Abstract
In this study, 99 strains of Aureobasidium species were isolated from various samples collected from different locations in China, among which 14 isolates showed different morphological characteristics to other strains identified as known Aureobasidium species. Based on morphological characteristics, those 14 strains were classified into four groups, represented by stains of KCL139, MDSC−10, XZY411−4, and MQL9−100, respectively. Molecular analysis of the internal transcriptional spacer (ITS) and part of the large ribosome subunit (D1/D2 domains) indicated that those four groups represent four new species in the Aureobasidium. Therefore, the names Aureobasidium insectorum sp. nov., A. planticola sp. nov., A. motuoense sp. nov., and A. intercalariosporum sp. nov. are proposed for KCL139, MDSC−10, XZY411−4, and MQL9−100, respectively. We also found that there were differences in the yield of exopolysaccharides (EPS) among and within species, indicating strain-related exopolysaccharide-producing diversity.
Keywords: black yeasts, four new species, molecular phylogeny
1. Introduction
Aureobasidium (Ascomycota: Dothideales) is a yeast-like fungal genus that is often called black yeast because of the production of melanin during its growth [1,2,3]. Species of Aureobasidium are widely distributed and normally possess multiple trophic modes. They are often found as saprophytes, endophytes, and pathogens in diverse environments, such as plant materials (roots, leaves, bark), water, marine sediments, swamps, soil, air, skin, and high osmotic environments (significant osmotic stress) [4,5,6,7,8,9,10,11]. The species of Aureobasidium produce one-celled conidia of various shapes from hyaline and terminal, lateral, or intercalary conidiogenous cells [4,9].
The genus Aureobasidium was first described by Viala and Boyer based on the isolates on grape leaves [10]. Hermanides-Nijhof assessed the phenotypic variety of Aureobasidium and related genera, and distinguished Aureobasidium from the related enteric Hormonema according to the mode of conidial production. Aureobasidium produced synchronous blastoconidia from undifferentiated, hyaline cells, whereas Hormonema produced conidia in basipetal succession from hyaline or dark hyphae [4]. De Hoog and Yurlova revised Aureobasidium taxonomy based on the morphology, physiology, and biochemistry, and thus the genus included three species: Aureobasidium pullulans, A. melanogenum, and A. aubasidani [5,6,7]. In 2008, Zalar et al. carried out a molecular analysis of A. pullulans and A. melanogenum, and identified the studied strains as A. pullulans, A. melanogenum, A. subglaciale, and A. namibiae [8,9]. In recent years, with the high accessibility of sequencing services and a large amount of available molecular data, the number of novel Aureobasidium species is increasing. Thirteen new species have been proposed, namely, Aureobasidium acericola [10], A. aerium [11], A. castaneae [12], A. iranianum [13], A. leucospermi [14], A. mangrovei [15], A. microtermitis [16], A. mustum [17], A. pini [18], A. thailandense [19], A. tremulum [20], and A. uvarum and A. vineae [17].
With the increasing number of Aureobasidium species, its functional activities have been explored. For example, Aureobasidium species are resistant to Botrytis cinerea and Rhizopus stolonifera as biological control agents, and they can also be used as sources of single-cell proteins [21,22]. Aureobasidium pullulans is often fermented to produce β-polymalic acid, laccase, liamocins, pullulan polysaccharides, and other commercial compounds [14,23,24,25,26,27,28]. Liamocins display pharmacological activities including anti-Streptococcus and anticancer [29]. Pullulan polysaccharides are non-toxic, tasteless, harmless, degradable, water-soluble, stable, film-forming, and present other excellent properties. As raw materials for food and cosmetics, pullulan polysaccharides are used for immune regulation, anti-tumor and anti-metastasis, relief of influenza and food allergy, and relief of stress [30].
Pullulan polysaccharides produced by A. pullulans have the properties of water retention, barrier formation, regeneration, whitening, hydrating, and repairing, and are added as an ingredient to cosmetic formulas. In order to screen an excellent A. pullulans to make a fermentation of excellent efficacy for cosmetics, we collected various samples from Tibet, Zhejiang, Yunnan, Shaanxi, Gansu, Hebei, and other areas in China for strain isolation. Ninety-nine strains belonging to Aureobasidium were obtained, among which 14 strains were identified as four new species, based on morphological characteristics and molecular analysis of the internal transcriptional spacer (ITS) and part of the large ribosome subunit (D1/D2 domains).
2. Materials and Methods
2.1. Sample Collection and Strain Isolation
Two hundred leaf samples of Sea buckthorn, willow, oak, crabapple, privet, camphor, and poplar collected from Motuo County in Tibet, and the Yunnan, Shaanxi, Gansu, and Zhoushan regions in Zhejiang Province, were cut into small pieces under sterile conditions and soaked in 0.1% Tween 80. The Tween solution from the leaves was diluted 10 times and directly spread on a potato dextrose agar medium (PDA; 200 g potatoes, 20 g glucose, 20 g agar per L) containing chloramphenicol (0.1 mg/mL) and cultured at 28 °C for 48 h.
Ten insect samples were collected from Xiaowutai Mountain, Zhangjiakou region, Hebei Province. Insect samples with 75% alcohol disinfection were removed from the head and ground with a grinder, then soaked in 0.1% Tween 80. The Tween solution from the inset was diluted 10 times and directly spread on PDA containing chloramphenicol (0.1 mg/mL) and cultured at 28 °C for 48 h.
2.2. DNA Isolation, PCR Amplification and Sequencing
DNA was extracted using the CTAB method [31]. ITS region of ribosomal DNA and the D1/D2 domains of the ribosome subunit (LSU) were amplified and sequenced with the primer pairs of ITS1/ITS4 (ITS1 5′ —GTC GTA ACA AGG TTT CCG TAG GTG— 3′; ITS4 5′ —TCC TCC GCT TAT TGA TAT GC— 3′) and NL1/NL4 (NL1 5′ —GCA TAT CAA TAA GCG GAG GAA AAG— 3′; NL4 5′ —GGT CCG TGT TTC AAG ACG G— 3′) [32,33].
The PCR reaction was performed in the 25 µL reaction mixture containing 0.5 µL of each primer (10 pM/µL), 1.0 µL of genomic DNA (10 ng/µL), and 23 µL of 1 × PCR Master Mix buffer (T3 Super PCR Mix, 10 × 1.125 mL, Tsingke Biotechnology Co., Ltd., Beijing, China). Amplification was performed in an AB 2720 thermal cycler (Applied Biosystems, Foster City, California, USA), with the program consisting of 98 °C for 2 min, 35 cycles of 98 °C for 10 s, 52 °C for 10 s, and 72 °C for 15 s, and the last elongation at 72 °C for 5 min.
2.3. Observation of Morphology
The isolates were cultured on PDA, oatmeal agar (OA; 30 g oatmeal, 20 g agar per L), and malt extract agar (MEA; 50 g malt extract, 20 g agar per L), and incubated in darkness at 25 °C for one week, in order to obtain morphological descriptions, including colony color and appearance. Fungal structures were transferred to microscope slides and mounted on 85% lactic acid drops. M40Y and M60Y media were prepared as described [15,19]. MEA media containing salt were prepared by adding analytical grade NaCl to the MEA prior to sterilization. Cultures were incubated at 25 °C unless otherwise noted.
The microscopes are equipped with LEICA DM2500 cameras (LECIA, Wetzlar, Germany) and use LASV4.13 software. At least 50 representative measurements were randomly selected and measured to calculate the average size.
2.4. Phylogenetic Analyses
For phylogenetic analyses, 28 new ITS and LSU sequences were obtained from the present study, and 56 reference sequences from GenBank (Table 1).
ITS and D1/D2 sequences were aligned with the Muscle program in MEGA7 [34], and minor gaps in all alignments were manually deleted. The most appropriate model of DNA substitution was searched with MEGA7 [35]. The model GTR + I + G was selected for Maximum likelihood (ML) and Bayesian inference (BI) analyses. ML analysis was carried out using MEGA7 [35] with 1000 bootstrap replicates. Bayesian inference (BI) analysis was conducted using MrBayes 3.1.2 [34] with 10,000,000 generations, and parameter settings were proposed by Wang et al. [36]. The phylogenetic tree and the alignments were deposited in TreeBASE (www.treebase.org, accessed on 21 December 2022, No. 30010).
2.5. Exopolysaccharides Production
The starter cultures of the 29 strains (Table 2) were prepared by cultivating the strains in 50 mL of inoculum medium containing 10 g yeast extract, 20 g peptone, and 20 g glucose per liter of distilled water at 25 °C for 3 days on a 150-rpm rotary shaker. Then, 5 mL of the starter culture was transferred to 100 mL synthetic medium containing 100 g sucrose, 1.7 g yeast extract, 5 g K2HPO4, 0.2 g MgSO4.7H2O, 0.6 g (NH4)2SO4 and 1.0 g NaCl per liter of deionized water at 28 °C for 6 days on a 150-rpm rotary shaker, the culture obtained was centrifuged at 7104 × g for 5 min, and the supernatant was collected. In order to precipitate exopolysaccharides, the cold ethanol was added to the obtained solution at a volumetric ratio of 2:1 v/v and the resulting mixture was kept in a refrigerator at 4 °C for 16 h [37]. The cold mixture was further centrifuged at 7104× g at 4 °C for 15 min and the pellet was dried overnight at 45 °C and then weighed to determine the yield of crude EPS production [38].
Table 2.
Exopolysaccharides production yield (EPY) of Aureobasidium.
| Strain | Species | Fermentation Liquid Color | Exopolysaccharides Yield (g/L) | Average Weight (g/L) |
|---|---|---|---|---|
| PTSL5−5 | A. thailandense | Light yellow | 8.47 | 6.17 |
| PTSL4−6 | A. thailandense | Light yellow | 8.09 | |
| PTSL5−3 | A. thailandense | Light yellow | 2.71 | |
| PTSL11−5 | A. thailandense | Light yellow | 5.42 | |
| PTSL9−106 | A. melanogenum | Pink | 1.53 | 39.06 |
| PTSL19−101 | A. melanogenum | Light yellow | 32.53 | |
| PTSL6−101 | A. melanogenum | Light yellow | 34.50 | |
| PTSL19−107 | A. melanogenum | Yellow | 41.84 | |
| PTSL19−104 | A. melanogenum | Yellow | 54.58 | |
| PTSL20−102 | A. melanogenum | Light yellow | 48.13 | |
| PTSL20−104 | A. melanogenum | Yellow | 52.33 | |
| PTSL19−104 | A. melanogenum | Light yellow | 45.71 | |
| PTSL19−104 | A. melanogenum | Light yellow | 45.71 | |
| PTSL17−4 | A. melanogenum | Light yellow | 34.36 | |
| PTSL9−100 | A. melanogenum | Light yellow | 38.45 | |
| LF75−2 | A. leucospermi | Light yellow | 0.92 | 17.24 |
| SXY35−16 | A. leucospermi | Light yellow | 28.94 | |
| SXY35−15 | A. leucospermi | Light yellow | 23.37 | |
| LF45−2 | A. leucospermi | Light yellow | 15.75 | |
| LPL−7A | A. insectorum | Light yellow | 27.67 | 14.7 |
| KCL139 | A. insectorum | Dark yellow | 8.64 | |
| XZY65−10 | A. insectorum | Dark yellow | 7.80 | |
| E26−4 | A. motuoense | Yellow | 15.74 | 26.57 |
| E31−1 | A. motuoense | Dark yellow | 21.39 | |
| XZY411−4 | A. motuoense | Dark yellow | 31.72 | |
| E82−2 | A. motuoense | Dark yellow | 37.43 | |
| MGL11−3 | A. intercalariosporum | Light yellow | 29.43 | 31.79 |
| MQL9−100 | A. intercalariosporum | Light yellow | 34.15 | |
| MDSC−10 | A. planticola | Black | 2.10 | 2.1 |
Table 1.
Names, strains, locations, and hosts, and corresponding GenBank numbers of the taxa used in this study.
| Species | Strain | Date | Location | Latitude and Longitude | Source | GenBank No. | References | |
|---|---|---|---|---|---|---|---|---|
| ITS | D1/D2 | |||||||
| Aureobasidium acericola | CDH 2020−10 | June 2020 | South Korea | 37°45′49.50″ N, 127°11′3.8″ E | Acer pseudosieboldianum | MT863788 | MT863787 | [10] |
| Aureobasidium aerium | CFCC 50324 | April 2015 | Sennon, Beijing, China | NA | air | ON007058 | ON007081 | [11] |
| Aureobasidium castanea | CFCC 54591 * | November 2021 | Jinjing Town, Changsha Hunan, China | 28°58′52″ N, 113°34′38″ E | Castanea heryi | NR_177551 | MW364275 | [12] |
| Aureobasidium caulivorum | CBS 242.64 | NA | Oregon, America | NA | Trifolium incarnatum | FJ150871 | FJ150944 | [39] |
| Aureobasidium insectorum sp. nov. | KCL139 | September 2021 | Zhangjiakou, Hebei, China | 39°30′ N, 113°50′ E | spittle insects | OP856707 | OP857208 | This study |
| LPL−1C | September 2022 | Zhoushan, Zhejiang, China | 29°53′28.86″ N, 122°24′59.35″ E | leaf | OP856705 | OP857207 | This study | |
| XZY65−10 | October 2019 | Shannan City, Tibet, China | 29°14′9.68 ″ N, 91°45′59.50″ E | leaf | OP856706 | OP857206 | This study | |
| L2PL−7A | September 2022 | Zhoushan, Zhejiang, China | 29°53′28.86″ N, 122°24′59.35″ E | leaf | OP856715 | OP857216 | This study | |
| T1−27−2 | November 2021 | Motuo County, Tibet, China | 29°19′37.128″ N, 95°19′53.76″ E | leaf | OP856714 | OP857215 | This study | |
| XZY249M1 | October 2019 | Nyingchi City, Tibet, China | 29°19′37.128″ N, 95°19′53.76″ E | deadwood | OP856713 | OP857214 | This study | |
| XZY63−10 | October 2019 | Shannan City, Tibet, China | 29°14′9.68″ N, 91°45′59.50″ E | leaf | OP856712 | OP857213 | This study | |
| Aureobasidium intercalariosporum sp. nov. | MGL11−3 | September 2022 | Zhoushan, Zhejiang, China | 29°53′28.86″ N, 122°24′59.35″ E | leaf | OP856703 | OP857204 | This study |
| MQL9−100 | September 2022 | Zhoushan, Zhejiang, China | 29°53′28.86″ N, 122°24′59.35″ E | leaf | OP856703 | OP857205 | This study | |
| Aureobasidium iranianum | CCTU 268 | June 2009 | Southern parts of Iran | NA | bamboo stems | NR_137598 | NG_057049 | [13] |
| Aureobasidium khasianum | NFCCI 4275 | December 2016 | Meghalaya, India | NA | litter samples | MH188305 | MH188306 | [40] |
| Aureobasidium leucospermi | CBS 130593 | April 2008 | South Africa | NA | leaves and stems of Proteaceae with cankers or leaf spots | NR_156246 | MH877257 | [14] |
| Aureobasidium lini | CBS 125.21T | NA | UK | NA | Linum usitatissimum | FJ150897 | FJ150946 | [8] |
| Aureobasidium mangrovei | IBRCM 30265T | January 2016 | Qeshm Island, Iran | 26°47′ N, 55°45′ E | mangrove trees (Avicennia marina) | NR_174637 | NG_078639 | [15] |
| Aureobasidium melanogenum | CBS 105.22 | NA | NA | NA | leaf | NR_159598 | NG_056960 | [8] |
| Aureobasidium microstictum | CBS 342.66 | NA | Germany | NA | dying or dead leaves | KT693743 | FJ150945 | [8] |
| Aureobasidium microstictum | CBS 114.64 | NA | Wageningen, The Netherlands | NA | Hemerocallis sp. | KT693744 | KT693986 | [8] |
| Aureobasidium microtermitis | NA | NA | NA | NA | NA | MW276135 | MW276136 | NA |
| Aureobasidium motuoense sp. nov. | E82−2 | October 2019 | Motuo County, Tibet, China | 29°19′37.128″ N, 95°19′53.76″ E | soil | OP856702 | OP857203 | This study |
| XZY411−4 | August 2019 | Motuo County, Tibet, China | 29°19′37.128″ N, 95°19′53.76″ E | leaf | OP856710 | OP857211 | This study | |
| E31−1 | October 2019 | Motuo County, Tibet, China | 29°19′37.128″ N, 95°19′53.76″ E | soil | OP856709 | OP857210 | This study | |
| E26−4 | October 2019 | Motuo County, Tibet, China | 29°19′37.128″ N, 95°19′53.76″ E | soil | OP856708 | OP857209 | This study | |
| Aureobasidium mustum | AWRI 4233 CO−2020 | NA | South Australia | NA | grape juice | NA | NA | [17] |
| Aureobasidium namibiae | CBS 147.97 | 1997 | Namib Desert, Namibia | NA | dolomitic marble | FJ150875 | FJ150937 | [8] |
| Aureobasidium pini | CFCC 52778 | May 2018 | Miyun District, Beijing, China | 40°41′18″ N, 116°55′21″ E | pine needles covered with mycelium | MK184533 | MK184535 | [18] |
| Aureobasidium planticola sp. nov. | MDSC−10 | September 2022 | Zhoushan, Zhejiang, China | 29°53′28.86″ N, 122°24′59.35″ E | leaf | OP856711 | OP857212 | This study |
| Aureobasidium proteae | CBS 114273 | February 2006 | Netherlands | NA | Protea sp. | JN712491 | JN712557 | [15] |
| Aureobasidium proteae | CPC 13701 | July 1998 | Hilly Lands Farm, Somerset West, South Africa | NA | Protea cv. ‘Sylvia’ | JN712490 | JN712556 | [15] |
| Aureobasidium pullulans | CBS 584.75 | 1974 | France | NA | fruit of Vitis vinifera | FJ150906 | FJ150942 | [8] |
| Aureobasidium pullulans | CBS 146.30 | NA | Germany, Ohlsdorf near Hamburg | NA | slime flux of Quercus sp. | FJ150902 | FJ150916 | [8] |
| Aureobasidium subglaciale | EXF−2481 | June and August 2001 | Norway, Svalbard, Kongsvegen | 79° N, 12° E | subglacial ice from seawater | FJ150895 | FJ150913 | [8] |
| Aureobasidium thailandense | NRRL 58539T | 2006 | Nakhonratchasima, Thailand | NA | leaf of Cerbera odollum | JX462674 | JX462674 | [19] |
| Aureobasidium thailandense | NRRL 58543 | 2006 | Prachuapkhirikhan, Thailand | NA | wood surface | JX462675 | JX462675 | [19] |
| Aureobasidium tremulum | UN 1 | NA | NA | NA | NA | MK503657 | MK503660 | NA |
| Aureobasidium uvarum | AWRI 4620 CO−2020 | NA | NA | NA | NA | NA | NA | [17] |
| Aureobasidium vineae | AWRI4619 CO−2020 | NA | NA | NA | NA | NA | NA | [17] |
| Selenophoma mahoniae | CBS 388.92 | NA | Colorado, America | NA | Mahonia repens, leaf | FJ150872 | FJ150943 | [8] |
| Sydowia polyspora | CBS 750.71 | September 1969 | Quebec, Lac Normand, Canada | NA | Pinus strobus, twig | MH872085 | MH872085 | [41] |
* Note: Generated sequences and new strains in this study are indicated in bold. NA: Not available.
3. Results
3.1. Phylogeny
The phylogenetic tree, based on a combined dataset of the ITS region and D1/D2 domain of the LSU sequences, was used to resolve the taxonomic position of the newly collected strains within Aureobasidium.
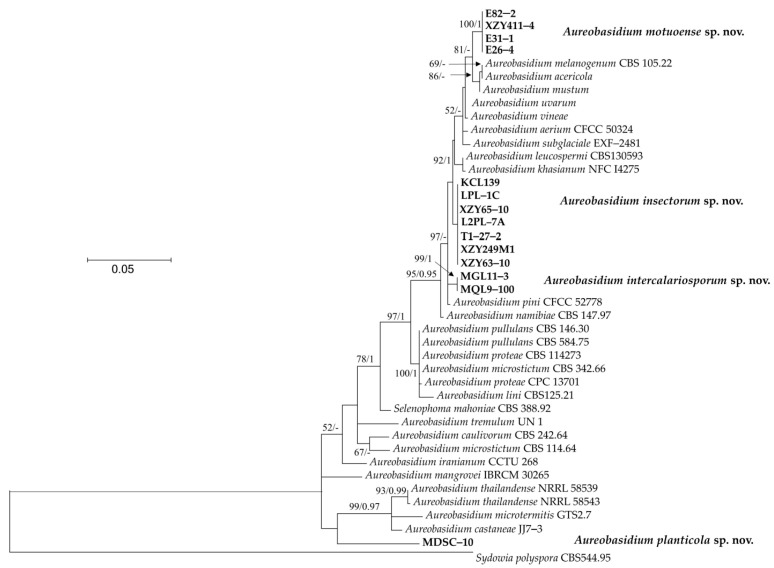
Fourteen newly isolated strains in this study were formed into four separate groups (Figure 1). Strains E82−2, XZY411−4, E31−1, and E26−4 were closely related to A. acericola, A. melanogenum and A. mustum, with 81% bootstrap support. Strains KCL139, LPL−1C, XZY65−10, L2PL−7A, T1−27−2, XZY249M1, and XZY63−10 formed a basal clade related to A. acericola, A. melanogenum, A. mustum, A. uvarum, A. vineae, A. aerium, A. subglaciale, A. leucospermi, and A. khasianum, but without support. MGL11−3 and MQL9−100 clustered together with a separate clade. Strain MDSC−10 located at a basal branch related to A. thailandense, A. microtermitis, and A. castaneae.
Figure 1.
The phylogenetic tree was inferred using the combined sequences of the ITS (including 5.8S rDNA) and LSU rDNA D1/D2 domains, depicting the phylogenetic positions of new taxa (in bold) within Aureobasidium. Bootstrap percentages of maximum likelihood analysis over 50% from 1000 bootstrap replicates and Bayesian inference higher than 0.9 (PP > 0.9) are shown on the deep and major branches. Bar = 0.05 substitutions per nucleotide position. Note: -, not supported (BP < 50% or PP < 0.9). The new taxa isolated in this study are shown in bold.
3.2. Taxonomy
3.2.1. Aureobasidium insectorum Q.M. Wang, F. Wu & M.M. Wang sp. nov.
Fungal names no: FN 571251
Etymology: Referring to the insect cicada, where the type of strain originated.
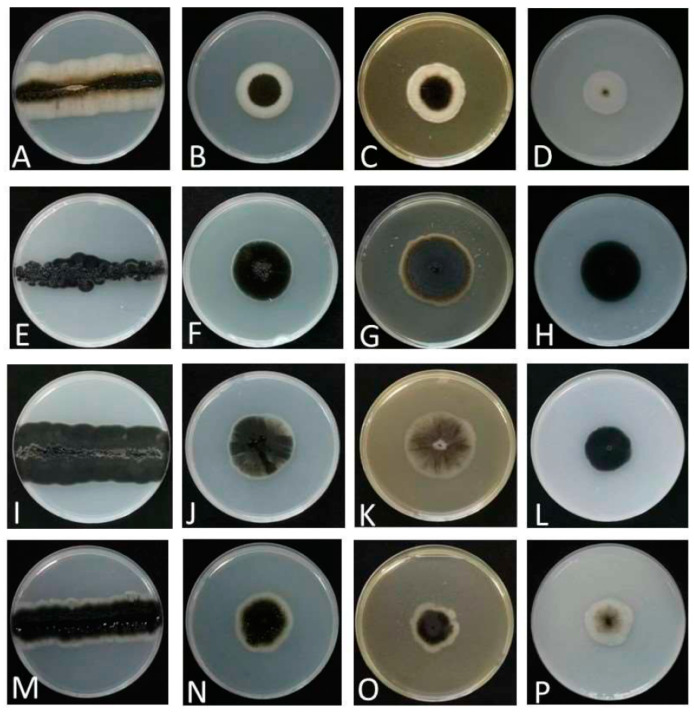
Colonies grew moderately on PDA, MEA, and OA (Figure 2), attaining 34 mm, 34 mm, and 27 mm diameters after 7 days of incubation at 25 °C, respectively. Colonies on PDA were flat, smooth, pitch black with white fimbriate margins, and lacking aerial mycelium. Colonies on MEA were flat, felty, and greenish-black with white fimbriate margins. Colonies on OA were flat, whitish, and olivaceous black in the center, with sparse aerial mycelium. The growth diameters, sugar and salt tolerance, and different cardinal growth temperature of A. insectorum are shown in Table 3. A. insectorum can grow at 4–30 °C, and the optimum growth temperature is 28 °C. On MEA supplemented with 15% (w/v) NaCl, the diameters of A. insectorum attained 8–9 mm, while A. mangrovei and A. pullulans were 5 mm and 7 mm, respectively [10,19]. Thus, A. insectorum grew stronger on concentrations of 15% NaCl than its closely related species. A. insectorum grew stronger on moderate-sugar-level media (PDA) and M40Y, and weaker on M60Y. This species is suitable to grow on M40Y and PDA.
Figure 2.
Colony characteristics of analyzed strains. (A–D): A. insectorum KCL139 on PDA, PDA, MEA, and OA, respectively. (E–H): A. planticola MDSC−10 on PDA, PDA, MEA, and OA, respectively. (I–L): A. motuoense XZY411−1 on PDA, PDA, MEA, and OA, respectively. (M–P): A. intercalariosporum MQL9−100 PDA, PDA, MEA, and OA, respectively.
Table 3.
Diameter (mm) of four strain colonies under different conditions and on different media. The growth temperature was 25 °C unless noted otherwise and incubation was for 7 d.
| Aureobasidium planticola | Aureobasidium intercalariosporum | Aureobasidium motuoense | Aureobasidium insectorum | |
|---|---|---|---|---|
| PDA | 22–25 | 32–36 | 34–38 | 32–35 |
| M40Y | 33–40 | 35–40 | 41–43 | 32–34 |
| M60Y | 27–34 | 29–34 | 36–40 | 25–27 |
| MEA + 5% NaCl | 14–15 | 14–17 | 13–15 | 9–12 |
| MEA + 10% NaCl | 7–8 | 8–11 | 10–11 | 8–8 |
| MEA + 15% NaCl | 0 | 9–13 | 5–8 | 8–9 |
| MEA + 20% NaCl | 0 | 0 | 0 | 0 |
| MEA at 4 °C | 0 | 0 | 0 | 5–5 |
| MEA at 17 °C | 10–13 | 13–15 | 8–13 | 12–15 |
| MEA at 28 °C | 27–27 | 24–25 | 32–35 | 28–28 |
| MEA at 30 °C | 11–10 | 13–14 | 38–44 | 7–8 |
| MEA at 37 °C | 0 | 0 | 7–8 | 0 |
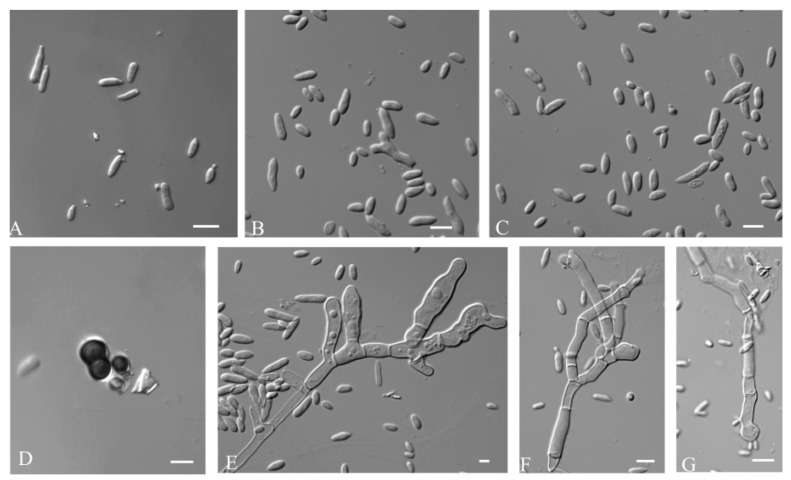
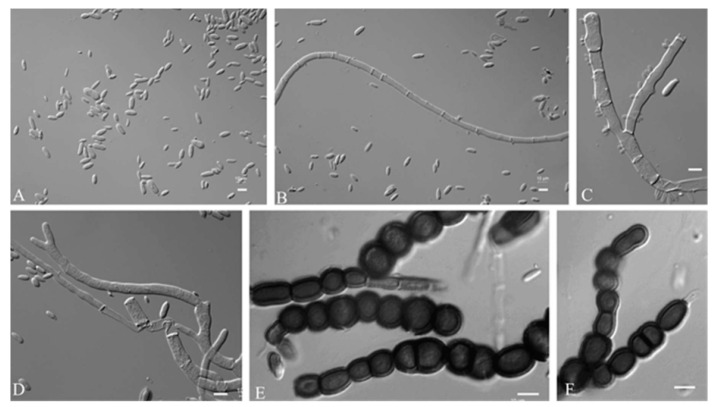
Mycelium hyaline were smooth, thin-walled, and 5.8–10.6 µm (av. = 8.0 µm) wide. Conidiophores were not developed. Conidiogenus cells were holoblastic, smooth, cylindrical, and 11.65–4.59 µm (av. = 7.16 µm) wide. Conidia were hyaline, aseptate, smooth walled, ellipsoidal to elongate-ellipsoidal, straight or slightly curved, 4.2–7.4 × 1.7–3.5 μm (av. = 5.2 × 2.8 μm), often polar or bipolar buds. Chlamydospore were 3.5–8.9 × 1.3–6.7 μm (av. = 6.5 × 5.4 μm), black, smooth, globose to ellipsoidal, septate or aseptate, and constricted at the septa (Figure 3).
Figure 3.
A. insectorum (CGMCC 2.7207- ex-type-culture). (A–C): Conidia. (D): Chlamydospores. (E–G): Conidiogenus cells. Scale bar: (A–G) = 10 μm.
Material examined: —CHINA, Hebei, Zhangjiakou, Xiaowutai Mountain, from cicada, L. Min, August 2021. (Holotype HMAS 352303; ex-type culture CGMCC 2.7207 = KCL139).
Notes—This species is phylogenetically close to A. aerium, A. intercalariosporum, A. leucospermi, and A. khasianum. These five species can be distinguished by the sizes of conidia (4.2–7.4 × 1.7–3.5 μm in A. insectorum, vs. 12.8–19.5 × 7.9–11.9 μm in A. aerium, vs. 8–13 × 5–9/8–24 × 2–10 μm in A. leucosperm, vs. 3–4 × 2–40 μm in A. khasianum, vs. 10.5–17.1 × 10.5–12.9 μm in A. intercalariosporum) [11,12,40].
3.2.2. Aureobasidium planticola Q.M. Wang, F. Wu & M.M. Wang sp. nov.
Fungal names no: FN 571262.
Etymology: Referring to the plant where the ex-type strain originated.
Colonies grew moderately on PDA, MEA, and OA (Figure 2), attaining 22 mm, 24 mm, and 30 mm diameters after 7 days of incubation at 25 °C, respectively. Colonies on PDA were flat, pitch black, and pale grey in the center. Colonies on MEA were flat, felty, and brown, with white fimbriate at the margin. Colonies on OA had a smooth margin, and were flat, olivaceous black, compact, and lacking aerial mycelium. The growth diameters, sugar and salt tolerance, and different cardinal growth temperature of A. planticola are shown in Table 3. A. planticola can grow at 17–30 °C, and the optimum growth temperature is 28 °C. On MEA supplemented with 10% (w/v) NaCl, the diameters of A. planticola attained 7–8 mm, while its closely related species A. iranianum was 5 mm [13]. A. planticola can grow on moderate-sugar-level media (PDA), and on both M40Y and M60Y. This species is suitable to grow on M40Y.
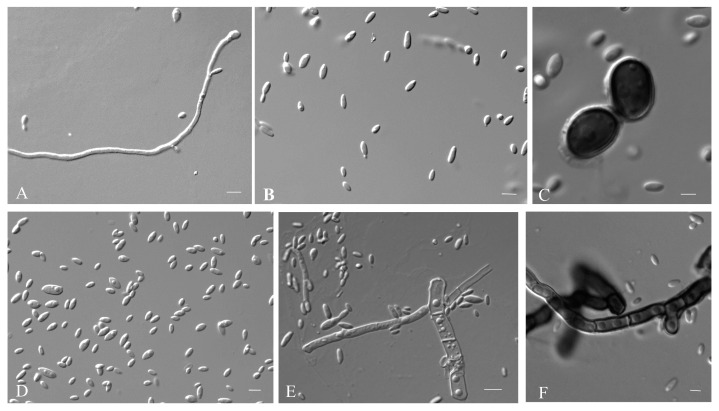
Mycelium was dark-pigmented, smooth, thick-walled, branched, and 5.8–10.6 µm (av. = 8.0 µm) wide. Conidiogenous cells were holoblastic, located laterally or terminally, single or in clusters, grey-black to black, and 4.75–2.70 μm (av. = 4.2 μm). Conidia were 5.7–7.7 × 1.5–2.7 μm (av. = 5.2 × 2.8 μm), hyaline, aseptate, smooth-walled, and ellipsoidal to ovoid (Figure 4).
Figure 4.
A. planticola (CGMCC 2.7199 ex-type culture). (A–F): Conidiogenous cells and thick-walled hyphae. Scale bar: (A–F) = 10 μm.
Material examined: —CHINA, Zhejiang, Zhoushan, Miaogen Mountain, from leaf, F, Zixuan, August 2022. (Holotype HMAS 352302; ex-type culture CGMCC 2.7199 = MDSC−10).
Notes—This species is phylogenetically related to A. thailandense and A. castaneae. These three species can be distinguished by the hyphae color (hyaline or brown in A. castaneae, vs. hyaline in A. thailandense, vs. dark black in A. planticola) [12,19].
3.2.3. Aureobasidium motuoense Q.M. Wang, F. Wu & M.M. Wang sp. nov.
Fungal names no: FN 571263.
Etymology: Referring to the location where the ex-type strain originated.
Colonies grew moderately on PDA, MEA, and OA (Figure 2), attaining 36 mm, 42 mm, and 27 mm diameters after 7 days of incubation at 25 °C, respectively. Colonies on PDA were dark brown, with irregular black zones, and sparse aerial mycelium. Colonies on MEA were flat, brownish olivaceous, and white near the margin. Colonies on OA were flat, compact, and pitch black. The growth diameters, sugar and salt tolerance, and different cardinal growth temperature of A. motuoense are shown in Table 3. A. motuoense can grow at 17–37 °C, and the optimum growth temperature is 30 °C. At 37 °C, the diameters of A. motuoense were 7–8 mm, while its relative A. mangrovei was 5 mm [15]. A. motuoense can tolerate concentrations of up to 15% NaCl. A. motuoense grew stronger on moderate-sugar-level media (PDA), and on both M40Y and M60Y. This species is suitable to grow on M40Y.
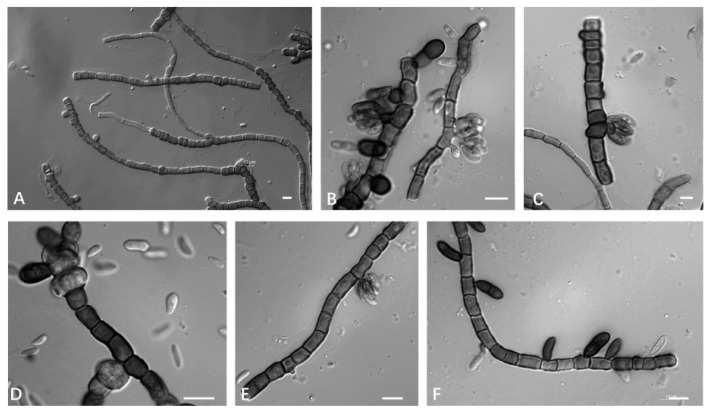
Mycelium were hyaline to dark brown, smooth-walled, branched, and 1.3–12.7 µm (av. =8.3 µm) wide. Conidiogenous cells were 6.4–12.2 × 3.6–4.5 μm (av. = 8.7× 3.9 μm). Conidia were hyaline, smooth-walled, terminal, and mono- or bipolar budding. Chlamydospores were 12.2–16.2 × 9.6–10.4 μm (av. = 13.6 × 10.0 μm), black, smooth, and globose to elliptic (Figure 5).
Figure 5.
A. motuoense (CGMCC 2.7206 ex-type culture). (A): Conidia. (B–D): Hyphae and conidiogenous cells. (E,F): Chlamydospores. Scale bars: (A–F) = 10 μm.
Material examined: —CHINA, Tibet, Motuo County, from leaf, W, Guishuang, August 2019. (Holotype HMAS 352304; ex-type culture CGMCC 2.7206 = XZY411−4).
Notes—This species is phylogenetically related to A. melanogenum and A. acericola. The colonies of A. melanogenum on MEA/PDA at 25 °C attained 25 mm diameters after 7 d, appearing smooth and slimy due to abundant sporulation and EPS formation, olive-brown to black in the centre, mustard yellow towards the margin, and at the margin were yellowish white. The colony morphology of A. acericola is fast-growing, attaining diameters of 65 mm in 14 days, rapidly turning to olivaceous black, with dark green, irregular margins, covered with slimy masses of conidia, and mycelium immersed or no aerial mycelium [8,10].
3.2.4. Aureobasidium intercalariosporum Q.M. Wang, F. Wu & M.M. Wang sp. nov.
Fungal names no: FN 571252
Etymology: Referring to the morphology of intercalary chlamydospores.
Colonies grew moderately on PDA, MEA, and OA (Figure 2), attaining 34 mm, 27 mm, and 28 mm diameters after 7 days of incubation at 25 °C, respectively. Colonies on PDA had an entire margin, and were floccose, greenish black, and white at the edge. Colonies on MEA had an undulate margin, and were flat, brownish olivaceous, grey at the centre, and white near the edge, with sparse aerial mycelium. Colonies on OA were flat, pale, and olivaceous brown to white from the middle to the edge.
The growth diameters, sugar and salt tolerance, and different cardinal growth temperature of A. intercalariosporum are shown in Table 3. A. intercalariosporum can grow at 17–30 °C, and the optimum growth temperature is 28 °C. On MEA supplemented with 15% (w/v) NaCl, the diameters of A. intercalariosporum attained 9–13 mm, while A. mangrovei and A. pullulans were 5 mm and 7 mm, respectively [8,16]. Therefore A. intercalariosporum grew stronger on concentrations of 15% NaCl than its closely related species. A. planticola grew on moderate-sugar-level media (PDA), and on both M40Y and M60Y. This species is suitable to grow on M40Y.
Mycelium was composed of branched, septate hyphae that occurred singly, and were verruculose to smooth, thin-walled, and 1.3–4.8 µm (av. = 3.2 µm) wide. Conidiogenous cells were undifferentiated, smooth, cylindrical, and 4.85–2.68 µm (av. = 3.54 µm) wide. Conidia were 10.5–17.1 × 10.5–12.9 μm (av. = 14.7 × 12.5 μm), smooth-walled hyaline, aseptate, ovoid, and ellipsoidal or elongated ellipsoidal. Chlamydospores were 10.5–17.1 × 10.5–12.9 μm (av. = 14.7 × 12.5 μm), deeply pigmented, smooth, thick walled, and globose to ellipsoidal (Figure 6).
Figure 6.
A. intercalariosporum (CGMCC 2.7208 ex-type culture). (A,E): Hyphae and conidiogenus cells. (B,D): Conidia. (C,F): Chlamydospores. Scale bars: (A–F) = 10 μm.
Material examined: —CHINA, Zhejiang, Zhoushan, Miaogen Mountain, from leaf, F, Zixuan, August 2022. (Holotype HMAS 352305; ex-type culture CGMCC 2.7208 = MQL9−100).
Notes—This species is phylogenetically related to A. pini, A. planticol, A. khasianum, A. leucospermi, A. namibiae, A. pullulans, and A. proteae.
The main difference between the eight strains is conidia (10.5–17.1 × 10.5–12.9 μm in A. intercalariosporu, vs. 4.2–7.4 × 1.7–3.5 μm in A. planticola, vs. 6.2–8.5 × 3.6–4.2 μm in A. pini, vs. 7–17 × 3.5–7 μm in A. namibiae, vs. 8–11 × 4–5 μm in A. leucospermi, vs. 7.5–16 × 3.5–7 μm in A. pullulans [8,14].
3.3. Exopolysaccharides Production
Ninety-nine Aureobasidium strains were isolated from different leaf and insect samples. Twenty-nine strains among them were selected to evaluate the exopolysaccharides production capacity (Table 3). PTSL19-104 and PTSL20-104 had the highest exopolysaccharide yield, which was 54.8 g/L and 52.33 g/L, respectively. The exopolysaccharides production capacity of Aureobasidium strains was different between species. A. melanogenum had a strong exopolysaccharide production capacity, with an average exopolysaccharide production of 39.06 g/L. A. thailandense was the weakest, with an average exopolysaccharide production of 6.17 g/L. As the interspecific hetero, the intraspecific differences also existed in the production capacity of exopolysaccharides. For example, the lowest sugar production of A. melanogenum strain was 1.53 g/L, and the highest sugar production was 54.58 g/L. The strains in the same branch of the phylogenetic tree have the same apparent and microscopic morphology, but the exopolysaccharides yields produced by fermentation are different. For example, the exopolysaccharides yields of four strains among A. motuoense are 15.74 g/L, 21.39 g/L, 31.72 g/L, and 37.43 g/L, respectively. In summary, there were differences in exopolysaccharides production capacity among species. Although strain morphology and molecular sequence were consistent within species, there were differences in physiological functions such as exopolysaccharides production capacity.
4. Discussion
The species of Aureobasidium are widely distributed globally in various habitats, such as house dust, air, tree surfaces (such as needles of Pinus tabuliformis, Acer pseudosieboldianum, Bintaro plants, Castanea henryi, and Castanea mollissima), plant interiors, seawater, sea ice and glacial meltwaters, water and sediment samples, soil, and subcutaneous phaeohyphomycosis from the US, Canada, Korea, Indonesia, China, the Arctic coast, and Brittany (France) [1,8,10,11,18,21,23]. In this study, many strains were isolated from soil and plant leaves, but strain KCL139 was isolated from the surface of a spittle insect. Yeasts occur commonly on insect species, and the number of insect-related yeasts had increased in the last 10 years reaching a ratio of 7.25% [42,43,44], compared with the report of Boekhout [45]. However, according to our knowledge, Aureobasidium species isolated from insects are rare. Our finding not only expands the ecology niches of Aureobasidium, but also supports the ‘dispersal–encounter’ hypothesis proposed by Madden et al. [46].
The four new species described in this study have different growth temperatures from each other (Table 2). They can grow at 17 °C, 28 °C and 30 °C; however, only A. motuoense can tolerate a high temperature of 37 °C, a feature associated with the climate characteristics of Motuo County where this new species was collected. Motuo County boasts a typical sub-tropical moist climate. The optimum growth temperature for A. planticola, A. intercalariosporum, and A. insectorum is 28 °C, whereas A. motuoens is 30 °C. A. planticola, A. intercalariosporum, and A. motuoense fail to grow at 4 °C, whereas A. insectorum grows at this temperature normally. Unfortunately, the psychrophilic character of A. insectorum cannot be explained based on the present data. A. planticola, A. motuoens, and A. insectorum can grow on MEA supplemented with 15% (w/v) NaCl, but A. planticola can only tolerate concentrations of up to 5% NaCl. All tested Aureobasidium species [10], can grow on 10% NaCl MEA, and most of them can tolerate concentrations of 10% NaCl. Only A. planticola is intolerant to more than 10% NaCl. A. planticola shows good growth on the M40Y medium, while the other strains grow well on the PDA medium.
The study of Zou et al. [47] showed that A. pullulans produced polymalic acid by fermentation, and strains ZD-3d, IP-1, Sp. P6, MCW, and ZX-10 produced 57.2 g/L, 57.4 g/L, 91.1 g/L, and 117.4 g/L, respectively, which indicated that different strains could produce different yields of polymalic acid at the same fermentation conditions. In this paper, we found that different strains of the same species, such as A. insectorum, A. leucospermi, A. motuoense, and A. melanogenum, produced pullulan ranging from 0.92 to 54.58 g/L (Table 3), which in agreement with the result from Haghighatpanah et al. [37], shows that there were differences in exopolysaccharides production within the same species. Our results also showed that different Aureobasidium species have great differences in their exopolysaccharides production capacity. Therefore, we should screen more diverse environments to isolate strains of certain species for comprehensive study in exploring Aureobasidium compounds for industrial application.
5. Conclusions
In this study, 14 strains of Aureobasidium isolated from Tibet, Hebei, and Zhejiang are proposed as four new species based on molecular analysis of their ITS and large ribosomal subunits of D1/D2 domains. The new species are named as Aureobasidium insectorum sp. nov., A. planticola sp. nov., A. motuoense sp. nov., and A. intercalariosporum sp. nov. We found differences in interspecific and intraspecific exopolysaccharides production between the species, indicating the diversity in strain-specific exopolysaccharides production. Aureobasidium has strong adaptability and wide distribution, and there may be many new Aureobasidium taxa in nature, which have the potential to produce new metabolites. Therefore, studies into the species diversity of this genus and the industrial application of its metabolites are of great significance.
Author Contributions
Q.W. conceived and designed the project. F.W. and Z.F. performed sampling and yeasts isolation. F.W., M.W., Z.F. and Q.W. performed phenotypic characterization and analyzed the molecular data. F.W., M.W. and Q.W. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All newly generated sequence data are available in NCBI GenBank. The phylogenetic tree and the alignments were deposited in TreeBASE.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was supported by grants No. 319611330200 and No. 31770018 from the National Natural Science Foundation of China (NSFC), No. 2021FY100900 from the Ministry of Science and Technology of China, and No. 521000981388 from the Advanced Talents Incubation Program of the Hebei University.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Hymphries Z., Seifert K.A., Hirooka Y., Visagie M. A new family and genus in Dothideales for Aureobasidium-like species isolated from house dust. IMA Fungus. 2017;8:299–315. doi: 10.5598/imafungus.2017.08.02.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thambugala K.M., Ariyawansa H.A., Li Y.M., Boonmee S., Hongsanan S., Tian Q., Singtripop C., Bhat D.J., Camporesi E., Jayawardena R., et al. Dothideales. Fungal Divers. 2014;68:105–158. doi: 10.1007/s13225-014-0303-8. [DOI] [Google Scholar]
- 3.Wijayawardene N.N., Crous P.W., Kirk P.M., Hawksworth D.L., Boonmee S., Braun U., Dai D.Q., D’souza M.J., Diederich P., Dissanayake A., et al. Naming and outline of Dothideomycetes–2014 including proposals for the protection or suppression of generic names. Fungal Divers. 2014;69:1–55. doi: 10.1007/s13225-014-0309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hermanides-Nijhof E.J. Aureobasidium and allied genera. Stud. Mycol. 1977;15:141–177. [Google Scholar]
- 5.De Hoog G.S., Yurlova N.A. Conidiogenesis, nutritional physiology and taxonomy of Aureobasidium and Hormonema. Antonie Van Leeuwenhoek. 1994;65:41–54. doi: 10.1007/BF00878278. [DOI] [PubMed] [Google Scholar]
- 6.De Hoog G.S., Zalar P., Urzi C., De Leo F., Yurlova N.A., Sterflinger K. Relationships of dothideaceous black yeasts and meristematic fungi based on 5.8 S and ITS2 rDNA sequence comparison. Stud. Mycol. 1999;43:31–37. [Google Scholar]
- 7.Yurlova N.A., Uijthof J.M.J., De Hoog G.S. Distinction of species in Aureobasidium and related genera by PCR-ribotyping. Antonie Van Leeuwenhoek. 1996;69:323. doi: 10.1007/BF00399621. [DOI] [PubMed] [Google Scholar]
- 8.Zalar P., Gostinčar C., De Hoog G.S., Uršič V., Sudhadham M., Gunde-Cimerman N. Redefinition of Aureobasidium pullulans and its varieties. Stud. Mycol. 2008;61:21–38. doi: 10.3114/sim.2008.61.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gostinčar C., Ohm R.A., Kogej T., Sonjak S., Turk M., Zajc J., Zalar P., Grube M., Sun H., Han J., et al. Genome sequencing of four Aureobasidium pullulans varieties: Biotechnological potential, stress tolerance, and description of new species. BMC Genom. 2014;15:549. doi: 10.1186/1471-2164-15-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee D.H., Cho S.E., Oh J.Y., Cho E.J., Kwon S. A novel species of Aureobasidium (Dothioraceae) recovered from Acer pseudosieboldianum in Korea. J. Asia-Pac. Biodivers. 2021;14:657–661. doi: 10.1016/j.japb.2021.08.005. [DOI] [Google Scholar]
- 11.Wang C.B., Jiang N., Zhu Y.Q., Xue H., Li Y. Aureobasidium aerium (Saccotheciaceae, Dothideales), a new yeast-like fungus from the air in Beijing, China. Phytotaxa. 2022;544:185–192. doi: 10.11646/phytotaxa.544.2.4. [DOI] [Google Scholar]
- 12.Jiang N., Fan X., Tian C. Identification and Characterization of Leaf-Inhabiting Fungi from Castanea Plantations in China. J. Fungi. 2021;18:64. doi: 10.3390/jof7010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arzanlou M. Aureobasidium iranianum, a new species on bamboo from Iran. Mycosphere. 2012;3:404–408. doi: 10.5943/mycosphere/3/4/2. [DOI] [Google Scholar]
- 14.Crous P.W., Summerell B.A., Swart L., Denman S., Taylor J.E., Bezuidenhout C.M., Palm M.E., Marincowitz S., Groenewald J.Z. Fungal pathogens of Proteaceae. Persoonia. 2011;27:20–45. doi: 10.3767/003158511X606239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nasr S., Mohammadimehr M., Geranpayeh Vaghei M., Amoozegar M.A., Shahzadeh Fazeli S.A. Aureobasidium mangrovei sp. nov., an ascomycetous species recovered from Hara protected forests in the Persian Gulf, Iran. Antonie Van Leeuwenhoek. 2018;111:1697–1705. doi: 10.1007/s10482-018-1059-z. [DOI] [PubMed] [Google Scholar]
- 16.Crous P.W., Cowan D.A., Maggs-Kölling G., Yilmaz N., Thangavel R., Wingfield M.J., Noordeloos M.E., Dima B., Brandrud T., Jansen G.M., et al. Fungal Planet description sheets. Persoonia. 2021;46:313–528. doi: 10.3767/persoonia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Onetto C.A., Schmidt S.A., Roach M.J., Borneman A.R. Comparative genome analysis proposes three new Aureobasidium species isolated from grape juice. FEMS Yeast Res. 2020;20:52. doi: 10.1093/femsyr/foaa052. [DOI] [PubMed] [Google Scholar]
- 18.Jiang N., Liang Y.M., Tian C.M. Aureobasidium pini sp. nov. from pine needle in China. Phytotaxa. 2019;402:10. doi: 10.11646/phytotaxa.402.4.3. [DOI] [Google Scholar]
- 19.Peterson S., Manitchotpisit P., Leathers T. Aureobasidium thailandense sp. nov. isolated from leaves and wooden surfaces. Int. J. Syst. Evol. Microbiol. 2012;63:790–795. doi: 10.1099/ijs.0.047613-0. [DOI] [PubMed] [Google Scholar]
- 20.Crous P.W., Carnegie A.J., Wingfield M.J., Sharma R., Mughini G., Noordeloos M.E., Santini A., Shouche Y.S., Bezerra J.D.P., Dima B., et al. Fungal Planet description sheets. Persoonia. 2019;44:291–473. doi: 10.3767/persoonia.2019.42.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prasongsuk S., Lotrakul P., Ali I., Bankeeree W., Punnapayak H. The current status of Aureobasidium pullulans in biotechnology. Folia Microbiol. 2018;63:129–140. doi: 10.1007/s12223-017-0561-4. [DOI] [PubMed] [Google Scholar]
- 22.Sharma R.R., Singh D., Singh R. Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A review. Biol. Control. 2009;50:205–221. doi: 10.1016/j.biocontrol.2009.05.001. [DOI] [Google Scholar]
- 23.Manitchotpisit P., Leathers T.D., Peterson S.W., Kurtzman C.P., Li X.L., Eveleigh D.E., Lotrakul P., Prasongsuk S., Dunlap C.A., Vermillion K.E., et al. Multilocus phylogenetic analyses, pullulan production and xylanase activity of tropical isolates of Aureobasidium pullulans. Mycol. Res. 2009;113:1107–1120. doi: 10.1016/j.mycres.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Kutleša M., Mlinarić-Missoni E., Hatvani L., Voncina D., Simon S., Lepur D., Baršić B. Chronic fungal meningitis caused by Aureobasidium proteae. Diagn. Microbiol. Infect. Dis. 2012;73:271–272. doi: 10.1016/j.diagmicrobio.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Leathers T.D. Biotechnological production and applications of pullulan. Appl. Microbiol. Biotechnol. 2002;62:468–473. doi: 10.1007/s00253-003-1386-4. [DOI] [PubMed] [Google Scholar]
- 26.Cheng K.C., Demirci A., Catchmark J.M. Pullulan: Biosynthesis, production, and applications. Appl. Microbiol. Biotechnol. 2011;92:29–44. doi: 10.1007/s00253-011-3477-y. [DOI] [PubMed] [Google Scholar]
- 27.Manitchotpisit P., Skory C.D., Peterson S.W., Price N.P.J., Vermillion K.E., Leathers T.D. Poly (β-L-malic acid) production by diverse phylogenetic clades of Aureobasidium pullulans. J. Ind. Microbiol. Biotechnol. 2012;39:125–132. doi: 10.1007/s10295-011-1007-7. [DOI] [PubMed] [Google Scholar]
- 28.Muthusamy S., Anandharaj S.J., Kumar P.S. Microbial pullulan for food, biomedicine, cosmetic, and water treatment: A review. Environ. Chem. Lett. 2022;20:3199–3234. doi: 10.1007/s10311-022-01460-7. [DOI] [Google Scholar]
- 29.Kang X.X., Jia S.L., Wei X., Zhang M., Liu G.L., Hu Z., Chi Z., Chi Z.M. Liamocins biosynthesis, its regulation in Aureobasidium spp., and their bioactivities. Crit. Rev. Biotechnol. 2022;42:93–105. doi: 10.1080/07388551.2021.1931017. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki T., Kusano K., Kondo N., Nishikawa K., Kuge T., Ohno N. Biological Activity of High-Purity β-1,3-1,6-Glucan Derived from the Black Yeast Aureobasidium pullulans: A Literature Review. Nutrients. 2021;13:242. doi: 10.3390/nu13010242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xin Z., Chen J. A high throughput DNA extraction method with high yield and quality. Plant Methods. 2012;8:26. doi: 10.1186/1746-4811-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White T.J., Bruns T., Lee S., Taylor J. Amplification, and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. Guide Methods Appl. 1990;18:315–322. [Google Scholar]
- 33.Lin D., Wu L.C., Rinaldi M.G., Lehmann P.F. Three distinct genotypes within Candida parapsilosis from clinical sources. J. Clin. Microbiol. 1995;33:1815–1821. doi: 10.1128/jcm.33.7.1815-1821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ronquist F., Teslenko M., Van Der Mark P., Ayres D.L., Darling A., Höhna S., Larget B., Liu L., Suchard M.A., Huelsenbeck J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurtzman C.P., Robnett C.J. Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5′ end of the large-subunit (26S) ribosomal DNA gene. J. Clin. Microbiol. 1997;35:1216–1223. doi: 10.1128/jcm.35.5.1216-1223.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Q.M., Begerow D., Groenewald M., Liu X., Theelen B., Bai F.Y., Boekhout T. Multigene phylogeny and taxonomic revision of yeasts and related fungi in the Ustilaginomycotina. Stud. Mycol. 2015;81:55–83. doi: 10.1016/j.simyco.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haghighatpanah N., Mirzaee H., Khodaiyan F., Kennedy J.F., Aghakhani A., Hosseini S.S., Jahanbin K. Optimization and characterization of pullulan produced by a newly identified strain of Aureobasidium pullulans. Int. J. Biol. Macromol. 2020;152:305–313. doi: 10.1016/j.ijbiomac.2020.02.226. [DOI] [PubMed] [Google Scholar]
- 38.Buksa K., Kowalczyk M., Boreczek J. Extraction, purification and characterisation of exopolysaccharides produced by newly isolated lactic acid bacteria strains and the examination of their influence on resistant starch for-mation. Food Chem. 2021;362:130221. doi: 10.1016/j.foodchem.2021.130221. [DOI] [PubMed] [Google Scholar]
- 39.Vu D., Groenewald M., de Vries M., Gehrmann T., Stielow B., Eberhardt U., Al-Hatmi A., Groenewald J.Z., Cardinali G., Houbraken J., et al. Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Stud. Mycol. 2019;92:135–154. doi: 10.1016/j.simyco.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prabhugaonkar A., Jalmi P. Aureobasidium khasianum (Aureobasidiaceae) a novel species with distinct morphology. Phytotaxa. 2018;374:257. doi: 10.11646/phytotaxa.374.3.7. [DOI] [Google Scholar]
- 41.Verkley G.J., Starink-Willemse M., van Iperen A., Abeln E.C. Phylogenetic analyses of Septoria species based on the ITS and LSU-D2 regions of nuclear ribosomal DNA. Mycologia. 2004;96:558–571. doi: 10.1080/15572536.2005.11832954. [DOI] [PubMed] [Google Scholar]
- 42.Groenewald M., Boundy-Mills K., Čadež N., Endoh R., Jindamorakot S., Pohl-Albertyn C., Rosa C.A., Turchetti B., Yurkov A. Census of yeasts isolated from natural ecosystem and conserved in worldwide collections. In: Buzzini P., Lachance M.A., Yurkov A., editors. Yeasts in Natural Ecosystems: Diversity. Springer; Berlin, Germany: 2017. pp. 455–476. [Google Scholar]
- 43.Blackwell M. Yeasts in Insects and Other Invertebrates. In: Buzzini P., Lachance M.A., Yurkov A., editors. Yeasts in Natural Ecosystems: Diversity. Springer; Berlin, Germany: 2017. pp. 397–433. [DOI] [Google Scholar]
- 44.Boekhout T., Amend A.S., EI Baidouri F., Gabaldón T., GemI J., Mittelbach M., Rober V., Tan C.S., Turchetti B., Vu D., et al. Trends in yeast diversity discovery. Fungal Divers. 2022;114:491–537. doi: 10.1007/s13225-021-00494-6. [DOI] [Google Scholar]
- 45.Boekhout T. Biodiversity: Gut feeling for yeasts. Nature. 2005;434:449–451. doi: 10.1038/434449a. [DOI] [PubMed] [Google Scholar]
- 46.Madden A.A., Epps M.J., Fukami T., Irwin R.E., Sheppard J., Sorger D.M., Dunn R.R. The ecology of insect-yeast relationships and its relevance to human industry. Proc. Biol. Sci. 2018;285:20172733. doi: 10.1098/rspb.2017.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zou X., Cheng C., Feng J., Song X., Lin M., Yang S.T. Biosynthesis of polymalic acid in fermentation: Advances and prospects for industrial application. Crit. Rev. Biotechnol. 2019;39:408–421. doi: 10.1080/07388551.2019.1571008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All newly generated sequence data are available in NCBI GenBank. The phylogenetic tree and the alignments were deposited in TreeBASE.