Abstract
Background and objectives: Bullous pemphigoid (BP), the most common subepidermal autoimmune skin blistering disease (AIBD) has an estimated annual incidence of 2.4 to 42.8 new cases per million in different populations, designating it an orphan disease. Characterized by disruption of the skin barrier combined with therapy-induced immunosuppression, BP could pose a risk for skin and soft tissue infections (SSTI). Necrotizing fasciitis (NF) is a rare necrotizing skin and soft tissue infection, with a prevalence of 0.40 cases per 100,000 to 15.5 cases per 100,000 population, often associated with immunosuppression. Low incidences of NF and BP classify them both as rare diseases, possibly contributing to the false inability of making a significant correlation between the two. Here, we present a systematic review of the existing literature related to the ways these two diseases correlate. Materials and methods: This systematic review was conducted according to the PRISMA guidelines. The literature review was conducted using PubMed (MEDLINE), Google Scholar, and SCOPUS databases. The primary outcome was prevalence of NF in BP patients, while the secondary outcome was prevalence and mortality of SSTI in BP patients. Due to the scarcity of data, case reports were also included. Results: A total of 13 studies were included, six case reports of BP complicated by NF with six retrospective studies and one randomized multicenter trial of SSTIs in BP patients. Conclusions: Loss of skin integrity, immunosuppressive therapy, and comorbidities commonly related to BP patients are risk factors for necrotizing fasciitis. Evidence of their significant correlation is emerging, and further studies are deemed necessary for the development of BP-specific diagnostic and treatment protocols.
Keywords: necrotizing soft tissue infection, LRINEC score, bullous pemphigoid, autoimmune skin blistering disease, immunosuppression
1. Introduction
Bullous pemphigoid (BP), the most common subepidermal autoimmune skin blistering disease (AIBD), has an estimated annual incidence of 2.4 to 42.8 new cases per million in different populations, designating it an orphan disease [1,2,3,4,5,6]. The circulating autoantibodies in bullous pemphigoid are targeted against epidermal proteins in the hemidesmosomal antigens BP180 and BP230, causing blistering at the dermal-epidermal junction [7]. Research has shown a steady increase in the incidence of bullous pemphigoid, while one group of authors found an average yearly increase in the incidence of 17% [1,8]. Bullous pemphigoid patients are found to have a genetic predisposition; still, environmental factors play an important role in disease development, such as viral infections, previous surgical procedures, trauma history, and the patient’s history of medications and vaccinations [9,10,11]. Drug-related bullous pemphigoid can manifest as a drug-induced or drug-triggered disease, usually affecting the younger population. While the cessation of medication use brings a resolution of the disease in drug-induced BP, in drug-triggered BP that is not the case [12]. Beta-blockers, diuretics, antibiotics, non-steroidal anti-inflammatory drugs (NSAIDs), neuroleptics, immune checkpoint inhibitors, as well as anti-tumor necrosis factor (TNF)-α, are described as triggers [13]. One recent study found that 6.28% of the world population, corresponding to 462 million individuals globally, are affected by diabetes, putting an especially heavy weight on the use of dipeptidyl peptidase 4 inhibitors (DPP-4) in diabetic patients’ therapy [14]. The use of DPP-4 inhibitors was found to be associated with a significant increase in the risk of developing BP by a group of authors in one case-control study with an adjusted odds ratio [aOR] of 1.58 and 95% CI, 1.25–2.00 [15]. Moreover, vaccine-induced BP has been previously described, with reports of COVID-19 vaccine-induced bullous pemphigoid cases emerging in the literature with both new onset as well as reactivation cases of BP. Mechanisms behind these findings are still unknown, while some authors indicate molecular mimicry or the activation of B and T cell immunity as the trigger in genetically predisposed individuals [16,17]. Consequently, the healthcare system of the future could expect a further increase in the number of BP patients, with autoimmune skin blistering diseases presenting a major healthcare burden and deserving more public concern.
One of the simplest classifications of disease severity depending on the total body surface area (TBSA) affected describes BP as mild (<10% TBSA), moderate (10 to 30% TBSA), or severe (>30% TBSA) [18]. Therapy for BP depends on the severity of the disease and relies mostly on long-term systemic and topical corticosteroids as well as other immunosuppressive agents [7,18]. In BP patients, given the disruption of the skin as the body’s main barrier, the finding of NF in individuals even after minor events such as abrasions and lacerations seems to place BP patients as hypothetically ideal candidates for such infections. Most studies addressing specific causes of death in BP patients found skin and soft tissue infections (SSTIs) to be one of the most common causes of death, and, additionally, that SSTIs affect virtually all BP patients to some extent.
Necrotizing fasciitis (NF) is a rare necrotizing skin and soft tissue infection (NSTI) with a prevalence of 0.40 cases per 100,000 to 15.5 cases per 100,000 population in the literature [19,20]. NF occurs more often in elderly patients with comorbidities such as diabetes mellitus and chronic renal failure [21]. Other risk factors include skin trauma, immunosuppression, immunosenescence, malnutrition, obesity, alcohol or drug abuse, and peripheral vascular disease. Causes of immunocompromise implicated include iatrogenic (therapy-related) or disease-related immunodeficiency [22,23]. Cases of NF were most commonly described after surgery, as well as spontaneous, without previous trauma. Most importantly, minor events such as tears, abrasions, lacerations, or insect bites as well as routine obstetrical and gynecologic procedures were all found to be associated with necrotizing fasciitis [24,25]. The main characteristic which defines necrotizing fasciitis and differentiates it from other SSTIs is its massive tissue destruction, followed by severe systemic toxicity, hemodynamic collapse, further organ failure, and ultimately, due to its rapid progression, high mortality [24,25].
Such infections in these conditions haven’t been systematically studied in the literature. Low incidences of NF and BP classify them both as rare diseases, possibly contributing to the false inability of making a significant correlation between the two, even though BP patients share an abundance of risk factors for NSTI, discussed in this review. As necrotizing fasciitis itself presents an often belatedly diagnosed, difficult to treat, and perilous soft tissue infection, it is plausible that such complications in BP patients are underreported and the risk of NF in these patients is underestimated [26]. Here, we present a systematic review of the existing literature related to the topic and the ways two diseases have a strong predilection of being highly correlated.
2. Materials and Methods
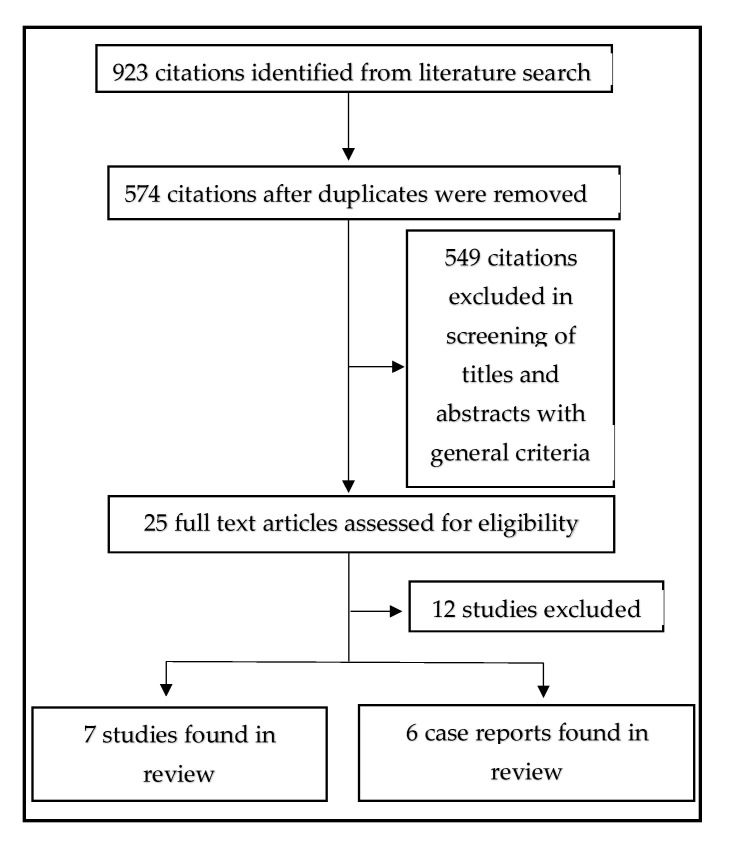
In this systematic review, the aim was to identify studies reporting the incidence, prevalence, and mortality of NF in patients with BP from the year 2000 onward. The systematic review was conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines [27]. The research was conducted by authors from 5 January 2022 to 15 December 2022. PubMed (MEDLINE), Google Scholar, and SCOPUS databases were searched for the studies including terms: [bullous pemphigoid OR autoimmune bullous disease] AND [necrotizing fasciitis OR skin and soft tissue infections] AND [mortality] used as keywords in various combinations. Publications were limited to English and French. The primary outcome was the prevalence of NF in BP patients, while the secondary outcome was the prevalence and mortality of SSTI in BP patients. Inclusion criteria were human studies addressing skin and soft tissue infections or NF in BP patients. Due to the scarcity of data, case reports were also included if they met the JBI Manual for Evidence Synthesis criteria [28]. Case reports and studies are presented in Table 1 and Table 2, separately.
Table 1.
A systematic review of all case reports included by the JBI Manual for Evidence Synthesis criteria.
| Authors | Age | Sex | Comorbidities | NF Onset (after BP Therapy) |
Control of BP | Previous Therapy | Current Therapy | Diagnostics | Bacteriology | NF Treatment | Evolution |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chamberlain 2003 [29] | 85 | F | HTA | 9 d | Incomplete | TCP, Erythromycin 250 mg ×2 | Prednisolone 30 mg/g + Betamethasone 500 µg × 2/d mouth wash, Erythromycin 250 mg ×2 |
Local findings of NF on the left leg, Laboratory, Microbiology, Ultrasound, |
Strep A | Successive surgical debridement, split thickness skin grafting Initial antimicrobe therapy: Vancomycin 1 g ×2 Meropenem 1 g ×3 Additional antimicrobial therapy: cefuroxime 1.5 g ×3 clindamycin 300 mg ×4 |
Survived (sequel) |
| Boughrara 2010 [30] |
73 | M | MI, benign vesical polyps | 2 years | Incomplete | ? | TCP | Local findings of NF in two localizations: left arm and dorsal side of the right hand, Microbiology |
Strep A, Pseudomonas sepsis |
Debridement of the left arm and amputation of the right index finger Antimicrobial therapy undisclosed. |
Died |
| Boughrara 2010 [30] |
64 | F | Obesety, insulin dependant DM | 14 d | Incomplete | ? | TCP, MTX 15 mg/week | Local findings of NF on the left leg, microbiology | Strep A, MRSA, Acinetobacter | Surgical debridement of the dorsal side of the foot and amputation of two toes. Antimicrobial therapy undisclosed. | Died |
| Boughrara 2010 [30] |
81 | M | HTA, insulin ndependat DM, preterminal CRI, COPD | 14 d | Incomplete | TCP | TCP, MMF | Local finding of NF on left leg and foot, Microbiology |
Strep A | Surgical debridement, Antimicrobial therapy undisclosed. |
Died |
| Doffoel-Hantz 2011 [31] |
86 | F | Acheimer’s dementia | 3 weeks | Incomplete | TCP | TCP | Retiform purpura of the ancle followed by development of necrosis. | Strep A | Broad-spectrum antibiotics, no surgical debridement was performed. | Died |
| Ekiz 2013 [32] | 78 | F | HTA | 3 weeks | Tetracyclin, Niacinamid, TCP, systemic steroids | Prednisolon 48 mg/d after 2 weeks 32 mg/d, | Local finding of NF on the left leg, laboratory, MRI, Microbiology Histopathology |
Strep A. | Surgical debridement, Sulbactam/ampicillin 1 g, oral ciprofloxacin 750 mg 2 × 1, tigecycline |
Died | |
| Sene 2014 [33] | 52 | M | Obesity | 35 d | Incomplete | TCP | Prednisolone 100 mg/d, after 2 weeks Prednisolone 60 mg/d and MMF 2 mg/d | Local finding of NF on the left leg, laboratory, Microbiology | Strep A | Surgical debridement, pipera- cillin-tazobactam and vancomycin | Survived |
| Sene 2014 [33] | 76 | F | HTA, DM | 2 weeks | Incomplete | TCP 30 g/d | TCP 10 g/d, MMF 2 g/d |
Local finding of NF on the left leg, laboratory, Microbiology | Strep A, MRSA | Surgical debridement piperacillin-tazobactam, gentamicin, and vancomycin | Died |
| Noguchi 2018 [34] | 69 | F | DM, | 4 d | Incomplete | Prednisolone 20 mg/d, Cyclosporine 100 mg/d | Prednisolone 40 mg/d, IVIG 4 days latter | Local finding of NF on the insertion point of CVC, Laboratory, Microbiology, CT scan |
MSSA | Surgical debridement piperacillin-tazobactam and vancomycin | Survived |
| Jurisic 2023 [35] |
51 | M | HTA, DM, Obesity | 3 weeks | Incomplete | Undisclosed corticosteroid therapy regimen | ? | Local finding of deep tissue necrosis due to advanced disease, CT scan, laboratory, microbiology |
Acinetobacter spp. Klebsiella-enterobacter Spp. Pseudomonas aeruginosa, Enterococcus spp. Acinetobacter baumannii complex sepsis (MDR) |
Successive surgical debridements, Vancomycin 2 × 1.5 g, Clindamycin 900 mg 3 × 1 and Meropenem 3 × 1 g Additional antimicrobial therapy: Colistimethate-sodium 3 × 3,000,000ij |
Died |
Abbreviations: Strep A—Streptococcus group A, MRSA—Methicillin-resistant Staphylococcus au reus, MSSA—Methicillin-sensitive Staphylococcus aureus, TCP—topical clobetasol propionate 0.5%, HTA—hypertension, MI—myocardial infarction, MTX—Methotrexate, CRI—chronic renal insufficiency, COPD—Chronic obstructive pulmonary disease, MMF—Mofetil mycophenolate, MRI—magnetic resonance imaging, CVC—central venous catheter, MDR—multi-drug resistant.
Table 2.
A systematic review of all original articles included.
| Author | Year | Type | No. Patients | Mean Age | Therapy | Events | Comorbidities | Risk Factors |
|---|---|---|---|---|---|---|---|---|
| Jolly | 2002 [36] | Randomized multicenter trial | 341 | 80 ± 11 | TCP Oral CS |
1 necrotizing Cellulitis in oral CS group |
Cardiovascular disease Neurologic disorder DementiaDiabetes mellitus Chronic lung condition | Oral corticosteroid therapy Low Karnofsky score Old age |
| Boughrara | 2010 [30] | Retrospective | 30 | 83.5 | TCP MTX MMF |
3 NF/10 SSTI | Diabetes Autoimmune diseases |
/ |
| Lehman | 2013 [37] | Retrospective | 54 | 75.8 | TCP Oral CS Cyclosporin MTX MMF Dapsone Rituximab Azathioprine |
43 SSTI’s | Diabetes Solid organ cancer/treatment Other autoimmune disorders |
Oral corticosteroid therapy |
| Cai | 2014 [38] | Retrospective | 359 | 75.7 ± 2.6 | Corticosteroids (88%), Doxycycline and/or nicotinamide (25.9%), Dapsone (13.9%) Azathioprine (39%), Combination therapy of corticosteroids and IMA 37.6% |
5 SSTI related causes of death | Heart failure Chronic renal disease Parkinson disease Stroke |
Concomitant neurologic disease Heart failure Parkinson disease |
| Phoon | 2015 [39] | Retrospective | 97 | 79 ± 11 | Prednisolone + adjuvant therapy in 53% of patients: dapsone 17% doxycycline and nicotinamide 34% azathioprine 3% MMF 6% |
7 SSTI’s | Hypertension Neurologic disorders Diabetes |
Low Karnofsky score, dementia, higher CCIS |
| Ren | 2018 [40] |
Retrospective | 13,342 | 77.3 | / | BP was associated with higher odds of necrotizing fasciitis, adjusted OR (95% CI) 2.91 (1.25–6.80), p = 0.0136 | Rheumatoid arthritis Systemic lupus erythematosus Diabetes Cushing’s Cancer |
Older age Higher number of chronic condition Diabetes Cushing’s Cancer |
| Chen | 2020 [41] | Retrospective | 252 | 67.2 | Corticosteroids 74.6% Other immunosuppressants 52.0% IVIG 3.6% |
40 SSTI’s | Diabetes, Mucosal involvement Respiratory comorbidities |
Maximal control dose of corticosteroids, Low serum albumin levels, Hospitalization, Diabetes |
TCP—topical clobetasol propionate, CS—corticosteroids, MTX—methotrexate, MMF—Mofetil mycophenolate, NF—necrotizing fasciitis, SSTI—skin and soft tissue infection, IMA—immunomodulatory agents, CCIS—Charlson Comorbidity Index Score, IVIG—intravenous immunoglobulins.
Two stages of the systematic review were conducted by two teams independently. The first team searched selected terms by title and abstract. Studies not reporting primary data were excluded; however, references were analyzed for additional eligible original studies.
Abstracts evaluated in the initial screen included in this systematic review had BP and NF or SSTI jointly. Abstracts meeting these criteria were eligible for full-text review by the second team. Duplicate studies and those without retrievable full texts were excluded. Regardless of the study design, all full-text studies meeting the inclusion criteria were included. The following information was collected: author, publication year, study design, reporting method, demographic features, therapy, data regarding SSTI or NF events, and risk factors.
During all stages of the screening and data extraction, team disagreements were resolved through consensus and discussion. All conflicts regarding the data entry process were resolved by senior or corresponding authors.
The flow chart of data collection and extraction is presented in Figure 1.
Figure 1.
The flow chart of data collection and extraction.
3. Results
In our search of BP complicated by NF, six case reports were included with a total of 10 patients, presented in Table 1 [29,30,31,32,33,34,35]. Patients aged from 51–86 years old, with a mean age of 71.5 years. 7/10 patients had a fatal outcome. At the time of NF diagnosis, all patients had incomplete control of the disease. The most common comorbidities described were diabetes mellitus, hypertension, and obesity. Most frequently isolated pathogens were Streptococcus group A (7/10), Methicillin-resistant Staphylococcus aureus (MRSA) (2/10), and Acinetobacter spp. (2/10) with Group A Streptococcus as a monomicrobial source of infections in most cases.
In cases of diseases with such small incidences, a possible publication bias of case reports could impede the gathering of important clinical events necessary for further investigation. Case reports are frequently discredited as low-evidence reports with a high risk of reporting bias and are rarely published due to their low citability. Still, rare diseases case reports could present the very beginning of high-impact scientific findings such as the correlation of Kaposi sarcoma and HIV in 1981, as well as a more recent finding regarding therapy of infantile hemangiomas with systemic propranolol, both of which began as case reports [42,43].
In the recent literature, 25 studies were found regarding the assessment of infection risk and/or specific causes of death. Studies not providing any information on skin and soft tissue infections (SSTIs) were excluded. The selected studies show conflicting data regarding skin and soft tissue infections, which are shown in Table 2. Most studies addressing specific causes of death in AIBD patients found infections to be the most frequent cause of death while skin and soft tissue infections are found to affect virtually all BP patients [37,38,39,40,41,44,45]. A small retrospective monocentric study by Boughrara et al. investigating infection in patients treated with topical clobetasol propionate (TCP) found that 30% of all participants had any SSTI, with three events of necrotizing fasciitis: one patient with a fatal NF on combination therapy of TCP and mofetil mycophenolate and one patient with fatal NF on combination therapy of TCP and methotrexate. One fatal outcome of NF was reported in a patient on TCP therapy exclusively [30]. Another retrospective study from Mayo Clinic found that 72% of patients studied developed at least one SSTI, and 82% of the cohort developed at least one systemic infection [41]. Although valued for their detailed data regarding infection, the limitations of these studies are their retrospective nature and small patient sample sizes. A study by Cai et al. found skin and soft tissue infections to be the third most common specific cause of death, while Phoon et al. registered only seven events of skin and soft tissue infections [38,46]. Chen et al. found cutaneous infections were one of the two most common complications with 40 SSTIs recorded [41]. A large retrospective study by Ren et al. including a total of 13,342 participants with primary and secondary bullous pemphigoid diagnoses is the only study that found a statistically significant correlation between bullous pemphigoid and diagnosis of necrotizing fasciitis [40]. Considering their incidences, both BP and necrotizing fasciitis have been placed among rare diseases, leaving only a paucity of clinical data for proper investigation and correlation. The large patient sample size of this retrospective study is most likely the contributing factor to eliciting these results. Limitations of all studies are their retrospective nature, bias due to possible differences in hospitalization criteria, and the use of data derived from registers such as death registers and International Classification of Diseases codes in some studies. Skin and soft tissue infections (SSTI) are most commonly divided into two groups: (1) uncomplicated, which affect the superficial layers of the skin and underlying soft tissue, and (2) complicated, necrotizing infections affecting deeper tissues, with systemic effects [26]. Another major factor contributing to reporting bias is clustering infections in one group, such as SSTI. Due to the different severity and outcome of different infections in the same group, this could be potentially misleading, underestimating the prevalence of necrotizing fasciitis in these patients. Not defining the source or the microorganisms in septic patients could also be contributing to reporting bias.
4. Discussion
To the best of our knowledge, this is the first systematic review of NF in BP patients.
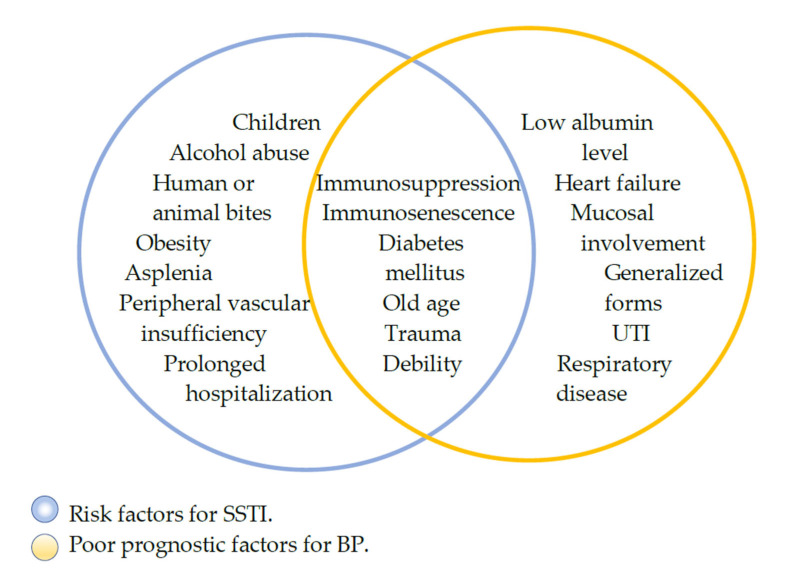
Given the disruption of the skin barrier combined with corticosteroid therapy, skin and soft tissue infections are one of the most common complications in these individuals, with impetigo, erysipelas, and cellulitis occurring most frequently [37]. If misdiagnosed or mismanaged, these infections can evolve into complicated, necrotizing infections [25,26]. Complicated, necrotizing soft tissue infections (NSTI) such as necrotizing fasciitis or Fournier’s gangrene commonly cause a systemic inflammatory response or sepsis and tend to rapidly spread into surrounding healthy tissues. Destructive, invasive, and especially fulminant in immunocompromised individuals, they can be fatal with the outcome mainly depending on timely diagnosis and management [47]. Previous studies of other autoimmune diseases such as rheumatoid arthritis, systemic lupus, and scleroderma have proven the major role immunosuppression plays in necrotizing fasciitis, but given the disruption of the skin as the main characteristic of BP and other chronic skin blistering diseases, this BP manifestation as well as comorbidities found in BP patients in the studies included in Table 1 and Table 2, such as diabetes mellitus, chronic renal disease, stroke, Parkinson’s disease, and dementia, could also be major contributing risk factors for NF [48,49,50,51]. Shared risk factors for poor outcomes in patients with bullous pemphigoid, the most common of the autoimmune bullous diseases, and necrotizing fasciitis are shown in Figure 2.
Figure 2.
Diagram of shared risk factors for NF and poor outcomes of BP.
4.1. Immunosuppression
Immunosuppression is an important risk factor for necrotizing skin and soft tissue infections and a contributing risk factor for lethal outcomes in BP patients [52]. The risk of infection in patients on systemic immunosuppressant therapy has been immensely researched, mostly following the development of transplant surgery and oncology [53]. Even though significant effort has been made in establishing new therapeutic regimens, systemic corticosteroids remain a cornerstone in BP therapy. They affect both innate and acquired immunity with their effects proven to be dose-dependent; thus, posing the risk of infection in a dose-dependent fashion [54,55]. One retrospective multicentric study by Rzany et al. found prednisolone doses greater than 37 mg/d at discharge a significant risk factor for lethal outcomes in BP patients. Chen et al. also found maximal control dose of corticosteroids a significant contributing factor to skin and soft tissue infection complications, while the same was found by Ren et al. for Cushing’s syndrome due to excessive use of corticosteroids as well as other immunocompromising diseases such as cancer [41,45,56]. A therapeutic milestone study from 2002 by Joly et al. showed that in patients with moderate to extensive disease topical 0.5% clobetasol propionate (TCP) yielded similar therapeutic results as systemic corticosteroids with longer overall survival in the topical corticosteroid group, and minimal to nonsystemic effects [36]. In this randomized controlled trial, in the oral corticosteroid group, one event of necrotizing cellulitis was reported. Concerning treatment, Lehman et al. found that all patients taking oral corticosteroids had a local or systemic infection (100%), while patients on other therapeutic regimens, though still frequently, were less likely to develop any of these infections (88%) [30]. In cases of bullous pemphigoid complicated by necrotizing fasciitis presented in Table 1, 9/10 patients received corticosteroid treatment with one patient’s medical history undisclosed. 5/10 of those patients received combined treatment while 4/10 received corticosteroids exclusively. Moreover, in most cases, the NSTI complication arose in the first month following initiation of BP treatment. Other cytotoxic antineoplastic agents are frequently used in BP therapy such as azathioprine, mofetil mycophenolate, and methotrexate. Working by myelosuppression, inhibition of B and T cell proliferation, and inhibition of antibody production, they are also known to be related to increased risk of infections in cancer patient studies [57]. In recent years, biologic therapy of skin blistering disease patients with rituximab has shown promising results [58,59]. Even though at a lower rate, in these studies infectious complications remained the most frequent adverse event. Necrotizing fasciitis has been a described complication in studies assessing the safety and effectiveness of rituximab therapy in systemic lupus erythematosus as well as in additional case series in recent years [48,49,50]. Keeping that in mind could be of significant importance for clinicians treating these patients while proper data collection and separation of necrotizing fasciitis from other skin and soft tissue infectious complications is deemed necessary for investigators in further studies.
4.2. Comorbidities
Diabetes mellitus is the most common underlying condition in necrotizing fasciitis, reported in 44.5% of patients in some studies, and the most frequent comorbidity in patients presented in Table 1 [60]. While in most studies in Table 2 diabetes is one of the most frequent comorbidities found in bullous pemphigoid patients, its influence as a risk factor could be underestimated. Diabetes is one of the most common side effects of corticosteroid therapy with hyperglycemia known to lead to poor wound healing on a cellular, metabolic, and biochemical level [61]. One study found that 40% of all inpatient endocrinology consultations in their hospital are new-onset diabetes cases induced by corticosteroid therapy [62]. This imposes the question of whether this corticosteroid treatment adverse effect is included in the diabetic patient’s group or whether the patients described in BP studies were diagnosed with diabetes prior to hospitalization for bullous pemphigoid, contributing to a certain level of reporting bias. A proper description of inclusion and exclusion criteria for these patients is imperative in assessing the impact of diabetes on bullous pemphigoid patients which would contribute to the development of better NF prevention strategies. Diabetes is a risk factor for uncomplicated skin and soft tissue infections leading to additional subsequent poor wound healing, as well as necrotizing skin and soft tissue infections with a potentially life-threatening outcome [26,63]. Another shared factor for necrotizing soft tissue infections in autoimmune bullous disorders could be neurologic disorders, primarily Parkinson’s disease, dementia, and stroke which would subsequently render patients in need of care and assistance due to debility [38,39,45,46,64]. In these patients, decubitus ulcers or perineal abscesses combined with paraplegia could lead to Fournier’s gangrene or necrotizing fasciitis of the lower abdominal wall [65].
4.3. Necrotizing Fasciitis in Immunocompromised Patients
In more recent literature, mortality rates of NF have been reported to be 8.7 to 76% [66]. A study assessing immunocompromised status in necrotizing fasciitis patients found that mortality was significantly higher in immunocompromised than in immunocompetent patients. Most importantly, the first surgical debridement and diagnosis were more often delayed in immunocompromised patients [52]. Urgent diagnosis and surgical debridement are imperative for patient survival. Impediment in treatment is found to increase the chances of fatal outcome 7.5 times in immunocompromised patients, while another study found that mortality rates were 9 times higher if surgical debridement was delayed more than 24 h after the onset of symptoms [67,68]. Necrotizing fasciitis type I is a polymicrobial infection usually described following trauma, caused by aerobe and anaerobe bacteria, most commonly associated with immunocompromised individuals as well as diabetic patients. According to available data, Staphylococcus aureus, β-hemolytic Streptococcus, coagulase-negative Staphylococcus, Escherichia coli, Enterobacter spp, Atropobium parvulum, Morganella morganii, and Klebsiella pneumoniae are the most often isolated bacteria in polymicrobial NF [69]. Type II infection is a monomicrobial infection often developing spontaneously, frequently caused by Streptococcus group A (GAS) and Methicillin-resistant Staphylococcus aureus. The source of infection in these patients is found to be asymptomatic pharyngitis, by systemically seeding as a transient bacteremia [26]. Some studies found that NF caused by gram-negative bacteria was associated with a higher incidence of bloodstream infections and the presence of hemorrhagic bullae, as well as a more rapid disease progression leading to a fatal outcome compared to NF caused by gram-positive bacteria [69].
The most important clinical symptom of necrotizing fasciitis is crescendo pain disproportionate to local findings, while other most common symptoms include local redness and tenderness, soft-tissue edema, and fever [26,65]. The presence of hemorrhagic bullae with bacteremia, purpura, and skin necrosis often follows as signs of advanced disease. Tachycardia, leukocytosis, acidosis, or hyperglycemia combined with the aforementioned local status and the presence of described risk factors should raise high suspicion of necrotizing fasciitis [26,69]. Still, necrotizing fasciitis was found to be misdiagnosed in a mean of 71.4% of 1463 patients by Goh et al. [60]. Several scoring systems have been developed as a diagnostic aide, such as the laboratory risk indicator for necrotizing fasciitis (LRINEC) score based on the following laboratory parameters: white blood cell count, hemoglobin, glucose, creatinine, sodium, and c-reactive protein [70,71]. A major difficulty in early diagnosis of necrotizing fasciitis in immunocompromised individuals is often its altered clinical presentation. Initial infection in these patients can seem subtle, often quickly taking a fulminant course. Due to myelosuppression, laboratory findings can show low white blood cell count, hematocrit, and platelet count. C-reactive protein can present as normal or mildly elevated; thus, compromising the results of LRINEC scoring [52,71]. In hospitalized patients, the use of NSAIDs as antipyretics or analgesics could attenuate pain, further diminishing another clinically important sign. Imaging tests such as MRI or CT scan have proven superior to X-ray, showing edema, fascial thickening, fat stranding, and/or gas in the subcutaneous tissues. Still, surgical debridement should always be given priority with an aggressive antimicrobial therapy covering gram-positive, gram-negative, and anaerobic bacteria administered empirically upon admission following suspicion of necrotizing fasciitis. These patients should be urgently managed by a surgical team experienced in the treatment of immunocompromised patients [25].
4.4. Surgical Reconstruction of Defects after Management of NF
Reconstruction of defects after necrotizing fasciitis management depends on the size of the defect, localization, the patient’s age, comorbidities, and the availability of healthy tissue. Defects of the abdominal wall were successfully reconstructed by abdominoplasty-type advancement flaps in individual reports, while reports of necrotizing fasciitis affecting the thoracic wall showed successful reconstruction of defects using a meshed bilaminar dermal regeneration template covered with a thin split-thickness skin graft in a patient with scleroderma [51,72]. One systematic review regarding flap coverage after NSTIs found that fasciocutaneous flaps were used most frequently (71%), followed by loco-regional muscle flaps (18%), while free flaps were used less frequently (8%), with rates of partial or complete flap loss of 3.3% [73]. Such reconstructions were performed in patients not eligible for skin grafting, while skin grafting reports are found as individual successful cases in the literature [68,74]. Still, various reports of skin grafting-induced eruptions of bullous pemphigoid on both skin graft and donor sites could be limiting factors for reconstruction of defects in these patients [75,76,77,78,79]. Additionally, in patients with >10 bullae per day or with the Bullous Pemphigoid Disease Area Index (BPDAI) severity score of ≥20 points equaling to a moderate or severe form of BP, in whom remission is not achieved, surgical reconstruction could be limited due to the insufficient healthy skin and soft tissue for flaps or donor sites. Prompt diagnosis and treatment in these patients is imperative for containing the infection from further tissue damage resulting in larger defects.
4.5. Intravenous Immunoglobulin (IVIG)
A small prospective study from 2001 showed all patients with severe BP, affecting 65% to 80% TBSA, obtained and sustained clinical remission receiving IVIG as monotherapy. IVIG was found to have corticosteroid-sparing effects, with no severe side effects [80]. Another more recent randomized double-blind trial found IVIG to reduce disease activity both clinically as well as by lowering anti-BP antibody titers [81]. Conversely, trials regarding IVIG therapy in NF patients showed conflicting results. One randomized control trial of NF patients showed promising results but was prematurely terminated due to slow patient recruitment, while another study found that IVIG was administered to NF patients too infrequently for any statistical significance [82]. Nevertheless, a meta-analysis regarding GAS-associated toxic shock syndrome found a reduction of mortality rates in IVIG-receiving patients after pooling data from studies that previously found no statistical significance independently [83].
The mechanism of action of IVIG is still unclear. Some studies of BP patients suggest intravenous immunoglobulins have a significant role in degrading circulating auto-antibodies such as anti–BP180, anti-Dsg1, and anti-Dsg3 IgG, as well as modulating functions of B and T cells and attenuating complement-mediated tissue damage. Whereas in vitro studies regarding IVIG therapy in sepsis found that IVIG preparations hold opsonins and neutralizing antibodies against staphylococcal and streptococcal superantigens and M-protein. So far, group A Streptococcal NF is the only etiology of NF with a clear indication for IVIG [18,81,84,85].
The significance of IVIG in NF patients is still unclear and relies only on a few studies and individual reports in the literature [86,87]. Treatment of NF patients on long-term corticosteroid therapy presents a challenge and tapering or discontinuation of immunosuppressive agents is often necessary. With this in mind, the use of IVIG in critically ill BP patients with NF caused by GAS or Staphylococcus could be justified, combined with aggressive surgical treatment and adequate antimicrobial therapy.
Side effects of IVIG therapy such as fever, nausea, headache, or malaise are found in about 10% of patients, while severe side effects such as pulmonary edema, thromboembolism, renal failure, and severe anaphylactic reactions are infrequent and are usually observed in patients with IgA deficiency [85]. Regardless of its seemingly many benefits, the use of IVIG is still limited due to its high cost and low availability.
Currently, ongoing studies are conducted in order to provide safe and effective target therapy for BP patients. Possible targets include CD20+ lymphocytes and interleukin inhibitors as well as inhibitors of the complement system [7]. A fully human monoclonal antibody dupilumab binds to IL-4 receptor and provides significant symptom control, especially in immunocompromised patients such as neoplastic and TBC-infected patients [7,87]. Eosinophils are found to play a crucial role in the pathogenesis of BP, with a positive correlation found between high IgE titers and TBSA affected by BP. Omalizumab, a monoclonal antibody that binds to the Cε3 domain of IgE, as well as bertilimumabin, a monoclonal antibody that targets eotaxin-1, shows promising results [7,88,89].
Many protocols have been developed for the management of infections in cancer or transplant patients, but possibly due to low incidences of bullous dermatoses, no protocols have been designed especially for these individuals. Studies of other autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis have confirmed that necrotizing fasciitis affects patients on long-term corticosteroid therapy [67,90]. So far, strong evidence correlating BP patients with necrotizing infections relies only on one large study and many other individual reports [45]. Given how timely diagnosis majorly influences survival, larger studies with detailed criteria for defining infections, as well as diabetes and other of the many sharing risk factors are necessary in assessing the correlation between BP and necrotizing fasciitis. Until a clearer picture is drawn, signs of erythema, swelling, crescendo pain, and local warmth together with changes in laboratory parameters should always raise a high suspicion of necrotizing fasciitis in BP patients.
5. Conclusions
Loss of skin integrity, immunosuppressive therapy, and comorbidities commonly related to BP patients are risk factors for necrotizing fasciitis. Evidence of their significant correlation is emerging, and further studies are deemed necessary for the development of BP-specific diagnostic and treatment protocols. Signs of erythema, swelling, crescendo pain and local warmth along with changes in laboratory parameters should always raise a high suspicion of necrotizing fasciitis in BP patients.
Author Contributions
All authors contributed to the study conception and design. M.S. and M.J. (Milana Jurišić) are in charge of the main idea and are the guarantors of integrity of the entire study; M.J. (Milana Jurišić), M.M., M.K., J.J., M.J. (Marko Jović), M.J. (Mladen Jovanović), M.J. (Milan Jovanović) and A.V. are in charge of the study concepts, design, manuscript preparation and editing. Material preparation, analysis and editing were performed by M.S., M.J. (Milan Jovanović), M.J. (Milana Jurišić), M.M., M.K., N.J., K.R. and D.V. All authors commented on previous versions of the manuscript. All authors declare that the details of any figure can be published. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Langan S.M., Smeeth L., Hubbard R., Fleming K.M., Smith C.J.P., West J. Bullous pemphigoid and pemphigus vulgaris--incidence and mortality in the UK: Population based cohort study. BMJ. 2008;337:a180. doi: 10.1136/bmj.a180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Milinković M., Janković S., Medenica L., Nikolić M., Reljić V., Popadić S. Incidence of autoimmune bullous diseases in Serbia: A 20-year retrospective study. J. Dtsch. Dermatol. Ges. 2016;14:995–1005. doi: 10.1111/ddg.13081. [DOI] [PubMed] [Google Scholar]
- 3.Joly P., Baricault S., Sparsa A., Bernard P., Bédane C., Duvert-Lehembre S., Courville P., Bravard P., Rémond B., Doffoel-Hantz V., et al. Incidence and Mortality of Bullous Pemphigoid in France. J. Investig. Dermatol. 2012;132:1998–2004. doi: 10.1038/jid.2012.35. [DOI] [PubMed] [Google Scholar]
- 4.Bertram F., Brocker E.B., Zillikens D., Schmidt E. Prospective analysis of the incidence of autoimmune bullous disorders in Lower Franconia, Germany. J. Dtsch. Dermatol. Ges. 2009;7:434–439. doi: 10.1111/j.1610-0387.2008.06976.x. [DOI] [PubMed] [Google Scholar]
- 5.Marazza G., Pham H., Schärer L., Pedrazzetti P., Hunziker T., Trüeb R., Hohl D., Itin P., Lautenschlager S., Naldi L., et al. Incidence of bullous pemphigoid and pemphigus in Switzerland: A 2-year prospective study. Br. J. Dermatol. 2009;161:861–868. doi: 10.1111/j.1365-2133.2009.09300.x. [DOI] [PubMed] [Google Scholar]
- 6.Hammers C.M., Stanley J.R. Mechanisms of Disease: Pemphigus and Bullous Pemphigoid. Annu. Rev. Pathol. 2016;11:175–197. doi: 10.1146/annurev-pathol-012615-044313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Agostino G.M., Rizzetto G., Marani A., Marasca S., Candelora M., Gambini D., Gioacchini H., De Simoni E., Maurizi A., Campanati A., et al. Bullous pemphygoid and novel therapeutic approaches. Biomedicines. 2022;10:2844. doi: 10.3390/biomedicines10112844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Persson M.S.M., Begum N., Grainge M.J., Harman K.E., Grindlay D., Gran S. The global incidence of bullous pemphigoid: A systematic review and meta-analysis. Br. J. Dermatol. 2022;186:414–425. doi: 10.1111/bjd.20743. [DOI] [PubMed] [Google Scholar]
- 9.Fang H., Shen S., Zheng X., Dang E., Zhang J., Shao S., Qiao P., Li Q., Wang H., Li C., et al. Association of HLA class I and class II alleles with bullous pemphigoid in Chinese Hans. J. Dermatol. Sci. 2018;89:258–262. doi: 10.1016/j.jdermsci.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Stavropoulos P.G., Soura E., Antoniou C. Drug-induced pemphigoid: A review of the literature. J. Eur. Acad. Dermatol. Venereol. 2014;8:1133–1140. doi: 10.1111/jdv.12366. [DOI] [PubMed] [Google Scholar]
- 11.Dănescu S., Chiorean R., Macovei V., Sitaru C., Baican A. Role of physical factors in the pathogenesis of bullous pemphigoid: Case report series and a comprehensive review of the published work. J. Dermatol. 2016;43:134–140. doi: 10.1111/1346-8138.13031. [DOI] [PubMed] [Google Scholar]
- 12.Yang M., Wu H., Zhao M., Chang C., Lu Q. The pathogenesis of bullous skin diseases. J. Transl. Autoimmun. 2019;2:100014. doi: 10.1016/j.jtauto.2019.100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moro F., Fania L., Sinagra J.L.M., Salemme A., Di Zenzo G. Bullous pemphigoid: Trigger and predisposing factors. Biomolecules. 2020;10:1432. doi: 10.3390/biom10101432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan M.A.B., Hashim M.J., King J.K., Govender R.D., Mustafa H., Al Kaabi J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health. 2020;10:107–111. doi: 10.2991/jegh.k.191028.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee S.G., Lee H.J., Yoon M.S., Kim D.H. Association of Dipeptidyl Peptidase 4 Inhibitor Use with Risk of Bullous Pemphigoid in Patients with Diabetes. JAMA Dermatol. 2019;155:172–177. doi: 10.1001/jamadermatol.2018.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomayko M.M., Damsky W., Fathy R., McMahon D.E., Turner N., Valentin M.N., Rallis T., Aivaz O., Fox L.P., Freeman E.E. Subepidermal blistering eruptions, including bullous pemphigoid, following COVID-19 vaccination. J. Allergy Clin. Immunol. 2021;148:750–751. doi: 10.1016/j.jaci.2021.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMahon D.E., Amerson E., Rosenbach M., Lipoff J.B., Moustafa D., Tyagi A., Desai S.R., French L.E., Lim H.W., Thiers B.H., et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: A registry-based study of 414 cases. J. Am. Acad. Dermatol. 2021;85:46–55. doi: 10.1016/j.jaad.2021.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu K.Y., Yu H.S., Yu S. Current and innovated managements for autoimmune bullous skin disorders: An overview. J. Clin. Med. 2022;11:528. doi: 10.3390/jcm11123528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaul R., McGeer A., Low D.E., Green K., Schwartz B., Simor A.E. Population-Based Surveillance for Group a Streptococcal Necrotizing Fasciitis: Clinical Features, Prognostic Indicators, and Microbiologic Analysis of Seventy-Seven Cases. Am. J. Med. 1997;103:18–24. doi: 10.1016/S0002-9343(97)00160-5. [DOI] [PubMed] [Google Scholar]
- 20.Tantraworasin A., Khamnuan P., Chongruksut W., Jearwattanakanok K., Patumanond J. Necrotizing fasciitis: Epidemiology and clinical predictors for amputation. Int. J. Gen. Med. 2015;14:195. doi: 10.2147/IJGM.S82999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sablone S., Lagouvardou E., Cazzato G., Carravetta F., Maselli R., Merlanti F. Necrotizing fasciitis of the thigh as unusual colonoscopic polypectomy complication: Review of the literature with case presentation. Medicina. 2022;58:131. doi: 10.3390/medicina58010131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puvanendran R., Huey J.C.M., Pasupathy S. Necrotizing fasciitis. Can. Fam. Physician. 2009;55:981–987. [PMC free article] [PubMed] [Google Scholar]
- 23.Wong C.H., Chang H.C., Pasupathy S., Khin L.W., Tan J.L., Low C.O. Necrotizing fasciitis: Clinical presentation, microbiology, and determinants of mortality. J. Bone Jt. Surg. Am. 2003;85:1454–1460. doi: 10.2106/00004623-200308000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Stevens D.L., Bryant A.E., Goldstein E.J. Necrotizing Soft Tissue Infections. Infect. Dis. Clin. North Am. 2021;35:135–155. doi: 10.1016/j.idc.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Stevens D.L., Bisno A.L., Chambers H.F., Dellinger E.P., Goldstein E.J.C., Gorbach S.L., Hirschmann J.V., Kaplan S.L., Montoya J.G., Wade J.C., et al. Practice Guidelines for the Diagnosis and Management of Skin and Soft Tissue Infections: 2014 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2014;59:e10–e52. doi: 10.1093/cid/ciu296. [DOI] [PubMed] [Google Scholar]
- 26.Stevens D.L., Bryant A.E. Necrotizing Soft-Tissue Infections. N. Engl. J. Med. 2017;377:2253–2265. doi: 10.1056/NEJMra1600673. [DOI] [PubMed] [Google Scholar]
- 27.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aromataris E., Munn Z. JBI Manual for Evidence Synthesis. 2020. [(accessed on 5 September 2022)]. Available online: https://synthesismanual.jbi.global. [DOI] [Google Scholar]
- 29.Chamberlain A.J., Wojnarowska F. Bullous pemphigoid complicated by nonfatal necrotizing fasciitis. Clin. Exp. Dermatol. 2003;28:448–449. doi: 10.1046/j.1365-2230.2003.01285_2.x. [DOI] [PubMed] [Google Scholar]
- 30.Boughrara Z., Ingen-Housz-Oro S., Legrand P., Duong T.A., Roujeau J.C. Cutaneous infections in bullous pemphigoid patients treated with topical corticosteroids. Ann. Dermatol. Venereol. 2010;137:345–351. doi: 10.1016/j.annder.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 31.Doffoel-Hantz V., Longueville C., Durox H., Bédane C., Sparsa A., Bonnetblanc J.M. Purpura rétiforme et pemphigoïde bulleuse: Une association fatale? Rev. Med. Interne. 2011;32:132–133. doi: 10.1016/j.revmed.2011.03.208. [DOI] [Google Scholar]
- 32.Ekiz Ö., Şen B.B., Rifaioğlu E.N., Özgür T., İnan M.U., Doğramacı Ç.A. Necrotizing fasciitis in a patient with bullous pemphigoid treating with systemic steroid. Cutan. Ocul. Toxicol. 2013;32:252–254. doi: 10.3109/15569527.2012.751391. [DOI] [PubMed] [Google Scholar]
- 33.Sené T., Regnier S., Robert N., Dupuy A., Morel P., Tancrede E., Rybojad M. Early necrotizing fasciitis following initiation of mycophenolate mofetil in two patients with bullous pemphigoid. Int. J. Dermatol. 2014;53:e236–e239. doi: 10.1111/ijd.12023. [DOI] [PubMed] [Google Scholar]
- 34.Noguchi E., Kamiya K., Maekawa T., Komine M., Murata S., Ohtsuki M. Bullous pemphigoid complicated by necrotising fasciitis successfully treated with systemic corticosteroids and antibiotics in combination with i.v. immunoglobulin. Australas J. Dermatol. 2018;59:e313–e314. doi: 10.1111/ajd.12859. [DOI] [PubMed] [Google Scholar]
- 35.Jurišić M., Nikolić G., Nikolić Živanović M., Stojičić M. Necrotizing fasciitis-a complication of autoimmune skin blistering diseases? Case report and literature review. J. Infect. Dev. Ctries. 2023;(in press) doi: 10.3855/jidc.17694. [DOI] [PubMed] [Google Scholar]
- 36.Joly P., Roujeau J.-C., Benichou J., Picard C., Dreno B., Delaporte E., Vaillant L., D'Incan M., Plantin P., Bedane C., et al. A Comparison of Oral and Topical Corticosteroids in Patients with Bullous Pemphigoid. N. Engl. J. Med. 2002;346:321–327. doi: 10.1056/NEJMoa011592. [DOI] [PubMed] [Google Scholar]
- 37.Lehman J.S., Khunger M., Lohse C.M. Infection in autoimmune bullous diseases: A retrospective comparative study. J. Dermatol. 2013;40:613–619. doi: 10.1111/1346-8138.12175. [DOI] [PubMed] [Google Scholar]
- 38.Cai S.C.S., Allen J.C., Lim Y.L., Chua S.H., Tan S.H., Tang M.B.Y. Mortality of bullous pemphigoid in Singapore: Risk factors and causes of death in 359 patients seen at the National Skin Centre. Br. J. Dermatol. 2014;170:1319–1326. doi: 10.1111/bjd.12806. [DOI] [PubMed] [Google Scholar]
- 39.Phoon Y.W., Fook-Chong S.M.C., Koh H.Y., Thirumoorthy T., Pang S.M., Lee H.Y. Infectious complications in bullous pemphigoid: An analysis of risk factors. J. Am. Acad. Dermatol. 2015;72:834–839. doi: 10.1016/j.jaad.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 40.Ren Z., Hsu D.Y., Brieva J., Silverberg N.B., Langan S.M., Silverberg J.I. Hospitalization, inpatient burden and comorbidities associated with bullous pemphigoid in the U.S.A. Br. J. Dermatol. 2017;176:87–99. doi: 10.1111/bjd.14821. [DOI] [PubMed] [Google Scholar]
- 41.Chen J., Mao X., Zhao W., Zhang B., Chen X., Yu C., Zheng Z., Jin H., Li L. Assessment of the Characteristics and Associated Factors of Infectious Complications in Bullous Pemphigoid. Front. Immunol. 2020;11:1607. doi: 10.3389/fimmu.2020.01607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gottlieb G.J., Ragaz A., Vogel J.V., Friedman-Kien A., Rywlin A.M., Weiner E.A., Ackerman A.B. A preliminary communication on extensively disseminated Kaposi’s sarcoma in young homosexual men. Am. J. Dermatopathol. 1981;3:111–114. doi: 10.1097/00000372-198100320-00002. [DOI] [PubMed] [Google Scholar]
- 43.Léauté-Labrèze C., de la Roque Dumas E., Hubiche T., Boralevi F. Propranolol for severe hemangiomas of infancy. N. Engl. J. Med. 2008;358:2649–2651. doi: 10.1056/NEJMc0708819. [DOI] [PubMed] [Google Scholar]
- 44.Gual A., Mascaró J.M., Rojas-Farreras S., Guilabert A., Julià M., Iranzo P. Mortality of bullous pemphigoid in the first year after diagnosis: A retrospective study in a Spanish medical centre. J. Eur. Acad. Dermatol. Venereol. 2014;28:500–506. doi: 10.1111/jdv.12065. [DOI] [PubMed] [Google Scholar]
- 45.Ren Z., Narla S., Hsu D.Y., Silverberg J.I. Association of serious infections with pemphigus and pemphigoid: Analysis of the Nationwide Inpatient Sample. J. Eur. Acad. Dermatol. Venereol. 2018;32:1768–1776. doi: 10.1111/jdv.14961. [DOI] [PubMed] [Google Scholar]
- 46.Barrick B.J., Lohse C.M., Lehman J.S. Specific causes of death in patients with bullous pemphigoid as measured by death certificate data: A retrospective cohort study. Int. J. Dermatol. 2015;54:56–61. doi: 10.1111/ijd.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramakrishnan K., Salinas R.C., Agudelo Higuita N.I. Skin and Soft Tissue Infections. Am. Fam. Physician. 2015;92:474–483. [PubMed] [Google Scholar]
- 48.Thatayatikom A., White A.J. Rituximab: A promising therapy in systemic lupus erythematosus. Autoimmun. Rev. 2006;5:18–24. doi: 10.1016/j.autrev.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 49.Looney R.J., Anolik J., Campbell D., Felgar R.E., Young F., Arend L.J., Sloand J.A., Rosenblatt J. B cell depletion as a novel treatment for systemic lupus erythematosus: A phase I/II dose-escalation trial of rituximab. Arthritis. Rheum. 2004;50:2580–2589. doi: 10.1002/art.20430. [DOI] [PubMed] [Google Scholar]
- 50.Abdulkareem A., D’Souza R.S., Shogbesan O., Donato A. A Case of Rituximab-Induced Necrotizing Fasciitis and a Review of the Literature. Case. Rep. Hematol. 2017;2017:1–5. doi: 10.1155/2017/6971027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rashid O.M., Nagahashi M., Takabe K. Management of massive soft tissue defects: The use of INTEGRA® artificial skin after necrotizing soft tissue infection of the chest. J. Thorac. Dis. 2012;4:331–335. doi: 10.3978/j.issn.2072-1439.2012.05.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keung E.Z., Liu X., Nuzhad A., Adams C., Ashley S.W., Askari R. Immunocompromised Status in Patients with Necrotizing Soft-Tissue Infection. JAMA Surg. 2013;148:419. doi: 10.1001/jamasurg.2013.173. [DOI] [PubMed] [Google Scholar]
- 53.Savin J.A., Noble W.C. Immunosuppression and skin infection. Br. J. Dermatol. 1975;93:115–120. doi: 10.1111/j.1365-2133.1975.tb06488.x. [DOI] [PubMed] [Google Scholar]
- 54.Ginzler E., Diamond H., Kaplan D., Weiner M., Schlesinger M., Seleznick M. Computer analysis of factors influencing frequency of infection in systemic lupus erythematosus. Arthritis Rheum. 1978;21:37–44. doi: 10.1002/art.1780210107. [DOI] [PubMed] [Google Scholar]
- 55.Stuck A.E., Minder C.E., Frey F.J. Risk of Infectious Complications in Patients Taking Glucocorticosteroids. Clin. Infect. Dis. 1989;11:954–963. doi: 10.1093/clinids/11.6.954. [DOI] [PubMed] [Google Scholar]
- 56.Rzany B., Partscht K., Jung M., Kippes W., Mecking D., Baima B., Prudlo C., Pawelczyk B., Messmer E.M., Schuhmann M., et al. Risk Factors for Lethal Outcome in Patients with Bullous Pemphigoid. Arch. Dermatol. 2002;138:903–908. doi: 10.1001/archderm.138.7.903. [DOI] [PubMed] [Google Scholar]
- 57.Dropulic L.K., Lederman H.M. Overview of Infections in the Immunocompromised Host. Microbiol. Spectr. 2016;4:3–50. doi: 10.1128/microbiolspec.DMIH2-0026-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Polansky M., Eisenstadt R., DeGrazia T., Zhao X., Liu Y., Feldman R. Rituximab therapy in patients with bullous pemphigoid: A retrospective study of 20 patients. J. Am. Acad. Dermatol. 2019;81:179–186. doi: 10.1016/j.jaad.2019.03.049. [DOI] [PubMed] [Google Scholar]
- 59.Lamberts A., Euverman H.I., Terra J.B., Jonkman M.F., Horváth B. Effectiveness and Safety of Rituximab in Recalcitrant Pemphigoid Diseases. Front. Immunol. 2018;19:9. doi: 10.3389/fimmu.2018.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goh T., Goh L.G., Ang C.H., Wong C.H. Early diagnosis of necrotizing fasciitis. Br. J. Surg. 2013;101:e119-25. doi: 10.1002/bjs.9371. [DOI] [PubMed] [Google Scholar]
- 61.Greenhalgh D.G. Wound healing and diabetes mellitus. Clin. Plast. Surg. 2003;30:37–45. doi: 10.1016/S0094-1298(02)00066-4. [DOI] [PubMed] [Google Scholar]
- 62.Donihi A.C., Raval D., Saul M., Korytkowski M.T., DeVita M.A. Prevalence and Predictors of Corticosteroid-Related Hyperglycemia in Hospitalized Patients. Endocr. Pract. 2006;12:358–362. doi: 10.4158/EP.12.4.358. [DOI] [PubMed] [Google Scholar]
- 63.Lipsky B.A., Tabak Y.P., Johannes R.S., Vo L., Hyde L., Weigelt J.A. Skin and soft tissue infections in hospitalised patients with diabetes: Culture isolates and risk factors associated with mortality, length of stay and cost. Diabetologia. 2010;53:914–923. doi: 10.1007/s00125-010-1672-5. [DOI] [PubMed] [Google Scholar]
- 64.Chen Y., Wu C., Lin M., Chen T., Liao K., Hwang C., Chu S., Chen C., Lee D., Chang Y., et al. Comorbidity profiles among patients with bullous pemphigoid: A nationwide population-based study. Br. J. Dermatol. 2011;165:593–599. doi: 10.1111/j.1365-2133.2011.10386.x. [DOI] [PubMed] [Google Scholar]
- 65.Misiakos E.P., Bagias G., Patapis P., Sotiropoulos D., Kanavidis P., Machairas A. Current concepts in the management of necrotizing fasciitis. Front. Surg. 2014;1:36. doi: 10.3389/fsurg.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goldstein E.J.C., Anaya D.A., Dellinger E.P. Necrotizing Soft-Tissue Infection: Diagnosis and Management. Clin. Infect. Dis. 2007;44:705–710. doi: 10.1086/511638. [DOI] [PubMed] [Google Scholar]
- 67.Mok M.Y., Wong S.Y., Chan T.M., Tang W.M., Wong W.S., Lau C.S. Necrotizing fasciitis in rheumatic diseases. Lupus. 2006;15:380–383. doi: 10.1191/0961203306lu2314cr. [DOI] [PubMed] [Google Scholar]
- 68.Roje Z., Roje Ž., Matić D., Librenjak D., Dokuzović S., Varvodić J. Necrotizing fasciitis: Literature review of contemporary strategies for diagnosing and management with three case reports: Torso, abdominal wall, upper and lower limbs. World J. Emerg. Surg. 2011;6:46. doi: 10.1186/1749-7922-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen H.-Y., Huang T.-Y., Chen J.-L., Kuo L.-T., Huang K.-C., Tsai Y.-H. Rational use of ceftriaxone in necrotizing fasciitis and mortality associated with bloodstream infection and hemorrhagic bullous lesions. Antibiotics. 2022;111:454. doi: 10.3390/antibiotics11111454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wong C.H., Khin L.W., Heng K.S., Tan K.C., Low C.O. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: A tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit. Care Med. 2004;32:1535–1541. doi: 10.1097/01.CCM.0000129486.35458.7D. [DOI] [PubMed] [Google Scholar]
- 71.Bechar J., Sepehripour S., Hardwicke J., Filobbos G. Laboratory risk indicator for necrotising fasciitis (LRINEC) score for the assessment of early necrotising fasciitis: A systematic review of the literature. Ann. R. Coll. Surg. Engl. 2017;99:341–346. doi: 10.1308/rcsann.2017.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brafa A., Grimaldi L., Brandi C., Nisi G., Calabrò M., Campa A., D’Aniello C. Abdominoplasty as a reconstructive surgical treatment of necrotising fasciitis of the abdominal wall. J. Plast. Reconstr. Aesthet. Surg. 2009;62:136–139. doi: 10.1016/j.bjps.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 73.Somasundaram J., Wallace D.L., Cartotto R., Rogers A.D. Flap coverage for necrotising soft tissue infections: A systematic review. Burns. 2021;47:1608–1620. doi: 10.1016/j.burns.2021.01.005. [DOI] [PubMed] [Google Scholar]
- 74.Tian Y., Liu T., Zhao Cq., Lei Y.-Z., Fan D.-L., Mao T-Ch. Negative pressure wound therapy and split thickness skin graft aided in the healing of extensive perineum necrotizing fasciitis without faecal diversion: A case report. BMC Surg. 2018;18:17. doi: 10.1186/s12893-018-0411-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Neri I., Antonucci V.A., Balestri R., Tengattini V., Iozzo I., Bardazzi F. Bullous pemphigoid appearing both on thermal burn scars and split-thickness skin graft donor sites. J. Dtsch. Dermatol. Ges. 2013;11:675–676. doi: 10.1111/ddg.12094. [DOI] [PubMed] [Google Scholar]
- 76.McGrath J., Black M. Split skin grafting and bullous pemphigoid. Clin. Exp. Dermatol. 1991;16:72–73. doi: 10.1111/j.1365-2230.1991.tb00306.x. [DOI] [PubMed] [Google Scholar]
- 77.Levin D.L., Sadhwani A. Blistering on a squamous cell carcinoma graft site in a patient with bullous pemphigoid. Cutis. 1994;54:40. [PubMed] [Google Scholar]
- 78.Ghura H.S., Johnston G.A., Milligan A. Development of a bullous pemphigoid after split-skin grafting. Br. J. Plast. Surg. 2001;54:447–449. doi: 10.1054/bjps.2001.3601. [DOI] [PubMed] [Google Scholar]
- 79.Hafejee A., Coulson I.H. Localized bullous pemphigoid 20 years after split skin grafting. Clin. Exp. Dermatol. 2005;30:187–188. doi: 10.1111/j.1365-2230.2004.01689.x. [DOI] [PubMed] [Google Scholar]
- 80.Razzaque A. Intravenous immunoglobulin therapy for patients with bullous pemphigoid unresponsive to conventional immunosuppressive treatment. J. Am. Acad. Dermatol. 2001;45:825–835. doi: 10.1067/mjd.2001.116337. [DOI] [PubMed] [Google Scholar]
- 81.Amagai M., Ikeda S., Hashimoto T., Mizuashi M., Fujisawa A., Ihn H., Matsuzaki Y., Ohtsuka M., Fujiwara H., Furuta J., et al. A randomized double-blind trial of intravenous immunoglobulin for bullous pemphigoid. J. Dermatol. Sci. 2017;85:77–84. doi: 10.1016/j.jdermsci.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 82.Darenberg J., Ihendyane N., Sjölin J., Aufwerber E., Haidl S., Follin P., Andersson J., Norrby-Teglund A. The Streptlg Study Group, Intravenous Immunoglobulin G Therapy in Streptococcal Toxic Shock Syndrome: A European Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Infect. Dis. 2003;37:333–340. doi: 10.1086/376630. [DOI] [PubMed] [Google Scholar]
- 83.Parks T., Wilson C., Curtis N., Norrby-Teglund A., Sriskandan S. Polyspecific Intravenous Immunoglobulin in Clindamycin-treated PAients With Streptococcal Toxic Shock Syndrome: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2018;67:1434–1436. doi: 10.1093/cid/ciy401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li N., Zhao M., Hilario-Vargas J., Prisayanh P., Warren S., Diaz L.A., Roopenian D.C., Liu Z. Complete FcRn dependence for intravenous Ig therapy in autoimmune skin blistering diseases. J. Clin. Investig. 2005;115:3440–3450. doi: 10.1172/JCI24394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hoffmann J.H.O., Enk A.H. High-dose intravenous immunoglobulin in skin autoimmune disease. Front. Immunol. 2019;10:1090. doi: 10.3389/fimmu.2019.01090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leo H.P., Volkmann E.R., Ohanian A.K. Necrotizing Soft Tissue Infection in a Patient with Bullous Systemic Lupus Erythematosus: Case Report. Ann. Arthritis Clin. Rheumatol. 2018;1:1002. [Google Scholar]
- 87.Berkani N., Joly P., Golinski M.-L., Colliou N., Lim A., Larbi A., Riou G., Caillot F., Bernard P., Bedane C., et al. B-cell depletion induces a shift in self antigen specific B-cell repertoire and cytokine pattern in patients with bullous pemphigoid. Sci. Rep. 2019;9:3525. doi: 10.1038/s41598-019-40203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kalowska M., Ciepiela O., Kowalewski C., Demkow U., Schwartz R.A., Wozniak K. Enzyme-linked immunoassay index for anti-NC16a IgG and IgE auto-antibodies correlates with severity and activity of bullous pemphigoid. Acta Derm. Venereol. 2016;96:191–196. doi: 10.2340/00015555-2101. [DOI] [PubMed] [Google Scholar]
- 89.Fiorino A.S., Baum S., Czernik A., Hall R., Zeeli T., Baniel A., Sinha A.A., Seiffert-Sinha K., Kolatch B., Zhang Z., et al. 570 Safety and efficacy of bertilimumab, a human anti-eotaxin-1 monoclonal antibody, in bullous pemphigoid in a phase 2a study. J. Investig. Dermatol. 2019;139:S98. doi: 10.1016/j.jid.2019.03.646. [DOI] [Google Scholar]
- 90.Tung C.Y., Isenberg D. Necrotising fasciitis in systemic lupus erythematosus: A case report and literature review. Lupus Sci. Med. 2014;1:e000008. doi: 10.1136/lupus-2014-000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.