Background:
Mitochondrial DNA (mtDNA)-induced myocardial inflammation is intimately involved in cardiac remodeling. ZBP1 (Z-DNA binding protein 1) is a pattern recognition receptor positively regulating inflammation in response to mtDNA in inflammatory cells, fibroblasts, and endothelial cells. However, the role of ZBP1 in myocardial inflammation and cardiac remodeling remains unclear. The aim of this study was to elucidate the role of ZBP1 in mtDNA-induced inflammation in cardiomyocytes and failing hearts.
Methods:
mtDNA was administrated into isolated cardiomyocytes. Myocardial infarctionwas conducted in wild type and ZBP1 knockout mice.
Results:
We here found that, unlike in macrophages, ZBP1 knockdown unexpectedly exacerbated mtDNA-induced inflammation such as increases in IL (interleukin)-1β and IL-6, accompanied by increases in RIPK3 (receptor interacting protein kinase 3), phosphorylated NF-κB (nuclear factor-κB), and NLRP3 (nucleotide-binding domain and leucine-rich-repeat family pyrin domain containing 3) in cardiomyocytes. RIPK3 knockdown canceled further increases in phosphorylated NF-κB, NLRP3, IL-1β, and IL-6 by ZBP1 knockdown in cardiomyocytes in response to mtDNA. Furthermore, NF-κB knockdown suppressed such increases in NLRP3, IL-1β, and IL-6 by ZBP1 knockdown in response to mtDNA. CpG-oligodeoxynucleotide, a Toll-like receptor 9 stimulator, increased RIPK3, IL-1β, and IL-6 and ZBP1 knockdown exacerbated them. Dloop, a component of mtDNA, but not Tert and B2m, components of nuclear DNA, was increased in cytosolic fraction from noninfarcted region of mouse hearts after myocardial infarction compared with control hearts. Consistent with this change, ZBP1, RIPK3, phosphorylated NF-κB, NLRP3, IL-1β, and IL-6 were increased in failing hearts. ZBP1 knockout mice exacerbated left ventricular dilatation and dysfunction after myocardial infarction, accompanied by further increases in RIPK3, phosphorylated NF-κB, NLRP3, IL-1β, and IL-6. In histological analysis, ZBP1 knockout increased interstitial fibrosis and myocardial apoptosis in failing hearts.
Conclusions:
Our study reveals unexpected protective roles of ZBP1 against cardiac remodeling as an endogenous suppressor of mtDNA-induced myocardial inflammation.
Keywords: cytokines; fibrosis; heart failure; inflammation; macrophages; nucleotides; receptors, pattern recognition
Novelty and Significance.
What Is Known?
Myocardial inflammation is intimately involved in cardiac remodeling and failure.
Mitochondrial DNA (mtDNA), as a damage-associated molecular pattern, induces myocardial inflammation.
ZBP1 (Z-DNA binding protein 1) negatively regulates inflammation in response to mtDNA in inflammatory cells, fibroblasts, and endothelial cells.
What New Information Does This Article Contribute?
ZBP1 unexpectedly attenuates mtDNA-induced inflammation in cardiomyocytes through suppression of the RIPK3 (receptor interacting protein kinase)-NF-κB (nuclear factor-κB) pathway, unlike in macrophages.
ZBP1 inhibits inflammation induced by TLR9 stimulation in cardiomyocytes.
ZBP1 protects against myocardial inflammation and cardiac remodeling after myocardial infarction.
Heart failure is increasing in prevalence, and its mortality is still high. Clinical studies suggested that inflammation is associated with heart failure. Previous studies have demonstrated that mtDNA was one of the sources of sterile inflammation in the pathogenesis of heart failure. ZBP1 can sense mtDNA to induce inflammatory responses. However, this study revealed that ZBP1 in cardiomyocytes, unlike in macrophages, had protective roles against mtDNA-induced inflammation. Upon mtDNA transfection into cardiomyocytes, TLR9 activated RIPK3-NF-κB-NLRP3 pathway. ZBP1 sequestrated mtDNA in cardiomyocytes, leading to inhibition of interaction between mtDNA and TLR9 and attenuation of RIPK3-NF-κB-NLRP3 pathway. ZBP1 knockout exacerbated postmyocardial infarction remodeling through activation of RIPK3-NF-κB-NLRP3 pathway. Our findings provide ambivalent roles of ZBP1 between cardiomyocytes and macrophages and identify ZBP1 in cardiomyocytes as a novel therapeutic target to prevent heart failure progression.
In This Issue, see p 1101
Meet the First Author, see p 1103
Heart failure (HF) is increasing in prevalence and incidence and has been defined as a global pandemic, affecting around 26 million people in the world.1 Despite significant therapeutic advances, morbidity and mortality in HF are still high. Therefore, novel insights into the pathophysiology and molecular mechanisms of HF are required to develop novel therapeutic approaches.
Previous studies have demonstrated that myocardial inflammation is critically involved in the pathophysiology of HF.2–7 In particular, sterile inflammation (noninfectious inflammation) plays a pivotal role in development of HF and is known to be mediated by mitochondrial DNA (mtDNA), as a part of damage-associated molecular patterns.8,9 mtDNA unprocessed by autophagy in cardiomyocytes induces cardiac inflammation and injury via stimulation of pattern recognition receptors, such as the TLR9 (Toll-like receptor 9).10 In addition, a recent study has demonstrated that mitochondria release mtDNA into cytoplasm during apoptosis.11 Furthermore, cytosolic mtDNA is directly taken up by lysosomes and degraded and TLR9 is rapidly recruited to lysosomes on activation.12,13 Stimulation of TLR9 activates NF-κB (nuclear factor-κB) and NLRP3 (nucleotide-binding domain and leucine-rich-repeat family pyrin domain containing 3) inflammasome, leading to increases in inflammatory cytokines, including IL (interleukin)-1β and IL-6.14,15 IL-1β and IL-6 are also intimately involved in development of HF.2,16,17 Therefore, the mechanisms of mtDNA-induced inflammation in cardiomyocytes are potential therapeutic targets for HF.
To date, several pattern recognition receptors other than TLR9 have been identified.18,19 Among them, ZBP1 (Z-DNA binding protein 1) is a nucleic acid sensing protein containing 2 Zα domains that directly binds to mitochondrial and nuclear double strand DNA20,21 and is known to positively regulate inflammation in response to mtDNA in inflammatory cells,22 fibroblasts,23 and endothelial cells.24 ZBP1 mediates activation of NF-κB by interacting with RIPK (receptor interacting protein kinase).25 It also induces inflammation via TBK1 (TANK [TRAF (tumor necrosis factor receptor-associated factor) family member-associated NF-κB activator]-binding kinase 1) activation.22,26 These findings raise a possibility that ZBP1 could be involved in mtDNA-induced inflammation in cardiomyocytes and hearts. However, the role of ZBP1 in cardiac inflammation and development of HF remains unknown. Furthermore, the interaction of ZBP1 with other mtDNA-sensing proteins, including TLR9, has not been elucidated yet.
In this study, we investigated the role of ZBP1 in mtDNA-induced inflammation in cardiomyocytes. We also examined the role of ZBP1 in HF model after myocardial infarction (MI) by using ZBP1 knockout mice. We found that, unexpectedly, ZBP1 negatively regulated mtDNA-induced myocardial inflammation by suppressing RIPK3-NF-κB pathway and played a protective role in cardiac dysfunction and HF. This study provides the first evidence for the anti-inflammatory and protective roles of ZBP1 in the heart.
Methods
Data Availability
All data and supporting materials have been provided with the published article. Please see the Detailed Methods and Major Resources Table in the Supplemental Materials.27–33 We showed the illustrative images for each Figure.
Results
mtDNA Increases ZBP1 and Activates Inflammatory Signaling in Cardiomyocytes
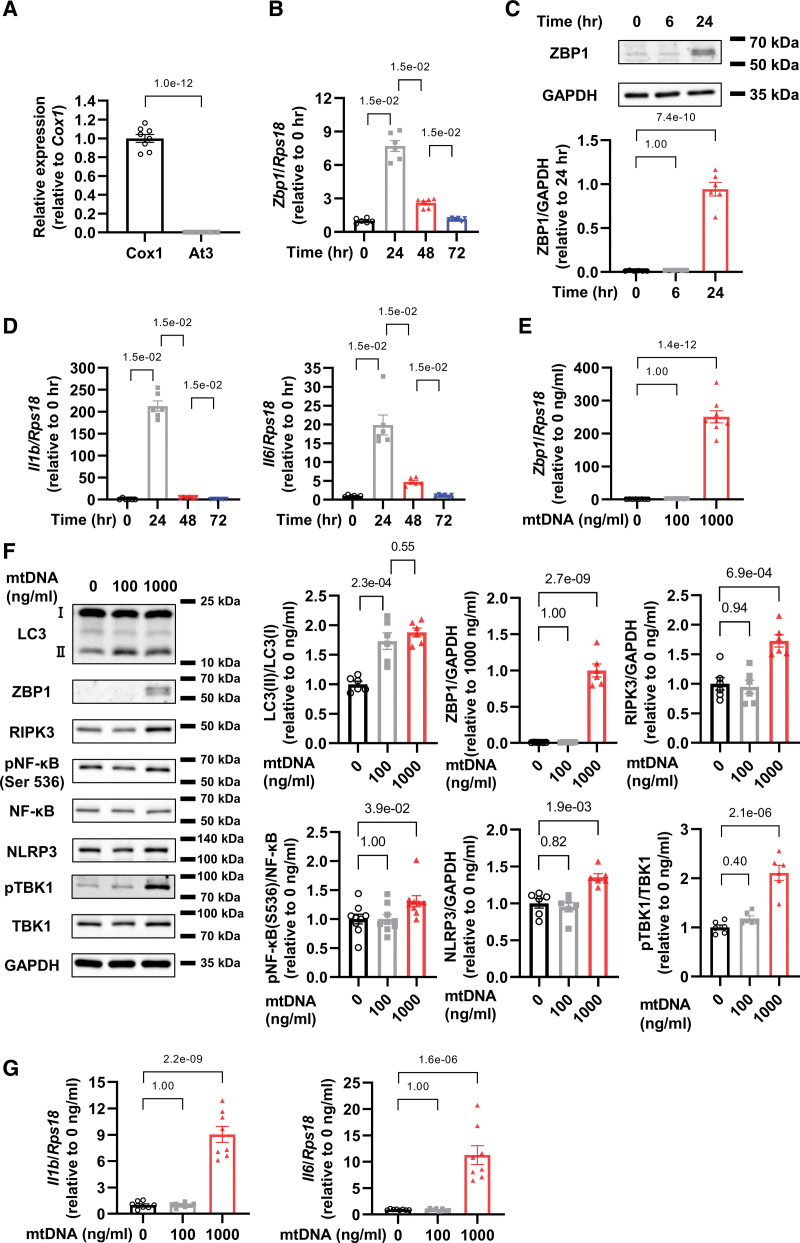
To evaluate ZBP1 expression in cardiomyocytes in response to mtDNA, we administered 1000 ng/mL of mtDNA extracted from rat liver into cardiomyocytes, the purity of which was validated by detecting Cox1, a part of mtDNA, but not At3, a part of nuclear DNA (Figure 1A). After 24 hours from mtDNA administration, we found that ZBP1 mRNA and protein levels were increased (Figure 1B and 1C) together with the increase of IL-1β and IL-6 mRNA levels (Figure 1D).
Figure 1.
Mitochondrial DNA (mtDNA) increases ZBP1 (Z-DNA binding protein 1) and activates inflammatory signaling in cardiomyocytes. A, Cox1 and At3 DNA levels in mtDNA extracted from rat livers was quantified by real-time polymerase chain reaction (n=8). The experiment was conducted 2×. B, mRNA levels of Zbp1 in cultured neonatal rat ventricular myocytes (NRVMs) treated with 1000 ng/mL of mtDNA in indicated time point (n=6). The experiment was conducted 2×. C, Representative immunoblots of ZBP1 and GAPDH in NRVMs treated with 1000 ng/mL of mtDNA in indicated time point (n=6). The experiment was conducted 2×. D, Il1b and Il6 mRNA levels in NRVMs treated with 1000 ng/mL of mtDNA in indicated time point (n=6). The experiment was conducted 2×. E, mRNA levels of Zbp1 in NRVMs treated with indicated concentrations of mtDNA (n=8). The experiment was conducted 2×. F, Representative immunoblots of LC3 (light chain 3; n=6), ZBP1 (n=6), RIPK3 (n=6), phosphorylated NF-κB (nuclear factor-κB; Ser 536), NF-κB (n=9), NLRP3 (n=6), phosphorylated TBK1 (TANK-binding kinase 1), TBK1 (n=12), and GAPDH in NRVMs treated with indicated concentrations of mtDNA. The experiment was conducted 2× except for NF-κB (3×). G, mRNA levels of Il1b and Il6 in NRVMs treated with indicated concentrations of mtDNA (n=8). The experiment was conducted 2×. Error bars denote standard errors. Data were analyzed using the Student t test (A), Wilcoxon rank sum test (B and D; adjust=3. F NF-κB; adjust=2), and 2-way ANOVA followed by Tukey multiple comparisons test (C, E, F except for NF-κB and G).
Furthermore, we investigated mtDNA-dose-dependent responses of ZBP1, and other proteins related to inflammation, including microtubule-associated protein 1A/1B- LC3 (light chain 3), RIPK3, phosphorylated NF-κB p65 subunit (Ser536), and NLRP3. Although a low dose of mtDNA (100 ng/mL) increased LC3-II but not ZBP1, a high dose of mtDNA (1000 ng/mL) increased both LC3-II and ZBP1 (Figure 1E and 1F). Importantly, only a high dose of mtDNA increased protein levels of RIPK3, phosphorylated NF-κB, NLRP3, and phosphorylated TBK1, which are major mediators of inflammation (Figure 1F). Additionally, a high dose of mtDNA increased mRNA levels of IL-1β and IL-6 whereas a low dose did not (Figure 1G). To investigate whether mtDNA from different tissues could induce the same response, we also used mtDNA extracted from rat hearts and conducted the same experiments. Above-mentioned results were replicated in the experiment using mtDNA extracted from rat hearts (Figure S1A through S1C). Hereafter, we used mtDNA extracted from liver due to its abundance. The real-time PCR showed 1.9× higher levels of Cox1 in cytosolic fraction in mtDNA 1000 ng/mL group in comparison with control group, while no detectable changes in Cox1 in 100 ng/mL group (Figure S1D). At3 was not detected in any group (Figure S1D). These data suggest that while a low dose of mtDNA activates and processed by autophagy, as demonstrated by the increase of LC3-II, a high dose of mtDNA not only induces autophagy but also increases ZBP1 and other inflammatory-related proteins, leading to myocardial inflammation.
ZBP1 Knockdown Exacerbates Increases in Inflammatory Cytokines in Cardiomyocytes Treated With mtDNA
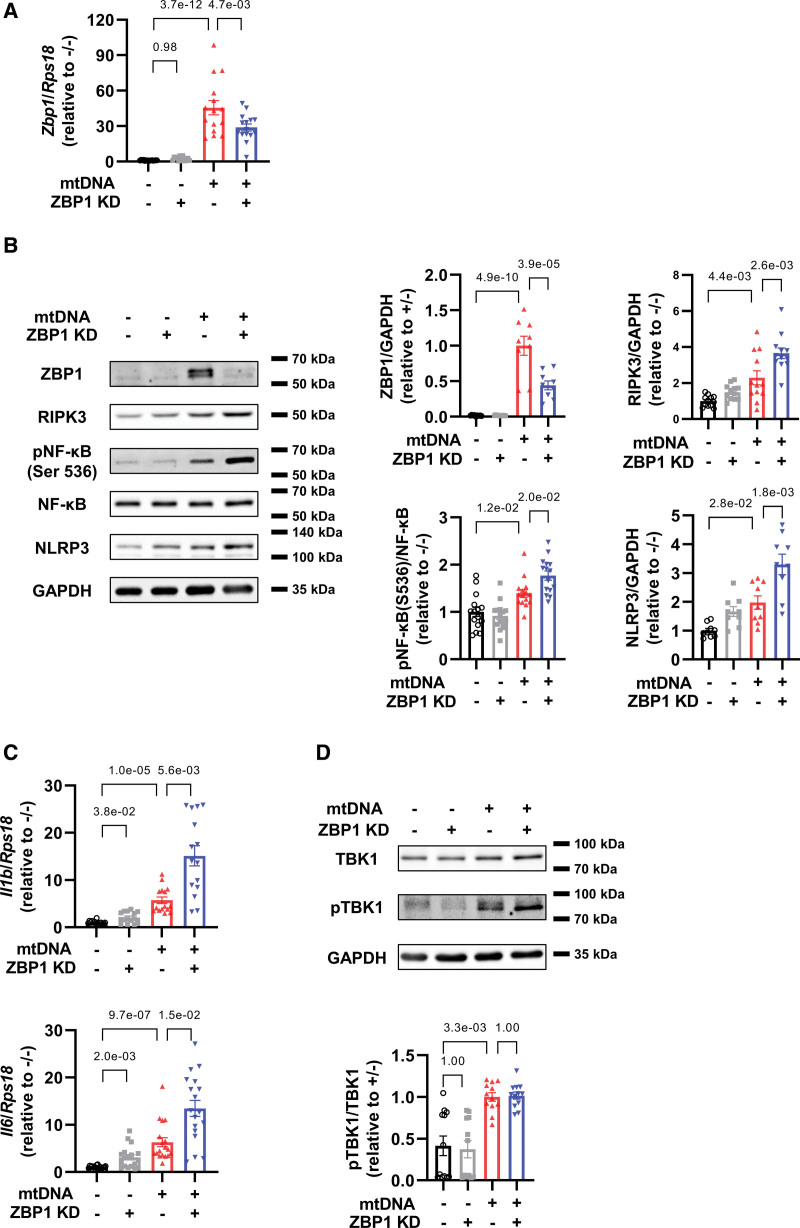
Next, we investigated the functional role of ZBP1 in cardiomyocytes in response to 1000 ng/mL of mtDNA. Knockdown by small interfering RNA efficiently suppressed mRNA and proteins levels of ZBP1 (Figure 2A and 2B). Unexpectedly, although ZBP-1 has been reported to mediate inflammation, ZBP1 knockdown exacerbated mtDNA-induced increases in RIPK3, phosphorylated NF-κB, and NLRP3 (Figure 2B). Consistent with these results, ZBP1 knockdown further increased mRNA levels of IL-1β and IL-6 in cardiomyocytes treated with mtDNA (Figure 2C). TBK1 is another downstream target of ZBP1.22,26 Although a high dose of mtDNA increased phosphorylation of TBK1 (Figure 1F), ZBP1 knockdown did not affect it (Figure 2D). These findings indicate that ZBP1 negatively regulates RIPK3, NF-κB, and inflammatory cytokines, but not TBK1, in cardiomyocytes.
Figure 2.
ZBP1 (Z-DNA binding protein 1) knockdown exacerbates increases in inflammatory cytokines in cardiomyocytes treated with mitochondrial DNA (mtDNA). A, mRNA levels of Zbp1 in neonatal rat ventricular myocytes (NRVMs) treated with or without small interfering RNA (siRNA) for ZBP1 (10 nmol/L) in the presence or absence of mtDNA (1000 ng/mL) for 24 hours (n=15). The experiment was conducted 5×. B, Representative immunoblots of ZBP1 (n=9), RIPK3 (receptor interacting protein kinase; n=12), phosphorylated NF-κB (nuclear factor-κB; Ser 536), NF-κB (n=15), NLRP3 (nucleotide-binding domain and leucine-rich-repeat family pyrin domain containing 3; n=9), and GAPDH in NRVMs treated with or without small interfering RNA (siRNA) for ZBP1 (10 nmol/L) in the presence or absence of mtDNA (1000 ng/mL) for 24 hours. The experiment was conducted 3 (ZBP1 and NLRP3), 4 (RIPK3), and 5× (NF-κB). C, mRNA levels of Il1b (n=15) and Il6 (n=18) in NRVMs treated with or without siRNA for ZBP1 (10 nmol/L) in the presence or absence of mtDNA (1000 ng/mL) for 24 hours. The experiment was conducted 5 (Il1b) or 6 (Il6) ×. D, Representative immunoblots of TBK1, phosphorylated TBK1, and GAPDH in NRVMs treated with or without siRNA for ZBP1 (10 nmol/L) in the presence or absence of mtDNA (1000 ng/mL) for 24 hours (n=12). The experiment was conducted 4×. Error bars denote standard errors. Data were analyzed using the Wilcoxon rank sum test (C and E; adjust=3), and 2-way ANOVA followed by Tukey multiple comparisons test (A, B, and D).
Overexpression of ZBP1 Attenuates Inflammatory Cytokines in Cardiomyocytes Treated With mtDNA
Overexpression of ZBP1 by adenovirus further increased mRNA and proteins levels of ZBP1 (Figure S2A and S2B). ZBP1 overexpression mitigated mtDNA-induced increases in RIPK3, phosphorylated NF-κB, and NLRP3 (Figure S2B), as well as mRNA levels of IL-1β and IL-6 (Figure S2C). These results support the protective role of ZBP1 in cardiomyocytes in response to mtDNA.
ZBP1 Knockdown Attenuates Increases in Inflammatory Cytokines in Macrophages Treated With mtDNA
The role of ZBP1 in macrophages have been extensively investigated as a positive mediator of inflammation and macrophage activation. We confirmed that mtDNA administration increased ZBP1 mRNA and proteins levels in RAW264.7 macrophages (Figure S3A and S3B). It also increased IL-1β and IL-6 in macrophages, whereas ZBP1 knockdown attenuated them (Figure S3C), demonstrating that ZBP1 has a proinflammatory role in response to mtDNA in macrophages, which is opposite to its role in cardiomyocytes as shown above.
RIPK3 Knockdown Cancels Increases in NF-κB-NLRP3 Axis by ZBP1 Knockdown in Cardiomyocytes Treated With mtDNA
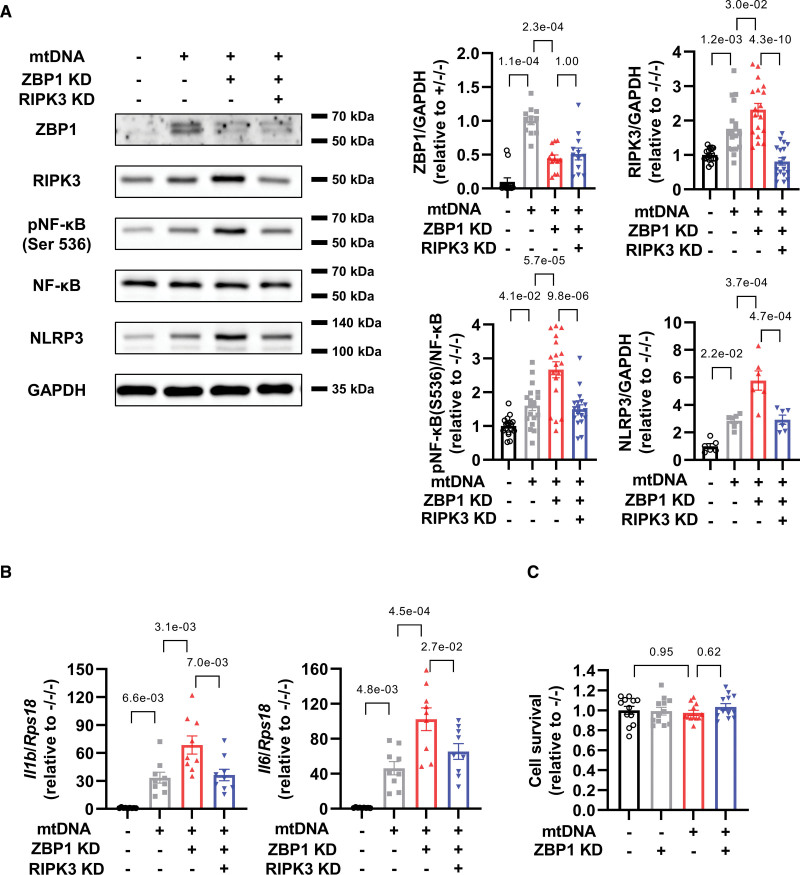
To investigate the relationship between ZBP1 and RIPK3 and to elucidate whether RIPK3 could mediate inflammation as a downstream target of ZBP1, we conducted an experiment of double knockdown of ZBP1 and RIPK3. The double knockdown efficiently decreased these proteins (Figure 3A). Importantly, RIPK3 knockdown attenuated exacerbation of increases in phosphorylated NF-κB and NLRP3 by ZBP1 knockdown in mtDNA-treated cardiomyocytes (Figure 3A). RIPK3 knockdown also decreased mRNA levels of IL-1β and IL-6 (Figure 3B). These findings suggest that RIPK3 positively regulates NF-κB and NLRP3 and that ZBP1 exerts anti-inflammatory effects by suppressing RIPK3. Conversely, both mtDNA and ZBP1 knockdown did not affect cell survival (Figure 3C), indicating that ZBP1-RIPK3 axis could not directly regulate cell death in cardiomyocytes.
Figure 3.
RIPK3 (receptor interacting protein kinase) knockdown cancels increases in NF-κB (nuclear factor-κB)-NLRP3 (nucleotide-binding domain and leucine-rich-repeat family pyrin domain containing 3) axis by ZBP1 (Z-DNA binding protein 1) knockdown in cardiomyocytes treated with mitochondrial DNA (mtDNA). A, Representative immunoblots of ZBP1 (n=12; number of experiments, 4), RIPK3 (n=18; number of experiments, 6), phosphorylated NF-κB (Ser 536), NF-κB (n=18; number of experiments, 6), NLRP3 (n=6; number of experiments, 2), and GAPDH in neonatal rat ventricular myocytes (NRVMs) treated with or without small interfering RNA (siRNA) for ZBP1 (10 nmol/L) and RIPK3 (10 nmol/L) in the presence or absence of mtDNA (1000 ng/mL) for 24 hours. B, mRNA levels of Il1b and Il6 in NRVMs treated with or without siRNA for ZBP1 (10 nmol/L) and RIPK3 (10 nmol/L) in the presence or absence of mtDNA (1000 ng/mL) for 24 hours (n=9). The experiment was conducted 3×. C, Cell viability was assessed using Cell Titer Blue assays in NRVMs treated with or without small interfering RNA for ZBP1 (10 nmol/L) in the presence or absence of mtDNA (1000 ng/mL) for 24 hours (n=12). The experiment was conducted 2×. Error bars denote standard errors. Data were analyzed using the Wilcoxon rank sum test (A ZBP1; adjust, 3), and 2-way ANOVA followed by Tukey multiple comparisons test (A except for ZBP1, B, and C).
NF-κB Knockdown Suppresses Increases in NLRP3 Axis by ZBP1 Knockdown in Cardiomyocytes Treated With mtDNA
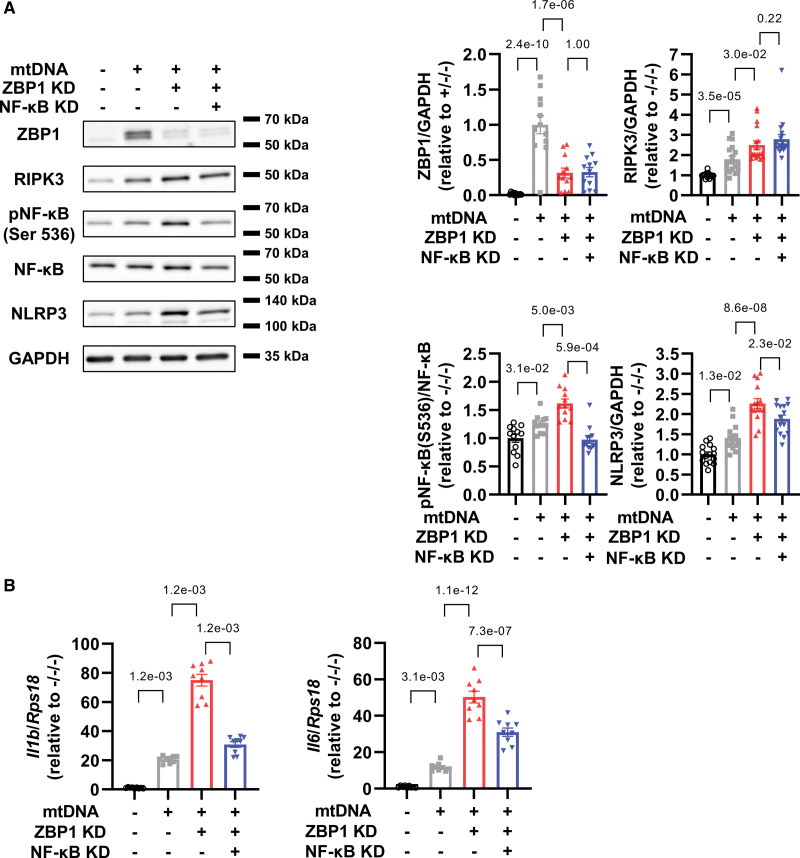
Next, we investigated whether NF-κB could mediate inflammation as a downstream target of ZBP1. We found that NF-κB knockdown attenuated exacerbation of increases in NLRP3, but not RIPK3, by ZBP1 knockdown in mtDNA-treated cardiomyocytes (Figure 4A). Importantly, these changes were accompanied by decreases in mRNA levels of IL-1β and IL-6 (Figure 4B). Taken together, these data indicate that ZBP1 exerts anti-inflammatory effects by suppressing NF-κB pathway.
Figure 4.
NF-κB (nuclear factor-κB) knockdown suppresses increases in NLRP3 (nucleotide-binding domain and leucine-rich-repeat family pyrin domain containing 3) axis by ZBP1 (Z-DNA binding protein 1) knockdown in cardiomyocytes treated with mitochondrial DNA (mtDNA). A, Representative immunoblots of ZBP1 (n=12; number of experiments, 4), RIPK3 (receptor interacting protein kinase; n=18; number of experiments, 6), phosphorylated NF-κB (Ser 536), NF-κB (n=12; number of experiments, 4), NLRP3 (n=15; number of experiments, 5), and GAPDH in neonatal rat ventricular myocytes (NRVMs) treated with or without small interfering RNA (siRNA) for ZBP1 (10 nmol/L) and NF-κB p65 subunit (10 nmol/L) in the presence or absence of mtDNA (1000 ng/mL) for 24 hours. B, mRNA levels of Il1b and Il6 in NRVMs treated with or without siRNA for ZBP1 (10 nmol/L) and NF-κB p65 subunit (10 nmol/L) in the presence or absence of mtDNA (1000 ng/mL) for 24 hours (n=9). The experiment was conducted 3×. Error bars denote standard errors. Data were analyzed using the Wilcoxon rank sum test (A, RIPK3 and NF-κB; B, Il1b; adjust=3), and 2-way ANOVA followed by Tukey multiple comparisons test (A, ZBP1 and NLRP3; B, Il6).
ZBP1 Negatively Regulates Inflammatory Responses in CpG-Oligodeoxynucleotides-Treated Cardiomyocytes
It has been known that ZBP1 could sense both mitochondrial and nuclear DNA.20,21 To identify the kind of DNA that stimulates ZBP1 in cardiomyocytes, we used CpG-oligodeoxynucleotides, which mimicked mtDNA, and control-oligodeoxynucleotides, which mimicked nuclear DNA. We found that fluorescein isothiocyanate (FITC)-labeled CpG-oligodeoxynucleotides colocalized with mcherry-tagged ZBP1 (Figure 5A). CpG-oligodeoxynucleotides increased ZBP1 mRNA and protein levels (Figure 5B and 5C) and ZBP1 knockdown further increased mRNA levels of IL-1β and IL-6 in cardiomyocytes treated with CpG-oligodeoxynucleotides (Figure 5D). Similarly, control-oligodeoxynucleotides increased ZBP1 mRNA and protein levels (Figure S4A and S4B) and ZBP1 knockdown further increased IL-1β and IL-6 in cardiomyocytes treated with control-oligodeoxynucleotides (Figure S4C). These findings suggest that ZBP1 could sense both mitochondrial and nuclear DNA in cardiomyocytes and negatively regulate inflammation in response to them.
Figure 5.
Interaction of ZBP1 (Z-DNA binding protein 1) and CpG-ODN (oligodeoxynucleotides) is involved in the anti-inflammatory effect of ZBP1 in CpG-ODN-treated cardiomyocytes. A, Representative images of neonatal rat ventricular myocytes (NRVMs) treated with adenovirus harboring mcherry-tagged ZBP1 in the presence of fluorescein isothiocyanate (FITC)-labeled CpG-oligodeoxynucleotide. Arrows indicated colocalized CpG-ODN and ZBP1. B, mRNA levels of Zbp1 in NRVMs treated with or without small interring (siRNA) for ZBP1 (10 nmol/L) in the presence or absence of CpG-ODN (1000 ng/mL) for 24 hours (n=6). The experiment was conducted 2×. C, Representative immunoblots of ZBP1 and GAPDH in NRVMs treated with or without siRNA for ZBP1 (10 nmol/L) in the presence or absence of CpG-ODN (1000 ng/mL) for 24 hours (n=9). D, mRNA levels of Il1b and Il6 in NRVMs treated with or without siRNA for ZBP1 (10 nmol/L) in the presence or absence of CpG-ODN (1000 ng/mL) for 24 hours (n=6). The experiment was conducted 2×. E, Representative immunoblots of RIPK3 (receptor interacting protein kinase) and GAPDH in NRVMs treated with CpG-ODN (1000 ng/mL) for 24 hours (n=6). The experiment was conducted 2×. F and G, mRNA levels of Tlr9 and Zbp1 in NRVMs treated with or without siRNA for TLR9 (Toll-like receptor 9; 10 nmol/L) in the presence or absence of mitochondrial DNA (mtDNA; 1000 ng/mL) for 24 hours (n=9). The experiment was conducted 3×. H, Representative immunoblots of ZBP1 and GAPDH in NRVMs treated with or without siRNA for TLR9 (10 nmol/L) in the presence or absence of mtDNA (1000 ng/mL) for 24 hours (n=6). The experiment was conducted 2×. I, Representative immunoblots of ZBP1 and GAPDH in NRVMs treated with or without siRNA for TLR9 (10 nmol/L) in the presence or absence of CpG-ODN (1000 ng/mL) for 24 hours (n=6). The experiment was conducted 2×. Error bars denote standard errors. Data were analyzed using the Student t test (E) and 2-way ANOVA followed by Tukey multiple comparisons test (B, C, D, F, G, H, and I).
TLR9 Knockdown Ameliorates Inflammatory Responses in mtDNA or CpG-Oligodeoxynucleotides-Treated Cardiomyocytes by Suppressing RIPK3, NF-κB, and NLRP3
Next, we sought to investigate the relationship between TLR9, ZBP1, and RIPK3 in mtDNA-treated cardiomyocytes. CpG-oligodeoxynucleotides, also known to be a TLR9 stimulator, was found to increase RIPK3 expression in cardiomyocytes (Figure 5E), indicating that RIPK3 is a downstream of TLR9. We confirmed that TLR9 knockdown efficiently decreased its mRNA levels (Figure 5F). Moreover, we found that TLR9 knockdown enhanced mtDNA-induced increases in ZBP1 mRNA and protein levels in cardiomyocytes (Figure 5G and 5H) and exacerbated CpG-oligodeoxynucleotides-induced increases in ZBP1 (Figure 5I) but not control-oligodeoxynucleotides (Figure S4D). TLR9 could be involved in ZBP1 regulation by stimulation of mtDNA but not nuclear DNA. Furthermore, TLR9 knockdown attenuated RIPK3, phosphorylated NF-κB, and NLRP3 (Figure S5A), as well as mRNA levels of IL1β and IL6 (Figure S5B). To investigate how ZBP1 protects against mtDNA-induced TLR9-inflammatory pathway, we assessed colocalization of TLR9 and FITC-labeled CpG-oligodeoxynucleotides. We used FITC not labeled CpG-oligodeoxynucleotides and rabbit isotype control as negative controls for FITC-labeled CpG-oligodeoxynucleotides and TLR9, respectively. Neither of them was detected (Figure S6A), which indicated that signals obtained from FITC-labeled CpG-oligodeoxynucleotides or anti-TLR9 antibody were genuine target staining. After transfection of CpG-labeled oligodeoxynucleotides, the colocalization was hardly observed, but it was increased by ZBP1 knockdown (Figure S6B). These data indicate that ZBP1 hampers the interaction between mtDNA and TLR9, thereby leading to prevention of downstream inflammatory signaling.
RIPK1 Knockdown Further Increases ZBP1 Expression and Exacerbates Downstream Inflammatory Signaling
RIPK1 have been shown to interact with both ZBP1 and RIPK3, however, the effects of RIPK1 on ZBP1 and RIPK3 were controversial. In our study, the expression of phosphorylated RIPK1 increased in response to mtDNA stimulation (Figure S7A). The RIPK1 knockdown increased ZBP1 and exacerbated downstream signaling, including RIPK3, phosphorylated NF-κB, and NLRP3 (Figure S7A), as well as IL-1β and IL-6 mRNA levels (Figure S7B). These findings indicate that RIPK1 suppresses mtDNA-induced inflammatory response independent from the effects of ZBP1.
STING Knockdown Suppresses ZBP1 Expression and Attenuates Inflammatory Signaling
STING is known to induce inflammatory response to cytosolic mtDNA. Indeed, mtDNA stimulation in cardiomyocytes increased expression of STING (Figure S8A), and its knockdown attenuated mRNA levels of IL-1β and IL-6 (Figure S8B). However, STING knockdown suppressed the expression of ZBP1 as well as RIPK3, phosphorylated NF-κB, and NLRP3 (Figure S8C). These findings indicate that STING knockdown mitigates mtDNA-induced inflammatory response independent from the effects of ZBP1.
ZBP1 and Cytosolic mtDNA Are Increased in Failing Hearts After MI
To evaluate the role of ZBP1 in failing hearts, we conducted in vivo experiments of MI in mice (Figure S9A). We validated infarction in MI model (Figure S9B). We found that ZBP1 protein levels increased in the noninfarcted area of post-MI hearts 3 days after MI operation (Figure 6A). We also investigated ZBP1 expression levels in another HF model. We created pressure overload model and validated pressure gradient and cardiac hypertrophy in this model (Figure S9C and S9D). ZBP1 was also increased in failing hearts induced by pressure overload (Figure S10A). These data indicate that ZBP1 is universally increased in failing hearts. Next, we analyzed DNA levels in cytosolic fraction in MI models. We validated the purity of cytosolic fraction by immunoblotting for GAPDH, a cytosolic fraction maker, COX4, a mitochondrial marker, and LC3, an autophagy marker (Figure 6B). Dloop, a component of mtDNA, in cytosolic fraction was abundantly detected in control hearts and increased in post-MI hearts (Figure 6B). On the contrary, Tert and B2m, components of nuclear DNA, were slightly detected in both control and post-MI hearts (Figure 6B). Same changes were observed in failing hearts in response to pressure overload by transverse aortic constriction operation (Figure S10B). Overall, these data suggest that ZBP1 is associated with an increase in cytosolic mtDNA in failing hearts.
Figure 6.
ZBP1 (Z-DNA binding protein 1) knockout (KO) enhances myocardial inflammation in failing hearts via RIPK3 (receptor interacting protein kinase)-NF-κB (nuclear factor-κB)-NLRP3 (nucleotide-binding domain and leucine-rich-repeat family pyrin domain containing 3) pathway. A, Representative immunoblots of ZBP1 and GAPDH in C57B/6J mouse hearts 3 days after left anterior descending artery (LAD) ligation or sham operation (n=6). The experiment was conducted 2×. *P<0.05: Student t test. B, Representative immunoblots of COX4 (cytochrome c oxidase subunit 4), LC3 (light chain 3), and GAPDH in cytosolic fraction of C57B/6J mouse hearts 3 days after LAD ligation or sham operation. DNA levels in this cytosolic fraction was quantified by real-time polymerase chain reaction (n=12 in sham group, and n=17 in LAD ligation group). The experiment was conducted 4×. C, Representative immunoblots of ZBP1 (n=9; number of experiments, 3), RIPK3 (n=9; number of experiments, 3), phosphorylated NF-κB (Ser 536), NF-κB (n=21; number of experiments, 7), NLRP3 (n=12; number of experiments, 4), and GAPDH in wild type (WT) and ZBP1 KO mice hearts 3 days after LAD ligation or sham operation. D, mRNA levels of Il1b (n=13) and Il6 (n=16) in WT and ZBP1 KO mice hearts 3 days after LAD ligation or sham operation. The experiment was conducted 2 (Il1b) or 3 (Il6) ×. E, Secretion levels of IL (interleukin)-1β (n=12) and IL-6 (n=6) measured with ELISA in WT and ZBP1 KO mice hearts 3 days after LAD ligation or sham operation. The experiment was conducted 2 (Il-6) or 3 (Il-1β) ×. F, Representative immunoblots of TBK1 (TANK-binding kinase 1), phosphorylated TBK1, and GAPDH in WT and ZBP1 knockout mice hearts 3 days after left anterior descending artery ligation or sham operation (n=6). The experiment was conducted 2×. Error bars denote standard errors. Data were analyzed using the Student t test (B, Dloop), Wilcoxon rank sum test (A and B, Tert and B2m; no adjustment; C, except for RIPK3; D adjust, 3), and 2-way ANOVA followed by Tukey multiple comparisons test (C, RIPK3; E; and F). MI indicates myocardial infarction.
ZBP1 Knockout Enhances Myocardial Inflammation in Failing Hearts Via RIPK3-NF-κB-NLRP3 Pathway
Next, we evaluated downstream signaling of ZBP1 in failing hearts. RIPK3, phosphorylated NF-κB, and NLRP3 were increased in post-MI hearts (Figure 6C). To investigate whether these changes are seen only in MI model, we assessed pressure overload model. These proteins were also found to increase in failing hearts in response to pressure overload (Figure S10A).
We investigated the role of ZBP1 in myocardial inflammation in noninfarcted area of post-MI hearts. ZBP1 was not detected in hearts from ZBP1 knockout mice (Figure 6C). The infarct size did not differ between wild type group and ZBP1 knockout group (Figure S9B), indicating that the severity of MI is the same between wild type and knockout mice. ZBP1 knockout mice demonstrated further increases in RIPK3, phosphorylated NF-κB, and NLRP3 (Figure 6C), which in turn exacerbated increases in mRNA and protein levels of IL-1β and IL-6 in post-MI hearts (Figure 6D and 6E). To assess whether this phenomenon took place within cardiomyocytes in noninfarcted area of post-MI hearts, we evaluated 2 key players in this signaling pathway, ZBP1 and RIPK3, with immunohistochemistry. When we used mouse or rabbit isotype control as a negative control for ZBP1 and RIPK3, respectively, no signals could be detected (Figure S11A). ZBP1 increased in cardiomyocytes of post-MI hearts and was not detected in ZBP1 knockout mice (Figure S11B). RIPK3 also increased in cardiomyocytes of post-MI hearts, and further increased in ZBP1 knockout mice (Figure S11C). It resulted in exacerbated infiltration of macrophages into post-MI hearts, and these signals could be distinguished from background (Figure S12A and S12B).
Interestingly, no statistically significant difference was observed in TBK1 protein levels and its phosphorylation in post-MI hearts and ZBP1 knockout did not significantly affect them (Figure 6F). These data indicate that ZBP1 negatively regulates myocardial inflammation via the RIPK3-NF-κB pathway, but not the TBK1 pathway. A previous study showed that mtDNA could be brought in by receptors. It is important to determine whether extracellular or intracellular mtDNA is a potential source of inflammation in cardiomyocytes. We transfected mtDNA without lipofectamine to cardiomyocytes to investigate whether extracellular mtDNA could be brought in and induce inflammation. As a result, no statistically significant difference was observed in ZBP1 pathway (Figure S13), indicating that extracellular mtDNA is less likely to be the source of cytosolic mtDNA in cardiomyocytes.
ZBP1 Knockout Exacerbates Cardiac Dysfunction and Remodeling After MI
ZBP1 knockout mice exhibited increased left ventricular (LV) diastolic and systolic diameters and decreased LV ejection fraction and fractional shortening in failing hearts after MI (Figure 7A). ZBP1 knockout also exacerbated increases in whole heart weight and LV weight (Figure 7B). In pathological analysis, ZBP1 knockout enhanced collagen volume, an index of cardiac fibrosis, but not cross-sectional area, an index of cardiomyocyte hypertrophy, in failing hearts (Figure 7C). Additionally, ZBP1 knockout increased apoptosis, evaluated by TUNEL staining, in failing hearts (Figure S14A). Consistent with this finding, ZBP1 knockout increased BAX (Bcl-2-associated X protein) to Bcl2 (B-cell chronic lymphocytic leukemia [CLL]/lymphoma 2) ratio (index of apoptosis) in failing hearts (Figure S15B). These data suggest that ZBP1 knockout exacerbates cardiac dysfunction and remodeling after MI.
Figure 7.
ZBP1 (Z-DNA binding protein 1) knockout (KO) exacerbates cardiac dysfunction and remodeling after myocardial infarction (MI). A, The representative echocardiographic images of ZBP1 KO and wild type (WT) mice hearts 28 days after left anterior descending artery (LAD) ligation (n=38) or sham operation (n=5). Long 2-way arrows and short 2-way arrows indicate left ventricular end-diastolic diameter (LVDd) and left ventricular end-systolic diameter (LVDs), respectively. B, Heart weight to tibial length (TL) ratio and left ventricle (LV) weight to TL ratio (n of sham, 8; n of LAD ligation, 41) in each group. C, Masson-trichrome-stained heart section in each group. Interstitial fibrosis was assessed by collagen volume in each group (n=5). Cardiomyocyte hypertrophy was assessed by cross-sectional area in each group (n=5). Error bars denote standard errors. Data were analyzed using the Wilcoxon rank sum test (A, LVDd; B and C, collagen volume; adjust, 3), and 2-way ANOVA followed by Tukey multiple comparisons test (A, except for LVDd and C, cross sectional area).
To elucidate whether ZBP1 could be involved in the formation of the infarcted region, we performed the echocardiographic analysis and measured organ weight at 3 days after MI. There were no significant changes in LV diameter, LV ejection fraction, and heart weight between wild type and ZBP1 knockout mice (Figure S15A and S15B).
Discussion
We here demonstrated that ZBP1 negatively regulated mtDNA-induced inflammation in cardiomyocytes by suppressing the RIPK3-NF-κB pathway. In addition, ZBP1 interfered with TLR9-related inflammation in cardiomyocytes. Furthermore, ZBP1 and cytosolic mtDNA were increased in failing hearts and ZBP1 protected against myocardial inflammation and cardiac remodeling. This study provides the first evidence for anti-inflammatory and protective roles of ZBP1 in cardiomyocytes and hearts (Figure 8).
Figure 8.
A schematic representation of the role of ZBP1 (Z-DNA binding protein 1) in myocardial inflammation and remodeling. Excessive amount of mitochondrial DNA (mtDNA) activates TLR9 (Toll-like receptor 9) and induces inflammation through RIPK3 (receptor interacting protein kinase)-NF-κB (nuclear factor-κB)-NLRP3 (nucleotide-binding domain and leucine-rich-repeat family pyrin domain containing 3) pathway. ZBP1 protects against adverse cardiac remodeling through suppressing this pathway.
Myocardial inflammation is intimately involved in cardiac remodeling and the pathophysiology of HF.2–7 Higher levels of inflammatory cytokines such as TNF-α (tumor necrosis factor α) and IL-6 are correlated with poor prognosis in patients with HF.2 Several animal experiments have demonstrated that inflammation mediates cardiac dysfunction and development of HF.3,34 However, in previous randomized trials, TNF-α inhibitors failed to improve clinical condition and prognosis of patients with HF.35,36 Conversely, recent studies demonstrated beneficial effects of IL-1β inhibitor or colchicine as anti-inflammatory therapy against ischemic heart disease.37,38 Therefore, it is important to further elucidate the regulatory mechanisms of myocardial inflammation and detect a novel therapeutic target. Recently, sterile inflammation induced by damage-associated molecular patterns has been shown to play crucial roles in cardiovascular diseases.8 Among several damage-associated molecular patterns, mtDNA critically mediates myocardial inflammation in HF. In particular, mtDNA unprocessed by autophagy activates TLR9, leading to myocardial inflammation and cardiac dysfunction.10 Thus, mtDNA-sensing and processing mechanism is a potential therapeutic target of HF. ZBP1 is a cytosolic pattern recognition receptor which senses double helical structures of the DNA20,21 and mtDNA triggers inflammation via the activation of ZBP1 in several types of cells.22–24 The present study demonstrated that mtDNA increased ZBP1 and inflammatory cytokines in cardiomyocytes (Figure 1B through 1D). Additionally, mtDNA, but not nuclear DNA, was increased in cytosolic fraction from failing hearts, accompanied by increases in ZBP1 and inflammatory cytokines (Figure 6A, 6B, 6D, and 6E). These data suggest that cytosolic mtDNA is intimately related to increases in ZBP1 and myocardial inflammation in cardiomyocytes.
To replicate the increase in cytosolic mtDNA in vivo model, we transfected 0 to 1000 ng/mL of mtDNA to cardiomyocytes. We found that 1000 ng/mL of mtDNA increased cytosolic mtDNA to the same extent as MI models (Figure S1D and Figure 6B). Then we conducted functional analysis of ZBP1 in the same condition. Based on previous studies, the proinflammatory roles of ZBP1 had been predicted even in cardiomyocytes and hearts. However, in the present study, unexpectedly, knockdown of ZBP1 exacerbated mtDNA-induced increases in inflammatory cytokines such as IL-1β and IL-6 (Figure 2C). Conversely, overexpression of ZBP1 protected against the same pathway (Figure S2A through S2C). Furthermore, knockout of ZBP1 exacerbated myocardial inflammation and cardiac dysfunction (Figure 6D, 6E, 7A, and 7B). Therefore, ZBP1 is multifunctional and its role is thought to depend on types of cells and stimuli. In fact, ZBP1 is known to promote an antiviral effect in neurons without inducing inflammation.39
Several downstream pathways of ZBP1 have been reported. Jiao et al23 demonstrated that, although ZBP1 induced apoptosis by interacting with RIPK1 and RIPK3, it induced inflammation and necroptosis by positively regulating RIPK3 in the absence of RIPK1. Szczesny et al24 reported that mtDNA bound to ZBP1 and triggered inflammation via the TBK1/interferon regulatory factor 3 signaling pathway. However, the present study demonstrated that ZBP1 negatively regulated RIPK3 protein levels, but did not affect TBK1 protein levels and its phosphorylation, in cardiomyocytes (Figure 2D). RIPK3 is identified as a necroptosis adaptor protein which induces inflammation by cell destruction.40,41 On the contrary, recently, RIPK3 has been reported to promote injury-induced cytokine expression independently of necroptosis.42 Furthermore, RIPK3 drives NF-κB and inflammasome activation independently of cell death.43 Consistent with these previous findings, the present study exhibited that NF-κB was a downstream of RIPK3 (Figure 3A) and positively regulated NLRP3 (Figure 4A), an inflammasome mediator, and inflammatory cytokines (Figure 4B) in cardiomyocytes. RIPK3, phosphorylation of NF-κB, and NLRP3 were also increased in ZBP1 knockout mouse hearts (Figure 6C). These findings provide strong evidence that ZBP1 induces inflammation through the RIPK3-NF-κB-inflammasome pathway in cardiomyocytes and hearts. Importantly, ZBP1 knockdown did not directly affect cardiomyocyte deaths in an in vitro experiment (Figure 3C). Therefore, increases in myocardial cell death in ZBP1 knockout mice were thought to be due to secondary effects of myocardial inflammation (Figure S14). RIPK1 is known to interact with both ZBP1 and RIPK3.44,45 Phosphorylated RIPK1 increased in response to mtDNA stimulation (Figure S7A). RIPK1 knockdown resulted in exacerbation of downstream inflammatory responses (Figure S7A and S7B). These results are supported by a previous study demonstrating the inhibitory effect of RIPK1 on RIPK3 and inflammation.44 Although RIPK1 knockdown exacerbated inflammatory responses, it further increased ZBP1 expression as well (Figure S7A). It indicated that RIPK1 knockdown exacerbated mtDNA-induced inflammatory responses independent from ZBP1, and it required anti-inflammatory effects of ZBP1. STING is one of pattern recognition receptors inducing inflammation as an upstream of RIPK3.46 mtDNA administration increased STING protein levels (Figure S8A). STING knockdown decreased ZBP1 and RIPK3 (Figure S8C). The suppressive effect of STING knockdown on inflammation might negate the requirement to increase ZBP1 in response to mtDNA. STING, as well as RIPK1, is thought to make complicated networks with ZBP1-RIPK3 pathway. Further investigations regarding this issue are needed.
Since we used systemic ZBP1 knockout mice, not cardiac-specific knockout mice, it does not rule out the possibilities that ZBP1 and RIPK3 in other cell types in the heart contribute to the worsening cardiac function and fibrosis in the setting of MI. ZBP1 expresses in not only cardiomyocytes but also inflammatory cells, including macrophages.47 In the present study, we confirmed that ZBP1 positively regulated mtDNA-induced increases in IL-1β and IL-6 in macrophages (Figure S3C) as shown in previous reports.47,48 On the contrary, ZBP1 negatively regulated inflammation in cardiomyocytes (Figure 2C). In terms of inflammation, the role of ZBP1 in macrophages is the exact opposite of its role in cardiomyocytes. Immunostaining showed that ZBP1 and RIPK3 mainly increased within cardiomyocytes and that ZBP1 knockout further increased RIPK3 within cardiomyocytes as well (Figure S11B and S11C). Given that ZBP1 knockout mice demonstrated exacerbation of increases in IL-1β and IL-6 in the heart after MI (Figure 6D and 6E), the effect of ZBP1 knockout in inflammation in the heart was thought to be primarily due to deletion of ZBP1 in cardiomyocytes, but not macrophages. Furthermore, our results indicated the divergent role of ZBP1 in the heart and myeloid cells.
ZBP1, cytosolic mtDNA, and inflammatory signaling such as RIPK3, phosphorylation of NF-κB, and NLRP3 were increased in several HF models, including post-MI (Figure 6C) and pressure overload due to transverse aortic constriction operation (Figure S10A). Thus, mtDNA-induced RIPK3-NF-κB-NLRP3 pathway is thought to be universally activated in failing hearts and mediates development of cardiac remodeling and failure. Furthermore, ZBP1 in cardiomyocytes could be an endogenous suppressor of this pathway.
Intriguingly, inhibition of TLR9 enhanced mtDNA-induced increases in ZBP1 mRNA and protein levels (Figure 5G and 5H). Although ZBP1 responds to both nuclear DNA and mtDNA, TLR9 is involved in ZBP1 regulation only in the context of mtDNA-induced myocardial inflammation. TLR9 is a major sensor of unprocessed mtDNA in autophagosome or lysosome and TLR9-related inflammation is involved in the pathophysiology of HF.10 In addition, RIPK3, NF-κB, and NLRP3 are known to be the downstream signaling of TLR949,50 and this pathway was confirmed in our experiments (Figure S5). Knockdown of ZBP1 exacerbated inflammation induced by CpG-oligodeoxynucleotides, a TLR9 stimulator (Figure 5D). Thus, our results raise an exciting possibility that ZBP1 suppresses inflammation induced by TLR9 stimulation in response to mtDNA. Of note, both ZBP1 and TLR9 can interact with mtDNA (Figure 5A and Figure S6B). The interaction between TLR9 and mtDNA significantly increased when ZBP1 was suppressed (Figure S6B). This result suggests that ZBP1 sequesters cytosolic mtDNA resulting in inhibition of TLR9-mtDNA interaction and its inflammatory responses.
Myocardial apoptosis was increased in ZBP1 knockout mice 3 days after MI (Figure S14). However, cardiac morphology and function were not statistically significantly different compared with WT mice (Figure S15). We speculate that, at this time point, an early stage of cell deaths does not yet impact cardiac morphology and dysfunction and that along with increased cell deaths, cardiac dysfunction becomes obvious later on (Figure 7A and 7B).
The source of cytosolic mtDNA in failing hearts remains unclear in the present study. No detectable changes in ZBP1 pathway were observed when we transfect 1000 ng/mL of mtDNA without lipofectamine (Figure S13), indicating that extracellular mtDNA is not the main source of cytosolic mtDNA in cardiomyocytes. Recently, mitochondria have been reported to release mtDNA into cytoplasm during apoptosis. Thus, in failing hearts, mitochondria might directly release mtDNA into cytoplasm, leading to myocardial inflammation. To elucidate precise mechanisms of interaction among cytosolic mtDNA, ZBP1, and TLR9 in failing hearts, further investigations are needed.
Myocardial inflammation facilitates cell deaths in infarcted areas after MI.51–54 Thus, ZBP1 might be involved in the formation of the infarcted region. However, the echocardiographic analysis demonstrated no significant changes in LV diameter, LV ejection fraction, and heart weight at 3 days after MI (Figure S15A and S15B), as well as infarct area at 28 days (Figure S9B) between wild type and ZBP1 knockout mice, suggesting that ZBP1 did not critically affect infarct size and cardiac function in its acute phase. Therefore, ZBP1 plays a pivotal role in myocardial inflammation in noninfarcted areas after acute phase.
In conclusions, ZBP1 plays a protective role in cardiac remodeling as an endogenous suppressor of myocardial inflammation via the RIPK3-NF-κB pathway. Therapeutic strategies designed to interfere with myocardial inflammation by ZBP1 might be beneficial in preventing cardiac remodeling and failure.
Article Information
Acknowledgments
We are very grateful to A. Hanada and M. Sato for technical support and to K. Funakoshi for statistical advice. We also appreciate the technical assistance from the Research Support Center, Faculty of Medical Sciences, Kyushu University. We appreciate the kind gift of ZBP1 (Z-DNA binding protein 1) knockout mice from professor Shizuo Akira in Osaka University and National Institutes of Biomedical Innovation, Health and Nutrition (NIBIOHN).
Author Contributions
N. Enzan and S. Matsushima contributed to conceptualization and data curation. R. Miyake and S. Kinugawa contributed to formal analysis. N. Enzan, S. Matsushima, K. Okabe, A. Ishikita, M. Sada, Y. Tsutsui, R. Nishimura, H.D. Miyamoto, K. Abe, M. Ikeda, and T. Ide participated in methodology. N. Enzan, S. Ikeda, T. Yamamoto, T. Toyohara, Y. Ikeda, Y. Shojima, and T. Tadokoro contributed to investigation. S. Ikeda and H.D. Miyamoto participated in visualization. S. Matsushima and H. Tsutsui contributed to funding acquisition, project administration, and supervision. S. Matsushima and S. Kinugawa participated in writing—original draft. N. Enzan, S. Matsushima, and H. Tsutsui participated in writing—review and editing.
Sources of Funding
This study was funded by Japan Society for the Promotion of Science (JSPS) KAKENHI (grant nos. 17K09581 and 21K08082 to S. Matsushima) and JSPS KAKENHI (grant nos. 19K22622 and 19H03655 to H. Tsutsui).
Disclosures
H. Tsutsui reports personal fees from Merck Sharp and Dohme (MSD), Astellas, Pfizer, Bristol-Myers Squibb, Otsuka Pharmaceutical, Daiichi-Sankyo, Mitsubishi Tanabe Pharma, Nippon Boehringer Ingelheim, Takeda Pharmaceutical, Bayer Yakuhin, Novartis Pharma, Kowa Pharmaceutical, Teijin Pharma, Medical Review Co, and Japanese Journal of Clinical Medicine; nonfinancial support from Actelion Pharmaceuticals, Japan Tobacco Inc, Mitsubishi Tanabe Pharma, Nippon Boehringer Ingelheim, Daiichi-Sankyo, IQVIA Services Japan, and Omron Healthcare Co; grants from Astellas, Novartis Pharma, Daiichi-Sankyo, Takeda Pharmaceutical, Mitsubishi Tanabe Pharma, and Teijin Pharma, MSD, outside the submitted work. The other authors report no conflicts.
Supplemental Material
Figures S1–15
Detailed Methods
Supplementary Material
Nonstandard Abbreviations and Acronyms
- FITC
- fluorescein isothiocyanate
- HF
- heart failure
- IL
- interleukin
- LC3
- light chain 3
- LV
- left ventricular
- MI
- myocardial infarction
- mtDNA
- mitochondrial DNA
- NF-κB
- nuclear factor-κB
- NLRP3
- nucleotide-binding domain and leucine-rich-repeat family pyrin domain containing 3
- RIPK3
- receptor interacting protein kinase 3
- TBK1
- TANK-binding kinase 1
- TLR9
- Toll-like receptor 9
- ZBP1
- Z-DNA binding protein 1
For Sources of Funding and Disclosures, see page 1125.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCRESAHA.122.322227.
References
- 1.Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, Jaarsma T, Krum H, Rastogi V, Rohde LE, et al. Heart failure: preventing disease and death worldwide. ESC Heart Fail. 2014;1:4–25. doi: 10.1002/ehf2.12005 [DOI] [PubMed] [Google Scholar]
- 2.Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST). Circulation. 2001;103:2055–2059. doi: 10.1161/01.cir.103.16.2055 [DOI] [PubMed] [Google Scholar]
- 3.Suematsu N, Tsutsui H, Wen J, Kang D, Ikeuchi M, Ide T, Hayashidani S, Shiomi T, Kubota T, Hamasaki N, et al. Oxidative stress mediates tumor necrosis factor-alpha-induced mitochondrial DNA damage and dysfunction in cardiac myocytes. Circulation. 2003;107:1418–1423. doi: 10.1161/01.cir.0000055318.09997 [DOI] [PubMed] [Google Scholar]
- 4.Westermann D, Lindner D, Kasner M, Zietsch C, Savvatis K, Escher F, von Schlippenbach J, Skurk C, Steendijk P, Riad A, et al. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail. 2011;4:44–52. doi: 10.1161/CIRCHEARTFAILURE.109.931451 [DOI] [PubMed] [Google Scholar]
- 5.Sobirin MA, Kinugawa S, Takahashi M, Fukushima A, Homma T, Ono T, Hirabayashi K, Suga T, Azalia P, Takada S, et al. Activation of natural killer T cells ameliorates postinfarct cardiac remodeling and failure in mice. Circ Res. 2012;111:1037–1047. doi: 10.1161/CIRCRESAHA.112.270132 [DOI] [PubMed] [Google Scholar]
- 6.Dick SA, Epelman S. Chronic heart failure and inflammation: what do we really know?. Circ Res. 2016;119:159–176. doi: 10.1161/CIRCRESAHA.116.308030 [DOI] [PubMed] [Google Scholar]
- 7.Takahashi M, Kinugawa S, Takada S, Kakutani N, Furihata T, Sobirin MA, Fukushima A, Obata Y, Saito A, Ishimori N, et al. The disruption of invariant natural killer T cells exacerbates cardiac hypertrophy and failure caused by pressure overload in mice. Exp Physiol. 2020;105:489–501. doi: 10.1113/EP087652 [DOI] [PubMed] [Google Scholar]
- 8.Nakayama H, Otsu K. Mitochondrial DNA as an inflammatory mediator in cardiovascular diseases. Biochem J. 2018;475:839–852. doi: 10.1042/BCJ20170714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liberale L, Montecucco F, Tardif JC, Libby P, Camici GG. Inflamm-ageing: the role of inflammation in age-dependent cardiovascular disease. Eur Heart J. 2020;41:2974–2982. doi: 10.1093/eurheartj/ehz961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, Oyabu J, Murakawa T, Nakayama H, Nishida K, et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485:251–255. doi: 10.1038/nature10992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McArthur K, Whitehead LW, Heddleston JM, Li L, Padman BS, Oorschot V, Geoghegan ND, Chappaz S, Davidson S, San Chin H, et al. BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science. 2018;359:eaa06047. doi: 10.1126/science.aao6047 [DOI] [PubMed] [Google Scholar]
- 12.Fujiwara Y, Kikuchi H, Aizawa S, Furuta A, Hatanaka Y, Konya C, Uchida K, Wada K, Kabuta T. Direct uptake and degradation of DNA by lysosomes. Autophagy. 2013;9:1167–1171. doi: 10.4161/auto.24880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, Lien E, Nilsen NJ, Espevik T, Golenbock DT. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5:190–198. doi: 10.1038/ni1028 [DOI] [PubMed] [Google Scholar]
- 14.Sasai M, Linehan MM, Iwasaki A. Bifurcation of Toll-like receptor 9 signaling by adaptor protein 3. Science. 2010;329:1530–1534. doi: 10.1126/science.1187029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16:407–420. doi: 10.1038/nri.2016.58 [DOI] [PubMed] [Google Scholar]
- 16.Abbate A, Toldo S, Marchetti C, Kron J, Van Tassell BW, Dinarello CA. Interleukin-1 and the inflammasome as therapeutic targets in cardiovascular disease. Circ Res. 2020;126:1260–1280. doi: 10.1161/CIRCRESAHA.120.315937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Markousis-Mavrogenis G, Tromp J, Ouwerkerk W, Devalaraja M, Anker SD, Cleland JG, Dickstein K, Filippatos GS, van der Harst P, Lang CC, et al. The clinical significance of interleukin-6 in heart failure: results from the BIOSTAT-CHF study. Eur J Heart Fail. 2019;21:965–973. doi: 10.1002/ejhf.1482 [DOI] [PubMed] [Google Scholar]
- 18.Bowie AG, Unterholzner L. Viral evasion and subversion of pattern-recognition receptor signalling. Nat Rev Immunol. 2008;8:911–922. doi: 10.1038/nri2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kameyama T, Takaoka A. Characterization of innate immune signalings stimulated by ligands for pattern recognition receptors. Methods Mol Biol. 2014;1142:19–32. doi: 10.1007/978-1-4939-0404-4_3 [DOI] [PubMed] [Google Scholar]
- 20.Fu Y, Comella N, Tognazzi K, Brown LF, Dvorak HF, Kocher O. Cloning of DLM-1, a novel gene that is up-regulated in activated macrophages, using RNA differential display. Gene. 1999;240:157–163. doi: 10.1016/s0378-1119(99)00419-9 [DOI] [PubMed] [Google Scholar]
- 21.Schwartz T, Behlke J, Lowenhaupt K, Heinemann U, Rich A. Structure of the DLM-1-Z-DNA complex reveals a conserved family of Z-DNA-binding proteins. Nat Struct Biol. 2001;8:761–765. doi: 10.1038/nsb0901-761 [DOI] [PubMed] [Google Scholar]
- 22.Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T, Lu Y, Miyagishi M, Kodama T, Honda K, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013 [DOI] [PubMed] [Google Scholar]
- 23.Jiao H, Wachsmuth L, Kumari S, Schwarzer R, Lin J, Eren RO, Fisher A, Lane R, Young GR, Kassiotis G, et al. Z-nucleic-acid sensing triggers ZBP1-dependent necroptosis and inflammation. Nature. 2020;580:391–395. doi: 10.1038/s41586-020-2129-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szczesny B, Marcatti M, Ahmad A, Montalbano M, Brunyanszki A, Bibli SI, Papapetropoulos A, Szabo C. Mitochondrial DNA damage and subsequent activation of Z-DNA binding protein 1 links oxidative stress to inflammation in epithelial cells. Sci Rep. 2018;8:914. doi: 10.1038/s41598-018-19216-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rebsamen M, Heinz LX, Meylan E, Michallet MC, Schroder K, Hofmann K, Vazquez J, Benedict CA, Tschopp J. DAI/ZBP1 recruits RIP1 and RIP3 through RIP homotypic interaction motifs to activate NF-kappaB. EMBO Rep. 2009;10:916–922. doi: 10.1038/embor.2009.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helgason E, Phung QT, Dueber EC. Recent insights into the complexity of Tank-binding kinase 1 signaling networks: the emerging role of cellular localization in the activation and substrate specificity of TBK1. FEBS Lett. 2013;587:1230–1237. doi: 10.1016/j.febslet.2013.01.059 [DOI] [PubMed] [Google Scholar]
- 27.Morisco C, Seta K, Hardt SE, Lee Y, Vatner SF, Sadoshima J. Glycogen synthase kinase 3beta regulates GATA4 in cardiac myocytes. J Biol Chem. 2001;276:28586–28597. doi: 10.1074/jbc.M103166200 [DOI] [PubMed] [Google Scholar]
- 28.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishii KJ, Kawagoe T, Koyama S, Matsui K, Kumar H, Kawai T, Uematsu S, Takeuchi O, Takeshita F, Coban C, et al. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature. 2008;451:725–729. doi: 10.1038/nature06537 [DOI] [PubMed] [Google Scholar]
- 30.Rathod KS, Kapil V, Velmurugan S, Khambata RS, Siddique U, Khan S, Van Eijl S, Gee LC, Bansal J, Pitrola K, et al. Accelerated resolution of inflammation underlies sex differences in inflammatory responses in humans. J Clin Invest. 2017;127:169–182. doi: 10.1172/JCI89429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ikeda M, Ide T, Fujino T, Matsuo Y, Arai S, Saku K, Kakino T, Oga Y, Nishizaki A, Sunagawa K. The Akt-mTOR axis is a pivotal regulator of eccentric hypertrophy during volume overload. Sci Rep. 2015;5:15881. doi: 10.1038/srep15881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto S, Yang G, Zablocki D, Liu J, Hong C, Kim SJ, Soler S, Odashima M, Thaisz J, Yehia G, et al. Activation of Mst1 causes dilated cardiomyopathy by stimulating apoptosis without compensatory ventricular myocyte hypertrophy. J Clin Invest. 2003;111:1463–1474. doi: 10.1172/JCI17459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsushima S, Ide T, Yamato M, Matsusaka H, Hattori F, Ikeuchi M, Kubota T, Sunagawa K, Hasegawa Y, Kurihara T, et al. Overexpression of mitochondrial peroxiredoxin-3 prevents left ventricular remodeling and failure after myocardial infarction in mice. Circulation. 2006;113:1779–1786. doi: 10.1161/CIRCULATIONAHA.105.582239 [DOI] [PubMed] [Google Scholar]
- 34.Kubota T, Bounoutas GS, Miyagishima M, Kadokami T, Sanders VJ, Bruton C, Robbins PD, McTiernan CF, Feldman AM. Soluble tumor necrosis factor receptor abrogates myocardial inflammation but not hypertrophy in cytokine-induced cardiomyopathy. Circulation. 2000;101:2518–2525. doi: 10.1161/01.cir.101.21.2518 [DOI] [PubMed] [Google Scholar]
- 35.Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT; Anti-TNF Therapy Against Congestive Heart Failure Investigators, Willerson JT and Anti TNFTACHFI. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107:3133–3140. doi: 10.1161/01.CIR.0000077913.60364.D2 [DOI] [PubMed] [Google Scholar]
- 36.Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, Djian J, Drexler H, Feldman A, Kober L, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation. 2004;109:1594–1602. doi: 10.1161/01.CIR.0000124490.27666.B2 [DOI] [PubMed] [Google Scholar]
- 37.Everett BM, Cornel JH, Lainscak M, Anker SD, Abbate A, Thuren T, Libby P, Glynn RJ, Ridker PM. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation. 2019;139:1289–1299. doi: 10.1161/CIRCULATIONAHA.118.038010 [DOI] [PubMed] [Google Scholar]
- 38.Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381:2497–2505. doi: 10.1056/NEJMoa1912388 [DOI] [PubMed] [Google Scholar]
- 39.Daniels BP, Kofman SB, Smith JR, Norris GT, Snyder AG, Kolb JP, Gao X, Locasale JW, Martinez J, Gale M, et al. The nucleotide sensor ZBP1 and kinase RIPK3 induce the enzyme IRG1 to promote an antiviral metabolic state in neurons. Immunity. 2019;50:64–76 e4. doi: 10.1016/j.immuni.2018.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021 [DOI] [PubMed] [Google Scholar]
- 41.Moriwaki K, Chan FK. RIP3: a molecular switch for necrosis and inflammation. Genes Dev. 2013;27:1640–1649. doi: 10.1101/gad.223321.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moriwaki K, Balaji S, McQuade T, Malhotra N, Kang J, Chan FK. The necroptosis adaptor RIPK3 promotes injury-induced cytokine expression and tissue repair. Immunity. 2014;41:567–578. doi: 10.1016/j.immuni.2014.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moriwaki K, Chan FK. Necroptosis-independent signaling by the RIP kinases in inflammation. Cell Mol Life Sci. 2016;73:2325–2534. doi: 10.1007/s00018-016-2203-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rickard JA, O’Donnell JA, Evans JM, Lalaoui N, Poh AR, Rogers T, Vince JE, Lawlor KE, Ninnis RL, Anderton H, et al. RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell. 2014;157:1175–1188. doi: 10.1016/j.cell.2014.04.019 [DOI] [PubMed] [Google Scholar]
- 45.Wegner KW, Saleh D, Degterev A. Complex pathologic roles of RIPK1 and RIPK3: moving beyond necroptosis. Trends Pharmacol Sci. 2017;38:202–225. doi: 10.1016/j.tips.2016.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang X, Wu J, Liu Q, Li X, Li S, Chen J, Hong Z, Wu X, Zhao Y, Ren J. mtDNA-STING pathway promotes necroptosis-dependent enterocyte injury in intestinal ischemia reperfusion. Cell Death Dis. 2020;11:1050. doi: 10.1038/s41419-020-03239-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muendlein HI, Connolly WM, Magri Z, Smirnova I, Ilyukha V, Gautam A, Degterev A, Poltorak A. ZBP1 promotes LPS-induced cell death and IL-1beta release via RHIM-mediated interactions with RIPK1. Nat Commun. 2021;12:86. doi: 10.1038/s41467-020-20357-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Du XK, Ge WY, Jing R, Pan LH. Necroptosis in pulmonary macrophages mediates lipopolysaccharide-induced lung inflammatory injury by activating ZBP-1. Int Immunopharmacol. 2019;77:105944. doi: 10.1016/j.intimp.2019.105944 [DOI] [PubMed] [Google Scholar]
- 49.Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, Sehon CA, Marquis RW, Bertin J, Mocarski ES. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem. 2013;288:31268–31279. doi: 10.1074/jbc.M113.462341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Y, Liu M, Zuo Z, Liu J, Yu X, Guan Y, Zhan R, Han Q, Zhang J, Zhou R, et al. TLR9 Regulates the NF-kappaB-NLRP3-IL-1beta pathway negatively in salmonella-induced NKG2D-mediated intestinal inflammation. J Immunol. 2017;199:761–773. doi: 10.4049/jimmunol.1601416 [DOI] [PubMed] [Google Scholar]
- 51.Abbate A, Salloum FN, Vecile E, Das A, Hoke NN, Straino S, Biondi-Zoccai GG, Houser JE, Qureshi IZ, Ownby ED, et al. Anakinra, a recombinant human interleukin-1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Circulation. 2008;117:2670–2683. doi: 10.1161/CIRCULATIONAHA.107.740233 [DOI] [PubMed] [Google Scholar]
- 52.Suzuki K, Murtuza B, Smolenski RT, Sammut IA, Suzuki N, Kaneda Y, Yacoub MH. Overexpression of interleukin-1 receptor antagonist provides cardioprotection against ischemia-reperfusion injury associated with reduction in apoptosis. Circulation. 2001;104:I308–13I3. doi: 10.1161/hc37t1.094871 [DOI] [PubMed] [Google Scholar]
- 53.Ing DJ, Zang J, Dzau VJ, Webster KA, Bishopric NH. Modulation of cytokine-induced cardiac myocyte apoptosis by nitric oxide, Bak, and Bcl-x. Circ Res. 1999;84:21–33. doi: 10.1161/01.res.84.1.21 [DOI] [PubMed] [Google Scholar]
- 54.Krown KA, Page MT, Nguyen C, Zechner D, Gutierrez V, Comstock KL, Glembotski CC, Quintana PJ, Sabbadini RA. Tumor necrosis factor alpha-induced apoptosis in cardiac myocytes. Involvement of the sphingolipid signaling cascade in cardiac cell death. J Clin Invest. 1996;98:2854–2865. doi: 10.1172/JCI119114 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and supporting materials have been provided with the published article. Please see the Detailed Methods and Major Resources Table in the Supplemental Materials.27–33 We showed the illustrative images for each Figure.