Abstract
The cell-bound cell envelope proteinase (CEP) of the mesophilic cheese-starter organism Lactococcus lactis subsp. cremoris SK11 is protected from rapid thermal inactivation at 25°C by calcium bound to weak binding sites. The interactions with calcium are believed to trigger reversible structural rearrangements which are coupled with changes in specific activity (F. A. Exterkate and A. C. Alting, Appl. Env. Microbiol. 65:1390–1396, 1999). In order to determine the significance of the rearrangements for CEP stability and the nature of the interactions involved, the effects of the net charge present on the enzyme and of different neutral salts were studied with the stable Ca-loaded CEP, the unstable so-called “Ca-free” CEP and with the Ca-free CEP which was stabilized nonspecifically and essentially in its native conformation by the nonionic additive sucrose. The results suggest that strengthening of hydrophobic interactions is conducive to stabilization of the Ca-free CEP. On the other hand, a hydrophobic effect contributes significantly to the stability of the Ca-loaded CEP; a phased salting-in effect by a chaotropic salt suggests a complex inactivation process of this enzyme due to weakening of hydrophobic interactions and involving an intermediate enzyme species. Moreover, a Ca-triggered increase of a relatively significant hydrophobic effect in the sucrose-stabilized Ca-free CEP occurs. It is suggested that in the Ca-free CEP the absence of both local calcium-mediated backbone rigidification and neutralization of negative electrostatic potentials in the weak Ca-binding sites, and in addition the lack of significant hydrophobic stabilization, increase the relative effectiveness of electrostatic repulsive forces on the protein to an extent that causes the observed instability. The conditions in cheese seem to confer stability upon the cell-bound enzyme; its possible involvement in proteolysis throughout the ripening period is discussed.
It is well-established that the cell envelope proteinase lactocepin (EC 3.4.21.96) (hereafter designated CEP) is the only extracellular cell surface proteinase of Lactococcus lactis and is essential for normal growth in milk. It is synthesized as a large pre-pro-protein of about 200 kDa, which is processed at the N terminus during or after membrane translocation (7, 14, 18). The mature, active CEP (±180 kDa) has an N-terminal domain of 512 residues which shows significant sequence similarity to the serine proteinases of the subtilisin family. A large C-terminal extension of more than 1,200 residues distinguishes it from the members of this family. Both the proteinase domain and the greater part of the extension, comprising the middle domain (886 residues) followed by a helical spacer domain (210 residues), are outside the cell wall, the latter domain being linked to a hydrophilic cell wall spacer (131 residues) (see references 14 and 21). The extreme C terminus of 36 residues shows the features of a hydrophobic membrane-spanning sequence which ends in a charged tail and is preceded by a signal sequence for cell wall sorting; both sequences are characteristic for a great number of cell surface proteins from gram-positive bacteria (17, 20, 21, 22, 26). It has been argued that a function of the part of the large C-terminal extension outside the cell wall (particularly the middle domain) is to act as a template on which the proteinase domain finds and maintains its basic conformation, which is then modulated and stabilized by the binding of Ca2+ (11). Several types of CEP occur among lactococcal strains; they can be distinguished on the basis of their specificity toward caseins (8, 24) as belonging to one of two classes, designated CEPI and CEPIII. A more detailed classification into several groups was possible on the basis of cleavage at pH 6.5 of peptide bonds in αs1-CN(f1–23), a peptide with features which makes it possible to distinguish small differences in specificity (10). The CEPs of strains SK11 and Wg2 represent two types in which the amino acid sequences differ at 44 positions (26). On the basis of the close sequence similarity of the proteinase domain of the CEP with the subtilisin family, the enzyme is predicted to possess at least three binding sites for Ca2+ ions (Ca1, Ca2, and Ca3) (22); each corresponding Ca site in the SK11- and Wg2-CEPs shows the same residues delivering the formal ligands or residues which, by their location and charge, can affect binding. Ca1 has a relatively strong affinity for Ca2+, while Ca2 and Ca3 are weak sites (7). Removal of weakly bound Ca2+ from the cell-bound CEP of strain SK11 results in an enzyme which shows exceptional conformational instability and a lower specific activity, the so-called “Ca-free” cell-bound CEP. In contrast, the consequences of Ca removal from the cell-bound Wg2-CEP are much less dramatic (11). However, unlike the Ca-free SK11-CEP, which at low cell densities is not released, the Ca-free Wg2-CEP is very sensitive to autoproteolytic release from the cell. Complete restoration of cell-bound CEP activity and stability was accomplished by the binding of at least two Ca2+ ions, probably involving Ca2 and Ca3; a simultaneous protection from release was observed already after binding of the first Ca2+ ion (11).
Cell-bound CEP activity is essential for initial gross proteolysis in cheese but the fate of the enzyme during ripening is unknown. The properties of the cell-bound CEP, especially its stability under cheese conditions (viz., a low pH, a high NaCl concentration, a relatively high Ca2+ concentration, and a restricted water activity) may give us a clue as to the involvement of this enzyme in proteolysis during later stages of ripening.
In order to further characterize the cell-bound SK11-CEP and to reveal the nature of the interactions involved in its structural stabilization by Ca2+, the effects of pH and neutral salts on the Ca-free and Ca-loaded CEP were studied as well as those on the Ca-free CEP which was stabilized in its native conformation by a nonionic agent. C-terminal deletion analysis of the gene coding for the SK11-CEP has demonstrated that deletion of the last 189 residues results in secretion of a truncated, but still catalytically active enzyme (3). This indicates proper folding of the enzyme molecule and confirms the involvement of the C terminus in membrane and/or cell wall anchoring. On the other hand, it indicates no further attachment of CEPIII to the cell wall, the proteinase domain being positioned in situ by the helical spacer and the middle domain away from the cell surface as predicted by domain analysis (21). Therefore, the proteinase is directly influenced by environmental changes, and monitored effects can be discussed with respect to protein structure only rather than also considering cell wall (surface) alterations which might have caused or influenced these effects. The specific binding of H+ ions to ionizable sites alters the electrostatic balance of a protein; neutral salts at concentrations at which they serve as a source of ionic strength may affect electrostatic interactions on a general charge-shielding basis. Both effects may influence the stability of the enzyme.
A more specific effect on the strength of hydrophobic interactions, independent of charge, may be expected at salt concentrations beyond the dilute level when electrostatic shielding effects have been saturated. The hydrophobic stability of proteins is based on the tendency of water lattices to reorganize around nonpolar residues of the unfolded molecule. This hydration is a thermodynamically unfavorable consequence of unfolding (25). It is generally accepted that ions can influence the availability of hydration water to these hydrophobic parts of the protein, thereby weakening (chaotropic ions, Ca2+, SCN−, I−, and Ba2+) or strengthening (SO42−) hydrophobic interactions (viz., “salting in” and “salting out,” respectively); K+, Na+, and Cl− are much less effective in both respects (15, 25). The results described here reveal the structural consequences of the presence of Ca2+ in the weak binding sites, which are responsible for the stability of the cell-bound CEP.
MATERIALS AND METHODS
Organism and growth and treatment of cells.
The nisin-negative L. lactis subsp. cremoris SK11, which produces the type III cell envelope proteinase (CEPIII), was used. The strain was grown in reconstituted skim milk (100 ml) at 25°C to the early stationary phase. The cells were harvested after clearing the milk culture with 1% (wt/vol) trisodium citrate at pH 6.5 and then routinely washed twice with ice-cold 50 mM imidazole buffer, pH 6.5 (40 ml), prepared with double-distilled water; they were finally resuspended in 40 ml of ice-cold buffer (or water) (i.e., the standard suspension). This procedure causes the complete removal of weakly bound Ca2+ from the cell-bound CEP (yielding Ca-free CEP), which results in a severe destabilization of the enzyme against thermal denaturation; owing to this effect, no activity can be detected with the enzyme at 25°C. To obtain the stable Ca-loaded CEP, the final suspending buffer was supplemented with 10 mM CaCl2. Resuspension of the washed cells therein instantaneously furnishes an optimally Ca-loaded enzyme which shows initial steady-state kinetics at 25°C without a lag time (11); this activity is referred to as the potential activity of the Ca-free CEP.
Proteinase assay.
CEP activity assays were performed using initial steady-state kinetics of the conversion of the substrate succinyl-alanyl-glutamyl-prolyl-phenylalanyl-p-nitroanilide (S-Glu) as described previously (11).
Effect of pH on activity and stability.
Washed cells were resuspended in ice-cold double-distilled water. Then, 100 μl of this cell suspension was added to 900 ml of each of the following buffers at 10 or 25°C containing 1 mM S-Glu and either 0 or 10 mM CaCl2: 50 mM sodium acetate, pH 4.0 to 6.0; 50 mM imidazole, pH 5.8 to 7.4; and 50 mM Tris, pH 7.4 to 8.8.
The mixtures were incubated at 10 or 25°C, and the reaction was stopped by adding 300 μl of 80% acetic acid.
The stability of the Ca-free CEP at different pH values was established by adding 100 μl of cell suspension to 900 μl of each of above buffers and incubating the mixture for 30 min at 25°C. After centrifugation, the cell pellet was resuspended in 1 ml of 50 mM imidazole (pH 6.5) containing 1 mM S-Glu and 10 mM Ca2+, and the residual activity was measured and expressed as a percentage of the initial activity.
Influence of salts on activity and stability.
The influence of different salts at the indicated concentrations on the activity of the Ca-free and Ca-loaded cell-bound CEP was tested in 50 mM imidazole buffer (pH 6.5; without or with 10 mM Ca2+, respectively) at 25°C and at 1/10 of the optical density (OD) of the standard cell suspension. In the case of stability measurements the different cell suspensions in buffer with salt (standard OD) were incubated for the indicated time period(s). Residual (potential) CEP activity was then measured by resuspension of the cells in 50 mM imidazole (pH 6.5) containing 10 mM Ca2+ and 1 mM S-Glu.
RESULTS
Effect of temperature and pH on the activity of the Ca-free and Ca-loaded CEP.
At 10°C the cell-bound Ca-free CEP appeared to be relatively stable and to exhibit linear initial progress curves over the pH range of 4 to 8; no release of active enzyme from the cell could be established. Figure 1 shows these initial activities, together with those of the Ca-loaded CEP, at 10 and 25°C. The optimum pH of the Ca-free enzyme (pH 5.8 to 6.0) is somewhat lower than that of the Ca-loaded CEP (pH 6.4). Ca affects the specific activity of the enzyme as well, the activity of the Ca-free CEP being lower (or zero at pH >8.5) relative to that of the Ca-loaded enzyme. This effect is maximal at a pH of >6.2, decreases toward more acidic values, and is virtually absent at pH values of <5.0; it has been related to a Ca-triggered structural rearrangement (11). The activities below pH 6.0 of the Ca-loaded CEP at 25°C relative to its optimum activity appear to be higher than those at 10°C, suggesting some temperature-dependent effect on enzyme structure.
FIG. 1.
pH dependence of the Ca-loaded (open symbols) and Ca-free (closed symbols) CEP activity at 10°C (——). The results obtained with two different cell suspensions with equal activities at pH 6.0 are shown. For comparison activities measured at 25°C are also shown (–––); the values of these activities were positioned on the vertical scale by normalizing them to an activity of the Ca-loaded CEP at pH 6.2 equal to that at 10°C. For the Ca-free CEP at 25°C, only activities in the pH range of 5.8 to 7.0 are shown in order to illustrate the effect removal of Ca2+ has at this temperature. The discontinuities in the curves (with or without a change in the symbol) represent the changes in the buffer system used. For further details, see Materials and Methods.
The stability and activity of the Ca-free CEP at 25°C and at different pH values are shown in Fig. 2 and 3. The results reveal that the enzyme is relatively stable at pH values of ca. 4.8; from pH 5.2 a remarkable destabilization is introduced until at alkaline pH values stability is increased again, reaching an optimum at pH 8.0 to 8.2 (Fig. 2). At all pH values no loss of activity was observed in the presence of Ca2+ (10 mM) (not shown). Also, in no case could the appearance of active enzyme in the supernatant be detected unless high-density suspensions were incubated (7, 11) and in that case only at a pH of >5.2 in the absence of Ca2+.
FIG. 2.
pH dependence of the instability of the Ca-free CEP at 25°C. For details, see Materials and Methods.
FIG. 3.
pH dependence of the Ca-loaded (○) and Ca-free (●) CEP activity at 25°C in the pH range of 3.8 to 5.4 (50 mM sodium acetate buffers). Means of activities obtained with three different cell suspensions are plotted with their extremes. All activities were positioned on the vertical scale by normalizing them to equal activities of the Ca-free CEP in the three suspensions at pH 4.8.
In contrast to its behavior at a pH of >5.2, at a pH of <5.2 the Ca-free CEP showed initial steady-state kinetics at 25°C, suggesting either the stabilization of its native structure as the enzyme-substrate complex or prevention of autoproteolytic inactivation. The differences in activities at these pH values and at 25°C between the Ca-free and the Ca-loaded enzyme (Fig. 3) are therefore due to a higher specific activity of the latter only; they are most obvious on either side of the pH region of 4.4 to 4.8, which is around the isoelectric point (pI) of the enzyme (9).
Effect of neutral salts on CEP activity and stability.
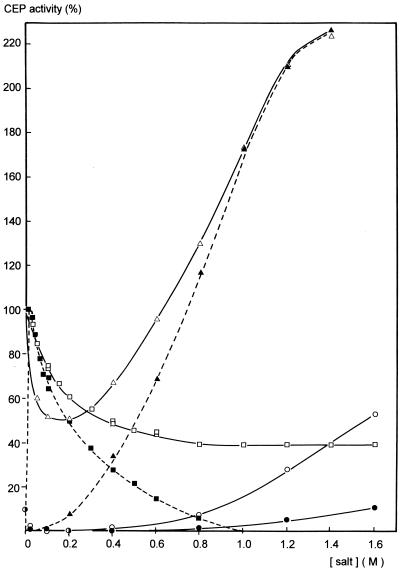
In order to establish the contribution of electrostatic and hydrophobic interactions to the stabilization of CEP, the effect of neutral salts on activity and stability was studied. In the case of CaCl2 a decrease in activity was observed at concentrations beyond the level when the enzyme is optimally loaded with Ca2+ ions (10 mM) (Fig. 4).
FIG. 4.
Effect of the concentration of different neutral salts on the activity of the Ca-free and Ca-loaded CEP at pH 6.5 and 25°C. Activities are plotted as percentages of the activity of the Ca-free CEP at 10 mM CaCl2. Symbols: ■, Ca-free CEP and CaCl2; ▴, Ca-free CEP and Na2SO4; ○, Ca-free CEP and NaCl; ●, Ca-free CEP and KCl; ▵, Ca-loaded CEP and Na2SO4; □, Ca-loaded CEP and NaCl.
Similar effects on the Ca-loaded CEP were observed with other salts up to the level when electrostatic shielding is saturated and electrostatic interactions are affected maximally (0.2 M). Beyond that concentration dominant effects of salt are on the properties of water (6, 25). A further decrease in activity was observed with the chaotropic salts such as CaCl2, KSCN, KI, and BaCl2.
In the presence of 1 M CaCl2 (Fig. 4) or 0.5 M KSCN at pH 6.5 or 0.5 M CaCl2 at pH 5.2 (data not shown), complete inactivation was observed. KCl and NaCl showed no or only a slight activating effect, respectively, while Na2SO4 caused a clear increase of activity. A similar effect of Na2SO4 was observed with the Ca-free CEP even at relatively low concentrations of this salt. A maximum activity was reached which was beyond that obtained with Ca2+ (Fig. 4). In this respect, NaCl rather than KCl is effective, albeit only slightly and only at relatively high concentrations (Fig. 4).
The stability of the Ca-loaded CEP at 25°C was not changed at relatively low salt concentrations at which predominantly an electrostatic effect is expected (not shown).
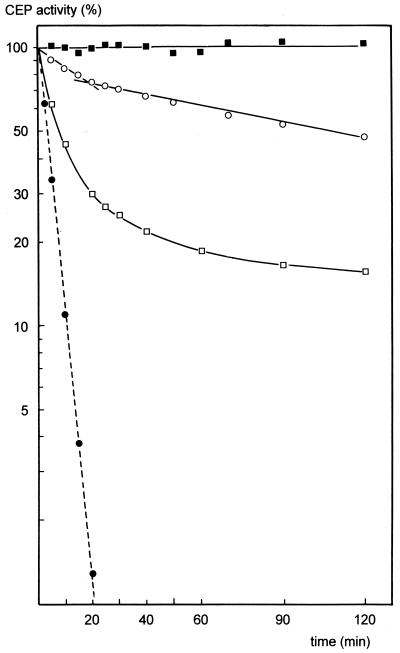
In order to investigate the possibility of a hydrophobic effect which contributes to the stabilization by Ca2+, the effect of relatively high salt concentrations on stability was studied with the Ca-free CEP. In the presence of NaCl or KCl at concentrations of >1 M the rate of loss of potential activity was slightly but significantly decreased. In fact, it was consistently established that in this respect again NaCl was somewhat more effective than KCl (Fig. 5).
FIG. 5.
(A) Effects of neutral salts on the stability of the Ca-free CEP at pH 6.5 and 25°C. Curves: --●--, without salt; --▵--, with 10 mM CaCl2; —■—, with 0.2 M NaCl; —▵—, with 0.2 M Na2SO4; —□—, with 1 M KCl; —▴—, with 1 M NaCl; and —●—, with 1 M Na2SO4. (B) Stability of the Ca-free CEP at pH 6.5 and 35°C (–––) in the presence of 10 mM CaCl2 (■), 1.6 M Na2SO4 (●), or 1.2 M sucrose (▴) and at 25°C (——) in the presence of 1 M (□), 1.6 M (▵), and 1.8 M (○) Na2SO4 or under “cheese-ripening conditions” (0.85 M NaCl, 150 mM Ca2+, pH 5.2) (▿).
Na2SO4 had a most striking stabilizing effect on the Ca-free CEP. At concentrations of >1 M the stability was comparable to that achieved by Ca binding, although an initial reduction of activity was always measured (Fig. 5); only Ca2+ ions could overcome this reduction, indicating Ca binding in spite of the high salt concentration. Also, no release of active enzyme in low- or high-density suspensions was observed in the presence of Na2SO4. This indicates protection by this salt from autoproteolysis also, as in the case of Ca binding (11).
Hydrophobic effect and stability of the Ca-loaded CEP.
The molecular basis for the Na2SO4-induced stabilization of the Ca-free enzyme most probably resides in the strengthening of essential hydrophobic interactions (15, 25). It suggests that strengthening of the hydrophobic effect is a condition to stabilize the Ca-free CEP in a catalytically active form. Hydrophobic stabilization may therefore also underlie the effect Ca2+ has on the enzyme. The essential involvement of hydrophobic interactions in the maintenance of the specific conformation of the Ca-loaded CEP is suggested by the effect of specific ions such as Ca2+ and SCN− at high concentrations. These ions, in contrast to SO42−, are particularly effective in destabilizing proteins (chaotropic ions which promote unfolding [i.e., salting-in]) by weakening hydrophobic interactions (25). In the presence of relatively high concentrations of these ions the Ca-loaded CEP not only is inactive (see above) but is irreversibly inactivated as well, with SCN− being more effective than Ca2+ (Fig. 6). With SCN−, typical biphasic inactivation kinetics were distinguished (Fig. 6A). Extrapolation of the second phase shows that with 1 M SCN− 60% of the Ca-loaded enzyme undergoes relatively rapid irreversible inactivation. In the case of 2 M Ca2+ this fraction is only ca. 10% (Fig. 6B).
FIG. 6.
(A) Stability of the Ca-free and Ca-loaded CEP at pH 6.5 and 25°C in the presence of KSCN. Symbols: ●, Ca-free CEP without addition; □, Ca-free CEP with 1 M KSCN; ■, Ca-free CEP with 0.5 M KSCN; ▵, Ca-loaded CEP with 1 M KSCN; ○, Ca-loaded CEP with 0.5 M KSCN; and ▴, Ca-loaded CEP with 0.2 M KSCN. (B) Effect of CaCl2 on the stability of CEP at pH 6.5 and 25°C. Symbols: ●, Ca-free CEP; ▴, Ca-loaded CEP (10 mM CaCl2); ○, Ca-loaded CEP, 1 M CaCl2; and ▵, Ca-loaded CEP, 2 M CaCl2.
Hydrophobic stabilization of the Ca-free CEP apparently is already minimal since no significant effect by SCN− ions on the initial rate of inactivation was seen. However, a second phase was observed which suggests the presence of a more stable enzyme fraction (Fig. 6A).
Ca-induced hydrophobic stabilization.
In order to reveal the supposed very weak hydrophobic effect in the Ca-free CEP and to further explore the view of a Ca-triggered structural change and strengthening of that hydrophobic effect, an attempt to stabilize the Ca-free CEP in its native form was made so that specific salt effects on this form could be reliably measured. It seemed probable that nonspecific stabilizers such as sugars and polyhydric alcohols might be suitable for this purpose. These compounds are water-structuring additives (depressors of water activity) which are known to stabilize proteins in solution in their native form. This stabilizing effect has been attributed mainly to their preferential exclusion from the hydration shell around the protein, thus favoring the minimization of protein surface area in a nonspecific way and reducing local backbone fluctuations away from the folded state (4, 12, 23). With sucrose it has been found that its stabilizing action did not affect the native conformation of three proteins studied (16).
In the case of the cell-bound CEP the addition of sucrose to the suspending buffer leads to stabilization of the cell-bound Ca-free enzyme, allowing the measurement of activity at pH 6.5 and 25°C. At 0.8 to 1.0 M sucrose, the activity was maximal, but it corresponded to only approximately 60% of the activity of the Ca-loaded CEP in the absence of sucrose (Fig. 7). Limited availability of solvent water, which hampers both mobility and diffusion of reactants, is most likely responsible for the slightly decreased activities measured at higher concentrations of sucrose. The stabilization by sucrose was less effective than that by Ca2+ or SO42−. Although relatively stable at 25°C over a period of 120 min, the enzyme in sucrose was inactivated much more rapidly at 35°C than the Ca-loaded (10 mM) or SO42−-stabilized (1.6 M) enzyme (Fig. 5B). The sucrose-stabilized enzyme could still be further stabilized, and be activated as well, by Ca2+, although activation was only to a maximum of 75 to 80% of the activity of the Ca-loaded enzyme in the absence of sucrose (data not shown). These results strongly suggest that the enzyme is stabilized by sucrose essentially in its Ca-free conformation without being significantly affected in its flexibility with respect to substrate binding and catalysis and to Ca2+-induced changes.
FIG. 7.
Activity of the Ca-free CEP at pH 6.5 and 25°C as a function of sucrose concentration and plotted as a percentage of the activity of the Ca-loaded CEP in the absence of sucrose.
The latter ability is further demonstrated by the difference in sensitivity toward high concentrations of SCN− (Fig. 8). In the presence of Ca2+ the sucrose-stabilized CEP showed the typical phasic inactivation kinetics, as observed in the absence of sucrose (Fig. 6A), although a higher concentration of the salt was needed to bring about a comparable initial inactivation rate. An effect of CNS− on the Ca-free CEP could also be clearly distinguished, showing that the initial inactivation rate was much higher than that with the Ca-loaded CEP. This would mean that Ca binding is indeed accompanied by increased hydrophobic stabilization.
FIG. 8.
Effect of KSCN on the stability of the sucrose (1 M)-stabilized Ca-free and Ca-loaded CEP at pH 6.5 and 25°C. Symbols: ■, sucrose-stabilized Ca-free CEP; □, sucrose-stabilized Ca-free CEP with 1 M KSCN; ○, sucrose-stabilized Ca-loaded CEP with 1 M KSCN; and ●, Ca-free CEP without addition (taken from Fig. 5A).
DISCUSSION
The results presented here show the essential protective role of weakly bound Ca2+ in the cell-bound CEP of strain SK11 against thermal inactivation under physiological conditions; a similar role of Ca2+ in the weak binding sites of related serine proteinases of the subtilisin family has been reported (13). It can be concluded that the differences in specific activity, stability, and pH dependency between the Ca-free CEP and the Ca-loaded CEP are related to a Ca-triggered structural rearrangement which involves the active site and affects hydrophobic stabilization and, in addition, to a Ca-triggered modulation of both charge repulsion by negative electrostatic potentials in the weak calcium binding sites and local backbone flexibility.
The instability changes observed with the Ca-free CEP going from an acid to an alkaline pH may be explained in terms of nonspecific charge repulsion and specific charge interactions on account of a progressive alteration of the electrostatic balance in the protein (6). Around the pI of the enzyme (at ca. 4.6 to 4.8) (9) there is little or no electrostatic repulsive force; this may give the enzyme its relative conformational stability. At lower or higher pH values the net charge on the protein increases and the resulting repulsive force destabilizes the folded protein significantly. Upon alkalinization an increase in the incidence of ion pairing may counteract the repulsive force successfully until at a pH of >8.0 ionic attractions are no longer sufficient to do this. At these high pH values the enzyme has lost its catalytic ability. Only at a pH of >5.5 is the enzyme subject to autoproteolytic release of a truncated but still active and relatively stable enzyme (11). Ca binding at 25°C by the Ca-free CEP results in an enzyme with a higher specific activity (or which at pH >8.5 is catalytically active again) and which is thermally stabilized as well as protected from autoproteolysis (viz., from release). This could mean that stabilization by Ca is twofold, viz., a reduction of local backbone flexibilities and a reduction in local repulsive force by shielding the charges on residues which are the formal ligands for binding. At pH <5.2 and 10°C the effect of Ca binding on specific activity is not observed, which suggests a temperature-induced rearrangement of the Ca-loaded CEP if the temperature is raised. Around pI this ultimate effect of Ca binding at 25°C is minimal.
The resistance for irreversible inactivation at 25°C of the Ca-loaded CEP in the presence of relatively low concentrations of salt would indicate that salt bridges are either not present or less important for enzyme stability or that salt has only little effect on the stability of salt bridges (6). It also indicates that Ca binding was not influenced to an extent which could lead to irreversible inactivation. The initial reductions in activity at these low salt concentrations may therefore reflect a shielding effect which results in the elimination of attractive charge-charge interactions between the enzyme and the substrate. At higher concentrations the different effects of salts on activity suggest specific actions on hydrophobic interactions in either substrate binding or the stability of the enzyme or both.
The SO42− ion is thought to introduce disorder into the tetrahedral coordination of water and thus makes more water available for ordering around hydrophobic parts of the enzyme. This decreases the water solubility of these parts and thus promotes hydrophobic interaction (salting out). The hydrophobic effect may explain why Na2SO4 not only stabilizes the catalytically active enzyme but also activates the catalytic reaction to an extent exceeding the maximal effect of Ca2+; it may promote hydrophobic interaction of the substrate residues with the binding sites as well. The drop in activity at >1.5 M Na2SO4 most probably occurs due to intermolecular interactions which affect the solubility of the substrate and/or cause aggregation of cells.
The salting-out effectiveness of NaCl and KCl at the indicated concentrations is equally poor (25). The apparent different effect on CEP activity and stability might therefore be related to the fact that Na+, unlike K+, is able to replace Ca2+ to some extent. This ability of Na+ can be explained in terms of its ion radius (0.98 Å), which is much smaller than that of K+ (1.33 Å) and very close to that of Ca2+ (0.99 Å). It might therefore occupy the Ca sites as, of all divalent cations tested apart from Ca2+, only Cd2+ (0.97) does most effectively (11).
The irreversible reduction of Ca-loaded CEP activity by chaotropic ions like SCN− and Ca2+ is supposed to be the reflection of weakening of hydrophobic interactions caused by the intrinsic specific action of these ions on water structure. These ions somehow make less water available for, and thus reduce the extent of the formation of, ordered-water structures around the nonpolar residues of proteins; consequently, they decrease the unfavorable consequences of unfolding (25). The results with particularly SCN− suggest that in fact denaturation (inactivation) is a rather complex process involving at least one intermediate enzyme species. The initial phase of reduction obviously represents two events which are the consequence of the weakening of the hydrophobic effect. An irreversible change to an inactive (denatured) enzyme occurs (ca. 60% in the presence of 1 M SCN−), while the remaining fraction of the enzyme has adopted a relatively stable conformation which can still be detected by removing the salt, most probably because of a restructuring to the original native Ca-loaded conformation. Similar events may occur at 0.5 M SCN− or at high Ca2+ concentrations, although in both cases initial irreversible inactivation concerns only a relatively small fraction (15 and 10%, respectively) of the enzyme population. The occurrence of a more stable fraction in case the Ca-free enzyme is incubated with SCN− may reflect a conformational adaptation which during the initial phase of inactivation has saved ca. 25% (at 0.5 M SCN−) from rapid inactivation. However, unlike stabilization by salts causing salting out, introduction of a significant hydrophobic effect is not likely to be responsible in this case. The significance of a hydrophobic factor for the stabilization of the Ca-free CEP and the occurrence of hydrophobic stabilization underlying the effect Ca binding has on the enzyme is confirmed with the Ca-free CEP stabilized by sucrose. The recognizably stronger hydrophobic effect in the Ca-free enzyme in the presence of sucrose compared to that in the enzyme in the absence of sucrose may indicate that sucrose acts not only by favoring a more compact and less flexible structure of the protein but that as a consequence of the increased packing efficiency in the compact structure a relatively weak hydrophobic effect is effectively increased as well (19). This hydrophobic effect is even further increased by the binding of Ca2+. In conclusion, the effects of different salt ions on the activity and stability of the Ca-free, the sucrose-stabilized Ca-free, and the Ca-loaded enzyme indicate that, on the one hand, stimulation of a hydrophobic effect stabilizes the Ca-free CEP and that, on the other hand, a hydrophobic effect contributes to the stability of the Ca-loaded CEP. It is therefore believed that adopting a new conformation upon Ca binding involves hydrophobic stabilization possibly as a consequence of a higher packing density. This is in line with the generally accepted view that the hydrophobic effect is the major factor in stabilizing the folded structure of a protein (6).
Based on foregoing considerations concerning the relationships between structure, interactions, and stability the following heuristic view on destabilization and denaturation of the cell-bound Ca-CEP at physiological temperatures can be proposed.
Removal of weakly bound Ca2+ and consequently exposure of negative charges increases local (loop) flexibilities and repulsive forces (destabilizes local conformation) and initiates a reversible structural rearrangement at the Ca-binding sites which is propagated to the active site. Such a Ca-triggered movement throughout the molecule has been shown to occur in the closely related proteinase K and to exert long-range effects on the geometry of both the substrate-binding site and the catalytic triad of this enzyme (2). The consequence of the rearrangement of cell-bound CEP structure is an enzyme which shows a reduced specific activity (or no activity at all [pH >8.5]) and is further destabilized by a significant reduction in the strength of the hydrophobic effect as well. At neutral pH it may incidentally undergo autoproteolytic release, but the cell-bound enzyme is principally denatured to the extent that the ability to be activated and stabilized by Ca2+ is lost.
With respect to ripening of cheese, the conditions seem conducive to a stable cell-bound enzyme which remains actively involved in proteolysis throughout the ripening period as long as an intact cell is concerned. If autolytic action occurs, Ca2+ in the cheese moisture may no longer protect the enzyme from autoproteolytic attack (5), which in the long run inactivates the enzyme—as was observed in vitro at pH 6.5 (unpublished data). Moreover, competition by intracellular endopeptidase (1) would severely limit the contribution by CEP provided the endopeptidase becomes accessible to its extracellular substrates through permeabilization or autolysis of cells.
ACKNOWLEDGMENT
This work was supported by contract no. AIR2-CT93-1531 of the Agriculture and Agro-Industry Research Programme of the Commission of European Communities.
REFERENCES
- 1.Baankreis R, van Schalkwijk S, Alting A C, Exterkate F A. The occurrence of two intracellular oligoendopeptidases in Lactococcus lactis and their significance for peptide conversion in cheese. Appl Microbiol Biotechnol. 1995;44:386–392. doi: 10.1007/BF00169933. [DOI] [PubMed] [Google Scholar]
- 2.Bajorath J, Raghunathan S, Hinrichs W, Saenger W. Long-range structural changes in proteinase K triggered by calcium ion removal. Nature. 1989;337:481–484. doi: 10.1038/337481a0. [DOI] [PubMed] [Google Scholar]
- 3.Bruinenberg P G, Vos P, de Vos W M. Proteinase overproduction in Lactococcus lactis strains: regulation and effect on growth and acidification in milk. Appl Environ Microbiol. 1992;58:78–84. doi: 10.1128/aem.58.1.78-84.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler S L, Falke J J. Effects of protein stabilizing agents on thermal backbone motions: a disulfide trapping study. Biochemistry. 1996;35:10595–10600. doi: 10.1021/bi961107v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coolbear T, Reid J R, Pritchard G G. Stability and specificity of the cell-wall-associated proteinase from Lactococcus lactis subsp. cremoris H2 released by treatment with lysozyme in the presence of calcium. Appl Environ Microbiol. 1992;58:3263–3270. doi: 10.1128/aem.58.10.3263-3270.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dill K A. Dominant forces in protein folding. Biochemistry. 1990;29:7133–7155. doi: 10.1021/bi00483a001. [DOI] [PubMed] [Google Scholar]
- 7.Exterkate F A. The lactococcal cell envelope proteinases: differences, Ca-binding effects and role in cheese ripening. Int Dairy J. 1995;5:995–1018. [Google Scholar]
- 8.Exterkate F A, de Veer G J C M. Partial isolation of and degradation of caseins by cell wall proteinases of Streptococcus cremoris HP. Appl Environ Microbiol. 1985;49:328–332. doi: 10.1128/aem.49.2.328-332.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Exterkate F A, de Veer G J C M. Characterization of the cell wall proteinase PIII of Lactococcus lactis subsp. cremoris strain AM1 and its relationship with the catalytically different cell wall proteinase PI/PII of strain HP. Syst Appl Microbiol. 1989;11:108–115. [Google Scholar]
- 10.Exterkate F A, Alting A C, Bruinenberg P G. Diversity of cell-envelope proteinase specificity among strains of Lactococcus lactis and its relationship to charge characteristics of the substrate-binding region. Appl Environ Microbiol. 1993;59:3640–3647. doi: 10.1128/aem.59.11.3640-3647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Exterkate F A, Alting A C. Role of calcium in activity and stability of the Lactococcus lactis cell envelope proteinase. Appl Environ Microbiol. 1999;65:1390–1396. doi: 10.1128/aem.65.4.1390-1396.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gekko K, Timasheff S N. Thermodynamic and kinetic examination of protein stabilization by glycerol. Biochemistry. 1981;20:4677–4686. doi: 10.1021/bi00519a024. [DOI] [PubMed] [Google Scholar]
- 13.Genov N, Filippi B, Dolashka P, Wilson K S, Betzel Ch. Stability of subtilisins and related proteinases (subtilases) Int J Peptide Prot Res. 1995;45:391–400. doi: 10.1111/j.1399-3011.1995.tb01054.x. [DOI] [PubMed] [Google Scholar]
- 14.Kunji E R S, Mierau I, Hagting A, Poolman B, Konings W N. The proteolytic systems of lactic acid bacteria. In: Venema G, Huis in't Veld J H J, Hugenholtz J, editors. Lactic acid bacteria: genetics, metabolism, and applications. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1996. pp. 187–221. [Google Scholar]
- 15.Leberman R, Soper A K. Effect of high salt concentrations on water structure. Nature. 1995;378:364–366. doi: 10.1038/378364a0. [DOI] [PubMed] [Google Scholar]
- 16.Lee J C, Timasheff S N. The stabilization of proteins by sucrose. J Biol Chem. 1981;256:7193–7201. [PubMed] [Google Scholar]
- 17.Navarre W W, Schneewind O. Proteolytic cleavage and cell wall anchoring at the LPXTG motif of surface proteins in gram-positive bacteria. Mol Microbiol. 1994;14:115–121. doi: 10.1111/j.1365-2958.1994.tb01271.x. [DOI] [PubMed] [Google Scholar]
- 18.Pritchard G G, Coolbear T. The physiology and biochemistry of the proteolytic system in lactic acid bacteria. FEMS Microbiol Rev. 1993;12:179–206. doi: 10.1111/j.1574-6976.1993.tb00018.x. [DOI] [PubMed] [Google Scholar]
- 19.Sandberg W S, Terwilliger T C. Influence of interior packing and hydrophobicity on the stability of a protein. Science. 1989;245:54–57. doi: 10.1126/science.2787053. [DOI] [PubMed] [Google Scholar]
- 20.Schneewind O, Mihaylova-Petkov D, Model P. Cell wall sorting signals in surface proteins of gram-positive bacteria. EMBO J. 1993;12:4803–4811. doi: 10.1002/j.1460-2075.1993.tb06169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siezen R J. Multidomain, cell-envelope proteinases of lactic acid bacteria. Antonie Leeuwenhoek. 1999;76:139–155. [PubMed] [Google Scholar]
- 22.Siezen R J, de Vos W M, Leunissen A M, Dijksta B W. Homology modelling and protein engineering strategy of subtilases, the family of subtilisin-like serine proteinases. Prot Eng. 1991;4:717–737. doi: 10.1093/protein/4.7.719. [DOI] [PubMed] [Google Scholar]
- 23.Timasheff S N. The control of protein stability and association by weak interactions with water: how do solvents affect these processes? Annu Rev Biophys Biomol Struct. 1993;22:67–97. doi: 10.1146/annurev.bb.22.060193.000435. [DOI] [PubMed] [Google Scholar]
- 24.Visser S, Exterkate F A, Slangen K J, de Veer G J C M. Comparative study of the action of cell wall proteinases from various strains of Streptococcus cremoris on bovine αs1, β- and κ-casein. Appl Environ Microbiol. 1986;52:1162–1166. doi: 10.1128/aem.52.5.1162-1166.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Von Hippel P H, Schleich T. The effects of neutral salts on the structure and conformational stability of macromolecules in solution. In: Timasheff S N, Fasman G D, editors. Structure and stability of biological molecules. New York, N.Y: Marcel Dekker, Inc.; 1969. pp. 417–574. [Google Scholar]
- 26.Vos P, Simons G, Siezen R J, de Vos W M. Primary structure and organization of the gene for a procaryotic, cell envelope-located serine proteinase. J Biol Chem. 1989;264:13579–13585. [PubMed] [Google Scholar]