks compared to <12 weeks were more effective in reducing ALT and AST levels. Conclusions: Our findings support the effectiveness of exercise in improving liver function markers but not in blood glucose control in NAFLD patients. Additional studies are needed to determine the exercise prescription to maximize health in these patients.
Keywords: non-alcoholic fatty liver disease, insulin resistance, exercise training, liver enzymes, meta-analysis
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is a prevalent hepatic disorder that is accompanied by the concentration of lipids in hepatocytes and usually arises when the concentration of fats in the liver exceeds 5% of the organ weight [1,2]. The disorder ranges from steatosis and non-alcoholic steatohepatitis (NASH) to advanced fibrosis and cirrhosis [3,4]. NAFLD is among the most prevalent forms of chronic hepatic diseases in the world and is the main reason for adults visiting hepatology clinics [5,6]. The disease affects almost 25% of the world population [7] and 23% of European citizens [8].
Over the past few years, NAFLD has attracted a lot of attention as a risk factor for the development of insulin resistance (IR) and type 2 diabetes mellitus (T2DM) [9,10]. Studies indicate that NAFLD is associated with obesity, insulin resistance, T2DM, and dyslipidemia, and its prevalence throughout the world appears to be increasing [11]. Currently, there is no pharmacological treatment for NAFLD, therefore the initial management focuses on lifestyle and dietary changes [12,13]. Engaging in adequate levels of physical activity plays a protective role against the emergence of T2DM, central (abdominal) obesity, and other risk factors related to NAFLD (including high blood pressure and dyslipidemia) [14]. Exercise interventions in the absence of dietary changes (with or without weight loss) have been shown to improve clinical markers in patients with NAFLD [12]. This may include improvement in markers such as intrahepatic lipids (IHLs), levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), and insulin resistance.
The European Association for the Study of the Liver (EASL) recommends 150–200 min per week of medium-intensity exercise for patients with NAFLD [15]. The effects of different exercise modalities in patients with NAFLD have produced conflicting results [16,17,18,19]. Two systematic reviews with meta-analyses in patients with NAFLD found that aerobic training, resistance training, and combined exercise training (aerobic training + resistance training) did not improve hepatic enzymes including ALT and AST [16,17]. A meta-analysis conducted by Xiong et al. including 1250 NAFLD patients showed that aerobic exercise led to reductions in more metabolic and liver function markers compared to resistance training and high-intensity [18]. Zhou et al. conducted a meta-analysis on 1627 NAFLD patients and found that combined exercise training produced the most favorable changes in total cholesterol (TC), ALT, and AST, compared to that with performing a single exercise modality (i.e., aerobic training, resistance training, or HIIT). However, it should be noted that for the previous meta-analysis, no significant differences between the exercise modalities were found for the hepatic enzymes [19].
Therefore, the most effective exercise modality for improvements in clinical markers of NAFLD is yet to be determined. Moreover, there are conflicting results concerning the effect of exercise on specific hepatic enzymes and their roles in the prevention and treatment of NAFLD [16,17,18,19]. To provide practitioners with the optimal exercise therapeutic approach for the management of NAFLD requires an up-to-date synthesis of evidence from randomized controlled trials (RCTs). Thus, the purpose of this study was to assess the effect of exercise on liver enzymes and insulin resistance markers in NAFLD patients via a systematic review and meta-analysis. A second aim was to examine whether the effects of an exercise intervention on liver enzymes and insulin resistance markers were influenced by exercise modality, intervention duration, and body mass index (BMI) status.
2. Materials and Methods
2.1. Search Strategy
This systematic review and meta-analysis was conducted according to the recommendations outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Supplementary Table S1) [20]. A detailed search was performed using six databases, including Web of Science, Embase, Cochrane, PubMed, Google Scholar, and Scopus. The search was conducted from the earliest record up to 5 October 2021, with an updated search up to and including 3 March 2022. The search strategy combined the terms: “liver steatosis”, “non-alcoholic fatty liver disease”, “NAFLD”, “randomized controlled trial”, “fatty liver”, “liver”, “hepatic”, “NASH”, “aminotransferase”, ‘‘liver enzymes’’, ‘‘AST’’, ‘‘ALT’’, ‘‘FBG’’, ‘‘fasting insulin’’, ‘‘HOMA-IR’’, “strength training”, “physical activity”, “combined training”, “exercise training”, “walking”, “aerobic training”, “resistance training”, “circuit training”, “circuit’’, ‘‘weight training”, “interval training”, “chronic training”, “ lifestyle activity”, “home training”, ‘‘water exercise’’, and ‘‘tai chi’’. All relevant studies and other available systematic reviews (including meta-analyses) and the reference lists of the identified articles were hand searched for additional eligible studies.
Where possible, searches were limited to studies involving human participants and published in the English language. Titles and abstracts of retrieved articles were individually evaluated by two reviewers (K.H. and D.H.) to assess their eligibility for review and meta-analysis. The reviewers were not blinded to the studies’ authors, institutions, or journals of publication. When abstracts did not provide sufficient information to examine study eligibility, the full-text was retrieved for evaluation. Duplicate publications were identified by comparing author names, treatment comparisons, publication dates, sample sizes, intervention, and outcomes.
2.2. Eligibility Criteria
Articles were eligible for inclusion if they met the following criteria: randomized controlled trials; peer-reviewed articles published in English; an exercise intervention of ≥4 weeks duration; adults (≥18 years) diagnosed with NAFLD or NASH; and reported post-intervention change in liver enzymes and insulin resistance markers. These markers specifically included the following: enzymes of the liver (e.g., ALT, AST), fasting blood glucose (FBG), insulin, and homeostatic model assessment for insulin resistance (HOMA-IR). The studies also required participants to be physically inactive (<150 min per week of moderate intensity aerobic exercise or <75 min per week of vigorous aerobic exercise) and no participation in an exercise program within the previous 6 months. Articles were deemed ineligible if only the abstract was available; did not involve humans (i.e., animal studies); and cohorts had secondary causes of fatty liver such as alcohol, hepatitis viruses, or medication.
2.3. Data Extraction
The information extracted comprised publication details (such as first author’s name, year of publication, and country), details of the study (i.e., sample size for the intervention and control group and health status), participants’ characteristics (i.e., mean age of participants), description of the intervention (i.e., type of exercise, intervention period, frequency, intensity, duration, sets, and repetition). Mean and standard deviation (SD) of the liver enzymes (ALT and AST) and insulin resistance marker (FBG and HOMA-IR) values at baseline, post-intervention, and/or changes between baseline and post-intervention were noted. If standard errors were reported, these values were converted to SD [21]. Any data presented in graphs were extracted using web-based software (GetData Graph Digitizer) [22]. If data was unavailable or could not be extracted from figures, the study’s corresponding author was emailed to request the data. All data were extracted by two authors (K.H. and D.H.).
2.4. Study Quality
The methodological quality of the studies were examined independently based on a fifteen-point scale, Tool for the Assessment of Study Quality and Reporting in Exercise (TESTEX), which is a validated tool for evaluating the quality (five points maximum) and reporting (10 points maximum) of exercise training studies [23]. Two authors (K.H. and D.H.) carried out this evaluation.
2.5. Statistical Analysis
All meta-analyses were conducted with Review Manager 5.3 (The Nordic Cochrane Centre, Copenhagen, Denmark). Effect size (ES) values were expressed as standardized mean difference (SMD) and 95% confidence interval (CI). The SMD was calculated using the formula shown below:
| (1) |
where DInt and DCtr are the post–pre differences in the exercise intervention and control groups, respectively, and SDpooled is the pooled standard deviation [24]. The ESs were interpreted by Cochrane guidelines, with SMD = 0.20–0.49 indicating a small ES, SMD = 0.50–0.79 indicating a medium ES, and SMD > 0.80 indicating a large ES [25].
When a study involved a control group and more than one exercise group, ESs were calculated for each exercise group and the sample size of the control group was divided by the number of exercise groups. Between-study variability was examined for heterogeneity using the I2 statistic for quantifying inconsistency. Heterogeneity thresholds included I2 = 25% (low), I2 = 50% (moderate), and I2 = 75% (high) [21]. A conservative random-effects model of meta-analysis was applied to the pooled data. Significance was set as p < 0.05. Subgroup analyses were employed to recognize potential causes of heterogeneity among the studies. Exercise training modality (i.e., aerobic, resistance, and combined aerobic + resistance); exercise duration (i.e., <12 weeks versus ≥12 weeks); and body mass index (BMI) (overweight: 25.0–29.9 kg/m2 versus obese: ≥30 kg/m2), were considered as the predefined sources of heterogeneity. Only one study that met the inclusion criteria investigated the effects of interval training on the outcomes of interest [26]. Therefore, it was decided that this study would not be included in any analysis. Publication bias was examined visually via funnel plots and statistically using Egger’s test (p < 0.05) [27].
3. Results
3.1. Study Selection
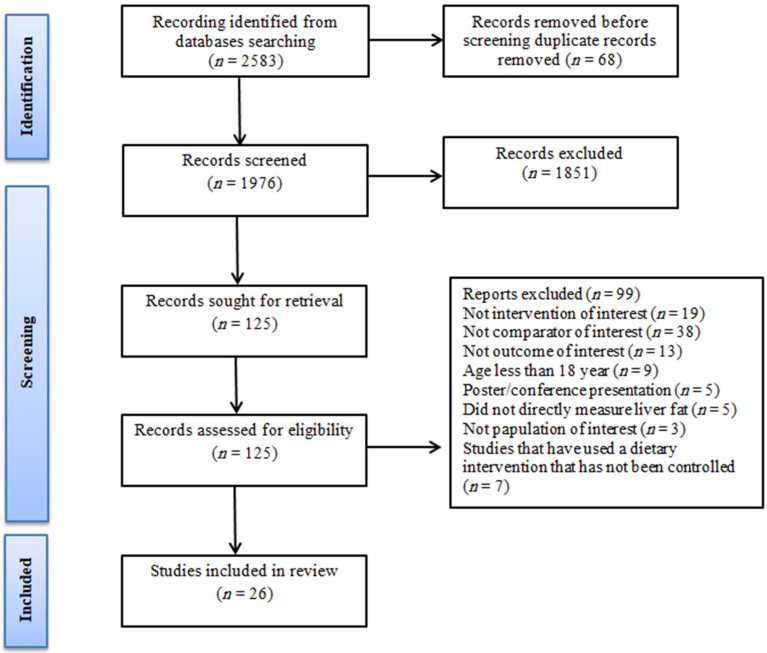
The initial search identified 2583 records. After removing duplicates and following title/abstract screening, 125 studies were included for full-text review. Of those 125 studies, 26 studies met the eligibility criteria. A flow diagram of the study search and selection, including reasons for exclusion, is shown in Figure 1.
Figure 1.
PRISMA flow diagram of the literature search.
3.2. Study and Participant Characteristics
Relevant characteristics of the included studies are presented in Table 1. Studies were carried out in Egypt (one study), India (one study), Brazil (one study), China (two studies), USA (two studies), Japan (one study), Australia (three studies), the UK (four studies), and Iran (11 studies).
There were 1316 participants included in this review (intervention groups: 800 participants; control groups: 516 participants). Of the 26 included studies, 10 studies exclusively recruited male subjects (n = 401) [28,29,30,31,32,33,34,35,36,37], 4 studies exclusively recruited female subjects (n = 303) [38,39,40,41], and 12 studies recruited both (male + female) (n = 507) [26,42,43,44,45,46,47,48,49,50,51,52].The mean age of subjects ranged from 18 to 66 years. The average BMI was 30.81 kg/m2. Furthermore, 11 studies involved overweight subjects (BMI of ≥25–29.9 kg/m2) [29,31,32,33,35,39,41,45,47,51,52], 14 studies involved obese subjects (BMI of ≥30 kg/m2) [30,34,36,37,38,40,42,43,44,46,48,49,50,53], and only one study did not report BMI [28].
Table 1.
Characteristics of the included studies.
| Participants | |||||||
|---|---|---|---|---|---|---|---|
| Author, Year | Country | Disease | Ex (CON) | BMI | Gender (M/F) |
Age | Outcome Assumed |
| Abdelbasset et al., 2019 [26] | Egypt | NAFLD | 16 (16) | 36.1 | M F |
54.8 | ALT, HBA1c, FBG, HOMA-IR |
| Cuthbertson et al., 2016 [42] | UK | NAFLD | 30 (20) | 30.15 | M F |
51 | ALT, AST, FBG, HOMA-IR, Insulin |
| Eckard et al., 2013 [37] | USA | NAFLD | 9 (11) | 33.3 | M | 51.5 | ALT, AST, FBG, HOMA-IR, Insulin |
| George et al., 2008 [36] | Australia | NAFLD | 109 (34) | 31.7 | M | 48.12 | ALT, AST, FBG, HOMA-IR, Insulin |
| Hallsworth et al., 2011 [43] | UK | NAFLD | 11 (8) | 32.3 | M F |
57 | ALT, FBG, HOMA-IR, Insulin |
| Hatami et al., 2016 [35] | Iran | NAFLD | 16 (16) | 28 | M | 32.93 | ALT, AST |
| Hoseini et al., 2020 [38] | Iran | NAFLD | 10 (10) | 34.11 | F | 62.3 | ALT, AST, FBG, HOMA-IR, Insulin |
| Houghton et al., 2017 [44] | Australia | NAFLD | 12 (12) | 33.00 | M F |
52.00 | ALT, AST, FBG, HOMA-IR |
| Javanmardi Fard et al., 2015 [45] | Iran | NAFLD | 30 (30) | 28.65 | M F |
19 | ALT, AST |
| Keating et al., 2015 [46] | Australia | NAFLD | 36 (12) | 33.42 | M F |
43.6 | ALT, AST, FBG, Insulin |
| Khaoshbaten et al., 2013 [47] | Iran | NAFLD | 45 (45) | 29.2 | M F |
37.6 | ALT, AST, FBG |
| Mohammadi et al., 2019 [34] | Iran | NAFLD | 10 (10) | 32.58 | M | 37.2 | ALT, AST |
| Moradi Kelardeh et al., 2017 [33] | Iran | NAFLD | 12 (12) | 29.27 | M | 38.24 | ALT, AST |
| Moradi Kelardeh et al., 2020 [39] | Iran | NAFLD | 12 (11) | 27.36 | F | 65.27 | ALT, AST |
| Nourian et al., 2020 [48] | Iran | NAFLD | 36 (33) | 32.19 | M F |
48.84 | ALT, AST |
| Pugh et al., 2014 [49] | UK | NAFLD | 34 (20) | 30.5 | M F |
47.5 | ALT, AST, FBG, HOMA-IR, Insulin |
| Rezende et al., 2016 [40] | Brazil | NAFLD | 19 (21) | 33.05 | F | 55.3 | ALT, AST, FBG, HOMA-IR, Insulin |
| Sadeghi et al., 2019 [32] | Iran | NAFLD | 11 (11) | 26.96 | M | 40.8 | ALT, AST |
| Shamsoddini et al., 2015 [31] | Iran | NAFLD | 20 (10) | 28.96 | M | 43.8 | ALT, AST, HOMA-IR |
| Shojaee-Moradie et al., 2016 [30] | UK | NAFLD | 15 (12) | 31.65 | M | 52.6 | ALT, AST, FBG, HOMA-IR, Insulin |
| Sreenivasa Baba et al., 2006 [29] | India | NAFLD | 44 (15) | 27.1 | M | 38.7 | ALT, AST |
| Sullivan et al., 2012 [50] | USA | NAFLD | 12 (6) | 38.1 | M F |
48.05 | ALT |
| Takahashi et al., 2015 [51] | Japan | NAFLD | 31 (22) | 28.35 | M F |
53.45 | ALT, AST, FBG, HOMA-IR, Insulin |
| Valizadeh et al., 2011 [28] | Iran | NAFLD | 12 (12) | NR | M | 32.5 | ALT, AST |
| Yao et al., 2018 [52] | China | NAFLD | 60 (31) | 26.26 | M F |
58.38 | ALT, FBG, Insulin |
| Zhang et al., 2016 [41] | China | NAFLD | 146 (74) | 28.0 | F | 53.9 | ALT, AST, FBG |
Note: The control group received no training. Abbreviations: M, male; F, female; NAFLD, nonalcoholic fatty liver disease; FBG, fasting blood glucose; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HOMA-IR, homeostatic model assessment for insulin resistance; n.r., not rated.
3.3. Intervention Characteristics
Exercise intervention characteristics are summarized in Table 2. The duration of exercise interventions ranged from 4 to 48 weeks, with the duration of individual exercise sessions ranging from 20 to 200 min. Frequency of exercise training ranged between two and seven sessions per week.
Table 2.
Exercise intervention details.
| Intervention | ||||||
|---|---|---|---|---|---|---|
| Study | Groups | Mode | Frequency (Days) | Intervention Duration (Weeks) |
Duration of Each Session (Min) |
Intensity |
| Abdelbasset et al., 2019 [26] | HIIT CON |
Exercise program was performed on a cycle ergometer | 3/7 | 8 | 40 min | HIIT group: 80–85% VO2max CON group: No intervention |
| Cuthbertson et al., 2016 [42] | AE CON |
Aerobic exercise (treadmill, cross-trainer, bike ergometer, rower) | 3–5/7 | 12 | 30–45 min | AE group: 30–60% HRmax CON group: No intervention |
| Eckard et al., 2013 [37] | AE CON |
Participants followed an 18-step program, including warm up, exercise bicycle, walking on a treadmill, various arm and leg strength exercises, and cool down, with gradual ramp up over the 6 months | 4–7/7 | 24 | 20–60 min | AE group: Moderate exercise CON group: No intervention |
| George et al., 2008 [36] | LI MI CON |
Participants were encouraged to increase both planned and incidental moderate-intensity PA to achieve at least 150 min/week for general health and to target more than 200 min/week for weight loss | 3/7 6/7 |
4 10 |
45–60 min | LI group: 35–40% VO2max MI group: 70–75% VO2max CON group: No intervention |
| Hallsworth et al., 2011 [43] | RT CON |
The program consisted of eight exercises: biceps curl; calf raise; triceps press; chest press; seated hamstrings curl; shoulder press; leg extension; and lateral pull down | 3/7 | 8 | 45–60 min | RT group: 50–70% 1RM CON group: No intervention |
| Hatami et al., 2016 [35] | AE + RT CON |
The aerobic training section consisted of rhythmic movements and the resistance training section consisted of biceps, triceps, pectoralis, quadriceps, and hamstring muscles in the position of dumbbell biceps (seated concentration curl), dumbbell triceps (triceps kickback), bench press, knee extension, and knee flexion | 3/7 | 8 | 40 min | AE + RT group: 3 sets/8–12 reps at 60–80% 1RM 65–85% HRmax CON group: No intervention |
| Hoseini et al., 2020 [38] | AE CON |
Walking and jogging/running | 3/7 | 8 | 20–40 min | AE group: 60–75%, HRmax CON group: No intervention |
| Houghton et al., 2017 [44] | AE + RT CON |
The exercise program consisted of aerobic (cycling) and resistance training | 3/7 | 12 | 60 min | AE + RT group: Moderate vigorous Activity CON group: No intervention |
| Javanmardi Fard et al., 2015 [45] | AE CON |
The physical activities included jogging, cycling, aerobic exercise or any other activity that is of a similar intensity | 4–5/7 | 12 | 30 min | AE group: Moderate physical activities CON group: No intervention |
| Keating et al., 2015 [46] | HILO LOHI LOLO CON |
Performed continuous cycling on the ergometer/brisk walk | 3–4/7 | 8 | 45–60 min | LO:HI group: 50% VO2peak HI:LO group: 70% VO2peak LO:LO group: 50% VO2peak CON group: No intervention |
| Khaoshbaten et al., 2013 [47] | AE CON |
Walking/running | 3/7 | 12 | 30 min | AE group: Maximal heart rate CON group: No intervention |
| Mohammadi et al., 2019 [34] | CRT CON |
The resistance training included three circles with nine stations per circle | 3/7 | 12 | 30–45 min | CRT group: 3 sets/10–20 reps at 40–80% 1RM CON group: No intervention |
| Moradi Kelardeh et al., 2017 [33] | RT CON |
Knee extension, bench press, incline bench press, seated row, dead lift, pulley crunches, lat pull-downs, calf raise, hamstring curl, press behind neck, upright row, arm curl | 3/7 | 12 | 45–60 min | RT group: 1–3 sets/2–20 reps at 40–95% 1RM CON group: No intervention |
| Moradi Kelardeh et al., 2020 [39] | RT CON |
Knee extension, bench press, incline bench press, seated row, dead lift, pulley crunches, lat pull-downs, calf raise, hamstring curl, press behind neck, upright row, arm curl | 3/7 | 12 | 60–70 min | RT group: 1–4 sets/2–20 reps at 40–95% 1RM CON group: No intervention |
| Nourian et al., 2020 [48] | AE CON |
The educational content of each session was presented to participants by using a short lecture, slide show, pamphlet, and discussion. | 5/7 | 8 | 30–60 min | AE group: Lifestyle modification education training CON group: No intervention |
| Pugh et al., 2014 [49] | AE CON |
Exercise training comprised a combination of treadmill and cycle ergometer-based exercise, which progressively increased in both intensity and duration throughout the course of the intervention | 3–5/7 | 12 | 30–45 min | AE group: 30–60 HRmax CON group: No intervention |
| Rezende et al., 2016 [40] | AE CON |
Aerobic exercise on treadmill | 2/7 | 24 | 120 min | AE group: Increased from VAT up to 10% below RCP CON group: No intervention |
| Sadeghi et al., 2019 [32] | RT CON |
These exercises include TRX plank on elbows, TRX T deltoid fly, TRX chest press, TRX high row, TRX triceps press, TRX biceps curl, TRX squat, and TRX hip press |
3/7 | 8 | 60 min | RT group: 3 sets/8 reps at 70–100% 1RM CON group: No intervention |
| Shamsoddini et al., 2015 [31] | AE RT CON |
Running on treadmill/seven exercises with machine: triceps press, biceps curl, calf raise, leg press, leg extension, lat pull down, and sit-ups | 3/7 | 8 | 45 min | AE group: 60–75% HRmax RT group: 50–70% 1RM CON group: No intervention |
| Shojaee-Moradie et al., 2016 [30] | AE CON |
Types of activities were either gym-based aerobic plus resistance exercise or outdoor aerobic activities and resistance exercise | 4–5/7 | 16 | 20–60 min | AE group: 40–60% HRmax CON group: No intervention |
| Sreenivasa Baba et al., 2006 [29] | AE CON |
Brisk walking/jogging or rhythmic aerobic exercises set to beat music | 5/7 | 12 | 20–45 min | AE group: 60–70% HRmax CON group: No intervention |
| Sullivan et al., 2012 [50] | AE CON |
Brisk walk/walking on a treadmill | 5/7 | 16 | 30–60 min | AE group: 45–55% VO2peak CON group: No intervention |
| Takahashi et al., 2015 [51] | RT CON |
Resistance exercise comprising push-ups and squats at the beginning of the study. | 3/7 | 12 | 20–30 min | RT group: 3 sets/10 reps CON group: No intervention |
| Valizadeh et al., 2011 [28] | AE CON |
Walking/running | 5/7 | 8 | 45 min | AE group: 50–70% VO2max CON group: No intervention |
| Yao et al., 2018 [52] | AE RT CON |
Walking/running Elastic band was used as the load instrument |
3/7 | 22 | 40–60 min | AE group: 60–70% HRmax RT group: 3 sets/10 reps; 60–70% 1RM CON group: No intervention |
| Zhang et al., 2016 [41] | ME VME CON |
Jogged on a treadmill and gradually increased exercise intensity | 5/7 | 48 | 30 min | ME group: 65–80% HRmax CON group: No intervention |
Note: The control group received no training. Abbreviations: VAT, ventilator anaerobic threshold; RCP, respiratory compensation point; CON, control; AE, aerobic; RT, resistance training; ME, moderate exercise; VME, vigorous–moderate exercise; HILO, high intensity, low volume aerobic exercise; LOHI, low to moderate intensity, high volume aerobic exercise; LOLO, low to moderate intensity, low volume aerobic exercise; LI, low intensity; MI, moderate intensity; HIIT, high-intensity interval training; HRmax, maximum heart rate; 1RM, 1 repetition maximum; VO2peak, peak oxygen uptake; VO2ma, maximal oxygen consumption.
Fifteen studies directly examined the effects of aerobic training [28,29,30,36,37,38,40,41,42,45,46,47,48,49,50], six directly examined the effects of resistance training [32,33,34,39,43,51], two studies directly investigated the effects of aerobic training versus resistance training [31,52], two studies directly investigated the effects of combined training (i.e., aerobic and resistance training) during sessions [35,44], and one study directly investigated the effects of interval training [26].
3.4. Effect of Exercise on Hepatic Enzyme Parameters
3.4.1. ALT Levels
Our meta-analysis of 25 studies revealed a significantly moderate effect of exercise training in reducing ALT levels (SMD: −0.59 [95% CI, −0.96 to −0.22], p = 0.002; (I2 = 88%, p < 0.001). The subgroup analysis of exercise type revealed that aerobic training had a significantly moderate effect on ALT levels (SMD: −0.63 [95% CI, −1.15 to −0.12], p = 0.02; I2 = 91%, p < 0.001). Resistance training had a significantly small effect on ALT levels (SMD: −0.45 [95% CI, −0.75 to −0.14], p < 0.004; I2 = 15%, p < 0.32). There was no significant effect of combined exercise training (aerobic + resistance) on AST levels (see Forest plot in Figure 2).
Figure 2.
Forest plot showing study precision against the mean difference effect estimate with 95% confidence interval for ALT [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52].
A subgroup analysis of exercise intervention duration indicated that a duration of ≥12 weeks significantly reduced ALT levels. Moderate effects were found for exercise training for ≥12 weeks (SMD: −0.69 [95% CI, −1.12 to −0.26], p = 0.002; I2 = 88%, p < 0.0001). However, no significant changes in ALT levels were found for exercise interventions of <12 weeks duration.
Subgroup analyses for BMI status revealed that the exercise intervention produced a significantly large effect in reducing ALT levels in patients with BMIs ≥30 kg/m2 (SMD: −0.93 [95% CI, −1.53 to −0.32], p = 0.003; I2 = 91%, p < 0.0001). A small effect (non-significant) on ALT levels was found following exercise in patients with a BMI of 25.0–29.9 kg/m2 (SMD: −0.13 [95% CI, −0.61 to 0.35], p = 0.60; I2 = 68%, p < 0.001).
3.4.2. AST Levels
Our meta-analysis of 22 studies revealed a significantly small effect of exercise training in reducing AST levels (SMD: −0.40 [95% CI, −0.71 to −0.09], p = 0.001; I2 = 74%, p < 0.001). The subgroup analysis by type of exercise revealed that resistance training had a significantly moderate effect on AST levels (SMD: −0.54 [95% CI, −0.95 to −0.13], p = 0.001; I2 = 31%, p < 0.20). There were no significant effects for either aerobic training or combined exercise training (aerobic + resistance) on AST levels (see Forest plot in Figure 3).
Figure 3.
Forest plot showing study precision against the mean difference effect estimate with 95% confidence interval for AST [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,44,45,46,47,48,49,51].
Subgroup analysis based on intervention duration indicated a significantly moderate effect on AST levels for intervention of ≥12 weeks (SMD: −0.59 [95% CI, −0.96 to −0.22], p < 0.002; I2 = 67%, p < 0.001). However, no significant changes in AST levels were found for exercise interventions of <12 weeks duration. Subgroup analyses for BMI status revealed that the exercise intervention produced a significantly small effect in reducing AST levels in patients with BMIs 25.0–29.9 kg/m2 (SMD: −0.27 [95% CI, −0.53 to −0.02], p = 0.04; I2 = 27%, p < 0.21). However, there were no significant effects on AST levels following exercise in patients with BMIs ≥30 kg/m2 (SMD: −0.14 [95% CI, −0.77 to 0.50], p = 0.67; I2 = 85%, p < 0.001).
3.5. Effect of Physical Activity on Glucose Metabolism Parameters
3.5.1. FBG Levels
Our meta-analysis of 13 studies revealed no significantly small effect of exercise training on FBG levels (SMD: −0.21 [95% CI, −0.48 to 0.07], p = 0.14; I2 = 49%, p < 0.02). From the subgroup analysis of exercise type, no significant effects on FBG levels were found following aerobic training (SMD: −0.23 [95% CI, −0.59 to 0.14], p = 0.22) and resistance training (SMD: −0.14 [95% CI, −0.51 to 0.24], p = 0.47) (see Forest plot in Figure 4).
Figure 4.
Forest plot showing study precision against the mean difference effect estimate with 95% confidence interval for FBG [30,36,37,38,40,41,42,43,46,47,49,51,52].
Subgroup analysis based on intervention duration indicated a significantly moderate effect on FBG levels for intervention of <12 weeks (SMD: −0.53 [95% CI, −0.99 to −0.07], p = 0.02; I2 = 57%, p < 0.02). However, no significant changes in FBG levels were found for exercise interventions of ≥12weeks duration. No significant effects of exercise on FBG levels were found from the subgroup analyses of BMI 25.0–29.9 kg/m2 (SMD: −0.10 [95% CI, −0.34 to 0.13], p = 0.38) and ≥30 kg/m2 (SMD: −0.34 [95% CI, −0.76 to 0.07], p = 0.11).
3.5.2. Insulin levels
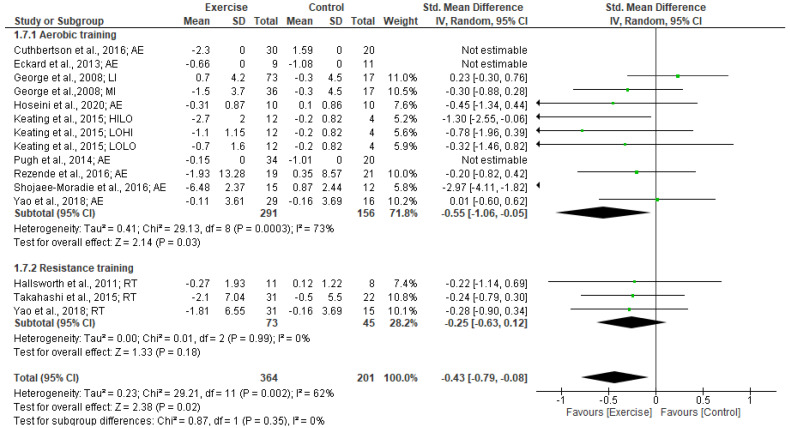
Our meta-analysis of 11 studies revealed a significantly small effect of exercise training in reducing insulin levels (SMD: −0.43 [95% CI, −0.79 to −0.08], p = 0.02; I2 = 62%, p < 0.002). The subgroup analysis by exercise type revealed that aerobic training had a significantly moderate effect in decreasing insulin (SMD: −0.55 [95% CI, −1.06 to −0.05], p = 0.03; I2 = 73%, p < 0.003). However, resistance training did not have a significantly small effect on insulin (SMD: −0.25 [95% CI, −0.63 to 0.12], p = 0.18; I2 = 0%, p < 0.99) (see Forest plot in Figure 5).
Figure 5.
Forest plot showing study precision against the mean difference effect estimate with 95% confidence interval for insulin [30,36,37,38,40,42,43,46,49,51,52].
Subgroup analysis based on intervention duration indicated no significant changes in insulin were found for exercise interventions for ≥12 weeks (SMD: −0.79 [95% CI, −1.68 to 0.1], p = 0.08; I2 = 85%, p < 0.002) and <12 weeks duration (SMD: −0.30 [95% CI, −0.70 to 0.1], p = 0.14; I2 = 28%, p < 0.22).
Subgroup analyses for BMI status revealed that the exercise intervention produced a significantly moderate effect on reducing insulin in patients with BMIs ≥ 30 kg/m2 (SMD: −0.60 [95% CI, −1.12 to 0.08], p = 0.02; I2 = 71%, p < 0.005). No significant effect of exercise on FBG levels were found from the subgroup analyses of BMI 25.0–29.9 kg/m2 (SMD: −0.18 [95% CI, −0.46 to 0.1], p = 0.21; I2 = 0%, p < 0.91).
3.5.3. HOMA-IR
Our meta-analysis of 10 studies revealed exercise training did not change HOMA-IR levels (SMD: −0.07 [95% CI, −0.64 to 0.05], p = 0.81; I2 = 80%, p < 0.001).The subgroup analysis by exercise type revealed that resistance training had a small effect for decreasing HOMA-IR, although this was not significant (SMD: −0.32 [95% CI, −0.75 to 0.11], p = 0.15; I2 = %, p < 0.98). Aerobic training did not significantly reduce HOMA-IR (SMD: −0.08 [95% CI, −0.78 to 0.94], p = 0.85; I2 = 87%, p < 0.001) (see Forest plot in Figure 6).
Figure 6.
Forest plot showing study precision against the mean difference effect estimate with 95% confidence interval for HOMA-IR [30,31,36,37,38,40,42,43,49,51].
Subgroup analysis based on intervention duration indicated a significantly small effect on HOMA-IR for <12 weeks (SMD: −0.46 [95% CI, −0.74 to −0.18], p = 0.001; I2 = 0%, p < 0.48). However, no significant changes in HOMA-IR were found for exercise interventions of ≥12 weeks duration (SMD: 0.97 [95% CI, −0.63 to 2.57], p = 0.24; I2 = 93%, p < 0.0001). Subgroup analyses for BMI status revealed that the exercise intervention produced a significantly small effect in reducing HOMA-IR in patients with BMIs 25.0–29.9 kg/m2 (SMD: −0.44 [95% CI, −0.78 to −0.1], p = 0.01; I2 = 0%, p < 0.57). No significant effect of exercise on HOMA-IR was found for patients with BMIs ≥30 kg/m2 (SMD: −0.04 [95% CI, −0.66 to 0.59], p = 0.90; I2 = 83%, p < 0.0001).
3.6. Study Quality
The overall methodological quality of the included studies was estimated to be moderate to good, with a median TESTEX score of 9 (range 8–14) out of a maximum of 15. One study scored 14, one study scored 13, one study scored 12, one study scored 11, four studies scored 10, ten studies scored 9, six studies scored 8, one study scored 7, and one study scored 6 (Supplementary Table S2). Of the TESTEX criteria, the following was done particularly poorly: randomization specified (13/26 studies); allocation concealment (8/26 studies); blinding of assessor (11/26 studies); intention to treat analyses (1/26 study); and physical activity monitoring in the control groups (7/26 studies). The other criteria were each met by at least 50% of included studies.
3.7. Heterogeneity and Publication Bias
Our analyses based on the effect of exercise demonstrated high heterogeneity in ALT levels (I2 = 88%, p = 0.0001) and HOMA-IR (I2 = 80%, p = 0.0001) and moderate heterogeneity in AST levels (I2 = 74%, p = 0.0001) and insulin levels (I2 = 62%, p = 0.002) However, FBG levels (I2 = 49%, p = 0.02) revealed low heterogeneity. Egger plots exhibited little to high evidence of publication bias as the standard error/mean difference plots were tightly grouped together (Supplementary Figures S1–S5). Furthermore, to show the quality of included studies, Figure 7 illustrates the results from the risk of bias assessment using the Cochrane risk of bias tool.
Figure 7.
Risk of bias assessment using Cochrane risk of bias tool. Top panel—Risk of bias summary showing review author’s judgment about each risk of bias item for each included study. Bottom panel—Risk of bias graph showing review author’s judgment about each risk of bias item presented as percentages across all included studies [26,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52].
4. Discussion
The purpose of the current systematic review and meta-analysis was to investigate the effects of exercise on liver function and insulin resistance markers in patients with non-alcoholic fatty liver disease. Overall, the pooled results from the 26 RCTs (with 1316 participants) indicated that exercise training significantly reduced liver enzymes (i.e., ALT and AST) and insulin in patients with non-alcoholic fatty liver disease.
Different exercise modalities were found to be effective in reducing liver enzymes (i.e., ALT and AST) and insulin. Aerobic training and resistance training were all effective in reducing ALT, while only resistance exercise training also led to reductions in AST levels. As for insulin resistance, this was improved following aerobic training but not after resistance training. There was no specific exercise training modality that effectively reduced FBG levels in the NAFLD patients. The exercise intervention duration appeared to influence its effectiveness with <12 weeks interventions favoring reductions in FBG and HOMA-IR, while ≥12 weeks interventions favored reduction in ALT and AST levels. Additionally, BMI status impacted the effectiveness of exercise with greater reductions in AST and HOMA-IR in overweight participants, whereas obese participants had a greater reduction in ALT and insulin levels. Overall, the quality of the literature included in the meta-analyses was moderate to good. Low to moderate heterogeneity and publication bias was found within most of the analyses.
The findings from the present review are consistent with the results of the meta-analysis conducted by Li et al. [54]. This previous meta-analysis in NAFLD patients showed that exercise was effective in reducing ALT and FBG. Another meta-analysis by Wang et al. in 951 patients with NAFLD showed that aerobic training as well as resistance training led to significant reductions in ALT and AST [55]. Smart et al. conducted a meta-analysis analyzing the effects of various exercise interventions (i.e., aerobic training, resistance training, and combined exercise training) on liver enzymes in 1530 patients with NAFLD. However, in contrast to the findings from the present review, their results showed no significant changes in the levels of ALT and AST following the exercise interventions [17]. One of the possible reasons for the discrepancy between the results of the present study and the findings of Smart et al. could be the diversity of RCTs included [17]. As an example, the characteristics of the RCTs included the participant characteristics (age, BMI, and sex) and study design. While the present review included 26 studies, the review by Smart et al. included 21 studies, which further supports the possibility of the diversity of RCTs, explaining the discrepancy between results [17].
Since there was evidence that the body weight of participants influenced the effectiveness of exercise on clinical markers of NAFLD, there may be altered responses for patients based on the amount of body fat. Alternatively, the ability to perform exercise may be influenced by the amount of excess body weight, which could also impact metabolic responses and adaptations. While further research is required to explore whether the degree of “fatness” impacts the effectiveness of exercise on clinical markers of NAFLD, it has been recognized that weight loss is associated with important pathophysiological changes, specifically increased insulin sensitivity, decreased fatty acids in the liver, decreased inflammatory mechanisms, and improved levels of ALT and AST enzymes [56]. In this regard, Katsagoni et al. reported that the effects of exercise on AST and ALT depend on weight loss. It has been shown that moderate intensity aerobic exercise with higher volume (above 180 min per week) is more effective in reducing intrahepatic triglyceride than moderate intensity aerobic exercise with low volume (volume 120 to 180 min per week) [57]. The mechanism attributed to the significant decreases in ALT and AST enzymes following exercise includes increased sensitivity of insulin, increased liver oxidation, decreased activity and inhibition of lipogenic enzymes, and thus reduced liver fat [58]. As for improvement of liver lipid composition, this may be mediated by adiponectin, lipid oxidation, and increased insulin sensitivity. Hallsworth et al. reported that insulin sensitivity, circulating lipids, and energy balance may be affected by mechanistic changes in liver fat after exercise training [43].
The results from the present meta-analysis are consistent with the findings of a meta-analysis conducted by Mohammad Rahimi et al. involving 2255 NAFLD patients. The results of this previous meta-analysis showed that aerobic exercise combined with diet led to a significant improvement in not only liver enzymes but also insulin sensitivity [59]. While diet-induced weight loss as well as exercise alone is associated with improvements in insulin sensitivity and glucose tolerance, a combination of diet and exercise is expected to promote the best results [60]. Calorie restriction and intensified physical activity (PA) are usually the basic components of intensive lifestyle (ILS) interventions causing weight loss. These interventions can hinder the inception of T2D in endangered populations and reduce the risk of cardiovascular disease (CVD) in patients with T2D. Physical activity is consistently reported to be as effective as weight loss, if not more so, for T2D prevention [61].
The review findings of both short and longer duration exercise interventions improving clinical markers in patients with NAFLD were not surprising. While longer duration exercise interventions would offer the advantage of potentially greater weight loss which would positively affect these markers (mentioned above), there is strong evidence for the effectiveness of exercise interventions of a shorter duration. According to some meta-analyses and systematic reviews, patients with T2D and fewer daily hyperglycemic excursions and 0.5%–0.7% reductions in hemoglobinA1C (A1C) show improved glycemia as a results of regular aerobic exercise training [62,63]. Furthermore, short-term aerobic exercise training leads to improvement in insulin sensitivity and mitochondrial function in patients with T2D [64]. In patients with obesity and T2D, short-term aerobic exercise increased peripheral insulin sensitivity more than hepatic insulin sensitivity and thus enhanced whole-body insulin action [65]. Vigorous aerobic exercise training for 7 days increased insulin-stimulated glucose disposal and suppression of hepatic glucose production, leading to improvement of glycemia without lowering body weight [66].
The present study has some limitations that should be considered when interpreting the results. One limitation was that our selection criteria was not sensitive to the geographical region where RCTs were conducted. Furthermore, many of the studies had small sample sizes (i.e., <20 participants per group), and only studies published in English were included. However, even with the strictest selection criteria for study inclusion, it is challenging to avoid some heterogeneity [67]. Factors contributing to the heterogeneity observed may include differences in study design and settings or factors more difficult to control, such as differences in response to exercise (i.e., responders versus non-responders) [68,69]. In addition, the exercise training protocols added to the present meta-analysis included aerobic and resistance exercises that were performed with varying intensity and duration. Therefore, variation in exercise components including duration and type of exercise may have played an important role in the adaptation of liver enzymes and insulin resistance indices to exercise. Finally, only one study met the inclusion criteria that used interval training as an exercise intervention [26]. We decided not to include this study in any analysis to ensure that conclusive results from the present review could be provided. However, it should be noted that there is evidence that high-intensity interval training (HIIT) is an efficacious exercise type for improvements in liver fat [70]. Furthermore, HIIT may elicit similar improvements in liver fat compared to continuous aerobic exercise, although requiring less energy and time commitment on the part of the patient.
5. Conclusions
The findings of this meta-analysis support the effectiveness of exercise in improving liver function markers but not blood glucose control in NAFLD patients. Aerobic training and resistance training appear to be of value in improving the health of patients with NAFLD. Intervention duration and BMI status may influence the effect of exercise on clinical markers of NAFLD. However, further studies are required to explore this topic as well as to determine the optimal exercise prescription to maximize health in patients with NAFLD.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12083011/s1, Figure S1: Funnel plot for ALT levels; Figure S2: Funnel plot for AST levels; Figure S3: Funnel plot for FBG levels; Figure S4: Funnel plot for insulin; Figure S5: Funnel plot for HOMA-IR; Table S1: PRISMA Checklist; Table S2. Study quality assessment; Table S3: Search strategy terms.
Author Contributions
Conceptualization, K.H. and D.H.; methodology, K.H. and D.H.; software, K.H. and D.H.; validation, K.H. and D.H.; formal analysis, K.H. and D.H.; investigation, K.H. and D.H.; resources, K.H. and D.H.; data curation, K.H. and D.H.; writing—original draft preparation, K.H.; writing—review and editing, K.H. and D.H.; visualization, K.H. and D.H.; supervision, K.H. and D.H.; project administration, K.H. and D.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Kneeman J.M., Misdraji J., Corey K.E. Secondary causes of nonalcoholic fatty liver disease. Ther. Adv. Gastroenterol. 2012;5:199–207. doi: 10.1177/1756283X11430859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muthiah M.D., Cheng Han N., Sanyal A.J. A clinical overview of non-alcoholic fatty liver disease: A guide to diagnosis, the clinical features, and complications—What the non-specialist needs to know. Diabetes Obes. Metab. 2022;24:3–14. doi: 10.1111/dom.14521. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez A., Valero-Breton M., Huerta-Salgado C., Achiardi O., Simon F., Cabello-Verrugio C. Impact of exercise training on the sarcopenia criteria in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Eur. J. Transl. Myol. 2021;31:9630. doi: 10.4081/ejtm.2021.9630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nseir W., Hellou E., Assy N. Role of diet and lifestyle changes in nonalcoholic fatty liver disease. World J. Gastroenterol. WJG. 2014;20:9338. doi: 10.3748/wjg.v20.i28.9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krasnoff J.B., Painter P.L., Wallace J.P., Bass N.M., Merriman R.B. Health-related fitness and physical activity in patients with nonalcoholic fatty liver disease. Hepatology. 2008;47:1158–1166. doi: 10.1002/hep.22137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.George A., Bauman A., Johnston A., Farrell G., Chey T., George J. Independent effects of physical activity in patients with nonalcoholic fatty liver disease. Hepatology. 2009;50:68–76. doi: 10.1002/hep.22940. [DOI] [PubMed] [Google Scholar]
- 7.Smith B.W., Adams L.A. Non-alcoholic fatty liver disease. Crit. Rev. Clin. Lab. Sci. 2011;48:97–113. doi: 10.3109/10408363.2011.596521. [DOI] [PubMed] [Google Scholar]
- 8.Younossi Z., Anstee Q.M., Marietti M., Hardy T., Henry L., Eslam M., George J., Bugianesi E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 9.Schindhelm R.K., Diamant M., Dekker J.M., Tushuizen M.E., Teerlink T., Heine R.J. Alanine aminotransferase as a marker of non-alcoholic fatty liver disease in relation to type 2 diabetes mellitus and cardiovascular disease. Diabetes/Metab. Res. Rev. 2006;22:437–443. doi: 10.1002/dmrr.666. [DOI] [PubMed] [Google Scholar]
- 10.Stefan N., Cusi K. A global view of the interplay between non-alcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol. 2022;10:284–296. doi: 10.1016/S2213-8587(22)00003-1. [DOI] [PubMed] [Google Scholar]
- 11.Leoni S., Tovoli F., Napoli L., Serio I., Ferri S., Bolondi L. Current guidelines for the management of non-alcoholic fatty liver disease: A systematic review with comparative analysis. World J. Gastroenterol. 2018;24:3361. doi: 10.3748/wjg.v24.i30.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babu A.F., Csader S., Lok J., Gómez-Gallego C., Hanhineva K., El-Nezami H., Schwab U. Positive Effects of Exercise Intervention without Weight Loss and Dietary Changes in NAFLD-Related Clinical Parameters: A Systematic Review and Meta-Analysis. Nutrients. 2021;13:3135. doi: 10.3390/nu13093135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thoma C., Day C.P., Trenell M.I. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: A systematic review. J. Hepatol. 2012;56:255–266. doi: 10.1016/j.jhep.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Qiu S., Cai X., Sun Z., Li L., Zügel M., Steinacker J.M., Schumann U. Association between physical activity and risk of nonalcoholic fatty liver disease: A meta-analysis. Ther. Adv. Gastroenterol. 2017;10:701–713. doi: 10.1177/1756283X17725977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Association for the Study of The Liver. European Association for the Study of Diabetes EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Keating S.E., Hackett D.A., George J., Johnson N.A. Exercise and non-alcoholic fatty liver disease: A systematic review and meta-analysis. J. Hepatol. 2012;57:157–166. doi: 10.1016/j.jhep.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 17.Smart N., King N., McFarlane J., Graham P., Dieberg G. Effect of exercise training on liver function in adults who are overweight or exhibit fatty liver disease: A systematic review and meta-analysis. Br. J. Sport. Med. 2018;52:834–843. doi: 10.1136/bjsports-2016-096197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiong Y., Peng Q., Cao C., Xu Z., Zhang B. Effect of different exercise methods on non-alcoholic fatty liver disease: A meta-analysis and meta-regression. Int. J. Environ. Res. Public Health. 2021;18:3242. doi: 10.3390/ijerph18063242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou B., Huang G., Wang W., Zhu L., Deng Y., He Y., Ma F. Intervention effects of four exercise modalities on nonalcoholic fatty liver disease: A systematic review and Bayesian network meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2021;25:7687–7697. doi: 10.26355/eurrev_202112_27615. [DOI] [PubMed] [Google Scholar]
- 20.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021;10:89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wojtyniak J.G., Britz H., Selzer D., Schwab M., Lehr T. Data digitizing: Accurate and precise data extraction for quantitative systems pharmacology and physiologically-based pharmacokinetic modeling. CPT Pharmacomet. Syst. Pharmacol. 2020;9:322–331. doi: 10.1002/psp4.12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smart N.A., Waldron M., Ismail H., Giallauria F., Vigorito C., Cornelissen V., Dieberg G. Validation of a new tool for the assessment of study quality and reporting in exercise training studies: TESTEX. Int. J. Evid.-Based Healthc. 2015;13:9–18. doi: 10.1097/XEB.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 24.Borenstein M., Hedges L.V., Higgins J.P., Rothstein H.R. Introduction to Meta-Analysis. John Wiley & Sons; Hoboken, NJ, USA: 2021. [Google Scholar]
- 25.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Routledge; London, UK: 2013. [Google Scholar]
- 26.Abdelbasset W.K., Tantawy S.A., Kamel D.M., Alqahtani B.A., Soliman G.S. A randomized controlled trial on the effectiveness of 8-week high-intensity interval exercise on intrahepatic triglycerides, visceral lipids, and health-related quality of life in diabetic obese patients with nonalcoholic fatty liver disease. Medicine. 2019;98:e14918. doi: 10.1097/MD.0000000000014918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valizadeh R., Nikbakht M., Davodi M., Khodadoost M. The effect of eight weeks elected aerobic exercise on the levels of (AST, ALT) enzymes of men patients with have fat liver. Procedia-Soc. Behav. Sci. 2011;15:3362–3365. doi: 10.1016/j.sbspro.2011.04.300. [DOI] [Google Scholar]
- 29.Sreenivasa Baba C., Alexander G., Kalyani B., Pandey R., Rastogi S., Pandey A., Choudhuri G. Effect of exercise and dietary modification on serum aminotransferase levels in patients with nonalcoholic steatohepatitis. J. Gastroenterol. Hepatol. 2006;21:191–198. doi: 10.1111/j.1440-1746.2005.04233.x. [DOI] [PubMed] [Google Scholar]
- 30.Shojaee-Moradie F., Cuthbertson D., Barrett M., Jackson N., Herring R., Thomas E., Bell J., Kemp G., Wright J., Umpleby A. Exercise training reduces liver fat and increases rates of VLDL clearance but not VLDL production in NAFLD. J. Clin. Endocrinol. Metab. 2016;101:4219–4228. doi: 10.1210/jc.2016-2353. [DOI] [PubMed] [Google Scholar]
- 31.Shamsoddini A., Sobhani V., Chehreh M.E.G., Alavian S.M., Zaree A. Effect of aerobic and resistance exercise training on liver enzymes and hepatic fat in Iranian men with nonalcoholic fatty liver disease. Hepat. Mon. 2015;15:e31434. doi: 10.5812/hepatmon.31434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sadeghi A., Pourrazi H., Yazdi H.-R. The Effect of Eight-Week Total Body Resistance Exercise on Liver Functional Parameters in Patients with Non-Alcoholic Fatty Liver Disease. Hormozgan Med. J. 2019;23:e97644. doi: 10.5812/hmj.97644. [DOI] [Google Scholar]
- 33.Moradi Kelardeh B., Keshavarz S., Karimi M. Effects of Nonlinear Resistance Training with Curcumin Supplement on Liver Enzymes in Men with Non-Alcoholic Fatty Liver Disease. Rep. Health Care. 2017;3:1–9. [Google Scholar]
- 34.Mohammadi F., Ghalavand A., Delaramnasab M. Effect of Circuit Resistance Training and L-Carnitine Supplementation on Body Composition and Liver Function in Men with Non-Alcoholic Fatty Liver Disease. Jundishapur J. Chronic Dis. Care. 2019;8:e90213. doi: 10.5812/jjcdc.90213. [DOI] [Google Scholar]
- 35.Hatami M.T., Eftekhari E. The Effect of Combined Aerobic and Resistance Training on Hepatic Enzymes in Males with Nonalcoholic Fatty Liver. Biotechnol. Health Sci. 2016;3:25–29. doi: 10.17795/bhs-36162. [DOI] [Google Scholar]
- 36.George A., Bauman A., Johnston A., Farrell G., Chey T., George J. Effect of a lifestyle intervention in patients with abnormal liver enzymes and metabolic risk factors. J. Gastroenterol. Hepatol. 2008;24:399–407. doi: 10.1111/j.1440-1746.2008.05694.x. [DOI] [PubMed] [Google Scholar]
- 37.Eckard C., Cole R., Lockwood J., Torres D.M., Williams C.D., Shaw J.C., Harrison S.A. Prospective histopathologic evaluation of lifestyle modification in nonalcoholic fatty liver disease: A randomized trial. Ther. Adv. Gastroenterol. 2013;6:249–259. doi: 10.1177/1756283X13484078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoseini Z., Behpour N., Hoseini R. Co-treatment with vitamin D supplementation and aerobic training in elderly women with Vit D deficiency and NAFLD: A single-blind controlled trial. Hepat. Mon. 2020;20:e96437. doi: 10.5812/hepatmon.96437. [DOI] [Google Scholar]
- 39.Moradi B., Rahmati-Ahmadabad S., Farzanegi P., Helalizadeh M., Azarbayjani M.-A. Effects of non-linear resistance training and curcumin supplementation on the liver biochemical markers levels and structure in older women with non-alcoholic fatty liver disease. J. Bodyw. Mov. Ther. 2020;24:154–160. doi: 10.1016/j.jbmt.2020.02.021. [DOI] [PubMed] [Google Scholar]
- 40.Rezende R.E., Duarte S., Stefano J.T., Roschel H., Gualano B., de Sá Pinto A.L., Vezozzo D.C., Carrilho F.J., Oliveira C.P. Randomized clinical trial: Benefits of aerobic physical activity for 24 weeks in postmenopausal women with nonalcoholic fatty liver disease. Menopause. 2016;23:876–883. doi: 10.1097/GME.0000000000000647. [DOI] [PubMed] [Google Scholar]
- 41.Zhang H.-J., He J., Pan L.-L., Ma Z.-M., Han C.-K., Chen C.-S., Chen Z., Han H.-W., Chen S., Sun Q. Effects of moderate and vigorous exercise on nonalcoholic fatty liver disease: A randomized clinical trial. JAMA Intern. Med. 2016;176:1074–1082. doi: 10.1001/jamainternmed.2016.3202. [DOI] [PubMed] [Google Scholar]
- 42.Cuthbertson D.J., Shojaee-Moradie F., Sprung V.S., Jones H., Pugh C.J., Richardson P., Kemp G.J., Barrett M., Jackson N.C., Thomas E.L. Dissociation between exercise-induced reduction in liver fat and changes in hepatic and peripheral glucose homoeostasis in obese patients with non-alcoholic fatty liver disease. Clin. Sci. 2016;130:93–104. doi: 10.1042/CS20150447. [DOI] [PubMed] [Google Scholar]
- 43.Hallsworth K., Fattakhova G., Hollingsworth K.G., Thoma C., Moore S., Taylor R., Day C.P., Trenell M.I. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut. 2011;60:1278–1283. doi: 10.1136/gut.2011.242073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Houghton D., Thoma C., Hallsworth K., Cassidy S., Hardy T., Burt A.D., Tiniakos D., Hollingsworth K.G., Taylor R., Day C.P. Exercise reduces liver lipids and visceral adiposity in patients with nonalcoholic steatohepatitis in a randomized controlled trial. Clin. Gastroenterol. Hepatol. 2017;15:96–102.e103. doi: 10.1016/j.cgh.2016.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Javanmardi Fard S., Ghodsbin F., Kaviani M.J., Jahanbin I., Bagheri Z. The effect of follow up (telenursing) on liver enzymes in patients with nonalcoholic fatty liver disease: A randomized controlled clinical trial. Int. J. Community Based Nurs. Midwifery. 2015;4:239. [PMC free article] [PubMed] [Google Scholar]
- 46.Keating S.E., Hackett D.A., Parker H.M., O’Connor H.T., Gerofi J.A., Sainsbury A., Baker M.K., Chuter V.H., Caterson I.D., George J. Effect of aerobic exercise training dose on liver fat and visceral adiposity. J. Hepatol. 2015;63:174–182. doi: 10.1016/j.jhep.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 47.Khaoshbaten M., Gholami N., Sokhtehzari S., Monazami A.H., Nejad M.R. The effect of an aerobic exercise on serum level of liver enzymes and liver echogenicity in patients with non alcoholic fatty liver disease. Gastroenterol. Hepatol. Bed Bench. 2013;6:S112. [PMC free article] [PubMed] [Google Scholar]
- 48.Nourian M., Askari G., Golshiri P., Miraghajani M., Shokri S., Arab A. Effect of lifestyle modification education based on health belief model in overweight/obese patients with non-alcoholic fatty liver disease: A parallel randomized controlled clinical trial. Clin. Nutr. ESPEN. 2020;38:236–241. doi: 10.1016/j.clnesp.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 49.Pugh C.J., Sprung V.S., Kemp G.J., Richardson P., Shojaee-Moradie F., Umpleby A.M., Green D.J., Cable N.T., Jones H., Cuthbertson D.J. Exercise training reverses endothelial dysfunction in nonalcoholic fatty liver disease. Am. J. Physiol.-Heart Circ. Physiol. 2014;307:H1298–H1306. doi: 10.1152/ajpheart.00306.2014. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan S., Kirk E.P., Mittendorfer B., Patterson B.W., Klein S. Randomized trial of exercise effect on intrahepatic triglyceride content and lipid kinetics in nonalcoholic fatty liver disease. Hepatology. 2012;55:1738–1745. doi: 10.1002/hep.25548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takahashi A., Abe K., Usami K., Imaizumi H., Hayashi M., Okai K., Kanno Y., Tanji N., Watanabe H., Ohira H. Simple resistance exercise helps patients with non-alcoholic fatty liver disease. Int. J. Sport. Med. 2015;94:848–852. doi: 10.1055/s-0035-1549853. [DOI] [PubMed] [Google Scholar]
- 52.Yao J., Meng M., Yang S., Li F., Anderson R.M., Liu C., Liu L., Yuan X., Fang Z., Lou Q. Effect of aerobic and resistance exercise on liver enzyme and blood lipids in Chinese patients with nonalcoholic fatty liver disease: A randomized controlled trial. Int. J. Clin. Exp. Med. 2018;11:4867–4874. [Google Scholar]
- 53.Pugh C.J., Cuthbertson D.J., Sprung V.S., Kemp G.J., Richardson P., Margot Umpleby A., Green D.J., Timothy Cable N., Jones H. Exercise training improves cutaneous microvascular function in nonalcoholic fatty liver disease. Am. J. Physiol.-Endocrinol. Metab. 2013;305:E50–E58. doi: 10.1152/ajpendo.00055.2013. [DOI] [PubMed] [Google Scholar]
- 54.Li J., Wang F., Chen K., Xia Y., Lu J., Zhou Y., Guo C. Effects of physical activity on liver function in patients with non-alcoholic fatty liver disease: A meta-analysis. So. J. Immunol. 2015;3:1–6. [Google Scholar]
- 55.Wang S.-T., Zheng J., Peng H.-W., Cai X.-L., Pan X.-T., Li H.-Q., Hong Q.-Z., Peng X.-E. Physical activity intervention for non-diabetic patients with non-alcoholic fatty liver disease: A meta-analysis of randomized controlled trials. BMC Gastroenterol. 2020;20:1–12. doi: 10.1186/s12876-020-01204-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brea A., Puzo J. Non-alcoholic fatty liver disease and cardiovascular risk. Int. J. Cardiol. 2013;167:1109–1117. doi: 10.1016/j.ijcard.2012.09.085. [DOI] [PubMed] [Google Scholar]
- 57.Katsagoni C.N., Georgoulis M., Papatheodoridis G.V., Panagiotakos D.B., Kontogianni M.D. Effects of lifestyle interventions on clinical characteristics of patients with non-alcoholic fatty liver disease: A meta-analysis. Metabolism. 2017;68:119–132. doi: 10.1016/j.metabol.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 58.Nikroo H., Attarzade Hosseini S., Sima H., Nematy M. Effects of diets with or without aerobic exercise program on anthropometric indices and cardiorespiratory fitness in patients with non-alcoholic steatohepatitis. J. North Khorasan Univ. Med. Sci. 2011;3:91–99. [Google Scholar]
- 59.Mohammad Rahimi G.R., Attarzadeh Hosseini S.R. Effect of Aerobic Exercise Alone or in Conjunction With Diet on Liver Function, Insulin Resistance and Lipids in Non-Alcoholic Fatty Liver Disease. Biol. Res. Nurs. 2022;24:259–276. doi: 10.1177/10998004211068026. [DOI] [PubMed] [Google Scholar]
- 60.Rice B., Janssen I., Hudson R., Ross R. Effects of aerobic or resistance exercise and/or diet on glucose tolerance and plasma insulin levels in obese men. Diabetes Care. 1999;22:684–691. doi: 10.2337/diacare.22.5.684. [DOI] [PubMed] [Google Scholar]
- 61.Kanaley J.A., Colberg S.R., Corcoran M.H., Malin S.K., Rodriguez N.R., Crespo C.J., Kirwan J.P., Zierath J.R. Exercise/physical activity in individuals with type 2 diabetes: A consensus statement from the American College of Sports Medicine. Med. Sci. Sport. Exerc. 2022;54:353–368. doi: 10.1249/MSS.0000000000002800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boulé N.G., Haddad E., Kenny G.P., Wells G.A., Sigal R.J. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: A meta-analysis of controlled clinical trials. JAMA. 2001;286:1218–1227. doi: 10.1001/jama.286.10.1218. [DOI] [PubMed] [Google Scholar]
- 63.Chudyk A., Petrella R.J. Effects of exercise on cardiovascular risk factors in type 2 diabetes: A meta-analysis. Diabetes Care. 2011;34:1228–1237. doi: 10.2337/dc10-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Phielix E., Meex R., Moonen-Kornips E., Hesselink M., Schrauwen P. Exercise training increases mitochondrial content and ex vivo mitochondrial function similarly in patients with type 2 diabetes and in control individuals. Diabetologia. 2010;53:1714–1721. doi: 10.1007/s00125-010-1764-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Winnick J.J., Sherman W.M., Habash D.L., Stout M.B., Failla M.L., Belury M.A., Schuster D.P. Short-term aerobic exercise training in obese humans with type 2 diabetes mellitus improves whole-body insulin sensitivity through gains in peripheral, not hepatic insulin sensitivity. J. Clin. Endocrinol. Metab. 2008;93:771–778. doi: 10.1210/jc.2007-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kirwan J.P., Solomon T.P., Wojta D.M., Staten M.A., Holloszy J.O. Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am. J. Physiol.-Endocrinol. Metab. 2009;297:E151–E156. doi: 10.1152/ajpendo.00210.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Melsen W., Bootsma M., Rovers M., Bonten M. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin. Microbiol. Infect. 2014;20:123–129. doi: 10.1111/1469-0691.12494. [DOI] [PubMed] [Google Scholar]
- 68.Pickering C., Kiely J. Do non-responders to exercise exist—And if so, what should we do about them? Sport. Med. 2019;49:1–7. doi: 10.1007/s40279-018-01041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sparks L.M. Exercise training response heterogeneity: Physiological and molecular insights. Diabetologia. 2017;60:2329–2336. doi: 10.1007/s00125-017-4461-6. [DOI] [PubMed] [Google Scholar]
- 70.Sabag A., Barr L., Armour M., Armstrong A., Baker C.J., Twigg S.M., Chang D., Hackett D.A., Keating S.E., George J., et al. The Effect of High-intensity Interval Training vs Moderate-intensity Continuous Training on Liver Fat: A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2022;107:862–881. doi: 10.1210/clinem/dgab795. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.