Abstract
Growing evidence suggest that there is a connection between Parkinson’s disease (PD) and insulin dysregulation in the brain, whilst the connection between PD and type 2 diabetes mellitus (T2DM) is still up for debate. Insulin is widely recognised to play a crucial role in neuronal survival and brain function; any changes in insulin metabolism and signalling in the central nervous system (CNS) can lead to the development of various brain disorders. There is accumulating evidence linking T2DM to PD and other neurodegenerative diseases. In fact, they have a lot in common patho-physiologically, including insulin dysregulation, oxidative stress resulting in mitochondrial dysfunction, microglial activation, and inflammation. As a result, initial research should focus on the role of insulin and its molecular mechanism in order to develop therapeutic outcomes. In this current review, we will look into the link between T2DM and PD, the function of insulin in the brain, and studies related to impact of insulin in causing T2DM and PD. Further, we have also highlighted the role of various insulin signalling pathway in both T2DM and PD. We have also suggested that T2DM-targeting pharmacological strategies as potential therapeutic approach for individuals with cognitive impairment, and we have demonstrated the effectiveness of T2DM-prescribed drugs through current PD treatment trials. In conclusion, this investigation would fill a research gap in T2DM-associated Parkinson’s disease (PD) with a potential therapy option.
Graphical Abstract
Keywords: Parkinson’s disease, Type 2 diabetes mellitus, Insulin, Pathophysiology, Therapeutics
Introduction
Parkinson’s disease (PD) is a movement disorder depicted by dopaminergic (DA) neuron depletion in the basal ganglia region [1]. It is characterised by four cardinal symptoms of rigidity, postural imbalance, bradykinesia, and tremor [2]. The cause and severity of PD may be influenced by a variety of demographic and environmental factors [3, 4]. PD is multi-factorial, but ageing is considered to one of the prime causative agent amongst others [5, 6]; however, the pathophysiology of PD is still elusive [7]. People living with PD are more likely to develop several other diseases, including heart disease [8], cancer [9], gastrointestinal disorders [10], type 2 diabetes mellitus (T2DM) [11], vitamin D deficiency [12], peripheral neuropathy [13], and musculoskeletal diseases [14]. Amongst these associated diseases, T2DM has been the most commonly associated disease with PD. In an earlier study, it was stated that lysosomal disruption, mitochondrial dysfunction, aberrant protein build-up, and chronic inflammation are some of the common possible signalling pathways that may cause protein misfolding and insulin resistance, in both PD and T2DM [15]. Interestingly, there is also an evidence for cross-seeding between islet amyloid polypeptide (IAPP) and αSyn [16]. Also, metabolically in T2DM, insufficient insulin production causes disruptions in glucose metabolism and chronic inflammation, whereas similar metabolic downregulation is seen in early PD conditions [17]. As a result, insulin is now widely recognised to play an important role in neuronal survival and brain function. Insulin action is vital for neuronal synaptic plasticity and aids learning and memory. Therefore, alterations in insulin metabolism and signalling in the central nervous system (CNS) can contribute to the development of many brain disorders [18]. The pathogenesis of these brain diseases suggested to be significantly influenced by both insulin resistance and reduced insulin action through many mechanisms [19]. Therefore, understanding the role of insulin and its signalling pathway is essential to investigate, in T2DM and PD [20]. Hence, in this review, we have discussed the link between T2DM and PD, by focusing the function of insulin in the brain, and its clinical and pre-clinical evidences on T2DM and neurodegeneration. We have also reported the efficiency of common therapeutic compounds on T2DM and PD, thereby concluding the need of research on the signalling pathways correlating T2DM and PD with its effectiveness on the development of therapeutic strategies for both.
Accompaniment Between T2DM and PD
The specific protein known as amylin misfolds may contribute to T2DM pathogenesis due to the loss of pancreatic beta cells, which in turn results in insulin insufficiency and hyperglycaemia and speed up of disease progression [21, 22]. Additionally, this amylin has been linked to PD pathophysiology since it interacts with alpha-synuclein (αSyn), which has been reported in an in vitro study[23]. This αSyn is also produced in pancreatic islets which plays a crucial role in glucose regulation [24]. Whereas in T2DM, IAPP aggregation takes place in the pancreatic cells [25]. As a result, it is important to maintain the balance of the protein levels between production, release, and clearance. Note that there may be an imbalance in the proportion of amylin or αSyn, which could encourage the formation of toxic aggregates in the brain and modulate pancreatic beta cells [26]. Ultimately, there is a possibility that these two proteins will interact indirectly or directly and result in T2DM and PD when their levels are altered [27]. Few reports have shown that oxidative stress, protein aggregation, neuroinflammation, insulin resistance, and mitochondrial dysfunction are involved in the aetiology of T2DM and PD. Almost all of the biomolecules in a cell can be damaged by oxidative stress, which can result in dysfunction and cell death. In T2DM and PD, oxidative stress damages pancreatic beta-cells as well as dopaminergic cells [28]. Another mechanism is insulin resistance, which inhibits insulin signal transduction which contributes to T2DM pathogenesis, also an imbalance in insulin inside the brain could also promote PD progression by impairing mitochondrial function [29] through an increase in reactive oxygen species (ROS) generation [30] [31]. Another important mechanism is neuroinflammation, where the microglia plays a crucial role in PD [32], whereas its activation plays a key role in the dysregulation of energy homeostasis, promotes excessive glucose levels, and has an influence on the occurrence of T2DM [33]. In this review, we have incorporated and depicted an overview of studies with epidemiological evidences supporting the association between T2DM and PD in Table 1.
Table 1.
Concomitance between T2DM and PD
| Study design | Study area | Objective | Sample size | Method | Result | Conclusion | References |
|---|---|---|---|---|---|---|---|
| Case–control | Italian population | Glucose metabolism abnormalities | 110 patients | • Oral-glucose-tolerance-test |
PD patients with dementia • ↑ disease duration • ↑ motor disability |
Insulin resistance • ↑PD with dementia • ↑PD without dementia |
[34] |
| Inter-group comparative analysis | South Korea | Effect of DM on PD patients | 671 patients |
• Neuroimaging analyses • DAT • Assessment of longitudinal changes in LED |
• ↓baseline DAT availability • ↓working memory • ↓frontal/executive function • ↑longitudinal changes in LED |
DM patients with PD • ↓baseline striatal dopamine • ↓cognitive function • ↓brain structural alterations |
[35] |
| Retrospective, case–control | Italy | Clinical features of patients with idiopathic PD (IPD) | PD (n = 89); control (n = 89) | • UPDRS |
• Higher UPDRS motor score • Severe Hoehn and Yahr staging |
The onset of DM before the onset of PD • ↑extreme PD symptoms |
[36] |
| Meta-analysis | Italy | Relationship between pre-existing DM and PD | Nine studies/1,947 citations | • Data obtained from electronic databases |
Onset of DM before the onset of PD • ↑risk for future PD |
• DM is a risk factor for PD | [37] |
| Cohort study | America | Association between T2DM and PD | 21,841 participants | Questionnaire | • ↑DM had an PD risk | No conclusive evidences, so more studies remain to be established | [38] |
| Cross-sectional study | Spain | Association between PD and DM | 79 PD and 4919 controls | NEDICES | • No association between prevalence of PD and DM | The risk of PD in DM might be limited to longer disease duration | [39] |
| Retrospective, cohort study | Spain | Association between T2DM and subsequent PD | 81,90,323 participants | Linked English national Hospital Episode Statistics and mortality data | • The relative increase was greater in those with complicated T2DM and when comparing younger individuals | ↑rate of subsequent PD following T2DM | [40] |
| Prospective | Northeast America | Obesity and DM are related to the risk of PD | 656 PD | Medical history and anthropometric variables | DM was not significantly associated with PD risk | No evidence between baseline DM and PD | [41] |
| Prospective | Singapore | Impact of DM in PD patients | PD, DM (n = 12); control (n = 65) | MRI imaging and neuropsychological assessments |
• ↓routine cognitive screening tests (MMSE and MOCA) • ↑atrophy in the cortical White matter |
DM in PD • ↑cognitive decline |
[42] |
| Retrospective, cohort study | Taiwan | Age-sex-specific incidence, relative risk of PD | DM (n = 603,416); control (n = 472,188) | Incidence rate and relative risk of PD evaluation | • DM elevated risk of PD patients especially in women and younger patients | A stronger link between DM and young-onset PD deserves further investigation | [43] |
| Cohort study meta-analysis | China | Associations between DM and the risk of PD | 1,761,632 individuals from 7 population | Data obtained from electronic databases (PubMed, Embase, and Scopus) | ↑DM associated with PD risk 38% | More large-scale prospective studies are warranted to further clarify this association and its mechanism | [44] |
| Case–control meta-analysis | China | Association between DM and the risk of PD | PD patients (n = 21,395); control (n = 84,579) | Data obtained from databases | ↓DM association with future PD | DM individuals may have a decreased incidence of PD despite significant heterogeneity | [45] |
| Cohort study | USA | Relationship between DM and future risk of PD | 288,662 with and without PD | Questionnaire | PD risk was higher amongst diabetic patients | ↑ PD with DM ∼40% | [46] |
PD, Parkinson’s disease; T2DM, type 2 DM mellitus; DM, DM mellitus; DAT, dopamine transporter; LED, levodopa-equivalent dose; UPDRS, Unified Parkinson’s Disease Rating Scale; NEDICES, Neurological Disorders in Central Spain study; HR, hazard ratio; CI, confidence interval; MMSE, Mini-Mental State Examination; MOCA, Montreal Cognitive Assessment
T2DM and Neurodegeneration: the Role of Insulin
The pancreatic beta-cells secrete insulin, a polypeptide hormone with a molecular weight of approximately 6000 Da [47]. The protein insulin is made up of two chains—an A chain, which has 21 amino acids, and a B chain, which has 30 amino acids—joined by sulphur atoms. Proinsulin, a prohormone molecule with 74 amino acids, is the precursor of insulin. Only a small amount of proinsulin is secreted normally since it is a relatively inert protein. The proinsulin molecule is split into two parts in the endoplasmic reticulum of beta cells, resulting in the A and B chains of insulin and an intervening, biologically inactive C peptide [48, 49]. Insulin enters the brain after being secreted by pancreatic b-cells, and this process is tightly controlled and may be altered by conditions like obesity, diabetes mellitus, fasting, and neurodegenerative diseases [50]. In fact, the brain has historically been assumed to be insensitive to insulin since it does not increase glucose absorption or metabolism in the brain. However, in the last two decades, studies in this domain have uncovered specific insulin effects in the brain, and there are growing evidences that reveals insulin to act in both central and peripheral brain regions and serves several functions [51, 52]. The development of the nervous system is found to be highly influenced by insulin action in the brain, which controls proliferation, specialisation, and nerve growth [18, 53]. Additionally, brain insulin functions as a neuroprotector by inhibiting the effects of apoptosis, β-amyloid toxicity, oxidative stress, and ischemia [31, 54, 55]. If the brain insulin metabolism is impaired, the protective effects of insulin may be reduced [56], which leads to cognitive impairment and depression as well as the lack of insulin action in the brain, and may increase the risk of neurodegenerative disorders [30, 57]. Insulin disturbances associated with the diabetic phenotype (hyperglycemia, hyperinsulinemia, and hypercholesterolemia) have been linked to brain atrophy and PD pathological features [58]. It is still unclear whether insulin resistance is a cause or a result of PD; however, insulin action has been shown to play an important role in PD pathogenesis. Emerging data highlights the significance of insulin dysregulation that may lead to neuropathophysiological conditions, given the fact that the role and processes behind insulin action in the human CNS still raise many issues and are far from being fully understood (Fig. 1).
Fig. 1.
Function of insulin in the brain. Insulin controls peripheral activities in the brain by autonomic nervous system (ANS) and the hypothalamic-pituitary axis (HPA). Insulin specifically acts in the hippocampus and prefrontal cortex to enhance cognitive function and lessen depression symptoms. Insulin reduces food intake and helps people lose weight by acting in the hypothalamic nuclei. Insulin functions in the brain to reduce hepatic glucose synthesis, enhance lipogenesis, reduce lipolysis, and boost the sympathoadrenal response to hypoglycemia via the efferent ANS to target organs. The hypothalamic-pituitary–gonadal axis is the pathway via which insulin improves reproductive competence
Insulin Signalling in Brain
In the brain, insulin will bind to its receptors causing the phosphorylation of substrates, Shc, and insulin receptor substrate (IRS) where IRS is responsible for the PI3K (Phosphatidylinositol-3-kinase)-Akt (also known as PKB (Protein kinase B) cascade and Shc for Ras-MAPK (mitogen-activated protein kinase) that functions in food behaviour, learning, memory, and neuromodulation [59]. For the activation of PI3K-AKT, initially, insulin binds to the α-subunit of the insulin receptor, leading to the formation of dimers and autophosphorylation of β-subunits [60]. PI3K first transforms the minor phospholipid from phosphatidylinositol (3,4)-bisphosphate (PIP2) to phosphatidylinositol (3,4,5)-trisphosphate (PIP3), and this conversion is important for engaging PKB or AKT to the membrane of the cell [61]. AKT is responsible for the regulation of several other proteins in the insulin signalling cascade, including mTORC1 and FOXO1 [62]. It is also an active participant in several different signalling pathways of cell proliferation, cell growth, and metabolism [63]. Insulin binding also activates another series of cascades leading to MAPK pathway [62]. Small G-protein Ras is activated by the phosphorylation of guanosine triphosphate which further results in the MAPK-ERK cascade signalling [64]. MAPK is the pathway through which insulin can affect both learning and memory [65]. It is also responsible for the initiation of synaptic plasticity and cell differentiation through the transcription of various genes [66]. From these studies, it is evident that the insulin plays a major role in the brain and the deviation in their levels do have an impact on the metabolic and cognitive functions (Fig. 2).
Fig. 2.
Signalling pathways involved in insulin signals in the brain. When insulin ligand binds to its receptor, the receptor substrates IRS and Shc were phosphorylated. IRS activates PI3K-Akt pathway and insulin signalling cascade such as mTORC and FOXO1. PI3K first converts PIP2 to PIP3 that helps in engaging the Akt to the cell membrane. Shc activates MAPK pathway by activating the Ras protein through phosphorylation. Insulin binding also helps in the expression and recruitment of other receptors like GABA, NMDA, and AMPA. IRS, insulin receptor substrate; mTORC, mammalian target of rapamycin complex; FOXO1, Forkhead box protein O1; MAPK, mitogen-activated protein kinase; PI3K, phosphatidylinositol-3-kinase; PIP2, phosphatidylinositol (3,4)-bisphosphate; PIP3, phosphatidylinositol (3,4,5)-trisphosphate; GABA, gamma-aminobutyric acid; NMDA, N-methyl-D-aspartate; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
Impact of Methylglyoxal in T2DM and PD
Methylglyoxal and T2DM
Certain conditions such as hyperglycaemia and glycation, a random event that typically takes place under biological circumstances, are generally aggravated in T2DM [67]. The key factor thought to be responsible for diabetes complications is hyperglycaemia, which occurs when there is an overwhelming quantity of glucose in the blood [68]. Methylglyoxal (MGO) is the main hazardous oxoaldehyde that is formed during hyperglycaemia in diabetes mellitus. In reality, the healthy levels of MGO in human plasma are about 150 nM, whilst T2DM individuals have been shown to have two to six times elevated levels [69, 70]. Prevailing thought is that methylglyoxal (MG), a reactive metabolite generated from glucose, and its breakdown by the glyoxalase pathway play a significant role in metabolic dysfunction, which connects hyperglycaemia to the onset of vascular problems in diabetes [71]. With a molecular weight of 72 Da, MGO is a-oxoaldehyde metabolite that is primarily produced as a by-product of glycolysis after the spontaneous breakdown of the triose phosphate intermediates glyceraldehyde-3-phosphate (G3P) and dihydroxyacetone phosphate (DHAP) [72]. Other minor sources of MGO production include the breakdown of glycated proteins [73], the threonine catabolism [74], and the metabolic activity of ketone bodies, where MGO is produced from the further oxidation of hydroxyacetone, which is generated from acetone hydroxylation [75, 76]. Nucleotides, lipids, and proteins undergo spontaneous chemical change as a result of MGO activity. It alters DNA primarily by forming the imidazopurinone adduct 3-(2'-deoxyribosyl)-6,7-dihydro-6,7-dihydroxy-6/7-methylimidazo-[2,3-b]purine-9(8)one (MGdG) by interacting with deoxyguanosine (dG) [77]. In addition, it can alter amino acids in proteins by forming advanced glycation end products (AGEs) by interacting with arginine, cysteine, and lysine residues in proteins [78]. The primary pathway for MGO detoxification in normoglycemic circumstances is the glyoxalase system, which also protects cells from MGO toxicity. During physiologic conditions, glyoxalases 1 and 2 (Glo-1 and Glo-2) are primarily responsible for the metabolism of MG, which results in the synthesis of D-lactate through a process that employs glutathione as a coenzyme. When reduced glutathione (GSH) is present, these enzymes react with MGO to create lactate [79]. In diabetic individuals, MGO plays a critical role in endothelial dysfunction that results in insulin resistance, hypertension, and nephropathy. In glycation processes, it is noticeably more sensitive than glucose. Additionally, AGEs are well-established, scientifically proven risk factors for the development of diabetes and associated consequences [78]. MG-modified proteins and MG-modified amino acids, also known as protein-bound AGEs and AGE-free adducts, correspondingly, are present in tissues and bodily fluids [80]. According to Stitt (2010), there were comparable increases in MG-H1 and CEL protein-bound and free adducts in plasma, suggesting that clinical translation may make MG-derived AGEs evaluated in plasma or serum biomarkers or risk indicators for vascular consequences in diabetes. These pieces of evidences show that AGEs and MGO produced from MG may be the best diagnostic biomarkers for the disease.
Methylglyoxal and PD
Researches have suggested that PD and T2DM may also be related [36, 81]. Here, it is hypothesised that PD and T2DM share a same fundamental causative mechanism that entails elevated levels of MG. One of the key characteristics of PD is a rapid decline in cellular reduced glutathione (GSH) levels in the initial phases of the disease. This leads to a reduction in the activity of the glyoxalase system, the primary catabolic pathway of the most significant glycation agent, MG [82]. The AGE levels rise as a result of the carbonyl stress, which eventually causes oxidative stress and further AGE formation. The loss of dopaminergic neurons and an increase in oxidative stress in the brain may both be caused by this harmful loop. According to Lee et al. [83], it was found that MGO could cause a-syn to oligomerize and also reduce the ability of glycated a-syn to bind to membrane in PD. Since PD is well recognised to be closely linked to the build-up and aggregation of α-synuclein. Additionally, beta-synuclein is highly glycation-susceptible, and beta-synuclein “knockout” animals had higher levels of MG, glycation stress, and glyoxalase activity [84]. Parkin affects energy metabolism, as well as a cell-based investigation has shown this [85]. Its over-expression was discovered to inhibit glucose uptake, reduce glycolytic rate, and promote oxygen consumption [86]. The generation of MG as a result of triosephosphate accumulation due to diminished NAD + accessibility or impaired glyceraldehyde-3-phosphate dehydrogenase activity is one effect of excessive glycolysis, as mentioned above. It is intriguing to learn that PD is linked to mitochondrial malfunction, which may impede NADH from being oxidised back to NAD + (through the malate or glycerol-phosphate shuttles) and enhance oxidative stress to glyceraldehyde-3-phosphate dehydrogenase in the cerebral cortex [87]. Therefore, these findings suggest that enhanced or inadequate glycolysis due to over production of MG leads to the PD phenotypes (Fig. 3).
Fig. 3.
Correlating the influence of methylglyoxal on T2DM and PD. This image depicts the possible influence of methylglyoxal on causing the T2DM and PD pathogenesis. In T2DM, glycolysis is the major factor for the release of MGO, whereas in PD in gets released due to low levels of GSH. Further in T2DM, it has a role in causing both phenotypic as well as genotypic changes. In PD, it impacts in two ways: (1) by increasing oxidative stress and (2) neuro-inflammation in the cells
Common Problems Observed in Both T2DM and PD
An association between T2DM and PD was first reported by Sandyk in 1993, where it was noted that PD patients with co-existent T2DM had worse motor symptoms and reduced response to treatment [88]. Also, a high prevalence of impaired glucose tolerance has been reported amongst PD patients (50–80%); however, a more recent estimate suggests that overtly impaired glucose metabolism occurs in only around 20% [89]. So there has to be a commonality between the characteristic features of the both the disease conditions in a similar pattern which has been explained below elaborately.
Insulin Dysregulation in PD
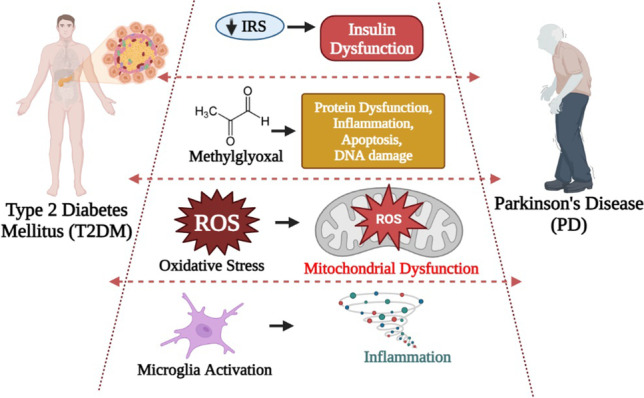
A peptide hormone called insulin is released into the bloodstream by pancreatic beta cells in response to postprandial hyperglycemia. Insulin receptors are expressed in the basal ganglia [90] and in the substantia nigra [91], which are the areas of the brain that are mostly affected in PD. Since a long time ago, it has been discovered that the hypothalamus, olfactory bulb, and midbrain all contain detectable levels of insulin [92]. According to experimental data, adult neuronal cells coming from the hippocampus and olfactory bulb as well as pyramidal neurons in the cortex produce insulin [93]. It has been discovered that there is a reduction in insulin signalling as a result of physiological changes brought on by ageing, particularly in PD patients. According to studies, patients with PD had much more insulin resistance and less insulin receptor mRNA in their substantia nigra pars compacta (SNpc) than age-matched controls [94, 95]. The loss of IGF-1 in the frontal cortex is substantially greater than that of insulin and IGF-1 signalling, and these changes are linked to higher levels of oxidative stress indicators and αSyn build-up in PD [96]. The suppression of IR-IRS-1-PI3K/AKT pathway in the hippocampus region of the brain, which in turn boosts the levels of phosphorylated IRS-1 at serine residues 636 and 616, would be the most likely molecular route by which insulin would be reduced in PD. Also, the phosphorylation of IRS-1 on serine residues, a crucial element of functional insulin signalling, prevents insulin/IGF-1 from binding to the IR and subsequent activation of downstream effectors. Further IR stimulates the aggregation of αSyn in PD [29]. Insulin resistance is linked to a more severe phenotype, a faster pace of disease progression, and a higher likelihood of cognitive deterioration in PD patients. These findings demonstrate that insulin dysregulation is a significant issue in both PD and T2DM. Figure 4a provides a brief explanation of the insulin dysregulation mechanisms between PD.
Fig. 4.
(a) Dysregulation of Insulin in PD pathogenesis. In PD, the production of insulin is hindered which directly or indirectly inhibits the IR-IRS-1-PI3K/AKT signalling pathway of insulin production. This results into consecutive production of either oxidative stress or aggregation of a-syn which is mostly observed in PD. (b) Impact of oxidative stress on T2DM and PD. This image lets us to easily compare the similarities and differences amongst the impact of oxidative stress on T2DM and PD. In both the cases, inflammation is the major role of disease pathogenesis. (c) Importance of microglia in T2DM and PD. Generally, the activation of microglia causes morphological as well as functional changes which results into disease conditions. This image enables us to understand the role of microglia activation in both T2DM and PD and how it triggers inflammation more easily
Oxidative Stress and Mitochondrial Dysfunction
An imbalance in the levels of ROS and difficulty of the body to outrage its toxic reactive intermediates leading to cellular damages are known as oxidative stress. A hyperglycaemic state can lead to an increase in the levels of oxidative stress-induced DNA damage markers such as 8-hydroxy-2′-deoxyguanosine (8-OHdG) and 8-oxo-7, 8-dihydro-2′-deoxyguanosine; lipid-peroxidation products measured as thiobarbituric acid-reactive substances (TBARS); protein oxidation products such as nitrotyrosine and carbonyl levels and also lower the activity of antioxidant enzymes. Exposure of β-cell line and isolated pancreatic islet cells to oxidative stress has been shown to inhibit the promoter activity and mRNA expression of the insulin gene therefore, decreasing insulin gene expression [97]. Oxidative stress is also strongly suspected to be involved in chronic hyperglycaemia-induced insulin resistance [98]. Increased ROS production in T2DM patients is thought to activate many detrimental pathways including hexosamine pathways, advanced glycation end-products (AGEs) formation, and PKCβ1/2 [99]. Hyperglycemia condition can induce oxidative stress by several mechanisms such as glucose autoxidation, polyol pathway, AGE formation, and PKCβ1/2 kinase. Elevated free fatty acids, leptin, and other circulating factors in T2DM patients may also contribute to cause ROS overproduction. Similarly, even in PD brains, the loss of dopaminergic neurons occurs mainly due to ROS levels which result from dopamine metabolism, low glutathione (GSH), and high levels of iron and calcium in the SNpc [100]. Additionally, the brain contains high concentrations of polyunsaturated fatty acids, which under oxidative stress conditions result in lipid peroxidation and the generation of toxic products [101]. Therefore, it is evident that excessive free radical generation, known to promote oxidative stress and cytokine production, appears to be the main trigger factors for cellular dysfunction including impaired insulin signalling and mitochondrial dysfunction resulting into T2DM and PD. In Fig. 4b, we have depicted the possible mechanism of oxidative stress in T2DM and PD in a comparable manner.
Microglial Activation and Inflammation
Microglial activation is a process of morphological and functional change that can be triggered by inflammation and vascular injury. Microglial stimulation and the consequent production of inflammatory mediators, which ultimately cause injury to brain tissue, are the principal manifestations of neuroinflammation. Microglial activation and inflammatory responses are modulated by signalling molecules, such as MAPKs and triggering receptor expressed on myeloid cells 2 (TREM-2) [102, 103]. The three main MAPKs are extracellular signal-regulated kinases 1 and 2 (ERK1/2), p38 MAPK, and c-Jun N-terminal kinases (JNK), which control a variety of cellular processes and are crucial in controlling the expression of pro-inflammatory cytokines like tumour necrosis factor-alpha (TNF-alpha) and interleukin-1 alpha (IL-1 alpha) [104]. According to a recent study, chronic cerebral hypoperfusion CCH-induced stimulation of microglia encourages the production of pro-inflammatory substances, which further contribute to cognitive dysfunction and long-term potentiation (LTP) impairment [105]. Additionally, in humans and rats with T2DM, microglial activation and an inflammation are also involved [106, 107]. Inflammation can be exacerbated by mitochondrial ROS brought on by hyperglycemia by activating the PKC, JNK, and p38 cascades [108]. JNK inhibition lessens the lipopolysaccharide (LPS)-induced activity of inflammatory cytokines in microglia, as activation of p38 is crucial for cytokine production and release [109]. In an in vitro model using microglial cell line, AGEs in diabetes trigger the MEK/ERK, PI3K/Akt, and NF-B pathways via RAGE, which causes the activation of pro-inflammatory cytokines [110]. These studies suggest that the JNK cascade is the most important mechanism contributing to oxidative/inflammatory stress and microglial activation during acute glucose fluctuations by evaluating the efficacy of all signalling pathway inhibitors especially in T2DM. In an effort to pinpoint a putative mechanism that might contribute to neuroinflammation and possibly underlying the aetiology of PD, the intricacy of microglial stimulation signals came under great scrutiny. In particular, areas of the SN and striatum in PD patients were found to have MHC-II immunoreactive microglia [111, 112]. M1 pro-inflammatory microglial cell activation encourages the production of substances that damage DA neurons in the brain. Followed which nuclear factor-kB is the primary initiator of inflammation and the first signal to do so. Other pro-inflammatory cytokines, such as tumour necrosis factor-alpha, interleukin 1b, and IL-6, are then released into the body [4]. Therefore, these results demonstrate that there is a tight relationship between microglia activation and inflammation in both T2DM and PD, which requires further study (Fig. 4c).
Drugs Available for T2DM
People with T2DM are probable to develop many health complications such as heart problems, retinal issues, and brain strokes. The finest kind of medication will depend on a number of factors, including the severity of diabetes, age, and whether with additional health issues. Here, we describe the drugs developed to treat T2DM, which has lack of scientific findings with convincing results (Table 2). As previously mentioned, T2DM and PD share several common characteristics, and it has been suggested that there is a list of drugs that have influenced the signalling pathways in both T2DM and PD, and are listed in detail in (Table 3).
Table 2.
List of drugs used in T2DM
| S. no | Plant/drug | Extract/compound | Mechanism of action | Organ function | Findings | Recommended/not recommended | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Moringa oleifera | Moringa seeds | Lessening the expression of iNOS, IL-1β, NO, TNF-α | Liver | ↓plasma glucose levels, HbA1c | Recommended | [113] |
| 2 | Garcinia macrophylla | 5,7,4′,5″,7″,3‴,4‴-heptahydroxyflavanone [3–6″] flavones | Antibacterial, antioxidant, and anti-T2DM | - | ↓ glucose level | Recommended | [114] |
| 3 | Albendazole lansoprazole | - | Activation of AMPK and PPAR pathways and incretin-like effect | - |
↑ glucose, HbA1c, triglyceride, LDL, leptin ↓insulin and HOMA-β |
Recommended | [115] |
| 4 | Remogliflozin etabonate (100 mg and 250 mg) | - | SGLT2 inhibition improves urinary glucose excretion thereby decreases blood glucose levels | ↓ glucose levels | Recommended | [116] | |
| 5 | Metformin hydrochloride | - | Lessens intestinal glucose absorption, declines hepatic glucose production, increases insulin sensitivity | Liver | ↓HbA1c levels, FBG, and weight loss | Recommended | [117] |
| 6 | Remogliflozin metformin teneligliptin | - | - | - | ↓glucose levels, weight | Recommended | [118] |
| 7 | Rhizoma coptidis | Berberine and polyphenols | Improves β cell dysfunction | Liver adipose tissues |
↓IL-6, TNFα ↑islet cell viability |
Recommended | [119] |
| 8 | Alkaline and flavone category compounds | MAPK1, STAT3, INSR, and 38 signalling pathways | - | Insulin resistance | Recommended | [120] | |
| 9 | Morus alba | Chlorogenic acid, isoquercitrin, and quercitrin | - | - | Glucose absorption, insulin secretion, antioxidant, anti-inflammatory, antihyperglycemic, and antihyperlipidemic activities, declined obesity | Recommended | [121] |
| 10 | Zingiber officinale | - | - | Heart |
↑diastole function and blood pressure ↓ HbA1c, triglycerides |
Recommended | [122] |
| 11 | Glargine/lixisenatide | - | GLP-1 RA, control postprandial glucose improved β-cell function | - |
↓ HbA1c, weight gain ↓ glucose levels |
Recommended | [123] |
| 12 | Gegen Qinlian decoction berberine | - | Proinflammatory cytokines Nfkb1, Stat1, and Ifnrg1 | Pancreas |
Enriched gut microflora ↓ glucose levels |
- | [124] |
| 13 | Gegen Qinlian decoction (meta-analysis study) | - | - | Pancreas |
↓ glucose levels ↑islet function ↓insulin resistance and sensitivity indexes |
A need for clinical prognosis and islet function | [125] |
| 14 | Huanglian Jiedu decoction | Decoction has Huanglian, Huangqin, Huangbo, and Zhizi mixture | Targets AKT1, IL-6, FOS, VEGFA, CASP3 | Pancreas | ↓ glucose levels, HbA1c | Recommended | [126] |
| 15 | - | Enhance GLUT4, INSR, MAPK1 | - | Improve in fasting glucose level, lipid level, insulin sensitivity index | Recommended | [127] | |
| 16 | Nigella sativa (meta-analysis study) | Thymol, thymohydroquinone, dithymoquinone, nigellone, alpha-hederin, flavonoids, and fatty acids | Antidiabetic, antioxidant, anticancer, hypolipidemic, and anti-inflammatory properties | - | ↓ HbA1c, glucose level, insulin resistance | Recommended | [128] |
| 17 | Metformin with Chinese traditional medicine | - | - | - | Lessened the hyperglycemia, altered gut microflora, improved insulin resistance | Recommended | [129] |
| 18 | GLP-1RAs and oral anti-diabetic drugs (meta-analysis) | - | - | Heart | Improvement in left ventricular diastolic function | Recommended | [130] |
| 19 | GLP-1 RA and SGLT-2i (meta-analysis) | - | - | Pancreas | 5 studies—GLP-1 RA showed decreasing effect in cardiovascular complications, glucose reduction | Recommended | [131] |
| 20 | Metformin and DDP-4 inhibitors | - | AMPK pathway activation, NFK-β, and mTOR suppression | - | Adverse outcome from COVID-19 | Not recommended | [132] |
| 21 | Statin, fibrates, and its combination | - | - | - | ↓lipid levels | Recommended | [133] |
| 22 | Incretin, DDP-4 inhibitors, and GLP-1 RA | - | - | - | ↓ HbA1c | Recommended | [134] |
| 23 | Anti-hyperglycaemic therapies (GLP-1 RA and SGLT2i) | - | - | - | No change in HbA1c levels | Recommended | [135] |
| 24 | Metformin (meta-analysis) | - | - | - |
↓ HbA1c ↓ glucose levels |
Recommended | [136] |
| 25 | Aspirin and statin | - | - | Heart | ↓cardiovascular complications in T2DM | Recommended | [133] |
| 26 | Tofogliflozin with DDP-4 inhibitors | - | - | Pancreas | ↓postprandial glucose | Recommended | [137] |
| 27 | Momordica charantia | - | - | Pancreas |
↑insulin secretion ↓glucose uptake |
Recommended | [138, 139] |
| 28 | Metformin | - | - | Pancreas | ↑epigenetic markers | Recommended for diagnosis | [140] |
| 29 | Chenpi | 5-OH PMFs |
Antidigestive and anti-inflammatory AMPK activation |
↓hepatic steatosis ↓glucose levels |
Recommended | [141] | |
| 30 | Aloe vera | - | - | - | ↓glucose levels | Recommended | [142] |
| 31 | Berberine | - | - | Pancreas |
↓ HbA1c levels ↓ cholesterol and triglyceride ↑insulin secretion |
Recommended | [143] |
| 32 | Pueraria lobata (meta-analysis) | - | - | Pancreas | Anti-diabetic activity | Recommended | [144] |
| 33 | SGLT-2i and GLP-1 RA | - | - | Heart | ↓cardiorenal problems, stroke | Recommended | [145] |
| 34 | - | - | Heart kidney |
↓renal risk No significance in cardio condition |
Recommended | [146, 147] | |
| 35 | Sulfonylureas, DPP-4 inhibitors, meglitinides | - | - | Heart | ↑myocardium infection | Recommended | [148] |
| 36 | Acupuncture with Chinese herbal medicine (meta-analysis) | - |
↓glucose levels ↑ insulin sensitivity and resistance |
Recommended | [149] | ||
| 37 | Ipragliflozin (meta-analysis) | - | - | - |
↑ genital infection No significance in glucose level |
Not recommended | [150] |
| 38 | Ginseng supplementation | - | - | - |
↑fasting glucose, postprandial insulin, and HOMA-IR Lipid levels were altered |
Recommended | [151] |
| 39 |
Shenqi Jiangtang Granules Baihu Jia Renshen Decoction Tianqi Jiangtang Capsule (meta-analysis) |
- | - | Pancreas | ↓hypoglycemia levels | Recommended | [152–154] |
| 40 | Chinese tradition medicine vs. Western medicine (meta-analysis) | - | - | Pancreas | Traditional medicine showed enhanced insulin sensitivity, decreasing body weight, protects from β cells | Recommended | [155] |
| 41 | Cotadutide (meta-analysis) | - | - | Pancreas | ↓glucose levels, HbA1c and body weight | Recommended | [156] |
| 42 | SGD | - | Influences PI3K-Akt, AMPK, and PPAR | Pancreas | ↓ glucose levels and lipid levels ↓ | Recommended | [157] |
| 43 | Sancai powder | - | - | Pancreas | ↓glucose levels, HbA1c, lipid levels | Recommended | [117] |
| 44 | Zhimu-Huangbai herb pair | - | - | Pancreas | ↓glucose levels | Recommended | [158] |
| 45 | Naoxintong capsule | - | Arachidonic acid metabolism, fatty acid β-oxidation, and glycerophospholipid metabolism | Pancreas | ↓hyperlipidemia, hyperglycemia, myocardial infarction, insulin resistance | Recommended | [159, p. 20] |
| 46 | Pioglitazone and canagliflozin | - | - | - | ↓ glucose levels, lipid levels, HbA1c | Recommended | [160] |
| 47 | Canagliflozin | - | - | Kidney | ↑ renal oxygen in T2DM | Recommended | [161] |
| 48 | Pine bark extract | - | - | Pancreas | ↓ HbA1c, VCAM-1, cholesterol levels, UACR | Recommended | [162] |
| 49 | Jinlida granules | - | - | Pancreas | ↓ HbA1c and glucose levels | Recommended | [163] |
iNOS, inducible nitric oxide synthase; IL-1β, interleukin-1 beta; NO, nitric oxide; TNF-α, tumour necrosis factor alpha; HbA1c, glycated haemoglobin; T2DM, type 2 diabetes mellitus; LDL, low density cholesterol; HOMA-β, homeostasis model assessment of β-cell function; AMPK, adenosine 5′ -monophosphate activated protein kinase; PPAR, peroxisome proliferator-activated receptor; SGLT-2, sodium glucose co-transporter-2; SGLT-2i, sodium-glucose cotransporter-2 inhibitors; FBG, fasting blood glucose; GLP-1 RA, glucagon-like peptide 1 receptor agonists; DDP-4, dipeptidyl peptidase-4 inhibitors; NFK-β, nuclear factor kappa B; mTOR, mammalian target of rapamycin; COVID-19, coronavirus disease-19; 5-OH PMFs, 5-demethylated poly methoxy flavones; HOMA-IR, homeostatic model assessment of insulin resistance; SGD, Shengmai-Yin and Ganmai dazao decoction; VCAM-1, vascular cell adhesion molecule 1; UACR, urinary albumin-to-creatinine ratio; PPAR, peroxisome proliferator-activated receptors; P13K-Akt, phosphatidylinositol 3-kinase-protein kinase B; GLUT4, glucose transporter type 4; INSR, insulin receptor; MAPK1, mitogen-activated protein kinase 1; IL-6, interleukin-6; FOS, Fos proto-oncogene; AP-1, transcription factor subunit; VEGFA, vascular endothelial growth factor A; CASP3, caspase 3; Stat1, signal transducer and activator of transcription 1; Ifngr1, interferon gamma receptor 1; STAT3, signal transducer and activator of transcription 3
Table 3.
Therapeutics targeting signalling pathways common in both T2DM and PD
| Therapeutic drug | Mechanism | Model | Dosage | Methods | Result | Conclusion | References |
|---|---|---|---|---|---|---|---|
| Metformin | Amyloid aggregation | Healthy human blood | 200 mmol/L |
• Spectrophotometry • In vitro assays |
• ↑anti-AChE • ↑anti-Aβ aggregation |
Metformin may be regarded as an effective adjuvant to donepezil | [233] |
| Oxidative stress and mitochondrial dysfunction | ASCs | 25 µmol/L |
• Western blot • Immunoblot • SDS-PAGE |
• ↓oxidative stress • ↓mitochondrial dysfunction • ↑adipogenicity • ↑insulin sensitivity |
Metformin may improve impaired adipogenesis and insulin sensitivity | [234] | |
| Microglial activation and chronic inflammation | BV-2 and bEnd3 cells | 50 mg/kg |
• Western blot • Immunohistochemistry • PCR |
• ↓p38 MAPK phosphorylation • ↓pro-inflammatory cytokines • ↓TLR-4 mRNA expression • ↓IL-6 • ↓TNF-α mRNA expression |
Metformin suppresses microglial activation by increasing AMPK phosphorylation | [235] | |
| GLP1R agonists/GIP receptor | Amyloid aggregation | Clonal mouse insulin-secreting cell line (min6) | 25 mM |
• RT-PCR • ELISA • Western blotting |
• ↓hIAPP-induced LC3II/I ratio • ↓hIAPP-induced cleaved caspase-3 • ↓p62 expression |
Exendin-4’s has the potential in the treatment of diabetic ß-cell failure | [236] |
| Microglial activation and chronic inflammation | Mice | 5.0 mg/ml |
• Immunofluorescence • Western blotting • Immunofluorescence staining |
• ↓Iba-1 • ↓IL-1β • ↓TNF-α release • ↓TNC microglial number • ↑allodynia • ↓CGRP and c-fos in the TNC |
GLP-1R agonist liraglutide might represent a new therapeutic approach for treating chronic migraine | [237] | |
| Impaired synaptic plasticity | Mice | _ |
• Open field assessment • Object recognition task • Water maze task |
Glp1r−/− mice • ↓motor activity • Normal exploratory activity and anxiety levels |
GLP-1R is crucial for some forms of memory formation and synaptic plasticity | [238, p. 20] | |
| Microglial activation and chronic inflammation | Mice | 25 nmol/kg | Rotarod and grip strength assessment |
• ↓increased levels of activated microglia and astrocytes ••••↑ GDNF |
GLP-1/GIP dual agonist has the potential to exhibit superior neuroprotective effects | [239] | |
| Impaired synaptic plasticity | Mice | 10 nmol/kg |
• Western blotting • Water maze • Probe test |
• ↓memory loss • ↑synaptic plasticity • ↓amyloid plaques • ↓pro-inflammatory cytokine levels |
GLP-1/GIP dual receptor agonist was more effective at reversing memory loss | [240] | |
| Insulin | Oxidative stress | Cultured cortical neurons | 10 M |
• MTT assay • SDS-PAGE • UV-spectrophotometry |
• ↓oxidative stress • ↓necrotic and apoptotic cell death • ↓ascorbate/Fe2 + -mediated lipid and protein oxidation |
Insulin may help reduce the damage caused by oxidative stress that develops in several neurodegenerative diseases | [31] |
| Impaired synaptic plasticity | Mice | 20 µl |
• Immunohistochemistry and image analysis • Western blotting |
• ↓rapamycin complex 1 (mTORC1) | Intranasal insulin prevents general anaesthesia-induced apoptosis of hippocampal cells and deficits in synaptic plasticity and memory | [241] | |
| Microglial activation and chronic inflammation | Microglial cell line | 125 ng/ml |
• Western blot • Immunocytochemistry • Phagocytosis assay |
• ↑ phagocytic activity • ↓NO • ↓TNF production • ↓ROS |
Insulin has beneficial effects on CNS injury or neurodegenerative conditions | [242] |
PI3K, phosphatidylinositol 3-kinase; Akt, protein kinase B; GSK-3 beta, glycogen synthase kinase-3 beta; FOXO1, Forkhead box protein O1; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; mTOR, mammalian target of rapamycin; DA4-JC, dual GLP-1/GIP receptor agonist; ASCs, adipose-derived stromal cells; HIAPP, human islet amyloid polypeptide; TNC, trigeminal nucleus caudalis; CM, chronic migraine; AMPK, 5' AMP-activated protein kinase; NO, nitric oxide; TNF, tumour necrosis factor; ROS, reactive oxygen species; iNOS, inducible NO synthase; AChE, human acetylcholinesterase; Aβ, amyloid beta; bEnd3, endothelial cells; TLR-4, Toll-like receptor-4; TNF-α, tumour necrosis factor-α; GDNF, glial derived neurotrophic factor
Mechanism of Action of Metformin in T2DM
Since a decade, metformin has been prescribed as a common drug to treat T2DM [174]. Metformin acts as an inhibitor of mitochondrial complex I, which ultimately results in the decrease in ATP synthesis and an increase in AMP levels activating the AMPK pathway [175]. AMPK regulates glucose metabolic pathway and energy balance [176]. As an AMPK activator, metformin regulates all the major metabolic pathways for glucose uptake, carbohydrate metabolism, and lipid metabolism [176]; and it was thought to be a key therapeutic target for obesity, T2DM, ageing, and neurodegeneration [215]. It functions by lowering the insulin-mediated hepatic glucose synthesis levels and by improving insulin sensitivity in T2DM patients [216], it is neuroprotective in human neural stem cells against amyloid-beta-induced mitochondrial dysfunction mediated through the PI3K/Akt pathway [217], and long-term treatment with metformin seems to decrease the risk of cognitive decline in diabetic patients [218] and improve depressive and cognitive performance, changing the glucose metabolism in depressed patients [164]. The inhibition of insulin and insulin growth factor receptor signalling by metformin results in alterations in metabolic balance. Metformin’s antidiabetic actions are primarily brought by the AMPK pathway [177] but it can also occur in an AMPK-independent mechanism [178]. Genes coding for the enzymes involved in glucogenesis are reduced by AMPK through phosphorylation of CREB binding protein (CBP) [179]. Activation of AMPK results in decreased adenosine monophosphate which inhibit fructose-1,6-bisphosphatase-1, a major enzyme in gluconeogenesis [180]. Similarly, metformin causes AMPK activation which involves in reduction of PD pathogenesis [181]. When AMPK deficiency is detected in Drosophila, the characteristics of PD was observed [182]. From the data, a possible mechanism of metformin for its neuroprotective role has also been revealed and it is reported to be a hotspot in linking PD and T2DM. Metformin functions similarly in PD too by inhibiting complex I which then activates the AMPK pathway. Complex I is one of the primary sources of ROS where metformin reduces the ROS generation through reverse electron transfer but will not boost their production [183]. Metformin therapy drastically reduced the amount of cytochrome C released from mitochondria into the cytosol, especially in the aged brain [35]. In the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated PD mice model, metformin exhibits neuroprotective benefits where it enhances mobility and muscular activity [184]. Another way through which ROS is reduced is by NAD(P)H inhibition and activation of AMPK [185]. Experimentally gathered data shows that ROS is the major factor in the death of dopaminergic neurons in PD patients [186]. A drop in ROS levels will result in a significant attenuation of DNA damage [187]. The incidence of cognitive decline in people with T2DM was considerably lowered by metformin therapy [188]. Metformin reduces neuroinflammation by an increase in the antiapoptotic protein Bcl-2 [189]. In addition, metformin mediated activation of AMPK also increases serine/threonine PP2A (protein phosphatase 2A) that dephosphorylates and reduce αSyn levels [190]. As a result, there are numerous molecular processes explaining metformin’s multiple beneficial effects, but there are limited studies conducted on therapeutic potential in PD; hence, more studies need to be elucidated in PD pathogenesis. Hence, from the studies, it is probable that AMPK targeted therapy might lessen the neurodegeneration, in PD where more research is required on therapeutics effects of metformin to confirm its neuroprotective role. The probable mechanism is depicted in Fig. 5.
Fig. 5.
Metformin: anti-diabetic and neuroprotective role. Metformin has a neuroprotective role through the AMPK pathway which is also their anti-diabetic pathway. AMPK is activated through inhibition of mitochondrial complex I and it drastically reduces the ROS levels causing neuronal survival. Metformin enhances autophagy by inhibiting mTORC1 which was phosphorylated by AMPK, anti-apoptosis by increasing anti-apoptotic protein Bcl-2, reduces DNA damage, and neuroinflammation hence accounting for neuronal protection. Metformin reduces the αSyn aggregates by increasing PP2A. AMPK, AMP-activated protein kinase; ROS, reactive oxygen species; mTORC1, mammalian target of rapamycin complex 1; Bcl-2, B-cell lymphoma 2; PP2A, protein phosphatase 2
Glucagon-Like Peptide-1 (GLP-1) Receptor Agonist
The GLP-1 receptor agonists are primarily known to be a group of anti-diabetic medications, and this GLP-1 has an intestinal peptide known as incretin that promotes glucose-dependent insulin secretion whilst inhibiting glucagon release to maintain blood sugar levels [191] and also GLP-1 exhibits potent and strictly glucose-dependent insulinotropic activity through unique GLP-1 receptors in the plasma membrane of pancreatic cells [192]. This inhibits glucagon secretion, which may be as clinically relevant as GLP-1’s insulinotropic action [193]. Furthermore, GLP-1 decreases food intake (most likely by activating GLP-1 receptors in the central nervous system and slows stomach voidance, resulting in lower postprandial glucose excursions and food intake) [192]. Few studies have also discovered that as one progresses from normal glucose tolerance to insulin resistance, plasma GLP-1 concentrations decreases [194, 195]. Also, GLP-1 is expressed in all regions of the brain, including the frontal cortex, hypothalamus, thalamus, hippocampus, cerebellum, and substantia nigra and has a key role in neuronal metabolism and neuroprotective effect in the brain through crossing the blood brain barrier (BBB) [196]. Additionally, GLP-1 exhibits regulatory effects that includes stimulation of cell neogenesis, growth, and differentiation; prevention of cell apoptosis; and boosting cell survival [197]. There are several GLP-1 analogues, particularly exenatide, liraglutide, and lixisenatide, which regulates cellular pathways that protect the neurons, maintain mitochondrial function, prevent apoptosis, and respond to oxidative stress in addition to their effects on glucose homeostasis [198, 199]. GLP-1 receptor agonists exhibits neuroprotective and neurorestorative characteristics in various PD experimental models. Exenatide-treated animal models can halt 6-hydroxydopamine (6-OHDA), MPTP, and LPS-induced dopaminergic degeneration and restore DA imbalance in PD, resulting in significant improvements in behaviour and motor function [200–202]. Longer-acting GLP-1 analogues (liraglutide and lixisenatide) have also shown protective benefits and enhanced motor function in MPTP induced PD mouse model [198]. Taken together, GLP-1 receptor agonists are already a proven fundamentally anti-diabetic medication, and also there is strong evidence that GLP-1 activity has a positive impact on the treatment of PD pathogenesis, as shown in numerous PD models.
Dipeptidyl Peptidase-4 Inhibitors (DPP-4i)
The anti-diabetic medication DPP-4i (gliptins) inhibits DPP-4, a proteolytic enzyme that has shown neuroprotective effects in the brain [203], and in T2DM, the inactivation of GLP-1 in the peripheral circulation is prevented by DPP-4 inhibitors by reducing DPP-4 activity in peripheral plasma thereby prevents T2DM condition and improves glucose metabolism [73]. DPP-4 inhibitors on elderly people with T2DM and mild cognitive impairment enhances glucose control, prevents deterioration of cognitive abilities, and also decreases β-amyloid toxicity and tau hyperphosphorylation in human neural cells [204]. DPP-4 inhibitors might improve synaptic function, lessen the loss of dopaminergic cells, and/or assist the regenerative process in PD by either increasing endogenous levels of GLP-1 and GIP both systemically and in the brain, or by acting directly on them [205]. Data from DPP-4 inhibitors in PD experimental models are inconsistent. In a rotarod test, rats were given saxagliptin before the induction of rotenone, which showed increased striatal DA production and decreased dopaminergic neuronal loss, resulting in improved motor performance and coordination [206]. However, rats given supramaximal doses of sitagliptin (a DPP-4 inhibitor with a significantly longer half-life than saxagliptin) were not protected against MPTP-induced striatal dopaminergic degeneration [207]. Thus, DPP-4 may be useful for T2DM with PD patients in terms of their initial dopamine degradation and in long-term effects it may also play a potential role in non-diabetic PD patients.
Insulin
Insulin is found to be beneficial in treating both T2DM and PD conditions. In general, insulin interacts with the brain in several ways, likely through the complex insulin/IR signalling pathway, including effects on cognition, memory, learning, and synaptic plasticity [208]; and importantly exogenous insulin can be administered nasally to prevent effects on peripheral glucose levels, and it may have benefits for PD patients [57]. At clinically significant levels, insulin enters the brain by passing the BBB, preventing neuronal death by activating protein kinase B, and regulating tau phosphorylation, beta-amyloid precursor protein metabolism, and beta-amyloid clearance in in vivo [209] [210]. Intranasal insulin administration increases mood and memory whilst preserving the volume of brain regions damaged by neuropathology, and it also improves cerebral glucose metabolism which is mediated through PI3K/Akt pathway [211]. Insulin reduced α-synuclein levels in MPP-positive C6 glial cells [212]. Insulin contributes in directing the autophagic process to protect the neurons by enhancing the autophagy of harmful proteins, by suppressing mTORC1 activity, activating Akt survival molecule, and increasing mTORC2 activity [213]. Injection of intranasal insulin was conducted in PD patients which revealed that the insulin-treated patients showed improvements in verbal fluency and motor severity compared to baseline scores at the end of the exposure period, and, more importantly, there were no reports of changes in serum glucose levels or hypoglycaemia [214]. Therefore, as we know that insulin is primarily known to regulate blood sugar levels, and with the evidence provided above, it is clear that insulin, through interactions with the complex insulin/IR signalling network, can help to treat multiple processes associated with the onset of PD.
Sulfonylureas
Sulfonylurea is primarily an antidiabetic drug and there are generations of drugs under the sulfonylurea class. The sulfonylurea glimepiride is linked to altered cell signalling and synaptic membranes and provides several protective advantages for neurons and is used to treat T2DM [220]. Gliclazide is a second-generation sulfonylurea hypoglycaemic medication. It reduces the free radical synthesis or increases their scavenging by lowering oxidative stress, protecting against DNA damage, and boosting antioxidant status, and may also help to regulate the physiological diseases that underlie both T2DM and ageing [221]. Glibenclamide is another generation of sulfonylurea, it is a KATP channel inhibitor where it decreases the amyloid-induced behavioural impairments and anxiety in a rat model, and it may be used as a therapeutic target for neurodegenerative diseases which is mediated through PI3K/Akt pathway [222]. In a rodent PD model, glibenclamide protected dopaminergic neurons by inhibiting inflammasome activation, microglial polarisation, and oxidative stress [223]. In both BV2 microglial cells and mouse PD models, glibenclamide was able to limit microglial activation and reduce excessive neuroinflammation. Furthermore, glibenclamide’s anti-neuroinflammation actions may be caused through mechanisms that regulate the c-Jun N-terminal kinase (JNK) and NF-B signalling pathways [224]. Together, these data suggest a possible role of sulfonylureas in treating both PD and T2DM.
Thiazolidinediones
Thiazolidinediones (TZDs), such as pioglitazone and rosiglitazone, are useful to improve insulin sensitivity in muscle, adipose, and hepatic tissue and reduce systemic insulin resistance. This includes the transfer of extra fatty acids to peripheral fat, and this improves insulin sensitivity by lowering the availability of fatty acids in the liver, muscles, and circulation to treat the T2DM condition [225]. Pioglitazone, the only commercially available TZD, can enter the brain, decrease glial activation, lessen neurodegenerative-related diseases [226], lessen spatial learning impairment, and prevent tau hyperphosphorylation by improving Akt signalling [227]. Various toxin induced PD models, including MPTP [228], LPS [229], 6-OHDA [230], and rotenone [231] showed that the pioglitazone and rosiglitazone have neuroprotective effects that improve behavioural and motor responses. The suppression of pro-inflammatory pathways, as well as the modification of mitochondrial activity and oxidative stress responses, is suggested to be the causes of these effects [232]. Considered together, there is evidence that TZD action has a beneficial impact on the treatment of both T2DM and the aetiology of PD.
Traditional Indian Medicinal Plants to Treat T2DM and PD
Traditionally, moringa seeds containing isothiocyanates reported to delay T2DM rat models [113]. Similarly, biflavonoid compound isolated from Garcinia macrophylla plant suggested to decline blood sugar level in diabetic rats [114]. Anti-diabetic activity by benzimidazole derivatives, albendazole, and lansoprazole in rats showed effective treatment strategy in curing T2DM [115]. In T2DM patients, remogliflozin etabonate reported to be an effective therapeutic option in lowering glucose levels [116]. In a comparative study using metformin sustainable-release tablet and original metformin tablet, metformin sustainable-release had significantly showed the therapeutic efficiency in T2DM than original tablet [164]. In participants with insulin glargine along with oral hypoglycemic therapy received remogliflozin which showed an alternative approach with drastic reduction of T2DM [118]. By using network pharmacology and docking analysis, mechanistic insights of Rhizoma coptidis were explored against T2DM which suggested to show anti-inflammatory effects thus proving to be competent in antidiabetic research [119, 120]. In a systemic review, Morus alba reported to be a potential therapeutic target for T2DM though it lacks more studies on synergistic effects [121]. Apart from drugs, exercise was recommended for glucose management and T2DM control [165]. In a clinical trial using Zingiber officinale as an add-on therapy in T2DM showed improved blood pressure, proper function of diastole, and maintenance of lipid profile [122]. In a 26-week treatment with insulin glargine/lixisenatide in Japanese T2DM patients inadequately controlled over oral antidiabetic drugs [123]. In a rat model, Gegen Qinlian Decoction and berberine showed the decline of blood glucose levels and alteration of gut microbiota [124]. Likewise, Ren et al. (2021) evaluated the effect of Gegen Qinlian Decoction on clinical prognosis and islet function in T2DM. The berberine effects in reducing glucose levels and maintenance of intestinal flora has revealed to be beneficial in T2DM [166]. Similarly, Huanglian Jiedu Decoction and its active compounds showed effective against T2DM [126, 127]. Studies have reviewed on beneficial aspects of curcumin and cinnamon on T2DM [167, 168]. Similarly, Nigella sativa and Taraxacum officinale revealed to show anti-diabetic properties on T2DM [128, 169]. In an open label clinical trial, metformin with Chinese traditional medicines alleviated T2DM along with alterations in gut microbiome [129]. Also, the Chinese traditional medicines effect on gut microflora reduced T2DM [124]. In a meta-analysis study, oral antidiabetic drugs and glucagon-like peptide-1 receptor agonists were assessed in heart failure occurring in T2DM, were the drug liraglutide showed a promising effect in preventing heart failure [130]. In a review, a placebo-controlled outcomes on lessening the glucose levels by glucagon-like peptide 1 receptor agonists, sodium-glucose cotransporter-2 inhibitors, and dipeptidyl peptidase-4 inhibitors were deliberated by Elbelt. (2018) and González-González et al. [132]. Recently, T2DM patients confirmed with COVID-19 infection showed that the usage of metformin and dipeptidyl peptidase-4 inhibitors had minimal contrary outcomes when compared to non-users. In T2DM patients receiving statin, fibrates and its combination showed decrease in lipid levels which shows the necessity in treating diabetes [170]. Therapeutic approaches using incretin and sodium-glucose cotransporter-2 inhibitors have made beneficial effect in renal function in T2DM [134]. Anti-hyperglycaemic drugs showed no significant change in haemoglobin A levels in glycaemic patients [135, p. 202]. In a meta-analysis study, the efficiency of metformin decreased the plasma glucose levels in T2DM patients [136]. The use of statins in T2DM patients has increased drastically since it has reduced the cardiovascular complications and mortality [133]. The add-on therapy using tofogliflozin to dipeptidyl peptidase-4 inhibitors has shown a reduced glucose level in T2DM [137]. In a review, Momordica charantia (bitter gourd) has been recommended as an anti-diabetic drug and more studies on its preparation, dosage, and duration were suggested to prove its efficiency in T2DM [138]. Momordica charantia has shown drastic reduction in glycaemic levels in T2DM patients [139]. Tolerance and intolerance of metformin was investigated in T2DM patients by measuring the epigenetic markers in blood which showed the effectiveness of epigenetics as a diagnostic tool in diabetes [170]. The extract of chenpi, 5-demethylated polymethoxyflavones (5-OH PMFs) reduced the T2DM as well as obesity with an enhancement in lipid profile [141]. In a meta-analysis study, aloe vera was reported to show an effective therapy in glycaemic control in T2DM and in pre-diabetic condition [142]. In diabetic rats, the effects of berberine were investigated to show a reduced lipid and glucose levels with an activation of adenosine 5′-monophosphate-activated protein kinase pathway [143]. In a meta-analysis, the root of Pueraria lobata has evidently showed that T2DM can be treated by increasing insulin levels and reducing glucose absorption [144]. By the use of sodium-glucose cotransporter-2 inhibitors in Japanese T2DM patients, the risk of heart failure and kidney related outcomes was reduced [145]. A comparison study was carried out using sodium-glucose cotransporter-2 inhibitors and glucagon-like peptide-1 receptor agonists in T2DM patients were the sodium-glucose cotransporter-2 inhibitors showed improvement on renal function with or without albuminuria [146]. Similar comparison study showed controlled effects on cardiorenal function in T2DM [147]. In a monotherapy study, metformin was compared with sulfonylureas and meglitinides showed increased risk to cardiovascular complications in T2DM [148]. In a meta-analysis study, acupuncture combined with Chinese herbal treatment showed positive therapeutic openings in T2DM [149]. The treatment using ipragliflozin has shown adverse effects causing genital infections in T2DM patients [150]. In a clinical trial, ginseng showed an efficient action on glucose levels with contradiction in lipid metabolites in T2DM [151]. The Chinese medicines, Shenqi Jiangtang Granules, Baihu Jia Renshen Decoction, and Tianqi Jiangtang Capsule reported as a safe therapy for T2DM [152–154]. In a Bayesian network meta-analysis study, the effective role of Chinese tradition medicine against T2DM was deliberated comparing with western medicines [152]. The cotadutide drug has been a potent drug in treating T2DM which has been effective in decreasing glucose levels, haemoglobin A, and body weight [156]. The Chinese medicine Astragali Radix-Coptis Rhizoma and Allium sativum L. was used to treat T2DM were its mechanistic action using network pharmacology was explained in a study [171, 172]. The combined effect of Shengmai-Yin and Ganmaidazao decoction (SGD) in T2DM showed declined insulin resistance [157]. In a review, the therapeutic effect of Cyclocarya paliurus was explained with mechanistic outcome in treating T2DM [173]. In a randomised clinical study, the Sancai powder showed effective results in T2DM patients in 12 weeks when compared with metformin [141]. In T2DM rats, Zhimu-Huangbai herb pair metabolites and ingredients reported with clinical efficacy in T2DM [158]. In T2DM rats treated with Naoxintong Capsule showed anti-diabetic activity with effective mechanistic strategy [159]. The blend of pioglitazone and canagliflozin treatment showed an improvement from T2DM and metabolites levels [160]. Canagliflozin improved the renal function in T2DM diagnosed patients [161]. The supplementation of pine bark extract was given to T2DM and has control on glycaemic levels and obesity [162]. The effect of Jinlida granules on glycemic levels was reported with a need in improvement in T2DM treatment [163]. From the studies discussed, it is well-known that ground-breaking discoveries have been coming up in treating T2DM. However, the mechanistic insights on previous drugs should be investigated for deliberating clinical assistance to the patients. Previously, it is known that many reviews, systemic reviews, and meta-analysis have been published on T2DM drugs; here, we have deliberated the commonly used western and traditional drugs involved in T2DM with recent outputs in Table 2.
Ongoing Clinical Trials About the Use of T2DM Drugs for PD
The relationship between PD and T2DM is supported by both epidemiological and experimental data. Both diseases are becoming more common worldwide and are strongly linked to ageing. At present, many clinical trials have been applied to repurpose prevailing drugs as well as to address the need for the development of vaccines and drugs against T2DM associated PD. Currently, the established T2DM drugs undergoing clinical trials has the probability to treat PD (Table 4). Additional research is needed to explore the efficiency of T2DM drugs on PD cure.
Table 4.
Ongoing clinical trials on anti-diabetic drugs for PD
| S. no | Study | Drug | Status | Organisation |
|---|---|---|---|---|
| 1 | Exenatide once weekly over 2 years as a potential disease modifying treatment for Parkinson's disease (exenatide-PD3) | Exenatide | Active, not recruiting | University College, London |
| 2 | Effects of exenatide on motor function and the brain | Exenatide | Active, not recruiting | University of Florida |
| 3 | A clinical study of NLY01 in patients with early Parkinson's disease | NLY01 | Active, not recruiting | Neuraly, Inc |
| 4 | SR-exenatide (PT320) to evaluate efficacy and safety in patients with early Parkinson's disease | PT320 | Active, not recruiting | Peptron, Inc |
| 5 | Safety and efficacy of liraglutide in Parkinson's disease | Liraglutide | Active, not recruiting | Cedars-Sinai Medical Centre |
| 6 | GLP1R in Parkinson's disease (GIPD) | Semaglutide | Not yet recruiting | Oslo University Hospital |
| 7 | Study to evaluate the effect of lixisenatide in patients with Parkinson's disease (LixiPark) | Lixisenatide | Active, not recruiting | University Hospital, Toulouse |
Future Perspective and Conclusion
It is now understood that insulin can have a significant impact on how the brain functions. The regulation of neuropathological characteristics of neurodegenerative diseases, such as PD, can be influenced by changes in insulin metabolism and signalling. Numerous in vivo and in vitro studies indicate the connection between T2DM and neurodegeneration and raise the possibility of therapeutic use of several anti-diabetics in the prevention or treatment of conditions like PD. The pathophysiological connections between T2DM and neurodegenerative diseases, as well as how anti-diabetics affect neurodegeneration, are not fully understood, and further studies are necessary to understand these connections and their therapeutic implications.
Acknowledgements
The authors would like to thank the Indian Council of Medical Research (ICMR), Department of Health Research—Grant‐In‐Aid (DHR‐GIA) (grant number: GIA/2019/000276/PRCGIA), Department of Zoology, Central University of Punjab, Bathinda—151401, Punjab, Government of India and Bharathiar University, Coimbatore, to carry out this review work.
Author Contribution
Balachandar Vellingiri, Mahalaxmi Iyer, and S Sri Sabari designed the review. S Sri Sabari, Dhivya Venkatesan, Kiruthika Balasubramani, Mahalaxmi Iyer, Abilash Valsala Gopalakrishnan, Arul N, Senthil Kumar Nachimuthu, and Balachandar Vellingiri wrote the manuscript. Balachandar Vellingiri conceived and supervised the review.
Funding
This work was supported by the Indian Council of Medical Research (ICMR), Department of Health Research—Grant‐In‐Aid (DHR‐GIA) (grant number: GIA/2019/000276/PRCGIA).
Data Availability
Data sharing is not applicable to this article as no new data were created or analysed in this study.
Declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All authors provide consent for publication.
Conflicts of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Venkatesan D, et al. Genotypic-phenotypic analysis, metabolic profiling and clinical correlations in Parkinson’s disease patients from Tamil Nadu population, India. J Mol Neurosci. 2022;72(8):1724–1737. doi: 10.1007/s12031-022-02028-4. [DOI] [PubMed] [Google Scholar]
- 2.Venkatesan D, Iyer M, Krishnan P, Wilson RS, Vellingiri B. A late-onset Parkinson’s disease in tribes in India – a case report. Brain Disord. 2021;3:100015. doi: 10.1016/j.dscb.2021.100015. [DOI] [Google Scholar]
- 3.Venkatesan D, Iyer M, Wilson R, Lakshmipathy G, Vellingiri B. The association between multiple risk factors, clinical correlations and molecular insights in Parkinson’s disease patients from Tamil Nadu population, India. Neurosci Lett. 2021;755:135903. doi: 10.1016/j.neulet.2021.135903. [DOI] [PubMed] [Google Scholar]
- 4.M. Iyer et al., “Role of RhoA-ROCK signaling in Parkinson’s disease,” European Journal of Pharmacology, vol. 894, p. 173815, Mar. 2021, 10.1016/j.ejphar.2020.173815. [DOI] [PubMed]
- 5.B. Vellingiri et al (2022) “Influence of heavy metals in Parkinson’s disease: an overview,” J Neurol10.1007/s00415-022-11282-w [DOI] [PubMed]
- 6.Vellingiri B, et al. Role of heavy metals (copper (Cu), arsenic (As), cadmium (Cd), iron (Fe) and lithium (Li)) induced neurotoxicity. Chemosphere. 2022;301:134625. doi: 10.1016/j.chemosphere.2022.134625. [DOI] [PubMed] [Google Scholar]
- 7.Vellingiri B, et al. Neurotoxicity of pesticides – a link to neurodegeneration. Ecotoxicol Environ Saf. 2022;243:113972. doi: 10.1016/j.ecoenv.2022.113972. [DOI] [PubMed] [Google Scholar]
- 8.Potashkin J, Huang X, Becker C, Chen H, Foltynie T, Marras C. Understanding the links between cardiovascular disease and Parkinson’s disease. Mov Disord. 2020;35(1):55–74. doi: 10.1002/mds.27836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ejma M, et al. The links between Parkinson’s disease and cancer. Biomed. 2020;8(10):416. doi: 10.3390/biomedicines8100416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fasano A, Visanji NP, Liu LWC, Lang AE, Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2015;14(6):625–639. doi: 10.1016/S1474-4422(15)00007-1. [DOI] [PubMed] [Google Scholar]
- 11.Hu G, Jousilahti P, Bidel S, Antikainen R, Tuomilehto J. Type 2 diabetes and the risk of Parkinson’s disease. Diabetes Care. 2007;30(4):842–847. doi: 10.2337/dc06-2011. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Z, Zhou R, Zhang Z, Li K. The association between vitamin D status, vitamin D supplementation, sunlight exposure, and Parkinson’s disease: a systematic review and meta-analysis. Med Sci Monit. 2019;25:666–674. doi: 10.12659/MSM.912840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Müller T, et al. Peripheral neuropathy in Parkinson’s disease: levodopa exposure and implications for duodenal delivery. Parkinsonism Relat Disord. 2013;19(5):501–507. doi: 10.1016/j.parkreldis.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Kim YE, Lee W, Yun JY, Yang HJ, Kim H-J, Jeon BS. Musculoskeletal problems in Parkinson’s disease: neglected issues. Parkinsonism Relat Disord. 2013;19(7):666–669. doi: 10.1016/j.parkreldis.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103(2):137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Pagano G, et al. Diabetes mellitus and Parkinson disease. Neurol. 2018;90(19):e1654–e1662. doi: 10.1212/WNL.0000000000005475. [DOI] [PubMed] [Google Scholar]
- 17.Adeghate E, Schattner P, Dunn E. An update on the etiology and epidemiology of diabetes mellitus. Ann N Y Acad Sci. 2006;1084(1):1–29. doi: 10.1196/annals.1372.029. [DOI] [PubMed] [Google Scholar]
- 18.Chiu S-L, Cline HT. Insulin receptor signaling in the development of neuronal structure and function. Neural Dev. 2010;5(1):7. doi: 10.1186/1749-8104-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biessels GJ, Strachan MWJ, Visseren FLJ, Kappelle LJ, Whitmer RA. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: towards targeted interventions. Lancet Diabetes Endocrinol. 2014;2(3):246–255. doi: 10.1016/S2213-8587(13)70088-3. [DOI] [PubMed] [Google Scholar]
- 20.White MF. Insulin signaling in health and disease. Sci. 2003;302(5651):1710–1711. doi: 10.1126/science.1092952. [DOI] [PubMed] [Google Scholar]
- 21.Obasse I, Taylor M, Fullwood NJ, Allsop D. Development of proteolytically stable N-methylated peptide inhibitors of aggregation of the amylin peptide implicated in type 2 diabetes. Interface Focus. 2017;7(6):20160127. doi: 10.1098/rsfs.2016.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vijay A, Ranganathan P, Vellingiri B. A survey to validate the traditional Siddha perception of diabetes mellitus. J Public Health. 2019;27(5):581–590. doi: 10.1007/s10389-018-0980-y. [DOI] [Google Scholar]
- 23.Poewe W, et al. Parkinson disease. Nat Rev Dis Primers. 2017;3(1):17013. doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Araujo G, et al. Low alpha-synuclein levels in the blood are associated with insulin resistance. Sci Rep. 2015;5(1):12081. doi: 10.1038/srep12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaikaran ETAS, Nilsson MR, Clark A. Pancreatic beta-cell granule peptides form heteromolecular complexes which inhibit islet amyloid polypeptide fibril formation. Biochem J. 2004;377(3):709–716. doi: 10.1042/bj20030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oueslati A, Ximerakis M, Vekrellis K. Protein transmission, seeding and degradation: key steps for α-synuclein prion-like propagation. Exp Neurobiol. 2014;23(4):324–336. doi: 10.5607/en.2014.23.4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horvath I, Wittung-Stafshede P. Cross-talk between amyloidogenic proteins in type-2 diabetes and Parkinson’s disease. Proc Natl Acad Sci USA. 2016;113(44):12473–12477. doi: 10.1073/pnas.1610371113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan MH, Wang X, Zhu X. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radical Biol Med. 2013;62:90–101. doi: 10.1016/j.freeradbiomed.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong C-T, et al. Insulin resistance promotes Parkinson’s disease through aberrant expression of α-synuclein, mitochondrial dysfunction, and deregulation of the polo-like kinase 2 signaling. Cells. 2020;9(3):740. doi: 10.3390/cells9030740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleinridders A, et al. Insulin resistance in brain alters dopamine turnover and causes behavioral disorders. Proc Natl Acad Sci USA. 2015;112(11):3463–3468. doi: 10.1073/pnas.1500877112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duarte AI, Santos MS, Seiça R, de Oliveira CR. Insulin affects synaptosomal GABA and glutamate transport under oxidative stress conditions. Brain Res. 2003;977(1):23–30. doi: 10.1016/S0006-8993(03)02679-9. [DOI] [PubMed] [Google Scholar]
- 32.Joers V, Tansey MG, Mulas G, Carta AR. Microglial phenotypes in Parkinson’s disease and animal models of the disease. Prog Neurobiol. 2017;155:57–75. doi: 10.1016/j.pneurobio.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maldonado-Ruiz R, Montalvo-Martínez L, Fuentes-Mera L, Camacho A. Microglia activation due to obesity programs metabolic failure leading to type two diabetes. Nutr & Diabetes. 2017;7(3):e254–e254. doi: 10.1038/nutd.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bosco D, et al. Dementia is associated with insulin resistance in patients with Parkinson’s disease. J Neurol Sci. 2012;315(1–2):39–43. doi: 10.1016/j.jns.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Chung SJ, et al. Detrimental effect of type 2 diabetes mellitus in a large case series of Parkinson’s disease. Parkinsonism Relat Disord. 2019;64:54–59. doi: 10.1016/j.parkreldis.2018.08.023. [DOI] [PubMed] [Google Scholar]
- 36.Cereda E, Barichella M, Cassani E, Caccialanza R, Pezzoli G. Clinical features of Parkinson disease when onset of diabetes came first: a case-control study. Neurol. 2012;78(19):1507–1511. doi: 10.1212/WNL.0b013e3182553cc9. [DOI] [PubMed] [Google Scholar]
- 37.Cereda E, et al. Diabetes and risk of Parkinson’s disease. Diabetes Care. 2011;34(12):2614–2623. doi: 10.2337/dc11-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Driver JA, Smith A, Buring JE, Gaziano JM, Kurth T, Logroscino G. Prospective cohort study of type 2 diabetes and the risk of Parkinson’s disease. Diabetes Care. 2008;31(10):2003–2005. doi: 10.2337/dc08-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Pablo-Fernandez E, Sierra-Hidalgo F, Benito-León J, Bermejo-Pareja F. Association between Parkinson’s disease and diabetes: data from NEDICES study. Acta Neurol Scand. 2017;136(6):732–736. doi: 10.1111/ane.12793. [DOI] [PubMed] [Google Scholar]
- 40.De Pablo-Fernandez E, Goldacre R, Pakpoor J, Noyce AJ, Warner TT. Association between diabetes and subsequent Parkinson disease: a record-linkage cohort study. Neurol. 2018;91(2):e139–e142. doi: 10.1212/WNL.0000000000005771. [DOI] [PubMed] [Google Scholar]
- 41.Palacios N, et al. Obesity, diabetes, and risk of Parkinson’s disease: obesity, diabetes, and risk of Parkinson’s disease. Mov Disord. 2011;26(12):2253–2259. doi: 10.1002/mds.23855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ong M, et al. Influence of diabetes mellitus on longitudinal atrophy and cognition in Parkinson’s disease. J Neurol Sci. 2017;377:122–126. doi: 10.1016/j.jns.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 43.Sun Y, Chang Y-H, Chen H-F, Su Y-H, Su H-F, Li C-Y. Risk of Parkinson disease onset in patients with diabetes. Diabetes Care. 2012;35(5):1047–1049. doi: 10.2337/dc11-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yue X, Li H, Yan H, Zhang P, Chang L, Li T. Risk of Parkinson disease in diabetes mellitus: an updated meta-analysis of population-based cohort studies. Medicine. 2016;95(18):e3549. doi: 10.1097/MD.0000000000003549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu L, Fu D, Li H, Liu A, Li J, Zheng G. Diabetes and risk of Parkinson’s disease: an updated meta-analysis of case-control studies. PLoS ONE. 2014;9(1):e85781. doi: 10.1371/journal.pone.0085781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu Q, et al. Diabetes and risk of Parkinson’s disease. Diabetes Care. 2011;34(4):910–915. doi: 10.2337/dc10-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marques RG, Fontaine MJ, Rogers J. C-peptide: much more than a byproduct of insulin biosynthesis. Pancreas. 2004;29(3):231–238. doi: 10.1097/00006676-200410000-00009. [DOI] [PubMed] [Google Scholar]
- 48.Jiménez JL, Nettleton EJ, Bouchard M, Robinson CV, Dobson CM, Saibil HR. The protofilament structure of insulin amyloid fibrils. Proc Natl Acad Sci USA. 2002;99(14):9196–9201. doi: 10.1073/pnas.142459399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joshi SR, Parikh RM, Das AK. Insulin–history, biochemistry, physiology and pharmacology. J Assoc Physicians India. 2007;55(Suppl):19–25. [PubMed] [Google Scholar]
- 50.Banks WA, Kastin AJ. Differential permeability of the blood–brain barrier to two pancreatic peptides: insulin and amylin. Peptides. 1998;19(5):883–889. doi: 10.1016/S0196-9781(98)00018-7. [DOI] [PubMed] [Google Scholar]
- 51.Agrawal R, Reno CM, Sharma S, Christensen C, Huang Y, Fisher SJ. Insulin action in the brain regulates both central and peripheral functions. Am J Physiol-Endocrinol Metab. 2021;321(1):E156–E163. doi: 10.1152/ajpendo.00642.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gray SM, Barrett EJ. Insulin transport into the brain. Am J Physiol Cell Physiol. 2018;315(2):C125–C136. doi: 10.1152/ajpcell.00240.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Apostolatos A, et al. Insulin promotes neuronal survival via the alternatively spliced protein kinase CδII isoform. J Biol Chem. 2012;287(12):9299–9310. doi: 10.1074/jbc.M111.313080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reno CM, et al. Antecedent glycemic control reduces severe hypoglycemia-induced neuronal damage in diabetic rats. Am J Physiol-Endocrinol Metab. 2013;304(12):E1331–E1337. doi: 10.1152/ajpendo.00084.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rensink AAM, et al. Insulin inhibits amyloid β-induced cell death in cultured human brain pericytes. Neurobiol Aging. 2004;25(1):93–103. doi: 10.1016/S0197-4580(03)00039-3. [DOI] [PubMed] [Google Scholar]
- 56.Soto M, Cai W, Konishi M, Kahn CR. Insulin signaling in the hippocampus and amygdala regulates metabolism and neurobehavior. Proc Natl Acad Sci USA. 2019;116(13):6379–6384. doi: 10.1073/pnas.1817391116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hölscher C. Insulin signaling impairment in the brain as a risk factor in Alzheimer’s disease. Front Aging Neurosci. 2019;11:88. doi: 10.3389/fnagi.2019.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kleinridders A, Ferris HA, Cai W, Kahn CR. Insulin action in brain regulates systemic metabolism and brain function. Diabetes. 2014;63(7):2232–2243. doi: 10.2337/db14-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stockhorst U, Defries D, Steingrueber H, Scherbaum W. Insulin and the CNS: effects on food intake, memory, and endocrine parameters and the role of intranasal insulin administration in humans. Physiol Behav. 2004;83(1):47–54. doi: 10.1016/S0031-9384(04)00348-8. [DOI] [PubMed] [Google Scholar]
- 60.Yip CC. The insulin-binding domain of insulin receptor is encoded by exon 2 and exon 3. J Cell Biochem. 1992;48(1):19–25. doi: 10.1002/jcb.240480105. [DOI] [PubMed] [Google Scholar]
- 61.Gabbouj S, et al. Altered insulin signaling in Alzheimer’s disease brain – special emphasis on PI3K-Akt pathway. Front Neurosci. 2019;13:629. doi: 10.3389/fnins.2019.00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim B, Feldman EL. Insulin resistance in the nervous system. Trends Endocrinol Metab. 2012;23(3):133–141. doi: 10.1016/j.tem.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Risso G, Blaustein M, Pozzi B, Mammi P, Srebrow A. Akt/PKB: one kinase, many modifications. Biochem J. 2015;468(2):203–214. doi: 10.1042/BJ20150041. [DOI] [PubMed] [Google Scholar]
- 64.Evans RM, Hui S, Perkins A, Lahiri DK, Poirier J, Farlow MR. Cholesterol and APOE genotype interact to influence Alzheimer disease progression. Neurol. 2004;62(10):1869–1871. doi: 10.1212/01.WNL.0000125323.15458.3F. [DOI] [PubMed] [Google Scholar]
- 65.Yao W-D, et al. Identification of PSD-95 as a regulator of dopamine-mediated synaptic and behavioral plasticity. Neuron. 2004;41(4):625–638. doi: 10.1016/S0896-6273(04)00048-0. [DOI] [PubMed] [Google Scholar]
- 66.Jeanneteau F, Deinhardt K. Fine-tuning MAPK signalling in the brain: the role of MKP-1. Commun Integr Biol. 2011;4(3):281–283. doi: 10.4161/cib.4.3.14766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahmed N, Thornalley PJ. Advanced glycation endproducts: what is their relevance to diabetic complications? Diabetes Obes Metab. 2007;9(3):233–245. doi: 10.1111/j.1463-1326.2006.00595.x. [DOI] [PubMed] [Google Scholar]
- 68.Jaganjac M, Tirosh O, Cohen G, Sasson S, Zarkovic N. Reactive aldehydes – second messengers of free radicals in diabetes mellitus. Free Radic Res. 2013;47(sup1):39–48. doi: 10.3109/10715762.2013.789136. [DOI] [PubMed] [Google Scholar]
- 69.Lu J, Randell E, Han Y, Adeli K, Krahn J, Meng QH. Increased plasma methylglyoxal level, inflammation, and vascular endothelial dysfunction in diabetic nephropathy. Clin Biochem. 2011;44(4):307–311. doi: 10.1016/j.clinbiochem.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 70.McLellan AC, Thornalley PJ, Benn J, Sonksen PH. Glyoxalase system in clinical diabetes mellitus and correlation with diabetic complications. Clin Sci. 1994;87(1):21–29. doi: 10.1042/cs0870021. [DOI] [PubMed] [Google Scholar]
- 71.Rabbani N. Methylglyoxal and glyoxalase 1—a metabolic stress pathway-linking hyperglycemia to the unfolded protein response and vascular complications of diabetes. Clin Sci. 2022;136(11):819–824. doi: 10.1042/CS20220099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sousa Silva M, Gomes RA, Ferreira AEN, Ponces Freire A, Cordeiro C. The glyoxalase pathway: the first hundred years… and beyond. Biochem J. 2013;453(1):1–15. doi: 10.1042/BJ20121743. [DOI] [PubMed] [Google Scholar]
- 73.Thornberry NA, Gallwitz B. Mechanism of action of inhibitors of dipeptidyl-peptidase-4 (DPP-4) Best Pract Res Clin Endocrinol Metab. 2009;23(4):479–486. doi: 10.1016/j.beem.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 74.Kazachkov M, Yu P. A novel HPLC procedure for detection and quantification of aminoacetone, a precursor of methylglyoxal, in biological samples. J Chromatogr B. 2005;824(1–2):116–122. doi: 10.1016/j.jchromb.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 75.Reichard GA, Skutches CL, Hoeldtke RD, Owen OE. Acetone metabolism in humans during diabetic ketoacidosis. Diabetes. 1986;35(6):668–674. doi: 10.2337/diab.35.6.668. [DOI] [PubMed] [Google Scholar]
- 76.Nigro C, et al. Dicarbonyl stress at the crossroads of healthy and unhealthy aging. Cells. 2019;8(7):749. doi: 10.3390/cells8070749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rabbani N, Thornalley PJ. Advanced glycation end products in the pathogenesis of chronic kidney disease. Kidney Int. 2018;93(4):803–813. doi: 10.1016/j.kint.2017.11.034. [DOI] [PubMed] [Google Scholar]
- 78.Thornalley PJ. Dicarbonyl intermediates in the Maillard reaction. Ann N Y Acad Sci. 2005;1043(1):111–117. doi: 10.1196/annals.1333.014. [DOI] [PubMed] [Google Scholar]
- 79.I. Allaman, M. Bélanger, and P. J. Magistretti (2015) “Methylglyoxal, the dark side of glycolysis,” Front. Neurosci., 9, 10.3389/fnins.2015.00023. [DOI] [PMC free article] [PubMed]
- 80.Gilbert RP, Brandt RB. Spectrophotometric determination of methyl glyoxal with 2,4-dinitrophenylhydrazine. Anal Chem. 1975;47(14):2418–2422. doi: 10.1021/ac60364a003. [DOI] [PubMed] [Google Scholar]
- 81.Aviles-Olmos I, Limousin P, Lees A, Foltynie T. Parkinson’s disease, insulin resistance and novel agents of neuroprotection. Brain. 2013;136(2):374–384. doi: 10.1093/brain/aws009. [DOI] [PubMed] [Google Scholar]
- 82.Thornalley PJ, Langborg A, Minhas HS. Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J. 1999;344(1):109–116. doi: 10.1042/bj3440109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wan OW, Chung KKK. The role of alpha-synuclein oligomerization and aggregation in cellular and animal models of Parkinson’s disease. PLoS ONE. 2012;7(6):e38545. doi: 10.1371/journal.pone.0038545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kurz A, et al. Alpha-synuclein deficiency leads to increased glyoxalase I expression and glycation stress. Cell Mol Life Sci. 2011;68(4):721–733. doi: 10.1007/s00018-010-0483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang C, et al. Parkin, a p53 target gene, mediates the role of p53 in glucose metabolism and the Warburg effect. Proc Natl Acad Sci USA. 2011;108(39):16259–16264. doi: 10.1073/pnas.1113884108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ferrer I, Martinez A, Blanco R, Dalfó E, Carmona M. Neuropathology of sporadic Parkinson disease before the appearance of parkinsonism: preclinical Parkinson disease. J Neural Transm. 2011;118(5):821–839. doi: 10.1007/s00702-010-0482-8. [DOI] [PubMed] [Google Scholar]
- 87.Gómez A, Ferrer I. Increased oxidation of certain glycolysis and energy metabolism enzymes in the frontal cortex in Lewy body diseases. J Neurosci Res. 2009;87(4):1002–1013. doi: 10.1002/jnr.21904. [DOI] [PubMed] [Google Scholar]
- 88.Sandyk R. The relationship between diabetes mellitus and Parkinson’s disease. Int J Neurosci. 1993;69(1–4):125–130. doi: 10.3109/00207459309003322. [DOI] [PubMed] [Google Scholar]
- 89.Marques A, et al. Glucose dysregulation in Parkinson’s disease: too much glucose or not enough insulin? Parkinsonism Relat Disord. 2018;55:122–127. doi: 10.1016/j.parkreldis.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 90.Athauda D, Foltynie T. Insulin resistance and Parkinson’s disease: a new target for disease modification? Prog Neurobiol. 2016;145–146:98–120. doi: 10.1016/j.pneurobio.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 91.Unger J, Livingston J, Moss A. Insulin receptors in the central nervous system: localization, signalling mechanisms and functional aspects. Prog Neurobiol. 1991;36(5):343–362. doi: 10.1016/0301-0082(91)90015-S. [DOI] [PubMed] [Google Scholar]
- 92.Baskin DG, Porte D, Guest K, Dorsa DM. Regional concentrations of insulin in the rat brain*. Endocrinol. 1983;112(3):898–903. doi: 10.1210/endo-112-3-898. [DOI] [PubMed] [Google Scholar]
- 93.Kuwabara T, et al. Insulin biosynthesis in neuronal progenitors derived from adult hippocampus and the olfactory bulb. EMBO Mol Med. 2011;3(12):742–754. doi: 10.1002/emmm.201100177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Duarte AI, Moreira PI, Oliveira CR. Insulin in central nervous system: more than just a peripheral hormone. J Aging Res. 2012;2012:1–21. doi: 10.1155/2012/384017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Morris JK, et al. Insulin resistance and gray matter volume in neurodegenerative disease. Neurosci. 2014;270:139–147. doi: 10.1016/j.neuroscience.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tong M, Dong M, de la Monte SM. Brain insulin-like growth factor and neurotrophin resistance in Parkinson’s disease and dementia with Lewy bodies: potential role of manganese neurotoxicity. JAD. 2009;16(3):585–599. doi: 10.3233/JAD-2009-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kawahito S, Kitahata H, Oshita S. Problems associated with glucose toxicity: role of hyperglycemia-induced oxidative stress. WJG. 2009;15(33):4137. doi: 10.3748/wjg.15.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Eriksson JW. Metabolic stress in insulin’s target cells leads to ROS accumulation - a hypothetical common pathway causing insulin resistance. FEBS Lett. 2007;581(19):3734–3742. doi: 10.1016/j.febslet.2007.06.044. [DOI] [PubMed] [Google Scholar]
- 99.Brownlee M. The pathobiology of diabetic complications. Diabetes. 2005;54(6):1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 100.Jenner P, Olanow CW. The pathogenesis of cell death in Parkinson’s disease. Neurol. 2006;66(10):24–36. doi: 10.1212/WNL.66.10_suppl_4.S24. [DOI] [PubMed] [Google Scholar]
- 101.Liu X, Yamada N, Maruyama W, Osawa T. Formation of dopamine adducts derived from brain polyunsaturated fatty acids. J Biol Chem. 2008;283(50):34887–34895. doi: 10.1074/jbc.M805682200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fan H, et al. Methionine sulfoxide reductase A negatively controls microglia-mediated neuroinflammation via inhibiting ROS/MAPKs/NF-κB signaling pathways through a catalytic antioxidant function. Antioxid Redox Signal. 2015;22(10):832–847. doi: 10.1089/ars.2014.6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang J, Zheng Y, Luo Y, Du Y, Zhang X, Fu J. Curcumin inhibits LPS-induced neuroinflammation by promoting microglial M2 polarization via TREM2/ TLR4/ NF-κB pathways in BV2 cells. Mol Immunol. 2019;116:29–37. doi: 10.1016/j.molimm.2019.09.020. [DOI] [PubMed] [Google Scholar]
- 104.Jayaraj RL, Azimullah S, Beiram R, Jalal FY, Rosenberg GA. Neuroinflammation: friend and foe for ischemic stroke. J Neuroinflammation. 2019;16(1):142. doi: 10.1186/s12974-019-1516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bhuvanendran S, Bakar SNS, Kumari Y, Othman I, Mohd FS, Hassan Z. Embelin improves the spatial memory and hippocampal long-term potentiation in a rat model of chronic cerebral hypoperfusion. Sci Rep. 2019;9(1):14507. doi: 10.1038/s41598-019-50954-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rajchgot T, et al. Neurons and microglia; a sickly-sweet duo in diabetic pain neuropathy. Front Neurosci. 2019;13:25. doi: 10.3389/fnins.2019.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu M, Gao L, Zhang N. Berberine reduces neuroglia activation and inflammation in streptozotocin-induced diabetic mice. Int J Immunopathol Pharmacol. 2019;33:205873841986637. doi: 10.1177/2058738419866379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sivitz WI, Yorek MA. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxid Redox Signal. 2010;12(4):537–577. doi: 10.1089/ars.2009.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ji R-R, Suter MR. p38 MAPK, microglial signaling, and neuropathic pain. Mol Pain. 2007;3:1744-8069-3-33. doi: 10.1186/1744-8069-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dukic-Stefanovic S, Gasic-Milenkovic J, Deuther-Conrad W, Münch G. Signal transduction pathways in mouse microglia N-11 cells activated by advanced glycation endproducts (AGEs): AGE signalling pathways in N-11 cells. J Neurochem. 2003;87(1):44–55. doi: 10.1046/j.1471-4159.2003.01988.x. [DOI] [PubMed] [Google Scholar]
- 111.Imamura K, Hishikawa N, Sawada M, Nagatsu T, Yoshida M, Hashizume Y. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol. 2003;106(6):518–526. doi: 10.1007/s00401-003-0766-2. [DOI] [PubMed] [Google Scholar]
- 112.McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38(8):1285–1285. doi: 10.1212/WNL.38.8.1285. [DOI] [PubMed] [Google Scholar]
- 113.Waterman C, et al. Moringa isothiocyanate-rich seed extract delays the onset of diabetes in UC Davis type-2 diabetes mellitus rats. Sci Rep. 2020;10(1):8861. doi: 10.1038/s41598-020-65722-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cane HPCA, et al. Macrophylloflavone: a new biflavonoid from Garcinia macrophylla mart. (Clusiaceae) for antibacterial, antioxidant, and anti-type 2 diabetes mellitus activities. Sci World J. 2020;2020:1–14. doi: 10.1155/2020/2983129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Di̇K B, Coşkun D, Bahçi̇Van E, Üney K. Potential antidiabetic activity of benzimidazole derivative albendazole and lansoprazole drugs in different doses in experimental type 2 diabetic rats. Turk J Med Sci. 2021;51(3):1578–1585. doi: 10.3906/sag-2004-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dharmalingam M, et al. Efficacy and safety of remogliflozin etabonate, a new sodium glucose co-transporter-2 inhibitor, in patients with type 2 diabetes mellitus: a 24-week, randomized, double-blind, active-controlled trial. Drugs. 2020;80(6):587–600. doi: 10.1007/s40265-020-01285-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Guo L, et al. Comparison of clinical efficacy and safety of metformin sustained-release tablet (II) (Dulening) and metformin tablet (Glucophage) in treatment of type 2 diabetes mellitus. Front Endocrinol. 2021;12:712200. doi: 10.3389/fendo.2021.712200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shankar A. An efficacy and safety study of remogliflozin in obese Indian type 2 diabetes mellitus patients who were inadequately controlled on insulin glargine plus other oral hypoglycemic agents. CDR. 2021;17(7):e122120189341. doi: 10.2174/1573399817666201222102520. [DOI] [PubMed] [Google Scholar]
- 119.An W, et al. Mechanisms of Rhizoma Coptidis against type 2 diabetes mellitus explored by network pharmacology combined with molecular docking and experimental validation. Sci Rep. 2021;11(1):20849. doi: 10.1038/s41598-021-00293-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sun Y, et al. Active ingredients and mechanism of action of Rhizoma Coptidis against type 2 diabetes based on network-pharmacology and bioinformatics. Curr Med Sci. 2020;40(2):257–264. doi: 10.1007/s11596-020-2182-4. [DOI] [PubMed] [Google Scholar]
- 121.Morales Ramos JG, Esteves Pairazamán AT, Mocarro Willis MES, Collantes Santisteban S, Caldas Herrera E. Medicinal properties of Morus alba for the control of type 2 diabetes mellitus: a systematic review. f1000Res. 2021;10:1022. doi: 10.12688/f1000research.55573.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nganou-Gnindjio CN, Ngati Nyonga D, Wafeu GS, Nga EN, Sobngwi E. Cardiometabolic effects of ZingiberOfficinale Roscoe extracts in type 2 diabetic Cameroonians patients after six weeks of add-on therapy : a single clinical-arm trial. Annales de Cardiologie et d’Angéiologie. 2022;71(3):160–165. doi: 10.1016/j.ancard.2021.09.010. [DOI] [PubMed] [Google Scholar]
- 123.Terauchi Y, Nakama T, Spranger R, Amano A, Inoue T, Niemoeller E. Efficacy and safety of insulin glargine/lixisenatide fixed-ratio combination ( iGlarLixi 1:1) in Japanese patients with type 2 diabetes mellitus inadequately controlled on oral antidiabetic drugs: a randomized, 26-week, open-label, multicentre study: the LixiLan JP-O2 randomized clinical trial. Diabetes Obes Metab. 2020;22(S4):14–23. doi: 10.1111/dom.14036. [DOI] [PubMed] [Google Scholar]
- 124.Xu X, et al. Antidiabetic effects of Gegen Qinlian decoction via the gut microbiota are attributable to its key ingredient berberine. Genomics Proteomics Bioinformatics. 2020;18(6):721–736. doi: 10.1016/j.gpb.2019.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ren L, Cheng Y, Qin F. Herbal formula Gegen-Qinlian decoction for type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Evid-Based Complement Altern Med. 2020;2020:1–11. doi: 10.1155/2020/3907920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yin B, Bi Y-M, Fan G-J, Xia Y-Q. Molecular mechanism of the effect of Huanglian Jiedu decoction on type 2 diabetes mellitus based on network pharmacology and molecular docking. J Diabetes Res. 2020;2020:1–24. doi: 10.1155/2020/5273914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pan L, Li Z, Wang Y, Zhang B, Liu G, Liu J. Network pharmacology and metabolomics study on the intervention of traditional Chinese medicine Huanglian decoction in rats with type 2 diabetes mellitus. J Ethnopharmacol. 2020;258:112842. doi: 10.1016/j.jep.2020.112842. [DOI] [PubMed] [Google Scholar]
- 128.Hamdan A, Haji Idrus R, Mokhtar MH. Effects of Nigella sativa on type-2 diabetes mellitus: a systematic review. IJERPH. 2019;16(24):4911. doi: 10.3390/ijerph16244911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tong X, et al. Structural alteration of gut microbiota during the amelioration of human type 2 diabetes with hyperlipidemia by metformin and a traditional Chinese herbal formula: a multicenter, randomized, open label clinical trial. mBio. 2018;9(3):02392–17. doi: 10.1128/mBio.02392-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ida S, et al. Effects of oral antidiabetic drugs and glucagon-like peptide-1 receptor agonists on left ventricular diastolic function in patients with type 2 diabetes mellitus: a systematic review and network meta-analysis. Heart Fail Rev. 2021;26(5):1151–1158. doi: 10.1007/s10741-020-09936-w. [DOI] [PubMed] [Google Scholar]
- 131.Doumas M, Imprialos Κ, Stavropoulos K, Reklou A, Sachinidis A, Athyros VG. Combination of SGLT-2 inhibitors and GLP-1 receptor agonists: potential benefits in surrogate and hard endpoints. CPD. 2018;24(17):1879–1886. doi: 10.2174/1381612824666180604113653. [DOI] [PubMed] [Google Scholar]
- 132.Luk AOY, et al. Glucose-lowering drugs and outcome from COVID-19 among patients with type 2 diabetes mellitus: a population-wide analysis in Hong Kong. BMJ Open. 2021;11(10):052310. doi: 10.1136/bmjopen-2021-052310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Machado-Duque ME, Garcia DA, Emura-Vélez MH, Gaviria-Mendoza A, Machado-Alba JE. Prevalence of the use of aspirin and statins for preventing cardiovascular events in the Colombian population with type 2 diabetes mellitus: comparison of 2008 and 2018. J Prim Care Community Health. 2021;12:215013272110070. doi: 10.1177/21501327211007015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mann JFE, Muskiet MHA. Incretin-based drugs and the kidney in type 2 diabetes: choosing between DPP-4 inhibitors and GLP-1 receptor agonists. Kidney Int. 2021;99(2):314–318. doi: 10.1016/j.kint.2020.08.036. [DOI] [PubMed] [Google Scholar]
- 135.Xiang AS, et al. Trends in glycaemic control and drug use in males and females with type 2 diabetes: Results of the Australian National Diabetes Audit from 2013 to 2019. Diabetes Obes Metab. 2021;23(12):2603–2613. doi: 10.1111/dom.14506. [DOI] [PubMed] [Google Scholar]
- 136.Piera-Mardemootoo C, Lambert P, Faillie J-L. Efficacy of metformin on glycemic control and weight in drug-naive type 2 diabetes mellitus patients: a systematic review and meta-analysis of placebo-controlled randomized trials. Therapies. 2021;76(6):647–656. doi: 10.1016/j.therap.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 137.T. Iijima et al (2021) “Acute effect of add‐on therapy with tofogliflozin, a sodium glucose co‐transporter 2 inhibitor, on 24‐hours glucose profile and glycaemic variability evaluated by continuous glucose monitoring in patients with type 2 diabetes receiving dipeptidyl peptidase‐4 inhibitors,” Int J Clin Pract, 75(11), 10.1111/ijcp.14732. [DOI] [PubMed]
- 138.Habicht S, Ludwig C, Yang R, Krawinkel M. Momordica charantia and type 2 diabetes: from in vitro to human studies. CDR. 2014;10(1):48–60. doi: 10.2174/1573399809666131126152044. [DOI] [PubMed] [Google Scholar]
- 139.Rauniyar GP, Sinha R, Chapagain K, Maskey R, Pandey DR. Effects of Momordica charantia (karela/bitterguord) in type 2 diabetic patients taking allopathic drugs: a pilot study. Kathmandu Univ Med J (KUMJ) 2021;19(74):143–147. [PubMed] [Google Scholar]
- 140.García-Calzón S, et al. Epigenetic markers associated with metformin response and intolerance in drug-naïve patients with type 2 diabetes. Sci. Transl. Med. 2020;12(561):eaaz1803. doi: 10.1126/scitranslmed.aaz1803. [DOI] [PubMed] [Google Scholar]
- 141.Guo J, Tao H, Cao Y, Ho C-T, Jin S, Huang Q. Prevention of obesity and type 2 diabetes with aged citrus peel ( Chenpi ) extract. J Agric Food Chem. 2016;64(10):2053–2061. doi: 10.1021/acs.jafc.5b06157. [DOI] [PubMed] [Google Scholar]
- 142.Suksomboon N, Poolsup N, Punthanitisarn S. Effect of aloe vera on glycaemic control in prediabetes and type 2 diabetes: a systematic review and meta-analysis. J Clin Pharm Ther. 2016;41(2):180–188. doi: 10.1111/jcpt.12382. [DOI] [PubMed] [Google Scholar]
- 143.Dong Y, et al. Metabolomics study of type 2 diabetes mellitus and the antidiabetic effect of berberine in Zucker diabetic fatty rats using Uplc-ESI-Hdms: metabolomics of T2DM and anti-diabetic effect of berberine in ZDF rats. Phytother Res. 2016;30(5):823–828. doi: 10.1002/ptr.5587. [DOI] [PubMed] [Google Scholar]
- 144.Yang L, et al. Pueraria lobata for diabetes mellitus: past, present and future. Am J Chin Med. 2019;47(07):1419–1444. doi: 10.1142/S0192415X19500733. [DOI] [PubMed] [Google Scholar]
- 145.Komuro I, Kadowaki T, Bodegård J, Thuresson M, Okami S, Yajima T. Lower heart failure and chronic kidney disease risks associated with sodium-glucose cotransporter-2 inhibitor use in Japanese type 2 diabetes patients without established cardiovascular and renal diseases. Diabetes Obes Metab. 2021;23(S2):19–27. doi: 10.1111/dom.14119. [DOI] [PubMed] [Google Scholar]
- 146.Kawai Y, et al. Comparison of effects of SGLT-2 inhibitors and GLP-1 receptor agonists on cardiovascular and renal outcomes in type 2 diabetes mellitus patients with/without albuminuria: a systematic review and network meta-analysis. Diabetes Res Clin Pract. 2022;183:109146. doi: 10.1016/j.diabres.2021.109146. [DOI] [PubMed] [Google Scholar]
- 147.Wei X-B, Wei W, Ding L-L, Liu S-Y. Comparison of the effects of 10 GLP-1 RA and SGLT2 inhibitor interventions on cardiovascular, mortality, and kidney outcomes in type 2 diabetes: a network meta-analysis of large randomized trials. Prim Care Diabetes. 2021;15(2):208–211. doi: 10.1016/j.pcd.2020.08.017. [DOI] [PubMed] [Google Scholar]
- 148.Herrera Comoglio R, Vidal Guitart X. Cardiovascular events and mortality among type 2 diabetes mellitus patients newly prescribed first-line blood glucose-lowering drugs monotherapies: a population-based cohort study in the Catalan electronic medical record database, SIDIAP, 2010–2015. Primary Care Diabetes. 2021;15(2):323–331. doi: 10.1016/j.pcd.2020.11.002. [DOI] [PubMed] [Google Scholar]
- 149.Bao P, et al. Efficacy and safety of acupuncture combined with Chinese herbal medicine in the treatment of type 2 diabetes mellitus: a protocol for a systematic review and meta-analysis. Medicine. 2021;100(43):e27658. doi: 10.1097/MD.0000000000027658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu D, Chen H, Song F, Ahmed MA, Wu H. Adverse drug events observed with the novel sodium/glucose co-transporter 2 inhibitor ipragliflozin for the treatment of patients with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized studies. Adv Ther. 2020;37(10):4356–4369. doi: 10.1007/s12325-020-01471-2. [DOI] [PubMed] [Google Scholar]
- 151.Gui Q, Xu Z, Xu K, Yang Y. The efficacy of ginseng-related therapies in type 2 diabetes mellitus: an updated systematic review and meta-analysis. Medicine. 2016;95(6):e2584. doi: 10.1097/MD.0000000000002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li T, et al. Efficacy and safety of Shenqi Jiangtang granules plus oral hypoglycemic agent in patients with type 2 diabetes mellitus: a systematic review and meta-analysis of 15 RCTs. Medicine. 2021;100(5):e23578. doi: 10.1097/MD.0000000000023578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tian Y, et al. Baihu Jia Renshen decoction for type 2 diabetic mellitus: a protocol for systematic review and meta-analysis. Medicine. 2020;99(19):20210. doi: 10.1097/MD.0000000000020210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Piao C, et al. Treatment of type 2 diabetes with Tianqi Jiangtang capsule: a systematic review and meta-analysis of randomized controlled trials. Medicine. 2020;99(21):e19702. doi: 10.1097/MD.0000000000019702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jingjing L, Hongmei Z, Yanping L, Bin W. Meta-analysis of the efficacy in treatment of primary sjögren’s syndrome: traditional Chinese medicine vs Western medicine. J Tradit Chin Med. 2016;36(5):596–605. doi: 10.1016/S0254-6272(16)30078-4. [DOI] [PubMed] [Google Scholar]
- 156.Ali MM, et al. Impact of Cotadutide drug on patients with type 2 diabetes mellitus: a systematic review and meta-analysis. BMC Endocr Disord. 2022;22(1):113. doi: 10.1186/s12902-022-01031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li S, et al. Exploring the protective effect of ShengMai-Yin and Ganmaidazao decoction combination against type 2 diabetes mellitus with nonalcoholic fatty liver disease by network pharmacology and validation in KKAy mice. J Ethnopharmacol. 2019;242:112029. doi: 10.1016/j.jep.2019.112029. [DOI] [PubMed] [Google Scholar]
- 158.Cao Y, Sun Z, Huang H, Lin A, Liu Y. Comparative analysis of absorbed ingredients and metabolites, and pharmacokinetic studies of Zhimu-Huangbai herb pair in the plasma of normal and type 2 diabetes mellitus rats by UHPLC-linear trap quadrupole-orbitrap MS and LC-MS/MS. J Sep Sci. 2022;45(3):664–676. doi: 10.1002/jssc.202100582. [DOI] [PubMed] [Google Scholar]
- 159.Yan Z, et al. Integrated metabolomics and gut microbiome to the effects and mechanisms of naoxintong capsule on type 2 diabetes in rats. Sci Rep. 2020;10(1):10829. doi: 10.1038/s41598-020-67362-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.E. Kutoh, A. N. Kuto, A. Wada, R. Kurihara, and R. Kojima (2021) “Complementary effects on glycaemic and non‐glycaemic parameters between responders and non‐responders treated with pioglitazone and canagliflozin in drug‐naive subjects with type 2 diabetes,” Int J Clinical Practice, 75(12), 10.1111/ijcp.14914. [DOI] [PubMed]
- 161.Zhou S, et al. Canagliflozin could improve the levels of renal oxygenation in newly diagnosed type 2 diabetes patients with normal renal function. Diabetes Metab. 2021;47(5):101274. doi: 10.1016/j.diabet.2021.101274. [DOI] [PubMed] [Google Scholar]
- 162.Navval-Esfahlan E, Rafraf M, Asghari S, Imani H, Asghari-Jafarabadi M, Karimi-Avval S. Effect of French maritime pine bark extract supplementation on metabolic status and serum vascular cell adhesion molecule-1 levels in patients with type 2 diabetes and microalbuminuria. Complement Ther Med. 2021;58:102689. doi: 10.1016/j.ctim.2021.102689. [DOI] [PubMed] [Google Scholar]
- 163.Pan J, et al. The effectiveness of traditional Chinese medicine Jinlida granules on glycemic variability in newly diagnosed type 2 diabetes: a double-blinded, randomized trial. J Diabetes Res. 2021;2021:1–8. doi: 10.1155/2021/6303063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.M. Guo et al (2014) “Metformin may produce antidepressant effects through improvement of cognitive function among depressed patients with diabetes mellitus,” Clin Exp Pharmacol Physiol, 10.1111/1440-1681.12265. [DOI] [PubMed]
- 165.Sgrò P, Emerenziani GP, Antinozzi C, Sacchetti M, Di Luigi L. Exercise as a drug for glucose management and prevention in type 2 diabetes mellitus. Curr Opin Pharmacol. 2021;59:95–102. doi: 10.1016/j.coph.2021.05.006. [DOI] [PubMed] [Google Scholar]
- 166.Li C, He J-Z, Zhou X-D, Xu X. Berberine regulates type 2 diabetes mellitus related with insulin resistance. Zhongguo Zhong Yao Za Zhi. 2017;42(12):2254–2260. doi: 10.19540/j.cnki.cjcmm.20170307.014. [DOI] [PubMed] [Google Scholar]
- 167.Silva ML, Bernardo MA, Singh J, de Mesquita MF. Cinnamon as a complementary therapeutic approach for dysglycemia and dyslipidemia control in type 2 diabetes mellitus and its molecular mechanism of action: a review. Nutrients. 2022;14(13):2773. doi: 10.3390/nu14132773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pivari F, Mingione A, Brasacchio C, Soldati L. Curcumin and type 2 diabetes mellitus: prevention and treatment. Nutrients. 2019;11(8):1837. doi: 10.3390/nu11081837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wirngo FE, Lambert MN, Jeppesen PB. The physiological effects of dandelion ( Taraxacum Officinale ) in type 2 diabetes. Rev Diabet Stud. 2016;13(2–3):113–131. doi: 10.1900/RDS.2016.13.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.García-Ulloa AC, et al. Clinician prescription of lipid-lowering drugs and achievement of treatment goals in patients with newly diagnosed type 2 diabetes mellitus. BMJ Open Diab Res Care. 2021;9(1):e001891. doi: 10.1136/bmjdrc-2020-001891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fu J-Y, Zhang Y. Mechanism of Astragali Radix-Coptis Rhizoma pair in treating type 2 diabetes mellitus based on network pharmacology. Zhongguo Zhong Yao Za Zhi. 2021;46(18):4808–4815. doi: 10.19540/j.cnki.cjcmm.20210609.705. [DOI] [PubMed] [Google Scholar]
- 172.K. K. Oh (2022) “A network pharmacology study to investigate bioactive compounds and signaling pathways of garlic ( Allium sativum L.) husk against type 2 diabetes mellitus,” Journal of Food Biochemistry, 46(7), 10.1111/jfbc.14106. [DOI] [PubMed]
- 173.Wang H, et al. Potential role of natural plant medicine Cyclocarya paliurus in the treatment of type 2 diabetes mellitus. J Diabetes Res. 2021;2021:1–12. doi: 10.1155/2021/1655336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bailey CJ. Metformin: historical overview. Diabetologia. 2017;60(9):1566–1576. doi: 10.1007/s00125-017-4318-z. [DOI] [PubMed] [Google Scholar]
- 175.Hawley SA, et al. Use of cells expressing γ subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11(6):554–565. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hardie DG. Minireview: The AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003;144(12):5179–5183. doi: 10.1210/en.2003-0982. [DOI] [PubMed] [Google Scholar]
- 177.Fullerton MD, et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med. 2013;19(12):1649–1654. doi: 10.1038/nm.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Foretz M, et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest. 2010;120(7):2355–2369. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.He L, et al. Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell. 2009;137(4):635–646. doi: 10.1016/j.cell.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Vincent MF, Marangos PJ, Gruber HE, van den Berghe G. Inhibition by AICA riboside of gluconeogenesis in isolated rat hepatocytes. Diabetes. 1991;40(10):1259–1266. doi: 10.2337/diab.40.10.1259. [DOI] [PubMed] [Google Scholar]
- 181.Curry DW, Stutz B, Andrews ZB, Elsworth JD. Targeting AMPK signaling as a neuroprotective strategy in Parkinson’s disease. JPD. 2018;8(2):161–181. doi: 10.3233/JPD-171296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hang L, et al. Conditional disruption of AMP kinase in dopaminergic neurons promotes Parkinson’s disease-associated phenotypes in vivo. Neurobiol Dis. 2021;161:105560. doi: 10.1016/j.nbd.2021.105560. [DOI] [PubMed] [Google Scholar]
- 183.Batandier C, et al. The ROS production induced by a reverse-electron flux at respiratory-chain complex 1 is hampered by metformin. J Bioenerg Biomembr. 2006;38(1):33–42. doi: 10.1007/s10863-006-9003-8. [DOI] [PubMed] [Google Scholar]
- 184.Patil SP, Jain PD, Ghumatkar PJ, Tambe R, Sathaye S. Neuroprotective effect of metformin in MPTP-induced Parkinson’s disease in mice. Neuroscience. 2014;277:747–754. doi: 10.1016/j.neuroscience.2014.07.046. [DOI] [PubMed] [Google Scholar]
- 185.Rotermund C, Machetanz G, Fitzgerald JC. The therapeutic potential of metformin in neurodegenerative diseases. Front Endocrinol. 2018;9:400. doi: 10.3389/fendo.2018.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Dias V, Junn E, Mouradian MM. The role of oxidative stress in Parkinson’s disease. J Parkinson’s Dis. 2013;3(4):461–491. doi: 10.3233/JPD-130230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Diehn M, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458(7239):780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang Q-Q, Li W-S, Liu Z, Zhang H-L, Ba Y-G, Zhang R-X. Metformin therapy and cognitive dysfunction in patients with type 2 diabetes: a meta-analysis and systematic review. Medicine. 2020;99(10):e19378. doi: 10.1097/MD.0000000000019378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Oda SS. Metformin protects against experimental acrylamide neuropathy in rats: metformin and acrylamide neuropathy. Drug Dev Res. 2017;78(7):349–359. doi: 10.1002/ddr.21400. [DOI] [PubMed] [Google Scholar]
- 190.Pérez-Revuelta BI, et al. Metformin lowers Ser-129 phosphorylated α-synuclein levels via mTOR-dependent protein phosphatase 2A activation. Cell Death Dis. 2014;5(5):e1209–e1209. doi: 10.1038/cddis.2014.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Nauck MA. Glucagon-like peptide 1 (GLP-1) in the treatment of diabetes. Horm Metab Res. 2004;36(11/12):852–858. doi: 10.1055/s-2004-826175. [DOI] [PubMed] [Google Scholar]
- 192.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 193.Orskov C, Holst JJ, Nielsen OV. Effect of truncated glucagon-like peptide-1 [proglucagon-(78–107) amide] on endocrine secretion from pig pancreas, antrum, and nonantral stomach. Endocrinology. 1988;123(4):2009–2013. doi: 10.1210/endo-123-4-2009. [DOI] [PubMed] [Google Scholar]
- 194.Rask E, et al. Insulin secretion and incretin hormones after oral glucose in non-obese subjects with impaired glucose tolerance. Metabolism. 2004;53(5):624–631. doi: 10.1016/j.metabol.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 195.Toft-Nielsen MB, et al. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab. 2001;86(8):3717–3723. doi: 10.1210/jcem.86.8.7750. [DOI] [PubMed] [Google Scholar]
- 196.Cork SC, Richards JE, Holt MK, Gribble FM, Reimann F, Trapp S. Distribution and characterisation of glucagon-like peptide-1 receptor expressing cells in the mouse brain. Mol Metab. 2015;4(10):718–731. doi: 10.1016/j.molmet.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Drucker DJ, Sherman SI, Gorelick FS, Bergenstal RM, Sherwin RS, Buse JB. Incretin-based therapies for the treatment of type 2 diabetes: evaluation of the risks and benefits. Diabetes Care. 2010;33(2):428–433. doi: 10.2337/dc09-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Liu X-Y, Wang L-X, Chen Z, Liu L-B. Liraglutide prevents beta-amyloid-induced neurotoxicity in SH-SY5Y cells via a PI3K-dependent signaling pathway. Neurol Res. 2016;38(4):313–319. doi: 10.1080/01616412.2016.1145914. [DOI] [PubMed] [Google Scholar]
- 199.Hunter K, Hölscher C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci. 2012;13(1):33. doi: 10.1186/1471-2202-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Bertilsson G, et al. Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson’s disease. J Neurosci Res. 2008;86(2):326–338. doi: 10.1002/jnr.21483. [DOI] [PubMed] [Google Scholar]
- 201.Harkavyi A, Abuirmeileh A, Lever R, Kingsbury AE, Biggs CS, Whitton PS. Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson’s disease. J Neuroinflammation. 2008;5(1):19. doi: 10.1186/1742-2094-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Li Y, et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci USA. 2009;106(4):1285–1290. doi: 10.1073/pnas.0806720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kosaraju J, et al. Saxagliptin: A dipeptidyl peptidase-4 inhibitor ameliorates streptozotocin induced Alzheimer’s disease. Neuropharmacology. 2013;72:291–300. doi: 10.1016/j.neuropharm.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 204.Rizzo MR, Barbieri M, Boccardi V, Angellotti E, Marfella R, Paolisso G. Dipeptidyl peptidase-4 inhibitors have protective effect on cognitive impairment in aged diabetic patients with mild cognitive impairment. J Gerontol A Biol Sci Med Sci. 2014;69(9):1122–1131. doi: 10.1093/gerona/glu032. [DOI] [PubMed] [Google Scholar]
- 205.Bayram E, Litvan I. Lowering the risk of Parkinson’s disease with GLP-1 agonists and DPP4 inhibitors in type 2 diabetes. Brain. 2020;143(10):2868–2871. doi: 10.1093/brain/awaa287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Nassar NN, Al-Shorbagy MY, Arab HH, Abdallah DM. Saxagliptin: a novel antiparkinsonian approach. Neuropharmacology. 2015;89:308–317. doi: 10.1016/j.neuropharm.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 207.C. Ribeiro, A. Silva, S. Viana, and F. Pereira (2012) Sitagliptin does not protect against MPTP-induced dopaminergic striatal toxicity.
- 208.Kern W, Peters A, Fruehwald-Schultes B, Deininger E, Born J, Fehm HL. Improving influence of insulin on cognitive functions in humans. Neuroendocrinology. 2001;74(4):270–280. doi: 10.1159/000054694. [DOI] [PubMed] [Google Scholar]
- 209.Freiherr J, et al. Intranasal insulin as a treatment for Alzheimer’s disease: a review of basic research and clinical evidence. CNS Drugs. 2013;27(7):505–514. doi: 10.1007/s40263-013-0076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Plum L, Schubert M, Brüning JC. The role of insulin receptor signaling in the brain. Trends Endocrinol Metab. 2005;16(2):59–65. doi: 10.1016/j.tem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 211.Craft S, et al. Effects of regular and long-acting insulin on cognition and Alzheimer’s disease biomarkers: a pilot clinical trial. JAD. 2017;57(4):1325–1334. doi: 10.3233/JAD-161256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ramalingam M, Kim S-J. Protective effects of activated signaling pathways by insulin on C6 glial cell model of MPP + -induced Parkinson’s disease. J Recept Signal Transduction. 2017;37(1):100–107. doi: 10.3109/10799893.2016.1171342. [DOI] [PubMed] [Google Scholar]
- 213.Heras-Sandoval D, Pérez-Rojas JM, Hernández-Damián J, Pedraza-Chaverri J. The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal. 2014;26(12):2694–2701. doi: 10.1016/j.cellsig.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 214.Novak P, Pimentel Maldonado DA, Novak V. Safety and preliminary efficacy of intranasal insulin for cognitive impairment in Parkinson disease and multiple system atrophy: a double-blinded placebo-controlled pilot study. PLoS ONE. 2019;14(4):0214364. doi: 10.1371/journal.pone.0214364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kwon KJ, Kim H-J, Shin CY, Han S-H. Melatonin potentiates the neuroprotective properties of resveratrol against beta-amyloid-induced neurodegeneration by modulating AMP-activated protein kinase pathways. J Clin Neurol. 2010;6(3):127. doi: 10.3988/jcn.2010.6.3.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hundal RS, et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49(12):2063–2069. doi: 10.2337/diabetes.49.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Chiang M-C, Cheng Y-C, Chen S-J, Yen C-H, Huang R-N. Metformin activation of AMPK-dependent pathways is neuroprotective in human neural stem cells against amyloid-beta-induced mitochondrial dysfunction. Exp Cell Res. 2016;347(2):322–331. doi: 10.1016/j.yexcr.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 218.Ng ACT, Delgado V, Borlaug BA, Bax JJ. Diabesity: the combined burden of obesity and diabetes on heart disease and the role of imaging. Nat Rev Cardiol. 2021;18(4):291–304. doi: 10.1038/s41569-020-00465-5. [DOI] [PubMed] [Google Scholar]
- 219.Agostini F, Masato A, Bubacco L, Bisaglia M. Metformin repurposing for Parkinson disease therapy: opportunities and challenges. IJMS. 2021;23(1):398. doi: 10.3390/ijms23010398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Osborne C, West E, Nolan W, McHale-Owen H, Williams A, Bate C. Glimepiride protects neurons against amyloid-β-induced synapse damage. Neuropharmacology. 2016;101:225–236. doi: 10.1016/j.neuropharm.2015.09.030. [DOI] [PubMed] [Google Scholar]
- 221.Alp H, et al. Protective effects of beta glucan and gliclazide on brain tissue and sciatic nerve of diabetic rats induced by streptozosin. Exp Diabetes Res. 2012;2012:1–7. doi: 10.1155/2012/230342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Esmaeili MH, Bahari B, Salari A-A. ATP-sensitive potassium-channel inhibitor glibenclamide attenuates HPA axis hyperactivity, depression- and anxiety-related symptoms in a rat model of Alzheimer’s disease. Brain Res Bull. 2018;137:265–276. doi: 10.1016/j.brainresbull.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 223.Qiu X, Wang Q, Hou L, Zhang C, Wang Q, Zhao X. Inhibition of NLRP3 inflammasome by glibenclamide attenuated dopaminergic neurodegeneration and motor deficits in paraquat and maneb-induced mouse Parkinson’s disease model. Toxicol Lett. 2021;349:1–11. doi: 10.1016/j.toxlet.2021.05.008. [DOI] [PubMed] [Google Scholar]
- 224.Ju Y-J, et al. Glibenclamide modulates microglial function and attenuates Aβ deposition in 5XFAD mice. Eur J Pharmacol. 2020;884:173416. doi: 10.1016/j.ejphar.2020.173416. [DOI] [PubMed] [Google Scholar]
- 225.Landreth G. Therapeutic use of agonists of the nuclear receptor PPARγ in Alzheimers disease. CAR. 2007;4(2):159–164. doi: 10.2174/156720507780362092. [DOI] [PubMed] [Google Scholar]
- 226.Heneka MT, et al. Acute treatment with the PPARγ agonist pioglitazone and ibuprofen reduces glial inflammation and Aβ1–42 levels in APPV717I transgenic mice. Brain. 2005;128(6):1442–1453. doi: 10.1093/brain/awh452. [DOI] [PubMed] [Google Scholar]
- 227.Yu Y, et al. Insulin sensitizers improve learning and attenuate tau hyperphosphorylation and neuroinflammation in 3xTg-AD mice. J Neural Transm. 2015;122(4):593–606. doi: 10.1007/s00702-014-1294-z. [DOI] [PubMed] [Google Scholar]
- 228.Quinn LP, et al. The PPARγ agonist pioglitazone is effective in the MPTP mouse model of Parkinson’s disease through inhibition of monoamine oxidase B: pioglitazone inhibits MAO-B activity in MPTP mice. Br J Pharmacol. 2008;154(1):226–233. doi: 10.1038/bjp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hunter RL, Choi D-Y, Ross SA, Bing G. Protective properties afforded by pioglitazone against intrastriatal LPS in Sprague-Dawley rats. Neurosci Lett. 2008;432(3):198–201. doi: 10.1016/j.neulet.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lee EY, Lee JE, Park JH, Shin IC, Koh HC. Rosiglitazone, a PPAR-γ agonist, protects against striatal dopaminergic neurodegeneration induced by 6-OHDA lesions in the substantia nigra of rats. Toxicol Lett. 2012;213(3):332–344. doi: 10.1016/j.toxlet.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 231.Corona JC, de Souza SC, Duchen MR. PPARγ activation rescues mitochondrial function from inhibition of complex I and loss of PINK1. Exp Neurol. 2014;253:16–27. doi: 10.1016/j.expneurol.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 232.Corona JC, Duchen MR. PPARγ and PGC-1α as therapeutic targets in Parkinson’s. Neurochem Res. 2015;40(2):308–316. doi: 10.1007/s11064-014-1377-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Markowicz-Piasecka M, Huttunen KM, Sikora J. Metformin and its sulphonamide derivative simultaneously potentiateanti-cholinesterase activity of donepezil and inhibit beta-amyloid aggregation. J Enzyme Inhib Med Chem. 2018;33(1):1309–1322. doi: 10.1080/14756366.2018.1499627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Le Pelletier L, et al. Metformin alleviates stress-induced cellular senescence of aging human adipose stromal cells and the ensuing adipocyte dysfunction. eLife. 2021;10:e62635. doi: 10.7554/eLife.62635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Pan Y, et al. Metformin reduces morphine tolerance by inhibiting microglial-mediated neuroinflammation. J Neuroinflammation. 2016;13(1):294. doi: 10.1186/s12974-016-0754-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.X. Chen et al (2018) “A GLP‑1 receptor agonist attenuates human islet amyloid polypeptide‑induced autophagy and apoptosis in MIN6 cells,” Mol Med Report10.3892/mmr.2018.9741 [DOI] [PubMed]
- 237.Jing F, Zou Q, Wang Y, Cai Z, Tang Y. Activation of microglial GLP-1R in the trigeminal nucleus caudalis suppresses central sensitization of chronic migraine after recurrent nitroglycerin stimulation. J Headache Pain. 2021;22(1):86. doi: 10.1186/s10194-021-01302-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Abbas T, Faivre E, Hölscher C. Impairment of synaptic plasticity and memory formation in GLP-1 receptor KO mice: interaction between type 2 diabetes and Alzheimer’s disease. Behav Brain Res. 2009;205(1):265–271. doi: 10.1016/j.bbr.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 239.Yuan Z, et al. A novel GLP-1/GIP dual agonist is more effective than liraglutide in reducing inflammation and enhancing GDNF release in the MPTP mouse model of Parkinson’s disease. Eur J Pharmacol. 2017;812:82–90. doi: 10.1016/j.ejphar.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 240.Maskery M, Goulding EM, Gengler S, Melchiorsen JU, Rosenkilde MM, Hölscher C. The dual GLP-1/GIP receptor agonist DA4-JC shows superior protective properties compared to the GLP-1 analogue liraglutide in the APP/PS1 mouse model of Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2020;35:153331752095304. doi: 10.1177/1533317520953041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Roque PS, et al. Intranasal insulin rescues repeated anesthesia-induced deficits in synaptic plasticity and memory and prevents apoptosis in neonatal mice via mTORC1. Sci Rep. 2021;11(1):15490. doi: 10.1038/s41598-021-94849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Brabazon F, Bermudez S, Shaughness M, Khayrullina G, Byrnes KR. The effects of insulin on the inflammatory activity of BV2 microglia. PLoS ONE. 2018;13(8):0201878. doi: 10.1371/journal.pone.0201878. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.







