Abstract
Artificial intelligence (AI), also known as machine intelligence, is widely utilized in the medical field, promoting medical advances. Malignant tumors are the critical focus of medical research and improvement of clinical diagnosis and treatment. Mediastinal malignancy is an important tumor that attracts increasing attention today due to the difficulties in treatment. Combined with artificial intelligence, challenges from drug discovery to survival improvement are constantly being overcome. This article reviews the progress of the use of AI in the diagnosis, treatment, and prognostic prospects of mediastinal malignant tumors based on current literature findings.
Keywords: artificial intelligence, mediastinal malignancy, deep learning, artificial neural network
1. Introduction
Artificial intelligence is a new intellectual ability that can simulate and expand human thinking and judgment [1]. As a comprehensive discipline developed through integrating computer science, psychology, linguistics, and other disciplines, artificial intelligence, alongside genetic engineering and nanoscience, is considered one of the three most state-of-the-art technologies in the world in the 21st century. Artificial intelligence was first proposed by the Dartmouth Institute in 1956 [2] and, in recent years, with advances in related technologies, it has reshaped every walk of life, encompassing numerous fields. The expansion of databases, the innovation of algorithms, and the improvement of hardware skills have laid a solid foundation for a wide range of applications of AI in the medical field.
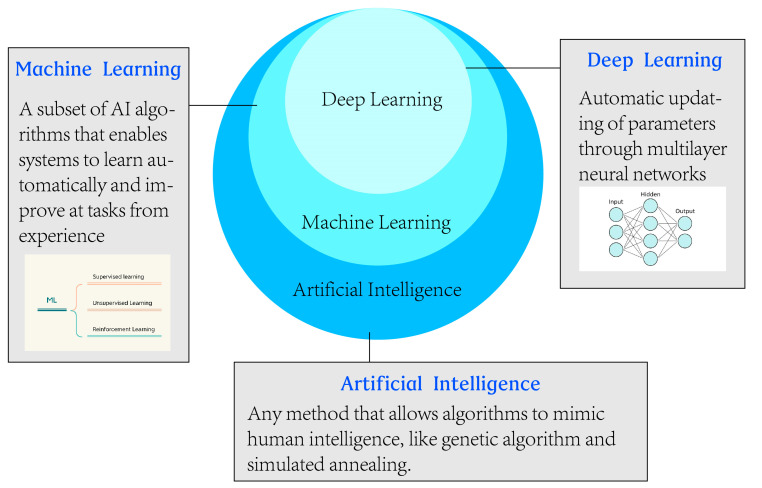
Machine learning (ML) is a branch of artificial intelligence that uses structured training data to teach machines so that the machine can learn and adapt to create and modify its algorithms for new data to solve problems (Figure 1) [3]. Meanwhile, deep learning is a new field and a part of machine learning, and directly processes unstructured data, such as images and sounds, with representation learning based on artificial neural networks. It is rich with powerful computing, automation, and variability [4]. In recent years, deep learning algorithms have brought remarkable accomplishments, even exceeding human intelligence [5,6,7]. Among various deep learning algorithm models, the convolutional neural network (CNN) is the most popular deep learning architecture in medical imaging [8]. CNN has the potential of representation learning and effectively extracts, organizes, and classifies internal features of imaging data.
Figure 1.
The differences and relationships among artificial intelligence (AI), machine learning (ML), and deep learning (DL).
Cancer is the second most common disease specific to morbidity and mortality, and up to 19.2 million new malignant tumor cases are diagnosed every year [9], endangering human health and prompting to seek long-term remedies. The mediastinum consists of the left and right mediastinal pleura, organs, other structures, and connective tissue [10]. A mediastinal malignant tumor refers to a malignancy found in the mediastinal area, including neurogenic tumor, teratoma, thymoma, and germinoma [11]. Clinical manifestations of mediastinal malignant tumors are severe chest tightness and pain, different degrees of respiratory tract compression, and nervous system and cardiovascular symptoms depending on their locations [12,13]. Moreover, prompt diagnosis and treatment are of utmost importance, considering the complex origin and anatomical position. Using artificial intelligence can substantially improve the efficiency of preliminary diagnosis to prognostic analysis. However, artificial intelligence comes with challenges, such as data security and patient privacy. Furthermore, mediastinal malignancy is less studied compared to lung, breast, and other major cancers, and requires more attention and exploration [14]. To recapitulate, there are still prospects of applying AI in the diagnosis and treatment of mediastinal malignancy in the future with addressing challenges.
2. Diagnosing Mediastinal Malignant Tumors
2.1. Diagnosis Using Imaging
Before the advent of the first CT machine in the 1970s, mediastinal tumors were mostly diagnosed using chest radiography with a qualitative diagnosis rate of 30%. CT improved the diagnostic accuracy of localized tumors to >90%. Currently, ultrasonography, magnetic resonance imaging, thoracoscopy, and other imaging technologies have been used in screening, staging, and evaluating the efficacy of mediastinal malignant tumors. Computer-aided diagnosis (CAD) has also been adopted since its development in the 1980s [15]. However, these traditional image technologies also come with limitations. Furthermore, diagnosis using imaging technologies depends on the level of experience of diagnosticians, which is highly subjective, inevitably leading to a misdiagnosis at times. Furthermore, with the increase in population and disease screening awareness, radiologists need to process huge image data and deal with an increasing workload. Radiologists can only afford 3 to 4 s to read an image [16], and this time demand undoubtedly results in a high error rate and serious consequences in the long run. With the presently available automated and reproducible assessment capabilities through AI, challenges in diagnostic accuracy and doctors’ workload could be easily solved.
Two AI approaches can be adapted to the diagnostic imaging of malignant tumors [17]. The first method predefines tumor characteristics, such as tumor texture, volume, and shape based on mathematical equations, and then quantifies using computer programs [18]. The second method is deep learning, attracting greater attention in the medical field. Esteva et al. [19] classified skin lesions from clinical images using a deep convolutional neural network and found similar diagnostic accuracy to that of 21 qualified dermatologists. Deep learning is a highly reliable powerful tool to integrate with imaging technologies to reduce the workload of doctors.
The learning ability and reliability of AI have been proven, compared with the outcomes of various mediastinal malignant tumor diagnostic assays. Ozkan et al. [20] proposed a machine learning model for positron emission tomography with 2-deoxy-2- [fluorine-18] fluoro-D-glucose integrated with computed tomographic (18F-FDG PET/CT) images using a multilayer perception (MLP) classifier (ANN), which successfully predicted the low-risk and high-risk thymomas (AUC = 0.830) (Table S1). Dai et al. [21] associated computed tomography features with pathological tumor characteristics, and they found the random forest model highly efficient in diagnosing thymic carcinoma and high-risk thymoma, with a predictive accuracy of 94.73% using test data. Lin et al. [22] developed an AI model to predict the pathological subtypes of prevascular mediastinal tumors (PMTs). The model was able to identify lymphoma, thymoma, and thymic carcinoma with sensitivities of 52.9%, 74.2%, and 92.8%, respectively. These are some examples demonstrating that an ML algorithm combined with CT, X-ray, MRI, and other conventional imaging diagnostic methods could greatly improve detection efficiency and accuracy, and reduce the pressure on diagnosticians.
Furthermore, recent years have witnessed remarkable contributions of various novel imaging technologies utilizing artificial intelligence in the diagnosis of malignant tumors [17]. For instance, Chowdhary et al. [23] developed an AI-based prognostic model for patients with thymic epithelial tumors, achieving an overall accuracy of 84.2% and 87.0% in the training and validation cohorts, respectively, with an AUC of 0.90. Advanced imaging technologies such as multi-physical coupled imaging and contrast-enhanced ultrasound are being continuously explored and updated, demonstrating significant potential for development [24]. Combining these methods with advanced machine learning would advance further. Diagnostic imaging of mediastinal tumors has long been a challenge due to their diverse clinical behaviors, including the primary and metastatic nature of tumors, complex mediastinal structure locations, and occult onset. These new technologies with AI can capture the features of tissues, and can accurately identify subtle characteristics, such as microvascular invasion and lymph node metastasis. Through accurate diagnosis, personalized treatment can be easily designed [25].
AI can also be applied to laboratory parameters assessment. Some clinical cases may be misjudged using laboratory diagnostic assay outcomes; for example, due to false positive and false negative results. Incorporating AI in analyzing the laboratory indices, employing big data for one-to-one comparison would accurately identify patients with mediastinal tumor risk.
AI still has some deficiencies with the application of diagnostic imaging. For example, the black-box nature of AI makes the outcome partially interpretable and makes it difficult to quantify the prediction [26]. Detection results may also be related to input data, image quality, and other factors, and errors may still exist. AI can hopefully be further developed to escort the diagnostic imaging of mediastinal tumors.
2.2. Diagnosis by Pathological Examinations
Despite the advent of non-invasive techniques such as liquid biopsy, traditional histopathology remains the gold standard for the diagnosis of mediastinal malignancies. Histopathological or cytopathological diagnosis confirms the presence of tumor cells in patient specimens, identifies biomarkers of cancers and describes the tumor characteristics, such as types, stages, and grades [27]. Compared to imaging and other diagnostic methods, histopathological/cytopathological assessment clearly describes the characteristics of tumors and is widely used in the qualitative evaluation and classification of mediastinal tumors before surgery and after surgery. However, diagnosis using biopsy samples requires extensive experience and knowledge of pathologists, but experts in this field are scarce, and do not guarantee diagnostic accuracy. On the other hand, histopathological evaluation from fixation to staining requires a series of complex processes, and there is no strict standardization for these processes. With the rapid development of AI in clinical image evaluation, natural language extraction, and other aspects, the use of AI for pathological diagnosis has become the focus of tumor diagnosis, which has been widely applied for differentiating benign tumors from malignant tumors, and the grading and typing of several tumors, including mediastinal tumors [28]. Kalra et al., reported that AI can aid pathologists in accurately diagnosing thymoma [29].
The first step in the use of AI in pathology was made in the early 1950s, when Mellors and Silver suggested the use of automated pre-screening machines (microfluorescence scanners) to evaluate Pap smears [30]. Bostrom et al., who represented the pioneering work of many scientists, used various experimental image analyzers to automatically scan the clinical images [31]. As of yet, AI is competent in pathological diagnosis, especially in glandular and tumor classifications [32,33].
The deep CNN model of artificial intelligence is particularly efficient in extracting primary features from high-resolution pathological specimen images to accurately diagnose [34]. Presently, a variety of ML algorithm models have been employed in the pathological diagnosis of thymic cancer, prostate cancer, colon cancer, breast cancer, and other tumors, with satisfactory accuracy [35,36,37,38,39,40]. Using raw input data from virtual images of lung cancer biopsy stained with H and E, Coudray et al. [41] trained a CNN model to accurately predict six different gene mutations by analyzing specific histological patterns associated with various molecular subtypes of lung adenocarcinoma. Nevertheless, research on mediastinal tumors is lacking, and the application of AI would help in managing mediastinal tumors. There are several types of mediastinal tumors, and a single high-magnification histopathological image may contain millions of cellular features. AI can process a large amount of complex information in a short time with the available data entered, and efficiently analyze the tissues with complex lesions or atypical tissues.
Mediastinal sampling can also be easier using AI. The sampling can be achieved by endoscopic techniques, including endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) and/or endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) for further histopathological analysis [42]. A presently available real-time endoscopic diagnostic support system based on deep learning could enhance endoscopic technology and achieve efficient tissue observation, sampling, and artificial intelligence [43]. Mediastinal tissue sampling is difficult because of sternal obstruction. A non-ideal sample results in misdiagnosis. Therefore, other mediastinal sampling methods using AI are currently being explored. In a prospective study by Kumar et al. [44], a biopsy needle was inserted into the target lesion with the assistance of an automated robotic arm, and tissue sampling was performed at the site of the highest metabolic activity after confirming the needle’s position by PET/CT imaging.
AI could also be applied in the emerging field of molecular diagnostic pathology. Differential diagnosis of some mediastinal malignancies is very complicated. For example, mediastinal sarcomas are difficult to diagnose because these tumors are relatively rare and possess overlapping clinical and histological features. Several previous studies found that different subtypes had diverse incidence rates, rendering the diagnosis more difficult [45,46,47,48]. Many of these tumors had unique, recurring genetic abnormalities, and cytogenetic and molecular testing assisted in accurate diagnosis [49]. Appropriate AI models could simplify and optimize the identification process. As demonstrated by Capper et al. [50], a specially trained random forest classifier using tumor DNA methylation profile significantly improved the prediction accuracy in hard-to-diagnose subclasses of central nervous system (CNS) cancers (AUC = 0.99).
Generally, the application of AI in managing mediastinal malignancies is very competent, given their associations with complex and recurrent mutations. Critical characteristics that are difficult to identify or quantify by the naked eye can be extracted, and mutations can be predicted from the histopathological images more cost-effectively compared to direct sequencing. Nevertheless, due to limited experience, the existing artificial intelligence may not accurately assess the relatively rare or abnormally classified mediastinal tumors, but it could be considered an auxiliary diagnostic tool. With the advancement of this technology and increasing the number of cases, artificial intelligence will become a powerful application in pathological diagnosis.
3. Treatment of Mediastinal Malignancies
3.1. Surgical Resection
Surgery is the main treatment option for mediastinal malignancies [51,52]. In traditional open surgery, the integrity of the sternum or chest wall is often altered following surgery and the incision is large, mostly a longitudinal split on the sternum or a large thoracotomy incision to completely expose the area, resulting in a long operation time, postoperative pain, and peri- and postoperative complications [53,54]. Since the 1990s, minimally invasive techniques such as VATS have gained popularity due to their advantages of smaller incisions and quicker recovery times [55]. However, its application has been limited by the distorted two-dimensional field of view and difficult lever-type procedure [56]. Additionally, the mediastinal space is narrow, requiring long thoracoscopic instruments. Hand tremor easily results in errors in judgment, and some areas cannot be reached, making the resection of tumor mass extremely laborious. Da Vinci robot-assisted surgery was invented, improving the accuracy and stability of surgical procedures. It provides a good ergonomic experience and high-definition 3D vision, which is preferred by surgeons. Presently, robotic systems have not yet used machine learning and deep learning, but have laid the foundation for the application of artificial intelligence in surgical procedures.
The current use of AI in handling mediastinal malignant tumors focuses on preoperative planning, covering the feasibility and scope of surgery. Clinical images provide apparent features for the analysis. Subsequently, fast and accurate feature extraction can be achieved through deep learning algorithms, and intelligent segmentation can be completed to promote high-precision identification and accurate matching of anatomical sites, achieving efficient and accurate preoperative planning [57,58,59]. Yohei et al. [60] created an artificial intelligence model using an automatic machine learning platform to predict the mediastinal lymph node metastasis, regulating the surgical procedure, with a prediction accuracy rate of 84%. Although the model had a low sensitivity value of 12%, AI had the ability and potential to complement the shortcomings of existing modalities.
A combination of robotic systems and AI is the future tool in the medical field. The complex structure of the mediastinum makes it easy to injure the spinal cord, cervical nerve roots, hilum of the lung, and unnamed vessels and nerve branches during the surgical approach, and renders fat removal during the surgical procedure difficult [61,62,63]. Completely depending on humans is not reliable enough. Uses of robotics have shown advantages over traditional mediastinal surgery pertinent to precision and stability [64,65]. AI has the potential to assist in determining the best surgical strategy and optimal surgical path [66], which may lead to a reduction in the incidence of complications such as muscle weakness, pneumonia, respiratory failure, and pulmonary edema [67,68,69].
There is still substantial room for improvement in the existing AI for handling mediastinal malignancies. The sensitivity is far from satisfactory. Furthermore, there is still a long way to go toward universal adoption. With the optimization of equipment and the improvement in operation technologies, AI-based robot-assisted surgery may soon become a routine surgical method.
3.2. Chemotherapy
The application of artificial intelligence in chemotherapy has many attributes. First, chemotherapeutic drugs can be improved. Some successes in the improvement of chemotherapeutics have been reported in cancer treatment. Berishvili et al., created a deep neural network algorithm to develop anticancer drugs inhibiting PI3Ks and tanker enzymes, promising targets for colorectal cancer (CRC) treatment [70]. Using DNN, Sakellaropoulous et al. [71] trained a model using cancer drug sensitivity genomics (GDSC) cell lines and then used it to predict the responses to paclitaxel in these cells and other mediastinal malignancies. Mediastinal tumors are diverse and suitable for different chemotherapeutic drugs. By improving the efficacies of drugs through artificial intelligence, database-based comparisons and analyses can be efficiently achieved, and the time of conducting research can be reduced. Artificial intelligence can also reduce the cost of research and development and enhance treatment efficacy [72].
AI can optimize individualized chemotherapy regimens to the greatest extent possible. Drug resistance is a serious problem in chemotherapy, resulting from insufficient concentrations of the drugs circulating through the bloodstream to reach the tumor. AI can predict the drug and its response using its learning ability, keeping the drug concentration above a threshold [73]. In 2008, an American biopharmaceutical company developed an AI platform to identify the potential drug targets in cancer patients and maximize the efficacies of cancer drugs [74,75]. A previous study successfully determined the optimal doses of ZEN-3694 and enzalutamide using “CURATE.AT”, an artificial intelligence platform created by the National University of Singapore using technologies, such as deep learning, to improve the efficacy and tolerability of combination therapies [76]. When chemotherapeutic drugs and nanorobots are functionally combined, nanoparticles act as drug carriers to accurately target the cancer cells to specifically bind, reducing the drug dosage and decreasing the side effects of chemotherapeutics [77]. Overall, artificial intelligence can simplify complex mediastinal tumor chemotherapy with the assistance of built robots, AI platforms, and other ways to develop the best-personalized management plan.
3.3. Radiotherapy
Many mediastinal tumors, such as thymoma, have a high risk of postoperative recurrence [78]. Radiotherapy is effective as an adjuvant treatment but poses many challenges, including a shortage of radiologists and patients’ intolerance to radiotherapy. AI could solve these problems in various ways.
The first is the determination of organs at risk (OARs) and the tumor size [79]. The goal of radiotherapy is to increase the benefit, but with a minimum possible radiation dose to non-diseased areas. Mediastinum is a narrow space, consisting of complex structures surrounding vital organs. With the insufficient target dose and deviated target structure, serious consequences, such as poor treatment effects and side effects, may emerge. Untoward consequences rely on the experience of the radiation oncologist, and precision radiation to the target mass is time-consuming and laborious. AI-based radiomics can achieve automatic delineation of mediastinal tumors, minimizing damage to the surrounding vital organ structures. Previous studies found that the average Dyce similarity coefficient (DSC) for manual delineation was 0.78–0.93, while that for automatic delineation was 0.97–0.99. The degree of variation of manual delineation was relatively high [80]. In a study by Lustberg et al. [81] with automatic segmentation of mediastinal tumors based on the atlas and deep learning, the DL took half of the time (10 min) compared with the manual delineation. AI had obvious advantages over manual target area delineation.
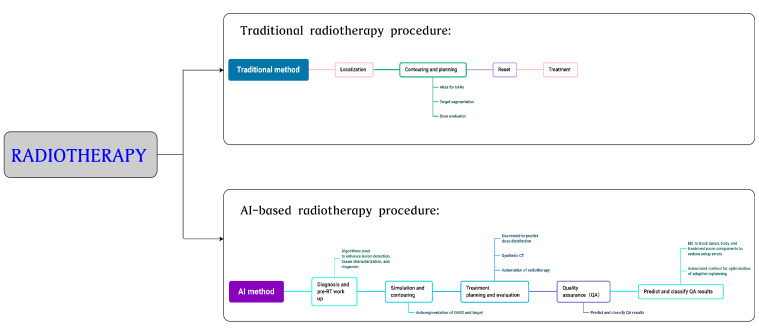
Second, AI can formulate radiotherapy plans. DL-based algorithms predict personalized 3D doses, which can be used to determine treatment dosages and precise locations of mediastinal malignant tumors and other tumors [82,83,84]. Individual bionic characteristics can be used in AI as a basis for making radiotherapy decisions. AI can also be used for assessing and adjusting radiotherapy. Precision radiotherapy, including adaptive radiotherapy (ART), has rapidly developed in recent years. Clinical images can be monitored, the radiotherapy method and prescribed dosage can be adjusted based on tissue changes, and radiotherapy planning can be optimized [85]. Schwaab et al. [86] integrated motion tracking based on ultrasound into heavy ion radiotherapy, used artificial neural networks to estimate the dosage distribution pixel by pixel, and compensated for the delay of about 200 ms between the target motion and position data, so that ultrasound could track the tumor location in real time, guaranteeing the accurate implementation of radiotherapy. Other applications of AI in radiotherapy include predicting radiation-induced toxicity, reconstructing images, and registering images [87,88,89,90]. Although AI is widely used in radiotherapy, it is focused on head and neck cancers and prostate cancers, but studies on mediastinal tumors are limited. Many studies are still in the theoretical stage with a lack of clinical knowledge. The use of AI can effectively increase treatment accuracy and efficiency, reduce complications, and accommodate the automation and intelligence of radiotherapeutic processes in the near future (Figure 2).
Figure 2.
Comparison of radiotherapy procedures between traditional and AI-based radiation methods.
4. Prognostic Analysis
Prognosis is the prediction and estimation of the consequences associated with the future recurrence of the disease [91]. Oncologists need to predict survival rate, survival time, and other consequences based on clinical evaluation. This prediction carries large contingency and uncertainty. The capability of the doctor, the cognitive level of the patient, the decision related to treatment, the patient’s age, and general health and psychological status are bound to the prognostic decision. Many force majeure events also increase the forecasting difficulty. With the help of AI, prognostic prediction accuracy can be improved. The DL algorithm that is used to automatically extract the features from medical data to build models may accurately predict the risk of tumor recurrence and patient response to treatment [92,93]. Many previous studies have achieved remarkable results in predicting tumor prognosis. The support vector machine system can be used to estimate the five-year survival rate of cancer patients. Zhong et al. [94] carried out a prognostic assessment of lung cancer-specific survival and the possibility of mediastinal lymph node metastasis using deep learning survival neural network models. Previous studies have also found that the prediction accuracy of AI was higher than that of linear regression analysis. In reference to Matsuo et al. [95], they utilized tumor characteristics and laboratory test data from 768 cancer patients to predict both progression-free survival (PFS) and overall survival (OS). The results indicated that the AI model had superior predictive ability compared to the Cox proportional hazards regression model. Sailer et al. [96] compared 10 common data mining algorithms to predict a binary target for five-year survival based on seven attributes, including sex and IACC stage. The average accuracy of ML was 67.7%, which was slightly higher than that of the clinician’s assessment of 59%.
Various studies have demonstrated the superiority of AI in prognostic analysis. Prognostic accuracy is not only useful for scientific research, but is also closely related to the survival status of patients. AI was better in predicting poor prognosis in patients with poor cancer-specific survival (adjusted hazard ratio = 3.04) compared to predicting good prognosis [97]. Intelligent health management can also, with the assistance of AI and data integration analysis, propose different intervention plans and follow-ups for medical services for patients, ensuring a smooth treatment process. AI also provided additional avenues for dynamically adjusting drug dosages with single or combination therapies in individual patients using patient-specific data points collected over time [98]. Furthermore, mediastinal malignant tumors are prone to relapse. Considering malignant thymomas as an example, they are difficult to treat, even by surgery, radiotherapy, and other comprehensive treatments, and result in greater odds of recurrence and metastasis. Moreover, emphysema and other complications, such as infection, are further problems. An accurate and efficient prognostic assessment undoubtedly improves the survival rate and prolongs the survival time in mediastinal malignant tumors.
Although AI makes breakthroughs in prognostic prediction, there are also disadvantages. First, although the prognostic accuracy exceeds that of humans in some studies, there is still a substantial need for improvement in achieving error-free results in diagnosis and other aspects. AI-related research is still in its infancy, the dataset is small in scale and single in type, and the knowledge of AI and clinical expertise are relatively limited. Therefore, it is difficult to develop models that fit the clinical situation and AI [99,100,101]. International multicenter studies should be performed in the future to construct robust algorithms and achieve in-depth communication across disciplines to organizationally combine clinical research [102].
5. Prospects of Using Artificial Intelligence
Artificial intelligence can be incorporated into all aspects of mediastinal tumor research and clinical management. At present, one of its potential applications in mediastinal malignancies is to design new anticancer therapeutics or to guide the development of therapeutics. Cancer drug development without doubt greatly benefits from the use of AI. Machine learning algorithms can screen relevant data from a large number of patients during treatment and assist to develop new drugs for improving treatment response, as well as drug resistance and drug side effects. Different drug combinations can be evaluated for their efficacies [103,104]. Drug development often requires the collection of several years of data, which is a complicated process. Machine learning, on the other hand, can process the data within a short period, greatly reducing the time to develop drugs [105,106,107,108]. Zhavoronkov et al., developed a DL algorithm model and found a powerful inhibitor of DDR1, a kinase target involved in a variety of cancers, in 21 days, compared with the time of about a year by conventional processes [109]. In addition to the neural network models that focus on drug molecule production, non-neural network models have remarkable power in predicting drug responses [110]. At present, multiple random forest models, support vector machine models, and other algorithm models have been developed with the capability of predicting various characteristics of drugs, including toxicities, adverse reactions, absorption, metabolism, and excretion [111,112]. AI could also enable the reuse of anti-cancer drugs. Antagonistic autoencoders are applied to full-dose response data measured in cell lines to develop deep-learning models. In addition, new methods such as integrated cell signature libraries (LINCS) have been used to develop transcriptional datasets to facilitate the reuse of datasets [113,114]. Some types of neural networks, including autoencoders, can elucidate the sets of molecules that represent certain activities and generate new knots with similar activities. The use of AI to understand the molecular mechanisms of drugs, estimate the effects of drugs, develop new drugs, and reuse drugs is critical to the future treatment of mediastinal tumors.
Furthermore, AI can sequence cancer genes and analyze the relationship between genotypic and phenotypic characteristics, to understand the biological basis of mediastinal tumors. Davis et al., found a role for F-box/WD repeat protein 7 (Fow7) in the oxidative metabolism of cancer cells through the analysis of gene expression characteristics of the cancer Gene Atlas dataset [115]. Gene mutations associated with mediastinal malignant tumors can be detected by using related algorithms. DeepVariant, a DNN-based method, first generates a read stack image for a candidate variant (making an image classification task) and then predicts its genotype status (homozygous reference, heterozygous variant, and homozygous variant) to detect the variants read by NGS [116].
The application of AI in cancer also includes the collection of comprehensive data, which is the basis of machine learning for cancer diagnosis, treatment, and prognostic analysis. Patients’ data, such as demographic information, family history, clinical symptoms, comorbidities, histopathological features, immunohistochemistry results, nucleic acid sequencing, biochemical analysis, digital images, and empirical measurements are collected using digital devices, and statistical and mathematical models are constructed [117]. Based on these data, data integration and analysis are performed to facilitate the tracking of long-term patient information [118,119].
Multi-omics integration will also be a key feature for the combination of mediastinal tumors and AI in the future. Data sources from different omics platforms are normalized, compared, and analyzed to establish the relationships among various groups, and a comprehensive and in-depth interpretation of biological processes at the gene, transcriptional, protein, and metabolic levels is carried out by integrating multiple omics data, to better understand the biological systems [120,121,122].
In conclusion, many pan-cancer events and machine learning combinations are currently being attempted, suggesting the infinite possibilities of application in handling mediastinal malignant tumors. However, mediastinal cancer is complex from its definition to its specific treatment. It is significant to use various learning methods and different data granularities to identify the right way to manage mediastinal malignancies in clinical practice. Breakthroughs can be made with the help of artificial intelligence in the diagnosis, treatment, and prognostic evaluation of mediastinal cancers [123,124].
6. Summary
Although AI has made rapid progress in recent years, its application in oncology is still full of unknowns and challenges. The application of AI in different cancers is disproportionate, and enough attention has not been paid to mediastinal tumors, showing a huge gap and potential for future development. The use of artificial intelligence in various fields, including the medical field, still presents many challenges, such as the lack of standardization, the immaturity of the technology, the high cost, the controversies in moral and ethical aspects, and poor supervision. It should be clear that AI could not become a remedy for all mediastinal tumors, or completely replace the analysis with human intelligence. However, AI can be gradually applied in the diagnosis, treatment, and prognosis assessment of mediastinal tumors to produce remarkable improvements. We firmly believe that there will be more AI-involved tasks in the medical field in the future, with generalization, accuracy, and stability, benefitting humans.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12082818/s1, Table S1: Application of artificial intelligence in diagnosing mediastinal malignant tumors.
Author Contributions
Conceptualization, supervision, and writing—review and editing, X.M.; methodology, investigation and formal analysis, W.X.; writing—original draft preparation and data curation, J.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This review study did not require ethical approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the materials and information will be available upon request by e-mail to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ramesh A.N., Kambhampati C., Monson J.R., Drew P.J. Artificial intelligence in medicine. Ann. R. Coll. Surg. Engl. 2004;86:334–338. doi: 10.1308/147870804290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jean A. A brief history of artificial intelligence. Med. Sci. 2020;36:1059–1067. doi: 10.1051/medsci/2020189. [DOI] [PubMed] [Google Scholar]
- 3.Choi R.Y., Coyner A.S., Kalpathy-Cramer J., Chiang M.F., Campbell J.P. Introduction to Machine Learning, Neural Networks, and Deep Learning. Transl. Vis. Sci. Technol. 2020;9:14. doi: 10.1167/tvst.9.2.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirschberg J., Manning C.D. Advances in natural language processing. Science. 2015;349:261–266. doi: 10.1126/science.aaa8685. [DOI] [PubMed] [Google Scholar]
- 5.Moravčík M., Schmid M., Burch N., Lisý V., Morrill D., Bard N., Davis T., Waugh K., Johanson M., Bowling M. DeepStack: Expert-level artificial intelligence in heads-up no-limit poker. Science. 2017;356:508–513. doi: 10.1126/science.aam6960. [DOI] [PubMed] [Google Scholar]
- 6.Milne-Ives M., de Cock C., Lim E., Shehadeh M.H., de Pennington N., Mole G., Normando E., Meinert E. The Effectiveness of Artificial Intelligence Conversational Agents in Health Care: Systematic Review. J. Med. Internet Res. 2020;22:e20346. doi: 10.2196/20346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrade F.A.D.A., Hovenburg A.R., de de Lima L.N., Rodin C.D., Johansen T.A., Storvold R., Correia C.A.M., Haddad D.B. Autonomous Unmanned Aerial Vehicles in Search and Rescue Missions Using Real-Time Cooperative Model Predictive Control. Sensors. 2019;19:4067. doi: 10.3390/s19194067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Litjens G., Kooi T., Bejnordi B.E., Setio A.A.A., Ciompi F., Ghafoorian M., van der Laak J.A.W.M., van Ginneken B., Sánchez C.I. A survey on deep learning in medical image analysis. Med. Image Anal. 2017;42:60–88. doi: 10.1016/j.media.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 10.Ruffini E., Oliaro A., Novero D., Campisi P., Filosso P.L. Neuroendocrine tumors of the thymus. Thorac. Surg. Clin. 2011;21:13–23. doi: 10.1016/j.thorsurg.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Marx A., Chan J.K.C., Chalabreysse L., Dacic S., Detterbeck F., French C.A., Hornick J.L., Inagaki H., Jain D., Lazar A.J., et al. The 2021 WHO Classification of Tumors of the Thymus and Mediastinum: What Is New in Thymic Epithelial, Germ Cell, and Mesenchymal Tumors? J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer. 2022;17:200–213. doi: 10.1016/j.jtho.2021.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Arrossi A.V., Dermawan J.K., Bolen M., Raymond D. Thymomas with Intravascular and Intracardiac Growth. Front. Oncol. 2022;12:881553. doi: 10.3389/fonc.2022.881553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koga K., Matsuno Y., Noguchi M., Mukai K., Asamura H., Goya T., Shimosato Y. A review of 79 thymomas: Modification of staging system and reappraisal of conventional division into invasive and non-invasive thymoma. Pathol. Int. 1994;44:359–367. doi: 10.1111/j.1440-1827.1994.tb02936.x. [DOI] [PubMed] [Google Scholar]
- 14.Vobugari N., Raja V., Sethi U., Gandhi K., Raja K., Surani S.R. Advancements in Oncology with Artificial Intelligence—A Review Article. Cancers. 2022;14:1349. doi: 10.3390/cancers14051349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aamir S., Rahim A., Aamir Z., Abbasi S.F., Khan M.S., Alhaisoni M., Khan K., Ahmad J. Predicting Breast Cancer Leveraging Supervised Machine Learning Techniques. Comput. Math. Methods Med. 2022;2022:5869529. doi: 10.1155/2022/5869529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDonald R.J., Schwartz K.M., Eckel L.J., Diehn F.E., Hunt C.H., Bartholmai B.J., Erickson B.J., Kallmes D.F. The effects of changes in utilization and technological advancements of cross-sectional imaging on radiologist workload. Acad. Radiol. 2015;22:1191–1198. doi: 10.1016/j.acra.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Hosny A., Parmar C., Quackenbush J., Schwartz L.H., Aerts H. Artificial intelligence in radiology. Nat. Rev. Cancer. 2018;18:500–510. doi: 10.1038/s41568-018-0016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castellino R.A. Computer aided detection (CAD): An overview. Cancer Imaging. 2005;5:17–19. doi: 10.1102/1470-7330.2005.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esteva A., Kuprel B., Novoa R.A., Ko J., Swetter S.M., Blau H.M., Thrun S. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115–118. doi: 10.1038/nature21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozkan E., Orhan K., Soydal C., Kahya Y., Tunc S.S., Celik O., Sak S.D., Cangir A.K. Combined clinical and specific positron emission tomography/computed tomography-based radiomic features and machine-learning model in prediction of thymoma risk groups. Nucl. Med. Commun. 2022;43:529–539. doi: 10.1097/MNM.0000000000001547. [DOI] [PubMed] [Google Scholar]
- 21.Dai H., Huang Y., Xiao G., Lan B., Jiang G., Tian J. Predictive Features of Thymic Carcinoma and High-Risk Thymomas Using Random Forest Analysis. J. Comput. Assist. Tomogr. 2020;44:857–864. doi: 10.1097/RCT.0000000000000953. [DOI] [PubMed] [Google Scholar]
- 22.Lin C.-Y., Yen Y.-T., Huang L.-T., Chen T.-Y., Liu Y.-S., Tang S.-Y., Huang W.-L., Chen Y.-Y., Lai C.-H., Fang Y.-H.D., et al. An MRI-Based Clinical-Perfusion Model Predicts Pathological Subtypes of Prevascular Mediastinal Tumors. Diagnostics. 2022;12:889. doi: 10.3390/diagnostics12040889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chowdhary R., Chufal K., Ahmad I., Chhabra A., Jwala M., Pahuja A., Sharma M., Gairola M. Artificial Intelligence Enabled Prognostic Modelling for Thymomas. Int. J. Radiat. Oncol. Biol. Phys. 2020;108:e787. doi: 10.1016/j.ijrobp.2020.07.256. [DOI] [Google Scholar]
- 24.Shen Y.-T., Chen L., Yue W.-W., Xu H.-X. Artificial intelligence in ultrasound. Eur. J. Radiol. 2021;139:109717. doi: 10.1016/j.ejrad.2021.109717. [DOI] [PubMed] [Google Scholar]
- 25.Seyyed-Kalantari L., Zhang H., McDermott M.B.A., Chen I.Y., Ghassemi M. Underdiagnosis bias of artificial intelligence algorithms applied to chest radiographs in under-served patient populations. Nat. Med. 2021;27:2176–2182. doi: 10.1038/s41591-021-01595-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bondeven P., Laurberg S., Hagemann-Madsen R.H., Pedersen B.G. Suboptimal surgery and omission of neoadjuvant therapy for upper rectal cancer is associated with a high risk of local recurrence. Color. Dis. 2015;17:216–224. doi: 10.1111/codi.12869. [DOI] [PubMed] [Google Scholar]
- 27.Tran K.A., Kondrashova O., Bradley A., Williams E.D., Pearson J.V., Waddell N. Deep learning in cancer diagnosis, prognosis and treatment selection. Genome Med. 2021;13:152. doi: 10.1186/s13073-021-00968-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vu T.H., Mousavi H.S., Monga V., Rao G., Rao U.K.A. Histopathological Image Classification Using Discriminative Feature-Oriented Dictionary Learning. IEEE Trans. Med. Imaging. 2016;35:738–751. doi: 10.1109/TMI.2015.2493530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalra S., Tizhoosh H.R., Shah S., Choi C., Damaskinos S., Safarpoor A., Shafiei S., Babaie M., Diamandis P., Campbell C.J.V., et al. Pan-cancer diagnostic consensus through searching archival histopathology images using artificial intelligence. NPJ Digit. Med. 2020;3:31. doi: 10.1038/s41746-020-0238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mellors R.C., Silver R. A micro-fluorometric scanner for the differential detection of cells; application of exfoliative cytology. Science. 1951;114:356–360. doi: 10.1126/science.114.2962.356. [DOI] [PubMed] [Google Scholar]
- 31.Tolles W.E., Bostrom R.C. Automatic screening of cytological smears for cancer: The instrumentation. Ann. N. Y. Acad. Sci. 1956;63:1211–1218. doi: 10.1111/j.1749-6632.1956.tb32131.x. [DOI] [PubMed] [Google Scholar]
- 32.Qiu H., Ding S., Liu J., Wang L., Wang X. Applications of Artificial Intelligence in Screening, Diagnosis, Treatment, and Prognosis of Colorectal Cancer. Curr. Oncol. 2022;29:146. doi: 10.3390/curroncol29030146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmitz R., Madesta F., Nielsen M., Krause J., Steurer S., Werner R., Rösch T. Multi-scale fully convolutional neural networks for histopathology image segmentation: From nuclear aberrations to the global tissue architecture. Med. Image Anal. 2021;70:101996. doi: 10.1016/j.media.2021.101996. [DOI] [PubMed] [Google Scholar]
- 34.DiPalma J., Suriawinata A.A., Tafe L.J., Torresani L., Hassanpour S. Resolution-based distillation for efficient histology image classification. Artif. Intell. Med. 2021;119:102136. doi: 10.1016/j.artmed.2021.102136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryu H.S., Jin M.-S., Park J.H., Lee S., Cho J., Oh S., Kwak T.-Y., Woo J.I., Mun Y., Kim S.W., et al. Automated Gleason Scoring and Tumor Quantification in Prostate Core Needle Biopsy Images Using Deep Neural Networks and Its Comparison with Pathologist-Based Assessment. Cancers. 2019;11:1860. doi: 10.3390/cancers11121860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimada Y., Okuda S., Watanabe Y., Tajima Y., Nagahashi M., Ichikawa H., Nakano M., Sakata J., Takii Y., Kawasaki T., et al. Histopathological characteristics and artificial intelligence for predicting tumor mutational burden-high colorectal cancer. J. Gastroenterol. 2021;56:547–559. doi: 10.1007/s00535-021-01789-w. [DOI] [PubMed] [Google Scholar]
- 37.Xu Y., Jia Z., Wang L.-B., Ai Y., Zhang F., Lai M., Chang E.I.-C. Large scale tissue histopathology image classification, segmentation, and visualization via deep convolutional activation features. BMC Bioinform. 2017;18:281. doi: 10.1186/s12859-017-1685-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kather J.N., Pearson A.T., Halama N., Jäger D., Krause J., Loosen S.H., Marx A., Boor P., Tacke F., Neumann U.P., et al. Deep learning can predict microsatellite instability directly from histology in gastrointestinal cancer. Nat. Med. 2019;25:1054–1056. doi: 10.1038/s41591-019-0462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El Achi H., Khoury J.D. Artificial Intelligence and Digital Microscopy Applications in Diagnostic Hematopathology. Cancers. 2020;12:797. doi: 10.3390/cancers12040797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bejnordi B.E., Mullooly M., Pfeiffer R.M., Fan S., Vacek P.M., Weaver D.L., Herschorn S., Brinton L.A., van Ginneken B., Karssemeijer N., et al. Using deep convolutional neural networks to identify and classify tumor-associated stroma in diagnostic breast biopsies. Mod. Pathol. 2018;31:1502–1512. doi: 10.1038/s41379-018-0073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coudray N., Ocampo P.S., Sakellaropoulos T., Narula N., Snuderl M., Fenyö D., Moreira A.L., Razavian N., Tsirigos A. Classification and mutation prediction from non–small cell lung cancer histopathology images using deep learning. Nat. Med. 2018;24:1559–1567. doi: 10.1038/s41591-018-0177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leiro-Fernández V., Fernández-Villar A. Mediastinal staging for non-small cell lung cancer. Transl. Lung Cancer Res. 2021;10:496–505. doi: 10.21037/tlcr.2020.03.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamada M., Saito Y., Imaoka H., Saiko M., Yamada S., Kondo H., Takamaru H., Sakamoto T., Sese J., Kuchiba A., et al. Development of a real-time endoscopic image diagnosis support system using deep learning technology in colonoscopy. Sci. Rep. 2019;9:14465. doi: 10.1038/s41598-019-50567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar R., Mittal B.R., Bhattacharya A., Singh H., Bal A., Prakash G., Singh N. 18F-FDG PET/CT-Guided Real-Time Automated Robotic Arm-Assisted Needle Navigation for Percutaneous Biopsy of Hypermetabolic Bone Lesions: Diagnostic Performance and Clinical Impact. Am. J. Roentgenol. 2019;212:W10–W18. doi: 10.2214/AJR.18.19698. [DOI] [PubMed] [Google Scholar]
- 45.Engelhardt K.E., DeCamp M.M., Yang A.D., Bilimoria K.Y., Odell D.D. Treatment Approaches and Outcomes for Primary Mediastinal Sarcoma: Analysis of 976 Patients. Ann. Thorac. Surg. 2018;106:333–339. doi: 10.1016/j.athoracsur.2018.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo D.X., Huang M.J., Xiong B., Li T., Xie K., Chen F.R., Che G.W., Wang J., Xu Y., Zhou X.J., et al. Primary mediastinal sarcoma: Surgical outcomes of 21 cases. Interact. Cardiovasc. Thorac. Surg. 2013;17:982–986. doi: 10.1093/icvts/ivt354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zehani A., Ayadi-Kaddour A., Daghfous H., Ridene I., Marghli A., Kilani T., El Mezni F. Primary mediastinal sarcomas. Rev. Des Mal. Respir. 2011;28:14–24. doi: 10.1016/j.rmr.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 48.Abdel-Rahman O. An analysis of clinical characteristics and patient outcomes in primary mediastinal sarcomas. Expert Rev. Anticancer. Ther. 2017;17:1071–1076. doi: 10.1080/14737140.2017.1378576. [DOI] [PubMed] [Google Scholar]
- 49.Suster D.I. The role of molecular pathology in mediastinal sarcomas. Mediastinum. 2020;4:33. doi: 10.21037/med-20-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Capper D., Jones D.T., Sill M., Hovestadt V., Schrimpf D., Sturm D., Koelsche C., Sahm F., Chavez L., Reuss D.E., et al. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555:469–474. doi: 10.1038/nature26000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wightman S.C., Shrager J.B. Non-Myasthenia Gravis Immune Syndromes and the Thymus: Is There a Role for Thymectomy? Thorac. Surg. Clin. 2019;29:215–225. doi: 10.1016/j.thorsurg.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 52.Issoufou I., Lakranbi M., Sani R., Belliraj L., Ammor F.Z., Ghalimi J., Ouadnouni Y., Smahi M. Neurogenic mediastinal tumors in adults. Rev. Pneumol. Clin. 2016;72:310–315. doi: 10.1016/j.pneumo.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 53.Friedant A.J., Handorf E.A., Su S., Scott W.J. Minimally Invasive versus Open Thymectomy for Thymic Malignancies: Systematic Review and Meta-Analysis. J. Thorac. Oncol. 2016;11:30–38. doi: 10.1016/j.jtho.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mehta C., Raparia K., Bharat A. Anterior Mediastinal Myelolipoma. Ann. Thorac. Surg. 2017;103:e81. doi: 10.1016/j.athoracsur.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 55.Nagahiro I., Andou A., Aoe M., Sano Y., Date H., Shimizu N. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: A comparison of VATS and conventional procedure. Ann. Thorac. Surg. 2001;72:362–365. doi: 10.1016/S0003-4975(01)02804-1. [DOI] [PubMed] [Google Scholar]
- 56.Dieter R.A., Jr., Kuzyçz G.B. Complications and contraindications of thoracoscopy. Int. Surg. 1997;82:232–239. [PubMed] [Google Scholar]
- 57.Raffort J., Adam C., Carrier M., Ballaith A., Coscas R., Jean-Baptiste E., Hassen-Khodja R., Chakfé N., Lareyre F. Artificial intelligence in abdominal aortic aneurysm. J. Vasc. Surg. 2020;72:321–333.e1. doi: 10.1016/j.jvs.2019.12.026. [DOI] [PubMed] [Google Scholar]
- 58.Huo J., Huang G., Han D., Wang X., Bu Y., Chen Y., Cai D., Zhao C. Value of 3D preoperative planning for primary total hip arthroplasty based on artificial intelligence technology. J. Orthop. Surg. Res. 2021;16:156. doi: 10.1186/s13018-021-02294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iftikhar P.M., Kuijpers M.V., Khayyat A., Iftikhar A., De Sa M.D. Artificial Intelligence: A New Paradigm in Obstetrics and Gynecology Research and Clinical Practice. Cureus. 2020;12:e7124. doi: 10.7759/cureus.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kawaguchi Y., Matsuura Y., Kondo Y., Ichinose J., Nakao M., Okumura S., Mun M. The predictive power of artificial intelligence on mediastinal lymphnode metastasis. Gen. Thorac. Cardiovasc. Surg. 2021;69:1545–1552. doi: 10.1007/s11748-021-01671-9. [DOI] [PubMed] [Google Scholar]
- 61.De Perrot M., McRae K. Evidence for thymectomy in myasthenia gravis: Getting stronger? J. Thorac. Cardiovasc. Surg. 2017;154:314–316. doi: 10.1016/j.jtcvs.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 62.Amore D., Cicalese M., Scaramuzzi R., Di Natale D., Casazza D., Curcio C. Hybrid robotic thoracic surgery for excision of large mediastinal masses. J. Vis. Surg. 2018;4:105. doi: 10.21037/jovs.2018.05.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen X., Ma Q., Wang S., Zhang H., Huang D. Surgical treatment of posterior mediastinal neurogenic tumors. J. Surg. Oncol. 2019;119:807–813. doi: 10.1002/jso.25381. [DOI] [PubMed] [Google Scholar]
- 64.Weksler B., Tavares J., Newhook T.E., Greenleaf C.E., Diehl J.T. Robot-assisted thymectomy is superior to transsternal thymectomy. Surg. Endosc. 2012;26:261–266. doi: 10.1007/s00464-011-1879-7. [DOI] [PubMed] [Google Scholar]
- 65.Seong Y.W., Kang C.H., Choi J.-W., Kim H.-S., Jeon J.H., Park I.K., Kim Y.T. Early clinical outcomes of robot-assisted surgery for anterior mediastinal mass: Its superiority over a conventional sternotomy approach evaluated by propensity score matching. Eur. J. Cardiothorac. Surg. 2014;45:e68–e73; discussion e73. doi: 10.1093/ejcts/ezt557. [DOI] [PubMed] [Google Scholar]
- 66.Loftus T.J., Tighe P.J., Filiberto A.C., Efron P.A., Brakenridge S.C., Mohr A.M., Rashidi P., Upchurch G.R., Bihorac A. Artificial Intelligence and Surgical Decision-making. JAMA Surg. 2020;155:148–158. doi: 10.1001/jamasurg.2019.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cakar F., Werner P., Augustin F., Schmid T., Wolf-Magele A., Sieb M., Bodner J. A comparison of outcomes after robotic open extended thymectomy for myasthenia gravis. Eur. J. Cardio-Thorac. Surg. 2007;31:501–504; discussion 504–505. doi: 10.1016/j.ejcts.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 68.Ricciardi S., Zirafa C.C., Davini F., Melfi F. How to get the best from robotic thoracic surgery. J. Thorac. Dis. 2018;10((Suppl. S8)):S947–S950. doi: 10.21037/jtd.2018.03.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gumbs A.A., Frigerio I., Spolverato G., Croner R., Illanes A., Chouillard E., Elyan E. Artificial Intelligence Surgery: How Do We Get to Autonomous Actions in Surgery? Sensors. 2021;21:5526. doi: 10.3390/s21165526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Berishvili V., Voronkov A.E., Radchenko E.V., Palyulin V.A. Palyulin, Machine Learning Classification Models to Improve the Docking-based Screening: A Case of PI3K-Tankyrase Inhibitors. Mol. Inform. 2018;37:e1800030. doi: 10.1002/minf.201800030. [DOI] [PubMed] [Google Scholar]
- 71.Sakellaropoulos T., Vougas K., Narang S., Koinis F., Kotsinas A., Polyzos A., Moss T.J., Piha-Paul S., Zhou H., Kardala E., et al. A Deep Learning Framework for Predicting Response to Therapy in Cancer. Cell Rep. 2019;29:3367–3373.e4. doi: 10.1016/j.celrep.2019.11.017. [DOI] [PubMed] [Google Scholar]
- 72.Janssen B.V., Theijse R., van Roessel S., de Ruiter R., Berkel A., Huiskens J., Busch O.R., Wilmink J.W., Kazemier G., Valkema P., et al. Artificial Intelligence-Based Segmentation of Residual Tumor in Histopathology of Pancreatic Cancer after Neoadjuvant Treatment. Cancers. 2021;13:5089. doi: 10.3390/cancers13205089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moussa H.G., Husseini G.A., Abel-Jabbar N., Ahmad S.E. Use of Model Predictive Control and Artificial Neural Networks to Optimize the Ultrasonic Release of a Model Drug from Liposomes. IEEE Trans. Nanobioscience. 2017;16:149–156. doi: 10.1109/TNB.2017.2661322. [DOI] [PubMed] [Google Scholar]
- 74.Mak K.-K., Pichika M.R. Artificial intelligence in drug development: Present status and future prospects. Drug Discov. Today. 2019;24:773–780. doi: 10.1016/j.drudis.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 75.Patel V., Shah M. Artificial intelligence and machine learning in drug discovery and development. Intell. Med. 2022;2:134–140. doi: 10.1016/j.imed.2021.10.001. [DOI] [Google Scholar]
- 76.Mukhopadhyay A., Sumner J., Ling L.H., Quek R.H.C., Tan A.T.H., Teng G.G., Seetharaman S.K., Gollamudi S.P.K., Ho D., Motani M. Personalised Dosing Using the CURATE.AI Algorithm: Protocol for a Feasibility Study in Patients with Hypertension and Type II Diabetes Mellitus. Int. J. Environ. Res. Public Health. 2022;19:8979. doi: 10.3390/ijerph19158979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hussain A., Malik A., Halim M.U., Ali A.M. The use of robotics in surgery: A review. Int. J. Clin. Pract. 2014;68:1376–1382. doi: 10.1111/ijcp.12492. [DOI] [PubMed] [Google Scholar]
- 78.Zhao J., Bhatnagar V., Ding L., Atay S.M., David E.A., McFadden P.M., Stamnes S., Lechtholz-Zey E., Wightman S.C., Detterbeck F.C., et al. A systematic review of paraneoplastic syndromes associated with thymoma: Treatment modalities, recurrence, and outcomes in resected cases. J. Thorac. Cardiovasc. Surg. 2020;160:306–314.e14. doi: 10.1016/j.jtcvs.2019.11.052. [DOI] [PubMed] [Google Scholar]
- 79.Thummerer A., Seller Oria C., Zaffino P., Visser S., Meijers A., Guterres Marmitt G., Wijsman R., Seco J., Langendijk J.A., Knopf A.C., et al. Deep learning-based 4D-synthetic CTs from sparse-view CBCTs for dose calculations in adaptive proton therapy. Med. Phys. 2022;49:6824–6839. doi: 10.1002/mp.15930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yuzhen N.W., Barrett S. A review of automatic lung tumour segmentation in the era of 4DCT. Rep. Pract. Oncol. Radiother. 2019;24:208–220. doi: 10.1016/j.rpor.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lustberg T., van Soest J., Gooding M., Peressutti D., Aljabar P., van der Stoep J., van Elmpt W., Dekker A. Clinical evaluation of atlas and deep learning based automatic contouring for lung cancer. Radiother. Oncol. 2018;126:312–317. doi: 10.1016/j.radonc.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 82.Nguyen D., Long T., Jia X., Lu W., Gu X., Iqbal Z., Jiang S. A feasibility study for predicting optimal radiation therapy dose distributions of prostate cancer patients from patient anatomy using deep learning. Sci. Rep. 2019;9:1076. doi: 10.1038/s41598-018-37741-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang C., Zhu X., Hong J.C., Zheng D. Artificial Intelligence in Radiotherapy Treatment Planning: Present and Future. Technol. Cancer Res. Treat. 2019;18:1533033819873922. doi: 10.1177/1533033819873922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu Z., Fan J., Li M., Yan H., Hu Z., Huang P., Tian Y., Miao J., Dai J. A deep learning method for prediction of three-dimensional dose distribution of helical tomotherapy. Med. Phys. 2019;46:1972–1983. doi: 10.1002/mp.13490. [DOI] [PubMed] [Google Scholar]
- 85.Wu Q.J., Li T., Wu Q., Yin F.F. Adaptive radiation therapy: Technical components and clinical applications. Cancer J. 2011;17:182–189. doi: 10.1097/PPO.0b013e31821da9d8. [DOI] [PubMed] [Google Scholar]
- 86.Schwaab J., Prall M., Sarti C., Kaderka R., Bert C., Kurz C., Parodi K., Günther M., Jenne J. Ultrasound tracking for intra-fractional motion compensation in radiation therapy. Phys. Med. 2014;30:578–582. doi: 10.1016/j.ejmp.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 87.Jiang Z., Chen Y., Zhang Y., Ge Y., Yin F.-F., Ren L. Augmentation of CBCT Reconstructed from Under-Sampled Projections Using Deep Learning. IEEE Trans. Med. Imaging. 2019;38:2705–2715. doi: 10.1109/TMI.2019.2912791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Madesta F., Sentker T., Gauer T., Werner R. Self-contained deep learning-based boosting of 4D cone-beam CT reconstruction. Med. Phys. 2020;47:5619–5631. doi: 10.1002/mp.14441. [DOI] [PubMed] [Google Scholar]
- 89.Maspero M., Savenije M.H.F., Dinkla A.M., Seevinck P.R., Intven M.P.W., Juergenliemk-Schulz I.M., Kerkmeijer L.G.W., Berg C.A.T.V.D. Dose evaluation of fast synthetic-CT generation using a generative adversarial network for general pelvis MR-only radiotherapy. Phys. Med. Biol. 2018;63:185001. doi: 10.1088/1361-6560/aada6d. [DOI] [PubMed] [Google Scholar]
- 90.Lei Y., Fu Y., Wang T., Liu Y., Patel P., Curran W.J., Liu T., Yang X. 4D-CT deformable image registration using multiscale unsupervised deep learning. Phys. Med. Biol. 2020;65:085003. doi: 10.1088/1361-6560/ab79c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pei Q., Luo Y., Chen Y., Li J., Xie D., Ye T. Artificial intelligence in clinical applications for lung cancer: Diagnosis, treatment and prognosis. Clin. Chem. Lab. Med. 2022;60:1974–1983. doi: 10.1515/cclm-2022-0291. [DOI] [PubMed] [Google Scholar]
- 92.Bychkov D., Linder N., Turkki R., Nordling S., Kovanen P.E., Verrill C., Walliander M., Lundin M., Haglund C., Lundin J. Deep learning based tissue analysis predicts outcome in colorectal cancer. Sci. Rep. 2018;8:3395. doi: 10.1038/s41598-018-21758-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Courtiol P., Maussion C., Moarii M., Pronier E., Pilcer S., Sefta M., Manceron P., Toldo S., Zaslavskiy M., Le Stang N., et al. Deep learning-based classification of mesothelioma improves prediction of patient outcome. Nat. Med. 2019;25:1519–1525. doi: 10.1038/s41591-019-0583-3. [DOI] [PubMed] [Google Scholar]
- 94.Zhong Y., She Y., Deng J., Chen S., Wang T., Yang M., Ma M., Song Y., Qi H., Wang Y., et al. Deep Learning for Prediction of N2 Metastasis and Survival for Clinical Stage I Non-Small Cell Lung Cancer. Radiology. 2022;302:200–211. doi: 10.1148/radiol.2021210902. [DOI] [PubMed] [Google Scholar]
- 95.Matsuo K., Purushotham S., Jiang B., Mandelbaum R.S., Takiuchi T., Liu Y., Roman L.D. Survival outcome prediction in cervical cancer: Cox models vs deep-learning model. Am. J. Obstet. Gynecol. 2019;220:381.e1–381.e14. doi: 10.1016/j.ajog.2018.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sailer F., Pobiruchin M., Bochum S., Martens U.M., Schramm W. Prediction of 5-Year Survival with Data Mining Algorithms. Stud. Health Technol. Inform. 2015;213:75–78. [PubMed] [Google Scholar]
- 97.Skrede O.J., De Raedt S., Kleppe A., Hveem T.S., Liestøl K., Maddison J., Askautrud H.A., Pradhan M., Nesheim J.A., Albregtsen F., et al. Deep learning for prediction of colorectal cancer outcome: A discovery and validation study. Lancet. 2020;395:350–360. doi: 10.1016/S0140-6736(19)32998-8. [DOI] [PubMed] [Google Scholar]
- 98.Blasiak A., Khong J., Kee T. CURATE.AI: Optimizing Personalized Medicine with Artificial Intelligence. SLAS Technol. 2020;25:95–105. doi: 10.1177/2472630319890316. [DOI] [PubMed] [Google Scholar]
- 99.Shen W.-C., Chen S.-W., Wu K.-C., Hsieh T.-C., Liang J.-A., Hung Y.-C., Yeh L.-S., Chang W.-C., Lin W.-C., Yen K.-Y., et al. Prediction of local relapse and distant metastasis in patients with definitive chemoradiotherapy-treated cervical cancer by deep learning from [(18)F]-fluorodeoxyglucose positron emission tomography/computed tomography. Eur. Radiol. 2019;29:6741–6749. doi: 10.1007/s00330-019-06265-x. [DOI] [PubMed] [Google Scholar]
- 100.Aramendía-Vidaurreta V., Cabeza R., Villanueva A., Navallas J., Alcázar J.L. Ultrasound Image Discrimination between Benign and Malignant Adnexal Masses Based on a Neural Network Approach. Ultrasound Med. Biol. 2016;42:742–752. doi: 10.1016/j.ultrasmedbio.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 101.Zhang L., Huang J., Liu L. Improved Deep Learning Network Based in combination with Cost-sensitive Learning for Early Detection of Ovarian Cancer in Color Ultrasound Detecting System. J. Med. Syst. 2019;43:251. doi: 10.1007/s10916-019-1356-8. [DOI] [PubMed] [Google Scholar]
- 102.Pergialiotis V., Pouliakis A., Parthenis C., Damaskou V., Chrelias C., Papantoniou N., Panayiotides I. The utility of artificial neural networks and classification and regression trees for the prediction of endometrial cancer in postmenopausal women. Public Health. 2018;164:1–6. doi: 10.1016/j.puhe.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 103.Simon A.B., Vitzthum L.K., Mell L.K. Challenge of Directly Comparing Imaging-Based Diagnoses Made by Machine Learning Algorithms with Those Made by Human Clinicians. J. Clin. Oncol. 2020;38:1868–1869. doi: 10.1200/JCO.19.03350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Goecks J., Jalili V., Heiser L.M., Gray J.W. How Machine Learning Will Transform Biomedicine. Cell. 2020;181:92–101. doi: 10.1016/j.cell.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vamathevan J., Clark D., Czodrowski P., Dunham I., Ferran E., Lee G., Li B., Madabhushi A., Shah P., Spitzer M., et al. Applications of machine learning in drug discovery and development. Nat. Rev. Drug Discov. 2019;18:463–477. doi: 10.1038/s41573-019-0024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nascimento A.C.A., Prudêncio R.B.C., Costa I.G. A Drug-Target Network-Based Supervised Machine Learning Repurposing Method Allowing the Use of Multiple Heterogeneous Information Sources. Methods Mol. Biol. 2019;1903:281–289. doi: 10.1007/978-1-4939-8955-3_17. [DOI] [PubMed] [Google Scholar]
- 107.Baskin I.I. The power of deep learning to ligand-based novel drug discovery. Expert Opin. Drug Discov. 2020;15:755–764. doi: 10.1080/17460441.2020.1745183. [DOI] [PubMed] [Google Scholar]
- 108.Ballester P.J. Machine Learning for Molecular Modelling in Drug Design. Biomolecules. 2019;9:216. doi: 10.3390/biom9060216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bhinder B., Gilvary C., Madhukar N.S., Elemento O. Artificial Intelligence in Cancer Research and Precision Medicine. Cancer Discov. 2021;11:900–915. doi: 10.1158/2159-8290.CD-21-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gayvert K.M., Madhukar N.S., Elemento O. A Data-Driven Approach to Predicting Successes and Failures of Clinical Trials. Cell Chem. Biol. 2016;23:1294–1301. doi: 10.1016/j.chembiol.2016.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Martins I.F., Teixeira A.L., Pinheiro L., Falcao A.O. A Bayesian approach to in silico blood-brain barrier penetration modeling. J. Chem. Inf. Model. 2012;52:1686–1697. doi: 10.1021/ci300124c. [DOI] [PubMed] [Google Scholar]
- 112.Shen J., Cheng F., Xu Y., Li W., Tang Y. Estimation of ADME properties with substructure pattern recognition. J. Chem. Inf. Model. 2010;50:1034–1041. doi: 10.1021/ci100104j. [DOI] [PubMed] [Google Scholar]
- 113.Subramanian A., Narayan R., Corsello S.M., Peck D.D., Natoli T.E., Lu X., Gould J., Davis J.F., Tubelli A.A., Asiedu J.K., et al. A Next Generation Connectivity Map: L1000 Platform and the First 1,000,000 Profiles. Cell. 2017;171:1437–1452.e17. doi: 10.1016/j.cell.2017.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kadurin A., Aliper A., Kazennov A., Mamoshina P., Vanhaelen Q., Khrabrov K., Zhavoronkov A. The cornucopia of meaningful leads: Applying deep adversarial autoencoders for new molecule development in oncology. Oncotarget. 2017;8:10883–10890. doi: 10.18632/oncotarget.14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Davis R.J., Gönen M., Margineantu D.H., Handeli S., Swanger J., Hoellerbauer P., Paddison P.J., Gu H., Raftery D., Grim J.E., et al. Pan-cancer transcriptional signatures predictive of oncogenic mutations reveal that Fbw7 regulates cancer cell oxidative metabolism. Proc. Natl. Acad. Sci. USA. 2018;115:5462–5467. doi: 10.1073/pnas.1718338115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Poplin R., Chang P.-C., Alexander D., Schwartz S., Colthurst T., Ku A., Newburger D., Dijamco J., Nguyen N., Afshar P.T., et al. A universal SNP and small-indel variant caller using deep neural networks. Nat. Biotechnol. 2018;36:983–987. doi: 10.1038/nbt.4235. [DOI] [PubMed] [Google Scholar]
- 117.Cirillo D., Núñez-Carpintero I., Valencia A. Artificial intelligence in cancer research: Learning at different levels of data granularity. Mol. Oncol. 2021;15:817–829. doi: 10.1002/1878-0261.12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Troyanskaya O., Trajanoski Z., Carpenter A., Thrun S., Razavian N., Oliver N. Artificial intelligence and cancer. Nat. Cancer. 2020;1:149–152. doi: 10.1038/s43018-020-0034-6. [DOI] [PubMed] [Google Scholar]
- 119.Liu S.-H., Shen P.-C., Chen C.-Y., Hsu A.-N., Cho Y.-C., Lai Y.-L., Chen F.-H., Li C.-Y., Wang S.-C., Chen M., et al. DriverDBv3: A multi-omics database for cancer driver gene research. Nucleic Acids Res. 2020;48:D863–D870. doi: 10.1093/nar/gkz964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chaudhary K., Poirion O.B., Lu L., Garmire L.X. Deep Learning-Based Multi-Omics Integration Robustly Predicts Survival in Liver Cancer. Clin. Cancer Res. 2018;24:1248–1259. doi: 10.1158/1078-0432.CCR-17-0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Malik V., Kalakoti Y., Sundar D. Deep learning assisted multi-omics integration for survival and drug-response prediction in breast cancer. BMC Genom. 2021;22:214. doi: 10.1186/s12864-021-07524-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Poirion O.B., Jing Z., Chaudhary K., Huang S., Garmire L.X. DeepProg: An ensemble of deep-learning and machine-learning models for prognosis prediction using multi-omics data. Genome Med. 2021;13:112. doi: 10.1186/s13073-021-00930-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 124.Topol E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019;25:44–56. doi: 10.1038/s41591-018-0300-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the materials and information will be available upon request by e-mail to the corresponding author.