Abstract
Diglycerol phosphate accumulates under salt stress in the archaeon Archaeoglobus fulgidus (L. O. Martins, R. Huber, H. Huber, K. O. Stetter, M. S. da Costa, and H. Santos, Appl. Environ. Microbiol. 63:896–902, 1997). This solute was purified after extraction from the cell biomass. In addition, the optically active and the optically inactive (racemic) forms of the compound were synthesized, and the ability of the solute to act as a protecting agent against heating was tested on several proteins derived from mesophilic or hyperthermophilic sources. Diglycerol phosphate exerted a considerable stabilizing effect against heat inactivation of rabbit muscle lactate dehydrogenase, baker's yeast alcohol dehydrogenase, and Thermococcus litoralis glutamate dehydrogenase. Highly homologous and structurally well-characterized rubredoxins from Desulfovibrio gigas, Desulfovibrio desulfuricans (ATCC 27774), and Clostridium pasteurianum were also examined for their thermal stabilities in the presence or absence of diglycerol phosphate, glycerol, and inorganic phosphate. These proteins showed different intrinsic thermostabilities, with half-lives in the range of 30 to 100 min. Diglycerol phosphate exerted a strong protecting effect, with approximately a fourfold increase in the half-lives for the loss of the visible spectra of D. gigas and C. pasteurianum rubredoxins. In contrast, the stability of D. desulfuricans rubredoxin was not affected. These different behaviors are discussed in the light of the known structural features of rubredoxins. The data show that diglycerol phosphate is a potentially useful protein stabilizer in biotechnological applications.
One of the most striking characteristics of extremophiles is their ability to thrive under environmental conditions that would be lethal to most organisms. In particular, hyperthermophiles, having optimal growth temperatures above 80°C (4), pose intriguing questions regarding the biochemical strategies used for the adaptation of their cellular structures to withstand such high temperatures. Maintaining protein performance at high temperature could be accomplished by a number of mechanisms: (i) intrinsic thermostability, (ii) increased turnover, (iii) improved action of molecular chaperones, and (iv) extrinsic stabilization by compatible solutes (13). Although most enzymes from thermophilic sources show an intrinsic thermostability higher than that of their mesophilic counterparts, several enzymes derived from hyperthermophilic sources show an unexpectedly low intrinsic stability in vitro (13, 14). Therefore, extrinsic factors, such as compatible solutes, may play a role in the thermostabilization of these cellular components.
Some compatible solutes, namely, glutamate, betaine, and trehalose, are widespread in mesophilic organisms, but compatible solutes unique to thermophiles and hyperthermophiles have also been identified in recent years (8; H. Santos and M. S. da Costa, submitted for publication). Newly discovered solutes from thermophilic and hyperthermophilic organisms include cyclic-2,3-bisphosphoglycerate (17), two isomers of di-myo-inositol phosphate (25, 31), mannosylglycerate and mannosylglyceramide (24, 28, 36), di-mannosyl-di-myo-inositol phosphate (25), diglycerol phosphate (DGP) (26), and galactosyl-5-hydroxylysine (20). Many of these solutes have only been identified in marine thermophiles and hyperthermophiles and may constitute an adaptive feature of these organisms to high temperatures (8; Santos and da Costa, submitted). This view is supported by the enlarged intracellular pools of organic solutes accumulating in response to growth at supraoptimal temperatures (14, 20, 24, 26, 36) and by the demonstration of the protecting effect of some of these solutes against heat inactivation of several enzymes in vitro (14, 18, 29, 31, 32).
A few years ago we discovered and characterized the new compound DGP (the correct chemical designation is 1,1′-diglyceryl phosphate) as the major solute accumulating in the hyperthermophilic archaeon Archaeoglobus fulgidus primarily in response to a salt stress (26). When A. fulgidus was grown in medium containing 4.5% NaCl, the intracellular levels reached approximately 1.4 μmol · mg of protein−1 (26), which corresponds to a concentration of 350 mM, providing that the value of 2.2 μl · mg of dry weight−1 determined for the internal volume of Pyrococcus furiosus (24) is appropriate. The present work was planned to assess the thermoprotective properties of DGP as applied to proteins. Three enzymes were selected as targets for this study: rabbit muscle lactate dehydrogenase (LDH) and baker's yeast alcohol dehydrogenase (ADH) from mesophilic sources and glutamate dehydrogenase (GDH) from the hyperthermophilic archaeon Thermococcus litoralis. In addition, three homologous and well-characterized rubredoxins (from Clostridium pasteurianum, Desulfovibrio gigas, and Desulfovibrio desulfuricans ATCC 27774) were used to obtain information on the extent to which the thermoprotection conferred by the solute was determined by specific structural features of the target protein.
The poor growth yields of A. fulgidus and the large amounts of solute required precluded the use of this organism as a natural source of DGP for these studies. Therefore, we decided to resort to chemical synthesis to obtain the compound. Both the optically active and the racemic forms were synthesized, and indirect evidence for the stereochemistry of the natural solute was obtained.
(Part of the data presented was included in European patent application 98670002.9-2105.)
MATERIALS AND METHODS
Organisms and growth conditions.
A. fulgidus (DSM 4304) cultures were grown as described by Martins et al. (26) at 76°C in a medium containing 4.5% NaCl. T. litoralis (DSM 5473) was grown as previously described by Xavier et al. (41) with maltose and peptone as the carbon sources.
Extraction and purification of DGP from A. fulgidus.
Ethanol extracts were obtained as previously described (24). The extract was freeze-dried, dissolved in 5 mM ammonium bicarbonate buffer (pH 8.0), and loaded onto a quaternary aminoethyl-Sephadex A-25 column (1.5 by 12 cm) previously equilibrated with the same buffer. Elution was carried out with a linear gradient of ammonium bicarbonate, pH 8.0 (5 mM to 1 M). Fractions were analyzed by 1H nuclear magnetic resonance (NMR). DGP-containing fractions were pooled, freeze-dried, and dissolved in the minimum volume of water. Ammonium bicarbonate was removed in a column of activated Dowex AG-50W-X8 (3 by 12 cm). The pH of the eluted fractions was adjusted to 3.5 with KOH prior to freeze-drying. The purity of the DGP preparations was assessed by 1H, 13C, and 31P NMR. All chromatographic steps were performed on a Hiload system (Pharmacia, Uppsala, Sweden).
Chemical synthesis of DGP.
The synthesis of 1,1′-bis(2,3-O-cyclohexylideneglycerol)phosphate triethylammonium salt was accomplished in the following manner. 1,2-O-Cyclohexylideneglycerol (2.0 g, 11.6 mmol) (prepared as described by Vogel [39]) in triethylamine (10 ml, 71.2 mmol) was added slowly to a vigorously stirred solution of phosphoryl chloride (0.56 ml, 6.0 mmol) in anhydrous tetrahydrofuran (10 ml) at −78°C. Upon complete addition of the substrate-triethylamine solution, the reaction mixture was stirred at −78°C for 10 min before being treated with water (10 ml) and then warmed to room temperature. Stirring was continued for about 10 min before the organic phase was separated, and the aqueous phase was extracted twice with ethyl acetate. The aqueous phase was evaporated to dryness, giving a white solid that was dissolved in approximately 20 ml of dichloromethane. Diethyl ether was then added to precipitate the triethylammonium hydrochloride, and the solution was filtered. Evaporation of solvent afforded a colorless gum, which by NMR analysis consisted of 1,1′-bis(2,3-O-cyclohexylideneglycerol) phosphate triethylammonium salt (1.458 g, 25%) only. In a second step, 1,1′-bis(2,3-O-cyclohexylideneglycerol) phosphate triethylammonium salt (1.458 g, 2.87 mmol) dissolved in distilled water was treated with about 15 g of activated Dowex 50W-X8 ion-exchange resin. The heterogeneous mixture was stirred vigorously overnight at room temperature, after which the ion-exchange resin was filtered and washed with distilled water and the combined filtrates were evaporated to dryness to furnish DGP (0.765 g, 85%).
Chemical synthesis of (R,R)-1,1′-diglyceryl phosphate.
(R)-1,2-O-Isopropylideneglyceraldehyde (0.425 g, 3.22 mmol) was prepared from d-mannitol by the procedures described by Vogel (p. 592 and 654 of reference 39). The aldehyde thus produced was then reduced to (S)-1,2-O-isopropylideneglycerol according to the procedure of Jung and Shaw (16). (S)-1,2-O-Isopropylideneglycerol presented a specific rotation ([α]d25) of +8.8 (C 9.46 in MeOH); the corresponding value for the R enantiomer was −8.73 (C 9.92 in MeOH) (16). In the next step, (S)-1,2-O-isopropylideneglycerol was converted into (R,R)-1,1′-di(2,3-O-isopropylideneglyceryl)phosphate triethylammonium in exactly the same manner as racemic triethylammonium 1,1′-di(2,3-O-cyclohexylideneglyceryl) phosphate was prepared from 1,2-O-cyclohexylideneglycerol (see above). (R,R)-1,1′-DGP (0.390 g, 49%), was obtained by the overnight treatment, at room temperature and under vigorous stirring, of the 1,1′-di(2,3-O-isopropylideneglyceryl) phosphate triethylammonium salt with 10 g (wet) of activated Dowex 50W-X8 ion-exchange resin. The ion-exchange resin was filtered and washed with distilled water, and the filtrate was evaporated to dryness under vacuum to furnish the (R,R)-1,1′-DGP. The specific rotation for this product ([α]36525) was +1.09 (C 0.46 in H2O). It should be pointed out that this could only be a residual value, since racemization of chiral hydroxylated organophosphates is a general phenomenon, especially in alkaline solutions. The compound was obtained as a colorless gum.
Structural stability of DGP.
The thermal stability of DGP was evaluated by incubating an aqueous solution of the pure compound (pH 3.5, 100 mM) at 95°C and recording 1H NMR spectra at intervals over a period of 180 min. The spectra presented no modification over this time period. To monitor the racemization process of (R,R)-1,1′-diglyceryl phosphate, a sample of this optically active compound was dissolved in distilled water and the pH of the solution was adjusted to 7.0 with KOH. This sample was incubated at 76°C, and its optical activity as a function of time was recorded on a Perkin-Elmer 241 polarimeter equipped with a thermally jacketed 10-cm cell at 25°C, using a 365-nm source obtained from a mercury vapor lamp.
Proteins.
D. gigas and D. desulfuricans (ATCC 27774) rubredoxins (RdDg and RdDd, respectively) were obtained and purified as described by LeGall and Dragoni (22) and Sieker et al. (33), respectively. Recombinant rubredoxins from D. gigas and C. pasteurianum (rRdDg and rRdCp, respectively) were obtained by cloning and overexpressing their genes in Escherichia coli; the proteins thus produced were purified (see below) and stored at −20°C in 50 mM Tris-HCl buffer at pH 7.6. LDH (Sigma type III; rabbit muscle) was purchased as a suspension in ammonium sulfate. For the enzymatic assays, this suspension was centrifuged, the supernatant was discarded, and the enzyme was suspended in 50 mM potassium phosphate (KPi), pH 7.5. ADH (Sigma; baker's yeast) was obtained in the lyophilized form and used without further purification. T. litoralis GDH was purified from biomass cultured as previously described (41). Fractions containing GDH activity that had been adsorbed on the red Sepharose column in the purification scheme used for the isolation of maltose phosphorylase from T. litoralis (41) were pooled, and the buffer was exchanged for 50 mM Tris-HCl, pH 7.6, by ultrafiltration (30-kDa cutoff membrane; YM30; Amicon, Beverly, Mass.). This preparation of GDH was at least 80% pure as judged by polyacrylamide gel electrophoresis.
Cloning of the coding unit of RdDg.
A 3.6-kb BamHI-BamHI DNA fragment containing both coding units of rubredoxin and rubredoxin-oxygen oxidoreductase from D. gigas was cloned and sequenced as described by Gomes et al. (12). In order to subclone the rubredoxin gene, we have included the restriction sites BamHI and NdeI at the 5′ end and HindIII at the 3′ end. Following PCR amplification, the resulting 175-bp DNA fragment was purified from a 2% agarose gel as described elsewhere (11), digested with BamHI and HindIII endonucleases, and then subcloned into pZErO-1 vector (Invitrogen). The obtained construct was named pZRd. DNA isolated from the recombinant clones was then analyzed by restriction analysis to confirm the sequence of the coding unit of rubredoxin and thereby to ensure that the amplification product did not contain any error.
Expression of RdDg and RdCp in E. coli.
Plasmid pZRd harboring the RdDg gene was digested with NdeI and EcoRI restriction enzymes. The obtained 175-bp DNA fragment was inserted into vector pCYTEXP1 (3) previously digested with the same restriction enzymes. The resulting plasmid was designated pRPPL1. The plasmid pCYTEXP1 contains the strong lambda PL promoter that is controlled by the temperature-sensitive CI repressor. The repressor is encoded by the same plasmid; therefore, the expression of the cloned gene becomes heat inducible. Overexpression of RdDg was performed by growing E. coli strain JM109 containing plasmid pRPPL1 in Luria-Bertani medium supplemented with ampicillin (100 μg/ml) at 28°C to an optical density at 600 nm (OD600) of 0.5. At this stage the temperature was quickly raised to 42°C, and the culture was allowed to grow until an OD of 1.5.
Plasmid pCPRD2 (27) harboring the RdCp gene was kindly supplied by J. M. Moulis, Grenoble, France. E. coli strain TG1 was transformed according to the procedure of Chung et al. (7). Cells were routinely plated on Luria broth supplemented with ampicillin (100 μg/ml). For expression of rubredoxin, cultures were grown at 36°C to an OD600 of 0.5; then isopropyl-1-thio-β-d-galactopyranoside (IPTG) was added to a final concentration of 25 μg/ml and the cultures were incubated for another 4 h.
Purification of the recombinant rubredoxins.
Transformed cells of E. coli grown in Luria-Bertani medium were centrifuged (7,000 × g, 10 min) and resuspended in 50 mM Tris-HCl buffer (pH 7.6) containing 1 mM phenylmethylsulfonyl fluoride. Cells were disrupted by two passages through a French pressure cell at 3.3 MPa; the resulting lysate was heated to 65°C for 10 min and centrifuged (20,000 × g, 40 min). The supernatant was loaded onto a DE-52 (Whatman, Maidstone, Kent, England) column equilibrated with 50 mM Tris-HCl, pH 7.6. Elution was performed using 50 mM Tris-HCl buffer containing 350 mM NaCl. Rubredoxin was concentrated by ultrafiltration using a YM3 membrane (Amicon) and loaded onto a Superdex 75 (Pharmacia) column equilibrated with 50 mM Tris-HCl buffer containing 150 mM NaCl. Rubredoxin-containing fractions were further purified by fast protein liquid chromatography on an anion-exchange column (Resource Q; Pharmacia) equilibrated with 50 mM Tris-HCl buffer. Elution with increasing salt concentrations gave rise to separate peaks of pure Fe and Zn rubredoxins with an approximate yield of 3 mg per liter of culture. The buffer was exchanged for 50 mM Tris-HCl, pH 7.6, and the protein solution was concentrated by ultrafiltration.
Thermal stability assays.
The stability of LDH (Sigma type III; rabbit muscle), ADH (Sigma; baker's yeast), and T. litoralis GDH against thermal inactivation in the presence or absence of several solutes was determined as described by Ramos et al. (29), except for the GDH activity, which was assayed by the procedure of Schmidt and Schmidt (30). The enzymes were incubated for 10 min at the temperatures at which examinations were made (LDH and ADH) or for different periods of time up to 80 min (GDH) in the experiments aiming to assess long-term inactivation. At the end of the incubation period, the solutions were cooled on ice and the activity was measured at 30°C. Surprisingly, after incubation of GDH at 90°C for 15 min, the residual activity was consistently higher than that of the enzyme that had not been subjected to heat treatment. It seems as though a conformational change that resulted in full activation was induced at high temperature. Therefore, the bulk of the enzyme was preheated at 95°C for 15 min prior to all experiments. It was also verified that freezing and thawing did not affect the specific activity of the heat-treated preparation.
The kinetics for disruption of the rubredoxin structure was monitored by UV-Vis absorption spectroscopy in a Shimadzu UV-1601 spectrophotometer equipped with a thermostated cell. A rubber septum was adapted to a quartz cell to allow measurements under anaerobic conditions. Unless otherwise stated the cell was flushed with argon before adding the assay solution and then was subjected to three vacuum-argon cycles (15 min per cycle). The assay solution consisted of 50 mM Tris-HCl buffer, pH 7.6, and the desired amount of a given solute. The temperature of the solution in the cuvette was checked with a thermocouple. Once thermal equilibrium was reached, approximately 50 μl of a concentrated protein solution was rapidly added (final concentration, 17 μM; final volume, 600 μl) and spectral scanning was started. Spectra were recorded for each time point and baseline corrected. The values of absorbance measured at 494 nm (A494) as a function of time (t) were fitted to the expression A494 = A exp(−kt), where A (absorbance at time zero) and k (the exponential decay constant) were treated as adjustable parameters.
NMR spectroscopy.
1H NMR spectra were recorded at 300.14 MHz on a Bruker AMX 300 spectrometer with a 5-mm probe head. Spectra were acquired with water presaturation, a 6-μs pulse width (corresponding to a 60° flip angle), and a repetition delay of 15 s. For quantification purposes formate was added as an internal concentration standard. Chemical shifts were referenced to external 3-(trimethylsilyl)propanesulfonic acid sodium salt, designated 0 ppm. 13C NMR spectra were recorded at 75.47 MHz on the same spectrometer with a broad-band inverse-detection 5-mm probe head. Proton decoupling was applied during the acquisition time only, using the wide-band alternating-phase low-power technique for zero-residue splitting sequence (WALTZ). Chemical shifts were referenced to the resonance of external methanol, defined as at 49.3 ppm.
RESULTS
Stability of the optically active DGP.
DGP was synthesized in both optically active and optically inactive forms. 1H, 13C, and 31P NMR spectra of the synthesized and of the natural compound were found to be identical. DGP (aqueous solution, potassium salt, pH 7) was a heat-stable compound resisting treatment at 95°C for at least 3 h. The natural compound purified by anion-exchange chromatography showed no optical activity. At this stage two hypotheses were put forward: either the natural compound was intrinsically inactive or it underwent racemization during the purification procedure. To clarify this point, we decided to study the configurational stability of the compound, which proved to be rather low. In fact, (R,R)-1,1′-DGP in aqueous solution at pH 7.0 and 76°C (the optimal growth temperature of A. fulgidus) lost all rotatory power in less than 2 h, and at room temperature the optical activity was completely lost overnight. Therefore, a non-optically active form could occur in the living cell. However, this conclusion should be regarded with caution since in a chiral environment, within a biological system, it is possible either that the racemization process does not occur or that one enantiomer is continuously reformed. The synthetic compound was used in the remaining part of this work.
Stabilization of enzymes by DGP.
The protecting effect of DGP against the heat inactivation of enzymes was examined with rabbit muscle LDH, baker's yeast ADH, and T. litoralis GDH. Incremental concentrations of DGP (50, 100, and 200 mM) resulted in increasing protection of LDH at 50°C, with a nearly linear dependence (Table 1). Full recovery of the enzyme activity was achieved at 200 mM concentration. The ability of DGP to confer thermal stability as a function of the incubation temperature (50, 55, and 60°C) was also evaluated. The beneficial effect of DGP is clearly apparent from the contrast between the drastic loss in the enzyme activity observed in the absence of solute and the relatively high recoveries achieved in the presence of DGP (Table 2). For example, at the highest temperature examined, the enzyme was virtually inactivated when DGP was not added, whereas 15% of the activity could still be recovered when the solute was present at 100 mM concentration.
TABLE 1.
Effect of several solutes on rabbit muscle LDH thermostability
| Solute | % Recovered activitya at solute concnb (mM) of:
|
|||
|---|---|---|---|---|
| 0 | 50 | 100 | 200 | |
| Glycerol | 31 ± 3 | 37 ± 6 | 42 ± 4 | 47 ± 3 |
| KCl | 37 ± 3 | 46 ± 1 | 54 ± 1 | |
| Trehalose | 47 ± 2 | 50 ± 2 | 55 ± 3 | |
| NaPic | 52 ± 2 | 65 ± 3 | 80 ± 2 | |
| KPi | 73 ± 6 | 83 ± 5 | 101 ± 7 | |
| KPi + glycerol | 75 ± 4 | 81 ± 4 | 99 ± 2 | |
| DGP | 62 ± 7 | 85 ± 8 | 97 ± 7 | |
After incubation for 10 min at 50°C. Values are means of at least three independent experiments.
The concentration of glycerol was double the indicated value to mimic the concentration of DGP.
NaPi, sodium phosphate.
TABLE 2.
Effect of DGP and related solutes (at 100 mM concentration) on rabbit muscle LDH and baker's yeast ADH thermostability
| Solute | % Recovered activitya for:
|
|||
|---|---|---|---|---|
| LDH at:
|
ADH at 50°C | |||
| 50°C | 55°C | 60°C | ||
| No additions | 31 ± 3 | 8 ± 2 | 2 ± 1 | 33 ± 3 |
| Glycerolb | 42 ± 4 | 8 ± 2 | 1 ± 1 | 31 ± 1 |
| KPi | 83 ± 5 | 29 ± 6 | 12 ± 3 | 85 ± 11 |
| KPi + glycerolb | 81 ± 4 | 31 ± 3 | 13 ± 2 | 88 ± 9 |
| DGP | 85 ± 8 | 35 ± 6 | 15 ± 2 | 88 ± 5 |
Mean values of at least three independent experiments. Values were obtained after incubations of 10 min at the indicated temperatures.
The concentration of glycerol was 200 mM to mimic the composition of DGP.
The efficiency of DGP as an enzyme stabilizer was compared with those of other solutes (Tables 1 and 2). Since the potassium salt of DGP was used in our studies, KPi was used for comparison in addition to glycerol (at a concentration twofold greater than that of DGP). Glycerol (100 to 400 mM) did not exert a significant protective effect against thermal inactivation of LDH. KPi, on the other hand, showed a protective effect comparable to that exerted by DGP, and the combination of glycerol and KPi in the assay mixture did not confer extra protection. Trehalose and KCl individually, at concentrations identical to that of DGP, were considerably less efficient. Sodium phosphate was a poorer stabilizer than KPi but better than trehalose, KCl, or glycerol.
As the incubation at the several temperatures was followed by cooling on ice and measurement at 30°C of the residual activity, the question arose whether DGP promoted correct refolding of the enzyme rather than conferring protection from denaturation. To investigate this point, two types of experiments were devised. In one experiment, the assay mixtures were cooled on ice immediately after the imposed heat stress (10 min at 50°C), and the activity was measured at 15-min intervals over a period of 1 h. No activity change was detected over this period in the presence or absence of DGP. In the other experiment, the thermal stress was applied in the absence of solute, which was added at the end of the incubation period, prior to cooling on ice. Again, no change in the extent of recovery was detected, showing that the solute did not promote refolding.
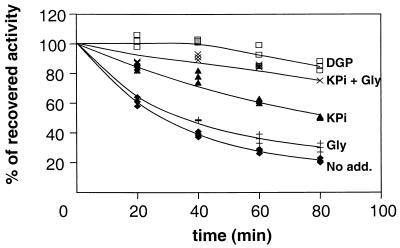
DGP also exerted a strong protective effect on baker's yeast ADH, and the effect of glycerol and KPi on this enzyme was very similar to that on rabbit muscle LDH (Table 2). Since DGP was isolated from a hyperthermophilic organism, A. fulgidus, we deemed it important to examine the protective effect of this solute on a thermostable enzyme also derived from a hyperthermophile. The thermostable T. litoralis GDH was selected for this purpose, and the protective effect of DGP was evaluated by measuring the enzyme activity after treatment at 95°C as a function of the incubation time. In the absence of solutes the activity decayed rapidly with a half-life (t1/2) of 35 min. In contrast, a remarkable protection was observed when the incubation was carried out in the presence of DGP: the activity was practically unaltered up to a 40-min incubation, and the recovered activity was still very high (92%) after 60 min (Fig. 1). KPi used at the same concentration (100 mM) showed a considerable, yet smaller, protective effect (62% of activity recovered after a 60-min incubation). Glycerol alone acted as a very poor protector, but the combined action of glycerol and KPi was only slightly less than that of DGP.
FIG. 1.
Effect of DGP and its chemical components on T. litoralis GDH thermostability at 95°C in the presence of no additions (⧫), 200 mM glycerol (+), 100 mM KPi (▴), 200 mM glycerol plus 100 mM KPi (×), 100 mM DGP (□). Individual points from three independent experiments are shown. Gly, glycerol.
Effect of DGP on rubredoxin thermostability.
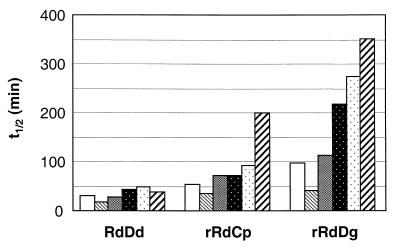
The UV-Vis absorption spectrum of rubredoxin presents signals with maxima centered at 380, 494, and 570 nm. These bands are bleached due to the disruption of the iron center when rubredoxin undergoes denaturation. Monitoring the loss of the metal center conformation from the decrease in A494 provides an expeditious way to evaluate the thermal stability of rubredoxins (5, 6, 9). The temperature dependence of the t1/2 for thermal denaturation of RdDg was investigated at temperatures between 80 and 95°C. Denaturation was very slow at 80°C (t1/2, 400 min) making impractical the implementation of a large number of experiments. At 85, 90, and 95°C the values of t1/2 were 130, 98, and 34 min, respectively. We decided to carry out all the remaining experiments at 90°C. All the rubredoxins examined exhibited a monoexponential behavior in regard to disruption of the iron center. RdDd had a t1/2 of 30 min, while rRdCp and rRdDg had t1/2s of 53 and 98 min, respectively (Fig. 2). RdDg showed a t1/2 similar to that of the recombinant protein (100 min). The effect of oxygen on the thermal stability of rubredoxins was examined in experiments where pure oxygen was bubbled through the assay mixtures for 10 min prior to heating. Under these oxygen-saturating conditions, a twofold decrease in the t1/2s for denaturation of all the rubredoxins was observed. This result is not surprising since the deleterious effects on the protein structure caused by the oxidation reactions were expected to be accentuated at the high working temperature. All the remaining experiments in this study were carried out under anaerobic conditions.
FIG. 2.
Effect of DGP on the t1/2 values for thermal denaturation at 90°C of RdDd, rRdCp, rRdDg, and RdDg.  , no additions and anaerobiosis;
, no additions and anaerobiosis;  , no additions and oxygen-saturating conditions;
, no additions and oxygen-saturating conditions;  , 200 mM glycerol;
, 200 mM glycerol;  , 100 mM KPi;
, 100 mM KPi;  , 100 mM KPi plus 200 mM glycerol;
, 100 mM KPi plus 200 mM glycerol;  , 100 mM DGP.
, 100 mM DGP.
The stability enhancement rendered by DGP was variable among the three rubredoxins. The enhancement was impressive for the rRdDg and rRdCp, the t1/2s increasing approximately fourfold in the presence of the solute. In contrast, no significant stabilization was observed for the RdDd. The efficiency of the solute as a rubredoxin stabilizer was compared with that exerted by KPi, glycerol, and a mixture of the two compounds intended to mimic the two chemical moieties in the DGP molecule. The addition of glycerol resulted only in a small stability enhancement of all rubredoxins examined, whereas the response to KPi differed from one rubredoxin to another. While the addition of KPi conferred only a slight stabilization to rRdCp and RdDd, the t1/2 values of rRdDg increased approximately threefold. The combined effect of glycerol and KPi was better than that of KPi alone, but DGP conferred the highest protection (Fig. 2). Under all conditions examined, the natural form of RdDg showed thermostability identical to that of the recombinant form and, therefore, only one set of data is illustrated in Fig. 2.
DISCUSSION
DGP exerted a considerable thermal stabilizing effect on three enzymes (LDH, ADH, and GDH) and on two of the rubredoxins tested. Solutes that are commonly used as enzyme stabilizers, such as glycerol and trehalose, are effective primarily in the molar range of concentration (1, 2, 42), whereas we showed that DGP is able to confer a high degree of protection at considerably lower concentrations (100 mM). Ectoine, another important solute derived from mesophiles, also shows negligible thermoprotection of LDH even at 1 M concentration (23). On the other hand, it is interesting to note the similar extents of protection of LDH rendered by DGP and phosphate, the ion with top salting-out ability in the Hofmeister series. However, KPi was a poorer protecting agent for T. litoralis GDH and the rubredoxins, indicating that phenomena other than those determined by the lyotropic series must play a role in the solute-protein stabilization mechanisms.
A comparison between the stabilizing effects exerted by DGP and those of its chemical components, glycerol and KPi, showed that glycerol had no significant protecting effect on all the proteins examined and that KPi was as good as DGP in the protection of LDH and ADH. Therefore, the negative charge in DGP seems to be the determinant for the observed stabilizing effect. The explanation, however, is probably not so simple or general. Glucosylglycerol, a neutral compound, is an efficient protecting agent of LDH against heat inactivation, despite the fact that glucose and glycerol are poor stabilizers of this enzyme (N. Borges, A. Ramos, and H. Santos, Abstr. Thermophiles '98, abstr. M-P26, 1998). The relative protective effects of KCl, KPi, and sodium phosphate on LDH follow the increasing salting-out ability predicted by the Hofmeister series. However, Scholz et al. (31) showed that sodium citrate is a better protector of Pyrococcus woesei glyceraldehyde-3-phosphate dehydrogenase than the corresponding potassium salt.
Altogether, these results corroborate the view that the protecting effect of a solute depends on the particular solute-protein pair involved (23). To obtain information on the extent to which the thermoprotection conferred by DGP was determined by specific structural features of the target protein, three homologous rubredoxins were examined. Despite the high structural homology of the rubredoxins, the extent of protection conferred by DGP was strikingly dependent on the specific rubredoxin considered. In particular, RdDd, the least thermostable protein, was not stabilized by DGP or by any other salt examined, whereas the two rubredoxins from other sources were considerably stabilized. This behavior is hard to explain in the light of the preferential exclusion model (1, 2), according to which protecting solutes, typically used in the molar range of concentrations, would act primarily by changing the physical properties of the solvent. Therefore, specific solute-protein interactions may play a role in the stabilization effect exerted by DGP.
RdDg and RdCp are structurally very similar (10, 34, 40). The overall folding, the iron environments, and the hydrogen bonding patterns of the two proteins are nearly identical; furthermore, the amino acid residues that build up the hydrophobic core are invariant (10, 35, 40). Despite this similarity, there must be fine structural differences that account for their distinct intrinsic thermostabilities (15). The least thermostable rubredoxin examined, RdDd, shows a clear structural difference originating in the absence of seven amino acids at positions 20 to 26 of the hairpin loop. This peculiarity exposes most of the hydrophobic core of the protein to the solvent and is only partially compensated for by a histidine residue at position 18, which shields part of the core (34). The low thermostability of RdDd could be related to this exposure of the core to the solvent. In fact, Lazaridis et al. (21) have suggested that rubredoxins unfold by first opening the loop region and exposing their hydrophobic core to the solvent. Unlike the other rubredoxins examined, RdDd is not stabilized by DGP. Perhaps, this anionic solute can interact preferentially with the hairpin loop present in the other rubredoxins, preventing them from unwinding and exposing the core to the solvent. Thus, the nature of the hairpin loop may be essential in determining rubredoxin thermostability and the extent of stabilization provided by DGP.
Although obtained from mesophilic organisms, the rubredoxins examined here showed considerably different intrinsic thermostabilities at 90°C. It is conceivable that small structural variations determined by amino acid differences, namely, those in the loop region, could result in a higher degree of spatial optimization of the weak interactions that determine protein stability (15, 19). In this context, it is interesting to note that optimization parameters calculated for the available rubredoxin structures by Spassov et al. (38) show an almost linear dependence between the charge-charge interaction optimization parameter (37) and the t1/2 values for loss of the visible spectra that we observed for RdDd, RdDg, and RdCp. On the other hand, only small differences in the hydrophobic interaction optimization parameter are found (38). Thus, our experimental results provide further support for the hypothesis that the optimization of charge-charge interactions on the protein surface is a determinant factor in the thermal stability of rubredoxins.
This work clearly shows the ability of DGP to act as a protein stabilizer in vitro. Since this solute accumulates in A. fulgidus to high levels, it is likely to play a role in the strategies of adaptation of this hyperthermophilic halophilic organism to high temperature. Work aiming to characterize at the molecular level the DGP-rubredoxin interaction is in progress.
ACKNOWLEDGMENTS
This work was supported by the European Community Biotech Programme (Extremophiles as Cell Factories, BIO4-CT96-0488), by PRAXIS XXI and FEDER, Portugal (PRAXIS/2/2.1/BIO/1109/95 to H.S., PRAXIS XXI 61/96 to J.L.G., NIH grant GM 56001 to J.L.G. and M.-Y.L., and PPRAXIS/PCNA/BIO32/96 to C.R.P.). P. Lamosa acknowledges a Ph.D. grant from PRAXIS XXI (BD/11474/97).
We thank J. M. Moulis for generously providing plasmid pCPRD2. We thank Isabel Pacheco for valuable assistance on protein purification.
REFERENCES
- 1.Arakawa T, Timasheff S N. Preferential interactions of proteins with solvent components in aqueous amino acid solutions. Arch Biochem Biophys. 1983;224:169–177. doi: 10.1016/0003-9861(83)90201-1. [DOI] [PubMed] [Google Scholar]
- 2.Arakawa T, Timasheff S N. The stabilization of proteins by osmolytes. Biophys J. 1985;47:411–414. doi: 10.1016/S0006-3495(85)83932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belev T N, Singh M, McCarthy J E G. A fully modular vector system for optimization of gene expression in Escherichia coli. Plasmid. 1991;26:147–150. doi: 10.1016/0147-619x(91)90056-3. [DOI] [PubMed] [Google Scholar]
- 4.Blöchl E, Burggraf S, Fiala G, Lauerer G, Huber G, Huber R, Rachel R, Segerer A, Stetter K O, Völkl P. Isolation, taxonomy and phylogeny of hyperthermophilic microorganisms. World J Microbiol Biotechnol. 1995;11:9–16. doi: 10.1007/BF00339133. [DOI] [PubMed] [Google Scholar]
- 5.Cavagnero S, Debe D A, Zhou Z H, Adams M W W, Chan S I. Kinetic role of electrostatic interactions in the unfolding of hyperthermophilic and mesophilic rubredoxins. Biochemistry. 1998;37:3369–3376. doi: 10.1021/bi9721795. [DOI] [PubMed] [Google Scholar]
- 6.Cavagnero S, Zhou Z H, Adams M W W, Chan S I. Unfolding mechanism of rubredoxin from Pyrococcus furiosus. Biochemistry. 1998;37:3377–3385. doi: 10.1021/bi9721804. [DOI] [PubMed] [Google Scholar]
- 7.Chung C T, Niemela S L, Miller R H. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci USA. 1989;86:2172–2175. doi: 10.1073/pnas.86.7.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.da Costa M S, Santos H, Galinski E A. An overview of the role and diversity of compatible solutes in Bacteria and Archaea. Adv Biochem Eng Biotechnol. 1998;61:117–153. doi: 10.1007/BFb0102291. [DOI] [PubMed] [Google Scholar]
- 9.Eidsness M K, Richie K A, Burden A E, Kurtz D M, Jr, Scott R A. Dissecting contributions to the thermostability of Pyrococcus furiosus rubredoxin: β-sheet chimeras. Biochemistry. 1997;36:10406–10413. doi: 10.1021/bi970110r. [DOI] [PubMed] [Google Scholar]
- 10.Frey M, Sieker L, Payan F, Haser R, Bruschi M, Pepe G, LeGall J. Rubredoxin from Desulfovibrio gigas a molecular model of the oxidized form at 1.4 Å resolution. J Mol Biol. 1987;197:525–541. doi: 10.1016/0022-2836(87)90562-6. [DOI] [PubMed] [Google Scholar]
- 11.Glenn T C, Glenn S J. Rapid elution of DNA from agarose gels using polyester plug spin inserts (PEPSIs) Trends Genet. 1994;10:344. doi: 10.1016/0168-9525(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 12.Gomes C M, Silva G, Oliveira S, LeGall J, Liu M Y, Xavier A V, Rodrigues-Pousada C, Teixeira M. Studies on the redox centers of the terminal oxidase from Desulfovibrio gigas and evidence for its interaction with rubredoxin. J Biol Chem. 1997;272:22502–22508. doi: 10.1074/jbc.272.36.22502. [DOI] [PubMed] [Google Scholar]
- 13.Hensel R. Proteins of extreme thermophiles. New Comp Biochem. 1993;26:209–221. [Google Scholar]
- 14.Hensel R, König H. Thermoadaptation of methanogenic bacteria by intracellular ion concentration. FEMS Microbiol Lett. 1988;49:75–79. [Google Scholar]
- 15.Jaenicke R, Böhm G. The stability of proteins in extreme environments. Curr Opin Struct Biol. 1999;8:738–748. doi: 10.1016/s0959-440x(98)80094-8. [DOI] [PubMed] [Google Scholar]
- 16.Jung M E, Shaw T J. Total synthesis of (R)-glycerol acetonide and the antiepileptic and hypotensive drug (−)-γ-amino-β-hydroxybutyric acid (GABOB): use of vitamin C as a chiral starting material. J Am Chem Soc. 1980;102:6304–6311. [Google Scholar]
- 17.Kanodia S, Roberts M F. Methanophosphagen: unique cyclic pyrophosphate isolated from Methanobacterium thermoautotrophicum. Proc Natl Acad Sci USA. 1983;80:5217–5221. doi: 10.1073/pnas.80.17.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein A R, Breitung J, Linder D, Stetter K O, Thauer R K. N5,N10-Methenyltetrahydromethanopterin cyclohydrolase from the extremely thermophilic sulphate reducing Archaeoglobus fulgidus: comparison of its properties with those of the cyclohydrolase from the extremely thermophilic Methanopyrus kandleri. Arch Microbiol. 1993;159:213–219. doi: 10.1007/BF00248474. [DOI] [PubMed] [Google Scholar]
- 19.Ladenstein R, Antranikian G. Proteins from hyperthermophiles: stability and enzymatic catalysis close to the boiling point of water. Adv Biochem Eng Biotechnol. 1998;61:37–85. doi: 10.1007/BFb0102289. [DOI] [PubMed] [Google Scholar]
- 20.Lamosa P, Martins L O, da Costa M S, Santos H. Effect of temperature, salinity, and medium composition on compatible solute accumulation by Thermococcus spp. Appl Environ Microbiol. 1998;64:3591–3598. doi: 10.1128/aem.64.10.3591-3598.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lazaridis T, Lee I, Karplus M. Dynamics and unfolding pathways of a hyperthermophilic and a mesophilic rubredoxin. Protein Sci. 1997;6:2589–2605. doi: 10.1002/pro.5560061211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LeGall J, Dragoni N. Dependence of sulfite-reduction on a crystallized ferredoxin from Desulfovibrio gigas. Biochem Biophys Res Commun. 1966;23:145–149. doi: 10.1016/0006-291x(66)90519-5. [DOI] [PubMed] [Google Scholar]
- 23.Lippert K, Galinski E A. Enzyme stabilization by ectoine-type compatible solutes: protection against heating, freezing and drying. Appl Microbiol Biotechnol. 1992;37:61–65. [Google Scholar]
- 24.Martins L O, Santos H. Accumulation of mannosylglycerate and di-myo-inositol-phosphate by Pyrococcus furiosus in response to salinity and temperature. Appl Environ Microbiol. 1995;61:3299–3303. doi: 10.1128/aem.61.9.3299-3303.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martins L O, Carreto L S, da Costa M S, Santos H. Novel compatible solutes related to di-myo-inositol-phosphate in the order Thermotogales. J Bacteriol. 1996;178:5644–5651. doi: 10.1128/jb.178.19.5644-5651.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martins L O, Huber R, Huber H, Stetter K O, da Costa M S, Santos H. Organic solutes in hyperthermophilic archaea. Appl Environ Microbiol. 1997;63:896–902. doi: 10.1128/aem.63.3.896-902.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathieu I, Meyer J-M, Moulis J. Cloning, sequencing and expression in Escherichia coli of the rubredoxin gene from Clostridium pasteurianum. Biochem J. 1992;285:255–262. doi: 10.1042/bj2850255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nunes O C, Manaia C M, da Costa M S, Santos H. Compatible solutes in the thermophilic bacteria Rhodothermus marinus and “Thermus thermophilus.”. Appl Environ Microbiol. 1995;61:2351–2357. doi: 10.1128/aem.61.6.2351-2357.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramos A, Raven N D H, Sharp R J, Bartolucci S, Rossi M, Cannio R, Lebbink J, van der Oost J, de Vos W M, Santos H. Stabilization of enzymes against thermal stress and freeze-drying by mannosylglycerate. Appl Environ Microbiol. 1997;63:4020–4025. doi: 10.1128/aem.63.10.4020-4025.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt E, Schmidt F W. Glutamate dehydrogenase. In: Bergmeyer H U, editor. Methods in enzymatic analysis. 3rd ed. III. Weinheim, Germany: VCH; 1987. pp. 216–227. [Google Scholar]
- 31.Scholz S, Sonnenbichler J, Schäfer W, Hensel R. Di-myo-inositol-1,1′-phosphate: a new inositol phosphate isolated from Pyrococcus woesei. FEBS Lett. 1992;306:239–242. doi: 10.1016/0014-5793(92)81008-a. [DOI] [PubMed] [Google Scholar]
- 32.Shima S, Herault D A, Berkessel A, Thauer R K. Activation and thermostabilization effects of cyclic 2,3-diphosphoglycerate on enzymes from the hyperthermophilic Methanopyrus kandleri. Arch Microbiol. 1998;170:469–472. doi: 10.1007/s002030050669. [DOI] [PubMed] [Google Scholar]
- 33.Sieker L C, Jensen L H, Prickril B, LeGall J. Crystallographic study of rubredoxin from the bacterium Desulfovibrio desulfuricans strain 27774. J Mol Biol. 1983;171:101–103. doi: 10.1016/s0022-2836(83)80316-7. [DOI] [PubMed] [Google Scholar]
- 34.Sieker L C, Stenkamp R E, Jensen L H, Prickril B, LeGall J. Structure of rubredoxin from the bacterium Desulfovibrio desulfuricans. FEBS Lett. 1986;208:73–76. doi: 10.1016/0014-5793(86)81535-6. [DOI] [PubMed] [Google Scholar]
- 35.Sieker L C, Stenkamp R E, LeGall J. Rubredoxin in the crystalline state. Methods Enzymol. 1994;246:203–216. doi: 10.1016/0076-6879(94)43016-0. [DOI] [PubMed] [Google Scholar]
- 36.Silva Z, Borges N, Martins L O, Wait R, da Costa M S, Santos H. Combined effect of the growth temperature and salinity of the medium on the accumulation of compatible solutes by Rhodothermus marinus and Rhodothermus obamensis. Extremophiles. 1999;3:163–172. doi: 10.1007/s007920050112. [DOI] [PubMed] [Google Scholar]
- 37.Spassov V Z, Karshikoff A D, Ladenstein R. The optimization of the electrostatic interactions in proteins of different functional and folding type. Protein Sci. 1994;3:1556–1569. doi: 10.1002/pro.5560030921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spassov V Z, Karshikoff A D, Ladenstein R. The optimization of protein-solvent interactions: thermostability and the role of hydrophobic and electrostatic interactions. Protein Sci. 1995;4:1516–1527. doi: 10.1002/pro.5560040809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vogel A I. Vogel's textbook of practical organic chemistry, fifth ed. Burnt Mill, Harlow, Essex, England: Longman Scientific Technical; 1989. [Google Scholar]
- 40.Watenpaugh K D, Sieker L C, Jensen L H. Crystallographic refinement of rubredoxin at 1.2 Å resolution. J Mol Biol. 1980;138:615–633. doi: 10.1016/s0022-2836(80)80020-9. [DOI] [PubMed] [Google Scholar]
- 41.Xavier K B, Peist R, Kossmann M, Boos W, Santos H. Maltose metabolism in the hyperthemophilic archaeon Thermococcus litoralis: purification and characterization of key enzymes. J Bacteriol. 1999;181:3358–3367. doi: 10.1128/jb.181.11.3358-3367.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie G, Timasheff S N. Mechanism of the stabilization of ribonuclease A by sorbitol: preferential hydration is greater for the denatured than for the native protein. Protein Sci. 1997;6:211–221. doi: 10.1002/pro.5560060123. [DOI] [PMC free article] [PubMed] [Google Scholar]