Abstract
Pseudomonas aeruginosa is a Gram-negative opportunistic pathogen that causes high morbidity and mortality in cystic fibrosis (CF) and immunocompromised patients, including patients with ventilator-associated pneumonia (VAP), severely burned patients, and patients with surgical wounds. Due to the intrinsic and extrinsic antibiotic resistance mechanisms, the ability to produce several cell-associated and extracellular virulence factors, and the capacity to adapt to several environmental conditions, eradicating P. aeruginosa within infected patients is difficult. Pseudomonas aeruginosa is one of the six multi-drug-resistant pathogens (ESKAPE) considered by the World Health Organization (WHO) as an entire group for which the development of novel antibiotics is urgently needed. In the United States (US) and within the last several years, P. aeruginosa caused 27% of deaths and approximately USD 767 million annually in health-care costs. Several P. aeruginosa therapies, including new antimicrobial agents, derivatives of existing antibiotics, novel antimicrobial agents such as bacteriophages and their chelators, potential vaccines targeting specific virulence factors, and immunotherapies have been developed. Within the last 2–3 decades, the efficacy of these different treatments was tested in clinical and preclinical trials. Despite these trials, no P. aeruginosa treatment is currently approved or available. In this review, we examined several of these clinicals, specifically those designed to combat P. aeruginosa infections in CF patients, patients with P. aeruginosa VAP, and P. aeruginosa–infected burn patients.
Keywords: ventilator-associated pneumonia, clinical trials, vaccines, cystic fibrosis, chronic lung infection, antibiotics, immunotherapy, bacteriophages, Pseudomonas aeruginosa virulence factors, biofilms
1. Introduction
Pseudomonas aeruginosa is one of the most prevalent pathogens leading to acute and chronic infections in wounds, lung, and urinary tract [1,2]. Pseudomonas species were the third most common cause of Gram-negative infections and accounted for 4% of cases in a prospective analysis of the SCOPE (Surveillance and Control of Pathogens of Epidemiologic Importance) database of 24,179 hospital-acquired infections that occurred in 49 hospitals in the United States between 1995 and 2002 [3]. In recent years, there has been an increase in P. aeruginosa infection rates among hospitalized patients, particularly in hospital-acquired infections (Figure 1), lower respiratory tract infection (Figure 2), and bloodstream infection (Figure 3). Furthermore, the rise of multi-drug-resistant P. aeruginosa species poses a morbidity and mortality risk on a global scale [4]. The World Health Organization classifies P. aeruginosa–resistant bacteria as critical-priority microorganisms (WHO). In fact, the combined P. aeruginosa’s resistance, multifactorial pathogenicity, and capacity for overadaptation make it particularly challenging to eradicate from infected patients. This review focuses on new treatments for P. aeruginosa infections that are presently undergoing human testing and could be made available to patients. We organized the current review as follows: First, we described the three main types of P. aeruginosa infections (ventilator-associated pneumonia (VAP), cystic fibrosis (CF) infection, and burn wound infection) that include the epidemiology, pathophysiology, and clinical presentation; second, we then described the different P. aeruginosa virulence factors that play a critical role in the above-described infections; lastly, we examined the different treatments/vaccines/immunotherapies that were used or are currently used in clinical trials.
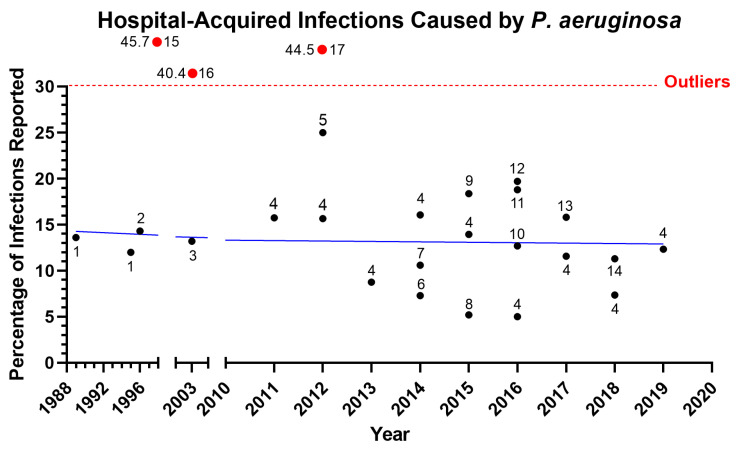
Figure 1.
Infection rates of P. aeruginosa in hospitalized patients over the past 3 decades (1989–2019). Hospital-acquired infections reported included bloodstream infections and catheter-associated bloodstream infections, catheter-associated urinary tract infection, surgical site infections and burn wound infections, and hospital-associated and ventilator-associated pneumonia; although not every article reported every category, all except two included three or more categories. The blue line is a regression line for the data excluding outliers, which are shown above the dotted red line. The numbers associated with the black circles indicate reference numbers; the numbers to the left of the red circles are the percentages, and those to the left are the reference numbers. References: 1. [5], 2. [6], 3. [7], 4. [8], 5. [9], 6. [10], 7. [11], 8. [12], 9. [13], 10. [14], 11. [15], 12. [16], 13. [17], 14. [18]. Outliers: 15. [19], 16. [20], 17. [21].
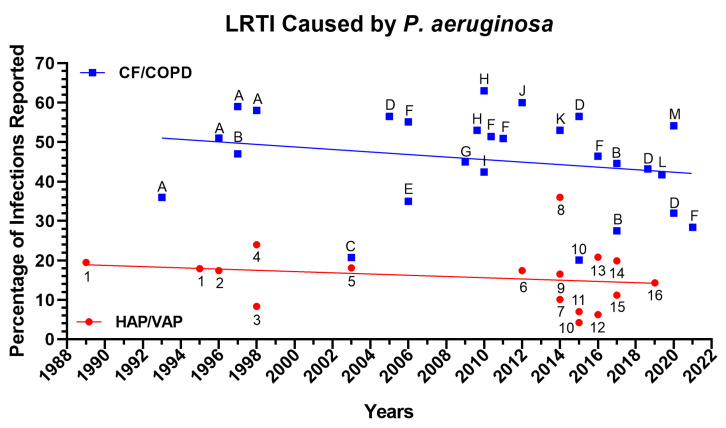
Figure 2.
Lower respiratory tract infection (LTRI) rates of P. aeruginosa. Reported rates of hospital-associated and ventilator-associated pneumonia (HAP/VAP) over the past 3 decades (1989–2019) and the regression line for the data are in red. The rates for patients with cystic fibrosis (CF) or chronic obstructive pulmonary disease (COPD) and the associated regression line are in blue. The numbers and letters associated with the red circles and blue squares, respectively, indicate the reference numbers. References for HAP/VAP: 1. [5], 2. [6], 3. [22], 4. [19], 5. [7], 6. [21], 7. [11], 8. [23], 9. [10], 10. [24], 11. [12], 12. [15], 13. [16], 14. [17], 15. [25], 16. [8]. References for CF/COPD: A. [26], B. [27], C. [28], D. [29], E. [30], F. [31], G. [32], H. [33], I. [34], J. [35], K. [36], L. [37], M. [38], 10. [24].
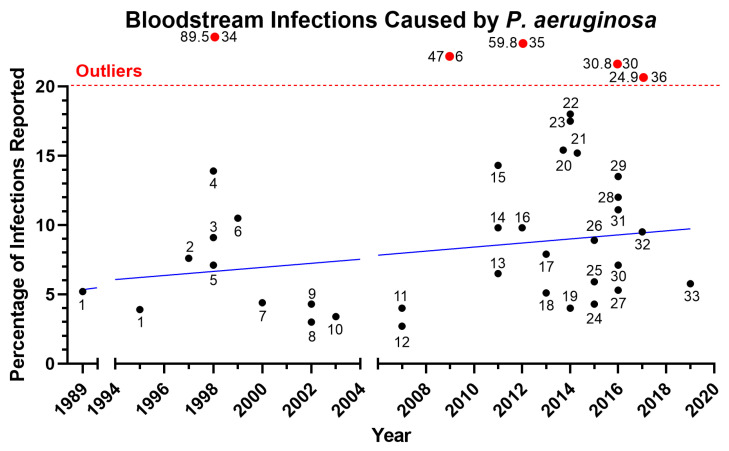
Figure 3.
Rates for bloodstream infections caused by P. aeruginosa in hospitalized patients over the past 3 decades (1989–2019). Hospital-acquired infections included bloodstream infections and catheter-associated bloodstream infections, catheter-associated urinary tract infection, surgical site infections and burn wound infections, and hospital-associated and ventilator-associated pneumonia; although not every article reported every category, most included at least three. Each circle represents the rate reported in the study, and the year corresponds to the last collection date of the data. The solid blue line represents the linear regression of the infection rate over the years excluding outliers, which are shown above the dotted red line. The numbers associated with the black circles indicate reference numbers; the numbers to the left of the red circles are the percentages, and those to the right are the reference numbers. References: 1. [5], 2. [39], 3. [22], 4. [19], 5. [40], 6. [41], 7. [42], 8. [43], 9. [3], 10. [7], 11. [44], 12. [45], 13. [46], 14. [47], 15. [48], 16. [49], 17. [50], 18. [51], 19. [10], 20. [52], 21. [11], 22. [53], 23. [54], 24. [55], 25. [56], 26. [57], 27. [58], 28. [59], 29. [60], 30. [61], 31. [16], 32. [17], 33. [8]. Outliers: 34. [62], 35. [21], 36. [63].
1.1. Ventilator-Associated Pneumonia due to P. aeruginosa Infection
Ventilator-associated pneumonia (VAP) is one of the most common infections reported in patients admitted to the intensive care units (ICUs). The most common infection identified is VAP in those with multi-drug-resistant organisms, particularly P. aeruginosa [64,65,66]. The biggest risk factors for P. aeruginosa patients with VAP include being placed on a mechanical ventilator longer than 5 days and previous antibiotic exposure [67,68,69]. Patients with chronic obstructive pulmonary disease (COPD) and other chronic respiratory disorders are at risk of developing a serious respiratory infection [67,68,69]. In intubated patients with a history of pneumonia as well as during the postoperative phase following lung transplantation, P. aeruginosa is the primary cause of pneumonia [70]. In addition, the most frequent pathogen isolated from individuals with health-care-associated pneumonia who needed ICU admission and mechanical ventilation is P. aeruginosa [71].
Despite clear risk factors for VAP, the treatment for VAP remains controversial. The original guidelines for treating VAP called for an antipseudomonal cephalosporin (cefepime, ceftazidime), carbapenem with antipseudomonal fluoroquinolone, or aminoglycoside [72]. However, the guidelines have changed with new clinical data indicating that this treatment regimen often used insufficient dosing, which led to increased morbidity, mortality, and recurrence of P. aeruginosa infections [72]. In various trials and meta-analyses, empiric combination therapy with a beta-lactam and an aminoglycoside was shown to be superior to monotherapy in the treatment of P. aeruginosa VAP, lowering mortality by up to 50% [73,74,75]. However, there is no difference between using one or two effective antibiotics; consequently, once microbiological data are obtained, monotherapy should be used instead of dual treatment [75]. The antibiotic of choice and the duration are also important considerations when treating P. aeruginosa VAP to prevent the emergence of multi-drug-resistant strains and reduce drug toxicity from prolonged antibiotic use [73,74,75]. With the rise in multi-drug-resistant strains, further research is being undertaken to address the need for safer and more effective antibiotics as well as vaccinations against P. aeruginosa VAP.
1.2. Cystic Fibrosis (CF) and Pneumonia Secondary to P. aeruginosa Infection
Cystic fibrosis (CF) is the most prevalent autosomal recessive genetic condition that is caused by a defect in the cystic fibrosis transmembrane conductance regulator (CFTR) protein and forms a chloride and bicarbonate channel [76,77,78]. The CFTR protein controls the ion transport and, ultimately, the hydration of the respiratory epithelial cells [79,80]. Mutations in CFTR, as seen in CF patients, cause dry and thick mucus discharges that affect multiple organ systems, including the hepatobiliary system, pancreas, gastrointestinal tract, and male reproductive tract [79,80]. The major cause of morbidity and death among CF patients is lung infection. Due to inadequate mucociliary clearance, the airways become vulnerable to bacterial and other infections, many of which develop into chronic and lifelong conditions [81]. With aging, the microbiological environment of CF lung tissue changes [81]. Younger pediatric patients are more likely to contract Haemophilus influenzae and Staphylococcus aureus, particularly methicillin-sensitive S. aureus and more recently methicillin-resistant strains [81]. Additional infections with built-in resistance to numerous antibiotics, such as Stenotrophomonas maltophilia, Achromobacter xylosoxidans, Burkholderia cepacia complex and nontuberculous mycobacteria, may predominate with advancing age or illness [82,83]. Pseudomonas aeruginosa often becomes the main bacteria cultured from the respiratory tract by adulthood. The incidence of P. aeruginosa infection in CF patients increases from 20% in individuals under the age of 5 to as high as 70% by the age of 18 [84,85]. Chronic lung infection with P. aeruginosa causes a rapid decline in pulmonary function that contributes to the death of many CF patients [84,85]. Thus, treatment of P. aeruginosa infection has become an integral part of CF care.
Pseudomonas aeruginosa isolates have unique characteristics that aid in their adaptability to and survival in the CF lung. The main one of these characteristics is the hypermutable genetic background, which leads to the production of P. aeruginosa species with distinct variations [86]. These variations include a mucoid phenotype, altered antigenic structures, and antibiotic resistance [86]. Extracellular alginate synthesis, which results in the mucoid phenotype, might lessen the efficacy of antibiotics by restricting drug penetration [86]. Additionally, available evidence suggests that P. aeruginosa forms biofilms in the lower respiratory system of P. aeruginosa infections in CF patients, making the organism immune to a variety of antibiotics [87,88]. High intrapulmonary drug concentrations can be achieved using aerosolized antibiotics with less systemic adverse effects. This treatment lessens inflammation and reduces the emergence of multi-drug-resistant P. aeruginosa density while maintaining lung function and reducing the frequency of acute pulmonary exacerbations [89,90]. Despite the wide variety of treatments for CF, a sizable portion of patients (25%) do not completely regain the lost lung function [91,92]. Therefore, there is a great need for more effective treatments to help reduce P. aeruginosa.
1.3. Pseudomonas aeruginosa Burn Wound Infections
The most frequent cause of morbidity and mortality in burn patients is still bacterial infection. Due to the numerous physiologic characteristics that make burn damage distinctive, the diagnosis and treatment of burn wound infections continues to be difficult [93]. Patients who experience a serious burn are at a high risk of having burn wound sepsis. Burn wound infection or sepsis is evident through an abrupt change in the burn wound’s appearance or the burn patient’s clinical status [93]. Several types of burn wound infection are classified based on the clinical characteristics and the level of invasion, which are assessed by analyzing the cultures and the histology of burn wound biopsies [93]. Although Staphylococcus and Pseudomonas are still the most common bacteria causing infections in burn wounds, the epidemiology of burn wound infections has evolved over time [93]. To properly treat burn wound infections, it is critical to be informed of the microbial flora and the antibiotic susceptibility in each burn unit and infected patient [93]. Depending on the kind of burn wound, a combination of wound cleaning, debridement, and topical or systemic antimicrobial medication is used to treat burn wound infection/sepsis [93].
In recent years, P. aeruginosa infections obtained from infected burn wounds were found to be resistant to several classes of antibiotics. For example, 20% of the P. aeruginosa isolates obtained from infected burn patients were resistant to meropenem, 76% were resistant to gentamicin and imipenem, and 89% were resistant to ticarcillin–clavulanate [94]. In another study, 71% of P. aeruginosa isolates were multidrug resistant [95]. In accordance with another study from a burn hospital in Sweden, 26% of P. aeruginosa isolates between 1994 and 2012 were carbapenem resistant [96]. Due to the emergence of multi-drug-resistant P. aeruginosa species, a combination of two antibiotics is recommended in burn patients with severe P. aeruginosa wound infections.
Several P. aeruginosa virulence factors dispersed into the environment or injected into host cells or other bacteria are responsible for P. aeruginosa’s pathogenesis [97]. These virulence factors alter or impair the signaling pathways of host cells, target the extracellular matrix, cause tissue damage, and allow P. aeruginosa to successfully compete with other pathogens and alter the local microbiota. P. aeruginosa virulence is combinatorial and multifaceted. Among the several well-characterized antibiotic resistance mechanisms in P. aeruginosa include the development of antibiotic-inactivating enzymes, intrinsic membrane permeability, drug efflux systems, loss of porin function, and drug efflux system [97]. The plasticity of the P. aeruginosa virulence factor gene expression, antibiotic resistance, and metabolism in response to selective pressure contributes to the ability of P. aeruginosa to shift its infection from acute to chronic. In the following section, we describe different P. aeruginosa cell–associated and extracellular virulence factors and their role in P. aeruginosa infections.
2. P. aeruginosa Pathogenesis and Virulence Factors
The ability of P. aeruginosa to successfully cause different types of infections is related to the bacteria’s large genome (5–7 megabases), which harbors a variety of virulence factors [98]. These virulence factors (i.e., type IV pili, flagella, exopolysaccharides (EPS), lipopolysaccharide (LPS), pyocyanin, siderophores, and secretion systems) serve specific functions in P. aeruginosa pathogenesis during infection. Many of these virulent factors are controlled by a complex regulatory network. This network is tightly regulated by a cell-to-cell communication system called quorum sensing (QS) [99]. P. aeruginosa has three well-studied QS systems, las, rhl, and pqs [100,101,102]. Type IV pili and flagella are important for P. aeruginosa in cell motility, adhesion, and colonization [103,104]. Different strains of P. aeruginosa produce three exopolysaccharides (EPS): Pel, Psl, and alginate. These proteins aid in cell adhesion and protect P. aeruginosa from the human immune cells [105,106]. Alginate is an overproduced EPS by mucoid strains of P. aeruginosa, which is a common characteristic of isolates from chronic lung infections [107,108]. LPS is a major part of the outer membrane of Gram-negative bacteria. It consists of three components: a membrane-anchored lipid A, a core oligosaccharide, and a highly variable O-antigen [109]. The components of LPS are necessary for cell motility, adhesion, and both eliciting and evading the immune system [110]. Among the secreted molecules, pyocyanin is a cytotoxic redox-active metabolite, while siderophores, such as pyoverdine, chelate iron for uptake into P. aeruginosa [111,112].
P. aeruginosa also has several secretion systems that are used to translocate proteins, known as effectors, to damage the host. Specifically, the type I secretion system of P. aeruginosa secretes a few virulence factors, such as an alkaline protease, that interfere with the activation of the complement system of the host immune system [113]. The type II secretion system secretes the largest number of proteins as virulence factors including elastases (LasB and LasA), protease IV, and exotoxin A [114]. LasB, LasA, and protease IV are important proteases in damaging host tissues [115,116]. LasB is an elastolytic metalloprotease associated with P. aeruginosa infections as an important virulence factor. This is due to its ability to degrade numerous constituents of the host tissue and immune system [117]. LasB has been also linked to biofilm formation [118,119]. LasA is a staphylolytic metallopeptidase with elastolytic activity [120]. It also enhances the elastolytic activity of LasB [121]. Protease IV is an important virulence factor because it alters immune response by degrading cytokines, such as IL-22 [122]. Exotoxin A is an adenosine diphosphate (ADP)–ribosylating toxin that inhibits the host elongation factor 2. This disrupts protein synthesis, leading to host cell death [123]. Exotoxin A also interferes with host immune response to P. aeruginosa infection [124]. In contrast with the type I and II secretion systems, P. aeruginosa uses the type III secretion system to translocate effectors, such as exoenzyme S (ExoS), exoenzyme T (ExoT), exoenzyme U (ExoU), and exoenzyme Y (ExoY), directly into the host cell [114,125]. ExoS inhibits phagocytosis and enhances the dissemination of P. aeruginosa during pneumonia [126,127]. ExoT is another effector that induces apoptosis in its target cell, while ExoU is another important virulence factor that is critical to P. aeruginosa pathogenesis during pneumonia [128,129]. In contrast, ExoY disrupts the endothelial barrier and enhances lung infiltration [130]. Lastly, the type VI secretion system is the most recently identified secretion system in P. aeruginosa that delivers several toxins that attack other pathogens as well as the host [131].
One major factor that contributes to the pathogenesis of P. aeruginosa within the bloodstream is its serum resistance. Some of the potentially involved cellular components in P. aeruginosa serum resistance include alterations in lipopolysaccharide components (e.g., O-antigen) and outer membrane proteins [132,133,134]. Several specific proteins were found to contribute to serum resistance, such as VacJ (involved in maintaining outer membrane lipid asymmetry), AmpD (beta-lactamase expression regulator), MexR (multidrug resistance operon repressor), OprD (outer membrane porin), HepP (a heparinase and a virulence factor), Wzz (necessary for LPS biosynthesis), and WaaL (necessary for LPS biosynthesis) [135,136,137,138]. Several of those identified virulence factors have been proposed as therapeutic targets against P. aeruginosa; however, further clinical research is needed to evaluate their efficacy. These identified virulence factors have been extensively studied in lab models prior to clinical trials.
3. Clinical Trials to Assess the Effectiveness of Anti-Pseudomonas aeruginosa Treatments
As it develops into a chronic stage, P. aeruginosa is extremely challenging to treat. Along with our understanding of the molecular processes that underlie P. aeruginosa, the different treatment algorithms and the list of potential targets for treatments or vaccine development are growing [139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186]. While many of the discoveries made in murine models have not yet been translated into clinical research, several clinical trials were conducted to investigate the translation of information learned from the murine models into treatment or prevention of human P. aeruginosa infections. Information on these clinical trials, discussed in more detail below, was obtained from the ClinicalTrials database [187]. We organized different clinical trials as follows: new antibiotics, bacteriophages, strategies targeting P. aeruginosa virulence (biofilm, quorum sensing, type III secretion system, and antimicrobial peptides), immunotherapy, outer membrane proteins as a vaccine, strategies targeting P. aeruginosa iron acquisition systems.
3.1. Antibiotics
3.1.1. New Antibiotics
Given the rise of P. aeruginosa multi-drug-resistant strains, there is an interest in developing new antimicrobial drugs using combinations of older medications or novel agents (Table 1A). A broad-spectrum combination antibiotic composed of tobramycin and fosfomycin (FTI) was investigated in a phase II trial to treat CF patients with persistent P. aeruginosa infections (NCT00794586) [188]. The inhaled FTI combination consisted of two antibiotics: tobramycin, an aminoglycoside antibiotic with a strong activity against Gram-negative pathogens, and fosfomycin, a broad-spectrum combination antibiotic with activity against both Gram-positive and Gram-negative bacteria [188]. Based on clinical studies that examined clinical isolates of P. aeruginosa, the concentration of tobramycin and fosfomycin together (4:1 (w/w) fosfomycin/tobramycin combination) was found to be the same or lower than the inhaled tobramycin alone [188,189]. For example, P. aeruginosa isolates from non-CF patients that were treated with the FTI combination versus tobramycin or fosfomycin had a minimum inhibitory concentration at 50% (MIC50) of 4 mg/dL (FTI), 1 mg/dL (tobramycin), and 32 mg/dL (fosfomycin) [189]. When the same experiment was repeated to determine the minimum inhibitory concentration at 90% (MIC90) for the FTI combination versus tobramycin or fosfomycin, the MIC90 for all three groups was the same [189]. A similar trend was observed for both the MIC50 and MIC90 when the FTI combination versus tobramycin or fosfomycin was tested against P. aeruginosa isolates from CF patients. The efficacy of the FTI combination and the tobramycin had similar efficacy, which suggests that the FTI combination may minimize long-term toxicity from repeated exposure to aminoglycosides such as tobramycin, which can cause nephrotoxicity and ototoxicity [188,189]. Therefore, a recent clinical trial assessed the safety and effectiveness of two dose combinations of fosfomycin/tobramycin (FTI) for inhalation in individuals with cystic fibrosis and P. aeruginosa lung infection [188]. The clinical trial had 120 adult CF patients with forced expiratory volume in 1 s (FEV1) greater than or equal to 25% and less than or equal to 75% predicted split into the control and treatment groups [188]. The treatment group consisted of two subgroups that received 80 mg/20 mg or 160 mg/40 mg inhaled FTI twice daily for 28 days [188]. The control group received an aztreonam for inhalation solution (AZLI) placebo inhaler twice daily for 28 days [188]. After 28 days, the relative change in lung function from baseline after 28 days was measured for both the treatment and control groups.
Table 1.
(A). Novel antibiotics against P. aeruginosa. (B). Beta-lactamase inhibitors targeting P. aeruginosa.
| (A) Novel antibiotics against P. aeruginosa. | ||||||
| Treatment/Study | Mechanism of Action | Population | Purpose | Phase | Number of Patients | Year, Reference |
| Inhaled fosfomycin/tobramycin (FTI) after aztreonam inhalation solution (AZLI) run-in (NCT00794586) |
Protein synthesis inhibition | Cystic fibrosis patients with P. aeruginosa respiratory tract infections | Efficacy, safety, and ability to maintain forced expiratory volume (FEV1) in 1 s | II | 120 | 2013, [188] |
| Fosfomycin IV (ZTI-01) and oral fosfomycin (NCT02178254) |
Protein synthesis inhibition | Hospital volunteers | Pharmacokinetics, safety | I | 30 | 2016, [190] |
| POL7080 (murepavadin) (NCT02096315) |
Outer membrane protein inhibitor | Non–cystic fibrosis patients with bronchiectasis | Safety, pharmacokinetics, pharmacodynamics | II | 20 | 2017, [191] |
| Murepavadin (NCT02110459) |
Outer membrane protein inhibitor | Healthy volunteers and renal failure patients | Pharmacokinetics, tolerability, safety | I | 32 | 2019, [192] |
| Murepavadin (PRISM-MDR) (NCT03409679) |
Outer membrane protein inhibitor | Ventilated ICU patients | Treating P. aeruginosa VAP | III | 41 | 2019, [193] |
| Murepavadin (PRISM-UDR) (NCT03582007) |
Outer membrane protein inhibitor | Hospital patients | Treating P. aeruginosa hospital-associated pneumonia | III | 2 | 2019, [194] |
| RC01 (NCT03832517) |
LPS binding | Healthy adult volunteers | Pharmacokinetics, tolerability, safety | I | 8 | 2019, [195] |
| Polymyxin B analogue SPR741 (NCT03376529) |
Plasma membrane disruption and/or antibiotic sensitization | Healthy adult volunteers | Pharmacokinetics, tolerability, safety | I | 27 | 2018, [196] |
| Polymyxin B analogue SPR741 (NCT03022175) |
Plasma membrane disruption and/or antibiotic sensitization | Healthy adult volunteers | Pharmacokinetics, tolerability, safety | I | 64 | 2017, [197] |
| Polymyxin B analogue SPR206 (NCT03792308) |
Plasma membrane disruption and/or antibiotic sensitization | Healthy human volunteers | Pharmacokinetics, tolerability, safety | I | 94 | 2020, [198] |
| Polymyxin B analogue SPR206 (NCT03376529) |
Plasma membrane disruption and/or antibiotic sensitization | Healthy human volunteers | Pharmacokinetics, tolerability, safety | I | 27 | 2018, [196] |
| Polymyxin B analogue MRX-8 (NCT04649541) |
Plasma membrane disruption and/or antibiotic sensitization | Healthy human volunteers | Pharmacokinetics, tolerability, safety | I | 116 | 2021, [199] |
| (B) Beta-lactamase inhibitors targeting P. aeruginosa. | ||||||
| Treatment/Study | Mechanism of Action | Population | Purpose | Phase | Number of Patients | Year, Reference |
| Imipenem/relebactam/cilastatin (NCT02452047 RESTORE-IMI 1) |
Beta-lactamase inhibitor | Patients with HABP, VAPB, urinary tract infections, and intra-abdominal infections | Efficacy at treating HABP, VAPB, urinary tract infections, and intra-abdominal infections | III | 31 | 2019, [200,201] |
| Imipenem/relebactam/cilastatin (NCT02493764-RESTORE-IMI 2) |
Beta-lactamase inhibitor | Patients with HABP or VAPB | Efficacy at treating HABP or VAPB | III | 264 | 2021, [202,203] |
| Imipenem/cilastatin/relebactam (NCT05561764) |
Beta-lactamase inhibitor | Patients with HABP and VAPB | Efficacy at treating HABP and VAPB | III | 274 | 2022, [204] |
| Imipenem/relebactam/Cilastatin (NCT03583333) |
Beta-lactamase inhibitor | Patients with CF pneumonia | Efficacy at treating with CF pneumonia | IV | 16 | 2023, [205] |
| Imipenem/cilastatin/XNW4107 (NCT05204563) |
Beta-lactamase inhibitor | Patients with HABP and VAPB | Efficacy at treating patients with HABP and VAPB | III | 450 | 2023, [206] |
| Nacubactam (NCT02134834, NCT02972255, and NCT03182504) |
Beta-lactamase inhibitor | Healthy human volunteers | Pharmacokinetics, tolerability, safety | I | 21 | 2018, [207,208,209] |
Another phase I clinical trial investigated the efficacy of fosfomycin IV (ZTI-01) against persistent P. aeruginosa infections (NCT02178254) [190]. ZTI-01 inhibits peptidoglycan assembly, thereby disrupting cell wall synthesis [190]. The intravenous form of fosfomycin is thought to be superior to oral dosing for CF patients infected with P. aeruginosa [190]. The study included 30 healthy individuals between the ages of 18 and 45 who were randomly assigned to one of three treatment sequences that lasted between 18 and 26 days [190]. The study was designed to determine the safety, tolerability, and pharmacokinetics of two single doses of ZTI-01 (1 and 8 g infused over 1 h) and a single dose of the reference label drug, Monurol® (oral sachet, 3 g) [190]. The first treatment sequence consisted of 10 patients receiving 1.0 g of intravenous ZTI-01 for period 1 (1 h infusion), 8.0 g IV ZTI-01 for period 2 (1 h infusion), and 3 g oral sachet of Monurol in period 3 [190]. For the second treatment sequence, 10 patients received 8.0 g IV ZTI-01 for period 1 (1 h infusion), 3 g oral sachet of Monurol in period 2, and 1.0 g of IV ZTI-01 for period 3 (1 h infusion) [190]. In the third treatment sequence, 10 patients received 3 g oral sachet of Monurol in period 1, 1.0 g of intravenous (IV) ZTI-01 for period 2 (1 h infusion), and 8.0 g IV ZTI-01 for period 3 [190].
One of the newly discovered antibiotics is murepavadin, which interferes with LPS transport in Gram-negative bacteria. Murepavadin (POL7080) belongs to a novel class of antibiotics, known as outer membrane protein targeting antibiotics (ompTAs) [191,210]. It was discovered through screening a large library of peptidomimetic macrocycles that were based on a truncated structure of the antimicrobial peptide protegrin I (PG-1) [211]. Protegrin-1 is a small peptide that contains 18 amino acid residences found in bacteria and fungi [211,212,213]. The structure of PG-1 contains six positively charged arginine residues and four positively charged cysteine residues that form two antiparallel β-sheets with a β-turn [211,212,213]. Murepavadin binds to the lipopolysaccharide transport protein D (LptD), an outer membrane protein involved in lipopolysaccharide biogenesis in Gram-negative bacteria. By binding to LptD, murepavadin inhibits the LPS transport function of LptD and causes lipopolysaccharide alterations in the outer membrane of the bacterium and, ultimately, cell death [210,211]. In vitro analysis demonstrated the strong bactericidal effects of murepavadin against 1219 P. aeruginosa isolates (many multi-drug-resistant) obtained from 112 medical clinical centers in the US, China, and Europe. These studies estimated the MIC50 of murepavadin against several P. aeruginosa isolates of 0.12 mg/liter [214].
Given its effectiveness in preclinical studies, a clinical trial (NCT02096315) was conducted to test whether murepavadin is effective in patients with exacerbation of non–cystic fibrosis bronchiectasis caused by P. aeruginosa infection [191]. The study administered murepavadin to 20 patients between 18 and 80 years of age with non–cystic fibrosis bronchiectasis caused by P. aeruginosa infection for a period of 20 days [191]. The primary outcome of the study was the reduction in CFU/mL (colony-forming units/mL) of P. aeruginosa [191].
Polyphor Ltd. followed up on this study with another clinical trial (NCT02110459) to investigate the pharmacokinetics and safety of a single dose of murepavadin in patients with mild, moderate, severe, and end-stage renal disease after a single intravenous infusion of murepavadin [192]. The study included 32 adults between ages 18 and 79 divided into four groups with either mild (group 1), moderate (group 2), and severe (group 3) renal function impairment or with normal renal function (group 4) [192]. All patients in the study received a single 2.2-mg/kg of body weight intravenous infusion of murepavadin administered over 3 h [192]. The study found that murepavadin was 2.0- to 2.5-fold higher in patients with renal function impairment compared with patients with normal renal function. Murepavadin was well tolerated in all groups. The study concluded that murepavadin was well tolerated by patients with renal disease with transient and mild adverse side effects [192].
Polyphor Ltd. conducted two clinical trials to evaluate the effectiveness of murepavadin (PRISM-MDR (ventilator-associated pneumonia) and PRISM-UDR (nosocomial pneumonia); NCT03409679 and NCT03582007, respectively). PRISM-MDR (NCT03409679) was a phase III randomized clinical trial that was designed to investigate the efficacy, stability, and tolerability as well as the pharmacokinetics of the intravenous injection of murepavadin with one of the other antipseudomonal antibiotics (piperacillin/tazobactam, ceftazidine, cefepime, meropenem, amikacin, ciprofloxacin, levofloxacin, colistin) to treat ventilator-associated bacterial (VAB) pneumonia [193]. The study included a total of 41 patients who developed VAB within 48 h after intubation to assess whether murepavadin increased the clinical cure rate 21–24 days after the start of treatment [193]. Patients in the study received either murepavadin every 8 h and one antipseudomonal antibiotic (piperacillin/tazobactam, ceftazidine, cefepime, meropenem, amikacin, ciprofloxacin, levofloxacin, colistin) and two antipseudomonal antibiotics (piperacillin/tazobactam, ceftazidine, cefepime, meropenem, amikacin, ciprofloxacin, levofloxacin, colistin) [193].
PRISM-UDR (NCT03582007) is a phase III, multicenter, open-labeled study to investigate the efficacy, stability, and tolerability of intravenous murepavadin given with ertapenem (beta-lactam antibiotic) versus one antipseudomonal antibiotic (meropenem or piperacillin/tazobactam) antibiotic in the treatment of nosocomial pneumonia [194]. The study included two patients who would receive either murepavadin and ertapenem or one antipseudomonal antibiotic (meropenem or piperacillin/tazobactam) to assess the all-cause mortality rates 28 days after the start of study treatment [194]. However, Polyphor Ltd. halted the enrollment due to the higher-than-expected acute kidney injury incidence (56%) observed in the murepavadin arm of the PRISM-UDR trial [194]. Investigators suggested that in patients with moderate or severely impaired renal functions, murepavadin’s dosage should be adjusted accordingly [194].
Another investigative drug is RC01, which, like murepavadin, functions by targeting LPS [195]. A phase I randomized clinical trial (NCT03832517) was designed to assess the safety, tolerability, and pharmacokinetics of RC01 in eight healthy volunteers. In the first part of the study, healthy patients received a single-dose escalation of increasing intravenous doses of RC-01. In the second part of the study, healthy patients received multiple-dose escalation of increasing intravenous doses of RC01 given either twice daily or three times daily.
3.1.2. Polymyxin B Derivatives
Despite their propensity for human nephrotoxicity and neurotoxicity, polymyxins are still a family of antibiotics that are available to treat infections caused by multidrug Gram-negative bacteria almost 60 years after their clinical approval [215]. Polymyxins are small cyclic cationic lipopeptides that interact with the anionic lipid A component of LPS in the Gram-negative bacteria’s outer membrane, causing disruption of the cytoplasmic membrane and bacterial cytotoxicity [215]. In recent years, the clinical usage of polymyxin B and polymyxin E has resumed. Many attempts have been made to alter the structure of polymyxins and enhance their safety profile. A polymyxin B derivative with lower nephrotoxicity is SPR741. SPR741 does not directly kill bacteria, but it increases the effectiveness of coadministered antibiotics, which by themselves would not reach their intracellular targets [216,217]. There are two ongoing clinical trials designed to examine the efficacy of SPR741 against P. aeruginosa (NCT03022175 and NCT03376529).
The first clinical trial (NCT03022175) is a phase I randomized control trial designed to evaluate the safety, tolerability, and pharmacokinetics of both single and multiple intravenous doses of SPR741 when given to 64 healthy adult volunteers. The trial consists of a single ascending dose (SAD) phase and a multiple ascending dose (MAD) phase. Participants in SAD will either receive a single dosage of SPR741 or a placebo. Over 14 days, participants in MAD will either receive repeated doses of SPR741 or a placebo. Sequential cohorts will experience escalating dosages of SPR741 in both phases. During the SAD phase, patients will receive single doses of SPR741 over 60 min intravenous infusion at different doses. In the MAD phase, patients will receive SPR741 over 60 min intravenous infusion three times a day [197]. The second clinical (NCT03376529) is a phase I randomized control trial designed to evaluate the drug–drug interaction, pharmacokinetics, safety, and tolerability of a single dose of SPR741 combined with each of three different antibiotics (ceftazidime or piperacillin/tazobactam or aztreonam) in 27 healthy volunteers [196]. Patients were administered either a single dose of SPR741 alone, a single dose of SPR741 in combination with one of three different partner antibiotics, or the partner antibiotic alone in a randomized sequence [196].
Another polymyxin B compound, SPR206 and SPR741, interacts with and increases the permeability of the P. aeruginosa outer membrane, thereby enhancing the accumulation of coadministered antibiotics [217,218]. The efficacy of SPR206 was examined in two randomized clinical trials (NCT03376529 and NCT03792308). In the first study, SPR206 was examined in NCT03792308 to assess the safety, tolerability, and pharmacokinetics of single and multiple intravenous doses of SPR206 when administered to 94 healthy adult volunteers [198]. During the SAD phase, patients received single doses of SPR206 via intravenous infusion for over 1 h. During the MAD phase, patients received SPR206 via IV infusion 1 h three times a day for 7 consecutive days or 1 h three times a day for 4 consecutive days. A second phase I clinical trial (NCT03376529) evaluated the drug–drug interaction, pharmacokinetics, safety, and tolerability of a single dose of SPR741 combined with each of three different partner antibiotics (ceftazidime or piperacillin/tazobactam or aztreonam) in healthy volunteers [196]. Both studies showed that upon coadministration, the pharmacokinetic profiles of SPR741 and the associated antibiotics remained unaltered [219]. In general, SPR741 was well tolerated. These findings support SPR741’s continued clinical development for the treatment of severe illnesses caused by P. aeruginosa bacteria [219].
Similar to the mechanism of SPR206, BRX-8 is designed to be less toxic to human cells and, thus, improve its application in patients [220]. A phase I randomized control trial (NCT04649541) was designed to assess the safety and tolerability of single and multiple intravenous doses of MRX-8. The study was focused on the pharmacokinetics of MRX-8 and its primary metabolite following single and multiple intravenous doses including the elimination rate of MRX-8 and its metabolite in urine [199].
3.1.3. Beta-Lactamase Inhibitors
A brand-new beta-lactamase inhibitor, relebactam, targets upon several beta-lactamase enzymes in several multi-drug-resistant bacteria (Table 1B) [221,222]. Relebactam was added to imipenem in vitro to restore its effectiveness against various imipenem-resistant bacteria, including P. aeruginosa. In response, there have been several clinical trials (NCT02493764, NCT02452047, NCT05561764, NCT03583333, and NCT05204563) to examine the use of relebactam against P. aeruginosa. A phase III randomized control trial (NCT02452047-RESTORE-IMI 1) was designed to evaluate the efficacy and safety of imipenem + cilastatin/relebactam versus colistimethate sodium + imipenem + cilastatin in the treatment of imipenem-resistant P. aeruginosa. Infections evaluated in the study included hospital-acquired bacterial pneumonia, ventilator-associated bacterial pneumonia, complicated intra-abdominal infection, and complicated urinary tract infection [200,201]. Patients with hospital-acquired/ventilator-associated pneumonia, complicated intra-abdominal infection, or complicated urinary tract infection caused by imipenem-nonsusceptible (but colistin- and imipenem/relebactam-susceptible) pathogens received for 5–21 days imipenem/relebactam or colistin and imipenem [200,201]. In the clinical trial, 16 patients received colistin plus imipenem, while 31 patients received imipenem/relebactam. The study found that 70% of patients taking colistin and imipenem and 71% of patients on imipenem/relebactam showed a favorable overall response. The mortality rate at 28 days was 10% among the imipenem/relebactam-treated group and 30% in the colistin/imipenem-treated group. In addition, 10% of imipenem/relebactam patients and 31% of colistin/imipenem patients experienced serious adverse effects, with the most frequent being nephrotoxicity [200,201]. Overall, imipenem/relebactam was found to be both an efficacious and well-tolerated treatment option for carbapenem-nonsusceptible infections, particularly among Pseudomonas species. A subsequent phase III clinical trial (NCT02493764-RESTORE-IMI 2 and NCT03583333) compared treatment with a fixed-dose combination of imipenem/relebactam/cilastatin with a fixed-dose combination of piperacillin/tazobactam in participants with hospital-acquired or ventilator-associated bacterial pneumonia (HABP or VAPB, respectively) [202,204]. The overall goal was to assess whether imipenem/relebactam/cilastatin was noninferior to piperacillin/tazobactam [202,204]. Patients with HABP/VABP were randomized to receive piperacillin/tazobactam intravenously every 6 h for 7–14 days or imipenem/cilastatin/relebactam. All-cause mortality at day 28 served as the main outcome [202,204]. Of the 264 imipenem/cilastatin/relebactam and 267 piperacillin/tazobactam patients included in the study, there was a similar improvement in mortality, morbidity, and clinical symptoms among patients who received either drug regimen. Overall, the study showed that patients infected with Gram-negative bacterial pathogens, including P. aeruginosa, can be treated effectively with imipenem/cilastatin/relebactam, even in critically ill, high-risk patients [202,204].
Given the results of the RESTORE-IMI 1 and 2 trials, another phase I clinical trial (NCT05561764) examined whether the combination of imipenem/cilastatin/relebactam was effective for P. aeruginosa infections in CF patients [205]. Specifically, the study assessed the pharmacokinetics and tolerability of imipenem/cilastatin/relebactam in 16 adolescent and adult patients with CF acute pulmonary exacerbations [205]. The CF patients received 10–14 days of imipenem/cilastatin/relebactam every 6 h with dose determined per renal function, with or without adjunctive aminoglycoside or fluoroquinolone therapy. The forced expiratory volume in 1 s (FEV1) was measured throughout the experiment to assess efficacy [205].
In addition, another novel beta-lactamase antibiotic, nacubactam, has shown promising results as an effective antibiotic against P. aeruginosa infections (NCT02134834, NCT02972255, and NCT03182504) [207,208,209]. In a single phase I clinical trial, the safety, tolerability, and pharmacokinetics of intravenous nacubactam were assessed using 40 healthy volunteers who received three different dose regiments. The study found that the use of nacubactam with other antibiotics, such as meropenem, did not affect its pharmacokinetics or side effects [207,208,209]. Overall, these findings support the continued clinical development of nacubactam and demonstrate the suitability of meropenem as a potential β-lactam partner for nacubactam.
3.2. Bacteriophages
The emergence of mutants that are resistant to most available antibiotics represents a serious global health crisis. As such, alternative approaches, such as phage therapy, have been extensively investigated. Phage therapy involves the purification of virulent lytic phages that infect and eliminate phage-sensitive bacteria [223]. Within the last several years, numerous clinical studies have been conducted to evaluate the effectiveness of phage therapy in treating specific P. aeruginosa infections.
A randomized, controlled, double-blind phase I/II trial was designed to test the efficacy of phage therapy for the treatment of P. aeruginosa wound infections in burned patients. The trial was given the name PhagoBurn (NCT02116010) (Table 2). In this study, a cocktail of 12 natural lytic P. aeruginosa bacteriophages (PP1131) was administered topically at very low concentrations and compared with the standard of care (1% sulfadiazine silver emulsion cream) [224]. Eligible participants were randomly assigned (1:1) to receive either the standard of care (1% sulfadiazine silver emulsion cream) or a cocktail of 12 natural lytic anti-P. aeruginosa bacteriophages (PP1131 at a concentration of 1 × 106 plaque-forming units (PFU) per mL) [224]. Both treatments were administered topically daily for 7 days with a 14-day follow-up period [224]. The main aim of the clinical trial was to determine the median time to a sustain and decrease in bacterial burden by at least two quadrants using a four-quadrant method [224]. This was performed using daily swabs on all participants who had a microbiologically confirmed infection on day 0 and who received at least one sulfadiazine silver or phage dressing. In total, 27 patients were enrolled across two recruiting periods totaling 13 months; 13 patients were randomly assigned to a phage therapy and 14 to the standard of care [224].
Table 2.
Bacteriophage therapy for P. aeruginosa.
| Treatment/Study | Mechanism of Action | Population | Purpose | Phase | Number of Patients | Reference/Year |
|---|---|---|---|---|---|---|
| MUCOPHAGES (10 Pseudomonas specific bacteriophages) (NCT01818206) |
Binding of bacteriophage to specific Pseudomonas targets to induce lysis | Cystic fibrosis sputa | Clinical study | -- | 59 | 2012, [225] |
| PhagoBurn (PP1131) 12 natural lytic Pseudomonas bacteriophages (NCT02116010) |
Binding of bacteriophage to specific Pseudomonas targets to induce lysis | Burn patients | Treat P. aeruginosa burn wound infection | I/II | -- | 2018, [224] |
| B-PAO1 (4 Pseudomonas-specific bacteriophages) (NCT03395743) |
Binding of bacteriophage to specific Pseudomonas targets to induce lysis | Cystic fibrosis patients | Prevent/treat P. aeruginosa infection | -- | -- | 2019, [226] |
| Bacteriophage cocktail spray (Phage cocktail-SPK) (NCT04323475) |
Binding of bacteriophage to specific Pseudomonas targets to induce lysis | Burn patients | Treatment of P. aeruginosa and infection of burn wounds | I | 12 | 2023, [227] |
| AP-PA02 (NCT04596319) |
Binding of bacteriophage to specific Pseudomonas targets to induce lysis | Chronic P. aeruginosa lung infections and CF | Treatment of chronic P. aeruginosa lung infections and CF | I/II | 29 | 2022, [228] |
| YPT-01 (NCT04684641) |
Binding of bacteriophage to specific Pseudomonas targets to induce lysis | Chronic P. aeruginosa lung infections and CF | Treatment of chronic P. aeruginosa lung infections and CF | I/II | 8 | 2023, [229] |
According to the PhagoBurn clinical trial and by the end of the phage treatment, half of the subjects successfully reduced their daily bacterial burden in most infected lesions by two quadrants or more [224]. However, the median time to reach this endpoint for those who received PP1131 was considerably longer than it was for those who received the standard of care [224]. The systemic administration of antibiotics that are effective against the infecting strain, whether they were started at day 0 or added later during the study therapy, had no impact on this outcome [224]. Investigators suggested that even though the phage multiplied on P. aeruginosa phage-sensitive strains within the infected burn wound, the phage cocktail titer was very low. In addition, due to challenges in manufacturing the cocktail, the patient sample size was small. The authors of the study suggested that additional studies using increasing phage concentrations and larger sample sizes may improve the outcomes for burn patients.
The study “MUCOPHAGES” (NCT01818206) examined the impact of a cocktail of 10 phages on P. aeruginosa strains isolated from CF patients’ sputum samples [225]. The trial’s aim was to evaluate the efficacy of bacteriophages in eliminating infecting P. aeruginosa strains present in sputum samples from CF patients who were 6 years and older [225]. Subsequently, a cocktail of 10 bacteriophages was applied directly to 60 sputum samples obtained from CF patients. The investigators determined that the number of P. aeruginosa (CFU) within each sample after 6 h alters the applications of the phages [225].
Another clinical trial is being conducted to assess the efficacy of a phage cocktail B-PAO1, which consists of four phages, against life-threatening P. aeruginosa infections (NCT03395743) [226]. The main aim of this clinical trial was to allow physicians to provide treatment with investigational drug, AB-PA01, for patients with serious or immediately life-threatening P. aeruginosa infections, for which no alternative treatments are currently available [226]. However, further studies with larger populations are still required to prove the efficacy of using bacteriophage therapy in treating P. aeruginosa infections. Based on the clinical trial data, investigators recommended modifications of both the composition and the application of the phage cocktail to improve therapeutic outcomes.
Another phase I clinical trial evaluated the safety and tolerability of a phage cocktail-SPK therapy compared with the standard of care (Xeroform and Kenacomb) for second-degree burn wounds infected by S. aureus, P. aeruginosa, or K. pneumoniae in adult patients (NCT04323475) [227]. The study included 12 adult patients who were split into control and treatment groups. The control group consisted of Xeroform primary dressing and Kenacomb topical antibiotic cream (for wounds with signs of localized infection) [227]. The experimental group consisted of dosage-metered airless spray containing a cocktail at a concentration of 1.4 × 108 PFU/mL for an effective dosage of 2.5 × 105 PFU/cm^2 of burned area [227].
A phase I randomized clinical trial (NCT04596319) was designed to evaluate the safety, tolerability, and phage recovery profile of inhaling the AP-PA02 multi-bacteriophage to treat 29 CF patients and/or chronic pulmonary P. aeruginosa infections for a period of 4 weeks [228]. Another phase II clinical trial (NCT04684641) examined whether YPT-01 phage therapy reduces sputum bacterial load in 8 CF subjects with P. aeruginosa for 7 days. In addition, the study evaluates the safety profile of phage therapy in CF patients [229].
3.3. Strategies Targeting P. aeruginosa Virulence (Biofilm, Quorum Sensing, Type III Secretion System, and Antimicrobial Peptides)
3.3.1. Quorum Sensing
The role of QS in P. aeruginosa virulence has been well established in animal models [230,231,232,233]. A clinical trial testing the ability of inhaled azithromycin to inhibit P. aeruginosa QS began in 2005 (Table 3). Previous preclinical studies showed that azithromycin inhibits the transcription of several genes involved in the function of QS in P. aeruginosa [234,235,236,237]. Specifically, azithromycin inhibits the 23S rRNA of the 50S ribosome unit, which decreased the production of genes essential for QS functions in P. aeruginosa [234,235,236,237]. The study was a prospective analysis of 92 intubated patients who were colonized with P. aeruginosa in intensive care units at 17 European hospitals [238]. Throughout the study, tracheal isolates were collected each day to estimate the total density of P. aeruginosa bacteria in the aspirates through genomic copy numbers [238]. The study showed that azithromycin, which has no bactericidal activity against P. aeruginosa but interferes with its QS, reduced QS-gene expression in tracheal aspirates of treated patients (NCT00610623) [238]. The study also showed that the prevalence of noncooperating (and hence less virulent) lasR P. aeruginosa mutants rose with time in the absence of azithromycin [238]. The LasR protein is a transcriptional activator that regulates the expression of several of the P. aeruginosa QS-controlled genes, including lasB, lasA, las, and phenazines [238]. In tracheal aspirates, direct QS-gene expression was considerably lowered by azithromycin. During azithromycin treatment, the benefit of lasR-mutants was lost, and virulent wild-type isolates predominated [238]. These in vitro findings were supported with the observation that the growth of the wild-type strain and not the LasR mutants decreased by azithromycin [238]. Furthermore, the absence of azithromycin prevented the lasR-mutant from successfully encroaching on wild-type populations [238]. The authors suggested that intervention based on QS blockade may increase the prevalence of P. aeruginosa strains with more virulent genotypes within the hospital environment [238]. A subsequent study by Welsh et al. corroborated these findings and suggested that alterations in virulence phenotype occur when only one compound is used to block a specific component of QS [239]. Despite these problems, it is likely that attempts to target QS to reduce P. aeruginosa virulence will continue.
Table 3.
Strategies targeting P. aeruginosa virulence.
| Treatment/Study | Mechanism of Action | Population | Purpose | Phase | Number of Patients | Reference/Year |
|---|---|---|---|---|---|---|
| Azithromycin to inhibit QS (NCT00610623) |
Biofilm formation inhibition | Ventilated patients | Reduced P. aeruginosa VAP | II | 92 | 2018, [238] |
| OligoG (NCT00970346, NCT03822455, and NCT03698448) |
Biofilm formation inhibition | Healthy volunteers | Pharmacokinetics, tolerability, safety | I | 26 | 2022, [240,241] |
| PLG0206 peptide (NCT05137314) |
Biofilm formation inhibition | Patients with prosthetic joints | Treatment for prosthetic joint infection | I | 14 | 2022, [242] |
| Fluorothiazinon (aka Ftortiazinon) (NCT03638830) |
Targeting the type III secretion system | Patients with P. aeruginosa complicated urinary tract infection | Adjunct treatment safety, efficacy | II | 777 | 2021, [243] |
3.3.2. Antibiofilm Agent
The antibiofilm agent OligoG has shown promise as an effective agent against P. aeruginosa infection [244,245]. By chelating calcium, the alginate oligosaccharide OligoG has the potential to reduce the viscosity of CF patients’ sputum, facilitate mucus removal from patient airways, and lower microbial burden and inflammation [244,245]. OligoG disrupted the biofilm structure of the P. aeruginosa mucoid phenotype, which may enhance the function of the host immune system and the efficacy of antibiotics. A phase I trial was conducted with a focus on pulmonary function and adverse events to investigate the safety and local tolerability of multiple dosages of OligoG administration of an inhaled fragment in 26 healthy volunteers (NCT00970346) [240,244,245]. Another phase II/III clinical trial (NCT03822455 and NCT03698448) assessed the safety, efficacy, and tolerability of OligoG in CF patients for 12 weeks [241,246].
3.3.3. Antimicrobial Peptides
Antimicrobial peptides are bioactive compounds that are extremely biocompatible and comparatively resistant to the development of bacterial resistance [247,248,249,250]. Most antimicrobial peptides eliminate bacteria by altering the normal permeability of the cell membrane [247,248,249,250]. In addition, there are several antimicrobial peptides that have been effectively covalently immobilized on a range of surfaces, including silicone, glass, titanium oxide, resin beads, and contact lenses [247,248,249,250]. In addition, PLG0206 (WLBU2), a broad-spectrum engineered cationic antimicrobial peptide with broad-spectrum activity, inhibits in vitro P. aeruginosa biofilm growth on airway epithelial cells [242]. PLG0206 is an engineered antibacterial peptide that is based on naturally occurring antimicrobial peptide and has shown effectiveness against P. aeruginosa and staphylococcus aureus biofilms [242]. A phase I trial was conducted in 14 healthy volunteers in 2018. In 2022, PLG0206 was entered into a phase I clinical trial to treat P. aeruginosa infections of the prosthetic joints (NCT05137314) [242].
3.3.4. Type III Secretion System
Numerous studies using both the murine models and the ex vivo model demonstrated the role of T3SS in the virulence of P. aeruginosa [127,251,252,253,254,255,256]. Several inhibitors of T3SS, including small molecule proteins and carbohydrates, have been discovered [257,258,259]. In general, these inhibitors target the regulation of different genes associated with essential functions of T3SS [257,258,259]. Fluorothiazinon is a small molecule that targets the function of T3SS [257,258,259]. Fluorothiazinon belongs to a class of 2,4-disubstituted-4H-[1,3,4]-thiadiazine-5-one molecules that inhibited the function of T3SS in chlamydia and salmonella [257]. Further analysis revealed that Fluorothiazinon inhibits ExoT and ExoY secretion in P. aeruginosa [257,258]. In addition, the molecule reduces bacterial cytotoxicity and enhances bactericidal internalization by both epithelioid and phagocytic cells [257,258]. Furthermore, Sheremet et al. demonstrated that Fluorothiazinon decreased bacterial load in the lung by eliminating P. aeruginosa and decreasing levels of IL-6, TNF α, and interferon γ [257,258]. In 2018, the Gamaleya Research Institute (Ministry of Health of the Russian Federation, Rakhmanovsky pereulok 3, Tverskoy District, Moscow) initiated a randomized placebo-controlled phase II trial consisting of 777 patients to evaluate the safety and efficacy of the drug Ftortiazinon (aka Fluorothiazinon) with cefepime in comparison with placebo and cefepime in the treatment of hospitalized adult patients with complicated urinary tract infections caused by P. aeruginosa (NCT03638830) [243]. The 777 patients were randomized into three separate groups: 150 mg Ftortiazinon, placebo, and cefepime; 300 mg Ftortiazinon and cefepime; and placebo and cefepime [243].
3.4. P. aeruginosa Virulence Factor Passive Immunotherapy
Originally, opsonic antibodies against the mucoid exopolysaccharide (MEP) of P. aeruginosa were shown to be protective in a murine model of chronic respiratory infection (Table 4) [260].
Table 4.
P. aeruginosa immunotherapy/monoclonal antibodies.
| Treatment/Study | Population | Purpose | Phase | Number of Patients | Reference/Year |
|---|---|---|---|---|---|
| MEP IGIV (mucoid exopolysaccharide Ab) (NCT00004747) |
Cystic fibrosis patients | Reduce frequency of acute pulmonary exacerbation and mucoid P. aeruginosa colonization | II | 170 | 2005, [261] |
| KB001 (anti-Pa PcrV Fab Ab; humanized, PEGylated recombinant) (NCT00691587) |
Ventilated patients | Prevent P. aeruginosa VAP | Pilot | 36 | 2009, [262] |
| KB001 (anti-Pa PcrV Fab Ab; humanized, PEGylated recombinant) (NCT00638365) |
Cystic fibrosis patients | Safety, tolerability, pharmacokinetics, pharmacodynamics, and prevent P. aeruginosa respiratory tract infection | I/II | 27 | 2014, [263] |
| KB001 (anti-Pa PcrV Fab Ab; humanized, PEGylated recombinant) (NCT01695343) |
Cystic fibrosis patients | Reduce P. aeruginosa infection | II | 169 | 2018, [264] |
| MEDI3902 (anti-PcrV + anti-Psl Mab) (NCT02255760) |
Healthy volunteers | Pharmacokinetics, safety | I | 56 | 2019, [265] |
| MEDI3902 (anti-PcrV + anti-Psl Mab) (NCT02696902) |
Ventilated patients | Prevent P. aeruginosa VAP | II | 168 | 2020, [266] |
| PseudIgY (avian Ab to Pa) (NCT00633191) |
Cystic fibrosis patients’ safety | Prevent P. aeruginosa respiratory tract colonization; preserve pulmonary function | I/II | 14 | 2012, [267] |
| PseudIgY (avian Ab to Pa) (NCT01455675) |
Cystic fibrosis patients | Reduce P. aeruginosa reinfection | III | 164 | 2017, [268] |
| KBPA-101 (panobacumab) human IgM anti-Pa O11 LPS Mab obtained from a volunteer immunized with LPS-toxin A conjugate vaccine (NCT00851435) |
Patients with P. aeruginosa hospital-acquired pneumonia | Safety, Pharmacokinetics, adjunct treatment of P. aeruginosa hospital-acquired pneumonia | I/II | 14 | 2014, [269,270,271] |
| AR-105 (Aerucin, anti-alginate Mab) (NCT03027609) |
Ventilated patients | Adjunct treatment of P. aeruginosa VAP | II | 158 | 2019, [272] |
| Pentaglobin (PENTALLO) (NCT03494959) |
Neutropenic acute leukemia or transplant patients with carbapenem-resistant Enterobacteriaceae | Decrease P. aeruginosa mortality |
II | 120 | 2023, [273] |
| TRL1068 monoclonal antibody (NCT04763759) |
Prosthetic joint infection patients secondary to P. aeruginosa | Safety and pharmacokinetics. Decrease C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), IL-6, and IL-10 |
I | 18 | 2023, [274] |
After opsonic P. aeruginosa MEP antibodies were successfully produced in mice [275], the immunogen was used to elicit opsonizing anti-MEP antibodies in rats [276]. These harvested antibodies, MEP IVIG, were used in a phase II clinical trial aimed at reducing acute pulmonary exacerbation of P. aeruginosa infection in CF patients. The trial was concluded in 2000, but no results ensued (NCT00004747) [261]. The clinical trial studied the efficacy of monthly intravenous mucoid exopolysaccharide P. aeruginosa immune globulin (MEP IGIV) given over 1 year in reducing the frequency of acute pulmonary exacerbation in patients with cystic fibrosis, mild to moderate pulmonary disease, and mucoid P. aeruginosa colonization [261]. The study examined whether MEP IGIV improved: forced expiratory volume in the first second (FEV1), reduced the density of mucoid P. aeruginosa colonies in the patient’s sputum, and improved the quality of life in patients infected with P. aeruginosa [261]. A total of 170 patients were included in the study to be randomized into three treatment groups: low-dose intravenous mucoid exopolysaccharide P. aeruginosa immune globulin (MEP IVIG), high-dose MEP IVIG, or placebo [261]. The treatments were administered every 28 days for a year [261].
Immunotherapeutic approaches targeting T3SS of P. aeruginosa have also been pursued. Passive immunization using PcrV, a protein critical for the translocation of T3SS effectors, significantly enhanced the survival of mice in lung and burn models of P. aeruginosa infection [277,278]. Subsequently, anti-PcrV MAb F(Ab′)2 monoclonal antibodies were generated and shown to be protective in murine models of lung infection [279,280]. Anti-PcrV MAb 166 has been developed for clinical use as KB001, an anti-PcrV PEGylated MAb F(Ab′)2 (NCT00638365, NCT00691587, and NCT01695343) [262,263,264,279]. A clinical trial (NCT00691587) evaluated KB001 in patients in the intensive care setting who were receiving ventilator therapy and suffering from P. aeruginosa lung infections. Patients received either the placebo or one of two dose levels of KB001. KB001 (Humaneered™), which was used to treat P. aeruginosa infections, is a modified, PEGylated, recombinant anti-P. aeruginosa PcrV Fab’ antibody that binds with high affinity to the PcrV protein and blocks its activity [262,263]. The transport of P. aeruginosa exotoxins into host immunological and epithelial cells depends on the protein PcrV, which is located close to the tip of the TTSS needle [263]. As such, this represents a novel therapeutic strategy for treating infection by reducing inflammation in CF patients through the inhibition of the PcrV protein’s function, thereby interfering the release of powerful cytotoxins involved in the initiation and maintenance of P. aeruginosa infections [263]. The clinical trial examined whether KB001 protects host epithelium and immune cells, and evaluated the reduction of pulmonary P. aeruginosa burden [262]. Patients were randomized to receive a low dose of KB001 monoclonal antibody, high dose of KB001 monoclonal antibody, or placebo [262].
A phase I/II clinical trial studied the safety, pharmacokinetic, and pharmacodynamic properties of KB001 in CF patients with chronic P. aeruginosa infections (NCT00638365) [263]. A single intravenous dosage of either KB001 (3 or 10 mg/kg) or a placebo was administered to 27 participants of at least 12 years of age [263]. In each patient, assessments were made concerning KB001’s safety and pharmacokinetics. The assessment also included an examination of the P. aeruginosa density, clinical results, and inflammatory markers [263]. KB001 had an acceptable safety profile. After a single dose, there were no appreciable differences between KB001 treated and the placebo groups in either: P. aeruginosa density, symptoms, or spirometry [263]. At day 28, sputum myeloperoxidase, IL-1, and IL-8, sputum neutrophile elastase, and neutrophil counts showed a dose-dependent reduction in the KB001 (10 mg/kg)-treated CF patients compared with the placebo group [263]. The clinical trial showed that KB001 targeted P. aeruginosa TTSS in CF patients with persistent P. aeruginosa infection; it also decreased both airway inflammation and lung damage.
A phase I/II clinical trial also examined whether 16 weeks of KB001 treatment improved the time required for the use of antibiotics in patients with worsening respiratory symptoms. In addition, the trial examined KB001’s safety and efficacy in improving inflammatory markers and spirometry for patients with a history of chronic P. aeruginosa infections (NCT01695343) [264]. At least one infusion of KB001 (n = 83) or a placebo (n = 86) was given to a total of 169 patients [264]. Except for one serious adverse event involving increased liver enzymes, KB001 was generally safe and well tolerated when compared with placebo [264]. The time before an antibiotic was required was the same for all groups. At week 16, KB001-A outperformed the placebo, resulting in an increase in percent predicted forced expiratory volume in 1 s [264]. At week 16, sputum neutrophil elastase showed a nonsignificant decline, whereas IL-8 concentrations were considerably lower in KB001-treated patients than in the placebo group [264].
The Psl exopolysaccharide is another virulence factor present in numerous strains of P. aeruginosa [281]. Antibodies against Psl were detected by phenotypic screening of human antibody phage display libraries constructed from peripheral blood B cells of healthy volunteers and individuals recovering from P. aeruginosa infections [282]. The Psl monoclonal antibody MAb Cam-003 was further evaluated in the acute lethal pneumonia murine model in which the antibody provided significant protection against P. aeruginosa strains expressing PsL [282]. It was later postulated that anti-PcrV would benefit by combination with another antivirulence factor antibody [283]. A bispecific antibody, BiS4αPa, targeting both PcrV and Psl was developed and shown to be protective in the murine pneumonia model, which led to the development of the clinical candidate MEDI3902 (NCT02255760 and NCT02696902) [265,266,283]. In a phase I trial (NCT02255760), healthy patients between the ages of 18 and 60 were given a single intravenous infusion of MEDI3902 to assess the drug’s safety, pharmacokinetics, antidrug antibody responses, ex vivo anticytotoxicity, and opsonophagocytic killing capabilities against P. aeruginosa. Using randomization, 56 patients were randomly assigned to receive either 250, 750, 1500, or 3000 mg of MEDI3902 or a placebo over a period of 60 days [265]. There were no major adverse events. The pharmacokinetics of MEDI3902 were roughly linear for doses of 250, 750, and 1500 mg and nonlinear for dosages of 1500 and 3000 mg. All doses of MEDI3902 were linked with serum anticytotoxicity antibody concentrations and opsonophagocytic killing activity [265]. The results of MEDI3902’s phase I study in healthy volunteers justify further investigation of the drug’s efficacy and safety in patients at risk for P. aeruginosa pneumonia.
A phase II clinical trial examined the efficacy, pharmacokinetics, and safety of MEDI3902 in mechanically ventilated ICU patients infected with P. aeruginosa (NCT02696902) [266]. The study included 168 patients with PCR-confirmed P. aeruginosa colonization of the lower respiratory tract who were randomized into either a single IV infusion of 1500 mg MEDI3902 (n = 85) or placebo (n = 83) [266]. The study showed that a single IV dosage of MEDI3902 increased the drug concentration over the desired amount, but it did not reduce P. aeruginosa pneumonia as the primary efficacy outcome.
In another study, eggs from chickens that have received a P. aeruginosa vaccination were used to create “anti-pseudomonas IgY”, called PseudIgY (avian Ab to P. aeruginosa). The trial assessed whether daily gargling with PsAer-IgY against P. aeruginosa prevents P. aeruginosa infections in CF patients (NCT00633191) [267]. The study included 14 CF patients who experienced sporadic P. aeruginosa infections. In the trial, patients received a brief course of antibiotics to eliminate P. aeruginosa infection. After that, they began gargling with a PseudIgY solution every night to ward off infection [267].
Another phase III double-blind placebo-controlled trial investigated whether PseudIgY prolongs the time to reinfection with P. aeruginosa following a successful treatment of acute or intermittent infection (NCT01455675) [268]. The PseudIgY and the control groups were told to gargle and ingest either solution [268]. In the treatment group, the patients were required to gargle for 2 min 70 cc of the IgY/placebo solution every night for 2 years. The study enrolled 164 patients to be randomized into either group [268].
Several immunotherapeutic trials have been conducted targeting different antigens of P. aeruginosa. One of the targeted antigens was the O-polysaccharide moiety of P. aeruginosa serotype O11. A human monoclonal IgM antibody, KBPA-101 or panobacumab, was developed and found to be safe and efficacious in preclinical in vitro and in vivo studies and in phase I clinical trials (NCT00851435) [269,270,271]. In the trial, 14 patients who did not receive the antibody were compared with 17 panobacumab patients (13 of whom received the complete course of treatment, consisting of three doses of 1.2 mg/kg). Within the group that received the complete three-course panobacumab treatment, adjunctive immunotherapy led to improved clinical outcomes [269,270,271]. Specifically, patients that received panobacumab had a greater resolution rate of 85% (11/13) compared with 64% (9/14) in the place group. Compared with the control, patients who received panobacumab also had a shorter time to clinical resolution [269,270,271]. In addition, panobacumab was safe and resulted in a high clinical cure and survival rates in patients developing nosocomial P. aeruginosa O11 pneumonia [269,270,271].
More recently, a phase II clinical trial was conducted to assess the efficacy, safety, and pharmacokinetics of a new human monoclonal antibody against P. aeruginosa alginate, AR-105 (Aerucin®), as adjunctive therapeutic treatment to standard-of-care antibiotics for P. aeruginosa pneumonia in mechanically ventilated patients (NCT03027609) [272]. The study was conducted at approximately 100 clinical sites across 17 countries [272]. The 158 patients included in the clinical trial were split into the control group or those given one intravenous infusion of AR-105 20 mg/kg.
Recent studies were conducted to elucidate the mechanism of action of IgM-enriched immunoglobulin (IgM-IVIg) [284]. The studies involved utilizing LPS from E. coli to cause sepsis in two animal models: Syrian golden hamsters [285] or the CLP model to induce sepsis in rats [286]. Further studies in humans have been conducted to elucidate the mechanism of action of IgM-IVIg [287]. All three studies concluded that the administration of IgM-IVIg (Pentaglobin®) attenuated the endotoxin (LPS) activity by reducing inflammation. A clinical trial examined the efficacy of IgM-enriched intravenous immunoglobulin (Pentaglobin®—5 mL/kg over a 12 h intravenous infusion for 3 consecutive days) to decrease mortality in 120 neutropenic acute leukemia or hematopoietic stem cell transplant patients colonized with carbapenem-resistant Enterobacteriaceae or P. aeruginosa (NCT03494959) [273].
Another promising novel treatment, TRL1068, a human monoclonal antibody that targets DNA-binding protein II (DNABII), is currently undergoing clinical testing. DNABII is an important structural element of biofilms because it stabilizes extracellular DNA [288,289,290]. This monoclonal antibody is being investigated for use in prosthetic joint infections, particularly with multi-drug-resistant P. aeruginosa species [288,291].Though rare, prosthetic joint infections caused by Gram-negative bacilli, such as P. aeruginosa, is a catastrophic consequence that leads to prolonged hospitalization and higher medical costs [292,293,294]. Specifically, P. aeruginosa is linked to osteomyelitis, septic arthritis, and prosthetic joint infections due to its ability to adhere to bone and fibrocartilaginous articular structures. A phase I randomized clinical trial (NCT04763759) was designed to assess the overall safety and pharmacokinetics of TRL1068 in 18 patients with prosthetic joints using three separate dosages: 6, 15, and 30 mg/kg [274]. The study will measure the number of P. aeruginosa and the level of C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), IL-6, and IL-10 [274].
3.5. P. aeruginosa Outer Membrane Proteins as a Vaccine
The first trial (NCT00778388) involved active immunization with a P. aeruginosa hybrid protein, OprF/I (Table 5). In the hybrid protein, the P. aeruginosa outer membrane OprF is fused with the lipoprotein OprI. Active immunization of neutropenic mice with OprF/I was protective against lethal P. aeruginosa [295,296]. In addition, the anti-OprF/I antibody protected mice with severe combined immunodeficiency from P. aeruginosa infection. Furthermore, OprF/I affected the P. aeruginosa QS system and the QS-related virulence in the plant and worm models [295,296].
Table 5.
P. aeruginosa outer membrane proteins as a vaccine.
| Treatment/Study | Mechanism of Action | Population | Purpose | Phase | Number of Patients | Reference/Year |
|---|---|---|---|---|---|---|
| IC43 vaccine (recombinant P. aeruginosa outer membrane protein OprF/I) (NCT00778388) |
Induce immune response against P. aeruginosa outer membrane protein (OprF/I) | Healthy volunteers | Safety pharmacokinetics immunogenicity | I | 163 | 2013, [297] |
| IC43 vaccine (recombinant P. aeruginosa outer membrane protein OprF/I) (NCT00876252) |
Induce immune response against P. aeruginosa outer membrane protein (OprF/I) | Ventilated patients | Safety immunogenicity | II | 400 | 2017, [298] |
| IC43 vaccine (recombinant P. aeruginosa outer membrane protein OprF/I) (NCT01563263) |
Induce immune response against P. aeruginosa outer membrane protein (OprF/I) | Ventilated ICU patients | Efficacy in preventing P. aeruginosa infection immunogenicity safety | II/III | 800 | 2020, [299] |
A phase I trial examined the immunogenicity, safety, and tolerance in a healthy adult immunized with three different dosages of IC43 compared with placebo (NCT00778388) [297]. Two intramuscular injections of IC43 were administered in the deltoid region 7 days apart, and the patients were randomly assigned to one of five treatment groups: 50, 100, or 200 g IC43 with adjuvant, 100 g IC43 without adjuvant, or placebo (0.9% sodium chloride) [297]. In healthy volunteers, IC43 dosages of 50 g or more were sufficient to cause a plateau in IgG antibody responses [297].
A subsequent phase II study in which IC43 was used to immunize mechanically ventilated ICU patients has been completed using doses of 100 and 200 μg with adjuvant and a dose of 100 μg without adjuvant (NCT00876252) [298]. Patients were initially randomized to IC43 100 μg, IC43 200 μg, or placebo. On day 0, patients were immunized. On day 7, a second immunization was administered. Up until day 90, clinical study visits were conducted. The OprF/I specific immunoglobulin IgG antibody titer was the primary objective in the immunogenicity evaluation at day 14 [298]. At visits made in the ICU, surveillance cultures were obtained for the identification of P. aeruginosa from the blood, wounds, respiratory tract, urine, and central venous catheter [298]. When medically necessary, the investigator collected samples for P. aeruginosa diagnosis between each visit up to day 90 [298]. All IC43 groups had higher OprF/I IgG antibody titers on day 14 compared with placebo. With 100 μg IC43 alone, seroconversion was greatest (80.6%) [298]. Pseudomonas aeruginosa infection rates were not significantly different, and the IC43 groups had a low incidence of invasive infections (11.2%–14.0%; pneumonia) [298]. In the group of patients receiving 100 μg of IC43 with adjuvant, 2 patients (1.9%) reported experiencing serious adverse effects thought to be connected to the treatment [298]. No fatalities were connected to trial therapy, and both serious adverse events resolved [298]. Overall, IC43 produced a significant immunogenic effect; however, P. aeruginosa infection rates did not differ significantly among the four treatment groups [298].
Another double-blind phase II/III clinical trial examined the efficacy, immunogenicity, and safety of IC43 recombinant P. aeruginosa vaccine in nonsurgical ICU patients (NCT01563263) [299]. Eight hundred patients between the ages of 18 and 80 who were admitted to the ICU and anticipated needing mechanical ventilation for less than 48 h were randomized 1:1 to receive two doses of IC43 100 g or a saline placebo, spaced 7 days apart [299]. All-cause death in individuals 28 days following the initial vaccination served as the primary efficacy outcome. Safety and immunogenicity were also assessed [299]. At day 28, the all-cause mortality rates in the IC43 and placebo groups were 29.2% and 27.7%, respectively [299]. Both groups’ overall survival rates and the percentage of patients with less than one confirmed P. aeruginosa invasive infection or respiratory tract infection were similar [299]. Compared with the IC43 100 μg vaccinated group (93.1%), more patients in the placebo group (96.5%) experienced one adverse event [299]. Respiratory failure (6.9% vs. 5.7%, respectively), septic shock (4.1% vs. 6.5%), cardiac arrest (4.3% vs. 5.7%), multiorgan failure (4.6% vs. 5.5%), and sepsis (4.6% vs. 4.2%) were the most frequently reported experienced serious adverse events in the IC43 and placebo groups [299]. In the IC43 treated group, there were no linked significant adverse events recorded [299]. The IC43 100 g vaccination was well tolerated [299]. High immunogenicity was achieved by the vaccine; however, there was no therapeutic advantage above placebo in terms of reducing total mortality [299].
3.6. Strategies Targeting P. aeruginosa Iron Acquisition Systems
In addition to the previous treatments, other alternatives for eliminating P. aeruginosa infections have been investigated, including inhaled gallium and sodium nitrite (Table 6) [157,300,301]. A previous preclinical study showed that gallium works by decreasing bacterial iron (Fe) uptake and interfering with Fe signaling [302]. A phase I clinical trial examined the pharmacokinetics, safety, and tolerability of an intravenous infusion of a drug called Ganite® (IV gallium nitrate) in P. aeruginosa infected CF (NCT01093521) [157]. The trial included CF patients with chronic P. aeruginosa respiratory infections [157]. The 20 patients involved in the study were randomized into two groups that received continuous IV gallium nitrate (Ganite®) infusion for 5 days with either 100 or 200 mg/m2.
Table 6.
Strategies targeting P. aeruginosa iron acquisition systems.
| Treatment/Study | Mechanism of Action | Population | Purpose | Phase | Number of Patients | Reference/Year |
|---|---|---|---|---|---|---|
| Gallium (GANITE), inhaled (NCT01093521) |
Decreasing bacterial Fe uptake and interfering with Fe signaling | Cystic fibrosis patients with chronic P. aeruginosa respiratory tract infection | Pharmacokinetics, safety, and reduce P. aeruginosa burden | I | 20 | 2013, [157] |
| Gallium (IGNITE) (NCT02354859) |
Decreasing bacterial Fe uptake and interfering with Fe signaling | Cystic fibrosis patients | Improve pulmonary function | II | 119 | 2018, [300] |
| Inhaled sodium nitrite (NCT02694393) |
Decreasing bacterial Fe uptake and interfering with Fe signaling | Cystic fibrosis patients with chronic P. aeruginosa infection | Safety and efficacy reduced P. aeruginosa bioburden | I/II | 35 | 2023, [301] |
| Cefiderocol (NCT03032380) |
Decreasing bacterial Fe uptake and interfering with Fe signaling | Nosocomial pneumonia secondary to P. aeruginosa | Efficacy against nosocomial pneumonia secondary to P. aeruginosa | III | 300 | 2020, [303] |
A subsequent phase II, multicenter, randomized, placebo-controlled trial in adult CF patients chronically infected with P. aeruginosa was conducted to evaluate the efficacy of IV gallium in improving pulmonary function as measured by a 5% or greater relative improvement in forced expiratory volume in 1 s (FEV1) from baseline to day 28 (NCT02354859) [300]. The 119 patients were randomized into two groups: continuous infusion of either gallium nitrate (200 mg/m2/day) or normal saline over 5 days [300].
In addition to gallium, another phase I/II study assessed the safety of inhaled sodium nitrite in 35 adults with CF and chronic Pseudomonas infections to reduce the burden of P. aeruginosa NCT02694393) [301]. Sodium nitrite was effective in eliminating P. aeruginosa within the lung of CF-infected patients by increasing the susceptibility of P. aeruginosa to antibiotics [304,305]. Patients inhaled 46 or 80 mg of sodium nitrite twice daily for 4 weeks.
Cefedrolor is a novel siderophore cephalosporin with broad activity and high potency against multi-drug-resistant Gram-negative bacteria [303,306]. A phase III randomized clinical trial (NCT03032380) was designed to compare all-cause mortality at day 14 in patients receiving cefiderocol or meropenem for HABP, VABP, or health-care-associated bacterial pneumonia (HCABP) caused by Gram-negative pathogens [303,306]. Between 7 to 14 days, patients received 3 h intravenous infusions of either cefiderocol (n = 148) or meropenem (n = 152) every 8 h. Moreover, open-label intravenous linezolid was administered to all patients for a minimum of 5 days [303,306]. In patients with Gram-negative nosocomial pneumonia, cefiderocol had similar tolerability and was noninferior to high-dose, extended-infusion meropenem in terms of all-cause mortality on day 14. The findings suggest that cefiderocol is a feasible therapy choice for individuals with nosocomial pneumonia, even those infected with multi-drug-resistant Gram-negative bacteria [303,306].
4. Conclusions
It is evident from the outcomes of the different clinical trials described in this review that the road to developing an effective anti-P. aeruginosa vaccine or therapy is long and challenging. Antibiotics will still be essential in combating P. aeruginosa infections. For example, other clinical trials have examined newer combinations of beta-lactamases, polymyxins, and alternative formulations, such as liposomal or inhalation, for traditional antibiotics against P. aeruginosa infections [201,306,307,308,309,310,311,312,313,314,315,316,317,318,319,320,321]. However, the continuous use of these antibiotics will likely lead to the emergence of multi-drug-resistant P. aeruginosa mutants. Therefore, developing and testing several nontraditional therapies is critical. These therapies may not be alternatives to or replace the current antibiotics. Rather, they may be used in conjunction with antibiotics to help reduce the dose of antibiotics required to treat infected patients, which will lessen the production of P. aeruginosa bioburden and/or reduce the rate of emergence of antibiotic resistant mutants.
Besides antibiotics, anti-P. aeruginosa treatments described in this review include: bacteriophage; vaccines targeting outer membrane proteins (opRF/opRI); and treatment targeting an essential virulence factor, such as the QS system, T3SS, and monoclonal antibodies. As we show in this review, many of these approaches produced encouraging results. In addition, and except for bacteriophages, these treatments are less likely to exert pressure on P. aeruginosa to develop resistant mutants. Bacteriophage therapy shares with antibiotics the inherit problem of emerging phage- or antibiotic-resistant mutants that may result from the overuse of these bacteriophages. However, unlike antibiotics, the application of multiple phages (cocktails) will significantly reduce the problem associated with the emergence of antibiotic resistant mutants.
Among the different nontraditional therapies described in this review, the most promising one is targeting a specific P. aeruginosa virulence factor, such as outer membrane proteins or a system/mechanism that could help the production of multiple virulence factors, such as the QS system, T3SS, and iron-acquisition system. However, a key factor that needs to be considered in developing these therapies is the pathogenesis of P. aeruginosa in different acute and chronic infections, including acute pneumonia, VAP, burn wound infections, urinary tract infections, and chronic lung infections in CF patients. Several previous studies have already demonstrated that the capacity of P. aeruginosa to cause different infections lies in its ability to tailor the production of different cell-associated and extracellular virulence factors in response to environmental cues within certain infection sites, including lung wounds, blood, or urinary tract. Therefore, to further develop current therapies and to identify new targets for further therapies, it is critical to define a specific virulent factor(s) that is produced in response to the in vivo environment. Previous studies analyzed P. aeruginosa pathogenesis utilizing growth conditions that mimic the in vivo conditions. For example, Palmer et al. [322] utilized CF sputum to analyze the pathogenesis of P. aeruginosa during chronic lung infections of CF patients, while Kruczek utilized whole blood to examine the pathogenesis of P. aeruginosa infections in severely burned patients [253]. Palmer et al. showed that compared with a laboratory medium, the growth of P. aeruginosa in CF sputum differentially expressed the iron-acquisition genes and flagella motility genes and enhanced the production of the cell-to-cell communication molecule, PQS [322]. In addition, CF sputum enhanced the production of the cell-to-cell communication molecule PQS [322]. As we showed in this review, gallium-related therapy that was assessed in clinical trials targeted the P. aeruginosa iron-acquisition system. Additional anti-P. aeruginosa therapies to treat lung infections in CF patients may target the synthesis and/or the secretion of the PQS molecule. Similarly, Kruczek et al. showed that compared with the growth in blood from healthy volunteers, the growth of P. aeruginosa in blood from severely burned patients significantly increased the expression of more than 1000 genes, including genes encoding the QS-controlled virulence factors and those encoding the transport heme and phosphate. Any of these systems is a likely target for further therapy to prevent bacteremia and sepsis in P. aeruginosa–infected severely burned patients.
As we demonstrated in this review, several clinical trials were focused on assessing the effectiveness of new antibiotics targeting the PcrV protein (Table 4). The 32.3 kDa PcrV is one of the several proteins that constitute T3SS in P. aeruginosa. The PcrV protein plays a pivotal role in the pathogenesis of P. aeruginosa infections. The production of several cell-associated and extracellular virulence factors strongly indicates the reliance of P. aeruginosa on many of these factors in establishing infection at different sites within the host. However, despite these factors, previous studies using the murine model of lung infection and the murine model of thermal injury clearly showed that specific PcrV antibodies only compromised the ability of P. aeruginosa to cause lung infection and bacteremia/sepsis, respectively [277,323]. Based on the result of these studies, more monoclonal antibiotics were developed, and their effectiveness in targeting P. aeruginosa lung infections in CF patients was assessed using the clinical trials described in Table 4. However, so far, the results of these trials are not encouraging (Table 4). In the murine model of thermal injury, PcrV antibodies eliminated bacteremia and sepsis in the thermally injured mice but did not significantly reduce the P. aeruginosa bacterial load at the infection site [277]. This suggests that in severely burned patients, PcrV antibodies interfere with the P. aeruginosa–induced bacteremia/sepsis by affecting the translocation of P. aeruginosa into the bloodstream. Therefore, it is possible that future clinical studies may prove that the PcrV monoclonal antibodies are more effective in preventing P. aeruginosa bacteremia in severely burned patients than effectively treating P. aeruginosa lung infections in CF patients. In addition, future studies may identify a potential target, similar to PcrV, that plays a critical role in the pathogenesis of P. aeruginosa infections. A treatment containing a combination of antibodies to PcrV and such a target may prove to be more effective than PcrV antibody alone in treating lung infections in CF patients. The antibodies may also be utilized in synergy with currently available antibiotics in treating P. aeruginosa lung infections.
Acknowledgments
The authors thank Joanna E. Swickard and Karishma Bisht for the critical reading of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Partial funding was provided by the Department of Surgery at the Texas Tech University Health Sciences Center, Lubbock, Texas, USA.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Al-Hasan M.N., Wilson J.W., Lahr B.D., Eckel-Passow J.E., Baddour L.M. Incidence of Pseudomonas aeruginosa bacteremia: A population-based study. Am. J. Med. 2008;121:702–708. doi: 10.1016/j.amjmed.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peleg A.Y., Hooper D.C. Hospital-acquired infections due to gram-negative bacteria. N. Engl. J. Med. 2010;362:1804–1813. doi: 10.1056/NEJMra0904124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wisplinghoff H., Bischoff T., Tallent S.M., Seifert H., Wenzel R.P., Edmond M.B. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 4.Telling K., Laht M., Brauer A., Remm M., Kisand V., Maimets M., Tenson T., Lutsar I. Multidrug resistant Pseudomonas aeruginosa in Estonian hospitals. BMC Infect. Dis. 2018;18:513. doi: 10.1186/s12879-018-3421-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fridkin S.K., Welbel S.F., Weinstein R.A. Magnitude and prevention of nosocomial infections in the intensive care unit. Infect. Dis. Clin. N. Am. 1997;11:479–496. doi: 10.1016/S0891-5520(05)70366-4. [DOI] [PubMed] [Google Scholar]
- 6.National Nosocomial Infections Surveillance (NNIS) report, data summary from October 1986-April 1997, issued May 1997. A report from the NNIS System. Am. J. Infect Control. 1997;25:477–487. doi: 10.1016/S0196-6553(97)90071-7. [DOI] [PubMed] [Google Scholar]
- 7.Gaynes R., Edwards J.R., National Nosocomial Infections Surveillance System Overview of nosocomial infections caused by gram-negative bacilli. Clin. Infect. Dis. 2005;41:848–854. doi: 10.1086/432803. [DOI] [PubMed] [Google Scholar]
- 8.Litwin A., Rojek S., Gozdzik W., Duszynska W. Pseudomonas aeruginosa device associated—Healthcare associated infections and its multidrug resistance at intensive care unit of University Hospital: Polish, 8.5-year, prospective, single-centre study. BMC Infect. Dis. 2021;21:180. doi: 10.1186/s12879-021-05883-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kepenekli E., Soysal A., Yalindag-Ozturk N., Ozgur O., Ozcan I., Devrim I., Akar S., Bakir M., Turkish P.-H.S.G. Healthcare-associated infections in pediatric intensive care units in Turkey: A national point-prevalence survey. Jpn. J. Infect. Dis. 2015;68:381–386. doi: 10.7883/yoken.JJID.2014.385. [DOI] [PubMed] [Google Scholar]
- 10.Weiner L.M., Fridkin S.K., Aponte-Torres Z., Avery L., Coffin N., Dudeck M.A., Edwards J.R., Jernigan J.A., Konnor R., Soe M.M., et al. Vital Signs: Preventing antibiotic-resistant infections in hospitals—United States, 2014. MMWR Morb. Mortal. Wkly. Rep. 2016;65:235–241. doi: 10.15585/mmwr.mm6509e1. [DOI] [PubMed] [Google Scholar]
- 11.Afhami S., Seifi A., Hajiabdolbaghi M., Bazaz N.E., Hadadi A., Hasibi M., Rezaie P., Mohamadnejad E., Ghahan A., Hajinoori M., et al. Assessment of device-associated infection rates in teaching hospitals in Islamic Republic of Iran. East Mediterr. Health J. 2019;25:90–97. doi: 10.26719/emhj.18.015. [DOI] [PubMed] [Google Scholar]
- 12.Magill S.S., O’Leary E., Janelle S.J., Thompson D.L., Dumyati G., Nadle J., Wilson L.E., Kainer M.A., Lynfield R., Greissman S., et al. Changes in prevalence of health care-associated infections in U.S. hospitals. N. Engl. J. Med. 2018;379:1732–1744. doi: 10.1056/NEJMoa1801550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yallew W.W., Kumie A., Yehuala F.M. Point prevalence of hospital-acquired infections in two teaching hospitals of Amhara region in Ethiopia. Drug Healthc. Patient Saf. 2016;8:71–76. doi: 10.2147/DHPS.S107344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelly D.C., Rizzo J., Yun H.C., Blyth D.M. Microbiology and clinical characteristics of industrial oil burns. Burns. 2020;46:711–717. doi: 10.1016/j.burns.2019.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Wardhana A., Djan R., Halim Z. Bacterial and antimicrobial susceptibility profile and the prevalence of sepsis among burn patients at the burn unit of Cipto Mangunkusumo Hospital. Ann. Burns Fire Disasters. 2017;30:107–115. [PMC free article] [PubMed] [Google Scholar]
- 16.European Centre for Disease Prevention and Control . Healthcare-Associated Infections Acquired in Intensive Care Units. ECDC; Stockholm, Sweden: 2018. [Google Scholar]
- 17.European Centre for Disease Prevention and Control . Healthcare-Associated Infections Acquired in Intensive Care Units. ECDC; Stockholm, Sweden: 2019. [Google Scholar]
- 18.Li J., Long D., Wu S., Wu X., Wei B., Chen D., Shao Y., Wang H., Cui L., Chen X., et al. Association of CFH polymorphism with susceptibility to sepsis caused by Pseudomonas aeruginosa in Chinese Han populations: A multi-center study. Gene. 2020;722:144127. doi: 10.1016/j.gene.2019.144127. [DOI] [PubMed] [Google Scholar]
- 19.Song W., Lee K.M., Kang H.J., Shin D.H., Kim D.K. Microbiologic aspects of predominant bacteria isolated from the burn patients in Korea. Burns. 2001;27:136–139. doi: 10.1016/S0305-4179(00)00086-3. [DOI] [PubMed] [Google Scholar]
- 20.Yildirim S., Nursal T.Z., Tarim A., Torer N., Noyan T., Demiroglu Y.Z., Moray G., Haberal M. Bacteriological profile and antibiotic resistance: Comparison of findings in a burn intensive care unit, other intensive care units, and the hospital services unit of a single center. J. Burn Care Rehabil. 2005;26:488–492. doi: 10.1097/01.bcr.0000185454.72237.c6. [DOI] [PubMed] [Google Scholar]
- 21.Oncul O., Oksuz S., Acar A., Ulkur E., Turhan V., Uygur F., Ulcay A., Erdem H., Ozyurt M., Gorenek L. Nosocomial infection characteristics in a burn intensive care unit: Analysis of an eleven-year active surveillance. Burns. 2014;40:835–841. doi: 10.1016/j.burns.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Garcia Bernal F.J., Torrero V., Regalado J., Gabilondo F.J. Bacteriology in burn patients undergoing mechanical ventilation. Burns. 2000;26:731–736. doi: 10.1016/S0305-4179(00)00055-3. [DOI] [PubMed] [Google Scholar]
- 23.Micek S.T., Chew B., Hampton N., Kollef M.H. A case-control study assessing the impact of nonventilated hospital-acquired pneumonia on patient outcomes. Chest. 2016;150:1008–1014. doi: 10.1016/j.chest.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Restrepo M.I., Babu B.L., Reyes L.F., Chalmers J.D., Soni N.J., Sibila O., Faverio P., Cilloniz C., Rodriguez-Cintron W., Aliberti S., et al. Burden and risk factors for Pseudomonas aeruginosa community-acquired pneumonia: A multinational point prevalence study of hospitalised patients. Eur. Respir. J. 2018;52:1701190. doi: 10.1183/13993003.01190-2017. [DOI] [PubMed] [Google Scholar]
- 25.Vesteinsdottir E., Helgason K.O., Sverrisson K.O., Gudlaugsson O., Karason S. Infections and outcomes after cardiac surgery-The impact of outbreaks traced to transesophageal echocardiography probes. Acta Anaesthesiol. Scand. 2019;63:871–878. doi: 10.1111/aas.13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tubbs D., Lenney W., Alcock P., Campbell C.A., Gray J., Pantin C. Pseudomonas aeruginosa in cystic fibrosis: Cross-infection and the need for segregation. Respir. Med. 2001;95:147–152. doi: 10.1053/rmed.2000.1009. [DOI] [PubMed] [Google Scholar]
- 27.Blanchard A.C., Waters V.J. Microbiology of cystic fibrosis airway disease. Semin. Respir. Crit. Care Med. 2019;40:727–736. doi: 10.1055/s-0039-1698464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lebecque P., Leal T., Zylberberg K., Reychler G., Bossuyt X., Godding V. Towards zero prevalence of chronic Pseudomonas aeruginosa infection in children with cystic fibrosis. J. Cyst. Fibros. 2006;5:237–244. doi: 10.1016/j.jcf.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Cystic Fibrosis Foundation . Patient Registry 2020 Annual Data Report. Cystic Fibrosis Foundation; Seattle, WA, USA: 2021. [Google Scholar]
- 30.Paixao V.A., Barros T.F., Mota C.M., Moreira T.F., Santana M.A., Reis J.N. Prevalence and antimicrobial susceptibility of respiratory pathogens in patients with cystic fibrosis. Braz. J. Infect. Dis. 2010;14:406–409. doi: 10.1016/S1413-8670(10)70083-0. [DOI] [PubMed] [Google Scholar]
- 31.Cystic Fibrosis Foundation . Patient Registry 2021 Annual Data Report. Cystic Fibrosis Foundation; Seattle, WA, USA: 2022. [Google Scholar]
- 32.Psoter K.J., De Roos A.J., Wakefield J., Mayer J., Rosenfeld M. Season is associated with Pseudomonas aeruginosa acquisition in young children with cystic fibrosis. Clin. Microbiol. Infect. 2013;19:E483–E489. doi: 10.1111/1469-0691.12272. [DOI] [PubMed] [Google Scholar]
- 33.Pittman J.E., Noah H., Calloway H.E., Davis S.D., Leigh M.W., Drumm M., Sagel S.D., Accurso F.J., Knowles M.R., Sontag M.K. Early childhood lung function is a stronger predictor of adolescent lung function in cystic fibrosis than early Pseudomonas aeruginosa infection. PLoS ONE. 2017;12:e0177215. doi: 10.1371/journal.pone.0177215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keating C., Poor A.D., Liu X., Chiuzan C., Backenroth D., Zhang Y., DiMango E. Reduced survival in adult cystic fibrosis despite attenuated lung function decline. J. Cyst. Fibros. 2017;16:78–84. doi: 10.1016/j.jcf.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 35.Ahlgren H.G., Benedetti A., Landry J.S., Bernier J., Matouk E., Radzioch D., Lands L.C., Rousseau S., Nguyen D. Clinical outcomes associated with Staphylococcus aureus and Pseudomonas aeruginosa airway infections in adult cystic fibrosis patients. BMC Pulm. Med. 2015;15:67. doi: 10.1186/s12890-015-0062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holm A.E., Schultz H.H.L., Johansen H.K., Pressler T., Lund T.K., Iversen M., Perch M. Bacterial re-colonization occurs early after lung transplantation in cystic fibrosis patients. J. Clin. Med. 2021;10:1275. doi: 10.3390/jcm10061275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erfanimanesh S., Emaneini M., Modaresi M.R., Feizabadi M.M., Halimi S., Beigverdi R., Nikbin V.S., Jabalameli F. Distribution and characteristics of bacteria isolated from cystic fibrosis patients with pulmonary exacerbation. Can. J. Infect. Dis. Med. Microbiol. 2022;2022:5831139. doi: 10.1155/2022/5831139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franciosi A.N., Wilcox P.G., Quon B.S. Cystic fibrosis respiratory microbiology monitoring during a global pandemic: Lessons learned from a shift to telehealth. Ann. Am. Thorac. Soc. 2022;19:498–500. doi: 10.1513/AnnalsATS.202101-087RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harbarth S., Ferriere K., Hugonnet S., Ricou B., Suter P., Pittet D. Epidemiology and prognostic determinants of bloodstream infections in surgical intensive care. Arch. Surg. 2002;137:1353–1359. doi: 10.1001/archsurg.137.12.1353. discussion 1359. [DOI] [PubMed] [Google Scholar]
- 40.Wisplinghoff H., Cornely O.A., Moser S., Bethe U., Stutzer H., Salzberger B., Fatkenheuer G., Seifert H. Outcomes of nosocomial bloodstream infections in adult neutropenic patients: A prospective cohort and matched case-control study. Infect. Control. Hosp. Epidemiol. 2003;24:905–911. doi: 10.1086/502158. [DOI] [PubMed] [Google Scholar]
- 41.Williams F.N., Herndon D.N., Hawkins H.K., Lee J.O., Cox R.A., Kulp G.A., Finnerty C.C., Chinkes D.L., Jeschke M.G. The leading causes of death after burn injury in a single pediatric burn center. Crit. Care. 2009;13:R183. doi: 10.1186/cc8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wisplinghoff H., Seifert H., Wenzel R.P., Edmond M.B. Current trends in the epidemiology of nosocomial bloodstream infections in patients with hematological malignancies and solid neoplasms in hospitals in the United States. Clin. Infect. Dis. 2003;36:1103–1110. doi: 10.1086/374339. [DOI] [PubMed] [Google Scholar]
- 43.Osmon S., Ward S., Fraser V.J., Kollef M.H. Hospital mortality for patients with bacteremia due to Staphylococcus aureus or Pseudomonas aeruginosa. Chest. 2004;125:607–616. doi: 10.1378/chest.125.2.607. [DOI] [PubMed] [Google Scholar]
- 44.Mikulska M., Del Bono V., Raiola A.M., Bruno B., Gualandi F., Occhini D., di Grazia C., Frassoni F., Bacigalupo A., Viscoli C. Blood stream infections in allogeneic hematopoietic stem cell transplant recipients: Reemergence of Gram-negative rods and increasing antibiotic resistance. Biol. Blood Marrow. Transplant. 2009;15:47–53. doi: 10.1016/j.bbmt.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 45.Vitkauskiene A., Skrodeniene E., Dambrauskiene A., Macas A., Sakalauskas R. Pseudomonas aeruginosa bacteremia: Resistance to antibiotics, risk factors, and patient mortality. Medicina. 2010;46:490–495. doi: 10.3390/medicina46070071. [DOI] [PubMed] [Google Scholar]
- 46.Satlin M.J., Soave R., Racanelli A.C., Shore T.B., van Besien K., Jenkins S.G., Walsh T.J. The emergence of vancomycin-resistant enterococcal bacteremia in hematopoietic stem cell transplant recipients. Leuk Lymphoma. 2014;55:2858–2865. doi: 10.3109/10428194.2014.896007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seo S.K., Xiao K., Huang Y.T., Jongwutiwes U., Chung D., Maloy M., Giralt S., Barker J.N., Jakubowski A.A., Papanicolaou G.A. Impact of peri-transplant vancomycin and fluoroquinolone administration on rates of bacteremia in allogeneic hematopoietic stem cell transplant (HSCT) recipients: A 12-year single institution study. J. Infect. 2014;69:341–351. doi: 10.1016/j.jinf.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Magret M., Lisboa T., Martin-Loeches I., Manez R., Nauwynck M., Wrigge H., Cardellino S., Diaz E., Koulenti D., Rello J. Bacteremia is an independent risk factor for mortality in nosocomial pneumonia: A prospective and observational multicenter study. Crit. Care. 2011;15:R62. doi: 10.1186/cc10036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trecarichi E.M., Pagano L., Candoni A., Pastore D., Cattaneo C., Fanci R., Nosari A., Caira M., Spadea A., Busca A., et al. Current epidemiology and antimicrobial resistance data for bacterial bloodstream infections in patients with hematologic malignancies: An Italian multicentre prospective survey. Clin. Microbiol. Infect. 2015;21:337–343. doi: 10.1016/j.cmi.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 50.Kuo F.C., Wang S.M., Shen C.F., Ma Y.J., Ho T.S., Chen J.S., Cheng C.N., Liu C.C. Bloodstream infections in pediatric patients with acute leukemia: Emphasis on gram-negative bacteria infections. J. Microbiol. Immunol. Infect. 2017;50:507–513. doi: 10.1016/j.jmii.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 51.Kikuchi M., Akahoshi Y., Nakano H., Ugai T., Wada H., Yamasaki R., Sakamoto K., Kawamura K., Ishihara Y., Sato M., et al. Risk factors for pre- and post-engraftment bloodstream infections after allogeneic hematopoietic stem cell transplantation. Transpl. Infect. Dis. 2015;17:56–65. doi: 10.1111/tid.12345. [DOI] [PubMed] [Google Scholar]
- 52.Calvo-Lon J., Landaverde D.U., Ramos-Esquivel A., Villalobos-Vindas J.M. Epidemiology and outcomes of bloodstream infections in patients with solid tumors in a Central American population at Mexico Hospital, San Jose, Costa Rica. J. Glob. Oncol. 2018;4:1–6. doi: 10.1200/JGO.17.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marin M., Gudiol C., Garcia-Vidal C., Ardanuy C., Carratala J. Bloodstream infections in patients with solid tumors: Epidemiology, antibiotic therapy, and outcomes in 528 episodes in a single cancer center. Medicine (Baltimore) 2014;93:143–149. doi: 10.1097/MD.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sousa D., Ceniceros A., Galeiras R., Pertega-Diaz S., Gutierrez-Urbon J.M., Rodriguez-Mayo M., Lopez-Suso E., Mourelo-Farina M., Llinares P. Microbiology in burns patients with blood stream infections: Trends over time and during the course of hospitalization. Infect. Dis. 2018;50:289–296. doi: 10.1080/23744235.2017.1397738. [DOI] [PubMed] [Google Scholar]
- 55.Buetti N., Lo Priore E., Sommerstein R., Atkinson A., Kronenberg A., Marschall J., Swiss Centre for Antibiotic resistance (ANRESIS) Epidemiology of subsequent bloodstream infections in the ICU. Crit. Care. 2018;22:259. doi: 10.1186/s13054-018-2148-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thaden J.T., Park L.P., Maskarinec S.A., Ruffin F., Fowler V.G., Jr., van Duin D. Results from a 13-year prospective cohort study show increased mortality associated with bloodstream infections caused by Pseudomonas aeruginosa compared to other bacteria. Antimicrob. Agents Chemother. 2017;61:e02671-16. doi: 10.1128/AAC.02671-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stoma I., Karpov I., Milanovich N., Uss A., Iskrov I. Risk factors for mortality in patients with bloodstream infections during the pre-engraftment period after hematopoietic stem cell transplantation. Blood Res. 2016;51:102–106. doi: 10.5045/br.2016.51.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Diekema D.J., Pfaller M.A., Jones R.N., Doern G.V., Winokur P.L., Gales A.C., Sader H.S., Kugler K., Beach M. Survey of bloodstream infections due to gram-negative bacilli: Frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, and Latin America for the SENTRY Antimicrobial Surveillance Program, 1997. Clin. Infect. Dis. 1999;29:595–607. doi: 10.1086/598640. [DOI] [PubMed] [Google Scholar]
- 59.Garcia-Vidal C., Cardozo-Espinola C., Puerta-Alcalde P., Marco F., Tellez A., Aguero D., Romero-Santana F., Diaz-Beya M., Gine E., Morata L., et al. Risk factors for mortality in patients with acute leukemia and bloodstream infections in the era of multiresistance. PLoS ONE. 2018;13:e0199531. doi: 10.1371/journal.pone.0199531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fily F., Ronat J.B., Malou N., Kanapathipillai R., Seguin C., Hussein N., Fakhri R.M., Langendorf C. Post-traumatic osteomyelitis in Middle East war-wounded civilians: Resistance to first-line antibiotics in selected bacteria over the decade 2006-2016. BMC Infect. Dis. 2019;19:103. doi: 10.1186/s12879-019-3741-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Djuric O., Markovic-Denic L., Jovanovic B., Bumbasirevic V. High incidence of multiresistant bacterial isolates from bloodstream infections in trauma emergency department and intensive care unit in Serbia. Acta Microbiol. Immunol. Hung. 2019;66:307–325. doi: 10.1556/030.66.2019.007. [DOI] [PubMed] [Google Scholar]
- 62.Lari A.R., Alaghehbandan R. Nosocomial infections in an Iranian burn care center. Burns. 2000;26:737–740. doi: 10.1016/S0305-4179(00)00048-6. [DOI] [PubMed] [Google Scholar]
- 63.Park J.J., Seo Y.B., Choi Y.K., Kym D., Lee J. Changes in the prevalence of causative pathogens isolated from severe burn patients from 2012 to 2017. Burns. 2020;46:695–701. doi: 10.1016/j.burns.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 64.Rello J., Jubert P., Vallés J., Artigas A., Rué M., Niederman M.S. Evaluation of outcome for intubated patients with pneumonia due to Pseudomonas aeruginosa. Clin. Infect. Dis. 1996;23:973–978. doi: 10.1093/clinids/23.5.973. [DOI] [PubMed] [Google Scholar]
- 65.Kollef M.H., Chastre J., Fagon J.Y., François B., Niederman M.S., Rello J., Torres A., Vincent J.L., Wunderink R.G., Go K.W., et al. Global prospective epidemiologic and surveillance study of ventilator-associated pneumonia due to Pseudomonas aeruginosa. Crit. Care Med. 2014;42:2178–2187. doi: 10.1097/CCM.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 66.Chastre J., Fagon J.Y. Ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 2002;165:867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 67.Rello J., Borgatta B., Lagunes L. Management of Pseudomonas aeruginosa pneumonia: One size does not fit all. Crit. Care. 2014;18:136. doi: 10.1186/cc13849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rello J., Lisboa T., Koulenti D. Respiratory infections in patients undergoing mechanical ventilation. Lancet Respir. Med. 2014;2:764–774. doi: 10.1016/S2213-2600(14)70171-7. [DOI] [PubMed] [Google Scholar]
- 69.Rello J., Ausina V., Ricart M., Puzo C., Quintana E., Net A., Prats G. Risk factors for infection by Pseudomonas aeruginosa in patients with ventilator-associated pneumonia. Intensive Care Med. 1994;20:193–198. doi: 10.1007/BF01704699. [DOI] [PubMed] [Google Scholar]
- 70.Rello J., Mariscal D., March F., Jubert P., Sanchez F., Valles J., Coll P. Recurrent Pseudomonas aeruginosa pneumonia in ventilated patients: Relapse or reinfection? Am. J. Respir. Crit. Care Med. 1998;157:912–916. doi: 10.1164/ajrccm.157.3.9703014. [DOI] [PubMed] [Google Scholar]
- 71.Vallés J., Mesalles E., Mariscal D., del Mar Fernández M., Peña R., Jiménez J.L., Rello J. A 7-year study of severe hospital-acquired pneumonia requiring ICU admission. Intensive Care Med. 2003;29:1981–1988. doi: 10.1007/s00134-003-2008-4. [DOI] [PubMed] [Google Scholar]
- 72.Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 73.Garnacho-Montero J., Sa-Borges M., Sole-Violan J., Barcenilla F., Escoresca-Ortega A., Ochoa M., Cayuela A., Rello J. Optimal management therapy for Pseudomonas aeruginosa ventilator-associated pneumonia: An observational, multicenter study comparing monotherapy with combination antibiotic therapy. Crit. Care Med. 2007;35:1888–1895. doi: 10.1097/01.CCM.0000275389.31974.22. [DOI] [PubMed] [Google Scholar]
- 74.Safdar N., Handelsman J., Maki D.G. Does combination antimicrobial therapy reduce mortality in Gram-negative bacteraemia? A meta-analysis. Lancet Infect. Dis. 2004;4:519–527. doi: 10.1016/S1473-3099(04)01108-9. [DOI] [PubMed] [Google Scholar]
- 75.Garnacho-Montero J., Corcia-Palomo Y., Amaya-Villar R., Martin-Villen L. How to treat VAP due to MDR pathogens in ICU patients. BMC Infect. Dis. 2014;14:135. doi: 10.1186/1471-2334-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ramsey B.W., Dorkin H.L., Eisenberg J.D., Gibson R.L., Harwood I.R., Kravitz R.M., Schidlow D.V., Wilmott R.W., Astley S.J., McBurnie M.A., et al. Efficacy of aerosolized tobramycin in patients with cystic fibrosis. N. Engl. J. Med. 1993;328:1740–1746. doi: 10.1056/NEJM199306173282403. [DOI] [PubMed] [Google Scholar]
- 77.Rowe S.M., Miller S., Sorscher E.J. Cystic fibrosis. N. Engl. J. Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 78.Rogan M.P., Stoltz D.A., Hornick D.B. Cystic fibrosis transmembrane conductance regulator intracellular processing, trafficking, and opportunities for mutation-specific treatment. Chest. 2011;139:1480–1490. doi: 10.1378/chest.10-2077. [DOI] [PubMed] [Google Scholar]
- 79.Mogayzel P.J., Jr., Naureckas E.T., Robinson K.A., Brady C., Guill M., Lahiri T., Lubsch L., Matsui J., Oermann C.M., Ratjen F., et al. Cystic Fibrosis Foundation pulmonary guideline. pharmacologic approaches to prevention and eradication of initial Pseudomonas aeruginosa infection. Ann. Am. Thorac. Soc. 2014;11:1640–1650. doi: 10.1513/AnnalsATS.201404-166OC. [DOI] [PubMed] [Google Scholar]
- 80.Saint-Criq V., Gray M.A. Role of CFTR in epithelial physiology. Cell. Mol. Life Sci. 2017;74:93–115. doi: 10.1007/s00018-016-2391-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Döring G., Flume P., Heijerman H., Elborn J.S. Treatment of lung infection in patients with cystic fibrosis: Current and future strategies. J. Cyst. Fibros. 2012;11:461–479. doi: 10.1016/j.jcf.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 82.Lipuma J.J. The changing microbial epidemiology in cystic fibrosis. Clin. Microbiol. Rev. 2010;23:299–323. doi: 10.1128/CMR.00068-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Esposito A., Pompilio A., Bettua C., Crocetta V., Giacobazzi E., Fiscarelli E., Jousson O., Di Bonaventura G. Evolution of Stenotrophomonas maltophilia in Cystic Fibrosis Lung over Chronic Infection: A Genomic and Phenotypic Population Study. Front. Microbiol. 2017;8:1590. doi: 10.3389/fmicb.2017.01590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ratjen F., Brockhaus F., Angyalosi G. Aminoglycoside therapy against Pseudomonas aeruginosa in cystic fibrosis: A review. J. Cyst. Fibros. 2009;8:361–369. doi: 10.1016/j.jcf.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 85.Stephenson A.L., Bell S.C. The Cystic Fibrosis Foundation Patient Registry. Design and Methods of a National Observational Disease Registry. Ann. Am. Thorac. Soc. 2016;13:1014–1015. doi: 10.1513/AnnalsATS.201604-250ED. [DOI] [PubMed] [Google Scholar]
- 86.Kenna D.T., Doherty C.J., Foweraker J., Macaskill L., Barcus V.A., Govan J.R.W. Hypermutability in environmental Pseudomonas aeruginosa and in populations causing pulmonary infection in individuals with cystic fibrosis. Microbiology. 2007;153:1852–1859. doi: 10.1099/mic.0.2006/005082-0. [DOI] [PubMed] [Google Scholar]
- 87.Ciofu O., Mandsberg L.F., Wang H., Høiby N. Phenotypes selected during chronic lung infection in cystic fibrosis patients: Implications for the treatment of Pseudomonas aeruginosa biofilm infections. FEMS Immunol. Med. Microbiol. 2012;65:215–225. doi: 10.1111/j.1574-695X.2012.00983.x. [DOI] [PubMed] [Google Scholar]
- 88.Drenkard E., Ausubel F.M. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature. 2002;416:740–743. doi: 10.1038/416740a. [DOI] [PubMed] [Google Scholar]
- 89.LiPuma J.J. Microbiological and immunologic considerations with aerosolized drug delivery. Chest. 2001;120:118s–123s. doi: 10.1378/chest.120.3_suppl.118S. [DOI] [PubMed] [Google Scholar]
- 90.Flume P.A., VanDevanter D.R. Clinical applications of pulmonary delivery of antibiotics. Adv. Drug Deliv. Rev. 2015;85:1–6. doi: 10.1016/j.addr.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Máiz L., Girón R.M., Olveira C., Quintana E., Lamas A., Pastor D., Cantón R., Mensa J. Inhaled antibiotics for the treatment of chronic bronchopulmonary Pseudomonas aeruginosa infection in cystic fibrosis: Systematic review of randomised controlled trials. Expert Opin. Pharmacother. 2013;14:1135–1149. doi: 10.1517/14656566.2013.790366. [DOI] [PubMed] [Google Scholar]
- 92.Sanders D.B., Hoffman L.R., Emerson J., Gibson R.L., Rosenfeld M., Redding G.J., Goss C.H. Return of FEV1 after pulmonary exacerbation in children with cystic fibrosis. Pediatr. Pulmonol. 2010;45:127–134. doi: 10.1002/ppul.21117. [DOI] [PubMed] [Google Scholar]
- 93.McManus A.T., Mason A.D., Jr., McManus W.F., Pruitt B.A., Jr. Twenty-five year review of Pseudomonas aeruginosa bacteremia in a burn center. Eur. J. Clin. Microbiol. 1985;4:219–223. doi: 10.1007/BF02013601. [DOI] [PubMed] [Google Scholar]
- 94.Ranjbar R., Owlia P., Saderi H., Mansouri S., Jonaidi-Jafari N., Izadi M., Farshad S., Arjomandzadegan M. Characterization of Pseudomonas aeruginosa strains isolated from burned patients hospitalized in a major burn center in Tehran, Iran. Acta Med. Iran. 2011;49:675–679. [PubMed] [Google Scholar]
- 95.de Almeida Silva K.C.F., Calomino M.A., Deutsch G., de Castilho S.R., de Paula G.R., Esper L.M.R., Teixeira L.A. Molecular characterization of multidrug-resistant (MDR) Pseudomonas aeruginosa isolated in a burn center. Burns. 2017;43:137–143. doi: 10.1016/j.burns.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 96.Fransén J., Huss F.R., Nilsson L.E., Rydell U., Sjöberg F., Hanberger H. Surveillance of antibiotic susceptibility in a Swedish Burn Center 1994-2012. Burns. 2016;42:1295–1303. doi: 10.1016/j.burns.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 97.Pang Z., Raudonis R., Glick B.R., Lin T.J., Cheng Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019;37:177–192. doi: 10.1016/j.biotechadv.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 98.Mathee K., Narasimhan G., Valdes C., Qiu X., Matewish J.M., Koehrsen M., Rokas A., Yandava C.N., Engels R., Zeng E., et al. Dynamics of Pseudomonas aeruginosa genome evolution. Proc. Natl. Acad. Sci. USA. 2008;105:3100–3105. doi: 10.1073/pnas.0711982105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Passador L., Cook J.M., Gambello M.J., Rust L., Iglewski B.H. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science. 1993;260:1127–1130. doi: 10.1126/science.8493556. [DOI] [PubMed] [Google Scholar]
- 100.Pesci E.C., Milbank J.B., Pearson J.P., McKnight S., Kende A.S., Greenberg E.P., Iglewski B.H. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA. 1999;96:11229–11234. doi: 10.1073/pnas.96.20.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pearson J.P., Passador L., Iglewski B.H., Greenberg E.P. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA. 1995;92:1490–1494. doi: 10.1073/pnas.92.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pearson J.P., Gray K.M., Passador L., Tucker K.D., Eberhard A., Iglewski B.H., Greenberg E.P. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc. Natl. Acad. Sci. USA. 1994;91:197–201. doi: 10.1073/pnas.91.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bucior I., Pielage J.F., Engel J.N. Pseudomonas aeruginosa pili and flagella mediate distinct binding and signaling events at the apical and basolateral surface of airway epithelium. PLoS Pathog. 2012;8:e1002616. doi: 10.1371/journal.ppat.1002616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van Delden C. Virulence Factors in Pseudomonas Aeruginosa. In: Ramos J.-L., editor. Virulence and Gene Regulation. Springer; Boston, MA, USA: 2004. pp. 3–45. [Google Scholar]
- 105.Leid J.G., Willson C.J., Shirtliff M.E., Hassett D.J., Parsek M.R., Jeffers A.K. The exopolysaccharide alginate protects Pseudomonas aeruginosa biofilm bacteria from IFN-gamma-mediated macrophage killing. J. Immunol. 2005;175:7512–7518. doi: 10.4049/jimmunol.175.11.7512. [DOI] [PubMed] [Google Scholar]
- 106.Ghafoor A., Hay I.D., Rehm B.H. Role of exopolysaccharides in Pseudomonas aeruginosa biofilm formation and architecture. Appl. Environ. Microbiol. 2011;77:5238–5246. doi: 10.1128/AEM.00637-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hentzer M., Teitzel G.M., Balzer G.J., Heydorn A., Molin S., Givskov M., Parsek M.R. Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J. Bacteriol. 2001;183:5395–5401. doi: 10.1128/JB.183.18.5395-5401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ramsey D.M., Wozniak D.J. Understanding the control of Pseudomonas aeruginosa alginate synthesis and the prospects for management of chronic infections in cystic fibrosis. Mol. Microbiol. 2005;56:309–322. doi: 10.1111/j.1365-2958.2005.04552.x. [DOI] [PubMed] [Google Scholar]
- 109.Maldonado R.F., Sa-Correia I., Valvano M.A. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol. Rev. 2016;40:480–493. doi: 10.1093/femsre/fuw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Huszczynski S.M., Lam J.S., Khursigara C.M. The Role of Pseudomonas aeruginosa Lipopolysaccharide in Bacterial Pathogenesis and Physiology. Pathogens. 2019;9:6. doi: 10.3390/pathogens9010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kang D., Kirienko D.R., Webster P., Fisher A.L., Kirienko N.V. Pyoverdine, a siderophore from Pseudomonas aeruginosa, translocates into C. elegans, removes iron, and activates a distinct host response. Virulence. 2018;9:804–817. doi: 10.1080/21505594.2018.1449508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lau G.W., Hassett D.J., Ran H., Kong F. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol. Med. 2004;10:599–606. doi: 10.1016/j.molmed.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 113.Laarman A.J., Bardoel B.W., Ruyken M., Fernie J., Milder F.J., van Strijp J.A., Rooijakkers S.H. Pseudomonas aeruginosa alkaline protease blocks complement activation via the classical and lectin pathways. J. Immunol. 2012;188:386–393. doi: 10.4049/jimmunol.1102162. [DOI] [PubMed] [Google Scholar]
- 114.Bleves S., Viarre V., Salacha R., Michel G.P., Filloux A., Voulhoux R. Protein secretion systems in Pseudomonas aeruginosa: A wealth of pathogenic weapons. Int. J. Med. Microbiol. 2010;300:534–543. doi: 10.1016/j.ijmm.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 115.Newman J.W., Floyd R.V., Fothergill J.L. The contribution of Pseudomonas aeruginosa virulence factors and host factors in the establishment of urinary tract infections. FEMS Microbiol. Lett. 2017;364:fnx124. doi: 10.1093/femsle/fnx124. [DOI] [PubMed] [Google Scholar]
- 116.Engel L.S., Hill J.M., Caballero A.R., Green L.C., O’Callaghan R.J. Protease IV, a unique extracellular protease and virulence factor from Pseudomonas aeruginosa. J. Biol. Chem. 1998;273:16792–16797. doi: 10.1074/jbc.273.27.16792. [DOI] [PubMed] [Google Scholar]
- 117.Zhu J., Cai X., Harris T.L., Gooyit M., Wood M., Lardy M., Janda K.D. Disarming Pseudomonas aeruginosa virulence factor LasB by leveraging a Caenorhabditis elegans infection model. Chem. Biol. 2015;22:483–491. doi: 10.1016/j.chembiol.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 118.Tielen P., Rosenau F., Wilhelm S., Jaeger K.E., Flemming H.C., Wingender J. Extracellular enzymes affect biofilm formation of mucoid Pseudomonas aeruginosa. Microbiology. 2010;156:2239–2252. doi: 10.1099/mic.0.037036-0. [DOI] [PubMed] [Google Scholar]
- 119.Park P.W., Pier G.B., Preston M.J., Goldberger O., Fitzgerald M.L., Bernfield M. Syndecan-1 shedding is enhanced by LasA, a secreted virulence factor of Pseudomonas aeruginosa. J. Biol. Chem. 2000;275:3057–3064. doi: 10.1074/jbc.275.5.3057. [DOI] [PubMed] [Google Scholar]
- 120.Spencer J., Murphy L.M., Conners R., Sessions R.B., Gamblin S.J. Crystal structure of the LasA virulence factor from Pseudomonas aeruginosa: Substrate specificity and mechanism of M23 metallopeptidases. J. Mol. Biol. 2010;396:908–923. doi: 10.1016/j.jmb.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 121.Kessler E., Safrin M., Abrams W.R., Rosenbloom J., Ohman D.E. Inhibitors and specificity of Pseudomonas aeruginosa LasA. J. Biol. Chem. 1997;272:9884–9889. doi: 10.1074/jbc.272.15.9884. [DOI] [PubMed] [Google Scholar]
- 122.Guillon A., Brea D., Morello E., Tang A., Jouan Y., Ramphal R., Korkmaz B., Perez-Cruz M., Trottein F., O’Callaghan R.J., et al. Pseudomonas aeruginosa proteolytically alters the interleukin 22-dependent lung mucosal defense. Virulence. 2017;8:810–820. doi: 10.1080/21505594.2016.1253658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Michalska M., Wolf P. Pseudomonas Exotoxin A: Optimized by evolution for effective killing. Front. Microbiol. 2015;6:963. doi: 10.3389/fmicb.2015.00963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schultz M.J., Speelman P., Zaat S.A., Hack C.E., van Deventer S.J., van der Poll T. The effect of pseudomonas exotoxin A on cytokine production in whole blood exposed to Pseudomonas aeruginosa. FEMS Immunol. Med. Microbiol. 2000;29:227–232. doi: 10.1111/j.1574-695X.2000.tb01527.x. [DOI] [PubMed] [Google Scholar]
- 125.Frank D.W. The exoenzyme S regulon of Pseudomonas aeruginosa. Mol. Microbiol. 1997;26:621–629. doi: 10.1046/j.1365-2958.1997.6251991.x. [DOI] [PubMed] [Google Scholar]
- 126.Rangel S.M., Logan L.K., Hauser A.R. The ADP-ribosyltransferase domain of the effector protein ExoS inhibits phagocytosis of Pseudomonas aeruginosa during pneumonia. mBio. 2014;5:e01080-14. doi: 10.1128/mBio.01080-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rangel S.M., Diaz M.H., Knoten C.A., Zhang A., Hauser A.R. The Role of ExoS in Dissemination of Pseudomonas aeruginosa during Pneumonia. PLoS Pathog. 2015;11:e1004945. doi: 10.1371/journal.ppat.1004945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Diaz M.H., Hauser A.R. Pseudomonas aeruginosa cytotoxin ExoU is injected into phagocytic cells during acute pneumonia. Infect. Immun. 2010;78:1447–1456. doi: 10.1128/IAI.01134-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wood S.J., Goldufsky J.W., Bello D., Masood S., Shafikhani S.H. Pseudomonas aeruginosa ExoT Induces Mitochondrial Apoptosis in Target Host Cells in a Manner That Depends on Its GTPase-activating Protein (GAP) Domain Activity. J. Biol. Chem. 2015;290:29063–29073. doi: 10.1074/jbc.M115.689950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sayner S.L., Frank D.W., King J., Chen H., VandeWaa J., Stevens T. Paradoxical cAMP-induced lung endothelial hyperpermeability revealed by Pseudomonas aeruginosa ExoY. Circ. Res. 2004;95:196–203. doi: 10.1161/01.RES.0000134922.25721.d9. [DOI] [PubMed] [Google Scholar]
- 131.Chen L., Zou Y., She P., Wu Y. Composition, function, and regulation of T6SS in Pseudomonas aeruginosa. Microbiol. Res. 2015;172:19–25. doi: 10.1016/j.micres.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 132.Schiller N.L., Hackley D.R., Morrison A. Isolation and characterization of serum-resistant strains ofPseudomonas aeruginosa derived from serum-sensitive parental strains. Curr. Microbiol. 1984;10:185–189. doi: 10.1007/BF01627252. [DOI] [Google Scholar]
- 133.Vitkauskiene A., Scheuss S., Sakalauskas R., Dudzevicius V., Sahly H. Pseudomonas aeruginosa strains from nosocomial pneumonia are more serum resistant than P. aeruginosa strains from noninfectious respiratory colonization processes. Infection. 2005;33:356–361. doi: 10.1007/s15010-005-5044-x. [DOI] [PubMed] [Google Scholar]
- 134.Hancock R.E., Mutharia L.M., Chan L., Darveau R.P., Speert D.P., Pier G.B. Pseudomonas aeruginosa isolates from patients with cystic fibrosis: A class of serum-sensitive, nontypable strains deficient in lipopolysaccharide O side chains. Infect. Immun. 1983;42:170–177. doi: 10.1128/iai.42.1.170-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Munguia J., LaRock D.L., Tsunemoto H., Olson J., Cornax I., Pogliano J., Nizet V. The Mla pathway is critical for Pseudomonas aeruginosa resistance to outer membrane permeabilization and host innate immune clearance. J. Mol. Med. 2017;95:1127–1136. doi: 10.1007/s00109-017-1579-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dzvova N., Colmer-Hamood J.A., Griswold J.A., Hamood A.N. Heparinase Is Essential for Pseudomonas aeruginosa Virulence during Thermal Injury and Infection. Infect. Immun. 2018;86:e00755-17. doi: 10.1128/IAI.00755-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Persyn E., Sassi M., Aubry M., Broly M., Delanou S., Asehnoune K., Caroff N., Cremet L. Rapid genetic and phenotypic changes in Pseudomonas aeruginosa clinical strains during ventilator-associated pneumonia. Sci. Rep. 2019;9:4720. doi: 10.1038/s41598-019-41201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Abeyrathne P.D., Lam J.S. WaaL of Pseudomonas aeruginosa utilizes ATP in in vitro ligation of O antigen onto lipid A-core. Mol. Microbiol. 2007;65:1345–1359. doi: 10.1111/j.1365-2958.2007.05875.x. [DOI] [PubMed] [Google Scholar]
- 139.Malekian A., Esmaeeli Djavid G., Akbarzadeh K., Soltandallal M., Rassi Y., Rafinejad J., Rahimi Foroushani A., Farhoud A., Bakhtiary R., Totonchi M. Efficacy of Maggot Therapy on Staphylococcus aureus and Pseudomonas aeruginosa in Diabetic Foot Ulcers: A Randomized Controlled Trial. J. Wound Ostomy Cont. Nurs. 2019;46:25–29. doi: 10.1097/WON.0000000000000496. [DOI] [PubMed] [Google Scholar]
- 140.Stone G.G., Newell P., Bradford P.A. In Vitro Activity of Ceftazidime-Avibactam against Isolates from Patients in a Phase 3 Clinical Trial for Treatment of Complicated Intra-abdominal Infections. Antimicrob. Agents Chemother. 2018;62:e02584-17. doi: 10.1128/AAC.02584-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Singh S., Hornick D., Fedler J., Launspach J.L., Teresi M.E., Santacroce T.R., Cavanaugh J.E., Horan R., Nelson G., Starner T.D., et al. Randomized controlled study of aerosolized hypertonic xylitol versus hypertonic saline in hospitalized patients with pulmonary exacerbation of cystic fibrosis. J. Cyst. Fibros. 2020;19:108–113. doi: 10.1016/j.jcf.2019.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ratjen F., Moeller A., McKinney M.L., Asherova I., Alon N., Maykut R., Angyalosi G. Eradication of early P. aeruginosa infection in children <7 years of age with cystic fibrosis: The early study. J. Cyst. Fibros. 2019;18:78–85. doi: 10.1016/j.jcf.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 143.Hagel S., Bach F., Brenner T., Bracht H., Brinkmann A., Annecke T., Hohn A., Weigand M., Michels G., Kluge S., et al. Effect of therapeutic drug monitoring-based dose optimization of piperacillin/tazobactam on sepsis-related organ dysfunction in patients with sepsis: A randomized controlled trial. Intensive Care Med. 2022;48:311–321. doi: 10.1007/s00134-021-06609-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Eklöf J., Misiakou M.A., Sivapalan P., Armbruster K., Browatzki A., Nielsen T.L., Lapperre T.S., Andreassen H.F., Janner J., Ulrik C.S., et al. Persistence and genetic adaptation of Pseudomonas aeruginosa in patients with chronic obstructive pulmonary disease. Clin. Microbiol. Infect. 2022;28:990–995. doi: 10.1016/j.cmi.2022.01.017. [DOI] [PubMed] [Google Scholar]
- 145.Cogen J.D., Onchiri F.M., Hamblett N.M., Gibson R.L., Morgan W.J., Rosenfeld M. Association of Intensity of Antipseudomonal Antibiotic Therapy With Risk of Treatment-Emergent Organisms in Children With Cystic Fibrosis and Newly Acquired Pseudomonas Aeruginosa. Clin. Infect. Dis. 2021;73:987–993. doi: 10.1093/cid/ciab208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Slekovec C., Robert J., Berthelot P., van der Mee-Marquet N., Rogues A.M., Derouin V., Cholley P., Bertrand X., Gbaguidi-Haore H. Do Contact Precautions Reduce the Incidence of Intensive Care Unit-Acquired Pseudomonas aeruginosa Infections? The DPCPYO (Detection and Contact Precautions for Patients With P. aeruginosa) Cluster-Randomized Crossover Trial. Clin. Infect. Dis. 2021;73:e2781–e2788. doi: 10.1093/cid/ciaa1663. [DOI] [PubMed] [Google Scholar]
- 147.Johnson M.G., Bruno C., Castanheira M., Yu B., Huntington J.A., Carmelitano P., Rhee E.G., De Anda C., Motyl M. Evaluating the emergence of nonsusceptibility among Pseudomonas aeruginosa respiratory isolates from a phase-3 clinical trial for treatment of nosocomial pneumonia (ASPECT-NP) Int. J. Antimicrob. Agents. 2021;57:106278. doi: 10.1016/j.ijantimicag.2021.106278. [DOI] [PubMed] [Google Scholar]
- 148.Portsmouth S., van Veenhuyzen D., Echols R., Machida M., Ferreira J.C.A., Ariyasu M., Tenke P., Nagata T.D. Cefiderocol versus imipenem-cilastatin for the treatment of complicated urinary tract infections caused by Gram-negative uropathogens: A phase 2, randomised, double-blind, non-inferiority trial. Lancet Infect. Dis. 2018;18:1319–1328. doi: 10.1016/S1473-3099(18)30554-1. [DOI] [PubMed] [Google Scholar]
- 149.Bouglé A., Tuffet S., Federici L., Leone M., Monsel A., Dessalle T., Amour J., Dahyot-Fizelier C., Barbier F., Luyt C.E., et al. Comparison of 8 versus 15 days of antibiotic therapy for Pseudomonas aeruginosa ventilator-associated pneumonia in adults: A randomized, controlled, open-label trial. Intensive Care Med. 2022;48:841–849. doi: 10.1007/s00134-022-06690-5. [DOI] [PubMed] [Google Scholar]
- 150.Fabre V., Amoah J., Cosgrove S.E., Tamma P.D. Antibiotic Therapy for Pseudomonas aeruginosa Bloodstream Infections: How Long Is Long Enough? Clin. Infect. Dis. 2019;69:2011–2014. doi: 10.1093/cid/ciz223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Eklöf J., Alispahic I.A., Sivapalan P., Wilcke T., Seersholm N., Armbruster K., Kjærgaard J.L., Saeed M.I., Nielsen T.L., Browatzki A., et al. Targeted AntiBiotics for Chronic pulmonary diseases (TARGET ABC): Can targeted antibiotic therapy improve the prognosis of Pseudomonas aeruginosa-infected patients with chronic pulmonary obstructive disease, non-cystic fibrosis bronchiectasis, and asthma? A multicenter, randomized, controlled, open-label trial. Trials. 2022;23:817. doi: 10.1186/s13063-022-06720-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Strouvalis I., Routsi C., Adamopoulou M., Raftogiannis M., Renieris G., Orfanos S.E., Kotanidou A., Sabracos L., Giamarellos-Bourboulis E.J. Early increase of VEGF-A is associated with resolution of ventilator-associated pneumonia: Clinical and experimental evidence. Respirology. 2018;23:942–949. doi: 10.1111/resp.13320. [DOI] [PubMed] [Google Scholar]
- 153.Mikamo H., Monden K., Miyasaka Y., Horiuchi T., Fujimoto G., Fukuhara T., Yoshinari T., Rhee E.G., Shizuya T. The efficacy and safety of tazobactam/ceftolozane in combination with metronidazole in Japanese patients with complicated intra-abdominal infections. J. Infect. Chemother. 2019;25:111–116. doi: 10.1016/j.jiac.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 154.Wach A., Dembowsky K., Dale G.E. Pharmacokinetics and Safety of Intravenous Murepavadin Infusion in Healthy Adult Subjects Administered Single and Multiple Ascending Doses. Antimicrob. Agents Chemother. 2018;62:e02355-17. doi: 10.1128/AAC.02355-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cheung S.W., Boost M.V., Cho P. Effect of povidone iodine contact lens disinfecting solution on orthokeratology lens and lens case contamination and organisms in the microbiome of the conjunctiva. Cont. Lens. Anterior. Eye. 2021;44:101412. doi: 10.1016/j.clae.2021.01.007. [DOI] [PubMed] [Google Scholar]
- 156.van Duijn P.J., Verbrugghe W., Jorens P.G., Spöhr F., Schedler D., Deja M., Rothbart A., Annane D., Lawrence C., Nguyen Van J.C., et al. The effects of antibiotic cycling and mixing on antibiotic resistance in intensive care units: A cluster-randomised crossover trial. Lancet Infect. Dis. 2018;18:401–409. doi: 10.1016/S1473-3099(18)30056-2. [DOI] [PubMed] [Google Scholar]
- 157.Goss C.H., Kaneko Y., Khuu L., Anderson G.D., Ravishankar S., Aitken M.L., Lechtzin N., Zhou G., Czyz D.M., McLean K., et al. Gallium disrupts bacterial iron metabolism and has therapeutic effects in mice and humans with lung infections. Sci. Transl. Med. 2018;10:eaat7520. doi: 10.1126/scitranslmed.aat7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gai X.Y., Bo S.N., Shen N., Zhou Q.T., Yin A.Y., Lu W. Pharmacokinetic-pharmacodynamic analysis of ciprofloxacin in elderly Chinese patients with lower respiratory tract infections caused by Gram-negative bacteria. Chin. Med. J. 2019;132:638–646. doi: 10.1097/CM9.0000000000000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Halimeh A., Farhad R.B., Naseh S., Karim N. Comparative efficacy of honey 12.5% and chlorhexidine 0.2% mouthwashes on the oropharyngeal bacterial colonization in mechanically-ventilated patients: A randomized controlled trial. J. Tradit. Chin. Med. 2020;40:440–446. doi: 10.19852/j.cnki.jtcm.2020.03.012. [DOI] [PubMed] [Google Scholar]
- 160.Mendes R.E., Castanheira M., Woosley L.N., Stone G.G., Bradford P.A., Flamm R.K. Characterization of β-Lactamase Content of Ceftazidime-Resistant Pathogens Recovered during the Pathogen-Directed Phase 3 REPRISE Trial for Ceftazidime-Avibactam: Correlation of Efficacy against β-Lactamase Producers. Antimicrob. Agents Chemother. 2019;63:e02655-18. doi: 10.1128/AAC.02655-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Singh S.B., Tan C.M., Kaelin D., Meinke P.T., Miesel L., Olsen D.B., Fukuda H., Kishii R., Takei M., Ohata K., et al. Structure activity relationship of N-1 substituted 1,5-naphthyrid-2-one analogs of oxabicyclooctane-linked novel bacterial topoisomerase inhibitors as broad-spectrum antibacterial agents (Part-9) Bioorg. Med. Chem. Lett. 2022;75:128808. doi: 10.1016/j.bmcl.2022.128808. [DOI] [PubMed] [Google Scholar]
- 162.Meesters K., Michelet R., Mauel R., Raes A., Van Bocxlaer J., Vande Walle J., Vermeulen A. Results of a Multicenter Population Pharmacokinetic Study of Ciprofloxacin in Children with Complicated Urinary Tract Infection. Antimicrob. Agents Chemother. 2018;62:e00517-18. doi: 10.1128/AAC.00517-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Surapat B., Montakantikul P., Malathum K., Kiertiburanakul S., Santanirand P., Chindavijak B. Microbial epidemiology and risk factors for relapse in gram-negative bacteria catheter-related bloodstream infection with a pilot prospective study in patients with catheter removal receiving short-duration of antibiotic therapy. BMC Infect. Dis. 2020;20:604. doi: 10.1186/s12879-020-05312-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Oesterreicher Z., Lackner E., Jäger W., Höferl M., Zeitlinger M. Lack of dermal penetration of topically applied gentamicin as pharmacokinetic evidence indicating insufficient efficacy. J. Antimicrob. Chemother. 2018;73:2823–2829. doi: 10.1093/jac/dky274. [DOI] [PubMed] [Google Scholar]
- 165.Ooi M.L., Jothin A., Bennett C., Ooi E.H., Vreugde S., Psaltis A.J., Wormald P.J. Manuka honey sinus irrigations in recalcitrant chronic rhinosinusitis: Phase 1 randomized, single-blinded, placebo-controlled trial. Int. Forum. Allergy Rhinol. 2019;9:1470–1477. doi: 10.1002/alr.22423. [DOI] [PubMed] [Google Scholar]
- 166.Shorr A.F., Bruno C.J., Zhang Z., Jensen E., Gao W., Feng H.P., Huntington J.A., Yu B., Rhee E.G., De Anda C., et al. Ceftolozane/tazobactam probability of target attainment and outcomes in participants with augmented renal clearance from the randomized phase 3 ASPECT-NP trial. Crit. Care. 2021;25:354. doi: 10.1186/s13054-021-03773-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sagel S.D., Khan U., Heltshe S.L., Clancy J.P., Borowitz D., Gelfond D., Donaldson S.H., Moran A., Ratjen F., VanDalfsen J.M., et al. Clinical Effectiveness of Lumacaftor/Ivacaftor in Patients with Cystic Fibrosis Homozygous for F508del-CFTR. A Clinical Trial. Ann. Am. Thorac. Soc. 2021;18:75–83. doi: 10.1513/AnnalsATS.202002-144OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Busse D., Simon P., Schmitt L., Petroff D., Dorn C., Dietrich A., Zeitlinger M., Huisinga W., Michelet R., Wrigge H., et al. Comparative Plasma and Interstitial Tissue Fluid Pharmacokinetics of Meropenem Demonstrate the Need for Increasing Dose and Infusion Duration in Obese and Non-obese Patients. Clin. Pharm. 2022;61:655–672. doi: 10.1007/s40262-021-01070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Deeb M.A., Alsahhaf A., Mubaraki S.A., Alhamoudi N., Al-Aali K.A., Abduljabbar T. Clinical and microbiological outcomes of photodynamic and systemic antimicrobial therapy in smokers with peri-implant inflammation. Photodiagnosis Photodyn Ther. 2020;29:101587. doi: 10.1016/j.pdpdt.2019.101587. [DOI] [PubMed] [Google Scholar]
- 170.van Duijn P.J., Verbrugghe W., Jorens P.G., Spöhr F., Schedler D., Deja M., Rothbart A., Annane D., Lawrence C., Jereb M., et al. The effects of antibiotic cycling and mixing on acquisition of antibiotic resistant bacteria in the ICU: A post-hoc individual patient analysis of a prospective cluster-randomized crossover study. PLoS ONE. 2022;17:e0265720. doi: 10.1371/journal.pone.0265720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sjövall F., Alobaid A.S., Wallis S.C., Perner A., Lipman J., Roberts J.A. Maximally effective dosing regimens of meropenem in patients with septic shock. J. Antimicrob. Chemother. 2018;73:191–198. doi: 10.1093/jac/dkx330. [DOI] [PubMed] [Google Scholar]
- 172.Dingemans J., Eyns H., Willekens J., Monsieurs P., Van Houdt R., Cornelis P., Malfroot A., Crabbé A. Intrapulmonary percussive ventilation improves lung function in cystic fibrosis patients chronically colonized with Pseudomonas aeruginosa: A pilot cross-over study. Eur. J. Clin. Microbiol. Infect. Dis. 2018;37:1143–1151. doi: 10.1007/s10096-018-3232-8. [DOI] [PubMed] [Google Scholar]
- 173.Caceres S.M., Sanders L.A., Rysavy N.M., Poch K.R., Jones C.R., Pickard K., Fingerlin T.E., Marcus R.A., Malcolm K.C., Taylor-Cousar J.L., et al. Blood mRNA biomarkers distinguish variable systemic and sputum inflammation at treatment initiation of inhaled antibiotics in cystic fibrosis: A prospective non-randomized trial. PLoS ONE. 2022;17:e0267592. doi: 10.1371/journal.pone.0267592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Genpeng L., Jinen S., Tao W., Zhihui L., Rixiang G., Jianyong L., Jingqiang Z. Intraoperative application of inactivated Pseudomonas aeruginosa in patients undergoing lateral neck dissection for metastatic thyroid cancer: A randomized, parallel group, placebo-controlled trial. Surgery. 2020;168:340–346. doi: 10.1016/j.surg.2020.03.020. [DOI] [PubMed] [Google Scholar]
- 175.Chu L., Acosta A.M., Aazami H., Dennis P., De Valle O., Ehmer D., Jr., Hedrick J.A., Ansley J.F. Efficacy and Safety of Ciprofloxacin Plus Fluocinolone Acetonide Among Patients With Acute Otitis Externa: A Randomized Clinical Trial. JAMA Netw. Open. 2022;5:e2221699. doi: 10.1001/jamanetworkopen.2022.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Shi M.M., Yang Q.Y., Monsel A., Yan J.Y., Dai C.X., Zhao J.Y., Shi G.C., Zhou M., Zhu X.M., Li S.K., et al. Preclinical efficacy and clinical safety of clinical-grade nebulized allogenic adipose mesenchymal stromal cells-derived extracellular vesicles. J. Extracell. Vesicles. 2021;10:e12134. doi: 10.1002/jev2.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wieërs G., Verbelen V., Van Den Driessche M., Melnik E., Vanheule G., Marot J.C., Cani P.D. Do Probiotics During In-Hospital Antibiotic Treatment Prevent Colonization of Gut Microbiota With Multi-Drug-Resistant Bacteria? A Randomized Placebo-Controlled Trial Comparing Saccharomyces to a Mixture of Lactobacillus, Bifidobacterium, and Saccharomyces. Front. Public Health. 2020;8:578089. doi: 10.3389/fpubh.2020.578089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.McCallin S., Sarker S.A., Sultana S., Oechslin F., Brüssow H. Metagenome analysis of Russian and Georgian Pyophage cocktails and a placebo-controlled safety trial of single phage versus phage cocktail in healthy Staphylococcus aureus carriers. Environ. Microbiol. 2018;20:3278–3293. doi: 10.1111/1462-2920.14310. [DOI] [PubMed] [Google Scholar]
- 179.Loose M., Naber K.G., Shields P., Reinhart H., Wagenlehner F.M.E. Urinary concentrations and antimicrobial activity of tobramycin in healthy volunteers receiving a single oral dose of a novel formulation for improved absorption. Int. J. Antimicrob. Agents. 2018;51:422–426. doi: 10.1016/j.ijantimicag.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 180.Loebinger M.R., Polverino E., Chalmers J.D., Tiddens H., Goossens H., Tunney M., Ringshausen F.C., Hill A.T., Pathan R., Angyalosi G., et al. Efficacy and safety of TOBI Podhaler in Pseudomonas aeruginosa-infected bronchiectasis patients: iBEST study. Eur. Respir. J. 2021;57:2001451. doi: 10.1183/13993003.01451-2020. [DOI] [PubMed] [Google Scholar]
- 181.Langton Hewer S.C., Smyth A.R., Brown M., Jones A.P., Hickey H., Kenna D., Ashby D., Thompson A., Sutton L., Clayton D., et al. Intravenous or oral antibiotic treatment in adults and children with cystic fibrosis and Pseudomonas aeruginosa infection: The TORPEDO-CF RCT. Health Technol. Assess. 2021;25:1–128. doi: 10.3310/hta25650. [DOI] [PubMed] [Google Scholar]
- 182.Durfey S.L., Pipavath S., Li A., Vo A.T., Ratjen A., Carter S., Morgan S.J., Radey M.C., Grogan B., Salipante S.J., et al. Combining Ivacaftor and Intensive Antibiotics Achieves Limited Clearance of Cystic Fibrosis Infections. mBio. 2021;12:e0314821. doi: 10.1128/mbio.03148-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Meijer L., Hery-Arnaud G., Leven C., Nowak E., Hillion S., Renaudineau Y., Durieu I., Chiron R., Prevotat A., Fajac I., et al. Safety and pharmacokinetics of Roscovitine (Seliciclib) in cystic fibrosis patients chronically infected with Pseudomonas aeruginosa, a randomized, placebo-controlled study. J. Cyst. Fibros. 2022;21:529–536. doi: 10.1016/j.jcf.2021.10.013. [DOI] [PubMed] [Google Scholar]
- 184.Puvvadi R., Mikkelsen H., McCahon L., Grogan S., Ditcham W., Reid D.W., Lamont I., Stick S.M., Clements B. Role of Tris-CaEDTA as an adjuvant with nebulised tobramycin in cystic fibrosis patients with Pseudomonas aeruginosa lung infections: A randomised controlled trial. J. Cyst. Fibros. 2021;20:316–323. doi: 10.1016/j.jcf.2020.12.004. [DOI] [PubMed] [Google Scholar]
- 185.Chastre J., François B., Bourgeois M., Komnos A., Ferrer R., Rahav G., De Schryver N., Lepape A., Koksal I., Luyt C.E., et al. Safety, efficacy, and pharmacokinetics of gremubamab (MEDI3902), an anti-Pseudomonas aeruginosa bispecific human monoclonal antibody, in P. aeruginosa-colonised, mechanically ventilated intensive care unit patients: A randomised controlled trial. Crit. Care. 2022;26:355. doi: 10.1186/s13054-022-04204-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bassetti M., Echols R., Matsunaga Y., Ariyasu M., Doi Y., Ferrer R., Lodise T.P., Naas T., Niki Y., Paterson D.L., et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): A randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect. Dis. 2021;21:226–240. doi: 10.1016/S1473-3099(20)30796-9. [DOI] [PubMed] [Google Scholar]
- 187.NLM ClinicalTrials.gov—A Database of Privately and Publicaly Funded Clinical Studies Conducted Around the World. [(accessed on 10 March 2023)]; Available online: https://clinicaltrials.gov/ct2/home.
- 188.Trapnell B.C., McColley S.A., Kissner D.G., Rolfe M.W., Rosen J.M., McKevitt M., Moorehead L., Montgomery A.B., Geller D.E. Fosfomycin/tobramycin for inhalation in patients with cystic fibrosis with pseudomonas airway infection. Am. J. Respir. Crit. Care Med. 2013;185:171–178. doi: 10.1164/rccm.201105-0924OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.MacLeod D.L., Barker L.M., Sutherland J.L., Moss S.C., Gurgel J.L., Kenney T.F., Burns J.L., Baker W.R. Antibacterial activities of a fosfomycin/tobramycin combination: A novel inhaled antibiotic for bronchiectasis. J. Antimicrob. Chemother. 2009;64:829–836. doi: 10.1093/jac/dkp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Safety, Tolerability and PK 3-Period Crossover Study Comparing 2 Single Doses of ZTI-01 and Monurol® in Healthy Subjects. [(accessed on 10 March 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02178254.
- 191.Polverino E., Hill A. Safety, Efficacy and PK/PD of POL7080 in Patients With Exacerbation of Non-Cystic Fibrosis Bronchiectasis. [(accessed on 10 March 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02096315.
- 192.Dale G.E., Halabi A., Petersen-Sylla M., Wach A., Zwingelstein C. Pharmacokinetics, Tolerability, and Safety of Murepavadin, a Novel Antipseudomonal Antibiotic, in Subjects with Mild, Moderate, or Severe Renal Function Impairment. Antimicrob. Agents Chemother. 2019;62:e00490-18. doi: 10.1128/AAC.00490-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.BioSpace Polyphor Temporarily Halts Enrollment in the Phase III Studies of Murepavadin for the Treatment of Patients with Nosocomial Pneumonia. [(accessed on 10 March 2023)]. Available online: https://www.biospace.com/article/polyphor-temporarily-halts-enrollment-in-the-phase-iii-studies-of-murepavadin-for-the-treatment-of-patients-with-nosocomial-pneumonia/
- 194.Pivotal Study in Nosocomial Pneumonia Suspected or Confirmed to be Due to Pseudomonas (PRISM-UDR) [(accessed on 10 March 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03582007.
- 195.Recida Therapeutics, Inc Single and Multiple Dose Escalation Trial of an Intravenous Antibiotic RC-01. [(accessed on 10 March 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03832517?term=NCT03832517&draw=2.
- 196.Koch A. Phase 1 Study to Evaluate DDI, PK, Safety, Tolerability of SPR741. [(accessed on 10 March 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03376529?cond=SPR741&draw=2&rank=1.
- 197.Farinola N. A First in Human Study of the Safety and Tolerability of Single and Multiple Doses of SPR741 in Healthy Volunteers. [(accessed on 10 March 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03022175?cond=SPR741&draw=2&rank=2.
- 198.Kuo J. A First in Human Study of the Safety and Tolerability of Single and Multiple Doses of SPR206 in Healthy Volunteers. [(accessed on 10 March 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03792308.
- 199.MicuRx. Biomedical Advanced Research and Development Authority. Wellcome Trust Study of the Safety, Tolerability, and PK of MRX-8 Administered Intravenously to HVs in SAD and MAD Cohorts. [(accessed on 10 March 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04649541?term=NCT04649541&draw=2.
- 200.Merck Sharp & Dohme LLC Efficacy and Safety of Imipenem+Cilastatin/Relebactam (MK-7655A) Versus Colistimethate Sodium+Imipenem+Cilastatin in Imipenem-Resistant Bacterial Infection (MK-7655A-013) (RESTORE-IMI 1) [(accessed on 10 March 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02452047.
- 201.Motsch J., Murta de Oliveira C., Stus V., Köksal I., Lyulko O., Boucher H.W., Kaye K.S., File T.M., Jr., Brown M.L., Khan I., et al. RESTORE-IMI 1: A Multicenter, Randomized, Double-blind Trial Comparing Efficacy and Safety of Imipenem/Relebactam vs Colistin Plus Imipenem in Patients With Imipenem-nonsusceptible Bacterial Infections. Clin. Infect. Dis. 2020;70:1799–1808. doi: 10.1093/cid/ciz530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Titov I., Wunderink R.G., Roquilly A., Rodríguez Gonzalez D., David-Wang A., Boucher H.W., Kaye K.S., Losada M.C., Du J., Tipping R., et al. A Randomized, Double-blind, Multicenter Trial Comparing Efficacy and Safety of Imipenem/Cilastatin/Relebactam Versus Piperacillin/Tazobactam in Adults With Hospital-acquired or Ventilator-associated Bacterial Pneumonia (RESTORE-IMI 2 Study) Clin. Infect. Dis. 2021;73:e4539–e4548. doi: 10.1093/cid/ciaa803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Merck Sharp & Dohme LLC Imipenem/Relebactam/Cilastatin Versus Piperacillin/Tazobactam for Treatment of Participants With Bacterial Pneumonia (MK-7655A-014) (RESTORE-IMI 2) [(accessed on 10 March 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02493764.
- 204.Merck Sharp & Dohme LLC Imipenem/Cilastatin/Relebactam (MK-7655A) Versus Piperacillin/Tazobactam in Participants With Hospital-Acquired or Ventilator-Associated Bacterial Pneumonia (MK-7655A-016) [(accessed on 28 March 2023)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT03583333.
- 205.Kuti J.L. Imipenem/Cilastatin/Relebactam Pharmacokinetics, Safety, and Outcomes in Adults and Adolescents With Cystic Fibrosis. [(accessed on 10 March 2023)]; Available online: https://www.clinicaltrials.gov/ct2/show/NCT05561764.
- 206.Pan Y. Evaluation of the Efficacy and Safety of Intravenous Imipenem/Cilastatin/XNW4107 in Comparison With Recarbrio in Adults With HABP/VABP (REITAB-2) [(accessed on 10 March 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT05204563.
- 207.Hoffmann-La Roche A Study to Investigate the Intrapulmonary Lung Penetration of Nacubactam in Healthy Participants. [(accessed on 10 March 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03182504.
- 208.Egawa M. A Phase I Study to Assess Safety, Tolerability and Pharmacokinetics of OP0595. [(accessed on 10 March 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02134834.
- 209.Hoffmann-La Roche A Study to Investigate the Safety, Tolerability, and Pharmacokinetics of RO7079901 and the Combination of RO7079901 With Meropenem in Adult Healthy Volunteers. [(accessed on 10 March 2023)];2017 Available online: https://clinicaltrials.gov/ct2/show/NCT02972255.
- 210.Martin-Loeches I., Dale G.E., Torres A. Murepavadin: A new antibiotic class in the pipeline. Expert Rev. Anti Infect. Ther. 2018;16:259–268. doi: 10.1080/14787210.2018.1441024. [DOI] [PubMed] [Google Scholar]
- 211.Luther A., Moehle K., Chevalier E., Dale G., Obrecht D. Protein epitope mimetic macrocycles as biopharmaceuticals. Curr. Opin. Chem. Biol. 2017;38:45–51. doi: 10.1016/j.cbpa.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 212.Kandasamy S.K., Larson R.G. Binding modes of protegrin-1, a beta-strand antimicrobial peptide, in lipid bilayers. Mol. Simul. 2007;33:799–807. doi: 10.1080/08927020701313737. [DOI] [Google Scholar]
- 213.Jang H., Ma B., Nussinov R. Conformational study of the protegrin-1 (PG-1) dimer interaction with lipid bilayers and its effect. BMC Struct. Biol. 2007;7:21. doi: 10.1186/1472-6807-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Sader H.S., Dale G.E., Rhomberg P.R., Flamm R.K. Antimicrobial Activity of Murepavadin Tested against Clinical Isolates of Pseudomonas aeruginosa from the United States, Europe, and China. Antimicrob. Agents Chemother. 2018;62:e00311-18. doi: 10.1128/AAC.00311-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Li J., Nation R.L., Kaye K.S. Polymyxin Antibiotics: From Laboratory Bench to Bedside. Springer; Berlin/Heidelberg, Germany: 2019. [Google Scholar]
- 216.French S., Farha M., Ellis M.J., Sameer Z., Côté J.-P., Cotroneo N., Lister T., Rubio A., Brown E.D. Potentiation of Antibiotics against Gram-Negative Bacteria by Polymyxin B Analogue SPR741 from Unique Perturbation of the Outer Membrane. ACS Infect. Dis. 2020;6:1405–1412. doi: 10.1021/acsinfecdis.9b00159. [DOI] [PubMed] [Google Scholar]
- 217.Corbett D., Wise A., Langley T., Skinner K., Trimby E., Birchall S., Dorali A., Sandiford S., Williams J., Warn P., et al. Potentiation of Antibiotic Activity by a Novel Cationic Peptide: Potency and Spectrum of Activity of SPR741. Antimicrob. Agents Chemother. 2017;61:e00200-17. doi: 10.1128/AAC.00200-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Vaara M., Siikanen O., Apajalahti J., Fox J., Frimodt-Møller N., He H., Poudyal A., Li J., Nation R.L., Vaara T. A novel polymyxin derivative that lacks the fatty acid tail and carries only three positive charges has strong synergism with agents excluded by the intact outer membrane. Antimicrob. Agents Chemother. 2010;54:3341–3346. doi: 10.1128/AAC.01439-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Eckburg P.B., Lister T., Walpole S., Keutzer T., Utley L., Tomayko J., Kopp E., Farinola N., Coleman S. Safety, Tolerability, Pharmacokinetics, and Drug Interaction Potential of SPR741, an Intravenous Potentiator, after Single and Multiple Ascending Doses and When Combined with β-Lactam Antibiotics in Healthy Subjects. Antimicrob. Agents Chemother. 2019;63:e00892-19. doi: 10.1128/AAC.00892-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lepak A.J., Wang W., Andes D.R. Pharmacodynamic Evaluation of MRX-8, a Novel Polymyxin, in the Neutropenic Mouse Thigh and Lung Infection Models against Gram-Negative Pathogens. Antimicrob. Agents Chemother. 2020;64:e01517-20. doi: 10.1128/AAC.01517-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Smith J.R., Rybak J.M., Claeys K.C. Imipenem-Cilastatin-Relebactam: A Novel β-Lactam-β-Lactamase Inhibitor Combination for the Treatment of Multidrug-Resistant Gram-Negative Infections. Pharmacotherapy. 2020;40:343–356. doi: 10.1002/phar.2378. [DOI] [PubMed] [Google Scholar]
- 222.Mansour H., Ouweini A.E.L., Chahine E.B., Karaoui L.R. Imipenem/cilastatin/relebactam: A new carbapenem β-lactamase inhibitor combination. Am. J. Health Syst. Pharm. 2021;78:674–683. doi: 10.1093/ajhp/zxab012. [DOI] [PubMed] [Google Scholar]
- 223.Viertel T.M., Ritter K., Horz H.P. Viruses versus bacteria-novel approaches to phage therapy as a tool against multidrug-resistant pathogens. J. Antimicrob. Chemother. 2014;69:2326–2336. doi: 10.1093/jac/dku173. [DOI] [PubMed] [Google Scholar]
- 224.Jault P., Leclerc T., Jennes S., Pirnay J.P., Que Y.A., Resch G., Rousseau A.F., Ravat F., Carsin H., Le Floch R., et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): A randomised, controlled, double-blind phase 1/2 trial. Lancet Infect. Dis. 2019;19:35–45. doi: 10.1016/S1473-3099(18)30482-1. [DOI] [PubMed] [Google Scholar]
- 225.RC R. Bacteriophage Effects on Pseudomonas Aeruginosa (MUCOPHAGES) [(accessed on 10 March 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT01818206.
- 226.Individual Patient Expanded Access for AB-PA01, an Investigational Anti-Pseudomonas Aeruginosa Bacteriophage Therapeutic. [(accessed on 28 March 2023)];2019 Available online: https://clinicaltrials.gov/ct2/show/NCT03395743%20.
- 227.Tawil N. Phage Therapy for the Prevention and Treatment of Wound Infections in Burned Patients. [(accessed on 28 March 2023)];2021 Available online: https://clinicaltrials.gov/ct2/show/NCT04323475.
- 228.Armata Pharmaceuticals, Inc Ph 1/2 Study Evaluating Safety and Tolerability of Inhaled AP-PA02 in Subjects With Chronic Pseudomonas Aeruginosa Lung Infections and Cystic Fibrosis (SWARM-Pa) [(accessed on 10 March 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04596319?term=NCT04596319&draw=2.
- 229.Koff J. CYstic Fibrosis bacterioPHage Study at Yale (CYPHY) [(accessed on 10 March 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04684641?term=NCT04684641&draw=2.
- 230.Pearson J.P., Feldman M., Iglewski B.H., Prince A. Pseudomonas aeruginosa cell-to-cell signaling is required for virulence in a model of acute pulmonary infection. Infect. Immun. 2000;68:4331–4334. doi: 10.1128/IAI.68.7.4331-4334.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Rumbaugh K.P., Griswold J.A., Hamood A.N. Contribution of the regulatory gene lasR to the pathogenesis of Pseudomonas aeruginosa infection of burned mice. J. Burn Care Rehabil. 1999;20:42–49. doi: 10.1097/00004630-199901001-00008. [DOI] [PubMed] [Google Scholar]
- 232.Rumbaugh K.P., Griswold J.A., Iglewski B.H., Hamood A.N. Contribution of quorum sensing to the virulence of Pseudomonas aeruginosa in burn wound infections. Infect. Immun. 1999;67:5854–5862. doi: 10.1128/IAI.67.11.5854-5862.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Wu H., Song Z., Hentzer M., Andersen J.B., Heydorn A., Mathee K., Moser C., Eberl L., Molin S., Hoiby N., et al. Detection of N-acylhomoserine lactones in lung tissues of mice infected with Pseudomonas aeruginosa. Pt 10Microbiology. 2000;146:2481–2493. doi: 10.1099/00221287-146-10-2481. [DOI] [PubMed] [Google Scholar]
- 234.Tateda K., Comte R., Pechere J.C., Köhler T., Yamaguchi K., Van Delden C. Azithromycin inhibits quorum sensing in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2001;45:1930–1933. doi: 10.1128/AAC.45.6.1930-1933.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Skindersoe M.E., Alhede M., Phipps R., Yang L., Jensen P.O., Rasmussen T.B., Bjarnsholt T., Tolker-Nielsen T., Høiby N., Givskov M. Effects of antibiotics on quorum sensing in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2008;52:3648–3663. doi: 10.1128/AAC.01230-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Nalca Y., Jänsch L., Bredenbruch F., Geffers R., Buer J., Häussler S. Quorum-sensing antagonistic activities of azithromycin in Pseudomonas aeruginosa PAO1: A global approach. Antimicrob. Agents Chemother. 2006;50:1680–1688. doi: 10.1128/AAC.50.5.1680-1688.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Köhler T., Dumas J.L., Van Delden C. Ribosome protection prevents azithromycin-mediated quorum-sensing modulation and stationary-phase killing of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2007;51:4243–4248. doi: 10.1128/AAC.00613-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Köhler T., Perron G.G., Buckling A., van Delden C. Quorum sensing inhibition selects for virulence and cooperation in Pseudomonas aeruginosa. PLoS Pathog. 2010;6:e1000883. doi: 10.1371/journal.ppat.1000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Welsh M.A., Eibergen N.R., Moore J.D., Blackwell H.E. Small molecule disruption of quorum sensing cross-regulation in Pseudomonas aeruginosa causes major and unexpected alterations to virulence phenotypes. J. Am. Chem. Soc. 2015;137:1510–1519. doi: 10.1021/ja5110798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Febbraro S. Safety and Efficacy of Inhaled OligoG CF-5/20 for the Treatment Cystic Fibrosis. [(accessed on 10 March 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT00970346?term=NCT00970346&draw=2.
- 241.Koningsbruggen-Rietschel S.v. A Dose-finding Study of Inhaled OligoG vs Placebo in Patients With Cystic Fibrosis (SMR3372) [(accessed on 10 March 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03698448?term=NCT03698448&draw=2.
- 242.Peptilogics Study in Patients Undergoing Debridement, Antibiotics, and Implant Retention (DAIR) for Treatment of a Periprosthetic Joint Infection (PJI) Occurring After Total Knee Arthroplasty (TKA) [(accessed on 10 March 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT05137314?term=NCT05137314&draw=2.
- 243.Shunkov V., Shvets A., Gorelov D., Kulagina L., Matevosyan E., Mozheyko M., Sinelnikov L., Bushara M., Yesayan A., Mangushlo A., et al. Safety and Efficacy Study of Ftortiazinon in the Treatment of Patients With Complicated Urinary Tract Infections Caused by P. aeruginosa. [(accessed on 28 March 2023)];2021 Available online: https://clinicaltrials.gov/ct2/show/NCT03638830.
- 244.Powell L.C., Pritchard M.F., Ferguson E.L., Powell K.A., Patel S.U., Rye P.D., Sakellakou S.M., Buurma N.J., Brilliant C.D., Copping J.M., et al. Targeted disruption of the extracellular polymeric network of Pseudomonas aeruginosa biofilms by alginate oligosaccharides. NPJ Biofilms Microbiomes. 2018;4:13. doi: 10.1038/s41522-018-0056-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Giorgetti M., Klymiuk N., Bähr A., Hemmerling M., Jinton L., Tarran R., Malmgren A., Åstrand A., Hansson G.C., Ermund A. New generation ENaC inhibitors detach cystic fibrosis airway mucus bundles via sodium/hydrogen exchanger inhibition. Eur. J. Pharmacol. 2021;904:174123. doi: 10.1016/j.ejphar.2021.174123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Wark P. A Phase 2b Randomised, Placebo Controlled Study of OligoG in Patients With Cystic Fibrosis. [(accessed on 10 March 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03822455.
- 247.Yasir M., Dutta D., Hossain K.R., Chen R., Ho K.K.K., Kuppusamy R., Clarke R.J., Kumar N., Willcox M.D.P. Mechanism of Action of Surface Immobilized Antimicrobial Peptides Against Pseudomonas aeruginosa. Front. Microbiol. 2020;10:3053. doi: 10.3389/fmicb.2019.03053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kim H., Jang J.H., Kim S.C., Cho J.H. Development of a novel hybrid antimicrobial peptide for targeted killing of Pseudomonas aeruginosa. Eur. J. Med. Chem. 2020;185:111814. doi: 10.1016/j.ejmech.2019.111814. [DOI] [PubMed] [Google Scholar]
- 249.Grishin S.Y., Domnin P.A., Kravchenko S.V., Azev V.N., Mustaeva L.G., Gorbunova E.Y., Kobyakova M.I., Surin A.K., Makarova M.A., Kurpe S.R., et al. Is It Possible to Create Antimicrobial Peptides Based on the Amyloidogenic Sequence of Ribosomal S1 Protein of P. aeruginosa? Int. J. Mol. Sci. 2021;22:9776. doi: 10.3390/ijms22189776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ridyard K.E., Elsawy M., Mattrasingh D., Klein D., Strehmel J., Beaulieu C., Wong A., Overhage J. Synergy between Human Peptide LL-37 and Polymyxin B against Planktonic and Biofilm Cells of Escherichia coli and Pseudomonas aeruginosa. Antibiotics. 2023;12:389. doi: 10.3390/antibiotics12020389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Holder I.A., Neely A.N., Frank D.W. Type III secretion/intoxication system important in virulence of Pseudomonas aeruginosa infections in burns. Burns. 2001;27:129–130. doi: 10.1016/S0305-4179(00)00142-X. [DOI] [PubMed] [Google Scholar]
- 252.Koh A.Y., Priebe G.P., Pier G.B. Virulence of Pseudomonas aeruginosa in a murine model of gastrointestinal colonization and dissemination in neutropenia. Infect. Immun. 2005;73:2262–2272. doi: 10.1128/IAI.73.4.2262-2272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Kruczek C., Kottapalli K.R., Dissanaike S., Dzvova N., Griswold J.A., Colmer-Hamood J.A., Hamood A.N. Major Transcriptome Changes Accompany the Growth of Pseudomonas aeruginosa in Blood from Patients with Severe Thermal Injuries. PLoS ONE. 2016;11:e0149229. doi: 10.1371/journal.pone.0149229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Plotkowski M.C., Estato V., Santos S.A., da Silva M.C., Miranda A.S., de Miranda P.E., Pinho V., Tibirica E., Morandi V., Teixeira M.M., et al. Contribution of the platelet activating factor signaling pathway to cerebral microcirculatory dysfunction during experimental sepsis by ExoU producing Pseudomonas aeruginosa. Pathog. Dis. 2015;73:ftv046. doi: 10.1093/femspd/ftv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Sawa T., Corry D.B., Gropper M.A., Ohara M., Kurahashi K., Wiener-Kronish J.P. IL-10 improves lung injury and survival in Pseudomonas aeruginosa pneumonia. J. Immunol. 1997;159:2858–2866. doi: 10.4049/jimmunol.159.6.2858. [DOI] [PubMed] [Google Scholar]
- 256.Hauser A.R. The type III secretion system of Pseudomonas aeruginosa: Infection by injection. Nat. Rev. Microbiol. 2009;7:654–665. doi: 10.1038/nrmicro2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Sheremet A.B., Zigangirova N.A., Zayakin E.S., Luyksaar S.I., Kapotina L.N., Nesterenko L.N., Kobets N.V., Gintsburg A.L. Small Molecule Inhibitor of Type Three Secretion System Belonging to a Class 2,4-disubstituted-4H-[1,3,4]-thiadiazine-5-ones Improves Survival and Decreases Bacterial Loads in an Airway Pseudomonas aeruginosa Infection in Mice. Biomed. Res. Int. 2018;2018:5810767. doi: 10.1155/2018/5810767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Sheremet A.B., Nesterenko L.N., Zigangirova N.A. The Type Three Secretion System of Pseudomonas aeruginosa as a Target for Development of Antivirulence Drugs. Mol. Genet. Microbiol. Virol. 2020;35:1–13. doi: 10.3103/S0891416820010073. [DOI] [Google Scholar]
- 259.Anantharajah A., Mingeot-Leclercq M.P., Van Bambeke F. Targeting the Type Three Secretion System in Pseudomonas aeruginosa. Trends Pharmacol. Sci. 2016;37:734–749. doi: 10.1016/j.tips.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 260.Pier G.B., Small G.J., Warren H.B. Protection against mucoid Pseudomonas aeruginosa in rodent models of endobronchial infections. Science. 1990;249:537–540. doi: 10.1126/science.2116663. [DOI] [PubMed] [Google Scholar]
- 261.Campbell P.W. Phase II Randomized, Double-Blind, Placebo-Controlled Study of Intravenous Mucoid Exopolysaccharide Pseudomonas Aeruginosa Immune Globulin for Cystic Fibrosis. [(accessed on 28 March 2023)];2005 Available online: https://clinicaltrials.gov/ct2/show/NCT00004747.
- 262.Chastre J. Pilot Trial of KB001 in Mechanically-Ventilated Patients Colonized With Pseudomonas Aeruginosa. [(accessed on 28 March 2023)];2009 Available online: https://clinicaltrials.gov/ct2/show/NCT00691587.
- 263.Milla C.E., Chmiel J.F., Accurso F.J., VanDevanter D.R., Konstan M.W., Yarranton G., Geller D.E. Anti-PcrV antibody in cystic fibrosis: A novel approach targeting Pseudomonas aeruginosa airway infection. Pediatr. Pulmonol. 2014;49:650–658. doi: 10.1002/ppul.22890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Jain R., Beckett V.V., Konstan M.W., Accurso F.J., Burns J.L., Mayer-Hamblett N., Milla C., VanDevanter D.R., Chmiel J.F. KB001-A, a novel anti-inflammatory, found to be safe and well-tolerated in cystic fibrosis patients infected with Pseudomonas aeruginosa. J. Cyst. Fibros. 2018;17:484–491. doi: 10.1016/j.jcf.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 265.Ali S.O., Yu X.Q., Robbie G.J., Wu Y., Shoemaker K., Yu L., DiGiandomenico A., Keller A.E., Anude C., Hernandez-Illas M., et al. Phase 1 study of MEDI3902, an investigational anti-Pseudomonas aeruginosa PcrV and Psl bispecific human monoclonal antibody, in healthy adults. Clin. Microbiol. Infect. 2019;25:629.e1–629.e6. doi: 10.1016/j.cmi.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 266.Chastre J., François B., Bourgeois M., Komnos A., Ferrer R., Rahav G., De Schryver N., Lepape A., Koksal I., Luyt C.E., et al. 635. Efficacy, Pharmacokinetics (PK), and Safety Profile of MEDI3902, an Anti-Pseudomonas aeruginosa Bispecific Human Monoclonal Antibody in Mechanically Ventilated Intensive Care Unit Patients; Results of the Phase 2 EVADE Study Conducted by the Public-Private COMBACTE-MAGNET Consortium in the Innovative Medicines Initiative (IMI) Program. [(accessed on 28 March 2023)];2020 Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7776862/
- 267.Hollsing A. Anti-pseudomonas IgY to Prevent Infections in Cystic Fibrosis (PseudIgY) [(accessed on 28 March 2023)];2016 Available online: https://clinicaltrials.gov/ct2/show/NCT00633191.
- 268.Schuster A. Efficacy Study of IgY (Antibody Against Pseudomonas) in Cystic Fibrosis Patients (PsAer-IgY) [(accessed on 28 March 2023)];2017 Available online: https://clinicaltrials.gov/ct2/show/NCT01455675.
- 269.Lu Q., Rouby J.J., Laterre P.F., Eggimann P., Dugard A., Giamarellos-Bourboulis E.J., Mercier E., Garbino J., Luyt C.E., Chastre J., et al. Pharmacokinetics and safety of panobacumab: Specific adjunctive immunotherapy in critical patients with nosocomial Pseudomonas aeruginosa O11 pneumonia. J. Antimicrob. Chemother. 2011;66:1110–1116. doi: 10.1093/jac/dkr046. [DOI] [PubMed] [Google Scholar]
- 270.Que Y.A., Lazar H., Wolff M., Francois B., Laterre P.F., Mercier E., Garbino J., Pagani J.L., Revelly J.P., Mus E., et al. Assessment of panobacumab as adjunctive immunotherapy for the treatment of nosocomial Pseudomonas aeruginosa pneumonia. Eur. J. Clin. Microbiol. Infect. Dis. 2014;33:1861–1867. doi: 10.1007/s10096-014-2156-1. [DOI] [PubMed] [Google Scholar]
- 271.Georgescu V. Safety and Pharmacokinetics of KBPA-101 in Hospital Acquired Pneumonia Caused by O11 Pseudomonas Aeruginosa. [(accessed on 28 March 2023)];2009 Available online: https://clinicaltrials.gov/ct2/show/NCT00851435.
- 272.Adjunctive Therapeutic Treatment With Human Monoclonal Antibody AR-105 (Aerucin®) in P. Aeruginosa Pneumonia. [(accessed on 28 March 2023)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT03027609.
- 273.Ciceri F. Pentaglobin in CRE and PA Neutropenic Infections (PENTALLO) [(accessed on 28 March 2023)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT03494959.
- 274.Bernthal N., Conway J., Stolarski E., Berkowitz R., Pulido L. Study to Evaluate Safety and Activity of TRL1068 in Prosthetic Joint Infections. [(accessed on 10 March 2023)]; Available online: https://www.clinicaltrials.gov/ct2/show/NCT04763759?term=TRL1068&draw=2.
- 275.Schreiber J.R., Pier G.B., Grout M., Nixon K., Patawaran M. Induction of opsonic antibodies to Pseudomonas aeruginosa mucoid exopolysaccharide by an anti-idiotypic monoclonal antibody. J. Infect. Dis. 1991;164:507–514. doi: 10.1093/infdis/164.3.507. [DOI] [PubMed] [Google Scholar]
- 276.Johansen H.K., Hoiby N., Pedersen S.S. Experimental immunization with Pseudomonas aeruginosa alginate induces IgA and IgG antibody responses. APMIS. 1991;99:1061–1068. doi: 10.1111/j.1699-0463.1991.tb01301.x. [DOI] [PubMed] [Google Scholar]
- 277.Holder I.A., Neely A.N., Frank D.W. PcrV immunization enhances survival of burned Pseudomonas aeruginosa-infected mice. Infect. Immun. 2001;69:5908–5910. doi: 10.1128/IAI.69.9.5908-5910.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Sawa T., Yahr T.L., Ohara M., Kurahashi K., Gropper M.A., Wiener-Kronish J.P., Frank D.W. Active and passive immunization with the Pseudomonas V antigen protects against type III intoxication and lung injury. Nat. Med. 1999;5:392–398. doi: 10.1038/7391. [DOI] [PubMed] [Google Scholar]
- 279.Frank D.W., Vallis A., Wiener-Kronish J.P., Roy-Burman A., Spack E.G., Mullaney B.P., Megdoud M., Marks J.D., Fritz R., Sawa T. Generation and characterization of a protective monoclonal antibody to Pseudomonas aeruginosa PcrV. J. Infect. Dis. 2002;186:64–73. doi: 10.1086/341069. [DOI] [PubMed] [Google Scholar]
- 280.Shime N., Sawa T., Fujimoto J., Faure K., Allmond L.R., Karaca T., Swanson B.L., Spack E.G., Wiener-Kronish J.P. Therapeutic administration of anti-PcrV F(ab’)(2) in sepsis associated with Pseudomonas aeruginosa. J. Immunol. 2001;167:5880–5886. doi: 10.4049/jimmunol.167.10.5880. [DOI] [PubMed] [Google Scholar]
- 281.Friedman L., Kolter R. Genes involved in matrix formation in Pseudomonas aeruginosa PA14 biofilms. Mol. Microbiol. 2004;51:675–690. doi: 10.1046/j.1365-2958.2003.03877.x. [DOI] [PubMed] [Google Scholar]
- 282.DiGiandomenico A., Warrener P., Hamilton M., Guillard S., Ravn P., Minter R., Camara M.M., Venkatraman V., Macgill R.S., Lin J., et al. Identification of broadly protective human antibodies to Pseudomonas aeruginosa exopolysaccharide Psl by phenotypic screening. J. Exp. Med. 2012;209:1273–1287. doi: 10.1084/jem.20120033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.DiGiandomenico A., Keller A.E., Gao C., Rainey G.J., Warrener P., Camara M.M., Bonnell J., Fleming R., Bezabeh B., Dimasi N., et al. A multifunctional bispecific antibody protects against Pseudomonas aeruginosa. Sci. Transl. Med. 2014;6:262ra155. doi: 10.1126/scitranslmed.3009655. [DOI] [PubMed] [Google Scholar]
- 284.Cui J., Wei X., Lv H., Li Y., Li P., Chen Z., Liu G. The clinical efficacy of intravenous IgM-enriched immunoglobulin (pentaglobin) in sepsis or septic shock: A meta-analysis with trial sequential analysis. Ann. Intensive Care. 2019;9:27. doi: 10.1186/s13613-019-0501-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Hoffman J.N., Fertmann J.M., Vollmar B., Laschke M.W., Jauch K.W., Menger M.D. Immunoglobulin M-enriched human intravenous immunoglobulins reduce leukocyte-endothelial cell interactions and attenuate microvascular perfusion failure in normotensive endotoxemia. Shock. 2008;29:133–139. doi: 10.1097/shk.0b013e318123e5a6. [DOI] [PubMed] [Google Scholar]
- 286.Ates I., Dogan N., Aksoy M., Halici Z., Gundogdu C., Keles M.S. The protective effects of IgM-enriched immunoglobulin and erythropoietin on the lung and small intestine tissues of rats with induced sepsis: Biochemical and histopathological evaluation. Pharm. Biol. 2015;53:78–84. doi: 10.3109/13880209.2014.910535. [DOI] [PubMed] [Google Scholar]
- 287.Wand S., Klages M., Kirbach C., Warszawska J., Meybohm P., Zacharowski K., Koch A. IgM-enriched immunoglobulin attenuates systemic endotoxin activity in early severe sepsis: A before-after cohort study. PLoS ONE. 2016;11:e0160907. doi: 10.1371/journal.pone.0160907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Thi M.T.T., Wibowo D., Rehm B.H.A. Pseudomonas aeruginosa Biofilms. Int. J. Mol. Sci. 2020;21:8671. doi: 10.3390/ijms21228671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Estellés A., Woischnig A.K., Liu K., Stephenson R., Lomongsod E., Nguyen D., Zhang J., Heidecker M., Yang Y., Simon R.J., et al. A High-Affinity Native Human Antibody Disrupts Biofilm from Staphylococcus aureus Bacteria and Potentiates Antibiotic Efficacy in a Mouse Implant Infection Model. Antimicrob. Agents Chemother. 2016;60:2292–2301. doi: 10.1128/AAC.02588-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Ryser S., Tenorio E., Estellés A., Kauvar L.M. Human antibody repertoire frequently includes antibodies to a bacterial biofilm associated protein. PLoS ONE. 2019;14:e0219256. doi: 10.1371/journal.pone.0219256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Shah N.B., Osmon D.R., Steckelberg J.M., Sierra R.J., Walker R.C., Tande A.J., Berbari E.F. Pseudomonas Prosthetic Joint Infections: A Review of 102 Episodes. J. Bone Jt. Infect. 2016;1:25–30. doi: 10.7150/jbji.15722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Hsieh P.H., Lee M.S., Hsu K.Y., Chang Y.H., Shih H.N., Ueng S.W. Gram-negative prosthetic joint infections: Risk factors and outcome of treatment. Clin. Infect. Dis. 2009;49:1036–1043. doi: 10.1086/605593. [DOI] [PubMed] [Google Scholar]
- 293.Marculescu C.E., Cantey J.R. Polymicrobial prosthetic joint infections: Risk factors and outcome. Clin. Orthop. Relat. Res. 2008;466:1397–1404. doi: 10.1007/s11999-008-0230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Zmistowski B., Fedorka C.J., Sheehan E., Deirmengian G., Austin M.S., Parvizi J. Prosthetic joint infection caused by gram-negative organisms. J. Arthroplast. 2011;26:104–108. doi: 10.1016/j.arth.2011.03.044. [DOI] [PubMed] [Google Scholar]
- 295.von Specht B.U., Knapp B., Muth G., Broker M., Hungerer K.D., Diehl K.D., Massarrat K., Seemann A., Domdey H. Protection of immunocompromised mice against lethal infection with Pseudomonas aeruginosa by active or passive immunization with recombinant P. aeruginosa outer membrane protein F and outer membrane protein I fusion proteins. Infect. Immun. 1995;63:1855–1862. doi: 10.1128/iai.63.5.1855-1862.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Fito-Boncompte L., Chapalain A., Bouffartigues E., Chaker H., Lesouhaitier O., Gicquel G., Bazire A., Madi A., Connil N., Veron W., et al. Full virulence of Pseudomonas aeruginosa requires OprF. Infect. Immun. 2011;79:1176–1186. doi: 10.1128/IAI.00850-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Westritschnig K., Hochreiter R., Wallner G., Firbas C., Schwameis M., Jilma B. A randomized, placebo-controlled phase I study assessing the safety and immunogenicity of a Pseudomonas aeruginosa hybrid outer membrane protein OprF/I vaccine (IC43) in healthy volunteers. Hum. Vaccin Immunother. 2014;10:170–183. doi: 10.4161/hv.26565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Rello J., Krenn C.G., Locker G., Pilger E., Madl C., Balica L., Dugernier T., Laterre P.F., Spapen H., Depuydt P., et al. A randomized placebo-controlled phase II study of a Pseudomonas vaccine in ventilated ICU patients. Crit. Care. 2017;21:22. doi: 10.1186/s13054-017-1601-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Adlbrecht C., Wurm R., Depuydt P., Spapen H., Lorente J.A., Staudinger T., Creteur J., Zauner C., Meier-Hellmann A., Eller P., et al. Efficacy, immunogenicity, and safety of IC43 recombinant Pseudomonas aeruginosa vaccine in mechanically ventilated intensive care patients-a randomized clinical trial. Crit. Care. 2020;24:74. doi: 10.1186/s13054-020-2792-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Goss C.H. A Pharmacokinetic and Safety Study of IV Gallium Nitrate (Ganite) in Cystic Fibrosis Patients. [(accessed on 28 March 2023)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT01093521.
- 301.Pilewski J. Inhaled Sodium Nitrite as an Antimicrobial for Cystic Fibrosis. [(accessed on 28 March 2023)];2022 Available online: https://clinicaltrials.gov/ct2/show/NCT02694393.
- 302.Kaneko Y., Thoendel M., Olakanmi O., Britigan B.E., Singh P.K. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J. Clin. Investig. 2007;117:877–888. doi: 10.1172/JCI30783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Shionogi Inc Clinical Study of Cefiderocol (S-649266) for the Treatment of Nosocomial Pneumonia Caused by Gram-negative Pathogens (APEKS-NP) [(accessed on 10 March 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03032380.
- 304.Zemke A.C., Shiva S., Burns J.L., Moskowitz S.M., Pilewski J.M., Gladwin M.T., Bomberger J.M. Nitrite modulates bacterial antibiotic susceptibility and biofilm formation in association with airway epithelial cells. Free Radic. Biol. Med. 2014;77:307–316. doi: 10.1016/j.freeradbiomed.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Major T.A., Panmanee W., Mortensen J.E., Gray L.D., Hoglen N., Hassett D.J. Sodium nitrite-mediated killing of the major cystic fibrosis pathogens Pseudomonas aeruginosa, Staphylococcus aureus, and Burkholderia cepacia under anaerobic planktonic and biofilm conditions. Antimicrob. Agents Chemother. 2010;54:4671–4677. doi: 10.1128/AAC.00379-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Wunderink R.G., Matsunaga Y., Ariyasu M., Clevenbergh P., Echols R., Kaye K.S., Kollef M., Menon A., Pogue J.M., Shorr A.F., et al. Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of Gram-negative nosocomial pneumonia (APEKS-NP): A randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect. Dis. 2021;21:213–225. doi: 10.1016/S1473-3099(20)30731-3. [DOI] [PubMed] [Google Scholar]
- 307.Bilton D., Fajac I., Pressler T., Clancy J.P., Sands D., Minic P., Cipolli M., Galeva I., Solé A., Quittner A.L., et al. Long-term amikacin liposome inhalation suspension in cystic fibrosis patients with chronic P. aeruginosa infection. J. Cyst. Fibros. 2021;20:1010–1017. doi: 10.1016/j.jcf.2021.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Ailiyaer Y., Wang X., Zhang Y., Li C., Li T., Qi Q., Li Y. A Prospective Trial of Nebulized Amikacin in the Treatment of Bronchiectasis Exacerbation. Respiration. 2018;95:327–333. doi: 10.1159/000486134. [DOI] [PubMed] [Google Scholar]
- 309.Loebinger M.R., Polverino E., Blasi F., Elborn S.J., Chalmers J.D., Tiddens H.A., Goossens H., Tunney M., Zhou W., Angyalosi G., et al. Efficacy and safety of tobramycin inhalation powder in bronchiectasis patients with P. aeruginosa infection: Design of a dose-finding study (iBEST-1) Pulm. Pharmacol. Ther. 2019;58:101834. doi: 10.1016/j.pupt.2019.101834. [DOI] [PubMed] [Google Scholar]
- 310.Bilton D., Pressler T., Fajac I., Clancy J.P., Sands D., Minic P., Cipolli M., Galeva I., Solé A., Quittner A.L., et al. Amikacin liposome inhalation suspension for chronic Pseudomonas aeruginosa infection in cystic fibrosis. J. Cyst. Fibros. 2020;19:284–291. doi: 10.1016/j.jcf.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Nichols D.P., Singh P.K., Baines A., Caverly L.J., Chmiel J.F., RL G.I., Lascano J., Morgan S.J., Retsch-Bogart G., Saiman L., et al. Testing the effects of combining azithromycin with inhaled tobramycin for P. aeruginosa in cystic fibrosis: A randomised, controlled clinical trial. Thorax. 2022;77:581–588. doi: 10.1136/thoraxjnl-2021-217782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Mayer-Hamblett N., Retsch-Bogart G., Kloster M., Accurso F., Rosenfeld M., Albers G., Black P., Brown P., Cairns A., Davis S.D., et al. Azithromycin for Early Pseudomonas Infection in Cystic Fibrosis. The OPTIMIZE Randomized Trial. Am. J. Respir. Crit. Care Med. 2018;198:1177–1187. doi: 10.1164/rccm.201802-0215OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Guan W.J., Xu J.F., Luo H., Xu X.X., Song Y.L., Ma W.L., Liang Z.A., Liu X.D., Zhang G.J., Zhang X.J., et al. A Double-Blind Randomized Placebo-Controlled Phase 3 Trial of Tobramycin Inhalation Solution in Adults With Bronchiectasis With Pseudomonas aeruginosa Infection. Chest. 2023;163:64–76. doi: 10.1016/j.chest.2022.07.007. [DOI] [PubMed] [Google Scholar]
- 314.Haworth C.S., Bilton D., Chalmers J.D., Davis A.M., Froehlich J., Gonda I., Thompson B., Wanner A., O’Donnell A.E. Inhaled liposomal ciprofloxacin in patients with non-cystic fibrosis bronchiectasis and chronic lung infection with Pseudomonas aeruginosa (ORBIT-3 and ORBIT-4): Two phase 3, randomised controlled trials. Lancet Respir. Med. 2019;7:213–226. doi: 10.1016/S2213-2600(18)30427-2. [DOI] [PubMed] [Google Scholar]
- 315.Preston R.A., Mamikonyan G., DeGraff S., Chiou J., Kemper C.J., Xu A., Mastim M., Yeole R., Chavan R., Patel A., et al. Single-Center Evaluation of the Pharmacokinetics of WCK 5222 (Cefepime-Zidebactam Combination) in Subjects with Renal Impairment. Antimicrob. Agents Chemother. 2019;63:e01484-18. doi: 10.1128/AAC.01484-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Huntington J.A., Yu B., Li L., Jensen E., Bruno C., Boakye M., Zhang Z., Gao W., Feng H.P., Rhee E. Outcomes in Participants with Renal Impairment from a Phase 3 Clinical Trial for Ceftolozane/Tazobactam Treatment of Nosocomial Pneumonia (ASPECT-NP) Antimicrob. Agents Chemother. 2020;64:e00731-20. doi: 10.1128/AAC.00731-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Stone G.G., Bradford P.A., Tawadrous M., Taylor D., Cadatal M.J., Chen Z., Chow J.W. In Vitro Activity of Ceftazidime-Avibactam against Isolates from Respiratory and Blood Specimens from Patients with Nosocomial Pneumonia, Including Ventilator-Associated Pneumonia, in a Phase 3 Clinical Trial. Antimicrob. Agents Chemother. 2020;64:e02356-19. doi: 10.1128/AAC.02356-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Timsit J.F., Huntington J.A., Wunderink R.G., Shime N., Kollef M.H., Kivistik Ü., Nováček M., Réa-Neto Á., Martin-Loeches I., Yu B., et al. Ceftolozane/tazobactam versus meropenem in patients with ventilated hospital-acquired bacterial pneumonia: Subset analysis of the ASPECT-NP randomized, controlled phase 3 trial. Crit. Care. 2021;25:290. doi: 10.1186/s13054-021-03694-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Torres A., Zhong N., Pachl J., Timsit J.F., Kollef M., Chen Z., Song J., Taylor D., Laud P.J., Stone G.G., et al. Ceftazidime-avibactam versus meropenem in nosocomial pneumonia, including ventilator-associated pneumonia (REPROVE): A randomised, double-blind, phase 3 non-inferiority trial. Lancet Infect. Dis. 2018;18:285–295. doi: 10.1016/S1473-3099(17)30747-8. [DOI] [PubMed] [Google Scholar]
- 320.Stone G.G., Newell P., Gasink L.B., Broadhurst H., Wardman A., Yates K., Chen Z., Song J., Chow J.W. Clinical activity of ceftazidime/avibactam against MDR Enterobacteriaceae and Pseudomonas aeruginosa: Pooled data from the ceftazidime/avibactam Phase III clinical trial programme. J. Antimicrob. Chemother. 2018;73:2519–2523. doi: 10.1093/jac/dky204. [DOI] [PubMed] [Google Scholar]
- 321.Bruss J., Lister T., Gupta V.K., Stone E., Morelli L., Lei Y., Melnick D. Single- and Multiple-Ascending-Dose Study of the Safety, Tolerability, and Pharmacokinetics of the Polymyxin Derivative SPR206. Antimicrob. Agents Chemother. 2021;65:e0073921. doi: 10.1128/AAC.00739-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Palmer K.L., Mashburn L.M., Singh P.K., Whiteley M. Cystic fibrosis sputum supports growth and cues key aspects of Pseudomonas aeruginosa physiology. J. Bacteriol. 2005;187:5267–5277. doi: 10.1128/JB.187.15.5267-5277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Imamura Y., Yanagihara K., Fukuda Y., Kaneko Y., Seki M., Izumikawa K., Miyazaki Y., Hirakata Y., Sawa T., Wiener-Kronish J.P., et al. Effect of anti-PcrV antibody in a murine chronic airway Pseudomonas aeruginosa infection model. Eur. Respir. J. 2007;29:965–968. doi: 10.1183/09031936.00147406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.