Abstract
Background: For a long time, trans-femoral venous pressure (FVP) measurement was considered a simple alternative for estimating intra-abdominal pressure (IAP). Since intravesical [IVP] and intragastric [IGP] pressure measurements are sometimes contraindicated for anatomical and pathophysiological reasons, FVP raised hopes, especially among pediatricians. Pediatric FVP validation studies have never been published; recent results from adult studies cast doubt on their interchangeability. Therefore, we compared for the first time the measurement agreement between FVP and IVP and IGP in children. Material and methods: We prospectively compared FVP with IVP and IGP, according to the Abdominal Compartment Society validation criteria. Additionally, we analyzed the agreement as a function of IAP or right heart valve regurgitation and pulmonary hypertension. Results: In a real-life PICU study design, n = 39 children were included (median age 4.8 y, LOS-PICU 23 days, PRISM III score 11). In n = 660 FVP–IGP measurement pairs, the median IAP was 7 (range 1 to 23) mmHg; in n = 459 FVP–IVP measurement pairs, the median IAP was 6 (range 1to 16) mmHg. The measurement agreement was extremely low with both established methods (FVP–IGP: r2 0.13, mean bias −0.8 ± 4.4 mmHg, limits of agreement (LOA) −9.6/+8.0, percentage error (PE) 55%; FVP–IVP: r2 0.14, bias +0.5 ± 4.2 mmHg, limit of agreement (LOA) −7.9/+8.9, percentage error (PE) 51%). No effect of the a priori defined influencing factors on the measurement agreement could be demonstrated. Conclusions: In a study cohort with a high proportion of critically ill children suffering from IAH, FVP did not agree reliably with either IVP or IGP. Its clinical use in critically ill children must therefore be strongly discouraged.
Keywords: femoral venous pressure, intra-abdominal pressure, intra-vesical pressure, intra-gastral pressure, intra-abdominal hypertension, children, pediatric
1. Introduction
The measurement of intra-abdominal pressure (IAP) is an important factor for the detection of abdominal compartment syndrome (ACS) in critical ill adults and children [1,2,3]. Early recognition and treatment are essential for prognosis and outcome [4,5]. In children, a consistently intra-abdominal hypertension (IAP > 10 mmHg) in combination with acute or worsening organ dysfunction attributed to the elevated IAP is defined as ACS [6].
There are different methods to measure IAP. The Abdominal Compartment Society (WSACS) currently advises bladder pressure measurement (synonym: intravesical pressure measurement (IVP)) as the benchmark technique for quantifying intra-abdominal pressure in non-adults [6]. The intra-vesical measurement is susceptible to disruptive factors such as patient positioning changes or artifact formation due to the fluid-filled system. A further disadvantage is that the measurements take place at certain points in time and not continuously, so that an acute increase in IAP could possibly be overlooked. In fact, regular the measurement of IAP is rather rare in clinical intensive care units [5,7,8].
Another option to evaluate IAP is the measurement of intra-gastric pressure via a nasogastric tube (IGP), which allows a continuous monitoring with similar susceptibility to faults. Recently, our research group validated a continuous intra-gastric measurement system for children and adolescents [9].
Transfemoral venous pressure (FVP) has been postulated for years as a measurement equivalent and trend monitor to assess IAP values [10,11,12,13]. In particular in preterm, neonates, babies and toddlers, the method was considered practicable, elegant and promising because the anatomy, pathophysiology and body size ratios of small children often make the use of established measurement methods via the bladder or the stomach (IVP, IGP) impossible or too risky. Nevertheless, based on study results from adult medicine, there has been increasing evidence in recent years that the measurement accuracy, sensitivity and reliability of the FVP method leave much to be desired. Remarkably, no form of FVP validation or usability studies have ever been performed in pediatrics and adolescent medicine.
Therefore, we conducted a single-center study to evaluate the reliability of the FVP measurement in relation to the detection of increased IAP. For this purpose, FVP measurements were compared with concurrent IVP (standard procedure) and IGP measurements in critically ill children and adolescents in a supraregional university pediatric intensive care unit (PICU), and the strength of the agreement was quantified.
2. Materials and Methods
2.1. Design of the Study
The study site of this prospective observational study—realized between January 2015 and February 2017—was the interdisciplinary pediatric intensive care unit of Hannover Medical School (MHH). The single-center clinical trial was authorized by the MHH local ethics board (ID 6677) and registered worldwide (ICTRP: DRKS00006556).
2.2. Patient Study Recruitment
All neonatal and pediatric patients who needed an intra-abdominal pressure (IAP) measurement via IVP or IGP and additionally a femoral central venous catheter were enrolled in the study. If the children did not need a stomach tube and/or a bladder catheter or a femoral central line due to their underlying disease, no devices were placed for study purposes; inclusion in the study was therefore not conceivable in such cases. The exclusion criteria comprised prematurity or immaturity at birth as well as any form of malformation, injury or other diseases of the mouth, pharynx, esophagus and stomach or urogenital tract that could make the insertion of a nasogastric tube or bladder catheter (or a catheter equivalent) difficult, dangerous or impossible. Prior to study participation, written informed consent was requested from the patients and/or their guardians.
2.3. Clinical Data Collection
In addition to the different measurement methods of IAP, defined biographical, anthropometric and prognostic data were collected and documented for each enrolled subject. These included, in particular, the diagnoses justifying admission, the duration of the stay at PICU (LOS-PICU) and the Pediatric Risk of Mortality III Score (PRISM III) [14]. This should facilitate the unmasking of possible influences on the agreement between the different measurement methods.
2.4. Intra-Abdominal Pressure Measurement (IAP)
Intra-abdominal hypertension (IAH) was defined as IAP > 10 mmHg in at least two consecutive IAP measurements, an abdominal compartment syndrome (ACS) as IAH accompanied by organ dysfunction (new or deteriorating) [6]. According to child-adapted WSACS definitions [5], IAH was classified into four grades (I°: IAP >10–12 mmHg, II°: 13–15 mmHg, III°: 16–18 mmHg, IV°: >18 mmHg).
2.5. Measurement Practice
Based upon the modified Kron technique [9,15], IVP measurements were performed using a transurethral catheter according to WSACS recommendations (emptying the bladder, filling with 1 mL/kg of BW normal saline (min. 3 mL, max. 25 mL) under aseptic conditions, waiting for at least 2 min to allow equilibration) with the midaxillary level as the zero reference (clinical standard). IVP is transmitted from the end-open transurethral catheter through the continuous liquid column in the catheter lumen to an outside pressure transducer (Codan, Germany). Transurethral catheters for IVP measurement were sizewise adjusted for weight and age (Norta-Nelaton 6–16 Charriére (Ch.) diameter, BSNmedical Company, Germany). For anatomical reasons, gastric tubes were used alternatively in small neonates (Flocare pursoft tube, 5 Ch., Nutricia Medical Devices, The Netherlands).
IGP was determined by air capsule-based measurement (Spiegelberg company, Germany) using a commercially available 9 French double-lumen nasogastric tube catheter with one lumen for continuous IGP measurement and another for regular feeding. The accurate intragastric or intravesical placement of the IGP and transurethral catheters was verified by ultrasound at minimum once a day and, in addition, whenever the IGP or IVP measurements did not reveal respiratory undulations [9].
The femoral vein pressure (FVP) was measured via the fluid-filled and flushed (1 mL/h), distal limb of a three-lumen central venous catheter (CVC) inserted into the femoral vein using the Seldinger technique (Vygon Multicath-3 Pädiatrie, Stolberg, Germany), with a pressure transducer and an A/D converter on the patient-monitoring unit from GE (General Electrics).
To assess the influence of possible tricuspid and/or pulmonary valve leakage on the FVP, an experienced pediatric cardiologist examined the subjects at least once by echocardiography and estimated the pulmonary arterial pressure that could be derived from the right cardiac valve insufficiency gradients [16].
2.6. Agreement of FVP with IVP/IGP
Simultaneous IAP readings were taken in all patients every hour during the day to assess compliance and to perform exploratory analyses to evaluate the possible effect of predefined predictive factors. The data were recorded either up to discharge from the pediatric intensive care unit or up to withdrawal of the IGP/IVP and FVP catheters, whichever occurred earlier. To avoid artefact-related false measurements, pressure measurements during the weaning of recovering patients were only used and analyzed if the patients did not show signs of agitation with or without mass movements but had stable and age-appropriate monitoring vital signs. Furthermore, IAP measures obtained during physician visits, nursing care and procedures, rehabilitative therapies and other examinations or procedures were eliminated and neither analyzed nor scored.
2.7. Explorative Analysis of Confounders
To identify possible factors influencing the robustness of the FVP measurements, the agreement of the measurement results depending on right ventricular valve insufficiencies and pulmonary arterial pressures on the one hand and on different IAP heights on the other hand was investigated in a second step in an explorative analysis.
(1) Right heart evaluation was performed by an experienced pediatric cardiologist using a Philips echocardiography device (CX50, Koningklijke Philips N.V, Eindhoven, The Netherlands). For the classification of valve insufficiencies into mild, moderate and severe forms, the definitions according to the guidelines and recommendations of the American (2017) [17] and European (2021) [18] professional societies for cardiology and echocardiography, respectively, were applied. According to the Nice Criteria of 2018, pulmonary hypertension (PHT) was defined in the case of an echocardiographically assessable mean pulmonary arterial pressure (mPAP) of mPAP > 20 mmHg [16].
The results were divided into 4 groups:
-
-
Group 1: no tricuspid valve (TI) and/or no pulmonary valve insufficiency/regurgitation (PI) and no pulmonary hypertension (PHT).
-
-
Group 2: mild TI and/or mild PI and no PHT
-
-
Group 3: mild to moderate TI and/or mild to moderate PI and signs of PHT
-
-
Group 4: no echocardiographic results available
-
-
No child showed signs of severe TI and/or PI.
(2) Depending on the IAP measured via IVP or IGP, the results were grouped into IAP classes:
-
-
Group 1: IAP < 7 mmHg (corresponds to normal IAP in children)
-
-
Group 2: IAP 7–9 mmHg (corresponds to “pre-IAH” especially in neonates and infants)
-
-
Group 3: IAP 10–12 mmHg (corresponds to IAH grade I in children)
-
-
Group 4: IAP 13–15 mmHg (corresponds to IAH grade II in children)
-
-
Group 5: IAP 16–18 mmHg (corresponds to IAH grade III in children)
-
-
Group 6: IAP > 16 mmHg (corresponds to IAH grade IV in children)
2.8. Data Processing and Statistical Analysis
Clinical data were collected using a digital patient data monitoring system (Copra®Systems, Berlin, Germany) and transferred to Excel®2016 (Microsoft® Corporation Redmond, WA, USA). The statistical analysis was performed using SPSS® Statistics 22.0 (IBM®, Armonk, North Castle, Armonk, NY, USA). The results of the simultaneous IAP readings were juxtaposed by means of a linear regression analysis. As there was no normal distribution (as per Shapiro–Wilk testing), the correlation analysis was carried out applying Spearman’s coefficient of determination. Following the WSACS guidelines (Table 1), the agreement and thus the exchangeability of the IAP assessment techniques was judged according to Bland–Altman, whereby the so-called precision corresponds to the standard deviation (SD) of the mean bias, and the percentage error (PE) to the quotient of limits of agreement (LOA) and the mean value of the IAP measured with both techniques at the same time [19]. Furthermore, a mean absolute percentage error (MAPE ± SD) was calculated according to de Myttenaere et al. [20]. The 1988 Cohen criteria were applied to interpret the Spearman’s correlation coefficients [21].
Table 1.
Amended * WSACS guidelines regarding the minimum requirements for the exchangeability of two competing IAP assessment techniques.
| No. of Enrolled Patients | r2 * | Bias [mmHg] | Precision [mmHg] | LOA [mmHg] | Percentage Error [%] | |
|---|---|---|---|---|---|---|
| Target values | ≥20 | ≥0.6 * | ≤|1| | ≤2 | −4 to +4 | ≤25 |
* The original WSACS recommendations do not consider a correlation coefficient (r2) as a parameter for assessing interchangeability. However, an r2 of at least 0.6 should be assumed. Abbreviations and definitions: FVP: femoral vein pressure; IGP intra-gastric pressure; IVP: intra-vesical pressure (bladder pressure); r2: Spearman’s correlation coefficient; bias: mean difference between two measures; precision: SD of the bias; LOA: limits of agreement (=upper and lower SD of the mean bias); percentage error: quotient of LOA and mean IAP.
3. Results
3.1. Patients Characteristics
Two groups, each comprising n = 19 (IVP group) and n = 20 patients (IGP group), were set up to compare the FVP with the reference method of IVP or IGP, respectively. Sex distribution (32% and 35% female) and average age, with a median of 4.9 years in the IVP group and 4.7 years in the IGP group, were almost identical (Table 2). In the cohort of the IGP measurement, with n = 660, the number of paired measurements was higher compared to the number of paired FVP measurements in the IVP cohort (n = 459). To illustrate the extensive homogeneity of both subgroups, the results of the descriptive statistics are summarized in Table 2. The PRISM III score, as an indicator for mortality in pediatric intensive care units, reflected similar disease severity in both groups at the beginning and end of the observation. One patient in the IGP group died.
Table 2.
Description of the patient population (presented as mean ± standard deviation [SD]).
| Parameter | FVP vs. IVP | FVP vs. IVP |
|---|---|---|
| Number of patients | 19 | 20 |
| Number of girls | 6 | 7 |
| Number of paired measures | 459 | 660 |
| Age [years] | 4.9 ± 5.3 | 4.7 ± 5.3 |
| Newborn [n = 1] | Newborn [n = 2] | |
| Sucklers [n = 5] | Sucklers [n = 5] | |
| Tots [n = 6] | Tots [n = 6] | |
| School [n = 5] | Schoolkids [n = 5] | |
| Teenagers [n = 2] | Teenagers [n = 2] | |
| Body mass index * | 16.7 ± 4.2 | 16.7 ± 4.1 |
| Duration of the stay at PICU [days] | 24.3 ± 41.6 | 21.8 ± 38.9 |
| Admission-justifying diagnoses (responsible clinical department) | Ped. Cardiology [n = 4] | Ped. Cardiology [n = 6] |
| Neurosurgery [n = 3] | Neurosurgery [n = 3] | |
| Pedosurgery [n = 5] | Pedosurgery [n = 4] | |
| Ped. pulmonology [n = 1] | Ped. pulmonology [n = 1] | |
| Ped. traumatology [n = 6] | Ped. traumatology [n = 6] | |
| PRISM-III-score | ||
| - First day of enrolment | 11.4 ± 6.5 | 11.3 ± 6.5 |
| - Last day of enrolment | 2.6 ± 2.8 | 2.6 ± 2.7 * |
| Lethality | 0% | 5% [1 deceased] ** |
* Definition: body mass index BMI = kg/m2. PICU: Pediatric intensive care unit; PRISM III Score: Pediatric Risk of Mortality Score III. ** The dataset of the child who died was not taken into account when assessing the PRISM score on the final day of trial participation.
3.2. Comparison between FVP and IGP
Table 3 shows the results for the comparison between FVP and IGP measurements.
Table 3.
Findings of the comparative statistics analyzing the FVP and IGP measurement agreement.
| No. of Measurements | IGP [mmHg] | FVP [mmHg] | r2 | Bias [mmHg] | Precision [mmHg] | LOA [mmHg] | Percentage Error [%] | Mean Absolute Percentage Error (SD) [%] | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Median [Range] | ||||||||||
| Overall Result | 660 | 7.0 (2.4–16.5) | 7.0 (1.0–23.0) | 0.13 | −0.8 | 4.4 | −9.6–8.0 | 117 | 55 (47) | |
| Explorative additional examination: influenceability by right cardiac valve insufficiency | ||||||||||
| Echo- findings | No TI, no PI and no PHT | 48 | 5.7 (4.0–10.9) | 3.0 (1.0–13.0) | 0.13 | 2.1 | 2.3 | −2.5 to 6.7 | 90 | 41 (29) |
| Mild TI, mild PI and no PHT | 271 | 7.0 (2.5–16.5) | 10.0 (1.0–23.0) | 0.10 | −2.2 | 4.2 | −10.6 to 6.2 | 102 | 62 (50) | |
| Mild to moderate TI and/or PI and/or PHT | 120 | 6.8 (3.5–12.9) | 10.0 (1.0–19.0) | 0.47 | −1.8 | 3.6 | −9.0 to 5.4 | 91 | 50 (27) | |
| No Echo findings available | 221 | 7.1 (2.4–14.2) | 5.0 (1.0–21.0) | 0.06 | 0.8 | 4.6 | −8.4 to 10.0 | 135 | 52 (53) | |
| Exploratory additional examination: influenceability by the height of intra-abdominal pressure (IAP) | ||||||||||
| IAP [mmHg] | <7 | 189 | 5.2 (2.4–6.9) | 3.0 (1.0–6.0) | 0.01 | 1.7 | 1.9 | −2.1 to 5.5 | 88 | 42 (25) |
| 7–9 | 189 | 7.8 (3.5–9.8) | 7.0 (1.0–9.0) | 0.08 | 1.6 | 3.1 | −4.6 to 7.8 | 93 | 40 (27) | |
| 10–12 | 162 | 7.8 (2.7–12.6) | 11.0 (2.0–12.0) | 0.15 | −2.3 | 3.6 | −9.5 to 4.9 | 80 | 57 (43) | |
| 13–15 | 83 | 8.3 (3.9–14.2) | 14.0 (7.0–15.0) | 0.02 | −5.2 | 2.7 | −10.6 to 0.2 | 49 | 76 (49) | |
| 16–18 | 23 | 8.4 (3.7–12.9) | 17.0 (16.0–18.0) | 0.05 | −8.5 | 2.9 | −14.3 to −2.7 | 46 | 123 (80) | |
| >16 | 14 | 8.0 (3.8–16.5) | 19.0 (19.0–23.0) | 0.05 | −11.5 | 3.2 | −17.9 to −5.3 | 46 | 170 (99) | |
Abbreviations: FVP: femoral vein pressure; IGP: intra-gastric pressure; r2: Spearman’s correlation coefficient; mean bias: difference between 2 measurements; precision: SD of the mean bias; LOA: limits of agreement; percentage error: Quotient of LOA and mean IAP; Echo: Echocardiography; TI: tricuspid regurgitation; PI: pulmonary regurgitation; PHT: pulmonary hypertonia; IAP intra-abdominal pressure.
3.2.1. Overall Results
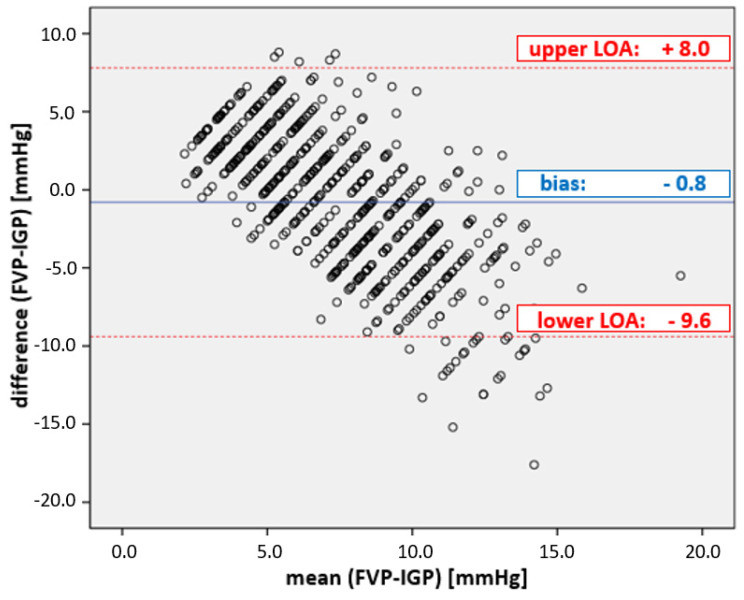
With a correlation coefficient of r2 = 0.13, the overall agreement between IGP and FVP measurements was very low. While the WSACS-defined compliance criteria for alternative measurement methods (Table 1) were met for bias (−0.8 mmHg), they were far outside the tolerable range for precision (with 4.4 mmHg) and limits of agreement (with results between −9.6 and 8.0 mmHg; see Figure 1). Furthermore, the percentage error was 117%, clearly above the cut-off.
Figure 1.
Plot of the agreement between FVP and IGP measurements. Bland–Altman diagram of FVP and IGP. The mean bias ± precision between FVP and IGP was −0.8 ± 4.4 mmHg; the limits of agreement (LOA) were from −9.6 to + 8.0 mmHg, well outside the acceptable boundaries set by WSACS (Table 1).
3.2.2. Explorative Additional Examinations
In the context of the explorative analysis of potential influencing factors, only children with elevated pulmonary arterial pressure showed a slightly improved correlation result according to Spearman, with r2 = 0.47 in this subgroup. In children without PHT, the Spearman’s correlation coefficient varied between just r2 = 0.1 and no more than r2 = 0.13, depending on the presence of right ventricular insufficiency (see Table 3).
A comparably poor agreement between r2 = 0.1 and r2 = 0.15 was also found for all IAP- and IAH-grade-dependent subgroup analyses (see Table 3). All other agreement criteria were far from being met in all subgroups (incl. PHT) (bias, precision, limits of agreement, percentage error).
3.3. Comparison between FVP and IVP
Table 4 shows the comparison between IVP and FVP measurements.
Table 4.
Findings of the comparative statistics analyzing the FVP and IVP measurement agreement.
| No. of Measurements | IVP [mmHg] | FVP [mmHg] | r2 | Bias [mmHg] | Precision [mmHg] | LOA [mmHg] | Percentage Error [%] | Mean Absolute Percentage Error (SD) [%] | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Median [Range] | ||||||||||
| Overall Result | 459 | 6.0 (1.0–16.0) | 5.0 (1.0–22.0) | 0.14 | 0.5 | 4.2 | −7.9 to 8.9 | 133 | 51 (55) | |
| Exploratory additional examination: influenceability by right cardiac valve insufficiency | ||||||||||
| Echo- findings | No TI, no PI and no PHT | 48 | 5.0 (3.0–12.0) | 3.0 (1.0–13.0) | 0.02 | 1.5 | 2.6 | −3.7 to 6.7 | 108 | 37 (32) |
| Mild TI, mild PI and no PHT | 117 | 6.0 (1.0–16.0) | 5.0 (1.0–22.0) | 0.30 | −0.4 | 4.0 | −8.4 to 7.6 | 123 | 56 (60) | |
| Mild to moderate TI and/or PI and/or PHT | 73 | 6.0 (2.0–11.0) | 6.0 (1.0–19.0) | 0.48 | −0.7 | 3.3 | −7.3 to 5.9 | 103 | 46 (35) | |
| No Echo findings available | 221 | 6.0 (3.0–14.0) | 5.0 (1.0–21.0) | 0.04 | 0.2 | 4.7 | −9.2 to 9.6 | 145 | 54 (61) | |
| Exploratory additional examination influenceability by the height of intra-abdominal pressure (IAP) | ||||||||||
| IAP [mmHg] | <7 | 203 | 5.0 (1.0–6.0) | 3.0 (1.0–6.0) | 0.06 | 1.3 | 1.8 | −2.3 to 4.9 | 87 | 38 (42) |
| 7–9 | 153 | 7.0 (3.0–9.0) | 6.0 (1.0–9.0) | 0.08 | 1.6 | 3.0 | −4.4 to 7.6 | 94 | 30 (40) | |
| 10–12 | 49 | 8.0 (3.0–12.0) | 10.0 (2.0–12.0) | 0.45 | −1.1 | 4.7 | −10.5 to 8.3 | 107 | 64 (62) | |
| 13–15 | 27 | 8.0 (4.0–15.0) | 14.0 (12.0–15.0) | 0.01 | −5.0 | 2.8 | −10.6 to 0.6 | 50 | 73 (50) | |
| 16–18 | 16 | 8.0 (4.0–9.0) | 17.0 (16.0–18.0) | 0.00 | −9.9 | 1.8 | −13.4 to −6.3 | 30 | 152 (73) | |
| >16 | 11 | 6.0 (4.0–16.0) | 19.0 (19.0–22.0) | 0.68 | −12.2 | 2.5 | −17.2 to −6.7 | 37 | 199 (93) | |
Abbreviations: FVP: femoral vein pressure; IVP: intra-vesical pressure (bladder pressure); r2: Spearman’s correlation coefficient; mean bias: difference between 2 measurements; precision: SD of the mean bias; LOA: limits of agreement; percentage error: Quotient of LOA and mean IAP; Echo: Echocardiography; TI: tricuspid regurgitation; PI: pulmonary regurgitation; PHT: pulmonary hypertonia; IAP intra-abdominal pressure.
3.3.1. Overall Results
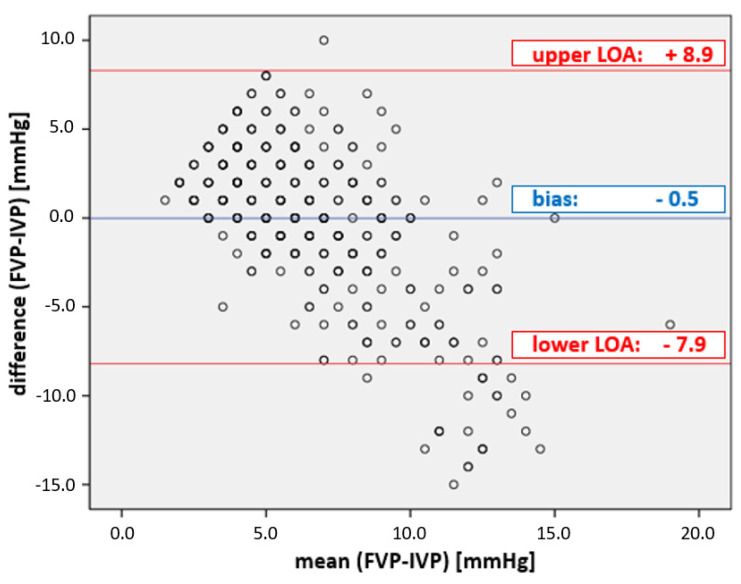
The overall correlation between IGP and FVP measurements was very low (r2 = 0.14). While the bias of −0.5 mmHg was in the given range (Table 1), the precision of 4.2 mmHg and the limits of agreement from −7.9 to 8.9 mmHg did not fulfill the given limits of the criteria (see Figure 2); the percentage error was 133%, clearly above the cut-off (25%).
Figure 2.
Plot of the agreement between FVP and IVP measurements. Bland–Altman diagram of FVP and IVP. The mean bias ± precision between FVP and IVP was −0.5 ± 4.2 mmHg; the limits of agreement (LOA) were from −7.9 to + 8.9 mmHg, well outside the acceptable boundaries set by WSACS (Table 1).
3.3.2. Exploratory Additional Examinations
In the context of the exploratory analysis of potential influencing factors, children with valvular insufficiencies or increased pulmonary arterial pressure showed a slightly better correlation result according to Spearman, with r2 = 0.3 and r2 = 0.47, respectively (Table 4), whereas children without right ventricular insufficiencies showed low correlations, from just r2 = 0.02 to r2 = 0.04 (see Table 4).
Similarly low correlations were found in the IAP- or IAH-grade-dependent subgroup analyses (Table 4). Only children with IVth-grade IAH (IAP > 18 mmHg) showed a stronger correlation, with r2 = 0.68.
All other WSACS agreement criteria for alternative measurement methods (Table 1: bias, precision, limits of agreement, percentage error) were far from achieved in all subgroups (incl. PHT and IAH grade IV) [Table 4].
4. Discussion
4.1. Study Design and Key Messages
After the reliability of FVP quantification—proposed as an alternative IAP measurement method in the 1980s and 1990s (Table 5)—had already been questioned by studies on adults, the main aim of the present monocentric study was to test the FVP measurement methodology for the very first time in children. For this reason, we performed a prospective cross-sectional validity evaluation to assess this IAP quantification technique in n = 39 children with critical illness. Remarkably, for the first time, this validation study fulfilled without exception all criteria postulated by the WSACS for the comparison of different IAP measurement methods in a clinical real-life pediatric ICU setting. We were able to collect reliable data across a broad pediatric age spectrum with a representative disease severity and distribution (Table 1). Our study cohort is of particular value in terms of its validation power based on the frequent occurrence of elevated abdominal pressures (IAH), ranging from 22% (IVP group) to 43% (IGP group) (i.e., cases with IAH grade III (16–18 mmHg) and IV (>16 mmHg) and IAP heights of up to 23 mmHg).
In this challenging population, we proved beyond doubt that IAP estimation by FVP quantification is useless in everyday clinical practice in the PICU. It is neither accurate, nor precise, reproducible or reliable against the actual medical benchmark method, bladder pressure measurement (IVP) and the validated measurement method alternative, namely, intra-gastric pressure measurement (IGP).
In an additional exploratory analysis, we also investigated clinical factors such as right cardiac valvular regurgitation and/or increased pulmonary arterial pressure (PHT), as well as IAP level per se, each of which could affect the agreement of the IAP measurement. Overall, we found neither positive nor negative influences of these potential confounders on the measurement agreement of FVP. Even if a somewhat stronger Spearman’s correlation was achieved in the presence of a PHT or with higher-grade IAH (max. r2 = 0.68), the other WSACS-postulated agreement criteria were still far from being achieved (see bias, precision, limits of agreement and percentage error, as shown in Table 3 and Table 4).
Based on these observations, the use of the FVP as a method for estimating the IAP in children must be strongly discouraged.
4.2. Medical Historical Motivations for This IAP Estimation Validation Study
In 1987, Lacey’s research group pioneered the idea of utilizing the trans-femoral venous pressure (FVP) for IAP estimation in neonates suffering from abdominal wall defects [22]. In an animal model, they compared the intraoperative agreement of different indirect IAP pressure measurement methods in 17 rabbits. The pressures measured intra-vesically and trans-femorally in the inferior vena cava (IVC) agreed best with the applied IAPs. In 2002, Gudmundson et al. wanted to verify the results in a large animal model and also found a strong correlation between IAP and FVP or IVP in eight pigs [11]. However, the applied pressure level of 15–40 mmHg was clearly above the IAP level that is regularly reached in clinical practice. When Jakob et al. [23] took this into account and tested the measurement agreement in 13 pigs in a more clinically relevant range between 0 and 22 mmHg, the first doubts arose about the clinical relevance and applicability of the trans-femoral measurement method, which appeared quite attractive due to its potentially continuous measurement methodology. In their pig study published in 2011, Regli et al. increasingly applied the agreement criteria for alternative IAP measurement methods defined by the WSACS and in particular objectified a far too large range with regard to the limits of agreement [24].
This temporal development from initial recommendation to interim doubt to final rejection of sufficient agreement and significance was also shown with a few years’ latency in the context of human clinical studies [12,13,25,26] (Table 5). The more the WSACS postulated criteria for agreement were applied over time, the more objective and clear the discrepancy between the alternative methods became. The working groups around De Keulenaer [25] and Howard [26] finally made the inadequacy of the FVP unmistakably clear.
Although the initial impetus for FVP measurement came from neonatal pediatric surgeons [22], any clinical translation and verification in a pediatric intensive care setting has been lacking. We wanted to take this into account and close the evidence gap that existed until then.
4.3. Clinical Implications
FVP has also proven to be useless in pediatric intensive care. IAP monitoring in the sensitive pediatric setting should therefore be limited to the established and validated methods of bladder and gastric pressure measurement until further notice [9]. Nonetheless, any increase in trans-femoral venous pressure should, of course, suggest the possibility of an acute increase in IAP and, if not already done, should result in immediate IAP quantification.
Recently, study results from various pilot projects and model validations of novel IAP measurement methods have been published [27,28,29,30]. Unfortunately, the majority of these methods have not even found their way out of preclinical testing into the clinical routine of adult medicine—let alone pediatrics. Even if there are advantages due to their non-invasiveness and continuous data collection, there are clear disadvantages in test series so far [27], such as
-
-
lack of accuracy, sensitivity and reliability
-
-
high susceptibility to errors (e.g., due to movement artefacts and sensitivity)
-
-
lack of standardization or even lack of proof of concept
-
-
high costs.
A further development of bladder pressure measurement via the Foley catheter could be the most promising new development, enabling the continuous and automated IAP assessment with the help of the novel so-called “SERENNO” equipment [30]. Apart from such conventional indirect pressure measurements via abdominal or retroperitoneal hollow organs, the microwave-based “transient radar method” seems to have the necessary potential, on the basis of which a translation into clinical routine could appear possible. However, due to the special anatomical and pathophysiological conditions in children and especially in premature and newborn infants, as well as the associated vulnerability, testing in pediatrics will only be conceivable once it has been successfully validated and established in adult medicine.
4.4. Study limitations
We pooled all the matched longitudinal measures used for the primary and the explorative assessments, ignoring the circumstance that some of them came from the same patients. We judge this to be feasible because the states of these subjects during their stay in ICU differed substantially in terms of hemodynamics, respiration, vigilance and several additional determinants. The variability resulting from these changing state combinations is likely to cause more influences than the fact that data sets were in part from identical subjects.
In sum, the findings of the primary and exploratory analyses did not reveal any important or significant difference. Therefore, targeted as well as not-targeted influences could not affect the poor measuring agreement of the tested FVP and IVP or IGP measurement methods.
Table 5.
Literature overview of publications concerning FVP measurements.
| Study Type/Model | Author Year [Ref.] |
Measure Methods | Methods–Statistics | No of Subjects Enrolled (No. of Paired Measures) |
IAP Range (mmHg) | Comparisons | Results | Conclusion | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Correlation Coefficient | WSACS Method Validation Criteria | |||||||||||||
| Bias (mmHg) | Precision (mmHg) | LOA (mmHg) | PE (%) |
|||||||||||
| in-vivo | Animals | Lacey 1987 [22] |
IVCP (FVP) IVP IGP |
Correlation coefficient (unspecified) |
17 rabbits (n.a.) |
0–30 | IAP versus IVCP (FVP) IAP versus IVP IAP versus IGP |
>0.87 >0.85 0.7 |
n.a. | n.a. | n.a. | n.a. | FVP usable | |
| Gudmundsson 2002 [11] |
FVP IVCP IVP |
Correlation coefficient (unspecified) |
8 pigs (n.a.) |
15–40 | IAP versus FVP IAP versus IVCP IAP versus IVP |
0.95 0.94 0.92 |
n.a. | n.a. | n.a. | n.a. | FVP usable | |||
| Jakob 2010 [23] |
IGP IVP IVCP (FVP) |
Pearson correlation coefficient (r²) Bias LOA |
12 pigs (n.a.) |
0–22 | IGP versus IVP IGP versus IVCP IVP versus IVCP |
0.60 0.63 0.52 |
n.a. | n.a. | n.a. | n.a. | FVP limited usable | |||
| Regli 2010 [24] |
FVP IVP |
Correlation coefficient (r²) Bias, precision |
13 pigs (n.a.) |
3–26 | IVP versus FVP | 0.89 | 5.0 | 3.8 | n.a. | n.a. | FVP limited usable | |||
| Human | Adults | Joynt 1996 [12] |
SVCP IVCP (FVP) |
Bias precision LOA |
19 (133) |
1–26 | SVCP versus IVCP | n.a. | 0.45 | 0.89 | −1.33 to 2.23 | n.a. | FVP usable | |
| Ho 1998 [13] |
SVCP IVCP |
Bias, precision LOA |
20 (140) |
n.a. | SVCP versus IVCP | n.a. | 0.1 | 1.06 | −2.04 to 2.2 | n.a. | FVP usable | |||
| Markou 2004 [25] |
IVCP (FVP) IVP |
Pearson correlation coefficient | 38 (151) |
n.a. | IVP versus IVCP 1. IAP < 10 mmHg 2. IAP 10–15 mmHg 3. IAP > 15 mmHg |
0.76 0.69 0.78 |
n.a. | n.a. | n.a. | n.a. | FVP not usable | |||
| De Keulenaer 2011 [26] |
IVP FVP |
Bias, precision LOA |
149 (866) |
6.7–22.4 | IVP versus FVP 1. pooled IAP 2. IAP ≥ 12mmHg 2. IAP > 20 mmHg |
n.a. |
−1.5 0.4 0.7 |
3.6 3.9 2 |
−8.6 to 5.7 −8.1 to 7.3 −3 to 4.6 |
n.a. | FVP not usable | |||
| Howard 2016 [31] |
IVP FVP |
All WSACS method validation criteria | 11 (53) |
0–25 | IVP versus FVP pooled without weight artificially increased IAP 5 kg artificially increased IAP 10 kg |
0.8 n.a. n.a. n.a. |
2.8 3.2 2.5 2.5 |
3.42 3.63 3.92 2.26 |
−4.1 to 9.6 −4.1 to 10.4 −5.4 to 10.3 −2.1 to 7 |
46.8 n.a. n.a. 27.1 |
FVP not usable | |||
| Children | Present study | FVP IVP IGP |
All WSACS method validation criteria | 39 (1119) |
1–23 | FVP versus IVP FVP versus IGP |
0.14 0.13 |
0.5 −0.8 |
4.2 4.4 |
−7.9 to 8.9 −9.6 to 8.0 |
133 117 |
FVP not usable | ||
The publications were subdivided according to study models. The last column contains the conclusion regarding the usefulness of the FVP method (color-coded according to the traffic light system). Abbreviations: FVP: femoral vein pressure; IAP: intra-abdominal pressure; IGP: intra-gastric pressure; IVCP: inferior vena cava pressure; IVP: intra-vesical pressure (bladder pressure); r2 degree of certainty (correlation coefficient); SVCP: superior vena cava pressure; bias: mean difference between 2 measures; precision: SD of the bias; LOA: limits of agreement; percentage error: Quotient of LOA and mean IAP.
5. Conclusions
Our data suggest that FVP does not reliably reflect IAP and that its use in clinical practice can lead to a fatal misjudgment in critically ill children. For the time being, IAP measurements in children should therefore continue to be made by quantifying IVP or IGP, without exception.
Acknowledgments
Our deepest gratitude to all children and their guardians for their readiness to join our clinical trial. We also want to express our gratefulness to the PICU multidisciplinary team for their fantastic assistance, teamwork and helpfulness throughout the study.
Abbreviations
| ACM | Air capsule-based measurement |
| ACS | Abdominal compartment syndrome |
| BMI | Body mass index |
| FVP | Femoral vein pressure |
| IAH | Intra-abdominal hypertension |
| IAP | Intra-abdominal pressure |
| IGP | intra-gastric pressure |
| IVCP | Inferior Vena cava pressure |
| IVP | Intra-vesical pressure |
| LA | Limits of agreement |
| LOS-PICU | Duration of stay at PICU |
| MHH | Medizinische Hochschule Hannover |
| PE | Percentage error |
| PHT | Pulmonary hypertension |
| PI | Pulmonary valve insufficiency |
| PICU | Pediatric intensive care unit |
| PRISM-III | Pediatric risk of mortality score III |
| SD | Standard deviation |
| SVCP | Superior vena cava pressure |
| TI | Tricuspid valve insufficiency |
| WHO | World Health Organisation |
| WSACS | Abdominal Compartment Society (formerly known as World Society of Abdominal Compartment Syndrome) |
Author Contributions
T.K. created the study concept and experimental design and implemented them in a project leadership role. T.K. as well as M.G. collected and analyzed the data. L.K., M.G. and T.K. drafted a first version of the manuscript. All authors contributed to revising the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The present clinical trial was conducted in accordance with the Helsinki Declaration principles. Permission was obtained by the Ethics Board of Hannover Medical School (ID 6677). The study was registered worldwide (ICTRP: DRKS00006556).
Informed Consent Statement
An informed consent agreement was obtained from each participant enrolled in the trial (or their representatives).
Data Availability Statement
Raw data and research findings can be obtained at the authors’ premises upon justified demand.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research project was funded by an in-house grant (MHH Clinical-Scientist Program 2015-2017[MHH: Medizinische Hochschule Hannover/Hannover Medical School]): Financial consumable grant of €15,000 plus temporary clinic duty release for research purposes (8/2015–5/2016).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Thabet F.C., Bougmiza I.M., Chehab M.S., Bafaqih H.A., Al Mohaimeed S.A., Malbrain M.L. Incidence, Risk Factors, and Prognosis of Intra-Abdominal Hypertension in Critically Ill Children: A Prospective Epidemiological Study. J. Intensive Care Med. 2016;31:403–408. doi: 10.1177/0885066615583645. [DOI] [PubMed] [Google Scholar]
- 2.Steinau G., Kaussen T., Bolten B., Schachtrupp A., Neumann U.P., Conze J., Boehm G. Abdominal compartment syndrome in childhood: Diagnostics, therapy and survival rate. Pediatr. Surg. Int. 2011;27:399–405. doi: 10.1007/s00383-010-2808-x. [DOI] [PubMed] [Google Scholar]
- 3.Ejike J.C., Mathur M., Moores D.C. Abdominal compartment syndrome: Focus on the children. Am. Surg. 2011;77((Suppl. S1)):S72–S77. [PubMed] [Google Scholar]
- 4.Ejike J.C., Humbert S., Bahjri K., Mathur M. Outcomes of children with abdominal compartment syndrome. Acta Clin. Belg. 2007;62((Suppl. S1)):141–148. doi: 10.1179/acb.2007.62.s1.018. [DOI] [PubMed] [Google Scholar]
- 5.Kaussen T., Steinau G., Srinivasan P.K., Otto J., Sasse M., Staudt F., Schachtrupp A. Recognition and management of abdominal compartment syndrome among German pediatric intensivists: Results of a national survey. Ann. Intensive Care. 2012;2((Suppl. S1)):S8. doi: 10.1186/2110-5820-2-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirkpatrick A.W., Roberts D.J., De Waele J., Jaeschke R., Malbrain M.L., De Keulenaer B., Duchesne J., Bjorck M., Leppaniemi A., Ejike J., et al. Intra-abdominal hypertension and the abdominal compartment syndrome: Updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;39:1190–1206. doi: 10.1007/s00134-013-2906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rezeni N., Thabet F. Awareness and management of intra-abdominal hypertension and abdominal compartment syndrome by paediatric intensive care physicians: A national survey. Anaesthesiol. Intensive Ther. 2022;54:315–319. doi: 10.5114/ait.2022.120366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newcombe J., Mathur M., Bahjri K., Ejike J.C. Pediatric critical care nurses’ experience with abdominal compartment syndrome. Ann. Intensive Care. 2012;2((Suppl. S1)):S6. doi: 10.1186/2110-5820-2-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaussen T., Gutting M., Lasch F., Boethig D., von Gise A., Dingemann J., Koeditz H., Jack T., Sasse M., Beerbaum P., et al. Continuous intra-gastral monitoring of intra-abdominal pressure in critically ill children: A validation study. Intensive Care Med. Exp. 2021;9:24. doi: 10.1186/s40635-021-00386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lacey S.R., Bruce J., Brooks S.P., Griswald J., Ferguson W., Allen J.E., Jewett T.C., Jr., Karp M.P., Cooney D.R. The relative merits of various methods of indirect measurement of intraabdominal pressure as a guide to closure of abdominal wall defects. J. Pediatr. Surg. 1987;22:1207–1211. doi: 10.1016/S0022-3468(87)80739-X. [DOI] [PubMed] [Google Scholar]
- 11.Gudmundsson F.F., Viste A., Gislason H., Svanes K. Comparison of different methods for measuring intra-abdominal pressure. Intensive Care Med. 2002;28:509–514. doi: 10.1007/s00134-001-1187-0. [DOI] [PubMed] [Google Scholar]
- 12.Joynt G.M., Gomersall C.D., Buckley T.A., Oh T.E., Young R.J., Freebairn R.C. Comparison of intrathoracic and intra-abdominal measurements of central venous pressure. Lancet. 1996;347:1155–1157. doi: 10.1016/S0140-6736(96)90611-X. [DOI] [PubMed] [Google Scholar]
- 13.Ho K.M., Joynt G.M., Tan P. A comparison of central venous pressure and common iliac venous pressure in critically ill mechanically ventilated patients. Crit. Care Med. 1998;26:461–464. doi: 10.1097/00003246-199803000-00015. [DOI] [PubMed] [Google Scholar]
- 14.Pollack M.M., Patel K.M., Ruttimann U.E. PRISM III: An updated Pediatric Risk of Mortality score. Crit. Care Med. 1996;24:743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Kron I.L. A simple technique to accurately determine intra-abdominal pressure. Crit. Care Med. 1989;17:714–715. doi: 10.1097/00003246-198907000-00039. [DOI] [PubMed] [Google Scholar]
- 16.Rosenzweig E.B., Abman S.H., Adatia I., Beghetti M., Bonnet D., Haworth S., Ivy D.D., Berger R. Paediatric pulmonary arterial hypertesion: Updates on definition, classification, diagnostics and management. Eur. Respir. J. 2019;53:1801916. doi: 10.1183/13993003.01916-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zoghbi W.A., Bonow R.O., Enriquez-Sarano M., Foster E., Grayburn P.A., Hahn R.T., Han Y., Hung J., Lang R.M., Little S., et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation—A Report from the American Society of Echocardiography. Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017;30:303–371. doi: 10.1016/j.echo.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Vahanian A.B.F., Praz F., Milojevic M., Baldus S., Bauersachs J., Capodanno D.C.L., De Bonis M., De Paulis R., Delgado V., Freemantle N., et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease—Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur. Heart J. 2022;43:561–632. doi: 10.1093/eurheartj/ehab395. [DOI] [PubMed] [Google Scholar]
- 19.Bland J.M., Altman D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- 20.De Myttenaere A.G.B., Le Grand B., Rossi F. Mean Absolute Percentage Error for regression models. Neurocomputing. 2016;192:10. doi: 10.1016/j.neucom.2015.12.114. [DOI] [Google Scholar]
- 21.Cohen J. In: Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Erlbaum L., editor. Lawrence Erlbaum Associates; Hillsdale, NJ, USA: 1988. [Google Scholar]
- 22.Lacey S.R., Carris L.A., Beyer A.J., Azizkhan R.G. Bladder pressure monitoring significantly enhances care of infants with abdominal wall defects: A prospective clinical study. J. Pediatr. Surg. 1993;28:1370–1374. doi: 10.1016/S0022-3468(05)80329-X. [DOI] [PubMed] [Google Scholar]
- 23.Jakob S.M., Knuesel R., Tenhunen J.J., Pradl R., Takala J. Increasing abdominal pressure with and without PEEP: Effects on intra-peritoneal, intra-organ and intra-vascular pressures. BMC Gastroenterol. 2010;10:70. doi: 10.1186/1471-230X-10-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Regli A., De Keulenaer B.L., Hockings L.E., Musk G.C., Roberts B., van Heerden P.V. The role of femoral venous pressure and femoral venous oxygen saturation in the setting of intra-abdominal hypertension: A pig model. Shock. 2011;35:422–427. doi: 10.1097/SHK.0b013e3181fddf45. [DOI] [PubMed] [Google Scholar]
- 25.Markou N., Grigorakos L., Myrianthefs P., Boutzouka E., Rizos M., Evagelopoulou P., Apostolakos H., Baltopoulos G. Venous pressure measurements in the superior and inferior vena cava: The influence of intra-abdominal pressure. Hepatogastroenterology. 2004;51:51–55. [PubMed] [Google Scholar]
- 26.De Keulenaer B.L., Regli A., Dabrowski W., Kaloiani V., Bodnar Z., Cea J.I., Litvin A.A., Davis W., Palermo A.-M., De Waele J., et al. Does femoral venous pressure measurement correlate well with intrabladder pressure measurement? A multicenter observational trial. Intensive Care Med. 2011;37:1620–1627. doi: 10.1007/s00134-011-2298-x. [DOI] [PubMed] [Google Scholar]
- 27.Tayebi S., Gutierrez A., Mohout I., Smets E., Wise R., Stiens J., Malbrain M.L.N.G. A concise overview of non-invasive intra-abdominal pressure measurement techniques: From bench to bedside. J. Clin. Monit. Comput. 2021;35:51–70. doi: 10.1007/s10877-020-00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tayebi S., Pourkazemi A., Malbrain M., Stiens J. Non-Invasive Intra-Abdominal Pressure Measurement by Means of Transient Radar Method: In Vitro Validation of a Novel Radar-Based Sensor. Sensors. 2021;21:18. doi: 10.3390/s21185999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malbrain M., De Keulenaer B.L., Khanna A.K. Continuous intra-abdominal pressure: Is it ready for prime time? Intensive Care Med. 2022;48:1501–1504. doi: 10.1007/s00134-022-06780-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tayebi S., Wise R., Pourkazemi A., Stiens J., Malbrain M. Pre-Clinical Validation of A Novel Continuous Intra-Abdominal Pressure Measurement Equipment (SERENNO) Life. 2022;12:1161. doi: 10.3390/life12081161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howard A.E., Regli A., Litton E., Malbrain M.M., Palermo A.M., De Keulenaer B.L. Can femoral venous pressure be used as an estimate for standard vesical intra-abdominal pressure measurement? Anaesth. Intensive Care. 2016;44:704–711. doi: 10.1177/0310057X1604400604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data and research findings can be obtained at the authors’ premises upon justified demand.