Abstract
Microbial phytosterol degradation is accompanied by the formation of steroid pathway intermediates, which are potential precursors in the synthesis of bioactive steroids. Degradation of these steroid intermediates is initiated by Δ1-dehydrogenation of the steroid ring structure. Characterization of a 2.9-kb DNA fragment of Rhodococcus erythropolis SQ1 revealed an open reading frame (kstD) showing similarity with known 3-ketosteroid Δ1-dehydrogenase genes. Heterologous expression of kstD yielded 3-ketosteroid Δ1-dehydrogenase (KSTD) activity under the control of the lac promoter in Escherichia coli. Targeted disruption of the kstD gene in R. erythropolis SQ1 was achieved, resulting in loss of more than 99% of the KSTD activity. However, growth on the steroid substrate 4-androstene-3,17-dione or 9α-hydroxy-4-androstene-3,17-dione was not abolished by the kstD gene disruption. Bioconversion of phytosterols was also not blocked at the level of Δ1-dehydrogenation in the kstD mutant strain, since no accumulation of steroid pathway intermediates was observed. Thus, inactivation of kstD is not sufficient for inactivation of the Δ1-dehydrogenase activity. Native polyacrylamide gel electrophoresis of cell extracts stained for KSTD activity showed that R. erythropolis SQ1 in fact harbors two activity bands, one of which is absent in the kstD mutant strain.
Rhodococcus species are well known for their catabolic potential (5, 40). Several Rhodococcus species degrade natural phytosterols. Microbial phytosterol degradation proceeds via the formation of steroids as pathway intermediates (16, 21, 22), i.e., 4-androstene-3,17-dione, 1,4-androstadiene-3,17-dione, and 9α-hydroxy-4-androstene-3,17-dione (Fig. 1). These steroid pathway intermediates may be used as precursors for the production of bioactive steroids (16). Degradation of the steroid ring skeleton is initiated by Δ1-dehydrogenation, inactivation of which is generally considered necessary to achieve accumulation of steroid pathway intermediates from phytosterols (16, 22). Rhodococcus and Mycobacterium strains treated with mutagens and/or incubated with enzyme inhibitors have been reported to convert sterols into 4-androstene-3,17-dione and 1,4-androstadiene-3,17-dione (21, 22). The industrial performance of these strains is generally inadequate, however, due to strain instability and low conversion efficiencies.
FIG. 1.
Proposed pathway for microbial phytosterol (e.g., β-sitosterol) degradation (16, 22). AD, 4-androstene-3,17-dione; ADD, 1,4-androstadiene-3,17-dione; 9OHAD, 9α-hydroxy-4-androstene-3,17-dione; 9OHADD, 9α-hydroxy-1,4-androstadiene-3,17-dione; KSTH, steroid 9α-hydroxylase.
Steroid accumulation by strains constructed via genetic engineering has not been reported thus far, probably due to a limited knowledge of the sterol catabolic pathway and the genetics of these microorganisms. The aims of our work are to develop genetic tools for Rhodococcus species, to apply these for a detailed molecular characterization of the sterol degradation pathway, and to disrupt selected target genes in order to achieve accumulation of steroid pathway intermediates from phytosterols.
The enzyme 3-ketosteroid Δ1-dehydrogenase (KSTD) [4-ene-3-oxosteroid:(acceptor)-1-ene-oxidoreductase; EC 1.3.99.4] performs the Δ1-dehydrogenation of the steroid polycyclic ring structure; its inactivation thus may lead to accumulation of 9α-hydroxy-4-androstene-3,17-dione from steroid compounds (Fig. 1). The enzyme has been characterized from several bacteria: Arthrobacter simplex (29), Pseudomonas spp. (19, 20), Nocardia restrictus (34), Nocardia corallina (13), Nocardia opaca (10), Mycobacterium fortuitum (41), and Rhodococcus erythropolis IMET7030 (15). Two seemingly distinct KSTD activities have been reported in M. fortuitum (41). Whether these activities actually represent separate enzymes was not elucidated. The KSTD of N. opaca has been characterized as a flavoprotein (18). Only the KSTD-encoding genes of A. simplex, Comamonas testosteroni, and Rhodococcus rhodochrous have been fully characterized (23, 24, 30). Although cloning of the gene encoding KSTD and expression of an inactive KSTD protein of R. erythropolis IMET7030 in Escherichia coli have been described (2, 38, 39) and a nucleotide sequence of the KSTD-encoding gene of N. opaca (10) (synonym, R. erythropolis IMET7030 [15]) is available (DDBJ/EMBL/GenBank accession no. U59422), no further characterization of this gene has been reported. Moreover, targeted disruption has not been carried out with either of the known KSTD-encoding genes.
Several methods for transformation of Rhodococcus cells have been developed (17). Homologous recombination events required for gene disruption are usually rare, necessitating higher transformation frequencies. Here we report an optimized electrotransformation protocol for R. erythropolis SQ1 (32), a detailed characterization of its kstD gene encoding KSTD, and the effects of a targeted kstD disruption on steroid degradation ability.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Plasmids and bacterial strains used are listed in Table 1. Rhodococcus strains were cultivated at 30°C and 250 rpm in liquid medium (LBP) containing 1% (wt/vol) Bacto Peptone (Difco, Detroit, Mich.), 0.5% (wt/vol) yeast extract (BBL Becton Dickinson and Company, Cockeysville, Md.), and 1% (wt/vol) NaCl. For growth on solid medium, LBP was supplemented with 1.5% (wt/vol) Bacto Agar (Difco). For steroid bioconversion experiments, strains were grown in YG15 medium (15 g of yeast extract · liter−1, 15 g of glucose · liter−1 [pH 7.0]) at 28°C (200 rpm). E. coli strains (Table 1) were grown in Luria-Bertani (LB) broth at 37°C. BBL agar (1.5% [wt/vol]) was added for growth on solid medium.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Reference or origin |
|---|---|---|
| Strains | ||
| Rhodococcus spp. | ||
| R. erythropolis SQ1 | Mutant of ATCC 4277-1 with increased transformability | 31 |
| R. erythropolis SDH420 | kstD mutant of R. erythropolis SQ1 | This study |
| R. erythropolis 141.93 | UV mutant of ATCC 4277 | Diosynth collection |
| R. erythropolis ATCC 11048 | Wild type able to degrade sterols | Diosynth collection |
| R. erythropolis ATCC 25544 | Wild type able to degrade sterols | Diosynth collection |
| R. rhodochrous 142.32 | UV mutant of ATCC 12674 | Diosynth collection |
| E. coli | ||
| DH5α | supE44 ΔlacU169 (φ80 lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | Bethesda Research Laboratories |
| JM110 | dam dcm supE44 hsdR17 thi leu rpsL lacY galK galT ara tonA thr tsx Δ(lac-proAB) F′[traD36 proAB+ lacIqlacZΔM15] | Bethesda Research Laboratories |
| Plasmids | ||
| pBlueScript(II) KS | bla lacZ | Stratagene |
| pDA71 | Rhodococcus-E. coli shuttle vector; cat ecoRI bla | 8 |
| pMVS301 | Rhodococcus-E. coli shuttle vector; tsr bla aphII sacB | 36 |
| pWJ5 | 14 | |
| pBsKm2 | aphII of pWJ5 in pBlueScript(II) KS | This study |
| pSDH100 | 6-kb BglII DNA fragment containing kstD ligated in BglII-digested pDA71 | This study |
| pSDH101 | ClaI/BamHI fragment of pSDH205 (Fig. 2) in pDA71 digested with SfuI and BglII; complements the kstD mutant | This study |
| pSDH200 | 6-kb BglII fragment of pSDH100 in the BamHI site of pBlueScript(II) KS | This study |
| pSDH205 | 2.9-kb EcoRV fragment of pSDH200 with kstD gene in the EcoRV site of pBlueScript | This study |
| pSDH305 | Expression in kstD by lac promoter; 1,756-bp ClaIBamHI fragment of pSDH205 (Fig. 2) ligated in ClaI/BamHI site of pBlueScript(II) KS | This study |
| pSDH309 | kstD in opposite direction of lac promoter; EcoRV/ClaI (Klenow fill-in) fragment of pSDH205 (Fig. 2) ligated in EcoRV site of pBlueScript(II) KS | This study |
| pSDH420 | kstD disruption vector containing aphII and a 741-bp Asp718/SalI kstD internal fragment | This study |
General cloning techniques.
Recombinant DNA techniques were used according to standard protocols (33). DNA isolation procedures for Rhodococcus plasmid DNA were performed as described by Vogt-Singer and Finnerty (37). DNA-modifying enzymes were purchased from Boehringer (Mannheim, Germany), New England Biolabs (Beverly, Mass.), or Amersham Pharmacia Biotech AB (Uppsala, Sweden) and were used as described by the manufacturer. Transformation of E. coli was performed as described by Chung et al. (6).
Electrotransformation of Rhodococcus.
LBP broth (300 ml) supplemented with 3% (wt/vol) glycine was inoculated with 5 ml of a 24-h Rhodococcus LBP culture and incubated until late-exponential phase (optical density at 600 nm [OD600], 2 to 3). Addition of 3% (wt/vol) glycine to the growth medium increased transformation frequencies 2.2-fold, and growth of R. erythropolis SQ1 was slightly inhibited. The cells were harvested by centrifugation for 10 min at 4,000 × g (4°C) and washed twice with cold distilled water. The pellet was resuspended in 2 to 3 ml of cold 30% (vol/vol) polyethylene glycol 1450 (PEG 1450). Higher-molecular-weight PEG compounds were also tested but resulted in lower transformation frequencies. Competent cells were divided into 100-μl portions and frozen at −80°C until use. Prior to transformation experiments, cells were thawed on ice. Plasmid DNA (1 μg) was added, and cells were kept on ice for 1 min. Electrotransformation was performed on a BTX600 electroporation apparatus (Biotechnology & Experimental Research Inc., San Diego, Calif.) in 2-mm gapped cuvettes with a single pulse. Both field strength and resistance influenced transformation efficiency, showing optima of 8.75 kV · cm−1 and 186 Ω (50 μF), respectively. These settings generally resulted in an observed field strength of 6.7 kV · cm−1 and time-pulse constants of approximately 8 ms. LBP medium (1 ml) was added immediately after the electropulse. The cell suspension was incubated for 4.5 h with shaking. Appropriate dilutions were plated on LBP agar supplemented with either 40 μg · ml−1 of chloramphenicol · ml−1 (pDA71) or 10 μg of thiostrepton · ml−1 (pMVS301). Transformants appeared after 3 days. The highest transformation frequencies were obtained for R. erythropolis SQ1 with pMVS301 (106 transformants · μg of DNA−1). The same protocol was used in the gene disruption experiment with pSDH420 (Fig. 2), using the antibiotic kanamycin at a concentration of 200 μg · ml−1.
FIG. 2.
Cloning strategies for the construction of pSDH205 and the nonreplicative vector pSDH420 used for kstD targeted gene disruption. ‘kstD’ in pSDH420 indicates the internal 741-bp Asp718/SalI fragment of the kstD gene used for targeted gene disruption of kstD in R. erythropolis SQ1. A 1,756-bp ClaI/BamHI fragment of pSDH205 (sites marked with asterisks) was used for the construction of pSDH101 (for complementation of the kstD mutant phenotype [Table 1]) and pSDH305 (heterologous kstD expression) [Table 1]). ORFs are indicated with arrows. Solid curved lines represent R. erythropolis SQ1 DNA. Open curved lines represent regions of the Rhodococcus-E. coli shuttle vector pDA71, encoding replication in Rhodococcus (rep) and chloramphenicol resistance (cat).
Colony PCR.
A Rhodococcus colony was resuspended in 25 μl of TE buffer (10 mM Tris–1 mM EDTA) and heated for 10 min in boiling water. A kstD PCR product (1,551 bp) was obtained with the kstD forward primer (5′ GCGCATATGCAGGACTGGACCAGCGAGTGC) and reverse primer (5′ GCGGGATCCGCGTTACTTCGCCATGTCCTG), annealing to the 5′ end (including start codon) and 3′ end (including stop codon) of the kstD gene, respectively. Primers were originally designed to include NdeI and BamHI restriction sites for KSTD expression in the T7 RNA polymerase pET3 expression system (Novagen, Madison, Wis.). PCR was performed using 5 cycles of 1 min at 95°C, 1.5 min at 60°C, and 1.5 min at 72°C, followed by 25 cycles of 1 min at 95°C, 1.5 min at 55°C, and 1.5 min at 72°C.
Southern hybridization.
Rhodococcus total DNA was isolated according to the procedure of Verhasselt et al. (36) as modified by Nagy et al. (26). Digested chromosomal DNA from R. erythropolis SQ1 was separated on a 1% (wt/vol) agarose gel and blotted onto a high-bond nylon membrane supplied by Qiagen (Basel, Switzerland), via an alkaline transfer method (33). Southern hybridizations were done at 68°C with a degenerate kstD oligonucleotide [5′ TTCGG (C/G)GG(C/G)AC(C/G)TC(C/G)GC(C/G)TACTC(C/G)GG(C/G)GC(C/G) TC(C/G)ATCTGG] labeled with the digoxigenin (DIG) oligonucleotide tailing kit from Boehringer. The kstD oligonucleotide was based on an amino acid sequence alignment of known KSTD proteins. The membrane was subsequently washed at 68°C with 2× SSC (1× SSC is 0.15 M NaCl and 0.015 M sodium citrate) containing 0.1% (wt/vol) sodium dodecyl sulfate (SDS) for 15 min and twice with 0.1× SSC containing 0.1% (wt/vol) SDS for 10 min. Labeling of the complete kstD gene obtained by PCR was done using the random primed labeling kit from Boehringer. Hybridization was performed at 60°C. Stringent washing (60°C) was done in 2× SSC containing 0.1% (wt/vol) SDS (twice for 5 min each time) and in 0.1× SSC with 0.1% (wt/vol) SDS (twice for 5 min each time).
Steroid bioconversion and steroid analysis.
Steroid bioconversions were done with Rhodococcus cultures grown in 75 ml of YG15 medium at 28°C (200 rpm). After growth overnight to late-exponential phase (OD600, 5 to 9), Generol (5 g · liter−1) or 4-androstene-3,17-dione (5 g · liter−1 in 0.1% [vol/vol] Tween 80) was added and bioconversion was monitored for several days. Steroids were extracted from the medium by methylene chloride extraction (for analysis by gas chromatography [GC]) or by methylene chloride-methanol (9:1) extraction (for analysis by thin-layer chromatography [TLC]). For high-performance liquid chromatography (HPLC) analysis, samples were diluted 5 times with methanol-water (70:30) and filtered (pore size, 0.45 μm). Steroids were analyzed by either HPLC (with a 250- by 3-mm reversed-phase Lichrosorb 10RP18 column [Varian Chrompack International, Middelburg, The Netherlands], UV detection at 254 nm, and a liquid phase of methanol-water [60:40] at 35°C), GC (with a J&W DB-5MS column measuring 30 m by 0.25 mm [inner diameter] with 0.25-μm film [Alltech, Deerfield, Ill.], a precolumn measuring 2 m by 0.25 mm [inner diameter], [InterSciences, Markham, Canada], and FID-40 detection at 300°C), or TLC (with a Kieselgel 60 F254 10- by 20-cm sheet [Merck, Darmstadt, Germany] in toluene-ethyl acetate [7:3]). The substrates used, 4-androstene-3,17-dione, 9α-hydroxy-4-androstene-3,17-dione, 1,4-androstadiene-3,17-dione, and Generol (sterol content, 82.7%, comprising mainly β-sitosterol [40.4%], stigmasterol [16.3%], and campesterol [22.4%]; Henkel, Düsseldorf, Germany), were supplied by Diosynth bv. (Oss, The Netherlands).
Preparation of cell extracts of Rhodococcus and KSTD activity staining on native PAGE.
Overnight cultures (250 ml) grown in YG15 medium were induced with 4-androstene-3,17-dione (0.25 g in 5 ml of dimethyl sulfoxide) for an additional 5 h. Cell pellets (30 min; 7,300 g; 4°C) were washed with 200 ml of phosphate buffer (KH2PO4, 2.72 g · liter−1; K2HPO4, 3.48 g · liter−1; MgSO4 · 7H2O, 2.46 g · liter−1 [pH 7.2]). Pellets were suspended in phosphate buffer in a 1:2 (wt/wt) ratio and sonicated 7 times for 10 s each time with 2-min cooling intervals. Cell extracts were centrifuged for 1 min at 14,000 rpm in an Eppendorf 5415C centrifuge to remove cell debris. The resulting supernatants (10 to 15 mg of protein · ml−1) either were used for analysis on native polyacrylamide gel electrophoresis (PAGE) gels (12.5% acrylamide) or for KSTD activity assays or were stored at −20°C. KSTD activity was visualized by incubating native PAGE gels in 100 ml of 66.7 mM Tris buffer containing 3.1 mg of phenazine methosulfate (PMS), 2.9 mg of a steroid (4-androstene-3,17-dione or 9α-hydroxy-4-androstene-3,17-dione dissolved in 500 μl of ethanol), and 41 mg of nitroblue tetrazolium (NBT) dissolved in 70% dimethyl formamide. Staining was allowed to proceed for several hours until clear activity bands were visible. The reaction was stopped by replacing the staining solution with 10% acetic acid. No KSTD activity staining was found in controls with 1,4-androstadiene-3,17-dione. 4-Androstene-3,17-dione and 9-hydroxy-4-androstene-3,17-dione were equally good steroid substrates for visualizing activity bands.
Heterologous expression of kstD in E. coli cells.
Recombinant E. coli cells were grown overnight and diluted 100-fold in LB broth (250 ml) supplemented with ampicillin (100 μg · ml−1). Isopropyl-β-d-thiogalactopyranoside (IPTG) was added after 3.5 h at a final concentration of 1 mM. After a 4-h induction period, cells were collected by centrifugation and E. coli cell extracts were prepared by sonic treatment as described above for Rhodococcus cell extracts. The resulting supernatants (10 to 15 mg of protein · ml−1) were used for KSTD activity staining.
KSTD enzyme activity assay.
Enzyme activities were measured spectrophotometrically at 25°C using PMS and NBT. The reaction mixture (1 ml) consisted of 0.86 M Tris (pH 9), 150 μM PMS, 550 μM NBT, cell extract, and 200 μM 4-androstene-3,17-dione in 2% methanol. Activities are expressed as milliunits per milligram of protein; 1 mU is defined as the formation of 1 nmol of diformazan · min−1 (ɛ570 = 13 cm2 · μmol−1) from NBT. No activity was observed in reaction mixtures without 4-androstene-3,17-dione.
DNA sequencing.
Nucleotide sequencing was done using dye primers by the cycle sequencing method (25) with the Thermosequenase kit RPN 2538 from Amersham Pharmacia Biotech AB. The samples were run on the ALF-Express sequencing robot. The nucleotide sequence was analyzed using CloneManager, version 4.01.
DDBJ/EMBL/GenBank database accession number.
Nucleotide sequence data have been submitted to the DDBJ/EMBL/GenBank database under accession number AF096929.
RESULTS
Rhodococcus strain selection.
R. erythropolis SQ1 (Table 1) was selected for these studies because it exhibits the highest transformation frequencies, maximizing the probability for successful targeted gene disruption, and is able to degrade phytosterols at a relatively high rate (Table 2). Electrotransformation of R. erythropolis SQ1 with pMVS301 DNA under optimized conditions generally resulted in 106 CFU · μg−1. Lower frequencies were found for pDA71 (Table 2). Evidently, pDA71 has a narrow host range, whereas pMVS301 is able to maintain itself in all Rhodococcus strains tested (Table 2). Vector pDA71, carrying several unique cloning sites within the ecoRI positive selection marker, was used in further work.
TABLE 2.
Bioconversion rates and electrotransformation frequenciesa of some phytosterol (Generol)-degrading Rhodococcus strains
| Strain | Generol degradationb | Transformation frequency (CFU · μg of DNA−1)
|
|
|---|---|---|---|
| pDA71 | pMVS301 | ||
| R. erythropolis SQ1 | ++ | 2.1 × 104 | 1 × 106 |
| R. erythropolis 141.93 | + | 0 | 1.7 × 104 |
| R. erythropolis ATCC 11048 | +++ | 0 | 2.3 × 102 |
| R. erythropolis ATCC 25544 | +++ | 0 | 1.6 × 104 |
| R. rhodochrous 142.32 | ++ | NA | 7.9 × 103 |
Electrotransformation conditions were as follows: 8.75 kV/cm, 186 Ω, 50 μF.
+, <100 mg/liter−1 · h−1; ++, 100 to 200 mg/liter−1 · h−1; +++, >200 mg/liter−1 · h−1.
NA, not applicable; this strain is chloramphenicol resistant.
Cloning and characterization of the kstD gene.
Alignment of the N-terminal parts of known KSTD protein sequences of A. simplex (23), C. testosteroni (30), and N. opaca (10) allowed development of a kstD oligonucleotide probe (see Materials and Methods). The known gene sequence of kstD of N. opaca was used as the primary template.
Southern analysis with the kstD oligonucleotide probe was performed on chromosomal DNA of strain SQ1 digested with several restriction enzymes. Following sucrose gradient centrifugation, a 6-kb BglII DNA fragment, hybridizing with the kstD oligonucleotide, was selected for cloning. This DNA fragment was cloned in the BglII site of pDA71 (pSDH100) and subsequently subcloned into BamHI-digested pBluescript(II) KS (pSDH200) (Fig. 2). Restriction mapping analysis and Southern hybridization showed that an approximately 2.9-kb EcoRV fragment of pSDH200 contained sequences homologous to that of the kstD oligonucleotide. Subcloning (pSDH205) and subsequent nucleotide sequencing of this fragment revealed two open reading frames (ORFs) of 1,533 nt (kstD) and 627 nt (ORF2) encoding putative proteins of 510 and 208 amino acids (aa), respectively. An overall GC content of 63.9% was found; this is relatively high but somewhat lower than previously reported for Rhodococcus spp. (67 to 73%) (11).
kstD (GC content, 64.5%) is preceded by a potential Shine-Dalgarno (S-D) nucleotide sequence (AGGACG-N5-ATG) (where N is any nucleotide) showing high similarity to the consensus S-D nucleotide sequence [(A/G)GGAGG] preceding genes found in the closely related genus Streptomyces (35). The deduced amino acid sequence with a calculated molecular weight of 53,054 showed similarity to known KSTD protein sequences of A. simplex, C. testosteroni, and R. rhodochrous. Also, a protein encoded by rv3537, a hypothetical gene present in Mycobacterium tuberculosis (7), displayed clear similarity with the KSTD-encoding genes. The first 147 aa of strain SQ1 KSTD showed 100% identity to the KSTD N-terminal sequences of N. opaca (10). The complete protein sequence of KSTD from N. opaca is available in databases (accession number Q04616) but contains several discrepancies compared to the strain SQ1 KSTD sequence. This appeared to be due to a large number of frameshifts in the nucleotide sequence of the former. KSTD from N. opaca therefore was not included in the KSTD alignment.
Alignment of the five KSTD amino acid sequences revealed an overall sequence identity (similarity) of 14% (35%). The R. erythropolis SQ1 KSTD protein showed a higher identity with the homologs of gram-positive bacteria (41 to 46%) than with the KSTD of the gram-negative bacterium C. testosteroni (34%). Strikingly, the degrees of identity and similarity for KSTD are much higher between R. rhodochrous and A. simplex than between the two different Rhodococcus species themselves: 68 and 88% versus 45 and 75%, respectively.
The active center motif of fumarate reductase flavocytochrome c of Shewanella putrefaciens (28) can be aligned with a conserved region in KSTD of R. erythropolis SQ1, as was previously shown by Molnár et al. (23) for KSTD of A. simplex. Other rather conserved regions can be distinguished in both the N-terminal (aa 1 to 100) and the C-terminal (aa 400 to 510) regions of the KSTD proteins. These regions most likely are of structural significance in flavin adenine dinucleotide (FAD) binding and binding of the substrate. In the KSTD of R. erythropolis SQ1 and the KSTD of C. testosteroni, the third glycine of the putative FAD-binding motif (GXGXXG) near the N terminus was replaced by an alanine. Furthermore, a conserved glutamic acid residue was found at position 37. A glutamic acid or aspartic acid residue at this position relative to the FAD-binding motif is thought to be involved in FAD binding as well (27). In summary, a consensus sequence for the putative FAD-binding pocket in KSTD enzymes can be deduced: GSG(G/A)(G/A)(G/A)X17E.
Southern analysis showed that no additional hybridizing sequences of kstD were present in the R. erythropolis SQ1 genome. Also, a reduced hybridization stringency did not reveal additional hybridization signals (data not shown).
Targeted disruption of the kstD gene.
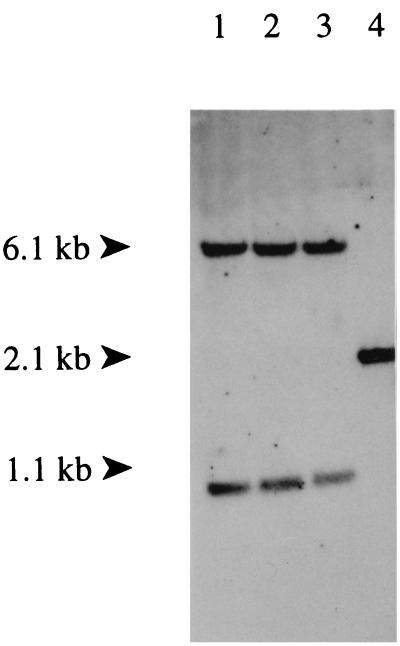
Construct pSDH420 (Table 1; Fig. 2), a nonreplicative vector in Rhodococcus containing the aphII marker of Tn5 (4) and a 741-bp Asp718/SalI internal DNA fragment of the kstD gene, was made for targeted gene disruption of kstD. After electrotransformation, a preliminary screening for integration of the vector at the kstD locus of R. erythropolis SQ1 was performed by colony PCR. Individual transformants were checked for loss of the wild-type kstD PCR fragment (1,551 bp) using forward and reverse kstD primers (Fig. 3). For 9 out of 13 transformants, no wild-type kstD PCR fragment was obtained, suggesting targeted disruption of kstD. Three of these nine transformants were used for further analysis. Confirmation of genuine targeted kstD gene disruption was obtained from Southern analysis (Fig. 4). Genomic DNA of the wild-type strain and three individual transformants was digested with ClaI and hybridized with the complete kstD gene (Fig. 4). A 2,095 bp DNA fragment of wild-type genomic DNA hybridizing with the kstD probe (Fig. 4, lane 4) was replaced by two DNA fragments of 1.06 and 6.06 kb in all three transformants (Fig. 4, lanes 1 to 3). This corresponds with what would be expected from integration of pSDH420 into the kstD gene by a single recombination event (Fig. 3). The kstD gene disruption mutant strain was designated R. erythropolis SDH420.
FIG. 3.
Schematic overview of targeted gene disruption of kstD by integration of pSDH420 into the R. erythropolis SQ1 chromosome. Single homologous recombination between the wild-type kstD gene on the R. erythropolis SQ1 chromosome and a 741-bp Asp718/SalI internal kstD fragment located on pSDH420 divides the wild-type ClaI DNA fragment (2,095 bp) into two ClaI DNA fragments of 1.06 and 6.05 kb. ORF2, encoding a TetR type of repressor protein, was identified upstream of kstD. Forward and reverse kstD primers, used for screening of potential kstD gene disruption mutants with colony PCR, are indicated by arrows.
FIG. 4.
Southern analysis of genomic DNA digested with ClaI of three individual kstD mutants (lanes 1 to 3) and wild-type R. erythropolis SQ1 (lane 4). The complete kstD gene, obtained with PCR using forward and reverse kstD primers, was used as a DIG-labeled probe.
The wild-type kstD PCR fragment was found in 4 out of 13 transformants, illustrating integration of the pSDH420 vector at locations other than the kstD locus. Integration of nonreplicative vector DNA due to illegitimate recombination has been described previously for Rhodococcus fascians (9) and for Rhodococcus globerulus (3).
Characterization of R. erythropolis strain SDH420.
Wild-type KSTD activity obtained after induction with 4-androstene-3,17-dione (855 ± 104 mU · mg−1) had become reduced significantly in the kstD mutant (2.7 ± 0.8 mU · mg−1). Targeted gene disruption of kstD thus does not result in complete loss of KSTD activity in R. erythropolis SDH420. Growth of the kstD mutant on mineral agar plates supplemented with either 4-androstene-3,17-dione or 9α-hydroxy-4-androstene-3,17-dione as a sole carbon source was not blocked. Also, bioconversion of phytosterols by R. erythropolis SDH420 remained virtually unaffected, and no accumulation of either of the expected steroid pathway intermediates 4-androstene-3,17-dione and 9α-hydroxy-4-androstene-3,17-dione from phytosterols (Fig. 1) was observed.
Reintroduction of kstD (pSDH101 [Table 1]) in the kstD mutant strain SDH420 restored KSTD activity to 476 ± 90 mU · mg−1, excluding the possibility that the KSTD-negative phenotype was due to some polar effect caused by genomic integration of the pSDH420 vector.
Heterologous expression of the kstD gene.
Exponential-phase cells of E. coli DH5α harboring pSDH305 (Table 1) expressed the kstD gene (1383 ± 140 mU · mg−1) under the control of the lac promoter (Fig. 5). No KSTD activity could be detected when the kstD gene was cloned in the direction opposite that of the lac promoter (pSDH309 [Table 1]), indicating that the kstD promoter is not functional in E. coli.
FIG. 5.
KSTD activity staining, using 4-androstene-3,17-dione as a substrate, on native PAGE gels loaded with cell extracts (approximately 35 μg of protein) of noninduced wild-type R. erythropolis SQ1 (lane 1), 4-androstene-3,17-dione-induced wild-type R. erythropolis SQ1 (lane 2), 4-androstene-3,17-dione-induced SDH420 (a kstD mutant) (lane 3), E. coli DH5α/pBlueScript(II) KS (lane 4), and E. coli DH5α/pSDH305 (lane 5). No activity bands could be visualized in controls using 1,4-androstadiene-3,17-dione as a substrate for staining.
KSTD activity staining.
The possible presence of additional KSTD enzymatic activities in R. erythropolis SQ1 was subsequently investigated. Staining for KSTD activity on native PAGE gels loaded with extracts of noninduced cells revealed two weak activity bands (Fig. 5, lane 1). The band with the highest electrophoretic mobility was expressed in E. coli DH5α/pSDH305 (KSTD; Fig. 5, lane 5). Cells of strain SQ1 induced with 4-androstene-3,17-dione showed induction of both activity bands (Fig. 5, lane 2). Targeted disruption of kstD abolished only the activity band with the highest electrophoretic mobility (KSTD), while the second activity band remained intact (Fig. 5, lane 3), indicating the presence of an additional KSTD enzymatic activity.
DISCUSSION
The ability to degrade phytosterols is widespread in nocardioform actinomycetes and requires a set of enzymes degrading the phytosterol aliphatic side chain and the steroid polycyclic ring structure (Fig. 1). Accumulation of steroids from phytosterols can be achieved only when the steroid skeleton is not enzymatically attacked. Steroid degradation may be blocked in mutants with inactivated KSTD and/or steroid 9α-hydroxylase enzymes isolated following UV irradiation or treatment with chemical mutagens. Alternatively, specific chelating agents may be used to inhibit steroid 9α-hydroxylase activity. Both these approaches, however, have some drawbacks. Chelating compounds are known to be inhibitory to sterol side chain cleavage as well (22), and mutagenized strains generally suffer from genetic instability during bioconversion processes. In our view, a molecular approach, in which stable and well-defined mutations are introduced by targeted disruption of selected genes involved in steroid ring degradation, will allow construction of Rhodococcus strains accumulating steroid pathway intermediates in efficient phytosterol bioconversion processes.
To our knowledge this is the first report on targeted disruption of an actinomycete kstD gene encoding a steroid catabolic enzyme, KSTD, and one of the first examples of targeted gene disruption in the genus Rhodococcus (17, 31). Disruption of the R. erythropolis SQ1 kstD gene resulted in a strong reduction in, but not in complete loss of, KSTD activity. Native PAGE of cell extracts stained for KSTD activity revealed that the remaining activity was not due to incomplete inactivation of the kstD-encoded KSTD activity. Instead, these studies provided evidence for the presence of two enzyme activities, one of which is responsible for the remaining activity observed in the kstD mutant strain (Fig. 5). This remaining activity appears to be more pronounced on native PAGE gels than in a direct KSTD assay. We believe this is due to the fact that native PAGE gel slabs are stained for several hours to visualize both activity bands. Activity staining does not proceed linearly during this period of time: when staining of KSTD is maximal, further staining of the other activity band still occurs. In addition, the remaining activity may be enhanced by electrophoresis, separating the enzyme from inhibitory factors. Disruption of kstD alone thus is insufficient for complete inactivation of KSTD activity in R. erythropolis strain SQ1 and explains why accumulation of steroid pathway intermediates from bioconversion of phytosterols did not occur in the kstD mutant strain. The absence of steroid accumulation in the kstD mutant, however, seems contradictory with the loss of more than 99% of KSTD activity (remaining KSTD activity, 2.7 mU · mg−1) observed in this mutant. This may imply that Δ1-dehydrogenation is not the rate-limiting step in phytosterol degradation, although alternative pathways of sterol degradation may very well exist. The observed growth of the kstD mutant on agar plates supplemented with the steroid substrate 4-androstene-3,17-dione or 9α-hydroxy-4-androstene-3,17-dione can also be explained by the presence of a second KSTD activity. These two KSTD enzymes in vivo could be responsible for the Δ1-dehydrogenation of either 4-androstene-3,17-dione or 9α-hydroxy-4-androstene-3,17-dione. KSTD activity staining of native PAGE gels showed, however, that 4-androstene-3,17-dione and 9α-hydroxy-4-androstene-3,17-dione are substrates of both enzymatic activities (data not shown), which suggests that these enzymes function as isoenzymes in vivo.
Upstream of the kstD gene a second, divergently transcribed putative regulatory gene (ORF2) was found that carries the consensus sequence of repressor proteins of the TetR family (1). Members of this family are generally transcribed divergently from the genes under their control, which suggests that the ORF2-encoded protein acts as a repressor of kstD expression. The ORF2-encoded protein shows no similarity with the putative DNA-binding regulator protein encoded by ksdR of A. simplex preceding ksdD (23). No regulatory gene has been found near ksdD in R. rhodochrous (24). No cotranscribed sequences were found downstream of kstD in R. erythropolis SQ1, as is the case in R. rhodochrous (24). Clustering of a few genes involved in steroid degradation has been reported for both A. simplex and C. testosteroni. The ksdD gene of A. simplex is thought to be translationally coupled to ksdI, which encodes a putative 3-ketosteroid-Δ5-isomerase (KS5IS). In C. testosteroni the gene encoding KSTD (Δ1dh) is cotranscribed with the gene encoding 3-ketosteroid Δ4-(5α)-dehydrogenase [Δ4-(5α)dh] (12). The molecular organization of steroid catabolic genes in R. erythropolis SQ1 thus differs from that in other microorganisms studied.
In this report we have shown that inactivation of KSTD activity, attempting to block steroid degradation with the aim of accumulating valuable steroid pathway intermediates, cannot be achieved by the inactivation of a single gene encoding this activity. Evidence is presented that a KSTD isoenzyme is present that prevents accumulation of steroid pathway intermediates from microbial phytosterol bioconversion. A more detailed understanding of the biological significance of the presence of KSTD isoenzymes in sterol-degrading bacteria clearly is of both scientific and applied interest. The effects of inactivation of both these enzymes will be investigated in more detail in further work.
ACKNOWLEDGMENTS
Thanks are due to I. Nagy and R. de Mot (University of Leuven, Leuven, Belgium) for their contributions in the early stages of the work on transformation of Rhodococcus. We are indebted to E. R. Dabbs (University of Witwatersrand, Johannesburg, South Africa) for providing R. erythropolis SQ1 and pDA71. We also thank Jörn Kalinowski for providing pWJ5.
This work was funded by the Programma Bedrijfsgerichte Technologie Stimulering (PBTS) grant BIO94049 in cooperation with Diosynth bv.
REFERENCES
- 1.Amaraki H, Yagi N, Suzuki M. Residues important for the function of a multihelical DNA binding domain in the new transcription factor family of Cam and Tet repressors. Protein Eng. 1995;8:1259–1266. doi: 10.1093/protein/8.12.1259. [DOI] [PubMed] [Google Scholar]
- 2.Atrat P, Curtiss R, Clark-Curtiss J E. High yield recombinant steroid-1-dehydrogenase production by culturing E. coli carrying plasmid containing rhodococcal gene for the enzyme under control of lac promoter. German patent DD285995. 1991. [Google Scholar]
- 3.Barnes M R, Duetz W A, Williams P A. A 3-(3-hydroxyphenyl)propionic acid catabolic pathway in Rhodococcus globerulus PWD1: cloning and characterization of the hpp operon. J Bacteriol. 1997;179:6145–6153. doi: 10.1128/jb.179.19.6145-6153.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck E, Ludwig G, Auerswald E A, Reiss B, Schaller H. Nucleotide sequence and exact localization of the neomycin phosphotransferase gene from Tn5. Gene. 1982;19:327–336. doi: 10.1016/0378-1119(82)90023-3. [DOI] [PubMed] [Google Scholar]
- 5.Bell K S, Philp J C, Aw D W J, Christofi N. The genus Rhodococcus. J Appl Microbiol. 1998;85:195–210. doi: 10.1046/j.1365-2672.1998.00525.x. [DOI] [PubMed] [Google Scholar]
- 6.Chung C T, Niemela S L, Miller R H. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci USA. 1989;86:2172–2175. doi: 10.1073/pnas.86.7.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry III C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail M A, Rajandream M-A, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston J E, Taylor K, Whitehead S, Barrell B G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 8.Dabbs E R, Gowan B, Quan S, Andersen S J. Development of improved Rhodococcus plasmid vectors and their use in cloning genes of potential commercial and medical importance. Biotechnologia. 1995;7–8:129–135. [Google Scholar]
- 9.Desomer J, Crespi M, Van Montagu M. Illegitimate integration of non-replicative vectors in the genome of Rhodococcus fascians upon electrotransformation as an insertional mutagenesis system. Mol Microbiol. 1991;5:2115–2124. doi: 10.1111/j.1365-2958.1991.tb02141.x. [DOI] [PubMed] [Google Scholar]
- 10.Drobnič K, Križaj L, Gubenšek F, Komel R. Improved purification of steroid 1:2-dehydrogenase from Nocardia opaca and partial characterization of its cloned gene sequence. Biochem Biophys Res Commun. 1993;190:509–515. doi: 10.1006/bbrc.1993.1077. [DOI] [PubMed] [Google Scholar]
- 11.Finnerty W R. The biology and genetics of the genus Rhodococcus. Annu Rev Microbiol. 1992;46:193–218. doi: 10.1146/annurev.mi.46.100192.001205. [DOI] [PubMed] [Google Scholar]
- 12.Florin C, Köhler T, Grandguillot M, Plesiat P. Comamonas testosteroni 3-ketosteroid-Δ4(5α)-dehydrogenase: gene and protein characterization. J Bacteriol. 1996;178:3322–3330. doi: 10.1128/jb.178.11.3322-3330.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Itagaki E, Wakabayashi T, Hatta T. Purification and characterization of 3-ketosteroid-Δ1-dehydrogenase from Nocardia corallina. Biochim Biophys Acta. 1990;1038:60–67. doi: 10.1016/0167-4838(90)90010-d. [DOI] [PubMed] [Google Scholar]
- 14.Jäger W, Schäfer A, Pühler A, Labes G, Wohlleben W. Expression of the Bacillus subtilis sacB gene leads to sucrose sensitivity in the gram-positive bacterium Corynebacterium glutamicum but not in Streptomyces lividans. J Bacteriol. 1992;174:5462–5465. doi: 10.1128/jb.174.16.5462-5465.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaufmann G, Thole H, Kraft R, Atrat P. Steroid-1-dehydrogenase of Rhodococcus erythropolis: purification and N-terminal amino acid sequence. J Steroid Biochem Mol Biol. 1992;43:297–301. doi: 10.1016/0960-0760(92)90164-e. [DOI] [PubMed] [Google Scholar]
- 16.Kieslich K. Microbial side-chain degradation of sterols. J Basic Microbiol. 1985;25:461–474. doi: 10.1002/jobm.3620250713. [DOI] [PubMed] [Google Scholar]
- 17.Larkin M J, De Mot R, Kulakov L A, Nagy I. Applied aspects of Rhodococcus genetics. Antonie Leeuwenhoek. 1998;74:133–153. doi: 10.1023/a:1001776500413. [DOI] [PubMed] [Google Scholar]
- 18.Lestrovaja N N, Dänhardt S, Hörhold C. Steroidumwandelnde Enzyme aus Mikroorganismen. V. Reinigung einer 4-En-3-oxosteroid: (Akzeptor)-1-en-oxidoreductase aus Nocardia opaca und Charakterisierung der prosthetischen Gruppe. Z Allg Mikrobiol. 1978;18:189–196. doi: 10.1002/jobm.3630180306. [DOI] [PubMed] [Google Scholar]
- 19.Levy H R, Talalay P. Bacterial oxidation of steroids. I. Ring A dehydrogenations by intact cells. J Biol Chem. 1959;234:2009–2013. [PubMed] [Google Scholar]
- 20.Levy H R, Talalay P. Bacterial oxidation of steroids. II. Studies on the enzymatic mechanism of ring A dehydrogenation. J Biol Chem. 1959;234:2014–2021. [PubMed] [Google Scholar]
- 21.Mahato S B, Garai S. Advances in microbial steroid biotransformation. Steroids. 1997;62:332–345. doi: 10.1016/s0039-128x(96)00251-6. [DOI] [PubMed] [Google Scholar]
- 22.Martin C K A. Microbial cleavage of sterol side chains. Adv Appl Microbiol. 1977;22:29–58. doi: 10.1016/s0065-2164(08)70159-x. [DOI] [PubMed] [Google Scholar]
- 23.Molnár I, Choi K-P, Yamashita M, Murooka Y. Molecular cloning, expression in Streptomyces lividans, and analysis of a gene cluster from Arthrobacter simplex encoding 3-ketosteroid-Δ1-dehydrogenase, 3-ketosteroid-Δ5-isomerase and a hypothetical regulatory protein. Mol Microbiol. 1995;15:895–905. doi: 10.1111/j.1365-2958.1995.tb02359.x. [DOI] [PubMed] [Google Scholar]
- 24.Morii S, Fujii C, Miyoshi T, Iwami M, Itagaki E. 3-Ketosteroid-Δ1-dehydrogenase of Rhodococcus rhodochrous: sequencing of the genomic DNA and hyperexpression, purification, and characterization of the recombinant enzyme. J Biochem. 1998;124:1026–1032. doi: 10.1093/oxfordjournals.jbchem.a022195. [DOI] [PubMed] [Google Scholar]
- 25.Murray V. Improved double-stranded DNA sequencing using the linear polymerase chain reaction. Nucleic Acids Res. 1989;17:8889. doi: 10.1093/nar/17.21.8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagy I, Schoofs G, Compernolle F, Proost P, Vanderleyden J, De Mot R. Degradation of the thiocarbamate herbicide EPTC (S-ethyl dipropylcarbamothioate) and biosafening by Rhodococcus sp. strain NI86/21 involve an inducible cytochrome P-450 system and aldehyde dehydrogenase. J Bacteriol. 1995;177:676–687. doi: 10.1128/jb.177.3.676-687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishiya Y, Imanaka T. Analysis of interaction between the Arthrobacter sarcosine oxidase and the coenzyme flavin adenine dinucleotide by site-directed mutagenesis. Appl Environ Microbiol. 1996;62:2405–2410. doi: 10.1128/aem.62.7.2405-2410.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pealing S L, Black A C, Manson F D C, Ward B, Chapman S K, Reid G A. Sequence of the gene encoding flavocytochrome c from Shewanella putrefaciens: a tetraheme flavoenzyme that is a soluble fumarate reductase related to the membrane-bound enzymes from other bacteria. Biochemistry. 1992;31:12132–12140. doi: 10.1021/bi00163a023. [DOI] [PubMed] [Google Scholar]
- 29.Penasse L, Peyre M. Studies of 3-oxo steroid Δ1-oxidoreductase of Arthrobacter simplex. Steroids. 1968;12:525–544. doi: 10.1016/s0039-128x(68)80116-3. [DOI] [PubMed] [Google Scholar]
- 30.Plesiat P, Grandguillot M, Harayama S, Vragar S, Michel-Briand Y. Cloning, sequencing, and expression of the Pseudomonas testosteroni gene encoding 3-oxosteroid Δ1-dehydrogenase. J Bacteriol. 1991;173:7219–7227. doi: 10.1128/jb.173.22.7219-7227.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Powell J A C, Archer J A C. Molecular characterisation of a Rhodococcus oph operon. Antonie Leeuwenhoek. 1998;74:175–188. doi: 10.1023/a:1001784702230. [DOI] [PubMed] [Google Scholar]
- 32.Quan S, Dabbs E R. Nocardioform arsenic resistance plasmid characterization and improved Rhodococcus cloning vectors. Plasmid. 1993;29:74–79. doi: 10.1006/plas.1993.1010. [DOI] [PubMed] [Google Scholar]
- 33.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 34.Sih C J, Bennet R E. Steroid 1-dehydrogenase of Nocardia restrictus. Biochim Biophys Acta. 1962;56:587–592. doi: 10.1016/0006-3002(62)90610-8. [DOI] [PubMed] [Google Scholar]
- 35.Strohl W R. Compilation and analysis of DNA sequences associated with apparent streptomycete promoters. Nucleic Acids Res. 1992;20:961–974. doi: 10.1093/nar/20.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verhasselt P, Poncelet F, Vits K, Van Gool A, Vanderleyden J. Cloning and expression of a Clostridium acetobutylicum α-amylase gene in Escherichia coli. FEMS Microbiol Lett. 1989;59:135–140. doi: 10.1111/j.1574-6968.1989.tb03097.x. [DOI] [PubMed] [Google Scholar]
- 37.Vogt-Singer M E, Finnerty W R. Construction of an Escherichia coli-Rhodococcus shuttle vector and plasmid transformation in Rhodococcus spp. J Bacteriol. 1988;170:638–645. doi: 10.1128/jb.170.2.638-645.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagner B, Atrat P G, Clark-Curtiss J E, Wagner M. Localization of the steroid 1-dehydrogenase in Rhodococcus erythropolis IMET 7030 by immunoelectron microscopy. J Basic Microbiol. 1992;32:65–71. doi: 10.1002/jobm.3620320119. [DOI] [PubMed] [Google Scholar]
- 39.Wagner M, Atrat P G, Wagner B, Hanemann V, Clark-Curtiss J E. Overexpression of a Rhodococcus erythropolis protein in Escherichia coli with immunological identity to the Rhodococcus steroid 1-dehydrogenase. Immunoelectron microscopic localization and electrophoretic studies. J Basic Microbiol. 1992;32:269–277. doi: 10.1002/jobm.3620320409. [DOI] [PubMed] [Google Scholar]
- 40.Warhurst A M, Fewson C A. Biotransformations catalyzed by the genus Rhodococcus. Crit Rev Biotechnol. 1994;14:29–73. doi: 10.3109/07388559409079833. [DOI] [PubMed] [Google Scholar]
- 41.Wovcha M G, Brooks K E, Kominek L A. Evidence for two steroid 1,2-dehydrogenase activities in Mycobacterium fortuitum. Biochim Biophys Acta. 1979;574:471–479. doi: 10.1016/0005-2760(79)90243-1. [DOI] [PubMed] [Google Scholar]