Abstract
Monkeypox has been declared a public health emergency by the World Health Organization. There is an urgent need for efficient and safe vaccines against the monkeypox virus (MPXV) in response to the rapidly spreading monkeypox epidemic. In the age of COVID-19, mRNA vaccines have been highly successful and emerged as platforms enabling rapid development and large-scale preparation. Here, we develop two MPXV quadrivalent mRNA vaccines, named mRNA-A-LNP and mRNA-B-LNP, based on two intracellular mature virus specific proteins (A29L and M1R) and two extracellular enveloped virus specific proteins (A35R and B6R). By administering mRNA-A-LNP and mRNA-B-LNP intramuscularly twice, mice induce MPXV specific IgG antibodies and potent vaccinia virus (VACV) specific neutralizing antibodies. Further, it elicits efficient MPXV specific Th-1 biased cellular immunity, as well as durable effector memory T and germinal center B cell responses in mice. In addition, two doses of mRNA-A-LNP and mRNA-B-LNP are protective against the VACV challenge in mice. And, the passive transfer of sera from mRNA-A-LNP and mRNA-B-LNP-immunized mice protects nude mice against the VACV challenge. Overall, our results demonstrate that mRNA-A-LNP and mRNA-B-LNP appear to be safe and effective vaccine candidates against monkeypox epidemics, as well as against outbreaks caused by other orthopoxviruses, including the smallpox virus.
Subject terms: Vaccines, Nucleic-acid therapeutics, Vaccines
Introduction
Monkeypox, caused by the monkeypox virus (MPXV), is a zoonotic viral disease. Since January 2022, there have been >85,000 confirmed MPXV cases and >89 deaths worldwide, and transmission has occurred in 110 countries. Monkeypox has been declared a public health emergency by the World Health Organization. According to phylogenetic analysis, MPXV can be divided into two distinct clades, Central Africa (also known as the Congo Basin) and West Africa, with a high degree of sequence similarity between the two clades.1 Among them, The Central African clade tended to be more virulent, with a mean mortality rate of 10.6 % compared to 3.6 % for the West African clade.2 This may be because several open reading frames encoding immune evasion genes are disrupted in the West African clade, resulting in lower virulence in this clade.3 Nevertheless, it is the West African clade that caused the cases that are circulating in this outbreak.4
As a family of double-stranded DNA viruses, orthopoxviruses are composed of various viruses, including the Variola virus, Vaccinia virus (VACV), MPXV, and Cowpox virus.5 Orthopoxvirus infection or immunization confers immunity against other viruses in the genus.6 As a result, two vaccines have been approved by the FDA for the prevention of MPXV: ACAM2000, a second-generation live VACV vaccine, and JYNNEOS, an attenuated third-generation vaccine based on Modified Vaccinia Ankara (MVA). It has been reported that these two VACV-based smallpox vaccines are cross-protective against MPXV.7 However, smallpox vaccination does not completely protect against MPXV during the current outbreak, according to a recent study.8 Additionally, ACAM2000 was a highly reproducing VACV vaccine that caused serious adverse events.9 These events include autoinoculation of the eye, generalized vaccinia, eczema vaccinatum, progressive vaccinia, myocarditis, and death.10 On the other hand, JYNNEOS, a replication-deficient vaccine, is expected to have the lowest incidence of severe adverse events.9 However, as live virus vaccines, JYNNEOS and ACAM2000 have undefined immune targets, and the impact of their gene products on immunity and adverse reactions remains unclear. Therefore, it is imperative to develop a vaccine specifically for MPXV to ensure complete protection.
In this study, the MPXV quadrivalent mRNA vaccines, mRNA-A-LNP and mRNA-B-LNP, were prepared using A29L, A35R, M1R, and B6R as antigenic targets. Orthopoxviruses can infect cells through two different mechanisms depending on whether they are intracellular mature viruses (IMV) or extracellular enveloped viruses (EEV).11 There are two IMV-specific proteins (A29L and M1R) in the quadrivalent mRNA vaccine, and two EEV-specific proteins (A35R and B6R). Among the orthologous homologous to VACV, the L1R and A27L are known neutralizing antibody targets for IMV; the B5R are known neutralizing antibody targets for EEV; however, the A33R is a target of complement-mediated cytolysis.12,13 The combination of IMV- and EEV-specific immunogens have been found to provide more protection than either immunogen alone.14 It was reported that a DNA vaccine expressing A27L, A33R, L1R and B5R provided protection against lethal monkeypox,12 rabbitpox15 and vaccinia16 virus challenges. In contrast, vaccination with a single L1R provided a degree of protection against the lethal MPXV challenge but not against severe disease.12
Since the outbreak of COVID-19, mRNA vaccines have received unprecedented attention. There is a better immune response and safety associated with mRNA vaccines than with live virus and DNA vaccines.17,18 Furthermore, as opposed to multiple punctures (scarification) percutaneous administration of live VACV vaccines6 and electroporation of DNA vaccines,15 intramuscular administration of mRNA vaccines has reduced healthcare worker training and healthcare costs. So far, successful mRNA immunization resulting in protection from orthopoxviruses has never been reported. Here, we targeted MPXV homologs rather than VACV as antigenic targets to prepare MPXV quadrivalent mRNA vaccine, which induced MPXV-specific IMV- and EEV-antigen-specific IgG and potent VACV live-virus neutralizing antibodies. Meanwhile, immunized mice elicited a durable cellular response. Furthermore, mRNA-A-LNP and mRNA-B-LNP protected mice from VACV challenge.
Results
In vitro characterization of mRNA-A-LNP and mRNA-B-LNP
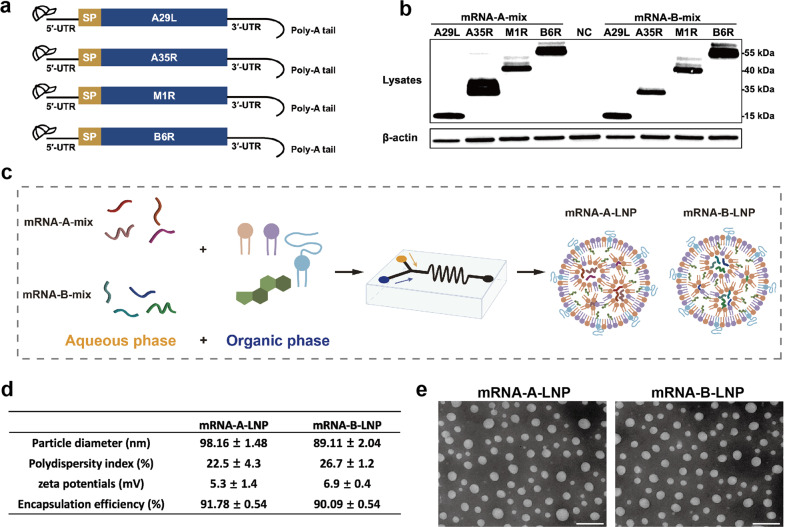
In this study, we developed potent MPXV quadrivalent mRNA vaccines that target two IMV-antigens and two EEV-antigens. Here, A29L, A35R, M1R and B6R, respectively, were chosen as antigenic targets for the mRNA coding sequences (Fig. 1a). The modified mRNA molecule begins with a 5'-cap 1, adheres to the 5'- and 3'-untranslated regions (UTRs), and then ends with a 120 nt Poly-A tail. First, based on different mRNA design platforms, we constructed two groups of mRNA sequences, mRNA-A-mix and mRNA-B-mix. All mRNAs have been authenticated by capillary electrophoresis using the Agilent 2100 Bioanalyzer System (Supplementary Fig. S1). Based on Western blot analysis, both mRNA-mix detected bands at positions corresponding to each of the four antigenic proteins. It indicated that both mRNA-A-mix and mRNA-B-mix could successfully express the proteins (Fig. 1b). Afterwards, the mixed mRNAs were processed into lipid nanoparticles (LNPs) formulations. The resulting two quadrivalent mRNA vaccines were named mRNA-A-LNP and mRNA-B-LNP, respectively (Fig. 1c). The final stored mRNA-A-LNP and mRNA-B-LNP showed average particle sizes of 98.16 nm and 89.11 nm, respectively, and potentials of 5.3 mV and 6.9 mV. Meanwhile, over 90% of encapsulation rates were achieved by both (Fig. 1d). The transmission electron microscopy (TEM) analysis also revealed that both mRNA-A-LNP and mRNA-B-LNP particles exhibited a homogeneous solid spherical morphology, which indicated that the morphology of the LNPs loaded with the four mRNAs remained stable (Fig. 1e). According to these results, mRNA-A-LNP and mRNA-B-LNP can efficiently express MPXV proteins A29L, A35R, M1R, and B6R in vitro.
Fig. 1.
Design and encapsulation of mRNA-A-LNP and mRNA-B-LNP. a The mRNA construct expresses the MPXV-specific antigen A29L, A35R, M1R, and B6R. b The MPXV-specific antigen A29L, A35R, M1R and B6R was expressed by mRNA in HEK293T cells. Cells were transfected with four mRNAs (1 μg/mL) each from mRNA-A-mix and mRNA-B-mix for 20 h using Lipofectamine 3000 transfection reagent. c Preparation mechanism of mRNA-A-LNP and mRNA-B-LNP. Briefly, the four mRNAs were mixed in an acidic aqueous solution, then injected with organic phase lipids, and the mixture was extruded through a microfluidic chip. d The physicochemical parameters of mRNA-A-LNP and mRNA-B-LNP. Data are shown as mean ± SEM. e A representative TEM image presented the morphology of mRNA-A-LNP and mRNA-B-LNP. Scale bar = 200 nm
The prophylactic administration of mRNA-A-LNP and mRNA-B-LNP induces potent humoral immunity in mice
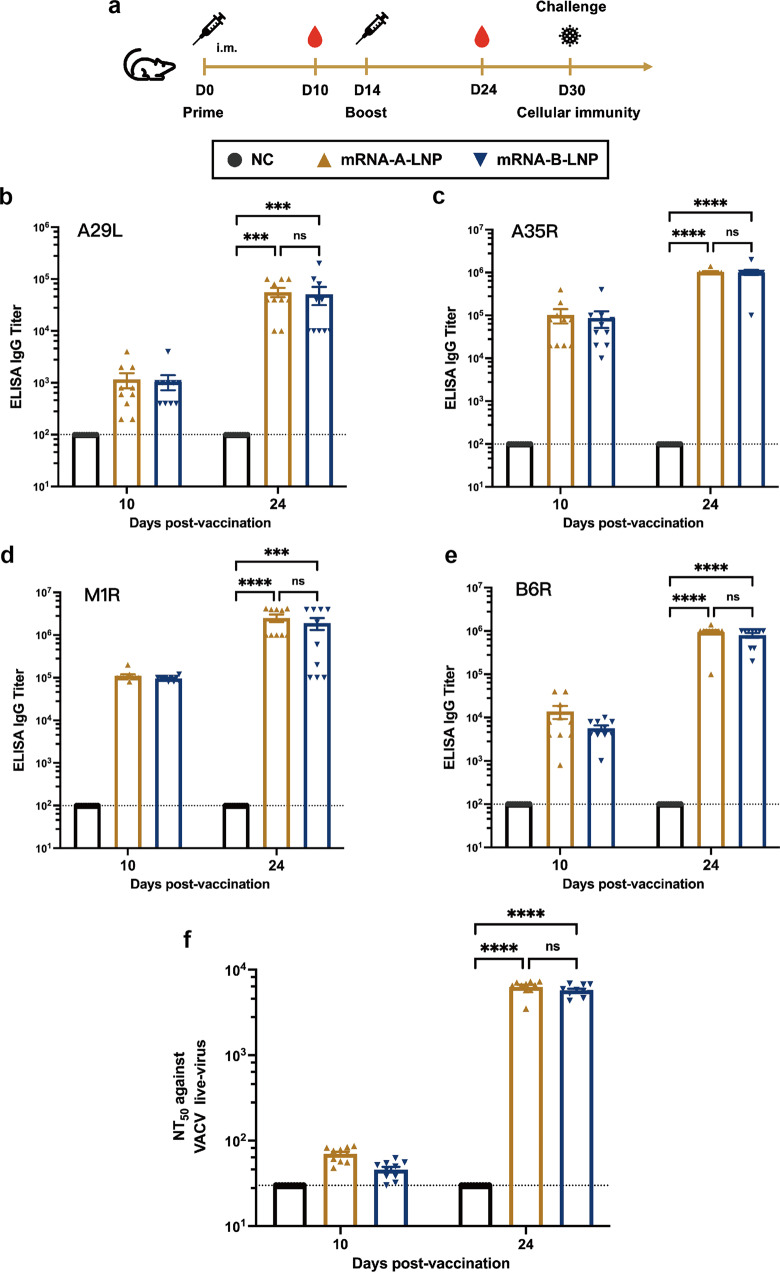
The immunogenicity and efficacy of mRNA-A-LNP and mRNA-B-LNP were assessed in mice (Fig. 2a). The mice were immunized with mRNA-A-LNP or mRNA-B-LNP, respectively, by twice intramuscular administration at intervals of 14 days; naive mice served as the control group. Sera were collected from all mice 10 and 24 days after their first immunization to assess humoral immunity. The binding antibody responses against MPXV antigens (A29L, A35R, M1R and B6R) were assessed by enzyme-linked immunosorbent assay (ELISA). Antibodies against all four target antigens were detected in all vaccinated mice and increased significantly over immunization frequency. The IgG titers against the four MPXV antigens at day 10 were 1/1160 (A29L), 1/102000 (A35R), 1/110000 (M1R), 1/13880 (B6R) for mRNA-A-LNP and 1/1060 (A29L), 1/87,000 (A35R), 1/96,000 (M1R), 1/5700 (B6R) for mRNA-B-LNP, respectively. After booster immunization with the same dose, the IgG titers against the four MPXV antigens were 1/56,000 (A29L), 1/1,040,000 (A35R), 1/2,520,000 (M1R), 1/950,000 (B6R) for mRNA-A-LNP and 1/51,000 (A29L), 1/1,010,000 (A35R), 1/1,910,000 (M1R), 1/800,000 (B6R) for mRNA-B-LNP, respectively (Fig. 2b-e). The results indicated that mRNA-A-LNP and mRNA-B-LNP induce robust IgG against MPXV antigens.
Fig. 2.
Humoral Immune Response in mRNA-A-LNP and mRNA-B-LNP-Vaccinated Mice. Female BALB/c mice were immunized with 40 μg mRNA-A-LNP vaccine or 40 μg mRNA-B-LNP vaccine or were designated negative controls (n = 10). Two intramuscular immunizations were on day 0 and day 14, respectively. Serum was collected 10 and 24 days after the initial vaccination. a Schematic diagram of immunization, sample collection, and challenge schedule. b–e The MPXV-specific antigen A29L, A35R, M1R and B6R IgG antibody titer was determined by ELISA. f The NT50 was determined by neutralizing antibody assay based on live VACV. The dashed line indicates the limit of detection of the assay. Data are shown as mean ± SEM. Significance was calculated using two-way ANOVA with multiple comparison tests (n.s., not significant; **p < 0.01)
The neutralizing antibody responses were assessed by live-virus neutralization tests using the VACV Tian Tan strain. After initial vaccination, neutralizing antibody titers were slightly above the detection limit, with median values of 1/70 and 1/46 for mRNA-A-LNP and mRNA-B-LNP, respectively (Fig. 2f). After booster immunization, neutralizing antibody titers increased by nearly 2 log10, with median values of 1/6327 and 1/5744 for mRNA-A-LNP and mRNA-B-LNP, respectively (Fig. 2f). Based on the MPXV antigen-specific ELISA and VACV live-virus neutralization tests, there was no statistical difference in the humoral response to mRNA-A-LNP or mRNA-B-LNP immunizations. In conclusion, these results suggest that the quadrivalent mRNA vaccine induced neutralizing antibodies against VACV and IgG antibodies against MPXV.
The mRNA-A-LNP and mRNA-B-LNP induce cellular immunity in mice
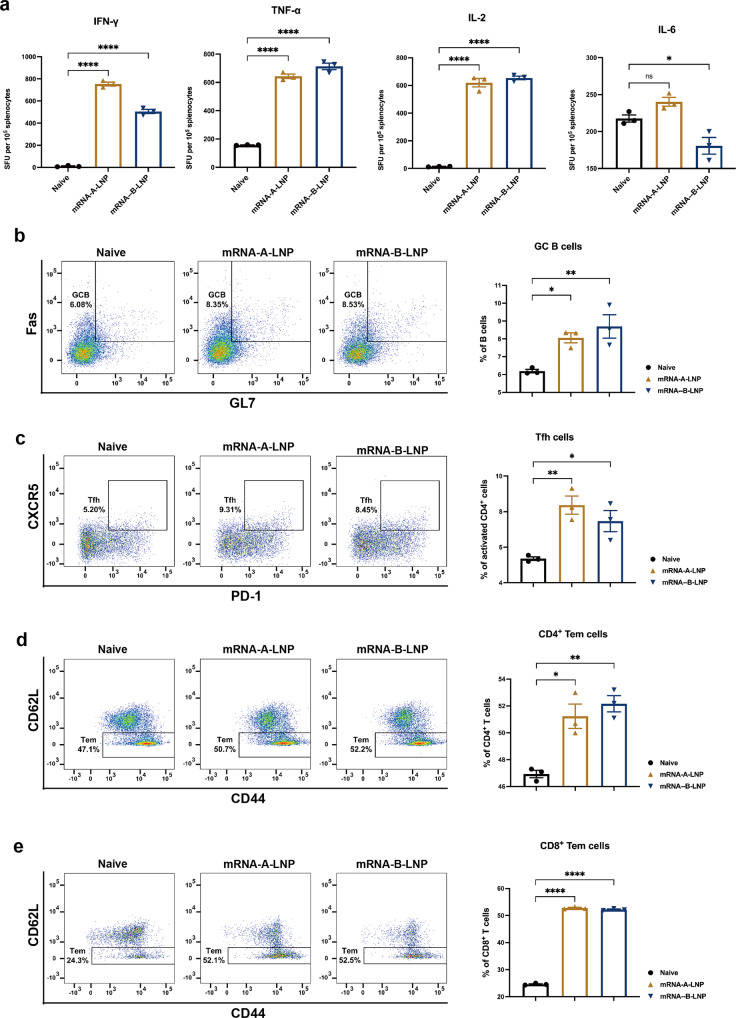
As T-cell immunity is essential for a robust anti-viral infection, we further investigated whether mRNA-A-LNP or mRNA-B-LNP immunization could evoke an MPXV-specific T cellular immunity. Enzyme-linked immune-spot (ELISPOT) analysis showed significant induction of IFN-γ (Th-1), TNF-α (Th-1) and IL-2 (Th-1) in splenocytes of vaccinated mice. In contrast, there was no significant difference in the level of IL-6 (Th-2) secretion between naive and vaccinated mice (Fig. 3a). The results showed that the mRNA-A-LNP and mRNA-B-LNP vaccines successfully induced Th1-based MPXV-specific cellular immune responses.
Fig. 3.
MPXV-Specific Cell Response in mRNA-A-LNP and mRNA-B-LNP-Vaccinated Mice. a ELISPOT detection of IFN-γ, TNF-α, IL-2 and IL-6 release from MPXV-A29L, A35R, M1R, and B6R peptide stimulated splenocytes 30 days after immunization. SFU spot forming units. Data are shown as mean ± SEM. b, c MPXV-specific GC B cells (b) and Tfh cells (c) in DLNs were detected by flow cytometry. d, e MPXV-specific CD4+ (d) and CD8+ (e) Tem cells in the spleen were detected by flow cytometry. Significance was calculated using one-way ANOVA with multiple comparison tests (n.s., not significant; *p < 0.05, **p < 0.01, ***p < 0.001)
The mRNA-A-LNP and mRNA-B-LNP induce memory cell responses in mice
MPXV infection is primarily controlled by antibodies, but memory B cells and memory T cells play a role in the development of the humoral response to the monkeypox vaccine.19 Here, through the measurement of MPXV-specific germinal center (GC) B cells, follicular helper T (Tfh) cells, as well as CD4+ and CD8+ effector memory T (Tem) cells, we assessed the ability of mRNA-A-LNP and mRNA-B-LNP to induce memory cell responses. The GC responses are responsible for generating high affinity neutralizing antibodies,20 while Tfh cells regulate the GC response.21,22 Furthermore, memory CD4+ T cells and memory CD8+ T cells may provide long-term protection. Flow cytometry results showed a significant increase in MPXV-specific GC B cells and Tfh cells in draining lymph nodes (DLNs) from mRNA-A-LNP, and mRNA-B-LNP-vaccinated mice compared with naive mice upon stimulation with MPXV-specific antigens (Fig. 3b-c). This suggests that mRNA-A-LNP and mRNA-B-LNP immunization can produce a long-lasting memory B cell effect. Then, specific CD4+ and CD8+ Tem cells in the spleen of immunized mice were further assessed. It was remarkable that mRNA-A-LNP and mRNA-B-LNP were more effective in induced MPXV-specific CD8+ Tem cells than CD4+ Tem cells (Fig. 3d-e). This demonstrated that our vaccine caused a memory T cell effect.
The mRNA-A-LNP and mRNA-B-LNP protected mice from VACV challenge
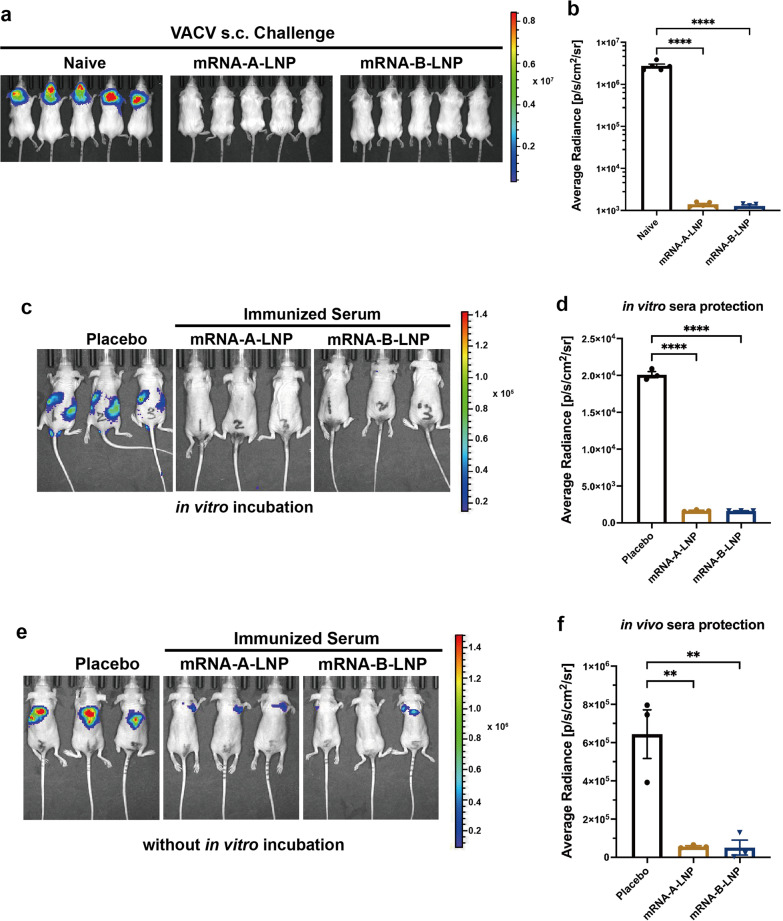
The VACV challenge model based on firefly luciferase expression23 was used to assess the protection of mRNA-A-LNP and mRNA-B-LNP in mice. Viral infection in mice was detected by bioluminescence imaging (BLI).24
First, we assessed the active protection of mRNA-A-LNP and mRNA-B-LNP. BALB/c mice immunized twice intramuscularly with mRNA-A-LNP or mRNA-B-LNP were challenged with VACV Tian Tan strain via the s.c. route. Viral load was measured in mice at 24 h following the challenge by using BLI. The bioluminescent signal was largely undetectable in immunized mice, whereas naive mice detected signal values as high as 6.5 log10, which suggested that VACV was rapidly cleared by vaccine-induced antibodies after the challenge (Fig. 4a-b). This suggests that prophylactic immunization with mRNA-A-LNP and mRNA-B-LNP is effective in protecting mice from s.c. VACV challenge.
Fig. 4.
Protection of mRNA-A-LNP and mRNA-B-LNP against VACV Challenge in Mice. a, b Thirty days after initial immunization, mice (n = 5) were s.c. challenged with VACV (4 × 105 TCID50). The viral load of mice was measured by bioluminescence imaging, and the bioluminescence signal of mice was measured 24 h after the challenge. c, d In vitro serum protection. Serum (13 μl) and virus (4 × 103 TCID50) were mixed for 1 h before the i.v. and i.p. challenges of the 4-week-old nude mice (n = 3). Bioluminescent signal measurements were taken 6 h after the viral challenge. e, f In vivo serum protection. Serum (50 μl) was first injected intravenously into the 4-week-old nude mice (n = 3), followed 1 h later by s.c. challenge with VACV (2.5 × 105 TCID50). Bioluminescent signal measurements were taken 24 h after the viral challenge. Data are shown as mean ± SEM. Significance was calculated using one-way ANOVA with multiple comparison tests (**p < 0.01, ****p < 0.0001)
In addition, we investigated the passive protection of mRNA-A-LNP and mRNA-B-LNP immunized mouse sera in vitro and in vivo. After incubation of sera with VACV for 1 h in vitro, the bioluminescence signal was significantly diminished in nude mice treated with immunized sera (Fig. 4c-d). It indicated that sera from mRNA-A-LNP and mRNA-B-LNP-immunized mice were effective in neutralizing VACV in vitro. Moreover, to verify the in vivo protection, mice were first injected with serum and then challenged with VACA. We found that only a slight luminescent signal was detected at the injection site in nude mice treated with immunized serum. In contrast, a significant luminescence signal was detected when treated with naive mouse serum. (Fig. 4e, f). In conclusion, these results demonstrated that mRNA-A-LNP and mRNA-B-LNP inoculated mouse sera had a passive protection against VACV challenge in immunodeficient young mice.
MPXV quadrivalent mRNA vaccine administered intramuscularly provides adequate safety
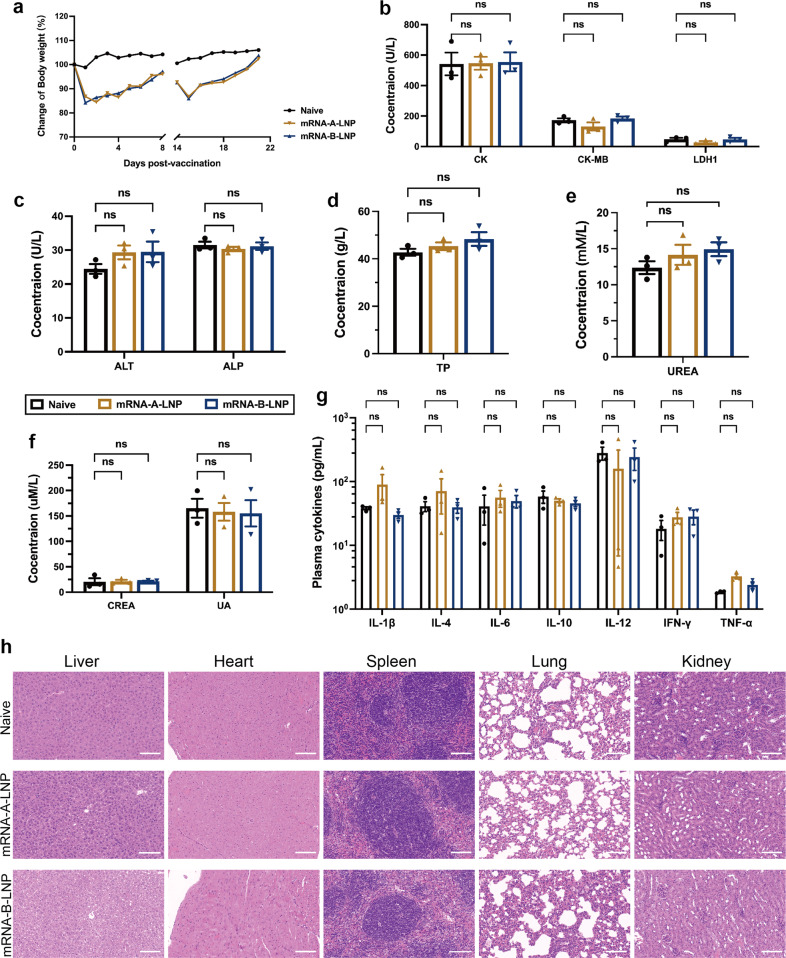
To assess the safety of our vaccine in vivo, we monitored several aspects, including mouse weight recordings, biochemical parameters, innate cytokine responses and histopathological changes. After mRNA-A-LNP or mRNA-B-LNP immunization, body weight rapidly recovered, and no weight loss occurred (Fig. 5a). Furthermore, no local skin reactions or inflammation were observed at the injection site. According to clinical trial data, VACV-based vaccines have caused adverse events, including a 0.6% risk of myocarditis.6 Therefore, we further assessed the heart, liver and kidney function based on blood biochemical parameters. The data were within normal limits in the immunized and control groups, and there were no significant differences (Fig. 5b-f). In addition, to assess whether the vaccine elicited an excessive inflammatory response, we isolated sera from all mice 48 h after the first vaccination to detect cytokines in the innate immune response. To detect the secretion levels of IL-6, TNF-α, IL-4, IL-10, IFN-γ, IL-12 and IL-1β in the serum of immunized and naive mice. In particular, the release of IL-10 is usually a strong indication of cytokine storm, which can lead to excessive inflammation.25,26 The inflammatory factors IL-6 and IL-1β were also evaluated. As a result, the levels of all cytokines in the serum of mRNA-A-LNP and mRNA-B-LNP mice were not significantly different from those of naive mice at 48 h (Fig. 5g). This implies that the injection of mRNA-A-LNP and mRNA-B-LNP did not trigger an inflammatory response in the mice. Finally, multiple tissues from the mice were extracted for histopathological examination. Since no difference among the H&E-stained histopathological tissue sections, the safety of mRNA-A-LNP and mRNA-B-LNP in mice was further confirmed (Fig. 5h). In conclusion, multiple lines of evidence demonstrate that our vaccine presents an adequate safety profile.
Fig. 5.
The safety evaluation of MPXV quadrivalent mRNA vaccine in mice. a The body weight records of mice on the first eight days after each vaccination. b–f Heart, liver and kidney function were determined by blood biochemical parameters (n = 5). CK, CK-MB and LDH1 represent heart function (b), ALT, ALP and TP represent liver function (c, d), while UREA, CREA and UA represent kidney function (e, f). g The immune activation effect of the vaccine was expressed by analysis of cytokines in innate immunity (n = 5). h Representative histopathology (H&E) of different tissues, liver, heart, spleen, lung and kidney from naive mice or mRNA vaccine-immunized mice. The H&E stained sections shown in the data are representative results from three test mice 48 h post-inoculation. Scale bar = 100 μm, 30×. Data are shown as mean ± SEM. Significance was calculated using two-way ANOVA with multiple comparison tests (n.s., not significant; *p < 0.05, **p < 0.01)
Discussion
Since the use of live VACV in the past century, smallpox has been completely eradicated, associated vaccination no longer occurs, and vaccine production has ceased accordingly. In this study, we report the immunogenicity and efficacy of the novel MPXV mRNA vaccine candidate, mRNA-A-LNP and mRNA-B-LNP. Two doses of immunization with mRNA-A-LNP or mRNA-B-LNP elicited robust antibody and cell responses in mice. High MPXV IgG titers were present in mice that received the vaccine, which implied that the vaccine induced potent MPXV antibody responses (Fig. 2b-e).
The genome’s central region of all orthopoxviruses showed over 90% homology, with 96.3% homology between MPXV and VACV, implying that they are highly genetically similar.27–29 In particular, MPXV A29L, A35R, M1R, and B6R genes exhibit high conservation with the orthologous genes of orthopoxviruses, including VACV and smallpox virus.16 Thus, we hypothesize that mRNA-A-LNP and mRNA-B-LNP have cross-neutralizing effects on orthopoxviruses. Interestingly, live-virus neutralizing antibody assays determined that our MPXV vaccine elicited potent VACV-neutralizing antibodies (Fig. 2f). This means that MPXV vaccines provide cross-neutralizing antibodies against VACV within orthopoxviruses.
The MPXV challenge in nonhuman primates (NHPs) and the VACV challenge in mice represent typical disease models that demonstrate the protective efficacy of orthopoxvirus countermeasures.15 Therefore, we chose the VACV mouse challenge model to assess the cross-protection of the MPXV vaccine candidate. First, two doses of prophylactic immunization with mRNA-A-LNP and mRNA-B-LNP protected mice from s.c. VACV challenge (Fig. 4a-b). Furthermore, antibodies induced by mRNA-A-LNP and mRNA-B-LNP neutralized VACV and provided protection in nude mice (Fig. 4c-f). And, vaccine-induced humoral responses were further confirmed in immunodeficient young mice by passive transfer tests. Our findings are consistent with a previous study, which shows that DNA vaccine-induced humoral immunity protects mice from VACV challenge.30
Using the mRNA vaccine platform, the MPXV quadrivalent mRNA vaccines, mRNA-A-LNP and mRNA-B-LNP, induced high titers of functional antibodies in mice while also boosting cellular immunity. The induction of cellular immunity may be a critical factor in explaining the remarkable protection provided by live attenuated vaccines compared with subunit vaccines.12,31 In the application of the SARS-CoV-2 vaccine, mRNA vaccines have been well demonstrated to induce cellular immunity.20,32 It is consistent with our findings that the MPXV quadrivalent mRNA vaccine was able to elicit excellent cellular immunity (Fig. 3a). In addition, live virus vaccines do not stop the virus from spreading.33 In contrast, the smallpox DNA vaccine, a nucleic acid vaccine, has been reported to prevent the shedding of infectious viruses in the oral cavity of vaccinated animals.34 As a result, it is reasonable to hypothesize that the MPXV mRNA vaccine, also as a nucleic acid vaccine, will prevent the shedding of infectious viruses in all inoculated animals. And we will conduct further studies to test this hypothesis.
In the orthopoxvirus route of infection, monocytes are first recruited to the site of infection as the initial target.35,36 The cytolytic T cells, meanwhile, kill infected monocytes to prevent virus spread. Memory CD8+ T cells have been reported effective in protecting susceptible mice from lethal orthopoxvirus challenges.37–39 Since mRNA-A-LNP and mRNA-B-LNP primarily elicit memory CD8+ T cells, this allows T cell-mediated lysis to prevent virus transmission during the early infection period of viral challenge (Fig. 3e). It is the long-lived plasma cells (LLPCs) and memory B cells that are responsible for most of the prolonged humoral immunity induced by vaccines.40 Memory B cells induced with the smallpox vaccine could respond quickly to infection and replenish LLPCs to maintain long-term antibodies levels in humans, according to a study.41 Therefore, considering that memory B cells are developed in the germinal center, the significant increase in MPXV-specific GC B cells and Tfh cells suggests that mRNA-A-LNP and mRNA-B-LNP can maintain protective antibody responses with high affinity and durability (Fig. 3b-c).
In vivo safety analysis revealed that mRNA-A-LNP and mRNA-B-LNP did not cause significant adverse reactions, demonstrating the safety of our MPXV quadrivalent mRNA vaccine. Meanwhile, we did not find any abnormal skin reactions at the injection site. This suggests, different from ACAM2000 (the damage at the vaccine site is often used as a marker of successful vaccination in highly replicating vaccines like ACAM2000), our vaccine does not undergo a significant cutaneous reaction, also known as “take”, which means no risks of autoinoculation or accidental vaccination.6
In summary, we report efficient and safe quadrivalent mRNA vaccine candidates against MPXV, based on MPXV-specific antigens A29L, A35R, M1R and B6R. The vaccines reported here are the first MPXV vaccines developed using an mRNA vaccine platform. As mRNA-A-LNP and mRNA-B-LNP induce solid humoral and cellular immunity, they could provide new ideas for orthopoxvirus vaccine development. Considering the rapidly spreading monkeypox epidemics, MPXV mRNA vaccines which can be rapidly developed and prepared on a large scale, are expected to protect against infection-related symptoms, hospitalizations, and death.
Materials and methods
Ethics statement
All animal studies, there were reviewed and approved by the Animal Experiment Committee of Laboratory Animal Center, Academy of Military Medical Sciences (AMMS), China (Assurance Number: IACUC-DWZX-2022-576). All animal studies were conducted strictly in accordance with the guidelines set by the Chinese Regulations of Laboratory Animals and Laboratory Animal Requirements of Environment and Housing Facilities.
Cells and viruses
HEK293T, Huh-7, RD, Vero and 143TK cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM; Thermo Fisher) supplemented with 10 % fetal bovine serum (FBS; Thermo Fisher) and penicillin (100 U/ml)-streptomycin (100 mg/ml) (Thermo Fisher). All cells were grown at 37 °C in a humidified 5% CO2 atmosphere.
The VACV Tian Tan strain expressing firefly luciferase was propagated in 143TK cells and titrated in Vero cells.23
Synthesis and characterization of MPXV mRNA
All mRNA sequences encoding MPXV proteins (A29L, A35R, M1R, B6R) were prepared by in vitro transcription, as described previously.22 Transcription was performed from linearized DNA templates using the T7-FlashScribe™ Transcription Kit (Cellscript). Further, the modified mRNA was synthesized by replacing UTP in the kit with pseudo-UTP (TriLink). Afterwards, the RNA was capped using the ScriptCap™ Cap 1 Capping System kit with ScriptCap™ Capping enzyme and 2'-O-methyltransferase (Cellscript) according to the manufacturer’s instructions. The mRNA product purified by ammonium acetate precipitation was then resuspended in RNase-free water for further analysis and application.
The concentration and quality of the synthesized MPXV mRNA were measured using an Agilent 2100 Bioanalyzer and RNA Nano 6000 Assay Kit (Agilent), according to the manufacturer’s instructions.
All mRNAs (2 μg) were transfected into HEK293T cells using Lipofectamine 3000 transfection reagent (Thermo Fisher) according to the manufacturer’s instructions, lysates were collected, and Western blotting was performed.
Formulation and characterization of mRNA-LNP
LNPs formulations were prepared using NanoAssemblr Ignite’s (Precision Nanosystems) NxGen Microfluidics technology. Briefly, lipids containing ionized lipids, 1, 2-distearoyl-sn-glycero-3-phosphocholine (DSPC), cholesterol and DMG-PEG2000 were dissolved in ethanol (with a molar ratio of 50:10:38.5:1.5). In an Ignite™ mixer, the lipid mixture was combined with 20 mM citrate buffer (pH 4.0) containing mRNA in a 1:3 volume ratio. It was then diluted through a 100 k MWCO PES membrane (Sartorius Stedim Biotech) against a 10-fold volume of DPBS (pH 7.4) and concentrated to the desired concentration.
The particle size and ζ-potential of mRNA-LNP were measured by a Litesizer 500 (Anton Paar). Particle size measurements were carried out using dynamic light scattering (DLS). The data were also analyzed using the Anton Paar Kalliope software package.
The morphology of mRNA-LNP was analyzed by transmission electron microscopy (Hitachi H-7800, Tokyo) using a negative staining technique. mRNA-LNP was absorbed into the copper mesh for 60 s and stained with phosphotungstic acid (1%) for 20 s before observation.
The Quant-iT RiboGreen RNA Reagent and Kit were used to detect the encapsulation of mRNA-LNP according to the manufacturer’s instructions.
Mouse vaccination
In this experiment, BALB/c mice (6–8 weeks of age, female, SPF) were randomly divided into three groups (n = 10). The mice were immunized with the mRNA-A-LNP vaccine or the mRNA-B-LNP vaccine or were designated negative controls. We administered the vaccine intramuscularly at 40 μg on day 0. And a booster immunization was administered on day 14. All mice were tested for IgG and neutralizing antibodies on days 10 and 24 after the initial immunization. The following flow cytometry analyses were performed 30 days after the initial vaccination.
Evaluation of serum antibody
IgG antibody titers to MPXV-specific antigens A29L, A35R, M1R and B6R were determined by ELISA. VACV-specific neutralizing antibody titers were determined by a live-virus based neutralization test.
-
ELISA assay.
IgG antibody titers against MPXV-specific antigens A29L, A35R, M1R and B6R were determined by ELISA. A 96-well plate was coated with 1 μg/ml of A29L (TSP-MV002, Tsingke Biotechnology), A35R (TSP-MV003, Tsingke Biotechnology), M1R (TSP-MV005, Tsingke Biotechnology) or B6R (40902-V08H, Sino Biological) protein respectively and incubated overnight at 4 °C. After incubation, plates were washed with 1 x TBST and blocked with BSA for 2 h at 37 °C. Then, serial 2-fold gradient dilutions of serum starting at 1:100 were added to the wells, diluted with casein block, and incubated for 1 h at 37 °C. Next, the plates were washed and treated with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Abclonal) for 1 h at 37 °C. Afterwards, the plates were washed and incubated with the substrate tetramethylbenzidine (TMB; TIANGEN) for 20 min at room temperature in the dark before the reaction was terminated with hydrochloric acid (2 M; Solarbio). Absorbance at 450/630 nm was recorded using an I-control Infinite 200 PRO microplate reader (TECAN). The ELISA endpoint titers were defined as the dilution of vaccinated serum, which resulted in absorbance no less than 2.1-fold that of the average negative serum (1:100).
Live-virus neutralization assay
Neutralizing antibody titers were determined by a live-virus neutralization assay. The VACV Tian Tan strain was designed to encode firefly luciferase protein.42 Sera were tested for neutralizing activity against the live virus via mixing serial 3-fold diluted sample, starting at 1:30, with 4 × 103 TCID50 of VACV. The serially diluted serum samples were mixed with the diluted virus in an equal volume. The antibody–virus and virus-only mixtures were then incubated at 37 °C with 5% CO2. After incubating for 1 h, we added Vero cells (40,000 cells/well) to each well at 37 °C with 5% CO2. After 48 h incubation, the cells were lysed, and the luciferase activity was measured via Bright-Glo Luciferase Assay System (Promega) according to the manufacturer’s specifications. Luciferase activity was then measured using an EnSight plate reader (PerkinElmer). Neutralizing activity was calculated by quantification of luciferase activity in relative light units (RLU). 50% live-virus neutralizing antibody titer (NT50) were calculated using a log (inhibitor) vs. normalized response (Variable slope) non-linear regression model in GraphPad Prism 8.0 (GraphPad Software).
Evaluation of cellular immune response
Cells from spleen and DLNs were isolated and analyzed by flow cytometry to determine the cellular immune response. DLNs were used to analyze Tfh cells responses and GC B cells responses, Spleen was used to analyze CD4+ or CD8+ Tem cells responses and for ELISPOT assays.
-
Flow cytometry assay
Briefly, cells from spleen or DLNs (1,000,000 cells/well) were stimulated with peptide pool for the MPXV-A29L, A35R, M1R, and B6R protein (2 μg/ml of each peptide) at 37 °C in 5% CO2 for 12 h. Brefeldin A (5 μg/ml; Biolegend) was incubated with cells for 4 h. Then, Fc receptors of cells were blocked using CD16/CD32 antibodies (Mouse BD Fc Block; BD Biosciences) for 15 min at 4 °C, and cells were stained with a cocktail of fluorescently conjugated antibodies to CD3-PE/Cyanine7 (Biolegend), CD4-FITC (Biolegend), CD8-PercP (Biolegend), CD44-PE (Biolegend), CD62L-APC (Biolegend), B220-FITC (Biolegend), CD4-PercP/Cyanine5.5 (Biolegend), CD44-APC (Biolegend), PD-1-PE/Cyanine7 (Biolegend), B220-PercP/Cyanine5.5 (Biolegend), Fas-PE (Biolegend) and GL7-FITC (Biolegend) for another 30 min at 4 °C in dark. Following washing with cell staining buffer (BD Biosciences), dead cells were stained with Fixable Viability Dye eFluor™ 780 (Thermo Fisher Scientific) for 30 min at 4 °C in the dark. A final wash with cell staining buffer, data were obtained by FACS Aria II flow cytometer (BD Biosciences) and analyzed by Flow J software. The GC B cell response was represented as live+/B220+/Fas+/GL7+. The Tfh cell response was represented as live+/B220-/CD4+/CD44+/PD-1+/CXCR5+. The CD4+ Tem cell response was represented as live+/CD3+/CD4+/CD44+/CD62L−. The CD8+ Tem cell response was represented as live+/CD3+/CD8+/CD44+/CD62L−.
ELISPOT assay
Cellular immune responses in the vaccinated mRNA vaccine mice were assessed using IFN-γ, TNF-α, IL-2 or IL-6 precoated ELISPOT kits (MabTech), according to the manufacturer’s protocol. Briefly, the plates were four washed with PBS and blocked using RPMI 1640 (Thermo Fisher Scientific) containing 10% FBS and incubated for 30 min at room temperature. Immunized mouse splenocytes (300,000 cells/well) were stimulated with peptide pool for the MPXV-A29L, A35R, M1R, and B6R protein (2 μg/ml of each peptide), PMA and Ionomycin (Dakewe) as positive control and RPMI 1640 media as negative control. Following incubation at 37 °C, 5% CO2 for 36 h, plates were washed with PBS and incubated with biotinylated anti-mouse IFN-γ, TNF-α, IL-2 or IL-6 antibody for 2 h at room temperature. Wash the plates and incubate Streptavidin-HRP for 1 h at room temperature. Finally, the plates were incubated with TMB substrate solution until spots exposed on the plates. Wash the plates with deionized water and dry in a dark place for 24 h. The spots were read by an automated VSR07 ELISPOT reader (AID). The numbers of spot-forming cells (SFU) per million cells were calculated by vSpot 7.0 software.
Serum protective test
The VACV challenge model is based on the VACV Tian Tan strain expressing firefly luciferase. Using 4-week-old BALB/c nude mice, the serum passive protection model was developed. Protective sera were collected from BALB/c mice immunized with mRNA-A-LNP and mRNA-B-LNP. Serum samples were collected 24 days after the initial immunization. To assess in vitro sera protection, serum (13 μl) and virus (4 × 103 TCID50) were mixed for 1 h before intravenous (i.v.) and intraperitoneal (i.p.) challenge of nude mice.
To assess in vivo sera protection, serum (50 μl) was first injected intravenously into nude mice, followed 1 h later by s.c. challenge with VACV (2.5 × 105 TCID50). Bioluminescent signal measurements were made following the viral challenge.
BLI was acquired and analyzed using the IVIS Lumina Series III imaging system (PerkinElmer). Briefly, luminescence was measured 5 min after intraperitoneal injection of the substrate D-luciferin (PerkinElmer). The bioluminescent signals in regions of interest (ROIs) were quantified using Living Image 3.5.
Mouse challenge
The VACV challenge model is based on the VACV Tian Tan strain expressing firefly luciferase. BALB/c mice (n = 5) immunized twice intramuscularly with 40 μg of mRNA-A-LNP or mRNA-B-LNP were challenged at day 30 with 4 × 105 median tissue culture infectious doses (TCID50) of the VACV Tian Tan strain via the s.c. route. Viral load was measured in mice at 24 h following the challenge by using BLI.
In vivo toxicity
To assess the in vivo toxicity of the vaccine, body weights were recorded after vaccination. Mice vaccinated with mRNA-A-LNP or mRNA-B-LNP (40 μg; n = 3) were analyzed for heart, liver and kidney function 48 h after immunization using a Chemray 240 and Chemray 800 (Rayto) automated biochemical analyzer. It was also used with IL-6 ELISA kits (Thermo Fisher), TNF-α ELISA kits (Thermo Fisher), IL-4 ELISA kits (MULTI), IL-10 ELISA kits (Thermo Fisher), IFN-γ ELISA kits (MULTI), IL-12 ELISA Kit (MULTI) and IL-1β ELISA Kit (Thermo Fisher) for the analysis of immune activated cytokines.
Organ tissues, including heart, liver, spleen, lung and kidney, were extracted 48 h after injection for histopathology and fixed in 4% neutral buffered formaldehyde. Afterwards, they were embedded in paraffin, sectioned and stained with hematoxylin and eosin (H&E). Images were taken with a NIKON Eclipse CI microscope.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 8.0 (GraphPad Software). All of the data are presented as the mean ± SEM. Statistical difference was analyzed by one-way or two-way ANOVA. All tests are accepted as statistically significant when the p-value is <0.05.
Supplementary information
Monkeypox virus quadrivalent mRNA vaccine induces immune response and protects against vaccinia virus
Acknowledgements
The study was supported by the National Key R&D Program of China (2021YFC2302405) and the National Natural Science Foundation of China (Grant No. 81830101).
Author contributions
J.Y. and S.W. conceived the project. Y.S., Z.Z. and H.L. synthesized the mRNA vaccine and performed the experiments. F.L., Y.W. and W.H. performed animal challenge experiments. C.Y., H.S., J.L., Y.C., J.M., Y.M., X.W., and J.F. provided experimental support. J.Y. designed the MPXV mRNA sequence. Y.S., Z.Z. and J.Y. analyzed all the data and wrote the manuscript. Y.S., J.Y. and S.W. edited and revised the manuscript. All authors have read and approved the article.
Data availability
The data used to support the findings of this study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Ye Sang, Zhen Zhang, Fan Liu, Haitao Lu
Contributor Information
Weijin Huang, Email: huangweijin@nifdc.org.cn.
Jing Yang, Email: jingyang0511@sina.com.
Shengqi Wang, Email: sqwang@bmi.ac.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41392-023-01432-5.
References
- 1.Likos AM, et al. A tale of two clades: monkeypox viruses. J. Gen. Virol. 2005;86:2661–2672. doi: 10.1099/vir.0.81215-0. [DOI] [PubMed] [Google Scholar]
- 2.Isidro J, et al. Addendum: Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus. Nat. Med. 2022;28:2220–2221. doi: 10.1038/s41591-022-02036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weaver JR, Isaacs SN. Monkeypox virus and insights into its immunomodulatory proteins. Immunol. Rev. 2008;225:96–113. doi: 10.1111/j.1600-065X.2008.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Otu A, Ebenso B, Walley J, Barcelo JM, Ochu CL. Global human monkeypox outbreak: atypical presentation demanding urgent public health action. Lancet Microbe. 2022;3:e554–e555. doi: 10.1016/S2666-5247(22)00153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lum FM, et al. Monkeypox: disease epidemiology, host immunity and clinical interventions. Nat. Rev. Immunol. 2022;22:597–613. doi: 10.1038/s41577-022-00775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen BW, Harms TJ, Reynolds MG, Harrison LH. Use of vaccinia virus smallpox vaccine in laboratory and health care personnel at risk for occupational exposure to orthopoxviruses—recommendations of the Advisory Committee on Immunization Practices (ACIP), 2015. MMWR Morb. Mortal Wkly Rep. 2016;65:257–262. doi: 10.15585/mmwr.mm6510a2. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed SF, Sohail MS, Quadeer AA, McKay MR. Vaccinia-virus-based vaccines are expected to elicit highly cross-reactive immunity to the 2022 monkeypox virus. Viruses. 2022;14:1960. doi: 10.3390/v14091960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thornhill JP, et al. Monkeypox virus infection in humans across 16 countries—April–June 2022. N. Engl. J. Med. 2022;387:679–691. doi: 10.1056/NEJMoa2207323. [DOI] [PubMed] [Google Scholar]
- 9.Rao AK, et al. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the advisory committee on immunization practices—United States, 2022. MMWR Morb. Mortal Wkly Rep. 2022;71:734–742. doi: 10.15585/mmwr.mm7122e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lane JM, Goldstein J. Adverse events occurring after smallpox vaccination. Semin Pediatr. Infect. Dis. 2003;14:189–195. doi: 10.1016/S1045-1870(03)00032-3. [DOI] [PubMed] [Google Scholar]
- 11.McFadden G. Poxvirus tropism. Nat. Rev. Microbiol. 2005;3:201–213. doi: 10.1038/nrmicro1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hooper JW, et al. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J. Virol. 2004;78:4433–4443. doi: 10.1128/JVI.78.9.4433-4443.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramirez JC, Tapia E, Esteban M. Administration to mice of a monoclonal antibody that neutralizes the intracellular mature virus form of vaccinia virus limits virus replication efficiently under prophylactic and therapeutic conditions. J Gen. Virol. 2002;83:1059–1067. doi: 10.1099/0022-1317-83-5-1059. [DOI] [PubMed] [Google Scholar]
- 14.Hooper JW, Custer DM, Schmaljohn CS, Schmaljohn AL. DNA vaccination with vaccinia virus L1R and A33R genes protects mice against a lethal poxvirus challenge. Virology. 2000;266:329–339. doi: 10.1006/viro.1999.0096. [DOI] [PubMed] [Google Scholar]
- 15.Mucker EM, et al. A nucleic acid-based orthopoxvirus vaccine targeting the vaccinia virus L1, A27, B5, and A33 proteins protects rabbits against lethal rabbitpox virus aerosol challenge. J. Virol. 2022;96:e0150421. doi: 10.1128/JVI.01504-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hooper JW, Custer DM, Thompson E. Four-gene-combination DNA vaccine protects mice against a lethal vaccinia virus challenge and elicits appropriate antibody responses in nonhuman primates. Virology. 2003;306:181–195. doi: 10.1016/S0042-6822(02)00038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pardi N, et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature. 2017;543:248–251. doi: 10.1038/nature21428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petsch B, et al. Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection. Nat. Biotechnol. 2012;30:1210–1216. doi: 10.1038/nbt.2436. [DOI] [PubMed] [Google Scholar]
- 19.Edghill-Smith Y, et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat. Med. 2005;11:740–747. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- 20.Lederer K, et al. SARS-CoV-2 mRNA vaccines foster potent antigen-specific germinal center responses associated with neutralizing antibody generation. Immunity. 2020;53:1281–1295.e1285. doi: 10.1016/j.immuni.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crotty S. T follicular helper cell biology: a decade of discovery and diseases. Immunity. 2019;50:1132–1148. doi: 10.1016/j.immuni.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sang Y, et al. An mRNA vaccine with broad-spectrum neutralizing protection against Omicron variant sublineages BA.4/5 -included SARS-CoV-2. Signal Transduct Target. Ther. 2022;7:362. doi: 10.1038/s41392-022-01207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu J, et al. Screening and evaluation of potential inhibitors against vaccinia virus from 767 approved drugs. J. Med. Virol. 2019;91:2016–2024. doi: 10.1002/jmv.25544. [DOI] [PubMed] [Google Scholar]
- 24.Zhang L, et al. A bioluminescent imaging mouse model for Marburg virus based on a pseudovirus system. Hum. Vaccin Immunother. 2017;13:1811–1817. doi: 10.1080/21645515.2017.1325050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu L, Zhang H, Dauphars DJ, He YW. A potential role of interleukin 10 in COVID-19 pathogenesis. Trends Immunol. 2021;42:3–5. doi: 10.1016/j.it.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohyagi H, et al. Monocyte-derived dendritic cells perform hemophagocytosis to fine-tune excessive immune responses. Immunity. 2013;39:584–598. doi: 10.1016/j.immuni.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 27.Gubser C, Hue S, Kellam P, Smith GL. Poxvirus genomes: a phylogenetic analysis. J. Gen. Virol. 2004;85:105–117. doi: 10.1099/vir.0.19565-0. [DOI] [PubMed] [Google Scholar]
- 28.Shchelkunova GA, Shchelkunov SN. Smallpox, monkeypox and other human orthopoxvirus infections. Viruses. 2022;15:103. doi: 10.3390/v15010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Y, Mu L, Wang W. Monkeypox: epidemiology, pathogenesis, treatment and prevention. Signal Transduct Target. Ther. 2022;7:373. doi: 10.1038/s41392-022-01215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Golden JW, et al. Polyclonal antibody cocktails generated using DNA vaccine technology protect in murine models of orthopoxvirus disease. Virol. J. 2011;8:441. doi: 10.1186/1743-422X-8-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakhatskyy P, Wang S, Chou TH, Lu S. Immunogenicity and protection efficacy of monovalent and polyvalent poxvirus vaccines that include the D8 antigen. Virology. 2006;355:164–174. doi: 10.1016/j.virol.2006.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turner JS, et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature. 2021;596:109–113. doi: 10.1038/s41586-021-03738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stittelaar KJ, et al. Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus. J. Virol. 2005;79:7845–7851. doi: 10.1128/JVI.79.12.7845-7851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Golden JW, et al. Side-by-side comparison of gene-based smallpox vaccine with MVA in nonhuman primates. PLoS One. 2012;7:e42353. doi: 10.1371/journal.pone.0042353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubins KH, et al. The host response to smallpox: analysis of the gene expression program in peripheral blood cells in a nonhuman primate model. Proc. Natl Acad. Sci. USA. 2004;101:15190–15195. doi: 10.1073/pnas.0405759101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaucha GM, Jahrling PB, Geisbert TW, Swearengen JR, Hensley L. The pathology of experimental aerosolized monkeypox virus infection in cynomolgus monkeys (Macaca fascicularis) Lab Invest. 2001;81:1581–1600. doi: 10.1038/labinvest.3780373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hickman HD, et al. Anatomically restricted synergistic antiviral activities of innate and adaptive immune cells in the skin. Cell Host Microbe. 2013;13:155–168. doi: 10.1016/j.chom.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Remakus S, Rubio D, Ma X, Sette A, Sigal LJ. Memory CD8+ T cells specific for a single immunodominant or subdominant determinant induced by peptide-dendritic cell immunization protect from an acute lethal viral disease. J. Virol. 2012;86:9748–9759. doi: 10.1128/JVI.00981-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu RH, Fang M, Klein-Szanto A, Sigal LJ. Memory CD8+ T cells are gatekeepers of the lymph node draining the site of viral infection. Proc. Natl Acad. Sci. USA. 2007;104:10992–10997. doi: 10.1073/pnas.0701822104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crotty S, Ahmed R. Immunological memory in humans. Semin Immunol. 2004;16:197–203. doi: 10.1016/j.smim.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 41.Crotty S, et al. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J. Immunol. 2003;171:4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 42.Liu Q, et al. A novel high-throughput vaccinia virus neutralization assay and preexisting immunity in populations from different geographic regions in China. PLoS One. 2012;7:e33392. doi: 10.1371/journal.pone.0033392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Monkeypox virus quadrivalent mRNA vaccine induces immune response and protects against vaccinia virus
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author on reasonable request.