Abstract
Oogenesis and folliculogenesis are considered as complex and species-specific cellular differentiation processes, which depend on the in vivo ovarian follicular environment and endocrine cues. Considerable efforts have been devoted to driving the differentiation of female primordial germ cells toward mature oocytes outside of the body. The recent experimental attempts have laid stress on offering a suitable microenvironment to assist the in vitro folliculogenesis and oogenesis. Despite developing a variety of bioengineering techniques and generating functional mature gametes through in vitro oogenesis in earlier studies, we still lack knowledge of appropriate microenvironment conditions for building biomimetic culture systems for female fertility preservation. Therefore, this review paper can provide a source for a large body of scientists developing cutting-edge in vitro culture systems for female germ cells or setting up the next generation of reproductive medicine as feasible options for female infertility treatment. The focal point of this review outlines advanced bioengineering technologies such as 3D biofabricated hydrogels/scaffolds and microfluidic systems utilized with female germlines for fertility preservation through in vitro folliculogenesis and oogenesis.
Keywords: female germ cells, in vitro folliculogenesis, in vitro oogenesis, advanced tissue engineering, female reproductive system, biomaterials, stem cells
Utilizing cutting-edge bioengineered culture microenvironments, it is possible to achieve successful in vitro folliculogenesis and oogenesis for therapeutic purposes.
Graphical Abstract
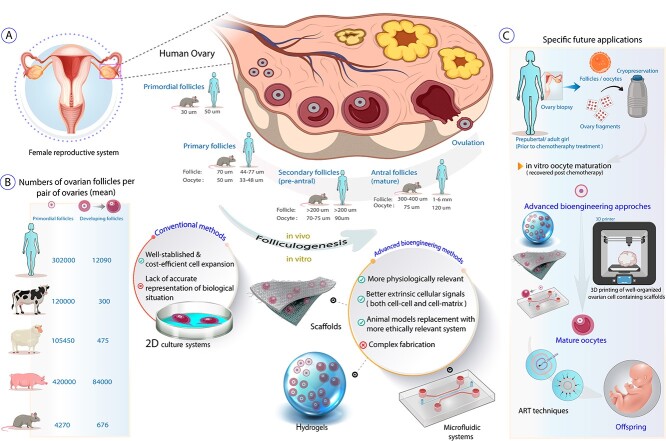
Schematic representation of species-specific differences in folliculogenesis between rodents and humans (A) plus estimates of the number of ovarian follicles in various species (B). The current and specific future applications of advanced technologies in female fertility preservation and in vitro folliculogenesis, utilizing 3D organ culture techniques, hydrogels, scaffolds, microfluidic systems, and bioprinters are illustrated and compared with the conventional culture systems (A and C). Some items created with BioRender.com
Graphical Abstract.
Introduction
Female infertility treatments have become increasingly important as a result of growing infertility with age and socioeconomic drives to delay having children. While infertility in males plays a role, female infertility remains the main cause of difficulties in conceiving. Significant recent progress has been made in bioengineering techniques which give promise of improved therapies for female infertility. Cryopreservation of ovarian tissue taken early in life can provide sources for in vitro fertilization at later time points; however, this is not always a viable option. In patients suffering from cancer who face the risk of metastasis in their ovaries, conventional ovarian tissue cryopreservation and re-transplantation of them to the patient after the treatment and generating healthy babies is in principle a possibility; however, risks of reestablishing cancer via returning tumor cells in the ovary complicate this option (1). In another patient group, fertility preservation is needed for patients with premature ovarian failure arising from several diseases such as pelvic inflammatory disease, ovarian endometriomas, and polycystic ovary syndrome (2). Here, culturing isolated follicles on an artificial ovary could be considered a safe approach to prevent cancer relapse in patients or an optimal option for others struggling with benign disease (3). The mammalian ovary's morphological and biofunctional unit, follicles, offers the proper microenvironment for oocyte growth, maturation, and ovulation. Follicles contain the oocyte being enveloped by granulosa cells connected through gap junctions. Such intercommunication leads to further proliferation of granulosa cells to develop a multilayered arrangement surrounding the oocyte, which both are encircled by theca cell layers (4).
The process of female germ cell nest breakdown during human fetal development and before birth in the rodent establishes primordial follicles consisting of a single circular layer of squamous granulosa cells embracing an oocyte (5). Primordial follicles dynamically either continue the developmental transition into primary follicles (an oocyte enclosed by one layer of cuboidal granulosa cells), one wave starts to develop right away, but most remain quiescent until sexual maturity (takes above 5 months in the human), or undergo atresia. Further proliferation of granulosa cells and oocyte growth, which both are covered by a basement membrane and the theca cells, form pre-antral follicles. These multilaminar follicles keep growing and convert into antral follicles and subsequently preovulatory follicles (6). During female reproductive life, most ovarian follicles (>99%) undergo a degenerative process termed atresia. While atretic degeneration can occur during any stage of ovarian development, granulosa cell apoptosis or insufficient follicle-stimulating hormone (FSH) are certain important factors leading to atresia (7).
Folliculogenesis is a sophisticated and multidirectional process that is governed by growth and signaling factors produced by follicular cells and the hypothalamic–pituitary–ovarian axis (8). Acquiring new fundamental knowledge on how folliculogenesis is controlled and regulated is crucial for the female's reproductive health. An in vitro system that can support folliculogenesis and oogenesis would shed more light on the mechanisms involved in oocyte maturation and ovulation to fulfill the objective.
Martinovitch in 1937 showed the first fruitful effort of in vitro ovarian tissue growth using the organ culture of whole mouse and rat ovaries on watch glasses (9). Attempts for improving the in vitro culture system for follicle and oocyte development outside of the body progressed with Eppig in 1977, showing the successful culture of isolated follicles (10). The conventional culture systems applying two-dimensional (2D) surfaces have been utilized for in vitro culture of follicles obtained from different species (11–14); however, only murine folliculogenesis has been able to produce mature oocytes and then live offspring (15, 16). The more complex 3D microenvironment is needed to precisely recapitulate the morphological and physiological properties of the mammalian ovaries along with folliculogenesis and oogenesis processes in vitro. The natural- or synthetic-based three-dimensional (3D) culture systems combined with follicular cells could maintain the appropriate cell-cell and cell-matrix signaling contributing in follicle and oocyte development. By selecting proper biomaterials and adding the necessary biochemical signals, 3D microenvironments could support oocyte maturation and preserve follicle architecture followed by ovulation.
This paper summarizes advanced tissue engineering strategies for bioengineering of the follicular cells for producing mature and functional oocytes, including different 3D cultures and microfluidic systems.
Hydrogels for in vitro ovarian follicle culture
In vitro 3D culture of the ovarian follicle has the potential to enable processes of folliculogenesis and preserve fertility by creating an adjustable milieu that can coordinate the cellular growth within the follicle. Maintaining the follicular shape along with regulating the physical and chemical features of the microenvironment can be used as a tool to understand the underlying biology of follicle maturation. In reproductive biology, hydrogels have been mainly utilized for encapsulating reproductive cells due to their adjustable properties and biocompatibility (17). Recapitulation of the ovarian follicular structure, consisting of the central oocyte enclosed by somatic cells, is strongly dependent on the surrounding microenvironment properties which are vital for preserving the oocyte–somatic cell connection and good functionality (18). Development of the hydrogel encapsulation technology in reproductive tissue regeneration may enable in vitro models to mimic functional tissue. Being encapsulated in a 3D hydrogel, follicles preserve their inherent structure and do not stick to culture platforms, while 2D culture vessels result in follicles spreading on the plate, and the granulosa cells migrate to the surface instead of surrounding the oocyte (19). Therefore, hydrogel culturing systems have the potential to regulate follicle development by providing the appropriate cell–cell and cell–matrix interactions (Figure 1) (20). To create an optimal 3D culture system for in vitro folliculogenesis, natural and synthetic hydrogels such as alginate, agar, collagen, and poly (ethylene glycol) (PEG) have been widely used. Matrigel and collagen that contain extracellular matrix (ECM) proteins are attractive hydrogels for culturing follicles due to mild crosslinking conditions, inherent bioactivity, optimal biocompatibility, and the proper mechanical properties which promote follicle expansion (21, 22). However, the main drawbacks of such hydrogel are follicle extraction using enzyme treatment that damages the ECM of follicles. Agarose and PEG seem to be suitable candidates for culturing follicles from the mechanical perspective, but during encapsulation, the gelation process may harm the follicle structure (23, 24). In contrast, alginate has been introduced as an appropriate natural polymer-based hydrogel for follicle culture owing to the straightforward and mild gelation condition with adjustable mechanical properties (25) (Table 1).
Figure 1.
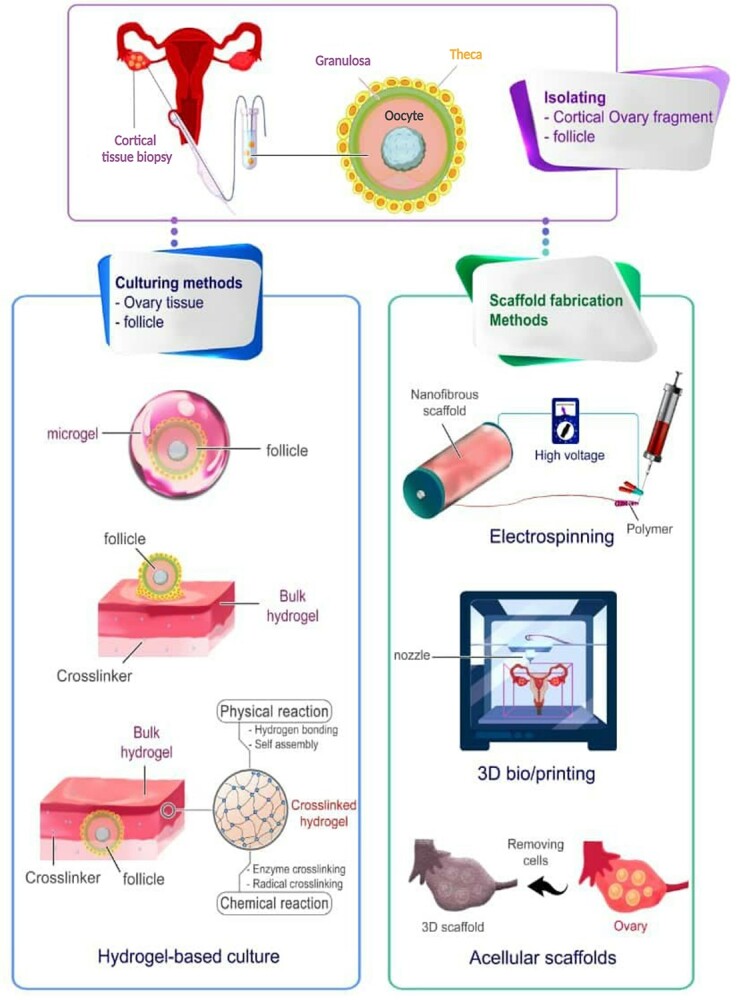
Schematic representation of applicable state-of-art technologies in female fertility preservation and in vitro folliculogenesis through utilizing various hydrogels and bioscaffolds. Bioscaffolds in forms of hydrogels, porous or fibrous structures can be fabricated via different approaches like acellularization of tissues, electrospinning, 3D printers, and so on using natural (collagen, alginate, agar, decellularized testis tissue-driven ECM, etc.) or synthetic (poly-L-lactide (PLLA), Poly(vinyl alcohol) (PVA), PCL, etc.) materials. The ovarian cortical fragments or isolated ovarian follicles could be cultured in vitro through the fabricated scaffolds to accomplish their goal for female fertility preservation.
Table 1.
Different types of hydrogels applied in supporting folliculogenesis and oogenesis
| Specie Family | Hydrogel and supplementary additives | Cell source | Stages of follicles and oocytes development | Main results | Ref. |
|---|---|---|---|---|---|
| Primate (Human) | Collagen type IX FSH | Primary follicles (29±11 years old) |
Ovulation | Histological findings from embedded follicles in collagen gel exhibited an increase in granulosa cell layer numbers and oocyte size in ~40% of the follicles within 24 h. TEM images indicated the formation of cellular outgrowths around growing follicles. Also, more extended culture led to follicle deterioration and oocyte release. | (26) |
| Primate (Human) | Collagen type I FSH | Fully and partially isolated follicles (30±7 years old) | - | Electron microscopy studies revealed detachment of partially isolated follicles from collagen. According to histological examinations, the fully isolated follicle size and granulosa cell numbers increased on collagen gel. Moreover, fully and partially follicles deteriorated within 24 h cultured on top of collagen. | (27) |
| Primate (Human)+ Rodent (Hamster) | Bacteriological Agar FSH | Pre-antral follicles (isolated from premenopausal human ovaries) +Large pre-antral follicles (7-8 layers of granulosa cells and thin theca cells) | Antral | Culturing human follicles for 168 h and their analysis by morphological test showed developia2ng pre-antral follicles to the antral stage creating an antral cavity with germinal vesicle oocyte. | (28) |
| Primate (Human) | Sodium alginate | Pre-antral follicles (25-35 years old) | Pre-antral | All 9 follicles incubated in an alginate-based 3D system after 7 days showed an increase in size with a survival rate of 90% according to morphological and histological assays. | (29) |
| Primate (Human) | Sodium alginate FSH | Secondary follicles (16-39 years old) | Antral | The 3D alginate hydrogel supported follicle development from early secondary to the antral stage containing healthy and growing oocytes with proper steroid production through hormonal and morphological investigations. | (30) |
| Primate (Human) | Sodium alginate | Primordial and primary follicles (21-31 years old) | Primordial and primary | Cryopreservation of follicles embedded in alginate beads with two other cryoprotectants (dimethyl sulfoxide (ME2SO) and ethylene glycol (EG)) showed better follicles preservation for 1.4 M ME2SO compared to 1.5 M EG after thawing and 7 days of culture according to viability and morphological analysis. | (31) |
| Primate (Human) | Alginate FSH | Primordial follicles and ovarian cortex tissue (2-41 years old (median age: 20.5 years)) | Pre-antral and antral |
Histological and morphological findings indicated that encapsulation and culture of ovarian cortical fragments in alginate hydrogel maintained the survival, differentiation, and growth of follicles up to growing pre-antral and antral stages within several weeks while the isolated follicles degenerated during 24 h. | (32) |
| Primate (Human) | Alginate FSH | Multilayered secondary follicles (72.7% younger than 20 years old, 20.5% between 20-30 and 6.8 % older than 30) | Antral MII oocytes |
The hydrogel system supported follicles developing from the pre-antral to the antral stage, producing meiotically competent metaphase II (MII) oocytes after in vitro maturation (IVM). | (33) |
| Primate (Human) | Alginate EGF, FSH, hCG, |
Secondary follicles (6-34 years old) | Ovulation | The alginate hydrogel system could support folliculogenesis and also the transformation accompanied with ovulation and luteinization. Follicles showed similar patterns of serum hormones identified through the menstrual cycle. | (34) |
| Primate (Monkey) | Sodium alginate FSH, LH | Pre-antral follicles (8-9 years old) | Antral | Based on the morphological result, different survival rates were seen in isolated follicles from the follicular phase of the menstrual cycle (higher) compared to those isolated from the luteal phase (lower). Follicles encapsulated in both alginate concentrations (0.5% and 0.25%) could grow and develop into the steroidogenesis phase by producing estradiol, androstenedione, and progesterone for ≤30 days. | (35) |
| Primate (Monkey) | Sodium alginate FSH | Primordial follicle (6.5, 9.5 and 12 years old) | Primordial | Utilizing various alginate concentrations, it was indicated that the follicle survival and morphology of encapsulated follicles in higher alginate concentrations (2%) were more optimal compared to softer hydrogel (0.5%) through histological assessment. | (36) |
| Primate (Monkey) | Fibrin-sodium alginate FSH | Pre-antral follicles (7–10 days postovulation) | Antral MII oocytes | Fibrin-alginate hydrogel enhanced the growth rate of pre-antral follicles and supported them to reach the small antral stage in the absence of FSH. | (37) |
| Primate (Monkey) | Fibrin-sodium alginate FSH | Primary and secondary follicles (7-14 years old) | Antral MII oocytes | The fibrin hydrogel could improve theDevelopment of only primary but not secondary follicles at week 1; however, the alginate encapsulation enhanced the growth properties of secondary follicles.. Moreover, only the fertilized oocyte from the secondary follicles reached the morula stage, while the oocyte from the primary follicle arrested without cell division. | (38) |
| Primate (Human) | PEG-fibrinogen and alginate FSH | Ovarian tissue (5–27 years old) | Primordial | PEG-fibrinogen hydrogels system supported the in vitro development of the follicles with less atretic follicles formation than alginate scaffolds. | (39) |
| Rodent (Mouse) | Collagen type I | Primordial to secondary follicles (10 days old) | Pre-antral | The 14-day culture in collagen gel matrix-maintained follicle 3D structure and follicle growth from unilaminar to multilaminar within 6 days based on morphological, and histological examinations. | (40) |
| Rodent (Mouse) | Collagen type I FSH | Pre-antral follicles (24-28 days old) | Pre-antral | Based on morphological and immunocytochemistry tests, the 3D gel-maintained follicle 3D structure and supported follicle growth and differentiation while 2D culture led to flattened follicles and caused spontaneous follicle disruption. | (41) |
| Rodent (Mouse) | Alginate-Collagen I FSH | Secondary follicles (12-16 days old) | Pre-antral | When cultivated in alginate-collagen I matrices, two-layered secondary follicles were FSH responsive, with FSH dose-dependent increases in follicle development, lactate generation, and steroid release according to histological and hormonal assessments. FSH, on the other hand, had a deleterious impact on the survival and Development of multilayered secondary follicles. | (42) |
| Rodent (Mouse) | Collagen I FSH | Primary and early secondary follicles (6 days old) | Ovulation | A multi-step follicle culture within collagen hydrogels indicated growing follicles and producing oocytes based on morphological and molecular analysis that were able to generate live birth after implantation in pseudo pregnant mice. | (43) |
| Rodent (Mouse) | Agar FSH | Early pre-antral follicles (14 days old) | Pre-antral MII oocytes | Based on the morphological assessment, in vitro growing pre-antral follicles in agar, oil, and hydrophobic ECM showed that agar led to follicular growth, although oocyte maturation percentage and oocytes reaching nuclear maturation was higher in oil culture. | (44) |
| Rodent (Mouse) | Sodium alginate FSH, EGF | Granulosa cell–oocyte complexes (12 days old) | - | The complex follicular architecture was maintained during the encapsulation confirmed through the TEM images. Besides, the granulosa cells proliferated, and the oocytes volume increased based on morphological findings. | (45) |
| Rodent (Mouse) | Sodium alginate FSH | Secondary follicles (12 days old) | Antral MII oocytes | Comparting cryopreserved and non-cryopreserved follicular culture on alginate hydrogel revealed a decrease in androstenedione levels in the cryopreservation group than in the other groups at day 12 of culture. Moreover, both individually isolated follicles or those within ovarian tissue could be successfully recovered and developed after slow-freezing and thawing | (46) |
| Rodent (Mouse) | Sodium alginate FSH | Secondary follicles (12-16 dpp) | Antral | Alginate hydrogels supported the Development of 2-layer and multi-layer secondary follicles within 12 days. Moreover, analysis of molecular markers involved in different stages of folliculogenesis for follicles grown in vitro compared to those grown in vivo showed 60% similarities among gene patterns during four days. | (47) |
| Rodent (Mouse) | Alginate FSH | Primary and early secondary follicles (isolated based on average initial diameter (70, 80, 90, and 100 μm)) | Antral | Co-culture of encapsulated follicles in alginate hydrogels with mouse embryonic fibroblasts (MEF) supported follicles survival and growth, which developed antral cavities within 14 days while culturing follicles without MEF resulted in cell degeneration within 6–10 days confirmed via morphological analysis. | (48) |
| Rodent (Mouse) | Alginate Insulin, IGF | Ovarian tissue | - | Adding insulin and IGF (potent proliferative factors) in culture media where ovarian tissue culturing in alginate hydrogels led to decreased follicular integrity with formation of the hyperplastic ovarian surface epithelium. | (49) |
| Rodent (Mouse) | Alginate FSH, hCG |
Primary follicles (12 days old) | Antral | The multiple cultured follicles in alginate hydrogels were survived and developed into the antral cavity formation stage, which contained meiotically competent gametes. | (50) |
| Rodent (Mouse) | Alginate FSH | Late primary and early secondary follicles (8-12 days old) |
Antral MII oocytes | The growth of encapsulated late primary and early secondary follicles was increased with antral cavities formation in the feeder-free medium containing αMEM/F12 (1:1) supplemented with fetuin, ITS, and FSH according to morphological assessments. | (51) |
| Rodent (Mouse) | Alginate FSH, Ascorbic acid | Primary and secondary follicles (7–10 days old) | Antral | The survival and growth of primary follicles were improved when they were cultured in a 3D system (alginate hydrogel) co-cultured with MEF and supplemented with ascorbic acid (50 μg/ml) for 18 days confirmed by morphological, immunohistological, and molecular examinations. | (52) |
| Rodent (Mouse) | Alginate FSH | Early secondary follicles (12 days old) | Pre-antral | Investigation of oxygen tension role on the early secondary follicles (110 μm) Development which were cultured on alginate hydrogel showed the close correlation between follicular survival/growth to hypoxia-mediated carbohydrate transport and metabolism (glycolysis). | (53) |
| Rodent (Mouse) | Sodium alginate hCG, Panax ginseng extract (PGE) | Pre-antral follicles (14 days old) | Pre-antral | Encapsulated follicles in sodium alginate showed variable growth rates and oocyte maturation depending on the concentration of PGE, demonstrating enhanced follicular function and development at the concentration of 100 μg/mL of supplemented PGE. | (54) |
| Rodent (Mouse) | Alginate FSH, EGF, hCG | Secondary follicles (18 days old) | Ovulation | The alginate hydrogel system could support folliculogenesis and also the transformation accompanied with ovulation and luteinization. Most of the cultured murine follicles ovulated in response to hCG. | (34) |
| Rodent (Mouse) | Alginate FSH, EGF, hCG | Secondary follicles (16 days old) | Ovulation MII oocytes | The percentage of MII oocytes for follicles encapsulated in alginate-based hydrogel was the highest (85%) on day 6 while this value increased for cultured follicles to a terminal diameter of 300–350 μm (93%) . | (55) |
| Rodent (Mouse) | Alginate FSH | Multilayered secondary follicles (16 days old) | Antral MII oocytes | The follicle growth, survival, and functions of encapsulated follicles in alginate beads were affected by the exposure levels of doxorubicin (DOX). Higher DOX concentration (200 nM) led to DNA damage and apoptosis, while the number of MII oocytes was increased at 20 nM DOX. | (56) |
| Rodent (Mouse) | Alginate Sodium alginate FSH, LH, EGF, hCG | Primary follicles (10-12 days old) | Antral | When compared to follicles cultivated in groups of 5, encapsulated follicles in groups of 10 demonstrated increased levels of survival, growth, and steroidogenesis. | (57) |
| Rodent (Mouse) | Alginate FSH, EGF, hCG | Pre-antral follicles (12-14 days old) | Ovulation MII oocytes | The 3D alginate-based system could support pre-antral follicles development outside of the body and approximately could recapitulate the in vivo situation which is useful as a model system for female reproductive toxicity testing system- | (58) |
| Rodent (Mouse) | Fibrin-alginate FSH, EGF, hCG | Multilayered secondary follicles (15-16 days old) | Antral MII oocytes | The encapsulation of both freshly harvested and vitrified follicles in alginate preserved the 3D structure of follicles. Moreover, alginate hydrogel supported folliculogenesis from multilayered secondary stage (day 0) to antral stage (day 8). | (59) |
| Rodent (Mouse) | Animal origin-free extracellular matrix-derived (ES) & alginate FSH, EGF, hCG | Two-layered secondary follicles (12 days old) | Antral MII oocytes | Based on morphological and hormonal assessments, interpenetrating fibrin–alginate matrices support follicle growth with a higher number of meiotically competent oocytes compared to either fibrin or alginate hydrogel. | (60) |
| Rodent (Mouse) | Pre-antral follicles (11-14 days old) | Ovulation MII oocytes | ES-hydrogel and alginate hydrogel could support follicle development. However, the pseudo-antrum formation, cumulus-oocyte complexes, MII oocyte, spindle rate, and E2 production level were higher in ES-hydrogel than 2D and alginate hydrogel groups. | (61) | |
| Rodent (Mouse) | Extracellular matrix-derived hydrogels (fibronectin, collagen, and laminin) & matrigel FSH, activin-A | Pre-antral follicles (14 days old) | Antral | Matrigel system could maintain the 3D structure of follicles for 7 days. Moreover, the matrigel system containing activin-A increased follicle growth, survival rate and formed antral space. | (62) |
| Rodent (Mouse) | Chitosan & alginate FSH, hCG | Pre-antral follicles (12 days old) | Antral MII oocytes | The chitosan-based culture system could support the growth of the follicle with a higher survival rate, follicle diameter, and chromosome alignment compared to alginate group. | (63) |
| Rodent (Mouse) | Polyethylene glycol (PEG) FSH, EGF, hCG | Immature secondary follicles (14-15 days old) | Antral MII oocytes | Applying trifunctional cross-linking peptides to 4-arm polyethylene glycol (PEG) hydrogels improved network formation and supported in vitro ovarian follicle maturation. | (17) |
| Rodent (Mouse) | Polyethylene glycol (PEG) FSH, EGF, hCG | Early secondary and pre-antral follicles (14 days old) | Antral MII oocytes | Comparing different gel softness (as reflected in swelling ratio) impacts on in vitro growth of follicles showed the swelling ratio above 21.4 resulted in better oocyte maturation than other levels. However, the highest competence to blastocyst formation was identified at 20.6 | (64) |
| Rodent (Mouse) | ECM binding peptide-functionalized polyethylene glycol (PEG) FSH, EGF, hCG | Secondary follicles (2-14 days old) | Antral MII oocytes | The PEG hydrogels functionalized with extracellular matrix (ECM)-sequestering peptides significantly enhanced follicle survival, growth, and maturation of ovarian follicles compared to the bioinert PEG hydrogel. | (65) |
| Rodent (Mouse) | RGD-modified dextran hydrogel FSH, EGF, Activin A | Immature ovary freagments (14 dpp) | Antral MII oocytes | By establishing an ECM-mimetic bioactive microenvironment to assist folliculogenesis in a 3D ovarian tissue culture platform, RGD-modified dextran hydrogels were capable to provide physical support as well as promote follicle expansion through in vitro culture system. Oocytes grown on RGD-modified dextran hydrogels were fertile and produced blastocysts. | (66) |
| Bovidae (Bovine) | Collagen FSH, LH, E2, bFGF, EGF | Primary follicles (50–70 mm) | Antral | Morphological results indicated increases in follicles diameter. Molecular findings showed the expression of follicular maturation markers was significantly altered and the antrum formation rate of follicles increased in presence of hormonal treatment | (67) |
| Bovidae (Goat) | Agar FSH, IGF-1 | Primary and secondary follicles | Antral | According to histological and morphological findings, agar culture-maintained follicle 3D structure and facilitated Development into secondary and antral follicles. Oocyte growth was observed and theca lamina and zona pellucida were formed. The survival rate of secondary follicles was ~7 times more than primary follicles. | (68) |
| Bovidae (Caprine) | Alginate FSH, LH, 17β-estradiol, EGF, IGF-1 | Pre-antral follicles (1-3 years old) | Antral MII oocytes | Culturing pre-antral follicles in a 3D hydrogel fabricated with different concentrations of alginate revealed that more morphologically normal follicles developed in stiffer hydrogel (0.5% and 1% alginate); however, follicles on the softer hydrogel (0.25%) showed higher levels of estradiol and progesterone with an enhanced rate of meiotic resumption compared to stiffer alginate hydrogel (0. 5% and 1%). | (69) |
| Bovidae (Caprine) | Fibrin–alginate FSH, LH, 17β-estradiol, EGF, IGF-1 | Multilayered secondary follicles (≥200 μm) (1-3 years old) | Antral MII oocytes | The fibrin–alginate hydrogel supported follicles growth and maturation up to producing parthenotes. | (70) |
| Suidae (Pig) | Collagen FSH | Pre-antral follicles (containing oocytes of 70-89.5 μm in diameter) | Antral MII oocytes | The morphological assessment showed increasing in follicles diameter. Based on molecular analysis, progression to metaphase II was observed in 40% of oocytes that were over 110 μm in diameter. | (71) |
| Suidae (Pig) | Collagen type I FSH, IGF-1 | Pre-antral follicles (12-15 weeks) | Antral | Follicles cultured for 30 days in collagen-coated 24-well plates by morphological assessment showed maintaining their 3D structure and oocyte viability. Follicle diameter increased while 29% of follicles formed an antrum. | (72) |
| Canidae (Dog) | Alginate FSH, LH | Pre- and early antral follicles (6 months-5 years old) | Antral | The alginate hydrogel supported the development of encapsulated follicles. Evaluation of physical (alginate rigidity) and hormonal influences (FSH concentration) of microenvironment on in vitro growth of follicles indicated that lower alginate concentration and increasing FSH amount stimulate in vitro follicle growth confirmed by hormonal and morphological assays. | (73) |
Hydrogel properties for in vitro folliculogenesis
To support the ovarian follicle, hydrogels should possess an optimized rigidity to preserve the 3D shape of the follicle along with maintaining an expansion ability because of oocyte growth, granulosa cell proliferation, and antrum formation (74). Mechanical features and density of the surrounded environment influence cellular activity and tissue formation. Follicle growth usually creates tension in the surrounding matrix resulting from the hydrogel stiffness and follicle-exerted force (63). Such reciprocated forces on the follicle need to be regulated because excess pressure causes follicular apoptosis and decreases the proliferation rate of the follicles (75). Hydrogel stiffness is related directly to the precursor concentration of biomaterials used for encapsulation. The hydrogel stiffness should be optimized to support cellular activity such as appropriate follicle growth, cellular differentiation (like theca cells), oocyte maturation, and acceptable secretion of hormones. Experimentally verification has shown that stiffer hydrogels lead to higher estradiol (E2) levels stimulated by androgen and progesterone, while hydrogels with lower stiffness induce estrogen production before the accumulation of androgen and progesterone (76). Hydrogel performance, from rigidity to responsive actions, follows the applied crosslinking techniques (77). To synthesize a 3D hydrogel for follicular cell culture, an appropriate crosslinking method should be used to minimize cell damage. Hydrogels can be crosslinked using different approaches where polymer chains are crosslinked by physical and chemical interaction (78). To achieve proper cell encapsulation with a reasonable degradation rate along with preserving the microgel integrity, such methods should be selected wisely as some have significant effects on the mechanical properties of the hydrogel and subsequently affect follicular cell proliferation, survival, differentiation, and migration.
Natural-based hydrogels are usually synthesized using physical crosslinking such as ionic crosslinking (79). Covalent crosslinking methods are tunable and have attracted broad attention because the hydrogel properties can be adjusted easily in terms of both mechanical properties and degradation rate; however, in some cases degradation products have shown cytotoxicity. Chemically crosslinked hydrogels present higher stiffness properties and adjustable swelling and degradation rates. Conversely, adding additional chemical agents risks causes toxicity and the hydrogel residue should be analyzed carefully after degradation (80). It should be noted that the specific choice of crosslinking methods can give, for example, injectable and/or self-healing hydrogels. Radical polymerization, which can enable injectability, is usually initiated by ultraviolet light, temperature, and chemical agents, which can harm the follicular cells, and even in some cases such crosslinked polymers are not degradable (78). Successful folliculogenesis and oocyte maturation in hydrogels strongly depend on matrix elasticity which supports follicle enlargement and ovulation.
Along with crosslinking method and density, monomer concentration and type of monomer/macromer significantly affect the mechanical properties of hydrogels (81). In a study by Shikanov et al., the crosslinking impacts on hydrogel integrity and consequently on follicle encapsulation were evaluated (17). For hydrogel fabrication, a four-arm PEG acrylate was used as a hydrogel precursor, and a trifunctional peptide as a crosslinking agent was exploited. Based on the obtained results, the trifunctional crosslinker decreased the swelling capacity compared with the bifunctional one and prevented the formation of dangling ends, while increased hydrogel elasticity. Bifunctional crosslinking agents caused cellular dehydration owing to the osmosis phenomenon in the period of the prolonged gelling process, while hydrogel based on trifunctional crosslinker promoted ovarian follicle maturation. It is noteworthy that the synthesized hydrogel could tolerate a 17-times expansion of the tissue which was regulated by the sensitivity of the crosslinker to the plasmin proteolytic activity. Such ideal hydrogels could preserve the intact follicle structure during gelation, giving improved fertility which can lay the basis for developing a technology for women encountering premature infertility from anticancer treatments (17).
One of the most prevalent shortcomings in hydrogel design is defect creation, which prolongs the gelling period, enhances the swelling ratio, and decreases elasticity. Defects can be formed from inadequate crosslinking, hydrogel heterogeneity, or intramolecular crosslinking creating a primary loop (17). For flawless hydrogel formation, a high concentration of precursors is used to carry out crosslinking reactions intramolecularly; although, for hydrogels used to encapsulate cells, a low concentration of precursors is required to avoid cytotoxicity, which can cause the formation of defects in the hydrogel (82).
Different strategies have been used to synthesize and functionalize the utilized hydrogel for cell culture but certain criteria should be considered in cell encapsulation using hydrogels. Before encapsulation, cells should be suspended in the liquid precursor (water-soluble) and such solution should be crosslinked in mild conditions to avoid damage to the cells. The aqueous solution should have an appropriate osmolality to avoid lysis of the cell (83). Hydrogels for cell encapsulation are usually synthesized from macromeres with >3 kDa (macromolecular monomers) derived from biocompatible polymers (i.e. typical hydrogels are synthesized from low molecular weight monomers causing toxicity) (84). Moreover, hydrogel chemistry should be biocompatible to carry and protect the cell and degrade with the well-orchestrated period of tissue formation, and the degradation products should not harm the cells (85).
Collagen
Collagen is one type of natural polymer that can be utilized for hydrogel fabrication. Torrance et al. first applied collagen gels for pre-antral follicle culture in 1989. The collagen-gel culture system supported normal follicle development for 2 weeks (40). Comparing flat culture vessels to 3D collagen gels for in vitro follicle growth in another study demonstrated that 3D collagen environments maintained mouse follicle organization and basal lamina compartments, which decreased the rate of follicular disruptions (41). In vitro follicular cell culture and maturation systems vary for each species or follicular stage. It has been demonstrated that a 16-day culture of large pre-antral follicles from pigs on collagen gels could mature to antral stages and obtain meiotic competence (71) while early follicles isolated from fresh or frozen-thawed human ovaries could survive and grow to pre-antral stages on collagen gels up to 24 h, but then started deterioration (26). Co-culturing granulosa cells with isolated follicles did not show any positive advantage, and only the fully isolated follicles could grow inside collagen hydrogel (27). In 2002, long-term culture of pig pre-antral follicles in a serum-free media utilizing collagen hydrogel was achieved. Collagen-based culture systems supported follicles for up to 30 days to grow and produce progesterone and E2 (72).
Follicle development and oocyte maturation are gonadotropin-dependent processes. In this regard, Sun et al. applied 3D collagen gel supplemented with FSH + luteinizing hormone (LH) + E2 + basic fibroblast growth factor + epidermal growth factor for the long-term growth of primary bovine follicles. Their optimized in vitro culture system could support follicular diameter enlargement, antral formation, and upregulation of oocyte maturation markers (growth differentiation factor-9 [GDF9] and bone morphogenetic protein-15 [BMP15]) during 21-day culture (67). Although the growth of animal middle/late secondary pre-antral follicles outside of the body exploiting collagen-based gels was reported, the culture of early growing pre-antral follicles (with one to three layers of somatic cells) needs further optimizations. Addressing the challenges, a multistep culture system was established to induce the growth of isolated mice primary/early secondary follicles. First, pre-antral follicles enclosed by one or a few layers of granulosa cells (<100 μm in diameter) were encapsulated in collagen microdroplets covered with mineral oil. Growing follicles were then separated from the gel on day 9 and were placed on collagen-coated membrane inserts for 8 days. The mature oocytes obtained from the surviving follicles cultured in this 3D system underwent in vitro fertilization, leading to two live offspring (43). FSH is broadly applied in assisted reproduction technologies owing to its beneficial effects on folliculogenesis which stimulates and improves in vitro oocyte maturation. To investigate the effects of FSH on mouse follicle development in a 3D cell culture microenvironment, Kreeger et al. utilized the two-layered and multi-layered secondary follicles encapsulated in alginate/collagen and alginate hydrogels. The two-layered follicles (100–130 μm; oocyte, 53–63 μm) encapsulated in alginate/collagen matrix were FSH dependent and they became significantly larger in the presence of FSH (5–25 mIU/ml); while the survival rate of multi-layered follicles (encapsulated in alginate hydrogel) was FSH dose-dependent, where doses greater than 25 mIU/ml of FSH adversely impacted follicle development (42).
Agar
Agar, a two-component mixture of the linear polysaccharide agarose and another polysaccharide agaropectin, is commonly used as a gelling agent in a microbial context for culturing bacteria. However, it can also be employed to fabricate hydrogels, making it a potential platform for research into the processes of folliculogenesis and oogenesis. In 1989, Roy and Greenwald first applied agar hydrogels to attempt a long-term culture method to investigate folliculogenesis regulating factors in pre-antral hamster follicles for up to 72 h (86). The same culture method was used to study isolation and culture methodologies for human, mouse, rat, pig, and hamster follicles for up to 168 h. The enzymatic separation procedure utilizing a collagenase and deoxyribonuclease mixture proved to be an efficient approach for separating follicles while at the same time preserving the basal lamina, especially in hamster and human specimens (28).
Furthermore, Huanmin and Yong investigated the in vitro growth and development of caprine pre-antral follicles cultured for 14 days to observe the growth and developmental pattern. The hydrogel supported the natural three-dimensional follicular structure, although the basal lamina started to decompose at the mid-stage, and granulosa cells began to spread. Additionally, some of the primary follicles successfully converted to secondary follicles; however, the pre-antral follicles' growth rate was faster than in an in vivo environment (68).
Fabricating and selecting an optimal microenvironment for in vitro culture of follicles/oocytes remains a significant challenge. In that respect, Mousset-Siméon et al. compared three different in vitro culture systems, namely oil, a hydrophobic membrane, and agar and evaluated follicular growth and meiotic maturation of oocytes or atresia. The follicles cultured on oil showed the highest follicle diameter, possibly caused by the lack of a three-dimensional structure and therefore did not reflect the natural ovarian environment (44).
Alginate
Alginate is a linear polysaccharide composed of β-d-mannuronic acid (M units) and α-l-guluronic acid (G units) extracted from brown algae. This polymer can be crosslinked using divalent (Ca2+, Ba2+, Fe2+, Sr2+, or so forth) or trivalent ions (like Al3+ ) participating in the interchain ionic binding between G units (known as an egg box) in the alginate chain leading to form a 3D network (87). Alginate provides a beneficial microenvironment to investigate the influences of the different factors on follicle development due to the lack of interaction with cellular signaling (88). It was demonstrated that the follicle culture in alginate microbeads preserved the follicle 3D shape and promoted the production of fertilizable oocytes. Ovarian cells can be encapsulated within alginate with minimal damage due to its mild gelation conditions; however, hydrogel degradation should be adjusted to allow extraction of the intact cultured cells within the hydrogel. Calcium binding to the chelator (like ethylene glycol bis (β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid) can control the alginate degradability; however, during retrieval, chelation effects on the follicle survival should be considered. An alternative method for alginate degradation to remove follicles is alginate lysis using enzymes.
Alginate properties such as gelation, mechanical properties, and the swelling ratio are related to the M/G proportion, where M-rich alginates are softer and more fragile with lower porosity (89). Mechanical properties such as stiffness and the mesh size can be modulated by changing the alginate formulation. In 2003, Pangas et al. developed an in vitro culture system with alginate hydrogel that provides a three-dimensional environment for the growth and development of mouse granulosa cell oocyte complexes with an incorporation efficiency of 50%. The cultured complexes supported oocyte maturation enabling them to resume meiosis (45). Furthermore, hydrogel properties were varied through altered solids concentration or molar mass of alginate to study their role on murine follicular growth and development (76). The biomechanical environment directly influenced follicle function, and changes in hormonal secretion revealed different behavioral patterns depending on the matrix stiffness. In general, a lower stiffness allowed for enhanced follicle growth and differentiation and better mimics the dense structure of ovarian tissue. A similar effect was observed by Hornick et al. (36) who could show that higher alginate concentration, which correlates with higher matrix stiffness, led to a better survival and growth outcome for primate primordial follicles. Based on these findings, Laronda et al. (32) applied an optimal alginate concentration in attempting to preserve human primordial follicles. The isolation and culture methods applied in the primate study, however, were not directly applicable to human tissue and therefore human primordial follicles had to be cultured in situ with ovarian tissue pieces. With an adapted protocol, survival, differentiation, and growth of human primordial follicles were accomplished for 6 weeks (32). In 2015, the production of human meiotically competent metaphase II (MII) oocytes with the help of alginate hydrogels was carried out for the first time. With an adapted two step-protocol, where the follicles were first cultured in a stiff alginate hydrogel and were later transferred to low-attachment plates at the antral stage, the terminal diameter of those follicles was greater than the ones grown only in alginate hydrogels. This result also indicated the requirements for correct follicular development in an in vitro environment changes over time which must be addressed in order to achieve the best possible outcome (33). It is noteworthy to mention for caprine follicles, a lower stiffness of the alginate hydrogel showed the best performance in terms of development into pre-antral follicles or the rate of meiotic resumption (69).
To identify appropriate microenvironments for 3D culture of ovarian follicles, all functions of cells from morphological aspect to gene profiles should be monitored. For instance, Parrish et al. compared the gene expression patterns, such as endocrine-related gene expression, of in vivo mouse ovarian follicles to those cultured on the alginate hydrogel. The study showed a similar, however not completely identical gene expression profile which provides important information for constructing better in vitro culture systems (47). In another study it was demonstrated that an alginate hydrogel could support the growth and survival of human pre-antral follicles after cryopreservation of ovarian tissue with a survival rate of 90% for 7 days (29). In the same manner, Camboni et al. (31) aimed to improve cryopreservation protocols and compared two different cryoprotectants, ME2SO (dimethyl sulfoxide) and EG (ethylene glycol), used for freezing and thawing human follicles embedded in alginate hydrogels. The viability of the cryopreserved follicles was compared with conventional in vitro culture (IVC) and freshly isolated primordial/primary follicles. While the MESO group showed high viability as freshly isolated follicles, the EG and IVC group displayed a significantly worse performance. Likewise, Xu et al. (46) cultured cryopreserved murine follicles on alginate matrices and additionally compared two different forms of cryopreserved tissues (either cortical strips of follicles with surrounding stroma or individually isolated follicles) with freshly isolated follicles. While there was no difference in survival, growth, or antrum formation in the 12-day culture period, the hormone and specific messenger ribonucleic acid (mRNA) levels varied between the three groups. Taken together, both methods provided successful cryopreservation, although individually isolated follicles showed signs of impaired oocyte development. The same group also reported the first in vitro growth of non-human primate antral follicles with the help of alginate encapsulation (35). The rhesus monkey-derived follicles were cultured for <30 days, and the influence of the matrix properties on follicle development was investigated. In reference to the obtained results, follicles cultured in a stiffer environment (0.50% alginate) showed a higher survival rate and larger diameter compared with the softer environment (0.25% alginate) (35).
Besides the matrix stiffness, the hormonal effects on the growth and steroidogenesis of canine follicles were also evaluated. In general, lower alginate and higher FSH concentrations positively induce follicle growth (73). An effective in vitro 3D culture system which recapitulates or supports normal ovary functions could be used for drug screening but also might help for testing hormone levels (90). From this perspective, King et al. (49) employed an alginate hydrogel culture system to monitor the influence of high concentrations of insulin and IGF (insulin-like growth factor) on the ovarian surface and folliculogenesis, which showed that ovarian surface epithelium proliferation and hyperplasia led to multiple cell layer formation through the phosphoinositide 3-kinase (PI3K) pathway. Developing a model system from alginate hydrogels to investigate hormonal patterns and mid-cycle ovulation as part of the ovarian cycle demonstrated that both human and murine follicles cultured in this system were able to produce follicular- and luteal-phase hormones (34).
Moreover, impacts of paracrine signals from the extracellular microenvironment were studied with the help of an alginate-based 3D culture environment. In that work, early-stage mouse primary follicles cultured in groups of either 5 or 10 follicles were used as a study object. A significant change between groups of 5 or 10 follicles regarding their phenotypical and functional appearance was observed which was determined by cytokine and transcriptional levels. This demonstrated a number-dependent manner of follicle development where a higher number of co-cultured follicles seemed to have a positive influence on survival rate due to increased paracrine signals (57). Among other possible external factors, it is known that ascorbic acid plays a role in ECM deposition/remodeling (Figure 2A) (52). Supplementing alginate-based culture media with ascorbic acid led to increases in the survival rate of murine follicles (up to 18 days) with developed antral cavities where the follicles’ diameter expanded up to 250 μm. In addition, gene expression analysis revealed an upregulation of ECM and cell adhesion proteins when ascorbic acid was involved.
Figure 2.
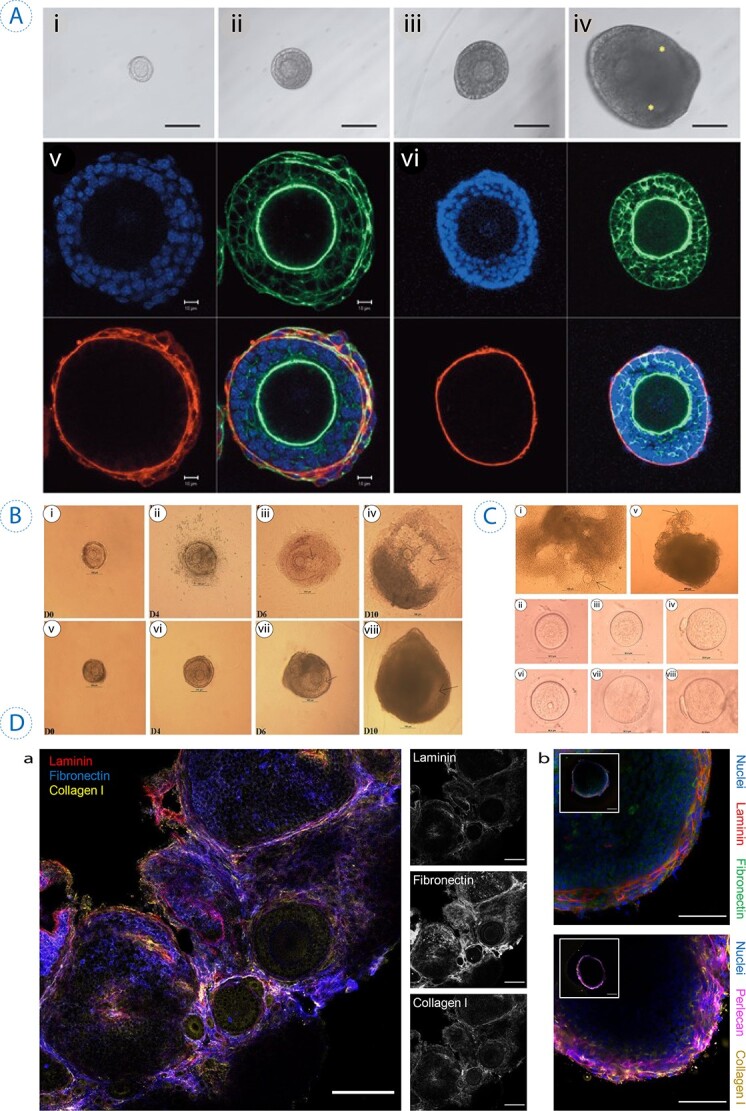
Several hydrogels applied to provide a suitable environment for in vitro folliculogenesis/oogenesis. (A) Survival of primary follicle cultured with ascorbic acid on days 0, 6, 10, and 16 (i–iv). Confocal images illustrate the growth of the follicle cultured in the presence of ascorbic acid after 6 days (v) and the control secondary follicle (vi) (staining represents red for laminin, green for f-actin, and blue for nucleus). It was observed that the survival rate increased by ascorbic acid, and primary follicles reached a diameter of around 250 μm. The scale bars: 100 μm for i–iv and 10 μm for iv. Reprinted with permission from Tagler et al. (52). (B) Morphological changes of rat follicle in the 2D (i–iv) and 3D (v–viii) in vitro growth (IVG). In both cultures at day 0, immature oocytes were observed at the center (i, v); however, the difference is distinguishable later as the follicles started to collapse in 2D (ii) compared with well-maintained follicles in 3D alginate bead (vi). During 10 days of culture, there were a few antrum-like cavities in 2D-cultured follicles (ii, iv), although in 3D condition, follicles were observed larger forming antrum-like cavities (vii, viii). (C) The IVG of rat oocytes in 2D (i–iv) and the 3D (v–viii) culture conditions. The release of COCs from follicles is shown by arrows (i, v), 20 h after exposure to hCG and EGF to induce ovulation. Germinal vesicles were detected in both of the cultures (ii, vi) and oocyte transition from a Germinal Vesicle (GV) to Germinal vesicle breakdown (GVBD) (iii, vii). Meiosis also continued as the mature oocytes released the first polar body (PB) (iv–viii). Reprinted with permission from Zhang et al. (58). (D) The ECM proteins and distribution visualized by a fluorescent microscope in ovarian tissue and antral follicles. (a) Staining represents blue for fibronectin and yellow for collagen I, throughout the stroma and within ovarian follicles, while laminin (in red) is only observed in the perifollicular space. Scale bars = 100 μm. (b) Staining represents green for fibronectin and yellow for collagen I, the granulosa cell layers of antral follicles isolated from ovarian tissue, but laminin (in red) and perlecan (in magenta) are detected in theca layers. Scale bars = 50 μm and 100 μm (inset). Reprinted with permission from Tomaszewski et al. (65).
One of the other purposes for conservation and in vitro follicle culture is to explore the possible adverse or even damaging effects of administered chemotherapeutics for cancer treatment. For this reason, a model system was developed to study the commonly used chemotherapeutic drug, doxorubicin (DOX), which was tested on alginate-embedded mouse follicles (56). The DOX presented dose-dependent toxicity on follicle development, hormone secretion, and oocyte maturation. Similarly, Zhang et al. developed an alginate-based platform for rat follicles that can be employed to monitor follicle and oocyte function when exposed to a potentially toxic substance, in this case, bisphenol A (Figures 2B and C) (58). Another protocol to enable fast and high-throughput ovotoxicity screening was implemented with optimized vitrification to create long-term storage and an easy-to-use system with alginate beads for mouse follicle culture that also allows for fertility preservation (59).
Ovarian follicles could be co-cultured with feeder cells like mouse embryonic fibroblasts (MEFs) to provide paracrine factors crucial for follicle survival and growth. The study by Tagler et al. showed that MEFs had a positive effect on follicle survival, where about 70% of the cultured follicles survived with better meiotic competence while the follicles cultures without MEFs only survived between 6 and 10 days (48). In another study focused on co-culturing follicles in which mouse primary follicles were cultured together with other growing follicles, the growth and survival rate increased, which were probably promoted by signaling factors released by neighboring cells (50). With this method, antral stage follicles could be obtained to produce meiotically competent gametes. To design a feeder-free culture system with similar properties to the feeder cell populations, Tagler et al. (51) cultured ovarian follicles encapsulated in alginate hydrogels and nourished with α minimum essential medium (αMEM)/F12 supplemented with fetuin, insulin, transferrin, selenium, and FSH. Culturing certain mouse follicles within this alginate-based microenvironment showed follicle development and maturation to the antral stage and MII eggs production from early secondary and late primary follicles, respectively.
Remarkable progress in order to achieve an ideal in vitro culture environment opened new avenues for fertility preservation of girls and women suffering from cancer (16, 45). In 2009, Xu et al. (30) cultured human ovarian follicles from cancerous patients in an alginate hydrogel. The successful maintenance of follicles for 30 days as well as a development from secondary to the antral stage were allowed through this 3D culture system. Apart from screening hormonal levels, medium composition, or testing various drugs, an optimal in vitro model system representing the biological situation of in vivo folliculogenesis could be utilized to probe the influence of oxygen tension on follicle function. An 8-day culture period with early-stage murine follicles comparing oxygen levels of 2.5 and 20% showed a significantly higher survival and growth rate for the lower oxygen concentration while they were encapsulated in alginate hydrogels (53). As mentioned earlier, certain sizes of follicles/oocytes exist in specific stages of the folliculogenesis process, which is used as a marker for specific follicle determination and selection (91). Accordingly, Xiao et al. cultured mouse follicles individually based upon their size rather than a defined time period. The reproductive outcomes were better in terms of oocyte quality and successful in vitro fertilization (IVF) rate and proposed using size-specific follicle selection as a marker rather than time (55).
Fibrin-alginate
Fibrin is a protein involved in the blood coagulation cascade that polymerizes into mesh-like structures upon activation from its monomeric precursor, fibrinogen (92). Unlike alginate, which is not a protein, fibrin can be easily degraded by proteases that can extend alginate hydrogels by providing an adaptive component. This can be advantageous when considering hydrogel systems for culturing/expanding cells or tissue such as follicles where the mechanical properties of the matrix need to change over time (60). Shikanov et al. provided a protocol for preparing a fibrin-alginate (FA) hydrogel that shows changes in mechanical properties over time via fibrin degradation caused by growing follicles, leaving an inert alginate scaffold, and thus mimicking the natural environment of ovaries (93). Using the protocol, successful development and maturation of mouse follicles were shown, leading to oocyte meiosis into MII (93). Another positive effect of fibrin on follicle development, observed by Xu et al., is the promotion of primary follicle development, indicated by the growth rate, size, steroidogenesis, or vascular endothelial growth factor production in macaque follicles; however, it harmed the growth rate of secondary follicles (38).
In a similar way, Brito et al. compared the effects of multiple goat follicle cultures in alginate, FA, and hyaluronate gels. Only pre-antral follicles cultured on FA hydrogels were able to result in oocytes that could resume meiosis and even produce embryos (70). Similar observations were made by Jin et al. (94), where they compared FA with alginate hydrogels in a two-step protocol in which the first step comprised whole ovaries tissue culture followed by secondary follicle culture. The FA approach was able to produce mature mouse oocytes that were even fertilization-competent.
FA hydrogel could also be applied for cell encapsulation, and Xu et al. (37) proved that luteal-phase baboon follicles could mature into meiotically competent oocytes when encircled by FA hydrogels. Taking such advantages into account, Zhou et al. (95) utilized FA hydrogels to develop a high-throughput screening system for studying ovarian toxicity. Fibrin degradation in FA encapsulated mouse follicles was exploited as an indicator for survival and growth to evaluate the toxicity of DOX. Moreover, the effects of mixed environmental toxicants, lindane, and 7,12-dimenthylbenz(a) anthracene (DMBA), on mouse follicles were studied with the help of 3D FA matrix ovarian toxicity and showed a greater toxicity for DMBA to encapsulated secondary follicles compared with lindane (96).
ECM-derived materials
The ECM has a wide-ranging effect on cell behavior and fate commitment. Cells recognize different proteins (in particular fibronectin, laminin, and collagen) integrated within the ECM composition via integrins receptors and then initiate specific cell responses (Figure 2D) (65, 97). In 2020, a new ECM-derived hydrogel was introduced and compared with an alginate hydrogel and a 2D culture system concerning its impacts on follicular growth (61). The ECM-hydrogel indeed showed an improved outcome in terms of pseudo antrum formation, cumulus–oocyte complexes, MII oocytes, normal spindle formation, or 17ß-E2 production, compared with alginate, which was possibly caused by a higher diffusion rate of hormones due to lower stiffness (61). Furthermore, Oktem and Oktay studied the function of murine follicles when cultured in an ECM-based environment and claimed that the normal 3D structure of follicles was preserved together with better growth and survival rate when supplemented with the growth hormone activin-A. Adding activin-A, which has para- and autocrine function in signal transduction, however, did not lead to an improvement of follicle growth while they were cultured on single ECM component matrixes when compared with the whole ECM mixture (62). A similar finding by Sadr et al. (22) who compared culturing mouse follicles on an ECM/alginate-based hydrogel with a fibrin-hydrogel demonstrated that the more complex ECM environment resulted in more desirable conditions in terms of maturation and survival rate compared with the less complex FA hydrogel. Moreover, comparing tyramine-based hyaluronan hydrogels with and without ECM proteins showed the resumption of meiosis and MII formation were possible for pre-antral mouse follicles cultured with ECM proteins (19). Such studies implied that complex growth conditions are necessary to mimic the natural ovarian environment.
Polyethylene glycol
PEG is a popular synthetic hydrogel material because of its high versatility and minimal biomolecular interactions. Mechanical tunability by protease degradation, biocompatibility, and functionalization with bioreactive modifications can be performed within a physiological range and therefore make PEG perfectly suited for in vitro follicle culture (98). The PEG-hydrogel stiffness could be adjusted by changing the reactive PEG concentration as appropriate stiffness is required for follicle culture. Although altering the PEG stiffness had no effect on murine follicular growth, a softer environment positively supported the developmental competencies of oocytes gained from pre-antral follicles (64). Further investigations revealed an enhanced network design in PEG hydrogel allowing tunability and providing an improved follicle culture environment (17, 24). In order to develop an even better imitation of an ovarian environment, Tomaszewski et al. incorporated ECM sequestering peptides into a PEG hydrogel. This composite microenvironment enabled matrix-cell interactions between follicles and the hydrogel, promoting the growth and maturation of early-stage small murine follicles (65). Moreover, a comparison of alginate with PEG-fibrinogen hydrogels performance in combination with substances that interfere with phosphatidylinositol-triphosphate (PIP3) production indicated PEG hydrogels as suitable environments for in vitro primordial follicle culture (39). However, increasing PIP3 production does not lead to proper folliculogenesis which brings certain concerns about considering PEG polymer as an available option for designing a favorable environment for in vitro culturing oocyte/follicle.
Bioscaffolds for female fertility restoration
Within an artificial ovary’s microenvironment, ovarian follicles should have proper proliferation and maturation to achieve their full development. The local microstructure also should contain interconnected pores that enable cells to infiltrate within, survive, and grow via vessel and capillary formation (99). During the last decade, different 3D scaffolds made of natural and synthetic biomaterials have been evaluated as platforms for female fertility preservation and are now reviewed here based on the published papers related to this subject (Table 2).
Table 2.
Different types of bioscaffolds applied in supporting folliculogenesis and oogenesis
| Spices | Material and media supplementary additives | Cell/tissue source | Stages of follicles and oocytes development | Main results | Ref. |
|---|---|---|---|---|---|
| Primate (human) + rodent (mouse) | Decellularized human ovary (DCT) | Pre-antral follicles (2–4 weeks old) |
Antral | In vivo transplantation, H&E staining, and Terminal deoxynucleotidyl transferase (TdT) dUTP Nick-End Labelin (TUNEL) assay showed that reseeded DCT could support the viability of human follicles and growth of murine follicles, as up to 39% of them grew to antral stages. | (100) |
| Primate (human) | Fibrin clot | Ovarian cells (26–51 years old) | - | Among nine combinations of fibrinogen (mg/ml) and thrombin (IU/ml), F12.5/ T1 and F25/T4 supported better distribution, survival, and proliferation of human ovarian cells characterized through TUNEL, H&E, and Ki67 antibody staining assays. | (101) |
| Primate (human) | Fibrin clot | Pre-antral follicles (28–34 years old) | Pre-antral | The fibrinogen (mg/ml) and thrombin (IU/ml) F50/T50 matrix with a modulus of ~3800 Pa, resembled physiological ovarian stiffness and exhibited similar fiber thickness to the native tissue. F30/T50, F50/T50, and F75/T75 samples showed similar follicle recovery and loss rates for encapsulated ovarian follicles using H&E staining. | (102) |
| Rodent (mouse) | DAM, IAM, FSH | Primary-secondary stages follicles (10 days old) | Pre-antral | The human IAM supports follicle development in terms of higher viability rate, larger follicular size, and higher estradiol production compared with DAM and 2D groups (control) based on their morphology, gene markers, and estradiol production evaluations. | (103) |
| Rodent (mouse) | Gelatin-methacryloyl (GelMA) bioink for bioprinting EGF, hCG | Two-layer secondary follicles (100–130 μm) and multi-layer secondary follicles (130–180 μm) (3 weeks old) | Antral MII oocytes | The 3D printed platform via GelMA bioink offered a suitable environment for the growth of follicles, which resulted in ovulation. MII oocytes were also obtained following in vitro oocyte maturation. | (104) |
| Rodent (mouse) | Decellularize mouse ovary tissue | - | - | SDC decellularized tissue exhibited more integrated and intact collagen, elastic fibers, and glycosaminoglycan compared with those treated with SDS and could be considered a potential option for ovarian bioengineering. | (105) |
| Rodent (mouse) | Decellularized human ovary (DCT) | Peritoneum mesenchymal stem cells | - | Comparing different types of cell culture on DCT scaffolds revealed higher cell penetration, proliferation, and ovarian differentiation in the rotational seeding method compared with static ones. | (106) |
| Rodent (mouse) | Bovine ovarian decellularized tissue | Pre-antral follicles (14 days old) and fibroblasts | Pre-antral | MTT assay revealed lower viability of fibroblasts seeded on tissues decellularized by SDS compared with those decellularized using Triton and Ammonium. Besides, the viability of cultured pre-antral follicles was observed to be 85.9% for SDS-Triton Ammonium decellularized scaffolds. | (107) |
| Rodent (mouse) | Bovine and human decellularized ovarian scaffold | Primary ovarian cells (3-4 weeks old) | Antral | The decellularized scaffolds seeded with primary ovarian cells could support cell growth and function, resulting in estradiol production in vitro. Furthermore, the recellularized grafts initiated puberty in ovariectomized mice. | (108) |
| Rodent (mouse) | Fibrin clot | Pre-antral follicles and ovarian cells (6 weeks old) | Antral | TUNEL assay, H&E staining, and Ki67 antibody staining showed that both formulations of fibrinogen (mg/ml) and thrombin (IU/mL) (F12.5/ T1 and F25/T4) exhibited low apoptotic cell death and a high rate of cell proliferation 1 week after autotransplantation. Neovascularization within the fibrin clots and endothelial cells was also observed around growing follicles one week after grafting, demonstrated by CD34 immunostaining. | (99) |
| Rodent (mouse) | Fibrin clot | Primordial, primary, and secondary follicles (6–25 weeks old) | Antral | After encapsulation in the fibrin matrix (fibrinogen 12.5 mg/ml/thrombin 1 IU/mL), a 100% viability was observed for primordial primary follicles compared with 92% in the secondary follicles group after 7 days demonstrated by live/dead and TUNEL assays. | (109) |
| Rodent (mouse) | PRF scaffold | Ovarian tissue (4–5 weeks old) | Antral | PRF scaffolds as a graft site for ovary autotransplantation supported follicle development. Moreover, the number of follicles, level of interleukin 10, progesterone, estradiol, and angiogenesis in the autografted+PRF bioscaffold group were higher in comparison with autografted group based on specific staining, biochemical analysis, and hormone assays. | (110) |
| Rodent (mouse) | Collagen-based DBM | Cumulus-free oocytes (6–8 weeks old) | MII oocyte | Assay of histone H1 kinase activity and zona pellucida (ZP) hardening showed normal maturation-promoting factor (MPF) and an increase in ZP hardening level, respectively, for 3D DBM co-culture system with cumulus cells. | (111) |
| Rodent (rat) | Collagen scaffold type I | ADSCs and a POI model | Antral | The collagen/ADSCs scaffolds demonstrated enhancement in the number of antral follicles, granulosa cell proliferation, estradiol (E2) level, and pregnancy rates compared with phosphate-buffered saline (PBS) treated groups according to immunostaining and hormone assay. | (112) |
| Rodent (rat) | Gelatin (pig origin) sponge (GS) | ADSCs (12 weeks old) | Antral | Estrous cycle resumption and an increase in estrogen receptors were observed using hormonal analysis in animals treated with ASCs-GS in comparison with the group treated with Gelfoam/culture medium. | (113) |
| Rodent (rat) | Porcine UBM | ADSCs (8 weeks old) | Primary | A higher number of primordial and growing follicles in ADSC/UBM group were observed compared with other groups after 28 days post-transplantation. | (114) |
| Rodent (rat) | DCT | Primary ovarian cell (8 weeks old) | - | A suitable cytocompatibility of scaffolds was demonstrated by the MTT test. Moreover, H&E staining showed that primary ovarian cells remained viable and reconstructed follicle-like structures. Besides, serum hormone assay revealed an increase in estradiol and progesterone levels in rats with ovarian cells-seeded grafts | (115) |
| Suidae (pig) | Decellularized porcine ovary | Skin fibroblast | - | The shape and homogeneity of ovary tissue were preserved after decellularization, and DNA quantification showed a reduction of 98% of DNA even though histological staining demonstrated the well-preserved intact ECM microarchitecture while supported fibroblasts adhesion and migration. | (116) |
| Suidae (pig) | Alginate porous scaffold loaded with BMP-4 FSH | Primordial follicles from prepubertal porcine and xenotransplantation into SCID mice | Antral | The developing follicles increased estradiol secretion and GDF-9/AMH gene expression based on live/dead, hormonal, and quantitative polymerase chain reaction analysis. Follicles grew to the antral size and secreted hormones related to restoration of ovarian function (estradiol and progesterone) after in vivo transplantation. | (117) |
| Suidae (pig) | PCL/gelatin fibrous scaffold FSH, ascorbic acid | Primordial to pre-antral follicles | - | Electrospun PCL/gelatin scaffolds could support ovarian follicles growth with higher secretion levels of estradiol and progesterone compared with pure PCL fibrous scaffold | (118) |
| Bovidae (buffalo) | Three-dimensional glass scaffold FSH | Oocyte | - | 3D glass scaffolds improved in vitro oocyte maturation followed by promoting the total cell numbers of blastocysts which could be related to higher expression of different ECM proteins (such as COL1A1, COL2A1, COL3A1, FN) and cell-cell related proteins (especially N-cadherin and E-cadherin) compared with 2D culture. | (119) |
| Bovidae (sheep) | Poly (epsilon-caprolactone) (PCL) electrospun scaffold hCG, Equine chorionic gonadotropin(eCG) | Pre-antral follicles (5 months old) | Antral MII oocytes | Applying different culture approaches such as two (7 days in 3D-oil and on scaffold) and one-step PCL-patterned-based (14 days on PCL-scaffold) showed the same ability to support follicle and oocyte development in resulting in antrum formation and oocyte meiotic competence. | (120) |
Decellularized scaffolds
Decellularization is a process in which the cells and cellular debris are removed from native tissue using different enzymes and detergents (121). The product of this procedure, ECM, is free of immunogenic components of the natural tissue while including different proteins, proteoglycans, and glycosaminoglycans (GAGs) crucial for normal cell functions such as cell attachment, migration, proliferation, and differentiation. It is also important to decellularize compositionally similar tissue to the same target tissue for regeneration. Thus, the structure and chemical composition of obtained ECM could precisely mimic the desired tissue (121).
In recent years, researchers reported different decellularization protocols used for in vitro and in vivo culture of follicles and evaluated their efficiency for use as female reproductive system mimicks (100, 105, 106, 116, 122). The amniotic membrane is considered a good option for fabricating decellularized scaffolds for reproductive biology. From this perspective, Motamed et al. decellularized the human amniotic membrane using trypsin and ethylene diamine tetra acetic acid and evaluated its potential for mouse ovarian follicle culture (103). The success of the decellularization procedure was confirmed by hematoxylin & eosin (H&E) staining as the removal of the cells was demonstrated. Mouse primary-secondary follicles were cultured in three groups, in base medium (control), on the intact amniotic membrane (IAM), and decellularized amniotic membrane (DAM). Results showed better viability and morphology of follicles and higher production of E2 and expression of Gdf9, Bmp15, and Cx37 genes for follicles cultured on the IAM group. The diameter of oocytes and follicles was also measured to be significantly larger with IAMs compared with other groups and with DAMs compared with the control group during 9 days (103). As reported in the literature, DAM exhibited a lower growth factor level than IAM, which could be the reason for the better follicular support that IAM possesses. Moreover, larger follicles could explain the high level of E2 production observed for IAM (Figure 3A) (103). In the other study by Hassanpour et al. (115), the human ovary was decellularized via sodium lauryl ester sulfate-treated process. Primary ovarian cells seeded on these well-preserved decellularized human ovarian tissue (DCT) scaffolds showed proper viability and primary follicle-like structures formed after transplantation of the scaffolds into ovariectomized rats. Furthermore, expression of steroid hormone receptors by surrounding somatic cells and expression of inhibin-α indicating the presence of granulosa cells within the grafts were detected through the immunostaining test (115). The decellularization approaches still need to be optimized to obtain a proper acellular ECM composition. On this subject, Pros et al. investigated an optimized method for decellularization of human ovarian tissue and showed that tissue incubation in 0.1% sodium dodecyl sulfate (SDS) solution for 18–24 h could effectively remove all cells and provide an intact ECM structure (100). Although DCT possessed 88–98% of the amount of collagen found in native ovarian tissue, DNA content of ovarian tissue decreased by >90% after the decellularization process. Survival of the seeded human pre-antral follicles on DCT in co-culture with human ovarian stromal cells was supported. After xenotransplantation, DCT scaffolds could preserve the viability of either human follicles or murine follicles, as up to 39% of them grew to antral stages (100). Comparing various protocols for decellularization of mouse ovary tissue using 0.5% SDS solution as protocol 1 (P), a 2% sodium deoxycholate (SDC) solution (P2), and a combination of both as the third protocol (P3) revealed that SDS can remove DNA and intracellular components effectively although substantially reduced the integrity of the ovarian ECM. By comparison, SDC, known as a milder detergent, conserved more integrated and intact collagen fibers and glycosaminoglycan visualized via histological staining. It was also noted that ovarian scaffolds made from SDC treatment exhibited higher content of DNA (105). Although in vitro folliculogenesis was not investigated using this scaffold, mesenchymal stem cells were used for scaffold recellularization and they distributed within all parts of the scaffolds, while it was deeper in the cortex region. Based on statistical analysis, the recellularization efficiency was not significantly different in three types of scaffolds, and cells remained viable and proliferative 14 days post cell seeding in all groups (105).
Figure 3.
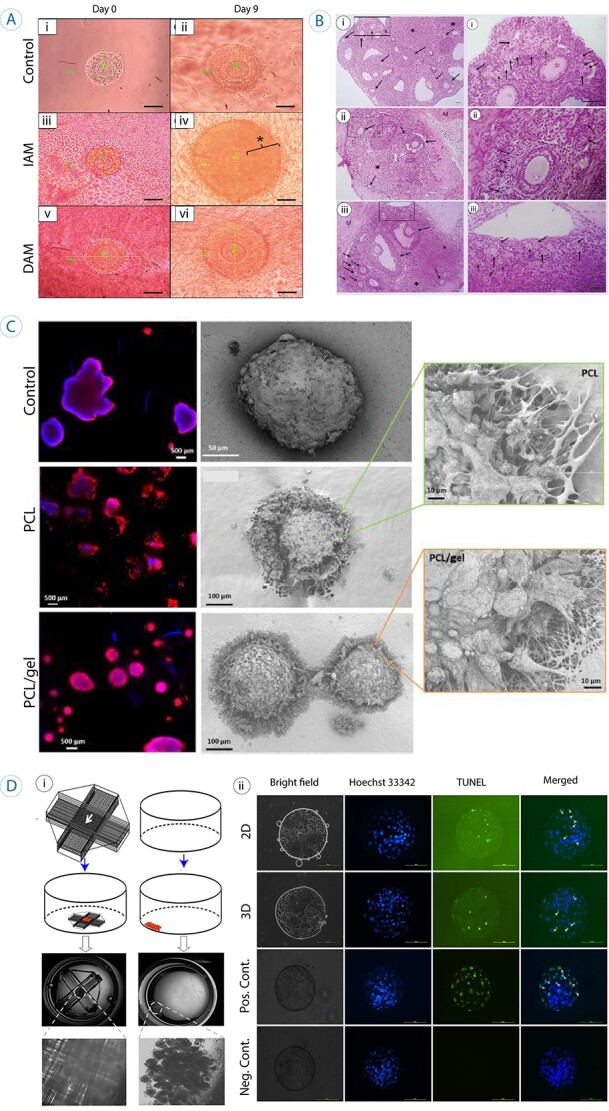
3D scaffolds used in supporting folliculogenesis and oogenesis. (A) In vitro culture of the primary follicle after 9 days on a decellularized scaffold. The control culture was in base medium, IAM on intact human amniotic membrane and DAM group represents the ones cultured on decellularized human amniotic membrane. Star represents hyperproliferation that occurred for granulosa cells in primary follicles cultured on IAM. Scale bar =50 μm. Reprinted with permission from Motamed et al. (103). (B) The histological sections of autotransplanted ovarian grafts in mice after 28 days (arrows show follicles). (i) Control group (mice without ovariectomy or grafting), (ii) autografted group which is detected by fewer follicles, (iii) autografted + platelet-rich fibrin bioscaffold group, which exhibited a significantly higher number of follicles. Scale bar: 100 μm. M: Muscle. Reprinted with permission from Shojafar et al. (110). (C) Porcine follicles seeded on electrospun PCL fibers, PCL/gel, and control (PET membrane) illustrated by fluorescence microscope (actin in red and nucleus in blue) and SEM images 10 days post-seeding. SEM micrographs with higher magnifications represented interaction between follicles and scaffolds, as no adhesion spots were observed for the control. Reprinted with permission from Liverani et al. (118). (D) i, The schematic represents the fabricated 3D glass scaffold and 2D culture methods. ii, Hoechst 33342 staining showed the total number of cells, and TUNEL assay exhibited apoptotic cells (the arrows point to TUNEL-positive nuclei). Reprinted with permission from Shen et al. (119).
In 2019, Pennarossa et al. (116) established a new protocol for whole ovary decellularization as a promising approach to obtain a bioengineered ovary. Porcine ovaries were decellularized via a freeze-thaw process accompanied by incubations in 0.5% SDS, 1% Triton X-100, and 2% SDC, respectively. The decellularization procedure not only preserved the shape and homogeneity of the ovary tissue but also histological staining revealed a reduction of 98% in the DNA level in comparison with the native tissue. Moreover, histochemical assessments and quantitative analysis showed that ECM microarchitecture, viewed via collagen, elastin, and GAG contents, was well preserved after decellularization. When re-seeded with homologous fibroblasts, these bioscaffolds supported cell adhesion, migration, and cluster-like structure forming (116). Recently, a research group decellularized bovine ovarian tissue using four different protocols. Two groups of tissues were decellularized using 0.1% SDS for two different durations (18 and 24 h) and two other protocols were implemented using 0.5% SDS for 3 h + 1% triton for 9 h (SDS-T group), 0.5% SDS for 2 h + 1% triton, and 0.1% ammonium hydroxide composition for 22 h (SDS-T-A group) (107). A DNA count assay revealed that the highest and the lowest DNA contents belonged to SDS-T and SDS-T-A groups, respectively. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay also demonstrated for the tissues exposed longer to SDS, the viability of fibroblasts was lower in comparison with SDS-T and SDS-T-A groups after 7 days. These results could be attributed to two main advantages of triton over SDS in decellularization. First, it caused less damage to ECM and second, it had greater efficiency in eliminating residual DNA, particularly when mixed with ammonium (123). Moreover, the viability of mouse prenatal follicles was assessed after 7 days in SDS-triton-ammonium decellularized scaffold and pure alginate as the control group, indicating no significant difference between these groups (85.9% and 81%, respectively) (107).
It should be noted that the urinary bladder matrix (UBM) is the other most promising decellularized ECM that has been utilized and commercialized for several regenerative medicine applications (124). Xing et al. assessed the effect of ovary autografting of UBM, which was seeded with adipose-derived stem cells (ADSCs), on the restoration of ovarian function. In this study, five groups of rats were evaluated, including an untreated control group, the groups underwent oophorectomy, autograft group, autograft accompanied with ADSCs, and autograft+UBM/ADSCs (UBM/ADSC) (114). The quantity of either growing and primordial follicles was significantly higher in the ADSC/UBM groups compared with the ADSC and the autograft group 28 days postautograft. Immunohistochemistry assessment after 28 days also revealed significantly improved cluster of differentiation 31 (CD31) expression in ADSC/UBM rats compared with the other groups. This could suggest that UBM is proangiogenic in ovarian graft, which is crucial for the survival of ADSCs and the process of folliculogenesis. Furthermore, hormonal analysis exhibited a higher serum concentration of anti-mullerian hormone (AMH) in the ADSC and ADSC/UBM groups (114).
Natural polymer-based scaffolds
Fibrin
A large number of studies revealed the great potential of fibrin gel for different tissue engineering applications (125). For the first time in the field of female reproductive systems, Luyckx et al. (101) developed a 3D fibrin scaffold using nine different concentrations of fibrinogen (F) and thrombin (T). It was demonstrated that two combinations of 12.5 mg/ml fibrinogen with 1 IU/ml thrombin (F12.5/ T1) and 25 mg/ml fibrinogen with 4 IU/ml thrombin (F25/T4) supported better distribution, survival, and proliferation of isolated human ovarian cells (101). The same group later investigated the ovarian cell-seeded fibrin clots with the mentioned concentrations as an artificial ovary transplanted to the peritoneal cavity (99). The grafts included ovarian follicles showed a total follicle recovery rate of around 31% for both F25/T4 and F12.5/T1 grafts. One week after transplantation, both formulations exhibited low apoptotic cell death and a high rate of cell proliferation. Furthermore, CD34 immunostaining demonstrated neovascularization within the fibrin clots along with observed endothelial cells around growing follicles (99). Fabricating a 3D environment using fibrin may affect both the survival and growth of follicles at specific stages. Considering such impacts, Chiti et al. (109) investigated the difference between survival and growth of primordial-primary and secondary follicles when impregnated within fibrin matrix and transplanted to severe combined immunodeficiency (SCID) mice as the recipient. After transplantation, histological analysis showed around 6% recovery rate for the primordial-primary group and 28% for the secondary follicles on day 7. The same group later used different fibrin formulations (F12.5/T1, F30/T50, F50/T50, F75/T75) to optimize and mimic the architecture of native human ovarian tissue. The human ovarian cortex of three patients was evaluated using scanning electron microscope (SEM) images and all were observed to be highly fibrous and structurally heterogeneous, with no difference in fiber thickness (~61.3 to 72.4 nm) (102). Although there was no significant difference in fiber thickness for F50/T50 and F75/T75 samples, the F12.5/T1 group exhibited considerably higher fiber thickness (~137.5 nm) compared with native tissue. Furthermore, mechanical characterization demonstrated that at higher concentrations of fibrin and thrombin, the storage modulus of samples was enhanced, with the F50/T50 matrix showing a modulus of ~3800 Pa, resembling physiological ovarian stiffness. Although the F50/T50 matrix mimicked well the fiber thickness, porosity, and rigidity of ovarian tissue, histological analysis of F30/T50, F50/T50, and F75/T75 samples impregnated with ovarian follicles showed comparable follicle recovery and loss rates among them (102).
Platelet-rich fibrin (PRF), a new generation of platelet concentrates, has been applied to fabricate bioscaffolds for ovarian graft application (110). A study applying this biopolymer (autografted+ PRF bioscaffold group) showed larger numbers of follicles and a higher level of superoxide dismutase activity along with a higher level of interleukin 10 (IL10), E2, and progesterone were achieved in comparison with the autografted group in conformity with serum biochemical and immunohistochemistry analysis. Enhanced angiogenesis in the autografted+PRF bioscaffold group was observed with a low apoptosis rate compared with the autografted group (Figure 3B). The observed result could be attributed to the potential of platelet-rich products such as platelet-rich plasma and PRF, which contain diverse growth factors and anti-inflammatory, antioxidant and angiogenic characteristics (110).
Collagen
Collagen is the most abundant protein in the ECM of mammalian tissue and has been extensively used in different tissue engineering applications such as bone, heart, muscle, cartilage, and skin (126, 127). Moreover, this biopolymer has been investigated as 3D scaffolds for the culture of oocytes and follicles. In 2007, Ma et al. evaluated the ability of collagen-based demineralized bone matrix (DBM) to improve in vitro maturation (IVM) of cumulus-free oocytes when co-cultured with cumulus cells. They also reported the denuded oocytes in the 3D DBM co-culture system with cumulus cells exhibited higher normal maturation-promoting factor (MPF) and zona pellucida (ZP) hardening levels and better preimplantation development through IVF, compared with in vivo matured oocytes (111).
Combination of stem cell therapy and tissue engineering may efficiently preserve female fertility, thanks to the great potential of stem cells for differentiation into distinct cell lines and secreting growth factors and paracrine factors crucial for tissue regeneration (113). Utilizing collagen scaffolds seeded by ADSCs compared with ADSCs alone to treat primary ovarian insufficiency (POI) in a rat model was assessed (112). The result for both collagen/ADSCs and ADSCs groups showed better cycle recovery, higher E2 level, increased number of antral follicles, and enhanced granulosa cell proliferation compared with a phosphate-buffered saline (PBS) group. It revealed that collagen could preserve ADSCs longer in vivo in ovaries and assist in long-term restoration of ovarian function as the pregnancy rate in POI models was increased (77.8% vs 72.7% for ADSCs alone group) (112).
Other natural polymers
Besides fibrin and collagen, there have been other natural biomaterials investigated as scaffolds for female reproductive organ culture. Gelatin is a natural polymer that could be derived from different sources of collagen such as skin, bone, and tendon (128). The broad variety of collagen sources and production methods led to several types of gelatin with different structural and compositional heterogeneity (129). Gelatin is widely used as a hydrogel for different tissue engineering applications, especially in female reproductive systems (130, 131). The gelatin-based Gelfoam® sponge and ADSCs (ADSCs-GS) as a scaffold were studied in cell therapy for rat frozen-thawed ovarian graft (113). Cellular viability was sustainable up to 120 h in the co-culture system and was higher compared with the control group. It was also shown that animals treated with ADSCs-GS exhibited faster estrous cycle resumption and increased levels of estrogen receptors in comparison with the group treated with Gelfoam/culture medium. Furthermore, this treatment with Gelfoam did not induce any change in morphology, inflammatory responses, enhanced fibrosis, or apoptosis.
Alginate is another natural component that has been investigated in ovarian tissue engineering (132–134). It is also stated that the porous structure of the alginate scaffold could provide well-organized cross-talk between follicular cells leading to proper exchange of paracrine signals and follicular development (135). Moreover, it has been demonstrated in several studies that affinity-bound BMP4 could increase the transition of primordial to primary follicular in rodent ovaries (117, 136). In this regard, a macroporous alginate scaffold loaded with BMP4 was recently developed as a scaffold for ovarian cell culture (117). Although affinity binding of BMP4 did not change the porous structure of the alginate scaffold and its interconnectivity, it exhibited an average pore size of 75 ± 26 μm which is relevant to the cortical area of the ovary. Porcine primordial follicles cultured within alginate scaffolds reached the pre-antral stage after 21 days, and scaffolds with affinity-bound BMP4 improved developing follicles, E2 secretion, and GDF-9/AMH gene expression compared with scaffolds with no affinity binding. In vivo xenotransplantation also revealed that follicles grew to the antral size and showed secretion of specific hormones associated with restoration of ovarian function (117).
Synthetic polymer-based scaffolds
As a result of the ease of selection of appropriate mechanical stiffness and flexibility and the ability to engineer controllable degradation and material reproducibility, synthetic polymers have been placed in the forefront of tissue engineering and regenerative medicine field typically before naturally derived materials (137, 138); however, they have not been studied widely for follicle culture and designing artificial ovaries. Among different methods of scaffold fabrication in tissue engineering, electrospinning is a versatile technique that provides micro and nanofibers capable of mimicking the fibrillar structure and morphology of ECM. Electrospun scaffolds made of different biomaterials have been shown to support cell attachment, spreading, and infiltration for different tissue engineering applications. One of the most studied polymers in tissue engineering via the electrospinning method is polycaprolactone (PCL) (139). This biodegradable polyester is highly available, inexpensive, and could be blended with other biopolymers (118). In a study by Liverani et al. (118), two types of electrospun scaffolds were fabricated using PCL and a combination of PCL with gelatin (PCL/gel) for porcine follicles culture. Live and dead staining analysis indicated more healthy follicles on both PCL and PCL/gel scaffolds after 10 days compared with PET substrate as the control group. In addition, it was reported that the PCL/gel supported more viable follicles compared with pure PCL fibrous scaffold. Follicle adhesion and growth were promoted by the gelatin-containing hydrogels, which supported follicle spheroidal shape, visualized by SEM (Figure 3C) (118). An increasing trend of E2 and progesterone levels was observed between days 2 and 10 which could be associated with follicle growth as it has been reported that follicle size has a direct correlation with the level of both estrogen and progesterone in porcine and humans (140, 141). Recently, Shen et al. (119) designed a novel three-dimensional glass scaffold to increase IVM of buffalo (Bubalus bubalis) oocytes. This 3D scaffold was designed in three layers of glass filaments with an outer diameter of about 100 μm and a length of 12 mm that intersect perpendicularly. The fabricated scaffold could inhibit apoptosis while improving the proliferation of cumulus cells in comparison with 2D culture. Furthermore, cumulus cells expressed higher levels of PI3K/AKT pathway proteins, known to be mediated via fibronectin (FN) and E-cadherin, including PI3K P85 and p-AKT in the 3D scaffold group compared with 2D dish culture (Figure 3D). The 3D culture regulates the expression of mRNA and other proteins of cumulus cells, leading to oocyte maturation to promote buffalo oocyte maturation (119).
Microfluidic applications in folliculogenesis and oogenesis
A wide range of scientific questions are now being addressed thanks to microfluidics—a science and technology of systems manipulating or processing small volumes of flows using micro-channels (142). Fluid mechanics governing this length scale considerably differ from those for the macroscale (143). Organ-on-a-chip devices are capable of simulating in vivo microenvironment characteristics such as physicochemical properties, 3D cellular architecture, cell–cell interactions, cell-ECM interplay, and cell signaling pathways. Another important advantage of the microfluidic organ-on-a-chip platform is the application of fluid flow in in vitro culture which is capable of modeling the mechanical stimuli induced by biological fluid flow over cells in vivo (144). Furthermore, microfluidic technology has made multi-organ or multi-tissue studies possible by connecting several cell culture chambers (145). All in all, the microscale handling of cells and media in microfluidic organ-on-a-chip devices has made them one of the promising 3D cell culture methods. In this section, we have reviewed articles on the application of microfluidics and organ-on-a-chip devices in tissue engineering of the female reproductive system.
Follicles play an important role in the development stages, folliculogenesis, and oocyte maturation through the secretion of different reproductive hormones. Aziz et al. (146) claimed that for the first time, they made a microfluidic device that was successfully used for the human follicle culture. Their device consists of three polydimethylsiloxane (PDMS) layers containing the channels and chamber fabricated by photolithography. The main part of the device is the middle PDMS layer consisting of five inlet channels to deliver injected fluids to the main channel, a serpentine-like section that mixes the five fluid streams, a circular chamber which is used to culture the follicle, and an outlet channel that evicts the fluids from the chamber to the outlet. Ovary follicles encapsulated with calcium alginate beads were placed in the culture chamber (Figure 4A). The measured follicle diameter and hormone secretion showed no significant difference between the two culture modes (the microfluidic cell culture and the conventional culture in dishes).
Figure 4.
Schematic representation of specific microfluidic systems applications in female fertility preservation and in vitro folliculogenesis. (A) Schematic representation of a microfluidic device containing five layers of which the middle one was used for follicle culture (reproduced with permission (146) ). (B) A schematic for a microfluidic chip including several inlets and outlets to create shelled hydrogel microtissue (reproduced with permission (20)). (C) A multiwell microfluidic device used for oocyte culture, cumulus cell removal, and contraceptive agents screening by (reproduced with permission (150)).
Single follicle culture is important due to the fact that there is only one follicle which reaches the maturation stage at a time in humans (13). Therefore, interactions between the follicles in an in vitro culture system could provide an incorrect picture (6). The relatively large numbers of inlets, along with the mixing section, provided this device with the ability to incorporate different fluids, media, drugs, and hormones. Other work from this group utilized the platform to carry out drug screening, hormone interaction studies, and disease simulations. The same group later used the microfluidic device to investigate the combined effect of DOX, Src, Ca2+, and PIM inhibitors in DOX-induced toxicity and its effect on ovarian follicles growth and 17β-E2 secretion (147). The growth of ovarian follicles (diameter increment) and hormone secretion were reduced due to DOX toxicity. While Src and PIM inhibitors increased the toxic effects of DOX on follicles (in terms of growth and hormone secretion), the endoplasmic reticulum calcium inhibitor reduced the toxicity of DOX.
One of the most prominent features of microfluidics platforms is their capability to replicate the relevant biomechanical environment for the cells, which is important since the ovarian follicles pass through heterogeneous mechanical environments on their way into the ovary during the estrous cycle (148). It has been proved that primordial follicles move from the cortex of ovaries with higher stiffness to the medulla with lower stiffness (149). Choi et al. (149) used a microfluidic device to encapsulate the early secondary pre-antral mammalian follicles in a spherical shell of a hydrogel. The shelled microcapsule consists of a softer core made of collagen and a harder surrounding shell of alginate. The proposed microfluidic device consisted of different inlets introducing fluids and hydrogels, different microfluidic modules, and outlets (Figure 4B). The first module of the microfluidic device is a flow-focusing module in which the core solution (i.e., follicles dispersed in collagen) is injected from the inlet I-1 and the shell solution (alginate) is provided by the inlet I-2 meets with the oil and calcium chloride solution entering from I-3 at the junction. The whole spherical shell hydrogel flows in an oil solution and reaches a serpentine-like module which is incorporated into the chip to increase the cross-linking of alginate with calcium chloride that is diffused in the oil.
The difference between the interfacial tension of hydrogel microcapsule-oil and hydrogel microcapsule-aqueous culture medium enforces the microtissue to move toward the aqueous culture medium layer. The results showed that the mechanical heterogeneity in the ovary plays an important role in follicle development. Almost 30% of pre-antral follicles devolved to the antral stage. However, the developmental percentage reduced to 0% when oxidized alginate was used instead of alginate in the shell while keeping the core from the collagen. The rheology measurements showed that oxidized alginate is significantly softer than alginate. These observations suggest that when there exists a mechanical heterogeneity in mechanical strength between core and shell materials, then cells show a greater developmental percentage (151, 152).
Oocyte IVM is one of the important fields of study aiming to facilitate infertility treatment for women with polycystic ovarian syndrome, women poorly responding to IVF, cases in which avoiding ovarian hyperstimulation is desired, or in which the male is the only reason of infertility (153). Microfluidics could be utilized as a platform for IVM allowing control and mimicking suitable culture environments. Zargari et al. (153) developed a simple microfluidic device that could trap oocytes in groups of 1, 3, 5, and 7 in specific locations of a microchannel. The wall shear stress was calculated to check if it is below a specified experimental value reported by Xie et al. (154) using an oversimplified numerical model in COMSOL multiphysics. However, the simulated wall shear stress of the chamber is not a good estimation of the magnitude of shear stress over the oocyte that is trapped in the chamber. They could have added oocytes as spheres to their computational domain to calculate a more accurate value for the shear stress as the presence of oocyte(s) changes the velocity profile and shear stress. They simply pumped the immature mouse oocytes with granulosa cells in the microchannels and the cells were trapped in the pits. Without providing any proof, they claimed that the maturation level of the oocytes was similar to the in vivo maturation level. Although they have designed the pits in the microchannel to accommodate groups of 1, 3, 5, and 7 mouse oocytes, they did not report any data about the differences between the groups.
The same microfluidic device with a 200-μm-depth chamber instead of a 120-μm-depth chamber was used in another study by Sadeghzadeh Oskouei et al. (155). Three different fluid flow (culture media) configurations were employed in this study. The peristaltic pump was utilized to provide a continuous fluid flow during the cell culture as the active dynamic group, whereas gravity was used to induce fluid flow in the passive dynamic group. They also cultured oocytes with several droplets of culture medium in a Petri dish as the static group. The dynamic groups reached a higher percentage of MII oocytes than the passive group. They also performed IVF on the oocytes. Although there was not a significant difference between all the groups, the active group had the highest fertilization rate. Interestingly, the dynamic groups were significantly more successful in terms of blastocyst formation rate.
Ovarian tissues and cells from other species (cat and dog) were also cultured via a simple three-layered microfluidic device that created three straight channels for simultaneous culture in each lane with the same conditions (156). The cat and dog cortical tissue and isolated follicles were cultured in three groups of static (conventional agarose gel culture), slow flow (2 μl/min), and fast flow (10 μl/min) microfluidic culture. There were notable differences between the results obtained from cat and dog groups. In cats, the number of morphologically normal follicles decreased in all cultured groups in comparison to the fresh uncultured tissue. Also, the fast flow microfluidic conditions showed a higher density of normal cells that was reduced as the flow rate increased in the microchannel. Although the differences in sizes of primordial cat follicles were not significant, the sizes were largest in the static culture and increased with increment in the flow rate of the microfluidic device. The trend was different in dogs, as the static group had almost the same size as fresh tissue and it reduced with an increase in the flow rate of the microfluidic device. They also cultured individual follicles in the microfluidic device with the static and the three dynamic groups. This report confirms the demand for more fundamental and profound studies on the effect of culture environment on cell evolution. It is worth noting that this study was one of the few investigations that examined large mammalian animal models, while almost all other studies have been done on small animal models, in particular murine.
Microfluidic platforms with good controllability features are suitable for physicochemical modeling of culture environments by incorporating endocrine and paracrine cytokines and chemicals (157). Xiao et al. (158) devised a device consisting of microfluidic modules which were capable of simulating the 28-day menstrual cycle. They used three devices with two distinct fluid pumping mechanisms: Solo-MFP (microfluidic platform) and Duet-MFP used pneumatic actuation, while Quintet-MFP used electromagnetic actuation for fluid handling. Solo-MFP had one donor, one acceptor, and one tissue chamber, while Duet-MFP had two tissue chambers. The Quintet-MFP containing five tissue chambers was designed to investigate tissue interactions. In the Solo-MFP experiment, the mouse follicles were treated with FSH for the first 14 days before day 0. The algorithm used in their study produced a surge in LH with a peak in human chorionic gonadotropin (hCG) which is similar to in vivo hormonal change follicle experience at different stages of development.
The Solo-MFP supported the follicle evolution from the primary/secondary stage to the antral stage. Besides, follicles released MII oocytes after the hCG stimulation (158). The device was capable of measuring hormone secretion from follicles during the 28-day menstrual cycle. The pattern of change in 17β- E2, progesterone, inhibin A, and inhibin B concentrations is consistent with healthy follicle performance (158).
Microfluidics platforms have better performance in chemical concentration measurement and simulation than some of the static cultures such as conventional 2D cultures in Petri dishes (159). Culturing cells in a 2D culture with a huge amount of culture medium with random mixing would dilute the chemicals to such a level that they might not even be sensed by adjacent cells (158). However, since the fluid volume incorporated in the microfluidic platforms is lower than most static cultures, the dilution effect of the media is lower. These naturally incorporated features in microfluidic platforms, due to their length scales, can be used to increase the cell–cell signaling in the culture medium and could provide concentration profiles more consistent with results to in vivo situations performances (160). Microfluidic devices have great capability for application in drug screening and testing. This is not only due to the aforementioned features of microfluidics in dynamic fluid handling or good microenvironment recapitulation but also its scalability, simplicity in manufacturing, ability to be integrated with other functional units (e.g., imaging and sensing systems), and capability to be automatized (161).
Li et al. developed a multi-well microfluidic device for screening contraceptive agents. They devised a transparent device capable of developing oocytes under contraceptive agents’ influence in a scalable and simple manner. Their device consisted of multi-wells for culturing and developing oocyte cells. Each well consists of two chambers separated by a filter membrane and oocytes are loaded into the device from the medium inlet and retained over the membrane and cultured for 16 h. This membrane facilitated oocyte incubation, medium exchange, enzymatic removal of cumulus cells, washing, and fluorescence staining in a single device (Figure 4C) (150). The cumulus cell expansion and oocyte maturation were quantitatively examined, and the impacts of three contraceptive agents of SB431542, N,N,N′,N′-tetrakis (2-pyridylmethyl)- 1,2-ethylenediamine, and pyrithione on cumulus–oocyte complex development were tested. With this transparent microfluidic device, imaging techniques could be integrated to observe the contraceptive agents’ influence on morphology, formation of polar bodies, cumulus cell expansion, etc.
All the microfluidic devices reviewed above were manufactured from PDMS and polymethylmethacrylate using lithography or micromachining. Alonso et al. (162), for the first time, used cyclic olefin copolymer (COC) as the material for fabricating a microfluidic device. COC is a group of polymers which are transparent, biocompatible, and have good chemical resistance (163). Various grades of COC have different glass transition temperatures ranging from 70 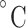 to 155
to 155 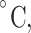 which is an advantage for thermal bonding in microfabrication. By incorporating micro-milling and hot embossing, the authors could design and build two COC microfluidic devices for bovine oocyte maturation and sperm characterization (162). A filter-like microfluidic device to trap and culture oocytes was demonstrated as well as a simple straight and long microchannel for mobility and viability characterization. Statically cultured cells in the microfluidic device showed lower cumulus cells expansion compared with the culture dish. While there were no discernible changes in the percentages of normal MII and cytoplasmic maturation between the groups, the microfluidic group had lower percentages. Furthermore, the fertilized matured oocytes showed a higher fertilization percentage in the culture dish. All things considered, they showed that a microfluidic device manufactured from COC is biocompatible and would not significantly alter oocyte maturation.
which is an advantage for thermal bonding in microfabrication. By incorporating micro-milling and hot embossing, the authors could design and build two COC microfluidic devices for bovine oocyte maturation and sperm characterization (162). A filter-like microfluidic device to trap and culture oocytes was demonstrated as well as a simple straight and long microchannel for mobility and viability characterization. Statically cultured cells in the microfluidic device showed lower cumulus cells expansion compared with the culture dish. While there were no discernible changes in the percentages of normal MII and cytoplasmic maturation between the groups, the microfluidic group had lower percentages. Furthermore, the fertilized matured oocytes showed a higher fertilization percentage in the culture dish. All things considered, they showed that a microfluidic device manufactured from COC is biocompatible and would not significantly alter oocyte maturation.
Outlook
The 2D cell culture platforms (on a culture dish) are the most commonly used platform for in vitro studies by researchers as they are simple and inexpensive. However, such culture platforms are unable to simulate the architecture, function, physiology, as well as dynamic 3D environment of the native cells and tissue. Bioengineering approaches using 3D culture microenvironments have shown significant potential in studying and treating female infertility, which can be classified into hydrogel, scaffolds, and microfluidic techniques. Such 3D microenvironments can better mimic ECM conditions and provide a more suitable environment for cell proliferation and differentiation both inside and outside of the body. Oogenesis is the process of the female gamete development from oogonium to oocyte. Due to the complex anatomical and physiological structure of the female reproductive system, the interaction between a material and a follicle during in vitro culture is complicated as well. To design an appropriate bioengineered platform recapitulating ovary, nutrient transport properties, physically relevant 3D support architectures and suitable biological and mechanical signaling must be considered. Some studies showed embryonic stem cells, induced pluripotent stem cells, adult mesenchymal cells, and other stem cell species can be used either alone or in combination with biomaterials such as biopolymeric hydrogels and natural or synthetic scaffolds to establish a 3D microenvironment for proliferation and differentiation of follicular cells. Providing such a state-of-the-art 3D microenvironment is challenging, and it has become a prime subject for advanced regenerative medicine. The ultimate potential of bioengineering applications for the female reproductive system will rely on a deeper understanding of organogenesis and the complicated physiological mechanisms of this system. Finally, reliable knowledge gained from these methodologies may one day assist in improving pregnancy outcomes in clinical settings.
Authors’ contributions
S.G. and H.E. developed the concept of the manuscript. S.G. coordinated the development of the manuscript and drew the figures. S.G. and H.E. wrote the “introduction” section. P.Z. and N.U. wrote the “Hydrogel” section. M.H.N. and E.M. wrote the “Bioscaffolds” section. A.M. and S.G. wrote all remaining parts. S.G., E.M., and D.S.S reviewed and revised the whole manuscript.
Acronyms
- AMH
anti-mullerian Hormone
- ADSCs
adipose derived stem cells
- ADSCs-GS
gelatin sponge-adipose derived stem cells
- BMP15
bone morphogenetic protein 15
- CD3
cluster of differentiation 31
- COC
cyclic olefin copolymer
- Cx37
Connexin 37
- DBM
demineralized bone matrix
- DCT
decellularized tissue
- DOX
doxorubicin
- DAM
decellularized amniotic membrane
- ECM
extracellular matrix
- ES-hydrogel
extracellular matrix-derived soft hydrogel
- F
fibrinogen
- FN
fibronectin
- FA
fibrin-alginate
- FSH
follicle-stimulating hormone
- GDF9
growth differentiation factor-9
- GAGs
glycosaminoglycans
- H&E
hematoxylin & eosin
- hCG
human chorionic gonadotropin
- IAM
intact amniotic membrane
- IVM
in vitro maturation
- IVF
in vitro fertilization
- IGF
insulin-like growth factor
- LH
luteinizing hormone
- MEFs
mouse embryonic fibroblasts
- mRNA
messenger ribonucleic acid
- MII
metaphase II
- MATLAB
Matrix Laboratory
- MFP
microfluidic platform
- MPF
maturation-promoting factor
- PIP3
phosphatidylinositol-triphosphate
- PRF
platelet-rich fibrin
- POI
primary ovarian insufficiency
- PBS
phosphate-buffered saline
- PCL
polycaprolactone
- PDMS
polydimethylsiloxane
- PI3K
phosphoinositide 3-kinase
- POF
premature ovarian failure
- PEG
polyethylene glycol
- SCID
severe combined immunodeficiency
- SDS
sodium dodecyl sulfate
- SDC
sodium deoxycholate
- SEM
scanning electron microscope
- UBM
urinary bladder matrix
- ZP
zona pellucida
- 2D
two-dimensional
- 3D
three-dimensional
- αMEM
α minimum essential medium
Acknowledgment
'S. G. and D.S.S. kindly acknowledge Danish National Research Foundation center grant CellPAT (DNRF135), for supporting of the authors throughout the study period and manuscript preparation. E.M. would like to acknowledge the support from the National Institute of Biomedical Imaging and Bioengineering (5T32EB009035).
Contributor Information
Sadegh Ghorbani, Interdisciplinary Nanoscience Center (iNANO), Aarhus University, Aarhus, Denmark.
Hossein Eyni, Cellular and Molecular Research Center, School of Medicine, Iran University of Medical Science, Tehran, Iran; Department of Anatomical Sciences, School of Medicine, Iran University of Medical Science, Tehran, Iran.
Mohammad Hadi Norahan, School of Engineering and Sciences, Tecnologico de Monterrey Unviersity, Monterrey, NL, Mexico.
Payam Zarrintaj, Department of Biomedical and Pharmaceutical Sciences, University of Montana, Missoula, MT, USA.
Nadine Urban, Freiburg Centre for Interactive Materials and Bioinspired Technology, University of Freiburg, Freiburg, Germany.
Alireza Mohammadzadeh, Department of Bioengineering, Universit y of Pittsburgh, Pittsburgh, PA, USA.
Ebrahim Mostafavi, Stanford Cardiovascular Institute, Stanford University School of Medicine, Stanford, CA, USA; Department of Medicine, Stanford University School of Medicine, Stanford, CA, USA.
Duncan S Sutherland, Interdisciplinary Nanoscience Center (iNANO), Aarhus University, Aarhus, Denmark.
Conflict of interest
The authors have declared that no conflict of interest exists.
References
- 1. Mahajan N. Fertility preservation in female cancer patients: an overview. J Hum Reprod Sci 2015; 8(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goswami D, Conway GS. Premature ovarian failure. Hum Reprod Update 2005; 11(4):391–410. [DOI] [PubMed] [Google Scholar]
- 3. Salama M, Woodruff TK. New advances in ovarian autotransplantation to restore fertility in cancer patients. Cancer Metastasis Rev 2015; 34(4):807–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Woodruff TK, Shea LD. The role of the extracellular matrix in ovarian follicle development. Reprod Sci 2007; 14(8_suppl):6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tingen C, Kim A, Woodruff TK. The primordial pool of follicles and nest breakdown in mammalian ovaries. Mol Hum Reprod 2009; 15(12):795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Telfer EE, Zelinski MB. Ovarian follicle culture: advances and challenges for human and nonhuman primates. Fertil Steril 2013; 99(6):1523–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaipia A, Hsueh AJ. Regulation of ovarian follicle atresia. Annu Rev Physiol 1997; 59(1):349–63. [DOI] [PubMed] [Google Scholar]
- 8. Rimon-Dahari N, Yerushalmi-Heinemann L, Alyagor L, Dekel N. Ovarian folliculogenesis. Mol Mech Cell Differ Gonad Dev 2016; 58:167–190. [DOI] [PubMed] [Google Scholar]
- 9. Martinovitch PN. The development in vitro of the mammalian gonad. Ovary and ovogenesis gonad. Proc R Soc Lond Ser B - Biol Sci 1938; 125(839):232–49. [Google Scholar]
- 10. Eppig JJ. Mouse oocyte development in vitro with various culture systems. Dev Biol 1977; 60(2):371–88. [DOI] [PubMed] [Google Scholar]
- 11. Abir R, Franks S, Mobberley MA, Moore PA, Margara RA, Winston RM. Mechanical isolation and in vitro growth of preantral and small antral human follicles. Fertil Steril 1997; 68(4):682–8. [DOI] [PubMed] [Google Scholar]
- 12. Gutierrez CG, Ralph JH, Telfer EE, Wilmut I, Webb R. Growth and antrum formation of bovine preantral follicles in long-term culture in vitro. Biol Reprod 2000; 62(5):1322–8. [DOI] [PubMed] [Google Scholar]
- 13. Roy SK, Treacy BJ. Isolation and long-term culture of human preantral follicles. Fertil Steril 1993; 59(4):783–90. [PubMed] [Google Scholar]
- 14. Tambe SS, Nandedkar TD. Steroidogenesis in sheeo ovarian antral follicles in culture: time course study and supplementation with a precursor. Steroids 1993; 58(8):379–83. [DOI] [PubMed] [Google Scholar]
- 15. Eppig JJ, O’Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod 1996; 54(1):197–207. [DOI] [PubMed] [Google Scholar]
- 16. O’Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod 2003; 68(5):1682–6. [DOI] [PubMed] [Google Scholar]
- 17. Shikanov A, Smith RM, Xu M, Woodruff TK, Shea LD. Hydrogel network design using multifunctional macromers to coordinate tissue maturation in ovarian follicle culture. Biomaterials 2011; 32(10):2524–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sutton M, Gilchrist R, Thompson J. Effects of in-vivo and in-vitro environments on the metabolism of the cumulus–oocyte complex and its influence on oocyte developmental capacity. Hum Reprod Update 2003; 9(1):35–48. [DOI] [PubMed] [Google Scholar]
- 19. Desai N, Abdelhafez F, Calabro A, Falcone T. Three dimensional culture of fresh and vitrified mouse pre-antral follicles in a hyaluronan-based hydrogel: a preliminary investigation of a novel biomaterial for in vitro follicle maturation. Reprod Biol Endocrinol 2012; 10(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Choi JK, Agarwal P, Huang H, Zhao S, He X. The crucial role of mechanical heterogeneity in regulating follicle development and ovulation with engineered ovarian microtissue. Biomaterials 2014; 35(19):5122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Berkholtz CB, Lai BE, Woodruff TK, Shea LD. Distribution of extracellular matrix proteins type I collagen, type IV collagen, fibronectin, and laminin in mouse folliculogenesis. Histochem Cell Biol 2006; 126(5):583–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sadr SZ, Fatehi R, Maroufizadeh S, Amorim CA, Ebrahimi B. Utilizing fibrin-alginate and matrigel-alginate for mouse follicle development in three-dimensional culture systems. Biopreser Biobank 2018; 16(2):120–7. [DOI] [PubMed] [Google Scholar]
- 23. Ihm JE, Lee ST, Han DK, Lim JM, Hubbell JA. Murine ovarian follicle culture in PEG-hydrogel: effects of mechanical properties and the hormones FSH and LH on development. Macromol Res 2015; 23(4):377–86. [Google Scholar]
- 24. Mendez U, Zhou H, Shikanov A. Synthetic PEG phydrogel for engineering the environment of ovarian follicles. In: Chawla K (ed). Biomaterials for Tissue Engineering [Internet]. New York, NY: Springer New York; 2018. [cited 2021 Jul 25]:–28. (Methods in Molecular Biology; vol. 1758). Available from: http://link.springer.com/10.1007/978-1-4939-7741-3_9 [DOI] [PubMed] [Google Scholar]
- 25. Kreeger PK, Deck JW, Woodruff TK, Shea LD. The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials 2006; 27(5):714–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Abir, R., P. Roizman, B. Fisch, S. Nitke, E. Okon, R. Orvieto, and Z. Ben Rafael. Pilot study of isolated early human follicles cultured in collagen gels for 24 hours. Hum Reprod 1999; 14(5):1299–301. [DOI] [PubMed] [Google Scholar]
- 27. Abir R, Fisch B, Nitke S, Okon E, Raz A, Ben Rafael Z. Morphological study of fully and partially isolated early human follicles. Fertil Steril 2001; 75(1):141–6. [DOI] [PubMed] [Google Scholar]
- 28. Roy SK, Greenwald GS. Methods of separation and in-vitro culture of pre-antral follicles from mammalian ovaries. Hum Reprod Update 1996;2(3):236–45. [DOI] [PubMed] [Google Scholar]
- 29. Amorim CA, Van Langendonckt A, David A, Dolmans MM, Donnez J. Survival of human pre-antral follicles after cryopreservation of ovarian tissue, follicular isolation and in vitro culture in a calcium alginate matrix. Hum Reprod 2009;24(1):92–9. [DOI] [PubMed] [Google Scholar]
- 30. Xu M, Barrett SL, West-Farrell E, Kondapalli LA, Kiesewetter SE, Shea LD et al. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod 2009;24(10):2531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Camboni A, Van Langendonckt A, Donnez J, Vanacker J, Dolmans MM, Amorim C. Alginate beads as a tool to handle, cryopreserve and culture isolated human primordial/primary follicles. Cryobiology 2013;67(1):64–9. [DOI] [PubMed] [Google Scholar]
- 32. Laronda MM, Duncan FE, Hornick JE, Xu M, Pahnke JE, Whelan KA et al. Alginate encapsulation supports the growth and differentiation of human primordial follicles within ovarian cortical tissue. J Assist Reprod Genet 2014;31(8):1013–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xiao S, Zhang J, Romero MM, Smith KN, Shea LD, Woodruff TK. In vitro follicle growth supports human oocyte meiotic maturation. Sci Rep 2015;5(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Skory RM, Xu Y, Shea LD, Woodruff TK. Engineering the ovarian cycle using in vitro follicle culture. Hum Reprod 2015;30(6):1386–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xu M, West-Farrell ER, Stouffer RL, Shea LD, Woodruff TK, Zelinski MB. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol Reprod 2009;81(3):587–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hornick J, Duncan F, Shea L, Woodruff T. Isolated primate primordial follicles require a rigid physical environment to survive and grow in vitro. Hum Reprod 2012;27(6):1801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu M, Fazleabas AT, Shikanov A, Jackson E, Barrett SL, Hirshfeld-Cytron J et al. In vitro oocyte maturation and preantral follicle culture from the luteal-phase baboon ovary produce mature oocytes. Biol Reprod 2011. ;84(4):689–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xu J, Lawson M, Yeoman R, Molskness T, Ting A, Stouffer R et al. Fibrin promotes development and function of macaque primary follicles during encapsulated three-dimensional culture. Hum Reprod 2013;28(8):2187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lerer-Serfaty G, Samara N, Fisch B, Shachar M, Kossover O, Seliktar D et al. Attempted application of bioengineered/biosynthetic supporting matrices with phosphatidylinositol-trisphosphate-enhancing substances to organ culture of human primordial follicles. J Assist Reprod Genet 2013. ;30(10):1279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Torrance C, Telfer E, Gosden R. Quantitative study of the development of isolated mouse pre-antral follicles in collagen gel culture. Reproduction 1989;87(1):367–74. [DOI] [PubMed] [Google Scholar]
- 41. Gomes JE, Correia SC, Gouveia-Oliveira A, Cidadão AJ, Plancha CE. Three-dimensional environments preserve extracellular matrix compartments of ovarian follicles and increase FSH-dependent growth. Mol Reprod Dev Inc Gamete Res 1999;54(2):163–72. [DOI] [PubMed] [Google Scholar]
- 42. Kreeger PK, Fernandes NN, Woodruff TK, Shea LD. Regulation of mouse follicle development by follicle-stimulating hormone in a three-dimensional in vitro culture system is dependent on follicle stage and dose. Biol Reprod 2005. ;73(5):942–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mochida N, Akatani-Hasegawa A, Saka K, Ogino M, Hosoda Y, Wada R et al. Live births from isolated primary/early secondary follicles following a multistep culture without organ culture in mice. Reproduction 2013;146(1):37–47. [DOI] [PubMed] [Google Scholar]
- 44. Mousset-Siméon N, Jouannet P, Le Cointre L, Coussieu C, Poirot C. Comparison of three in vitro culture systems for maturation of early preantral mouse ovarian follicles. Zygote 2005;13(2):167–75. [DOI] [PubMed] [Google Scholar]
- 45. Pangas SA, Saudye H, Shea LD, Woodruff TK. Novel approach for the three-dimensional culture of granulosa cell–oocyte complexes. Tissue Eng 2003;9(5):1013–21. [DOI] [PubMed] [Google Scholar]
- 46. Xu M, Banc A, Woodruff TK, Shea LD. Secondary follicle growth and oocyte maturation by culture in alginate hydrogel following cryopreservation of the ovary or individual follicles. Biotechnol Bioeng 2009;103(2):378–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Parrish EM, Siletz A, Xu M, Woodruff TK, Shea LD. Gene expression in mouse ovarian follicle development in vivo versus an ex vivo alginate culture system. Reproduction 2011;142(2):309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tagler D, Tu T, Smith RM, Anderson NR, Tingen CM, Woodruff TK et al. Embryonic fibroblasts enable the culture of primary ovarian follicles within alginate hydrogels. Tissue Eng Part A 2012;18(11–12):1229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. King SM, Modi DA, Eddie SL, Burdette JE. Insulin and insulin-like growth factor signaling increases proliferation and hyperplasia of the ovarian surface epithelium and decreases follicular integrity through upregulation of the PI3-kinase pathway. J Ovarian Res. 2013;6(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hornick J, Duncan F, Shea L, Woodruff T. Multiple follicle culture supports primary follicle growth through paracrine-acting signals. Reproduction. 2013;145(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tagler D, Makanji Y, Anderson NR, Woodruff TK, Shea LD. Supplemented αMEM/F12-based medium enables the survival and growth of primary ovarian follicles encapsulated in alginate hydrogels. Biotechnol Bioeng 2013;110(12):3258–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tagler D, Makanji Y, Tu T, Bernabé BP, Lee R, Zhu J et al. Promoting extracellular matrix remodeling via ascorbic acid enhances the survival of primary ovarian follicles encapsulated in alginate hydrogels. Biotechnol Bioeng 2014;111(7):1417–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Makanji Y, Tagler D, Pahnke J, Shea LD, Woodruff TK. Hypoxia-mediated carbohydrate metabolism and transport promote early-stage murine follicle growth and survival. Am J Physiol-Endocrinol Metab 2014;306(8):E893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Majdi Seghinsara A, Shoorei H, Hassanzadeh Taheri MM, Khaki A, Shokoohi M, Tahmasebi M et al. Panax ginseng extract improves follicular development after mouse preantral follicle 3D culture. Cell J. 2019;21(2):210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xiao S, Duncan FE, Bai L, Nguyen CT, Shea LD, Woodruff TK. Size-specific follicle selection improves mouse oocyte reproductive outcomes. Reproduction. 2015;150(3):183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xiao S, Zhang J, Liu M, Iwahata H, Rogers HB, Woodruff TK. Doxorubicin has dose-dependent toxicity on mouse ovarian follicle development, hormone secretion, and oocyte maturation. Toxicol Sci 2017;157(2):320–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhou H, Decker JT, Lemke MM, Tomaszweski CE, Shea LD, Arnold KB et al. Synergy of paracrine signaling during early-stage mouse ovarian follicle development in vitro. Cell Mol Bioeng 2018;11(5):435–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang X, Jiang L, Tian Y, Xia Y, Yan L, Wu C et al. Establishment of in-vitro three dimensional rat follicle culture system and validation of the applicability as an in vitro female reproductive toxicity testing system. Toxicol In Vitro 2019; 58:161–169. [DOI] [PubMed] [Google Scholar]
- 59. Wang Y, Xu J, Stanley JE, Xu M, Brooks BW, Scott GI et al. A closed vitrification system enables a murine ovarian follicle bank for high-throughput ovotoxicity screening, which identifies endocrine disrupting activity of microcystins. Reprod Toxicol 2020; 93:118–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shikanov A, Xu M, Woodruff TK, Shea LD. Interpenetrating fibrin–alginate matrices for in vitro ovarian follicle development. Biomaterials 2009;30(29):5476–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kim EJ, Yang C, Lee J, Youm HW, Lee JR, Suh CS et al. The new biocompatible material for mouse ovarian follicle development in three-dimensional in vitro culture systems. Theriogenology 2020; 144:33–40. [DOI] [PubMed] [Google Scholar]
- 62. Oktem O, Oktay K. The role of extracellular matrix and activin-a in in vitro growth and survival of murine preantral follicles. Reprod Sci 2007. ;14(4):358–66. [DOI] [PubMed] [Google Scholar]
- 63. Hassani F, Ebrahimi B, Moini A, Ghiaseddin A, Bazrafkan M, Hassanzadeh G et al. Chitosan hydrogel supports integrity of ovarian follicles during in vitro culture: a preliminary of a novel biomaterial for three dimensional culture of ovarian follicles. Cell J 2020;21(4):479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ahn JI, Kim GA, Kwon HS, Ahn JY, Hubbell JA, Song YS et al. Culture of preantral follicles in poly(ethylene) glycol-based, three-dimensional hydrogel: a relationship between swelling ratio and follicular developments. J Tissue Eng Regen Med 2015. ;9(3):319–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tomaszewski CE, DiLillo KM, Baker BM, Arnold KB, Shikanov A. Sequestered cell-secreted extracellular matrix proteins improve murine folliculogenesis and oocyte maturation for fertility preservation. Acta Biomater 2021; S1742706121001914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. RGD-modified dextran hydrogel promotes follicle growth in three-dimensional ovarian tissue culture in mice - ScienceDirect [Internet]. [cited 2022. Jul 27]. Available from: https://www.sciencedirect.com/science/article/pii/S0093691X22000565?via%3Dihub [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sun J, Li X. Growth and antrum formation of bovine primary follicles in long-term culture in vitro. Reprod Biol 2013. ;13(3):221–8. [DOI] [PubMed] [Google Scholar]
- 68. Huanmin Z, Yong Z. In vitro development of caprine ovarian preantral follicles. Theriogenology 2000;54(4):641–50. [DOI] [PubMed] [Google Scholar]
- 69. Brito I, Silva C, Duarte A, Lima I, Rodrigues G, Rossetto R et al. Alginate hydrogel matrix stiffness influences the in vitro development of caprine preantral follicles. Mol Reprod Dev 2014;81(7):636–45. [DOI] [PubMed] [Google Scholar]
- 70. Brito I, Silva G, Sales A, Lobo C, Rodrigues G, Sousa R, Moura AAA, Calderón CEM, Bertolini M, Campello CC, Smitz J, Figueiredo JR. Fibrin-alginate hydrogel supports steroidogenesis, in vitro maturation of oocytes and parthenotes production from caprine preantral follicles cultured in group. Reprod Domest Anim 2016;51(6):997–1009. [DOI] [PubMed] [Google Scholar]
- 71. Hirao Y, Nagai T, Kubo M, Miyano T, Miyake M, Kato S. In vitro growth and maturation of pig oocytes. Reproduction 1994;100(2):333–9. [DOI] [PubMed] [Google Scholar]
- 72. Shuttleworth G, Broughton Pipkin F, Hunter M. In vitro development of pig preantral follicles cultured in a serum-free medium and the effect of angiotensin II. Reproduction 2002; 1:807–818. [PubMed] [Google Scholar]
- 73. Songsasen N, Woodruff T, Wildt D. In vitro growth and steroidogenesis of dog follicles as influenced by the physical and hormonal microenvironment. Reproduction 2011;142(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Smith RM, Woodruff TK, Shea LD. Designing Follicle–Environment Interactions with Biomaterials. In: Woodruff TK, Zoloth L, Campo-Engelstein L, Rodriguez S, editors. Oncofertility [Internet]. Boston, MA: Springer US; 2010. [cited 2021 Aug 12]. p. 11–24. (Cancer Treatment and Research; vol. 156). Available from: http://link.springer.com/10.1007/978-1-4419-6518-9_2 [Google Scholar]
- 75. Xu M, West E, Shea LD, Woodruff TK. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod 2006;75(6):916–23. [DOI] [PubMed] [Google Scholar]
- 76. West ER, Xu M, Woodruff TK, Shea LD. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials 2007;28(30):4439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hennink WE, van Nostrum CF. Novel crosslinking methods to design hydrogels. Adv Drug Deliv Rev 2012; 64:223–236. [DOI] [PubMed] [Google Scholar]
- 78. Hu W, Wang Z, Xiao Y, Zhang S, Wang J. Advances in crosslinking strategies of biomedical hydrogels. Biomater Sci 2019;7(3):843–55. [DOI] [PubMed] [Google Scholar]
- 79. Akhtar MF, Hanif M, Ranjha NM. Methods of synthesis of hydrogels: a review. Saudi Pharm J 2016;24(5):554–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Echalier C, Valot L, Martinez J, Mehdi A, Subra G. Chemical cross-linking methods for cell encapsulation in hydrogels. Mater Today Commun 2019; 20:100536. [Google Scholar]
- 81. Kloxin AM, Kloxin CJ, Bowman CN, Anseth KS. Mechanical properties of cellularly responsive hydrogels and their experimental determination. Adv Mater 2010;22(31):3484–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Elliott JE, Bowman CN. Monomer functionality and polymer network formation. Macromolecules 2001;34(13):4642–9. [Google Scholar]
- 83. Bakhshandeh B, Zarrintaj P, Oftadeh MO, Keramati F, Fouladiha H, Sohrabi-Jahromi S et al. Tissue engineering; strategies, tissues, and biomaterials. Biotechnol Genet Eng Rev 2017;33(2):144–72. [DOI] [PubMed] [Google Scholar]
- 84. Peppas NA. Hydrogels in Medicine and Pharmacy: Fundamentals, vol. 1. CRC Press; 2019. [Google Scholar]
- 85. Nicodemus GD, Bryant SJ. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng Part B Rev 2008;14(2):149–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Roy SK, Greenwald G. Hormonal requirements for the growth and differentiation of hamster preantral follicles in long-term culture. Reproduction 1989;87(1):103–14. [DOI] [PubMed] [Google Scholar]
- 87. Zhang H, Cheng J, Ao Q. Preparation of alginate-based biomaterials and their applications in biomedicine. Mar Drugs 2021;19(5):264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Jamalzaei P, Valojerdi MR, Montazeri L, Baharvand H. Applicability of hyaluronic acid-alginate hydrogel and ovarian cells for in vitro development of mouse preantral follicles. Cell J 2020;22(Suppl 1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Rezende RA, Bártolo P, Mendes A, Filho RM. Experimental characterisation of the alginate gelation process for rapid prototyping. Chem Eng Trans 2007; 11:509–514. [Google Scholar]
- 90. Xu Y, Duncan FE, Xu M, Woodruff TK. Use of an organotypic mammalian in vitro follicle growth assay to facilitate female reproductive toxicity screening. Reprod Fertil Dev 2016;28(9):1295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Dunlop CE, Anderson RA. The regulation and assessment of follicular growth. Scand J Clin Lab Invest 2014;74(sup244):13–7. [DOI] [PubMed] [Google Scholar]
- 92. Sierra DH. Fibrin sealant adhesive systems: a review of their chemistry, material properties and clinical applications. J Biomater Appl 1993. ;7(4):309–52. [DOI] [PubMed] [Google Scholar]
- 93. Shikanov A, Xu M, Woodruff TK, Shea LD. A method for ovarian follicle encapsulation and culture in a proteolytically degradable 3 dimensional system. J Vis Exp 2011;(49). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Jin SY, Lei L, Shikanov A, Shea LD, Woodruff TK. A novel two-step strategy for in vitro culture of early-stage ovarian follicles in the mouse. Fertil Steril 2010. ;93(8):2633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zhou H, Malik MA, Arab A, Hill MT, Shikanov A. Hydrogel based 3-dimensional (3D) system for toxicity and high-throughput (HTP) analysis for cultured murine ovarian follicles. PLOS ONE. 2015;10(10):e0140205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhou H, Young CJ, Loch-Caruso R, Shikanov A. Detection of lindane and 7,12-dimethylbenz[a]anthracene toxicity at low concentrations in a three-dimensional ovarian follicle culture system. Reprod Toxicol 2018; 78:141–149. [DOI] [PubMed] [Google Scholar]
- 97. Giancotti FG, Tarone G. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu Rev Cell Dev Biol 2003. ;19(1):173–206. [DOI] [PubMed] [Google Scholar]
- 98. Zhu J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010. ;31(17):4639–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Luyckx V, Dolmans MM, Vanacker J, Legat C, Fortuño Moya C, Donnez J et al. A new step toward the artificial ovary: survival and proliferation of isolated murine follicles after autologous transplantation in a fibrin scaffold. Fertil Steril 2014. ;101(4):1149–56. [DOI] [PubMed] [Google Scholar]
- 100. Pors S, Ramløse M, Nikiforov D, Lundsgaard K, Cheng J, Andersen CY et al. Initial steps in reconstruction of the human ovary: survival of pre-antral stage follicles in a decellularized human ovarian scaffold. Hum Reprod 2019;34(8):1523–35. [DOI] [PubMed] [Google Scholar]
- 101. Luyckx V, Dolmans MM, Vanacker J, Scalercio SR, Donnez J, Amorim CA. First step in developing a 3D biodegradable fibrin scaffold for an artificial ovary. J Ovarian Res 2013;6(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Chiti MC, Dolmans MM, Mortiaux L, Zhuge F, Ouni E, Shahri PAK et al. A novel fibrin-based artificial ovary prototype resembling human ovarian tissue in terms of architecture and rigidity. J Assist Reprod Genet 2018;35(1):41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Motamed M, Sadr Z, Valojerdi M, Moini A, Oryan S, Totonchi M et al. Tissue engineered human amniotic membrane application in mouse ovarian follicular culture. Ann Biomed Eng 2017;45(7):1664–75. [DOI] [PubMed] [Google Scholar]
- 104. Wu T, Gao Y, Su J, Tang X, Chen Q, Ma L et al. Three-dimensional bioprinting of artificial ovaries by an extrusion-based method using gelatin-methacryloyl bioink. Climacteric 2022;25(2):170–8. [DOI] [PubMed] [Google Scholar]
- 105. Alshaikh AB, Padma AM, Dehlin M, Akouri R, Song MJ, Brännström M et al. Decellularization of the mouse ovary: comparison of different scaffold generation protocols for future ovarian bioengineering. J Ovarian Res 2019;12(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Mirzaeian L, Eivazkhani F, Hezavehei M, Moini A, Esfandiari F, Valojerdi MR et al. Optimizing the cell seeding protocol to human decellularized ovarian scaffold: application of dynamic system for bio-engineering. Cell J 2020;22(2):227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Nikniaz H, Zandieh Z, Nouri M, Daei-Farshbaf N, Aflatoonian R, Gholipourmalekabadi M et al. Comparing various protocols of human and bovine ovarian tissue decellularization to prepare extracellular matrix-alginate scaffold for better follicle development in vitro. BMC Biotechnol 2021;21(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Laronda MM, Jakus AE, Whelan KA, Wertheim JA, Shah RN, Woodruff TK. Initiation of puberty in mice following decellularized ovary transplant. Biomaterials 2015. 1;50:20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Chiti MC, Dolmans MM, Orellana R, Soares M, Paulini F, Donnez J et al. Influence of follicle stage on artificial ovary outcome using fibrin as a matrix. Hum Reprod 2016;31(2):427–35. [DOI] [PubMed] [Google Scholar]
- 110. Shojafar E, Mehranjani MS, Shariatzadeh SM. Utilizing platelet-rich fibrin bioscaffold at the graft site improves the structure and function of mice ovarian grafts. Regen Med 2019;14(5):409–22. [DOI] [PubMed] [Google Scholar]
- 111. Ma S, Lin H, Miao Y, Liu X, Wang B, Dai J. The effect of three-dimensional demineralized bone matrix on in vitro cumulus-free oocyte maturation. Biomaterials 2007;28(21):3198–207. [DOI] [PubMed] [Google Scholar]
- 112. Su J, Ding L, Cheng J, Yang J, Li X, Yan G et al. Transplantation of adipose-derived stem cells combined with collagen scaffolds restores ovarian function in a rat model of premature ovarian insufficiency. Hum Reprod 2016;31(5):1075–86. [DOI] [PubMed] [Google Scholar]
- 113. Damous LL, Nakamuta JS, de Carvalho AETS, Carvalho KC, Soares-Jr JM, de Jesus Simões M et al. Scaffold-based delivery of adipose tissue-derived stem cells in rat frozen-thawed ovarian autografts: preliminary studies in a rat model. J Assist Reprod Genet 2015;32(8):1285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Xing Y, Zhao W, Li W et al. Effect of adipose-derived mesenchymal stem cells combined with urinary bladder matrix scaffold on the structure and function of autografted rat ovaries. 2021, PREPRINT (Version 1) available at Research Square [ 10.21203/rs.3.rs-422334/v1] [DOI]
- 115. Hassanpour A, Talaei-Khozani T, Kargar-Abarghouei E, Razban V, Vojdani Z. Decellularized human ovarian scaffold based on a sodium lauryl ester sulfate (SLES)-treated protocol, as a natural three-dimensional scaffold for construction of bioengineered ovaries. Stem Cell Res Ther 2018;9(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Pennarossa G, Ghiringhelli M, Gandolfi F, Brevini TA. Whole-ovary decellularization generates an effective 3D bioscaffold for ovarian bioengineering. J Assist Reprod Genet 2020;37(6):1329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Felder S, Masasa H, Orenbuch A, Levaot N, Goldenberg MS, Cohen S. Reconstruction of the ovary microenvironment utilizing macroporous scaffold with affinity-bound growth factors. Biomaterials 2019; 205:11–22. [DOI] [PubMed] [Google Scholar]
- 118. Liverani L, Raffel N, Fattahi A, Preis A, Hoffmann I, Boccaccini AR et al. Electrospun patterned porous scaffolds for the support of ovarian follicles growth: a feasibility study. Sci Rep 2019;9(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Shen P, Xu J, Wang P, Zhao X, Huang B, Wu F et al. A new three-dimensional glass scaffold increases the in vitro maturation efficiency of buffalo (Bubalus bubalis) oocyte via remodelling the extracellular matrix and cell connection of cumulus cells. Reprod Domest Anim 2020;55(2):170–80. [DOI] [PubMed] [Google Scholar]
- 120. Di Berardino C, Liverani L, Peserico A, Capacchietti G, Russo V, Bernabò N et al. When electrospun fiber support matters: in vitro ovine long-term folliculogenesis on poly (epsilon caprolactone) (PCL)-patterned fibers. Cell 2022;11(12):1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Heath DE. A review of decellularized extracellular matrix biomaterials for regenerative engineering applications. Regen Eng Transl Med 2019;5(2):155–66. [Google Scholar]
- 122. Hassanpour A, Talaei-Khozani T, Kargar-Abarghouei E, Razban V, Vojdani Z. Decellularized human ovarian scaffold based on a sodium lauryl ester sulfate (SLES)-treated protocol, as a natural three-dimensional scaffold for construction of bioengineered ovaries. Stem Cell Res Ther. 2018;9(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Gilpin A, Yang Y. Decellularization strategies for regenerative medicine: from processing techniques to applications. Biomed Res Int 2017; 2017:9831534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Sadtler K, Sommerfeld SD, Wolf MT, Wang X, Majumdar S, Chung L et al. Proteomic composition and immunomodulatory properties of urinary bladder matrix scaffolds in homeostasis and injury. 2017; 29:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Li Y, Meng H, Liu Y, Lee BP. Fibrin gel as an injectable biodegradable scaffold and cell carrier for tissue engineering. Sci World J 2015; 2015:685690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Ehterami A, Salehi M, Farzamfar S, Vaez A, Samadian H, Sahrapeyma H et al. In vitro and in vivo study of PCL/COLL wound dressing loaded with insulin-chitosan nanoparticles on cutaneous wound healing in rats model. Int J Biol Macromol 2018; 117:601–609. [DOI] [PubMed] [Google Scholar]
- 127. Tebyanian H, Norahan MH, Eyni H, Movahedin M, Mortazavi SJ, Karami A et al. Effects of collagen/β-tricalcium phosphate bone graft to regenerate bone in critically sized rabbit calvarial defects. J Appl Biomater Funct Mater 2019;17(1):2280800018820490. [DOI] [PubMed] [Google Scholar]
- 128. Wang X, Ao Q, Tian X, Fan J, Tong H, Hou W et al. Gelatin-based hydrogels for organ 3D bioprinting. Polymers 2017;9(9):401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Hoque ME, Nuge T, Yeow TK, Nordin N, Prasad R. Gelatin based scaffolds for tissue engineering-a review. Polym Res J 2015;9(1):15. [Google Scholar]
- 130. Naseri-Nosar M, Farzamfar S, Sahrapeyma H, Ghorbani S, Bastami F, Vaez A et al. Cerium oxide nanoparticle-containing poly (ε-caprolactone)/gelatin electrospun film as a potential wound dressing material: in vitro and in vivo evaluation. Mater Sci Eng C 2017; 81:366–372. [DOI] [PubMed] [Google Scholar]
- 131. Farzamfar S, Naseri-Nosar M, Vaez A, Esmaeilpour F, Ehterami A, Sahrapeyma H et al. Neural tissue regeneration by a gabapentin-loaded cellulose acetate/gelatin wet-electrospun scaffold. Cellulose 2018;25(2):1229–38. [Google Scholar]
- 132. Laronda MM, Rutz AL, Xiao S, Whelan KA, Duncan FE, Roth EW et al. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat Commun 2017;8(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Vanacker J, Luyckx V, Dolmans MM, Des Rieux A, Jaeger J, Van Langendonckt A et al. Transplantation of an alginate–matrigel matrix containing isolated ovarian cells: first step in developing a biodegradable scaffold to transplant isolated preantral follicles and ovarian cells. Biomaterials 2012;33(26):6079–85. [DOI] [PubMed] [Google Scholar]
- 134. Liu G, Li S, Yuan H, Hao M, Yun Z, Zhao J et al. Effect of sodium alginate on mouse ovary vitrification. Theriogenology 2018; 113:78–84. [DOI] [PubMed] [Google Scholar]
- 135. Hsueh AJ, Kawamura K, Cheng Y, Fauser BC. Intraovarian control of early folliculogenesis. Endocr Rev 2015;36(1):1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Nilsson EE, Skinner MK. Bone morphogenetic protein-4 acts as an ovarian follicle survival factor and promotes primordial follicle development. Biol Reprod 2003;69(4):1265–72. [DOI] [PubMed] [Google Scholar]
- 137. Liu X, Holzwarth JM, Ma PX. Functionalized synthetic biodegradable polymer scaffolds for tissue engineering. Macromol Biosci 2012;12(7):911–9. [DOI] [PubMed] [Google Scholar]
- 138. Asghari F, Samiei M, Adibkia K, Akbarzadeh A, Davaran S. Biodegradable and biocompatible polymers for tissue engineering application: a review. Artif Cells Nanomedicine Biotechnol 2017;45(2):185–92. [DOI] [PubMed] [Google Scholar]
- 139. Malikmammadov E, Tanir TE, Kiziltay A, Hasirci V, Hasirci N. PCL and PCL-based materials in biomedical applications. J Biomater Sci Polym Ed 2018;29(7–9):863–93. [DOI] [PubMed] [Google Scholar]
- 140. McNATTY KP, Makris A, Degrazia C, Rapin O, Ryan KJ. The production of progesterone, androgens, and estrogens by granulosa cells, thecal tissue, and stromal tissue from human ovaries in vitro. J Clin Endocrinol Metab 1979;49(5):687–99. [DOI] [PubMed] [Google Scholar]
- 141. Haney A, Schomberg DW. Estrogen and progesterone production by developing porcine follicles in vitro: evidence for estrogen formation by theca. Endocrinology 1981;109(3):971–7. [DOI] [PubMed] [Google Scholar]
- 142. Whitesides GM. The origins and the future of microfluidics. Nature 2006;442(7101):368–73. [DOI] [PubMed] [Google Scholar]
- 143. Sackmann EK, Fulton AL, Beebe DJ. The present and future role of microfluidics in biomedical research. Nature 2014;507(7491):181–9. [DOI] [PubMed] [Google Scholar]
- 144. Homan KA, Kolesky DB, Skylar-Scott MA, Herrmann J, Obuobi H, Moisan A et al. Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Sci Rep 2016;6(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Weber EJ, Chapron A, Chapron BD, Voellinger JL, Lidberg KA, Yeung CK et al. Development of a microphysiological model of human kidney proximal tubule function. Kidney Int 2016;90(3):627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Aziz AUR, Fu M, Deng J, Geng C, Luo Y, Lin B et al. A microfluidic device for culturing an encapsulated ovarian follicle. Micromachines 2017. ;8(11):335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Aziz A Ur R, Yu X, Jiang Q, Zhao Y, Deng S, Qin K et al. Doxorubicin-induced toxicity to 3D-cultured rat ovarian follicles on a microfluidic chip. Toxicol In Vitro 2020. 1;62:104677. [DOI] [PubMed] [Google Scholar]
- 148. Hopkins TI, Bemmer VL, Franks S, Dunlop C, Hardy K, Dunlop IE. Mapping the mechanical microenvironment in the ovary. bioRxiv 2021.01.03.425098; 10.1101/2021.01.03.425098. [DOI] [Google Scholar]
- 149. Choi JK, Agarwal P, Huang H, Zhao S, He X. The crucial role of mechanical heterogeneity in regulating follicle development and ovulation with engineered ovarian microtissue. Biomaterials 2014. ;35(19):5122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Li H, Garner T, Diaz F, Wong PK. A multiwell microfluidic device for analyzing and screening nonhormonal contraceptive agents. Small 2019;15(28):1901910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. He X. Microfluidic encapsulation of ovarian follicles for 3D culture. Ann Biomed Eng 2017. ;45(7):1676–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Agarwal P, Choi JK, Huang H, Zhao S, Dumbleton J, Li J et al. A biomimetic core–shell platform for miniaturized 3D cell and tissue engineering. Part Part Syst Charact 2015;32(8):809–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Zargari S, Veladi H, Sadeghzadeh B, Shahabi P, Frounchi J. A microfluidic chip for in vitro oocyte maturation. Sens Lett 2016;14(4):435–40. [Google Scholar]
- 154. Xie Y, Wang F, Zhong W, Puscheck E, Shen H, Rappolee DA. Shear stress induces preimplantation embryo death that is delayed by the zona pellucida and associated with stress-activated protein kinase-mediated apoptosis. Biol Reprod 2006;75(1):45–55. [DOI] [PubMed] [Google Scholar]
- 155. Sadeghzadeh Oskouei B, Pashaiasl M, Heidari MH, Salehi M, Veladi H, Ghaderi Pakdel F et al. Evaluation of mouse oocyte in vitro maturation developmental competency in dynamic culture systems by design and construction of a lab on a chip device and its comparison with conventional culture system. Cell J 2016;18(2):205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Nagashima JB, El Assal R, Songsasen N, Demirci U. Evaluation of an ovary-on-a-chip in large mammalian models: species specificity and influence of follicle isolation status. J Tissue Eng Regen Med 2018;12(4):e1926–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Gupta N, Liu JR, Patel B, Solomon DE, Vaidya B, Gupta V. Microfluidics-based 3D cell culture models: utility in novel drug discovery and delivery research. Bioeng Transl Med 2016;1(1):63–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Xiao S, Coppeta JR, Rogers HB, Isenberg BC, Zhu J, Olalekan SA et al. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat Commun 2017. ;8(1):14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Silva PN, Green BJ, Altamentova SM, Rocheleau JV. A microfluidic device designed to induce media flow throughout pancreatic islets while limiting shear-induced damage. Lab Chip 2013;13(22):4374–84. [DOI] [PubMed] [Google Scholar]
- 160. Nahavandi S, Tang S, Baratchi S, Soffe R, Nahavandi S, Kalantar-zadeh K et al. Microfluidic platforms for the investigation of intercellular signalling mechanisms. Small 2014;10(23):4810–26. [DOI] [PubMed] [Google Scholar]
- 161. Dittrich PS, Manz A. Lab-on-a-chip: microfluidics in drug discovery. Nat Rev Drug Discov 2006;5(3):210–8. [DOI] [PubMed] [Google Scholar]
- 162. Berenguel-Alonso M, Sabés-Alsina M, Morató R, Ymbern O, Rodríguez-Vázquez L, Talló-Parra O et al. Rapid prototyping of a cyclic olefin copolymer microfluidic device for automated oocyte culturing. SLAS Technol Transl Life Sci Innov 2017;22(5):507–17. [DOI] [PubMed] [Google Scholar]
- 163. Tsao C, Hromada L, Liu J, Kumar P, DeVoe D. Low temperature bonding of PMMA and COC microfluidic substrates using UV/ozone surface treatment. Lab Chip 2007;7(4):499–505. [DOI] [PubMed] [Google Scholar]