Abstract
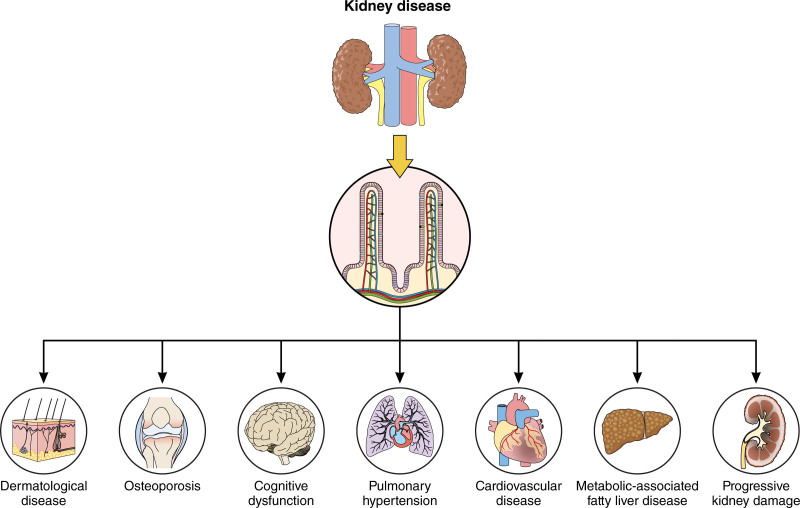
Kidney disease is associated with adverse consequences in many organs beyond the kidney, including the heart, lungs, brain, and intestines. The kidney-intestinal cross talk involves intestinal epithelial damage, dysbiosis, and generation of uremic toxins. Recent studies reveal that kidney injury expands the intestinal lymphatics, increases lymphatic flow, and alters the composition of mesenteric lymph. The intestinal lymphatics, like blood vessels, are a route for transporting potentially harmful substances generated by the intestines. The lymphatic architecture and actions are uniquely suited to take up and transport large macromolecules, functions that differentiate them from blood vessels, allowing them to play a distinct role in a variety of physiological and pathological processes. Here, we focus on the mechanisms by which kidney diseases result in deleterious changes in intestinal lymphatics and consider a novel paradigm of a vicious cycle of detrimental organ cross talk. This concept involves kidney injury–induced modulation of intestinal lymphatics that promotes production and distribution of harmful factors, which in turn contributes to disease progression in distant organ systems.
Keywords: intestines; kidney; lymphatic; renal insufficiency, chronic
Kidney disease is a growing global epidemic with significant health care and economic consequences. Even modest kidney impairment dramatically increases extrarenal morbidity, most notably as it relates to cardiovascular disease (CVD) but also infection, bone disease, cognitive dysfunction, and cancer.1 For example, impaired kidney function is a more important predictor of CVD than traditional cardiovascular risks such as elevated blood pressure, cholesterol, and diabetes.2 Nonetheless, questions remain regarding the mechanistic basis of how kidney disease affects distant organs.
Recent studies have demonstrated a central role for the gut in mediating kidney disease–related complications.3 Kidney disease disrupts the intestinal barrier and modulates the composition and metabolism of the intestinal microbiome, producing bioactive metabolites and toxins.4 Traditionally, blood vessels and nerves were thought to be the primary pipelines by which disease-associated factors could spread from the gut to distant organs. Only recently have researchers turned their attention to the lymphatics, which route fluids, macromolecules, and cells from the interstitial compartments of virtually every organ for return into the systemic circulation. The intestinal lymphatics are unique within the lymphatic vascular network because, in addition to the usual functions, they are responsible for absorption, remodeling, and transport of dietary lipids and intestinally generated lipoproteins, making them a potentially significant player in the dissemination of gut-borne disease-associated factors. Thus, disruptions in lymph transport and lymphatic vessel integrity are powerful potentiators of disease, including CVD, inflammatory bowel disease, bone disease, cognitive dysfunction, and chronic kidney disease.
This review examines the novel paradigm of a vicious cycle of detrimental organ cross talk by which kidney injury–induced modulation of intestinal lymphatics contributes to systemic organ disease progression.
Lymphatic System
Lymphatic Form and Function
The lymphatic system comprises an extensive vascular network punctuated by lymphoid organs, which plays an essential role in interstitial fluid balance, immune surveillance, and lipid transport. Recently, the lymphatic system has also been recognized as a facilitator of interorgan cross talk under physiological and pathological conditions.3,5,6 Divided into 3 classes, lymphoid organs are central components of the body’s immune response. Primary lymphoid organs (bone marrow and thymus) contain lymphoid progenitor cells required for lymphocyte differentiation. Secondary lymphoid organs, also called mucosa-associated lymphoid tissues, include lymph nodes, the spleen, tonsils, Peyer patches, and the appendix. These organs facilitate adaptive immune responses by providing the environment in which naive lymphocytes are activated and maintained, resulting in the antigen-driven expansion of memory T cells, effector B cells, and plasma cells.7 Tertiary lymphoid organs include collections of immune cells that resemble secondary organs but form in peripheral tissues in response to chronic inflammation or tissue injury.8 While lymphoid organs provide cellular components of the body’s immune response, lymphatic vessels facilitate their trafficking.
The lymphatic vessel network courses through every vascularized organ of the body, with the exception of bone marrow.9 In the interstitium, immune cells, macromolecules, and excess fluid (collectively referred to as lymph) enter highly permeable, blind-ended lymphatic capillaries that measure 30 to 80 μm in diameter. Lymph then drains into larger precollector and collecting vessels. These vessels differ from lymphatic capillaries in that they are considerably less permeable, become increasingly covered by smooth muscle cells, and contain intraluminal valves that help facilitate unidirectional pumping of lymph toward the ≈600 to 800 lymph nodes dispersed throughout the body. Within the node, lymph is screened for antigens that mediate adaptive immune responses and self-antigen tolerance10 and then funneled toward the thoracic duct, where it reenters the systemic circulation at the junction of the left subclavian and internal jugular veins.
Lymphatic Endothelial Cells
Lymphatic endothelial cells (LECs) line lymphatic vessels and lymph nodes and form the intraluminal valves of collecting vessels. During development, most LECs are derived from a subpopulation of LYVE-1 (lymphatic vessel endothelial hyaluronan receptor 1)/PROX1 (prospero homeobox 1)-positive venous endothelial cells located in the cardinal vein.11 Discrete nonvenous and hematogenic sources of LEC progenitor cells have also been described and are thought to give rise to portions of the dermal, mesenteric, and cardiac lymphatic vasculature.12–14 Once specified, LECs migrate and proliferate, forming rudimentary lymph sacs and a primitive plexus of vessels that undergo extensive remodeling to establish the complex network of lymphatic vessels and organs. The initial stimulus for lymphatic progenitor cell exit from veins is dependent on VEGFC (vascular endothelial growth factor C) signaling.15 VEGFC activates both VEGFR2 (vascular endothelial growth factor receptor 2) and VEGFR3 (vascular endothelial growth factor receptor 3); however, only VEGFR3 is required for developmental lymphangiogenesis.16 In LECs, VEGFR3-mediated signaling can be finely tuned by the formation of VEGFR2 heterodimers and the binding of coreceptors (neuropilin-1 and 2) and other proteins including syndecan 4 and β1 integrin.17 Expressed in both blood and lymphatic endothelium, VEGFR2 plays a pivotal role in angiogenesis but is less important for lymphangiogenesis, primarily acting to indirectly restrict lymphatic vessel development by sequestering VEGFC in blood endothelial cells.18
Mature LECs are a heterogenous population of cells, genetically distinct from blood endothelial cells, that can be divided into subpopulations based on their location and function within the lymphatic system. Structurally, all LECs express the following endothelial-specific junctional proteins: VE-cadherin (vascular endothelial-cadherin), claudin-5, and PECAM-1 (platelet endothelial cell adhesion molecule-1), but the organization of these proteins within cell-cell junctions is variable based on the location of the LEC within the lymphatic network.19 Most capillary-forming LECs have button-like point connections between cells, while LECs that line collecting vessels have a zipper-like continuous connections between neighboring cells. LEC junctional complexes have a high degree of plasticity and can switch between button and zipper confirmations during development and in pathophysiologic settings as discussed in more detail below.
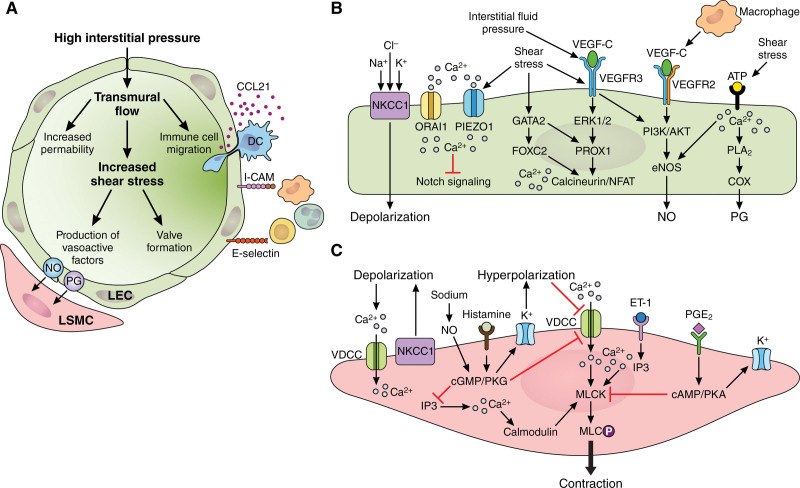
Single-cell RNA sequencing studies have identified unique transcriptomic signatures for various LEC subpopulations, providing new insights into the specialized functions of each group.20 LECs that line lymph nodes are distinct from LEC populations of peripheral (extranodal) lymphatics in that they express genes involved in attracting and transporting lymphocytes (ITGA2B, MIP-3A, MADCAM-1, and GLYCAM-1), screening for microorganisms (PTX3), attracting neutrophils to fight off lymph-borne pathogens (CXCL5, CXCL1, and C-type lectins), and immunological tolerance (TNFRSF9, PDL1, and IFNGR).20,21 Similarly, a subset of collecting vessel LECs gives rise to the intraluminal valves that prevent the backflow of lymph. These LECs are unique in that they are highly mechanosensitive and initiate valve formation gene programs in response to the mechanical forces generated by the flow of lymph.22 The molecular and biomechanical pathways involved in LEC development and function are described in Figure 1A and 1B.
Figure 1.
Molecular and biomechanical pathways involved in lymphatic vessel function. A, High interstitial fluid pressure stretches lymphatic endothelial cells (LECs) opening up gaps between overlapping cells, which promotes influx of fluid, molecules, and cells into the capillary lumen. Transmural flow also alters the organization of LEC junctional proteins and upregulates the leukocyte homing chemokine, CCL21 (C-C motif chemokine ligand 21), and the adhesion molecules, ICAM (intercellular adhesion molecule-1), and E-selectin, thereby increasing leukocyte transmigration into lymphatic capillaries. Lymph accumulation and flow generates shear stress necessary for valve morphogenesis and maintenance, as well as production of LEC-derived vasoactive factors including NO and PGs (prostaglandins), which regulate lymphatic smooth muscle cell (LSMC) contractility. B, LECs integrate biomechanical and molecular signals to coordinate transcription factor (PROX1 [prospero homeobox 1], GATA2 [Gata binding protein 2], and FOXC2 [forkhead box C2]) and receptor tyrosine kinase (VEGFR3 [vascular endothelial growth factor receptor 3]) activity necessary for the regulation of lymphatic cell specification, survival, and maintenance. PROX1 is the master regulator of LEC identity and other LEC-related genes including VEGFR3. VEGFR3 can be activated by biomechanical forces or by binding of VEGFC (vascular endothelial growth factor C). In PROX1-positive LECs, VEGFR3 activation is necessary for embryonic and injury-induced lymphangiogenesis. Similarly, high levels of PROX1 and FOXC2 are required for endothelial cell alignment and elongation necessary for intraluminal valve formation. Calcium (Ca2+) signaling has a major role in modulating LEC signaling cascades. Intracellular Ca2+ accumulation occurs via cation channel activation (PIEZO1 [Piezo1 type mechanosensitive ion channel component 1] and ORAI1 [ORAI calcium release-activated calcium modulator 1]) or by inositol 1,4,5-triphosphate (IP3)–mediated release of Ca2+ from the endoplasmic reticulum. Increased intracellular Ca2+ promotes sprouting via inhibition of Notch signaling, valve formation via calcineurin/NFAT (Nuclear factor of activated T cells) signaling, production of eNOS (endothelial-specific NO)-derived NO, and prostaglandin synthesis via PLA2 (phospholipase A2)-mediated release of arachidonic acid, which is converted to PG metabolites via COX (cyclooxygenase). LECs can also exert vasoactive effects on neighboring LSMCs via transferred membrane depolarization at myoendothelial junctions. C, Intracellular Ca2+ signaling also regulates the magnitude and frequency of LSMC contractions. Ca2+ can enter cells via activation of ion channels/transporters including NKCC1 (Na+-K+-2CI− cotransporter 1) that depolarize the membrane, activating voltage-dependent calcium channels (VDCCs). ET-1 (endothelin-1) binding to its receptor promotes IP3-mediated release of Ca2+ from the sarcoplasmic reticulum. In contrast, factors including sodium, NO, PGE2, and histamine inhibit Ca2+ accumulation by activating cGMP/PKG (protein kinase G) and cAMP/PKA (protein kinase A) signaling, which activates hyperpolarizing potassium (K+) channels, inhibiting VDCCs. Accumulation of intracellular Ca2+ binds to calmodulin, which facilitates activation of MLCK (myosin light chain kinase) and phosphorylation of MLC (myosin light chain), allowing for myosin and actin interaction and subsequent muscle contraction. Illustration credit: Sceyence Studios.
Lymphatic Smooth Muscle
As capillaries coalesce into precollector and collecting vessels, they acquire 1 to 3 layers of lymphatic smooth muscle cells (LSMCs) intertwined with collagen and elastin fibers. Additionally, they become studded with the unidirectional, bileaflet valves that delineate the borders of lymphangions. Although aided by extrinsic forces such as skeletal and visceral smooth muscle contractions and respiratory movements, the independent and spontaneous contraction of LSMCs within individual lymphangions serves as the main driver of lymph transport against unfavorable pressure gradients.23,24
Spontaneous lymphangion contractions occur within the greater context of the myogenic tone of the vessel. Similar to the cascade of events needed to generate arterial tone, intracellular calcium accumulation occurs via 2 mechanisms (membrane depolarization–based activation of L-type voltage-dependent calcium channels and inositol 1,4,5-triphosphate–mediated release of sarcoplasmic calcium stores) and drives muscle contraction. Elevated levels of intracellular calcium bind to and activate calmodulin, which in turn activate MLCK (myosin light chain kinase) and subsequent phosphorylation of myosin light chains. This then facilitates myosin and actin interaction and crossbridge cycling, ultimately leading to vasoconstriction (Figure 1C).25,26 Lymphatic tone is regulated by many of the same biochemical vasodilators, vasoconstrictors, and biomechanical factors that modify arteriole tone.27 This has fueled the notion that lymphatic muscle is a form of vascular smooth muscle. However, lymphatic muscle is a hybrid muscle type that integrates smooth, skeletal, and cardiac muscle contractile proteins.28,29 Furthermore, there is regional variability in the expression of specific contractile proteins among lymphatic vessels that can be correlated to differences in pumping capacity. Muthuchamy et al showed that mesenteric vessels expressed higher levels of specific cardiac, visceral, and skeletal actin isoforms compared with thoracic ducts. In addition, mesenteric lymphatic vessels were enriched in SMB (smooth muscle myosin heavy chain B) and the fetal cardiac/skeletal slow twitch β-myosin heavy chain isoform—a non–smooth muscle isoform that confers relatively fast contractile properties. In functional studies, thoracic duct contractions were 30% to 50% less frequent and 60% to 75% less powerful than mesenteric vessel contractions.29 These biochemical and functional variations are likely due to differences in the local environment of each vessel type: the thoracic duct is situated between the aorta and esophagus, each capable of exerting external forces onto the duct that would aid in the movement of lymph, while mesenteric vessels are not subject to the same degree of external compression by external forces and, therefore, must rely on powerful intrinsic contractions to pump lymph.
Lymphatic muscle also has unique electrophysical properties. LSMCs fire spontaneous action potentials that initiate waves of contraction that must be coordinated over the length of a lymphangion. Analogous to the cardiac cycle, LSMC contractions produce a systolic phase where lymph is actively pushed through an open valve into the adjacent lymphangion. This is followed by a diastolic phase where LSMC relaxation and closure of the previously open valve allow the lymphangion to refill. Modulation of membrane potential is integral to generating action potential spontaneous LSMC contractions. Several classes of ion channels including large-conductance calcium-activated potassium channels, inward-rectified potassium channels, delayed rectifier potassium channels, ATP-gated potassium channels, and calcium-activated chloride channels set the resting membrane potential of lymphatic muscle.30–32 Similarly, symporters play a role in regulating tone and contractility as inhibition of the NKCC (Na+-K+-2CI− cotransporter) cotransporter with furosemide or bumetanide caused LSMC hyperpolarization, vasorelaxation, and a decrease in contraction frequency.33,34
The rhythmic nature of lymphatic contractions suggests a pacemaking property. Small transient depolarizations (STDs) are thought to set the timing of lymphatic contractions, although the exact nature of the lymphatic pacemaker is still unresolved. STDs can be generated without neuronal innervation or the presence of an intact endothelium, which suggests that they originate within LSMCs. Another line of evidence that points to the pacemaking function of STDs comes via the observation that factors that increase the frequency of lymphatic contractions (eg, histamine, ET-1 [endothelin-1], and the thromboxane mimetic U46619) also increase STD activity. However, STDs can be abolished using the calcium-activated chloride channel blocker niflumic acid, without significantly altering the contraction frequency, making it unlikely that STDs are the only regulator of lymphatic pacemaking.35 Contraction frequency can adapt to subtle changes in the microenvironment due to alterations in transmural hydraulic pressure, fluid sheer stress, lymph osmolarity, and surrounding tissue temperature.23 LECs sense and respond to the shear stress generated by lymph flow by releasing vasoactive second messengers that modulate the basal tone and pacemaker function of adjacent LSMCs.23,36,37 For instance, increased shear stress causes release of LEC-derived NO, which affects the basal tone and pacemaker function of LSMCs.23
Of note, the local interstitial environment exerts different effects on lymphatic vessels based on the quantity and composition of fluid, cells, and solutes leaked from surrounding blood vessels. Numerous diseases are associated with deranged blood vessel endothelial barrier function, increased permeability, and extravasation into the microenvironment surrounding lymphatic vessels. This is especially common in diseases with an inflammatory component where the capillary leakage of blood-derived fluid, cells, and proteins then becomes a critical driver of lymphatic flow and lymphatic dysfunction.23,28 Such extravasation may be especially prominent in kidney diseases accompanied by hypoalbuminemia that lessens intravascular oncotic pressure promoting further transudation. In addition to these general principles of lymphatic vessel structure and function, the lymphatics of the gut have unique properties discussed below.
Gut Lymphatic Homeostasis
Lymphatic vasculature is heterogenous and plastic, acquiring specializations based on regional functional requirements and specific properties of the local microenvironment. The lymphatic vessels of the gut have distinctive structural features directly related to the specialized functions of this system, which include absorption of dietary lipids and fat-soluble vitamins, cholesterol transport, and gut immunosurveillance.
Intestinal Lymphatic Anatomy
The intestinal lymphatic network is uniquely patterned and partitions the organ into 2 distinct vascular plexuses: one that runs throughout the outer muscular layers of the gut and the other that is restricted to the inner mucosal and submucosal regions.38–40 Lymphatic capillaries in the gut, known as lacteals, are exclusively located in the mucosal region, extending into the center of each villus in the small intestine and connecting to underlying vessels in the submucosal space. These, along with the vessels of the muscular layer and vessels stemming from Peyer patches, coalesce and drain into collecting vessels located in the mesentery, which propel lymph toward the thoracic duct and back into the systemic circulation.38,39 Of note, this transportation route bypasses the liver, providing a path by which molecules carried in the lymph, including lipid-soluble drugs, can avoid first-pass metabolism and circulate systemically before degradation.40
In contrast to other lymphatic capillary beds, lacteals are surrounded by smooth muscle fibers that extend longitudinally from the submucosal region to the tip of each villus.41 Contraction of these fibers allows lacteals to actively propel lymph—a process that can be modulated by neurohumoral factors released by the autonomic nervous system.42 Furthermore, contraction of visceral smooth muscle in the intestinal wall also aids in this process and may play a larger role in facilitating lymph movement as lipid concentration within the lymph increases. This was demonstrated using a rat model, in which ingesting a high-fat meal resulted in increased lymph viscosity and flow rate, while conversely decreasing the amplitude and frequency of spontaneous mesenteric vessel contractions. One explanation for these seemingly contradictory outputs is that the resulting increase in shear stress generated by increased lymph viscosity and flow triggered a signaling cascade (perhaps NO production) that contributed to the observed decrease in pumping dynamics. This scenario may provide broader mechanistic insight for the lymphatic dysfunction that is often associated with lipid-related pathologies including obesity, diabetes, and other metabolic disorders.43
Lipoprotein Transport and Immunosurveillance
The absorption of dietary fat takes place primarily in the proximal duodenum where lipids pass from the intestinal lumen into enterocytes where they are packaged into triglyceride-rich chylomicrons. Chylomicrons can also contain fat-soluble vitamins and drugs, microbiota components such as bacterial lipopolysaccharide, and food antigens, gut hormones, and chylomicron-specific lipoproteins. Dietary cholesterol is also absorbed by enterocytes and transported into the lamina propria either by incorporation into chylomicrons or by direct secretion in the form of HDL (high-density lipoprotein) bound with the lipoprotein apoAI (apolipoprotein AI).38,44 Once formed, chylomicrons are released from the enterocyte’s basolateral membrane into the lamina propria, where they can enter into lacteals by diffusion through LEC junctions or by intracellular transport across LECs via vesicles.45,46 Chylomicron transport from the lamina propria into lacteals is affected by the rate of lymph formation and flow. As fat absorption occurs, fluid from the intestinal lumen is also absorbed, causing glycosaminoglycans in the lamina propria to become increasingly hydrated. This enhances lymph formation and flow, which helps to facilitate chylomicron transport by either decreasing extracellular matrix resistance to particle movement or increasing the convective fluid movement of chylomicrons through LEC junctions.47,48
Additionally, gut lymphatics play an important role in immunosurveillance and maintaining barrier function. The lumen of the gut serves as an interface between the outside world and the inside of the body. As such, it is continually exposed to vast amounts of antigens originating from pathogens, as well as innocuous substances including food and the commensal bacteria of the microbiome. This presents a complex challenge in which antigen-presenting cells (namely macrophages and dendritic cells) must strike a balance between inducing an appropriate immune response and developing immune tolerance. Failure to do so can result in chronic inflammatory conditions such as Crohn disease and ulcerative colitis or food allergies and celiac disease. The importance of gut lymphatics to immunosurveillance is further highlighted by the fact that lacteals are required for maintaining villi structure and intestinal barrier function. When lymphatic vessels in the small intestine of transgenic mice were ablated by diphtheria toxin, there was complete collapse of villus architecture, which allowed for pathogen invasion and entry into the systemic circulation, resulting in subsequent septic shock and death.49
Kidney Disease Modifies Gut Lymphatics
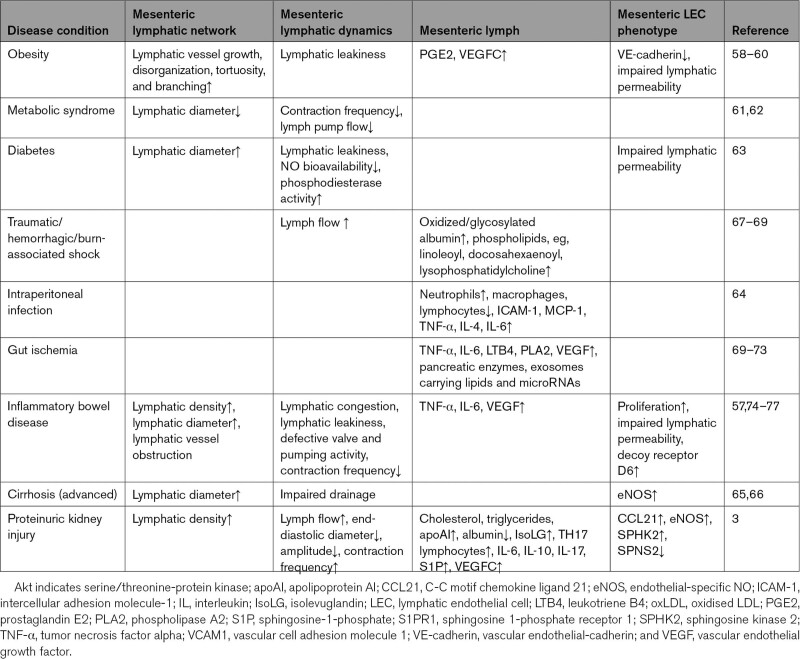
Although the gut is an increasingly recognized player in many diseases, recent studies have identified the intestinal lymphatic network as a key participant in the pathophysiology of diverse conditions ranging from cardiovascular and central nervous system disease to eczema.28,50–54 Disruption and impairment of intestinal lymphatics occurs not only in diseases primarily affecting the gut but also in systemic conditions and diseases in other organ systems. Thus, primary infectious or inflammatory bowel disease stimulate lymphangiogenesis, lymphangiectasia, and intralymphatic stasis that contribute to interstitial intestinal edema, infiltration of fat into the intestinal wall, and a sustained inflammatory response.55–57 In addition, intestinal lymphatic changes have also been documented in obesity, metabolic syndrome, diabetes, peritonitis, and cirrhosis.58–66 Table 1 summarizes the intestinal lymphatic perturbations in extraintestinal diseases. Importantly, kidney injury can now be added to the list of conditions that disrupt intestinal lymphatics.3
Table 1.
Disease Conditions That Modify Intestinal Lymphatics
Proteinuric kidney injury causes significant expansion of the intestinal lymphatics. Kidney injury also increased the mesenteric lymph flow and modulated pumping dynamics in the mesenteric bed. Mesenteric lymphatic vessels isolated from animals with kidney injury had a significant increase in ejection fraction and a marked decrease in contraction amplitude and end-diastolic diameter compared with vessels from uninjured kidneys. Mesenteric lymph of proteinuric rats had reduced albumin output but increased output of cholesterol, triglycerides, and apoAI—the major structural protein in HDL. These changes in lymph composition could have affected shear stress triggering NO production similar to lymphatic disruption associated with lipid-related pathologies noted above.43 Importantly, the mesenteric lymph of kidney-injured animals was enriched in lipid peroxides, specifically, the highly reactive isolevuglandin (IsoLG), which cross-links apoAI (see below). The number of Th17 cells and cytokines including IL (interleukin)-6, IL-10, and IL-17 was increased in mesenteric lymph from the kidney-injured animals. S1P (sphingosine-1-phosphate), the immune cell trafficking factor and VEGFC, the prolymphangiogenesis factor also increased. Proteinuric kidney injury altered the transcriptome of intestinal LECs, affecting the expression of genes involved in lymphangiogenesis, vasodilation, and immune cell chemoattraction, for example, CCL21 (C-C motif chemokine ligand 21), eNOS (endothelial-specific NO), SPHK2 (sphingosine kinase 2), and SPNS2 (sphingolipid transporter 2). These are interesting observations in view of the recent findings that the composition of lymph, including lymphatic cells and lipoproteins, is modified during trafficking through the lymphatic network and that the modifications are organ specific.78 Thus, kidney injury–induced changes in intestinal lymphatic growth, dynamics, and lymph composition may significantly impact the systemic and organ disorders accompanying kidney disease. Although the mechanisms driving lymphatic remodeling, dysfunction, or composition are unclear, kidney disease presents a fertile circumstance for understanding these pathways.
Mechanisms by Which Kidney Disease Can Modify the Lymphatic Network
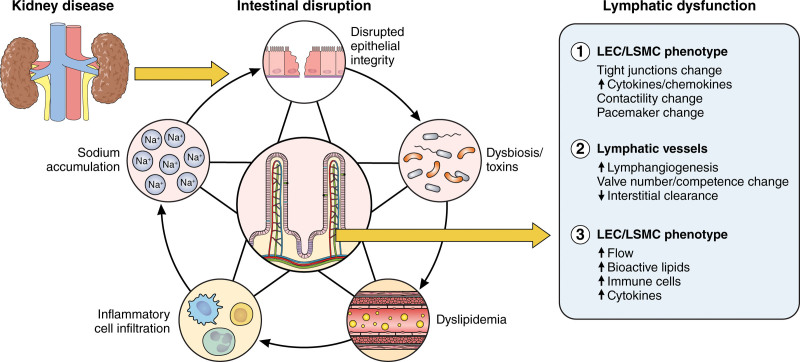
This section considers several kidney disease–linked complications that can disorder the lymphatic network including disruption of the intestinal epithelial barrier, dysbiosis and production of toxins, altered absorption and modification of lipids, low-grade inflammation, and sodium accumulation (Figure 2).
Figure 2.
Kidney disease disrupts intestinal lymphatics. Kidney disease damages intestinal architecture and function by causing disruption of epithelial integrity, dysbiosis, dyslipidemic metabolism, inflammatory cell infiltration, and sodium accumulation. The subsequent intestinal damage then impacts lymphatics by (1) increasing lymphatic flow and altering lymph composition; (2) activating lymphatic endothelial cells (LECs) that modulate tight junction organization (button-to-zipper ratio), augmenting production of cytokines/chemokines with vasoactive properties, and altering lymphatic smooth muscle cell (LSMC) contractility and pacemaker functions; (3) stimulating lymphatic growth but negatively affecting interstitial clearance and valve homeostasis. Illustration credit: Sceyence Studios.
Disruption of the Intestinal Epithelial Barrier
Kidney disease causes significant proteomic changes to the intestinal epithelium, resulting in reduced expression of proteins required for tight junction formation (claudin-1 and 2) and maintenance of barrier integrity (HSP70 [heat shock protein 70]), which compromise barrier function.79 Increasing urea levels occurring with progressive kidney disease directly deplete tight junction proteins including occludin, claudin-1, and zona occludens, further degrading the barrier structure and increasing local levels of oxidative stress.80 Progressive disruption of the epithelium and underlying intestinal wall stimulates influx of immune cells, causing local inflammation and increased cytokine production, which results in further retraction and endocytosis of claudins and occludin.81–83 An obvious consequence of increased epithelial permeability is the increased risk of translocation of bacterial products and toxins into the systemic circulation. Perhaps equally wide-reaching consequences of barrier degradation are the deleterious effects that local enrichment of invading bacteria and toxins impose on the structure and function of intestinal lymphatic vessels.
Dysbiosis/Toxins
The gut microbiome has emerged as a modulable factor affecting health and disease. Many diseases disrupt the normal gut microbiome, causing dysbiosis that can dictate the disease phenotype. Patients with kidney disease have dysbiosis that includes lower colonization of Bifidobacteriaceae families, mainly Bifidobacterium, Lactobacillaceae, Bacteroidaceae, and Prevotellaceae genera and higher intestinal levels of Enterobacteriaceae, Enterobacter, Klebsiella, Enterococci, and Clostridium perfringes.84–86 The imbalance of these bacterial species underlies chronic inflammation that prevails across the entire spectrum of kidney disease.87,88 Factors linked to adverse transformation of the gut microbiome in chronic kidney disease include (1) reduced consumption of dietary fibers, (2) slow intestinal transit time that contributes to the imbalance between saccharolytic and proteolytic microbiota, (3) increased urea that supports bacterial families generating specific uremic toxins, (4) gut ischemia, and (5) edema.89–91 Critically, the chronic kidney disease patient population is also routinely exposed to medications such as phosphate binders, iron-containing compounds, and especially, antibiotics that further disrupt normal intestinal structure and function, as well as disordering the normal microbiome.
The profound dysbiosis and toxins prevailing in kidney disease patients is relevant because intestinal microbiome is a critical regulator of development and maintenance of gut lymphatics.23,56,92,93 Lacteals appear within the intestinal villi at postnatal day 7 and continue to grow and remodel after weaning.94 Germ-free mice lacking endogenous microbiota have decreased lacteal length, lower number of Prox1-positive LECs in their villi, and reduced VEGFR3.56 The microbiome also regulates junctional plasticity. The proportion of button-like junctions decreased while zipper-like junctions increased between LECs of antibiotic-treated mice versus vehicle-treated animals.94 The magnitude of the effect varied by intestinal segment and paralleled the presence of microbiota, being most pronounced in the jejunum and ileum and least in the duodenum, which is the less colonized segment of the intestine.56
The microbiome also maintains lacteal length, integrity, and functionality in adulthood. As in immature animals, depletion of microbiota by antibiotic treatment in adults reduced the lacteal length in jejunum and ileum, with little change in the duodenum.56 The remodeling was driven by continuous activation of Notch ligand delta-like 4 and adrenomedullin-calcitonin receptor-like receptor signaling.38,95 Lacteal regression was not due to decreased survival of LECs nor a direct toxic effect of the antibiotics on LECs and instead reflected reduced expression of VEGFC, thereby reiterating its critical role in maintaining lacteal integrity.56,96 There are 2 primary sources of VEGFC around lacteals, the longitudinal smooth muscle cells surrounding lacteals and villus macrophages. Antibiotic treatment did not alter abundance or distribution of the smooth muscle cells.56 However, a marked reduction in macrophage-expressed VEGFC was observed in antibiotic-treated mice, supporting a critical role of villus macrophages in VEGFC production and maintenance of lacteal structure. It follows then that alterations to the microbiota would also have functional consequences on lacteals. Indeed, depletion of gut microbiota resulted in reduced triglyceride, chylomicron, and free fatty acid transport attributed to structural defects assessed by intravital imaging.56 The detrimental effects of depleting the intestinal microbiome on lacteal phenotype were reversed by conventionalizing the germ-free animals into a normal environment.56 It is notable that only the intestinal lymphatics were affected by the antibiotic depletion of intestinal microbiota as lymphatic vessels in ear skin, trachea, diaphragm, and inguinal lymph nodes showed no discernible effects.56 These findings highlight the unique characteristics of intestinal lymphatics. Unlike LECs in lymphatics of other organs, they have filopodia and positive staining for Ki67 proliferation markers, demonstrating their measurable proliferative capacity and ability to remodel under steady-state conditions.23,97,98
While strong evidence supports microbiome regulation of intestinal lymphatics, there is also evidence for the reverse relationship, whereby lymphatics modulate the gut microbiome.56 In a murine colitis model, supplementation of VEGFC promoted lymphangiogenesis, increased intestinal drainage, and significantly increased the abundance of Bacterioidate and decreased the abundance of Firmicutes at phylum level in fecal samples.99 In a primate model of Crohn disease, obstruction of lymphatic outflow dramatically altered intestinal microbial subgroups, including greater abundance of Prevotellaceae and Bacteroidetes-Prevotella-Porphyromonas.100 These results illustrate a cycle whereby metabolic changes associated with disease, including kidney disease, disrupt the intestinal microbiome, which impairs the lymphatic vascular network, which, in turn, intensifies dysbiosis and intestinal damage, thus furthering disease.
Dyslipidemia
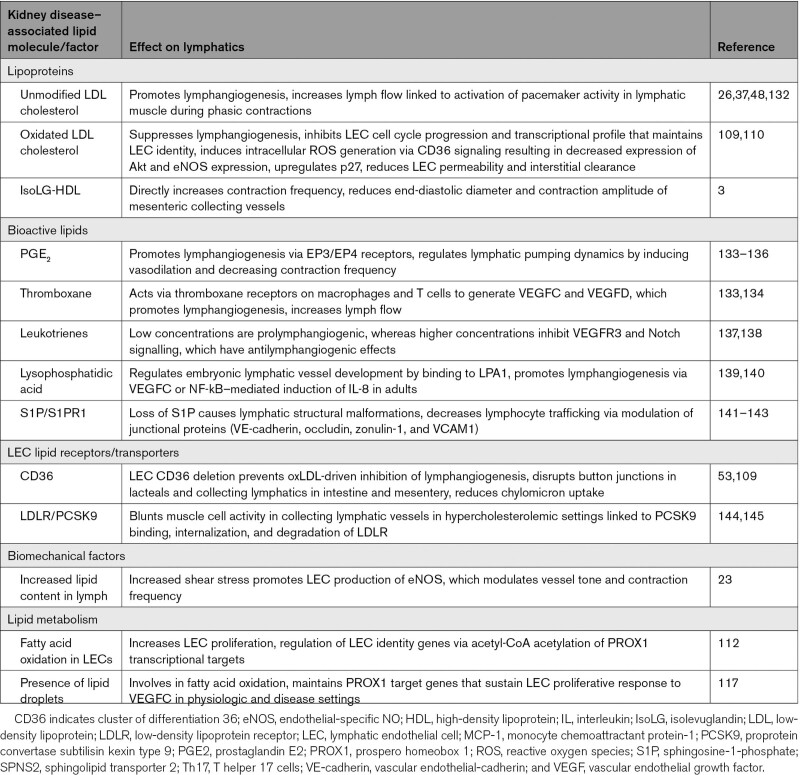
Lipids have a variety of functions beyond their traditional roles in building cell membranes and energy storage. They also participate in physiologic and pathologic signaling cascades that regulate cellular metabolism, cell fate determination, proliferation, and immune response, and their activity is tied to specific modifications of their basic structure. Kidney disease causes abnormalities in the lipid profile. The characteristics of these abnormalities vary depending on the degree of kidney impairment, the underlying etiology, and whether proteinuria, especially nephrotic syndrome, is present.101–107 Aside from altering lipid levels, a low-grade chronic proinflammatory and high oxidative state prevails across the entire spectrum of kidney disease. This creates a milieu that promotes formation of oxidatively and enzymatically modified lipoproteins and lipid metabolites (eg prostaglandins, lysophosphatidic acid) that directly modulate LEC phenotype and lymphatic growth, barrier function, and contractility.43,108–110
Although interaction between lipids and lymphatics is established, recent studies have uncovered previously unappreciated lipid metabolism in LECs. As in other cells, LECs use mitochondrial fatty acid oxidation for their energy needs. Fatty acid oxidation is also key in promoting LEC proliferation by providing acetyl-CoA that sustains the Krebs cycle and deoxyribonucleotide synthesis needed for cellular proliferation and division.111,112 Inhibition of fatty acid oxidation in LECs reduces expression of the rate-limiting enzyme, CTP1A (carnitine palmitoyltransferase 1A), which significantly decreases endothelial cell proliferation and lymphatic differentiation via PROX1-dependent regulation of VEGFR3 expression.111,112 These findings suggest a therapeutic potential of lowering fatty acid oxidation to inhibit pathophysiologic lymphangiogenesis (ie, cancer). Conversely, supplementation of fatty acids (eg, acetate) rescues the acetyl-CoA, VEGFR3 expression, and lymphatic sprouting and restores lymphangiogenesis after corneal injury. These findings suggest fatty acids or their metabolites can be used to modulate LECs and lymphatic growth in pathophysiologic settings.
The pathophysiological implication of LEC lipid metabolism was further illuminated by the demonstration that LECs contain lipid droplets. Lipid droplets have traditionally been considered static energy storage deposits that increase with impaired cellular homeostasis.113,114 Increasingly, however, lipid droplets are recognized as dynamic and inducible platforms that not only sequester fatty acids and prevent lipotoxicity but also regulate energy metabolism, membrane trafficking, immune responses, and cytokine production.115,116 In view of the specialized function of lymphatic vessels in lipid trafficking, finding lipid droplets in LECs uncovers the cells’ potential to regulate fatty acid storage and degradation and, therefore, lymphatic functions. Strong support of this concept comes from a recent study showing that genetic deficiency in lipid droplet degradation by autophagy causes significant LEC damage.117 Autophagy blockade caused accumulation of lipid droplets linked to defective trafficking of lipid droplets to mitochondria. This in turn reduced mitochondrial ATP production, fatty acid oxidation that interrupted the mitochondrial-PROX1–driven transcriptional circuit blunting the normal response to VEGFC. The net result was impaired lymphangiogenesis documented in cultured cells and in an in vivo corneal injury model. It is interesting that ketogenic diets that provide short-chain fatty acids increase lymphangiogenesis and improve lymphatic function after myocardial infarction and in a lymphedema mouse model.117–119 Dietary fatty acids also affected accumulation of lipid droplets and cellular injury in proximal tubules of mice with diabetic nephropathy.120 Whether the diabetic kidney injury affected the formation of lipid droplets in LECs and whether dietary fatty acids can modulate LEC lipid droplets is currently unknown. Nonetheless, there is strong support for the concept that lipid droplet degradation is an important source of fatty acid oxidation driving lymphangiogenesis and that dietary lipids can maintain LEC responsiveness to VEGFC in physiological conditions and following injury-stimulated lymphangiogenesis.117
Modified lipids have specific roles in regulating lymphatics. This is relevant because kidney disease–associated inflammation promotes lipid oxidation, including oxidation of LDL (low-density lipoprotein) cholesterol, which profoundly affects lymphatic growth and function. Comparison of normal unmodified LDL with oxLDL (oxidized LDL) illustrates the differences: normal LDL stimulated lymphangiogenesis while oxLDL inhibited lymphangiogenesis.109 Oxidized, but not normal, LDL blocked cell cycle progression in cultured LECs, reduced their expression of Akt (serine/threonine-protein kinase) and eNOS, increased p27 (an inhibitor of the cell cycle) expression, and induced intracellular oxidative stress.54,109 Knockdown of the lipid transporter, CD36 (cluster of differentiation 36), in LECs prevented oxLDL-induced suppression of lymphangiogenesis via AKT/eNOS pathway and cell cycle. These findings suggest blockade of LEC CD36 signaling may promote lymphangiogenesis and, therefore, clearance of cells and potentially harmful molecules.109
A critical role for oxLDL was recently shown in humans and mice with fatty liver disease. The study revealed hepatic fatty infiltration affects lymphangiogenesis and impairs the ability of LECs to maintain a lymphatic-specific transcriptional profile.110 These changes culminated in diminished lymphatic draining of the liver. The findings complement observations that fatty acid oxidation in LECs is critical in promoting lymphatic growth and lymphatic-specific chromatin modifications.112 OxLDL also altered the permeability of the lymphatic vasculature. Single-cell analysis of liver LECs from mouse model of fatty liver disease and cultured LECs exposed to oxLDL skewed the ratio of Vegfr2/Vegfr3 and the cells became significantly less permeable. Importantly, treatment with recombinant VEGFC (cys156ser) targeting VEGFR3 resulted in increased hepatic lymphatic vessel density but also improved liver inflammation and progressive hepatic fibrosis. Together, these observations show that oxLDL directly impairs LEC phenotype, growth, permeability, and interstitial clearance by the lymphatic vessels, which may present a therapeutic target to lessen disease.
Lipoprotein oxidation generates a variety of lipid peroxides including the most reactive dicarbonyl IsoLGs, which covalently modify proteins, nucleic acids, and other lipids, causing dysfunction in cells and tissues. Among lipoproteins, HDL is especially susceptible to IsoLG adduction. Elevation in IsoLG adducts has been linked to the oxidative damage in atherosclerosis, cardiac arrhythmia, hypertension, kidney damage associated with systemic lypus erythematosus, Alzheimer disease, macular degeneration, and cancer.121–125 Different cells, including antigen-presenting immune cells, platelets, cardiac myocytes, and epithelial cells in lung and liver, can generate IsoLG.121–128 IsoLG adducts have recently been shown to be increased in gastrointestinal epithelial cells of humans and animal models with gastritis and colitis associated with gastrointestinal carcinogenesis.125 We show that kidney injury also stimulates intestinal epithelial production of IsoLG and that the IsoLG colocalizes with apoAI in lymphatic lacteals. Since about one-third of apoAI is synthesized by the ileum,129,130 it is possible that enterically produced IsoLG adducts local apoAI and modulates the mesenteric lymphatic bed. Indeed, IsoLG-apoAI directly affected lymphatic growth and contractility. Moreover, IsoLG-apoAI increased NOS3 (nitric oxide synthase 3) in cultured LECs, and IsoLG-apoAI directly blunted vasoactivity and increased contraction frequency versus unmodified apoAI. Critically, treatment to scavenge IsoLGs significantly reduced intestinal lymphangiogenesis, albuminuria, and interstitial fibrosis supporting the concept that intestinal lymphatic growth associated with kidney injury is linked to IsoLG.3,131 Thus, the mesenteric lymphatic network appears to serve as both target and perpetrator of IsoLG-HDL’s effects by augmenting lymphangiogenesis, lymphatic vessel contractions, LEC activation, and increased lymph flow.
Injury releases arachidonic acid from cell membrane phospholipids, providing precursors for bioactive mediators including prostaglandins, thromboxanes, and leukotrienes that have important roles in inflammation, cellular proliferation, fibrosis, and regulation of vascular tone, including lymphatics (Table 2). PGE2 stimulates lymphangiogenesis and increases lymph flow by inducing VEGFC/D transcription.133 Similarly, thromboxane 2, acting via the TP (thromboxane prostenoid receptor) on macrophages and T cells and not LECs, promotes lymphangiogenesis by inducing VEGFC/D.134 Acting through the TP receptor, thromboxane 2 also increases lymphatic flow rate that reflects expansion of lymphatic density, greater reabsorptive capacity, and stronger pumping by collecting vessels.134 Leukotriene modulation of lymphatics depends on their levels whereby lower leukotriene levels prompt lymphangiogenesis while higher concentrations inhibit VEGFR3, lymphatic growth, and Notch signaling, all critical to lymphatic development and maintenance.137,138,146,147 Studies with the nonsteroidal anti-inflammatory drugs ketoprofen and ibuprofen have added to our understanding of the relative effects of prostaglandins and leukotrienes on lymphatics.148 Ketoprofen inhibits cyclooxygenase enzymes, which generates prostaglandins, and 5-LO (5-lipoxygenase), which generates leukotrienes. Ketoprofen enhanced postsurgical lymphedema, indicating that cyclooxygenase-derived prostaglandins or 5-LO–derived leukotrienes have a role in lymphedema formation.137 Ibuprofen—a cyclooxygenase inhibitor—exacerbated edema, indicating beneficial effects of prostaglandins in lymphedema.137 Indeed, pharmacological inhibitors and genetic targeting of the 5-LO pathway lessened edema and improved lymphatic vascular function.137,149,150
Table 2.
Lipid-Related Modulators of Lymphatic Vessels
Lysophosphatidic acid, which encompass bioactive phosphoglycerides synthesized from membrane phospholipids, is involved in embryogenic development of lymphatic vessels.139,140,151 Lysophosphatidic acid also activates expression of VEGFC that potentiates lymphangiogenesis following injury in adults though NF-κB (nuclear factor kappa B)–mediated induction of IL-8.139 Lysophosphatidic acid in human and animal atheroma acts as an LEC chemoattractant to induce lymphangiogenesis.152–154
S1P is synthesized from ceramide, phosphorylated by SPHK1 and SPHK2, and carried primarily by HDL. S1P regulates endothelial cell proliferation and migration.141,142,155,156 LYVE-1-driven depletion of SPHK1/SPHK2 reduces S1P in LECs, leading to abnormal lymphatic morphology and reduced lymphocyte egress from peripheral tissues. This suggests LEC-derived S1P has a key role in lymphatic patterning and lymphocyte trafficking.47,143 Indeed, our studies showed that mesenteric lymph of kidney-injured animals contained increased levels of S1P and that intestinal LECs had more SPHK2 mRNA, indicating S1P signaling may be an attractive therapeutic target to improve lymphocyte trafficking.3
Inflammation
Persistent low-grade inflammation prevails across all stages of kidney damage and is evident not only in plasma and kidney parenchyma but also in extrarenal tissues. Immune cells and their cytokines are powerful mediators of lymphatic vessel growth, permeability, and pumping. Proteinuric kidney injury led to a 3-fold increase in the number of CD68-positive cells in the ileum compared with normal controls.3 Macrophages in particular are an important source of VEGFC,157 and macrophage depletion reduced lymphangiogenesis in acute colitis,158 hypertension,159 and proteinuric kidney injury.160 Dendritic cells and neutrophils have also emerged as sources of lymphangiogenic signals.161 In addition to VEGFC, IL-17 and IL-8 have also been shown to induce lymphangiogenesis.162,163 IFN-γ (interferon gamma) produced by activated T cells decreased lymphatic vessel formation.164,165 Inflammatory mediators impact other lymphatic functions. IL-6, TNFα (tumor necrosis factor alpha), and IFN-γ increase lymphatic permeability in association with reduced expression of VE-cadherin.166 Inflammatory cytokines also modulate lymphatic pumping activity. Prostaglandins, IL-1β, IL-6, and TNF-α reduced lymphatic pumping frequency.167 Blocking TNF-α increased lymphatic contractions and reduced joint inflammation.168 Substance P—a neuropeptide also secreted by inflammatory cells—increases the pumping frequency of rat mesenteric vessels.169
Sodium
Sodium accumulation accompanies kidney disease and other edema-forming diseases such as congestive heart failure, cirrhosis, and hypertension.170 Over the last 2 decades, studies have suggested that sodium accumulation within the interstitium is linked to lymphangiogenesis. The sodium-lymphatic relationship has been most extensively studied in the skin of hypertensive animals and humans and involves transcription factor TonEBP (tonicity-responsive enhancer protein)-induced macrophage secretion of VEGFC secreted by infiltrating macrophages.159,171,172 Depletion of mononuclear phagocytes or blockade of VEGFC during high salt intake increased density and hyperplasia of lymphatic capillaries in the skin in association with hypertension, although recent studies from the same groups have questioned the singular effect of lymphangiogenesis on blood pressure.159,171,173 It is, therefore, notable that aside from lymphatic growth, sodium can also modulate lymphatic dynamics, which may have a role in hypertension and other diseases.5,174,175 Mizuno et al175 showed high-sodium diet suppressed the amplitude, ejection fraction, and stroke volume that markedly reduced pumping activity in afferent lymphatics while efferent lymphatics were resistant to the effects high-salt diet and preserved pumping reflected increased contraction frequency. High-salt diet or DOCA treatment increased skin and muscle lymphatic vessel contraction frequency while reducing contraction amplitude.174 Similarly, we showed that direct exposure of lymphatic vessels to a high-sodium environment increases the frequency of contraction of collecting lymphatic vessels and reduced the contraction amplitude and, to a lesser extent, the ejection fraction.5 Currently, there are no studies specifically assessing the effects of sodium on mesenteric lymphatics; however, it is known that this lymphatic bed adapts to physiologic and pathophysiologic stimuli. For example, oxidative stress, fructose feeding, aging, and intestinal inflammation impair intrinsic contractility of the mesenteric collecting lymphatic vessels.74,176,177 These are important observations because, unlike blood vessels, which depend on the heart to pump blood, lymph flow is propelled by forces in the surrounding tissues and intrinsic contractions of the lymphatic vessels, which are exquisitely sensitive to the microenvironment.37,178 Thus, local sodium concentration may be a significant regulator of lymphatic dynamics.
Sodium can also function as a signaling molecule. We show the Na-K-2Cl cotransporter NKCC1, but not NKCC2, is expressed in lymphatic vessels.34 Thus, while NKCC2 is best known for its actions on tubular epithelial cells responsible for maintenance of sodium homeostasis, we find NKCC1 modulates the tone in collecting lymphatic vessels.34 Of potential clinical relevance are data showing that the NKCC1 inhibitor, furosemide, dilates lymphatic vessels and decreases contraction frequency, amplitude, and ejection fraction. High-sodium environment also decreases lymphatic vessel contraction amplitude and ejection fraction, reduces phosphorated NKCC1, phosphorylated SPAK (SPS1-related proline/alanine-rich kinase), an upstream kinase, and phosphorylated eNOS, a downstream vasoactive factor, and blunts lymphatic response in injured kidneys.5 These results suggest sodium accumulation accompanying kidney disease can contribute to heretofore unrecognized impairment in lymphatic function that may accompany sodium-avid states and impaired lymphatic function. These findings also suggest that resistance to the diuretic effects of furosemide sometimes observed in sodium-avid conditions may reflect dampening of the contractile and pacemaker functions of lymphatic vessels.
Clinical Implications and Therapeutic Interventions
Kidney disease is associated with many complications including hypertension, CVD, bone disease, neurologic and cognitive dysfunctions, and progression of kidney scarring, even when the original inciting disease has abated. Each of these clinical conditions has been linked to changes in the gut and led to new nomenclature to encompass the concept of interorgan cross talk via terms such as gut-hypertension axis, gut-CVD axis, gut-bone axis, gut-cognition axis, and gut-kidney axis. Mediators in these interorgan communication networks include intestinally originating metabolites, immune cells activated within the intestinal wall, and lipid particles absorbed from the diet or generated within the intestines and then modified within the local environment. Until now, blood vessels and nerves were thought to be the primary pathways by which metabolites and toxins affect distant organs. It now appears that intestinal lymphatics constitutes an additional pathway in the gut-organ axis (Figure 3).
Figure 3.
Intestinal lymphatics mediates the adverse consequences of kidney disease in other organ systems. Kidney disease causes intestinal damage and lymphatic dysfunction due to epithelial disruption, alterations of the microbiome and lipid profile, inflammation, and sodium accumulation. This results in the production of disease-associated molecules (bioactive metabolites, modified lipoproteins, activated immune cells, and cytokines) that can then be dispersed throughout the body via the lymphatic vasculature where they have secondary pathogenic effects. This model represents a novel mechanism by which lymphatics mediates the production and distribution of kidney disease–derived factors that subsequently contribute to disease progression in other organ systems. Illustration credit: Sceyence Studios.
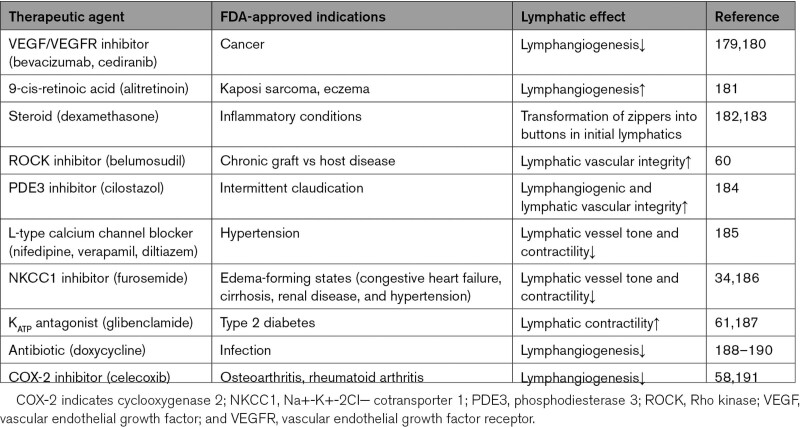
Currently, therapeutic focus for kidney disease is the pathophysiologic processes affecting the kidneys with additional therapies aimed at treating specific systemic complications such as anemia and bone disease. Here, we focus on the lymphatic network and the possibility that future therapies may target the absorptive capacity, contractile dynamics, and clearance competence of the intestinal lymphatics, which could lessen the adverse consequences associated with kidney disease. Heretofore, no drug has been developed for clinical use that specifically targets lymphatics. However, the increasing number of Food and Drug Administration–approved drugs are being recognized to have effects on lymphatic vessel number or function (Table 3). Pharmacotherapeutic agents that modify the lymphatic network can be broadly categorized by their proposed mechanism or functional target, for example, VEGFC activators/inhibitors to modulate lymphangiogenesis, ROCK (Rho kinase) inhibitors to modulate lymphatic integrity, ATP-gated potassium antagonists to modulate lymphatic contractility, anti-inflammatory/antioxidant agents to modify the lymphatic environment.
Table 3.
Clinically Used Drugs With Lymphatic Effects
VEGFC/VEGFR3 is a critical axis in lymphangiogenesis. Much of the pharmacologic efforts have focused on stimulation or inhibition of lymphangiogenesis in disease models such as inflammatory bowel disease, atherosclerotic/congestive cardiac failure, joint and skin inflammation, and cancer. Lymphactin—an adeno-associated virus vector–based overexpression of VEGFC gene therapy—is currently in a phase II clinical trial in patients with breast cancer-associated secondary lymphedema.192 Lymphangiogenesis can also be induced by retinoic acid via the FGFR (fibroblast growth factor receptor) pathway. The 9-cis retinoic acid—a Food and Drug Administration–approved drug for treating Kaposi sarcoma and chronic eczema—stimulates LEC migration and differentiation in vitro and reduces lymphedema via increased lymphangiogenesis in preclinical animal models.181,193 Since VEGFC also binds VEGFR2, VEGFC modulators can also affect blood vessels.194 Future therapeutics will need to consider ways that limit the potentially undesirable vascular side effects such as angiogenesis or capillary leakage.
Lymphatic integrity is another contributor and target of lymphatic dysfunction. For example, the anti-inflammatory agent dexamethasone, acting through glucocorticoid receptors, inhibits VEGFC–induced lymphangiogenesis and also promotes zipper to button junction formation, shown to resolve inflammation.182,183,195 FOXC1 (Forkhead box C1) or FOXC2 (Forkhead box C2) induces hyperactivation of contractile stress fibers in LECs, which is rescued by the ROCK inhibitor Y27632.196 ROCK inhibition also enhances lacteal zipper junction formation and improves LEC barrier integrity in mesenteric collecting vessels, which has been proposed as a therapeutic approach to treat obesity/metabolic dysfunction.60 In type 2 diabetes, reduced NO bioavailability and consequent PDE3 (phosphodiesterase 3) activation cause a lymphatic permeability defect.63 Oral administration of cilostazol—a selective PDE3 inhibitor approved by the Food and Drug Administration for treating intermittent claudication—reversed genetically and surgically induced lymphedema in mice, which was attributed to both the lymphangiogenic and lymphatic vascular integrity effects.184 These examples illustrate that therapeutic targets can affect multiple lymphatic functions and different lymphatic segments, for example, capillaries and collecting vessels that may have complementary or opposing effects on the lymphatic network.
Ion channels and transporters are significant mediators of the membrane potential in lymphatic vessels. This is important because many currently used medications belong to this class of drugs. A recent review of >200 drugs with potential lymphatic vessel effects found that the most frequently used drug with inhibitory effects on lymphatic pumping was the calcium channel blocker.197 Notably, most of the identified drugs dampen lymphatic contractile functions.197 These observations highlight the possibility that adverse consequences such as refractory edema with diuretic use may be linked to effects on lymphatics. We found that NKCC1 inhibition by the diuretic furosemide decreased both lymphatic vessel tone and contractility.34 Furosemide has been reported to impair human thoracic duct contractility in vitro.186 ATP-gated potassium openers, such as pinacidil and diazoxide used to treat hypertension, induce severe peripheral edema, which is contributed by the hyperpolarization in the lymphatic muscle cell and reduced lymphatic contractility and lymph flow.30,198 Conversely, glibenclamide (Food and Drug Administration–approved ATP-gated potassium antagonist) rescued lymphatic contractile dysfunction in a mouse model with gain-of-function mutations in the Kir6.1 gene, which mimics the lymphoedema observed in patients with Cantú syndrome.187 Glibenclamide also partially restored the mesenteric lymphatic contractility in the metabolic syndrome model.61 Cumulatively, these studies illustrate the previously underrecognized effects of current drugs on lymphatics. The observations also highlight the need for therapies that specifically target the lymphatic function.
Since local inflammation is a key modulator of lymphatics, anti-inflammatory interventions have been used to affect lymphatic functions. The antibiotic doxycycline not only impeded monocyte recruitment and polarization of alternatively activated macrophage but also inhibited lymphangiogenesis via VEGFC/VEGFR3 signaling.188,189 In a clinical study of 162 patients with filarial lymphedema, doxycycline treatment also improved lymphedema compared with amoxicillin or placebo.190 The benefit of doxycycline treatment was independent of ongoing infection and linked to activation of the VEGFC/VEGFR3 pathway. Inhibition of proinflammatory metabolites of arachidonic acid such as leukotrienes affects lymphatics in animals and humans. Bestatin—a selective leukotriene B4 antagonist—increased lymphatic function and restored lymphatic architecture in the murine tail model of lymphedema.137,191 A phase II randomized and placebo-controlled study in patients with lower extremity lymphedema is underway, and results are pending.137,191 Because inflammatory modulation of lymphatic function is local, there is increasing focus on delivery of anti-inflammatory therapeutics into the relevant tissue.
Recognition of the importance of the lymphatic system on disease progression has led to new strategies to access lymphatics and design biomaterials that specifically enter lymphatics. Currently, this approach includes the route of administration and drug formulation. Gastrointestinal, subcutaneous, or pulmonary administration appear to provide better access to the lymphatic network than intravenous administration of a therapeutic. Here, we focus on gastrointestinal lymphatics. Targeting the gastrointestinal lymphatics increases target specificity by bypassing first-pass metabolism in the liver, which reduces systemic concentrations of a drug. For example, compared with intravenous administration, drug-containing nanoparticles delivered to the intestines were shown to have a >20-fold increase in drug bioavailability, a 30-fold increase in the elimination half-life, and less accumulation in the heart, lungs, spleen, and kidneys.199,200 This approach also reduces off-target effects, which is a serious problem in patients with kidney disease. Specific drug formulations can also target lymphatic entry. For example, while small molecules (<5 nm in diameter) are easily taken up by blood capillaries, middle-size molecular species (5–100 nm) are shuttled to lymphatics.201,202 Lymphatic uptake can be improved by making lipophilic nanomaterial formulations of small-molecule drugs.203 A nucleoside modified VEGFC mRNA delivered in lipid nanoparticles significantly increased lymphangiogenesis without blood vessel proliferation in an experimentally induced lymphedema.204 Another approach is to use drug-triglyceride formulations that undergo hydrolysis into a drug-monoglyceride, which is then assembled into a lipoprotein that enters the mesenteric lymph. The approach was used for a celecoxib prodrug that linked celecoxib to a glyceride backbone and enhanced drug transport into mesenteric lymph and concentrated celecoxib at a site of lymph leakage.58 Compared with nontargeted celecoxib, lymph-targeted celecoxib prodrug was more effective in reversing mesenteric lymphatic vessel branching and lymph leakage, reduced visceral obesity and inflammation, and restored glycemic control in obese mice. Nanomaterial-based strategy can be more narrowly directed to target the lymphatic endothelium when the carrier is conjugated with agents targeting expressed receptors, such as LYVE-1.205
Perspectives
New evidence indicates that kidney injury causes intestinal lymphangiogenesis, disrupted lymphatic contractility, altered composition of lymph, and activated LECs lining these vessels. These observations underscore the distinct qualities of the lymphatic pathway including the capacity to take up and convey large bioactive metabolites, modified lipoproteins, and activated immune cells generated within the intestinal lumen and wall and disperse them throughout the body, contributing to the adverse consequences associated with chronic kidney disease. Therefore, we propose a novel paradigm of disease progression in which a cycle of detrimental organ cross talk, fueled by kidney injury–induced modulation of intestinal lymphatics, contributes to systemic organ disease progression, which then perpetuates intestinal anomalies (Figure 3). An additional implication of the intestinal-lymphatic axis is its emergence as a novel avenue for drug delivery in the treatment of the serious clinical complications associated with kidney disease. Specifically, the intestinal lymphatic network may provide new drug targets and added advantage by bypassing liver-mediated degradation before delivery. Thus, in addition to specific treatments for kidney disease, future therapies may also target the absorptive capacity, contractile dynamics, and clearance competence of the intestinal lymphatics that could lessen the associated adverse consequences.
Article Information
Sources of Funding
This work was supported by NIH 1P01HL116263 to V. Kon; K01HL130497, R03HL155041, and R01HL144941 to A. Kirabo; and R01HD099777 to E.L. Shelton.
Disclosures
None.
Nonstandard Abbreviations and Acronyms
- apoAI
- apolipoprotein AI
- CVD
- cardiovascular disease
- HDL
- high-density lipoprotein
- IL
- interleukin
- IsoLG
- isolevuglandin
- LDL
- low-density lipoprotein
- LEC
- lymphatic endothelial cell
- LSMC
- lymphatic smooth muscle cell
- NKCC
- Na+-K+-2CI− cotransporter
- S1P
- sphingosine-1-phosphate
- SPHK
- sphingosine kinase
- STD
- small transient depolarization
- VEGFC
- vascular endothelial growth factor C
- VEGFR2
- vascular endothelial growth factor receptor 2
- VEGFR3
- vascular endothelial growth factor receptor 3
For Sources of Funding and Disclosures, see page 1240.
References
- 1.Wang AA, Cai X, Srivastava A, Prasad PV, Sprague SM, Carr J, Wolf M, Ix JH, Block GA, Chonchol M, et al. Abnormalities in cardiac structure and function among individuals with CKD: the COMBINE trial. Kidney360. 2022;3:258–268. doi: 10.34067/KID.0005022021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsushita K, Coresh J, Sang Y, Chalmers J, Fox C, Guallar E, Jafar T, Jassal SK, Landman GW, Muntner P, et al. ; CKD Prognosis Consortium. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3:514–525. doi: 10.1016/S2213-8587(15)00040-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhong J, Yang HC, Yermalitsky V, Shelton EL, Otsuka T, Wiese CB, May-Zhang LS, Banan B, Abumrad N, Huang J, et al. Kidney injury-mediated disruption of intestinal lymphatics involves dicarbonyl-modified lipoproteins. Kidney Int. 2021;100:585–596. doi: 10.1016/j.kint.2021.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lohia S, Vlahou A, Zoidakis J. Microbiome in chronic kidney disease (CKD): an omics perspective. Toxins (Basel). 2022;14:176. doi: 10.3390/toxins14030176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J, Shelton EL, Crescenzi R, Colvin DC, Kirabo A, Zhong J, Delpire EJ, Yang HC, Kon V. Kidney injury causes accumulation of renal sodium that modulates renal lymphatic dynamics. Int J Mol Sci. 2022;23:1428. doi: 10.3390/ijms23031428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kon V, Shelton EL, Pitzer A, Yang HC, Kirabo A. Inflammation, lymphatics, and cardiovascular disease: amplification by chronic kidney disease. Curr Hypertens Rep. 2022;24:455–463. doi: 10.1007/s11906-022-01206-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruddle NH, Akirav EM. Secondary lymphoid organs: responding to genetic and environmental cues in ontogeny and the immune response. J Immunol. 2009;183:2205–2212. doi: 10.4049/jimmunol.0804324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bery AI, Shepherd HM, Li W, Krupnick AS, Gelman AE, Kreisel D. Role of tertiary lymphoid organs in the regulation of immune responses in the periphery. Cell Mol Life Sci. 2022;79:359. doi: 10.1007/s00018-022-04388-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breslin JW, Yang Y, Scallan JP, Sweat RS, Adderley SP, Murfee WL. Lymphatic vessel network structure and physiology. Compr Physiol. 2018;9:207–299. doi: 10.1002/cphy.c180015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clement CC, Wang W, Dzieciatkowska M, Cortese M, Hansen KC, Becerra A, Thangaswamy S, Nizamutdinova I, Moon JY, Stern LJ, et al. Quantitative profiling of the lymph node clearance capacity. Sci Rep. 2018;8:11253. doi: 10.1038/s41598-018-29614-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler MG, Isogai S, Weinstein BM. Lymphatic development. Birth Defects Res C Embryo Today. 2009;87:222–231. doi: 10.1002/bdrc.20155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klotz L, Norman S, Vieira JM, Masters M, Rohling M, Dube KN, Bollini S, Matsuzaki F, Carr CA, Riley PR. Cardiac lymphatics are heterogeneous in origin and respond to injury. Nature. 2015;522:62–67. doi: 10.1038/nature14483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez-Corral I, Ulvmar MH, Stanczuk L, Tatin F, Kizhatil K, John SW, Alitalo K, Ortega S, Makinen T. Nonvenous origin of dermal lymphatic vasculature. Circ Res. 2015;116:1649–1654. doi: 10.1161/CIRCRESAHA.116.306170 [DOI] [PubMed] [Google Scholar]
- 14.Stanczuk L, Martinez-Corral I, Ulvmar MH, Zhang Y, Lavina B, Fruttiger M, Adams RH, Saur D, Betsholtz C, Ortega S, et al. cKit lineage hemogenic endothelium-derived cells contribute to mesenteric lymphatic vessels. Cell Rep. 2015;10:1708–1721. doi: 10.1016/j.celrep.2015.02.026 [DOI] [PubMed] [Google Scholar]
- 15.Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5:74–80. doi: 10.1038/ni1013 [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Zhou F, Han W, Shen B, Luo J, Shibuya M, He Y. VEGFR-3 ligand-binding and kinase activity are required for lymphangiogenesis but not for angiogenesis. Cell Res. 2010;20:1319–1331. doi: 10.1038/cr.2010.116 [DOI] [PubMed] [Google Scholar]
- 17.Secker GA, Harvey NL. Regulation of VEGFR signalling in lymphatic vascular development and disease: an update. Int J Mol Sci. 2021;22:7760. doi: 10.3390/ijms22147760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abhinand CS, Raju R, Soumya SJ, Arya PS, Sudhakaran PR. VEGF-A/VEGFR2 signaling network in endothelial cells relevant to angiogenesis. J Cell Commun Signal. 2016;10:347–354. doi: 10.1007/s12079-016-0352-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baluk P, McDonald DM. Buttons and zippers: endothelial junctions in lymphatic vessels. Cold Spring Harb Perspect Med. 2022;12:a041178. doi: 10.1101/cshperspect.a041178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.den Braanker H, van Stigt AC, Kok MR, Lubberts E, Bisoendial RJ. Single-cell RNA sequencing reveals heterogeneity and functional diversity of lymphatic endothelial cells. Int J Mol Sci. 2021;22:11976. doi: 10.3390/ijms222111976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeda A, Hollmen M, Dermadi D, Pan J, Brulois KF, Kaukonen R, Lonnberg T, Bostrom P, Koskivuo I, Irjala H, et al. Single-cell survey of human lymphatics unveils marked endothelial cell heterogeneity and mechanisms of homing for neutrophils. Immunity. 2019;51:561–572.e5. doi: 10.1016/j.immuni.2019.06.027 [DOI] [PubMed] [Google Scholar]
- 22.Sabine A, Agalarov Y, Maby-El Hajjami H, Jaquet M, Hagerling R, Pollmann C, Bebber D, Pfenniger A, Miura N, Dormond O, et al. Mechanotransduction, PROX1, and FOXC2 cooperate to control connexin37 and calcineurin during lymphatic-valve formation. Dev Cell. 2012;22:430–445. doi: 10.1016/j.devcel.2011.12.020 [DOI] [PubMed] [Google Scholar]
- 23.Solari E, Marcozzi C, Negrini D, Moriondo A. Lymphatic vessels and their surroundings: how local physical factors affect lymph flow. Biology (Basel). 2020;9:463. doi: 10.3390/biology9120463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zweifach BW, Prather JW. Micromanipulation of pressure in terminal lymphatics in the mesentery. Am J Physiol. 1975;228:1326–1335. doi: 10.1152/ajplegacy.1975.228.5.1326 [DOI] [PubMed] [Google Scholar]
- 25.Nepiyushchikh ZV, Chakraborty S, Wang W, Davis MJ, Zawieja DC, Muthuchamy M. Differential effects of myosin light chain kinase inhibition on contractility, force development and myosin light chain 20 phosphorylation of rat cervical and thoracic duct lymphatics. J Physiol. 2011;589:5415–5429. doi: 10.1113/jphysiol.2011.218446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang W, Nepiyushchikh Z, Zawieja DC, Chakraborty S, Zawieja SD, Gashev AA, Davis MJ, Muthuchamy M. Inhibition of myosin light chain phosphorylation decreases rat mesenteric lymphatic contractile activity. Am J Physiol Heart Circ Physiol. 2009;297:H726–H734. doi: 10.1152/ajpheart.00312.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breslin JW. Mechanical forces and lymphatic transport. Microvasc Res. 2014;96:46–54. doi: 10.1016/j.mvr.2014.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von der Weid PY. Lymphatic vessel pumping. Adv Exp Med Biol. 2019;1124:357–377. doi: 10.1007/978-981-13-5895-1_15 [DOI] [PubMed] [Google Scholar]
- 29.Muthuchamy M, Gashev A, Boswell N, Dawson N, Zawieja D. Molecular and functional analyses of the contractile apparatus in lymphatic muscle. FASEB J. 2003;17:920–922. doi: 10.1096/fj.02-0626fje [DOI] [PubMed] [Google Scholar]
- 30.Telinius N, Kim S, Pilegaard H, Pahle E, Nielsen J, Hjortdal V, Aalkjaer C, Boedtkjer DB. The contribution of K(+) channels to human thoracic duct contractility. Am J Physiol Heart Circ Physiol. 2014;307:H33–H43. doi: 10.1152/ajpheart.00921.2013 [DOI] [PubMed] [Google Scholar]
- 31.Toland HM, McCloskey KD, Thornbury KD, McHale NG, Hollywood MA. Ca(2+)-activated Cl(-) current in sheep lymphatic smooth muscle. Am J Physiol Cell Physiol. 2000;279:C1327–C1335. doi: 10.1152/ajpcell.2000.279.5.C1327 [DOI] [PubMed] [Google Scholar]
- 32.von der Weid PY. ATP-sensitive K+ channels in smooth muscle cells of guinea-pig mesenteric lymphatics: role in nitric oxide and beta-adrenoceptor agonist-induced hyperpolarizations. Br J Pharmacol. 1998;125:17–22. doi: 10.1038/sj.bjp.0702026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee Y, Zawieja SD, Muthuchamy M. Lymphatic collecting vessel: new perspectives on mechanisms of contractile regulation and potential lymphatic contractile pathways to target in obesity and metabolic diseases. Front Pharmacol. 2022;13:848088. doi: 10.3389/fphar.2022.848088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shelton EL, Yang HC, Zhong J, Salzman MM, Kon V. Renal lymphatic vessel dynamics. Am J Physiol Renal Physiol. 2020;319:F1027–F1036. doi: 10.1152/ajprenal.00322.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von der Weid PY, Lee S, Imtiaz MS, Zawieja DC, Davis MJ. Electrophysiological properties of rat mesenteric lymphatic vessels and their regulation by stretch. Lymphat Res Biol. 2014;12:66–75. doi: 10.1089/lrb.2013.0045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawai Y, Yokoyama Y, Kaidoh M, Ohhashi T. Shear stress-induced ATP-mediated endothelial constitutive nitric oxide synthase expression in human lymphatic endothelial cells. Am J Physiol Cell Physiol. 2010;298:C647–C655. doi: 10.1152/ajpcell.00249.2009 [DOI] [PubMed] [Google Scholar]
- 37.Solari E, Marcozzi C, Bartolini B, Viola M, Negrini D, Moriondo A. Acute exposure of collecting lymphatic vessels to low-density lipoproteins increases both contraction frequency and lymph flow: an in vivo mechanical insight. Lymphat Res Biol. 2020;18:146–155. doi: 10.1089/lrb.2019.0040 [DOI] [PubMed] [Google Scholar]
- 38.Bernier-Latmani J, Petrova TV. Intestinal lymphatic vasculature: structure, mechanisms and functions. Nat Rev Gastroenterol Hepatol. 2017;14:510–526. doi: 10.1038/nrgastro.2017.79 [DOI] [PubMed] [Google Scholar]
- 39.Unthank JL, Bohlen HG. Lymphatic pathways and role of valves in lymph propulsion from small intestine. Am J Physiol. 1988;254:G389–G398. doi: 10.1152/ajpgi.1988.254.3.G389 [DOI] [PubMed] [Google Scholar]
- 40.Trevaskis NL, Kaminskas LM, Porter CJ. From sewer to saviour - targeting the lymphatic system to promote drug exposure and activity. Nat Rev Drug Discov. 2015;14:781–803. doi: 10.1038/nrd4608 [DOI] [PubMed] [Google Scholar]
- 41.Cifarelli V, Eichmann A. The intestinal lymphatic system: functions and metabolic implications. Cell Mol Gastroenterol Hepatol. 2018;7:503–513. doi: 10.1016/j.jcmgh.2018.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choe K, Jang JY, Park I, Kim Y, Ahn S, Park DY, Hong YK, Alitalo K, Koh GY, Kim P. Intravital imaging of intestinal lacteals unveils lipid drainage through contractility. J Clin Invest. 2015;125:4042–4052. doi: 10.1172/JCI76509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kassis T, Yarlagadda SC, Kohan AB, Tso P, Breedveld V, Dixon JB. Postprandial lymphatic pump function after a high-fat meal: a characterization of contractility, flow, and viscosity. Am J Physiol Gastrointest Liver Physiol. 2016;310:G776–G789. doi: 10.1152/ajpgi.00318.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dash S, Xiao C, Morgantini C, Lewis GF. New insights into the regulation of chylomicron production. Annu Rev Nutr. 2015;35:265–294. doi: 10.1146/annurev-nutr-071714-034338 [DOI] [PubMed] [Google Scholar]
- 45.Dobbins WO, 3rd. Intestinal mucosal lacteal in transport of macromolecules and chylomicrons. Am J Clin Nutr. 1971;24:77–90. doi: 10.1093/ajcn/24.1.77 [DOI] [PubMed] [Google Scholar]
- 46.Dixon JB. Mechanisms of chylomicron uptake into lacteals. Ann N Y Acad Sci. 2010;1207:E52–E57. doi: 10.1111/j.1749-6632.2010.05716.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiong Y, Piao W, Brinkman CC, Li L, Kulinski JM, Olivera A, Cartier A, Hla T, Hippen KL, Blazar BR, et al. CD4 T cell sphingosine 1-phosphate receptor (S1PR)1 and S1PR4 and endothelial S1PR2 regulate afferent lymphatic migration. Sci Immunol. 2019;4:eaav1263. doi: 10.1126/sciimmunol.aav1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tso P, Balint JA. Formation and transport of chylomicrons by enterocytes to the lymphatics. Am J Physiol. 1986;250:G715–G726. doi: 10.1152/ajpgi.1986.250.6.G715 [DOI] [PubMed] [Google Scholar]
- 49.Jang JY, Koh YJ, Lee SH, Lee J, Kim KH, Kim D, Koh GY, Yoo OJ. Conditional ablation of LYVE-1+ cells unveils defensive roles of lymphatic vessels in intestine and lymph nodes. Blood. 2013;122:2151–2161. doi: 10.1182/blood-2013-01-478941 [DOI] [PubMed] [Google Scholar]
- 50.Ahlawat S, Asha , Sharma KK. Gut-organ axis: a microbial outreach and networking. Lett Appl Microbiol. 2021;72:636–668. doi: 10.1111/lam.13333 [DOI] [PubMed] [Google Scholar]
- 51.Witkowski M, Weeks TL, Hazen SL. Gut microbiota and cardiovascular disease. Circ Res. 2020;127:553–570. doi: 10.1161/CIRCRESAHA.120.316242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oliver G, Kipnis J, Randolph GJ, Harvey NL. The lymphatic vasculature in the 21(st) century: novel functional roles in homeostasis and disease. Cell. 2020;182:270–296. doi: 10.1016/j.cell.2020.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cifarelli V, Appak-Baskoy S, Peche VS, Kluzak A, Shew T, Narendran R, Pietka KM, Cella M, Walls CW, Czepielewski R, et al. Visceral obesity and insulin resistance associate with CD36 deletion in lymphatic endothelial cells. Nat Commun. 2021;12:3350. doi: 10.1038/s41467-021-23808-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singla B, Aithabathula RV, Kiran S, Kapil S, Kumar S, Singh UP. Reactive oxygen species in regulating lymphangiogenesis and lymphatic function. Cells. 2022;11:1750. doi: 10.3390/cells11111750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.D’Alessio S, Correale C, Tacconi C, Gandelli A, Pietrogrande G, Vetrano S, Genua M, Arena V, Spinelli A, Peyrin-Biroulet L, et al. VEGF-C-dependent stimulation of lymphatic function ameliorates experimental inflammatory bowel disease. J Clin Invest. 2014;124:3863–3878. doi: 10.1172/JCI72189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suh SH, Choe K, Hong SP, Jeong SH, Makinen T, Kim KS, Alitalo K, Surh CD, Koh GY, Song JH. Gut microbiota regulates lacteal integrity by inducing VEGF-C in intestinal villus macrophages. EMBO Rep. 2019;20:e46927. doi: 10.15252/embr.201846927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rehal S, Stephens M, Roizes S, Liao S, von der Weid PY. Acute small intestinal inflammation results in persistent lymphatic alterations. Am J Physiol Gastrointest Liver Physiol. 2018;314:G408–G417. doi: 10.1152/ajpgi.00340.2017 [DOI] [PubMed] [Google Scholar]
- 58.Cao E, Watt MJ, Nowell CJ, Quach T, Simpson JS, De Melo Ferreira V, Agarwal S, Chu H, Srivastava A, Anderson D, et al. Mesenteric lymphatic dysfunction promotes insulin resistance and represents a potential treatment target in obesity. Nat Metab. 2021;3:1175–1188. doi: 10.1038/s42255-021-00457-w [DOI] [PubMed] [Google Scholar]
- 59.Escobedo N, Proulx ST, Karaman S, Dillard ME, Johnson N, Detmar M, Oliver G. Restoration of lymphatic function rescues obesity in Prox1-haploinsufficient mice. JCI Insight. 2016;1:e85096. doi: 10.1172/jci.insight.85096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang F, Zarkada G, Han J, Li J, Dubrac A, Ola R, Genet G, Boye K, Michon P, Kunzel SE, et al. Lacteal junction zippering protects against diet-induced obesity. Science. 2018;361:599–603. doi: 10.1126/science.aap9331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zawieja SD, Wang W, Chakraborty S, Zawieja DC, Muthuchamy M. Macrophage alterations within the mesenteric lymphatic tissue are associated with impairment of lymphatic pump in metabolic syndrome. Microcirculation. 2016;23:558–570. doi: 10.1111/micc.12307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zawieja SD, Wang W, Wu X, Nepiyushchikh ZV, Zawieja DC, Muthuchamy M. Impairments in the intrinsic contractility of mesenteric collecting lymphatics in a rat model of metabolic syndrome. Am J Physiol Heart Circ Physiol. 2012;302:H643–H653. doi: 10.1152/ajpheart.00606.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scallan JP, Hill MA, Davis MJ. Lymphatic vascular integrity is disrupted in type 2 diabetes due to impaired nitric oxide signalling. Cardiovasc Res. 2015;107:89–97. doi: 10.1093/cvr/cvv117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Y, Zhang S, Tsui N. Mesenteric lymph duct drainage attenuates acute lung injury in rats with severe intraperitoneal infection. Inflammation. 2015;38:1239–1249. doi: 10.1007/s10753-014-0091-z [DOI] [PubMed] [Google Scholar]
- 65.Kumar R, Kumar T, Anand U, Priyadarshi RN. Intestinal lymphangiectasia associated with refractory ascites in a cirrhosis patient. Cureus. 2021;13:e12567. doi: 10.7759/cureus.12567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ribera J, Pauta M, Melgar-Lesmes P, Tugues S, Fernandez-Varo G, Held KF, Soria G, Tudela R, Planas AM, Fernandez-Hernando C, et al. Increased nitric oxide production in lymphatic endothelial cells causes impairment of lymphatic drainage in cirrhotic rats. Gut. 2013;62:138–145. doi: 10.1136/gutjnl-2011-300703 [DOI] [PubMed] [Google Scholar]
- 67.Davidson MT, Deitch EA, Lu Q, Osband A, Feketeova E, Nemeth ZH, Hasko G, Xu DZ. A study of the biologic activity of trauma-hemorrhagic shock mesenteric lymph over time and the relative role of cytokines. Surgery. 2004;136:32–41. doi: 10.1016/j.surg.2003.12.012 [DOI] [PubMed] [Google Scholar]
- 68.Kaiser VL, Sifri ZC, Dikdan GS, Berezina T, Zaets S, Lu Q, Xu DZ, Deitch EA. Trauma-hemorrhagic shock mesenteric lymph from rat contains a modified form of albumin that is implicated in endothelial cell toxicity. Shock. 2005;23:417–425. doi: 10.1097/01.shk.0000160524.14235.6c [DOI] [PubMed] [Google Scholar]
- 69.Jordan JR, Moore EE, Sarin EL, Damle SS, Kashuk SB, Silliman CC, Banerjee A. Arachidonic acid in postshock mesenteric lymph induces pulmonary synthesis of leukotriene B4. J Appl Physiol (1985). 2008;104:1161–1166. doi: 10.1152/japplphysiol.00022.2007 [DOI] [PubMed] [Google Scholar]
- 70.Cavriani G, Domingos HV, Soares AL, Trezena AG, Ligeiro-Oliveira AP, Oliveira-Filho RM, Sudo-Hayashi LS, Tavares de Lima W. Lymphatic system as a path underlying the spread of lung and gut injury after intestinal ischemia/reperfusion in rats. Shock. 2005;23:330–336. doi: 10.1097/01.shk.0000157303.76749.9b [DOI] [PubMed] [Google Scholar]
- 71.Kojima M, Gimenes-Junior JA, Langness S, Morishita K, Lavoie-Gagne O, Eliceiri B, Costantini TW, Coimbra R. Exosomes, not protein or lipids, in mesenteric lymph activate inflammation: unlocking the mystery of post-shock multiple organ failure. J Trauma Acute Care Surg. 2017;82:42–50. doi: 10.1097/TA.0000000000001296 [DOI] [PubMed] [Google Scholar]
- 72.Rosario HS, Waldo SW, Becker SA, Schmid-Schonbein GW. Pancreatic trypsin increases matrix metalloproteinase-9 accumulation and activation during acute intestinal ischemia-reperfusion in the rat. Am J Pathol. 2004;164:1707–1716. doi: 10.1016/S0002-9440(10)63729-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Breithaupt-Faloppa AC, Vitoretti LB, Coelho FR, dos Santos Franco AL, Domingos HV, Sudo-Hayashi LS, Oliveira-Filho RM, Tavares de Lima W. Nitric oxide mediates lung vascular permeability and lymph-borne IL-6 after an intestinal ischemic insult. Shock. 2009;32:55–61. doi: 10.1097/SHK.0b013e31818bb7a1 [DOI] [PubMed] [Google Scholar]
- 74.Wu TF, Carati CJ, Macnaughton WK, von der Weid PY. Contractile activity of lymphatic vessels is altered in the TNBS model of guinea pig ileitis. Am J Physiol Gastrointest Liver Physiol. 2006;291:G566–G574. doi: 10.1152/ajpgi.00058.2006 [DOI] [PubMed] [Google Scholar]
- 75.Zhang L, Ocansey DKW, Liu L, Olovo CV, Zhang X, Qian H, Xu W, Mao F. Implications of lymphatic alterations in the pathogenesis and treatment of inflammatory bowel disease. Biomed Pharmacother. 2021;140:111752. doi: 10.1016/j.biopha.2021.111752 [DOI] [PubMed] [Google Scholar]
- 76.Vetrano S, Borroni EM, Sarukhan A, Savino B, Bonecchi R, Correale C, Arena V, Fantini M, Roncalli M, Malesci A, et al. The lymphatic system controls intestinal inflammation and inflammation-associated colon cancer through the chemokine decoy receptor D6. Gut. 2010;59:197–206. doi: 10.1136/gut.2009.183772 [DOI] [PubMed] [Google Scholar]
- 77.Ge Y, Li Y, Gong J, Zhu W. Mesenteric organ lymphatics and inflammatory bowel disease. Ann Anat. 2018;218:199–204. doi: 10.1016/j.aanat.2018.03.006 [DOI] [PubMed] [Google Scholar]
- 78.Gracia G, Cao E, Johnston APR, Porter CJH, Trevaskis NL. Organ-specific lymphatics play distinct roles in regulating HDL trafficking and composition. Am J Physiol Gastrointest Liver Physiol. 2020;318:G725–G735. doi: 10.1152/ajpgi.00340.2019 [DOI] [PubMed] [Google Scholar]
- 79.Yang J, Lim SY, Ko YS, Lee HY, Oh SW, Kim MG, Cho WY, Jo SK. Intestinal barrier disruption and dysregulated mucosal immunity contribute to kidney fibrosis in chronic kidney disease. Nephrol Dial Transplant. 2019;34:419–428. doi: 10.1093/ndt/gfy172 [DOI] [PubMed] [Google Scholar]
- 80.Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, Ni Z, Nguyen TH, Andersen GL. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013;83:308–315. doi: 10.1038/ki.2012.345 [DOI] [PubMed] [Google Scholar]
- 81.Vaziri ND, Goshtasbi N, Yuan J, Jellbauer S, Moradi H, Raffatellu M, Kalantar-Zadeh K. Uremic plasma impairs barrier function and depletes the tight junction protein constituents of intestinal epithelium. Am J Nephrol. 2012;36:438–443. doi: 10.1159/000343886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vaziri ND, Yuan J, Norris K. Role of urea in intestinal barrier dysfunction and disruption of epithelial tight junction in chronic kidney disease. Am J Nephrol. 2013;37:1–6. doi: 10.1159/000345969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Al-Sadi R, Boivin M, Ma T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front Biosci (Landmark Ed). 2009;14:2765–2778. doi: 10.2741/3413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wilkins LJ, Monga M, Miller AW. Defining dysbiosis for a cluster of chronic diseases. Sci Rep. 2019;9:12918. doi: 10.1038/s41598-019-49452-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jazani NH, Savoj J, Lustgarten M, Lau WL, Vaziri ND. Impact of gut dysbiosis on neurohormonal pathways in chronic kidney disease. Diseases. 2019;7:21. doi: 10.3390/diseases7010021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sircana A, De Michieli F, Parente R, Framarin L, Leone N, Berrutti M, Paschetta E, Bongiovanni D, Musso G. Gut microbiota, hypertension and chronic kidney disease: recent advances. Pharmacol Res. 2019;144:390–408. doi: 10.1016/j.phrs.2018.01.013 [DOI] [PubMed] [Google Scholar]
- 87.Castillo-Rodriguez E, Fernandez-Prado R, Esteras R, Perez-Gomez MV, Gracia-Iguacel C, Fernandez-Fernandez B, Kanbay M, Tejedor A, Lazaro A, Ruiz-Ortega M, et al. Impact of altered intestinal microbiota on chronic kidney disease progression. Toxins (Basel). 2018;10:300. doi: 10.3390/toxins10070300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Salguero MV, Al-Obaide MAI, Singh R, Siepmann T, Vasylyeva TL. Dysbiosis of Gram-negative gut microbiota and the associated serum lipopolysaccharide exacerbates inflammation in type 2 diabetic patients with chronic kidney disease. Exp Ther Med. 2019;18:3461–3469. doi: 10.3892/etm.2019.7943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mafra D, Fouque D. Gut microbiota and inflammation in chronic kidney disease patients. Clin Kidney J. 2015;8:332–334. doi: 10.1093/ckj/sfv026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vangay P, Ward T, Gerber JS, Knights D. Antibiotics, pediatric dysbiosis, and disease. Cell Host Microbe. 2015;17:553–564. doi: 10.1016/j.chom.2015.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rukavina Mikusic NL, Kouyoumdzian NM, Choi MR. Gut microbiota and chronic kidney disease: evidences and mechanisms that mediate a new communication in the gastrointestinal-renal axis. Pflugers Arch. 2020;472:303–320. doi: 10.1007/s00424-020-02352-x [DOI] [PubMed] [Google Scholar]
- 92.Ocansey DKW, Pei B, Xu X, Zhang L, Olovo CV, Mao F. Cellular and molecular mediators of lymphangiogenesis in inflammatory bowel disease. J Transl Med. 2021;19:254. doi: 10.1186/s12967-021-02922-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Magold AI, Swartz MA. Pathogenic exploitation of lymphatic vessels. Cells. 2022;11:979. doi: 10.3390/cells11060979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim KE, Sung HK, Koh GY. Lymphatic development in mouse small intestine. Dev Dyn. 2007;236:2020–2025. doi: 10.1002/dvdy.21200 [DOI] [PubMed] [Google Scholar]
- 95.Bernier-Latmani J, Cisarovsky C, Demir CS, Bruand M, Jaquet M, Davanture S, Ragusa S, Siegert S, Dormond O, Benedito R, et al. DLL4 promotes continuous adult intestinal lacteal regeneration and dietary fat transport. J Clin Invest. 2015;125:4572–4586. doi: 10.1172/JCI82045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nurmi H, Saharinen P, Zarkada G, Zheng W, Robciuc MR, Alitalo K. VEGF-C is required for intestinal lymphatic vessel maintenance and lipid absorption. EMBO Mol Med. 2015;7:1418–1425. doi: 10.15252/emmm.201505731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schulte-Merker S, Sabine A, Petrova TV. Lymphatic vascular morphogenesis in development, physiology, and disease. J Cell Biol. 2011;193:607–618. doi: 10.1083/jcb.201012094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang Y, Zhang Y, Cao Z, Ji H, Yang X, Iwamoto H, Wahlberg E, Lanne T, Sun B, Cao Y. Anti-VEGF- and anti-VEGF receptor-induced vascular alteration in mouse healthy tissues. Proc Natl Acad Sci USA. 2013;110:12018–12023. doi: 10.1073/pnas.1301331110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang X, Zhao J, Qin L. VEGF-C mediated enhancement of lymphatic drainage reduces intestinal inflammation by regulating IL-9/IL-17 balance and improving gut microbiota in experimental chronic colitis. Am J Transl Res. 2017;9:4772–4784. [PMC free article] [PubMed] [Google Scholar]
- 100.Becker F, Gavins FNE, Fontenot J, Jordan P, Yun JY, Scott R, Polk PR, Friday RE, Boktor M, Musso M, et al. Dynamic gut microbiome changes following regional intestinal lymphatic obstruction in primates. Pathophysiology. 2019;26:253–261. doi: 10.1016/j.pathophys.2019.06.004 [DOI] [PubMed] [Google Scholar]
- 101.Vaziri ND. HDL abnormalities in nephrotic syndrome and chronic kidney disease. Nat Rev Nephrol. 2016;12:37–47. doi: 10.1038/nrneph.2015.180 [DOI] [PubMed] [Google Scholar]
- 102.Marsche G, Heine GH, Stadler JT, Holzer M. Current understanding of the relationship of HDL composition, structure and function to their cardioprotective properties in chronic kidney disease. Biomolecules. 2020;10:1348. doi: 10.3390/biom10091348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vaziri ND. Molecular mechanisms of lipid disorders in nephrotic syndrome. Kidney Int. 2003;63:1964–1976. doi: 10.1046/j.1523-1755.2003.00941.x [DOI] [PubMed] [Google Scholar]
- 104.Moradi H, Vaziri ND. Molecular mechanisms of disorders of lipid metabolism in chronic kidney disease. Front Biosci (Landmark Ed). 2018;23:146–161. doi: 10.2741/4585 [DOI] [PubMed] [Google Scholar]
- 105.Goldberg IJ. Lipoprotein metabolism in normal and uremic patients. Am J Kidney Dis. 1993;21:87–90. doi: 10.1016/s0272-6386(12)80728-1 [DOI] [PubMed] [Google Scholar]
- 106.Li M, Ellis E, Johansson H, Nowak G, Isaksson B, Gnocchi D, Parini P, Axelsson J. Changes in gluconeogenesis and intracellular lipid accumulation characterize uremic human hepatocytes ex vivo. Am J Physiol Gastrointest Liver Physiol. 2016;310:G952–G961. doi: 10.1152/ajpgi.00379.2015 [DOI] [PubMed] [Google Scholar]
- 107.Chapagain A, Caton PW, Kieswich J, Andrikopoulos P, Nayuni N, Long JH, Harwood SM, Webster SP, Raftery MJ, Thiemermann C, et al. Elevated hepatic 11beta-hydroxysteroid dehydrogenase type 1 induces insulin resistance in uremia. Proc Natl Acad Sci USA. 2014;111:3817–3822. doi: 10.1073/pnas.1312436111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Majima M, Hosono K, Ito Y, Amano H. Biologically active lipids in the regulation of lymphangiogenesis in disease states. Pharmacol Ther. 2022;232:108011. doi: 10.1016/j.pharmthera.2021.108011 [DOI] [PubMed] [Google Scholar]
- 109.Singla B, Lin HP, Ahn W, White J, Csanyi G. Oxidatively modified LDL suppresses lymphangiogenesis via CD36 signaling. Antioxidants (Basel). 2021;10:331. doi: 10.3390/antiox10020331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Burchill MA, Finlon JM, Goldberg AR, Gillen AE, Dahms PA, McMahan RH, Tye A, Winter AB, Reisz JA, Bohrnsen E, et al. Oxidized low-density lipoprotein drives dysfunction of the liver lymphatic system. Cell Mol Gastroenterol Hepatol. 2021;11:573–595. doi: 10.1016/j.jcmgh.2020.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schoors S, Bruning U, Missiaen R, Queiroz KC, Borgers G, Elia I, Zecchin A, Cantelmo AR, Christen S, Goveia J, et al. Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature. 2015;520:192–197. doi: 10.1038/nature14362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wong BW, Wang X, Zecchin A, Thienpont B, Cornelissen I, Kalucka J, Garcia-Caballero M, Missiaen R, Huang H, Bruning U, et al. The role of fatty acid beta-oxidation in lymphangiogenesis. Nature. 2017;542:49–54. doi: 10.1038/nature21028 [DOI] [PubMed] [Google Scholar]
- 113.Jung JI, Cho HJ, Jung YJ, Kwon SH, Her S, Choi SS, Shin SH, Lee KW, Park JH. High-fat diet-induced obesity increases lymphangiogenesis and lymph node metastasis in the B16F10 melanoma allograft model: roles of adipocytes and M2-macrophages. Int J Cancer. 2015;136:258–270. doi: 10.1002/ijc.28983 [DOI] [PubMed] [Google Scholar]
- 114.Boschi F, Rizzatti V, Zamboni M, Sbarbati A. Models of lipid droplets growth and fission in adipocyte cells. Exp Cell Res. 2015;336:253–262. doi: 10.1016/j.yexcr.2015.06.001 [DOI] [PubMed] [Google Scholar]
- 115.Cruz ALS, Barreto EA, Fazolini NPB, Viola JPB, Bozza PT. Lipid droplets: platforms with multiple functions in cancer hallmarks. Cell Death Dis. 2020;11:105. doi: 10.1038/s41419-020-2297-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.den Brok MH, Raaijmakers TK, Collado-Camps E, Adema GJ. Lipid droplets as immune modulators in myeloid cells. Trends Immunol. 2018;39:380–392. doi: 10.1016/j.it.2018.01.012 [DOI] [PubMed] [Google Scholar]
- 117.Mece O, Houbaert D, Sassano ML, Durre T, Maes H, Schaaf M, More S, Ganne M, Garcia-Caballero M, Borri M, et al. Lipid droplet degradation by autophagy connects mitochondria metabolism to Prox1-driven expression of lymphatic genes and lymphangiogenesis. Nat Commun. 2022;13:2760. doi: 10.1038/s41467-022-30490-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xu L, Kanasaki M, He J, Kitada M, Nagao K, Jinzu H, Noguchi Y, Maegawa H, Kanasaki K, Koya D. Ketogenic essential amino acids replacement diet ameliorated hepatosteatosis with altering autophagy-associated molecules. Biochim Biophys Acta. 2013;1832:1605–1612. doi: 10.1016/j.bbadis.2013.05.003 [DOI] [PubMed] [Google Scholar]
- 119.Garcia-Caballero M, Zecchin A, Souffreau J, Truong AK, Teuwen LA, Vermaelen W, Martin-Perez R, de Zeeuw P, Bouche A, Vinckier S, et al. Role and therapeutic potential of dietary ketone bodies in lymph vessel growth. Nat Metab. 2019;1:666–675. doi: 10.1038/s42255-019-0087-y [DOI] [PubMed] [Google Scholar]
- 120.Perez-Marti A, Ramakrishnan S, Li J, Dugourd A, Molenaar MR, De La Motte LR, Grand K, Mansouri A, Parisot M, Lienkamp SS, et al. Reducing lipid bilayer stress by monounsaturated fatty acids protects renal proximal tubules in diabetes. Elife. 2022;11:e74391. doi: 10.7554/eLife.74391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tao H, Huang J, Yancey PG, Yermalitsky V, Blakemore JL, Zhang Y, Ding L, Zagol-Ikapitte I, Ye F, Amarnath V, et al. Scavenging of reactive dicarbonyls with 2-hydroxybenzylamine reduces atherosclerosis in hypercholesterolemic Ldlr(-/-) mice. Nat Commun. 2020;11:4084. doi: 10.1038/s41467-020-17915-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Boutaud O, Ou JJ, Chaurand P, Caprioli RM, Montine TJ, Oates JA. Prostaglandin H2 (PGH2) accelerates formation of amyloid beta1-42 oligomers. J Neurochem. 2002;82:1003–1006. doi: 10.1046/j.1471-4159.2002.01064.x [DOI] [PubMed] [Google Scholar]
- 123.Li W, Laird JM, Lu L, Roychowdhury S, Nagy LE, Zhou R, Crabb JW, Salomon RG. Isolevuglandins covalently modify phosphatidylethanolamines in vivo: detection and quantitative analysis of hydroxylactam adducts. Free Radic Biol Med. 2009;47:1539–1552. doi: 10.1016/j.freeradbiomed.2009.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Prinsen JK, Kannankeril PJ, Sidorova TN, Yermalitskaya LV, Boutaud O, Zagol-Ikapitte I, Barnett JV, Murphy MB, Subati T, Stark JM, et al. Highly reactive isolevuglandins promote atrial fibrillation caused by hypertension. JACC Basic Transl Sci. 2020;5:602–615. doi: 10.1016/j.jacbts.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gobert AP, Boutaud O, Asim M, Zagol-Ikapitte IA, Delgado AG, Latour YL, Finley JL, Singh K, Verriere TG, Allaman MM, et al. Dicarbonyl electrophiles mediate inflammation-induced gastrointestinal carcinogenesis. Gastroenterology. 2021;160:1256–1268.e9. doi: 10.1053/j.gastro.2020.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, Bikineyeva AT, Dikalov S, Xiao L, Chen W, et al. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest. 2014;124:4642–4656. doi: 10.1172/JCI74084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dixon KB, Davies SS, Kirabo A. Dendritic cells and isolevuglandins in immunity, inflammation, and hypertension. Am J Physiol Heart Circ Physiol. 2017;312:H368–H374. doi: 10.1152/ajpheart.00603.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Krishnan J, de la Visitacion N, Hennen EM, Amarnath V, Harrison DG, Patrick DM. IsoLGs (isolevuglandins) drive neutrophil migration in hypertension and are essential for the formation of neutrophil extracellular traps. Hypertension. 2022;79:1644–1655. doi: 10.1161/HYPERTENSIONAHA.122.19305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Brunham LR, Kruit JK, Iqbal J, Fievet C, Timmins JM, Pape TD, Coburn BA, Bissada N, Staels B, Groen AK, et al. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J Clin Invest. 2006;116:1052–1062. doi: 10.1172/JCI27352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Forester GP, Tall AR, Bisgaier CL, Glickman RM. Rat intestine secretes spherical high density lipoproteins. J Biol Chem. 1983;258:5938–5943. [PubMed] [Google Scholar]
- 131.Zhong J, Yang HC, Shelton EL, Matsusaka T, Clark AJ, Yermalitsky V, Mashhadi Z, May-Zhang LS, Linton MF, Fogo AB, et al. Dicarbonyl-modified lipoproteins contribute to proteinuric kidney injury. JCI Insight. 2022;7:e161878. doi: 10.1172/jci.insight.161878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Miura S, Sekizuka E, Nagata H, Oshio C, Minamitani H, Suematsu M, Suzuki M, Hamada Y, Kobayashi K, Asakura H, et al. Increased lymphocyte transport by lipid absorption in rat mesenteric lymphatics. Am J Physiol. 1987;253:G596–G600. doi: 10.1152/ajpgi.1987.253.5.G596 [DOI] [PubMed] [Google Scholar]
- 133.Matsuda H, Hosono K, Tsuru S, Kurashige C, Sekiguchi K, Akira S, Uematsu S, Okamoto H, Majima M. Roles of mPGES-1, an inducible prostaglandin E synthase, in enhancement of LPS-induced lymphangiogenesis in a mouse peritonitis model. Life Sci. 2015;142:1–7. doi: 10.1016/j.lfs.2015.10.008 [DOI] [PubMed] [Google Scholar]
- 134.Matsuda H, Ito Y, Hosono K, Tsuru S, Inoue T, Nakamoto S, Kurashige C, Hirashima M, Narumiya S, Okamoto H, et al. Roles of thromboxane receptor signaling in enhancement of lipopolysaccharide-induced lymphangiogenesis and lymphatic drainage function in diaphragm. Arterioscler Thromb Vasc Biol. 2021;41:1390–1407. doi: 10.1161/ATVBAHA.120.315507 [DOI] [PubMed] [Google Scholar]
- 135.Hosono K, Suzuki T, Tamaki H, Sakagami H, Hayashi I, Narumiya S, Alitalo K, Majima M. Roles of prostaglandin E2-EP3/EP4 receptor signaling in the enhancement of lymphangiogenesis during fibroblast growth factor-2-induced granulation formation. Arterioscler Thromb Vasc Biol. 2011;31:1049–1058. doi: 10.1161/ATVBAHA.110.222356 [DOI] [PubMed] [Google Scholar]
- 136.Hosono K, Isonaka R, Kawakami T, Narumiya S, Majima M. Signaling of prostaglandin E receptors, EP3 and EP4 facilitates wound healing and lymphangiogenesis with enhanced recruitment of M2 macrophages in mice. PLoS One. 2016;11:e0162532. doi: 10.1371/journal.pone.0162532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tian W, Rockson SG, Jiang X, Kim J, Begaye A, Shuffle EM, Tu AB, Cribb M, Nepiyushchikh Z, Feroze AH, et al. Leukotriene B4 antagonism ameliorates experimental lymphedema. Sci Transl Med. 2017;9:eaal3920. doi: 10.1126/scitranslmed.aal3920 [DOI] [PubMed] [Google Scholar]
- 138.Murtomaki A, Uh MK, Kitajewski C, Zhao J, Nagasaki T, Shawber CJ, Kitajewski J. Notch signaling functions in lymphatic valve formation. Development. 2014;141:2446–2451. doi: 10.1242/dev.101188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mu H, Calderone TL, Davies MA, Prieto VG, Wang H, Mills GB, Bar-Eli M, Gershenwald JE. Lysophosphatidic acid induces lymphangiogenesis and IL-8 production in vitro in human lymphatic endothelial cells. Am J Pathol. 2012;180:2170–2181. doi: 10.1016/j.ajpath.2012.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lee SJ, Chan TH, Chen TC, Liao BK, Hwang PP, Lee H. LPA1 is essential for lymphatic vessel development in zebrafish. FASEB J. 2008;22:3706–3715. doi: 10.1096/fj.08-106088 [DOI] [PubMed] [Google Scholar]
- 141.Mukhopadhyay P, Ramanathan R, Takabe K. S1P promotes breast cancer progression by angiogenesis and lymphangiogenesis. Breast Cancer Manag. 2015;4:241–244. doi: 10.2217/bmt.15.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nagahashi M, Yamada A, Aoyagi T, Allegood J, Wakai T, Spiegel S, Takabe K. Sphingosine-1-phosphate in the lymphatic fluid determined by novel methods. Heliyon. 2016;2:e00219. doi: 10.1016/j.heliyon.2016.e00219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pham TH, Baluk P, Xu Y, Grigorova I, Bankovich AJ, Pappu R, Coughlin SR, McDonald DM, Schwab SR, Cyster JG. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J Exp Med. 2010;207:17–27. doi: 10.1084/jem.20091619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Milasan A, Dallaire F, Mayer G, Martel C. Effects of LDL receptor modulation on lymphatic function. Sci Rep. 2016;6:27862. doi: 10.1038/srep27862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vachon L, Smaani A, Tessier N, Jean G, Demers A, Milasan A, Ardo N, Jarry S, Villeneuve L, Alikashani A, et al. Downregulation of low-density lipoprotein receptor mRNA in lymphatic endothelial cells impairs lymphatic function through changes in intracellular lipids. Theranostics. 2022;12:1440–1458. doi: 10.7150/thno.58780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lynch KR, O’Neill GP, Liu Q, Im DS, Sawyer N, Metters KM, Coulombe N, Abramovitz M, Figueroa DJ, Zeng Z, et al. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature. 1999;399:789–793. doi: 10.1038/21658 [DOI] [PubMed] [Google Scholar]
- 147.Peters-Golden M, Henderson WR, Jr. Leukotrienes. N Engl J Med. 2007;357:1841–1854. doi: 10.1056/NEJMra071371 [DOI] [PubMed] [Google Scholar]
- 148.Nakamura K, Radhakrishnan K, Wong YM, Rockson SG. Anti-inflammatory pharmacotherapy with ketoprofen ameliorates experimental lymphatic vascular insufficiency in mice. PLoS One. 2009;4:e8380. doi: 10.1371/journal.pone.0008380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kashiwagi S, Hosono K, Suzuki T, Takeda A, Uchinuma E, Majima M. Role of COX-2 in lymphangiogenesis and restoration of lymphatic flow in secondary lymphedema. Lab Invest. 2011;91:1314–1325. doi: 10.1038/labinvest.2011.84 [DOI] [PubMed] [Google Scholar]
- 150.Tager AM, Bromley SK, Medoff BD, Islam SA, Bercury SD, Friedrich EB, Carafone AD, Gerszten RE, Luster AD. Leukotriene B4 receptor BLT1 mediates early effector T cell recruitment. Nat Immunol. 2003;4:982–990. doi: 10.1038/ni970 [DOI] [PubMed] [Google Scholar]
- 151.Sumida H, Noguchi K, Kihara Y, Abe M, Yanagida K, Hamano F, Sato S, Tamaki K, Morishita Y, Kano MR, et al. LPA4 regulates blood and lymphatic vessel formation during mouse embryogenesis. Blood. 2010;116:5060–5070. doi: 10.1182/blood-2010-03-272443 [DOI] [PubMed] [Google Scholar]
- 152.Tanaka H, Zaima N, Sasaki T, Yamamoto N, Inuzuka K, Yata T, Iwaki T, Umemura K, Sano H, Suzuki Y, et al. Lysophosphatidylcholine acyltransferase-3 expression is associated with atherosclerosis progression. J Vasc Res. 2017;54:200–208. doi: 10.1159/000473879 [DOI] [PubMed] [Google Scholar]
- 153.Bot M, de Jager SC, MacAleese L, Lagraauw HM, van Berkel TJ, Quax PH, Kuiper J, Heeren RM, Biessen EA, Bot I. Lysophosphatidic acid triggers mast cell-driven atherosclerotic plaque destabilization by increasing vascular inflammation. J Lipid Res. 2013;54:1265–1274. doi: 10.1194/jlr.M032862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rademakers T, van der Vorst EP, Daissormont IT, Otten JJ, Theodorou K, Theelen TL, Gijbels M, Anisimov A, Nurmi H, Lindeman JH, et al. Adventitial lymphatic capillary expansion impacts on plaque T cell accumulation in atherosclerosis. Sci Rep. 2017;7:45263. doi: 10.1038/srep45263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Levkau B. HDL-S1P: cardiovascular functions, disease-associated alterations, and therapeutic applications. Front Pharmacol. 2015;6:243. doi: 10.3389/fphar.2015.00243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Weichand B, Popp R, Dziumbla S, Mora J, Strack E, Elwakeel E, Frank AC, Scholich K, Pierre S, Syed SN, et al. S1PR1 on tumor-associated macrophages promotes lymphangiogenesis and metastasis via NLRP3/IL-1beta. J Exp Med. 2017;214:2695–2713. doi: 10.1084/jem.20160392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gousopoulos E, Proulx ST, Scholl J, Uecker M, Detmar M. Prominent lymphatic vessel hyperplasia with progressive dysfunction and distinct immune cell infiltration in lymphedema. Am J Pathol. 2016;186:2193–2203. doi: 10.1016/j.ajpath.2016.04.006 [DOI] [PubMed] [Google Scholar]
- 158.Becker F, Kurmaeva E, Gavins FN, Stevenson EV, Navratil AR, Jin L, Tsunoda I, Orr AW, Alexander JS, Ostanin DV. A critical role for monocytes/macrophages during intestinal inflammation-associated lymphangiogenesis. Inflamm Bowel Dis. 2016;22:1326–1345. doi: 10.1097/MIB.0000000000000731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, Park JK, Beck FX, Muller DN, Derer W, et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med. 2009;15:545–552. doi: 10.1038/nm.1960 [DOI] [PubMed] [Google Scholar]
- 160.Yazdani S, Hijmans RS, Poosti F, Dam W, Navis G, van Goor H, van den Born J. Targeting tubulointerstitial remodeling in proteinuric nephropathy in rats. Dis Model Mech. 2015;8:919–930. doi: 10.1242/dmm.018580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Baluk P, Tammela T, Ator E, Lyubynska N, Achen MG, Hicklin DJ, Jeltsch M, Petrova TV, Pytowski B, Stacker SA, et al. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest. 2005;115:247–257. doi: 10.1172/JCI22037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chauhan SK, Jin Y, Goyal S, Lee HS, Fuchsluger TA, Lee HK, Dana R. A novel pro-lymphangiogenic function for Th17/IL-17. Blood. 2011;118:4630–4634. doi: 10.1182/blood-2011-01-332049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Choi I, Lee YS, Chung HK, Choi D, Ecoiffier T, Lee HN, Kim KE, Lee S, Park EK, Maeng YS, et al. Interleukin-8 reduces post-surgical lymphedema formation by promoting lymphatic vessel regeneration. Angiogenesis. 2013;16:29–44. doi: 10.1007/s10456-012-9297-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kataru RP, Kim H, Jang C, Choi DK, Koh BI, Kim M, Gollamudi S, Kim YK, Lee SH, Koh GY. T lymphocytes negatively regulate lymph node lymphatic vessel formation. Immunity. 2011;34:96–107. doi: 10.1016/j.immuni.2010.12.016 [DOI] [PubMed] [Google Scholar]
- 165.Chaitanya GV, Franks SE, Cromer W, Wells SR, Bienkowska M, Jennings MH, Ruddell A, Ando T, Wang Y, Gu Y, et al. Differential cytokine responses in human and mouse lymphatic endothelial cells to cytokines in vitro. Lymphat Res Biol. 2010;8:155–164. doi: 10.1089/lrb.2010.0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cromer WE, Zawieja SD, Tharakan B, Childs EW, Newell MK, Zawieja DC. The effects of inflammatory cytokines on lymphatic endothelial barrier function. Angiogenesis. 2014;17:395–406. doi: 10.1007/s10456-013-9393-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Aldrich MB, Sevick-Muraca EM. Cytokines are systemic effectors of lymphatic function in acute inflammation. Cytokine. 2013;64:362–369. doi: 10.1016/j.cyto.2013.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bouta EM, Kuzin I, de Mesy Bentley K, Wood RW, Rahimi H, Ji RC, Ritchlin CT, Bottaro A, Xing L, Schwarz EM. Brief report: treatment of tumor necrosis factor-transgenic mice with anti-tumor necrosis factor restores lymphatic contractions, repairs lymphatic vessels, and may increase monocyte/macrophage egress. Arthritis Rheumatol. 2017;69:1187–1193. doi: 10.1002/art.40047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Davis MJ, Lane MM, Davis AM, Durtschi D, Zawieja DC, Muthuchamy M, Gashev AA. Modulation of lymphatic muscle contractility by the neuropeptide substance P. Am J Physiol Heart Circ Physiol. 2008;295:H587–H597. doi: 10.1152/ajpheart.01029.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ellison DH, Welling P. Insights into salt handling and blood pressure. N Engl J Med. 2021;385:1981–1993. doi: 10.1056/NEJMra2030212 [DOI] [PubMed] [Google Scholar]
- 171.Wiig H, Schroder A, Neuhofer W, Jantsch J, Kopp C, Karlsen TV, Boschmann M, Goss J, Bry M, Rakova N, et al. Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J Clin Invest. 2013;123:2803–2815. doi: 10.1172/JCI60113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rossitto G, Mary S, Chen JY, Boder P, Chew KS, Neves KB, Alves RL, Montezano AC, Welsh P, Petrie MC, et al. Tissue sodium excess is not hypertonic and reflects extracellular volume expansion. Nat Commun. 2020;11:4222. doi: 10.1038/s41467-020-17820-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Thowsen IM, Reikvam T, Skogstrand T, Samuelsson AM, Muller DN, Tenstad O, Alitalo K, Karlsen T, Wiig H. Genetic engineering of lymphangiogenesis in skin does not affect blood pressure in mouse models of salt-sensitive hypertension. Hypertension. 2022;79:2451–2462. doi: 10.1161/HYPERTENSIONAHA.122.19777 [DOI] [PubMed] [Google Scholar]
- 174.Karlsen TV, Nikpey E, Han J, Reikvam T, Rakova N, Castorena-Gonzalez JA, Davis MJ, Titze JM, Tenstad O, Wiig H. High-salt diet causes expansion of the lymphatic network and increased lymph flow in skin and muscle of rats. Arterioscler Thromb Vasc Biol. 2018;38:2054–2064. doi: 10.1161/ATVBAHA.118.311149 [DOI] [PubMed] [Google Scholar]
- 175.Mizuno R, Isshiki M, Ono N, Nishimoto M, Fujita T. A high-salt diet differentially modulates mechanical activity of afferent and efferent collecting lymphatics in murine iliac lymph nodes. Lymphat Res Biol. 2015;13:85–92. doi: 10.1089/lrb.2014.0043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.DeLano FA, Balete R, Schmid-Schonbein GW. Control of oxidative stress in microcirculation of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2005;288:H805–H812. doi: 10.1152/ajpheart.00696.2004 [DOI] [PubMed] [Google Scholar]
- 177.Thangaswamy S, Bridenbaugh EA, Gashev AA. Evidence of increased oxidative stress in aged mesenteric lymphatic vessels. Lymphat Res Biol. 2012;10:53–62. doi: 10.1089/lrb.2011.0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Aukland K, Reed RK. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev. 1993;73:1–78. doi: 10.1152/physrev.1993.73.1.1 [DOI] [PubMed] [Google Scholar]
- 179.Hos D, Koch KR, Bucher F, Bock F, Cursiefen C, Heindl LM. Serum eyedrops antagonize the anti(lymph)angiogenic effects of bevacizumab in vitro and in vivo. Invest Ophthalmol Vis Sci. 2013;54:6133–6142. doi: 10.1167/iovs.13-12460 [DOI] [PubMed] [Google Scholar]
- 180.Heckman CA, Holopainen T, Wirzenius M, Keskitalo S, Jeltsch M, Yla-Herttuala S, Wedge SR, Jurgensmeier JM, Alitalo K. The tyrosine kinase inhibitor cediranib blocks ligand-induced vascular endothelial growth factor receptor-3 activity and lymphangiogenesis. Cancer Res. 2008;68:4754–4762. doi: 10.1158/0008-5472.CAN-07-5809 [DOI] [PubMed] [Google Scholar]
- 181.Choi I, Lee S, Kyoung Chung H, Suk Lee Y, Eui Kim K, Choi D, Park EK, Yang D, Ecoiffier T, Monahan J, et al. 9-cis retinoic acid promotes lymphangiogenesis and enhances lymphatic vessel regeneration: therapeutic implications of 9-cis retinoic acid for secondary lymphedema. Circulation. 2012;125:872–882. doi: 10.1161/CIRCULATIONAHA.111.030296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yao LC, Baluk P, Feng J, McDonald DM. Steroid-resistant lymphatic remodeling in chronically inflamed mouse airways. Am J Pathol. 2010;176:1525–1541. doi: 10.2353/ajpath.2010.090909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yao LC, Baluk P, Srinivasan RS, Oliver G, McDonald DM. Plasticity of button-like junctions in the endothelium of airway lymphatics in development and inflammation. Am J Pathol. 2012;180:2561–2575. doi: 10.1016/j.ajpath.2012.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kimura T, Hamazaki TS, Sugaya M, Fukuda S, Chan T, Tamura-Nakano M, Sato S, Okochi H. Cilostazol improves lymphatic function by inducing proliferation and stabilization of lymphatic endothelial cells. J Dermatol Sci. 2014;74:150–158. doi: 10.1016/j.jdermsci.2014.01.001 [DOI] [PubMed] [Google Scholar]
- 185.Telinius N, Mohanakumar S, Majgaard J, Kim S, Pilegaard H, Pahle E, Nielsen J, de Leval M, Aalkjaer C, Hjortdal V, et al. Human lymphatic vessel contractile activity is inhibited in vitro but not in vivo by the calcium channel blocker nifedipine. J Physiol. 2014;592:4697–4714. doi: 10.1113/jphysiol.2014.276683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mohanakumar S, Majgaard J, Telinius N, Katballe N, Pahle E, Hjortdal V, Boedtkjer D. Spontaneous and alpha-adrenoceptor-induced contractility in human collecting lymphatic vessels require chloride. Am J Physiol Heart Circ Physiol. 2018;315:H389–H401. doi: 10.1152/ajpheart.00551.2017 [DOI] [PubMed] [Google Scholar]
- 187.Davis MJ, Kim HJ, Zawieja SD, Castorena-Gonzalez JA, Gui P, Li M, Saunders BT, Zinselmeyer BH, Randolph GJ, Remedi MS, et al. Kir6.1-dependent KATP channels in lymphatic smooth muscle and vessel dysfunction in mice with Kir6.1 gain-of-function. J Physiol. 2020;598:3107–3127. doi: 10.1113/JP279612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Furlong-Silva J, Cross SD, Marriott AE, Pionnier N, Archer J, Steven A, Merker SS, Mack M, Hong YK, Taylor MJ, et al. Tetracyclines improve experimental lymphatic filariasis pathology by disrupting interleukin-4 receptor-mediated lymphangiogenesis. J Clin Invest. 2021;131:e140853. doi: 10.1172/JCI140853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Han L, Su W, Huang J, Zhou J, Qiu S, Liang D. Doxycycline inhibits inflammation-induced lymphangiogenesis in mouse cornea by multiple mechanisms. PLoS One. 2014;9:e108931. doi: 10.1371/journal.pone.0108931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mand S, Debrah AY, Klarmann U, Batsa L, Marfo-Debrekyei Y, Kwarteng A, Specht S, Belda-Domene A, Fimmers R, Taylor M, et al. Doxycycline improves filarial lymphedema independent of active filarial infection: a randomized controlled trial. Clin Infect Dis. 2012;55:621–630. doi: 10.1093/cid/cis486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cribb MT, Sestito LF, Rockson SG, Nicolls MR, Thomas SN, Dixon JB. The kinetics of lymphatic dysfunction and leukocyte expansion in the draining lymph node during LTB4 antagonism in a mouse model of lymphedema. Int J Mol Sci. 2021;22:4455. doi: 10.3390/ijms22094455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hartiala P, Suominen S, Suominen E, Kaartinen I, Kiiski J, Viitanen T, Alitalo K, Saarikko AM. Phase 1 lymfactin() study: short-term safety of combined adenoviral VEGF-C and lymph node transfer treatment for upper extremity lymphedema. J Plast Reconstr Aesthet Surg. 2020;73:1612–1621. doi: 10.1016/j.bjps.2020.05.009 [DOI] [PubMed] [Google Scholar]
- 193.Daneshgaran G, Paik CB, Cooper MN, Sung C, Lo A, Jiao W, Park SY, Kim GH, Hong YK, Wong AK. Prevention of postsurgical lymphedema via immediate delivery of sustained-release 9-cis retinoic acid to the lymphedenectomy site. J Surg Oncol. 2020;121:100–108. doi: 10.1002/jso.25587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Visuri MT, Honkonen KM, Hartiala P, Tervala TV, Halonen PJ, Junkkari H, Knuutinen N, Yla-Herttuala S, Alitalo KK, Saarikko AM. VEGF-C and VEGF-C156S in the pro-lymphangiogenic growth factor therapy of lymphedema: a large animal study. Angiogenesis. 2015;18:313–326. doi: 10.1007/s10456-015-9469-2 [DOI] [PubMed] [Google Scholar]
- 195.Yao L, Wright MF, Farmer BC, Peterson LS, Khan AM, Zhong J, Gewin L, Hao CM, Yang HC, Fogo AB. Fibroblast-specific plasminogen activator inhibitor-1 depletion ameliorates renal interstitial fibrosis after unilateral ureteral obstruction. Nephrol Dial Transplant. 2019;34:2042–2050. doi: 10.1093/ndt/gfz050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Norden PR, Sabine A, Wang Y, Demir CS, Liu T, Petrova TV, Kume T. Shear stimulation of FOXC1 and FOXC2 differentially regulates cytoskeletal activity during lymphatic valve maturation. Elife. 2020;9:e53814. doi: 10.7554/eLife.53814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Russell PS, Hong J, Trevaskis NL, Windsor JA, Martin ND, Phillips ARJ. Lymphatic contractile function: a comprehensive review of drug effects and potential clinical application. Cardiovasc Res. 2022;118:2437–2457. doi: 10.1093/cvr/cvab279 [DOI] [PubMed] [Google Scholar]
- 198.Garner BR, Stolarz AJ, Stuckey D, Sarimollaoglu M, Liu Y, Palade PT, Rusch NJ, Mu S. KATP channel openers inhibit lymphatic contractions and lymph flow as a possible mechanism of peripheral edema. J Pharmacol Exp Ther. 2021;376:40–50. doi: 10.1124/jpet.120.000121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Harivardhan Reddy L, Sharma RK, Chuttani K, Mishra AK, Murthy RS. Influence of administration route on tumor uptake and biodistribution of etoposide loaded solid lipid nanoparticles in Dalton’s lymphoma tumor bearing mice. J Control Release. 2005;105:185–198. doi: 10.1016/j.jconrel.2005.02.028 [DOI] [PubMed] [Google Scholar]
- 200.Alderfer L, Hall E, Hanjaya-Putra D. Harnessing biomaterials for lymphatic system modulation. Acta Biomater. 2021;133:34–45. doi: 10.1016/j.actbio.2021.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kobayashi H, Kawamoto S, Bernardo M, Brechbiel MW, Knopp MV, Choyke PL. Delivery of gadolinium-labeled nanoparticles to the sentinel lymph node: comparison of the sentinel node visualization and estimations of intra-nodal gadolinium concentration by the magnetic resonance imaging. J Control Release. 2006;111:343–351. doi: 10.1016/j.jconrel.2005.12.019 [DOI] [PubMed] [Google Scholar]
- 202.McCright J, Skeen C, Yarmovsky J, Maisel K. Nanoparticles with dense poly(ethylene glycol) coatings with near neutral charge are maximally transported across lymphatics and to the lymph nodes. Acta Biomater. 2022;145:146–158. doi: 10.1016/j.actbio.2022.03.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Schudel A, Francis DM, Thomas SN. Material design for lymph node drug delivery. Nat Rev Mater. 2019;4:415–428. doi: 10.1038/s41578-019-0110-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Szoke D, Kovacs G, Kemecsei E, Balint L, Szotak-Ajtay K, Aradi P, Styevkone Dinnyes A, Mui BL, Tam YK, Madden TD, et al. Nucleoside-modified VEGFC mRNA induces organ-specific lymphatic growth and reverses experimental lymphedema. Nat Commun. 2021;12:3460. doi: 10.1038/s41467-021-23546-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Guo Q, Liu Y, Xu K, Ren K, Sun W. Mouse lymphatic endothelial cell targeted probes: anti-LYVE-1 antibody-based magnetic nanoparticles. Int J Nanomedicine. 2013;8:2273–2284. doi: 10.2147/IJN.S45817 [DOI] [PMC free article] [PubMed] [Google Scholar]