Abstract
Background
Animal experiments have shown the anticancer activity of Toxoplasma gondii (T. gondii), but its effect on the prognosis of cancer patients is unclear. Thus, the present study aimed to investigate the prognostic role of anti-T. gondii IgG in breast cancer patients and the modification effect of cytokines.
Methods
A total of 1121 breast cancer patients were recruited between 2008 and 2018 and followed up until December 31, 2021. Anti-T. gondii IgG and cytokines were measured using an enzyme-linked immunosorbent assay (ELISA) kit and a multiplex assay platform. Endpoints were overall survival (OS) and progression-free survival (PFS). Survival and multiplicative interaction analyses were performed using multivariate Cox regression models.
Results
According to the cutoff value of optical density (OD=0.111), 900 (80.29%) and 221 (19.71%) patients were divided into two groups: low or high anti-T. gondii IgG. Compared to patients with a low anti-T. gondii IgG level, the adjusted hazard ratios (HRs) of OS and PFS for patients with high anti-T. gondii IgG levels were 0.60 (95% confidence interval (CI): 0.37–0.99) and 0.67 (0.46–0.98), respectively. These associations were profound among patients with a high cytokine score (HR=0.29, 95% CI: 0.10–0.82 for OS; HR=0.30, 95% CI: 0.13–0.69 for PFS), accompanied by a significant interaction between the level of anti-T. gondii IgG and the cytokine score (Pinteraction=0.019 for PFS); interleukin-17 (IL-17) and interleukin-9 (IL-9) were the main contributors to the interaction.
Conclusion
Anti-T. gondii IgG was found to be beneficial to breast cancer survival, especially in women with systematic inflammation and high IL-17 or IL-9 levels, suggesting the potential of T. gondii as a prognostic marker and a novel immunotherapy approach for cancer patients.
Keywords: Toxoplasma gondii, breast cancer, prognosis, cytokines
Introduction
Toxoplasma gondii (T. gondii) is an obligate intracellular protozoan that infects approximately one-third of the global population.1 T. gondii can be acquired through food or water contaminated with the parasite or by vertical transmission from mother to child.2 Typically, T. gondii infection is asymptomatic or self-limiting but can cause severe toxoplasmosis in immunocompromised hosts and severe damage to the fetus during pregnancy.3,4 Interestingly, T. gondii possesses anticancer activity, as shown in animal models, by secreting cancer-associated mucin-type O-glycans, rhoptry proteins (ROPs), and dense granule antigens (GRAs) that activate the host antigen-presenting system to inhibit tumor growth.5,6 After efficiently promoting activation of cellular immunity,6 it also inhibits progression of various cancers in animal models, such as melanoma,7 ovarian cancer,8 pancreatic cancer,9 and breast cancer.10,11 A recent study revealed that T. gondii DNA is present in malignant breast tissues of humans.12 Several studies have shown a higher prevalence of T. gondii infection in cancer patients than in healthy controls, including breast cancer patients,13–15 but whether infection by T. gondii might affect the prognosis of cancer patients has yet to be explored.16
In addition, T. gondii infection has been shown to modify cytokine levels among breast cancer patients,17 which are associated with prognosis,18 suggesting that T. gondii infection might affect survival in breast cancer by regulating cytokine-related signaling pathways. Therefore, we investigated the impact of T. gondii infection on the survival of breast cancer patients using Guangzhou Breast Cancer Study (GZBCS) data and further explored whether the association is modified by cytokines.
Materials and Methods
Study Population and Serum Sample Collection
Patients with breast cancer identified by pathology were recruited from the First and Second Affiliated Hospitals of Sun Yat-sen University in Guangzhou, China, from October 2008 to January 2018.19 Serum samples from 1775 patients were collected at diagnosis and stored at −80 °C. Next, we excluded patients for whom any of the following information was missing: clinical stage (N=243), estrogen receptor (ER) status (N=144), progesterone receptor (PR) status (N=147), human epidermal growth receptor (HER2) status (N=207), and follow-up (N=108). After excluding patients due to poor serum quality (N=204), we obtained an analytic sample size of 1121 cases. Written informed consent was obtained from all participants. This study was conducted in accordance with the Declaration of Helsinki. The protocol was approved by the Ethics Committee of the School of Public Health at Sun Yat-sen University (Institutional Review Board approval number: 2012-8).
Data Collection
Baseline information was obtained through a structured questionnaire by trained investigators. The following information was collected: demographic characteristics, reproductive history, menstrual history, lifestyles, and family history of cancer. Clinical and pathological characteristics, such as tumor size, nodal status, and clinical stage, were collected from medical records. The status of ER, PR, and HER2 was determined based on immunohistochemistry and the definitions of these biomarkers, as described previously.20
Follow-Up
Follow-up was conducted at least every 3 months during the first year after diagnosis and every 6 months in the second and third years. Thereafter, the patients were followed up annually. The median follow-up time was 63.91 months. Follow-up information, including recurrence, metastasis, and death, was obtained through phone calls and outpatient visits. The primary and secondary endpoints of this study were OS and PFS, respectively, as described previously.19 Survival statuses were censored at the latest follow-up date or December 31, 2021.
Serological Assay
Anti-T. gondii IgG in serum samples was detected using a commercial enzyme-linked immunosorbent assay (ELISA) kit (Haitai Biological Pharmaceuticals Co., Ltd, Zhuhai, China), which is a routine method for T. gondii infection screening and diagnosis in China.21 The assays were performed according to the manufacturer’s instructions. The kit’s positive, negative, and critical controls were placed on each test plate; optical density (OD) values were measured at 450 nm using an automatic microplate reader (800 TS, BIO-TEK, Winooski, Vermont, USA). The results were considered positive if the OD was equal to or greater than the threshold value (determined by the mean of three critical controls in each test). Total IgG was measured using an immunoglobulin kit (CUSABIO, Wuhan, China) according to the manufacturer’s instructions. All samples were tested in duplicate.
Cytokine Measurement
A total of 794 serum samples were randomly selected to detect cytokines. A total of 27 cytokines were measured using the Bio-Plex Pro Human Cytokine 27-plex assay (Bio-Rad, M500KCAF0Y)22 and Luminex 200 platform (Luminex Corporation, Austin, TX, USA). The assays included a series of known concentrations to generate standard curves constructed by Bio-Plex Manager 6.0 (Bio-Rad Laboratories). For samples with values below the assay’s lower limit of detection, values equal to half of the lower limit of detection were used.23
Statistical Analysis
To determine potential confounders, we estimated associations of clinicopathological and demographic factors with breast cancer survival. Next, X-tile software version 3.6.1 (Yale University, New Haven, CT, USA) was applied to obtain the optimal OD cutoff value for dividing the patients into low, middle, or high anti-T. gondii IgG level groups.24 Then, Cox proportional hazards regression models were used to evaluate the association between anti-T. gondii IgG levels and prognosis.
Cytokines were analyzed separately and used as a composite indicator based on a previously developed cytokine score.25 Briefly, after log-transforming and standardizing the values of all cytokines, a Cox proportional hazards regression model was constructed to test the association between OS (dependent variable) and all cytokines (independent variables) while considering the regression coefficients as weights. The processed cytokine levels mentioned above were weighted by these regression coefficients and summed for a total cytokine score (formula: 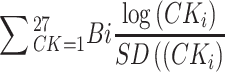 , where Bi is the regression coefficient and CK is the cytokine). The cytokine score was input into a Cox regression model using restricted cubic splines to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs). The cytokine score was divided into two groups (low and high) according to the restricted cubic spline results.
, where Bi is the regression coefficient and CK is the cytokine). The cytokine score was input into a Cox regression model using restricted cubic splines to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs). The cytokine score was divided into two groups (low and high) according to the restricted cubic spline results.
Furthermore, stratified analyses were performed to assess whether associations between anti-T. gondii IgG and breast cancer prognosis are modified by the cytokine score, individual cytokines, or other clinical characteristics. Multiplicative interaction for survival was estimated by the product terms in the model.
Additionally, mediation analyses were performed to assess whether the association between anti-T. gondii IgG and breast cancer survival is mediated by individual cytokines and cytokine scores. Sensitivity analysis was conducted excluding patients without cytokine data (N=327). All statistical tests were two-tailed, with P<0.05 indicating significance. All statistical analyses were conducted using R software version 4.1.3.
Results
Demographic and Clinicopathological Characteristics and Their Associations with Breast Cancer Prognosis
At the time of diagnosis, the mean age of the patients was 49.35±10.88 years, and 59.3% of them were premenopausal (Table 1). Most of the patients were ER positive (76.8%), and >75% were diagnosed with early-stage cancer (stage I/II: 78.5%) (Table 1). Age at diagnosis, menopausal status, ER status, PR status, Ki-67, tumor size, nodal status, and clinical stage were significantly associated with breast cancer survival (Table 1).
Table 1.
Demographic and Clinicopathologic Characteristics and the Associations with Breast Cancer Prognosis
| Characteristics | Total (%) | Overall Survival | Progression-Free Survival | ||
|---|---|---|---|---|---|
| Events | HR (95% CI) | Events | HR (95% CI) | ||
| Age at diagnosis (yr) | |||||
| ≤40 | 244 (21.8) | 30 | 1.00 (reference) | 53 | 1.00 (reference) |
| 41–60 | 714 (63.7) | 86 | 1.00 (0.66, 1.52) | 138 | 0.90 (0.65, 1.23) |
| >60 | 163 (14.5) | 34 | 1.71 (1.05, 2.80) | 50 | 1.41 (0.96, 2.07) |
| Education status | |||||
| Below Junior school | 451 (43.6) | 66 | 1.00 (reference) | 106 | 1.00 (reference) |
| Senior high school | 317 (30.6) | 36 | 0.73 (0.48, 1.09) | 62 | 0.79 (0.58, 1.09) |
| College or above | 267 (25.8) | 29 | 0.73 (0.47, 1.12) | 47 | 0.73 (0.52, 1.04) |
| Marital statusa | |||||
| Unmarried | 91 (8.3) | 18 | 1.00 (reference) | 27 | 1.00 (reference) |
| Married | 1007 (91.7) | 122 | 0.67 (0.41, 1.10) | 201 | 0.72 (0.48, 1.07) |
| Age at menarche (yr) | |||||
| ≤12 | 136 (12.5) | 20 | 1.00 (reference) | 31 | 1.00 (reference) |
| >12 | 952 (87.5) | 119 | 0.84 (0.52, 1.34) | 198 | 0.89 (0.61, 1.30) |
| Menopausal status | |||||
| Premenopausal | 643 (59.3) | 74 | 1.00 (reference) | 124 | 1.00 (reference) |
| Postmenopausal | 442 (40.7) | 69 | 1.43 (1.03, 1.99) | 108 | 1.33 (1.03, 1.72) |
| Parity | |||||
| 0 | 62 (5.7) | 11 | 1.00 (reference) | 17 | 1.00 (reference) |
| ≥1 | 1029 (94.3) | 128 | 0.73 (0.39, 1.35) | 211 | 0.76 (0.47, 1.25) |
| Breast feeding | |||||
| Absent | 79 (8.0) | 7 | 1.00 (reference) | 11 | 1.00 (reference) |
| Present | 908 (92.0) | 117 | 1.53 (0.71, 3.28) | 193 | 1.60 (0.87, 2.93) |
| Family history of breast cancer | |||||
| Absent | 1024 (94.2) | 139 | 1.00 (reference) | 219 | 1.00 (reference) |
| Present | 63 (5.8) | 4 | 0.47 (0.17, 1.27) | 11 | 0.79 (0.43, 1.45) |
| BMI (kg/m2) | |||||
| <18.5 | 68 (6.3) | 12 | 1.32 (0.72, 2.42) | 17 | 1.16 (0.70, 1.92) |
| 18.5–23.9 | 663 (61.1) | 84 | 1.00 (reference) | 136 | 1.00 (reference) |
| ≥24.0 | 354 (32.6) | 46 | 1.06 (0.74, 1.51) | 77 | 1.08 (0.82, 1.43) |
| ER status | |||||
| Negative | 260 (23.2) | 45 | 1.00 (reference) | 70 | 1.00 (reference) |
| Positive | 861 (76.8) | 105 | 0.64 (0.45, 0.91) | 171 | 0.68 (0.51, 0.89) |
| PR status | |||||
| Negative | 365 (32.6) | 67 | 1.00 (reference) | 102 | 1.00 (reference) |
| Positive | 756 (67.4) | 83 | 0.54 (0.39, 0.74) | 139 | 0.58 (0.45, 0.75) |
| HER2 | |||||
| Negative | 650 (58.0) | 85 | 1.00 (reference) | 138 | 1.00 (reference) |
| Positive/equivocal | 471 (42.0) | 65 | 1.32 (0.96, 1.83) | 103 | 1.26 (0.97, 1.62) |
| Triple-negative breast cancer | |||||
| No | 990 (90.7) | 128 | 1.00 (reference) | 205 | 1.00 (reference) |
| Yes | 102 (9.3) | 16 | 1.16 (0.69, 1.96) | 24 | 1.08 (0.71, 1.66) |
| Ki67 index | |||||
| <14%+ | 246 (23.9) | 25 | 1.00 (reference) | 40 | 1.00 (reference) |
| ≥14%+ | 783 (76.1) | 107 | 1.51 (0.97, 2.33) | 175 | 1.54 (1.10, 2.18) |
| Tumor size (cm) | |||||
| ≤2.0 | 472 (42.3) | 37 | 1.00 (reference) | 64 | 1.00 (reference) |
| >2.0 | 644 (57.7) | 111 | 2.51 (1.73, 3.65) | 174 | 2.35 (1.76, 3.13) |
| Nodal status | |||||
| Negative | 623 (55.9) | 49 | 1.00 (reference) | 93 | 1.00 (reference) |
| Positive | 491 (44.1) | 97 | 2.87 (2.03, 4.05) | 142 | 2.26 (174, 2.93) |
| Clinical stage | |||||
| I | 319 (28.5) | 17 | 1.00 (reference) | 35 | 1.00 (reference) |
| II | 567 (50.6) | 49 | 1.80 (1.03, 3.12) | 93 | 1.66 (1.13, 2.45) |
| III | 180 (16.1) | 50 | 6.84 (3.94, 11.87) | 73 | 5.09 (3.40, 7.62) |
| IV | 55 (4.9) | 34 | 24.27 (13.44, 43.84) | 40 | 16.15 (10.15, 25.67) |
Notes: Bold character indicates statistically significant result. aUnmarried status included never married, separated, divorced, and widowed; married status included living as married.
Abbreviations: BMI, body mass index; CI, confidence interval; HR, hazard ratio.
Association of Anti-T. gondii IgG with Breast Cancer Survival
According to the ELISA kit’s criteria, 69 (6.16%) of the patients were seropositive for anti-T. gondii IgG. After adjustment for total IgG and potential prognostic factors of breast cancer, patients seropositive for anti-T. gondii IgG exhibited a significantly decreased risk of progression compared to seronegative patients (HR=0.41, 95% CI: 0.18–0.92; Table 2). Based on the direction of these HRs and the small sample size of the seropositive patients, we used X-tile software to obtain an optimal cutoff dividing anti-T. gondii IgG OD into three levels. As shown in Table 2, compared to patients with anti-T. gondii IgG OD<0.020, those (anti-T. gondii IgG OD>0.111) had a significantly decreased risk of death (HR=0.48, 95% CI: 0.24–0.93) and a nonsignificantly decreased risk of progression (HR=0.65, 95% CI: 0.38–1.11); a trend of dose‒response association between anti-T. gondii IgG and OS was also observed (P=0.024). When the two lower levels of anti-T. gondii IgG were combined as the reference level, a higher level of anti-T. gondii IgG was associated with a significantly decreased risk of death or progression (HR=0.60, 95% CI: 0.37–0.99 for OS; HR= 0.67, 95% CI: 0.46–0.98 for PFS; Table 2). In contrast, no significant associations between anti-T. gondii IgG and clinicopathological characteristics were observed in addition to age at diagnosis (Table 3). Patients with a high anti-T. gondii IgG level were more likely to be younger than those with a low anti-T. gondii IgG level (Table 3). Stratified analyses were performed to explore whether demographic or clinicopathological characteristics modify the effect of anti-T. gondii IgG on prognosis. As shown in Table S1, no significant differential associations were detected among different ages, ER, PR, and HER2 statuses, or clinical stages.
Table 2.
Univariate and Multivariate Cox Regression for Anti-T. gondii IgG and Cytokine Score with Breast Cancer OS and PFS
| Variables | Total | Overall Survival | Progression-Free Survival | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Events | HR (95% CI)a | HR (95% CI)b | HR (95% CI)c | Events | HR (95% CI)a | HR (95% CI)b | HR (95% CI)c | ||
| Anti-T. gondii IgG (ELISA) | |||||||||
| Negative | 1052 | 145 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 231 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Positive | 69 | 5 | 0.51 (0.21, 1.24) | 0.44 (0.16, 1.21) | 0.46 (0.17, 1.26) | 10 | 0.62 (0.33, 1.17) | 0.39 (0.17, 0.89) | 0.41 (0.18, 0.92) |
| Anti-T. gondii IgG (X-tile) | |||||||||
| < 0.020 | 120 | 21 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 27 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 0.020 ~ 0.111 | 780 | 105 | 0.63 (0.39, 1.00) | 0.76 (0.45, 1.28) | 0.76 (0.45, 1.29) | 173 | 0.83 (0.56, 1.25) | 0.97 (0.62, 1.51) | 0.96 (0.62, 1.50) |
| > 0.111 | 221 | 24 | 0.51 (0.28, 0.91) | 0.48 (0.25, 0.94) | 0.48 (0.24, 0.93) | 41 | 0.68 (0.42, 1.11) | 0.64 (0.38, 1.10) | 0.65 (0.38, 1.11) |
| P for trend | 0.032 | 0.025 | 0.024 | 0.113 | 0.056 | 0.065 | |||
| Anti-T. gondii IgG (X-tile) | |||||||||
| Low | 900 | 126 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 200 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| High | 221 | 24 | 0.76 (0.49, 1.17) | 0.61 (0.38, 1.00)d | 0.60 (0.37, 0.99) | 41 | 0.80 (0.57, 1.12) | 0.66 (0.46, 0.96) | 0.67 (0.46, 0.98) |
| Cytokine scoree | |||||||||
| ≤ −12.89 | 397 | 36 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 65 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| > −12.89 | 397 | 67 | 1.88 (1.25, 2.82) | 1.56 (1.01, 2.43) | 1.56 (1.01, 2.43) | 98 | 1.56 (1.14, 2.14) | 1.40 (1.00, 1.96) | 1.40 (1.00, 1.96) |
Notes: Bold character indicates statistically significant result. aThe univariate COX model. bAdjusted for age at diagnosis (continuous variable), ER status, PR status, HER2 status, Ki67 index, tumor size, nodal status, and clinical stage (early stage VS advanced stage). cAdjusted for age at diagnosis (continuous variable), ER status, PR status, HER2 status, Ki67 index, tumor size, nodal status, clinical stage (early stage VS advanced stage), and total IgG. dP = 0.049. eA total of 794 serum samples were randomly selected to detect cytokines.
Table 3.
Relationships Between Anti-T. gondii IgG and Clinicopathologic Characteristics of Breast Cancer
| Characteristics | Anti-T. gondii IgG | P* | |
|---|---|---|---|
| Low (%) | High (%) | ||
| Age at diagnosis | 0.006 | ||
| ≤40 | 210 (23.3) | 34 (15.4) | |
| 41–59 | 553 (61.4) | 161 (72.9) | |
| ≥60 | 137 (15.2) | 26 (11.8) | |
| Menopausal status | 0.093 | ||
| Premenopausal | 527 (60.5) | 116 (54.2) | |
| Postmenopausal | 344 (39.5) | 98 (45.8) | |
| Family history of breast cancer | 0.240 | ||
| No | 826 (94.6) | 198 (92.5) | |
| Yes | 47 (5.4) | 16 (7.5) | |
| BMI (kg/m2) | 0.801 | ||
| <18.5 | 57 (6.5) | 11 (5.3) | |
| 18.5–23.9 | 534 (61.0) | 129 (61.7) | |
| ≥24.0 | 285 (32.5) | 69 (33.0) | |
| ER | 0.963 | ||
| Negative | 209 (23.2) | 51 (23.1) | |
| Positive | 691 (76.8) | 170 (76.9) | |
| PR | 0.995 | ||
| Negative | 293 (32.6) | 72 (32.6) | |
| Positive | 607 (67.4) | 149 (67.4) | |
| HER2 | 0.982 | ||
| Negative | 522 (58.0) | 128 (57.9) | |
| Positive/equivocal | 378 (42.0) | 93 (42.1) | |
| Triple-negative breast cancer | 0.791 | ||
| No | 797 (90.8) | 193 (90.2) | |
| Yes | 81 (9.2) | 21 (9.8) | |
| Ki-67 | 0.603 | ||
| < 14% | 201 (24.2) | 45 (22.5) | |
| ≥14% | 628 (75.8) | 155 (77.5) | |
| Tumor size (cm) | 0.885 | ||
| ≤2.0 | 378 (42.2) | 94 (42.7) | |
| >2.0 | 518 (57.8) | 126 (57.3) | |
| Lymph nodes status | 0.360 | ||
| Negative | 506 (56.6) | 117 (53.2) | |
| Positive | 388 (43.4) | 103 (46.8) | |
| Clinical stage | 0.219 | ||
| I/II | 718 (79.8) | 168 (76.0) | |
| III | 182 (20.2) | 53 (24.0) | |
Note: *Chi‐square test; Bold character indicate statistically significant result.
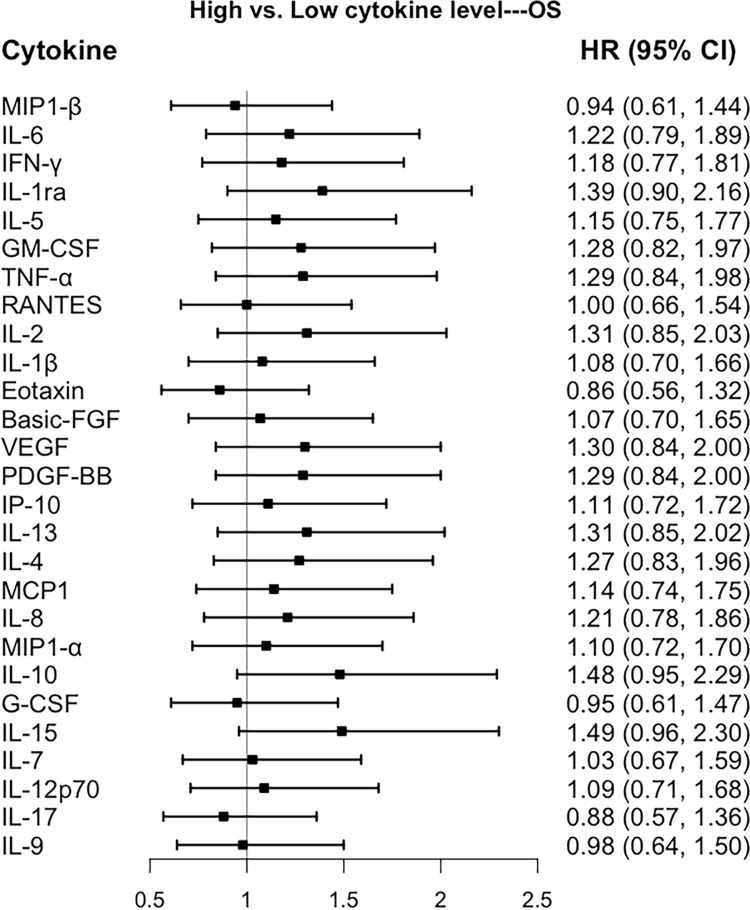
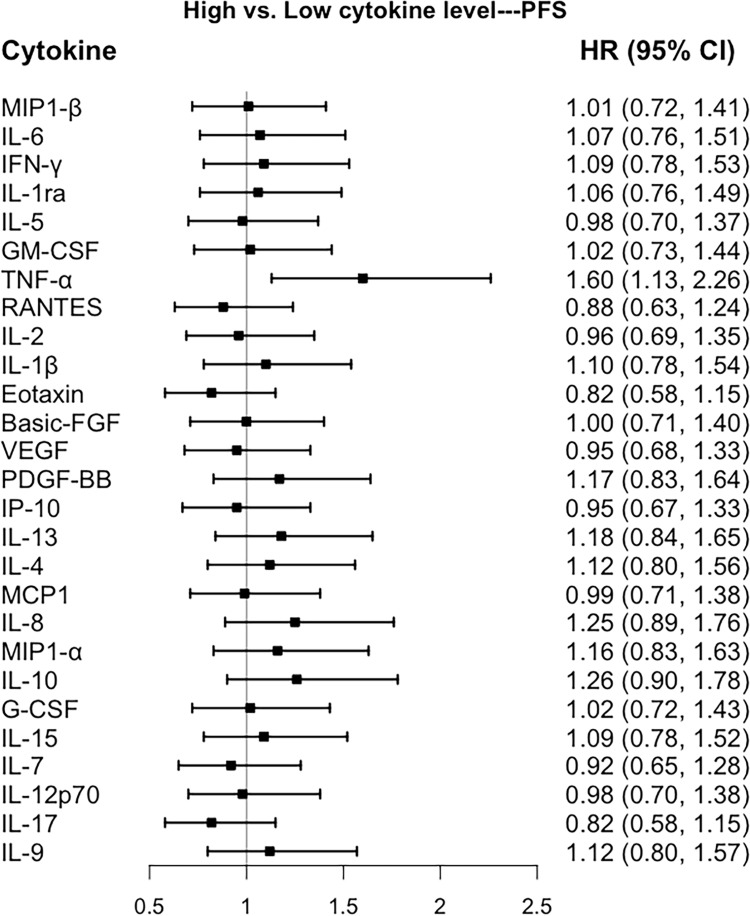
Associations of Cytokines with Prognosis of Breast Cancer
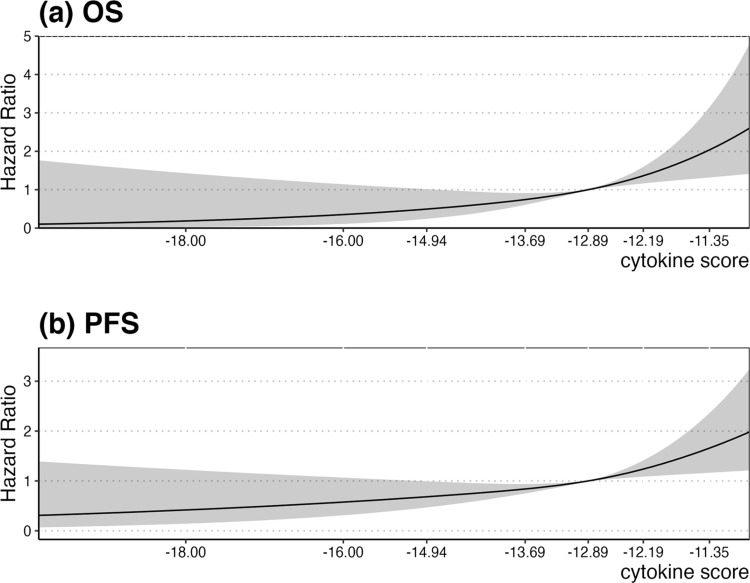
As shown in Figure 1, none of the cytokines were associated with OS. However, in multivariate Cox regression analyses, TNF-α was associated with poor breast cancer PFS (HR=1.60, 95% CI: 1.13–2.26; Figure 2). As the composite measure of multiple cytokines, the continuous cytokine score was associated with prognosis in breast cancer. With regard to OS, risk of death increased slightly until the cytokine score of approximately −12.89 sharply increased to the highest level, which was accompanied by a significant association between the cytokine score and OS (P<0.001; P nonlinear=0.648; Figure 3a). A similar significant pattern was observed for PFS (P<0.001; P nonlinear=0.385; Figure 3b). Based on the results of restricted cubic splines of the cytokine score, we categorized the cytokine score into two levels using a cutoff point of −12.89. After adjusting for potential prognostic factors, patients whose cytokine score was >−12.89 exhibited a significantly increased risk of death compared to those with a score ≤−12.89 (HR=1.56, 95% CI: 1.01–2.43; Table 2).
Figure 1.
Forest plot of the associations between individual cytokines with overall survival in a multivariable model adjusted with age at diagnosis (continuous variable), ER status, PR status, HER2 status, Ki67 index, tumor size, nodal status, and clinical stage (early stage VS advanced stage). All cytokines were modeled as binary variables according to the median value of the cytokines.
Figure 2.
Forest plot of the associations between individual cytokines with progression-free survival in a multivariable model adjusted with age at diagnosis (continuous variable), ER status, PR status, HER2 status, Ki67 index, tumor size, nodal status, and clinical stage (early stage VS advanced stage). All cytokines were modeled as binary variables according to the median value of the cytokines.
Figure 3.
Restricted cubic splines of cytokine score with breast cancer (a) OS and (b) PFS. Solid black lines were hazard ratios, with gray area showing 95% CIs derived from restricted cubic spline regressions with three knots.
Interaction Between Anti-T. gondii IgG and Cytokines and Prognosis of Breast Cancer
The protective effect of a high anti-T. gondii IgG level on breast cancer PFS was more evident in patients with a cytokine score >−12.89 (HR=0.30, 95% CI: 0.13–0.69) than in those with a cytokine score ≤−12.89 (HR=0.96, 95% CI: 0.51–1.80), a significant interaction (P=0.019, Table 4). Moreover, the negative association between high anti-T. gondii IgG and risk of death (HR=0.29, 95% CI=0.10–0.82) was observed among patients with a cytokine score >−12.89 but not among those with a cytokine score ≤−12.89 (HR=0.85, 95% CI 0.35–2.04; Table 4), though the interaction was not significant.
Table 4.
Association Between Anti-T. gondii IgG Level and the Prognosis of Breast Cancer Stratified by Cytokine Score
| Cytokine Score | Anti-T. gondii IgG Level | Total | Overall Survival | Progression-Free Survival | ||||
|---|---|---|---|---|---|---|---|---|
| Events | HR (95% CI)a | HR (95% CI)b | Events | HR (95% CI)a | HR (95% CI)b | |||
| ≤ −12.89 | Low | 326 | 29 | 1.00 (reference) | 1.00 (reference) | 51 | 1.00 (reference) | 1.00 (reference) |
| High | 71 | 7 | 1.16 (0.51, 2.65) | 0.85 (0.35, 2.04) | 14 | 1.22 (0.68, 2.21) | 0.96 (0.51, 1.80) | |
| > −12.89 | Low | 320 | 59 | 1.00 (reference) | 1.00 (reference) | 86 | 1.00 (reference) | 1.00 (reference) |
| High | 77 | 8 | 0.50 (0.24, 1.05) | 0.29 (0.10, 0.82) | 12 | 0.51 (0.28, 0.94) | 0.30 (0.13, 0.69) | |
| P for interaction | 0.151 | 0.095 | 0.036 | 0.019 | ||||
Notes: Bold character indicates statistically significant result. aUnivariate analysis. bAdjusted for age at diagnosis (continuous variable), ER status, PR status, HER2 status, Ki67 index, tumor size, nodal status, and clinical stage (early stage VS advanced stage).
To determine the cytokines contributing maximally to the interaction, we performed stratified analyses according to the median value of cytokines. As shown in Table 5, a high anti-T. gondii IgG level significantly improved PFS among breast cancer patients with high IL-17 (HR=0.40, 95% CI=0.19–0.84) but not among those with low IL-17 (HR=1.09, 95% CI=0.64–1.86); this interaction was significant (P=0.032). A high anti-T. gondii IgG level also significantly reduced risk of death among patients with high IL-17 (HR=0.25, 95% CI: 0.08–0.72) but not among those with low IL-17 (HR=1.15, 95% CI=0.59–2.26); this interaction was also significant (P=0.027; Table 5). Similarly, the protective effect of high anti-T. gondii IgG on PFS was observed among patients with high IL-9 (HR=0.37, 95% CI: 0.17–0.81) but not among those with low IL-9 (HR=1.08, 95% CI: 0.63–1.84), and this interaction was significant (P=0.032; Table 5). Other cytokines did not significantly interact with anti-T. gondii IgG levels with respect to breast cancer prognosis (data not shown). In addition, no significant mediation by cytokines was detected in the association between anti-T. gondii IgG level and survival (Tables S2 and S3).
Table 5.
Association Between Anti-T. gondii IgG Level and the Prognosis of Breast Cancer Stratified by IL-17 and IL-9
| Cytokines | Anti-T.gondii IgG Level | Total | Overall Survival | Progression-Free Survival | ||||
|---|---|---|---|---|---|---|---|---|
| Events | HR (95% CI)a | HR (95% CI)b | Events | HR (95% CI)a | HR (95% CI)b | |||
| IL-17 | ||||||||
| Low | Low | 322 | 46 | 1.00 (reference) | 1.00 (reference) | 72 | 1.00 (reference) | 1.00 (reference) |
| High | 76 | 11 | 0.97 (0.50, 1.88) | 1.15 (0.59, 2.26) | 18 | 1.00 (0.60, 1.68) | 1.09 (0.64, 1.86) | |
| High | Low | 324 | 42 | 1.00 (reference) | 1.00 (reference) | 65 | 1.00 (reference) | 1.00 (reference) |
| High | 72 | 4 | 0.40 (0.14, 1.12) | 0.25 (0.08, 0.72) | 8 | 0.51 (0.24, 1.06) | 0.40 (0.19, 0.84) | |
| P for interaction | 0.170 | 0.027 | 0.146 | 0.032 | ||||
| IL-9 | ||||||||
| Low | Low | 320 | 45 | 1.00 (reference) | 1.00 (reference) | 63 | 1.00 (reference) | 1.00 (reference) |
| High | 77 | 10 | 0.91 (0.46, 1.81) | 0.73 (0.35, 1.49) | 19 | 1.24 (0.74, 2.07) | 1.08 (0.63, 1.84) | |
| High | Low | 326 | 43 | 1.00 (reference) | 1.00 (reference) | 74 | 1.00 (reference) | 1.00 (reference) |
| High | 71 | 5 | 0.49 (0.19, 1.24) | 0.47 (0.18, 1.19) | 7 | 0.38 (0.18, 0.83) | 0.37 (0.17, 0.81) | |
| P for interaction | 0.286 | 0.525 | 0.012 | 0.032 | ||||
Notes: Bold character indicates statistically significant result. All cytokines were modeled as binary variables according to the median value of the cytokines. aUnivariate COX regression. bAdjusted for age at diagnosis (continuous variable), ER status, PR status, HER2 status, tumor size, nodal status, and clinical stage (early stage VS advanced stage).
Discussion
The present hospital-based cohort study reports that a high anti-T. gondii IgG level is associated with improved breast cancer OS and PFS, independent of multiple well-known prognostic factors. The protective effect of anti-T. gondii IgG was evident among patients with a high cytokine score, accompanied by a significant interaction (between anti-T. gondii IgG and the cytokine score) for breast cancer PFS. Furthermore, IL-17 and IL-9 were identified as the main contributors to this interaction.
In agreement with the protective effects of T. gondii in tumor-bearing mouse models, we observed beneficial effects in breast cancer patients, indicating great potential for application of T. gondii in tumor therapy.6 Recently, Xu et al confirmed the antitumor effect of T. gondii on breast cancer by injecting artificial attenuated strains into mice inoculated with 4T1 cells (murine triple-negative breast cancer cells).11 The mechanisms underlying such effects include hypoxia, avascular necrosis, and anti-angiogenesis.26 Moreover, the parasite can remain viable in the form of tissue cysts throughout the life of the host, maintaining long-lasting effects.27 Therefore, T. gondii may persistently play a protective role in cancer patients and improve their prognosis, especially for those with recurrence-prone and refractory types, such as invasive papillary carcinoma.28,29 Notably, a previous study showed the rate of T. gondii infection to be higher in cancer patients than in healthy controls, indicating that infection by T. gondii is a possible risk factor for cancer.30 However, these were retrospective studies, and a reverse causality could not be ruled out.13
Inflammation is a hallmark of cancer that contributes to angiogenesis and activation of the epithelial-mesenchymal transition (EMT) by supplying several cytokines.31 In the current study, we observed a harmful effect of higher levels of TNF-α on PFS in breast cancer, consistent with previous results.32 For example, Kim et al reported that a high level of TNF-α at baseline is associated with a significantly high risk of disease progression.33 As a composite measure of individual cytokine values, the cytokine score showed a significant association with breast cancer prognosis in our study. The direction of this association was consistent with that of individual cytokines and breast cancer prognosis, with its magnitude being slightly greater than that of individual cytokines. These results are in accordance with previous studies, in which circulating systemic inflammatory markers were found to be poor prognosis indicators in breast cancer patients.34,35
Furthermore, we observed marked protective effects of anti-T. gondii IgG on breast cancer prognosis among women with a higher cytokine score than those with a lower score, a significant interaction (mainly contributed by IL-17 and IL-9). Patients with a high cytokine score might experience systemic inflammation, which induces angiogenesis,36 a well-established driver of breast cancer aggressiveness and poor prognosis,37 yet T. gondii infection inhibits angiogenesis.11,38 Therefore, patients with a higher cytokine score might have better prognosis because tumor neovascularization in these patients is more effectively inhibited by T. gondii than in patients with a lower cytokine score; IL-17 and IL-9 promote angiogenesis and are major contributors to the interaction.39,40 However, the precise mechanisms underlying the interaction have yet to be explored.
Nevertheless, the present study has some limitations. First, only patients with serum samples were included, making it difficult to avoid selection bias. However, the distribution of the subjects’ clinicopathological characteristics in the present study was similar to that from the same cohort as reported previously.19 Moreover, the seroprevalence of anti-T. gondii IgG (69/1121, 6.16%) in this study was in line with that found in a previous study (1/56, 1.79%).41 Second, the modification effect of cytokines was assessed among only a subset of 794 individuals, indicating that the results might not be generalizable to all study subjects. Nonetheless, the protective effects of anti-T. gondii IgG in this subset of patients was significant among all patients (Table S4). In addition, the patients with cytokine data were randomly selected from the whole study population, suggesting that the modification effect may be maintained in all patients. Although we did not collect information on treatment, which is crucial for prognosis, we adjusted clinicopathological characteristics to partially control for the confounding effect of treatment.
Conclusions
In conclusion, the current results suggest a protective effect of anti-T. gondii IgG on prognosis in breast cancer, and this effect is modified by serum cytokine levels, particularly IL-17 and IL-9. The current study demonstrates that T. gondii infection confers a beneficial effect on the prognosis of breast cancer patients, which has only been found in mice thus far, indicating that attenuated T. gondii has significant potential as a novel immunotherapy for the treatment of breast cancer.42 However, additional studies are required to elucidate the exact mechanisms.
Acknowledgments
We sincerely thank the patients who participated in this study, the staff who conducted the baseline and the follow‐up data collection, and the medical staff in the breast departments of the First Affiliated Hospital, the Second Affiliated Hospital, and the Cancer Center of Sun Yat‐Sen University.
Funding Statement
This work was supported by the National Natural Science Foundation of China [Grant Numbers 81973115] and the Science and Technology Planning Project of Guangdong Province, China [Grant Number 2019B030316002].
Data Sharing Statement
The datasets used and analyzed during this study were available from the corresponding author on request.
Ethics Approval and Informed Consent
This study was approved by the Ethics Committee of the School of Public Health at Sun Yat-sen University (number of Institutional Review Board approval: 2012-8) and performed in accordance with the Declaration of Helsinki. The written informed consents were obtained from all the participants.
Consent for Publication
Written informed consent for publication was obtained from all participants.
Author Contributions
All authors contributed to study design, data collection, statistical analysis and interpretation, visualization, drafting or revising the article; have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363(9425):1965–1976. doi: 10.1016/s0140-6736(04)16412-x [DOI] [PubMed] [Google Scholar]
- 2.de Barros RAM, Torrecilhas AC, Marciano MAM, Mazuz ML, Pereira-Chioccola VL, Fux B. Toxoplasmosis in human and animals around the world. diagnosis and perspectives in the one health approach. Acta Trop. 2022;231:106432. doi: 10.1016/j.actatropica.2022.106432 [DOI] [PubMed] [Google Scholar]
- 3.Megli CJ, Coyne CB. Infections at the maternal-fetal interface: an overview of pathogenesis and defence. Nat Rev Microbiol. 2022;20(2):67–82. doi: 10.1038/s41579-021-00610-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robert-Gangneux F, Dardé ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. 2012;25(2):264–296. doi: 10.1128/cmr.05013-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darani HY, Yousefi M. Parasites and cancers: parasite antigens as possible targets for cancer immunotherapy. Future Oncol. 2012;8(12):1529–1535. doi: 10.2217/fon.12.155 [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Liao W, Peng H. Toxoplasma gondii infection possibly reverses host immunosuppression to restrain tumor growth. Front Cell Infect Microbiol. 2022;12:959300. doi: 10.3389/fcimb.2022.959300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baird JR, Byrne KT, Lizotte PH, et al. Immune-mediated regression of established B16F10 melanoma by intratumoral injection of attenuated Toxoplasma gondii protects against rechallenge. J Immunol. 2013;190(1):469–478. doi: 10.4049/jimmunol.1201209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baird JR, Fox BA, Sanders KL, et al. Avirulent Toxoplasma gondii generates therapeutic antitumor immunity by reversing immunosuppression in the ovarian cancer microenvironment. Cancer Res. 2013;73(13):3842–3851. doi: 10.1158/0008-5472.Can-12-1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders KL, Fox BA, Bzik DJ. Attenuated Toxoplasma gondii stimulates immunity to pancreatic cancer by manipulation of myeloid cell populations. Cancer Immunol Res. 2015;3(8):891–901. doi: 10.1158/2326-6066.Cir-14-0235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu G, Zhou J, Zhao YH, Li QL, Gao YY, Wang L. Transcriptome sequencing investigated the tumor-related factors changes after T. gondii infection. Front Microbiol. 2019;10:181. doi: 10.3389/fmicb.2019.00181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu LQ, Yao LJ, Jiang D, et al. A uracil auxotroph Toxoplasma gondii exerting immunomodulation to inhibit breast cancer growth and metastasis. Parasit Vectors. 2021;14(1):601. doi: 10.1186/s13071-021-05032-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalantari N, Ahangar Darabi Z, Siadati S, et al. Detection of Toxoplasma gondii DNA in malignant breast tissues in breast cancer patients. Int J Mol Cell Med. 2017;6(3):190–196. doi: 10.22088/acadpub.BUMS.6.3.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anvari D, Sharif M, Sarvi S, et al. Seroprevalence of Toxoplasma gondii infection in cancer patients: a systematic review and meta-analysis. Microb Pathog. 2019;129:30–42. doi: 10.1016/j.micpath.2019.01.040 [DOI] [PubMed] [Google Scholar]
- 14.Cong W, Liu GH, Meng QF, et al. Toxoplasma gondii infection in cancer patients: prevalence, risk factors, genotypes and association with clinical diagnosis. Cancer Lett. 2015;359(2):307–313. doi: 10.1016/j.canlet.2015.01.036 [DOI] [PubMed] [Google Scholar]
- 15.Kalantari N, Ghaffari S, Bayani M, et al. Preliminary study on association between toxoplasmosis and breast cancer in Iran. Asian Pac J Trop Biomed. 2015;5(1):44–47. doi: 10.1016/S2221-1691(15)30169-6 [DOI] [Google Scholar]
- 16.Thirugnanam S, Rout N, Gnanasekar M. Possible role of Toxoplasma gondii in brain cancer through modulation of host microRNAs. Infect Agent Cancer. 2013;8(1):8. doi: 10.1186/1750-9378-8-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Assim MM, Saheb EJ. Serum levels of Il-12 and Il-23 in breast cancer patients infected with toxoplasma gondii: a case-control study. Iran J Parasitol. 2020;15(4):466–474. doi: 10.18502/ijpa.v15i4.4850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esquivel-Velázquez M, Ostoa-Saloma P, Palacios-Arreola MI, Nava-Castro KE, Castro JI, Morales-Montor J. The role of cytokines in breast cancer development and progression. J Interferon Cytokine Res. 2015;35(1):1–16. doi: 10.1089/jir.2014.0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye H, Tang LY, Liang ZZ, et al. Effects of infection-induced fever and the interaction with IL6 rs1800796 polymorphism on the prognosis of breast cancer. Cancer Epidemiol Biomarkers Prev. 2022;31(11):2030–2037. doi: 10.1158/1055-9965.Epi-22-0498 [DOI] [PubMed] [Google Scholar]
- 20.He JR, Tang LY, Yu DD, et al. Epstein-Barr virus and breast cancer: serological study in a high-incidence area of nasopharyngeal carcinoma. Cancer Lett. 2011;309(2):128–136. doi: 10.1016/j.canlet.2011.05.012 [DOI] [PubMed] [Google Scholar]
- 21.Wang L, He LY, Meng DD, et al. Seroprevalence and genetic characterization of Toxoplasma gondii in cancer patients in Anhui Province, Eastern China. Parasit Vectors. 2015;8:162. doi: 10.1186/s13071-015-0778-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jabeen S, Espinoza JA, Torland LA, et al. Noninvasive profiling of serum cytokines in breast cancer patients and clinicopathological characteristics. Oncoimmunology. 2019;8(2):e1537691. doi: 10.1080/2162402x.2018.1537691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang MJ, Guo J, Ye YF, et al. Decreased macrophage inflammatory protein (MIP)-1α and MIP-1β increase the risk of developing nasopharyngeal carcinoma. Cancer Commun (Lond). 2018;38(1):7. doi: 10.1186/s40880-018-0279-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–7259. doi: 10.1158/1078-0432.Ccr-04-0713 [DOI] [PubMed] [Google Scholar]
- 25.Hughes-Austin JM, Deane KD, Derber LA, et al. Multiple cytokines and chemokines are associated with rheumatoid arthritis-related autoimmunity in first-degree relatives without rheumatoid arthritis: Studies of the Aetiology of Rheumatoid Arthritis (SERA). Ann Rheum Dis. 2013;72(6):901–907. doi: 10.1136/annrheumdis-2012-201505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunter CA, Yu D, Gee M, et al. Cutting edge: systemic inhibition of angiogenesis underlies resistance to tumors during acute toxoplasmosis. J Immunol. 2001;166(10):5878–5881. doi: 10.4049/jimmunol.166.10.5878 [DOI] [PubMed] [Google Scholar]
- 27.Weiss LM, Dubey JP. Toxoplasmosis: a history of clinical observations. Int J Parasitol. 2009;39(8):895–901. doi: 10.1016/j.ijpara.2009.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verras GI, Tchabashvili L, Mulita F, et al. Micropapillary breast carcinoma: from molecular pathogenesis to prognosis. Breast Cancer (Dove Med Press). 2022;14:41–61. doi: 10.2147/bctt.S346301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verras GI, Mulita F, Tchabashvili L, et al. A rare case of invasive micropapillary carcinoma of the breast. Prz Menopauzalny. 2022;21(1):73–80. doi: 10.5114/pm.2022.113834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdollahi A, Razavian I, Razavian E, et al. Toxoplasma gondii infection/exposure and the risk of brain tumors: a systematic review and meta-analysis. Cancer Epidemiol. 2022;77:102119. doi: 10.1016/j.canep.2022.102119 [DOI] [PubMed] [Google Scholar]
- 31.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 32.Cruceriu D, Baldasici O, Balacescu O, Berindan-Neagoe I. The dual role of tumor necrosis factor-alpha (TNF-α) in breast cancer: molecular insights and therapeutic approaches. Cell Oncol (Dordr). 2020;43(1):1–18. doi: 10.1007/s13402-019-00489-1 [DOI] [PubMed] [Google Scholar]
- 33.Kim JW, Lee S, Kim HS, et al. Prognostic effects of cytokine levels on patients treated with taxane and zoledronic acid for metastatic breast cancer in bone (BEAT-ZO) (KCSG BR 10-13). Cytokine. 2021;142:155487. doi: 10.1016/j.cyto.2021.155487 [DOI] [PubMed] [Google Scholar]
- 34.Savioli F, Morrow ES, Dolan RD, et al. Prognostic role of preoperative circulating systemic inflammatory response markers in primary breast cancer: meta-analysis. Br J Surg. 2022;109:1206–1215. doi: 10.1093/bjs/znac319 [DOI] [PubMed] [Google Scholar]
- 35.Verras GI, Tchabashvili L, Chlorogiannis DD, Mulita F, Argentou MI. Updated clinical evidence on the role of adipokines and breast cancer: a review. Cancers (Basel). 2023;15(5):1572. doi: 10.3390/cancers15051572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ono M. Molecular links between tumor angiogenesis and inflammation: inflammatory stimuli of macrophages and cancer cells as targets for therapeutic strategy. Cancer Sci. 2008;99(8):1501–1506. doi: 10.1111/j.1349-7006.2008.00853.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Heer EC, Jalving M, Harris AL. HIFs, angiogenesis, and metabolism: elusive enemies in breast cancer. J Clin Invest. 2020;130(10):5074–5087. doi: 10.1172/jci137552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim JO, Jung SS, Kim SY, et al. Inhibition of Lewis lung carcinoma growth by Toxoplasma gondii through induction of Th1 immune responses and inhibition of angiogenesis. J Korean Med Sci. 2007;22:S38–46. doi: 10.3346/jkms.2007.22.S.S38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chung AS, Wu X, Zhuang G, et al. An interleukin-17-mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nat Med. 2013;19(9):1114–1123. doi: 10.1038/nm.3291 [DOI] [PubMed] [Google Scholar]
- 40.He J, Wang L, Zhang C, et al. Interleukin-9 promotes tumorigenesis through augmenting angiogenesis in non-small cell lung cancer. Int Immunopharmacol. 2019;75:105766. doi: 10.1016/j.intimp.2019.105766 [DOI] [PubMed] [Google Scholar]
- 41.Mao F, Yang Y, Chen Y, et al. Seroprevalence and risk factors of toxoplasma gondii infection among high-risk populations in Jiangsu Province, Eastern China. Front Cell Infect Microbiol. 2021;11:783654. doi: 10.3389/fcimb.2021.783654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu YC, Elsheikha HM, Wang JH, et al. Synergy between Toxoplasma gondii type I Δ GRA17 immunotherapy and PD-L1 checkpoint inhibition triggers the regression of targeted and distal tumors. J Immunother Cancer. 2021;9(11):e002970. doi: 10.1136/jitc-2021-002970 [DOI] [PMC free article] [PubMed] [Google Scholar]