Abstract
1-Hydroxybenzotriazole, violuric acid, and N-hydroxyacetanilide are three N-OH compounds capable of mediating a range of laccase-catalyzed biotransformations, such as paper pulp delignification and degradation of polycyclic hydrocarbons. The mechanism of their enzymatic oxidation was studied with seven fungal laccases. The oxidation had a bell-shaped pH-activity profile with an optimal pH ranging from 4 to 7. The oxidation rate was found to be dependent on the redox potential difference between the N-OH substrate and laccase. A laccase with a higher redox potential or an N-OH compound with a lower redox potential tended to have a higher oxidation rate. Similar to the enzymatic oxidation of phenols, phenoxazines, phenothiazines, and other redox-active compounds, an “outer-sphere” type of single-electron transfer from the substrate to laccase and proton release are speculated to be involved in the rate-limiting step for N-OH oxidation.
Laccases (EC 1.10.3.2) are multi-Cu oxidases that can catalyze the oxidation of a range of reducing substances with the concomitant reduction of O2 (for recent reviews, see reference 24 and references therein). Because of their capability of catalyzing the oxidation of aromatic compounds, laccases are receiving increasing attention as potential industrial enzymes in various applications, such as pulp delignification, wood fiber modification, dye or stain bleaching, chemical or medicinal synthesis, and contaminated water or soil remediation (15, 37).
Laccases contain one type 1 (T1) Cu center, one type 2 (T2) Cu center, and one type 3 (T3) Cu center. The T2 and T3 sites form a trinuclear Cu cluster onto which O2 is reduced. The T1 Cu oxidizes the reducing substrate and transfers electrons to the T2 and T3 Cu. Laccase is able to oxidize certain phenols with E0 values higher than its own (0.5 to 0.8 V versus the normal hydrogen electrode [NHE]) (36). However, many inorganic and organic compounds with comparable E0 values (such as 1,2,3,5-tetramethoxybenzene [18]) are not laccase substrates due to unfavorable kinetics. Under certain conditions, however, these compounds can be indirectly oxidized by laccase through the mediation of small, redox-active laccase sub- strates. 2,2′-Azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) was the first compound found capable of efficiently mediating the laccase oxidation of high-E0, nonsubstrate lignin model compounds (such as veratryl alcohol and nonphenolic lignin model dimers) (8). Based on product structure analysis, it has been proposed that laccase-oxidized ABTS can abstract an H atom from the lignin model compounds, leading to indirect laccase catalysis upon the oxidation of the compounds (25). To date, other types of mediators, particularly phenoxazines and N-OH compounds, also have been recognized for their mediation function in laccase catalysis (1, 6, 17, 29).
Mediated laccase catalysis has been applied to a wide range of applications, such as pulp delignification (9, 10, 12, 22, 32), textile dye bleaching (31), polycyclic aromatic hydrocarbon degradation (16, 23), pesticide or insecticide degradation (1, 29), and organic synthesis (13, 28). For the paper and pulp industry, novel biological or enzymatic bleaching technologies (including mediated laccase catalysis) have attracted increasing attention (9, 10, 12, 14, 22, 27, 32) because of concerns regarding the environmental impact of the chlorine-based oxidants currently being used in delignification or bleaching.
Detailed, comparative information on the interaction between mediator and laccase remains to be reported (22), although various physical and chemical characterizations have been performed on several well-known laccase mediators (2, 4, 7, 11, 21, 35). For N-OH-type mediators, it has not been clear whether their oxidation by laccase involves H abstraction or electron transfer, similar to that found with the oxidation of phenol (38). To better understand the mechanism that governs the oxidation of these compounds by laccase, we studied the interactions of three N-OH compounds (Fig. 1) with seven fungal laccases. The observed dependence of the reaction rate on ΔE0 suggests that the laccase-catalyzed oxidation of N-OH compounds is governed by a mechanism similar to that reported for phenols, phenoxazines, and phenothiazines.
FIG. 1.
Structures of HBT, VA, and NHA.
MATERIALS AND METHODS
Materials.
The chemicals used were commercial products of at least reagent grade. Botrytis cinerea laccase (BcL) (22), Coprinus cinereus laccase-1 (CcL) (30), Myceliophthora thermophila laccase (MtL) (5), Myrothecium verrucaria bilirubin oxidase (MvBO) (39), Pycnoporus cinnabarinus laccase (PcL) (22), Rhizoctonia solani laccase 4 (RsL) (34), Scytalidium thermophilum laccase (StL) (39), and Trametes villosa (Polyporus pinsitus) laccase 1 (TvL) (40) were purified as previously reported. Violuric acid (VA) and 1-hydroxybenzotriazole (HBT) were purchased from Aldrich. Promazine and chloropromazine were purchased from Sigma. N-Hydroxyacetanilide (NHA) and phenothiazine-10-propionic acid (PP) were synthesized as described previously (26, 33). 10-Methyl phenothiazine, 3,10-dimethyl phenothiazine, 10-ethyl phenothiazine, 10-(2-hydroxyethyl) phenothiazine, phenothiazine 10-methylpropionate, phenothiazine 10-propionamide, phenothiazine 10-propionitrile, 10-methyl-1-carboxylic acid phenothiazine, 10-methyl-2-carboxylic acid phenothiazine, 10-methyl-3-carboxylic acid phenothiazine, 10-ethyl-4-carboxylic acid phenothiazine, 10-(3-hydroxypropyl) phenothiazine, 10-(2-ethoxy-2′-hydroxyethyl) phenothiazine, 2-acetyl-10-methyl phenothiazine, 10-methyl-3-(2-hydroxyethyl) phenothiazine, 2-chloro-10-methyl phenothiazine, 2-methoxy-10-methyl phenothiazine, 10-methyl phenoxazine, 10-(2-hydroxyethyl) phenoxazine, and phenoxazine 10-propionic acid were synthesized as described elsewhere (20a).
Instruments.
UV-visible absorption spectroscopy (including kinetic spectral measurements) was performed either on a spectrophotometer (Shimadzu UV160U or Gilford Instruments 2600) and a quartz cuvette or on a microplate reader (Molecular Devices Thermomax) and 96-well microplates (Costar tissue culture plates). Cyclic and differential pulse voltammetry analyses were performed on a computer-controlled electroanalytical system (Cypress Systems), with a glass carbon working electrode (Cypress Systems model CS-1087), a KCl-saturated calomel reference electrode (Radiometer model K-401), and a platinum wire counterelectrode (0.2-mm diameter, 4-cm length, mounted on the end of the reference electrode). Surface cleansing of the working electrode was carried out by polishing with alumina and washing with water.
Electrochemistry.
To determine the E0 of the N-OH compounds, cyclic voltammetry was performed at 25°C in (aerobic) solutions containing 1 mM N-OH compound, 0.1 M KCl, 33 mM sodium phosphate, 33 mM sodium borate, and 33 mM sodium carbonate (pH 4 to 10). The scanning rate was 0.1 V/s. Measured potentials were compared to the NHE by considering the E0 of the KCl-saturated calomel reference electrode to be 0.242 V against the NHE.
The E0 values of the phenoxazines and phenothiazines were measured by differential pulse voltammetry in 50 mM sodium phosphate (pH 7) at room temperature, with a 50-mV pulse height, a 2-mV step height, a 40-ms pulse width, an 0.8- to 0.3-V electrode potential change, and a 12 to 21 μM concentration. E0 values of 0.66 to 0.95 V were observed (20a).
O2 electrode-based enzymatic assays.
Laccase activity was measured in 10 mM morpholineethanesulfonic acid (MES)-NaOH (pH 5.5) at 20°C with a Hansatech O2 cell (38). ABTS was used as a calibrator. The N-OH substrate stock solutions were made in dimethylformamide (1 M for HBT, 0.5 M for VA, and 0.1 M for NHA). At the tested level (≤10%), the dimethylformamide introduced along with the substrate did not alter the kinetic measurements (as tested by ABTS oxidation). The laccase concentrations were 0.8 μM for TvL, 1 to 15 μM for RsL, 2 to 37 μM for MtL, 4 to 55 μM for StL, 3 to 19 μM for CcL, 0.4 to 4 μM for PcL, and 1 to 3 μM for BcL.
The pH-activity profile was measured at 20°C in Britton-Robinson buffer, made by mixing 0.1 M boric acid, 0.1 M acetic acid, 0.1 M phosphoric acid, and 0.5 M NaOH. The substrate concentrations were 40 to 100 mM for HBT, 33 to 58 mM for VA, and 6.7 mM for NHA. The laccase concentrations were 0.8 μM for TvL, 2 to 15 μM for RsL, 2 to 11 μM for MtL, 2 to 16 μM for StL, 3 to 19 μM for CcL, 0.7 μM for PcL, and 3 to 6 μM for BcL.
PP oxidation was performed with 0.01 to 1 mM PP in 10 mM MES (pH 5.5). The concentrations of laccase were 0.1 μM for TvL, 2 μM for RsL, 2 μM for MtL, 5 μM for StL, and 1 μM for MvBO. The stock solution of PP (0.1 M) was made in 0.1 M NaOH.
Spectrophotometric enzymatic assays.
Spectrophotometric enzymatic assays were performed with 50 mM sodium acetate (pH 5.5) at 25°C. The oxidation of VA or NHA by laccase was monitored at 310 nm with a molar absorption (ɛ) value of 13.9 or 8.9 mM−1 cm−1, respectively. Substrate concentration ranges were 10 to 120 μM for VA and 25 to 200 μM for NHA. Laccase concentration ranges were 0.2 to 1 μM (when oxidizing VA) or 0.2 μM (when oxidizing NHA) for TvL, 1 μM for CcL, and 1 μM (when oxidizing NHA) or 10 μM (when oxidizing VA) for MtL. Third-order polynomial [c = a + b(t) + c(t2) + d(t3)], where c is the concentration and b is the initial rate constant) nonlinear regression was applied (using the MathCad program) to the kinetic data to extract the apparent rate constant.
For phenoxazines and phenothiazines, their oxidation (into cation radicals) by TvL was monitored at 525 and 514 nm with ɛ values of 16 and 8.9 mM−1 cm−1, respectively. The reactions were carried out with 1.5 to 80 μM substrate and 2.5 to 40 nM TvL in 50 mM sodium acetate (pH 5.3) and 1% ethanol at 25°C. Km values from 6 to 678 μM and kcat (Vmax/[laccase]) values from 120 to 8,580 min−1 were observed (20a).
RESULTS
Electrochemistry and redox potentials of N-OH compounds.
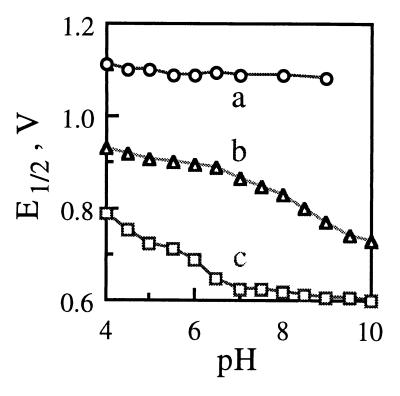
Under the conditions used in this study, the cyclic voltammetry of HBT exhibited irreversible oxidation, similar to the observation previously reported (7, 19). Depending on pH, an anodic peak was observed near a peak potential [Epa] of 1.1 to 1.2 V, with a peak current intensity (Ipa) corresponding to 2.1 to 2.4 electrons transferred per HBT molecule. Within the scanning rate range, only a small cathodic peak (with a peak potential [Epc] near 0.54 V and a peak current intensity [Ipc] ≤10% that of Ipa) was detected, indicating the residual reduction of oxidized HBT. As shown in Fig. 2, the pH dependence of Epa for HBT was not significant.
FIG. 2.
Formal redox potentials of the N-OH compounds as a function of pH. Traces a, b, and c represent the pH dependence of Epa of HBT, E1/2 of VA, and E1/2 of NHA, respectively. For HBT, there was no significant acid-base transition for its Epa; thus, no apparent pKa was extracted from trace a. For VA, two apparent pKa values of 6.4 and 8.6 were extracted from trace b. For NHA, two apparent pKa values of 3.7 and 6.3 were extracted from trace c. E1/2 values of 0.83 and 0.91 V at pH 4 have been reported for NHA and VA, respectively, by R. Bourbonnais et al. (Oxidative Enzymes for Lignocellulose Processing, Symp. Am. Chem. Soc. 217th Nat. Meet., Anaheim, Calif., 21 to 25 March 1999).
Unlike HBT, VA showed a well-shaped cathodic peak, indicating apparent reversibility. The differences between the anodic and cathodic peak potentials (ΔEp = Epa − Epc) were ∼70, 80, and 140 mV for pHs 4 to 8, 9 and 10, respectively. Based on the Ipa, ∼1.4, 1.5, 1.6, 1.4, 1.3, and 1.1 electrons were transferred per VA molecule during oxidation at pHs 4, 5, 6 to 7, 8, 9, and 10, respectively.
Like VA, NHA had a quasi-reversible cyclic voltammogram. The differences (ΔEp) were ∼130, 110, and 80 mV for pHs 4 to 5, 6 to 7, and 8 to 10, respectively. Based on the Ipa, ∼1.1, 1.0, 0.8, 0.9, and 1.1 electrons were transferred per NHA molecule during oxidation at pHs 4 to 6, 7, 8, 9, and 10, respectively. As shown in Fig. 2, the formal redox potentials {E1/2 = [(Epa + Epc)/2]} of both VA and NHA were pH dependent. For pH ranges of 6 to 9 and 4 to 7, the E1/2-pH plot of VA or NHA had an apparent slope of 50 or 56 mV per pH unit, respectively. For a given pH, the redox potentials of these three N-OH compounds were on the order of HBT > VA > NHA.
Laccase-catalyzed oxidation of N-OH compounds.
Serving as a reducing substrate for laccase, the three N-OH compounds exhibited typical Michaelis-Menten kinetics, as monitored by concomitant O2 reduction (Fig. 3). Table 1 shows the Km and kcat values observed in 10 mM MES (pH 5.5) for the seven laccases and the three N-OH compounds. For MtL, up to 40 mM NHA could not lead to saturation of the initial oxidation rate, thus not allowing an accurate measurement of Km and kcat values.
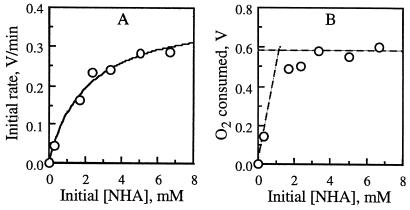
FIG. 3.
O2 consumption in RsL-catalyzed oxidation of NHA at pH 5.5. Plotted against the initial NHA concentration [NHA] are the initial O2 consumption rate (as the output voltage change rate) (A) and the final O2 consumed (as the final output voltage change) (B). For graph clarity, two sets of data, obtained with 0.05 and 0.3 mM NHA, are omitted from panel B. Their symbols overlap with those for 0 and 0.33 mM NHA (shown), and their values were included in the graph fitting. In panel A, the solid curve shows the fit to the Michaelis-Menten equation {v = −Vmax[NHA]/(Km + [NHA])} with a Km of 2.0 ± 0.5 mM and a Vmax of 0.39 ± 0.03 V min−1 or 0.19 ± 0.01 mM min−1 (corresponding to a kcat of 150 ± 10 min−1 (mean ± standard deviation). In panel B, the horizontal broken line represents the voltage change (0.58 ± 0.03 V, averaged over the data obtained with 3.3, 5.0, and 6.7 mM NHA) corresponding to maximal O2 consumption. Its cross point with the other broken line (voltage change, 0.5 × [NHA]; r2, 0.92), obtained by fitting the data obtained with 0, 0.05, 0.30, and 0.33 mM NHA, yielded a saturating [NHA] of 1.2 mM. By dividing 1.2 mM by 0.28 mM, the dissolved [O2] in water, we estimated an oxidation stoichiometry number of 4.1.
TABLE 1.
Kinetic properties of the laccases on HBT, VA, and NHA at pH 5.5a
| Laccase | Substrate | Mean ± SD
|
Optimal pH | |
|---|---|---|---|---|
| Km (mM) | kcat (min−1) | |||
| TvL | HBT | 15 ± 3 | 84 ± 6 | 5–6 |
| VA | 5 ± 1 | 260 ± 20 | 6 | |
| NHA | 0.9 ± 0.3 | 470 ± 60 | 5 | |
| BcL | HBT | 12 ± 4 | 10 ± 1 | 6 |
| VA | 11 ± 1 | 40 ± 2 | 5 | |
| NHA | 1.5 ± 0.5 | 160 ± 20 | 5 | |
| PcL | HBT | 29 ± 7 | 22 ± 2 | 6 |
| VA | 9 ± 1 | 370 ± 20 | 4–5 | |
| NHA | 2.2 ± 0.6 | 1,500 ± 200 | 4 | |
| RsL | HBT | 10 ± 2 | 0.57 ± 0.02 | 5 |
| VA | 2.7 ± 0.4 | 46 ± 2 | 4 | |
| NHA | 2.0 ± 0.5 | 150 ± 10 | 7 | |
| CcL | HBT | 7 ± 2 | 0.45 ± 0.05 | 6 |
| VA | 5 ± 1 | 10 ± 1 | 5 | |
| NHA | 3 ± 2 | 17 ± 5 | 6 | |
| StL | HBT | 31 ± 16 | 1.3 ± 0.3 | 5 |
| VA | 0.35 ± 0.08 | 3.2 ± 0.3 | 6 | |
| NHA | 12 ± 3 | 6 ± 1 | 7 | |
| MtL | HBT | 10 ± 8 | 0.12 ± 0.05 | 6 |
| VA | 18 ± 2 | 27 ± 1 | 4 | |
| NHA | ≥20 | ≥36 | 7 | |
The Km and kcat data for TvL-, BcL-, PcL-, and MtL-catalyzed HBT and VA oxidations are taken from reference 22.
The oxidation of VA and NHA by TvL, CcL, and MtL was also monitored spectrophotometrically. Under laccase catalysis, the oxidation of VA led to a decrease of the absorbance centered at 310 nm. The oxidation of NHA increased the absorbances at 220 to 230 and 266 to 370 nm (with maxima at 229, 283, and 308 nm) and decreased the absorbance centered at 245 nm (with two apparent isobestic points at 230 and 266 nm). Before the full formation of the apparently stable product (peak wavelengths [λmax] at 229, 283, and 308 nm; trough wavelengths [λmin] at 261 and 290 nm), a transient product seemed to be formed, as demonstrated by a spectrum with λmax at 245 and 323 nm and λmin at 293 nm. A linear dependence of rate on substrate concentration was observed at the selected concentration ranges. For VA, apparent rate constants of ∼350, 170, and 5.9 M−1 s−1 were observed for TvL, CcL, and MtL, respectively. For NHA, apparent rate constants of ∼2,100, 330, and 30 M−1 s−1 were observed for TvL, CcL, and MtL, respectively.
Dependence on E0 and pH.
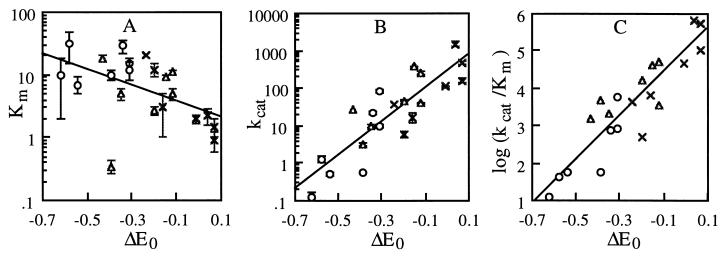
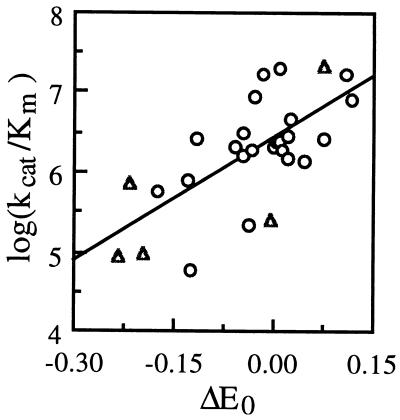
Figure 4 shows the dependence of kcat, Km, and kcat/Km on ΔE0 {E0 [laccase (T1 Cu)] − E0 [substrate]} at pH 5.5. For HBT, VA, and NHA, the Epa, E1/2, and E1/2 determined from cyclic voltammetry, respectively, were used to calculate ΔE0. For HBT, the use of Epa would slightly overestimate ΔE0, since the irreversible decay of the immediately oxidized HBT would yield Epa greater than or equal to E1/2 (∼1.11 V at pH 4 [7]), according to the kinetic characteristics of a homogeneous redox catalysis electrochemical reaction (3). An apparently linear, positive correlation was observed between log(kcat) and ΔE0 as well as between log(kcat/Km) and ΔE0, while an apparently linear, negative correlation was observed between log(Km) and ΔE0. All three N-OH substrates showed bell-shaped pH-rate profiles with an optimal pH ranging from 4 to 7 (Table 1).
FIG. 4.
Dependence of Km, kcat, and kcat/Km on ΔE0. Symbols: ○, HBT; ▵, VA; ×, NHA. Correlation lines: A, log(Km) = −1.3(ΔE0) + 0.46 (r2, 0.26); B, log(kcat) = 4.5(ΔE0) + 2.5 (r2, 0.74); C, log(kcat/Km) = 5.8(ΔE0) + 5.0 (r2, 0.82). Units: A, Km, mM; B, kcat, min−1; C, kcat/Km, M−1 min−1. ΔE0 is reported in V. E0 values for laccase are taken from reference 36. Error bars indicate standard deviations.
Laccase-catalyzed oxidation of phenoxazines and phenothiazines.
Serving as a reducing substrate for laccase, the oxidation of PP exhibited typical Michaelis-Menten kinetics, as monitored by concomitant O2 reduction. The Km and kcat values (mean ± standard deviation) observed in 10 mM MES (pH 5.5) were 120 ± 50 μM and 2,500 ± 400 min−1 for TvL, 32 ± 5 μM and 8 ± 1 min−1 for RsL, 120 ± 40 μM and 11 ± 4 min−1 for MtL, 47 ± 5 μM and 4.7 ± 0.1 min−1 for StL, and 30 ± 5 μM and 21 ± 1 min−1 for MvBO, respectively. An apparently linear correlation was observed between log(kcat/Km) and ΔE0 (Fig. 5), similar to the data obtained for the TvL-catalyzed oxidation of more than 20 phenothiazines and phenoxazines (20a).
FIG. 5.
Correlations between log(kcat/Km) and ΔE0 for phenothiazines and phenoxazines. Symbols: ○, oxidation by TvL of 3 phenoxazines and 20 phenothiazines (see Materials and Methods for their formulas) (20a); ▵, oxidation of PP (E0, 0.71 V) by TvL, RsL, MtL, StL, and MvBO at pH 5.3 to 5.5. Correlation: log(kcat/Km) = 5.1(▵E0) + 6.4 (r2, 0.47). Units: kcat/Km, M−1 min−1; ΔE0, V.
DISCUSSION
Redox chemistry of N-OH compounds.
It is known that the oxidation of HBT generates a highly unstable intermediate, putatively an N-O· radical, that quickly decays into catalytically inactive secondary product(s), including benzotriazole (21). An apparent E1/2 of ∼1.1 V has been reported for a two-electron electrochemical oxidation of HBT at pH 4 (7). In our study, instability of the putative HBT radical was observed over the pH range of 4 to 10. The better stability observed for the immediate oxidation products (likely N-O· in nature) of VA and NHA could be related to their E1/2 values, which were 0.2 to 0.3 V lower than that of HBT. The reduction in E1/2 might decrease the oxidative potency or activity of N-O; thus enhancing stability.
As shown in Fig. 2, the E1/2 of VA and NHA decreased when pH increased. Since phenyl-N-OH is a heteroatomic homolog of phenol, the oxidation of an aromatic N-OH compound could lead to H+ release (N-OH → N-O⋅ + e− + H+), as for phenol (C-OH → C-O⋅ + e− + H+). According to the Nernst equation, E0 = E0 + {RT/F} ln {[N-O⋅][H+]/[N-OH]} = E0 + {RT/F} ln {[N-O⋅]/[N-OH]} − RT/{F log(e)}pH, such H+ release would lead to a lower potential at a higher pH (∼0.06-V reduction per pH unit at room temperature), reflecting the fact that the quenching of H+ by OH− at a higher pH facilitates thermodynamically the N-OH oxidation. Thus, the decrease in E1/2 of VA and NHA with an increase in pH indicated the involvement of H+ release and the concomitant production of N-O· during the oxidation.
Electron transfer from N-OH compounds to laccases.
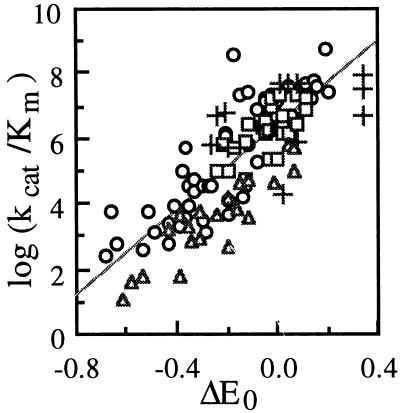
At steady state, the rate-limiting step for phenol oxidation by laccase involves the Marcus “outer-sphere” mechanism. In this mechanism, ΔE0 (together with reorganization energy and transmission coefficient) determines the electron transfer rate, distinguishing it from other oxidation mechanisms (i.e., H abstraction), where energetic factors related to covalent bond are most important (i.e., homolytic O—H bond dissociation energy). As shown in Fig. 4, a linear correlation existed between log(kcat) or log(kcat/Km) (in which kcat/Km could be approximated as the second-order rate constant of the oxidation) and ΔE0 (the driving force for electron transfer from the N-OH compound to laccase) for laccase-catalyzed oxidation of the N-OH compounds. When the data for a wide variety of phenols, phenothiazines, phenoxazines, N-OH compounds, and other inorganic and organic redox-active molecules are analyzed together, a common linear correlation between log(kcat/Km) and ΔE0 can be found (Fig. 6). Thus, as for other laccase substrates, the rate-limiting step of laccase-catalyzed N-OH oxidation involves electron transfer from the substrate to the T1 Cu site in laccase. It is ΔE0 that dominates the oxidation rate. The higher E0 (laccase) or the lower E0 (N-OH) is, the faster the oxidation rate tends to be. Other factors (such as the composition, structure, or pKa of the substrate) seem to be minor, but they could fine-tune the activity for a given ΔE0 (an effect that might contribute to the scattering shown in Fig. 6).
FIG. 6.
Correlations between log(kcat/Km) and ΔE0 for laccase catalysis. Symbols: ○, oxidation of 24 phenols by 10 fungal on plant laccases (36); □, oxidation of 3 phenoxazines and 22 phenothiazines by TvL, as well as oxidation of PP by 5 fungal laccases (data from Fig. 5); ▵, oxidation of three N-OH compounds by 7 fungal laccases (data from Table 1 and Fig. 4C); +, oxidation of ABTS, K4Fe(CN)6 and morpholinoaniline by up to 10 fungal or plant laccases (20, 36). Other conditions: pH, 5.3 to 5.5; temperature, 20 to 25°C. Correlation: log(kcat/Km) = 6.4(ΔE0) + 6.4 (r2, 0.65). Units: kcat/Km, M−1 min−1; ΔE0, V.
The apparent negative correlation between log(Km) and ΔE0 suggests that substrate affinity tends to increase when ΔE0 increases (realized by either E0 [laccase] increase or E0 [substrate] decrease) (Fig. 4A), a phenomenon also observed for phenolic substrates (36, 39). Prior to electron transfer, the filled (valence) molecular orbitals of N-O in the N-OH compounds (or the phenoxy-O in phenols) overlap with the half-occupied molecular orbitals (HOMO) of T1 Cu when the substrate is bound to laccase. A larger ΔE0 could create a transitional energy state more favorable for the molecular orbital interaction, resulting in better substrate binding and consequently faster electron transfer.
Dependence of activity on pH.
When oxidizing a phenolic substrate, laccase generally possesses a bell-shaped pH-activity profile. Two opposing factors, ΔE0 (involving substrate and laccase T1 Cu) and OH− inhibition (involving T2 Cu in laccase), are suggested to play important roles in determining the pH-activity profile (38). Like phenols, HBT, VA, and NHA have redox potentials that decrease when pH increases (Fig. 2). Since the E0 of laccase is often quite insensitive to pH change (38), the decrease in the E0 of N-OH as pH increases would increase ΔE0, which in turn would enhance the oxidation rate through the correlation shown in Fig. 4. However, the OH− inhibition of laccase would become overwhelming at an alkaline pH. The combination of these two effects might contribute to the bell-shaped pH-activity profiles of N-OH compounds.
The speculation of an H+ release step during laccase-catalyzed N-OH oxidation, together with the observation that the reduction of one O2 was accompanied by the oxidation of about four N-OH groups, indicates that the reaction N-OH → N-O· + e− + H+ might be involved in the rate-limiting step, similar to the reaction C-OH → C-O· + e− + H+, which is involved in laccase-catalyzed phenol oxidation (36).
Overall remarks.
The results of this study suggest that the initial oxidation of a phenol (aryl C-OH) compound by laccase is quite similar to the oxidation of an aryl N-OH (phenol homolog) compound in terms of the dependence of the initial rate on E0 and pH. In general, phenol is first oxidized to a highly unstable phenoxy radical (aryl C-O·), which then surrenders an additional e− (at a rate faster than that of the first e− transfer) to yield a stable, but much less active, quinone. Oxidation of N-OH compounds also involves a single e− transfer at the initial oxidation step. N-O· could be less active but more stable than a phenoxy radical. In laccase-catalyzed pulp delignification, a desirable redox mediator should be a good laccase substrate, have a half-life at its oxidized form long enough to permit diffusion to heterogeneous lignin, and possess high oxidation potency to effectively oxidize lignin. In comparison with those of a phenoxy radical, the activity and stability of N-O· seem to be better balanced, which could contribute to the better performance of the latter as a mediator for laccase-based delignification.
ACKNOWLEDGMENTS
We thank Alan V. Klotz and Henrik Bisgård-Frantzen of Novo Nordisk for critical reading of the manuscript and helpful suggestions.
REFERENCES
- 1.Amitai G, Adani R, Sod-Moriah G, Rabinovitz I, Vincze A, Leader H, Chefetz B, Leibovitz-Persky L, Friesem D, Hadar Y. Oxidative biodegradation of phosphorothiolates by laccase. FEBS Lett. 1998;438:195–200. doi: 10.1016/s0014-5793(98)01300-3. [DOI] [PubMed] [Google Scholar]
- 2.Ander P, Messner K. Oxidation of 1-hydroxybenzotriazole by laccase and lignin peroxidase. Biotechnol Techniques. 1998;12:191–195. [Google Scholar]
- 3.Andrieux C P, Saveant J-M. Homogeneous redox catalysis of electrochemical reactions: electron transfers followed by a very fast chemical step. J Electroanal Chem. 1986;205:43–58. [Google Scholar]
- 4.Aurich H G, Bach G, Hahn K, Küttner G, Weiss W. Aminyloxide (Nitroxide). XXV. Reaktionen von Benzotriazolyloxid-radikalen mit Aromaten. J Chem Res. 1977;1997:1537–1545. [Google Scholar]
- 5.Berka R M, Schneider P, Golightly E J, Brown S H, Madden M, Brown K M, Halkier T, Mondorf K, Xu F. Characterization of the gene encoding an extracellular polyphenoloxidase of Myceliophthora thermophila and analysis of the recombinant enzyme expressed in Aspergillus oryzae. Appl Environ Microbiol. 1997;63:3151–3157. doi: 10.1128/aem.63.8.3151-3157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Böhmer S, Messner K, Srebotnik E. Oxidation of phenanthrene by a fungal laccase in the presence of 1-hydroxybenzotriazole and unsaturated lipids. Biochem Biophys Res Commun. 1998;244:233–238. doi: 10.1006/bbrc.1998.8228. [DOI] [PubMed] [Google Scholar]
- 7.Bourbonnais R, Leech D, Paice M G. Electrochemical analysis of the interactions of laccase mediators with lignin model compounds. Biochim Biophys Acta. 1998;1379:381–390. doi: 10.1016/s0304-4165(97)00117-7. [DOI] [PubMed] [Google Scholar]
- 8.Bourbonnais R, Paice M G. Oxidation of non-phenolic substrates. An expanded role for laccase in lignin biodegradation. FEBS Lett. 1990;267:99–102. doi: 10.1016/0014-5793(90)80298-w. [DOI] [PubMed] [Google Scholar]
- 9.Bourbonnais R, Paice M G. Enzymatic delignification of kraft pulp using laccase and a mediator. Tappi J. 1996;79:199–204. [Google Scholar]
- 10.Call H P, Mücke I. History, overview and applications of mediated lignolytic systems, especially laccase-mediator-systems (Lignozym®-process) J Biotechnol. 1997;53:163–202. [Google Scholar]
- 11.Collins P J, Dobson A D W, Field J A. Reduction of the 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonate) cation radical by physiological organic acids in the absence and presence of manganese. Appl Environ Microbiol. 1998;64:2026–2031. doi: 10.1128/aem.64.6.2026-2031.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crestini C L, Argyropoulos D S. The early oxidative biodegradation steps of residual kraft lignin models with laccase. Bioorg Med Chem. 1998;6:2161–2169. doi: 10.1016/s0968-0896(98)00173-4. [DOI] [PubMed] [Google Scholar]
- 13.Fritz-Langhals E, Kunath B. Synthesis of aromatic aldehydes by laccase-mediator assisted oxidation. Tetrahedron Lett. 1998;39:5955–5956. [Google Scholar]
- 14.Fujita K, Kondo R, Sakai K, Kashino Y, Nishida T, Takahara Y. Biobleaching of kraft pulp using white-rot fungus IZU-154. Tappi J. 1991;74:123–127. [Google Scholar]
- 15.Gianfreda L, Xu F, Bollag J-M. Laccases: a useful group of oxidoreductive enzymes. Bioremediation J. 1999;3:1–25. [Google Scholar]
- 16.Johannes C, Majcherczyk A, Hüttermann A. Degradation of anthracene by laccase of Trametes versicolor in the presence of different mediator compounds. Appl Microbiol Biotechnol. 1996;46:313–317. doi: 10.1007/s002530050823. [DOI] [PubMed] [Google Scholar]
- 17.Kawai S, Umezawa T, Higuchi T. Oxidation of methoxylated benzyl alcohols by laccase of Coriolus versicolor in the presence of syringaldehyde. Wood Res. 1989;76:10–16. [Google Scholar]
- 18.Kersten P J, Kalyanaraman B, Hammel K, Reinhammar B, Kirk T K. Comparison of lignin peroxidase, horseradish peroxidase and laccase in the oxidation of methoxybenzenes. Biochem J. 1990;268:475–480. doi: 10.1042/bj2680475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krikstopaitis K, Kulys J, Palaima A. Fungal peroxidase- and laccase-catalyzed oxidation of 1-hydroxybenzotriazole. Biologija. 1996;4:33–38. [Google Scholar]
- 20.Kulys J, Drungiliene A, Wollenberger U, Krikstopaitis K, Scheller F. Electroanalytical determination of peroxidases and laccases on carbon paste electrodes. Electroanalysis. 1997;9:213–218. [Google Scholar]
- 20a.Kulys, J., P. Schneider, S. Ebdrup, A. H. Pedersen, K. Krikstopaitis, and A. Ziemys. J. Biol. Inorg. Chem., in press. [DOI] [PubMed]
- 21.Li K, Helm R F, Erikssen K-E L. Mechanistic studies of the oxidation of a non-phenolic lignin model compound by the laccase/1-hydroxybenzotriazole redox system. Biotechnol Appl Biochem. 1998;27:239–243. [Google Scholar]
- 22.Li K, Xu F, Erikssen K-E L. Comparison of fungal laccases and redox mediators in oxidation of a non-phenolic lignin model compound. Appl Environ Microbiol. 1999;65:2654–2660. doi: 10.1128/aem.65.6.2654-2660.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majcherczyk A, Johannes C, Hüttermann A. Oxidation of polycyclic aromatic hydrocarbons (PAH) by laccase of Trametes versicolor. Enzyme Microb Technol. 1998;22:335–341. [Google Scholar]
- 24.Messerschmidt A. Multi-copper oxidases. Singapore, Singapore: World Scientific; 1997. [Google Scholar]
- 25.Muheim A, Fiechter A, Harvey P J, Schoemaker H E. On the mechanism of oxidation of non-phenolic lignin model compounds by the laccase-ABTS couple. Holzforschung. 1992;46:121–126. [Google Scholar]
- 26.Oxley P W, Adger B M, Sasse M J, Forth M A. N-acetyl-N-phenylhydroxylamine via catalytic transfer hydrogenation of nitrobenzene using hydrazine and rhodium on carbon. Org Synth. 1989;67:187–192. [Google Scholar]
- 27.Paice M G, Jurasek L, Ho C, Bourbonnais R. Direct biological bleaching of hardwood kraft pulp with the fungus Coriolus versicolor. Tappi J. 1989;72:217–221. [Google Scholar]
- 28.Potthast A, Rosenau T, Chen C L, Gratzl J S. A novel method for the conversion of benzyl alcohols to benzaldehydes by laccase-catalysed oxidation. J Mol Catal A. 1996;108:5–9. [Google Scholar]
- 29.Sariaslani F S, Beale J M, Jr, Rosazza P. Oxidation of rotenone by Polyporus anceps laccase. J Nat Prod. 1984;47:692–697. doi: 10.1021/np50034a021. [DOI] [PubMed] [Google Scholar]
- 30.Schneider P, Caspersen M B, Mondorf K, Halkier T, Skov L K, Østergaard P R, Brown K M, Brown S H, Xu F. Characterization of a Coprinus cinereus laccase. Enzyme Microb Technol. 1999;25:502–508. [Google Scholar]
- 31.Schneider P, Pedersen A H. Enhancement of laccase reactions. PCT world patent WO 95/01426. January 1995. [Google Scholar]
- 32.Sealey J, Ragauskas A J. Residual lignin studies of laccase-delignified kraft pulps. Enzyme Microb Technol. 1998;23:422–426. [Google Scholar]
- 33.Smith N L. Synthesis of phenothiazine derivatives for use as antioxidants. J Org Chem. 1950;15:1125–1129. [Google Scholar]
- 34.Wahleithner J A, Xu F, Brown K M, Brown S H, Golightly E J, Halkier T, Kauppinen S, Pederson A, Schneider P. The identification and characterization of four laccases from the plant pathogenic fungus Rhizoctonia solani. Curr Genet. 1996;29:395–403. doi: 10.1007/BF02208621. [DOI] [PubMed] [Google Scholar]
- 35.Wolfenden B S, Willson R L. Radical-cations as reference chromogens in kinetic studies of one-electron transfer reactions: pulse radiolysis studies of 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulphonate) J Chem Soc Perkin Trans 2. 1982;1982:805–812. [Google Scholar]
- 36.Xu F. Oxidation of phenols, anilines, and benzenethiols by fungal laccases: correlation between activity and redox potentials as well as halide inhibition. Biochemistry. 1996;35:7608–7614. doi: 10.1021/bi952971a. [DOI] [PubMed] [Google Scholar]
- 37.Xu F. Recent progress in laccase study: properties, enzymology, production, and applications. In: Flickinger M C, Drew S W, editors. The encyclopedia of bioprocessing technology: fermentation, biocatalysis, and bioseparation. New York, N.Y: John Wiley & Sons, Inc.; 1999. pp. 1545–1554. [Google Scholar]
- 38.Xu F. Effects of redox potential and hydroxide inhibition on the pH activity profile of fungal laccases. J Biol Chem. 1997;272:924–928. doi: 10.1074/jbc.272.2.924. [DOI] [PubMed] [Google Scholar]
- 39.Xu F, Shin W, Brown S H, Wahleithner J A, Sundaram U M, Solomon E I. A study of a series of recombinant fungal laccases and bilirubin oxidase that exhibit significant differences in redox potential, substrate specificity, and stability. Biochim Biophys Acta. 1996;1292:303–311. doi: 10.1016/0167-4838(95)00210-3. . (Corrigendum, 1341:99, 1997.) [DOI] [PubMed] [Google Scholar]
- 40.Yaver D S, Xu F, Golightly E J, Brown K M, Brown S H, Rey M W, Schneider P, Halkier T, Mondorf K, Dalbøge H. Purification, characterization, molecular cloning, and expression of two laccase genes from the white rot basidiomycete Trametes villosa. Appl Environ Microbiol. 1996;62:834–841. doi: 10.1128/aem.62.3.834-841.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]