Abstract
Expression of luminescence in the Penaeus monodon pathogen Vibrio harveyi is regulated by an intercellular quorum sensing mechanism involving the synthesis and detection of two signaling molecules, one of which is N-hydroxy butanoyl-l-homoserine lactone and the other of which is uncharacterized. Indirect evidence has suggested that virulence, associated with a toxic extracellular protein, and luminescence in V. harveyi are coregulated. In this study the effects of an acylated homoserine lactone antagonist produced by the marine alga Delisea pulchra on luminescence and toxin production in a virulent strain of V. harveyi were analyzed. Luminescence and toxin production were both inhibited by the signal antagonist at concentrations that had no impact on growth. Toxin production was found to be prematurely induced in V. harveyi cultures incubated in a 10% conditioned medium. Additionally, a significant reduction in the toxicity of concentrated supernatant extracts from V. harveyi cultures incubated in the presence of the signal antagonist, as measured by in vivo toxicity assays in mice and prawns, was observed. These results suggest that intercellular signaling antagonists have potential utility in the control of V. harveyi prawn infections.
Vibrio harveyi is a gram-negative, luminescent, marine bacterium isolated both in a free-living state (16, 17, 20) and as a commensal organism in the enteric contents of marine animals (11, 22). Recognized as a primary pathogen of many commercially cultured invertebrate species, such as the black tiger prawn (Penaeus monodon), V. harveyi can cause up to 100% mortality of larvae in the hatchery stage of penaeid culture (12, 16).
Virulence in V. harveyi (strain 47666-1) has been attributed to the production of an extracellular protein referred to as toxin T1 with a molecular mass of approximately 100 kDa (10, 19). The extracellular protein is produced during the mid-exponential phase of growth and has sequence similarity to virulence-associated proteins in Salmonella, Shigella, and Bacillus species (10).
As suggested by the name of the disease phenomenon commonly referred to as luminous vibriosis, the expression of luminescence in V. harveyi has long been associated with virulence in pathogenic strains of this organism (14). Expression of the luminescence phenotype in V. harveyi is controlled at the transcriptional level by an atypical quorum sensing system (1). In the model quorum sensing system of Vibrio fischeri, a diffusible N-acylated-l-homoserine lactone (AHL) molecule is employed to link expression of the luminescence phenotype to bacterial population density. AHL, synthesized by the LuxI protein, accumulates throughout the population and at high concentrations interacts with a regulatory protein (LuxR), which then transcriptionally activates expression of the structural genes (luxCDABEG) encoding the luminescence phenotype (23). Other AHL quorum sensing systems, such as those regulating expression of virulence factors in Pseudomonas aeruginosa and Erwinia carotovora, also rely on LuxI and LuxR homologues for AHL production and detection, respectively (8).
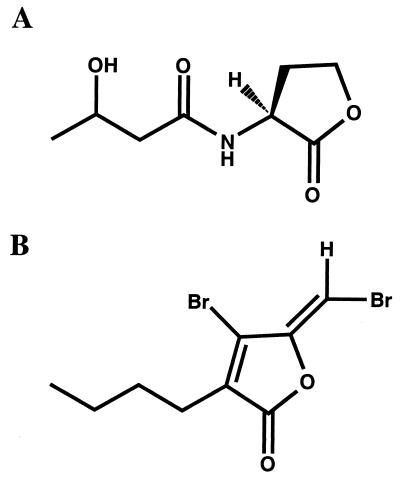
In contrast, synthesis of the AHL N-hydroxy butanoyl-l-homoserine lactone (HBHL) (Fig. 1A) by V. harveyi is dependent on the products of two genes (luxLM) which have no sequence similarity to luxI-type AHL synthase genes (2). Additionally, instead of binding a transcriptional regulator directly, HBHL derepresses transcription of the lux structural genes (luxCDABEGH) by stimulating phosphatase activity in a sensory protein (LuxN), which acts to dephosphorylate the transcriptional repressor LuxO (6) via a phosphorelay protein named LuxU (7). A second, uncharacterized quorum sensing signal, known as AI-2, stimulates phosphatase activity in a second sensory protein named LuxQ, the activity of which converges at LuxU with the HBHL-regulated phosphorelay channel (7). Following inactivation of the LuxO repressor protein, transcription of the lux structural genes is stimulated by a signal-independent transcriptional activator protein (24).
FIG. 1.
Structures of HBHL produced by V. harveyi (A) and a halogenated furanone produced by D. pulchra (B).
Recently it has been shown that halogenated furanones produced by the red, benthic, marine macroalga Delisea pulchra interfere with AHL-regulated gene expression. These algal metabolites are active against the luminescence phenotype of V. fischeri (9). Halogenated furanones are thought to act by displacing AHL from its receptor protein (LuxR or LuxR homologue), thus inhibiting transcriptional activation of genes encoding the quorum sensing phenotype (18).
In this study we investigated the ability of a halogenated furanone (Fig. 1B) to inhibit the quorum sensing regulated luminescence phenotype of the pathogenic V. harveyi strain 47666-1. Given the association between luminescence and virulence, we also tested the effect of the algal compound on production of toxin T1 by this strain. We found that the halogenated furanone from D. pulchra inhibited the luminescence phenotype without affecting the growth of the organism. Additionally, extracellular toxin production was inhibited by the halogenated furanone. Furthermore, the toxicity of cell supernatant extracts of furanone-treated V. harveyi cultures was reduced in in vivo toxicity assays with P. monodon specimens and CBA mice. These results demonstrate the potential for the use of halogenated furanones in the management of V. harveyi infection in aquaculture and indirectly suggest that virulence, like luminescence, is dependent on an intercellular signaling mechanism for expression.
MATERIALS AND METHODS
Strains and culture conditions.
V. harveyi strain 47666-1 (Australian Collection of Marine Organisms, Townsville, Australia) was grown on luminous agar at 28°C or in luminous broth (21) with shaking at 200 rpm, at 28°C unless otherwise stated.
Extraction of halogenated furanone and synthesis of AHL.
(5Z)-4-Bromo-5-(bromomethylene)-3-butyl-2(5H)-furanone was extracted and purified from D. pulchra as previously described (4). The purified furanone was stored in ethanol at −20°C before use.
HBHL was synthesized by the coupling of 3-hydroxybutyric acid with (S)-homoserine lactone as previously described (3). HBHL was stored in ethanol at −20°C before use.
Bioluminescence assay and growth.
Aliquots (100 μl) of overnight cultures of V. harveyi adjusted to an optical density at 600 nm (OD600) of 0.1 were inoculated into 5-ml volumes of luminous broth. Halogenated furanone was added to treatment cultures to final concentrations of 50, 100, or 200 μM. An appropriate volume of ethanol (furanone solvent) was added to control cultures. Cultures were incubated with shaking at 28°C. Aliquots (200 μl each) were removed hourly for determination of luminescence and OD600. Luminescence was quantified on a Wallac Microbeta Plus liquid scintillation counter and reported in relative light units.
Preparation of concentrated cell supernatant extracts and analysis of toxin T1 production.
To determine the effect of the algal metabolites on toxin T1 production, 100-ml duplicate cultures of V. harveyi were grown to an OD600 of 0.4 in the presence or absence of 100 μM halogenated furanone. Cells were then pelleted, and supernatants were filtered through a 0.22-μm-pore-size filter. The resulting cell-free supernatants were then concentrated 100-fold with Ultrafree 15 centrifugal filters, which retain proteins with masses above 10 kDa (Millipore). Native polyacrylamide gel electrophoresis (PAGE) with a 10% separating gel (15) was used to visualize the concentrated cell supernatant extracts from furanone-treated and untreated cultures. Relative concentrations of toxin T1 were quantified by standard densitometry using Multi-Analyst software (Bio-Rad). To control for a direct effect of the furanone on toxin T1 activity, 100 μM halogenated furanone was added to a previously untreated filtered culture supernatant, which was then concentrated as described above.
To determine the effect of HBHL and AI-2 on toxin T1 production, 100-ml duplicate cultures of V. harveyi were grown to an OD600 of 0.1 in the presence and absence of 200 nM HBHL or a 10% filtered high-density (OD600 = 0.4) V. harveyi culture supernatant. Toxin T1 preparations were made from these cultures and analyzed as described above. To ensure that the presence of toxin T1 in low-density cultures was not left over from addition of the 10% high-density supernatant, a 10-fold dilution of concentrated supernatant extract prepared from the filtered high-density (OD600 = 0.4) V. harveyi culture employed was included in the PAGE analysis.
Toxicity of concentrated cell supernatant extracts in CBA mice and juvenile P. monodon specimens.
A dilution series in phosphate-buffered saline of the concentrated cell supernatant extracts, prepared as described above, was used to inoculate male CBA mice by intraperitoneal injection. In an initial experiment, groups of 3 mice with less than 1 g of variation in weight were used per treatment. In the final experiment, groups of 10 mice with less than 1 g of variation in weight were used. For both experiments, a dose volume of 100 μl was used per mouse. Following inoculation, the animals were observed for gross behavioral changes, including loss of activity, reduced grooming, and loss of appetite, every 12 h for up to 4 days.
Similarly, a dilution series of the concentrated supernatant extracts was used to inoculate the juvenile P. monodon species by intramuscular injection into the third abdominal segment anterior to the telson. Groups of 7 or more prawns with less than 2 g of variation in weight were used per treatment. Dose volumes of 50 μl were used per animal. Following inoculation, the animals were observed for gross behavioral changes, including loss of motility, every 12 h for up to 4 days.
Control treatment groups of CBA mice and P. monodon specimens were injected with 100 μl and 50 μl, respectively, of phosphate-buffered saline as a diluent control and an appropriate volume of a 4-mg/ml bovine serum albumin solution as an inert protein control. Following inoculation, the animals were observed every 12 h for up to 4 days.
Pairwise comparisons of treatment groups according to dose and time were used to analyze survival data for significant differences (P < 0.05) between treatment groups, where G, calculated as the log-likelihood ratio, approximates the chi-squared distribution. Fifty percent lethal doses (LD50s) were calculated by probit analysis (logistic regression analysis) of dose-response models. SPSS for Windows (SPSS Inc., Chicago, Ill.) was used to perform both analyses.
RESULTS
Inhibition of luminescence in V. harveyi by a halogenated furanone.
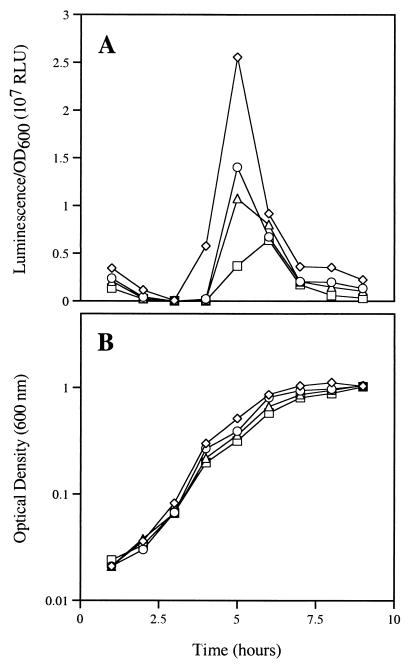
Given the ability of halogenated furanones to interfere with AHL-mediated gene expression in other organisms such, as V. fischeri and Serratia liquefaciens (9, 18), it was of interest to test the effect of the algal metabolites on the genetically distinct signal-mediated gene expression of V. harveyi. Cultures of V. harveyi were grown from low to high cell density in the presence and absence of 50, 100, or 200 μM halogenated furanone (Fig. 1B). In this strain, the characteristic autoinduced increase in the expression of the luminescence phenotype is associated with an OD600 of 0.2 to 0.4. Figure 2A shows the concentration-dependent inhibition of the luminescence phenotype, most apparent after 4 h of incubation at an OD600 of approximately 0.3, by the halogenated furanone. At the concentrations tested, no alteration in the growth of the bacterium was observed as measured by optical density (Fig. 2B). This suggested that the AHL-dependent luminescence phenotype of V. harveyi, as with V. fischeri, is susceptible to regulation by halogenated furanones.
FIG. 2.
Effect of the halogenated furanone at 0 μM (diamonds), 50 μM (circles), 100 μM (triangles), and 200 μM (squares) on specific luminescence response reported in relative light units (RLU) (A) and growth as determined by OD600 of V. harveyi (B).
Inhibition of toxin T1 production.
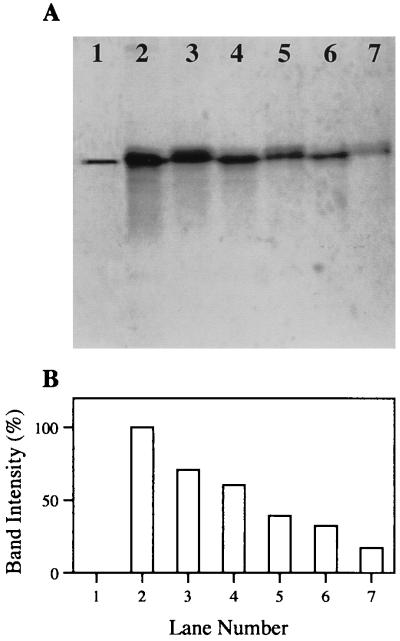
Virulence in V. harveyi has been attributed to a partially characterized 100-kDa protein referred to as toxin T1 (10). PAGE analysis of concentrated cell supernatant extracts has shown that the toxin appears in culture supernatants at an optical density between 0.2 and 0.4, corresponding with the density at which luminescence is expressed. The putative link between expression of luminescence and virulence prompted us to test the effect of a halogenated furanone on the production of toxin T1 in V. harveyi. Cultures were grown in the presence and absence of 100 μM halogenated furanone to an optical density of 0.4. Concentrated cell supernatant extracts of the untreated and furanone-treated cultures contained 3.9 μg and 3.8 μg of total protein/ml, respectively. These concentrates were analyzed by PAGE to assess the resultant effect of the furanone on toxin production (Fig. 3A). Densitometric analysis revealed a 50% reduction in the presence of toxin T1 in the supernatants of cultures treated with the halogenated furanone (Fig. 3B). The simultaneous down-regulation of luminescence and toxin T1 production adds further indirect evidence to the putative coregulation of luminescence and virulence in this pathogenic V. harveyi strain.
FIG. 3.
Effect of 100 μM halogenated furanone on the presence of toxin T1 in V. harveyi supernatants as determined by native PAGE and densitometry. (A) The gel shows purified toxin T1 (lane 1), a twofold-dilution series of extracts prepared from control cultures (lanes 2, 4, and 6), and a twofold-dilution series of extracts prepared from halogenated furanone-treated cultures (lanes 3, 5, and 7). (B) Bar graph representing the band intensity of toxin T1 in each lane as a percentage of the untreated, undiluted toxin T1 band (lane 2).
In vivo toxicity of cell supernatant extracts is reduced when the extracts are prepared from halogenated furanone-treated V. harveyi cultures.
Harris and Owens (10) have previously found that cell supernatant extracts from V. harveyi, administered by intraperitoneal injection, are toxic to CBA mice, with an LD50 of 3.9 μg of total protein per g of body weight. Similarly, such extracts, administered by intramuscular injection, are toxic to P. monodon, with an LD50 of 3.5 μg per g of prawn. To determine whether in vivo toxicity of V. harveyi cell supernatant extracts to mice and prawns is reduced by furanones, extracts were prepared from V. harveyi cultures incubated in the presence and absence of 100 μM halogenated furanone.
CBA mice were challenged in triplicate with 100 μl of 1-in-4-dilution, 1-in-2-dilution, or undiluted extracts from untreated or furanone-treated cultures of V. harveyi. The resultant survival and symptoms of treated mice were recorded every 12 h for 96 h after injection. All mice treated with undiluted extracts (13.7 μg/g) or 1-in-2 dilutions of extracts (∼7.5 μg/g) from untreated or furanone-treated cultures died within 96 h of injection (Table 1). It was noted that mice injected with a 1-in-2 dilution of extract from the halogenated furanone-treated culture had delayed mortality (1 mouse deceased after 96 h, 2 survivors recovered after 96 h) compared with mice injected with equivalent dilutions of extract from the untreated culture (3 mice deceased after 48, 48, and 60 h). Mice injected with a 1-in-4 dilution of an extract (4.5 μg/g) from the halogenated furanone-treated culture displayed a higher survival rate (100%) than mice treated with the same dilution of extract (3.6 μg/g) from an untreated V. harveyi culture (33%). Based on these data, groups of 10 mice were injected with a 1-in-4 dilution (∼4.5 μg/g) of freshly prepared extracts from untreated or furanone-treated cultures and were observed as described above. Table 1 reveals a significant reduction in mortality rates for CBA mice treated with a 1-in-4 dilution of extracts prepared from furanone-treated (90% survival) versus untreated (20% survival) V. harveyi cultures (G = 9.404; df = 1; P = 0.0022).
TABLE 1.
Survival rates of CBA mice and juvenile P. monodon specimens challenged with concentrated cell supernatant extracts prepared from cultures of V. harveyi 47666-1 grown in the presence and absence of 100 μM halogenated furanone
| Subjects | Supernatant extracts from:
|
|||
|---|---|---|---|---|
| Untreated culturea
|
Furanone-treated cultureb
|
|||
| No. surviving/no. injected (% surviving) | Dose (μg/g) | No. surviving/no. injected (% surviving) | Dose (μg/g) | |
| CBA mice | 0/3 (0) | 13.7 | 0/3 (0) | 13.7 |
| 0/3 (0) | 7.5 | 0/3 (0) | 7.7 | |
| 1/3 (33) | 3.6 | 3/3 (100) | 4.5 | |
| 2/10 (20) | 4.4 | 9/10 90) | 4.5 | |
| P. monodon specimens | 0/7 (0) | 16.5 | 0/7 (0) | 14.3 |
| 1/7 (14) | 7.4 | 4/7 (57) | 7.1 | |
| 4/11 (36) | 3.5 | 9/11 (82) | 3.6 | |
| 6/8 (75) | 1.5 | 7/7 (100) | 1.9 | |
Total protein concentration = 3.8 μg/ml.
Total protein concentration = 3.9 μg/ml.
As with the CBA mice, P. monodon specimens were challenged with extracts from furanone-treated and untreated V. harveyi cultures. Significant differences in survival were not observed between prawns injected with pure extracts (∼15 μg/g) or 1-in-2 dilutions of extracts (∼7 μg/g) from untreated or furanone-treated cultures (Table 1). A significant difference was observed, however, when prawns were injected with a 1-in-4 dilution (∼3.5 μg/g) of the extracts (G = 4.487, df = 1, P = 0.1698). Eighty-two percent of prawns challenged with a 1-in-4 dilution of extract from the furanone-treated culture survived the treatment, compared with a 36% survival rate for prawns treated with the equivalent dilution of extract prepared from untreated V. harveyi cultures. No significant differences were observed when 1-in-8 dilutions of the two extracts were used.
The LD50 of the dilution series of extracts from furanone-treated cultures for P. monodon was calculated to be 7.11 μg of protein per g of prawn, which was significantly higher than that with the extract prepared from untreated cultures, calculated to be 3.45 μg/g. These data suggest that the production, stability, or activity of the extracellular virulence factor produced by V. harveyi is reduced by the presence of 100 μM halogenated furanone.
To ensure that this reduction in in vivo toxicity was not the result of the halogenated furanone interfering directly with the stability or activity of the extracellular virulence factor, samples of cell supernatant from an untreated V. harveyi culture were treated with 100 μM halogenated furanone, and others were left untreated. These samples were then concentrated and injected into CBA mice and P. monodon specimens. At all dilutions tested, no significant differences in toxicity for mice or prawns were observed between furanone-treated and untreated cell supernatants (Table 2). This suggests that the halogenated furanone acts on the production of the virulence factor.
TABLE 2.
Survival rates of CBA mice and juvenile P. monodon specimens challenged with concentrated cell supernatant extracts prepared from cultures of V. harveyi 47666-1 and treated postextraction with 100 μM halogenated furanone
| Subjects | Untreated extracta
|
Furanone-treated extractb
|
||
|---|---|---|---|---|
| No. surviving/no. injected (% surviving) | Dose (μg/g) | No. surviving/no. injected (% surviving) | Dose (μg/g) | |
| CBA mice | 0/3 (0) | 10.6 | 0/3 (0) | 10.4 |
| 1/3 (33) | 6.1 | 0/3 (0) | 6.0 | |
| 1/3 (33) | 4.2 | 1/3 (33) | 4.2 | |
| 0/3 (0) | 2.9 | 1/3 (33) | 3.1 | |
| P. monodon specimens | 2/9 (22) | 13.9 | 0/7 (0) | 14 |
| 3/9 (33) | 6.1 | 2/8 (25) | 7.4 | |
| 5/9 (56) | 5.6 | 5/8 (63) | 5.5 | |
| 4/7 (57) | 4.1 | 6/7 (86) | 4.5 | |
| 7/7 (100) | 3.5 | 6/7 (86) | 3.4 | |
Total protein concentration = 4.0 μg/ml.
Total protein concentration = 3.8 μg/ml.
Stimulation of toxin T1 production with the supernatant of a high-density culture.
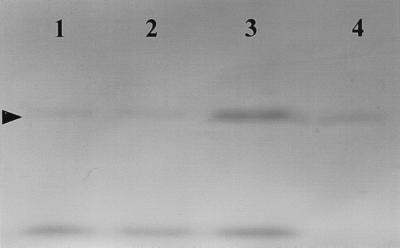
Based on indirect evidence suggesting that toxin T1 production is regulated by the quorum sensing mechanism of V. harveyi, the effects of HBHL and spent-culture supernatants on toxin T1 production were tested. HBHL at 200 nM or 10% conditioned media were added to V. harveyi cultures, which were analyzed by PAGE for the presence of toxin T1 at a low density to check for an early induction. As shown in Fig. 4, toxin T1 was barely detectable in extracts from untreated cultures at low densities (OD600 = 0.1). Culturing in the presence of HBHL did not increase toxin T1 production at this density. Addition of 10% high-density culture supernatant, however, caused production of toxin T1 to be induced even at this low cell density (Fig. 4). This strongly suggests that toxin T1 production is regulated by intercellular signals.
FIG. 4.
Effect of culturing Vibrio harveyi in the presence of 200 nM HBHL and 10% high-density culture supernatant on the appearance of toxin T1 in low-density (OD600 = 0.1) culture supernatants as determined by native PAGE. The gel shows supernatant extract from untreated (OD600 = 0.1) culture (lane 1), supernatant extract from HBHL-treated (OD600 = 0.1) culture (lane 2), supernatant extract from 10% high-density supernatant-treated (OD600 = 0.1) culture (lane 3), and a 10-fold dilution of high-density supernatant extract from untreated (OD600 = 0.4) culture (lane 4). The arrowhead indicates toxin T1.
DISCUSSION
The quorum sensing mechanism regulating luminescence in V. harveyi differs from that of V. fischeri, as initially was indicated by the inability of culture supernatants from either species to stimulate luminescence in the other (5). It was found that intercellular signals (HBHL and AI-2) of V. harveyi do not interact with a transcriptional activator directly but rather stimulate a phosphorelay, which culminates in the deactivation of a transcriptional repressor protein (2, 6).
Because HBHL is unable to stimulate transcriptional activation in the LuxR protein of V. fischeri and because the intercellular signal receptors of V. harveyi are not LuxR homologues, it was of interest to see if halogenated furanones, known to displace N-3-oxohexanoyl-l-homoserine lactone from the LuxR protein of V. fischeri, could interfere with quorum sensing in V. harveyi. This was tested in V. harveyi strain 47666-1, a known pathogen of the commercially farmed prawn species P. monodon.
Structural similarities between HBHL and halogenated furanones suggest that the two molecules could interact with receptor proteins in similar fashions. The halogenated furanone tested has a molecular weight of 309.97, whereas the molecular weight of HBHL is 187.18. Both molecules have acyl chains of four carbons extending from five-membered lactone rings. The differences between the structures are that the algal metabolite possesses a furan ring rather than a homoserine lactone ring and that the two possess different side groups in different positions. HBHL has a ketone and a hydroxyl group extending from carbons 1 and 3 of the acyl chain, respectively. The halogenated furanone has a bromine atom and a brominated exocyclic double bond extending from atoms 4 and 5 of the ring, respectively. Such differences do not render the furanones incapable of antagonizing AHL activity but do reduce their affinity for AHL receptor proteins relative to the native signals (18).
This study reveals that in the presence of the halogenated furanone, the luminescence phenotype of V. harveyi was reduced with no impact on the growth of the organism. This result, in conjunction with similar results in V. fischeri and other bacterial species, may indicate that halogenated furanones possess broader antagonist activity than AHLs. The present study also demonstrates that the production of an extracellular toxin, which appears in the supernatant of V. harveyi cultures concurrently with expression of the luminescence phenotype, is inhibited by the halogenated furanone.
Cell density-dependent expression of virulence factors is not uncommon (18). In support of the putative regulatory link between expression of luminescence and virulence in this organism, we found that the addition of high-density culture supernatant of V. harveyi to low-density cultures causes an early induction of toxin T1 synthesis. HBHL did not have this effect.
If indeed the toxin T1 structural gene, like the lux cassette, is under transcriptional repression by the LuxO protein, then toxin T1 production is dependent on switching the kinase activity of the signal receptors, LuxN and LuxQ, to phosphatase activity (6). Based on the inhibition of toxin T1 production by the halogenated furanone, we initially expected addition of HBHL to affect this switch, causing an early induction of toxin T1 synthesis at low cell density. Closer examination of the V. harveyi quorum sensing mechanism, however, suggested that this would not be the case.
Freeman and Bassler (6) have demonstrated that a luxN mutant has the wild-type capacity to phosphorylate LuxU, producing a wild-type luminescence response, and that a luxQ mutant, in contrast, has much-reduced kinase activity. This suggests that at low cell densities, repression of the lux cassette and any other LuxO-repressed genes is largely mediated by the kinase activity of phosphorylated LuxQ and that derepression requires AI-2. This potentially explains why, at low cell density, toxin T1 production is not responsive to HBHL alone but requires the addition of both signals, in the form of a high-density culture supernatant. Reduced toxin T1 production, via interference with HBHL binding by the halogenated furanone at a higher cell density, is then as expected. Alternatively, the halogenated furanone may exert its effect on luminescence and toxin T1 synthesis through interference with AI-2 activity. This interpretion, however, remains highly speculative, given that the structure of AI-2 is unresolved.
In vivo assays demonstrated that the toxicity of V. harveyi supernatant extracts is reduced when cultures contain the halogenated furanone. This reduction in toxicity was found not to be the result of a direct furanone interaction with the toxin leading to inactivation or degradation; it is most probable that it is due to inhibition of transcription of the toxin T1 structural gene. These data suggest that there exists potential for the use of furanones to limit V. harveyi infections in P. monodon. Delivery and efficacy of the algal metabolites at the site of V. harveyi infection in P. monodon are currently under investigation.
The results of this study have shown that a halogenated furanone from the alga D. pulchra inhibits the concurrent expression of luminescence and toxin production in the prawn pathogen V. harveyi. Additionally, toxin production was found to be induced at low density by a high-density culture supernatant. Besides the potential biotechnological application, these findings suggest that virulence in V. harveyi, like luminescence, is at least partially regulated by the quorum sensing mechanism.
ACKNOWLEDGMENTS
Equal contributions to this study were made by Lachlan Harris and Michael Manefield, and thus both are recognized as primary authors of this publication.
This research was funded by the Cooperative Research Centre for Aquaculture. Funding support was also given by the Australian Research Council and the Centre for Marine Biofouling and Bio-Innovation. Lachlan Harris was funded by a Cooperative Research Centre for Aquaculture scholarship, and Rocky de Nys was funded by an ARC postdoctoral fellowship.
The contributions of William Lao and Naresh Kumar to this study are gratefully acknowledged.
REFERENCES
- 1.Bassler B L. A multichannel two-component signaling relay controls quorum sensing in Vibrio harveyi. In: Dunny G M, Winans S C, editors. Cell-cell signaling in bacteria. Washington, D.C.: American Society for Microbiology; 1999. pp. 259–273. [Google Scholar]
- 2.Bassler B L, Wright M, Showalter R E, Silverman M R. Intercellular signaling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol Microbiol. 1993;9:773–786. doi: 10.1111/j.1365-2958.1993.tb01737.x. [DOI] [PubMed] [Google Scholar]
- 3.Cao J-G, Meighen E A. Biosynthesis and stereochemistry of the autoinducer controlling luminescence in Vibrio harveyi. J Bacteriol. 1993;175:3856–3862. doi: 10.1128/jb.175.12.3856-3862.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Nys R, Wright A D, Konig G M, Sticher O. New halogenated furanones from the marine alga Delisea pulchra (cf. fimbriata) Tetrahedron. 1993;49:11213–11220. [Google Scholar]
- 5.Eberhard A. Inhibition and activation of bacterial luciferase synthesis. J Bacteriol. 1972;109:1101–1105. doi: 10.1128/jb.109.3.1101-1105.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freeman J, Bassler B L. A genetic analysis of the function of LuxO, a two-component response regulator involved in quorum sensing in Vibrio harveyi. Mol Microbiol. 1999;31:665–677. doi: 10.1046/j.1365-2958.1999.01208.x. [DOI] [PubMed] [Google Scholar]
- 7.Freeman J A, Bassler B L. Sequence and function of LuxU: a two-component phosphorelay protein that regulates quorum sensing in Vibrio harveyi. J Bacteriol. 1999;181:899–906. doi: 10.1128/jb.181.3.899-906.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuqua C, Winans S C, Greenberg E P. Census and consensus in bacterial ecosystems: the LuR-LuxI family of quorum sensing transcriptional regulators. Annu Rev Microbiol. 1996;50:727–751. doi: 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- 9.Givskov M, de Nys R, Manefield M, Gram L, Maximilien R, Eberl L, Molin S, Steinberg P D, Kjelleberg S. Eukaryotic interference with homoserine lactone-mediated prokaryotic signaling. J Bacteriol. 1996;178:6618–6622. doi: 10.1128/jb.178.22.6618-6622.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris L J, Owens L. Production of exotoxins by two luminous Vibrio harveyi strains known to be primary pathogens of Penaeus monodon larvae. Dis Aquat Org. 1999;38:11–22. [Google Scholar]
- 11.Hoyt P R, Sizemore R K. Competitive dominance by a bacteriocin-producing Vibrio harveyi strain. Appl Environ Microbiol. 1982;44:653–658. doi: 10.1128/aem.44.3.653-658.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiravanichpaisal P, Miyazaki T, Limsuwan C. Histopathology, biochemistry and pathogenicity of Vibrio harveyi infecting black tiger prawn Penaeus monodon. J Aquat Anim Health. 1994;6:27–35. [Google Scholar]
- 13.Jones S, Yu B, Bainton N J, Birdsall M, Bycroft B W, Chahabra S R, Cox A J R, Golby P, Reeves P J, Stephens S, Winson M K, Salmond G P C, Stewart G S A B, Williams P. The lux autoinducer regulates the production of exoenzyme virulence determinants in Erwinia carotovora and Pseudomonas aeruginosa. EMBO J. 1993;12:2477–2482. doi: 10.1002/j.1460-2075.1993.tb05902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karunasagar I, Pai R, Malathi G R, Karunasagar I. Mass mortality of Penaeus monodon larvae due to antibiotic-resistant Vibrio harveyi infection. Aquaculture. 1994;128:203–209. [Google Scholar]
- 15.Laemmli U K. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Lavilla-Pitogo C R, Baticados M C L, Cruz-Lacierda E R, de la Pena L D. Occurrence of the luminous bacterial disease of Penaeus monodon larvae in the Philippines. Aquaculture. 1990;91:1–13. [Google Scholar]
- 17.Makemson J C, Hastings J W. Luciferase-dependent growth of cytochrome-deficient Vibrio harveyi. FEMS Microbiol Ecol. 1986;38:79–85. [Google Scholar]
- 18.Manefield M, de Nys R, Kumar N, Read R, Givskov M, Steinberg P D, Kjelleberg S. Evidence that halogenated furanones from Delisea pulchra inhibit acylated homoserine lactone (AHL)-mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology. 1999;145:283–291. doi: 10.1099/13500872-145-2-283. [DOI] [PubMed] [Google Scholar]
- 19.Pizzutto M, Hirst R G. Classification of isolates of Vibrio harveyi virulent to Penaeus monodon larvae by protein profile analysis and M13 DNA fingerprinting. Dis Aquat Org. 1995;21:61–68. [Google Scholar]
- 20.Ramesh A, Loganathan B G, Venugopalan V K. Seasonal distribution of luminous bacteria in the sediments of a tropical estuary. J Gen Appl Microbiol. 1989;35:363–368. [Google Scholar]
- 21.Reichelt J L, Baumann P. Taxonomy of the marine luminous bacteria. Arch Microbiol. 1973;94:283–330. [Google Scholar]
- 22.Ruby E G, Morin J G. Luminous enteric bacteria of marine fishes: a study of their distribution, densities, and dispersion. Appl Environ Microbiol. 1979;38:406–411. doi: 10.1128/aem.38.3.406-411.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevens A M, Greenberg E P. Quorum sensing in Vibrio fischeri: essential elements for activation of the luminescence genes. J Bacteriol. 1997;179:557–562. doi: 10.1128/jb.179.2.557-562.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swartzman E, Silverman M, Meighen E A. The luxR gene product of Vibrio harveyi is a transcriptional activator of the lux promoter. J Bacteriol. 1992;174:7490–7493. doi: 10.1128/jb.174.22.7490-7493.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]