Abstract
Deinococcus radiodurans is an exceptionally radiation-resistant microorganism capable of surviving acute exposures to ionizing radiation doses of 15,000 Gy and previously described as having a strictly aerobic respiratory metabolism. Under strict anaerobic conditions, D. radiodurans R1 reduced Fe(III)-nitrilotriacetic acid coupled to the oxidation of lactate to CO2 and acetate but was unable to link this process to growth. D. radiodurans reduced the humic acid analog anthraquinone-2,6-disulfonate (AQDS) to its dihydroquinone form, AH2DS, which subsequently transferred electrons to the Fe(III) oxides hydrous ferric oxide and goethite via a previously described electron shuttle mechanism. D. radiodurans reduced the solid-phase Fe(III) oxides in the presence of either 0.1 mM AQDS or leonardite humic acids (2 mg ml−1) but not in their absence. D. radiodurans also reduced U(VI) and Tc(VII) in the presence of AQDS. In contrast, Cr(VI) was directly reduced in anaerobic cultures with lactate although the rate of reduction was higher in the presence of AQDS. The results are the first evidence that D. radiodurans can reduce Fe(III) coupled to the oxidation of lactate or other organic compounds. Also, D. radiodurans, in combination with humic acids or synthetic electron shuttle agents, can reduce U and Tc and thus has potential applications for remediation of metal- and radionuclide-contaminated sites where ionizing radiation or other DNA-damaging agents may restrict the activity of more sensitive organisms.
Deinococcus radiodurans is the most radiation-resistant organism discovered to date, exhibiting the ability to withstand doses of ionizing radiation to 15,000 Gy without lethality (8). D. radiodurans, at this dose of radiation, incurs a large number of double-stranded DNA breaks, 130 per chromosome (8). Extremely efficient DNA repair mechanisms in operation during recovery in the absence of radiation are responsible for the extreme radiation resistance observed in this organism (6, 7, 25). Resistance to such high levels of ionizing radiation probably does not represent a direct response adaptation, since there are no natural terrestrial environments that generate such high fluxes of ionizing radiation (25). It is more likely that the efficient DNA repair system in this bacterium represents an adaptation to prolonged desiccation as dehydration of cells results in DNA damage, double-stranded DNA breaks, similar to that resulting from exposure to ionizing radiation (24).
The remarkable ability to withstand high doses of radiation including chronic or continuous doses, periods of extended desiccation, and numerous other DNA-damaging agents coupled with the relative ease of genetic manipulation (33) has made D. radiodurans an attractive candidate for genetic manipulation for enhancing organopollutant degradation. Such organisms could have potential applications at contaminated sites where mixed wastes are problematic. To this end, Lange et al. (17) constructed strains of D. radiodurans that expressed toluene dioxygenase activity. The resulting D. radiodurans constructs could oxidize toluene, chlorobenzene, 3,4-dichloro-1-butene, and indole; the engineered strain also grew and synthesized toluene dioxygenase while being exposed to ionizing radiation at a dose of 60 Gy h−1. More recently, a mercuric ion reductase gene (merA) was cloned and expressed in D. radiodurans (3) with the goal of using such constructs for bioremediation in high-radiation environments. Such environments exist at U.S. Department of Energy (DOE) sites previously involved in the production of nuclear materials.
Recently, we isolated a Thermus sp. from a groundwater sample collected from an ultradeep (3.2-km) gold mine in South Africa that could utilize Fe(III) as an electron acceptor coupled to the oxidation of lactate (15). Due to the interest in using highly radiation-resistant D. radiodurans for remediation of radioactive metal- and radionuclide-contaminated sites and because of the close phylogenetic relationship between members of the genera Thermus and Deinococcus (14), we investigated the potential for metal reduction by D. radiodurans strain R1.
MATERIALS AND METHODS
Cultures and media.
D. radiodurans R1 was routinely cultured in TYG medium containing 5 g of tryptone, 3 g of yeast extract, and 1 g of dextrose per liter of deionized water. Cultures were incubated at 30°C on a rotary shaker. Cells were harvested by centrifugation and washed three times in either sterile, pH 7, phosphate-buffered 0.85% saline or bicarbonate buffer containing 2.5 g of NaHCO3 and 0.1 g of KCl per liter of deionized water.
The ability of D. radiodurans R1 to reduce Fe(III) and other electron acceptors was evaluated using a defined basal medium consisting of 0.42 g of KH2PO4, 0.22 g of K2HPO4, 0.2 g of NH4Cl, 0.38 g of KCl, 0.36 g of NaCl, 0.04 g of CaCl2 · 2H2O, 0.1 g of MgSO4 · 7H2O, 1.8 g of NaHCO3, 0.5 g of Na2CO3, 0.19 mg of Na2SeO4, 10 ml of trace element solution, and 15 ml of a vitamin mixture per liter of deionized water. The trace element solution contained 2.14 g of nitrilotriacetic acid (NTA), 0.1 g of MnCl2 · 4H2O, 0.3 g of FeSO4 · 7H2O, 0.03 g of CuCl2 · 2H2O, 0.17 g of CoC2 · 6H2O, 0.2 g of ZnSO4 · 7H2O, 5 mg of AlK(SO4)2 · 12H2O, 5 mg of H3BO3, 0.09 g of Na2MoO4, 0.11 g of NiSO4 · 6H2O, and 0.02 g of Na2WO4 · 2H2O per liter of deionized water. The vitamin mixture contained 2.0 mg of biotin, 2.0 mg of folic acid, 10.0 mg of pyridoxine HCl, 5.0 mg of riboflavin, 5.0 mg of thiamine, 5.0 mg of nicotinic acid, 5.0 mg of pantothenic acid, 0.1 mg of cyanocobalamin, 5.0 mg of 4-aminobenzoic acid, and 5.0 mg of thioctic acid per liter of deionized water. Media were autoclaved or filter sterilized and purged with an O2-free gas mix comprised of 80% N2 and 20% CO2.
Electron acceptors and donors.
The ability of D. radiodurans to reduce Fe(III) was examined in cultures containing 10 mM Fe(III)-NTA and 10 mM lactate in the basal medium described above at 30°C. Stocks of 100 mM Fe(III)-NTA were prepared by dissolving 1.64 g of NaHCO3, 2.56 g of trisodium NTA, and 2.7 g of FeCl3 · 6H2O in 100 ml of deionized water. Acetate, pyruvate, succinate, ethanol, l-lactate, and d-lactate at 10 mM each or H2 at 33 mM was evaluated as an electron donor supporting Fe(III) reduction. Other forms of Fe(III) evaluated as electron acceptors for D. radiodurans R1 included hydrous ferric oxide (HFO) (12), ferric pyrophosphate, ferric citrate, and goethite (α-FeOOH) (41) at 10 mM each. Other metals evaluated for reduction coupled to lactate included U(VI), Tc(VII), and Cr(VI). Anthraquinone-2,6-disulfonate (AQDS; Sigma Chemical Co., St. Louis, Mo.), 0.1 mM, was also evaluated as an electron acceptor. HFO, goethite, Tc(VII), U(VI), and Cr(VI) were also tested for reduction with 0.1 mM AQDS as an electron shuttle (21). Leonardite humic acid was purchased from the International Humic Substances Society (University of Minnesota, St. Paul) and was used at a concentration of 2 mg ml−1. Fe(II) generated in experiments with Fe oxides was extracted with 0.5 N HCl before determining the concentration using the ferrozine assay, as previously described (12).
For experiments with AQDS, Tc, U, and Cr, pressure tubes containing bicarbonate buffer (30 mM NaHCO3 and 1.3 mM KCl) and 10 mM sodium lactate as electron donor were purged with O2-free N2:CO2 (80:20) and sealed with thick butyl rubber stoppers. Washed D. radiodurans cells suspended in anaerobic bicarbonate buffer were added using a needle and syringe and purged with N2:CO2, to a final density of ∼5 × 107 cells ml−1. Filter-sterilized (0.2-μm pore size) AQDS was purged with O2-free N2 and added to obtain a final concentration of 0.1 mM. Technetium, as NH499Tc(VII)O4 (Amersham Life Sciences Products, Arlington Heights, Ill.), and uranium, as U(VI) acetate (Fluka Chemical Co., Milwaukee, Wis.), were also purged with O2-free N2 and added to achieve final solution concentrations of 5 to 200 μM. All subsequent incubations and sampling were conducted in an anoxic atmosphere (Forma Scientific Inc., Marietta, Ohio, or Coy Laboratory Products, Ann Arbor, Mich.) containing Ar (95%) and H2 (5%) and (<1 × 10−4)% O2. Controls consisted of identical treatments either without cells or without AQDS. All treatments were carried out in duplicate or triplicate.
At selected time points, samples were withdrawn from pressure tubes using a needle and syringe and filtrates (<0.2-μm pore size) were collected. Total Tc activity in filtrates was determined by liquid scintillation counting (model 1411; Wallac Inc., Gaithersburg, Md.). Pertechnetate (TcO41−) remaining in the solution was determined by direct extraction (36) and liquid scintillation counting. A decrease in TcO41− concentration indicated that Tc(VII) was reduced, presumably to other lower oxidation states. Soluble U(VI) was analyzed using a kinetic phosphorescence analyzer (4) (KPA-10; Chemchek Instruments, Inc., Richland, Wash.). Cr(VI) was quantified by mixing filtrates (pore size, 0.2 μm) with sym-diphenylcarbizide reagent (0.25% in acetone) and measuring the absorbance of solutions at 540 nm (37). Reduction of AQDS was measured using a scanning spectrophotometer (model DU-50; Beckman Instruments, Palo Alto, Calif.). AQDS and its reduced form, AH2DS, were measured at 325 and 405 nm, respectively.
Fe reduction coupled to lactate oxidation.
Mineralization of lactate to CO2 was measured in conjunction with the reduction of Fe(III)-NTA in HCO3-buffered medium at neutral pH, and in cultures lacking Fe(III), using uniformly labeled Na lactate (150 mCi mmol−1, 99% radiopurity; American Radiolabeled Chemicals, Inc., St. Louis, Mo.). Ethanol was removed from the lactate before use by purging with N2. The lactate was added to sterile anaerobic water and diluted in the basal medium prior to addition to the cultures. Cultures for measuring mineralization of lactate consisted of 10 ml containing 2.8 × 108 cells ml−1, 3 mM sodium lactate (Sigma), 10 mM Fe(III)-NTA (except in controls), and approximately 0.4 μCi of 14C-labeled lactate in basal medium. Cultures were incubated in 30-ml serum bottles with 80:20 N2:CO2 headspace at 30°C without shaking. A 2-ml cryovial (Nalgene) was suspended inside the serum bottle to trap evolved CO2. A 1.0 N KOH (0.1-ml) solution was added to each cryovial at a volume sufficient to trap both the evolved 14CO2 and the CO2 in the headspace. At each sample point, duplicate cultures were sacrificed by adding 1.0 ml of 5.5 N HCl to each culture and 1.0 ml of KOH to each trap. KOH, 0.95 ml, was removed and added to Opti-Fluor scintillation fluid (Packard Instrument, Downers Grove, Ill.) for liquid scintillation counting. These cultures were also analyzed for Fe(II) using the ferrozine assay (34). Additionally, identical 10-ml cultures containing 10 mM Fe(III)-NTA with and without 3 mM lactate were prepared. At the same time points that the cultures containing [14C]lactate were sampled, a duplicate set of cultures containing unlabeled lactate were also sacrificed and analyzed for Fe(II) and concentrations of lactate and acetate. Loss of lactate as well as oxidation products in cultures with and without Fe(III)-NTA was measured from culture filtrates with a DX 500 ion chromatography system (Dionex, Sunnyvale, Calif.) using an Ion Pac AS 11 (Dionex) analytical column and a CD 20 conductivity detector (Dionex). The eluent gradient was programmed to result in a 0.2 mM NaOH solution during equilibration and analysis and a 35 mM NaOH solution during column regeneration. The flow rate was 1 ml min−1, and the injection volume was 50 μl.
Growth measurements.
The ability of D. radiodurans populations to grow with Fe(III)-NTA as the sole electron acceptor was examined. These cultures were composed of the same medium described above amended with tryptone at 50 mg liter−1 and yeast extract at 30 mg liter−1 and contained 3 mM lactate. Initial cell concentrations were 5.7 × 106 ml−1. Lactate consumption and evolution of other metabolic products were analyzed by ion chromatography as described above. Aerobic cultures of D. radiodurans in the same medium described above but without Fe(III)-NTA were also tested for growth. Cell concentrations were determined by microscopic counting of acridine orange-stained cells.
Electron microscopy and X-ray diffraction (XRD).
Solids associated with HFO-AQDS cultures were imaged and analyzed using scanning electron microscopy. All samples were prepared in an anaerobic glove box to avoid oxidation of reduced solids. Whole mounts were prepared by placing a drop of cell suspension on a Formvar-coated copper transmission electron microscopy grid. Grids were dried and stored in a sealed vial under an anaerobic atmosphere until they were transferred to the high vacuum of the electron microscope. Samples were analyzed using a LEO 982 field emission scanning electron microscope. Elemental analyses were accomplished using an Oxford-Link Isis energy-dispersive spectrometer, equipped with a SiLi detector.
For XRD analysis, settled mineral residue was removed from the pressure tubes to minimize liquid transfer and dried under anaerobic conditions; the dried solid was smeared on a glass slide. The slides were maintained under an anoxic atmosphere until the time of analysis. The XRD apparatus consisted of two Philips wide-range vertical goniometers with incident-beam 2-theta compensating slits, soller slits, fixed 2-mm receiving slits, diffracted beam graphite monochromators, and scintillation counter detectors. The X-ray source was a Philips XRG3100 X-ray generator operating a fixed-anode, long-fine-focus Cu tube at 45 kV, 40 mA (1,800 W). Instrument control was by means of Databox NIMBIM modules (Materials Data, Inc., Livermore, Calif.).
RESULTS
Fe(III)-NTA reduction coupled to oxidation of organic substrates.
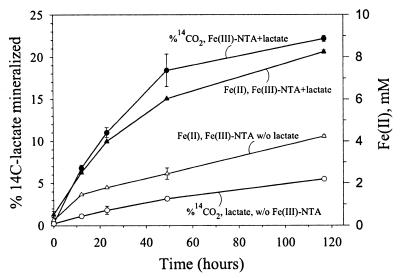
D. radiodurans effectively coupled the oxidation of [14C]lactate to CO2 with the reduction of Fe(III)-NTA to Fe(II) in the absence of O2 (Fig. 1). The stoichiometry of Fe(III) reduced to lactate oxidized in this experiment was 12.8:1. However, if the Fe(II) and percent [14C]lactate mineralized were corrected for controls lacking either electron donor (lactate) or electron acceptor (Fe-NTA), the ratio was 8.6:1. In separate cultures with unlabeled lactate, ion chromatography analyses revealed that one-half (1.45 mM) of the lactate was consumed and 0.8 mM acetate was produced over the same incubation period (116 h). D. radiodurans cultures containing 3 mM lactate were unable to reduce either ferric citrate or ferric pyrophosphate.
FIG. 1.
Mineralization of 14C-labeled lactate coupled to Fe(III)-NTA reduction by D. radiodurans. All values represent the means of three replicates, and the absence of error bars indicates that the magnitude of the error is less than the size of the symbol.
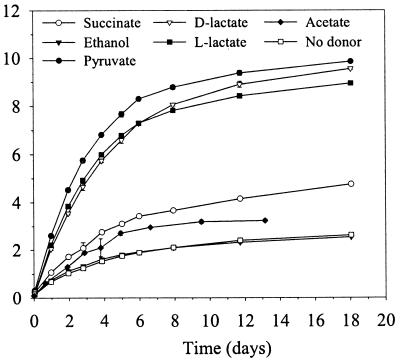
d- and l-lactate were equally effective as electron donors for Fe(III)-NTA reduction (Fig. 2). Pyruvate was as effective as lactate, and succinate was approximately 48% as effective as pyruvate. Cultures with ethanol did not result in more Fe(III) reduced than was observed in the no-donor control (Fig. 2). Acetate also promoted Fe(III)-NTA reduction (3.2 mM Fe2+) over an incubation period of 18 days by D. radiodurans compared to no-donor control (1.7 mM Fe2+) although H2 did not (data not shown). These results, in combination with the acetate analyses from the lactate cultures described above, indicate that acetate is incompletely oxidized by D. radiodurans coupled to Fe(III)-NTA reduction. Additional studies are required to more thoroughly examine the oxidation of acetate coupled to Fe(III)-NTA reduction by D. radiodurans.
FIG. 2.
Effectiveness of various organic electron donors for the reduction of Fe(III)-NTA by D. radiodurans.
Growth experiments.
The ability of D. radiodurans to grow anaerobically with Fe(III)-NTA (10 mM) as the sole electron acceptor was investigated using bicarbonate-buffered medium containing 3 mM lactate amended with tryptone (50 mg liter−1) and yeast extract (30 mg liter−1). Although D. radiodurans cells reduced Fe(III) (1.43 mM) in excess of that in the cultures without lactate (0.54 mM), there was no increase in cell numbers over the period during which Fe reduction was observed. The same medium without Fe(III)-NTA supported the growth of D. radiodurans in aerated liquid cultures as indicated by a visible increase in turbidity. These results indicate that the lack of growth in the anaerobic cultures was not due to the absence of one or more necessary growth factors. Also, D. radiodurans did not grow in anaerobic TYG broth or TYG supplemented with SO42−, S0, or NO3−, nor was there any evidence of these potential electron acceptors being reduced.
Reduction of AQDS and electron shuttling to Fe oxides.
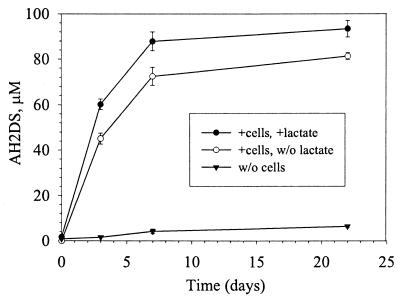
AQDS is a model humic acid compound (35) that can be utilized as an electron acceptor for respiration and growth by a variety of dissimilatory metal-reducing bacteria including Geobacter spp. and Shewanella spp. (21). As an electron acceptor, AQDS is reduced to the corresponding dihydroquinone (AH2DS) (31). Suspensions of D. radiodurans cells (6 × 107 cells ml−1) reduced 0.1 mM AQDS to AH2DS regardless of whether lactate was added or not, although more was reduced in the cultures with lactate (Fig. 3). In the absence of cells, less than 7% of the initial AQDS was reduced. These results are consistent with the observation that cultures of D. radiodurans cells, at similar densities, reduced >2 mM Fe(III)-NTA in the absence of any exogenous electron donor (Fig. 2). Reduction of electron acceptors in the absence of exogenous electron donor is likely due to respiration with endogenous carbon reserves. This phenomenon has been observed for other metal-reducing organisms including Thermus strain SA (15).
FIG. 3.
Reduction of AQDS to AH2DS by D. radiodurans.
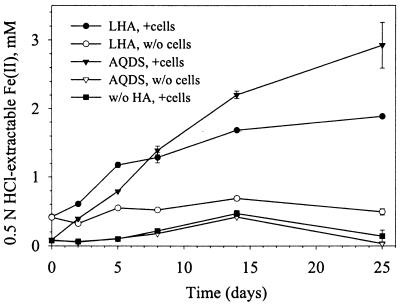
To determine whether D. radiodurans could reduce Fe(III) oxides with AQDS or humic acid functioning as an electron shuttle (22, 41), the reduction of freshly precipitated HFO and a medium-surface-area (∼55 m2 g−1) goethite by D. radiodurans in the presence and absence of 0.1 mM AQDS was investigated. The results of this experiment demonstrate that D. radiodurans can effectively reduce HFO in the presence of AQDS and leonardite humic acid but not in their absence (Fig. 4).
FIG. 4.
D. radiodurans reduction of HFO promoted by AQDS or leonardite humic acid (LHA) as electron shuttles.
The reduction of HFO in the presence of AQDS resulted in the precipitation of white solids that were identified as vivianite [Fe3(PO4)2 · 8H2O]. These solids were large (50 to 150 μm) lathe-shaped crystallites and were composed primarily of Fe, O, and P. The XRD pattern of these solids was consistent with the d-spacings for vivianite specimens. D. radiodurans also reduced 451 μM Fe(III) of the synthetic goethite over a 19-day period in the presence of AQDS but only 9.6 μM Fe(III) in its absence over the same time period.
Reduction of dissolved metals and radionuclides.
Because D. radiodurans could couple the reduction of AQDS to the reduction of Fe oxides, we hypothesized that it may also be able to couple its reduction to other metals and radionuclides. Previous studies have shown that microbially reduced AQDS is an effective reductant of U(VI) (13). In the presence of 0.1 mM AQDS, D. radiodurans effectively reduced 95 to 100% of Tc(VII) and U(VI) at concentrations ranging from 5 to 100 μM within a period of 21 days (Table 1). At the highest concentrations evaluated (>200 μM), the percentage of Tc(VII) or U(VI) that was reduced was slightly lower, 82 and 89%, respectively, than the lower starting concentrations of the radionuclides. D. radiodurans was unable to directly reduce Tc(VII) at 100 μM or U(VI) at 100 or 500 μM in the absence of AQDS with lactate as the electron donor (data not shown). In the U(VI) reduction experiments, 30 mM NaHCO3 was used as a buffer and therefore the predominant U(VI) species in solution would have been UO2(CO3)34− and UO2(CO3)22− (13).
TABLE 1.
Reduction of varying concentrations of Tc(VII) and U(VI) by D. radiodurans in the presence of 0.1 mM AQDS
| Element | Incubation time (days) | Tc(VII) or U(VI) concn (μM)a for expt no.:
|
|||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Tc(VII) | 0 | 5.4 (0.1) | 55.7 (1.7) | 110.4 (2.0) | 222.8 (4.9) |
| 3 | 0.4 (0.1) | 0.5 (0.2) | 27.7 (0.1) | 144.7 (2.7) | |
| 21 | 0.3 (0.1) | 1.3 (0.3) | 1.8 (0.1) | 39.0 (7.2) | |
| U(VI) | 0 | 4.7 (0.1) | 56.5 (3.2) | 117.5 (0.4) | 235.5 (6.5) |
| 21 | BDb | BD | 2.5 (3.6) | 26.7 (2.4) | |
Values in parentheses are the standard errors of the means of duplicate samples.
BD, below the analytical detection limit.
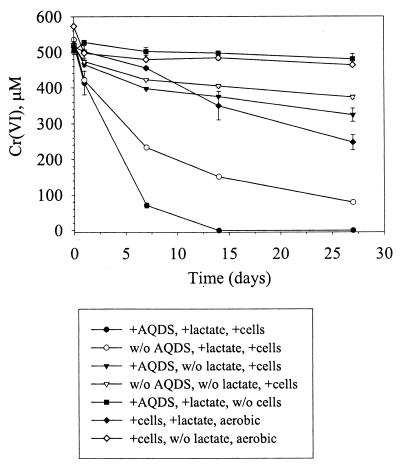
In contrast to U(VI) and Tc(VII), D. radiodurans was able to directly reduce Cr(VI) with approximately 72% of the initial Cr(VI) being reduced after 14 days under anaerobic conditions (Fig. 5). Consistent with the results for U(VI) and Tc(VII), AQDS enhanced the rate and extent of Cr(VI); after 14 days the initial Cr(VI) was reduced to below the detection level. Some Cr(VI) was also reduced by cells in the absence of lactate, but the amount was much less (22 to 28%) than when lactate was included (Fig. 5). Cells incubated aerobically with Cr(VI) and lactate but without AQDS reduced >50% of the Cr(VI) within the same time period as the anaerobic incubations (27 days). Growth with Cr(VI) as the electron acceptor was not tested.
FIG. 5.
Reduction of Cr(VI) by D. radiodurans, with or without AQDS, as measured by loss of Cr(VI) from solution.
DISCUSSION
Species of the genus Deinococcus, including D. radiodurans, have been described as having a strictly aerobic metabolism (11) with no known capacity for either fermentative or respiratory metabolism in the absence of O2. The results presented here demonstrate that D. radiodurans strain R1 can oxidize lactate to CO2 coupled to the reduction of Fe(III)-NTA under strictly anaerobic conditions. Although other bacteria, including several Thermus spp. that, interestingly, are phylogenetically related to Deinococcus (10), have been shown to conserve energy for growth from the oxidation of lactate coupled to Fe(III)-NTA reduction (15), D. radiodurans was unable to grow with this electron acceptor-electron donor combination. This same medium without Fe(III)-NTA supported vigorous growth in air, suggesting that the reduction of Fe(III)-NTA under anaerobic conditions with lactate is a fortuitous catabolic reaction.
D. radiodurans also effectively utilized pyruvate and succinate to reduce Fe(III)-NTA; the ability to reduce Fe(III) was limited to the Fe-NTA complex; other forms of Fe(III) including ferric citrate, ferric pyrophosphate, HFO, and goethite were not directly reduced. For dissimilatory iron-reducing bacteria, such as Shewanella putrefaciens, dissolved Fe(III)-organic complexes including Fe(III)-NTA are more readily reduced than solid-phase Fe oxides (9), although some crystalline Fe oxides are also extensively reduced (29, 41). The reduction of Fe(III)-organic complexes might be expected to be more rapid than that of Fe oxides given the relative insolubility of the latter at circumneutral pH values. This, however, does not completely explain the inability of D. radiodurans to reduce the solid-phase Fe oxides. S. putrefaciens MR-1, recently reclassified as Shewanella oneidensis (38), as well as other Shewanella spp., can reduce Fe(III) associated with a variety of oxides including HFO (12), goethite and hematite (41), and magnetite (16). The ability to reduce solid-phase Fe(III) has been attributed to the localization of Fe(III)-reducing cytochromes to the outer membrane (26, 27) where they can potentially come in direct contact with the solid-phase oxides. The presence of an extracellular c-type cytochrome in Geobacter sulfurreducens that could reduce ferrihydrite and manganese dioxide led Seeliger et al. (32) to speculate that this protein is involved in electron transfer from cells to the solid-phase oxides. However, the specific mechanisms by which dissimilatory iron-reducing bacteria transfer electrons to solid Fe oxides remain poorly understood. One possible explanation for the inability of D. radiodurans to reduce solid-phase Fe oxides is that this organism lacks surface-associated or extracellular electron transfer factors, accounting for its inability to reduce solid-phase Fe oxides such as HFO or goethite in the absence of an electron shuttle.
The reasons for the inability of D. radiodurans to reduce Fe(III)-citrate are also unclear, although intrinsic differences in the ability to utilize different forms of soluble, complexed Fe(III) have been noted among various dissimilatory iron-reducing bacteria. For example, the reduction of Fe(III)-NTA by S. putrefaciens (ATCC 8071) was over fivefold greater than that of Fe(III)-citrate; the anaerobic growth of this organism with lactate and Fe(III)-citrate was also noted to be poor (9).
D. radiodurans reduced AQDS to AH2DS and could couple this reaction, in addition to the reduction of humic acids, to the reduction of solid-phase Fe oxides including HFO and goethite. Since more Fe(III) (1.56 mM) was reduced than could be accounted for even if all of the 0.1 mM AQDS was initially reduced (Fig. 4) in these experiments, AQDS must have cycled between the oxidized and reduced forms, functioning as an electron shuttle (during the oxidation of lactate or endogenous carbon reserves) between D. radiodurans and the oxides. A major end product of HFO reduction was vivianite, also observed as a biogenic mineral resulting from HFO reduction in P-containing cultures of S. putrefaciens strain CN32 in bicarbonate buffer with lactate and 0.1 mM AQDS (12).
Several dissimilatory iron-reducing bacteria including Geobacter metallireducens and Shewanella algae are capable of utilizing humic acids as electron acceptors during respiration (21). The quinone moieties of humic acids have been identified as the electron acceptors for bacterial respiration (20, 31). Microbially reduced humic acids, and the model humic acid compound AQDS, are facile reductants of Fe oxides. The presence of humic acids or AQDS in cultures of metal-reducing bacteria has been shown to enhance the rate and extent of reduction of Fe oxides (12, 22) including crystalline phases such as goethite and hematite (41). This effect has been attributed to an electron shuttle mechanism whereby the bacteria reduce soluble humic acids that in turn reduce Fe(III) associated with the solid-phase oxide (21).
The reduction of HFO in the presence of 0.1 mM AQDS by D. radiodurans was relatively slow, 22% reduction of 10 mM HFO in 14 days (Fig. 3), in comparison to the dissimilatory metal-reducing bacterium G. metallireducens and to S. putrefaciens strain CN32. These bacteria reduced approximately 50% of 6 mM HFO within 3 h (21) and >50% of 50 mM HFO within 72 h (12), respectively, in the presence of 0.1 mM AQDS. The differences in the rates of HFO reduction coupled to AQDS electron shuttling may be due to an inherently lower rate of AQDS reduction by D. radiodurans. In D. radiodurans cultures with lactate and 0.1 mM AQDS, 88% of the AQDS was reduced within 7 days (Fig. 3) whereas S. putrefaciens CN32 lactate cultures completely reduced 0.1 mM AQDS in approximately 1 h (J. Fredrickson, unpublished data).
The standard potential (E°) for the AQDS-AH2DS couple (0.23 V) is significantly below that for the couple UO2(CO3)34−-UO2 (0.69 V) (13) or TcO4−-TcO2 (0.75 V) (40) and is considerably lower than that for CrO42−-Cr(OH)3 (1.28 V) (1). Thus, the transfer of electrons from AH2DS to each of these metals is thermodynamically feasible. This possibility was supported by experiments demonstrating the reduction of U(VI) and Tc(VII) in D. radiodurans cultures containing, but not in those lacking, AQDS. Although D. radiodurans directly reduced Cr(VI) under anaerobic conditions, the rate and extent of Cr(VI) reduction were greater when AQDS was present (Fig. 5).
The inability of D. radiodurans to directly reduce U or Tc may be due to enzyme substrate specificity or enzyme inhibition rather than lack of a sufficiently low enzyme midpoint potential since AQDS was reduced to AH2DS. The redox potential of cultures in which AQDS was present is readily calculated from the ratio of the oxidized to reduced species (12). In the experiment with 0.1 mM AQDS (Fig. 3), the calculated Eh in the solution incubated with D. radiodurans and lactate for 22 days was −0.227 V. This potential is sufficiently reducing that both U (13) and Tc (40) would be expected to exist predominantly in the +4 oxidation state. Additional research is required to identify the enzyme(s) in D. radiodurans that is responsible for Fe(III)-NTA and AQDS reduction, determine whether it is part of the organism's electron transport system, and determine if metal reduction is more broadly distributed throughout the deinococci. The entire genome of D. radiodurans R1 has recently been sequenced (39), potentially facilitating the identification and study of genes involved in Fe(III)-NTA and AQDS reduction.
The enzymatic reduction of multivalent metals and radionuclides can have a major impact on their solubility and, hence, mobility in the environment. Such changes in solubility make microbial metal reduction an attractive process for removing metals and radionuclides from contaminated waters via ex situ processes or for immobilizing these contaminants in situ (19). As a result of the production of weapons-grade nuclear materials between 1945 and 1986, the DOE is facing challenging cleanup problems at more than 18 facilities across the United States. The most common inorganic contaminants at these sites are uranium, strontium, cesium, plutonium, technetium, chromium, lead, and mercury (28). Among these, U, Pu, Tc, and Cr are less mobile when reduced, and all can be reduced by microorganisms (5, 18, 23, 30). Localized contaminated sediments and soils at DOE sites, particularly beneath leaking waste storage tanks, can have radiation levels that exceed those that can be tolerated by most microorganisms. Under such conditions where ionizing radiation fields are especially high and other DNA-damaging agents are present at inhibitory concentrations, D. radiodurans may provide a means for limiting the migration of multivalent radionuclides and heavy metals. Such a remediation strategy would require the presence of humic acids as electron shuttles. Because they function as catalysts for bacterial metal reduction, relatively small concentrations are required to facilitate reduction (22). Humic acids, because of their recalcitrance to biodegradation, are common to many soils and sediments (21). However, in their absence, humic acids or other quinone-containing organic compounds could be added to stimulate metal reduction at contaminated sites.
The deinococci appear to be widely distributed in soils and have been routinely isolated from organically rich as well as dry, nutrient-poor environments (2). Therefore, it is possible that deinococci capable of reducing metal and radionuclides may be native to some contaminated environments. Additional research is required to better understand the ecology of the deinococci and the potential for naturally occurring strains to reduce metals.
ACKNOWLEDGMENTS
We acknowledge the technical contributions of Alice Dohnalkova for the analysis of the biominerals by electron microscopy.
This research was supported by the Natural and Accelerated Bioremediation Research Program (NABIR), Office of Biological and Environmental Research (OBER), U.S. Department of Energy (DOE). Pacific Northwest National Laboratory is operated for the DOE by Battelle Memorial Institute under contract DE-AC06-76RLO 1830. USUHS research was supported by DOE-OBER grants FG02-97ER62492 from NABIR and FG07-97ER20293 from the Environmental Management Sciences Program.
REFERENCES
- 1.Ball J W, Nordstrom D K. Critical evaluation and selection of standard state thermodynamic properties for chromium metal and its aqueous ions, hydrolysis species, oxides, and hydroxides. J Chem Eng Data. 1998;43:895–918. [Google Scholar]
- 2.Battista J R. Against all odds: the survival strategies of Deinococcus radiodurans. Annu Rev Microbiol. 1997;51:203–224. doi: 10.1146/annurev.micro.51.1.203. [DOI] [PubMed] [Google Scholar]
- 3.Brim H, McFarlan S C, Fredrickson J K, Minton K W, Zhai M, Wackett L P, Daly M. Engineering Deinococcus radiodurans for metal remediation in radioactive mixed waste environments. Nat Biotechnol. 2000;18:85–90. doi: 10.1038/71986. [DOI] [PubMed] [Google Scholar]
- 4.Brina R, Miller A G. Direct detection of trace levels of uranium by laser-induced kinetic phosphorimetry. Anal Chem. 1992;64:1413–1418. [Google Scholar]
- 5.Campos J, Martinez-Pacheco M, Cervantes C. Hexavalent-chromium reduction by a chromate-resistant Bacillus sp. strain. Antonie Leeuwenhoek. 1995;68:203–208. doi: 10.1007/BF00871816. [DOI] [PubMed] [Google Scholar]
- 6.Daly M J, Minton K W. An alternative pathway of recombination of chromosomal fragments precedes recA-dependent recombination in the radioresistant bacterium Deinococcus radiodurans. J Bacteriol. 1996;178:4461–4471. doi: 10.1128/jb.178.15.4461-4471.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daly M J, Minton K W. Interchromosomal recombination in the extremely radioresistant bacterium Deinococcus radiodurans. J Bacteriol. 1995;177:5495–5505. doi: 10.1128/jb.177.19.5495-5505.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daly M J, Ouyang L, Fuchs P, Minton K W. In vivo damage and recA-dependent repair of plasmid and chromosomal DNA in the radiation-resistant bacterium Deinococcus radiodurans. J Bacteriol. 1994;176:3508–3517. doi: 10.1128/jb.176.12.3508-3517.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobbin P S, Powell A K, McEwan A G, Richardson D J. The influence of chelating agents upon the dissimilatory reduction of Fe(III) by Shewanella putrefaciens. BioMetals. 1995;8:163–173. [Google Scholar]
- 10.Embley T M, Thomas R H, Williams R A D. Reduced thermophilic bias in the 16S rDNA sequence from Thermus ruber provides further support for a relationship between Thermus and Deinococcus. Syst Appl Microbiol. 1993;16:25–29. [Google Scholar]
- 11.Ferreira A C, Nobre M F, Rainey F A, Silva M T, Wait R, Burghardt J, Chung P, da Costa M S. Deinococcus geothermalis sp. nov. and Deinococcus murrayi sp. nov., two extremely radiation-resistant and slightly thermophilic species from hot springs. Int J Syst Bacteriol. 1997;47:939–947. doi: 10.1099/00207713-47-4-939. [DOI] [PubMed] [Google Scholar]
- 12.Fredrickson J K, Zachara J M, Kennedy D W, Dong H, Onstott T C, Hinman N W, Li S W. Biogenic iron mineralization accompanying the dissimilatory reduction of hydrous ferric oxide by a groundwater bacterium. Geochim Cosmochim Acta. 1998;62:3239–3257. [Google Scholar]
- 13.Fredrickson, J. K., J. M. Zachara, D. W. Kennedy, M. C. Duff, Y. A. Gorby, S. W. Li, and K. M. Krupka. Reduction of U(VI) in goethite (α-FeOOH) suspensions by a dissimilatory metal-reducing bacterium. Geochim. Cosmochim. Acta, in press.
- 14.Hensel R, Demharter W, Kandler O, Kroppenstedt R M, Stackebrandt E. Chemotaxonomic and molecular-genetic studies of the genus Thermus: evidence for a phylogenetic relationship of Thermus aquaticus and Thermus ruber to the genus Deinococcus. Int J Syst Bacteriol. 1986;36:444–453. [Google Scholar]
- 15.Kieft T L, Fredrickson J K, Onstott T C, Gorby Y A, Kostandarithes H M, Bailey T J, Kennedy D W, Li S W, Plymale A E, Spadoni C M, Gray M S. Dissimilatory reduction of Fe(III) and other electron acceptors by a Thermus isolate. Appl Environ Microbiol. 1999;65:1214–1221. doi: 10.1128/aem.65.3.1214-1221.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kostka J E, Nealson K H. Dissolution and reduction of magnetite by bacteria. Environ Sci Technol. 1995;29:2535–2540. doi: 10.1021/es00010a012. [DOI] [PubMed] [Google Scholar]
- 17.Lange C C, Wackett L P, Minton K W, Daly M J. Engineering a recombinant Deinococcus radiodurans for organopollutant degradation in mixed waste environments. Nat Biotechnol. 1998;16:929–933. doi: 10.1038/nbt1098-929. [DOI] [PubMed] [Google Scholar]
- 18.Lloyd J R, Macaskie L E. A novel phosphorimager-based technique for monitoring the microbial reduction of technetium. Appl Environ Microbiol. 1996;62:578–582. doi: 10.1128/aem.62.2.578-582.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lovley D. Bioremediation of organic and metal contaminants with dissimilatory metal reduction. J Ind Microbiol. 1995;14:85–93. doi: 10.1007/BF01569889. [DOI] [PubMed] [Google Scholar]
- 20.Lovley D R, Blunt-Harris E L. Role of humic-bound iron as an electron transfer agent in dissimilatory Fe(III) reduction. Appl Environ Microbiol. 1999;65:4252–4254. doi: 10.1128/aem.65.9.4252-4254.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lovley D R, Coates J D, Blunt-Harris E L, Phillips E J P, Woodward J C. Humic substances as electron acceptors for microbial respiration. Nature. 1996;382:445–448. [Google Scholar]
- 22.Lovley D R, Fraga J L, Blunt-Harris E L, Hayes L A, Phillips E J P, Coates J D. Humic substances as a mediator for microbially catalyzed metal reduction. Acta Hydrochim Hydrobiol. 1998;26:152–157. [Google Scholar]
- 23.Lovley D R, Phillips E J P, Gorby Y A, Landa E R. Microbial reduction of uranium. Nature. 1991;350:413–416. [Google Scholar]
- 24.Mattimore V, Battista J R. Radioresistance of Deinococcus radiodurans: functions necessary to survive ionizing radiation are also necessary to survive prolonged desiccation. J Bacteriol. 1996;178:633–637. doi: 10.1128/jb.178.3.633-637.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minton K W. Repair of ionizing-radiation damage in the radiation resistant bacterium Deinococcus radiodurans. Mutat Res. 1996;363:1–7. doi: 10.1016/0921-8777(95)00014-3. [DOI] [PubMed] [Google Scholar]
- 26.Myers C, Myers J. Outer membrane cytochromes of Shewanella putrefaciens MR-1: spectral analysis, and purification of the 83-kDa c-type cytochrome. Biochim Biophys Acta. 1997;1326:307–318. doi: 10.1016/s0005-2736(97)00034-5. [DOI] [PubMed] [Google Scholar]
- 27.Myers C R, Myers J M. Localization of cytochromes to the outer membrane of anaerobically grown Shewanella putrefaciens MR-1. J Bacteriol. 1992;174:3429–3438. doi: 10.1128/jb.174.11.3429-3438.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riley R G, Zachara J M. Chemical contaminants on DOE lands and selection of contaminant mixtures for subsurface science research. DOE/ER-0547T. U.S. Washington, D.C.: Department of Energy; 1992. [Google Scholar]
- 29.Roden E E, Zachara J M. Microbial reduction of crystalline Fe(III) oxides: influence of oxide surface area and potential for cell growth. Environ Sci Technol. 1996;30:1618–1628. [Google Scholar]
- 30.Rusin P A, Quintana L, Brainard J R, Strietelmeier B A, Tait C D, Ekberg S A, Palmer P D, Newton T W, Clark D L. Solubilization of plutonium hydrous oxide by iron-reducing bacteria. Environ Sci Technol. 1994;28:1686–1690. doi: 10.1021/es00058a021. [DOI] [PubMed] [Google Scholar]
- 31.Scott D T, McKnight D M, Blunt-Harris E L, Kolesar S E, Lovley D R. Quinone moieties act as electron acceptors in the reduction of humic substances by humics-reducing microorganisms. Environ Sci Technol. 1998;32:2984–2989. [Google Scholar]
- 32.Seeliger S, Cord-Ruwisch R, Schink B. A periplasmic and extracellular c-type cytochrome of Geobacter sulfurreducens acts as a ferric iron reductase and as an electron carrier to other acceptors or to partner bacteria. J Bacteriol. 1998;180:3686–3691. doi: 10.1128/jb.180.14.3686-3691.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith M D, Lennon E, McNeil L B, Minton K W. Duplication insertion of drug resistance determinants in the radioresistant bacterium Deinococcus radiodurans. J Bacteriol. 1988;170:2126–2135. doi: 10.1128/jb.170.5.2126-2135.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stookey L L. Ferrozine—a new spectrophotometric reagent for iron. Anal Chem. 1970;42:779–781. [Google Scholar]
- 35.Tratnyek P G, Macalady D L. Abiotic reduction of nitro aromatic pesticides in anaerobic laboratory systems. J Agric Food Chem. 1989;37:248–254. [Google Scholar]
- 36.Tribalat S, Beydon J. Isoelement du technetium. Anal Chim Acta. 1953;8:22–28. [Google Scholar]
- 37.Urone P F. Stability of colorimetric reagent for chromium, s-diphenylcarbizide, in various solvents. Anal Chem. 1955;27:1354–1355. [Google Scholar]
- 38.Venkateswaran K, Moser D P, Dollhopf M E, Lies D P, Saffarini D A, MacGregor B J, Ringelberg D B, White D C, Nishijima M, Sano H, Burghardt J, Stackebrandt E, Nealson K H. Polyphasic taxonomy of the genus Shewanella and description of Shewanella oneidensis sp. nov. Int J Syst Bacteriol. 1999;49:705–724. doi: 10.1099/00207713-49-2-705. [DOI] [PubMed] [Google Scholar]
- 39.White O, Eisen J A, Heidelberg J F, Hickey E K, Peterson J D, Dodson R J, Haft D H, Gwinn M L, Nelson W C, Richardson D L, Moffat K S, Qin H, Jiang L, Pamphile W, Crosby M, Shen M, Vamathevan J J, Lam P, McDonald L, Utterback T, Zalewski C, Makarova K S, Aravind L, Daly M J, Minton K W, Fleischmann R D, Ketchum K A, Nelson K E, Salzberg S, Smith H O, Venter J C, Fraser C M. Genome sequence of the radioresistant bacterium Deinococcus radiodurans R1. Science. 1999;286:1571–1577. doi: 10.1126/science.286.5444.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wildung, R. E., Y. A. Gorby, K. M. Krupka, N. J. Hess, S. W. Li, A. E. Plymale, J. P. McKinley, and J. K. Fredrickson. Effect of electron donor and solution chemistry on the products of the dissimilatory reduction of technetium by Shewanella putrefaciens. Appl. Environ. Microbiol., in press. [DOI] [PMC free article] [PubMed]
- 41.Zachara J M, Fredrickson J K, Li S W, Kennedy D W, Smith S C, Gassman P L. Bacterial reduction of crystalline Fe3+ oxides in single phase suspensions and subsurface materials. Am Mineral. 1998;83:1426–1443. [Google Scholar]