Abstract
Soil-borne beneficial microbes establish symbioses with plant hosts and play key roles during growth and development therein. In this study, two fungal strains, FLP7 and B9, were isolated from the rhizosphere microbiome associated with Choy Sum (Brassica rapa var. parachinensis) and barley (Hordeum vulgare), respectively. Sequence analyses of the internal transcribed spacer and 18S ribosomal RNA genes combined with colony and conidial morphology identified FLP7 and B9 to be Penicillium citrinum strains/isolates. Plant–fungus interaction assays revealed that isolate B9 showed significant growth promotion effects in Choy Sum plants cultivated in normal soil, as well as under phosphate-limiting conditions. In comparison to the mock control, B9-inoculated plants showed a 34% increase in growth in aerial parts and an 85% upsurge in the fresh weight of roots when cultivated in sterilized soil. The dry biomass of such fungus-inoculated Choy Sum increased by 39% and 74% for the shoots and roots, respectively. Root colonization assays showed that P. citrinum associates directly with the root surface but does not enter or invade the root cortex of the inoculated Choy Sum plants. Preliminary results also indicated that P. citrinum can promote growth in Choy Sum via volatile metabolites too. Interestingly, we detected relatively higher amounts of gibberellins and cytokinins in axenic P. citrinum culture filtrates through liquid chromatography–mass spectrometry analyses. This could plausibly explain the overall growth induction in P. citrinum-inoculated Choy Sum plants. Furthermore, the phenotypic growth defects associated with the Arabidopsis ga1 mutant could be chemically complemented by the exogenous application of P. citrinum culture filtrate, which also showed accumulation of fungus-derived active gibberellins. Our study underscores the importance of transkingdom beneficial effects of such mycobiome-assisted nutrient assimilation and beneficial fungus-derived phytohormone-like metabolites in the induction of robust growth in urban farmed crops.
Keywords: Choy Sum, Penicillium citrinum, growth promotion, gibberellin, cytokinin, phytohormone
1. Introduction
Cruciferous (Brassicaceae family) species represent a large number of economically important crops in global agriculture and food markets and constitute about 12% of the annual cultivation of leafy vegetables [1,2]. Brassicas are well recognized for being rich in antioxidants, carotenoids, polyphenols and more importantly for the presence of a unique group of anti-carcinogenic bioactive compounds termed glucosinolates. Brassica rapa subsp. chinensis var parachinensis (Choy Sum), a green leafy vegetable also known as Chinese flowering cabbage is a prolific and important cash crop in the tropics. It is widely cultivated and consumed in many Asian countries such as China, Japan and Singapore [2,3]. Recent studies on Choy Sum have reported the nutritional shifts of various metabolites depending on the growth stage and the effects of light intensity on the growth parameters and glucosinolate content [2,4,5]. However, the microbiome that shapes the rhizosphere environment of Choy Sum remains largely unexplored.
Plants harbor diverse microorganisms that engage in a continuum of interactions ranging from beneficial plant growth-promoting (PGP) to adverse pathogenic outcomes. PGP microbes play a key role in soil health and in the proper development of crop plants. Current trends in agriculture are focused towards reducing the input of inorganic fertilizers and the use of PGP microbes to achieve sustainability. Mentioned hitherto, the world of PGP microbes remains unexplored as far as Choy Sum is concerned, although some reports have described such beneficial microbes and endophytic fungi in other Brassicaceae species [6,7,8,9,10]. Plant growth-promoting rhizobacteria (PGPR) colonize roots and enhance plant growth directly and indirectly [11,12]. We aimed to explore the PGP mycobiome—fungi that are important components of soil microbiota and biomass—of Choy Sum. Rhizosphere- and root-associated fungi that are able to promote plant growth upon root colonization or through their secreted metabolites are functionally designated as plant growth-promoting fungi (PGPF) [13,14] and have gained significant attention in recent years. Over the decades, a growing list of PGPF from diverse genera including Trichoderma, Penicillium, Phoma, Piriformospora, Fusarium, Aspergillus and sterile non-sporulating fungi has been identified [15,16,17].
Previous reports showed that Penicillium oxalicum P4 and Aspergillus niger P85 isolates can produce seven and four organic acids, respectively, and can solubilise phosphate (Pi) and promote maize growth [18]. The fungal derived gibberellins and IAA by Penicillium sp. play a vital role in plant growth and development [19,20]. Similarly, in Arabidopsis, three fungal endophytes from water mint can increase the fresh and dry weight of Arabidopsis at 14 and 21 days post inoculation. Among them, Phoma macrostoma can increase both root area and depth at 21 days [21]. Active gibberellins (GAs) such as GA1, GA3, GA4, and GA7 were detected from pure cultures of Phoma glomerata and Penicillium species and they significantly increased the chlorophyll content in leaves and fresh and dry weight of shoots [22]. Penicillium pinophilum formed arbuscular mycorrhizae, which increase the plant dry weight, nitrogen content, P content and photosynthesis rate by 31%, 47%, 57% and 71%, respectively [23]. Talaromyces pinophilus, an endophytic fungus isolated from halophytic plants of Korea, can increase the plant height in comparison with the uninoculated wild-type [24].
Working on the hypothesis that the outcome of the host–PGPF interaction depends on the plant and microbial species, numerous fungi were isolated from the rhizosphere of Choy Sum and other plants and we describe here, for the first time, the association of a Penicillium species therein and its ability to promote plant growth. Penicillium species are known for their occurrences in diverse habitats and have received significant attention in the production of bioactive compounds. Investigations in recent years, including the research presented here, have reported Penicillium species as potent PGPFs that are capable of inducing plant growth by one or several direct or indirect mechanisms that include production of PGP phytohormones, solubilization of minerals, alleviation of drought and salinity stresses and antagonism against phytopathogens [20,25].
We report here two isolates of Penicillium citrinum B9 and FLP7 that showed promising growth phenotypes in Choy Sum in the laboratory as well as under soil-based greenhouse cultivation. Such induced plant growth was found to be due to nutrient acquisition enabled by the beneficial fungus and in part through fungus-derived and secreted phytohormones such as GAs and cytokinin(s). Our results demonstrate that symbiotic interactions with beneficial fungi play an important role in promoting plant growth and in sustainably increasing agricultural productivity.
2. Materials and Methods
2.1. Fungus Culture Conditions and Molecular Identification
The two fungal isolates, FLP7 and B9, used in this study are from our laboratory culture collection of beneficial fungal strains isolated from different environments, and possess plant growth promoting characteristics. The fungal strain FLP7 was isolated from the rhizosphere of Choy Sum grown under Pi-limiting conditions (12.5 µM), whereas B9 was from the roots of two-week-old barley seedlings grown in the soil. The GFP-labeled strain B9GFP was further constructed in this study. The fungal strains were routinely cultivated in a prune juice agar (PA) medium [26] or in Potato Dextrose agar (PDA; VWR, Singapore) at 28 °C for 2 days in the dark and transferred to light at room temperature and grown for 7 days. Fungal cultures were grown in a complete medium (CM; 0.6% yeast extract, 0.6% casein hydrolysate and 1% sucrose) [27] for DNA extraction or for obtaining the culture filtrate or mycelial extract. Spore suspensions were prepared by rinsing the colony surface with sterile water, gently scraping the spores and hyphae off the medium followed by filtration through two layers of sterile Calbiochem Miracloth (Millipore, Burlington, MA, USA). The spore concentration was determined using a haemocytometer and adjusted with sterile water to the required concentration for plant assays.
To identify the fungal isolates, genomic DNA from mycelia grown in a PA medium was extracted using the MasterPureTM Yeast DNA purification kit (Lucigen Corporation, Middleton, WI, USA) and used subsequently for PCR amplification using standard primers for the ITS or rDNA repeats. The PCR products were purified and sequenced. The sequences were used for NCBI BLAST analyses. The PCR primer pairs are listed in Table S1. The PCR protocol used was 95 °C for 5 min; 35 cycles of 95 °C for 30 s, 48 °C for 30 s, and 72 °C for 1.5 min, 72 °C for 5 min; 95 °C for 5 min, 35 cycles of 95 °C for 30 s, 52 °C for 30 s, and 72 °C for 1.5 min, 72 °C for 5 min and 95 °C for 5 min, 35 cycles of 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s, 72 °C for 5 min, respectively.
2.2. Plant Growth-Promotion Assays
Seeds from Choy Sum or the Arabidopsis ga1 mutant were surface sterilized and placed on a Murashige Skoog medium for germination. The Choy Sum seedlings were transplanted to autoclaved or non-autoclaved soil at 4 dpi (days post inoculation), respectively. The ga1 mutant seedlings were transplanted to Phytatray II boxes containing the MS medium supplemented with cell-free culture filtrate from FLP7 or B9. The conidia were collected and diluted to a 1–5 × 105 spores/mL suspension for inoculation. The fungus-inoculated plants were placed in a growth chamber for 2 days and then cultivated in a greenhouse until 21 dpi. The experiments were repeated three times each using 10–20 seedlings in each instance.
To assay for growth-promoting volatile metabolites, Choy Sum seeds were surface sterilized and grown on an MS medium as described above. The four-day-old seedlings in triplicate were transferred to Phytatray II boxes with the MS medium, together with fungal strains grown on prune agar, and the boxes were incubated at 25 °C, 70% relative humidity (RH) (day) and 23 °C, 50% RH (night) for 10 days. Barium hydroxide was added to the experimental set-up to quench excess carbon dioxide (CO2) in order to rule out its indirect beneficial effects in plant growth.
2.3. Agrobacterium-Mediated Transformation of B9
Agrobacterium-mediated transformation of target fungus was performed as described previously [28]. Agrobacterium tumefacians strain AGL1 carrying the appropriate Transfer-DNA vector/plasmid was grown at 28 °C in a LB medium containing 100 μg/mL Kanamycin overnight. The overnight AGL1 culture was diluted to OD600 = 0.15 using a standard induction medium [K salts (10 mMK2HPO4, 10 mM KH2PO4); M salts (2 mM MgSO4-7H2O, 2.5 mM NaCl, 4 mM NH4NO3, 0.7 mM CaCl2, 10 μM FeSO4); with glucose 5 mM, MES 40 mM, glycerol 0.5%, 100 μg/mL Kanamycin, 200 μg/mL acetosyringone] and incubated at 28 °C with gentle shaking at 160 rpm for 6 h. Simultaneously, conidia (fungal spores) were harvested from fully grown cultures of P. citrinum B9 strain (on a prune agar medium under light for about 1 week) and re-suspended to 1 × 106/mL in distilled water. A sterile 0.45 μm nitrocellulose filter membrane was placed on an induction medium containing 200 μg/mL acetosyringone. A mixture of equal volume (100 μL each) of the AGL1 culture and the fungal conidial suspension was spotted and air-dried on the filter membrane. The plate was then incubated at 28 °C for 48 h. After the co-culture, all the growth on the filter membrane was scraped into 2 mL of sterile PBS (containing 200 μg/mL Cefotaxime, 60 mg/mL Streptomycin and 100 mg/mL Ampicillin) and was vortexed briefly. The resuspension in PBS was plated equally (200 μL) onto ten CM selection medium plates containing 200 μg/mL Cefotaxime (to kill Agrobacteria), 60 μg/mL Streptomycin, 100 μg/mL Ampicillin and 250 μg/mL Hygromycin. The selection plates were incubated at 28 °C until the transformed fungal colonies appeared (typically 3–5 days). The individual colonies were selected for mycelium preparation and DNA extraction as above. The primer pairs used for PCR amplification are listed in Table S1.
2.4. Fungal Interaction/Colonization Assays in Choy Sum Roots
The Choy Sum seed germination and seedling preparation were as described above. The GFP-expressing B9 strain was prepared as above. The seedlings were submerged in the conidial suspension containing 1 × 104 spores/mL. The interaction between Choy Sum and the cytosolic GFP-expressing B9 was analyzed using laser scanning confocal microscopy (Exciter, Zeiss) using the 10× water-immersion and 63× oil objectives. The excitation/emission wavelength (Ex/Em) was 488 nm/505–550 nm.
2.5. Plant Growth Assay under Low Pi Using P. citrinum Isolate(s)
The Choy Sum seeds were sterilized and germinated on a Murashige Skoog medium with low Pi (12.5 µM) for 4 days. The germinated seedlings were transplanted to autoclaved rice soil (with 0.11% (w/w) Pi) and grew for 21 days, as above. During the growth, no fertilizer was added. The average leaf area (cm2), root length (cm), dry weight of the roots and aerial parts (mg) were calculated for both control and B9- or FLP7-treated seedlings.
2.6. Extraction and Purification Methods for Detection of Phytohormones in P. citrinum
Certified standards of GAs (GA1, GA4, GA20) were purchased from OIChemim Ltd. (Olomouc, Czech Republic). Certified GA3 standard and formic acid were purchased from Sigma-Aldrich (Darmstadt, Germany). Trans-zeatin and trans-zeatin riboside standards were provided by Prakash Kumar (National University of Singapore, Singapore). Acetonitrile with 0.1% formic acid (Optima LC-MS grade) and methanol (Optima LC-MS grade) were obtained from Fluka Honeywell (Charlotte, NC, USA). Milli-Q water was used for preparation of the mobile phase (Millipore, Burlington, MA, USA). The Prime HLB SPE cartridge (200 mg, 6 cc) and Oasis MCX (200 mg, 6 cc) cartridge were supplied by Waters Corporation (Wilmslow, UK).
Standard stock solutions (1000 μg/mL) of GA1, GA3, GA4, GA20, trans-zeatin and trans-zeatin riboside were prepared individually in methanol and stored at −20 °C in the dark. Stock solutions were used to prepare working standard solutions for analytical experiments.
Extraction procedures for GA were adopted from the methods described previously [22,29,30]. Briefly, the liquid complete medium inoculated with P. citrinum isolates FLP7 or B9 was incubated at 28 °C at 180 rpm for 7 days. The purification of fungal samples was achieved via solid-phase extraction (SPE) with a reverse phase C-18 cartridge. Initially, fungal samples were extracted with ethyl acetate containing 1% (v/v) formic acid and centrifuged at 3000 rpm for 15 min. The supernatant of the mixture was then transferred and evaporated to dryness. For SPE purification, samples were reconstituted in 100% methanol and loaded onto the C-18 cartridge preconditioned with 6 mL methanol and then underwent equilibration with 6 mL distilled water. The cartridges were washed with 5 mL distilled water and the retained phytohormones (GAs) were eluted with 6 mL 100% methanol containing 1% (v/v) formic acid. The eluted extract was evaporated to dryness and reconstituted in 50% (v/v) methanol for the following LC-MS/MS analysis.
Extraction of cytokinins (trans-zeatin and trans-zeatin riboside) and subsequent sample clean-up and purification were performed using methods adapted from Morrison et al. (2015) [31]. Briefly, cell-free filtrates were snap frozen, lyophilized and subsequently homogenized in a cold (−20 °C)-modified Bieleski extraction buffer (Methanol/Water/Formic Acid, 15/4/1). Samples were allowed to extract passively, twice at −20 °C, and pooled supernatants were dried in a speed vacuum concentrator at ambient temperature (UVS400, Thermo Fisher Scientific, Waltham, MA, USA). Dried supernatant residues were reconstituted in 1 mL 1 M HCO2H and subjected to solid phase extraction on a mixed mode, reverse-phase, cation-exchange cartridge (Oasis MCX 6 cc; Waters, Wilmslow, UK). Trans-zeatin and trans-zeatin riboside were eluted with 0.35 M NH4OH in 60% CH3OH. Samples were evaporated and stored at −80 °C prior to analyses. Samples were reconstituted in initial mobile phase conditions (95:5 H2O:CH3OH with 0.08% acetic acid (CH3CO2H)) prior to analyses.
Headspace solid-phase microextraction (HS-SPME) sampling was used to collect volatile compounds emitted from P. citrinum [32]. The SPME fiber (50/30 DVB/CAR/PDMS, Agilent, USA) was exposed into the headspace of glass vials or Phytatrays II containing P. citrinum at 28 °C for 5–7 days in the dark. An uninoculated PA medium served as a negative/mock control for comparison. Determination of volatile profiles of P. citrinum was performed using untargeted gas chromatography coupled with electron impact ionization/time-of-flight mass spectrometry (GC-EI/TOF-MS) using Agilent 7890A platform following the manufacturer’s instructions.
2.7. Liquid Chromatography–Mass Spectrometry
LC–MS data were acquired on an Agilent 1290 Infinity coupled to an Agilent 6400 series Triple Quadrupole (Agilent, Santa Clara, CA, USA). An ultra-high performance liquid chromatography (UHPLC) system was integrated with Agilent 6490 controlled by MassHunter software B.06.00 (Agilent, Santa Clara, CA, USA).
For detection of GAs, 10 µL of extracts was chromatographed on a Zorbax RRHD SB-C18 (50 mm length × 2.1 mm diameter, 1.8 µm particle size) (Agilent, USA) with the column temperature set at 50 °C and auto-sampler temperature set at 4 °C. The mobile phase consisted of water acidified with 0.1% formic acid (Solvent A) and acetonitrile acidified with 0.1% formic acid (Solvent B). A gradient elution (flow rate 300 µL/min) consisting of 5% solvent B for 1 min followed by a linear gradient of 100% solvent B at 10.5 min was maintained until 13.4 min, followed by 5% solvent B at 13.5 min to 16.5 min for re-equilibration. Mass spectrometric detection was performed with a Triple Quadrupole in a negative mode with an Agilent Jet Stream ESI (G1958-65138) ion source using optimized monitoring reactions (Supplementary Figures S3 and S4; Supplementary Table S2).
For detection of trans-zeatin and trans-zeatin riboside, 10 µL of extracts were chromatographed on a Kinetex C18 column (2.6 µm C18 100 Å, 100 × 2.1 mm) (Phenomenex, Torrance, CA, USA) with the column temperature set at 50 °C and auto-sampler temperature set at 4 °C. The mobile phase consisted of water acidified with 0.08% acetic acid (Solvent A) and methanol (Solvent B). A gradient elution (flow rate 300 µL/min) was used consisting of 5% of solvent B at 0 min followed 45% solvent B at 4 min, 75% B at 5 min followed by 95% B at 5.1 and was maintained until 6.1 min, followed by 5% solvent B at 6.2 min to 8.2 min for re-equilibration. Mass spectrometric detection was performed with a Triple Quadrupole in a positive mode with an Agilent Jet Stream ESI (G1958-65138) ion source using optimized monitoring conditions (Supplementary Figure S5; Supplementary Table S2).
The mass spectrometer settings were as follows for all the phytohormones: source temperature 250 °C, gas flow 12 L/min, nebulizer gas pressure 35 psi, sheath gas temperature 350 °C and sheath gas flow 11 L/min. Data were recorded in the multiple reaction monitoring mode. All data collection, mass spectrometric and statistical analyses were carried out with the Mass Hunter Workstation software package: MH Acquisition B.05.00, MH Qualitative Analysis B.06.00. (Agilent Technologies, Santa Clara, CA, USA). All samples were randomized before LC-MS analyses.
2.8. Statistical Analysis
The data and comparison with controls were represented by using means with standard error. The significance of differences between the control and treatments was statistically evaluated using GraphPad (https://www.graphpad.com/quickcalcs/ttest1.cfm; accessed on 20 November 2022). Differences were considered significant at a probability level of p < 0.05 (*) or p < 0.01(***).
3. Results
3.1. Morphological Characteristics and Identification of Fungal Isolates B9 and FLP7
The morphological characteristics of the colonies and spores produced by B9 and FLP7 were analyzed by growing the isolates on a PA medium for seven days. The colonies were greyish green, moderately deep, with a raised center and with low or entire margins. On the PA medium, the conidiophores produced were mono-verticillate and sometimes bi-verticillate, with stipes smooth, phialides bottle-like, the conidia smooth walled, with globose to sub-globose and appearing in chains on the heads of phialides (Supplementary Figure S1a,b). The sequencing results of the internal transcribed spacer (ITS), large subunit (LSU) and small subunit (SSU) of 18S nuclear ribosomal RNA genes identified B9 and FLP7 to be Penicillium citrinum. As shown in Supplementary Figure S1c, the nucleotide sequence of the ITS region of B9 shared 100% nucleotide identity to the P. citrinum-type strain when searched for orthologous sequences in the NCBI Genbank database.
3.2. P. citrinum Improves Choy Sum Growth under Nutrient Rich Conditions
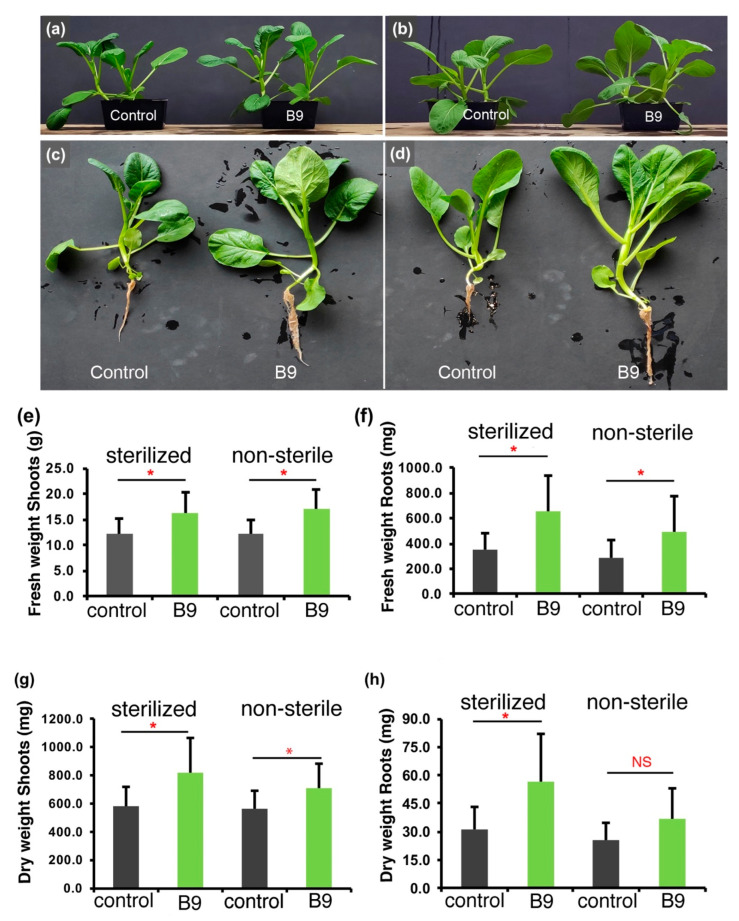
Standard plant–fungus interaction assays were conducted to check if Penicillium citrinum strains can induce or promote growth in green leafy vegetables. Inoculation of the spores of B9 to Choy Sum at the seedling stage could significantly increase the growth of plants both in sterilized soil as well as in non-autoclaved soil. Overall, the B9-inoculated plants were larger and grew taller than the uninoculated controls (Figure 1a–d). Compared with the mock control, the fresh and dry weight of aerial parts in the B9-inoculated plants increased by 34.8%, 39.5%, 41.2% and 25.4% in the sterilized or non-sterile soil, respectively (Figure 1e–h; n = 24, p < 0.05). Similarly, the fresh and dry weight of roots increased by 85.4%, 74.9%, 83% and 42.4% under the respective conditions (Figure 1e–h). These data helped us conclude that the B9 isolate of P. citrinum can significantly promote growth in Choy Sum both in the presence or absence of resident commensal microbiota in the rhizosphere. However, unlike B9, FLP7 did not promote growth in Choy Sum (Supplementary Figure S2a–f). The morphological traits of Choy Sum inoculated with FLP7 were comparable to the uninoculated controls. Furthermore, the fresh and dry weight of shoots and roots between FLP7 and its corresponding mock control did not show any significant differences (Supplementary Figure S2a–c) in nutrient rich conditions.
Figure 1.
The morphological traits of Choy Sum inoculated with P. citrinum and the plant growth promotion effect in sterilized or non-sterilized soil (b,d). (a,c) The morphological traits of Choy Sum grown for 21 days in autoclaved soil (left, water; right, inoculated with B9 conidia); (b,d) The morphology and growth characteristics of Choy Sum plants grown in non-autoclaved soil for 21 days (left, water; right, inoculated B9 spores). (e–h) Bar charts showing quantification of the fresh and dry weight of shoots/aerial parts (e,g) and roots (f,h) under the two growth conditions, respectively. Data represents means ± SDs from 3 replicates consisting of 16 plants in each instance. Differences were considered significant at a probability level of p < 0.05 (*). NS: not significant.
3.3. P. citrinum Improves Choy Sum Growth under Pi-Limiting Conditions
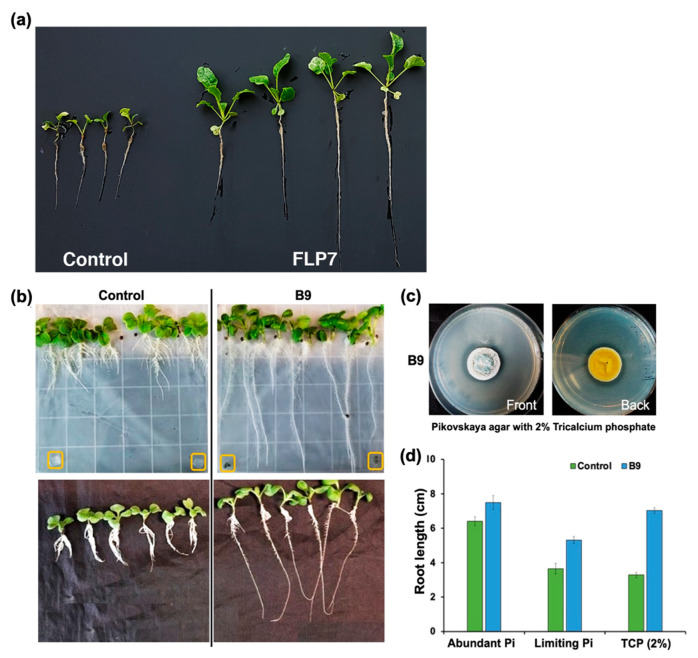
A previous report showed that the root endophyte Colletotrichum tofieldiae (Ct) promotes Arabidopsis growth under Pi-deficient conditions, though Ct did not display growth promotion under Pi-replete conditions [33]. Since the P. citrinum strain FLP7 was isolated from seedlings cultivated in a growth medium containing low levels of Pi, we tested whether the ability (if any) to promote growth in the host plants is restricted to Pi-limiting conditions. To further investigate this, sterilized soil with very low Pi content (0.11% (w/w)) was used to test this hypothesis. In comparison to mock treatment with sterile water, the Choy Sum seedlings grown in low Pi soil inoculated with FLP7 conidia showed a significant increase in overall growth and size of the plants. The average leaf area, root length, dry weight of the roots and shoots were 5.7, 2.0, 3.3 and 3.9 times that of the control, respectively (Figure 2a). We infer that the FLP7 isolate of P. citrinum can indeed improve the overall growth of Choy Sum, likely via facilitating the availability and/or uptake of phosphate in the host under low Pi conditions. To further check if the isolate B9 could also perform the same function under nutrient-limiting conditions, we used MS media with limiting amounts of Pi and checked the growth of Choy Sum grown along with B9. P. citrinum (B9) was able to induce Choy Sum growth under Pi-limiting conditions (Figure 2b). Furthermore B9 was found to possess a significant phosphate-solubilising activity as it was able to solubilize Ca3(PO4)2 in vitro (Figure 2c). As quantified in the bar chart, such Pi bioavailability and consequent root growth was further enhanced in planta in the presence of additional tricalcium phosphate (Ca3(PO4)2) (Figure 2d). We infer that P. citrinum can indeed improve the overall growth, specifically the root development in Choy Sum, likely via facilitating the bioavailability and/or uptake of Pi in the host under low Pi conditions.
Figure 2.
P. citrinum enables robust root and shoot growth in Choy Sum under phosphate (Pi)-limiting conditions. (a) The morphological traits of Choy Sum treated with FLP7 conidia or with water as control. The average leaf area (cm2), root length (cm), the dry weight of roots and aerial parts (mg) determined from both FLP7-treated seedlings and the mock control plants were determined and mentioned in the Section 3. Data represents means ± SDs from 3 replicates consisting of 8 plants in each instance. (b) The morphological traits of Choy Sum treated with B9, or with water as control in phosphate-limiting conditions in MS agar (top panels; B9 provided as mycelial plugs (boxed in yellow) or in soil (lower; B9 provided as conidia suspension). Control refers to treatment with water or uninoculated medium plugs (only) in the absence of the fungal mycelia/conidia. The average leaf area (cm2), root length (cm), the dry weight (mg) of roots and aerial parts were determined from both B9-treated seedlings and the mock control plants. Data represents means ± SDs from 3 replicates consisting of 16 plants in each instance. (c) P. citrinum helps assimilate phosphate and enables its bioavailability to the host plants. B9 is capable of solubilizing tricalcium phosphate, as judged by the zone of clearance around edges of the B9 colony cultivated on Pikovskaya agar containing 2% Ca3(PO4)2. (d) P. citrinum provides solubilized phosphate to host plants to induce root growth in Pi-limiting conditions. Bar graph quantifying average root length in Choy Sum plants grown in the presence of phosphate-replete or phosphate-limiting conditions in the presence or absence of the P. citrinum B9 strain.
3.4. P. citrinum Isolates Enhance Choy Sum Growth via Volatile Secondary Metabolites
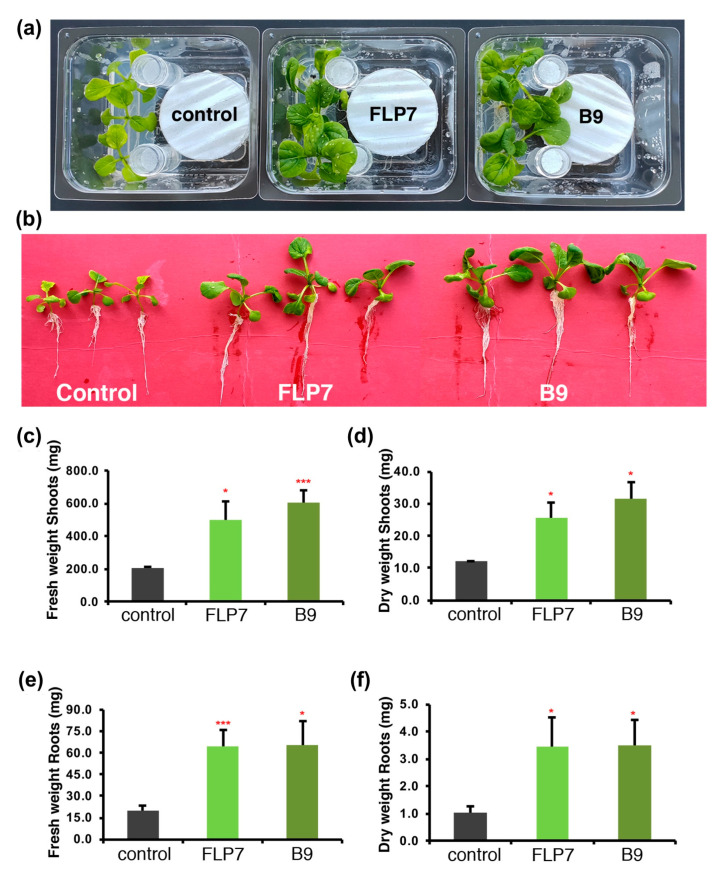
Previous studies demonstrated that some beneficial fungi could secrete volatile organic compounds/metabolites to trigger plant growth and development [34,35,36]. To investigate if P. citrinum isolates B9 and FLP7 can induce similar VOC-based growth stimulation, we co-incubated 4-day-old Choy Sum seedlings with B9 or FLP7 colonies in Phytatrays for 10 days. Barium hydroxide was added to the experimental set-up to quench excess CO2 in order to rule out its indirect beneficial effects on plant growth. The tests for such volatile compounds indicated that compared to the mock control (the prune agar medium only), the size of the seedlings co-cultivated in Phytatrays with FLP7 or B9 colonies was significantly larger (Figure 3a–c; n = 18, p < 0.05). The fresh and dry weight of the shoots and roots of seedlings incubated with FLP7 (or B9) was 1.46, 1.14, 2.22 and 2.28 times higher than that of the respective mock controls (Figure 3c–f). Furthermore, the fresh and dry weight of shoots and roots of the seedlings incubated with B9 was 1.98, 1.63, 2.28 and 2.35 times than that of the uninoculated control plants (Figure 3c–f). Our preliminary analysis using solid-phase microextraction coupled with GC-MS identified seven differentially produced VOCs (Supplementary Table S3). The chemically identified compounds with individual retention time and the standards used for comparison (with CAS number) are mentioned. These results confirmed that P. citrinum secretes/exudes several putative volatile compound(s) which are likely responsible for indirectly imparting such beneficial effects on overall plant growth and development in Choy Sum.
Figure 3.
Volatile organic compounds from P. citrinum isolates stimulate robust seedling growth in Choy Sum. (a) The morphological traits of Choy Sum seedlings grown in tripartite Phytatray II for 10 days; (b) the seedlings from (a); (c–f) quantification of the fresh and dry weight of shoots (c,d) and roots (e,f) from the seedlings incubated individually with P. citrinum B9 or FLP7 conidia. Data represents means ± SDs from 3 replicates each consisting of 8 plants. Barium hydroxide was used to quench excess CO2 produced by fungal growth and/or metabolism. Control (mock) inoculation utilized the growth medium (PA, prune agar) without any fungus. Differences were deemed significant at a probability level of p < 0.05 (*) or p < 0.01(***).
3.5. Analysing the Colonization of Plant Roots by P. citrinum Isolate B9
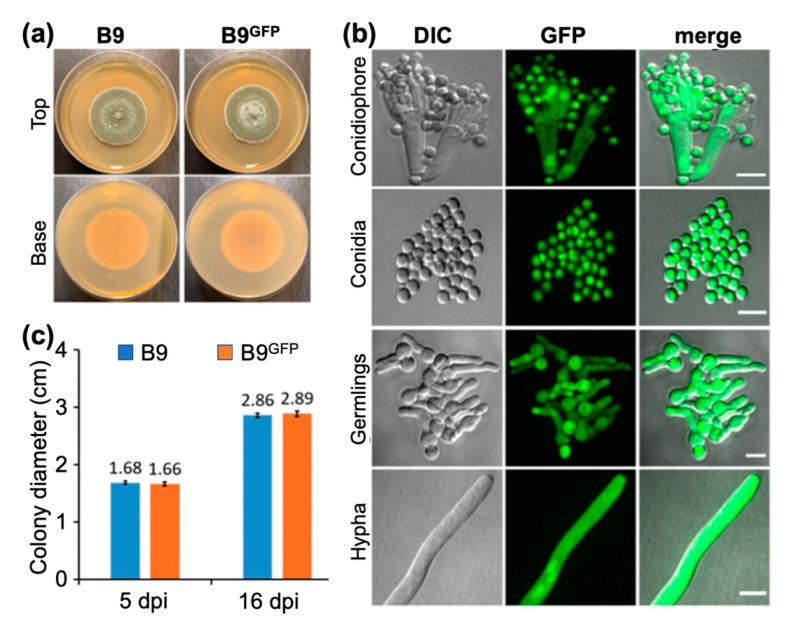
To further investigate the mode of interaction of P. citrinum in the rhizosphere of plants, isolate B9 that showed promising effects in the conducted assays was transformed with the gene expressing a cytosolic enhanced green fluorescent protein (GFP). The resultant transformants were verified using PCR and sequencing, and the confirmed cytosolic GFP-expressing B9 strain tested for growth and conidiation (Figure 4a–c) was used for Choy Sum root inoculation assays. The host-associated hyphal growth of eGFP-expressing B9 was visualized at 12 h after inoculation.
Figure 4.
Generation and comparative analysis of the cytosolic GFP-expressing P. citrinum isolate B9. (a) Colony morphology and growth characteristics of the P. citrinum B9 and B9GFP strain. Photographs were taken at 5 dpi (b) Confocal microscopy-based confirmation of cytosolic GFP expression in the B9GFP strain at the indicated stages of fungal development. Scale bar equals 5 μm. (c) Cytosolic GFP expression does not affect mycelial growth and asexual development or conidiation in the P. citrinum B9GFP strain. Conidiation was assessed at day 5 post inoculation, as was growth under constant illumination.
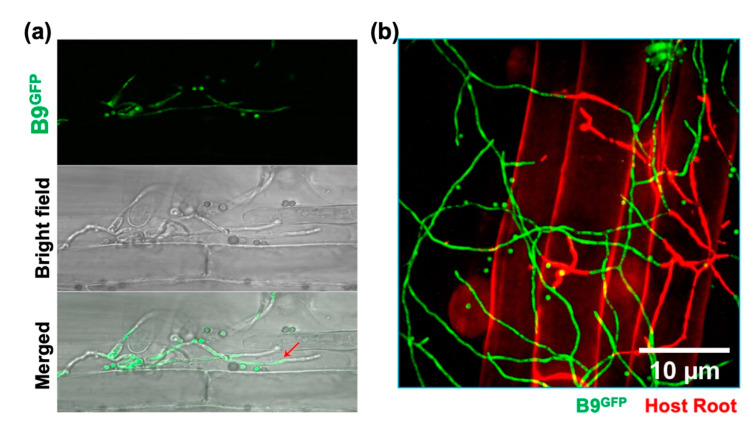
However, no intracellular invasion or colonization within the roots was evident, even at 3 days post inoculation (Figure 5a). Similarly, the eGFP-tagged B9 strain was used to co-incubate with the roots of 4-day-old Choy Sum seedlings. Confocal microscopy revealed that the hyphae of B9 strain of P. citrinum also make contact with and adhere to the surface of Choy Sum roots (Figure 5b) but do not enter or colonize the root epidermal cells per se. These results demonstrated that the P. citrinum imparts beneficial effects via hyphopodial surface attachment and biotrophic interactions with Choy Sum roots.
Figure 5.
Confocal micrographs of Choy Sum roots incubated with cytosolic eGFP-expressing B9 strain of P. citrinum. Choy Sum roots were incubated with 1 × 104 spores/mL at day 5 post germination. The root colonization was analyzed at 1, 2 and 3 days after incubation. Confocal microscopic images of Choy Sum roots incubated with B9GFP strain (a,b). Root tissue was counter-stained with Propidium iodide. GFP, green fluorescent protein; bright field, host root, Propidium iodide (PI) staining, merge, composite of the GFP, PI and bright field channels.
3.6. P. citrinum Produces the Phytohormones Gibberellin and Cytokinin
Given the strong growth-enhancing effect on the host plants, we decided to evaluate whether P. citrinum isolate B9 produces and secretes any growth-promoting secondary metabolites or phytohormones. Towards this end, the axenic culture filtrates of B9 and FLP7 were analyzed using liquid chromatography–mass spectrometry (LC-MS) together with the requisite standards for two major classes of growth-promoting plant hormones: gibberellins (GAs) and cytokinins. The phytohormone detection was performed using optimized reaction monitoring conditions for the requisite standards as detailed in Supplementary Figures S3–S5 and Supplementary Table S2.
Analysis of the cell-free extracts of B9 showed the presence of bioactive GAs including GA1 and GA3 and the inactive GA20 while in FLP7; the inactive GA20 can alone be detected even in the absence of the host plants (Supplementary Figure S6). However, the results were variable, possibly due to the low abundance and/or instability of GAs produced and secreted by the fungus. Furthermore, GA-related inter-conversions should be considered, since only selective GAs were monitored in this study. On the contrary, GAs were absent or undetectable in the mock control (uninoculated growth medium) or within the mycelial extracts, thus indicating that the GAs detected in the fungal culture filtrates were most likely secreted by P. citrinum and not sourced from the growth medium per se, thus suggesting that the fungal GAs are most likely secreted extracellular metabolites.
To determine if the GA-related compounds produced by P. citrinum are, indeed, functionally active, an Arabidopsis GA-deficient mutant, ga1, and its isogenic wild-type Col-0 accession were germinated on a growth medium lacking or containing the culture filtrate of P. citrinum B9 or FLP7 (Figure 6). After 9 days, the ga1 mutant supplemented with FLP7 or B9 culture filtrates showed shoot elongation and early flowering, with FLP7 showing comparatively better results (Figure 6a). Nevertheless, the wild-type Arabidopsis plants treated with the cell-free exudate of P. citrinum isolates showed early flowering compared to the control plants grown in the medium lacking the fungal culture filtrate (Figure 6b). Paclobutrazol is a known inhibitor of GA biosynthesis in plants [37]. In line with this, during our analysis, GAs were undetectable in culture filtrates and/or the corresponding mycelia from B9 treated with 10 µM Paclobutrazol. Hence, we used Paclobutrazol to further confirm if the fungal-produced GAs play a role in plant growth promotion. Interestingly, Choy Sum plants were unable to produce the effect of the culture filtrate and unable to establish when the inhibitor of GAs was added to the media (Figure 6c). This result indicates that the biosynthesis of fungal GAs is blocked in Paclobutrazol-treated P. citrinum. In contrast, the control set without the GA inhibitor showed the presence of GAs and consequent growth induction in the culture filtrates of both the beneficial isolates of P. citrinum. Taken together, these results revealed that the beneficial P. citrinum isolates FLP7 and B9 indeed produce a minor, albeit functionally significant amount of functional GAs, which is sufficient in trans to rescue the growth defects in the ga1 mutant of Arabidopsis.
Figure 6.
The effect of P. citrinum on the growth of Arabidopsis ga1 mutant and wild-type accession Col-0. (a) The morphological traits of Arabidopsis ga1 mutant in the presence or absence of exudates from beneficial P. citrinum cultures (B9 and FLP7) grown in a complete medium. (b) Wild-type Arabidopsis Col-0 treated with the culture filtrate from P. citrinum. Control refers to the mock inoculation with equivalent amount of growth medium without the fungus. (c) Gibberellin(s) produced by P. citrinum contribute in part to growth promotion in Choy Sum. The culture filtrate from the indicated P. citrinum isolate grown in a complete medium in the presence or absence of Paclobutrazol (the gibberellin biosynthesis inhibitor) was inoculated on Choy Sum seeds. Control refers to mock inoculation with the sterile growth medium in the absence of the fungus.
Apart from GAs, we evaluated the presence of cytokinins in the cell-free culture filtrates of these two isolates (B9 and FLP7) as well as in media extracts. We detected two cytokinins: trans-zeatin and trans-zeatin ribosides in the culture filtrates of P. citrinum B9 and FLP7. Interestingly, B9 produced higher amounts of these cytokinins compared to their presence within FLP7 (Supplementary Figure S5c, upper and lower panels). Taken together, we infer that P. citrinum (B9 isolate) produces relatively higher amounts of the two cytokinins, which are secreted; in addition, it also generates active GA derivatives, which are together likely responsible for the cross-kingdom increase in growth in B9- and/or FLP7-inoculated Choy Sum plants. However, detailed analytical studies are warranted to obtain further conclusive insights into the biogenesis and transport/uptake of P. citrinum-derived phytohormones or derivatives that are capable of inducing growth in the Brassicaceae host Choy Sum.
Overall, we conclude that the beneficial isolates of P. citrinum transiently associate with the host root surface, and physical entry or root colonization per se is likely not mandated or required for the observed beneficial effects in Choy Sum. The fungus-derived phytohormones, GAs and cytokinins likely play a key role in promoting robust growth and increased biomass in Choy Sum, an economically important urban vegetable crop in Singapore and Asia.
4. Discussion
The mode of action of rhizosphere fungi on plant growth promotion is complex. A wide range of factors interacting with multiple targets characterizes the response of beneficial fungi in plant growth and development. Our results demonstrate that P. citrinum is a plant growth-promoting fungus (PGPF) on the green leafy vegetable Choy Sum. P. citrinum IR-3-3 from the sand dune has previously been described as a beneficial fungal isolate in cereal plants [38]. More importantly, the analyses of IR-3-3 culture filtrate reports the presence of bioactive GAs, which displays growth-promoting activities on Atriplex gemelinii. The study of P. citrinum B9 and FLP7 is, from our perspective, an extended demonstration of a fungal species from different origins that leads to growth in different plant hosts. As an urban farm crop, Choy Sum is simple to grow in outdoor conditions. Improved shoot and root biomass are observed when Choy Sum plants are inoculated with P. citrinum, while no discernible symptoms during fungal colonization within plant roots are observed, indicating a biotrophic and beneficial interaction. To investigate the potential mechanisms of P. citrinum induced plant growth promotion, we evaluate the phytohormones in a culture filtrate. Intriguingly, the presence of GA1, GA3 and GA20 is detected either in the culture filtrate of B9 or FLP7. GAs are major plant growth stimulators that regulate cell elongation. They also play critical roles in seed germination, stem elongation and floral transition [39,40]. Complementation analyses reveals three lines of evidence for the bioactive function of P. citrinum-derived GAs in plant growth and development: (1) the rescue of the dwarf and flowering-defective phenotypes of ga-1 mutant lines; (2) the upregulation of Arabidopsis plant size upon exogenous supply of fungal culture filtrate; and (3) the growth retardant by GA inhibitor to the Choy Sum inoculated with culture filtrates of B9 or FLP7. Thus, the growth-promoting effect of culture filtrate inoculation on plants highlights the bioactive GA production capacity of P. citrinum.
Our study adds to the emerging role and importance of mycobiota-derived phytohormones and/or derivatives that contribute to the functional aspects of growth benefits in the host plants. Many fungi have been shown to produce phytohormones such as auxin, jasmonates and/or GAs [19,22,30,41,42,43]. GAs play key roles in root colonization and are well known for their role in various developmental processes in plants, including germination and stem elongation [44]. Several studies have documented that production of IAA and GAs by P. citrinum and other Penicillium sp. were most effective in promoting plant growth [20,38]. Apart from GAs, the presence of cytokinins is also observed in the culture filtrate of P. citrinum, the other important phytohormones involved in maintaining cellular proliferation and differentiation. Zeatin production has been documented in Piriformospora indica and T. harzianum [45,46], and it has been shown that trans-zeatin cytokinin biosynthesis is crucial for P. indica-mediated growth stimulation in Arabidopsis [46]. This evidence suggests that P. citrinum could mediate plant growth and development at different stages by influencing the phytohormone balance in the host plants.
In addition to phytohormones, volatile secondary metabolites produced by rhizosphere microbes are one of the important chemical stimuli involved in plant growth promotion. In our study, we have shown the increased shoot and root biomass of Choy Sum, even in the absence of physical contact with P. citrinum. CO2 has been verified as a constituent of the plant growth-promoting volatiles produced by microorganisms in a hermetic system [47]. Interestingly, the enhanced plant biomass is not affected by the exclusion of CO2 in the hermetic system, suggesting a minor role for fungus-derived CO2 in plant growth promotion. Recent studies have identified the role of VOCs as the signaling molecules in the plant–fungus systems [34,48]. In general, VOCs are thought to be good candidates for below-ground communication due to their great diffusivity in soil [49]. Consistent with this, several fungal VOCs display growth-promoting effects on plants. For example, ectomycorrhizal fungus Laccaria bicolor produces sesquiterpenes (SQTs) as bioactive agents and promotes lateral root formation in Populus and Arabidopsis plants [50]. Our preliminary analysis using solid-phase microextraction coupled with GC-MS identified seven differentially produced volatile metabolites, including the sesquiterpene Longifolene-(V4) as one of the VOCs produced by P. citrinum. However, it remains to be confirmed whether the growth-promotion effect of P. citrinum in Choy Sum is caused by such volatile compound(s)/sesquiterpene. Interestingly, methyl salicylate (MeSA), which is a volatile plant defense compound produced in response to pathogen attacks [51] and plays phytohormone-like regulatory roles [52] was also detected in the P. citrinum-associated VOCs. MeSA is derived from SA by an SA carboxyl methyltransferase. A previous study confirmed that SA can regulate processes such as seed germination, vegetative growth and photosynthesis, in addition to its role as a regulatory signal mediating plant response to abiotic stresses such as drought and chilling [53]. Moreover, only a few compounds could be detected in the shared atmospheric space of P. citrinum. It is possible that most of these VOCs are produced in meagre amounts that are below the detection limits, and/or are labile and produced only during a specific developmental stage such as during direct physical interaction with plant roots. Newer methods could be utilized in the future for the capture and identification of such VOCs. Longer capture times such as 10–14 days could also be tested in future experiments. Lastly, much older, i.e., 2–3-week-old fungus could be harnessed for VOC collection to improve the quantity and quality of the emitted VOCs [54]. Although we have shown that VOCs produced by P. citrinum likely promote the host plant growth, their detailed mode(s) of action and ecological benefits have not been fully elucidated. Thus, further identification and functional characterization of growth-enhancing VOCs produced by P. citrinum will be necessary to fully understand such beneficial interactions in fungus–plant systems.
5. Conclusions
Under natural conditions, rhizosphere microbes have different interactions with the plant hosts, ranging from commensalism to mutualism. Reciprocally, plants can also shape the rhizosphere microbiome for their growth, development and abiotic and biotic stress tolerance. Although many studies have been conducted, the understanding of molecular mechanisms associated with the beneficial microbe and host interactions is still far from complete. Next-generation sequencing technology, combined with the development of metatranscriptomics, metaproteomics and metabolomics, will push forward the understanding between hosts and the rhizosphere microbes. In this study, we showed that beneficial P. citrinum isolates can promote growth in Choy Sum (an important urban crop for food and nutritional security) via nutrient assimilation, and via secreted phytohormone(s) and/or putative volatile compounds. We demonstrated that rhizosphere fungi can be considered as a useful bioresource, enhancing soil fertility and promoting sustainable plant growth. Integrating the knowledge of mycobiome community composition, beneficial microbial consortia, volatile signals and mutual interactions could aid in sustainable agriculture in urban settings. Future studies will be directed at understanding the physiology and mechanism-of-action of these fungal phytohormone(s) in such cross-kingdom growth promotion and resilience (if any) in other important food crops in traditional and urban agriculture.
Acknowledgments
We thank the Fungal Pathobiology group for useful discussions and suggestions. We thank Yu Hao for sharing the Arabidopsis ga1 mutant; and Prakash Kumar for providing the cytokinin standards. We would also like thank Zhang Hui and Liu Yu (NUS Environmental Research Institute, Singapore) for technical support in metabolite analysis.
Supplementary Materials
The following supplementary data are available online at www.mdpi.com/article/10.3390/jof9040420/s1. Figure S1: Morphometric and taxonomic identification of beneficial fungal isolates. Figure S2: Effect of P. citrinum strain FLP7 (and/or B9) on the growth of Choy Sum. Figure S3: Total Ion Chromatograms (TIC) for the chemical standards for two important classes of phytohormones i.e Gibberellins and Cytokinins. Figure S4: Multiple Reaction Monitoring (MRM) for gibberellins and cytokinins, together with the precursor ions and fragment ion transitions, respectively. Figure S5: MRM transitions for cytokinins showing the respective precursor ion and the fragment ion transitions. Figure S6: Overlay total ion chromatogram (TIC) for cell free exudates of P. citrinum FLP7 isolate showing three specific gibberellin derivatives. Table S1: Oligonucleotide primers used in this study. Table S2: Selected reaction monitoring conditions for protonated or deprotonated forms of the indicated plant hormones ([M+H]+ or [M−H]−). Table S3: List of volatile organic compounds emitted during P. citrinum-derived growth promotion of Choy Sum.
Author Contributions
K.G. and N.I.N. designed the experiments. K.G., C.-Y.C., S.P., P.S., W.Z. and Y.T.Y. performed the experiments; and P.S. co-wrote the manuscript with N.I.N. Whereas S.P. and C.-Y.C. helped in metabolite analyses and manuscript revision. K.G., C.-Y.C., S.S., P.S. and N.I.N. analyzed the data. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All datasets for this study are included in the manuscript and/or the Supplementary Files. All data that support the findings of this study are available from the corresponding authors upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
This work was supported by grants from the National Research Foundation (Prime Minister’s Office; NRF-CRP16-2015-04) to S.S. and N.I.N.; and by intramural funds from the Temasek Life Sciences Laboratory (Singapore) to N.I.N.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Poveda J., Zabalgogeazcoa I., Soengas P., Rodriguez V.M., Cartea M.E., Abilleira R., Velasco P. Brassica oleracea var. acephala (kale) improvement by biological activity of root endophytic fungi. Sci. Rep. 2020;10:20224. doi: 10.1038/s41598-020-77215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan W.K., Goenadie V., Lee H.W., Liang X., Loh C.S., Ong C.N., Tan H.T.W. Growth and glucosinolate profiles of a common Asian green leafy vegetable, Brassica rapa subsp. chinensis var. parachinensis (choy sum), under LED lighting. Sci. Hortic. 2020;261:108922. doi: 10.1016/j.scienta.2019.108922. [DOI] [Google Scholar]
- 3.Gupta S.K. Biotechnology of Crucifers. 1st ed. Springer; New York, NY, USA: 2013. [Google Scholar]
- 4.Zou L., Tan W.K., Du Y., Lee H.W., Liang X., Lei J., Striegel L., Weber N., Rychlik M., Ong C.N. Nutritional metabolites in Brassica rapa subsp. chinensis var. parachinensis (choy sum) at three different growth stages: Microgreen, seedling and adult plant. Food Chem. 2021;357:129535. doi: 10.1016/j.foodchem.2021.129535. [DOI] [PubMed] [Google Scholar]
- 5.Huang J.J., D’Souza C., Zhou W. Light-Time-Biomass Response Model for Predicting the Growth of Choy Sum (Brassica rapa var. parachinensis) in Soil-Based LED-Constructed Indoor Plant Factory for Efficient Seedling Production. Front. Plant Sci. 2021;12:623682. doi: 10.3389/fpls.2021.623682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poveda J., Díaz-González S., Díaz-Urbano M., Velasco P., Sacristán S. Fungal endophytes of Brassicaceae: Molecular interactions and crop benefits. Front. Plant Sci. 2022;13:932288. doi: 10.3389/fpls.2022.932288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poveda J., Rodríguez V.M., Díaz-Urbano M., Sklenář F., Saati-Santamaría Z., Menéndez E., Velasco P. Endophytic fungi from kale (Brassica oleracea var. acephala) modify roots-glucosinolate profile and promote plant growth in cultivated Brassica species. First description of Pyrenophora gallaeciana. Front. Microbiol. 2022;13:981507. doi: 10.3389/fmicb.2022.981507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiruma K., Kobae Y., Toju H. Beneficial associations between Brassicaceae plants and fungal endophytes under nutrient-limiting conditions: Evolutionary origins and host-symbiont molecular mechanisms. Curr. Opin. Plant Biol. 2018;44:145–154. doi: 10.1016/j.pbi.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Jiang L., Seo J., Peng Y., Jeon D., Park S.J., Kim C.Y., Kim P.I., Kim C.H., Lee J.H., Lee J. Genome insights into the plant growth-promoting bacterium Saccharibacillus brassicae ATSA2(T) AMB Express. 2023;13:9. doi: 10.1186/s13568-023-01514-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia R., Chen J., Hu L., Liu X., Xiao K., Wang Y. Alcaligenes faecalis Juj3 alleviates Plasmodiophora brassicae stress to cabbage via promoting growth and inducing resistance. Front. Sustain. Food Syst. 2022;6:942409. doi: 10.3389/fsufs.2022.942409. [DOI] [Google Scholar]
- 11.Vacheron J., Desbrosses G., Bouffaud M.L., Touraine B., Moenne-Loccoz Y., Muller D., Legendre L., Wisniewski-Dye F., Prigent-Combaret C. Plant growth-promoting rhizobacteria and root system functioning. Front. Plant Sci. 2013;4:356. doi: 10.3389/fpls.2013.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mabood F., Zhou X., Smith D.L. Microbial signaling and plant growth promotion. Can. J. Plant Sci. 2014;94:1051–1063. doi: 10.4141/cjps2013-148. [DOI] [Google Scholar]
- 13.Hyakumachi M. Plant-Growth-Promoting Fungi from Turfgrass Rhizosphere with Potential for Disease Suppression. Soil Microorg. 1994;44:53–68. doi: 10.18946/jssm.44.0_53. [DOI] [Google Scholar]
- 14.Hossain M.M., Sultana F., Miyazawa M., Hyakumachi M. Plant growth-promoting fungus Penicillium spp. GP15-1 enhances growth and confers protection against damping-off and anthracnose in the cucumber. J. Oleo Sci. 2014;63:391–400. doi: 10.5650/jos.ess13143. [DOI] [PubMed] [Google Scholar]
- 15.Shoresh M., Harman G.E., Mastouri F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 2010;48:21–43. doi: 10.1146/annurev-phyto-073009-114450. [DOI] [PubMed] [Google Scholar]
- 16.Hossain M., Sultana F., Islam S. Plant-Microbe Interactions in Agro-Ecological Perspectives. Springer; Singapore: 2017. Plant Growth-Promoting Fungi (PGPF): Phytostimulation and Induced Systemic Resistance; pp. 135–191. [Google Scholar]
- 17.Murali M., Naziya B., Ansari M.A., Alomary M.N., AlYahya S., Almatroudi A., Thriveni M.C., Gowtham H.G., Singh S.B., Aiyaz M., et al. Bioprospecting of Rhizosphere-Resident Fungi: Their Role and Importance in Sustainable Agriculture. J. Fungi. 2021;7:314. doi: 10.3390/jof7040314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin Z., Shi F., Jiang H., Roberts D.P., Chen S., Fan B. Phosphate solubilization and promotion of maize growth by Penicillium oxalicum P4 and Aspergillus niger P85 in a calcareous soil. Can. J. Microbiol. 2015;61:913–923. doi: 10.1139/cjm-2015-0358. [DOI] [PubMed] [Google Scholar]
- 19.Choi W.Y., Rim S.O., Lee J.H., Lee J.M., Lee I.J., Cho K.J., Rhee I.K., Kwon J.B., Kim J.G. Isolation of Gibberellins-Producing Fungi from the Root of Several Sesamum indicum Plants. J. Microbiol. Biotechnol. 2005;15:22–28. [Google Scholar]
- 20.Babu A.G., Kim S.W., Yadav D.R., Hyum U., Adhikari M., Lee Y.S. Penicillium menonorum: A Novel Fungus to Promote Growth and Nutrient Management in Cucumber Plants. Mycobiology. 2015;43:49–56. doi: 10.5941/MYCO.2015.43.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dovana F., Mucciarelli M., Mascarello M., Fusconi A. In Vitro Morphogenesis of Arabidopsis to Search for Novel Endophytic Fungi Modulating Plant Growth. PLoS ONE. 2015;10:e0143353. doi: 10.1371/journal.pone.0143353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waqas M., Khan A.L., Kamran M., Hamayun M., Kang S.M., Kim Y.H., Lee I.J. Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules. 2012;17:10754–10773. doi: 10.3390/molecules170910754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan Y., Luan Y., An L., Yu K. Arbuscular mycorrhizae formed by Penicillium pinophilum improve the growth, nutrient uptake and photosynthesis of strawberry with two inoculum-types. Biotechnol. Lett. 2008;30:1489–1494. doi: 10.1007/s10529-008-9691-8. [DOI] [PubMed] [Google Scholar]
- 24.Khalmuratova I., Kim H., Nam Y.J., Oh Y., Jeong M.J., Choi H.R., You Y.H., Choo Y.S., Lee I.J., Shin J.H., et al. Diversity and Plant Growth Promoting Capacity of Endophytic Fungi Associated with Halophytic Plants from the West Coast of Korea. Mycobiology. 2015;43:373–383. doi: 10.5941/MYCO.2015.43.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radhakrishnan R., Kang S.-M., Baek I.-Y., Lee I.-J. Characterization of plant growth-promoting traits of Penicillium species against the effects of high soil salinity and root disease. J. Plant Interact. 2014;9:754–762. doi: 10.1080/17429145.2014.930524. [DOI] [Google Scholar]
- 26.Selvaraj P., Shen Q., Yang F., Naqvi N.I. Cpk2, a Catalytic Subunit of Cyclic AMP-PKA, Regulates Growth and Pathogenesis in Rice Blast. Front. Microbiol. 2017;8:2289. doi: 10.3389/fmicb.2017.02289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soundararajan S., Jedd G., Li X., Ramos-Pamploña M., Chua N.H., Naqvi N.I. Woronin body function in Magnaporthe grisea is essential for efficient pathogenesis and for survival during nitrogen starvation stress. Plant Cell. 2004;16:1564–1574. doi: 10.1105/tpc.020677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng W., Zhou J., He Y., Xie Q., Chen A., Zheng H., Shi L., Zhao X., Zhang C., Huang Q., et al. Retromer Is Essential for Autophagy-Dependent Plant Infection by the Rice Blast Fungus. PLoS Genet. 2015;11:e1005704. doi: 10.1371/journal.pgen.1005704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan A., Hamayun M., Kim Y.-H., Kang S.-M., Lee J.-H., Lee I.-J. Gibberellins producing endophytic Aspergillus fumigatus sp. LH02 influenced endogenous phytohormonal levels, isoflavonoids production and plant growth in salinity stress. Process Biochem. 2011;46:440–447. doi: 10.1016/j.procbio.2010.09.013. [DOI] [Google Scholar]
- 30.Khan A.L., Hamayun M., Kang S.M., Kim Y.H., Jung H.Y., Lee J.H., Lee I.J. Endophytic fungal association via gibberellins and indole acetic acid can improve plant growth under abiotic stress: An example of Paecilomyces formosus LHL10. BMC Microbiol. 2012;12:3. doi: 10.1186/1471-2180-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrison E.N., Knowles S., Hayward A., Thorn R.G., Saville B.J., Emery R.J.N. Detection of phytohormones in temperate forest fungi predicts consistent abscisic acid production and a common pathway for cytokinin biosynthesis. Mycologia. 2015;107:245–257. doi: 10.3852/14-157. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y., Zhang H., Umashankar S., Liang X., Lee H.W., Swarup S., Ong C.N. Characterization of Plant Volatiles Reveals Distinct Metabolic Profiles and Pathways among 12 Brassicaceae Vegetables. Metabolites. 2018;8:94. doi: 10.3390/metabo8040094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hiruma K., Gerlach N., Sacristan S., Nakano R.T., Hacquard S., Kracher B., Neumann U., Ramirez D., Bucher M., O’Connell R.J., et al. Root Endophyte Colletotrichum tofieldiae Confers Plant Fitness Benefits that Are Phosphate Status Dependent. Cell. 2016;165:464–474. doi: 10.1016/j.cell.2016.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hung R., Lee S., Bennett J.W. Arabidopsis thaliana as a model system for testing the effect of Trichoderma volatile organic compounds. Fungal Ecol. 2013;6:19–26. doi: 10.1016/j.funeco.2012.09.005. [DOI] [Google Scholar]
- 35.Naznin H.A., Kimura M., Miyazawa M., Hyakumachi M. Analysis of volatile organic compounds emitted by plant growth-promoting fungus Phoma sp. GS8-3 for growth promotion effects on tobacco. Microbes Environ. 2013;28:42–49. doi: 10.1264/jsme2.ME12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jalali F., Zafari D., Salari H. Volatile organic compounds of some Trichoderma spp. increase growth and induce salt tolerance in Arabidopsis thaliana. Fungal Ecol. 2017;29:67–75. doi: 10.1016/j.funeco.2017.06.007. [DOI] [Google Scholar]
- 37.Fletcher R.A., Sopher C.R., Vettakkorumakankav N.N. Modulation of gibberellins protects plants from environmental stresses. Indian J. Plant Physiol. 2000;5:115–126. [Google Scholar]
- 38.Khan S.A., Hamayun M., Yoon H., Kim H.Y., Suh S.J., Hwang S.K., Kim J.M., Lee I.J., Choo Y.S., Yoon U.H., et al. Plant growth promotion and Penicillium citrinum. BMC Microbiol. 2008;8:231. doi: 10.1186/1471-2180-8-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwechheimer C. Gibberellin signaling in plants—The extended version. Front. Plant Sci. 2011;2:107. doi: 10.3389/fpls.2011.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta R., Chakrabarty S.K. Gibberellic acid in plant: Still a mystery unresolved. Plant Signal. Behav. 2013;8:e25504. doi: 10.4161/psb.25504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hasan H.A.H. Gibberellin and auxin production by plant root-fungi and their biosynthesis under salinity-calcium interaction. Plant Soil Environ. 2002;48:101–106. doi: 10.17221/4207-PSE. [DOI] [PubMed] [Google Scholar]
- 42.Nassar A.H., El-Tarabily K.A., Sivasithamparam K. Promotion of plant growth by an auxin-producing isolate of the yeast Williopsis saturnus endophytic in maize (Zea mays L.) roots. Biol. Fertil. Soils. 2005;42:97–108. doi: 10.1007/s00374-005-0008-y. [DOI] [Google Scholar]
- 43.Rim S.O., Lee J.H., Choi W.Y., Hwang S.K., Suh S.J., Lee I.J., Rhee I.K., Kim J.G. Fusarium proliferatum KGL0401 as a New Gibberellin-Producing Fungus. J. Microbiol. Biotechnol. 2005;15:809–814. [Google Scholar]
- 44.Claeys H., De Bodt S., Inze D. Gibberellins and DELLAs: Central nodes in growth regulatory networks. Trends Plant Sci. 2014;19:231–239. doi: 10.1016/j.tplants.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Martinez-Medina A., Roldan A., Albacete A., Pascual J.A. The interaction with arbuscular mycorrhizal fungi or Trichoderma harzianum alters the shoot hormonal profile in melon plants. Phytochemistry. 2011;72:223–229. doi: 10.1016/j.phytochem.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 46.Vadassery J., Ritter C., Venus Y., Camehl I., Varma A., Shahollari B., Novak O., Strnad M., Ludwig-Muller J., Oelmuller R. The role of auxins and cytokinins in the mutualistic interaction between Arabidopsis and Piriformospora indica. Mol. Plant Microbe Interact. 2008;21:1371–1383. doi: 10.1094/MPMI-21-10-1371. [DOI] [PubMed] [Google Scholar]
- 47.Kai M., Piechulla B. Plant growth promotion due to rhizobacterial volatiles--an effect of CO2? FEBS Lett. 2009;583:3473–3477. doi: 10.1016/j.febslet.2009.09.053. [DOI] [PubMed] [Google Scholar]
- 48.Müller A., Faubert P., Hagen M., Zu Castell W., Polle A., Schnitzler J.P., Rosenkranz M. Volatile profiles of fungi—Chemotyping of species and ecological functions. Fungal Genet. Biol. 2013;54:25–33. doi: 10.1016/j.fgb.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 49.Hiltpold I., Turlings T.C. Belowground chemical signaling in maize: When simplicity rhymes with efficiency. J. Chem. Ecol. 2008;34:628–635. doi: 10.1007/s10886-008-9467-6. [DOI] [PubMed] [Google Scholar]
- 50.Ditengou F.A., Muller A., Rosenkranz M., Felten J., Lasok H., van Doorn M.M., Legue V., Palme K., Schnitzler J.P., Polle A. Volatile signalling by sesquiterpenes from ectomycorrhizal fungi reprogrammes root architecture. Nat. Commun. 2015;6:6279. doi: 10.1038/ncomms7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kobayashi K. Plant methyl salicylate induces defense responses in the rhizobacterium Bacillus subtilis. Environ. Microbiol. 2015;17:1365–1376. doi: 10.1111/1462-2920.12613. [DOI] [PubMed] [Google Scholar]
- 52.Gaspar T., Kevers C., Penel C., Greppin H., Reid D.M., Thorpe T.A. Plant hormones and plant growth regulators in plant tissue culture. In Vitro Cell. Dev. Biol.—Plant. 1996;32:272–289. doi: 10.1007/BF02822700. [DOI] [Google Scholar]
- 53.Rivas-San Vicente M., Plasencia J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011;62:3321–3338. doi: 10.1093/jxb/err031. [DOI] [PubMed] [Google Scholar]
- 54.Lee S., Hung R., Yap M., Bennett J.W. Age matters: The effects of volatile organic compounds emitted by Trichoderma atroviride on plant growth. Arch. Microbiol. 2015;197:723–727. doi: 10.1007/s00203-015-1104-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets for this study are included in the manuscript and/or the Supplementary Files. All data that support the findings of this study are available from the corresponding authors upon reasonable request.