Dear Sirs,
Eating epilepsy is an uncommon type of reflex epilepsy in which seizure are often, or even always, triggered by eating-related stimuli [1]. Seizures related to eating most commonly originate from temporo-limbic structures and they are often of unknown etiology because no focal lesion is identified [1]. Herein we describe the case of an adult patient developing new-onset eating epilepsy with refractory seizures shortly after SARS-CoV-2 vaccination in whom no structural, infectious, or metabolic etiologies were found. A possible immune-mediated etiology was suspected and a complete control of seizures was achieved with periodic intravenous immunoglobulin (IVIg) therapy.
A 40-year-old woman was admitted to our Department for a first unprovoked seizure. She has no previous personal or family history of neurological disorders, seizures, head trauma or central nervous system (CNS) infection. She received two Pfizer-BioNTech COVID-19 vaccine doses between June and July 2021 (first and second dose given 21 days apart), with no associated flu-like symptoms. Four days after the second injection, she started experiencing episodes of loss of contact, impaired speech, face flushing, mydriasis, and diaphoresis. The episodes were very frequent since the beginning (multiple daily), lasted only few minutes with complete recovery, and were almost always associated with the beginning of a meal (breakfast, lunch, or dinner). Twenty days after the second injection she experienced a probable tonic–clonic seizure with unknown onset: she was alone having breakfast and suddenly she woke up with signs of head trauma, vomit, and tongue biting, without recollection of the events.
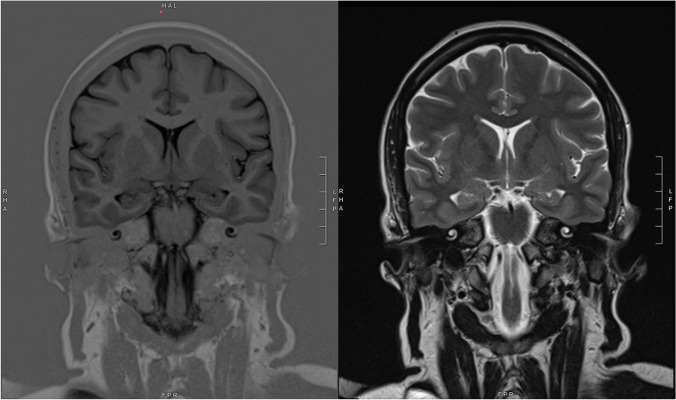
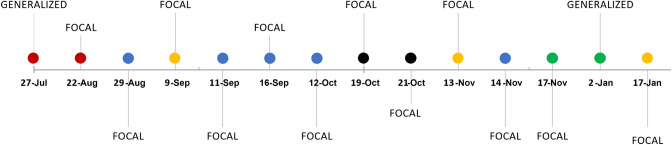
She was admitted to the Emergency Department, where she underwent a head computed tomography (CT) scan, showing no sign of acute brain damage, and an electroencephalogram (EEG), demonstrating a bilateral temporal theta slowing with rare epileptic abnormalities (spikes and sharp waves), predominantly over the left temporal lobe. A brain magnetic resonance imaging (MRI) was performed, which showed only mild nonspecific gliosis of the white matter. No hippocampal abnormalities were noted (Fig. 1). As suggested, she started keeping record of seizures in a diary. The dose of anti-seizure medication (ASM) was not enough to control the seizures, even though the frequency was lower. Seizures continued to be related to the beginning of meals (Fig. 2). Considering the persistency of seizures, levetiracetam was increased up to 2500 mg daily, without achieving complete resolution of the episodes.
Fig. 1.
Brain magnetic resonance imaging (MRI): coronal T1-weighted (left) and T2-weighted (right) sections at the level of the hippocampi, showing no significant abnormalities
Fig. 2.
Seizure diary from July 2021 to January 2022. Red dot: seizure at breakfast. Blue dot: seizure at lunch. Yellow dot: seizure at dinner. Green dot: spontaneous seizure. Black dot: more than one seizure at meals
She was therefore admitted to the Epilepsy Monitoring Unit in November 2021. Metabolic screening showed no abnormalities. CSF samples were analyzed, including testing for oligoclonal bands, antibodies against neuronal cell surface and onconeuronal antigens, as well as anti-MOG and anti-aquaporin-4 antibodies. A low-title positivity for anti-AMPA1-R/AMPA2-R Ab was detected on serum (Autoimmune Encephalitis Mosaic 6, Euroimmun, Lübeck, Germany), but it was not confirmed neither using CSF nor with indirect immunofluorescence on primate cerebellum. CSF sample was also tested at the French Reference Center for Paraneoplastic Neurological Syndromes and Autoimmune Encephalitis (Lyon, France) with negative results. An oligoclonal band type-4 pattern was identified (i.e. identical oligoclonal bands in CSF and serum, indicating systemic immune reaction with passive transfer to the intrathecal compartment). A large panel of cytokines (IL-1b, IL-6, IL-8, TNFa, sIL-2Ra, CXCL10, IL-10, IFNg) was analyzed on serum and CSF samples (using an ultrasensitive multiplex immunoenzymatic assays on Ella instrument, Bio-Techne, USA): all the biomarkers resulted within the normal ranges, apart from a mild increase of sIL2-Ra concentration in serum (1474 pg/ml; reference range 440–1435) and a mild increase of IL-6 in CSF (4.3 pg/ml, reference range 1–3.1 pg/ml). A neuropsychological evaluation was performed, but no significant abnormalities were described.
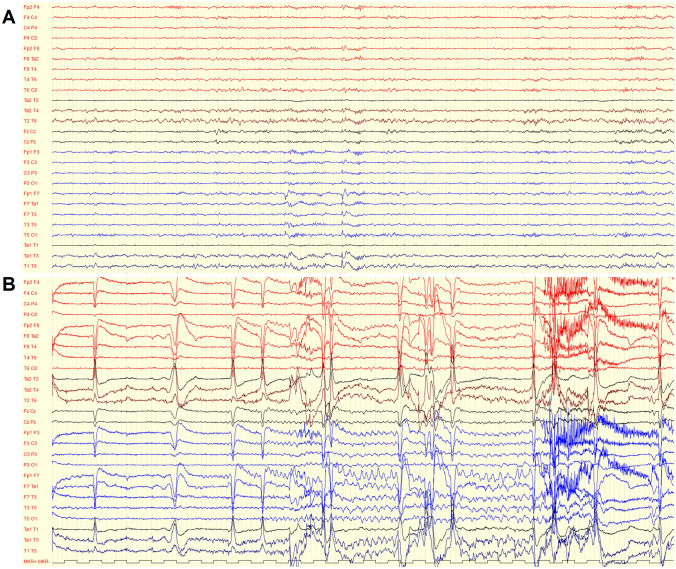
A video-EEG monitoring was performed for 5 days, which confirmed an inter-ictal epileptic focus over the left temporal region (Fig. 3A). A single seizure was captured: ictal EEG pattern showed monomorphic theta activity, localized on the left temporal regions, progressively increasing in amplitude and changing in morphology, then diffusing on the rest of the left hemisphere and on the homologous right regions, ending abruptly after 1 min (Fig. 3B). Clinically, the patient was unable to answer any questions while she was repeating “I don’t know”. The video-EEG permitted to disclose that not all the seizures were related to eating, and it is likely that other similar events occurred at home without recall by the patient. In fact, although the patient received three meals per day during video-EEG monitoring, the only seizure recorded was triggered by questioning the patient on the eating-related factors able to induce the events, which permitted her to re-experience the pre-seizure feelings.
Fig. 3.
A Interictal activity: theta slowing and sharp waves over the left temporal region. B Ictal activity: monomorphic theta activity on the left temporal regions, progressively increasing in amplitude and changing in morphology, then diffusing on the rest of the left hemisphere and on the homologous right regions
A diagnosis of a left temporal lobe epilepsy of unknown origin associated with food-intake reflex focal seizures with impaired awareness was established, and the patient was discharged with an increased dose of levetiracetam (3000 mg daily). An additional brain MRI in December 2021 showed no changes or abnormalities. Nevertheless, focal seizures continued with the same features, and the patient experienced a spontaneous focal to bilateral tonic–clonic seizure in January 2022. It was necessary to add lacosamide as an add-on therapy up to a daily dose of 200 mg but it could not completely control the epilepsy.
Using 2 ASMs, seizure frequency was approximately 2 per month. Clinical features changed over time, becoming briefer, associated with flushing and subjective heat sensation, but without speech arrest and staring. Diffuse ictal piloerection involving the limbs (mostly unilaterally) was documented during some episodes (Fig. 4). A further neuropsychological evaluation, after 1 year from epilepsy onset, demonstrated the presence of memory impairment, which was initially absent.
Fig. 4.
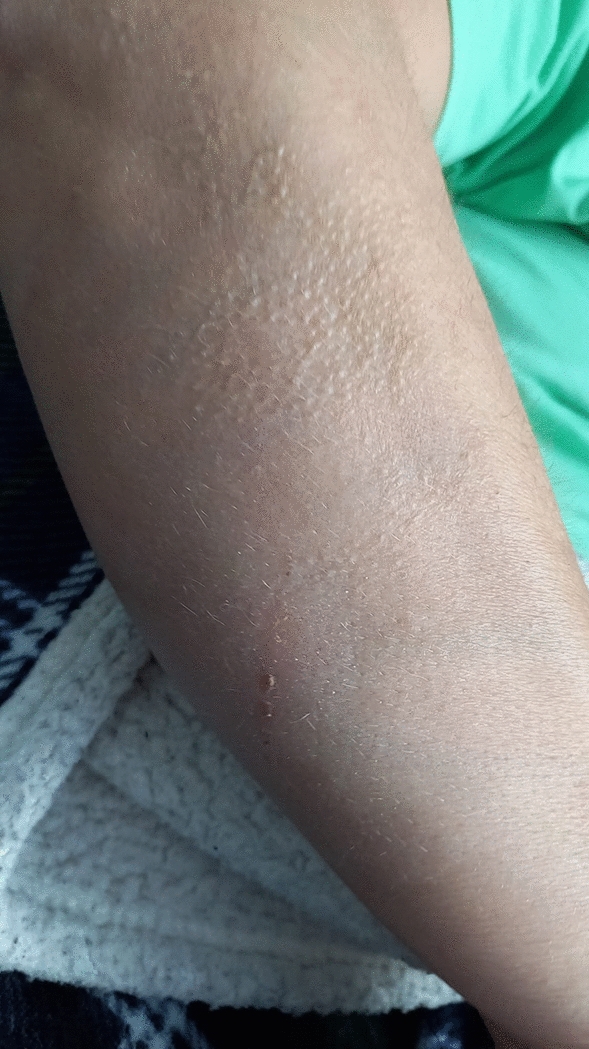
Ictal diffuse right arm piloerection
Given the suspicion of an immune-mediated origin of the epilepsy, we decided the administration of immunotherapy with intravenous immunoglobulins (IVIg), with a total standard dose of 2 g per kg divided in 5 days of infusion (September 2022), followed by monthly infusions. A dramatic benefit was seen after IVIg administration, with no seizures reported from September 2022 to February 2023, at last follow-up.
Eating epilepsy is a well-known, although rare, type of reflex epilepsy. About half of the patients suffering from eating epilepsy experiences seizures only when eating, while the other half also has spontaneous seizures. The most common type of seizure is focal with impaired awareness, although focal to bilateral seizures are also described [1].
Regarding the etiology, approximately 30% of cases described in the literature is symptomatic, and it is caused by an identifiable lesion, while the rest of them has no evident alterations [1].
The majority of cases of eating epilepsies were reported from South Asia, suggesting a role for both genetic and environmental factors, like dietary habits. Interestingly, two cases of encephalitis-related eating epilepsy were described in the literature [1].
Vaccines can rarely provoke seizure by inducing an immune-mediated encephalopathy. mRNA COVID-19 vaccination was administered worldwide with excellent safety data. Very few neurological adverse events were reported, including few reports of encephalitis [2]. No cases of reflex epilepsies were related to COVID-19 vaccination so far.
In favor of an immune-mediated etiology, we found evidence of systemic immune activation with likely subsequent induction of CNS inflammation, such as (1) the oligoclonal band type-4 pattern, (2) the increased serum concentration of sIL2Ra and (3) the upregulation of IL-6 CSF concentration.
IL2 is a well-known marker of T-cell activation and can be elevated in epileptic conditions such as new-onset refractory status epilepticus (NORSE) or febrile infection-related epilepsy syndrome (FIRES) [3]. The IL-2–IL-2R pathway displays a paradoxical but crucial role in the balance between tolerance and immunity. In particular, there are several indications that sIL-2Ra acts as an antagonist or possibly decoy receptor, affecting in particular the T-reg function, while producing an imbalanced immune activation towards autoimmunity. In a murine model of experimental autoimmune encephalomyelitis (EAE) the administration of sIL-2Ra exacerbated the disease [4] and more elevated sIL-2Ra correlated with malignant course in relapsing remitting multiple sclerosis [5]. Among a large panel of cytokine and chemokines examined in a recent meta-analysis, only IL-6 and IL-10 CSF levels were significantly higher in both infectious and autoimmune encephalitis compared to controls [6]. IL-6 represents a crucial inflammatory cytokine [7], but also an important factor for B-cell proliferation and antibody production. Interestingly, a case series of patients with NORSE due to autoimmune origin in which CSF showed increased IL-6, TNF-α, IL-2, and IL-4 levels demonstrated improvement in seizure activity and return to normal cytokine levels after receiving tocilizumab therapy, an anti-IL-6R antibody [8].
Of note, the case herein reported shares some elements with immune-mediated epilepsies: there was evidence of systemic immune activation and there was also a drug resistance using two well-tolerated ASMs; in addition, memory impairment developed over time.
Furthermore, seizures were characterized by speech alteration and autonomic symptoms, such as face flushing, diaphoresis, mydriasis, and piloerection. Ictal piloerection is an uncommon epileptic manifestation, which was linked to autoimmune (typically limbic) encephalitis [9].
Using the criteria to assess causality between vaccine immunization and neurological manifestation, our case was labeled as “probable adverse event” (typical time frame, no alternative etiology, and no risk factors) [10].
The APE score [11] assigned to our patient is borderline, so it is unlikely that the patient dysimmune condition is sustained by autoantibodies. Indeed, most of the neurological syndromes developing after infection or vaccinations are T cell mediated [12].
In addition, if we consider the APE score as a tool to identify patients suffering from immune-mediated epilepsy [11], we propose that antecedent vaccination should be considered similarly to recent infection in the calculation of the score, along with flushing and piloerection in the group of autonomic manifestations.
Finally, we could assume the autoimmune etiology of the epilepsy by considering the dramatic positive response to IVIg administration as an ex adiuvantibus criterion. Generally, IVIg infusions do not control seizures in other drug‐resistant epilepsies, but they are an effective treatment of seizure due to autoimmune etiology [9, 13, 14].
Notwithstanding the case presented here, it should be underlined that the beneficial effects of vaccination largely outweigh potential risks at the population level, especially in the context of COVID-19 where the frequency of reported neurological complications is high after the infection [15], while extremely rare after vaccination.
We described a case of drug-resistant eating epilepsy developing in close temporal association with SARS-CoV-2 vaccination. Clinical features and laboratory findings, as well as the clear therapeutic response to IVIg, suggest an immune-mediated origin. A possible causal association with the vaccine administration may be considered, although it is difficult to prove conclusively. Clinicians should be aware of this rare epileptic phenotype, which is typically associated with focal (temporal) lobe involvement, and in exceptional circumstances can be the expression of an underlying autoimmune (potentially treatable) encephalitis.
Data availability
Anonymized data will be shared on reasonable request from any qualified investigator.
Declarations
Conflicts of interest
The authors declare they have no known competing financial interests or personal relationships which could have influenced the work hereby reported.
Ethical approval
The patient provided written consent. All procedures were performed in accordance with the local ethics committee and the Declaration of Helsinki.
Footnotes
Alberto Vogrig and Salvatore Versace: Co-first authors.
References
- 1.Girges C, Vijiaratnam N, Wirth T, et al. Seizures triggered by eating—zs rare form of reflex epilepsy: a systematic review. Seizure. 2020;83:21–31. doi: 10.1016/j.seizure.2020.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Zuhorn F, Graf T, Klingebiel R, et al. Postvaccinal encephalitis after ChAdOx1 nCov-19. Ann Neurol. 2021;90:506–511. doi: 10.1002/ana.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan TH, Perucca P, O’Brien TJ, et al. Inflammation, ictogenesis, and epileptogenesis: an exploration through human disease. Epilepsia. 2021;62:303–324. doi: 10.1111/epi.16788. [DOI] [PubMed] [Google Scholar]
- 4.Russell SE, Moore AC, Fallon PG, Walsh PT. Soluble IL-2Rα (sCD25) exacerbates autoimmunity and enhances the development of Th17 responses in mice. PLoS ONE. 2012;7:e47748. doi: 10.1371/journal.pone.0047748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maier LM, Anderson DE, Severson CA, et al. (2009) Soluble IL-2RA levels in multiple sclerosis subjects and the effect of soluble IL-2RA on immune responses. J Immunol Baltim Md. 1950;182:1541–1547. doi: 10.4049/jimmunol.182.3.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soltani Khaboushan A, Pahlevan-Fallahy M-T, Shobeiri P, et al. Cytokines and chemokines profile in encephalitis patients: a meta-analysis. PLoS ONE. 2022;17:e0273920. doi: 10.1371/journal.pone.0273920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aguilar-Castillo MJ, Cabezudo-García P, Ciano-Petersen NL, et al. Immune mechanism of epileptogenesis and related therapeutic strategies. Biomedicines. 2022;10:716. doi: 10.3390/biomedicines10030716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jun J-S, Lee S-T, Kim R, et al. Tocilizumab treatment for new onset refractory status epilepticus. Ann Neurol. 2018;84:940–945. doi: 10.1002/ana.25374. [DOI] [PubMed] [Google Scholar]
- 9.Vogrig A, Joubert B, André-Obadia N, et al. Seizure specificities in patients with antibody-mediated autoimmune encephalitis. Epilepsia. 2019;60:1508–1525. doi: 10.1111/epi.16282. [DOI] [PubMed] [Google Scholar]
- 10.Butler M, Tamborska A, Wood GK, et al. Considerations for causality assessment of neurological and neuropsychiatric complications of SARS-CoV-2 vaccines: from cerebral venous sinus thrombosis to functional neurological disorder. J Neurol Neurosurg Psychiatry. 2021;92:1144–1151. doi: 10.1136/jnnp-2021-326924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubey D, Alqallaf A, Hays R, et al. Neurological autoantibody prevalence in epilepsy of unknown etiology. JAMA Neurol. 2017;74:397. doi: 10.1001/jamaneurol.2016.5429. [DOI] [PubMed] [Google Scholar]
- 12.Pilli D, Zou A, Tea F, et al. Expanding role of T cells in human autoimmune diseases of the central nervous system. Front Immunol. 2017;8:652. doi: 10.3389/fimmu.2017.00652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Britton JW, Flanagan EP, McKeon A, Pittock SJ. Autoimmune epilepsy disorders. In: Cascino GD, Sirven JI, Tatum WO, editors. Epilepsy. 1. Oxford: Wiley; 2021. pp. 249–273. [Google Scholar]
- 14.Vogrig A, Gigli GL, Nilo A, et al. Seizures, epilepsy, and NORSE secondary to autoimmune encephalitis: a practical guide for clinicians. Biomedicines. 2022;11:44. doi: 10.3390/biomedicines11010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ariño H, Heartshorne R, Michael BD, et al. Neuroimmune disorders in COVID-19. J Neurol. 2022;269:2827–2839. doi: 10.1007/s00415-022-11050-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared on reasonable request from any qualified investigator.