INTRODUCTION:
Although the 9-minute mean withdrawal time (m-WT) is often reported to be associated with the optimal adenoma detection rate (ADR), no randomized trials of screening colonoscopy have confirmed the impact of a 9-minute m-WT on adenoma miss rate (AMR) and ADR.
METHODS:
A multicenter tandem trial was conducted in 11 centers. Seven hundred thirty-three asymptomatic participants were randomized to receive segmental tandem screening colonoscopy with a 9-minute withdrawal, followed by a 6-minute withdrawal (9-minute-first group, 9MF, n = 366) or vice versa (6-minute-first group, 6MF, n = 367). The primary outcome was the lesion-level AMR.
RESULTS:
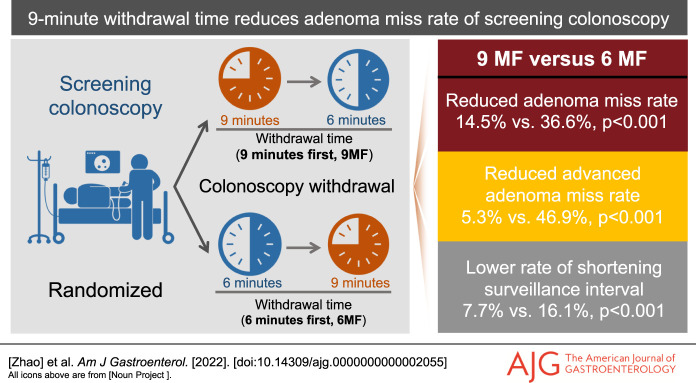
The intention-to-treat analysis revealed that 9MF significantly reduced the lesion-level (14.5% vs 36.6%, P < 0.001) and participant-level AMR (10.9% vs 25.9%, P < 0.001), advanced adenoma miss rate (AAMR, 5.3% vs 46.9%, P = 0.002), multiple adenomas miss rate (20.7% vs 56.5%, P = 0.01), and high-risk adenomas miss rate (14.6% vs 39.5%, P = 0.01) of 6MF without compromising detection efficiency (P = 0.79). In addition, a lower false-negative rate for adenomas (P = 0.002) and high-risk adenomas (P < 0.05), and a lower rate of shortening surveillance schedule (P < 0.001) were also found in 9MF, accompanying with an improved ADR in the 9-minute vs 6-minute m-WT (42.3% vs 33.5%, P = 0.02). The independent inverse association between m-WT and AMR remained significant even after adjusting ADR, and meanwhile, 9-minute m-WT was identified as an independent protector for AMR and AAMR.
DISCUSSION:
In addition to increasing ADR, 9-minute m-WT also significantly reduces the AMR and AAMR of screening colonoscopy without compromising detection efficiency.
INTRODUCTION
The incidence and mortality of colorectal cancer (CRC) have been proven to be decreased by screening colonoscopy, whose inspecting quality is dependent on the adenoma detection rate (ADR) (1). Nevertheless, ADRs vary greatly among colonoscopists, and colonoscopy is unable to detect all colorectal lesions (2). The adenoma miss rates (AMRs) of qualified colonoscopists are reported to range between 26% and 62% (3), which are main causes of interval CRCs that occur in the screening or surveillance interval and contribute to 8%–9% of total CRCs (4,5). Therefore, several quality indicators, including withdrawal time (WT), are introduced to improve inspecting quality and reduce missed adenomas (6).
In 2002, 6–10 minutes was first introduced as the standard for mean WT of negative colonoscopies (m-NWTs) (7), mainly based on that 2 colonoscopists with >8-minute m-NWTs showed the lowest AMRs (8). Afterward, a simple >6-minute indicator was established for higher ADRs was observed in colonoscopists with >6-minute m-NWTs vs those with shorter m-NWTs (6,9). However, subsequent studies further identified a positive association between m-NWT and ADR over 6-minute m-NWT, where 9 minutes were frequently found to be with the optimal ADR (10–13). Compared with the 6-minute mean WT (m-WT), we previously reported that 9-minute m-WT exhibited an improved ADR (14). Nevertheless, the finding was based on the insufficient WT of 9-minute group and mixed-indication patients whose ADRs varied greatly, whereas no randomized controlled trials (RCTs) have confirmed the ADR improvement of 9 minutes in screening population. Moreover, no trials have been conducted to demonstrate the effect of 9-minute m-WT on AMR, which is clinically relevant in evaluating colonoscopists with high ADRs (2,12,15).
Therefore, this screening-colonoscopy based RCT is aimed to determine the effect of 9-minute m-WT on AMR and ADR.
METHODS
Overview
This multicenter trial was initiated by the National Clinical Research Center for Digestive Diseases (Shanghai) and National Quality Control Center of Digestive Endoscopy of China. It was conducted by 15 gastroenterologists of 11 tertiary hospitals from March 2021 to December 2021. The trial was approved by the ethics committees of all participating centers and is registered at ClinicalTrials.gov as NCT04797065. Informed consent was obtained from all participants, and all authors had access to the study data and approved the final version of the manuscript. The related definitions, including advanced adenomas (AAs), high-risk adenomas (HRAs), and others, are illustrated in the Supplementary Materials (see Supplementary Digital Content 1, http://links.lww.com/AJG/C737).
Inclusion and exclusion criteria
Considering the increasing risk of early-onset CRCs and advanced neoplasia in people aged 40 years or older (16), 40 was selected as the initiating age of inclusion, which also serves as the starting year of CRC screening in China, Australia, and other countries (17,18). Therefore, consecutive asymptomatic population aged 40–75 years without fecal immunochemical test as the primary screening was invited to participate. Patients with alarming symptoms and signs including hematochezia, melena, weight loss or anemia without specific causes, abdominal mass, and positive digital rectal examination, indications of surveillance or diagnosis, and therapeutic colonoscopy for existing lesions were excluded. Other exclusions included a history of colonic resection or abdominal surgery, suspected or diagnosed CRCs, inflammatory bowel diseases, hereditary CRC syndromes, coagulopathy or receiving antiplatelet or anticoagulant within 7 days before the colonoscopy, failed cecal intubation, poor bowel preparation quality (BPQ) with inadequate Aronchick score in the insertion, pregnancy, severe chronic cardiopulmonary and other comorbidities, and refusal to participate. The details of bowel preparation and withdrawal techniques are illustrated in the Supplementary Materials (see Supplementary Digital Content 1, http://links.lww.com/AJG/C737).
Randomization and masking
A computer-generated randomized sequence with block design (6 participants per block) was used to assign participants to the 9-minute-first group (9MF) or 6-minute-first group (6MF) at a 1:1 ratio. The randomization lists were stratified by colonoscopists and were generated by the Center of Clinical Epidemiology (Naval Medical University) for each center. The opaque and sealed envelopes containing random sequence were prepared by the investigators who did not participate in data collection and analysis, and the randomization ratio and block design were blinded to all colonoscopists for each center. The envelopes were opened for colonoscopists only after cecal intubation was confirmed, and participants were blinded to group allocation. All data were collected by the investigators at each center who did not participate in data analysis.
Intervention procedures
The 6-minute and 9-minute m-WT protocols as previously described (14) is adopted for a steady withdrawal speed. Briefly, the colorectum was classified into 3 segments: right colon, transverse colon, and left colon, using the hepatic and splenic flexures as the dividing landmarks. As adopted previously (12), segmental tandem withdrawal was used to reduce the difficulty and pain of reinsertion and facilitate the immediate comparison of twice withdrawals where the landmarks of anatomical locations were pictured to confirm the same locations reached. Except for the protocol and pilot trials, colonoscopists were not given additional specific training or instructions for the 6-minute or 9-minute withdrawal and used routine clinical practice. During the inspection, washing and suctioning were allowed in the 6-minute or 9-minute withdrawal at the discretion of the colonoscopists.
The designated 6-/9-minute WT was equally divided into 3 parts (two-thirds minutes) for each segment withdrawal, with actual WTs recorded. To remind the WT spent, the timer was set to ring 1 minute before the designated end point of WT for each segment (see Supplementary Figure 1, Supplementary Digital Content 1, http://links.lww.com/AJG/C737). Any ≥1-minute violation of predesigned WT in each segment led to the exclusion from per-protocol set. The reinsertion time and related time for biopsy or polypectomy was subtracted from WT. The colonoscopists adopted the same inspecting techniques for each withdrawal, and the size of the polyp was estimated using open biopsy forceps or snares. All polyps included for analysis were biopsied by forceps or resected by snares, with histopathology diagnosed by the guidelines of the Fourth World Health Organization Classification of Tumors, whereas the optically diagnosed hyperplastic diminutive rectosigmoid polyps and those detected only in the insertion process were not included.
Outcome measures
The primary outcome measure was the lesion-level AMR, with its subgroups analyzed. The secondary outcome measures included advanced AMR (AAMR), ADR, participant-level AMR, advanced ADR (AADR), and others.
Sample size estimation
According to our finding of pilot trial (3), the lesion-level AMR was estimated to be 30% in the 6MF and could be halved to 15% by the 9MF. When calculated with a 2-sided significance of 0.05 and a power of 80%, the number of adenomas needed to be ≥132. With 0.53 adenomas per colonoscopy (APC) (14), 498 participants were needed. Although we observed a decreased AMR of 9MF in the interim analysis, considering that tandem studies were probably inclined to be positive (19) and the increased APC in the trial, we therefore adopted a more conservative method (a 2-sided significance of 0.01 and a power of 90%) with a 0.74 APC (data from interim analysis) to estimate sample size, which require adenomas to be ≥239 and participants to be ≥644. Considering an 8% dropout rate, >700 participants should be invited.
Statistical analysis
Both intention-to-treat and per-protocol analyses were performed. A planned interim analysis was conducted to assess the rationality of the used parameters and re-estimate sample size. Continuous variables were described as the mean with SD and compared using Student t test, whereas categorical data were expressed as numbers with percentages and analyzed using the χ2 test or Fisher exact test. The interaction between subgroups and primary comparison was analyzed by the Mantel–Haenszel test, and adjusted P values with Bonferroni correction were used for multiple comparisons. The Spearman rank-correlation coefficient was used to measure the relationship between the m-WTs and ADR, AADR, AMR, and AAMR of individual colonoscopists. Multiple linear regression was adopted to identify independent predictors of AMR, whereas multivariate logistic regressions, including generalized estimating equations, were used to identify the independent factors of missed adenomas and AAs. Odds ratios (ORs) or relative risks with 95% confidence intervals (CIs) are presented, and 2-sided P values <0.05 were considered statistically significant. All statistical analyses were performed with SPSS Statistics 21 (IBM, Armonk, NY) or R software.
RESULTS
Baseline characteristics
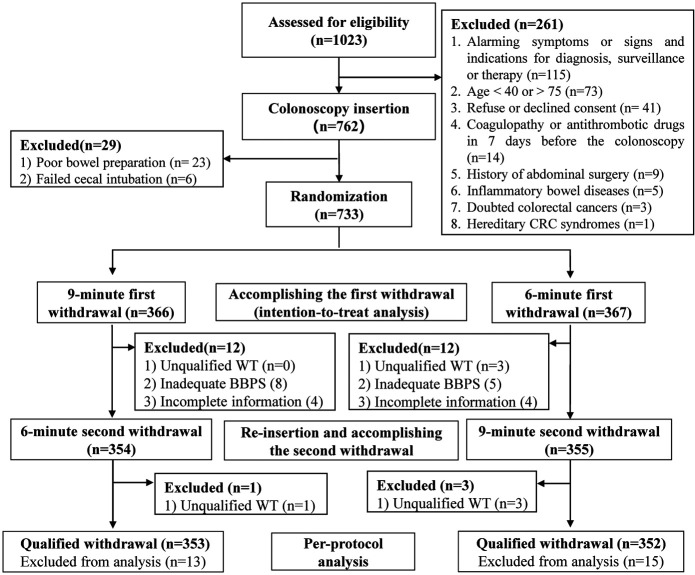
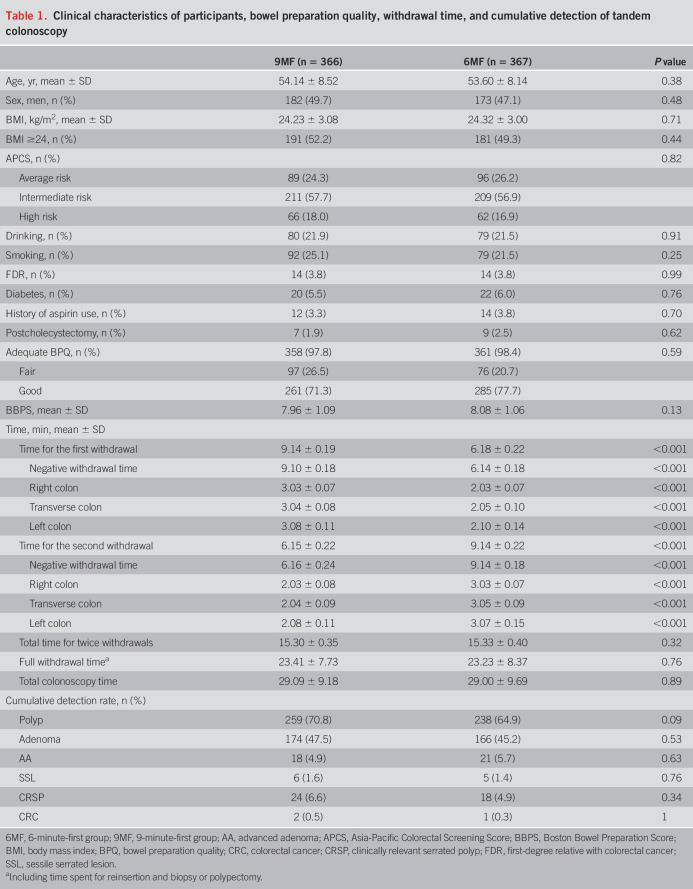
A total of 1,023 participants were invited, and 733 included participants were randomized to the 6MF (n = 367) and 9MF (n = 366) (intention-to-treat set), with excluded participants illustrated in Figure 1; 7, 13 and 8 participants were excluded from per-protocol set because of unqualified WT, inadequate BPQ, and incomplete data, respectively. The baseline characteristics, CRC risk profiles, and colonoscopy findings between 2 groups were comparable (Table 1, see Supplementary Table 1, Supplementary Digital Content 1, http://links.lww.com/AJG/C737). The m-WT and m-NWT of 9MF were longer in the first withdrawal (9.14 vs 6.18, P < 0.001; 9.10 vs 6.14, P < 0.001, respectively) and shorter in the second withdrawal (6.15 vs 9.14, P < 0.001; 6.16 vs 9.14, P < 0.001, respectively) than those of 6MF. Similar findings were also found in the m-WTs of the right, transverse, and left colon (all P < 0.001, Table 1). No adverse events were found.
Figure 1.
Flow diagram of participant enrollment and colonoscopy. BBPS, Boston Bowel Preparation Score; WT, withdrawal time.
Table 1.
Clinical characteristics of participants, bowel preparation quality, withdrawal time, and cumulative detection of tandem colonoscopy
Lesion-level detection and miss rate
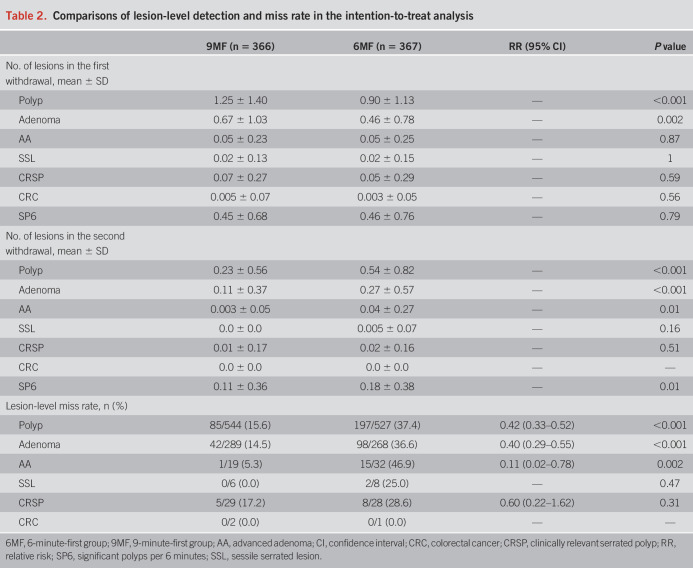
Table 2 indicates that with 9-minute vs 6-minute m-WT, more adenomas were detected in the first (0.67 vs 0.46, P = 0.002) and second withdrawal (0.27 vs 0.11, P < 0.001), more AAs were detected in the second withdrawal (0.04 vs 0.003, P = 0.01), and the significant polyps per 6 minutes remained similar in the first withdrawal (0.45 vs 0.46, P = 0.79) and increased in the second withdrawal (0.18 vs 0.11, P = 0.01). 9MF showed lower miss rates for adenomas (14.5% vs 36.6%, P < 0.001) and advanced adenomas (5.3% vs 46.9%, P = 0.002) than 6MF (Table 2). 9MF tended to show lower AMRs and AAMRs in all subgroups; no subgroups showed significant interactions with the WT grouping (all P > 0.05; see Supplementary Tables 2 and 3, Supplementary Digital Content 1, http://links.lww.com/AJG/C737). Consistent lesion-level findings were confirmed in the per-protocol analysis (see Supplementary Table 4, Supplementary Digital Content 1, http://links.lww.com/AJG/C737).
Table 2.
Comparisons of lesion-level detection and miss rate in the intention-to-treat analysis
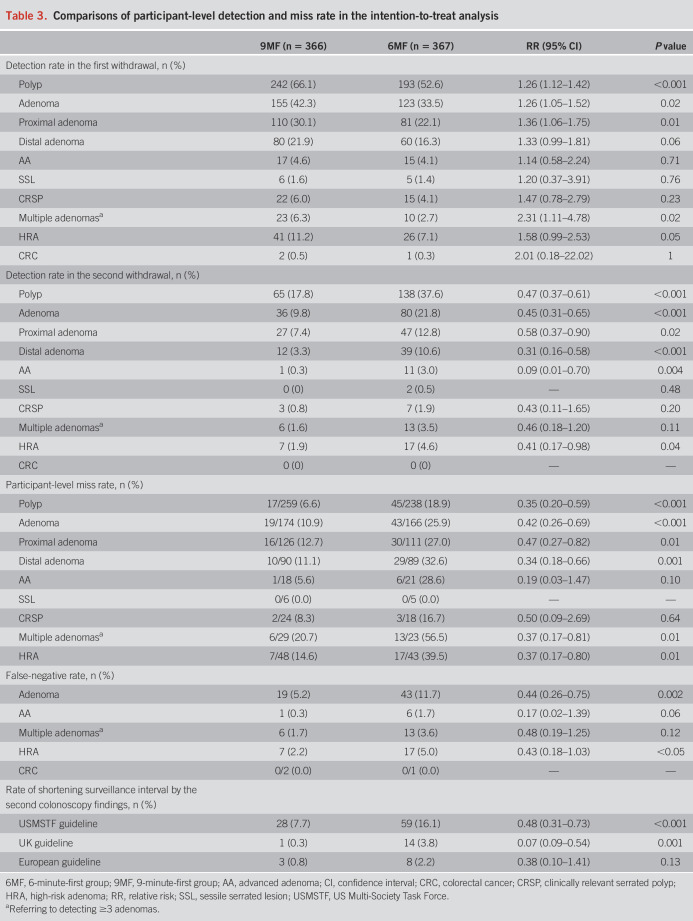
Participant-level detection and miss rate
Compared with 6-minute m-WT, 9-minute m-WT improved the ADR both in the first (42.3% vs 33.5%, P = 0.02) and second withdrawal (21.8% vs 9.8%, P < 0.001; Table 3). With the 9-minute m-WT, an increased multiple adenomas detection rate was found in the first withdrawal (6.3% vs 2.7%, P = 0.02). Compared with 6MF, 9MF reduced the participant-level AMR (10.9% vs 25.9%, P < 0.001), multiple adenomas miss rate (20.7% vs 56.5%, P = 0.01), and HRA miss rate (HMR, 14.6% vs 39.5%, P = 0.01; Table 3). The ADR improvement was associated with diminutive (P = 0.01), flat (P = 0.01), and tubular adenomas (P = 0.02, see Supplementary Table 5, Supplementary Digital Content 1, http://links.lww.com/AJG/C737). Colonoscopists with high ADR in the 6-minute m-WT might not benefit from the 9-minute m-WT (38.9% vs 40.6%, P = 0.75, P for interaction = 0.02), whereas no other subgroups showed significant interactions with WT grouping (all P > 0.05; see Supplementary Table 6, Supplementary Digital Content 1, http://links.lww.com/AJG/C737). Consistent participant-level findings were also confirmed in the per-protocol analysis (see Supplementary Table 7, Supplementary Digital Content 1, http://links.lww.com/AJG/C737).
Table 3.
Comparisons of participant-level detection and miss rate in the intention-to-treat analysis
False-negative rate, rate of shortening surveillance schedule, and actual-WT analysis
Compared with 6MF, 9MF reduced false-negative rate for adenomas (5.2% vs 11.7%, P = 0.002) and HRAs (2.2% vs 5.0%, P < 0.05) (Table 3). The rate of shortening surveillance schedule, caused by lesions detected in the second withdrawal, was significantly lower in 9MF vs 6MF according to the guidelines of the United States (7.7% vs 16.1%, P < 0.001) and United Kingdom (0.3% vs 3.8%, P = 0.001; Table 3). Colonoscopies with ≥ 9-minute WTs showed a proportionately lower AMR (P < 0.001) and AAMR (P = 0.001) than those colonoscopies with shorter WTs; consistent findings occurred in the proximal (P < 0.001; P = 0.04) and distal colon (P < 0.001; P = 0.03, see Supplementary Table 8, Supplementary Digital Content 1, http://links.lww.com/AJG/C737).
Independent predictors for AMR
All m-WTs of colonoscopists ranged correspondingly during 6–7 or 9–10 minutes (see Supplementary Table 9, Supplementary Digital Content 1, http://links.lww.com/AJG/C737). The lower AMRs and AAMRs in 9MF remained significant through the sensitivity analysis (see Supplementary Table 10, Supplementary Digital Content 1, http://links.lww.com/AJG/C737). Longer m-WTs were positively associated with ADR (ρ = 0.40, P = 0.03) and AADR (ρ = 0.41, P = 0.03) and inversely associated with AMR (ρ = −0.50, P = 0.01) and AAMR (ρ = −0.50, P = 0.03); ADR, APC, adenomas per positive colonoscopy, ADR-plus, and significant polyps per 6 minutes were also inversely associated with AMR (see Supplementary Table 11, Supplementary Digital Content 1, http://links.lww.com/AJG/C737), whereas only m-WTs (coefficient = −5.08, P = 0.01) and ADR (coefficient = −0.64, P = 0.002) were the independent predictors of AMR (see Supplementary Table 12, Supplementary Digital Content 1, http://links.lww.com/AJG/C737).
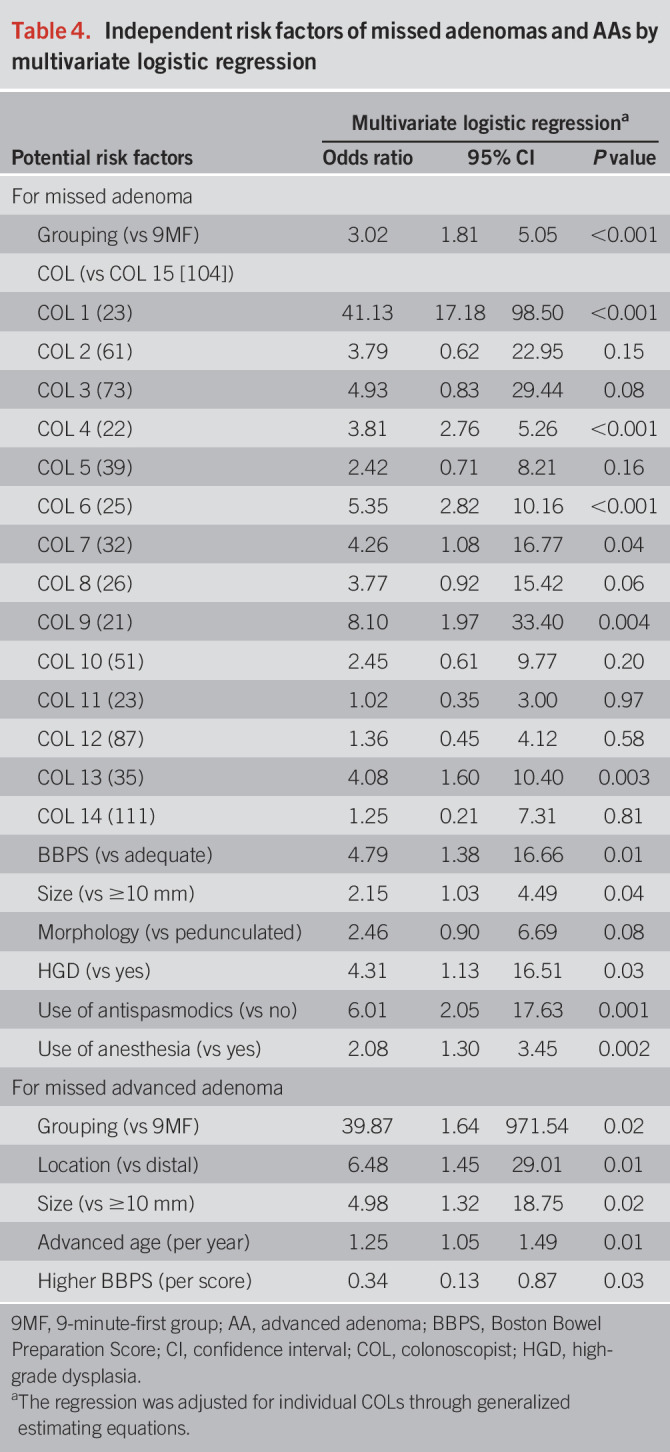
Independent risk factors for AMR and AAMR
6MF (OR = 3.02, P < 0.001), individual colonoscopists (OR = 1.02–41.13, P < 0.001), inadequate BPQ (OR = 4.79, P = 0.01), smaller size (OR = 2.15, P = 0.04), no high-grade dysplasia (OR = 4.31, P = 0.03), use of antispasmodics (OR = 6.01, P = 0.001), and lack of anesthesia (OR = 2.08, P = 0.002) were independent risk factors of missed adenomas (Table 4). Independent risk or protective factors of missed AAs included 6MF (OR = 39.87, P = 0.02), proximal location (OR = 6.48, P = 0.01), smaller size (OR = 4.98, P = 0.02), advanced age (OR = 1.25, P = 0.002), and higher Boston Bowel Preparation Score (OR = 0.34, P = 0.03) (Table 4).
Table 4.
Independent risk factors of missed adenomas and AAs by multivariate logistic regression
DISCUSSION
To our best knowledge, the RCT is the first trial to demonstrate a reduced AMR, AAMR, HMR, and rate of shortening surveillance schedule with 9MF vs 6MF. With better compliance, the trial further confirmed an improved ADR and APC in the screening population (14). Longer WTs might be associated with higher ADRs and AADRs, as well as lower AMRs and AAMRs, whose inverse association (with AMR) remained independent even after adjusting for ADR. 9MF was the independent protective factor for the missed adenomas and AAs, which was consistent with sensitivity analysis and actual-WT analysis.
Although 9-minute m-WT were often observed to be of the highest ADR (10,11,20), inconsistencies still exist (13). Compared with our trial with insufficient m-WT (8.88 minutes) in 9-minute group and with mixed-indication population (14), the study further confirmed the ADR improvement in screening population with adequate m-WTs in all participating colonoscopists. Notably, the colonoscopists with qualified ADR (≥25%) in routine practice also showed a tendency of ADR improvement (41% vs 34.8%; P for interaction = 0.62) through a 3-minute prolongation. This improvement is clinically relevant because the risk of interval CRC could be further reduced from a 35% or higher ADR (1,21,22) without a “ceiling effect” (22–24) or extra expense (25). Besides, 9-minute m-WTs were also identified to be positively associated with higher AADRs, which is supported by previous reports (26,27). However, for colonoscopists reaching high ADRs (≥33.5%) in 6-minute m-WTs, the benefit of WT prolongation might be limited in ADR. Besides previous finding (14), the high baseline ADR (33.5%) reached in the current trial was comparable with other reports from Chinese (28,29), indicating that the adenoma prevalence of Chinese might have increased along with the CRC incidence. The insufficient m-NWT (6.38 vs 4.76) (30), inadequate bowel preparation with 2 L polyethylene glycol (31), and young population (age range 18–65, mean 50 (31); age range 18–75; mean 49.5 (30)) might lead to the low-reported ADRs. Consistently, the latest RCT of Chinese population with high-dose polyethylene glycol and WT also achieved a high ADR (38.5% vs 33.0%) in relatively young population (age range 35–75, mean 54) (32). In addition, we also observed an approximately 30% ADR in the low-risk population (simultaneously with nonhigh CRC risk profile (33), negative fecal immunochemical test and negative stool DNA methylation test) in our multicenter community-based screening study (NCT04786704). Presumably, the Chinese ADR in screening might be previously underestimated and should be cautiously redefined. In our previous work, the detection rate of sessile serrated lesions (SSLs) was found to be improved in the per-protocol analysis (4.0% vs 1.3%, P = 0.04) but not in the intention-to-treat analysis (4.0% vs 2.2%, P = 0.15) (14), where the compliance of trial protocol was suboptimal and only 5 participating centers were able to diagnosis SSLs. Therefore, the improvement of SSL detection rate (SDR) might be greatly influenced by the protocol compliance and the selection of participating centers, for which we did not confirm the improvement of SDR in the conclusion (14). In addition, because the variation in pathological standards of SSL is great (23) and the pathological diagnosis of SSL has not reached an extensive consensus in Chinese medical centers, the SDR in China is relatively low and variable (14,28,32,34). Therefore, the difference of SDR might be confounded by pathologists' factor and random chance (owning to less precision of the estimates), which might contribute to the comparable SDRs between 2 groups in current trial.
In addition, ADR is not a perfect quality indicator because of the “one and done” effect (2) and colonoscopists with high ADRs are also likely to miss multiple adenomas (2). Despite the well-established ADR standards, interval CRCs still contribute to 8%–9% CRCs (4,5) and are mainly originated from missed adenomas (5), whose rate could be over 40% for flat lesions (3). Therefore, in addition to ADR, the current quality measures also put high priority in reducing missed adenomas (3,5). For the first time, the tandem trial demonstrated a reduced AMR in the 9-minute m-WT and the independent inverse association between m-WT and AMR even remained after adjusting ADR, indicating that ADR might not be the only mediator of low AMR and WT should also be monitored to reduce AMR. Moreover, although 9-minute m-WT may not improve ADR of colonoscopists who reach high ADRs in the 6-minute m-WT, yet it decreased their AMR (P = 0.003) and AAMR (P = 0.01), showing the complementary value of WT to ADR in monitoring colonoscopy quality and weakening the “one and done” effect (2). Therefore, 9-minute m-NWT is gradually recommended as an aspirational WT target by the American Gastroenterological Association and the American Society for Gastrointestinal Endoscopy for the best clinical practice (35,36).
AAs represent higher risks of CRC progression even with more intensive surveillance frequency (37) and are estimated to progress to CRCs at a rate of 1% per year (38); their missed diagnosis will falsely reassure the population of interval CRC risk (39). Notably, 9MF also decreased the AAMR and no AAs were missed in ≥9-minute actual WT. We believe the finding is convincing for following points: (i) 9MF not only reduced the AAMR of large adenomas, which might be gamed by overestimating size, but also reduced the AAMR of (tubulo)villous adenomas and high-grade dysplasia; (ii) the missed AAs occurred in 6 colonoscopists of different centers and the difference remained significant after sensitivity analysis; and (iii) both the multivariate regression and correlation analysis supported the lower AAMR in 9MF. Besides, the finding is also supported by the previous reports with more AAs detected in longer WT (27,40). Nevertheless, the participant-level AAMR and AADR remained unchanged, possibly indicating that the missed AAs probably tend to occur meantime with detected AAs. Therefore, longer WTs should be recommended in withdrawal with AAs detected to identify all synchronous advanced lesions (41). In addition, our finding might also explain the reasons underlying the independent inverse association between interval CRC incidence and m-NWT after adjusting ADR (42); it probably lies in that 9-minute WT not only improves ADR but also reduces the rate of missed HRAs, including AAs, large SSLs, or multiple adenomas, which cannot be reflected by ADR. Notably, compared with the estimated 10% AAMR in the meta-analysis (3), the magnitude of AAMR might be overestimated by the limited sample size as previously reported (43,44), which needs more data to accurately determine. Notably, we found the lack of anesthesia was an independent risk factor for missed adenomas, which is consistent with the finding of several latest reports from China and the United States (45,46), indicating an improved ADR with sedated colonoscopy vs unsedated colonoscopy. However, a recent meta-analysis (47) (not including the above 2 latest reports (45,46)) reported similar ADRs between propofol sedation vs opioid/benzodiazepine sedation. Presumably, the difference of using sedation or not using might actually be the critical factor, whereas the choice of sedation type would not affect ADR and inspecting quality (48). The lower AMR of sedated colonoscopy in our trial supported the speculation, and further validation is needed.
Our study has several limitations. First, the trial was an open-label RCT for colonoscopists, and the same colonoscopists performed tandem withdrawal, which could have introduced subjective bias and Hawthorne effect, whereas such limitation is almost unavoidable for endoscopic trial. Second, although the validated segmental tandem withdrawal (12) is beneficial for providing direct comparisons between twice withdrawals and reducing difficulty and pain in the reinsertion, the anatomic landmarks of hepatic and splenic flexures might be vague and nontypical for accurate locating, whereas it might influence the effect of 9-minute WT as a whole or in each colonic segment. Third, the long-term effect of prolonging WT from 6 to 9 minutes on CRC incidence is unknown. Finally, although superiority of 9-minute WT were observed in the Chinese population and the subgroup of over 50-year population, future validation for Caucasian population in accord with the local CRC screening strategy is valuable to promote generalizability.
In summary, with 9-minute vs 6-minute m-WT, the RCT is the first trial to demonstrate a reduced AMR and AAMR, as well as an improved ADR in screening colonoscopy. Individual colonoscopists may acquire significant benefits in the ADR, AMR, AAMR, and HMR from a 3-minute WT prolongation without compromising detection efficiency. Along with the evidence from current trial and previous findings (14,42), we suggest that in addition to ADR, the 9-minute WT deserves to be considered as a potential quality indicator to further optimize colonoscopy quality.
CONFLICTS OF INTEREST
Guarantor of the article: Zhaoshen Li, MD, PhD.
Specific author contributions: L.Z.S., B.Y., and Z.S.B.: study concept and design. Z.S.B., S.Y.H., W.S.L., P.P., W.R., F.Z.J., G.A.X., Y.X., Y.D.M., Z.J.W., B.J.H., L.T., R.X., T.X.F., L.Q., Y.D., Z.J.H., S.B., W.P., C.W.G., S.G.C., X.Y.Q., X.P., L.Y.X., X.H.H., Y.X.M., S.J.Q., T.Y.R., L.X.H., Z.Y.B., M.Y.C., S.H.Z., Z.X.F., W.F.Y., W.L., and W.X.F.: acquisition of data. B.Y., Z.S.B., S.Y.H., and W.S.L.: analysis and interpretation of data. Z.S.B. and S.Y.H.: drafting of the manuscript. Z.S.B., B.Y., L.Z.S., S.Y.H., and W.S.L.: critical revision of the manuscript. Z.S.B., S.Y.H., and W.S.L.: statistical analysis. B.Y. and L.Z.S.: administrative, technical, or material support. B.Y. and L.Z.S.: study supervision.
Financial support: This work was supported by the National Science and Technology Plan Project of the Ministry of Science and Technology of China (grant no. 2015BAI13B08). B.Y. is supported by the National Natural Science Foundation of China (No.82170567, 81873546), Program of Shanghai Academic/Technology Research Leader (No. 22XD1425000), the “Shu Guang” project of Shanghai Municipal Education Commission and Shanghai Education Development Foundation (No. 19SG30, China), and 234 Discipline Climbing Plan of Changhai Hospital, Naval Medical University (No. 2019YXK004, China).
Potential competing interests: There are no conflicts of interest for each author.
Data sharing: Data from deidentified individual patients can be shared with investigators who propose to use the data under the approval of an independent review committee. Data can only be used for the goals specified in the proposal. Data sharing will be implemented between 6 and 18 months after article publication with a data-sharing agreement signed. Zhaoshen Li can be contacted at the corresponding email.
Registration: This trial is registered at ClinicalTrials.gov as NCT04797065.
Study Highlights.
WHAT IS KNOWN
✓ Colonoscopy is reported to miss over one-fourth of adenomas and almost one-tenth of advanced adenomas.
✓ Nine-minute mean withdrawal time (m-WT) was often reported to be associated with the optimal adenoma detection rate (ADR).
✓ No randomized trials have confirmed the effect of 9-minute m-WT on the ADR and adenoma miss rate (AMR) in screening colonoscopy.
WHAT IS NEW HERE
✓ When compared with the conventional 6-minute m-WT, the study first demonstrated a reduced miss rate for adenoma, advanced adenoma, and high-risk adenoma without compromising detection efficiency in the 9-minute m-WT, along with a lower rate of shortening surveillance schedule caused by the missing lesions.
✓ For individual colonoscopists, 9-minute m-WT remained lower miss rates after sensitivity analysis and showed an independent inverse association with the AMR even after adjusting ADR, which also served as an independent protective factor for missed adenomas and advanced adenomas.
✓ Nine-minute WT deserves to be incorporated into the current panel of indicators to optimize colonoscopy quality.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/AJG/C737
Shengbing Zhao, Yihang Song, Shuling Wang, Rong Wang, Zhijie Feng, Aixia Gong, and Xia Yang are co-first authors.
Contributor Information
Shengbing Zhao, Email: zhaoshengbing@hotmail.com.
Yihang Song, Email: noah_ir77@163.com.
Shuling Wang, Email: wangshuling0000@126.com.
Rong Wang, Email: wangxiongzai@126.com.
Zhijie Feng, Email: zhijiefeng2005@126.com.
Aixia Gong, Email: gong0825@aliyun.com.
Xia Yang, Email: lily-xia@163.com.
Peng Pan, Email: panpeng211@126.com.
Dongmei Yao, Email: ydm8080@126.com.
Jingwen Zhang, Email: 277232639@qq.com.
Yaqin Zhu, Email: 170512052@qq.com.
Tao Li, Email: 491222383@qq.com.
Junhua Bi, Email: 554694881@qq.com.
Xu Ren, Email: hljxhy2001@126.com.
Xiufen Tang, Email: 13796616601@126.com.
Qiang Li, Email: asss2002@163.com.
Dan Yu, Email: 15846591014@163.com.
Jinghua Zheng, Email: Yantaizhjha2007@126.com.
Bo Song, Email: 595640111@qq.com.
Ping Wang, Email: 641001240@qq.com.
Weigang Chen, Email: 13579456959@126.com.
Guochen Shang, Email: 276851127@qq.com.
Yanqiu Xu, Email: 1425658022@qq.com.
Ping Xu, Email: sjzxxp@yeah.net.
Yuexing Lai, Email: 18918288995@163.com.
Huanhai Xu, Email: xhh19992000@163.com.
Xiaomin Yang, Email: xm503xm@163.com.
Jianqiu Sheng, Email: jianqiu@263.net.
Yurong Tao, Email: mmroll@126.com.
Xinghua Li, Email: lixinghua2002@aliyun.com.
Yangbei Zhu, Email: zhubei965@126.com.
Xiaofeng Zhang, Email: 837837@zju.edu.cn.
Hongzhang Shen, Email: shz@zcmu.edu.cn.
Yingcai Ma, Email: mayingcai0271@sina.com.
Fangyu Wang, Email: wangfy65@nju.edu.cn.
Lin Wu, Email: wlandecho@163.com.
Xianfei Wang, Email: 408309620@qq.com.
Yu Bai, Email: changhaibaiyu@smmu.edu.cn.
REFERENCES
- 1.Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med 2014;370(14):1298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aniwan S, Orkoonsawat P, Viriyautsahakul V, et al. The secondary quality indicator to improve prediction of adenoma miss rate apart from adenoma detection rate. Am J Gastroenterol 2016;111(5):723–9. [DOI] [PubMed] [Google Scholar]
- 3.Zhao S, Wang S, Pan P, et al. Magnitude, risk factors, and factors associated with adenoma miss rate of tandem colonoscopy: A systematic review and meta-analysis. Gastroenterology 2019;156(6):1661–74.e11. [DOI] [PubMed] [Google Scholar]
- 4.Adler J, Robertson DJ. Interval colorectal cancer after colonoscopy: Exploring explanations and solutions. Am J Gastroenterol 2015;110(12):1657–64; quiz 1665. [DOI] [PubMed] [Google Scholar]
- 5.Robertson DJ, Lieberman DA, Winawer SJ, et al. Colorectal cancers soon after colonoscopy: A pooled multicohort analysis. Gut 2014;63(6):949–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rex DK, Petrini JL, Baron TH, et al. Quality indicators for colonoscopy. Am J Gastroenterol 2006;101(4):873–85. [DOI] [PubMed] [Google Scholar]
- 7.Rex DK, Bond JH, Winawer S, et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: Recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol 2002;97(6):1296–308. [DOI] [PubMed] [Google Scholar]
- 8.Rex DK, Cutler CS, Lemmel GT, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology 1997;112(1):24–8. [DOI] [PubMed] [Google Scholar]
- 9.Barclay RL, Vicari JJ, Doughty AS, et al. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med 2006;355(24):2533–41. [DOI] [PubMed] [Google Scholar]
- 10.Lee TJW, Blanks RG, Rees CJ, et al. Longer mean colonoscopy withdrawal time is associated with increased adenoma detection: Evidence from the Bowel Cancer Screening Programme in England. Endoscopy 2012;45(1):20–6. [DOI] [PubMed] [Google Scholar]
- 11.Butterly L, Robinson CM, Anderson JC, et al. Serrated and adenomatous polyp detection increases with longer withdrawal time: Results from the New Hampshire Colonoscopy Registry. Am J Gastroenterol 2014;109(3):417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar S, Thosani N, Ladabaum U, et al. Adenoma miss rates associated with a 3-minute versus 6-minute colonoscopy withdrawal time: A prospective, randomized trial. Gastrointest Endosc 2017;85(6):1273–80. [DOI] [PubMed] [Google Scholar]
- 13.Sawhney MS, Cury MS, Neeman N, et al. Effect of institution-wide policy of colonoscopy withdrawal time ≥7 minutes on polyp detection. Gastroenterology 2008;135(6):1892–8. [DOI] [PubMed] [Google Scholar]
- 14.Zhao S, Yang X, Wang S, et al. Impact of 9-minute withdrawal time on the adenoma detection rate: A multicenter randomized controlled trial. Clin Gastroenterol Hepatol 2022;20(2):e168–81. [DOI] [PubMed] [Google Scholar]
- 15.van den Broek FJC, Kuiper T, Dekker E, et al. Study designs to compare new colonoscopic techniques: Clinical considerations, data analysis, and sample size calculations. Endoscopy 2013;45(11):922–7. [DOI] [PubMed] [Google Scholar]
- 16.Butterly LF, Siegel RL, Fedewa S, et al. Colonoscopy outcomes in average-risk screening equivalent young adults: Data from the New Hampshire Colonoscopy Registry. Am J Gastroenterol 2021;116(1):171–9. [DOI] [PubMed] [Google Scholar]
- 17.Schreuders EH, Ruco A, Rabeneck L, et al. Colorectal cancer screening: A global overview of existing programmes. Gut 2015;64(10):1637–49. [DOI] [PubMed] [Google Scholar]
- 18.Chinese Society of Gastroenterology, Cancer Collaboration Group of Chinese Society of Gastroenterology, Chinese Medical Association. Chinese consensus on prevention of colorectal neoplasia (2021, Shanghai). Chin J Dig 2021;41:726–59. [Google Scholar]
- 19.Zimmermann-Fraedrich K, Pohl H, Rösch T, et al. Designs of colonoscopic adenoma detection trials: More positive results with tandem than with parallel studies: An analysis of studies on imaging techniques and mechanical devices. Gut 2021;70(2):268–75. [DOI] [PubMed] [Google Scholar]
- 20.Coghlan E, Laferrere L, Zenon E, et al. Timed screening colonoscopy: A randomized trial of two colonoscopic withdrawal techniques. Surg Endosc 2020;34(3):1200–5. [DOI] [PubMed] [Google Scholar]
- 21.Lam AY, Li Y, Gregory DL, et al. Association between improved adenoma detection rates and interval colorectal cancer rates after a quality improvement program. Gastrointest Endosc 2020;92(2):355–64.e5. [DOI] [PubMed] [Google Scholar]
- 22.Schottinger JE, Jensen CD, Ghai NR, et al. Association of physician adenoma detection rates with postcolonoscopy colorectal cancer. JAMA 2022;327(21):2114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdeljawad K, Vemulapalli KC, Kahi CJ, et al. Sessile serrated polyp prevalence determined by a colonoscopist with a high lesion detection rate and an experienced pathologist. Gastrointest Endosc 2015;81(3):517–24. [DOI] [PubMed] [Google Scholar]
- 24.Kaminski MF, Wieszczy P, Rupinski M, et al. Increased rate of adenoma detection associates with reduced risk of colorectal cancer and death. Gastroenterology 2017;153(1):98–105. [DOI] [PubMed] [Google Scholar]
- 25.Meester RGS, Doubeni CA, Lansdorp-Vogelaar I, et al. Variation in adenoma detection rate and the lifetime benefits and cost of colorectal cancer screening: A microsimulation model. JAMA 2015;313(23):2349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhurwal A, Rattan P, Sarkar A, et al. A comparison of 9-min colonoscopy withdrawal time and 6-min colonoscopy withdrawal time: A systematic review and meta-analysis. J Gastroenterol Hepatol 2021;36(12):3260–7. [DOI] [PubMed] [Google Scholar]
- 27.Jover R, Zapater P, Polania E, et al. Modifiable endoscopic factors that influence the adenoma detection rate in colorectal cancer screening colonoscopies. Gastrointest Endosc 2013;77(3):381–9.e1. [DOI] [PubMed] [Google Scholar]
- 28.Wang P, Liu P, Glissen Brown JR, et al. Lower adenoma miss rate of computer-aided detection-assisted colonoscopy vs routine white-light colonoscopy in a prospective tandem study. Gastroenterology 2020;159(4):1252–61.e5. [DOI] [PubMed] [Google Scholar]
- 29.Tang RSY, Lee JWJ, Chang LC, et al. Two vs one forward view examination of right colon on adenoma detection: An international multicenter randomized trial. Clin Gastroenterol Hepatol 2022;20(2):372–80.e2. [DOI] [PubMed] [Google Scholar]
- 30.Gong D, Wu L, Zhang J, et al. Detection of colorectal adenomas with a real-time computer-aided system (ENDOANGEL): A randomised controlled study. Lancet Gastroenterol Hepatol 2020;5(4):352–61. [DOI] [PubMed] [Google Scholar]
- 31.Bai Y, Fang J, Zhao SB, et al. Impact of preprocedure simethicone on adenoma detection rate during colonoscopy: A multicenter, endoscopist-blinded randomized controlled trial. Endoscopy 2018;50(2):128–36. [DOI] [PubMed] [Google Scholar]
- 32.Li J, Zhang D, Wei Y, et al. Colorectal sessile serrated lesion detection using linked color imaging: A multicenter, parallel randomized controlled trial. Clin Gastroenterol Hepatol 2022;S1542-3565(22)00308-1. doi: 10.1016/j.cgh.2022.03.033 [DOI] [PubMed] [Google Scholar]
- 33.Yeoh K-G, Ho K-Y, Chiu H-M, et al. The Asia-Pacific Colorectal Screening score: A validated tool that stratifies risk for colorectal advanced neoplasia in asymptomatic Asian subjects. Gut 2011;60(9):1236–41. [DOI] [PubMed] [Google Scholar]
- 34.Jia H, Pan Y, Guo X, et al. Water exchange method significantly improves adenoma detection rate: A multicenter, randomized controlled trial. Am J Gastroenterol 2017;112(4):568–76. [DOI] [PubMed] [Google Scholar]
- 35.Keswani RN, Crockett SD, Calderwood AH. AGA clinical practice update on strategies to improve quality of screening and surveillance colonoscopy: Expert review. Gastroenterology 2021;161(2):701–11. [DOI] [PubMed] [Google Scholar]
- 36.Shaukat A, Tuskey A, Rao VL, et al. Interventions to improve adenoma detection rates for colonoscopy. Gastrointest Endosc 2022;96(2):171–83. [DOI] [PubMed] [Google Scholar]
- 37.Gupta S, Lieberman D, Anderson JC, et al. Recommendations for follow-up after colonoscopy and polypectomy: A consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2020;158(4):1131–53.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stryker SJ, Wolff BG, Culp CE, et al. Natural history of untreated colonic polyps. Gastroenterology 1987;93(5):1009–13. [DOI] [PubMed] [Google Scholar]
- 39.Zorzi M, Zappa M. Synthetic indicator of the impact of colorectal cancer screening programmes on incidence rates. Gut 2020;69(2):311–6. [DOI] [PubMed] [Google Scholar]
- 40.Barclay RL, Vicari JJ, Greenlaw RL. Effect of a time-dependent colonoscopic withdrawal protocol on adenoma detection during screening colonoscopy. Clin Gastroenterol Hepatol 2008;6(10):1091–8. [DOI] [PubMed] [Google Scholar]
- 41.Papatheodoridis GV, Triantafyllou K, Tzouvala M, et al. Characteristics of rectosigmoid adenomas as predictors of synchronous advanced proximal colon neoplasms. Am J Gastroenterol 1996;91(9):1809–13. [PubMed] [Google Scholar]
- 42.Shaukat A, Rector TS, Church TR, et al. Longer withdrawal time is associated with a reduced incidence of interval cancer after screening colonoscopy. Gastroenterology 2015;149(4):952–7. [DOI] [PubMed] [Google Scholar]
- 43.Gralnek IM, Siersema PD, Halpern Z, et al. Standard forward-viewing colonoscopy versus full-spectrum endoscopy: An international, multicentre, randomised, tandem colonoscopy trial. Lancet Oncol 2014;15(3):353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paggi S, Mogavero G, Amato A, et al. Linked color imaging reduces the miss rate of neoplastic lesions in the right colon: A randomized tandem colonoscopy study. Endoscopy 2018;50(4):396–402. [DOI] [PubMed] [Google Scholar]
- 45.Khan F, Hur C, Lebwohl B, et al. Unsedated colonoscopy: Impact on quality indicators. Dig Dis Sci 2020;65(11):3116–22. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Q, Dong Z, Jiang Y, et al. The impact of sedation on adenoma detection rate and cecal intubation rate in colonoscopy. Gastroenterol Res Pract 2020;2020:3089094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aziz M, Weissman S, Fatima R, et al. Impact of propofol sedation versus opioid/benzodiazepine sedation on colonoscopy outcomes: A systematic review with meta-analysis. Endosc Int Open 2020;8(6):E701–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tarhini H, Alrazim A, Ghusn W, et al. Impact of sedation type on adenoma detection rate by colonoscopy. Clin Res Hepatol Gastroenterol 2022;46(7):101981. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.