INTRODUCTION:
We aimed to evaluate the real-world effectiveness and safety of tofacitinib for the treatment of ulcerative colitis (UC).
METHODS:
REMIT-UC is a Canadian multicenter cohort study. Standardized data collection was performed on 334 consecutive adult outpatients with UC treated with tofacitinib. The primary outcomes were achievement of clinical and endoscopic remission. Safety outcomes were reported using incidence rates (events/100 patient-years of exposure). A multivariable Cox proportional hazards model was used to evaluate predictors of loss of response after tofacitinib dose de-escalation to 5 mg twice daily (BID).
RESULTS:
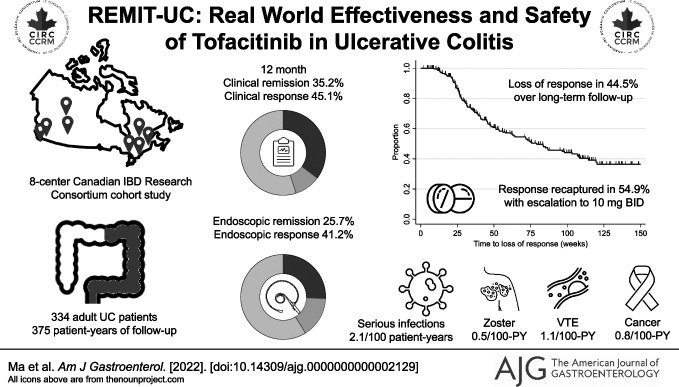
Clinical remission was achieved by 35.3% (106/300), 36.0% (104/289), and 35.2% (93/264) of patients at weeks 12, 24, and 52, respectively. Endoscopic remission was achieved by 18.5% (15/81), 23.0% (28/122), and 25.7% (35/136) of patients at weeks 12, 24, and 52, respectively. Incidence of serious infections, herpes zoster, and venous thromboembolism were 2.1 [0.9–4.2], 0.5 [0.1–1.9], and 1.1 [0.3–2.7], respectively. Among responders, 44.5% (109/245) lost response during follow-up, which was recaptured in 54.9% (39/71) of patients who re-escalated to 10 mg BID. Patients with a baseline Mayo endoscopic score of 3 (adjusted hazard ratio 3.60 [95% confidence interval: 1.70–7.62]) and prior biologic failure (adjusted hazard ratio 3.89 [95% confidence interval: 1.28–11.86]) were at a higher risk for losing response after dose reduction.
DISCUSSION:
One-third of patients with UC treated with tofacitinib achieved clinical remission with few serious adverse events. However, half of patients lost response with de-escalation, which was only partially recaptured with increasing the maintenance dose. Those with negative prognostic factors should be counselled about the risks and benefits of continuing high doses of tofacitinib.
KEYWORDS: colitis, effectiveness, Janus kinase, safety, ofacitinib
INTRODUCTION
Targeting Janus kinase (JAK) has emerged as an important therapeutic strategy for patients with inflammatory bowel disease (IBD) (1–4). Tofacitinib is a potent oral small molecule JAK inhibitor, predominantly selective for JAK1 and JAK3, which was demonstrated in the phase III OCTAVE (A Study Evaluating The Efficacy And Safety Of CP-690,550 In Patients With Moderate To Severe Ulcerative Colitis) program to be more effective than placebo for inducing and maintaining clinical remission and endoscopic improvement in patients with moderate-to-severely active ulcerative colitis (UC) (5). The US Food and Drug Administration subsequently approved the use of tofacitinib for the treatment of UC in 2018, and it has been available in many jurisdictions, including Canada, for the past 4 years.
Despite high efficacy rates, concerns regarding the safety profile of JAK inhibitors have been identified, particularly relating to infections such as herpes zoster (HZ), venous thromboembolism (VTE), major adverse cardiovascular events (MACE), and malignancy (6). In older patients (aged 50 years or older) with active rheumatoid arthritis (RA) and at least 1 additional cardiovascular risk factor, results from the randomized, open-label ORAL Surveillance (Phase 3B/4 Randomized Safety Endpoint Study of 2 Doses of Tofacitinib in Comparison to a Tumor Necrosis Factor Inhibitor in Subjects with Rheumatoid Arthritis) trial failed to demonstrate noninferiority of tofacitinib compared with tumor necrosis factor inhibitors for the coprimary end point of adjudicated MACE and cancer (7). There was also an increased risk of opportunistic infections, HZ, and VTE (in the tofacitinib 10 mg twice daily [BID] group) (7), prompting the US Food and Drug Administration to add a black box warning to the JAK inhibitor class, limiting tofacitinib use to patients failing a tumor necrosis factor antagonist, and recommending the lowest dose required to maintain remission (8).
Whether results from the ORAL Surveillance trial are generalizable to the UC population is unclear (9,10). Furthermore, approximately 25% of patients will lose response to tofacitinib after dose de-escalation from 10 to 5 mg BID (11,12). Given these considerations, robust real-world data are required to inform the appropriate positioning, dosing, and safety profile of tofacitinib in patients with UC. Although several open-label cohorts have been previously reported (13,14), most studies to date have been limited by selection bias from tertiary care referral centers, relatively small sample sizes, and short duration of follow-up. In this study, we report results from REMIT-UC, a Canadian multicenter cohort study examining the effectiveness and long-term safety of tofacitinib in patients with UC.
METHODS
Study design
This is a multicenter, retrospective observational cohort study, conducted in partnership with the CIRC. CIRC is a national collaborative, nonprofit research group that provides independent scientific review and support for Canadian, investigator-initiated IBD studies (https://circ-ccrm.ca/). Eight centers across Canada participated, reflecting geographic diversity and different practice settings, ranging from community IBD clinics to tertiary care referral centers. Institutional review board approval was obtained at each site.
Study population
Consecutive adult (age 18 years or older) outpatients with UC treated with tofacitinib between January 15, 2015, and February 8, 2022, were included. Eligibility criteria were as follows: (i) confirmed diagnosis of UC based on clinical, endoscopic, and/or histologic criteria during tofacitinib induction; (ii) active symptoms or endoscopic disease activity attributable to UC before tofacitinib induction; and (iii) at least 1 clinical or endoscopic follow-up visit after tofacitinib induction. Patients treated with tofacitinib solely for the control of extraintestinal manifestations or for non-UC indications; patients with Crohn's disease, indeterminate colitis, or pouchitis; and patients receiving tofacitinib as part of a clinical trial or in-hospital for acute severe UC were excluded.
Data collection
All clinical assessments from up to 6 weeks before the initiation of tofacitinib until tofacitinib discontinuation or last available follow-up were captured using a standardized electronic case report form. All investigators attended a study-specific training session before site activation to ensure consistent definitions of all covariables and outcomes were applied. Specifically, training about the Mayo Clinic Score and Mayo Endoscopic subscore (MES) was conducted to ensure consistent disease activity evaluations. Data quality checks were performed by the central site. Covariables included the following: patient factors (e.g., sex, age, smoking status, thrombosis-related risk factors, and HZ vaccination); disease-related factors (disease extent and duration, clinical and endoscopic disease activity, C-reactive protein [CRP] and/or fecal calprotectin at baseline, prior hospitalizations, and Clostridioides difficile infection); and treatment-related characteristics (dosing, prior and concomitant therapy).
Outcomes
The primary outcome was the proportion of patients achieving clinical remission and endoscopic remission at week 52 after tofacitinib induction. Clinical outcomes were preferentially assessed using the partial Mayo score (PMS) incorporating rectal bleeding, stool frequency, and physician global assessment (PGA) subscores. Endoscopic outcomes were evaluated using the MES. In situations where the rectal bleeding or stool frequency subscores could not be calculated, the PGA was used, similar to previous real-world UC studies (15). Clinical remission was defined by a PMS ≤2 with resolution of rectal bleeding or resolution of all UC-related symptoms based on PGA; endoscopic remission was defined by an MES = 0.
Secondary outcomes were clinical and endoscopic remission at 12 and 24 weeks, clinical response (defined by reduction in PMS ≥2 points compared with baseline), endoscopic improvement (MES = 0 or 1), corticosteroid-free clinical remission, normalization of CRP and fecal calprotectin, loss of response among initial tofacitinib responders (defined by increase in PMS ≥2 points with accompanying biomarker or endoscopic evidence of inflammation), UC-related hospitalization or colectomy, and treatment-emergent adverse events (AE). Safety outcomes of specific interest included infections, HZ, thrombosis, MACE, or malignancy. Serious AEs (SAE) were defined as those that were life-threatening, resulted in prolonged hospitalization >24 hours, caused permanent disability, resulted in death, or were otherwise judged as serious by the investigator. A data collection window of ±6 weeks was permitted for week 12, 24, and 52 outcomes, given the real-world nature of this study. There were patients who did not have a follow-up until weeks 12, 24, and 52 but were still on tofacitinib: these patients were censored for time-to-event analyses and not included in the denominator for dichotomous outcomes (e.g., if a patient was on tofacitinib and the last available follow-up was at week 30, they were included in the denominator for week 24 clinical and endoscopic response/remission outcomes, but not counted in the denominator for week 52 outcomes).
Statistical methods
Baseline demographic characteristics were summarized using proportions for categorical covariables and mean with SD or median with interquartile range (IQR) for continuous covariables. Baseline characteristics were compared using the Pearson χ2 test, independent sample t test, or Mann-Whitney-U test, as appropriate. The proportion of patients achieving clinical response and remission was calculated as the observed number of patients achieving the respective end point divided by the total number of patients with follow-up to the time point or who discontinued treatment before the time point (treated as nonresponders). Timing of endoscopy evaluations was not protocolized: consistent with the real-world design, there was variability in both why and when patients underwent endoscopy. The denominator consisted of all patients who underwent endoscopy at the time point or who had discontinued therapy (all patients discontinuing treatment were considered nonresponders) to ensure that response and remission rates were not overestimated. Kaplan-Meier survival methods were used to evaluate the cumulative probability of patients achieving biomarker normalization, among those with an elevated CRP ≥5 mg/L and/or fecal calprotectin ≥250 μg/g at baseline. Nonresponder imputation was used for patients with missing laboratory values to minimize the risk of attrition bias. Kaplan-Meier survival methods were also used to evaluate the risk of UC-related hospitalization, colectomy, and loss of response among initial tofacitinib responders. Log-rank tests were used to compare survival between subgroups. For safety outcomes, the incidence rate (IR) for all AE, SAE, and AE of specific interest per 100 patient-years (PY) of tofacitinib exposure was calculated.
Predictors of loss of response among patients de-escalating to tofacitinib 5 mg BID were evaluated using a multivariable Cox proportional hazards model. Covariables for modeling were selected a priori based on clinical plausibility, including disease extent and duration, baseline clinical and endoscopic severity, achievement of early clinical remission (within 12 weeks of induction), achievement of endoscopic remission vs endoscopic improvement, and prior biologic exposure. The proportional hazards assumption was evaluated using Schoenfeld residuals. All statistical analyses were performed using STATA 17.0 (StataCorp LLC, College Station, TX).
RESULTS
Baseline disease characteristics
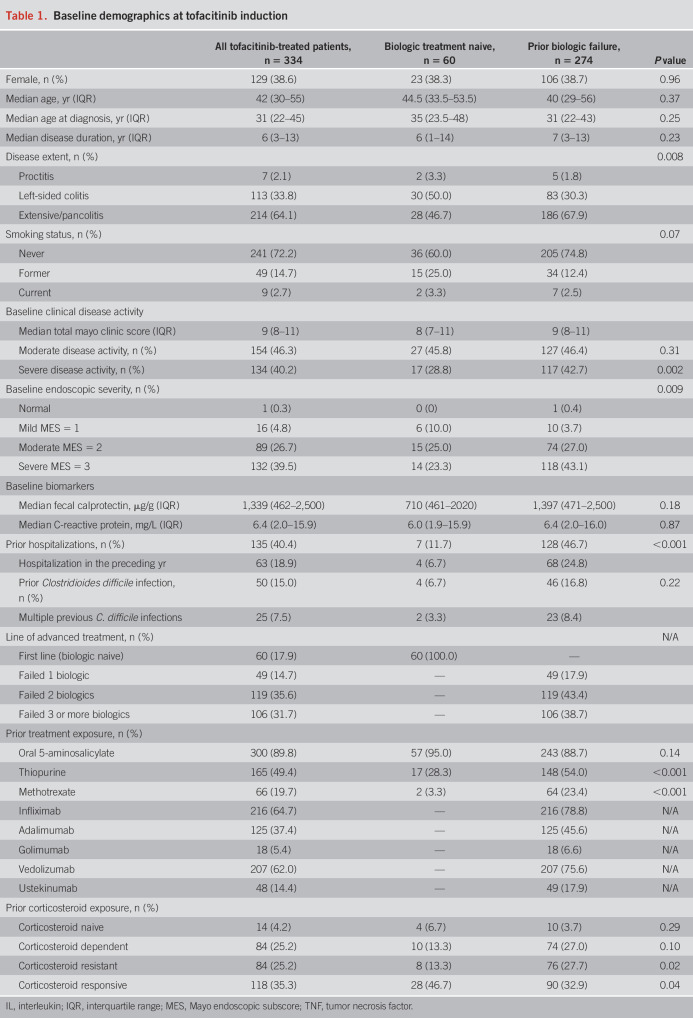
A total of 334 patients treated with UC treated with tofacitinib were included (Table 1), followed up for a total duration of 375 PY (see Supplemental Figure 1, http://links.lww.com/AJG/C826). Most of the patients (82.0%, 274/334) had previously been exposed to a biologic, with 82.1% (225/274) having failed at least 2 prior biologics. Approximately two-thirds of patients had pancolitis (64.1%, 214/334) with moderate-to-severe endoscopic disease activity at baseline. Most patients received tofacitinib 10 mg BID (94.3%, 315/334) as induction dosing; approximately two-thirds of patients de-escalated to 5 mg BID (222/334) for maintenance dosing. Data for vaccination against HZ were available for 230 patients: 86.1% (198/230) of patients had been vaccinated. A total of 148 patients (44.3%) received concurrent corticosteroids at tofacitinib induction (123 prednisone, 25 budesonide MMX) and 20 patients (6.0%) were on concomitant thiopurines or methotrexate.
Table 1.
Baseline demographics at tofacitinib induction
Treatment effectiveness
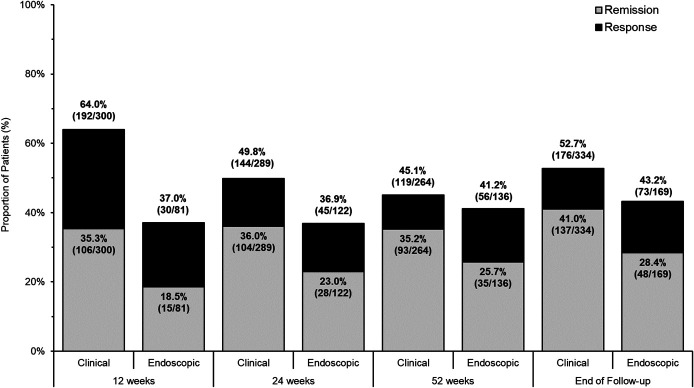
Clinical and endoscopic response and remission to tofacitinib at 12, 24, and 52 weeks and at end of follow-up are presented in Figure 1. Clinical remission was achieved by 35.3% (106/300), 36.0% (104/289), and 35.2% (93/264) of patients at weeks 12, 24, and 52, respectively. Most patients who achieved clinical remission did so without corticosteroids: corticosteroid-free clinical remission rates were achieved by 31.3% (94/300), 32.9% (95/289), and 33.3% (88/264) of patients at 12, 24, and 52 weeks, respectively. Endoscopic remission was achieved by 18.5% (15/81), 23.0% (28/122), and 25.7% (35/136) of patients at weeks 12, 24, and 52, respectively. Corticosteroid-free endoscopic remission was achieved in 17.3% (14/81), 18.9% (23/122), and 21.5% (29/135) of patients at weeks 12, 24, and 52, respectively. The median time between visits at weeks 12 and 24 was 12.4 weeks (IQR 11.2–16.8 weeks); the median time between visits at weeks 24 and 52 was 24.4 weeks (IQR 20.4–27.4 weeks).
Figure 1.
Clinical and endoscopic effectiveness of tofacitinib for treatment of ulcerative colitis.
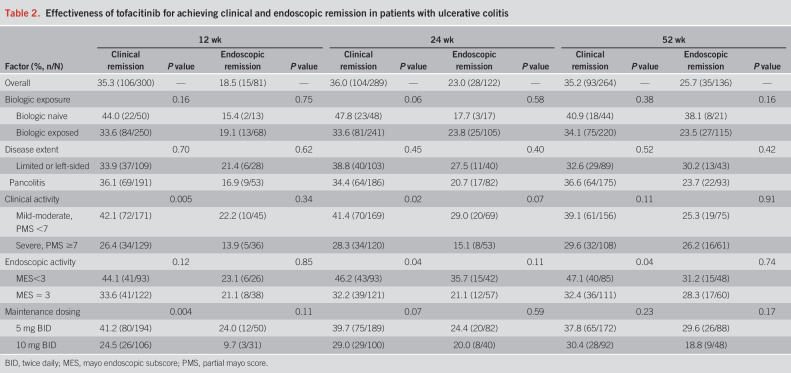
Subgroup analyses of treatment effectiveness are summarized in Table 2. Lower clinical and endoscopic disease activity scores at baseline were generally associated with higher treatment response, although at week 52, there was no association between endoscopic remission and prior biologic exposure, disease extent, baseline clinical or endoscopic disease activity, or initial maintenance dosing.
Table 2.
Effectiveness of tofacitinib for achieving clinical and endoscopic remission in patients with ulcerative colitis
At baseline, 58.1% (168/289) of patients had an elevated CRP ≥5 mg/L and 84.1% (106/126) had an elevated fecal calprotectin ≥250 μg/g. Among 20 patients with a fecal calprotectin <250 μg/g at baseline, 11/20 had fecal calprotectin ≥150 μg/g, 6 patients had an MES = 2, and 5 patients had an MES = 3. The cumulative proportion of patients with CRP normalization was 32.0% and 63.4% at 24 and 52 weeks, respectively (see Supplemental Figure 2, http://links.lww.com/AJG/C826). The cumulative proportion of patients with fecal calprotectin normalization was 31.9% and 59.3% at 24 and 52 weeks, respectively.
The probability of treatment persistence at 12, 24, and 52 weeks was 88.9%, 78.4%, and 64.2%, respectively. A total of 88 patients (26.4%) had no clinical response to tofacitinib therapy and discontinued treatment after a median duration of 12.9 weeks (IQR 7.6–21.7). During follow-up, a total of 131 patients discontinued treatment. The most common reason for discontinuation was nonresponse to tofacitinib (90.8%, 119/131). One patient discontinued treatment because of pregnancy.
Hospitalization and colectomy
UC-related hospitalizations were observed in 42 patients (12.6%) at a median time of 15.1 week (IQR 5.7–27.4) after tofacitinib induction (see Supplemental Figure 3, http://links.lww.com/AJG/C826). The risk of hospitalization was significantly higher for biologic-experienced patients (hazard ratio [HR] 4.46, 95% confidence interval [CI]: 1.08–18.47], P = 0.04) and patients with PMS ≥7 at baseline (HR 2.48 [95% CI: 1.33–4.62], P = 0.004). Colectomy was required in 27 patients (8.1%) at a median time of 14.4 weeks (IQR 6.7–26.7) after tofacitinib induction, exclusively in biologic-experienced patients and almost always for medically refractory disease (96.3%, 26/27). Severe clinical disease activity (PMS ≥7) at baseline was significantly associated with an increased risk for colectomy (HR 4.28 [95% CI: 1.81–10.14], P = 0.001).
Loss of response and dose optimization
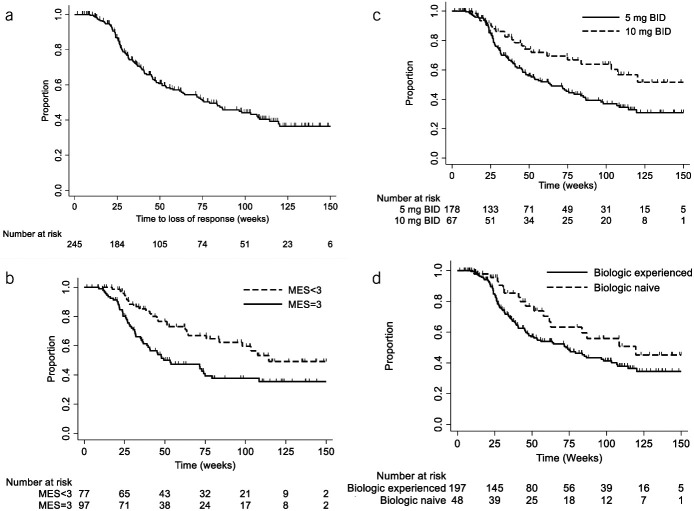
Among 245 patients with UC with an initial clinical response to tofacitinib, loss of response occurred in 44.5% (109/245) of patients over long-term follow-up (Figure 2). Dose re-escalation to 10 mg BID was attempted in 71 patients, and response was recaptured in 54.9% (39/71). The median time to dose de-escalation was 8 weeks (IQR 8–10.5 weeks); the median time to dose re-escalation was 22 weeks (IQR 9.9–45.8 weeks) after initial de-escalation. In multivariable Cox proportional hazards modeling, patients undergoing dose reduction were at more than 3-fold increased risk for loss of response if they had a baseline MES = 3 (adjusted HR [aHR] 3.60 [95% CI: 1.70–7.62], P = 0.001) or had failed a previous biologic (aHR 3.89 [95% CI: 1.28–11.86], P = 0.02), irrespective of whether they achieved early clinical or corticosteroid-free clinical remission. By contrast, achievement of endoscopic remission (MES = 0) was protective (aHR 0.41 [95% CI: 0.20–0.80], P = 0.009), but endoscopic improvement alone (MES = 1) was not associated with a reduced hazard for loss of response (aHR 1.26 [95% CI: 0.61–2.60], P = 0.53) (see Supplemental Table 1, http://links.lww.com/AJG/C826).
Figure 2.
Loss of response over time to tofacitinib, overall (a) and by: (b) baseline Mayo endoscopic subscore (MES; log-rank P value = 0.004), (c) maintenance dosing (P = 0.008), and (d) biologic exposure (P = 0.07). BID, twice daily.
Safety
A total of 97 patients (29.0%) experienced 119 AE (Table 3). Almost all SAE were related to hospitalization or colectomy for worsening UC despite tofacitinib. The most reported AE were bloodwork abnormalities, including elevated liver enzymes, elevated creatinine kinase, or abnormal lipids, occurring in 35 cases (29.4%), although none were reported as severe or required treatment discontinuation. Two cases of HZ (IR 0.5 events/100 PY [95% CI: 0.1–1.9]) were reported; both patients had been previously vaccinated against HZ. The most frequently reported infections were C. difficile (n = 9 cases) and upper or lower respiratory tract infections (n = 5 cases). Other infections were rare or single cases of viral gastroenteritis, traveler's diarrhea, folliculitis, otitis media, urinary tract infections, cellulitis, and a dental infection. One patient developed a supralevator abscess that required surgical drainage.
Table 3.
Safety of tofacitinib for treatment of ulcerative colitis
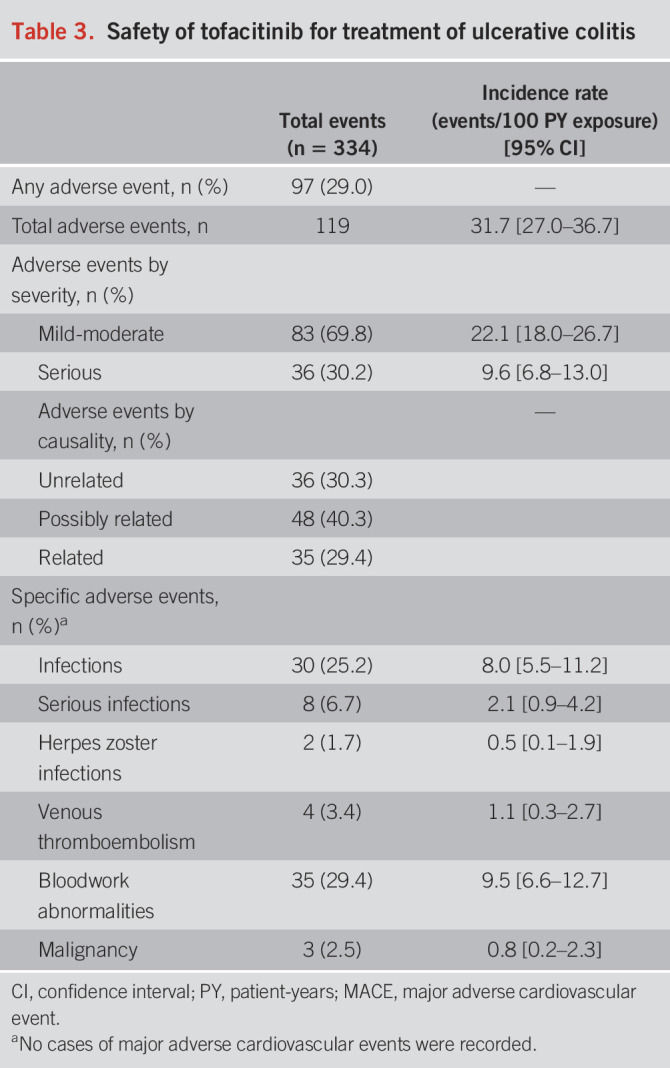
Three malignancies were reported (IR 0.8 events/100 PY [95% CI: 0.2–2.3]): 1 case of Kaposi sarcoma in a 50-year-old man with human immunodeficiency virus, 1 case of an incidentally discovered small bowel neuroendocrine tumor in a 28-year-old woman, and 1 case of multiple myeloma in a 35-year-old man. There were 4 cases of VTE (IR 1.1 events/100 PY [95% CI: 0.3–2.7]). One case was related to superficial thrombophlebitis in a 74-year-old woman. One case was judged to be a provoked subsegmental pulmonary embolism occurring immediately postoperatively in a 30-year-old man admitted to hospital with acute severe UC and undergoing colectomy. One case of subclavian vein thrombosis occurred in a 63-year-old man with active tonsillar squamous cell carcinoma. One case of acute pulmonary embolism occurred in a 71-year-old female ex-smoker with a personal history of prior VTE not on anticoagulation, but who was treated with tofacitinib as rescue therapy after failure of multiple prior biologics. No cases of MACE were reported.
DISCUSSION
Robust real-world studies are required to inform our understanding of treatment effectiveness and safety of JAK inhibitors in a generalizable patient population with UC and to help guide clinical decisions. REMIT-UC is the largest, real-world, multicenter cohort study of patients with UC treated with tofacitinib, capturing nationally representative data from geographically and clinically diverse practice settings throughout Canada. Our study highlights several novel findings. First, we show that although a substantial proportion of patients, including patients who failed other advanced agents, will achieve clinical and endoscopic remission, nearly half will lose response with dose de-escalation, and this is only partially recaptured with increasing the dose. Patients who are at a high risk for losing response include those with prior biologic failure and severe baseline endoscopic activity. In addition, those who only achieve endoscopic improvement but not enter complete endoscopic remission are not protected from losing response during follow-up. Dose de-escalation in this group must be considered carefully, especially for patients who have already failed other biologics and may be using tofacitinib last line before colectomy. Finally, we present reassuring safety data specific to patients with UC from 375 PY of drug exposure: in our population, treatment-related AE seem to be distinct from those observed in older, more comorbid patients enrolled in randomized controlled trials (RCTs) from other disease areas (7).
Several other real-world tofacitinib studies have been published (16–20), including multicenter cohorts from the Dutch Initiative on Crohn and Colitis registry (20), the Spanish ENEIDA (Estudio Nacional en Enfermedad Inflamatoria intestinal sobre Determinantes genéticos y Ambientales) registry (19), and the United States Tofacitinib Real-world Outcomes in Patients with UC and Crohn's disease consortium (18). Limitations of the existing literature include the following: (i) small sample sizes with short duration of follow-up, resulting in imprecise estimates of effectiveness and safety; (ii) selection bias toward patients enrolled from tertiary care centers that may not be reflective of routine practice; and (iii) inconsistent definitions of clinical and endoscopic outcomes, with subsequent heterogeneity in treatment effects. We attempted to mitigate these limitations by capturing a representative national sample of consecutive patients with UC treated with tofacitinib and with long-term follow-up, using relevant, guideline-endorsed outcomes (21,22).
In a meta-analysis of real-world tofacitinib studies, Taxonera et al (13) reported a week-12 to week-16 clinical remission rate of 47.0% and a 6-month clinical remission rate of 38.3%, although with substantial heterogeneity (I2 59%–61%). Overall, clinical remission rates in our cohort were similar, albeit slightly more conservative, given that nonresponder methods were used for missing data. We did not demonstrate a difference in tofacitinib effectiveness when used as first-line therapy vs after biologic failure, although numerically higher rates of remission were observed at all time points for patients naive to biologic therapy. Most patients in our cohort were treated with tofacitinib after failure of other advanced agents, and therefore, we are likely underpowered to definitively evaluate differential efficacy by line of therapy. Efficacy of JAK inhibitors is dose dependent: in the OCTAVE trials, greater efficacy was demonstrated for tofacitinib 10 vs 5 mg BID (23), and a recent post hoc analysis of the upadacitinib phase 3 trial program demonstrated greater rates of remission in patients maintained on 30 vs 15 mg daily (24). However, we observed that a higher proportion of patients on 5 mg BID were in clinical remission at week 12 compared with patients on 10 mg BID. This reflects the nonrandomized study design because in clinical care, patients are less likely to be dose reduced to 5 mg BID if they have not achieved remission.
In clinical care, deciding whether and/or when tofacitinib dosing should be de-escalated is an important decision for patients and providers. The Canadian product monograph recommends 8 weeks of induction treatment at 10 mg BID, followed by de-escalation to maintenance dosing at 5 mg BID. Stepping down to the lowest effective dose during maintenance is recommended, but the appropriate dose depends on the initial treatment response. Nearly half of patients lost response in our study when reducing to 5 mg BID, and this loss of response was only partially recaptured with re-escalation of therapy. By contrast, only 25% of patients de-escalating in the OCTAVE Open long-term extension study lost remission (12), and 77.1% of patients randomized to 5 mg BID step-down dosing in the RIVETING de-escalation trial remained in remission at 6 months (11). Higher loss of response rates in our cohort likely reflect shorter time to dose reduction (median time to de-escalation only 8 weeks in our cohort) and the relative depth of remission before de-escalation. By contrast, patients stepped down in the OCTAVE Open study after 52 weeks, and patients in the RIVETING (A Phase 3B/4, Multi-center, Double-Blind, Randomized, Parallel Group Study of Tofacitinib (CP-690,550) In Subjects with Ulcerative Colitis in Stable Remission) trial had already received at least 2 consecutive years of tofacitinib 10 mg BID before de-escalation. We hypothesize these trial populations represent a highly selected group of patients with sustained remission on tofacitinib, who are more likely to be able to tolerate dose reduction. Another important finding from REMIT-UC is that patients with prior biologic failure or severe endoscopic disease activity at baseline are at over 3-fold higher risk for loss of response. Dose de-escalation should be considered cautiously in this population, especially if complete endoscopic remission has not been achieved.
REMIT-UC also provides the most comprehensive real-world evaluation of tofacitinib safety to date, with 375 PY of follow-up (more than double that was previously reported in the US Tofacitinib Real-world Outcomes in Patients with UC and Crohn's disease Consortium) (18). Several key safety observations should be highlighted. First, the IR of HZ in our cohort (0.5 events/100 PY) was substantively lower than that reported in integrated safety analyses of RCT data from the UC (IR 3.30–3.38/100 PY) and RA (IR 3.6/100 PY) development programs, which we hypothesize may relate to protective effects of HZ vaccination (25,26). Only 1.1% (23/2062) of patients in the OCTAVE program treated with tofacitinib had received an HZ vaccine, when compared with >85% in our study. Vaccination against HZ should be considered a priority for this population. Second, we observed 1 superficial and 3 deep VTE in our cohort, all of which occurred in patients with preexisting risk factors (prior thrombosis, active malignancy, and postsurgery). These findings are reassuring and consistent with the thromboembolic risk observed in OCTAVE (IR approximately 0.20 events per 100 PY) (27). Although the IR for adjudicated VTE in the ORAL Surveillance trial for an enriched population of patients with RA treated with tofacitinib 10 mg BID was numerically higher (IR 0.70 [95% CI: 0.49–0.99]), this does not seem to be reflective of the risk observed in other RA, psoriasis, or psoriatic arthritis studies (IR approximately 0.13–0.38) nor the UC population (28,29). However, our results do highlight that gastroenterologists must be diligent in identifying and mitigating other potential thromboembolic risk factors for patients treated with tofacitinib. Finally, there were reassuringly no observed cases of MACE. Other AE such as dyslipidemia were mild, did not require treatment discontinuation, and were in keeping with the known safety profile of tofacitinib (30).
Our study has some important strengths. This is the largest cohort published to date, including 8 Canadian IBD centers with varied and diverse practice patterns. This allowed us to generate more representative estimates of effectiveness and safety. We focused on answering relevant clinical questions, such as the risk for relapse after dose de-escalation and the probability of achieving guideline-recommended outcomes. Finally, our results are strengthened by the inclusion of patients receiving tofacitinib as both their first-line advanced agent and as second-line therapy. Biologic-naive patients may not have access to tofacitinib in other jurisdictions.
However, we also acknowledge some key limitations. First, this is a retrospective study, with potential variability in disease assessments. Furthermore, decisions in routine care, including when and why patients will start treatment, adjust dose, or stop therapy, are often based on clinical judgment, which is difficult to adjust for. This contrasts with the strict inclusion/exclusion criteria and protocolled management observed in RCT settings. For example, only one-third of patients completed a fecal calprotectin at baseline and 17 patients did not have either endoscopy or biomarker assessment immediately within the 6 weeks before starting treatment. These patients were likely treated because of their clinical symptoms (15/17 had rectal bleeding or a stool frequency subscore of 3), which reflects the reality of routine care where not all patients will undergo comprehensive investigations prior to starting therapy. This introduces heterogeneity in the study population for assessing effectiveness. Second, in contrast to controlled trial settings, “real-world” circumstances such as endoscopy availability, scheduling variability, and patient adherence with follow-up tests all affect when disease evaluations can occur. Accordingly, not every patient contributed clinical, endoscopic, and biomarker data at every time point, and we specifically caution against making comparisons between time points or between assessment methods in this study. Third, potentially important confounders of treatment response and safety such as medication adherence are challenging to capture in a retrospective design. Fourth, there exists the risk of observation bias in retrospective studies. This is especially relevant for abstraction of endoscopic end points (which were not blindly or centrally read) or patient-reported outcome data and for measures with a high degree of subjectivity such as the PGA. We attempted to mitigate this using standardizing data collection language, implementation of study-specific training, and rigorous monitoring of the quality and accuracy of data entry.
In conclusion, REMIT-UC is a large, real-world, multicenter Canadian study that builds on the literature supporting the effectiveness of tofacitinib for achieving clinical, endoscopic, and biomarker remission, as both first-line and subsequent-line therapies in UC. Key clinical takeaways include the following: (i) tofacitinib is generally safe and well-tolerated in patients with UC, although clinicians must be cognizant of potential infectious and thrombotic risk factors; (ii) nearly half of patients lose response to tofacitinib after dose de-escalation, and this is only partly recaptured with dose re-escalation; (iii) patients with severe endoscopic activity and prior biologic failure are at an approximately 3-fold higher risk for losing response after dose de-escalation; (iv) achievement of endoscopic improvement alone is not protective against losing response after de-escalation; and (v) there remains a substantial risk for hospitalization and colectomy, particularly in patients receiving tofacitinib after multiple biologic failures. Taken together, these findings will help inform decisions about starting and optimizing JAK inhibitor therapy in patients with UC.
CONFLICTS OF INTEREST
Guarantor of the article: Christopher Ma, MD, MPH.
Specific author contributions: Study conceptualization and design: C.M., R.P., F.P., B.B., V.J., T.B. Data collection: C.M., Y.X., Y.K., E.C.L.W., C.T., M.R.F., S.Y.S., R.S. Data analysis: C.M. Manuscript drafting: C.M. Manuscript editing for important intellectual content: all authors. All authors have approved the final version of this manuscript.
Financial support: This study was cofunded by Canadian IBD Research Consortium (CIRC) and Pfizer. Pfizer and associated employees did not have access to any data. CIRC provided comments on the study design and input on data interpretation. All data collection was performed by CIRC investigators at each site; all data analyses were performed centrally at the University of Calgary.
Potential competing interests: C.M. has received consulting fees from AbbVie, Alimentiv, Amgen, AVIR Pharma Inc, BioJAMP, Bristol Myers Squibb, Celltrion, Ferring, Fresenius Kabi, Janssen, McKesson, Mylan, Takeda, Pendopharm, Pfizer, and Roche; speaker's fees from AbbVie, Amgen, AVIR Pharma Inc, Alimentiv, Ferring, Janssen, Takeda, and Pfizer; research support from Ferring and Pfizer. R.P. has received consulting fees from Abbott, AbbVie, Alimentiv Inc Amgen, Arena Pharmaceuticals, AstraZeneca, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Celltrion, Cosmos Pharmaceuticals, Eisai, Elan, Eli Lilly, Ferring, Galapagos, Fresenius Kabi, Genentech, Gilead Sciences, Glaxo-Smith Kline, BioJAMP, Janssen, Merck, Mylan, Oppilan, Organon Pharma, Pandion Pharma, Pendopharm, Pfizer, Progenity, Protagonist Therapeutics, Roche, Sandoz, Satisfai Health, Shire, Sublimity Therapeutics, Takeda Pharmaceuticals, Theravance Biopharma, and Viatris; speaker's fees from AbbVie, Amgen, Arena Pharmaceuticals, Bristol-Myers Squibb, Celgene, Eli Lilly, Fresenius Kabi, Ferring, Gilead Sciences, Janssen, Merck, Pfizer, Roche, Sandoz, Shire, and Takeda Pharmaceuticals; advisory boards fees from AbbVie, Amgen, Arena Pharmaceuticals, Bristol-Myers Squibb, Celgene, Celltrion, Eli Lilly, Ferring, Galapagos, Genentech, Gilead Sciences, Glaxo-Smith Kline, Janssen, Merck, Mylan, Oppilan Pharma, Pandion Pharma, Pfizer, Sandoz, Shire, Sublimity Therapeutics, Takeda Pharmaceuticals, and Theravance Biopharma; and research/educational support from AbbVie, Ferring, Janssen, Pfizer, and Takeda Pharmaceuticals. Y.X., Y.K., S.M., and E.C.L.W. have no conflicts of interest. N.N. has received consulting fees from Iterative Scopes, Abbvie, Takeda, Janssen, and Pfizer; lecture fees from Janssen, Abbvie, Takeda, and Pfizer. C.T. has no conflicts of interest. F.P. has received speaker/advisory board fees from Janssen, AbbVie, Takeda, Pfizer, and Pharmascience. M.R.F. has no conflicts of interest. B.B. has received advisor/speaker fees: Ferring, Janssen, Abbvie, Takeda, Pfizer, Novartis, BMS, and Merck. Advisor: Alimentiv, Gilead, Iterative Scopes, AMT, Celgene, Microbiome Insights, Merck, Amgen, Pendopharm, Genentech, BMS, Allergan, Protagonist, Fresenius Kabi, Mylan, and Bausch Health; research support: Janssen, Abbvie, GSK, BMS, Amgen, Genentech, Merck, BI, Qu Biologic, Celgene, and Alvine; and stock options: Qu Biologic. S.Y.S. has no conflicts of interest. D.L. has received consulting fees for Abbvie, Amgen, Bristol Myers Squibb, Ferring, Fresenius-Kabi, Gilead, Janssen, McKesson, Merck, Organon, Pendopharm, Pfizer, Sandoz, Shire, Samsung, and Takeda and speaker fees from Abbvie, Amgen, Bausch, Ferring, Fresenius Kabi, Janssen, Lupin, Organon, Mylan, Pfizer, Sandoz, Shire, and Takeda. R.S. has no conflicts of interest to declare. V.J. has received advisory board/consulting fees from AbbVie, Alimentiv Inc. (formerly Robarts Clinical Trials Inc), Amgen, Applied Strategic, Arena Pharmaceuticals, Asahi Kasei Pharma, Asieris, Astra Zeneca, Celgene/BMS, Celltrion, Eli Lilly, Ferring, F. Hoffman-La Roche Ltd, Flagship Pioneering, Fresenius Kabi, Galapagos, Genentech, Gilead, GlaxoSmithKline, Janssen, Organon (Merck), Mylan, Pandion, Pendopharm, Pfizer, Reistone Biopharma, Sandoz, Second Genome, Takeda, Teva, Topivert, Ventyx, and Vividion Therapeutics; speaker fees from Abbvie, Ferring, Galapagos, Janssen, Pfizer, and Takeda; and research support from Celgene/BMS, Pfizer, Abbvie, Boehringer Ingelheim, Eli Lilly, Janssen, Takeda, Gilead Sciences, and Tigenix. T.B. has received consulting or speaker fees for Abbvie, Alimentiv, Amgen, Bristol Myers Squibb, Ferring, Janssen, Merck, Pfizer, Roche, Sandoz, Takeda, Gilead, Viatris, Fresenius, and Kabi.
Study Highlights.
WHAT IS KNOWN
✓ Tofacitinib, a pan-Janus kinase inhibitor, is an effective treatment for patients with moderate-to-severely active ulcerative colitis (UC).
✓ Concerns regarding the safety profile of Janus kinase inhibitors have been identified, although adverse events occurring in trial populations may not be generalizable to real-world practice.
✓ During maintenance, the lowest dose of tofacitinib required to maintain remission is recommended, but some patients who dose reduce from 10 to 5 mg twice daily will lose response.
WHAT IS NEW HERE
✓ REMIT-UC is the largest multicenter, real-world cohort study of patients with UC treated with tofacitinib to date, including more than 375 patient-years of follow-up.
✓ Nearly half of patients who de-escalate from 10 to 5 mg lost response, and this was only recaptured in half of patients who re-escalated treatment.
✓ Patients with severe endoscopic activity and prior biologic failure are at over 3-fold increased risk for loss of response after dose reduction. Dose de-escalation must be considered judiciously in this population.
✓ The safety profile of tofacitinib in this real-world cohort of younger patients with UC who are predominantly vaccinated against herpes zoster was reassuring, with low rates of venous thromboembolism and infections.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jane Castelli for her support as the project manager for this study.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/AJG/C826
Contributor Information
Remo Panaccione, Email: rpanacci@ucalgary.ca.
Yasi Xiao, Email: yasi.xiao@gmail.com.
Yuvan Khandelwal, Email: yuvankhandelwal@gmail.com.
Sanjay K. Murthy, Email: smurthy@toh.ca.
Emily C.L. Wong, Email: wonge12@mcmaster.ca.
Neeraj Narula, Email: neeraj.narula@medportal.ca.
Catherine Tsai, Email: catherinetsai@ualberta.ca.
Farhad Peerani, Email: peerani@ualberta.ca.
Marica Reise-Filteau, Email: marica_rf@hotmail.com.
Brian Bressler, Email: brian_bressler@hotmail.com.
Samantha Y. Starkey, Email: sstark3y@student.ubc.ca.
Dustin Loomes, Email: dloomes@ualberta.ca.
Rocio Sedano, Email: rocio.sedano@alimentiv.com.
Vipul Jairath, Email: vjairath@uwo.ca.
Talat Bessissow, Email: talat.bessissow@mcgill.ca.
REFERENCES
- 1.Lovato P, Brender C, Agnholt J, et al. Constitutive STAT3 activation in intestinal T cells from patients with Crohn's disease. J Biol Chem 2003;278(19):16777–81. [DOI] [PubMed] [Google Scholar]
- 2.Schreiber S, Rosenstiel P, Hampe J, et al. Activation of signal transducer and activator of transcription (STAT) 1 in human chronic inflammatory bowel disease. Gut 2002;51(3):379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shuai K, Liu B. Regulation of JAK–STAT signalling in the immune system. Nat Rev Immunol 2003;3(11):900–11. [DOI] [PubMed] [Google Scholar]
- 4.Ma C, Lee JK, Mitra AR, et al. Systematic review with meta-analysis: Efficacy and safety of oral Janus kinase inhibitors for inflammatory bowel disease. Aliment Pharmacol Ther 2019;50(1):5–23. [DOI] [PubMed] [Google Scholar]
- 5.Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;376(18):1723–36. [DOI] [PubMed] [Google Scholar]
- 6.Ma C, Jairath V, Vande Casteele N. Pharmacology, efficacy and safety of JAK inhibitors in Crohn's disease. Best Pract Res Clin Gastroenterol 2019;38-39:101606. [DOI] [PubMed] [Google Scholar]
- 7.Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med 2022;386(4):316–26. [DOI] [PubMed] [Google Scholar]
- 8.US Food & Drug Administration. FDA Drug Safety Communication: FDA Requires Warnings about Increased Risk of Serious Heart-Related Events, Cancer, Blood Clots, and Death for JAK Inhibitors that Treat Certain Chronic Inflammatory Conditions. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death. Published February 4, 2021. Updated December 7, 2021. Accessed December 26, 2022. [Google Scholar]
- 9.Sandborn WJ, Panes J, D'Haens GR, et al. Safety of tofacitinib for treatment of ulcerative colitis, based on 4.4 years of data from global clinical trials. Clin Gastroenterol Hepatol 2019;17(8):1541–50. [DOI] [PubMed] [Google Scholar]
- 10.Sandborn WJ, Lawendy N, Danese S, et al. Safety and efficacy of tofacitinib for treatment of ulcerative colitis: Final analysis of OCTAVE open, an open-label, long-term extension study with up to 7.0 years of treatment. Aliment Pharmacol Ther 2022;55(4):464–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vermeire S, Su C, Lawendy N, et al. Outcomes of tofacitinib dose reduction in patients with ulcerative colitis in stable remission from the randomised RIVETING trial. J Crohns Colitis 2021;15(7):1130–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sands BE, Armuzzi A, Marshall JK, et al. Efficacy and safety of tofacitinib dose de-escalation and dose escalation for patients with ulcerative colitis: Results from OCTAVE open. Aliment Pharmacol Ther 2020;51(2):271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taxonera C, Olivares D, Alba C. Real-world effectiveness and safety of tofacitinib in patients with ulcerative colitis: Systematic review with meta-analysis. Inflamm Bowel Dis 2022;28(1):32–40. [DOI] [PubMed] [Google Scholar]
- 14.Lucaciu LA, Constantine-Cooke N, Plevris N, et al. Real-world experience with tofacitinib in ulcerative colitis: A systematic review and meta-analysis. Therap Adv Gastroenterol 2021;14:175628482110640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narula N, Peerani F, Meserve J, et al. Open: Vedolizumab for ulcerative colitis: Treatment outcomes from the VICTORY consortium. Am J Gastroenterol 2018;113(9):1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weisshof R, Aharoni Golan M, Sossenheimer PH, et al. Real-world experience with tofacitinib in IBD at a tertiary center. Dig Dis Sci 2019;64(7):1945–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honap S, Chee D, Chapman TP, et al. Real-world effectiveness of tofacitinib for moderate to severe ulcerative colitis: A multicentre UK experience. J Crohns Colitis 2020;14(10):1385–93. [DOI] [PubMed] [Google Scholar]
- 18.Deepak P, Alayo QA, Khatiwada A, et al. Safety of tofacitinib in a real-world cohort of patients with ulcerative colitis. Clin Gastroenterol Hepatol 2021;19(8):1592–601 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaparro M, Garre A, Mesonero F, et al. Tofacitinib in ulcerative colitis: Real-world evidence from the ENEIDA registry. J Crohns Colitis 2021;15(1):35–42. [DOI] [PubMed] [Google Scholar]
- 20.Biemans VBC, Sleutjes JAM, de Vries AC, et al. Tofacitinib for ulcerative colitis: Results of the prospective Dutch initiative on Crohn and colitis (ICC) registry. Aliment Pharmacol Ther 2020;51(9):880–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma C, Hanzel J, Panaccione R, et al. CORE-IBD: A multidisciplinary international consensus initiative to develop a core outcome set for randomized controlled trials in inflammatory bowel disease. Gastroenterology. 2022;163(4):950–64. [DOI] [PubMed] [Google Scholar]
- 22.Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: An update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): Determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology 2021;160(5):1570–83. [DOI] [PubMed] [Google Scholar]
- 23.Mukherjee A, Hazra A, Smith MK, et al. Exposure-response characterization of tofacitinib efficacy in moderate to severe ulcerative colitis: Results from a dose-ranging phase 2 trial. Br J Clin Pharmacol 2018;84(6):1136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feagan B, Parkes G, Juillerat P, et al. OP197 benefits of high versus low dose upadacitinib as maintenance treatment in ulcerative colitis patients WHO were responders to 8-WEEK induction with upadacitinib: Results from the U-achieve phase 3 maintenance trial. United Eur Gastroenterol J 2022;10:151. [PMC free article] [PubMed] [Google Scholar]
- 25.Winthrop KL, Vermeire S, Long MD, et al. Long-term risk of herpes zoster infection in patients with ulcerative colitis receiving tofacitinib. Inflamm Bowel Dis 2022:izac063. Available at: https://pubmed.ncbi.nlm.nih.gov/35648151/. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen SB, Tanaka Y, Mariette X, et al. Long-term safety of tofacitinib up to 9.5 years: A comprehensive integrated analysis of the rheumatoid arthritis clinical development programme. RMD Open 2020;6(3):e001395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandborn WJ, Panes J, Sands BE, et al. Venous thromboembolic events in the tofacitinib ulcerative colitis clinical development programme. Aliment Pharmacol Ther 2019;50(10):1068–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burmester GR, Curtis JR, Yun H, et al. An integrated analysis of the safety of tofacitinib in psoriatic arthritis across phase III and long-term extension studies with comparison to real-world observational data. Drug Saf 2020;43(4):379–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mease P, Charles-Schoeman C, Cohen S, et al. Incidence of venous and arterial thromboembolic events reported in the tofacitinib rheumatoid arthritis, psoriasis and psoriatic arthritis development programmes and from real-world data. Ann Rheum Dis 2020;79(11):1400–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sands BE, Taub PR, Armuzzi A, et al. Tofacitinib treatment is associated with modest and reversible increases in serum lipids in patients with ulcerative colitis. Clin Gastroenterol Hepatol 2020;18(1):123–32.e3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.