Abstract
Wound healing (WH) is a complex multistep process in which a failure could lead to a chronic wound (CW). CW is a major health problem and includes leg venous ulcers, diabetic foot ulcers, and pressure ulcers. CW is difficult to treat and affects vulnerable and pluripathological patients. On the other hand, excessive scarring leads to keloids and hypertrophic scars causing disfiguration and sometimes itchiness and pain. Treatment of WH includes the cleaning and careful handling of injured tissue, early treatment and prevention of infection, and promotion of healing. Treatment of underlying conditions and the use of special dressings promote healing. The patient at risk and risk areas should avoid injury as much as possible. This review aims to summarize the role of physical therapies as complementary treatments in WH and scarring. The article proposes a translational view, opening the opportunity to develop these therapies in an optimal way in clinical management, as many of them are emerging. The role of laser, photobiomodulation, photodynamic therapy, electrical stimulation, ultrasound therapy, and others are highlighted in a practical and comprehensive approach.
Keywords: chronic wound, electromagnetic fields, hypertrophic scar, keloid, laser, physical therapies, photobiomodulation, photodynamic therapy, radiofrequency, ultrasound therapy, wound healing
1. Introduction
Wound healing (WH) is a main health problem in current society. Firstly, acute wounds could lead to scars and disfiguring lesions, and secondly, chronic wounds (CW) cause morbidity and high economic cost. AWs occur, in general, after surgery, trauma, or burns, whereas in CWs occur, in general, with an underlying systemic condition, such as diabetes, elderly, vascular alterations, or malnutrition.
Guidelines for care in wounds are useful, clear, and concise [1]. They represent the principal approach in clinical practice. The main CWs presented in clinical practice include leg ulcers (LU), diabetic foot ulcer (DFU), and pressure ulcer (PU). The main treatment of CWs include adequate dressing, debridement, and pressure control. Nevertheless, undoubtedly, there is an uncovered gap in this pathology, as scarring is sometimes unavoidable, and CWs could persist for months. The focus of this review is to provide a tool for clinicians, and a useful guide from the basic science, to develop and improve physical therapies in WH.
Lots of research works are nowadays focused on solving the problem of WH, most of them searching for very advanced therapies, such as cellular transplantation therapy [2,3], vascular enhancers [4], regenerative materials [5], or nanoparticles in hydrogels [6]. Despite the highly anticipated novel therapies in development, right now, they are very far from being used in real practice.
Physical therapy (PT) is present in daily clinical consultations and has demonstrated a certain utility in WH [7]. This review came across different techniques such as laser, low-level laser light therapy, photodynamic therapy, or electrical stimulation, among others, and their role in WH.
2. General Approach to Wounds
2.1. Epidemiology
The data in the literature referring to failure of WH show the seriousness of the problem.
WH failure, dermal fibrosis and scarring affect all ethnicities, while keloids or hypertrophic scars are more prevalent in American, African, and Asian populations, which can reach up to 16% of the population [8]. Factors associated with excessive scarring include genetic predisposition, hypertension, endocrine dysfunction, autoimmune diseases, and endocrine alterations [9]. The genetic factors described have been found to be associated with polymorphism alterations in genes such as TGF-beta, which evolved in fibrosis formation, opening, and interested therapeutic target [10]. The subsequent endothelial malfunction in hypertension has recently been associated with the risk of scarring and other diseases which have fibrosis and remodeling in their pathogenic [11].
On the other hand, the type of injury has also been associated with the risk of scarring and other factors often seen in clinical practice [12] (See Table 1). Two types of scars are described: keloids and hypertrophic scars (HS). HS are limited to the wound with an increase in cicatricial tissue, whereas keloids are invasive, going through the limit of the wound [12]. Table 1 summarized the differences between keloids and HS. The interaction between the environment of keloids and the scar is complex, and diet, smoking, stress, and physical exercise could influence the process [11].
Table 1.
Clinical differences between keloids and hypertrophic scars.
| Characteristic | Keloids | Hypertrophic Scars |
|---|---|---|
| Trauma | Non-severe (acne, folliculitis) | Burns, incision |
| Body sites | Chest, upper back, earlobe | Any |
| Symptoms | Erythema, itch, pain | Erythema, itch |
| Exploration | Beyond the limit of the trauma | Limited to the initial wound |
| Treatment | Combined therapies with frequent recurrence |
Good response |
| Surgical excision | Contraindicated due to recurrence |
Without recurrences, could be considered a treatment |
Conversely, the failure of healing a wound also produces a high impact on the patients. A chronic wound (CW) is a wound that fails to repair and restore the skin in three months [13]. It is estimated that 1–2% of the population suffer from CWs [14], for example, in the United States more than 6.5 million patients are affected [15].
2.2. Process and Stages of Wound Healing
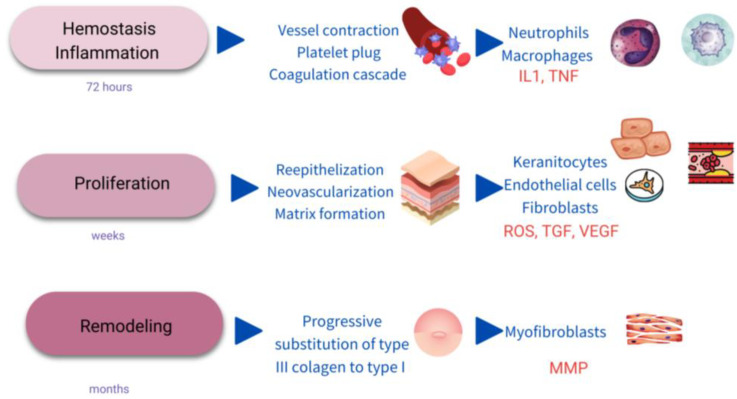
WH is a complex process evolving multiple biological pathways and mechanisms. Classically, it is divided into different phases, including hemostasis/inflammatory stage, proliferation, and remodeling (Figure 1) [2,16].
Figure 1.
Scheme of the stages of wound healing. IL1: interkeukin1; TNF: Tumor necrosis factor; ROS: single oxygen radicals; TGF: transforming growth factor; VEGF: vascular endothelial growth factor; MMP: metalloproteinases.
2.2.1. Hemostasis/Inflammatory Stage
The first response to an injury is the constriction of the affected vessels and platelet activation to form a fibrin clot and stop the bleeding [3]. Platelets are activated for the exposure to collagen of the subendothelial matrix in the so-called primary hemostasis. Next, secondary hemostasis produces the activation of the coagulation cascade [17]. Local mast cell degranulation release occurs in the following minutes, and mediators such as histamine and TNF-alfa are released [18].
The next cells to appear in the wound scenario are the neutrophils which are not usually present on normal skin. Neutrophils represent an innate inflammation [19] and are recruited from damaged vessels and attracted by interleukin 1 (IL1), tumor necrosis factor alfa (TNFα) and bacterial toxins [20].
Activated neutrophils destroy bacteria and cell debris and provide a good environment for WH through the liberation of reactive oxygen species (ROS), antimicrobial peptides and proteolytic enzymes [16]. Clearance of neutrophils occurs by apoptosis or necrosis and ulterior phagocytosis by macrophages. Complete hemostasis and inflammatory phase in WH usually last 72 h [2].
2.2.2. Proliferation
The clot is substituted by connective tissue or granulation tissue, meanwhile neovascularization, re-epithelialization, and immunomodulation appear in parallel lasting days or weeks [16]. Many cytokines and growth-factors participate in this phase, such as the transforming growth factor-beta (TGF-β) family, and vascular epidermal growth-factors (VEGF) [2,16]. Most of these mediators support the mechanism of action of physical therapies in WH and are the focus to work in with. The duration of this phase is as follows: 3–21 days [21].
2.2.3. Maturation/Remodeling
Finally, a progressive substitution of the existing cells in the initial fibrin clot led to a wound contraction. This event is related to the maturation of type I collagen and the elimination of type III immature collagen, and by apoptosis of the myofibroblast during several weeks and months after the injury [22]. This change is regulated by metalloproteases (MMPs), collagenases express and secrete by macrophages, myofibroblasts, and keratinocytes [3,16]. The duration of this phase is as follows: 3 weeks–6 months [21].
2.3. Chronic Wounds
A chronic wound (CW) is described as a wound that fails to repair itself or remains unhealed after 12 weeks [1].
Most of the CW are classified as diabetic ulcers (DU), pressure ulcers (PU), or venous leg ulcers (VU), in relation to their clinical findings and cause.
2.3.1. Diabetic Ulcer
DU is a deleterious complication representing the first cause of amputation of the lower limb [23]. DU are located on the foot and are caused by neuropathy and vascular illness, which causes the inability to detect pain and injuries. In general, DUs are deep, similar to a crater and expose the tendon and the bone. A surrounded hyperkeratotic tissue is put in place, forming a callus-like ring. Imaging testing could be necessary to exclude osteomyelitis [1,13].
2.3.2. Pressure Ulcer
PU appears on areas under pressure, usually over a bony prominence such as the sacrum or the heels. The pression on the vessels decreases irrigation of the skin resulting in an initial dermatitis, which if prolonged leads to a loss of tissue. The cause is multifactorial, e.g., immobilization in bed, nutrition alteration, systemic diseases, or being elderly [1]. PUs varies in severity, and are classified in four different stages, IV being the more severe, which implies the loss of the full thickness of the skin. In those cases, the management of the PU should be surgical, and PT would not play a role.
2.3.3. Venous Ulcer
A VU typically appears in the lower limb over the medial supramalleolar area. The risk factors for VUs include obesity and venous insufficiency. About 75% of chronic ulcers are VU, being the most frequent, affecting 1–5% of the population [24]. VU are associated with more signs of venous malfunction in clinical exploration than oedema, hemosiderosis, cutaneous atrophy, lipodermatosclerosis or annexal absence. If necessary, further examination with a duplex ultrasonography confirms the diagnosis.
The management of a VU includes the general treatment of CW, adding compressive therapy and healing the venous system if possible, with surgery [13,25]. Adjuvant therapies include nutritional balance and supplementation, diet, physical exercise and improving blood circulation with drugs such as pentoxifylline. Despite using the correct treatment, a VU could take 6 to 12 months to heal, and relapsing is very frequent in the following year [13,24,25].
2.4. General Management of Chronic Wounds
WH and scarring is a complex process with multiple influencing and interacting factors. Additionally, some of those factors are not under the control of the dermatologist, such as age, vascular abnormalities, comorbidities, malnutrition, or smoking [1]. The management is challenging, and multiple approaches and visits are needed with the implication of different health care workers [1] and arisen important indirect costs.
All CW should be treated according to the TIME principles: tissue debridement, infection control, moisture balance and edges of the wound [13]. Debridement is the first step in the treatment of a CW, it must be carried out weekly and it increases the speed of healing by over 72% [26].
Biofilm is presented in the extracellular matrix and is considered the cause of 80% of the infections in CW [27]. Biofilm is invisible to the naked eye, and different techniques to assess its presence are being developed, apart from a cutaneous biopsy. Nevertheless, the biofilm must be removed because it maintains the CW in the inflammatory stage [28]. The risk of infection is usually controlled by topical antibiotics, silver dressing, or with other topical components.
The wound should not be exposed to air, and if the skin appears dry, moisturizer should be added to the dressing. On the other hand, if excessive drainage is present, it needs to be clean and dried. The wound edge, in case of overgrowth, must be excised for epithelization [29].
Table 2 describes the local cellular response alterations underlying a CW. CW are characterized by excessive inflammation, a decrease in growth factors secretion, and a disbalance in the proteolytic enzymes and cellular senescence which perpetuates the wound unhealed [25]. Therefore, a high number of mast cells, neutrophils and dendritic cells are found in CW with an increase in pro-inflammatory cytokines and proteases (see Figure 1). These inflammatory cells not only prolong the wound but also increase susceptibility to infections [22]. The alteration of the expression and activation of MMPs is strongly associated with CW, damaging the granulation tissue, and producing exudates in the wound [30]. Cells implicated in the remodeling and re-epithelization are dysfunctional too. Fibroblasts are senescent and the excess of wound proteases (MMPs, elastase, cathepsin G, and urokinase-type plasminogen activator (uPA)) activities degrade the extracellular matrix, the growth factors (VEGF, TGF-beta) and cytokines (TNF-alfa) [22]. Keratinocytes hyper proliferate at the edge of the wound, so hyperkeratosis appears, and the subsequent wound fails to close. The microbiome profiles of aged and diabetic patients with CW have been found to have a decrease in alfa-diversity [3,20,30].
Table 2.
Mechanisms found in failure to heal wound (FHW).
| Wound | Cellular Mechanisms | Mediators |
|---|---|---|
| Inflammation Exudates Infection |
Neutrophils’ excessive number and function Defective macrophages High number of mast cells Loss of microbiome diversity |
Oxidative stress Wound proteases (MMPs, elastase, cathepsin G, and urokinase-type plasminogen activator (uPA)) Increase in inflammatory cytokines |
| Hyperkeratotic edge of the wound | Keratinocyte hyperproliferation and malfunction | Elevated b-catenin and c-myc |
| Failure to heal and close | Senescent fibroblasts | Degradation of VEGF, TGF-beta, and TNF-alfa |
MMP: metalloproteinases; VEGF: vascular endothelial growth factor; TGF: transforming growth factor; TNF: tumoral necrosis factor.
2.5. General Prevention of Scarring
Excess WH or scarring is caused by an overproduction of extracellular matrix generated by myofibroblasts, which in this type of lesion are not replaced by fibroblasts during the proliferative phase. In this fibrosis, matrix proteins such as alpha-smooth muscle actin (alpha-SMA) are overexpressed, and the expression of MMP decreases, which induces an accumulation of collagen [12].
Keloid and HS are clinical expressions, and both can be considered successive stages of the same proliferative disorder. The initial common process is a purulent inflammatory skin lesion, the hyperfunction of the fibroblasts and excessive extracellular matrix deposition. HS consists of mainly type III collagen, whereas keloids contain type I and III [31].
The general strategy for the prevention of scarring is summarized in Table 3. The early recognition of the alteration is considered of cardinal importance for early treatment [32]. The healing process varies from one patient to another; thus, controlling the procedure, preventing the infection, and providing personalized wound care are the main prevention and treatment methods [33].
Table 3.
Prevention and treatment of scarring, keloids, and hypertrophic scars (HS).
| Prevention | Treatment | Alternative Therapies |
|---|---|---|
| Early diagnosis | Silicone gel or dressing Topical retinoids |
Peelings |
| Careful wound care | Topical Imiquimod Topical 5-Fluorouracil Intralesional Bleomicin |
Microneedling Dermabrasion Radiotherapy |
| Prevent infection | ||
| Sun protection Avoid risk areas if possible Avoid risk patients if possible |
3. The Role of Physical Therapies in Hard-To-Heal Chronic Wounds
Principles and Basis
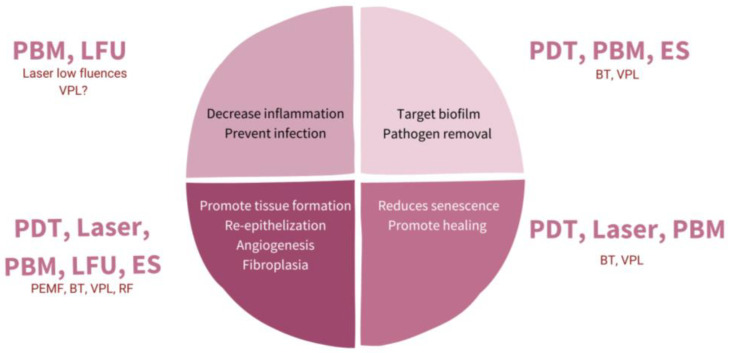
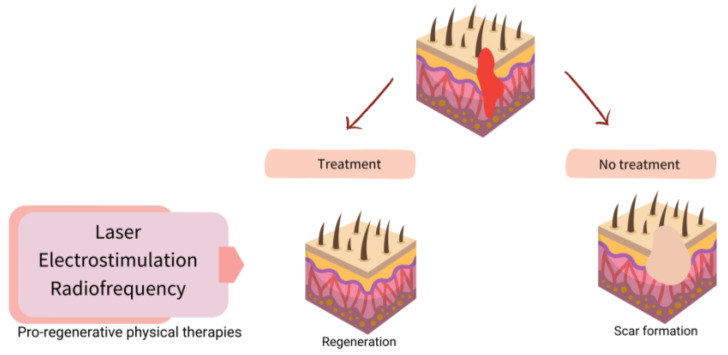
Once it is known what fails in WH, the possibility of understanding the role of physical therapies arises more clearly. Figure 2 shows a scheme of possible targets for increasing WH, and Figure 3 shows how to promote regeneration rather than scarring with physical therapies (PT). It is of notice that with their theoretical mechanisms, we can impact in all the phases completing and fostering traditional treatments with innocuity.
Figure 2.
Diagram representing different targets with physical therapies (PT) for promoting wound healing (WH). PBM: photobiomodulation; LFU: low frequent ultrasound; PDT: photodynamic therapy; ES: electrostimulation; VPL: visible pulsed light; PEMF: pulsed electromagnetic fields; BT: biophotonic therapy; RF: radiofrequency.
Figure 3.
Scheme of strategies for physical therapies (PT) in assisted well-scaring.
The guidelines for the management of CW are extensive, but the pillars are promoting patient adherence to treatment, debridement control of the possible infection, covering with an appropriate dressing and effective compression if necessary [1].
Two options appear when PT are introduced in the treatment, either CW or scarring. One is proactive management, starting the treatment in the initial phases of the wound, as a prevention or adjuvant therapy. The other one is using those therapies when a CW, HS or keloid has appeared. Both situations not only depend on the physician but also the patient consultation.
4. Physical Therapies in Assisted Healing and Scar Prevention
4.1. Laser
The main target of laser therapy is the treatment and prevention of scarring, and there are few studies published in its assistance in WH. Among the different issues presented in an HS or keloids, different lasers could be used to target each objective [34] (Table 4). Laser treatment is flexible and allows for their combination in a single treatment session. The most widely applied are fractional lasers in combination with vascular lasers and lasers targeting melanin [35]. Basically, there are two different types of fractional laser: ablative (wavelengths of 2790–10,600 nm) and non-ablative (1320–1927 nm). Both ablative and non-ablative lasers have become the gold standard for the treatment of scarring, although ablative lasers are probably the most used [36].
Table 4.
Targeting lasers according to clinical exploration in hypertrophic scars and keloids.
| Skin Alteration | Type of Laser |
|---|---|
| Erythema | Pulsed dye laser Intense pulsed light Neodimio-Yag laser |
| Skin thickness/Hypertrophic | Erbio laser CO2 laser Non-ablative laser |
| Hyperpigmentation | Alejandrita laser Intense pulsed light KTP 532 nm Q-switched laser |
Erbio and CO2 lasers are ablative lasers that target water, producing a selective burn in the skin. In a fractional mode, they work in separated columns, allowing for a better regeneration throughout the non-damage columns of skin. Both induce selective thermal necrosis in the skin, increasing in the first weeks of the inflammatory stage of the scar, but after three months, collagen remodeling in a thin bundle due to collagen III appears [37]. The clinical results show a decreasing dermis thickness and increasing skin flexibility [35]. On the other hand, vascular lasers target small vessels and are used for decreasing erythema in HS and keloids, causing excessive neovascularization. Pulsed dye laser (PDL) is probably the most used. PDL has been demonstrated to decrease connective tissue growth factor expression in keloids, despite targeting vessels [38] and inhibiting fibroblast proliferation in vitro [39]. After the vessel coagulation and subsequent hypoxia, PDL leads to an increase in collagen type III [40].
Apart from the treatment of scars, some studies of lasers in assisted WH and preventing scarring from the first day of surgery have been published showing different results. Curiously, the immediate application of lasers differs from other physical therapies, which need some healing days before they start to be applied. In a split-face study, no differences were found in the area treated with CO2 laser immediately after surgery, but in other similar studies the scars treated exhibit better healing and cosmetic outcome [41,42,43]. PDL and non-ablative fractional laser have also been shown to improve scaring when used early; however, the differences with the untreated area were not statistically significant [44]. Different types of lasers could be applied in the same session, PDL plus ablative fractional CO2 laser have been suggested to be the best combination [45].
An early start of the treatment is recommended in the literature reviewed when lasers are used to assist scarring. The optimal interval between sessions has been found to be 5 to 6 weeks during a period of months [46]. All the lasers applied in early treatment times have also been used under lower parameters [40]. Further clinical trials with long-term follow-up are needed to support the evidence of laser treatment in HS, keloids and WH alone or in combination with other options for treatment [47]; however, lasers are recommended for expert panels as a first-line therapy in scarring [48].
4.2. Photobiomodulation (Low-Level Light Therapy-LLLT)
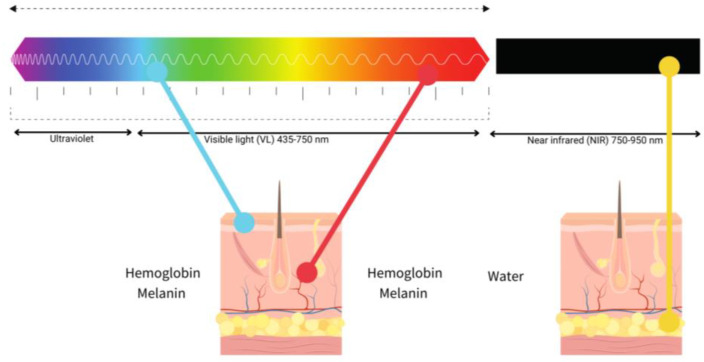
LLLT has been intensely studied in WH, the near-infrared light (NIR) between 800 and 900 nm and red light (600–700 nm) being the most used. The use of light in a non-thermal effect is supported by the photon’s absorption of the cells’ receptors. The main three chromophores in the skin are melanin in the epidermis, hemoglobin in the dermis and water in all the skin [49] and longer wavelengths achieve deeper penetration (Figure 4).
Figure 4.
Diagram of the relationship between visible light (blue, red) and near-infrared (NIR), penetration and chromophores.
Hormesis responses occur in WH in response to low doses of light (LLLT) or photobiomodulation (PBM). Hormesis or biomodulation are terms used to describe a natural biological process in which low doses of an input, for example, light or energy, induce activation, but high doses produce an inhibition [50]. PBM induces the production of nitric oxide (NO), a vasodilator, and anti-inflammatory agent (Table 5) [51]. LLLT can trigger natural mechanisms involved in WH, including TGF-beta families of molecules, transforming growth platelet factor, interleukins (IL6, 13, 15), and matrix metalloproteinases (MMPs) associated with alterations in WH. TGF-beta is crucial in fibroblast proliferation [7,50,51]. Thus, PBM has been demonstrated to be useful in all the steps of WH.
Table 5.
Summary of the beneficial effects of photobiomodulation (PBM) in wound healing (WH) and chronic wound (CW).
| Effect | Mediator | Phase of WH/CW |
|---|---|---|
| Anti-inflammatory | ROS, NO, IL | Inflammation |
| Vasodilatation | NO | Proliferation |
| Matrix formation | TGF-beta and MMPs | Proliferation and remodeling |
| Promote epithelial cell function | Cyclin D1 | Proliferation and remodeling |
ROS: radical oxygen singlet; NO: nictric oxide; IL: interleukins; MMPs: metaloproteinases.
In animal models, LLLT increases collagen and reduces oxidative and nitroxidative stress in diabetic wounded mouse skin [51]. In vitro studies have also found an increased expression in keratinocytes after LLLT of cyclin D1 and cytokeratin, suggesting an increase in proliferation and maturation [52,53].
LLLT is not as widely used as laser despite being safer, without adverse reactions such as swelling, crusting or purpura. With respect to laser, LLLT is easy to apply, allows the treatment of bigger areas, a wearable device is available, self-treating is an opportunity and it is not as expensive. The main disadvantage of LLLT is the necessity of near-daily repeated sessions [54].
There are few studies of LLLT in WH with different results. In VU, red light did not demonstrate any additional benefit to conventional treatment [55]. Whereas in PU and DU, red light increases healing with better outcomes when compared with NIR [56]. A prophylactic treatment in the prevention of keloid in three patients was shown effective with NIR (LED 805 nm). In this small study, patients self-treated at home daily for one month [57].
LLLT improves inflammation, releases pain, and fosters healing in clinical practice. Even though it has been deeply investigated, further studies in the daily clinical application are necessary as no standard protocol has been developed [54].
Blue Light Emission Diode
LED technology greatly benefited from the pioneering research conducted during the gallium nitride crystal boom of the 1980s by Akasaki, Amano, and Nakamura, which led to the invention of the blue LED. This discovery was extremely important as it made it possible to obtain white light from LED sources, paving the way for revolutionary uses of radiation [58].
Blue-light PBM triggers a cascade of events attributable to the absorption of photons by intracellular photoreceptors. Among these effects, the impact of light on cytochrome-C oxidase can be observed: it induces an increase in cell proliferation, migration and differentiation, cytokine modulation, growth factor synthesis, and anti-inflammatory effects; thus, stimulating the improvement of the healing process [59,60].
In wounds treated with blue light, a faster healing process and better deposition and morphology of dermal collagen are observed when compared to wounds not treated with blue light. Furthermore, treated wounds show better modulation of the inflammatory response where mast cells assume a central role [50].
4.3. Photodynamic Therapy
Photodynamic therapy (PDT) is a safe and easy procedure to enhance WH, nevertheless, further studies are necessary to determine an exact protocol. Anyhow, PDT is versatile, with the limitation of pain during the treatment and repeat sessions.
PDT is indicated in dermatology for the treatment of actinic keratosis, basal cell carcinoma and Bowen disease [61]. PDT has been explored in WH and prevents scarring, whereas no results have been found in the treatment of keloids and HS. The main difference between PDT and other PT in WH is the ability to scope infections without resistance to antibiotics.
PDT consists of the combination of a photosensitizer (PS) in the target tissue and the subsequent illumination of an adequate light source for inducing necrosis and apoptosis of the tissue. Through the literature, a variety of lights and PS have been tested in WH. Nowadays, PS are preferred to be used topically, as they have lower side effects. A lot of optimal light sources could be used in the PpIX absorption spectrum; however, LEDs are mostly explored for their simplicity and lower side effects. Table 6 summarized which PS could be used in WH and different light devices [62,63]. Most of the light sources are in the red spectrum [63], although there are studies with green light. The protocols and doses for the use of PDT in WH are very different, which is a limitation when trying to come up with a conclusion [64].
Table 6.
Summary of some of the photosensitizers (PS) and light sources used in photodynamic therapy (PDT) in wound healing (WH).
| Group of Photosensitizers | Molecule | Light Sources (570–800 nm) | Protocol |
|---|---|---|---|
| Hematoporphyrin derivates | Photofrin® | LED (red and NIR) | 37–100 J/cm2/session |
| PpIX precursors | ALA, MAL | Laser (Vascular and Diode) | |
| Clorins | Foscan® (mTHPC) | 1–2 sessions/week/1 month |
PpIX: Protoporphyrin IX; ALA: aminolaevulinic acid; MAL: Methylaminolaevulinic acid; mTHPC: meso-tetra-hydroyphenyl chlorin; LED: ligh emitting diodes; NIR: near infrared.
The PS increases the intracellular production of Protoporphyrin IX, which absorbs the light and produces the reaction. Destruction is mediated by the production of excessive intracellular ROS (radical oxygen singlet).
The mechanisms of action of PDT are well known; besides the necrosis of the tumors, a lot of parallel biological phenomena are produced, which lead to exploring other indications of WH (Table 7) [65].
Table 7.
Summary of the mechanism of action revised of PDT in WH.
| Effect | Mediator | Phase of Wound Healing |
|---|---|---|
| Activation/suppression of the immune system | TNF-alfa IL1, IL6, IL10 |
Inflammation |
| Antibacterial activity | ROS | Chronic inflammation |
| Reepithelization, matrix formation |
MMPs | Regeneration and remodeling |
| Neovascularization | VEGF | Regeneration and remodeling |
TNF: tumoral necrosis factor; IL: interleukins; ROS: radical oxygen singlet; MMPs: metalloproteinases; VEGF: vascular endothelial growth factor.
PDT produces the activation of acute inflammation in WH, fostering the natural process, and consequently, the neutrophils, TNF-alfa, and IL6 become increased [66]. PDT also induces neovascularization induced by VEGF needed for remodeling [30].
Additionally, studies have indicated that the early activation of fibroblasts and re-epithelization and increase in degranulation index by mast cells play a crucial role in the healing of chronic wounds. It is worth remembering how interactions of the immune system with the nervous system are important in the regulation of wound healing processes. Recent studies have demonstrated that MC interactions with neuronal cells containing neurotransmitters involved in wound healing processes, such as CGRP, NGF, NKA, NPY, SP, PGP 9.5, and VIP, are common in chronic wounds. This fact can be related to other facts such as the secretion of extracellular matrix by fibroblasts, as well as increases in TGF beta levels and the response of cellular infiltrates [18,30].
Afterwards, PDT a negative regulation of the inflammation appears with IL10 expression and down regulation of IL1 and IL6 [67]. It has been suggested that the modulatory effect of PDT in the immune system and the necrosis versus apoptosis induction depends on the intensity of the protocol [68], more specifically on the ROS levels. Therefore, high intracellular production of ROS could change the activation into destruction (hormesis) [69].
PDT increases the levels of MMPs after three weeks, and the histological improvement appears at nine months. On the other hand, PDT has antibacterial activity, targeting the biofilm, which is responsive to chronic inflammation [30,70].
4.4. Electrical Stimulation
Endogenous bioelectric fields (EBF) take place during WH, produced by the cells generated by the Na+/K+ ATPase of the epidermis. EBFs influence cell migration, proliferation, and function, but also gene and protein expression [71]. The underlying mechanisms presented in a CW could be targeted with electrical stimulation (ES) mimicking the natural process (Figure 2). Table 8 summarized the effects of ES in WH outlining in which part of the process this mechanism is working. Theoretically, ES offers benefits in WH after some days in the wound improving the proliferation and remodeling phase. Moreover, if a CW is established, ES could decrease inflammation and the risk of infection. In vitro studies have demonstrated a decrease in Staphylococcus aureus, Pseudomonas aeruginosa and Escherichia coli with ES [72]. Positive scattered results with ES have been reported in CW, VU, and LU, with a possible increase of 30–42% in the reduction in the wound area [72,73]. ES is a safe, simple, cheap, and easy procedure to use without adverse effects.
Table 8.
Beneficial effects reported of electrical stimulation (ES) in wound healing (WH) and chronic wound (CW).
| Effect | Mediator | Phase of WH/CW |
|---|---|---|
| Angiogenesis | VEGF | Proliferation |
| Fibroblast proliferation | FGF | Proliferation and remodeling |
| Reduces bacterial colonization | PH alteration | Persistent inflammation and risk of infection |
EGF: vascular endothelial growth factor; FGF: fibroblast growth factor.
There are different forms of ES, including direct current, alternating current and pulsed current on mono or bipolar devices. That huge variability limits knowing the real exact beneficial protocol. Moreover, no comparative study between those modalities has been conducted, whereas it is supposed that the pulsed current is the most similar to the physiological(25) [25,71,72]. Theoretically, not all forms of ES are beneficial in all phases of WH, the alternative current only being useful in the first days [25]. There is also a lack of literature about standard protocols [73].
According to the mechanism of action of ES, it would be more effective in the proliferative and remodeling phase of WH, that implies, from days to months after the injury, either in acute wounds (AW) to prevent scarring or in CW to enhance healing [73].
If ES is applied, it should be added to the conventional treatment of the wound as a complementary treatment (Table 9). The ES devices are usually applied by setting electrodes around the wound. Repeated-weekly sessions are necessarily, lasting from 45 min to hours [25]. The therapy could last months, which is a great limitation due to time consumption and displacement. Therefore, ES might be used in selected patients with a risk of failure in WH. Novel devices are emerging, offering different possibilities such as home devices, electric dressings, or electric fields, providing a practical future [71].
Table 9.
Summary of the practical initial application of electrical stimulation (ES) in wound healing (WH).
| Modality | Type of Wound | Not yet Studied/Not Beneficial |
|---|---|---|
| Pulsed current Electrodes around the wound From 30 min to hours |
Acute current in CW | |
| From 5 to 7 days a week | DU | Scarring prevention or treatment |
| After 2–5 days of the injury | LU |
DU: diabetic ulcer; LU: Leg ulcer.
4.5. Others
4.5.1. Ultrasound Therapy
Ultrasound therapy (UT) consists of sound waves that cause thermal and non-thermal effects in tissues. When UT is strongly applied to the skin, the temperature will rise to 40 Celsius degrees and produces an increase in vessel flow, cell proliferation, collagen synthesis, and tissue regeneration. Moreover, UT has anti-inflammatory properties. The non-thermal effects comply with acoustic streaming with a displacement of the particles and cavitation with the generation of microenvironmental gases [74]. Cavitation cleans necrotic tissue preserving the healthy one [7].
UT accelerates the decrease in the wound area with respect to controls in LU, and it is approved as an adjuvant therapy in WH by the FDA [75,76].
Two types of therapeutic US exits are low frequency ultrasound (LFU) from 30 to 40 kHz and high-frequency ultrasound (HFU), ranging from 1 to 3 MHz. HFU has been used for decades for the treatment of muscular diseases in sports medicine. A variant of HFU, micro focused ultrasound in high intense mode (MFU, HIFU), is being widely studied because of its benefits in aesthetic medicine reducing wrinkles and laxity of the skin [75]. In contrast, LFU has demonstrated efficacy in WH and has been applied with good results in LU.
LFU is used directly on the skin, around the wound for 5 to 10 min (Table 10). A topical gel is usually needed between the skin surface and the applicator [74]. UT is contraindicated in a patient carrying a metal prosthesis in the leg, neuropathy, infection, or thrombophlebitis [75].
Table 10.
Summary of the practical protocols reviewed in ultrasound therapy (UT) in wound healing (WH).
| Modality | Type of Wound | Not yet Studied/Not Beneficial |
|---|---|---|
| LFU Around the wound |
LU | HFU Contraindicated metal prosthesis, infection, or neuropathy |
| From 5 to 10 min Repeated sessions in a week |
Prevent or treat keloids or HS | |
| After 2–5 days of the injury |
LU: Leg ulcer; HFU: high-frequency ultrasound; HS: Hypertrophic scars.
UT has a possible application in WH; nevertheless, there are no clinical studies of the effects of UT in WH or scar prevention, most of the evidence is limited to LU and further randomized clinical essays and protocols are necessary [75,76].
4.5.2. Electromagnetic Fields
Low frequency pulsed electromagnetic fields (PEMF) can accelerate WH, generating connective tissue, enhancing the VEGF pathway and the production of collagen type I. There are some published studies with good results in PU, VLU and DU. PEMF are possible to apply at home on portable devices as multiple sessions are necessary [77,78].
4.5.3. Biophotonic Therapy
Biophotonic therapy (BT) consists of the application of the PBM applying a special gel over the CW containing the chromophores. Afterwards, an LED lamp with a hyper pulsed beam and low energy is used to activate the photoconverter gel. One concrete device known as “Lumihel®” was evaluated showing improvement in the healing of the CW, increasing the life quality of the patient without adverse events. The main limitations of the study were the simultaneous inclusion of VU, LU and PU and the weekly treatment sessions lasting 8 weeks [79].
4.5.4. Visible Polarized Light
Visible polarized light (VPL) has been used as a complementary therapy in WH. The device used emitted light like the sun but without ultraviolet radiation. Thus, the light used was safe, low energy light, polychromatic, incoherent, and polarized. The polarization allows it to work on flat surfaces and enhances light penetration. The molecular mechanism of action of VL is not well documented; however, some studies showing improvement in treated CW have been published [80,81]. PVL seems a promising possible treatment for WH but needs to be more deeply studied [82].
4.5.5. Radiofrequency
Radiofrequency therapies consist of the application of a high-frequency electromagnetic field (3 kHz and 300 GHz) that induces oscillation and friction in the molecules of the target tissue, which causes tissue hyperthermia. This electrically induced hyperthermia can degrade collagen, which stimulates neocollagenogenesis and tissue remodeling [83]. The main indicators of RF are skin tightening, the reduction of wrinkles and the treatment of scars. There are some studies assessing the efficacy of RF in WH with good results in releasing pain. Nevertheless, multiple sessions are necessary for 2–4 weeks. RF technology is rapidly developing, with new micro-needling devices and fractionated delivery, which shows good results in acne scars, HS, and keloids [21,84].
5. Summary of Clinical Trials of Physical Therapies in Wound Healing
Finally, it is important to take into consideration that there are few clinical trials published about interventions with PT in WH. When the term “Physical therapies skin wounds” is introduced in “Pubmed” and filters added as, “last 10 years”, “clinical trials” a total of 66 results appeared. After having been selected, only 8 works were focused on skin and only 6 in CW (Figure S1).
PT are not included in clinical Guidelines of management of CW, and they are used as adjuvant therapies [1]. Polak treated with electrical stimulation (ES) areas of pressure injuries in patients with neurological damage. A total of 43 patients were divided into three groups, anodal, cathodal and placebo. A diminution of proinflammatory blood cytokines was found in the patients treated in correlation with an improvement in the clinical peripheric inflammation of the wound and a significant reduction in the size of the wound was assessed, in comparison to the placebo group. The same therapy was used for other authors [85] to explore the effect in CW in combination with silver dressing. Ten patients were treated only in one wound and the rest were used as controls, leading to significant differences. ES has also been proved to alleviate pain in CW, in addition to accelerating healing [86]. In a study of 10 patients treated with ES compared with 10 patients treated with placebo, ES improved in DU the blood levels of VEGF and NO [87].
In an interesting study, ultrasound (US) and electrical stimulation (ES) were compared in the treatment of PU. Both treatments improved WH in 27 patients treated without significant differences between them [88]. Another comparative study was carried out by Polak in 77 patients with PU using standard wound care, US, and ES. Patients treated with PT had a significant decrease in the ulcer area compared with placebo without differences between US and ES [89]. The main limitation of all those clinical trials is control over others factor that could influence the healing of the wound.
6. Conclusions and Future Perspectives
WH and pathological scars are important problems in daily practice causing pain and morbidity and are difficult to manage. PT arises as a possible safe complementary treatment that might improve the results of the traditional treatment. PT has been demonstrated to improve tissue healing with different grades or evidence; however, further studies are necessary to develop practical protocols in clinical practice based on the theoretical mechanism of action of the therapy. The main limitations of PT are the lack of clinical trials, the availability, the variability of the parameters used in different conditions and the lack of comparable results. Probably, electrical stimulation and ultrasound are the most studied. The scope of this review was to offer a complete view of PT for clinicians in WH so they can start working up new adjuvant protocols.
Abbreviations
| AU | Atypical ulcer |
| BT | Biophotonic therapy |
| CW | Chronic wound |
| DU | Diabetic ulcer |
| EBF | Endogenous bioelectric fields |
| ES | Electrical stimulation |
| FHW | Fail to heal wound |
| HS | Hypertrophic scar |
| LLLT | Low level laser light therapy |
| PDL | Pulsed dye laser |
| PEMF | Pulsed electromagnetic field |
| PT | Physical therapies |
| PU | Pressure ulcer |
| US | Ultrasound |
| UT | Ultrasound therapy |
| VU | Venous leg ulcer |
| WH | Wound healing |
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24087487/s1.
Author Contributions
Conceptualization, M.F.-G. and M.L.H.-B.; methodology, S.B., M.F.-G. and M.L.H.-B.; software, M.F.-G.; investigation, M.B.-M. and A.C.-M.; resources, M.F.-G.; writing—original draft preparation, M.F.-G. and S.B.; writing—review and editing, M.F.-G., L.A.P.G. and S.B.; visualization, M.F.-G., M.L.H.-B. and S.B.; supervision, M.L.H.-B. and S.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data reviewed in the manuscript are published in the articles mentioned in the references and selected for being writing in English and indexed in PubMed.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Gupta S., Andersen C., Black J., Fife C., Lantis J.I., Niezgoda J., Snyder R., Sumpio B., Tettelbach W., Treadwell T., et al. Management of Chronic Wounds: Diagnosis, Preparation, Treatment, and Follow-Up. Wounds Compend. Clin. Res. Pract. 2017;29:S19–S36. [PubMed] [Google Scholar]
- 2.Wang P.H., Huang B.S., Horng H.C., Yeh C.C., Chen Y.J. Wound healing. J. Chin. Med. Assoc. 2018;81:94–101. doi: 10.1016/j.jcma.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Rodrigues M., Kosaric N., Bonham C.A., Gurtner G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019;99:665–706. doi: 10.1152/physrev.00067.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veith A.P., Henderson K., Spencer A., Sligar A.D., Baker A.B. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv. Drug Deliv. Rev. 2019;146:97–125. doi: 10.1016/j.addr.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monavarian M., Kader S., Moeinzadeh S., Jabbari E. Regenerative Scar-Free Skin Wound Healing. Tissue Eng. Part B Rev. 2019;25:294–311. doi: 10.1089/ten.teb.2018.0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bai Q., Han K., Dong K., Zheng C., Zhang Y., Long Q., Lu T. Potential Applications of Nanomaterials and Technology for Diabetic Wound Healing. Int. J. Nanomed. 2020;15:9717–9743. doi: 10.2147/IJN.S276001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmieri B., Vadalà M., Laurino C. Electromedical devices in wound healing management: A narrative review. J. Wound Care. 2020;29:408–418. doi: 10.12968/jowc.2020.29.7.408. [DOI] [PubMed] [Google Scholar]
- 8.Lu W.-S., Zheng X.-D., Yao X.-H., Zhang L.-F. Clinical and epidemiological analysis of keloids in Chinese patients. Arch. Dermatol. Res. 2015;307:109–114. doi: 10.1007/s00403-014-1507-1. [DOI] [PubMed] [Google Scholar]
- 9.Berman B., Maderal A., Raphael B. Keloids and Hypertrophic Scars: Pathophysiology, Classification, and Treatment. Dermatol. Surg. 2017;43:S3–S18. doi: 10.1097/DSS.0000000000000819. [DOI] [PubMed] [Google Scholar]
- 10.He Y., Deng Z., Alghamdi M., Lu L., Fear M.W., He L. From genetics to epigenetics: New insights into keloid scarring. Cell Prolif. 2017;50:e12326. doi: 10.1111/cpr.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang C., Ogawa R. Systemic factors that shape cutaneous pathological scarring. FASEB J. 2020;34:13171–13184. doi: 10.1096/fj.202001157R. [DOI] [PubMed] [Google Scholar]
- 12.Huang C., Ogawa R. Keloidal pathophysiology: Current notions. Scars Burn. Health. 2021;7 doi: 10.1177/2059513120980320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowers S., Franco E. Chronic Wounds: Evaluation and Management. Am. Fam. Physician. 2020;101:159–166. [PubMed] [Google Scholar]
- 14.Gottrup F. A specialized wound-healing center concept: Importance of a multidisciplinary department structure and surgical treatment facilities in the treatment of chronic wounds. Am. J. Surg. 2004;187:S38–S43. doi: 10.1016/S0002-9610(03)00303-9. [DOI] [PubMed] [Google Scholar]
- 15.Fife C.E., Carter M.J. Wound Care Outcomes and Associated Cost among Patients Treated in US Outpatient Wound Centers: Data from the US Wound Registry. Wounds A Compend. Clin. Res. Pract. 2012;24:10–17. [PubMed] [Google Scholar]
- 16.Gurtner G.C., Werner S., Barrandon Y., Longaker M.T. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 17.Furie B., Furie B.C. Mechanisms of thrombus formation. N. Engl. J. Med. 2008;359:938–949. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 18.Bacci S. Fine Regulation during Wound Healing by Mast Cells, a Physiological Role Not Yet Clarified. Int. J. Mol. Sci. 2022;23:1820. doi: 10.3390/ijms23031820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jorch S.K., Kubes P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat Med. 2017;23:279–287. doi: 10.1038/nm.4294. [DOI] [PubMed] [Google Scholar]
- 20.Kolaczkowska E., Kubes P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 21.Akbik D., Ghadiri M., Chrzanowski W., Rohanizadeh R. Curcumin as a wound healing agent. Life Sci. 2014;116:1–7. doi: 10.1016/j.lfs.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Wilkinson H.N., Hardman M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020;10:200223. doi: 10.1098/rsob.200223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jupiter D.C., Thorud J.C., Buckley C.J., Shibuya N. The impact of foot ulceration and amputation on mortality in diabetic patients. I: From ulceration to death, a systematic review. Int. Wound J. 2016;13:892–903. doi: 10.1111/iwj.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meulendijks A.M., de Vries F.M.C., van Dooren A.A., Schuurmans M.J., Neumann H.A.M. A systematic review on risk factors in developing a first-time Venous Leg Ulcer. J. Eur. Acad. Dermatol. Venereol. 2019;33:1241–1248. doi: 10.1111/jdv.15343. [DOI] [PubMed] [Google Scholar]
- 25.Aleksandrowicz H., Owczarczyk-Saczonek A., Placek W. Venous Leg Ulcers: Advanced Therapies and New Technologies. Biomedicines. 2021;9:1569. doi: 10.3390/biomedicines9111569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilcox J.R., Carter M.J., Covington S. Frequency of debridements and time to heal: A retrospective cohort study of 312 744 wounds. JAMA Dermatol. 2013;149:1050–1058. doi: 10.1001/jamadermatol.2013.4960. [DOI] [PubMed] [Google Scholar]
- 27.Azevedo M.-M., Lisboa C., Cobrado L., Pina-Vaz C., Rodrigues A.G. Hard-to-heal wounds, biofilm and wound healing: An intricate interrelationship. Br. J. Nurs. 2020;29:S6–S13. doi: 10.12968/bjon.2020.29.5.S6. [DOI] [PubMed] [Google Scholar]
- 28.Sun F., Qu F., Ling Y., Mao P., Xia P., Chen H., Zhou D. Biofilm-associated infections: Antibiotic resistance and novel therapeutic strategies. Futur. Microbiol. 2013;8:877–886. doi: 10.2217/fmb.13.58. [DOI] [PubMed] [Google Scholar]
- 29.Pastar I., Stojadinovic O., Yin N.C., Ramirez H., Nusbaum A.G., Sawaya A., Patel S.B., Khalid L., Isseroff R.R., Tomic-Canic M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care. 2014;3:445–464. doi: 10.1089/wound.2013.0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grandi V., Corsi A., Pimpinelli N., Bacci S. Cellular Mechanisms in Acute and Chronic Wounds after PDT Therapy: An Update. Biomedicines. 2022;10:1624. doi: 10.3390/biomedicines10071624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang C., Akaishi S., Hyakusoku H., Ogawa R. Are keloid and hypertrophic scar different forms of the same disorder? A fibroproliferative skin disorder hypothesis based on keloid findings. Int. Wound J. 2014;11:517–522. doi: 10.1111/j.1742-481X.2012.01118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khetarpal S., Kaw U., Dover J.S., Arndt K.A. Laser advances in the treatment of burn and traumatic scars. Semin. Cutan. Med. Surg. 2017;36:185–191. doi: 10.12788/j.sder.2017.030. [DOI] [PubMed] [Google Scholar]
- 33.Jourdan M., Madfes D.C., Lima E., Tian Y., Seité S. Skin Care Management for Medical and Aesthetic Procedures to Prevent Scarring. Clin. Cosmet. Investig. Dermatol. 2019;12:799–804. doi: 10.2147/CCID.S218134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kauvar A.N.B., Kubicki S.L., Suggs A.K., Friedman P.M. Laser Therapy of Traumatic and Surgical Scars and an Algorithm for Their Treatment. Lasers Surg. Med. 2020;52:125–136. doi: 10.1002/lsm.23171. [DOI] [PubMed] [Google Scholar]
- 35.Altemir A., Boixeda P. Laser Treatment of Burn Scars. Actas Dermosifiliogr. 2022;113:T938–T944. doi: 10.1016/j.ad.2022.10.009. [DOI] [PubMed] [Google Scholar]
- 36.Clementoni M.T., Pedrelli V., Zaccaria G., Pontini P., Motta L.R., Azzopardi E.A. New Developments for Fractional CO2 Resurfacing for Skin Rejuvenation and Scar Reduction. Facial Plast. Surg. Clin. N. Am. 2020;28:17–28. doi: 10.1016/j.fsc.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Azzam O.A., Bassiouny D.A., El-Hawary M.S., El Maadawi Z.M., Sobhi R.M., El-Mesidy M.S. Treatment of hypertrophic scars and keloids by fractional carbon dioxide laser: A clinical, histological, and immunohistochemical study. Lasers Med. Sci. 2016;31:9–18. doi: 10.1007/s10103-015-1824-4. [DOI] [PubMed] [Google Scholar]
- 38.Yang Q., Ma Y., Zhu R., Huang G., Guan M., Avram M.M., Lu Z. The effect of flashlamp pulsed dye laser on the expression of connective tissue growth factor in keloids. Lasers Surg. Med. 2012;44:377–383. doi: 10.1002/lsm.22031. [DOI] [PubMed] [Google Scholar]
- 39.Zhibo X., Miaobo Z. Molecular mechanism of pulsed-dye laser in treatment of keloids: An in vitro study. Adv. Skin Wound Care. 2010;23:29–33. doi: 10.1097/01.ASW.0000363486.94352.41. [DOI] [PubMed] [Google Scholar]
- 40.Lv K., Xia Z., Chinese Consensus Panel on the Prevention and Treatment of Scars Chinese expert consensus on clinical prevention and treatment of scar+ Burn. Trauma. 2018;6:27. doi: 10.1186/s41038-018-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sobanko J.F., Vachiramon V., Rattanaumpawan P., Miller C.J. Early postoperative single treatment ablative fractional lasing of Mohs micrographic surgery facial scars: A split-scar, evaluator-blinded study. Lasers Surg. Med. 2015;47:1–5. doi: 10.1002/lsm.22314. [DOI] [PubMed] [Google Scholar]
- 42.Shin H.W., Suk S., Chae S.W., Yoon K.C., Kim J. Early postoperative treatment of mastectomy scars using a fractional carbon dioxide laser: A randomized, controlled, split-scar, blinded study. Arch. Plast. Surg. 2021;48:347–352. doi: 10.5999/aps.2020.02495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee S.H., Zheng Z., Roh M.R. Early Postoperative Treatment of Surgical Scars Using a Fractional Carbon Dioxide Laser: A Split-Scar, Evaluator-Blinded Study. Dermatol. Surg. 2013;39:1190–1196. doi: 10.1111/dsu.12228. [DOI] [PubMed] [Google Scholar]
- 44.Kim D.H., Ryu H.J., Choi J.E., Ahn H.H., Kye Y.C., Seo S.H. A Comparison of the Scar Prevention Effect between Carbon Dioxide Fractional Laser and Pulsed Dye Laser in Surgical Scars. Dermatol. Surg. 2014;40:973–978. doi: 10.1097/01.DSS.0000452623.24760.9c. [DOI] [PubMed] [Google Scholar]
- 45.Liu X.-J., Liu W.-H., Fang S.-W., Zhou X.-L., Xu J.-X., Li G.-S. Lasers and Intense Pulsed Light for the Treatment of Pathological Scars: A Network Meta-Analysis. Aesthetic Surg. J. 2022;42:NP675–NP687. doi: 10.1093/asj/sjac175. [DOI] [PubMed] [Google Scholar]
- 46.Brewin M.P., Lister T.S. Prevention or treatment of hypertrophic burn scarring: A review of when and how to treat with the Pulsed Dye Laser. Burns. 2014;40:797–804. doi: 10.1016/j.burns.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 47.Leszczynski R., da Silva C.A., Pinto A., Kuczynski U., da Silva E.M. Laser therapy for treating hypertrophic and keloid scars. Cochrane Database Syst. Rev. 2022;9:CD011642. doi: 10.1002/14651858.CD011642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seago M., Shumaker P.R., Spring L.K., Alam M., Al-Niaimi F., Anderson R.R., Artzi O., Bayat A., Cassuto D., Chan H.H., et al. Laser Treatment of Traumatic Scars and Contractures: 2020 International Consensus Recommendations. Lasers Surg. Med. 2020;52:96–116. doi: 10.1002/lsm.23201. [DOI] [PubMed] [Google Scholar]
- 49.Mosca R.C., Ong A.A., Albasha O., Bass K., Arany P. Photobiomodulation Therapy for Wound Care: A Potent, Noninvasive, Photoceutical Approach. Adv. Ski. Wound Care. 2019;32:157–167. doi: 10.1097/01.ASW.0000553600.97572.d2. [DOI] [PubMed] [Google Scholar]
- 50.Calabrese E.J., Dhawan G., Kapoor R., Agathokleous E., Calabrese V. Hormesis: Wound healing and fibroblasts. Pharmacol. Res. 2022;184:106449. doi: 10.1016/j.phrs.2022.106449. [DOI] [PubMed] [Google Scholar]
- 51.Tatmatsu-Rocha J.C., Ferraresi C., Hamblin M.R., Maia F.D., do Nascimento N.R., Driusso P., Parizotto N. Low-level laser therapy (904 nm) can increase collagen and reduce oxidative and nitrosative stress in diabetic wounded mouse skin. J. Photochem. Photobiol. B Biol. 2016;164:96–102. doi: 10.1016/j.jphotobiol.2016.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sperandio F.F., Simões A., Corrêa L., Aranha A.C.C., Giudice F.S., Hamblin M.R., Sousa S.C. Low-level laser irradiation promotes the proliferation and maturation of keratinocytes during epithelial wound repair. J. Biophotonics. 2014;8:795–803. doi: 10.1002/jbio.201400064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Calabrese E.J., Dhawan G., Kapoor R., Agathokleous E., Calabrese V. Hormesis: Wound healing and keratinocytes. Pharmacol. Res. 2022;183:106393. doi: 10.1016/j.phrs.2022.106393. [DOI] [PubMed] [Google Scholar]
- 54.Heiskanen V., Hamblin M.R. Photobiomodulation: Lasers vs. light emitting diodes? Photochem. Photobiol. Sci. 2018;17:1003–1017. doi: 10.1039/c8pp00176f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vitse J., Bekara F., Byun S., Herlin C., Teot L. A Double-Blind, Placebo-Controlled Randomized Evaluation of the Effect of Low-Level Laser Therapy on Venous Leg Ulcers. Int. J. Low. Extremity Wounds. 2017;16:29–35. doi: 10.1177/1534734617690948. [DOI] [PubMed] [Google Scholar]
- 56.Taradaj J., Halski T., Kucharzewski M., Urbanek T., Halska U., Kucio C. Effect of Laser Irradiation at Different Wavelengths (940, 808, and 658 nm) on Pressure Ulcer Healing: Results from a Clinical Study. Evid. Based Complement. Altern. Med. 2013;2013:960240. doi: 10.1155/2013/960240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barolet D., Boucher A. Prophylactic low-level light therapy for the treatment of hypertrophic scars and keloids: A case series. Lasers Surg. Med. 2010;42:597–601. doi: 10.1002/lsm.20952. [DOI] [PubMed] [Google Scholar]
- 58.Akasaki I. Blue Light: A Fascinating Journey (Nobel Lecture) Angew. Chem. Int. Ed. Engl. 2015;54:7750–7763. doi: 10.1002/anie.201502664. [DOI] [PubMed] [Google Scholar]
- 59.Dini V., Romanelli M., Oranges T., Davini G., Janowska A. Blue light emission in the management of hard-to-heal wounds. G. Ital. Dermatol. E Venereol. 2021;156:703–713. doi: 10.23736/S2784-8671.20.06691-2. [DOI] [PubMed] [Google Scholar]
- 60.Ngoc L.T.N., Moon J.Y., Lee Y.C. Utilization of light-emitting diodes for skin therapy: Systematic review and meta-analysis. Photodermatol. Photoimmunol. Photomed. 2022 doi: 10.1111/phpp.12841. [DOI] [PubMed] [Google Scholar]
- 61.Morton C.A., Szeimies R.M., Basset-Seguin N., Calzavara-Pinton P., Gilaberte Y., Haedersdal M., Hofbauer G.F.L., Hunger R.E., Karrer S., Piaserico S., et al. European Dermatology Forum guidelines on topical photodynamic therapy 2019 Part 1: Treatment delivery and established indications—Actinic keratoses, Bowen’s disease and basal cell carcinomas. J. Eur. Acad. Dermatol. Venereol. 2019;33:2225–2238. doi: 10.1111/jdv.16017. [DOI] [PubMed] [Google Scholar]
- 62.Oyama J., Ramos-Milaré Á.C.F.H., Lera-Nonose D.S.S.L., Nesi-Reis V., Demarchi I.G., Aristides S.M.A., Teixeira J.J.V., Silveira T.G.V., Lonardoni M.V.C. Photodynamic therapy in wound healing in vivo: A systematic review. Photodiagnosis Photodyn. Ther. 2020;30:101682. doi: 10.1016/j.pdpdt.2020.101682. [DOI] [PubMed] [Google Scholar]
- 63.Nesi-Reis V., Lera-Nonose D., Oyama J., Silva-Lalucci M.P.P., Demarchi I.G., Aristides S.M.A., Teixeira J.J.V., Silveira T.G.V., Lonardoni M.V.C. Contribution of photodynamic therapy in wound healing: A systematic review. Photodiagnosis Photodyn. Ther. 2017;21:294–305. doi: 10.1016/j.pdpdt.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 64.Kang K., Bacci S. Photodynamic Therapy. Biomedicines. 2022;10:2701. doi: 10.3390/biomedicines10112701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fernández-Guarino M., García-Morales I., Harto A., Montull C., Pérez-García B., Jaén P. Photodynamic Therapy: New Indications. Actas Dermo-Sifiliográficas Engl. Ed. 2007;98:377–395. doi: 10.1016/S0001-7310(07)70091-1. [DOI] [PubMed] [Google Scholar]
- 66.Li L., Yang Y., Yang Z., Zheng M., Luo G., He W., Yin R. Effects of ALA-PDT on the macrophages in wound healing and its related mechanisms in vivo and in vitro. Photodiagnosis Photodyn. Ther. 2022;38:102816. doi: 10.1016/j.pdpdt.2022.102816. [DOI] [PubMed] [Google Scholar]
- 67.Choi J.Y., Park G.T., Na E.Y., Wi H.S., Lee S.-C., Lee J.-B. Molecular changes following topical photodynamic therapy using methyl aminolaevulinate in mouse skin. J. Dermatol. Sci. 2010;58:198–203. doi: 10.1016/j.jdermsci.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 68.Mroz P., Hamblin M.R. The immunosuppressive side of PDT. Photochem. Photobiol. Sci. 2011;10:751–758. doi: 10.1039/c0pp00345j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khorsandi K., Hosseinzadeh R., Esfahani H., Zandsalimi K., Shahidi F.K., Abrahamse H. Accelerating skin regeneration and wound healing by controlled ROS from photodynamic treatment. Inflamm. Regen. 2022;42:40. doi: 10.1186/s41232-022-00226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Oliveira A.B., Ferrisse T.M., Fontana C.R., Basso F.G., Brighenti F.L. Photodynamic therapy for treating infected skin wounds: A systematic review and meta-analysis from randomized clinical trials. Photodiagnosis Photodyn Ther. 2022;40:103118. doi: 10.1016/j.pdpdt.2022.103118. [DOI] [PubMed] [Google Scholar]
- 71.Rajendran S.B., Challen K., Wright K.L., Hardy J.G. Electrical Stimulation to Enhance Wound Healing. J. Funct. Biomater. 2021;12:40. doi: 10.3390/jfb12020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ud-Din S., Bayat A. Electrical Stimulation and Cutaneous Wound Healing: A Review of Clinical Evidence. Healthcare. 2014;2:445–467. doi: 10.3390/healthcare2040445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koel G., Houghton P.E. Electrostimulation: Current Status, Strength of Evidence Guidelines, and Meta-Analysis. Adv. Wound Care. 2014;3:118–126. doi: 10.1089/wound.2013.0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cullum N., Nelson E.A., Flemming K., Sheldon T. Systematic reviews of wound care management: (5) beds; (6) compression; (7) laser therapy, therapeutic ultrasound, electrotherapy and electromagnetic therapy. Health Technol. Assess. 2001;5:9. doi: 10.3310/hta5090. [DOI] [PubMed] [Google Scholar]
- 75.Beheshti A., Shafigh Y., Parsa H., Zangivand A.A. Comparison of High-Frequency and MIST Ultrasound Therapy for the Healing of Venous Leg Ulcers. Adv. Clin. Exp. Med. 2014;23:969–975. doi: 10.17219/acem/37353. [DOI] [PubMed] [Google Scholar]
- 76.Cullum N., Liu Z. Therapeutic ultrasound for venous leg ulcers. Cochrane Database Syst. Rev. 2017;5:CD001180. doi: 10.1002/14651858.CD001180.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guerriero F., Botarelli E., Mele G., Polo L., Zoncu D., Renati P., Sgarlata C., Rollone M., Ricevuti G., Maurizi N., et al. Effectiveness of an Innovative Pulsed Electromagnetic Fields Stimulation in Healing of Untreatable Skin Ulcers in the Frail Elderly: Two Case Reports. Case Rep. Dermatol. Med. 2015;2015:576580. doi: 10.1155/2015/576580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kwan R.L., Wong W.C., Yip S.L., Chan K.L., Zheng Y.P., Cheing G.L. Pulsed electromagnetic field therapy promotes healing and microcirculation of chronic diabetic foot ulcers: A pilot study. Adv. Skin Wound Care. 2015;28:212–219. doi: 10.1097/01.ASW.0000462012.58911.53. [DOI] [PubMed] [Google Scholar]
- 79.Romanelli M., Piaggesi A., Scapagnini G., Dini V., Janowska A., Iacopi E., Scarpa C., Fauverghe S., Bassetto F., EUREKA Study Group Evaluation of fluorescence biomodulation in the real-life management of chronic wounds: The EUREKA trial. J. Wound Care. 2018;27:744–753. doi: 10.12968/jowc.2018.27.11.744. [DOI] [PubMed] [Google Scholar]
- 80.Taha M.M., El-Nagar M.M., Elrefaey B.H., Elkholy R.M., Ali O.I., Alkhamees N., Felaya E.-S.E.E.-S. Effect of Polarized Light Therapy (Bioptron) on Wound Healing and Microbiota in Diabetic Foot Ulcer: A Randomized Controlled Trial. Photobiomodulation Photomed. Laser Surg. 2022;40:792–799. doi: 10.1089/photob.2021.0175. [DOI] [PubMed] [Google Scholar]
- 81.M. Allam N., Eladl H.M., Eid M.M. Polarized Light Therapy in the Treatment of Wounds: A Review. Int. J. Low Extrem. Wounds. 2022:15347346221113991. doi: 10.1177/15347346221113991. [DOI] [PubMed] [Google Scholar]
- 82.Feehan J., Burrows S.P., Cornelius L., Cook A.M., Mikkelsen K., Apostolopoulos V., Husaric M., Kiatos D. Therapeutic applications of polarized light: Tissue healing and immunomodulatory effects. Maturitas. 2018;116:11–17. doi: 10.1016/j.maturitas.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 83.Ekelem C., Thomas L., Van Hal M., Valdebran M., Lotfizadeh A., Mlynek K., Mesinkovska N.A. Radiofrequency Therapy and Noncosmetic Cutaneous Conditions. Dermatol. Surg. 2019;45:908–930. doi: 10.1097/DSS.0000000000001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cucu C., Butacu A., Niculae B.D., Tiplica G.S. Benefits of fractional radiofrequency treatment in patients with atrophic acne scars—Literature review. J. Cosmet. Dermatol. 2021;20:381–385. doi: 10.1111/jocd.13900. [DOI] [PubMed] [Google Scholar]
- 85.Zhou K., Krug K., Stachura J., Niewczyk P., Ross M., Tutuska J., Ford G. Silver-Collagen Dressing and High-voltage, Pulsed-current Therapy for the Treatment of Chronic Full-thickness Wounds: A Case Series. J. Wound Ostomy Cont. Nurs. 2016;62:36–44. [PubMed] [Google Scholar]
- 86.Fraccalvieri M., Salomone M., Zingarelli E.M., Rivarossa F., Bruschi S. Electrical stimulation for difficult wounds: Only an alternative procedure? Int. Wound J. 2015;12:669–673. doi: 10.1111/iwj.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mohajeri-Tehrani M.R., Nasiripoor F., Torkaman G., Hedayati M., Annabestani Z., Asadi M.R. Effect of low-intensity direct current on expression of vascular endothelial growth factor and nitric oxide in diabetic foot ulcers. J. Rehabil. Res. Dev. 2014;51:815–824. doi: 10.1682/JRRD.2013.08.0174. [DOI] [PubMed] [Google Scholar]
- 88.Bora Karsli P., Gurcay E., Karaahmet O.Z., Cakci A. High-Voltage Electrical Stimulation Versus Ultrasound in the Treatment of Pressure Ulcers. Adv. Skin Wound Care. 2017;30:565–570. doi: 10.1097/01.ASW.0000526606.72489.99. [DOI] [PubMed] [Google Scholar]
- 89.Polak A., Taradaj J., Nawrat-Szoltysik A., Stania M., Dolibog P., Blaszczak E., Zarzeczny R., Juras G., Franek A., Kucio C. Reduction of pressure ulcer size with high-voltage pulsed current and high-frequency ultrasound: A randomised trial. J. Wound Care. 2016;25:742–754. doi: 10.12968/jowc.2016.25.12.742. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data reviewed in the manuscript are published in the articles mentioned in the references and selected for being writing in English and indexed in PubMed.