Abstract
The oral cavity is very diverse, wherein saliva plays an important role in maintaining oral health. The metabolism of saliva has been used to investigate oral diseases as well as general diseases, mainly to detect diagnostic biomarkers. There are many sources of salivary metabolites in the mouth. Online English language sources and the PubMed database were searched to retrieve relevant studies on oral salivary metabolites. The physiological balance of the mouth is influenced by many factors that are reflected in the salivary metabolite profile. Similarly, the dysbiosis of microbes can alter the salivary metabolite profile, which may express oral inflammation or oral diseases. This narrative review highlights the factors to be considered when examining saliva and its use as a diagnostic biofluid for different diseases. Salivary metabolites, mainly small-molecule metabolites may enter the bloodstream and cause illness elsewhere in the body. The importance of salivary metabolites produced in the oral cavity as risk factors for general diseases and their possible relationship to the body’s function are also discussed.
Keywords: saliva, gingival crevicular fluid, metabolites, oral disease, inflammation, oral microbiota
1. Introduction
The metabolic analysis of human biofluids, including saliva, has progressed rapidly over the last decade evolving to obtain new biological information about different diseases and salivary biomarkers in diagnostics. Analysis of salivary metabolites has already become a common task in saliva research due to continuous development in analytical techniques, so that low-molecular-weight metabolites are easily accessible in different biofluids, including saliva. Mass spectrometry (MS) and nuclear magnetic resonance (NMR) spectroscopy are the most commonly used methods for the study of salivary metabolites [1]. Other less used methods in saliva research include Fourier-transform infrared (FTIR), photoacoustic spectroscopy, and Raman spectroscopy [2]. These new methods allow the same metabolites to be found in the saliva as in the blood, even though they are lesser quantitatively in the saliva.
Whole-mouth (WMS) saliva is the most-used biofluid in the salivary metabolic analyses. The collection of saliva samples has many advantages because it is noninvasive, painless, and safe without piercing the skin. Saliva is easy to collect without complex equipment and does not require special expertise. Furthermore, the storage of saliva samples is simple and cheap. Saliva contains both endogenous and exogenous metabolites, which can tell us about biological pathways in the oral cavity. However, their role as biomarkers in the diagnostics of oral and systemic diseases is also investigated as reviewed by Hyvärinen et al. [3]. Saliva allows the measuring the levels of metabolites, including nucleic acids, lipids, amino acids, peptides, vitamins, organic acids, thiols, and carbohydrates, representing a useful tool for the detection of biomarkers for various oral diseases and monitoring disease progression. Salivary metabolites are involved in a variety of cellular functions, such as direct regulation of gene expression and they function as the effectors of molecular events that contribute to disease [4]. A minor part role for salivary metabolites could be as a potential source of systemic biomarkers, however, the main sources of salivary metabolites are productions of oral metabolic pathways, especially produced by microorganisms [5,6].
The oral cavity is a complicated organ with different niches for the colonization of millions of microorganisms including bacteria, fungi, viruses, protozoa, and archaea [7,8]. Saliva represents a major role in the maintenance of oral homeostasis with its multifactorial defense system and lubrication of different oral surfaces [9]. Oral inflammatory diseases and oral microorganisms contribute to the salivary metabolic fingerprint [5]. It is suggested that due to the high degree of connectivity in cellular metabolism, any disease will carry a metabolic signature that can be identified through the analysis of the metabolome in saliva and other tissue fluids. Furthermore, the multifunctional defense components of the saliva come from blood or serum via salivary glands, from gingival crevicular fluid (GCF) and epithelial cells.
Microorganisms play an important role in the metabolism of the oral cavity but it is not known how their diversity relates to salivary metabolites in patients with different oral or systemic diseases. However, the health of the oral cavity and oral diseases alter the saliva metabolism profile, and they must be considered in the investigations of systemic diseases. Hence, this narrative review aims to provide a general overview of salivary metabolites associated with the oral cavity and oral diseases. Metabolites produced in the oral cavity as risk factors for general diseases and their possible relationship to the body’s function are also discussed.
This narrative review is based on a literary search, which was performed on the PubMed database with the keywords “saliva”, “oral diseases”, “metabolites” and “oral cavity”. In addition, this article is based on the long-time clinical experience of two authors (BK, AMK).
2. Sources of Salivary Metabolites in Healthy Subjects
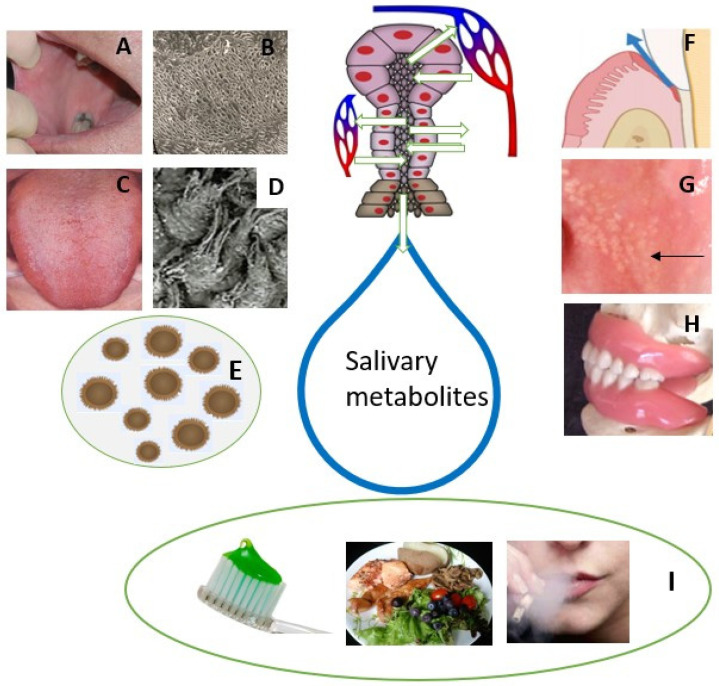
Saliva is an oral fluid secreted by the major and minor salivary glands. After entering the oral cavity, it is referred to as mixed or whole saliva, supplemented with many constituents originating from blood, mucosal cells, immune cells, and microorganisms [9]. Whole saliva represents a complex mixture of a variety of molecules and hence, it is valuable to use in research. The composition of saliva and oral microbiota differ in healthy subjects most likely due to age, gender, habits, diet, oral hygiene, medication, and different oral niches (prothesis, tooth fillings, tongue disorders, sebaceous glands = Fordyce granules) (Figure 1).
Figure 1.
Summary of oral metabolite sources that may take part in salivary metabolic fingerprint in healthy subjects. Saliva is secreted by salivary glands in salivon with acinar and ductal cells. Metabolites are obtained also from oral mucosal cells (A–D) and gingival crevicular fluid (F). Most of salivary metabolites are produced by oral microorganisms (E). Sebaceous glands referred as Fordyce granules (arrow) are located in the buccal mucosa (G). Dentition and dentures (H) form different niches for attaching of microorganisms. Oral hygiene, diet, and habits, including smoking and alcohol consumption, differ salivary metabolite profile (I).
The oral microbiome is a set of diverse microorganisms that inhabit different niches of the oral cavity. They, together with salivary defense, play a key role in the oral balance between health and diseases. Oral microbes communicate with oral epithelial cells via Toll-like receptors (TLRs), which play a key role in the oral immune system producing inflammatory cytokines. Studies using scanning electron microscopy (SEM; Figure 1B) showed no or very few microorganisms on the buccal mucosal surface. Instead, most of the mucosal microorganisms are located on the dorsal tongue surface (Figure 1C), especially on the surface of rough hyphae of filiform papillae (Figure 1D).
GCF is another oral fluid and an inflammatory exudate derived from the blood vessels of gingival plexus, adjacent to the gingival epithelium. The bacterial degradation product in the GCF promotes the binding of calculus formation subgingivally [10]. GCF provides ease in collection and sampling of multiple sites of the oral cavity simultaneously due to its close interaction with periodontal cells and bacterial biofilm.
Fordyce granules (FG) are tubular–acinar sebaceous glands (Figure 1G), most often located in the lip and buccal mucosa and are more common in males. The ductus of FG opens into the oral cavity and a lipase-containing secretion is possibly passed into saliva. However, their significance in the salivary metabolic profile is likely to be limited.
Dentures present different niches for the colonization of microorganisms (Figure 1H). Candidiasis without any symptoms is a quite common oral disorder in denture wearers. This also indicates dysbiosis of oral microbiota that further changes the salivary metabolic profile in denture-wearing patients. Furthermore, the denture can be colonized by respiratory pathogens, which can even be a risk of respiratory infection [11]. Individuals with appliances for orthodontic treatment are advised to practice proper oral hygiene. Failure in such practices results in plaque and calculus deposition superimposed with the bacterial degradation product causing gingival and periodontal inflammation. This further raises the possibility of changes in salivary metabolites.
3. Salivary Metabolites in Patients with Oral Inflammation
The oral cavity contains a complex array of diverse microorganism that is tightly controlled by their host via metabolic machinery, substrate-specific or salivary secretory products. A mutually beneficial equilibrium exists between the host and oral microbiota until it is disturbed by external factors [12]. Several oral disorders, including caries, gingivitis, periodontitis, and oral ulcerations, relate to oral microbiota dysbiosis wherein generation of metabolites can result in inflammation-mediated tissue destruction (Table 1).
Table 1.
Salivary metabolites associated with most common oral inflammatory diseases, caries and periodontitis, based on NMR spectroscopic studies.
| Oral Diseases N (HC/D) * |
Saliva | Elevated Salivary Metabolites | Lowered Salivary Metabolites | Reference |
|---|---|---|---|---|
| Periodontitis 54 (22/32) |
WS | acetate, c-aminobutyrate, n-butyrate, succinate, trimethylamine, propionate, valine | pyruvate, N-acetyl groups | [13] |
| Periodontitis 52 (26/26) |
SWS | butyrate | fucose, lactate, acetate, N-acetyl of glycoprotein, GABA, 3-hydroxybutyrate, pyruvate, methanol, threonine, ethanol | [14] |
| Aggressive chronic periodontitis 100 (39/61) |
USWS | proline, phenylalanine, isoleucine, valine, tyrosine | pyruvate, N-acetyl groups, lactate | [15] |
| Chronic periodontitis 130 (120/10) |
USWS | caproate, isocaproate, butyrate, isovalerate, isoleucine, isopropanol, methanol, 4-aminobutyrate, choline, sucrose, glycine, lysine, lactate, proline | [16] | |
| Chronic periodontitis, post-surgery 176 (52/124) |
WS | lactate, ethanol, succinate, glutamate | [17] | |
| Chronic periodontitis 45 (15/30) |
US | propylene glycol, ethanol, lactate, acetoin, succinate, methanol, glycerol, formate | valine, propionate, isopropanol, alanine, pyruvate, acetate, acetone, choline, taurine, glycine, glucose | [18] |
| Periodontitis after therapy 23 (11/12) |
USWS | alanine, glycine, taurine, proline, leucine, valine, isovalerate, tyrosine, methylamine, phenylalanine, isoleucine, lactate, formate, glucose, sarcosine, hypoxanthine, uracil | acetate, propionate, butyrate, ethanol, succinate, acetoin, galactose, aspartate, creatine, choline, methanol, pyruvate, isopropanol | [19] |
| Periodontitis 221 (92/129) |
SWS | taurine, glucose, butyrate, isovalerate, glycolate, formate | ethanol, acetate, acetone, acetoin, choline, pyruvate, proline, lysine, propionate | [20] |
| Dental caries 30 (10/20) |
USWS | butyrate, ambiguous, lysine, saccharide region, phenylalanine, and propionate. | [21] | |
| Dental caries in children 38 (38/NM) |
USWS SWS |
alanine, aspartate, glutamine, glycine, isoleucine, leucine, proline, taurine, tyrosine, fucose, galactose, glucose, xylose, choline, dimethylsulfone, hypoxanthine, menthol, N-acetyls, uracil | butyrate, acetone | [22] |
* N = total number of subjects; HC = healthy controls; D = diseased; NM = not mentioned; WS = whole saliva; USWS = unstimulated whole saliva; SWS = stimulated whole saliva; GABA = γ-aminoglutamate.
Caries and periodontitis are the most common oral inflammatory diseases in humans globally. Caries is more common in children and older people while periodontitis is the most common oral disease in the middle-aged population. Therefore, the age of individuals participating in a study may affect the metabolic profile. For example, in a comparative study of caries-related metabolites, a difference was found between adults’ and children’s salivary metabolites [21,22].
Both caries and periodontitis are induced by bacterial dysbiosis in the oral cavity. Dental caries results in an imbalance in the microbiota. Most of the microorganisms associated with tooth decay acquire a selective advantage over other species by changing the homeostatic balance of the salivary biofilm. The main source of carbohydrates for caries-causing microorganisms is consumed food. These carbohydrates usually leave the mouth within about an hour due to saliva’s lubrication effect of the saliva. Of course, this washout is affected by the saliva secretion rate, which means that hyposalivation in patients does not wash their mouth out in the same way as in subjects with normal salivary flow rate [23]. Microorganisms in the oral biofilm can metabolize dietary carbohydrates to produce organic acids, which will decrease pH and initiate the demineralization of dental hard tissue, developing caries [24]. High levels of free amino acids have been linked to increased protein hydrolysis activity by oral bacteria. High proline and glycine levels are the possible result of the hydrolysis of dentin-collagen in caries active individuals [25]. The high level of lipids on the salivary pellicle of tooth surfaces can inhibit the acid diffusion and accelerate to caries development [26]. Salivary metabolites produced by bacterial metabolism, including lactate, acetate and n-butyrate have been shown in patients with caries. These metabolites can reduce the pH and increase the porosity of the dental plaque matrix [27].
Periodontopathogenic bacteria contribute to periodontal diseases. The oral microbes release salivary metabolites as a product of multifactorial interactions between host, oral bacteria, and altered cellular metabolism of the host. The change in the metabolite concentration is correlated with the products of the pathogenic bacterial population. The change in the subgingival environment with regard to oxygen tension, redox potential, pH, and availability of host-derived macromolecules has been shown previously. Such changes are responsible in a cause-and-effect way for modulation of the bacterial composition [14] (Figure 2). Gingival crevicular fluid (GCF), whose composition is close to that of serum, flows into the oral cavity from periodontal pockets and varies in gingival inflammation [28]. Some anaerobic periodontal bacteria can produce very short chain fatty acid (SCFA) metabolites that are released from infection sites into the microenvironment. This can further contribute to the periodontal pathogenesis further through impairment of immune cells or fibroblasts and epithelial cell functions [29]. SCFAs, the end-products of bacterial metabolism such as butyrate, caproate, isocaproate, propionate, isovalerate and lactate have been linked to deep periodontal pockets, loss of insertion, bleeding, and inflammation. These metabolites are significantly decreased following periodontal treatment and gradually increase over time, which makes them possible indicators of periodontal disease development and progression [16].
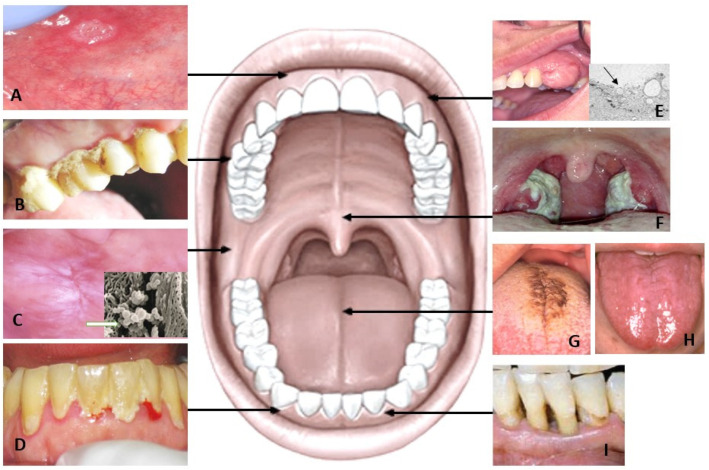
Figure 2.
Aphthous ulcer presents erythematous halo surrounding round yellowish ulcer (A). Caries (B) is one of the most common individual illnesses. Leukoplakia of the buccal mucosa (C) can colonize more microorganisms than the healthy mucosa (studied with SEM). Dental plaque and bleeding of the gingiva (D) are sources of different metabolites in the whole mouth saliva. Oral tumors (E) and their small vesicles (arrow) produce metabolites, which can be used in diagnostics. Pharyngitis in tonsils (F) must also be remembered in saliva studies. Changes of the dorsal tongue surface, including hairy tongue (G) and atrophic tongue without hairs of filiform papillae (H) affect the biofilm of the oral cavity. Parodontitis (I) causes deep pockets around the teeth with many anaerobic bacteria altering the oral microbiome.
In recent years, the relationship of periodontitis with systemic diseases such as cardiovascular diseases, diabetes mellitus, and problems during pregnancy, rheumatoid arthritis, chronic obstructive pulmonary disease, pneumonia, obesity, chronic kidney disease, metabolic syndrome and cancer has been amply demonstrated [30]. Several mechanisms have been proposed, including the transient bacteremia resulting in bacterial colonization in extraoral sites, systemic injury by release of free toxins and systemic inflammation triggered by soluble antigens of oral pathogens [31]. However, controversy exists between different studies due to the heterogeneity in the definitions and identification of periodontitis.
4. Salivary Metabolomics in Oral Mucosal Diseases and Oral Cancer
In the oral cavity, there are many kinds of tissue forming a very complex milieu. There are also many diseases in the mouth, of which only the most important are presented.
Oral ulcerations are the most common oral mucosal lesions. Recurrent aphthous stomatitis (RAS) is an inflammatory disorder, which is characterized by recurring and painful ulcers on the surface of the oral mucosa (Figure 2A). Typically, aphthous ulcers occur in adolescents and young adults, with the majority of patients affected being under 30 years of age and seldom in adults older than 40 years [32]. Ulcerations associated with aphthous stomatitis (RAU) are thought to represent a dysfunction of the oral immune system [33,34] Several studies have reported different etiology factors for RAU including the presence of certain oral microbial communities, immunological factors, endocrinopathies, and psychological and hereditary factors [35]. The difference in the oxidant/antioxidant status in the blood and saliva of patients with and without RAU has been previously described [36]). Only one study has been conducted on changes in the metabolite related to aphthous stomatitis [37]. An imbalance of tryptophan metabolism and steroid hormone biosynthesis has been shown to be correlated with increased incidence of oral ulcers [37]. Salivary metabolites have been shown to increase in serotonin, which influences psychological factors including depression and stress in patients with aphthous ulcers. However, the exact molecular mechanism remains unclear.
4.1. Tongue Disorders
Variation in color and structure of the tongue surface can be considered normal variation or caused by different diseases. These changes are easy to diagnose clinically because there are visible changes in filiform papillae (Figure 2G,H). Changes in these papillae may greatly alter the concentration of salivary metabolites, as oral microbes are harbored on these papillae normally in the healthy mouth. Geographic tongue (GT), also known as migratory glossitis, is a benign chronic inflammatory tongue condition. The typical clinical appearance is characterized by erythematous lesions with filiform papillae atrophy, surrounded by a white limited zone, producing a map-like appearance. Furthermore, fissured tongue (FT) is a disorder manifested as grooves and presents many enlarged, smooth papillae without hairs [38]. In patients with GT and FT, salivary calprotectin was elevated indicating the activation of neutrophils. Two opposite tongue lesions are hairy tongue (HT; Figure 2G) and atrophic tongue (AT; Figure 2H). On the surface of HT, there are a lot of long threads, onto which many microorganisms and food leftovers attach. Unlike HT, the filiform papillae have disappeared partially or completely in AT. It can be assumed that in both cases the biofilm of the tongue, like the whole oral microbial system, has changed.
4.2. Leukoplakia and Lichen Planus
Most of the oral cancers are preceded by asymptomatic or symptomatic clinical lesions with increased potential for malignant transformation, referred to as oral potentially malignant disorder (OPMD). Oral leukoplakia (OL) (Figure 2C) and oral lichen planus (OLP) are OPMDs [39]. Salivary metabolites studies have presented different metabolites in OL and OLP compared to oral cancer due to differences in pathophysiological stimuli (Table 2). The common elevated metabolites reported in OL are c- aminobutyric acid (GABA), phenylalanine, valine, lactate, eicosane, 4-nitroquinoline-1-oxide, and in OLP the metabolites are indole-3-acetate and ethanolamine phosphate. The metabolites of OL and OLP are shared with oral cancers suggesting their metabolic alterations are associated with the development of cancer. Increased glycolysis, products of glycolysis, carbohydrate metabolism, altered tricarboxylic cycle, phospholipid metabolism, lysine metabolic pathway and increased metabolic utilization are leading factors in the alteration in salivary metabolites[40,41,42,43]. This implies increased risk of OMPDs and oral cancers. As, salivary metabolomics provides a noninvasive approach for early detection of diseases by employing a novel and unique strategy, it can provide a complete metabolic response of organisms and their modification. However, such factors as the clinical appearance of OMPDs, age, gender, socioeconomic status, smoking and alcohol use might influence the salivary metabolites and must be considered in future studies.
Table 2.
Salivary metabolites associated with oral mucosal lesions and oral squamous cell carcinoma (OSCC).
| Oral Diseases N (HC/D) * |
Saliva | Elevated Salivary Metabolites | Lowered Salivary Metabolites | Reference |
|---|---|---|---|---|
| OSCC 87/215 |
WS | alanine, taurine, pipecolic acid, leucine, isoleucine, histidine, valine, tryptophan, glutamic acid, threonine, carnitine | [44] | |
| OSCC/OL 34/69 |
NM | alanine, lactic acid, 3-indolepropionic acid, n-eicosanoic acid | valine, proline, isoleucine, leucine, n-tetradecanoic acid, proline, threonine, phenylalanine, γ-aminobutyric acid | [40] |
| OSCC 30/30 |
USWS | lactic acid, hydroxyphenynactic acid, N-nonanoylglycine, 5-hydroxymethyluracil, succinic acid, ornithine, hexanoylcarnitine, propionylcholine | carnitine, 4-hydroxy-L-glutamic acid, acetylphenylalanine, spihingarine, phytosphingosine, S-carboxymethyl-L-cystein | [45] |
| Oral cancer 44/24 |
USWS | 3PG, pipecolate, spermidine, met, SAM, 2 AB, trp, val, hypoxanthine, gly-gly, trimetrhylamine N-oxide, guanine, guanosine, tautine, choline, cadaverine, thr | [46] | |
| OSCC 35/101 |
NM | glycine, proline, citrulline, ornithine | [47] | |
| OSCC (OED, PSOML) NA/48 |
USWS | ornithine, carnitine, arginine, o-hydroxybenzoate, N-acetylglucosamine-1-phosphate, and ribose 5-phosphate (R5P) | [48] | |
| OL and OSCC 18/43 |
USWS | d-glycerate-2-phosphate, estrone-3-glucuronide, 4-nitroquinoline-1-oxide, sphin-ganine-1 phosphate,1-methyl histidine, inositol 1,3,4-triphosphate, d-glycerate-2-phosphate, 2-oxoarginine, norcocaine nitroxide, pseudouridine | S-ureidoglycolic acid, p-chlorphenylalanine, d-urobilinogen, N-(3-Indolylacetyl)-l-isoleucine, tetradecanedioic acid, 1-hexadecyl hexadecanoate, l- homocysteic acid, ubiquinone, neuraminic acid, and estradiol valerate. | [41] |
| OSCC 124/249 |
USWS | putrescine, cadaverine, thymidine, adenosine, 5-aminopentoate | hippuric acid, phosphocholine, glucose, serine, adrenic acid. | [49] |
| OLP/OSCC NA/60 |
USWS | trimethylamine N-oxide, putrescine, creatinine, 5-aminovalerate, pipecolate, N-acetylputrescine, gamma-butyrobetaine, indole-3-acetate, N1-acetylspermine, 2′-deoxyinosine, ethanolamine phosphate, N-acetylglucosamine | N-acetylhistidine, o-acetylcarnitine | [42] |
| OLP 125/120 |
USWS | 6 amino acid metabolites, 2 carnitines, 2 lipid metabolites and 9 other metabolites. | [50] | |
| HNC with RT NA/9 |
USWS | histidine, tyrosine, urocanate, glycine, glutamic acid, aspartic acid, tryptophan, lysine, methionine, gamma-aminobutyric acid (GABA), butyrate, 2-isopropaylate, 2-aminobutyric acids | [51] | |
| Oral cancer and OL 30/60 | USWS | decanedioic acid, 2-methyloctacosane, Eicosane, Octane, 3,5-dimethyl, pentadecane, hentriacontane, 5,5-diethylpentadecane, nonadecane, oxalic acid, 6-phenylundecanea, L-proline, 2-furancarboxamide, 2-isopropyl-5-methyl-1-heptanol, pentanoic acid, docosane. | [43] |
* N = total number of subjects; HC = healthy controls; D = diseased; NM = not mentioned; WS = whole saliva; USWS = unstimulated whole saliva; SWS = stimulated whole saliva; LC/MS = liquid chromatography-mass spectrometry; GC/MS = gas chromatography mass spectrometry; CE-TOF-MS = capillary electrophoresis time-of-flight mass spectrometry; UHPLC-MS/MS = ultrahigh performance liquid chromatography and tandem mass spectrometry; NMR = NMR-spectroscopy; OSCC = oral squamous cell carcinoma; OL = oral leukoplakia; OLP = oral lichen planus; PSOML = persistent suspicious oral mucosal lesions; HNC-head and neck carcinoma; RT-radiotherapy.
4.3. Oral Cancer
The most common oral cancer is oral squamous cell carcinoma (OSCC). Despite current treatment methods, its five-year survival forecast is lower when compared to other cancers. Oral cancer is diagnosed worldwide every year and it causes the death in about 177,384 people per year [52]. Oral cancer is another condition to be considered when addressing the microbiome’s role in oral health. Most oral cancers are related to smoking habits and alcohol consumption; however, some studies reported other etiological factors, namely genetic susceptibility, external agents, and viral infections [53]. Recently, increasing evidence has indicated the role of bacteria in oral cancer. Oral dysbiosis was the term described in patients with oral cancer [54,55]. It was also suggested that metabolic bacterial products can be used as salivary markers for early detection of oral cancer [46]).
The mechanisms underlying the link between the oral microbiome metabolites and carcinogenesis are still unknown. It was shown previously that oral bacteria have the capacity to convert ethanol to acetaldehyde, which is a carcinogen [56]. Other proposed mechanisms suggest that the release of proinflammatory mediators that can disturb cellular cycling, disrupt signaling mechanisms, and act as tumor promoters [57].
The most frequently reported metabolites are amino acids (alanine, valine, leucine, threonine, proline, glutamic acid, phenylalanine, and choline), carbohydrates (N-acetylneuraminate (sialic acid), and the fatty acids (hexanoic acid and 2-hydroxypentanoate) (Table 2). The metabolites observed in oral cancer patients are related to the arginine and proline-, or cysteine and methionine-, or glycine and serine-, and glycerophospholipid and purine pathways [58]. Oral cancers are in direct contact with the saliva and tumor cell by-products are released into the saliva. Hence, the presence of hydrolyzed products by local tissue destruction or tumor cells in the saliva of oral cancer patients, enables identification of salivary metabolite identification.
5. Discussion
This article describes the most common oral factors that may affect the salivary metabolite profile. The challenge of salivary research is that both oral health and different habits and changes in human physiology affect the composition and volume of whole saliva.
It is well-known that many factors, including dietary behavior, oral hygiene, physical exercise, smoking, alcohol consumption and oral dysbiosis, influence oral health and metabolism in many ways. Salivary components attach and concentrate on the oral mucosal surface as a mucosal pellicle, which protects the oral mucosa from bacterial invasion and other irritants [59]. Hence, there are only a few microorganisms attached to oral healthy buccal mucosa (Figure 1B). Instead, the dorsal tongue surface contains many microorganisms on the surface of rough filiform papillae, which cover the entire tongue surface [38]. Morphological tongue changes, such as atrophic, hairy, geographic, and fissured tongue, can alter the oral biofilm considerably and further the salivary metabolic fingerprint. Investigation of the tongue surface may reveal much about the mouth and general health.
Oral diseases reduce well-being, and their role as a risk factor for many systemic diseases, including cardiovascular diseases, dementia, different cancers, and inflammatory bowel disease, have been shown in many studies. Further, periodontitis is involved in numerous systemic diseases, including cardiovascular disease, bacterial pneumonia, diabetes mellitus, dementia, and low birth weight [60,61,62]. The most extensive salivary studies on oral disorders have been conducted on the metabolism of periodontitis. However, the relationship between periodontitis and systemic diseases and their connection mechanisms is unknown.
The metabolic profile of the oral fluid was clearly identified for periodontitis. Some anaerobic bacteria from the supragingival and subgingival plaque can produce butyrate, one of the SCFAs. Butyrate is released from infection sites and contributes to the pathogenesis of periodontitis. In a chromatography analysis, the concentration of butyric acid was shown to decrease in patients with chronic periodontitis receiving periodontal treatment. However, gradually increasing concentrations of butyric acids were observed in the long-term after treatment, emphasizing the recolonization of periodontal pathogens [63]. The increase in the salivary butyrate, an important indicator of the pathogenic microorganism’s growth and periodontal tissue destruction was reported by some authors (Table 1). In four of the eight studies, salivary butyrate was elevated in patients with periodontitis, whereas one study showed a decrease in butyrate concentration after treatment [13,14,16,20]. Additionally, it was previously shown that with prolonged retention of butyrate within the gingival tissue, it will gradually enter the bloodstream [64]. Thus, butyrate related periodontal destruction could serve as a health risk factor capable of inducing systemic manifestations, which requires further elucidation.
Other SCFAs such as acetate, formate, propionate, caproate, isocaproate, propionate, isovalerate and lactate can also play important roles in periodontal pathology. These metabolites can promote an inflammatory response with the release of cytokines, hence, preventing repair at the cellular level [63]. Lactate, a product of lactic acid bacteria, forms a symbiotic relationship between bacteria and the host and can be metabolized to acetate and propionate [13]. In periodontitis, the tissue damage liberates different enzymes such as lactate dehydrogenase, related to cell death and destruction. Increased lactate levels in individuals with periodontitis have been reported [65]. Decrease in salivary lactate in periodontitis indicates a shift in the microbial composition of commensal bacteria in the oral cavity and other mucosal sites. Microbial species found between the teeth and the gums that inhibit the growth and colonization of exogenous pathogens have been recognized as beneficial [14]. Propionate levels can increase with an increase in the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors that have a biological role in tumor invasion [66]. The rise in acetate levels in saliva indicate a restricted supply of carbohydrates and was correlated with caries in subjects [21]. Most oral cavity microorganisms are unable to utilize the carbohydrate energy sources, and this causes an increase in amylase activity and monosaccharide levels in periodontitis. The salivary amylase hydrolyzes starch to glucose, and high levels of glucose indicate excessive amylase activity in periodontitis [20].
Succinate, itaconate, fumarate and citrate, are the intermediate metabolites of the Krebs cycle. These metabolites can regulate immune responses and have a role in disease progression [67]. Succinate is synthesized within the mitochondrial matrix and is a well-described immunemetabolites. Succinate is an essential intermediate of the tricarboxylic acid (TCA) cycle that exerts pleiotropic roles beyond metabolism in both physiological and pathological conditions. The increase in succinate can induce secretion of inflammatory cytokines by activating the hypoxia-induced factor 1 (HIF-1) transcriptional pathway, which leads to inflammatory diseases’ development [68]. Succinate aggravates periodontitis through the succinate receptor, SUCNR1 [69]. In another study, a small-compound gel formulation was developed that specifically blocks SUCNR1 to prevent and treat periodontitis by inhibiting dysbiosis, inflammation, and bone loss [70].
These studies indicate that butyrate plays an important role in untreated periodontitis and may lead to a general low-grade infection throughout the whole body. The increase in butyrate and succinate and decrease in lactate are considered the salivary metabolic markers of periodontitis (Table 1).
Generally, cancer cells utilize energy via the glycolysis pathway, and this results in high production of lactate in the body fluids. This is shown in many tumors as an increased level of lactic acid and such increased lactic acid is associated with a decrease in pyruvate [71]. Pyruvate is an essential component for entering the TCA cycle, but due to decreased pyruvate supply, the TCA cycle is supplemented with other amino acids such as valine, leucine, isoleucine, and phenylalanine. Leucine, isoleucine, and valine have important effects on protein synthesis, glucose homeostasis, and nutrient-sensitive signaling pathways. With an increase in the metabolic utilization in transformed cancer cells, increased or decreased variations in salivary metabolites were observed and reported in several studies (Table 2). In one study, the salivary metabolite gamma-aminobutyric acid (GABA), one of the TCA intermediates, was found at a decreased level, which correlates with an increased recurrence of OSCC [40]. Glutamine, another TCA intermediate, increases the utilization of GABA by cancer cells and decreases GABA levels in saliva; hence, it is regarded as an important diagnostic marker in some of the cancers [72,73].
Another study reported an increase in sphinganine-1-phosphate (SIP) and 4-nitroquinoline-1-oxide in OL and OSCC saliva. SIP is a known pro-inflammatory, promitogenic, and/or chemotaxic lipids that enables cancer progression [41]. It can interact with different receptors such as epidermal growth factor and transforming growth factor beta to accelerate carcinogenesis [74], while 4-nitroquinoline-1-oxide is a strong carcinogen known to elicit its carcinogenic potential by producing free radical oxidative damage to DNA [75]. Subsequent damage to DNA and oxidative stress affects the estrogen metabolism and estrogen related metabolites are either upregulated or downregulated in saliva [76]. The nucleotide biosynthesis pathway is also affected in OL and OSCC. Inositol 1,3,4-triphosphate (IP3R) is another important salivary biomarker in OSCC as IP3R plays an important role in growth, aggressiveness, and drug resistance via modulation of different signaling pathways [77].
Indole-3-acetate and ethanolamine phosphate were reported to be discriminating factors between OLP and OSCC [42]. The high level of salivary indole-3-acetate is correlated with its production by cancer cells, and it promotes tumor growth and progression. The intermediate of the phospholipid metabolism is ethanolamine phosphate which forms a main part of sphingolipids. Sphingolipids are phosphorylated and transferred as SIP, a known carcinogenic factor [41]. Hence, we can speculate that the heterogeneous nature of OPMDs and oral cancers produce various salivary metabolite profiles. Also, the complexity of systemic networks in the human body allows some communications between diseases and salivary glands that alter gene expression and protein metabolism, and this is reflected as salivary biomarker profiles.
A causal association has been established between alcohol consumption and oral cancers. One of the diagnostic markers for oral cancer is acetaldehyde (C2H4O). It is an oxidative form of ethyl alcohol and describes the metabolism of the oral cavity related to alcohol use. According to the International Cancer Research Institute (IARC), acetaldehyde is classified as a class I carcinogen, i.e., it has carcinogenic effects. Exposure to acetaldehyde affects oral microbial activity. Poor oral hygiene and smoking have also been shown to increase the concentration of acetaldehyde in saliva [78]. Understanding the metabolism of acetaldehyde in the oral cavity can give new information about oral metabolism related to oral cancer.
OSCC tumor cells are in contact with saliva. In this case, the metabolites produced by the cells via small vesicles (Figure 2E) are reflected in the saliva metabolism profile. Fucose (6-deoxy-L-galactose) is known to occur during the development of cancers, when the tumor cells modulate their surface by increasing fucosylation [79]. It has been shown that blood fucosis levels are high in different cancers [80,81], and also in oral cancers [82,83]. Only one preliminary study showed raised fucosis in saliva [84]. This relationship should be further investigated using a larger patient data set.
In the future, oral health will be one of the key factors to be considered in saliva metabolic studies. Therefore, the subjects to be examined should be selected according to oral health if the whole saliva is used for the determination of metabolites. There are also wide variations in different studies on salivary metabolites [85]. When collecting saliva for studies, certain factors should be considered such as timing (circadian rhythm), the stimulation of saliva flow, physical stress, and the abstention period of eating and drinking before the saliva collection. Other important factors include consistent methods of saliva collection and methods of analysis to assess the effects of general disorders on the saliva metabolism profile. A comparison between the different studies can be made after the same methods and a study protocol can be carried out.
6. Conclusions
Oral health is one of the key factors to be considered in studies on salivary metabolomics. Oral bacteria and their metabolites, and inflammatory agents produced by the bacteria, may pass through microvessels from the invaded gingiva into the bloodstream, and then cause disease directly or indirectly. However, some of the salivary metabolites are transferred into the bloodstream via gingival pockets or via endocrine mechanisms of salivary glands communicating with other organs throughout the body.
Salivary metabolomics has a high potential for the diagnosis of systemic diseases. To validate the salivary metabolite data, a comparison between the different studies can be made when the same methods and a study protocol can be carried out. However, oral health condition should be taken into consideration in these studies.
Author Contributions
Conceptualization, A.M.K.; writing—original draft preparation, E.H.; writing—review and editing, B.K. and A.M.K.; supervision, B.K. and A.M.K.; project administration, A.M.K. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Mikkonen J.J., Singh S.P., Herrala M., Lappalainen R., Myllymaa S., Kullaa A.M. Salivary metabolomics in the diagnosis of oral cancer and periodontal diseases. J. Periodontal Res. 2016;51:431–437. doi: 10.1111/jre.12327. [DOI] [PubMed] [Google Scholar]
- 2.Mikkonen J.J., Raittila J., Rieppo L., Lappalainen R., Kullaa A.M., Myllymaa S. Fourier Transform Infrared Spectroscopy and Photoacoustic Spectroscopy for Saliva Analysis. Appl. Spectrosc. 2016;70:1502–1510. doi: 10.1177/0003702816654149. [DOI] [PubMed] [Google Scholar]
- 3.Hyvärinen E., Savolainen M., Mikkonen J.J.W., Kullaa A.M. Salivary Metabolomics for Diagnosis and Monitoring Diseases: Challenges and Possibilities. Metabolites. 2021;11:587. doi: 10.3390/metabo11090587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nielsen J. Systems Biology of Metabolism. Annu. Rev. Biochem. 2017;86:245–275. doi: 10.1146/annurev-biochem-061516-044757. [DOI] [PubMed] [Google Scholar]
- 5.Gardner A., Parkes H.G., Carpenter G.H., So P.W. Developing and standardizing a protocol for quantitative proton nuclear magnetic resonance (1H NMR) spectroscopy of saliva. J. Proteome Res. 2018;17:1521–1531. doi: 10.1021/acs.jproteome.7b00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gardner A., Parkes H.G., So P.W., Carpenter G.H. Determining bacterial and host contributions to the human salivary metabolome. J. Oral Microbiol. 2019;11:1617014. doi: 10.1080/20002297.2019.1617014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sampaio-Maia B., Caldas I.M., Pereira M.L., Pérez-Mongiovi D., Araujo R. The Oral Microbiome in Health and Its Implication in Oral and Systemic Diseases. Adv. Appl. Microbiol. 2016;97:171–210. doi: 10.1016/bs.aambs.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Mosaddad S.A., Tahmasebi E., Yazdanian A., Rezvani M.B., Seifalian A., Yazdanian M., Tebyanian H. Oral microbial biofilms: An update. Eur. J. Clin. Microbiol. Infect. Dis. 2019;38:2005–2019. doi: 10.1007/s10096-019-03641-9. [DOI] [PubMed] [Google Scholar]
- 9.Fábián T.K., Hermann P., Beck A., Fejérdy P., Fábián G. Salivary defense proteins: Their network and role in innate and acquired oral immunity. Int. J. Mol. Sci. 2012;13:4295–4320. doi: 10.3390/ijms13044295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barros S.P., Williams R., Offenbacher S., Morelli T. Gingival crevicular fluid as a source of biomarkers for periodontitis. Periodontol 2000. 2016;70:53–64. doi: 10.1111/prd.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Donnell L.E., Smith K., Williams C., Nile C.J., Lappin D.F., Bradshaw D., Lambert M., Robertson D.P., Bagg J., Hannah V., et al. Dentures are a Reservoir for Respiratory Pathogens. J. Prosthodont. 2016;25:99–104. doi: 10.1111/jopr.12342. [DOI] [PubMed] [Google Scholar]
- 12.Tuganbaev T., Yoshida K., Honda K. The effects of oral microbiota on health. Science. 2022;376:934–936. doi: 10.1126/science.abn1890. [DOI] [PubMed] [Google Scholar]
- 13.Aimetti M., Cacciatore S., Graziano A., Tenori L. Metabonomic analysis of saliva reveals generalized chronic periodontitis signature. Metabolomics. 2012;8:465–474. doi: 10.1007/s11306-011-0331-2. [DOI] [Google Scholar]
- 14.Rzeznik M., Triba M.N., Levy P., Jungo S., Botosoa E., Duchemann B., Le Moyec L., Bernaudin J.F., Savarin P., Guez D. Identification of a discriminative metabolomic fingerprint of potential clinical relevance in saliva of patients with periodontitis using 1H nuclear magnetic resonance (NMR) spectroscopy. PLoS ONE. 2017;12:e0182767. doi: 10.1371/journal.pone.0182767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romano F., Meoni G., Manavella V., Baima G., Tenori L., Cacciatore S., Aimetti M. Analysis of salivary phenotypes of generalized aggressive and chronic periodontitis through nuclear magnetic resonance-based metabolomics. J. Periodontol. 2018;89:1452–1460. doi: 10.1002/JPER.18-0097. [DOI] [PubMed] [Google Scholar]
- 16.García-Villaescusa A., Morales-Tatay J.M., Monleón-Salvadó D., González-Darder J.M., Bellot-Arcis C., Montiel-Company J.M., Almerich-Silla J.M. Using NMR in saliva to identify possible biomarkers of glioblastoma and chronic periodontitis. PLoS ONE. 2018;13:e0188710. doi: 10.1371/journal.pone.0188710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh M.P., Saxena M., Saimbi C.S., Siddiqui M.H., Roy R. Post-periodontal surgery propounds early repair salivary biomarkers by 1 H NMR based metabolomics. Metabolomics. 2019;15:141. doi: 10.1007/s11306-019-1593-3. [DOI] [PubMed] [Google Scholar]
- 18.Gawron K., Wojtowicz W., Łazarz-Bartyzel K., Łamasz A., Qasem B., Mydel P., Chomyszyn-Gajewska M., Potempa J., Mlynarz P. Metabolomic status of the oral cavity in chronic periodontitis. In Vivo. 2019;33:1165–1174. doi: 10.21873/invivo.11587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Citterio F., Romano F., Meoni G., Iaderosa G., Grossi S., Sobrero A., Dego F., Corana M., Berta C.N., Tenori L., et al. Changes in the salivary metabolic profile of generalized periodontitis patients after non-surgical periodontal therapy: A metabolomic analysis using nuclear magnetic resonance spectroscopy. J. Clin. Med. 2020;9:3977. doi: 10.3390/jcm9123977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim S., Kim H.J., Song Y., Lee H.A., Kim S., Chung J. Metabolic phenotyping of saliva to identify possible biomarkers of periodontitis using proton nuclear magnetic resonance. J. Clin. Periodontol. 2021;48:1240–1249. doi: 10.1111/jcpe.13516. [DOI] [PubMed] [Google Scholar]
- 21.Fidalgo T.K.S., Freitas-Fernandes L.B., Angeli R., Muniz A.M.S., Gonsalves E., Santos R., Nadal J., Almeida F.C.L., Valente A.P., Souza I.P.R. Salivary metabolite signatures of children with and without dental caries lesions. Metabolomics. 2013;9:657–666. doi: 10.1007/s11306-012-0484-7. [DOI] [Google Scholar]
- 22.Pereira J.L., Duarte D., Carneiro T.J., Ferreira S., Cunha B., Soares D., Costa A.L., Gil A.M. Saliva NMR metabolomics: Analytical issues in pediatric oral health research. Oral Dis. 2019;25:1545–1554. doi: 10.1111/odi.13117. [DOI] [PubMed] [Google Scholar]
- 23.Jakubovics N.S. Saliva as the Sole Nutritional Source in the Development of Multispecies Communities in Dental Plaque. Microbiol. Spectr. 2015;3:263–277. doi: 10.1128/microbiolspec.MBP-0013-2014. [DOI] [PubMed] [Google Scholar]
- 24.Marsh P.D. Microbial Ecology of Dental Plaque and its Significance in Health and Disease. Adv. Dent. Res. 1994;8:263–271. doi: 10.1177/08959374940080022001. [DOI] [PubMed] [Google Scholar]
- 25.Schulz A., Lang R., Behr J., Hertel S., Reich M., Kümmerer K., Hannig M., Hannig C., Hofmann T. Targeted metabolomics of pellicle and saliva in children with different caries activity. Sci. Rep. 2020;10:697. doi: 10.1038/s41598-020-57531-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomita Y., Miyake N., Yamanaka S. Lipids in human parotid saliva with regard to caries experience. J. Oleo. Sci. 2008;57:115–121. doi: 10.5650/jos.57.115. [DOI] [PubMed] [Google Scholar]
- 27.Van Houte J. Role of micro-organisms in caries etiology. J. Dent. Res. 1994;73:672–681. doi: 10.1177/00220345940730031301. [DOI] [PubMed] [Google Scholar]
- 28.Estreicher A., Broggiato A., Duroux P., Andersen E., Cimasoni G. Low molecular-weight proteins in human gingival crevicular fluid. Arch. Oral Biol. 1996;41:733–738. doi: 10.1016/S0003-9969(96)00076-3. [DOI] [PubMed] [Google Scholar]
- 29.Niederman R., Zhang J., Kashket S. Short-chain carboxylic-acid-stimulated, PMN-mediated gingival inflammation. Crit. Rev. Oral Biol. Med. 1997;8:269–290. doi: 10.1177/10454411970080030301. [DOI] [PubMed] [Google Scholar]
- 30.Linden G.J., Lyons A., Scannapieco F.A. Periodontal systemic associations: Review of the evidence. J. Periodontol. 2013;84:S8–S19. doi: 10.1902/jop.2013.1340010. [DOI] [PubMed] [Google Scholar]
- 31.Han Y.W., Wang X. Mobile microbiome: Oral bacteria in extra-oral infections and inflammation. J. Dent. Res. 2013;92:485–491. doi: 10.1177/0022034513487559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cui R.Z., Bruce A.J., Rogers R.S., 3rd Recurrent aphthous stomatitis. Clin. Dermatol. 2016;34:475–481. doi: 10.1016/j.clindermatol.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 33.Akintoye S.O., Greenberg M.S. Recurrent aphthous stomatitis. Dent. Clin. N. Am. 2014;58:281–297. doi: 10.1016/j.cden.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pärssinen M., Jäsberg H., Mikkonen J.J.W., Kullaa A.M. Oral mucosal pellicle as an immune protection against micro-organisms in patients with recurrent aphthous stomatitis: A hypothesis. Med. Hypotheses. 2021;146:110449. doi: 10.1016/j.mehy.2020.110449. [DOI] [PubMed] [Google Scholar]
- 35.Slebioda Z., Szponar E., Kowalska A. Etiopathogenesis of recurrent aphthous stomatitis and the role of immunologic aspects: Literature review. Arch. Immunol. Ther. Exp. 2014;62:205–215. doi: 10.1007/s00005-013-0261-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Babaee N., Hosseinkazemi H., Pouramir M., Baboli O.K., Salehi M., Khadir F., Bijani A., Mehryari M. Salivary oxidant/ antioxidant status and hematological parameters in patients with recurrent aphthous stomatitis. Caspian. J. Intern. Med. 2016;7:13–18. [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y., Wang D., Zeng C., Liu Y., Huang G., Mei Z. Salivary metabolomics profile of patients with recurrent aphthous ulcer as revealed by liquid chromatography-tandem mass spectrometry. J. Int. Med. Res. 2018;46:1052–1062. doi: 10.1177/0300060517745388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kullaa-Mikkonen A., Sorvari T.E. A scanning electron microscopic study of fissured tongue. J. Oral Pathol. 1986;15:93–97. doi: 10.1111/j.1600-0714.1986.tb00584.x. [DOI] [PubMed] [Google Scholar]
- 39.Wetzel S.L., Wollenberg J. Oral Potentially Malignant Disorders. Dent. Clin. N. Am. 2020;64:25–37. doi: 10.1016/j.cden.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Wei J., Xie G., Zhou Z., Shi P., Qiu Y., Zheng X., Chen T., Su M., Zhao A., Jia W. Salivary metabolite signatures of oral cancer and leukoplakia. Int. J. Cancer. 2011;129:2207–2217. doi: 10.1002/ijc.25881. [DOI] [PubMed] [Google Scholar]
- 41.Sridharan G., Ramani P., Patankar S., Vijayaraghavan R. Evaluation of salivary metabolomics in oral leukoplakia and oral squamous cell carcinoma. J. Oral Pathol. Med. 2019;48:299–306. doi: 10.1111/jop.12835. [DOI] [PubMed] [Google Scholar]
- 42.Ishikawa S., Sugimoto M., Edamatsu K., Sugano A., Kitabatake K., Iino M. Discrimination of oral squamous cell carcinoma from oral lichen planus by salivary metabolomics. Oral Dis. 2020;26:35–42. doi: 10.1111/odi.13209. [DOI] [PubMed] [Google Scholar]
- 43.Tantray S., Sharma S., Prabhat K., Nasrullah N., Gupta M. Salivary metabolite signatures of oral cancer and leukoplakia through gas chromatography-mass spectrometry. J. Oral Maxillofac. Pathol. 2022;26:31–37. doi: 10.4103/jomfp.jomfp_335_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sugimoto M., Wong D.T., Hirayama A., Soga T., Tomita M. Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics. 2010;6:78–95. doi: 10.1007/s11306-009-0178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Q., Gao P., Wang X., Duan Y. The early diagnosis and monitoring of squamous cell carcinoma via saliva metabolomics. Sci. Rep. 2014;4:6802. doi: 10.1038/srep06802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ishikawa S., Sugimoto M., Kitabatake K., Sugano A., Nakamura M., Kaneko M., Ota S., Hiwatari K., Enomoto A., Soga T., et al. Identification of salivary metabolomic biomarkers for oral cancer screening. Sci. Rep. 2016;19:31520. doi: 10.1038/srep31520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lohavanichbutr P., Zhang Y., Wang P., Gu H., Nagana Gowda G.A., Djukovic D., Buas M.F., Raftery D., Chen C. Salivary metabolite profiling distinguishes patients with oral cavity squamous cell carcinoma from normal controls. PLoS ONE. 2018;13:e0204249. doi: 10.1371/journal.pone.0204249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishikawa S., Wong D.T.W., Sugimoto M., Gleber-Netto F.O., Li F., Tu M., Zhang Y., Akin D., Iino M. Identification of salivary metabolites for oral squamous cell carcinoma and oral epithelial dysplasia screening from persistent suspicious oral mucosal lesions. Clin. Oral Investig. 2019;23:3557–3563. doi: 10.1007/s00784-018-2777-3. [DOI] [PubMed] [Google Scholar]
- 49.Song X., Yang X., Narayanan R., Shankar V., Ethiraj S., Wang X., Duan N., Ni Y.H., Hu Q., Zare R.N. Oral squamous cell carcinoma diagnosed from saliva metabolic profiling. Proc. Natl. Acad. Sci. USA. 2020;117:16167–16173. doi: 10.1073/pnas.2001395117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang X., Liu L., Du Q., Sun Z., Yue E., Xue P., Zhao H. Human Saliva Metabolome for Oral Lichen Planus Biomarker Identification. Recent Pat. Anticancer Drug Discov. 2021;16:417–425. doi: 10.2174/1574892816666210224160120. [DOI] [PubMed] [Google Scholar]
- 51.Yatsuoka W., Ueno T., Miyano K., Enomoto A., Ota S., Sugimoto M., Uezono Y. Time-Course of Salivary Metabolomic Profiles during Radiation Therapy for Head and Neck Cancer. J. Clin. Med. 2021;10:2631. doi: 10.3390/jcm10122631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer tatistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 53.Chocolatewala N., Chaturvedi P., Desale R. The role of bacteria in oral cancer. Indian J. Med. Paediatr. Oncol. 2010;31:126–131. doi: 10.4103/0971-5851.76195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tuominen H., Rautava J. Oral Microbiota and Cancer Development. Pathobiology. 2021;88:116–126. doi: 10.1159/000510979. [DOI] [PubMed] [Google Scholar]
- 55.Yang T., Santisteban M.M., Rodriguez V., Li E., Ahmari N., Carvajal J.M., Zadeh M., Gong M., Qi Y., Zubcevic J., et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65:1331–1340. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahn J., Chen C.Y., Hayes R.B. Oral microbiome and oral and gastrointestinal cancer risk. Cancer Causes Control. 2012;23:399–404. doi: 10.1007/s10552-011-9892-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meurman J.H. Oral microbiota and cancer. J. Oral Microbiol. 2010;10:2. doi: 10.3402/jom.v2i0.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Assad D.X., Mascarenhas E.C.P., de Lima C.L., de Toledo I.P., Chardin H., Combes A., Acevedo A.C., Guerra E.N.S. Salivary metabolites to detect patients with cancer: A systematic review. Int. J. Clin. Oncol. 2020;25:1016–1036. doi: 10.1007/s10147-020-01660-7. [DOI] [PubMed] [Google Scholar]
- 59.Hannig C., Hannig M., Kensche A., Carpenter G. The mucosal pellicle—An underestimated factor in oral physiology. Arch. Oral Biol. 2017;80:144–152. doi: 10.1016/j.archoralbio.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 60.Li X., Kolltveit K.M., Tronstad L., Oslen I. Systemic diseases caused by oral infection. Clin. Microbiol. Rev. 2000;13:547–558. doi: 10.1128/CMR.13.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hajishengallis G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015;15:30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Asher S., Stephen R., Mäntylä P., Suominen A.L., Solomon A. Periodontal health, cognitive decline, and dementia: A systematic review and meta-analysis of longitudinal studies. J. Am. Geriatr. Soc. 2022;70:2695–2709. doi: 10.1111/jgs.17978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qiqiang L., Huanxin M., Xuejun G. Longitudinal study of volatile fatty acids in the gingival crevicular fluid of patients with periodontitis before and after nonsurgical therapy. J. Periodontal. Res. 2012;47:740–749. doi: 10.1111/j.1600-0765.2012.01489.x. [DOI] [PubMed] [Google Scholar]
- 64.Cueno M.E., Ochiai K. Gingival Periodontal Disease (PD) Level-Butyric Acid Affects the Systemic Blood and Brain Organ: Insights Into the Systemic Inflammation of Periodontal Disease. Front. Immunol. 2018;4:1158. doi: 10.3389/fimmu.2018.01158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De La Peña V.A., Diz Dios P., Tojo Sierra R. Relationship between lactate dehydrogenase activity in saliva and oral health status. Arch. Oral Biol. 2007;52:911–915. doi: 10.1016/j.archoralbio.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 66.Piao Y., Lu L., de Groot J. AMPA receptors promote perivascular glioma invasion via beta1 integrin-dependent adhesion to the extracellular matrix. Neuro. Oncol. 2009;11:260–273. doi: 10.1215/15228517-2008-094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lyssiotis C.A., Kimmelman A.C. Metabolic Interactions in the Tumor Microenvironment. Trends Cell Biol. 2017;27:863–875. doi: 10.1016/j.tcb.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tannahill G.M., Curtis A.M., Adamik J., Palsson-McDermott E.M., McGettrick A.F., Goel G., Frezza C., Bernard N.J., Kelly B., Foley N.H., et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harber K.J., de Goede K.E., Verberk S.G.S., Meinster E., de Vries H.E., van Weeghel M., de Winther M.P.J., Van den Bossche J. Succinate Is an Inflammation-Induced Immunoregulatory Metabolite in Macrophages. Metabolites. 2020;10:372. doi: 10.3390/metabo10090372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guo Y., Xu F., Thomas S.C., Zhang Y., Paul B., Sakilam S., Chae S., Li P., Almeter C., Kamer A.R., et al. Targeting the succinate receptor effectively inhibits periodontitis. Cell Rep. 2022;40:111389. doi: 10.1016/j.celrep.2022.111389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sonveaux P., Végran F., Schroeder T., Wergin M.C., Verrax J., Rabbani Z.N., De Saedeleer C.J., Kennedy K.M., Diepart C., Jordan B.F., et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Investig. 2008;118:3930–3942. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schuller H.M., Al-Wadei H.A., Majidi M. Gamma-aminobutyric acid, a potential tumor suppressor for small airway-derived lung adenocarcinoma. Carcinogenesis. 2008;29:1979–1985. doi: 10.1093/carcin/bgn041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Joseph J., Niggemann B., Zaenker K.S., Entschladen F. The neurotransmitter gamma-aminobutyric acid is an inhibitory regulator for the migration of SW 480 colon carcinoma cells. Cancer Res. 2002;62:6467–6469. [PubMed] [Google Scholar]
- 74.Tamashiro P.M., Furuya H., Shimizu Y., Iino K., Kawamori T. The impact of sphingosine kinase-1 in head and neck cancer. Biomolecules. 2013;3:481–513. doi: 10.3390/biom3030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arima Y., Nishigori C., Takeuchi T., Oka S., Morimoto K., Utani A., Miyachi Y. 4-Nitroquinoline 1-oxide forms 8-hydroxydeoxyguanosine in human fibroblasts through reactive oxygen species. Toxicol. Sci. 2006;91:382–392. doi: 10.1093/toxsci/kfj161. [DOI] [PubMed] [Google Scholar]
- 76.Roy D., Cai Q., Felty Q., Narayan S. Estrogen-induced generation of reactive oxygen and nitrogen species, gene damage, and estrogen-dependent cancers. J. Toxicol. Environ. Health B Crit. Rev. 2007;10:235–257. doi: 10.1080/15287390600974924. [DOI] [PubMed] [Google Scholar]
- 77.Parys J.B., Decuypere J.P., Bultynck G. Role of the inositol 1,4,5-trisphosphate receptor/Ca2+-release channel in autophagy. Cell Commun. Signal. 2012;10:17. doi: 10.1186/1478-811X-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Salaspuro M. Acetaldehyde and gastric cancer. J. Dig. Dis. 2011;12:51–59. doi: 10.1111/j.1751-2980.2011.00480.x. [DOI] [PubMed] [Google Scholar]
- 79.Miyoshi E., Moriwaki K., Nakagawa T. Biological function of fucosylation in cancer biology. J. Biochem. 2008;143:725–729. doi: 10.1093/jb/mvn011. [DOI] [PubMed] [Google Scholar]
- 80.Manjula S., Monteiro F., Rao Aroor A., Rao S., Annaswamy R., Rao A. Assessment of serum L-fucose in brain tumor cases. Ann. Indian Acad. Neurol. 2010;13:33–36. doi: 10.4103/0972-2327.61274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Listinsky J.J., Siegal G.P., Listinsky C.M. The emerging importance of α-L-fucose in human breast cancer: A review. Am. J. Transl. Res. 2011;3:292–322. [PMC free article] [PubMed] [Google Scholar]
- 82.Shah M., Telang S., Raval G., Shah P., Patel P.S. Serum fucosylation changes in oral cancer and oral precancerous conditions: Alpha-L-fucosidase as a marker. Cancer. 2008;113:336–346. doi: 10.1002/cncr.23556. [DOI] [PubMed] [Google Scholar]
- 83.Shetty R.K., Bhandary S.K., Kali A. Significance of Serum L-fucose Glycoprotein as Cancer Biomarker in Head and Neck Malignancies without Distant Metastasis. J. Clin. Diagn. Res. 2013;7:2818–2820. doi: 10.7860/JCDR/2013/6681.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mikkonen J.J.W., Singh S.P., Akhi R., Salo T., Lappalainen R., González-Arriagada W.A., Ajudarte Lopes M., Kullaa A.M., Myllymaa S. Potential role of nuclear magnetic resonance spectroscopy to identify salivary metabolite alterations in patients with head and neck cancer. Oncol. Lett. 2018;16:6795–6800. doi: 10.3892/ol.2018.9419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Martina E., Campanati A., Diotallevi F., Offidani A. Saliva and Oral Diseases. J. Clin. Med. 2020;9:466. doi: 10.3390/jcm9020466. [DOI] [PMC free article] [PubMed] [Google Scholar]