Abstract
Vaginal dysbiosis can lead to serious infections in asymptomatic women. Lactobacillus probiotics (LBPs) are being investigated as a promising therapy for reversing vaginal microbiota dysbiosis. This study aimed to investigate whether administering LBPs could improve vaginal dysbiosis and facilitate the colonization of Lactobacillus species in asymptomatic women. 36 asymptomatic women were classified based on the Nugent score as Low-NS (n = 26) and High-NS (n = 10) groups. A combination of Lactobacillus acidophilus CBT LA1, Lactobacillus rhamnosus CBT LR5, and Lactobacillus reuteri CBT LU4 was administered orally for 6 weeks. The study found that among women with a High-NS, 60% showed improved vaginal dysbiosis with a Low-NS after LBP intake, while four retained a High-NS. Among women with a Low-NS, 11.5 % switched to a High-NS. Genera associated with vaginal dysbiosis were positively correlated with the alpha diversity or NS, while a negative correlation was observed between Lactobacillus and the alpha diversity and with the NS. Vaginal dysbiosis in asymptomatic women with an HNS improved after 6 weeks of LBP intake, and qRT-PCR revealed the colonization of Lactobacillus spp. in the vagina. These results suggested that oral administration of this LBP could improve vaginal health in asymptomatic women with an HNS.
Keywords: Lactobacillus, probiotics, cervicovaginal fluid, vaginal microbiota, vaginal dysbiosis, bacterial vaginosis, Nugent score
1. Introduction
The composition of the vaginal microbiota is associated with beneficial or detrimental effects on vaginal health [1,2]. Vaginal microbiota ecology, especially the high relative abundance of Lactobacillus spp., is considered crucial for vaginal health in preventing the invasion of pathogens and decreasing susceptibility to gynecological infections, such as sexually transmitted infections (STIs), including bacterial vaginosis (BV) [3,4,5]. Lactobacillus spp. produces lactic acid via metabolic processes, thus, contributes to an acidic environment (low pH) in the vagina, where they exert antimicrobial, antiviral, and immunomodulatory effects [6]. Lactobacillus crispatus, Lactobacillus gasseri, Lactobacillus iners, and Lactobacillus jensenii are the most frequently detected microbial species in a healthy vagina and may be involved in the prevention of pathogenic infections by maintaining richness in the vaginal microbiota’s abundance [7]. A high abundance of Lactobacillus spp. in the vaginal microbiota indicates vaginal eubiosis and is associated with a healthy vaginal environment [8,9]. In contrast, vaginal dysbiosis is characterized by decreased levels of Lactobacillus spp. and increased levels of anaerobic bacteria, primarily Gardnerella spp., Atopobium spp., Prevotella spp., and Ureaplasma spp. [10,11,12]. Vaginal dysbiosis dynamics can lead to a significant increase in the risk of BV, preterm birth, and urinary tract infections [13,14,15,16]. In terms of BV, the following eight genera have been reported to be associated with vaginal dysbiosis: Gardnerella, Atopobium, Megasphaera, Eggerthella, Aerococcus, Leptotrichia/Sneathia, Prevotella, and Papillibacter [17].
Probiotics are “living microorganisms” that have beneficial health effects when consumed in appropriate amounts, as defined by the World Health Organization and the United Nations Food and Agriculture Organization in 2002 [18,19]. Probiotic intake plays a role in maintaining and improving the diversity and dynamics of the gut microbiota profile [20,21]. In addition, probiotics prevent diseases through functions such as stabilizing the gut microbiota, generating short-chain fatty acids, and inhibiting the settlement of pathogenic microbiota [22]. The gut microbiota significantly influences metabolic pathways of distant organs, including reproductive homeostasis [23,24,25]. Although the gut microbiota and vaginal microbiota represent two different ecosystems, the gut microbiota is considered an extravaginal reservoir that might influence the risk of vaginal dysbiosis through dysbiotic gut microbiota [26]. Specific probiotics, such as Lactobacillus probiotics (LBPs), are suggested to have safe and promising therapeutic effects to modulate microbiota homeostasis in the general population [27]. Studies have shown that oral LBP intake not only improves vaginal microbiota dysbiosis significantly, but also improves leucorrhea, itching, and vulvo-vaginal erythema/edema [28,29]. In addition, Lactobacillus surface active molecules (peptidoglycan, lipoteichoic acid, exopolysaccharides, etc.) antagonize the pathogenic microbes [30]. Therefore, specific LBP combinations could be novel treatments against pathogenic microbes that cause vaginal dysbiosis by increasing Lactobacillus abundance.
Symptomatic vaginal dysbiosis, such as the STI BV, is characterized by symptoms such as vulvovaginal itching, burning, irritation, bad odor, rashes, unusual discharge, and pain in the vagina [5]. In addition, approximately half of BV-positive women have no clear symptoms and are considered to have asymptomatic vaginal dysbiosis [31,32]. The Nugent score (NS) is used as the gold standard tool for screening for asymptomatic BV and identifying suitable treatments, such as antibiotics [33,34]. Limited data exist concerning the effect of LBPs and effectiveness of molecular-based diagnosis methods, such as STI-PCR and 16S rRNA amplicon sequencing, in asymptomatic vaginal dysbiosis [35,36,37,38]. To obtain accurate information on pathogenicity, molecular-based diagnostic validation can better confirm a diagnosis and improve understanding of how to manage a microbial dysbiosis. In the present study, it was hypothesized that LBP intake could improve vaginal dysbiosis and facilitate the colonization of Lactobacillus spp. in the vagina in women with asymptomatic vaginal dysbiosis. To investigate whether the LBP modulates vaginal dysbiosis and changes the microbiota dynamics to improve vaginal dysbiosis and maintain a normal vaginal environment, the NS was used to first categorize the study subjects. Then, 16S rRNA gene amplicon microbiota analysis (NGS) and quantitative real-time polymerase chain reaction (qRT-PCR) were performed for validation of the vaginal microbiota in cervicovaginal fluid (CVF) samples of women with asymptomatic vaginal dysbiosis.
2. Materials and Methods
2.1. Enrolled Subject Criteria and Sample Collection
Overall, 57 premenopausal women aged between 19 and 55 years old who visited the Obstetrics and Gynecology outpatient clinic or the Health Examination Center at Ewha Womans University Mokdong Hospital from June 2021 to September 2021 were enrolled. Several subjects were excluded based on the following exclusion criteria: undergoing immune or hormonal therapy; taking probiotics or antibiotics; having a history of alcohol or drug addiction; or being at risk of pregnancy. Finally, after counseling, women who agreed to participate in the trial/study provided written informed consent prior to enrolment.
The CVF samples of the participants were collected using an NBG-S5V Swab kit (Noble Biosciences, Suwon-si, Gyeonggi-Do, Republic of Korea) by swabbing the exocervix, and the swabs were then dipped into buffer. Collected samples were immediately transferred to a laboratory and stored at −80 °C for further microbiome analysis. The CVF samples were stored under strict regulations for research and analysis institutes.
The Institutional Research Board (IRB) of Ewha Womans University (Mokdong Hospital, Seoul, Republic of Korea) approved this prospective study (IRB approval no. 2020-11-035-007) of LBP oral intake by healthy women with asymptomatic vaginal dysbiosis. The study was conducted in accordance with the approved guidelines.
2.2. Probiotic Combination and Intervention
The LBP used in the study contained a combination of Lactobacillus acidophilus CBT LA1 (LA1, KCTC 11906BP), Lactobacillus rhamnosus CBT LR5 (LR5, KCTC 12202BP), and Lactobacillus reuteri CTB LU4 (LU4, KCTC 12397BP) strains isolated from Korean human feces by Cell Biotech (Gimpo-si, Republic of Korea). The CBT of LA1, LR5, and LU4 was prepared with dextrose, fructooligosaccharide, xylitol, pomegranate powder, pomegranate spice, malic acid, corn starch, and Starch 1500, which are known as safe materials used in the preparation of various asymptomatic functional foods.
The intake method was one packet of probiotics (2 g) per day, which contained 1 × 1010 CFU of total bacterial strains, either directly or with water. After the collection of CVF on the first visit (the initial visit), probiotics were administered for 3 weeks, and Gram staining, NGS, and qRT-PCR were performed. After 3 weeks, i.e., on the second visit (mid-visit), CVF samples were again collected, probiotics were administered for another 3 weeks, and Gram staining, NGS, and qRT-PCR were performed on the collected sample. Finally, on the third visit (the final visit), which was the endpoint of the study, CVF samples were collected, and Gram staining, NGS, qRT-PCR, and STI-PCR (only on the third visit) were performed on the collected sample. In addition, on the second and third visits, the subject’s medication compilation was recorded after LBP intake.
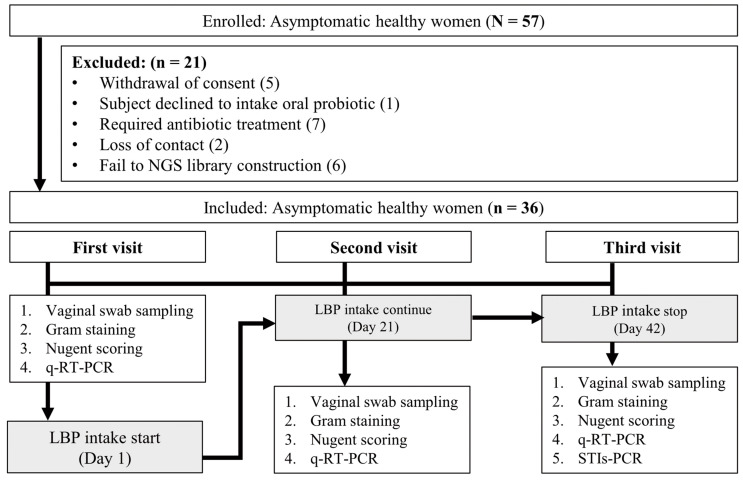
The study subjects who were diagnosed with vaginitis according to the Gram stain results of the CVF sample collected on the first visit and ones who needed treatment were subjected to additional testing with the genital mycoplasma culture or culture and sensitivity test. The subjects were then guided to appropriate treatment and were not included in the study. In total, 142 samples were collected from 57 women across three visits; however, only 108 samples from 36 women were included in the final analysis (Figure 1).
Figure 1.
Flow chart of subject analysis. qRT-PCR, quantitative real-time polymerase chain reaction; LBP, Lactobacillus probiotic; STI-PCR, sexually transmitted infection polymerase chain reaction.
2.3. Gram Staining and NS
For Gram staining, one drop of CVF sample was smeared on a glass slide, dried, stained with crystal violet for 1–2 min, and bleached, after which the slides were observed under a microscope for the presence of gram-positive bacteria. The presence and type of microorganisms in the vagina were examined to compare the microbiota present before and after LBP intake. Therefore, not only were changes in the microorganisms in the vagina noted, but also whether the vaginal environment was asymptomatic or more vulnerable to vaginitis or other infections/diseases. Slide smears were examined by three independent microbiologists who scored and interpreted the slides independently using the NS method. Using the Gram stain of CVF samples, the NS was applied as follows: 0–3, normal; 4–6, intermediate; and 7–10, BV. Based on the NS at first visit, the study subjects with a Low NS (LNS ≤ 3) were designated as Group A (normal group), and those with a High NS (HNS ≥ 4–10) were designated as Group B (abnormal group).
2.4. DNA Extraction and STI-PCR
For STI-PCR, the CVF samples were vigorously agitated in a buffer to dislodge the cells. Microbial DNA was extracted using the FastDNA SPIN Kit for Soil (MP Biochemicals, Santa Ana, CA, USA) according to the manufacturer’s instructions. The extracted microbial DNA was purified using a DNeasy PowerClean Pro Cleanup Kit (Qiagen, Hilden, Germany), and the DNA quality was assessed using a QuickDrop (Molecular Devices, San Jose, CA, USA). The concentration of the purified DNA was measured using the Qubit dsDNA BR Assay kit (Thermo Fisher Scientific, Waltham, MA, USA).
2.5. V4–V5 Targeted 16S rRNA Gene Sequencing
Sequencing of 16S rRNA was performed to determine the relative abundance of Lactobacillus spp. and to assess the shift in the presence and type of each bacterial species before and after LBP intake. Extracted DNA was used for PCR amplification. The amplicon (5 µL) of each participant was subjected to electrophoretic separation on a 1.5% agarose gel (Cosmogentech, Ltd., Seoul, Republic of Korea), and the product (~490 bp) was visualized under UV light (Daihan Scientific, Ltd., Wonju, Republic of Korea). A sequencing library with subsequent steps (purification, sample indexing, sample quantification, and pooling) was prepared according to the Illumina 16S metagenomic sequencing library preparation guide (Illumina, San Diego, CA, USA). The V4–V5 region of the bacterial 16S rRNA gene was amplified using 16S rRNA gene sequencing using the following primers: a forward primer in the V4 region (CCA GCM GCC GCG GTA ATW C) and a reverse primer in the V5 region (CC GTC AAT TYY TTT RAG TTT). The amplified sequencing library was purified using Agencourt® AMPure XP beads (Beckman Coulter, Brea, CA, USA), and the quality of the library was assessed using a 2100 Bioanalyzer (Agilent, Santa Clara, CA, USA). The library pool was sequenced with 250 bp paired-end reads on the MiSeq platform (Illumina, San Diego, CA, USA) using the MiSeq reagent kit V2 (Illumina, San Diego, CA, USA).
2.6. qRT-PCR
qRT-PCR was performed to confirm whether the three Lactobacillus spp. in the LBP colonized the vagina or not. After culturing the three bacterial strains that were ingested by the subject, the cells were counted and used as a standard for the experiment. Then, DNA was extracted using the FastDNA SPIN Kit for Soil (MP Bio-chemicals) following the manufacturer’s instructions. The extracted microbial DNA was purified using the DNeasy PowerClean Pro Cleanup Kit (Qiagen), and the DNA quality was assessed using QuickDrop (Molecular Devices). The concentration of the purified DNA was measured using the Qubit dsDNA BR Assay kit (Thermo Fisher Scientific). Purified DNA was used as a qRT-PCR standard. Values of cells/mL were converted to DNA concentration values (cells/ng) and used for calculations. Prime Q-Master mix (Genet Bio, Chungnam, Republic of Korea) was used for qRT-PCR. The primers used for qRT-PCR are listed in Supplementary Table S1 and the amplification conditions are listed in Supplementary Table S2.
2.7. Data Processing and Statistical Analysis
Targeted sequencing of the V4–V5 region of the microbiota was performed using Illumina MiSeq. Raw sequencing data were processed using the Quantitative Insight Into Microbial Ecology software package 2 (QIIME2, v2021.11, http://qiime2.org, accessed on 6 February 2023). Denoising was performed using DADA2 and a taxonomy table was created using the SILVA database (v138). Data were normalized to a depth of 14,000, which was the minimum depth of the sample that was used for alpha (amplicon sequence variants [ASVs], Shannon diversity, and Pielou’s evenness) and beta diversity analyses. The results following data processing, analysis, and visualization were analyzed using the ggplot2 package of R (v4.1.3), and statistical analysis was performed using the Wilcoxon rank-sum test, Wilcoxon signed-rank test, Kruskal–Wallis test, Mann–Whitney test, and PERMANOVA using the vegan package. Linear discriminant analysis effect size was performed using Galaxy (https://huttenhower.sph.harvard.edu/galaxy. accessed on 6 February 2023). The data were then filtered and normalized. For clinical parameter statistical analysis, the two-tailed p-value was applied, and a p-value < 0.05 was considered to be significant.
3. Results
3.1. Demographic Profile, Gram Stain, and NS
A total of 57 asymptomatic women aged 19–55 years were enrolled in this study. Of the 57 subjects, five withdrew their consent, one declined to take the oral LBP, seven had to be treated with antibiotics, two were lost to follow-up, and six had samples that failed during NGS library construction. Therefore, only 36 women were included in the final analysis. The study subjects with an LNS ≤ 3 were designated as the normal group, and those with an HNS ≥ 4–10 were designated as the abnormal group, after analysis of the NS of the Gram stain results performed at the first visit (Table 1). The median age was 41.0 years, and the median BMI was 23.2 on the first visit for all 36 subjects. There were no significant differences in age or BMI between the two groups. In the LNS group (n = 26), four women had positive gram stains on the second visit, and three women had positive gram stains on the third visit, whereas in the HNS group (n = 10), four women had positive gram stains on the second and third visits (Table 1). There was a 60% reduction in the HNS group (n = 6) after LBP intake. Concerning the NS, at the first visit, the difference was significantly high (p < 0.001) between groups, while the difference decreased at the second visit (p < 0.04) and the third visit (p < 0.06) with the decrement of the NS after LBP intake.
Table 1.
Clinical characteristics of asymptomatic women after Nugent scoring.
| Parameters | Women with an LNS (n = 26) |
Women with an HNS (n = 10) |
p Value |
|---|---|---|---|
| Age (Median) | 40.50 (11) | 46.50 (11) | NSD |
| BMI (Median) | 22.71 (3.71) | 24.88 (4.86) | NSD |
| Gram stain (number of positive subjects) | |||
| First visit | 0 | 10 | <0.05 |
| Second visit | 4 | 4 | NSD |
| Third visit | 3 | 4 | NSD |
| Nugent score (Mean ± SD) | |||
| First visit | 0.81 ± 0.94 | 5.70 ± 1.83 | <0.001 * |
| Second visit | 1.54 ± 1.98 | 2.80 ± 2.44 | <0.04 * |
| Third visit | 1.69 ± 1.91 | 3.80 ± 3.33 | NSD * |
BMI, body mass index; LNS, Low Nugent score (LNS ≤ 3); HNS, High Nugent score (HNS ≥ 4–10); NSD, no significant difference. * Kruskal–Wallis test.
3.2. Alpha Diversity and Vaginal Microbiota Taxonomy of the Normal and Abnormal Groups
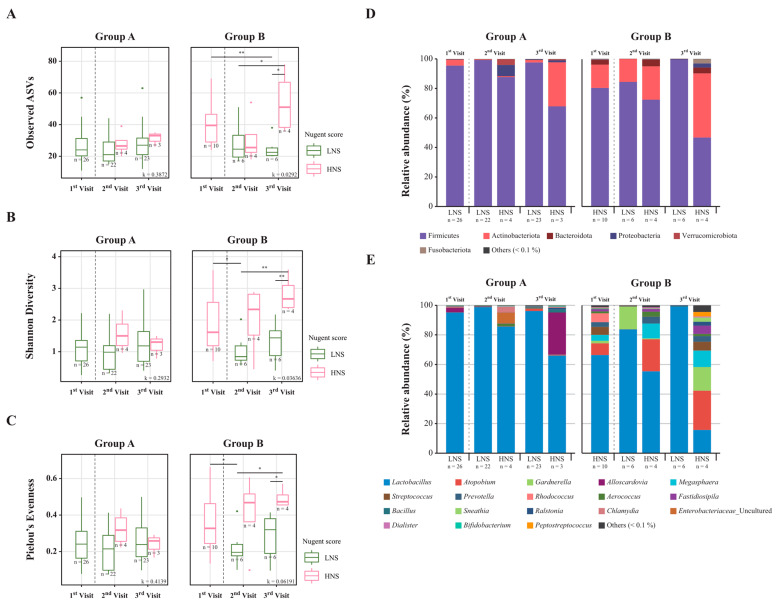
Amplicon sequencing of 16S rRNA was performed to compare the taxonomic composition of the vaginal microbiota in the samples collected on the first, second, and third visits. Alpha diversity was measured using three indices: observed ASVs, Shannon diversity, and Pielou’s evenness, at the different visits. At the initial visit, samples classified as LNS were categorized into Group A, and samples classified as HNS were categorized into Group B. It was confirmed that in Group B, samples with a decreased NS (samples that changed from an HNS to an LNS) showed a significant difference in alpha diversity as the number of visits increased. Furthermore, it was observed that the alpha diversity values of 60% of the samples that changed from an HNS in Group B to an LNS became similar to those of Group A; however, for the LNS, there was no significant difference between the visits at any indices of alpha diversity (Figure 2A–C). Observably, the indices of alpha diversity decreased significantly in six women with an HNS who were classified as having an LNS on the second and third visits (Figure 2A–C).
Figure 2.
Changes in alpha diversity and taxonomy composition for each visit in the two groups divided by the Nugent score of the first visit. Boxplot showing (A) observed amplicon sequence variants (ASVs), (B) Shannon diversity, and (C) Pielou’s evenness in Group A and B. Relative abundance at the (D) phylum and (E) genus levels in Group A and B. Each group was classified as LNS or HNS based on the NS at the first visit. * p < 0.05, ** p < 0.01 (Wilcoxon rank-sum test). An average of <1% was labeled as other.
The taxonomic composition of six phyla in the vaginal microbiota: Firmicutes, Actinobacteria, Bacteroidota, Proteobacteria, Verrucomicrobiota, and Fusobacteriota, was observed (>1%) at each visit. It was found that Firmicutes were the dominant bacteria in most of the vaginal samples analyzed. Additionally, it was observed that Actinobacteriota were predominantly dominant in HNS samples (Figure 2D). At the genus level, 17 genera: Lactobacillus, Atopobium, Megasphaera, Prevotella, Alloscardovia, Rhodococcus, Gardnerella, Streptococcus, Bacillus, Aerococcus, Fastidiosipila, Enterobacteriaceae, Ralstonia, Chlamydia, Sneathia, Dialister, and Peptostreptococcus, were observed (>1%) on all visits (Figure 2E). The relative abundance of the genus Lactobacillus belonging to the phylum Firmicutes was assessed in all samples from Groups A and B. After LBP oral intake, in Group B, six samples that changed from HNS to LNS were dominated by Lactobacillus at the second and third visits, while three samples in Group A that changed from LNS to HNS at the third visit showed that the relative abundance of Lactobacillus decreased and the ecosystem appeared destroyed, but alpha diversity did not increase significantly (Figure 2E).
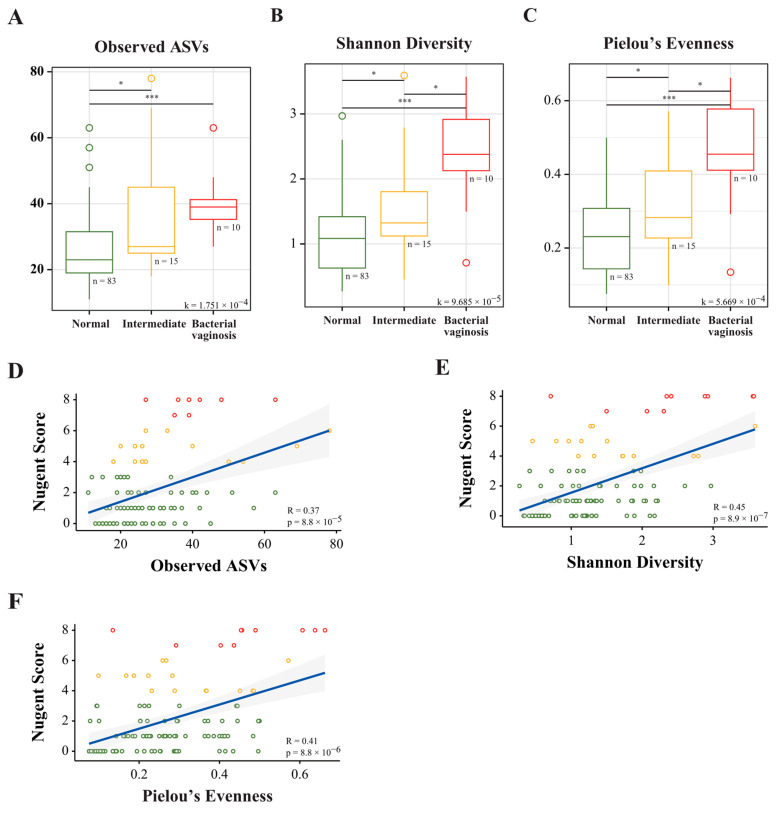
3.3. Correlation between NS and the Vaginal Microbiota Taxonomy of Normal, Intermediate, and BV Groups
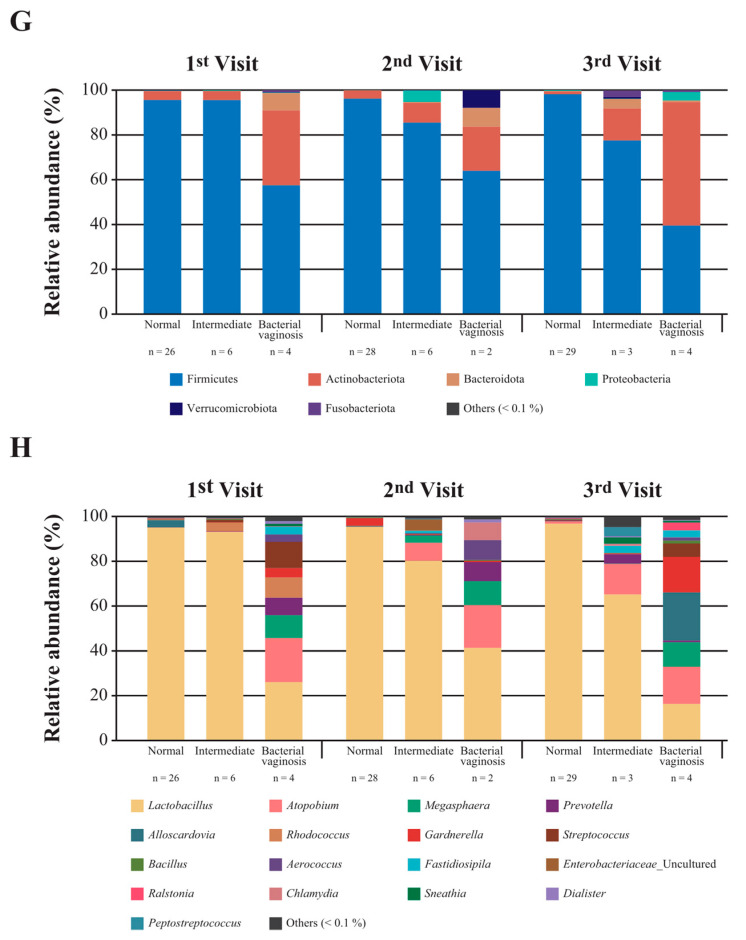
The correlation between alpha diversity and the NS was analyzed to compare the composition of microbiota as assessed by the NS and 16S rRNA amplicon sequencing. The HNS group was further classified into intermediate and BV groups, and the LNS group was considered the normal group. The mean alpha diversity indices of the intermediate and BV groups were significantly different from those of the LNS group (p < 0.05; Figure 3A–C). Moreover, there was a positive correlation between the NS and alpha diversity for each index of alpha diversity (p < 0.05; Figure 3D–F). At the phylum level, a high abundance of Firmicutes (~>80–90%) was observed in the normal and intermediate groups compared to that in the BV group (>40–60%) (Figure 3G). At the genus level, Lactobacillus was dominant at >90% and slightly improved among visits in the normal group after LBP intake. The abundance of Lactobacillus in the intermediate group was ~70–90%, while that in the BV group was ~20–40% due to the increased abundance of other genera across all visits compared with that in the normal group (Figure 3H). The genera Atopobium, Megasphaera, Prevotella, Gardnerella, and Streptococcus were highly distributed in the BV group (Figure 3H).
Figure 3.
Alpha diversity and correlation between the Nugent score and alpha diversity, and the taxonomic composition of the vaginal microbiota between groups at different visits. Boxplot illustrating the (A) observed amplicon sequence variants (ASVs), (B) Shannon diversity, and (C) Pielou’s evenness. Spearman’s correlation between the Nugent score with the (D) observed ASVs, (E) Shannon diversity, and (F) Pielou’s evenness. Relative abundance by visit order at the (G) phylum and (H) genus levels. * p < 0.05, *** p < 0.001 (Wilcoxon rank-sum test).
3.4. Correlation between Alpha Diversity and the NS with Dominant Vaginal Microbiota
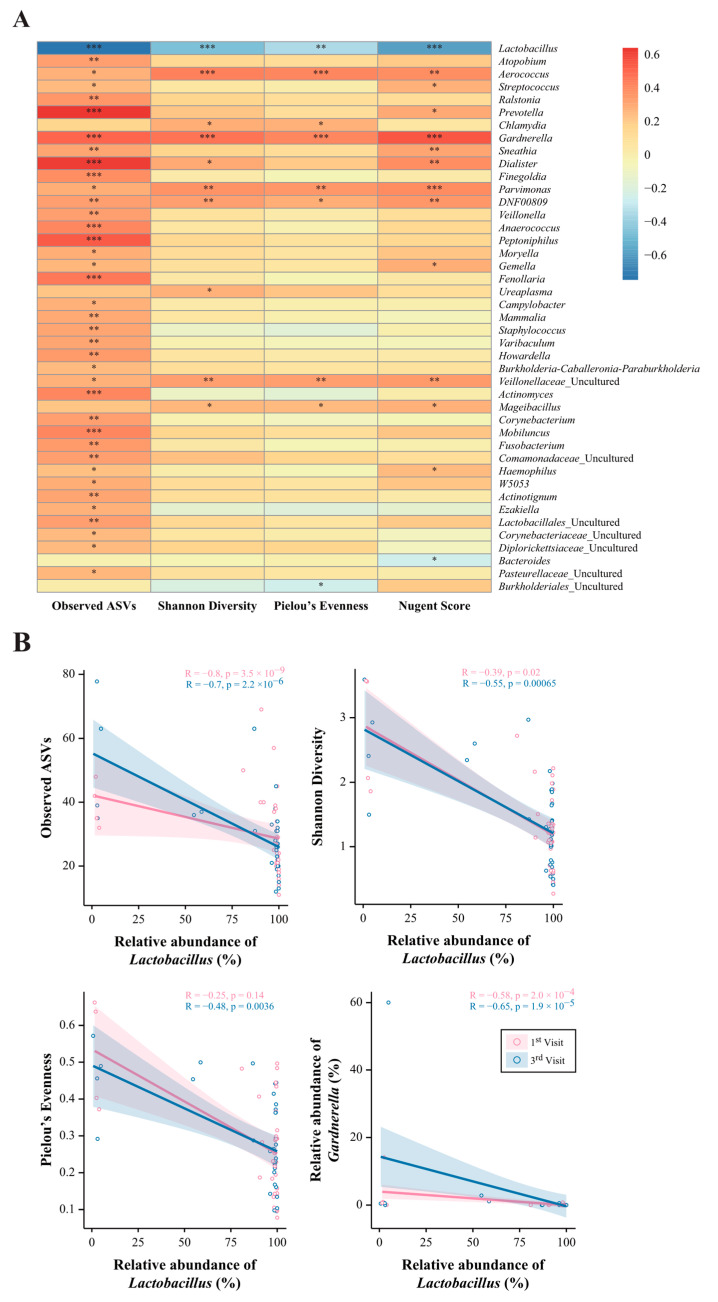
A correlation analysis was performed between the parameters alpha-diversity, NS, and vaginal microbiota, and a heatmap was constructed. A positive correlation was observed between all indices of alpha diversity and dominant vaginal microbiota, such as Aerococcus, Gardnerella, Parvimonas, DNF00809, Veillonellaceae, and Mageibacillus. In addition, genera Prevotella, Sneathia, Dialister, Anaerococcus, Peptoniphilus, Fenollaria, Actinomyces, and Mobiluncus showed a highly significant positive correlation for the ASV index of alpha diversity only (p < 0.01). As well as Aerococcus, Prevotella, Gardnerella, Sneathia, Dialister, Parvimonas, DNF00809, Veillonellaceae, and Mageibacillus, three genera, Streptococcus, Gemella, and Haemophilus, showed a significant positive correlation with the NS (p < 0.05). As expected, Lactobacillus showed a significant negative correlation with all indices of alpha diversity and the NS (p < 0.05) (Figure 4A). Concerning a highly significant negative correlation of beneficial Lactobacillus and a highly significant positive correlation of pathogenic Gardnerella with alpha diversity and NS, correlation analysis was performed with the first and third visits (p < 0.001) (Figure 4B). The correlation between Lactobacillus and parameters, including Shannon diversity, Pielou’s evenness, and Gardnerella, at the third visit showed much greater significance than that at the first visit. It was evident that LBP intake resulted in a significant effect on asymptomatic vaginal dysbiosis (Figure 4B).
Figure 4.
Correlation between parameters, including alpha diversity, Nugent score, and vaginal microbiota at the first and third visits. (A) Heatmap showing the correlation between parameters and the vaginal microbiota. (B) The correlation between parameters and the abundance of Lactobacillus at the first and third visits. * p < 0.05, ** p < 0.01, *** p < 0.001.
3.5. Beta Diversity and Microbiota Shift after 6 Weeks of LBP Intake
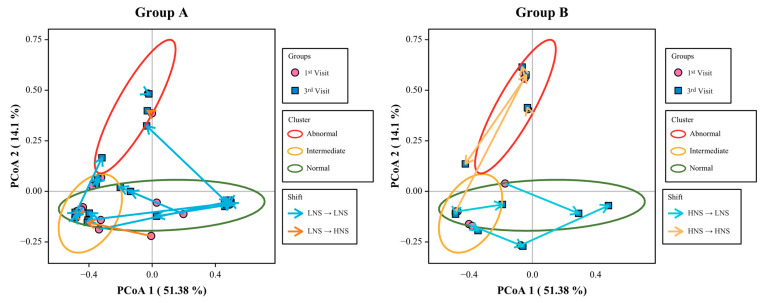
Changes in the vaginal microbiota composition between the normal, intermediate, and BV groups were analyzed after six weeks of LBP intake using Bray–Curtis dissimilarity. Clusters were observed to demonstrate dissimilarity between the normal, intermediate, and BV groups (Supplementary Figure S1). In the group-wise analysis of the first and third visits, a pattern was observed after LBP intake; a shift from LNS to HNS clusters was observed in two of the 26 women in the LNS group (normal) (Figure 5A). Whereas in the HNS (abnormal), six of the 10 women showed a shift from HNS to LNS clusters (Figure 5B). The arrow represents the shift from the first to the third visit.
Figure 5.
Beta diversity and shift after 6 weeks of LBP intake. The principal coordinate analysis illustrates the beta diversity shift in Group A and B. Arrows represent the shift from the first visit to the third visit. Group A, LNS at first visit; Group B, HNS at first visit. PCoA, principal coordinate analysis.
3.6. Quantitative Expression of Lactobacillus Species
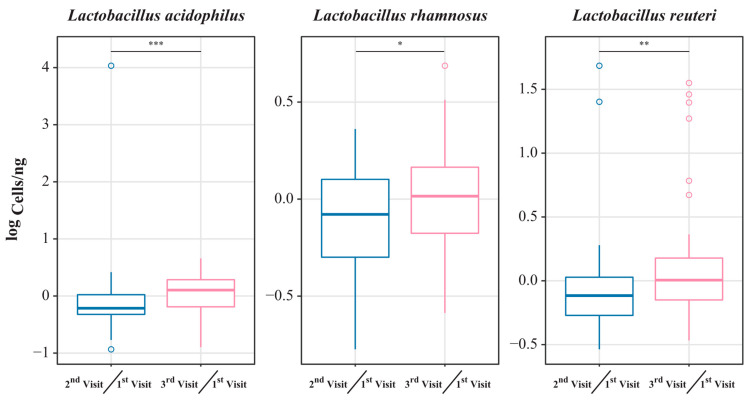
qRT-PCR was performed to assess differences in the transcripts of probiotic Lactobacillus spp. in CVF samples among the three visits. The ratio of expression was calculated by dividing the expression in CVF samples collected on the second and third visits by that collected on the first visit. After LBP intake, by comparing the expression ratio of the ingested species, it was found that the total cell of L. acidophilus (p < 0.001), L. rhamnosus (p < 0.05), and L. reuteri (p = 0.01) increased significantly (Figure 6). Overall, the results showed that probiotic Lactobacillus spp. colonized the vagina after LBP intake. In Group B, the majority of samples showed a downward shift on PCoA2 (y-axis) and a rightward shift on PCoA1 (x-axis).
Figure 6.
Expression of Lactobacillus spp. present in the oral probiotic in cervicovaginal fluid samples, as assessed by qRT-PCR. The expression ratio at the second and third visit compared to that of the first visit. qRT-PCR quantification was expressed as log cells/ng. * p < 0.05, ** p < 0.01, *** p < 0.001 (Wilcoxon signed-rank test).
4. Discussion
Vaginal microbiota diversity is important for maintaining vaginal health and preventing disease, and this fluctuates with ethnicity, age, and lifestyle [39]. In addition, vaginal microbiota diversity fluctuates with hormone levels from puberty to menopause [40]. According to the World Health Organization, in women of reproductive age (15–49 years), Lactobacillus spp. is the dominant species in the vagina [12]. Herein, the study subjects were asymptomatic women with vaginal dysbiosis aged between 19 and 55 years. Their vaginal health was analyzed, and the improvement of vaginal dysbiosis by LBP intake for 6 weeks was evaluated (Figure 1). After applying the exclusion criteria, 36 asymptomatic women were divided into two groups: the LNS group (n = 26; as normal) and the HNS group (n = 10; as abnormal), after determining the NS on the first visit. The study aimed to investigate the status of vaginal dysbiosis of asymptomatic women using the NS and validate it using molecular diagnostic tools through 16S rRNA sequencing and qRT-PCR in CVF samples. The results suggested a potential therapeutic effect of LBP intake on vaginal health improvement over the 6-week intervention period through decreased NS and increased colonization of Lactobacillus spp. in the vagina.
As discussed previously, the NS is considered the gold standard technique for evaluating BV status, especially during pregnancy [34,41]. In this study, the NS was used to group the asymptomatic women into the LNS (n = 26) and HNS (n = 10) groups, and vaginal dysbiosis was evaluated after 6 weeks of LBP intake. More than 27% (n = 10) of women fell into the HNS group showing asymptomatic vaginal dysbiosis at the first visit, and 60% (n = 6) of that 27% shifted to the LNS group after 6 weeks of LBP intake, as revealed through Gram staining (Figure 2). The sample size of 10 individuals in Group B may not have been representative of the entire population of the HNS group. But Group B was a divided group among 36 asymptomatic women, and 10 out of 36, or 28%, were applicable. The effect needs to be verified by making changes in future studies, such as using a larger number of samples, including patients with vaginitis, and having a longer period. However, the LNS group had a high relative abundance of Lactobacillus spp. before LBP intake, which was slightly changed after intake as three women in the LNS group shifted to the HNS group. This was potentially due to host hormonal or immunological factors or metabolic pathway differences [42,43,44]. In a shift from an HNS to an LNS, the vaginal microbiota diversity decreases with an increased relative abundance of Lactobacillus, which is an indication of vaginal dysbiosis normalization [45]. As observed, the abundance of Lactobacillus spp. was high (>90%) in the LNS group, and the high abundance of Gardnerella spp. in the HNS group suggested potential for the diagnosis of BV and a correlation with the NS [17,46]. The improvement in the abundance of Lactobacillus spp. in the vaginal microbiota from an HNS to an LNS, i.e., a normal condition, might have been due to the translocation of Lactobacillus from the gut to the vagina after intake of the LBP.
The normal vaginal microbiota is composed of gram-positive bacilli (Doderlein’s bacilli), which are thought to translocate from the gut, and are of the genus Lactobacillus [45,47]. The six common bacterial phyla were Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, and Fusobacteria, with a high relative abundance of Lactobacillus spp. present in both the gut and vagina, as observed through vaginal microbiota analysis [40,48,49,50]. The Lactobacillus spp. can colonize the vagina after oral administration through the gut pathway [51]. In a previous study, a combination of L. rhamnosus GR-1 and L. reuteri RC-14 was administered as an LBP, and four different samples (buccal mucosa, tongue coat, feces, and vagina) were analyzed. The longitudinal dataset revealed that there was very limited probiotic translocation to the vagina [52]. Although LBP spp. were detected more frequently in the feces of healthy women, an increased abundance of probiotic strains was not observed in the oral or vaginal samples, suggesting that L. rhamnosus GR-1 and L. reuteri RC-14 do not translocate from the gut to the vagina [26,52]. In contrast, another study showed that Lactobacillus spp. recover the asymptomatic or intermediate BV through the gut [53]. A different study reported that Lactobacillus spp. not only significantly altered the vaginal flora but also reduced the colonization of pathogenic microbes [54]. In the present study, a significant increase in Lactobacillus spp. was found in the CVF sample of the HNS group after LBP intake. By analyzing the sample dataset from the first, second, and third visits in the normal, intermediate, and BV groups, significant vaginal microbiota dynamics and LBP efficacy were found based on a comprehensive analysis of the normalization of BV levels in the HNS, which also showed a positive correlation between the alpha diversity and NS (Figure 3).
Lactobacillus spp. are known as potential biomarkers of a healthy vaginal microbiota, while vaginal dysbiosis can increase the colonization of anaerobic bacterial species associated with STIs, such as Gardnerella, Prevotella, Sneathia, Ralstonia, Dialister, and Streptococcus [17,35,55]. Herein, Lactobacillus, which was the dominant genera in the LNS group, showed a significant negative correlation with the NS, and genera related to BV, such as Gardnerella, Streptococcus, Prevotella, Ralstonia, and Dialister, showed a positive correlation [17]. In the present study of healthy women, it was found that the dominant genus in the vaginal microbiota was Lactobacillus, showing a negative correlation with alpha diversity (Figure 4). It also showed a negative correlation with the NS, which was related to Gardnerella. Alpha diversity showed the diversity of the vaginal microbiota, while NS represented the vaginal environment with some representative species. According to Wessels et al., high microbial diversity was observed in female sex workers regardless of the NS, suggesting that the NS may not represent vaginal microbial diversity [56]. Dols et al. suggested that microbial diversity in the vagina, like the NS, can be used to diagnose BV [57]. Therefore, it is believed that showing the vaginal microbiota for both markers will give a clearer indication of the healthy female flora. Although Ureaplasma is one of the genera most frequently associated with BV, which is further associated with complications, such as preterm birth, herein, a positive correlation of Ureaplasma only with Shannon diversity was observed [58]. Additionally, in our recent study, it was found that during pregnancy Ureaplasma and Prevotella colonization with Lactobacillus abundance facilitates term birth [59]. Thus, it might be possible that a high abundance of Ureaplasma with Lactobacillus in asymptomatic women carries a risk of preterm birth. Moreover, Gardnerella is normally present in the healthy vagina; consistently, a significant negative correlation was observed between Gardnerella with the NS [60]. However, a significant positive correlation of Haemophilus, which is significantly associated with obesity, with NS was observed [61]. Gestational obesity is associated with a greater risk of invasive Haemophilus infection and a poor pregnancy outcome [62]. STI-related bacteria, such as Gardnerella, Atopobium, Prevotella, Streptococcus, and Ureaplasma, can co-exist with Lactobacillus spp. and not cause any problems but can cause infections, such as BV, when the balance is disturbed [32]. In contrast, when Lactobacillus spp. and Gardnerella spp. co-existed, a negative correlation was observed between these two spp. that maintain vaginal health or susceptibility to pathogenic infections, respectively [63].
A shift in beta-diversity was confirmed following probiotic intake (Figure 5). Specifically, in Group B, the majority of samples showed a downward shift on the y-axis and a rightward shift on the x-axis. The vaginal environment often changes due to various factors, such as menstruation. As female hormone levels change, so does the vaginal environment. Although 12% (n = 3) of Group A’s LNS changed to an HNS at visit three, there was no significant change observed in alpha diversity (Figure 2A–C). Moreover, the taxa composition (Figure 2D,E) was also found to be different from that of Group B’s HNS. Considering that 88% (n = 26) of Group A did not experience a change in the LNS, and 60% (n = 6) of Group B changed to an LNS, it was considered that the probiotics may have a positive impact on maintaining and modifying the vaginal environment.
Lactobacillus species are considered as candidate probiotics for human health, including vaginal health [64,65]. Recent studies have shown the potential effects of a single specific species and/or combination of Lactobacillus species on dysbiotic conditions. In a previous study, oral intake of a combination of L. rhamnosus GR-1 with L. reuteri RC-14 during pregnancy was associated with a low risk for premature birth, which was directly associated with vaginal health and vaginal microbiota eubiosis [44]. In another study, the administration of probiotics, including the combination of 10 species, reduced the vaginal pH in women with an intermediate NS [66]. Here, the combination of L. acidophilus CBT LA1, L. rhamnosus CBT LR5, and L. reuteri CBT LU4 as an LBP was explored for vaginal dysbiosis. L. acidophilus, L. rhamnosus, and L. reuteri are used as raw materials in healthy functional foods (daily intake of 108–1010 CFU) according to Health Functional Food Standards, Ministry of Food and Drug Safety. In another study, administering a 1:1 combination of L. rhamnosus IMC 501® and Lactobacillus paracasei IMC 502® (5 × 109 CFU/capsule/day for 15 consecutive days) improved leucorrhea, itching, and vulvo-vaginal erythema/edema [29]. In the present study, L. acidophilus, L. rhamnosus and L. reuteri abundance showed a decrease at visit 2 (Figure 6). This may mean competition in the vaginal microbial community of Lactobacillus species. Figure 3 confirms that there was a change in the flora. At visits 2 and 3, there was a significant increase in Lactobacillus species abundance, such as for L. acidophilus, L. rhamnosus, and L. reuteri, suggesting that the vaginal environment was altered by lowering the pH through the secretion of lactic acid. This suggests an environment conducive to the settlement of Lactobacillus species. In addition, there was a tendency for Lactobacillus species abundance to increase between visit 1 and 3 through 6 weeks of intake, and a significant difference would be observed if intake occurred over a longer period of time.
Studies using probiotics have confirmed changes in vaginal dysbiosis as well as changes in nitrogen quality, vaginal secretion, odor, and NS [66]. Thus, consistent with the observations of the present study, consumption of LBPs is believed to inhibit an increase in alpha diversity and prevent vaginal dysbiosis in asymptomatic women. In addition, the results of short- and long-term interventions are dependent on the combination of spp. in the LBP. For example, short-term (1 week) intervention of L. brevis (CD2), L. salivarius subsp. salicinius (FV2), and L. plantarum (FV9) as an LBP resolved BV infections and improved vaginal health [67]. On the other hand, long-term (6 months) oral intake of L. rhamnosus GR-1 and L. reuteri RC-14 as an LBP did not enhance the curing of BV in Chinese women [68]. In the present study, improvement in the vaginal microbiota in women with LBP intake for just 6 weeks was observed. These findings suggested that the results of LBP intake may vary according to ethnicity, age, and lifestyle [12,69].
However, there were some limitations in this study. First, compared to 60% of participants who showed improvements, 40% with an HNS ≥ 4–10 as the abnormal group did not demonstrate improvement of vaginal dysbiosis. Therefore, STI-PCR, a multiplex PCR used to identify the presence of STI-related bacteria, including spp. of Gardnerella, Ureaplasma, and Chlamydia, was performed with third visit samples to assess the severity of the infection. An attempt was made to determine whether the levels of these species were reduced, but no significant results could be obtained. Consequently, additional research on the variations in the vaginal microbiota at a large scale is required. Second, even though the effect of LBP intake for a period of 6 weeks was validated, it was not possible to assess how long the effect would last once oral administration was stopped. Future studies should establish a prolonged study period and include longitudinal research. Finally, there was no placebo group in the final study to allow for comparisons. Moreover, subjects with vaginitis were excluded from the study. However, this study did not aim to investigate patients suffering from severe vaginitis, but rather those that were able to function normally. Therefore, the results of this study on the consumption of probiotics and their effects on the vaginal microbiota may be more relevant to healthy women.
The robust data produced in this study suggest that LBP consumption may assist with maintaining and increasing the population of Lactobacillus spp., as well as with maintaining and decreasing microbial diversity for asymptomatic vaginal dysbiosis in the short term. During the 6 week intake period, colonization of Lactobacillus spp. and a decrease in the NS were confirmed. This implied that LBP consumption enhanced the colonization of Lactobacillus spp. in the vagina via translocation from the gastrointestinal tract, as confirmed by qRT-PCR. Collectively, this microbiota-based study allowed the LNS and HNS vaginal microbiota to be distinguished in asymptomatic women, thereby facilitating the development of therapies and treatment options for vaginal dysbiosis. This study found that oral administration of an LBP containing L. acidophilus CBT LA1, L. rhamnosus CBT LR5, and L. reuteri CBT LU4 could promote vaginal health in asymptomatic women.
Acknowledgments
We are grateful to all our colleagues at Ewha Womans University and the Cell Biotech Co., Ltd. for their assistance in collecting samples, conducting experiments, and analyzing data, as well as their valuable suggestions that improved the quality of this manuscript.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15081862/s1, Figure S1: Changes in the microbial diversity at each visit based on the levels of different species in the vaginal microbiota. The principal component analysis shows the change in the beta diversity distance of the samples according to levels. Abbreviations: PCoA, principal component analysis; Table S1: qRT-PCR primers used in this study; Table S2: Composition of the reaction mixture for qRT-PCR and PCR thermal cycling conditions. References [70,71,72] are cited in the supplementary materials.
Author Contributions
Conceptualization, S.L. and Y.J.K.; investigation, A.A., D.S., S.K., S.L. and Y.J.K.; resources, Y.M.H., S.P., S.M.K. and G.L.; data curation, A.A. and D.S.; visualization, D.S.; writing—original draft preparation, A.A. and D.S.; writing—review and editing, A.A, D.S., Y.-A.Y., Y.C. and S.L.; project administration, D.S. and S.K.; supervision, Y.-A.Y. and Y.C.; funding acquisition: Y.-A.Y. and Y.J.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Ewha Womans University (protocol code 2020-11-035-007 and 21 November 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The sequence data that support the findings of this study are available via BioProject, accession number PRJNA899123 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA899123, accessed on 12 April 2023).
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) No. NRF-2020R1I1A1A01074518 and No. NRF-2020R1A2C3011850), and by the BK21 FOUR (Fostering Outstanding Universities for Re-search) funded by the Ministry of Education (MOE, Korea) and National Research Foundation of Korea (NRF-5199990614253, Education Research Center for 4IR-Based Health Care).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Martin D.H. The microbiota of the vagina and its influence on women’s health and disease. Am. J. Med. Sci. 2012;343:2–9. doi: 10.1097/MAJ.0b013e31823ea228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gajer P., Brotman R.M., Bai G., Sakamoto J., Schütte U.M., Zhong X., Koenig S.S.K., Fu L., Ma Z., Zhou X., et al. Temporal dynamics of the human vaginal microbiota. Sci. Transl. Med. 2012;4:ra52–ra132. doi: 10.1126/scitranslmed.3003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng N., Guo R., Wang J., Zhou W., Ling Z. Contribution of Lactobacillus iners to vaginal health and diseases: A systematic review. Front. Cell. Infect. Microbiol. 2021;11:1177. doi: 10.3389/fcimb.2021.792787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenbaum S., Greenbaum G., Moran-Gilad J., Weintraub A.Y. Ecological dynamics of the vaginal microbiome in relation to health and disease. Am. J. Obstet. Gynecol. 2019;220:324–335. doi: 10.1016/j.ajog.2018.11.1089. [DOI] [PubMed] [Google Scholar]
- 5.Workowski K.A., Bachmann L.H., Chan P.A., Johnston C.M., Muzny C.A., Park I., Reno H., Zenilman J.M., Bolan G.A. Sexually transmitted infections treatment guidelines. MMWR Recomm. Rep. 2021;70:1–187. doi: 10.15585/mmwr.rr7004a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tachedjian G., Aldunate M., Bradshaw C.S., Cone R.A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Microbiol. Res. 2017;168:782–792. doi: 10.1016/j.resmic.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Smith S.B., Ravel J. The vaginal microbiota, host defence and reproductive physiology. Physiol. J. 2017;595:451–463. doi: 10.1113/JP271694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrova M.I., Lievens E., Malik S., Imholz N., Lebeer S. Lactobacillus species as biomarkers and agents that can promote various aspects of vaginal health. Front. Physiol. 2015;6:81. doi: 10.3389/fphys.2015.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aldunate M., Srbinovski D., Hearps A.C., Latham C.F., Ramsland P.A., Gugasyan R., Cone R.A., Tachedjian G. Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front. Physiol. 2015;6:164. doi: 10.3389/fphys.2015.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou X., Brown C.J., Abdo Z., Davis C.C., Hansmann M.A., Joyce P., Forney L.J. Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. ISME J. 2007;1:121–133. doi: 10.1038/ismej.2007.12. [DOI] [PubMed] [Google Scholar]
- 11.Verhelst R., Verstraelen H., Claeys G., Verschraegen G., Delanghe J., Van Simaey L., De Ganck C., Temmerman M., Vaneechoutte M. Cloning of 16S rRNA genes amplified from normal and disturbed vaginal microflora suggests a strong association between Atopobium vaginae, Gardnerella vaginalis and bacterial vaginosis. BMC Microbiol. 2004;4:16. doi: 10.1186/1471-2180-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ravel J., Gajer P., Abdo Z., Schneider G.M., Koenig S.S.K., McCulle S.L., Karlebach S., Gorle R., Russell J., Tacket C.O., et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA. 2011;108:4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Onderdonk A.B., Delaney M.L., Fichorova R.N. The human microbiome during bacterial vaginosis. Clin. Microbiol. Rev. 2016;29:223–238. doi: 10.1128/CMR.00075-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anahtar M.N., Gootenberg D.B., Mitchell C.M., Kwon D.S. Cervicovaginal microbiota and reproductive health: The virtue of simplicity. Cell Host Microbe. 2018;23:159–168. doi: 10.1016/j.chom.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Fettweis J.M., Serrano M.G., Brooks J.P., Edwards D.J., Girerd P.H., Parikh H.I., Huang B., Arodz T.J., Edupuganti L., Glascock A.L., et al. The vaginal microbiome and preterm birth. Nat. Med. 2019;25:1012–1021. doi: 10.1038/s41591-019-0450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hur Y.M., Kang M.N., Kim Y.J. Vaginal health in women and the possibility of predicting preterm birth through microbiome analysis. J. Korean Med. Assoc. 2021;64:833–840. doi: 10.5124/jkma.2021.64.12.833. [DOI] [Google Scholar]
- 17.Ling Z., Kong J., Liu F., Zhu H., Chen X., Wang Y., Li L., Nelson K.E., Xia Y., Xiang C. Molecular analysis of the diversity of vaginal microbiota associated with bacterial vaginosis. BMC Genom. 2010;11:488. doi: 10.1186/1471-2164-11-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bested A.C., Logan A.C., Selhub E.M. Intestinal microbiota, probiotics and mental health: From Metchnikoff to modern advances: Part II–contemporary contextual research. Gut Pathog. 2013;5:3. doi: 10.1186/1757-4749-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morelli L., Capurso L. FAO/WHO guidelines on probiotics: 10 years later. J. Clin. Gastroenterol. 2012;46:S1–S2. doi: 10.1097/MCG.0b013e318269fdd5. [DOI] [PubMed] [Google Scholar]
- 20.Cho I., Blaser M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012;13:260–270. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams N.T. Probiotics. AJHP. 2010;67:449–458. doi: 10.2146/ajhp090168. [DOI] [PubMed] [Google Scholar]
- 22.Veljović K., Dinić M., Lukić J., Mihajlović S., Tolinački M., Živković M., Begović J., Mrvaljević I. Promotion of early gut colonization by probiotic intervention on microbiota diversity in pregnant sows. Front. Microbiol. 2017;8:1–12. doi: 10.3389/fmicb.2017.02028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X.Q., Zhang A.H., Miao J.H., Sun H., Yan G.L., Wu F.F., Begović J., Mrvaljević I., Golić N., Terzić-Vidojević A. Gut microbiota as important modulator of metabolism in health and disease. RSC Adv. 2018;8:42380–42389. doi: 10.1039/C8RA08094A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qi X., Yun C., Pang Y., Qiao J. The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microbes. 2021;13:e1894070. doi: 10.1080/19490976.2021.1894070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clemente J.C., Ursell L.K., Parfrey L.W., Knight R. The impact of the gut microbiota on human health: An integrative view. Cell. 2012;148:1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marrazzo J.M., Fiedler T.L., Srinivasan S., Thomas K.K., Liu C., Ko D., Xie H., Saracino M., Fredricks D.N. Extravaginal reservoirs of vaginal bacteria as risk factors for incident bacterial vaginosis. J. Infect. Dis. 2012;205:1580–1588. doi: 10.1093/infdis/jis242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gareau M.G., Sherman P.M., Walker W.A. Probiotics and the gut microbiota in intestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2010;7:503–514. doi: 10.1038/nrgastro.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim E.Y., Song E.J., Kim J.G., Jung S.Y., Lee S.Y., Shin H.S., Nam Y.D., Kim Y.T. Lactobacillus intestinalis YT2 restores the gut microbiota and improves menopausal symptoms in ovariectomized rats. Benef. Microbes. 2021;12:503–516. doi: 10.3920/BM2020.0217. [DOI] [PubMed] [Google Scholar]
- 29.Pino A., Rapisarda A.M.C., Vaccalluzzo A., Sanfilippo R.R., Coman M.M., Grimaldi R.L., Caggia C., Randazzo C.L., Russo N., Panella M.M., et al. Oral Intake of the Commercial Probiotic Blend Synbio® for the Management of Vaginal Dysbiosis. J. Clin. Med. 2023;12:27. doi: 10.3390/jcm12010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleerebezem M., Hols P., Bernard E., Rolain T., Zhou M., Siezen R.J., Bron P.A. The extracellular biology of the lactobacilli. FEMS Microbiol. Rev. 2010;34:199–230. doi: 10.1111/j.1574-6976.2009.00208.x. [DOI] [PubMed] [Google Scholar]
- 31.Srinivasan S., Fredricks D. The Human Vaginal Bacterial Biota and Bacterial Vaginosis. Interdiscip. Perspect. Infect. Dis. 2008;2008:750479. doi: 10.1155/2008/750479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee S.-J. Diagnosis and treatment of women with recurrent urinary tract infection. J. Korean Med. Assoc. 2022;65:594–603. doi: 10.5124/jkma.2022.65.9.594. [DOI] [Google Scholar]
- 33.Lewis A.L., Laurent L.C. USPSTF 2020 recommendations on screening for asymptomatic bacterial vaginosis in pregnancy. JAMA. 2020;323:1253–1255. doi: 10.1001/jama.2019.22311. [DOI] [PubMed] [Google Scholar]
- 34.Nugent R.P., Krohn M.A., Hillier S.L. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J. Clin. Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van de Wijgert J.H. The vaginal microbiome and sexually transmitted infections are interlinked: Consequences for treatment and prevention. PLoS Med. 2017;14:e1002478. doi: 10.1371/journal.pmed.1002478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiu S.-F., Huang P.-J., Cheng W.-H., Huang C.-Y., Chu L.J., Lee C.-C., Lin H.-C., Chen L.-C., Lin W.-N., Tsao C.-H., et al. Vaginal Microbiota of the Sexually Transmitted Infections Caused by Chlamydia trachomatis and Trichomonas vaginalis in Women with Vaginitis in Taiwan. Microorganisms. 2021;9:1864. doi: 10.3390/microorganisms9091864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deurenberg R.H., Bathoorn E., Chlebowicz M.A., Couto N., Ferdous M., García-Cobos S., Kooistra-Smid A.M.D., Raangs E.C., Rosema S., Veloo A.C.M., et al. Application of next gener-ation sequencing in clinical microbiology and infection prevention. J. Biotechnol. 2017;243:16–24. doi: 10.1016/j.jbiotec.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 38.Poretsky R., Rodriguez-R L.M., Luo C., Tsementzi D., Konstantinidis K.T. Strengths and Limitations of 16S rRNA Gene Amplicon Sequencing in Revealing Temporal Microbial Community Dynamics. PLoS ONE. 2014;9:e93827. doi: 10.1371/journal.pone.0093827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karkman A., Lehtimäki J., Ruokolainen L. The ecology of human microbiota: Dynamics and diversity in health and disease. Ann. New York Acad. Sci. 2017;1399:78–92. doi: 10.1111/nyas.13326. [DOI] [PubMed] [Google Scholar]
- 40.Kaur H., Merchant M., Haque M.M., Mande S.S. Crosstalk Between Female Gonadal Hormones and Vaginal Microbiota Across Various Phases of Women’s Gynecological Lifecycle. Front. Microbiol. 2020;11:551. doi: 10.3389/fmicb.2020.00551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forsum U., Holst E., Larsson P.G., Vasquez A., Jakobsson T., Mattsby-Baltzer I. Bacterial vaginosis–a microbiological and immu-nological enigma. Apmis. 2005;113:81–90. doi: 10.1111/j.1600-0463.2005.apm1130201.x. [DOI] [PubMed] [Google Scholar]
- 42.Cervantes-Barragan L., Chai J.N., Tianero M.D., Di Luccia B., Ahern P.P., Merriman J., Cortez V.S., Caparon M.G., Donia M.S., Gilfillan S., et al. Lactobacillus reuteri induces gut intraepithelial CD4+ CD8αα+ T cells. Science. 2017;357:806–810. doi: 10.1126/science.aah5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amabebe E., Anumba D.O. Female gut and genital tract microbiota-induced crosstalk and differential effects of short-chain fatty acids on immune sequelae. Front. Immunol. 2020;11:2184. doi: 10.3389/fimmu.2020.02184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang S., Reid G., Challis J.R., Gloor G.B., Asztalos E., Money D., Seney S., Bocking A.D. Effect of oral probiotic Lactobacillus rham-nosus GR-1 and Lactobacillus reuteri RC-14 on the vaginal microbiota, cytokines and chemokines in pregnant women. Nutrients. 2020;12:368. doi: 10.3390/nu12020368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amabebe E., Anumba D.O.C. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front. Med. 2018;5:181. doi: 10.3389/fmed.2018.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cox C., McKenna J.P., Watt A.P., Coyle P.V. New assay for Gardnerella vaginalis loads correlates with Nugent scores and has potential in the diagnosis of bacterial vaginosis. J. Med. Microbiol. 2015;64:978–984. doi: 10.1099/jmm.0.000118. [DOI] [PubMed] [Google Scholar]
- 47.Weinstein L., Bogin M., Howard J.H., Finkelstone B.B. A survey of the vaginal flora at various ages, with special reference to the Döderlein bacillus. Am. J. Obstet. Gynecol. 1936;32:211–218. doi: 10.1016/S0002-9378(36)90120-5. [DOI] [Google Scholar]
- 48.Kobyliak N., Virchenko O., Falalyeyeva T. Pathophysiological role of host microbiota in the development of obesity. Nutr. J. 2015;15:43. doi: 10.1186/s12937-016-0166-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amabebe E., Robert F.O., Agbalalah T., Orubu E.S. Microbial dysbiosis-induced obesity: Role of gut microbiota in ho-moeostasis of energy metabolism. BJN. 2020;123:1127–1137. doi: 10.1017/S0007114520000380. [DOI] [PubMed] [Google Scholar]
- 50.Fudaba M., Kamiya T., Tachibana D., Koyama M., Ohtani N. Bioinformatics Analysis of Oral, Vaginal, and Rectal Microbial Profiles during Pregnancy: A Pilot Study on the Bacterial Co-Residence in Pregnant Women. Microorganisms. 2021;9:1027. doi: 10.3390/microorganisms9051027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DE Leo V., Lazzeri E., Governini L., Cuppone A.M., Colombini L., Teodori L., Ciprandi G., Iannelli F., Pozzi G. Vaginal colonization of women after oral administration of Lactobacillus crispatus strain NTCVAG04 from the human microbiota. Minerva Obstet. Gynecol. 2022 doi: 10.23736/S2724-606X.22.05087-4. [DOI] [PubMed] [Google Scholar]
- 52.Chen C., Hao L., Zhang Z., Tian L., Zhang X., Zhu J., Jie Z., Tong X., Xiao L., Zhang T., et al. Cervicovaginal microbiome dynamics after taking oral probiotics. J. Genet. Genom. 2021;48:716–726. doi: 10.1016/j.jgg.2021.03.019. [DOI] [PubMed] [Google Scholar]
- 53.Reid G., Bruce A.W., Fraser N., Heinemann C., Owen J., Henning B. Oral probiotics can resolve urogenital infections. FEMS Microbiol. Immunol. 2001;30:49–52. doi: 10.1111/j.1574-695X.2001.tb01549.x. [DOI] [PubMed] [Google Scholar]
- 54.Reid G., Charbonneau D., Erb J., Kochanowski B., Beuerman D., Poehner R., Bruce A.W. Oral use of Lactobacillus rhamnosus GR-1 and L. fermentum RC-14 significantly alters vaginal flora: Randomized, placebo-controlled trial in 64 healthy women. FEMS Microbiol. Immunol. 2003;35:131–134. doi: 10.1016/S0928-8244(02)00465-0. [DOI] [PubMed] [Google Scholar]
- 55.Georgijević A., Cjukić-Ivancević S., Bujko M. Bacterial vaginosis. Epidemiology and risk factors. Srpski arhiv za celokupno lekarstvo. 2000;128:29–33. [PubMed] [Google Scholar]
- 56.Wessels J.M., Lajoie J., Vitali D., Omollo K., Kimani J., Oyugi J., Cheruiyot J., Kimani M., Mungai J.N., Akolo M., et al. Association of high-risk sexual behaviour with di-versity of the vaginal microbiota and abundance of Lactobacillus. PLoS ONE. 2017;12:e0187612. doi: 10.1371/journal.pone.0187612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dols J.A.M., Molenaar D., Van Der Helm J.J., Caspers M.P.M., Angelino-Bart A.D.K., Schuren F.H.J., Speksnijder A.G.C.L., Westerhoff H.V., Richardus J.H., Boon M.E., et al. Molecular assessment of bacterial vaginosis by Lactobacillus abundance and species diversity. BMC Infect. Dis. 2016;16:180. doi: 10.1186/s12879-016-1513-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park S., Moon J., Kang N., Kim Y., You Y.A., Kwon E., Ansari A., Hur Y.M., Park T., Kim Y.J. Predicting preterm birth through vaginal microbiota, cervical length and WBC using a machine learning model. Front. Microbiol. 2022;13:912853. doi: 10.3389/fmicb.2022.912853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park S., You Y.A., Kim Y.H., Kwon E., Ansari A., Kim S.M., Lee G., Hur Y.M., Jung Y.J., Kim K., et al. Ureaplasma and Prevotella Colonization with Lactoba-cillus Abundance During Pregnancy Facilitates Term Birth. Sci. Rep. 2022;12:10148. doi: 10.1038/s41598-022-13871-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hickey R.J., Forney L.J. Gardnerella vaginalis does not always cause bacterial vaginosis. J. Infect. Dis. 2014;210:1682–1683. doi: 10.1093/infdis/jiu303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen X., Sun H., Jiang F., Shen Y., Li X., Hu X., Shen X., Wei P. Alteration of the gut microbiota associated with childhood obesity by 16S rRNA gene sequencing. PeerJ. 2020;8:e8317. doi: 10.7717/peerj.8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Collins S., Ramsay M., Slack M.P.E., Campbell H., Flynn S., Litt D., Ladhani S.N. Risk of Invasive Haemophilus influenzae Infection During Pregnancy and Association With Adverse Fetal Outcomes. JAMA. 2014;311:1125–1132. doi: 10.1001/jama.2014.1878. [DOI] [PubMed] [Google Scholar]
- 63.Boris S., Barbés C. Role played by lactobacilli in controlling the population of vaginal pathogens. Microbes Infect. 2000;2:543–546. doi: 10.1016/S1286-4579(00)00313-0. [DOI] [PubMed] [Google Scholar]
- 64.Pino A., Vaccalluzzo A., Caggia C., Balzaretti S., Vanella L., Sorrenti V., Ronkainen A., Satokari R., Randazzo C.L. Lacticaseibacillus rhamnosus CA15 (DSM 33960) as a Candidate Probiotic Strain for Human Health. Nutrients. 2022;14:4902. doi: 10.3390/nu14224902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coman M.M., Verdenelli M.C., Cecchini C., Silvi S., Orpianesi C., Boyko N., Cresci A. In vitro evaluation of antimicrobial activity of Lactobacillus rhamnosus IMC 501®, Lactobacillus paracasei IMC 502® and SYNBIO® against pathogens. J. Appl. Microbiol. 2014;117:518–527. doi: 10.1111/jam.12544. [DOI] [PubMed] [Google Scholar]
- 66.Martoni C.J., Frederiksen A.K.S., Damholt A., Leyer G. Effects of a 10-Strain Oral Probiotic on Parameters of Vaginal Health and Microbial Community: A Pilot Clinical Study. Int. J. Women Heal. 2022;ume 14:29–39. doi: 10.2147/IJWH.S341046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mastromarino P., Macchia S., Meggiorini L., Trinchieri V., Mosca L., Perluigi M., Midulla C. Effectiveness of Lactobacil-lus-containing vaginal tablets in the treatment of symptomatic bacterial vaginosis. Clin. Microbiol. Infect. 2009;15:67–74. doi: 10.1111/j.1469-0691.2008.02112.x. [DOI] [PubMed] [Google Scholar]
- 68.Hummelen R., Changalucha J., Butamanya N.L., Cook A., Habbema J.D.F., Reid G. Lactobacillus rhamnosus GR-1 and L. reuteri RC-14 to prevent or cure bacterial vaginosis among women with HIV. Int. J. Gynecol. Obstet. 2010;111:245–248. doi: 10.1016/j.ijgo.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 69.Hotkani Z.G., Ghaedmohammadi S., Mozdoori N. Meta-analysis of race and age influence on the vaginal microbiome in pregnant and nonpregnant healthy women. Futur. Microbiol. 2022;17:1147–1159. doi: 10.2217/fmb-2021-0209. [DOI] [PubMed] [Google Scholar]
- 70.Haarman M., Knol J. Quantitative Real-Time PCR Analysis of Fecal Lactobacillus Species in Infants Receiving a Prebiotic Infant Formula. Appl. Environ. Microbiol. 2006;72:2359–2365. doi: 10.1128/AEM.72.4.2359-2365.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Song Y.L., Kato N., Liu C.X., Matsumiya Y., Kato H., Watanabe K. Rapid identification of 11 human intestinal Lacto-bacillus species by multiplex PCR assays using group-and species-specific primers derived from the 16S–23S rRNA intergenic spacer region and its flanking 23S rRNA. FEMS Microbiol. Lett. 2000;187:167–173. doi: 10.1111/j.1574-6968.2000.tb09155.x. [DOI] [PubMed] [Google Scholar]
- 72.Wang C., Wei S., Liu B., Wang F., Lu Z., Jin M., Wang Y. Maternal consumption of a fermented diet protects offspring against intestinal inflammation by regulating the gut microbiota. Gut Microbes. 2022;14:2057779. doi: 10.1080/19490976.2022.2057779. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequence data that support the findings of this study are available via BioProject, accession number PRJNA899123 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA899123, accessed on 12 April 2023).