Abstract
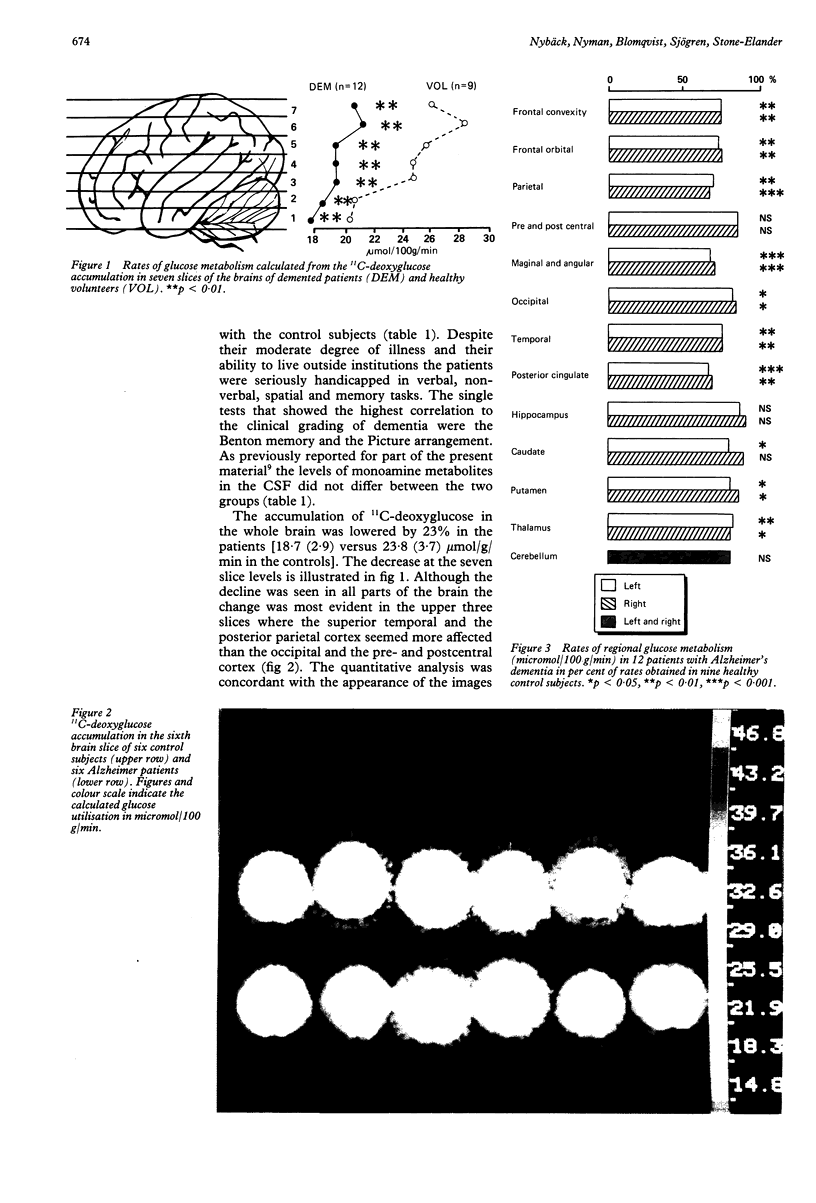
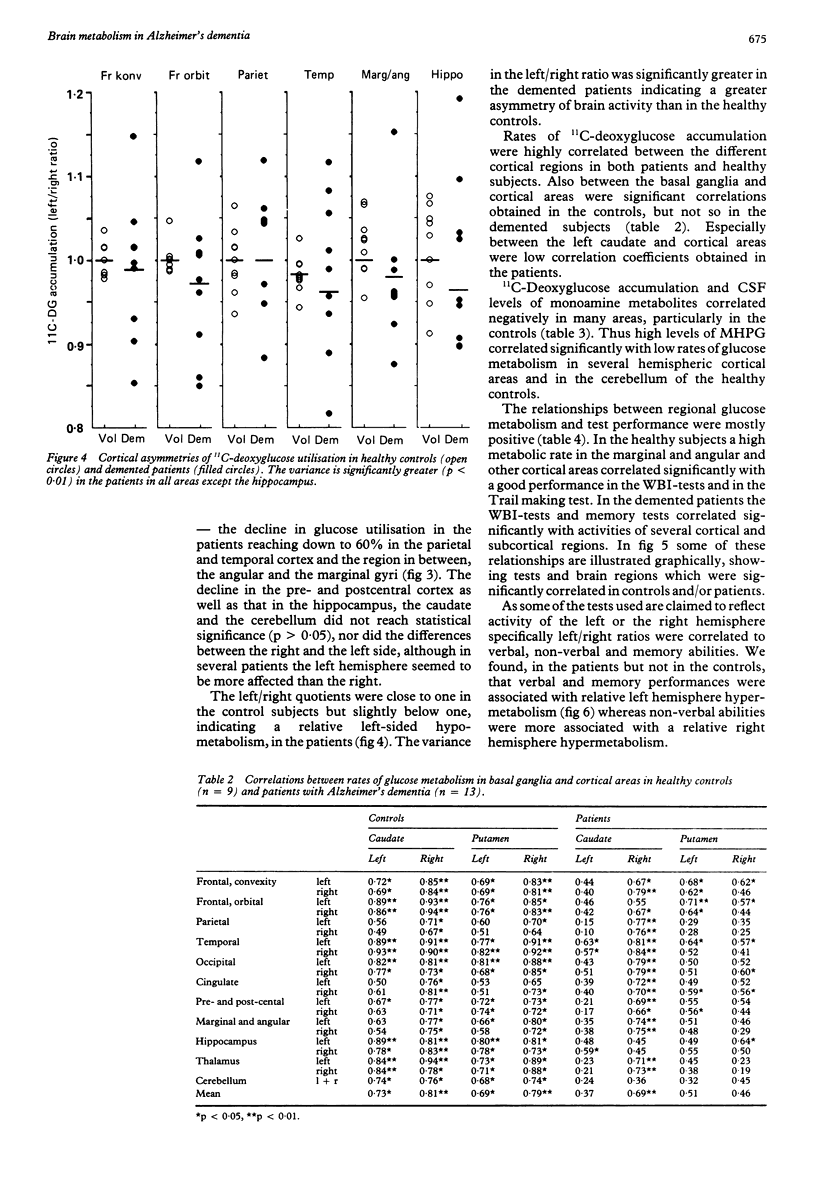
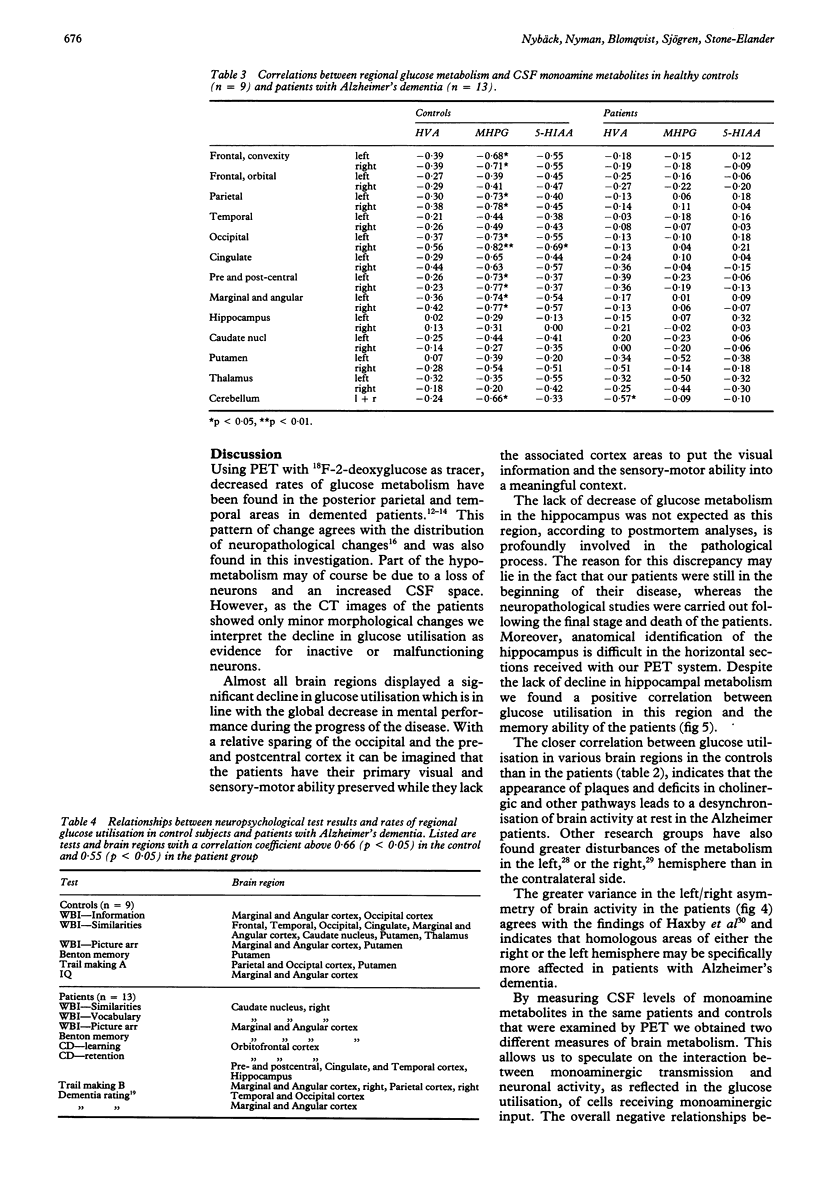
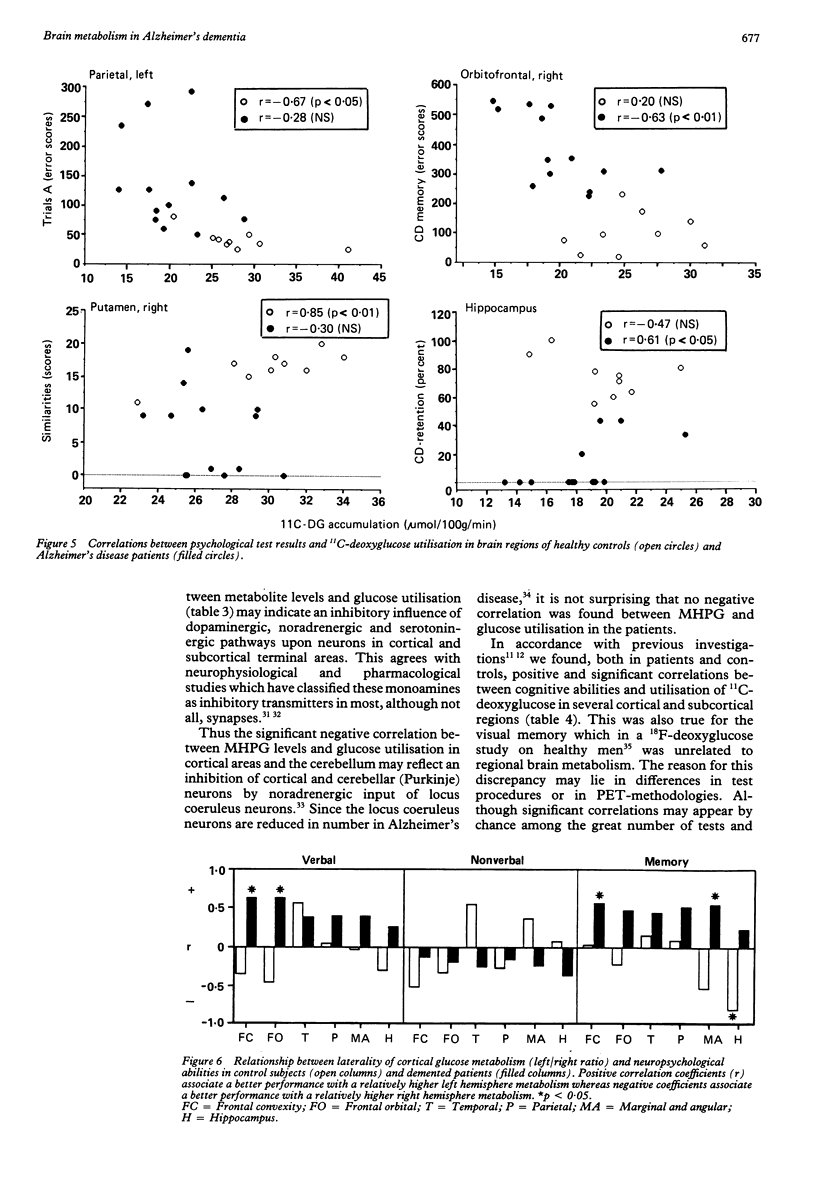
Thirteen patients with dementia of Alzheimer type and nine age-matched control subjects were examined by a battery of neuropsychological tests and by positron emission tomography (PET) with 11C-deoxyglucose as a tracer for regional glucose metabolism in the brain. Concentrations of the monoamine metabolites HVA, MHPG and 5-HIAA were determined in the CSF from patients and controls. In the patients there was a diminished glucose metabolism in posterior parietal and superior temporal cortex areas to 60% of control levels. Other cortical areas showed similar changes, whereas the pre- and postcentral area, the cerebellum, the hippocampus and the basal ganglia showed less or no change. The decline in cortical metabolism in the patients was symmetrical but the variation in the left/right ratio was greater than in the controls. The CSF levels of monoamine metabolites did not differ between patients and controls. High levels of the metabolites were associated with low rates of glucose metabolism, possibly due to inhibitory influences of monoaminergic pathways upon cortical and subcortical neurons. The rate of glucose metabolism correlated positively with the neuropsychological test performance in both patients and controls. Verbal and memory performances were associated with greater left hemisphere metabolism in the patients, but not in the controls, whereas non-verbal abilities tended to be associated with right hemisphere metabolic dominance.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adolfsson R., Gottfries C. G., Nyström L., Winblad B. Prevalence of dementia disorders in institutionalized Swedish old people. The work load imposed by caring for these patients. Acta Psychiatr Scand. 1981 Mar;63(3):225–244. doi: 10.1111/j.1600-0447.1981.tb00670.x. [DOI] [PubMed] [Google Scholar]
- Adolfsson R., Gottfries C. G., Roos B. E., Winblad B. Changes in the brain catecholamines in patients with dementia of Alzheimer type. Br J Psychiatry. 1979 Sep;135:216–223. doi: 10.1192/bjp.135.3.216. [DOI] [PubMed] [Google Scholar]
- Bergström M., Boëthius J., Eriksson L., Greitz T., Ribbe T., Widén L. Head fixation device for reproducible position alignment in transmission CT and positron emission tomography. J Comput Assist Tomogr. 1981 Feb;5(1):136–141. doi: 10.1097/00004728-198102000-00027. [DOI] [PubMed] [Google Scholar]
- Bloom F. E., Costa E., Salmoiraghi G. C. Anesthesia and the responsiveness of individual neurons of the caudate nucleus of the cat to acetylcholine, norepinephrine and dopamine administered by microelectrophoresis. J Pharmacol Exp Ther. 1965 Nov;150(2):244–252. [PubMed] [Google Scholar]
- Bowen D. M., Smith C. B., White P., Davison A. N. Neurotransmitter-related enzymes and indices of hypoxia in senile dementia and other abiotrophies. Brain. 1976 Sep;99(3):459–496. doi: 10.1093/brain/99.3.459. [DOI] [PubMed] [Google Scholar]
- Foote S. L., Freedman R., Oliver A. P. Effects of putative neurotransmitters on neuronal activity in monkey auditory cortex. Brain Res. 1975 Mar 21;86(2):229–242. doi: 10.1016/0006-8993(75)90699-x. [DOI] [PubMed] [Google Scholar]
- Foster N. L., Chase T. N., Fedio P., Patronas N. J., Brooks R. A., Di Chiro G. Alzheimer's disease: focal cortical changes shown by positron emission tomography. Neurology. 1983 Aug;33(8):961–965. doi: 10.1212/wnl.33.8.961. [DOI] [PubMed] [Google Scholar]
- Frackowiak R. S., Pozzilli C., Legg N. J., Du Boulay G. H., Marshall J., Lenzi G. L., Jones T. Regional cerebral oxygen supply and utilization in dementia. A clinical and physiological study with oxygen-15 and positron tomography. Brain. 1981 Dec;104(Pt 4):753–778. doi: 10.1093/brain/104.4.753. [DOI] [PubMed] [Google Scholar]
- Friedland R. P., Budinger T. F., Ganz E., Yano Y., Mathis C. A., Koss B., Ober B. A., Huesman R. H., Derenzo S. E. Regional cerebral metabolic alterations in dementia of the Alzheimer type: positron emission tomography with [18F]fluorodeoxyglucose. J Comput Assist Tomogr. 1983 Aug;7(4):590–598. doi: 10.1097/00004728-198308000-00003. [DOI] [PubMed] [Google Scholar]
- Gottfries C. G., Gottfries I., Roos B. E. Homovanillic acid and 5-hydroxyindoleacetic acid in the cerebrospinal fluid of patients with senile dementia, presenile dementia and parkinsonism. J Neurochem. 1969 Sep;16(9):1341–1345. doi: 10.1111/j.1471-4159.1969.tb05984.x. [DOI] [PubMed] [Google Scholar]
- HONIGFELD G., KLETT C. J. THE NURSES' OBSERVATION SCALE FOR INPATIENT EVALUATION: A NEW SCALE FOR MEASURING IMPROVEMENT IN CHRONIC SCHIZOPHRENIA. J Clin Psychol. 1965 Jan;21:65–71. doi: 10.1002/1097-4679(196501)21:1<65::aid-jclp2270210122>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Haxby J. V., Duara R., Grady C. L., Cutler N. R., Rapoport S. I. Relations between neuropsychological and cerebral metabolic asymmetries in early Alzheimer's disease. J Cereb Blood Flow Metab. 1985 Jun;5(2):193–200. doi: 10.1038/jcbfm.1985.25. [DOI] [PubMed] [Google Scholar]
- Haxby J. V., Grady C. L., Duara R., Robertson-Tchabo E. A., Koziarz B., Cutler N. R., Rapoport S. I. Relations among age, visual memory, and resting cerebral metabolism in 40 healthy men. Brain Cogn. 1986 Oct;5(4):412–427. doi: 10.1016/0278-2626(86)90043-6. [DOI] [PubMed] [Google Scholar]
- Hoffer B. J., Siggins G. R., Oliver A. P., Bloom F. E. Activation of the pathway from locus coeruleus to rat cerebellar Purkinje neurons: pharmacological evidence of noradrenergic central inhibition. J Pharmacol Exp Ther. 1973 Mar;184(3):553–569. [PubMed] [Google Scholar]
- Koss E., Friedland R. P., Ober B. A., Jagust W. J. Differences in lateral hemispheric asymmetries of glucose utilization between early- and late-onset Alzheimer-type dementia. Am J Psychiatry. 1985 May;142(5):638–640. doi: 10.1176/ajp.142.5.638. [DOI] [PubMed] [Google Scholar]
- Litton J., Bergström M., Eriksson L., Bohm C., Blomqvist G., Kesselberg M. Performance study of the PC-384 positron camera system for emission tomography of the brain. J Comput Assist Tomogr. 1984 Feb;8(1):74–87. doi: 10.1097/00004728-198402000-00016. [DOI] [PubMed] [Google Scholar]
- Mann J. J., Stanley M., Neophytides A., de Leon M. J., Ferris S. H., Gershon S. Central amine metabolism in Alzheimer's disease: in vivo relationship to cognitive deficit. Neurobiol Aging. 1981 Spring;2(1):57–60. doi: 10.1016/0197-4580(81)90060-9. [DOI] [PubMed] [Google Scholar]
- Nybäck H., Nyman H., Schalling D. Neuropsychological test performance and CSF levels of monoamine metabolites in healthy volunteers and patients with Alzheimer's dementia. Acta Psychiatr Scand. 1987 Dec;76(6):648–656. doi: 10.1111/j.1600-0447.1987.tb02935.x. [DOI] [PubMed] [Google Scholar]
- Palmer A. M., Sims N. R., Bowen D. M., Neary D., Palo J., Wikstrom J., Davison A. N. Monoamine metabolite concentrations in lumbar cerebrospinal fluid of patients with histologically verified Alzheimer's dementia. J Neurol Neurosurg Psychiatry. 1984 May;47(5):481–484. doi: 10.1136/jnnp.47.5.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin P. J., Kendall B. E., Marshall J., McDonald W. I. Amaurosis fugax: some aspects of management. J Neurol Neurosurg Psychiatry. 1982 Jan;45(1):1–6. doi: 10.1136/jnnp.45.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry E. K., Tomlinson B. E., Blessed G., Bergmann K., Gibson P. H., Perry R. H. Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. Br Med J. 1978 Nov 25;2(6150):1457–1459. doi: 10.1136/bmj.2.6150.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisberg B., Ferris S. H., de Leon M. J., Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982 Sep;139(9):1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- Rossor M. N., Iversen L. L., Reynolds G. P., Mountjoy C. Q., Roth M. Neurochemical characteristics of early and late onset types of Alzheimer's disease. Br Med J (Clin Res Ed) 1984 Mar 31;288(6422):961–964. doi: 10.1136/bmj.288.6422.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff L., Reivich M., Kennedy C., Des Rosiers M. H., Patlak C. S., Pettigrew K. D., Sakurada O., Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977 May;28(5):897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Stone-Elander S., Nilsson J. L., Blomqvist G., Ehrin E., Eriksson L., Garmelius B., Greitz T., Johnström P., Sjögren I., Widén L. 11C-2-deoxy-D-glucose: synthesis and preliminary comparison with 11C-D-glucose as a tracer for cerebral energy metabolism in PET studies. Eur J Nucl Med. 1985;10(11-12):481–486. doi: 10.1007/BF00252737. [DOI] [PubMed] [Google Scholar]
- Swahn C-G, Sandgárde B., Wiesel F-A, Sedvall G. Simultaneous determination of the three major monoamine metabolites in brain tissue and body fluids by a mass fragmentographic method. Psychopharmacology (Berl) 1976 Jul 28;48(2):147–153. doi: 10.1007/BF00423253. [DOI] [PubMed] [Google Scholar]