Abstract
Disturbances that threaten homeostasis elicit activation of the sympathetic nervous system (SNS) and the adrenal medulla. The effectors discharge as a unit to drive global and immediate changes in whole-body physiology. Descending sympathetic information is conveyed to the adrenal medulla via preganglionic splanchnic fibers. These fibers pass into the gland and synapse onto chromaffin cells, which synthesize, store, and secrete catecholamines and vasoactive peptides. While the importance of the sympatho-adrenal branch of the autonomic nervous system has been appreciated for many decades, the mechanisms underlying transmission between presynaptic splanchnic neurons and postsynaptic chromaffin cells have remained obscure. In contrast to chromaffin cells, which have enjoyed sustained attention as a model system for exocytosis, even the Ca2+ sensors that are expressed within splanchnic terminals have not yet been identified. This study shows that a ubiquitous Ca2+-binding protein, synaptotagmin-7 (Syt7), is expressed within the fibers that innervate the adrenal medulla, and that its absence can alter synaptic transmission in the preganglionic terminals of chromaffin cells. The prevailing impact in synapses that lack Syt7 is a decrease in synaptic strength and neuronal short-term plasticity. Evoked excitatory postsynaptic currents (EPSCs) in Syt7 KO preganglionic terminals are smaller in amplitude than in wild-type synapses stimulated in an identical manner. Splanchnic inputs also display robust short-term presynaptic facilitation, which is compromised in the absence of Syt7. These data reveal, for the first time, a role for any synaptotagmin at the splanchnic-chromaffin cell synapse. They also suggest that Syt7 has actions at synaptic terminals that are conserved across central and peripheral branches of the nervous system.
Keywords: Synaptotagmin-7, Synaptotagmin, Preganglionic synapses, Adrenal medulla
1. Introduction
The adrenal medulla is a core effector of the sympathetic nervous system in the periphery [8]. When activated, it discharges a cocktail of powerful hormones (epinephrine, norepinephrine, and vasoactive peptides) into the suprarenal vein for circulation throughout the body [51]. These hormones modulate cardiac, pulmonary, and metabolic functions in ways that favor survival or preserve internal conditions when they are likely to be disturbed [8,16,17]. Thus, many of the “emergency” measures encompassed in the term fight-or-flight, rely on the adrenal medulla.
Secretion from the adrenal medulla is dependent on input from preganglionic, sympathetic fibers which pass into the gland via the splanchnic nerves [14,18]. The fibers terminate on adrenomedullary chromaffin cells which secrete hormones contained in dense core granules [9]. Due to their experimental accessibility, a great deal is now known about the mechanisms underlying dense core granule exocytosis in chromaffin cells [1,3]. On the other hand, little is known about the molecular operation of exocytosis in splanchnic neurons.
The purpose of this study was to characterize the role of one synaptic protein in particular – synaptotagmin-7 (Syt7) – in regulating exocytosis at the splanchnic-chromaffin cell synapse. Syt7 belongs to a large family of proteins, numbering 17 in total, many of which couple calcium influx to vesicle fusion [40,44,45]. Our interest in Syt7 at splanchnic terminals has developed because of recent discoveries concerning its function at central nervous system (CNS) synapses. There, Syt7 is now generally acknowledged to regulate synaptic transmission in important ways, and to be required for forms of synaptic plasticity that are driven by subtle variations in calcium levels, including asynchronous release and facilitation [2,29,35,37,38,46]. To date, no synaptotagmins have been identified in splanchnic neurons.
The experiments described here were performed on adrenal slices obtained from wild-type (WT) animals or animals in which the Syt7 gene had been deleted (hereafter referred to as Syt7 KO) [11]. The absence of Syt7 had several readily identifiable effects on splanchnic synaptic transmission, which was evoked by stimulating preganglionic input to chromaffin cells via electrical stimulation. Specifically, evoked EPSCs in KO slices are smaller in amplitude than those in WT slices. Decreases in the coefficient of variation (CV −2) in evoked EPSCs in KO slices compared to WT, without changes in the decay, is consistent with a presynaptic modulation of acetylcholine release probability that involves Syt7. Moreover, facilitation, which is ordinarily a robust property of this synapse, was abrogated in the absence of Syt7. The magnitude of tonic currents on which synchronous EPSCs are imposed was substantially smaller in KO synapses than in WT synapses. These functional data are supported by fluorescent imaging of adrenal sections, in which Syt7-positive puncta are found to be associated with a marker of splanchnic neurons, choline acetyltransferase (ChAT).
In sum, we demonstrate here that Syt7 is indeed expressed within the splanchnic fibers that innervate the adrenal medulla and has a role in evoked release and in facilitation at their synaptic terminals. These data are the first to document a function for Syt7 in regulating short-term synaptic plasticity in the autonomic nervous system.
2. Materials and methods
2.1. Animals
Litters of adult male and female Syt7−/− (gift of Dr. Joel Swanson; [11] and Syt7+/+ (from a C57BL/6J background and obtained from Jackson Labs, Bar Harbor, ME) were used in these studies. Animals were group housed (2 to 5 per ventilated cage) with 24 hr (12/12 dark/light cycle) access to food and water. All animal procedures and experiments were conducted in accordance with the Institutional Animal Care and Use Committee. No randomization was performed to allocate subjects in the study.
2.2. In situ electrophysiology recordings and analysis
Syt7 +/− mice were crossed to generate Syt7−/− or +/+ littermates. Mice were genotyped according to instructions provided by Jackson Labs (https://www.jax.org/strain/004950). All electrophysiological studies were performed on littermates by experimenters blinded to the genotype of the animal.
3–4-month-old animals (male and female; 18 to 25 g) were gas anesthetized using an isoflurane drop jar technique and sacrificed by guillotine decapitation (all procedures are in accordance with approved UM IACAC protocol PRO000009265). Chromaffin cells are responsible for releasing catecholamines in response to stress, including hypoxia. Hence, isoflurane is used to induce a faster loss of consciousness compared to CO2 euthanasia (30 s to 1 min versus several minutes) and reduce animal stress.
Adrenal glands were then quickly removed from the kidney and placed in ice cold (4 °C) slicing solution containing, in mM: 62.5 NaCl, 2.5 KCl, 1.25 KH2PO4, 26 NaHCO3, 5 MgCl2, 0.5 CaCl2, 20 Glucose and 100 Sucrose (pH maintained at 7.4 by saturation with O2/CO2, 95/5% respectively) at an osmolarity of ~ 315 milliosmolar (mOsm). Glands were subsequently embedded in 3.5 % agarose block solution at 4 °C. Approximately 300 μm thick sections were cut with a microtome (VF-300, CompresstomeTM; Precisionary instruments, Natick MA). Slices were transferred to a stabilization chamber where they were maintained at room temperature for 60 min in artificial cerebrospinal fluid (ACSF) containing in mM: 125 NaCl, 2.5 KCl, 1.25 KH2PO4, 26 NaHCO3, 1 MgCl2, 2 CaCl2 and 20 Glucose, pH 7.4 (with 95 % O2 and 5 % CO2 bubbling through the solution, ~300 mOsm). Then individual slices were transferred to the microscope to the recording chamber (~300 μL volume) continuously super-fused with ACSF (1–2 mL/min) at room temperature.
The adrenal gland was visualized at in the microscope (Nikon Eclipse FN-1) at X10 to determine the recording and stimulation areas. The cholinergic nerve terminals of preganglionic neurons were activated using a focal stimulating FHC tungsten metal electrode (2–3 MΩ). The electrode was placed in the border between the adrenal cortex and the adrenal medulla around 50–100 μm away from the recording pipette.
Recording micropipettes were pulled (P-97; Sutter Instruments, Novato, CA) from borosilicate glass capillaries (1.5 mm O.D.; Harvard Apparatus, Holliston, MA) for a final resistance of 3–6 MΩ. Pipettes were filled with a cesium-based internal solution of the composition in mM: 135 CsCl, 4 NaCl, 0.4 GTP, 2 Mg-ATP,0.5 CaCl2, 5 EGTA and 10 HEPES pH 7.25 – 7.3 (290 mOsm). Currents were recorded with Axon Instruments, Multiclamp 700B (Axon Instruments, Union City, CA), low pass filtered at 2 kHz. Chromaffin cells in medullary slices were identified using a Nikon Eclipse FN-1 microscope with a X40 water-immersion objective and a DAGE-MTI IR-1000 video camera. Whole-cell recordings (>8 GΩ before break-in) were obtained in voltage-clamp configuration, acquired at 2 kHz fixing the voltage at −20 mV. Series resistance was monitored throughout the experiment and experiments were aborted if changes >20 % occurred. The cells were chosen according to the access resistance and visual examination of their membranes. The EPSCs were evoked by stimulating the preganglionic input at 0.1 Hz and were distinguished by their all-or-none response to presynaptic stimulation and fast kinetics [4,22,50]. The reciprocal of the squared coefficient of variation (CV) of the synaptic response amplitude was quantified as (CV) −2 = 1/[(SD/mean)2] [12,33,50]. Once a “synapse” was identified, to evaluate short-term synaptic plasticity the preganglionic input was stimulated at 5–50 V intensity (0.5 ms pulse duration) every 15 s at intervals of 60, 100, 200 and 500 ms or during high frequency trains (20 Hz). In some experiments the calcium concentration in the ACSF was reduced 2 mM to 0.5 mM, to avoid calcium saturation. Stimulation waveforms were introduced via a Grass S48 stimulator (Quincy, MA) that was triggered using Clampex9 software. Paired-pulse ratio (PPR) of EPSCs were calculated by dividing the amplitude of the second EPSC2 by that of the first EPSC1 (PPR = EPSC2/EPSC1); Differences assessed by PPR parameter will indicate changes in neurotransmitters release mediated presynaptically [7,32,52]. For experiments involving the application of hexamethonium (hexane-1,6-bis (trimethylammonium bromide)) a non-depolarizing nicotinic acetylcholine receptor (nAChR) antagonist at concentration of 100 μM was added to the bath, and stimulation was conducted at 0.1 Hz (unless otherwise indicated) the slice was perfused with ACSF (1–2 mL/min) in presence of the drug for 5 min before washout and for before 5 min to obtain a baseline response. Peak current amplitudes were searched identified manually using pCLAMP 10 (Molecular Devices, San Jose, CA), and visually monitored to exclude the erroneous noise. The current response was fit by a single exponential equation to obtain the time constant (tau) of decay. Tonic current (during high frequency stimulation) is measured as the difference between the sustained currents reached during the train and the overall baseline current of the record [38].
2.3. Immunofluorescence
3–4 month-old mice (male and female) were anaesthetized in a drop jar with 200 μL isoflurane and subsequently euthanized by cervical dislocation. After cardiac perfusion with PBS, adrenal glands were removed and trimmed. Glands were immediately immersed in OCT compound and snap-frozen with dry ice. Glands were sectioned to 8 mm on a cryostat. Adrenal sections were fixed with ice-cold methanol for 20 min and washed 3x with PBS. Following fixation, slices were permeabilized using 0.5 % triton X-100 in PBS for 15 min. Slices were then blocked with 20 % horse serum and 0.1 % tween in PBS for one hour, before incubating with primary antibodies (rabbit anti-Syt-7 (SySy, Goettingen, Germany), guinea pig anti-ChAT (SySy, Goettingen, Germany)) at a 1:100 dilution and at 4 °C overnight. The next day, slices were washed 3× for 15 min each with PBST and incubated with secondary antibodies diluted in blocking buffer for 1hr at room temperature. Fluorescently conjugated secondary antibodies Alexa 488 and 647 (1:400, ThermoFisher, Waltham, MA) were used as secondaries. Sections were then washed three times with PBST for 15 min each and mounted on glass slides with DAPI Fluoromount-G (SouthernBiotech, Birmingham, AL).
Imaging was performed using a Leica TCS SP5 Multiphoton laser scanning confocal microscope with a 63x oil immersion objective. The images were taken in a sequential scan mode. Sections were excited by 405-, 488-, and 633-nm lasers. All images were further processed in Adobe CS6 Photoshop Software.
2.4. Statistical analysis
All data were analyzed using GraphPad Prism (Version 8.0) Software, San Diego CA, USA. The Shapiro-Wilk test was used to ensure normal (Gaussian) distribution of the samples followed by a Bartlett’s test to check the homogeneity of the variances based on the means. Changes in amplitude of EPSC and PPR were analyzed using two-way ANOVA followed by Sidak’s multiple comparisons test to compare differences between experimental conditions, or one-way ANOVA following by Dunn’s multiple comparisons test, as appropriate. When only two conditions were compared Student’s t-test or Mann-Whitney test was used. A significance level of 0.05 was used for all statistical tests. For box and whisker plots, boxes represent the 25–75 % confidence interval, horizontal lines are the median value, the plus symbol represents the mean, and the whiskers show the full data range.
3. Results
3.1. Synaptotagmin-7 regulates exocytosis at the splanchnic-chromaffin cell synapse
To test the contribution of Syt7 in splanchnic-chromaffin cell synapse and determine whether a presynaptic mechanism was involved, we measured cholinergic synaptic responses in adrenal gland slices. A stimulation electrode was placed at the border between the cortex and medulla in an adrenal section prepared as described in the Methods (Fig. 1A). Electrical pulses were applied to stimulate splanchnic processes while recording from chromaffin cells, which were voltage-clamped in the whole-cell configuration. Excitatory postsynaptic currents (EPSCs), evoked by splanchnic stimulation, were measured in glands obtained from both WT and Syt7 KO animals (Fig. 1). These currents are reversibly inhibited by the nicotinic receptor antagonist, hexamethonium (Fig. 1B) [50]. The amplitude of evoked EPSCs and synaptic charge transfer were significantly smaller in slices that lack Syt7 compared to WT slices (Fig. 1C–E). No difference was detected in the single exponential decay time constant of the EPSC (Fig. 1F), which indicated that the currents were mediated by a similar pool of acetylcholine receptors [4,24]. Because single exponential fits of EPSC decay may not reflect changes in asynchronous release, we estimated the skewness of the data by normalizing charge transfer to peak EPSC amplitude (Fig. 1G). This analysis did not reveal any additional differences between WT and Syt7 KO preparations. The inverse of the coefficient of variation (CV −2) of synaptic current amplitude was smaller in Syt7 KO than WT adrenals (Fig. 1H). This is consistent with a presynaptic modulation of neurotransmitter release probability, but not quantal content [12,33,50].
Fig. 1. Comparison of evoked EPSCs in WT and Syt7 KO synapses.
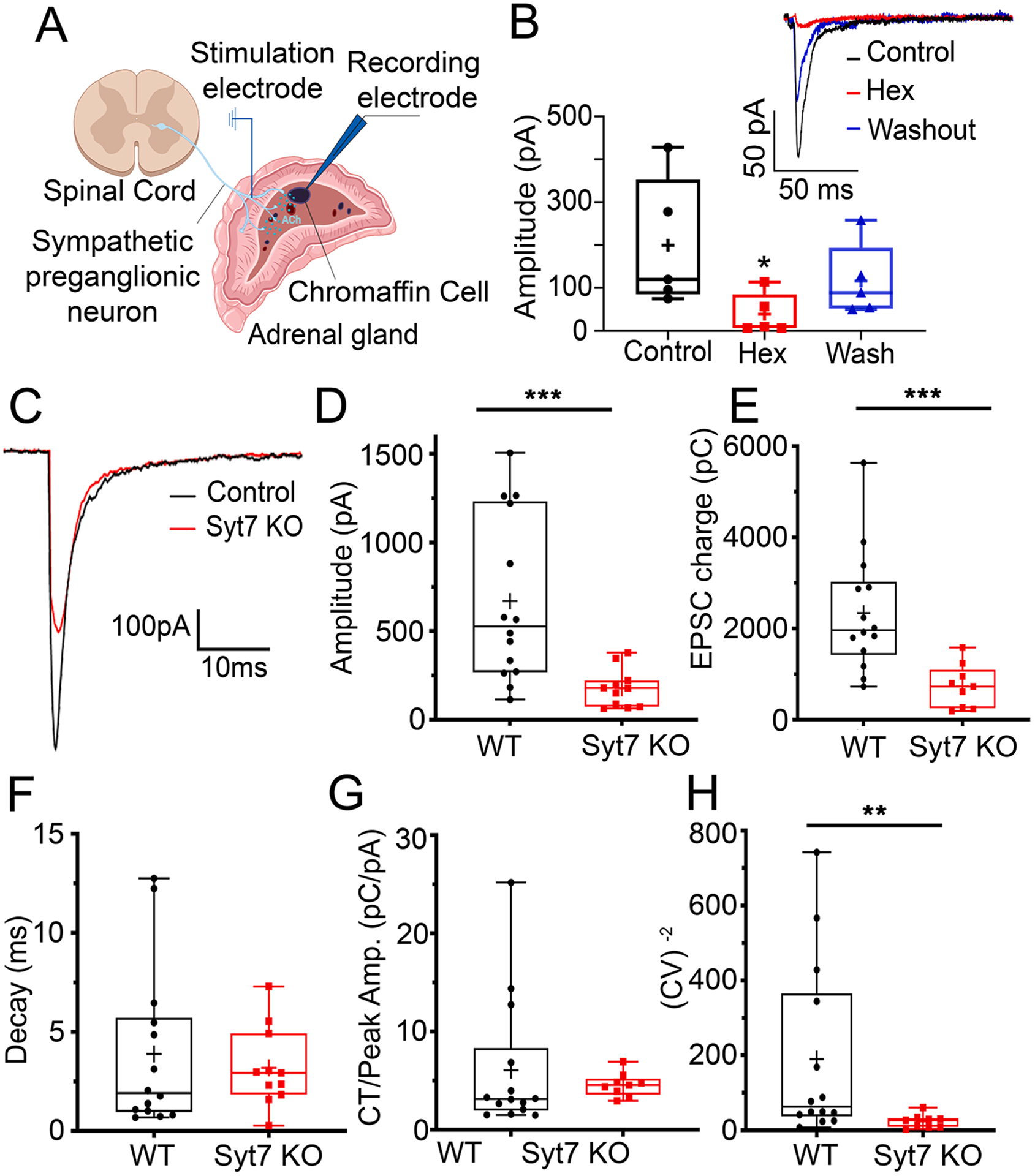
EPSCs were evoked by stimulating preganglionic input to the adrenal medulla with a bipolar stimulating electrode. Models created with BioRender (https://www.biorender.com). B. EPSCs recorded in a chromaffin cell evoked by stimulating the preganglionic nerve terminals (black) were blocked by the cholinergic antagonist hexamethonium (red), and recovered during washout (blue), *p = 0.032, Kruskal-Wallis, Dunn’s multiple comparisons (n = 5 slices; 3 independent preps). Representative EPSCs at WT synapses: Control (black), during block by Hexamethonium (Hex, red), and after Washout (blue) (inset). C. EPSCs recorded in chromaffin cells by stimulating the WT (black) and Syt7 KO (red) preganglionic nerve terminals. D. Averaged peak amplitudes of evoked EPSCs from Syt7 KO (red) are decreased compared to WT (black). ***p < 0.001, Mann Whitney test (n = 14 wt and n = 9 KO slices; > 6 independent preps). E. Average EPSC charge transfer from Syt7 KO (red) mice are decreased compared to WT (black) mice. ***p < 0.001, Mann Whitney test (n = 14 wt and n = 9 KO slices; > 6 independent preps) F. Average decay time constants of evoked EPSCs in chromaffin cells after stimulation of WT (black) and Syt7 KO (red) axons. p = ns, Mann Whitney test (n = 14 wt and n = 9 KO slices; >6 independent preps). G. Charge transfer normalized to peak EPSC amplitude from evoked EPSCs are not different in WT (black) mice compared to Syt7 KO (red) mice. p = ns, Mann Whitney test (n = 14 wt and n = 9 KO slices; >6 independent preps). H. CV−2 of the EPSC amplitude was significantly greater in chromaffin cells from WT (black) compared to Syt7 KO (red) mice. **p < 0.05, Mann Whitney test (n = 14 wt and n = 9 KO slices; > 6 independent preps, those records were obtained at 2 mM external calcium).
3.2. Paired-pulse facilitation is eliminated in synapses lacking Syt7
Syt7 has been reported to function as a specialized calcium sensor that mediates synaptic facilitation in several types of synapses in the brain [29,47–48]. Next, we tested whether Syt7 serves a similar function within splanchnic nerve terminals. Fig. 2 shows that facilitation is indeed a property of synapses within the adrenal medulla. This was demonstrated by applying two successive depolarizing pulses and calculating the paired-pulse ratio (PPR, the amplitude of the second EPSC divided by the amplitude of the first). Interstimulus intervals (ISIs) ranging from 60 ms to 200 ms consistently resulted in PPRs above 1 (Fig. 2A). On the other hand, PPRs above 1 were not observed at synapses lacking Syt7, irrespective of the ISI (Fig. 2A). To rule out that this was not a consequence of the first pulse releasing so much transmitter that the terminals were already partly exhausted, we reduced evoked release by lowering extracellular calcium from 2.0 mM to 0.5 mM [29] (Fig. 2B). Even under these conditions of low release probability, Syt7-deficient synapses did not exhibit facilitation.
Fig. 2. Synaptic facilitation is evident in WT but not Syt7 KO medullae.
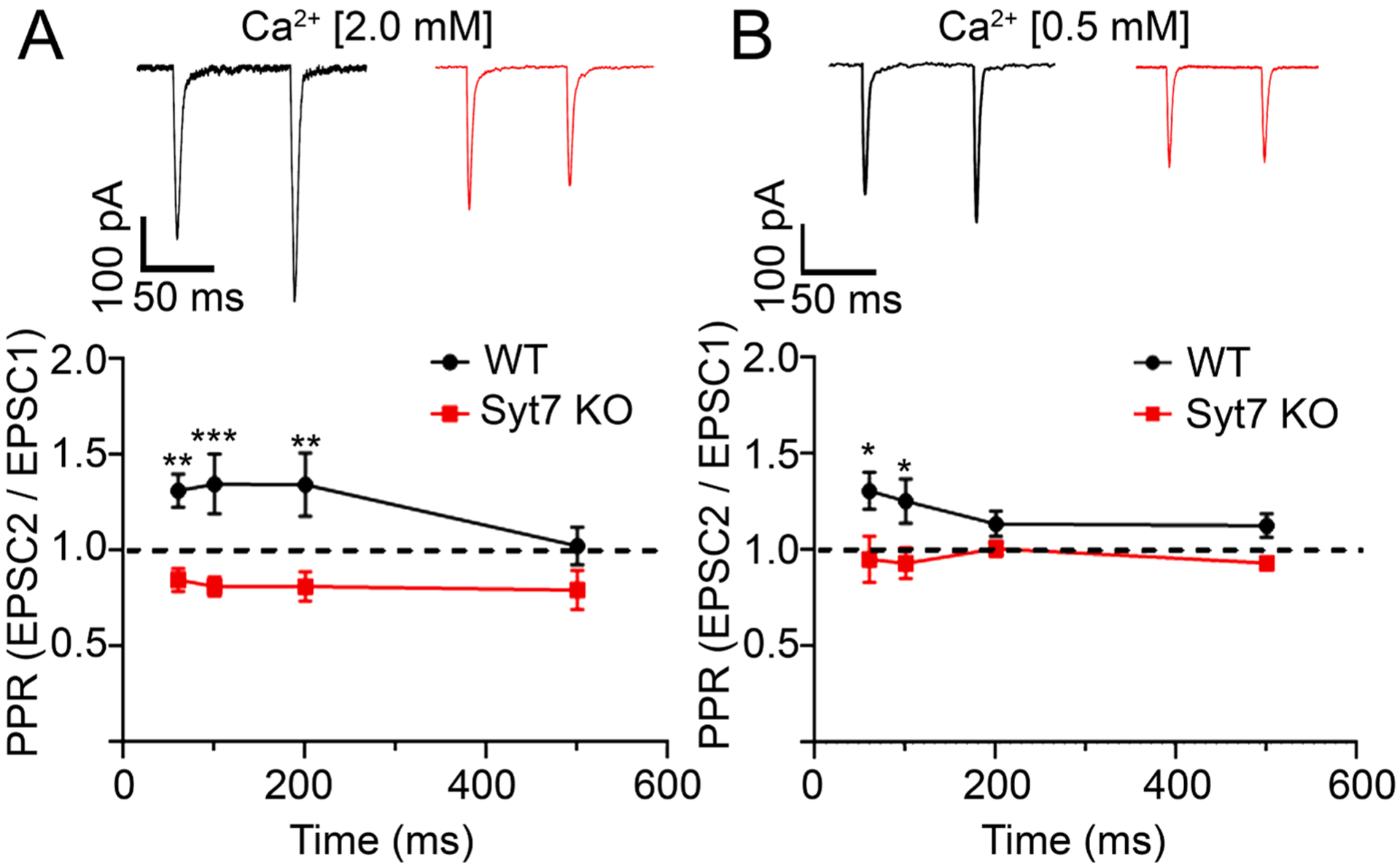
A. Representative traces (top) and averaged paired-pulse ratio (PPRs) ± SEM from evoked EPSCs at different interstimulus intervals (ISIs). PPRs at WT (black) and Syt7 KO (red) synapses are significantly different at ISIs of 60, 100 and 200 ms intervals but not at 500 ms. **p = 0.002, ***p < 0.001, Two-way ANOVA (n = 13 wt and n = 13 KO slices; >6 independent preps). B. Experiments were repeated at low extracellular Ca2+ (0.5 mM) and PPRs calculated as in A. *p = 0.01, Two-way ANOVA (n = 13 wt and n = 15 KO slices; >6 independent preps). Experiments at different calcium concentrations were performed on independent samples.
3.3. High frequency trains result in facilitating EPSCs in WT but not Syt7 KO synapses
The splanchnic fibers that innervate the medulla can vary dramatically in the rate at which they fire, depending on the level of sympathetic activity [13,19]. To model “full activation” of the medulla, a 20 Hz stimulus train was applied to WT and Syt7 KO splanchnic fibers [13]. Under these experimental conditions, two components are distinguishable: a phasic component consisting of the synchronous EPSCs and a tonic component on top of which the synchronous EPSCs ride [2,35,36,37,41,42]. The amplitude of the synchronous EPSCs increased monotonically during the stimulus train (Fig. 3A, C). The net increase in EPSC size may arise from several factors, including facilitation, receptor desensitization, and spillover [10]. Conversely, high frequency stimulus trains applied to Syt7 KO fibers result in a net decrease in EPSC size in chromaffin cells (Fig. 3B, C, and E). Another notable difference between the records shown in Fig. 3A and 3B is that the tonic current, is markedly smaller in the absence of Syt7 (Fig. 3D). The PPR, obtained by dividing the second EPSC in the train by the first, is also smaller at Syt7KO synapses compared to WT synapses (Fig. 3E).
Fig. 3. Tonic current is reduced at Syt7 KO synapses.
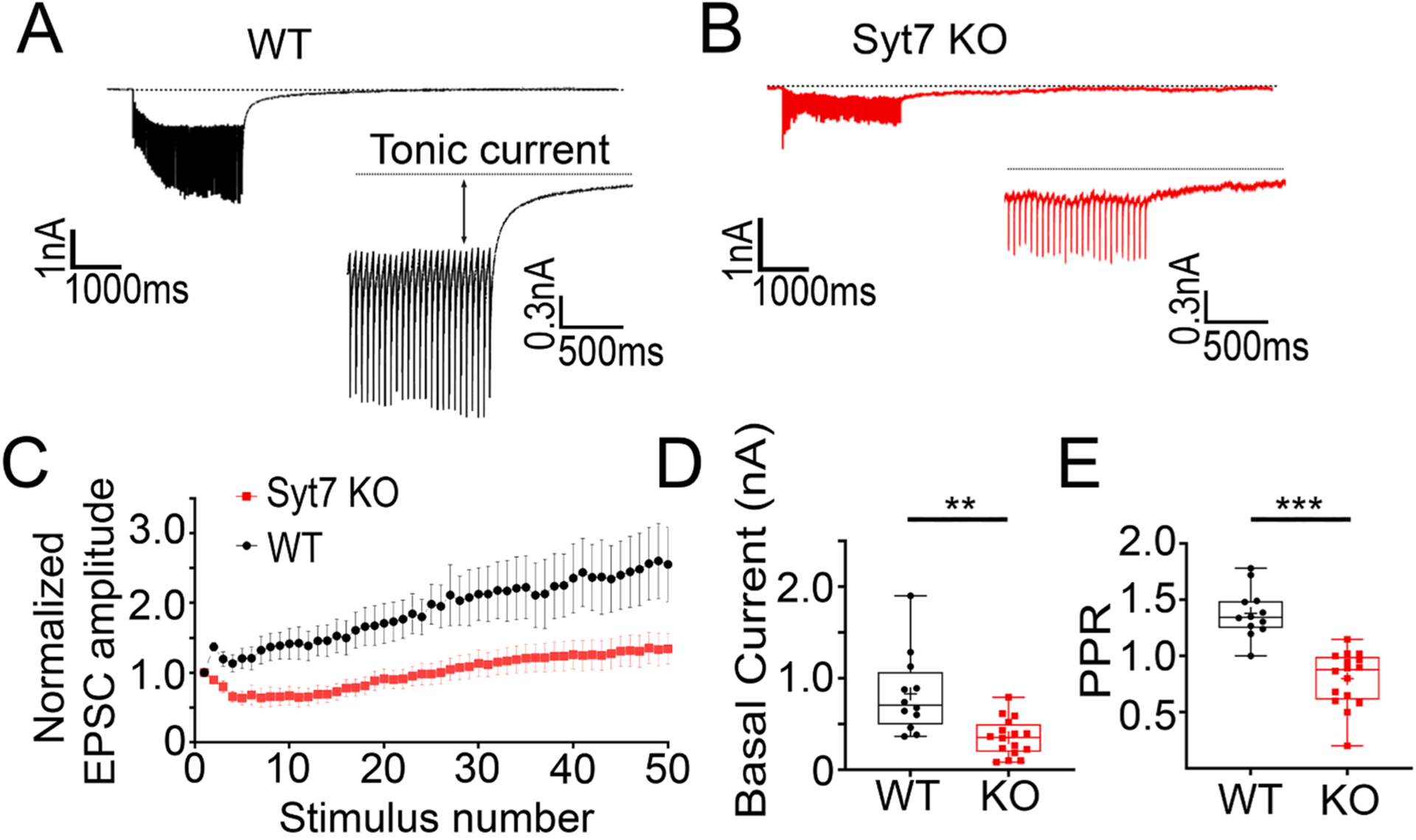
A, B. Synaptic responses to 20 Hz stimulation recorded from WT (black) and Syt7 KO (red) preparations. Expanded traces show the tonic current. C. Summary graph of individual EPSC amplitudes normalized to the first EPSC amplitude during a train (WT in black and Syt7 KO in red). D. Summary graph of the average tonic current, tonic current amplitudes was significantly greater in chromaffin cells from WT (black) compared to Syt7 KO (red) mice***p < 0.001, t = 3.840, df = 26 Student’s t-test (n = 12 wt and n = 16 KO slices; > 6 independent preps). (n = 7 wt and n = 11 KO slices; >4 independent preps). E. Average of PPRs calculated by dividing the second EPSC in the train by the first is. Facilitation is reduced in in chromaffin cells from Syt7 KO (red) compared with WT(black) mice ***p = 0.005, t = 6.515, df = 26 Student’s t-test (n = 12 wt and n = 16 KO slices; >6 independent preps.
3.4. Synaptotagmin-7 is present in preganglionic, cholinergic axons that innervate the adrenal medulla
Aspects of synaptic transmission in the adrenal medulla are compromised in animals lacking Syt7 (Figs. 1–3). Therefore, it was important to verify that Syt7 is present in the neurons responsible for releasing ACh onto chromaffin cells. To this end, immunocytochemical imaging was performed on adrenal sections which were exposed to antibodies for choline acetyltransferase (ChAT) – to label ACh-producing neurons – in addition to Syt7.
In WT medullae, Syt7 exhibits a wide distribution (Fig. 4A, C). On the other hand, Syt7-positive fluorescence in KO medullae is barely detectable (Fig. 4A, C). Syt7 is frequently colocalized with ChAT-positive axon fibers (Fig. 4B, 4E). An example of co-local Syt7 and ChAT is illustrated in Fig. 4D. Syt7 puncta are boxed in yellow within ChAT axons whose boundaries are indicated by the dashed, white lines. To demonstrate that such regions of fluorescent overlap are unlikely to occur at random, Syt7 fluorescence was measured in a different field within the region masked by the ChAT object, as defined in Fig. 4D. This process was repeated in many pairs of images and used to generate the data presented in Fig. 4E.
Fig. 4. Syt7 is expressed in splanchnic processes and chromaffin cells in the adrenal medulla.
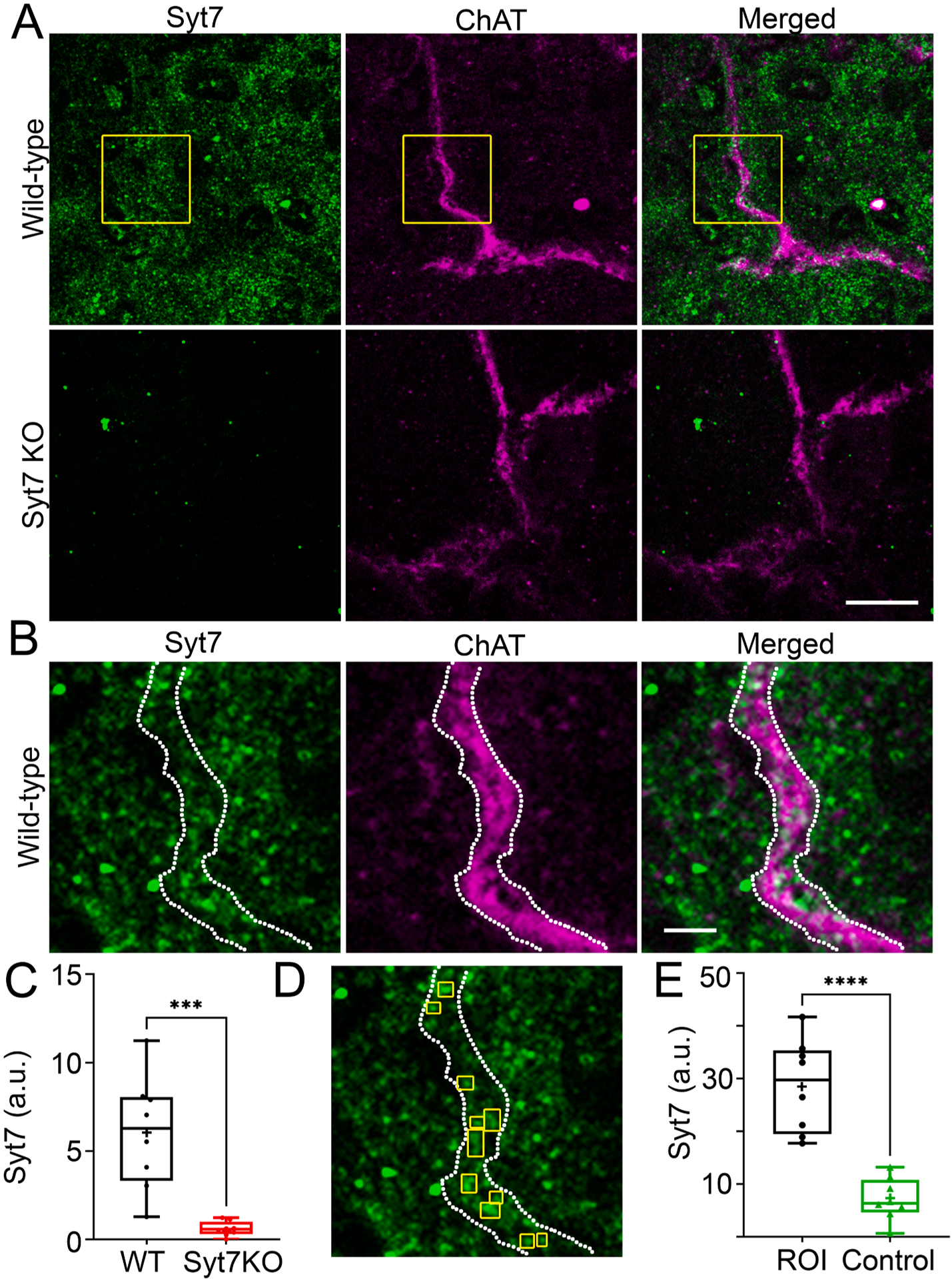
A. A WT and Syt7 KO adrenal sections were stained with antibodies against Syt7 and ChAT, then imaged as shown. B. Boxed region in A is expanded. C. Fluorescence emission from the Syt7 channel was averaged across 8 wt and KO fields. Significantly less Syt7-positive fluorescence was detected in KO sections compared to WT sections. *** p < 0.001 Student’s t test, t = 4.765, df = 14 (n = 8 wt and n = 8 KO sections; 2 independent preps) D. The fluorescence intensity of Syt7 in discrete ROIs within a ChAT-positive object (i.e., axon) was measured. These ROIs were positioned in an image of a different Syt7-labeled field. Syt7 fluorescence was measured in these ROIs and used to define the null hypothesis (labeled “Control”). This process was repeated across 8 pairs of images on separate experimental days from sections obtained from two WT mice. E. The experimental distribution of intensities was compared to the null distribution of intensities. ****p < 0.0001 Student’s t test, t = 6.281, df = 14 (n = 8 ROI and n = 8 Ctrl images; 2 independent preps).
4. Discussion
We have shown here that a ubiquitous Ca2+-binding protein, Syt7, is expressed within the neurons that innervate the adrenal medulla. These data are the first to implicate a role for any synaptotagmin in regulating neurotransmission at the splanchnic-chromaffin cell synapse. As is the case in the CNS, the functions of Syt7 in the periphery are closely tied to a property that sets it apart from the other Ca2+-binding members of the synaptotagmin family – its exceptionally high affinity for Ca2+ [6,27]. What situations might demand such a Ca2+ sensor? Two closely related forms of synaptic plasticity are thought to rely on submicromolar Ca2+ – asynchronous release and facilitation [30,43]. Facilitation is a robust property of the splanchnic-chromaffin cell synapse (Fig. 2). And, consistent with published studies in central synapses [29,47], facilitation is prevented in the absence of Syt7, whether it is driven by a pair of closely spaced depolarizing pulses, or a high frequency stimulus train (Figs. 2 and 3). It remains to be seen whether Syt7is involved in regulating asynchronous release as our experiments did not directly test this possibility.
The properties of unitary currents we describe here are consistent with those previously reported in identical gland preparations [4,25,31,50]. However, the decrease in evoked EPSCs amplitude at KO compared to WT medullae differs from previous studies with respect to the impact of Syt7 on baseline synaptic responses [29,35]. Differences were not detected in the decay time constant of EPSCs evoked by a single stimulus (Fig. 1F). This suggests that a similar group of receptors is being activated in both WT and Syt7 KO medullae. The similar decay time courses indicate that transmitter vesicles are released with a high degree of synchrony with stimulation, irrespective of Syt7 expression. The fact that evoked currents are prevented by hexamethonium indicate that synaptic stimulation activates nicotinic receptors in both cases [31,50]. Thus, differences in the amplitude of stimulated currents are most likely due to alterations in the amount of neurotransmitter coming from presynaptic terminals [41,42].
There are number of potential explanations for the difference in baseline EPSCs in Syt7 KO splanchnic terminals. It may be that fewer presynaptic axons are activated by the stimulation electrode in Syt7 KO adrenal sections, possibly resulting from a different arrangement of fibers entering the medulla. Altered EPSC amplitudes in the KO could also be due to a reduction in postsynaptic nicotinic receptor expression. We previously shown that nicotinic currents in dissociated WT and Syt7 KO chromaffin cells are not discernibly different, rendering such a possibility unlikely [5]. Note as well that robust synchronous EPSCs are still evident in Syt7 KO medullae, which does suggest other “fast” synaptotagmins (e.g., Syt1, Syt2, Syt3, etc.) are likely expressed in splanchnic neurons and collaborate with Syt7 to regulate exocytosis.
Our results strongly suggest that Syt7 has an important role in regulating neurotransmitter release in the adrenal medulla. Syt7 may also interact directly or indirectly with other pathways for release in the splanchnic neurons. For example, it was recently shown that Neuropeptide Y (NPY) secreted by postsynaptic chromaffin cells, can diffuse to and bind Y5 receptors on splanchnic neurons, thereby stimulating signaling cascades strengthening synaptic vesicle exocytosis [50]. This paracrine pathway for regulating release may be of particular importance when sympathetic activity is heightened [26]. Differences in evoked EPSCs observed in the Syt7KO could be influenced by a decrease in NPY release from postsynaptic chromaffin cells, especially in experiments where stimulation trains were applied.
Differences between WT and Syt7 KO synapses were detected beyond the synchronous component of the evoked EPSC. The tonic current was also substantially reduced in synapses that lacked Syt7. Although tonic current has frequently been attributed to an asynchronous component of release (i.e., where secretion is not time-locked to the arrival of an action potential;) [38], its origins at the splanchnic-chromaffin cell synapse are not immediately obvious. For example, the tonic current may report on the stimulation of chromaffin cells by neurotransmitter that has “spilled over” from neighboring terminals – a phenomenon that has been extensively studied at central synapses [10,15,34] – or it may reflect the direct activation of non-canonical (e.g., GPCR-dependent), slow postsynaptic conductances known to be active in the medulla [22]. Splanchnic neurons are known to house and secrete a multitude of peptide cargos [19]. We cannot yet account for the various ways, subtle or otherwise, in which peptidergic neurotransmission contributes to the phenomena measured here. The tonic current may also reflect electrotonic current spread from one depolarized chromaffin cell to another. Electric coupling is believed to be enhanced in conditions of increased splanchnic nerve activity [20,23].
It is also possible that differences in tonic current amplitude between WT and KO medullae would be even larger than we measured, but for desensitization of postsynaptic nicotinic receptors. However, the evidence for nicotinic receptor desensitization in the setting of high frequency splanchnic stimulation, is mixed. Parkington and colleagues reported that prolonged periods of repetitive stimulation at frequencies up to 30 Hz produced little to no desensitization of nicotinic receptors [25]. On the other hand, Wakade reported a substantial rundown of catecholamine secretion when the splanchnic input to the medulla was stimulated at 10 Hz [49]. This rundown was taken to be a consequence of nicotinic desensitization. It may be that there is a species-specific component to desensitization, as different model systems (guinea pig vs rat) were used to generate the afore-mentioned data.
Overall, this study provides strong evidence that basic functions of splanchnic-chromaffin cell synapse operation depend on Syt7, including a form of short-term synaptic plasticity termed facilitation. It has been suggested that facilitating synapses signal high frequency information to target cells, thereby modulating their excitability [28]. However, the physiological role of facilitation has remained elusive. In the context of the sympatho-adrenal system, facilitation may have a role in amplifying epinephrine discharge from chromaffin cells during conditions that increase sympathetic tone, including hypoglycemia [39,50]. The resulting increase in circulating epinephrine would then be expected to increase blood glucose via multiple metabolic pathways [21,39,50]. A hypothesis, which future studies should test, is that regulated physiological responses to metabolic stressors (e.g., fasting) will require release driven by splanchnic Syt7. Such studies may have to wait until Syt7 expression can be abrogated solely in the periphery, and in a tissue-specific manner. In fact, tissue-specific deletion of Syt7 will be necessary to definitively disentangle its functions in controlling CNS drive of the sympathetic nervous system, from pre- and postsynaptic functions of Syt7 in the adrenal medulla. While these sorts of efforts will not be trivial, the data presented here encourage deeper investigations into the molecular mechanisms of release at these and other autonomic synapses about which very little is known.
Acknowledgements
We would like to thank Dr. Will Birdsong (University of Michigan), Dr. Michael Roberts (University of Michigan), and Dr. Kevin Bender (UCSF) for critical discussion of our data.
Funding
This work was support by NIH R01NS122534 (AA), as well as the Pharmacological Sciences Training Program T32GM007767 (JMP and AM), and the Rackham Predoctoral Fellowship, Charles W. Edmunds Fellowship, and Maas Fellowship (JMP).
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
References
- [1].Anantharam A, Kreutzberger AJB, Unraveling the mechanisms of calcium-dependent secretion, J. Gen. Physiol 151 (4) (2019) 417–434, 10.1085/jgp.201812298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bacaj T, Wu D, Yang X, Morishita W, Zhou P, Xu W, Malenka RC, Sudhof TC, Synaptotagmin-1 and synaptotagmin-7 trigger synchronous and asynchronous phases of neurotransmitter release, Neuron 80 (4) (2013) 947–959, 10.1016/j.neuron.2013.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bader MF, Holz RW, Kumakura KONO, Vitale NICO, Exocytosis: The Chromaffin Cell As a Model System, Ann. N. Y. Acad. Sci 971 (1) (2002) 178–183. NOT IN FILE. [DOI] [PubMed] [Google Scholar]
- [4].Barbara JG, Takeda K, Quantal release at a neuronal nicotinic synapse from rat adrenal gland, PNAS 93 (18) (1996) 9905–9909, 10.1073/pnas.93.18.9905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bendahmane M, Morales A, Kreutzberger AJB, Schenk NA, Mohan R, Bakshi S, Philippe JM, Zhang S, Kiessling V, Tamm LK, Giovannucci DR, Jenkins PM, Anantharam A, Synaptotagmin-7 enhances calcium-sensing of chromaffin cell granules and slows discharge of granule cargos, J. Neurochem 154 (6) (2020) 598–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bhalla A, Tucker WC, Chapman ER, Synaptotagmin Isoforms Couple Distinct Ranges of Ca 2+, Ba 2+, and Sr 2+ Concentration to SNARE-mediated Membrane Fusion, MBoC 16 (10) (2005) 4755–4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Caballero-Floran RN, Conde-Rojas I, Chavez AO, Cortes-Calleja H, Lopez-Santiago LF, Isom LL, Aceves J, Erlij D, Floran B, Cannabinoid-induced depression of synaptic transmission is switched to stimulation when dopaminergic tone is increased in the globus pallidus of the rodent, Neuropharmacology 110 (2016) 407–418, 10.1016/j.neuropharm.2016.08.002. [DOI] [PubMed] [Google Scholar]
- [8].Cannon WB, The Adrenal Medulla, Bull. N. Y. Acad. Med 16 (1) (1940) 3–13. https://www.ncbi.nlm.nih.gov/pubmed/19312138. [PMC free article] [PubMed] [Google Scholar]
- [9].Carmichael SW, Winkler H, The adrenal chromaffin cell, Sci. Am 253 (2) (1985) 40–49. https://www.ncbi.nlm.nih.gov/pubmed/3161180. [DOI] [PubMed] [Google Scholar]
- [10].Carter AG, Regehr WG, Prolonged synaptic currents and glutamate spillover at the parallel fiber to stellate cell synapse, J. Neurosci 20 (12) (2000) 4423–4434. https://www.ncbi.nlm.nih.gov/pubmed/10844011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chakrabarti S, Kobayashi KS, Flavell RA, Marks CB, Miyake K, Liston DR, Fowler KT, Gorelick FS, Andrews NW, Impaired membrane resealing and autoimmune myositis in synaptotagmin VII-deficient mice, J. Cell Biol 162 (4) (2003) 543–549, 10.1083/jcb.200305131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chaveznoriega LE, Stevens CF, Increased Transmitter Release at Excitatory Synapses Produced by Direct Activation of Adenylate-Cyclase in Rat Hippocampal Slices, J. Neurosci 14 (1) (1994) 310–317. <Go to ISI>://WOS: A1994MQ11800027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].de Diego AM, Gandia L, & Garcia AG (2008). A physiological view of the central and peripheral mechanisms that regulate the release of catecholamines at the adrenal medulla [Research Support, Non-U.S. Gov’t Review]. Acta Physiol (Oxf), 192(2), 287–301. doi: 10.1111/j.1748-1716.2007.01807.x. [DOI] [PubMed] [Google Scholar]
- [14].De Robertis E, Ferreira AV, Submicroscopic changes of the nerve endings in the adrenal medulla after stimulation of the splanchnic nerve, J. Biophys. Biochem. Cytol 3 (4) (1957) 611–614, 10.1083/jcb.3.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Diamond JS, A broad view of glutamate spillover, Nat. Neurosci 5 (4) (2002) 291–292, 10.1038/nn0402-291. [DOI] [PubMed] [Google Scholar]
- [16].Goldstein DS, Adrenal responses to stress, Cell. Mol. Neurobiol 30 (8) (2010) 1433–1440, 10.1007/s10571-010-9606-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Goldstein DS, Kopin IJ, Evolution of concepts of stress, Stress 10 (2) (2007) 109–120, 10.1080/10253890701288935. [DOI] [PubMed] [Google Scholar]
- [18].Grynszpan-Winograd O, Adrenaline and noradrenaline cells in the adrenal medulla of the hamster: a morphological study of their innervation, J. Neurocytol 3 (3) (1974) 341–361, 10.1007/bf01097918. [DOI] [PubMed] [Google Scholar]
- [19].Gúerineau NC, Cholinergic and peptidergic neurotransmission in the adrenal medulla: A dynamic control of stimulus-secretion coupling, IUBMB Life 72 (4) (2020) 553–567. [DOI] [PubMed] [Google Scholar]
- [20].Gúerineau NC, Desarménien MG, Carabelli V, Carbone E, Functional Chromaffin Cell Plasticity in Response to Stress: Focus on Nicotinic, Gap Junction, and Voltage-Gated Ca(2+) Channels, J. Mol. Neurosci 48 (2) (2012) 368–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gupta R, Ma Y, Wang M, Whim MD, AgRP-Expressing Adrenal Chromaffin Cells Are Involved in the Sympathetic Response to Fasting, Endocrinology 158 (8) (2017) 2572–2584, 10.1210/en.2016-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hill J, Chan SA, Kuri B, Smith C, Pituitary adenylate cyclase-activating peptide (PACAP) recruits low voltage-activated T-type calcium influx under acute sympathetic stimulation in mouse adrenal chromaffin cells, J. Biol. Chem 286 (49) (2011) 42459–42469, 10.1074/jbc.M111.289389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hill J, Lee SK, Samasilp P, & Smith C (2012). Pituitary adenylate cyclase-activating peptide enhances electrical coupling in the mouse adrenal medulla. Am. J. Physiol. Cell Physiol, 303(3), C257–266. doi: 10.1152/ajpcell.00119.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Holman ME, Coleman HA, Tonta MA, Parkington HC, Synaptic Transmission from Splanchnic Nerves to the Adrenal-Medulla of Guinea-Pigs, J. Physiol.-London 478 (1) (1994) 115–124, 10.1113/jphysiol.1994.sp020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Holman ME, Coleman HA, Tonta MA, Parkington HC, Synaptic transmission from splanchnic nerves to the adrenal medulla of guinea-pigs, J. Physiol 478 (Pt 1) (1994) 115–124, 10.1113/jphysiol.1994.sp020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Holz RW, Anantharam A, Food deprivation induces presynaptic plasticity in the autonomic nervous system, PNAS 113 (21) (2016) 5766–5767, 10.1073/pnas.1605618113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hui E, Bai J, Wang P, Sugimori M, Llinas RR, Chapman ER, Three distinct kinetic groupings of the synaptotagmin family: candidate sensors for rapid and delayed exocytosis, PNAS 102 (14) (2005) 5210–5214, 10.1073/pnas.0500941102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jackman SL, Regehr WG, The Mechanisms and Functions of Synaptic Facilitation, Neuron 94 (3) (2017) 447–464, 10.1016/j.neuron.2017.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jackman SL, Turecek J, Belinsky JE, Regehr WG, The calcium sensor synaptotagmin 7 is required for synaptic facilitation, Nature 529 (7584) (2016) 88–91, 10.1038/nature16507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kaeser PS, Regehr WG, Molecular mechanisms for synchronous, asynchronous, and spontaneous neurotransmitter release, Annu. Rev. Physiol 76 (2014) 333–363, 10.1146/annurev-physiol-021113-170338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kajiwara R, Sand O, Kidokoro Y, Barish ME, Iijima T, Functional organization of chromaffin cells and cholinergic synaptic transmission in rat adrenal medulla, Jpn. J. Physiol 47 (5) (1997) 449–464, 10.2170/jjphysiol.47.449. [DOI] [PubMed] [Google Scholar]
- [32].Kamiya H, Zucker RS, Residual Ca2+ and Short-Term Synaptic Plasticity, Nature 371 (6498) (1994) 603–606, 10.1038/371603a0. [DOI] [PubMed] [Google Scholar]
- [33].Lachamp PM, Liu Y, Liu SJ, Glutamatergic Modulation of Cerebellar Interneuron Activity Is Mediated by an Enhancement of GABA Release and Requires Protein Kinase A/RIM1 alpha Signaling, J. Neurosci 29 (2) (2009) 381–392, 10.1523/Jneurosci.2354-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lamotte d’Incamps B, Krejci E, Ascher P, Mechanisms shaping the slow nicotinic synaptic current at the motoneuron-renshaw cell synapse, J. Neurosci 32 (24) (2012) 8413–8423, 10.1523/JNEUROSCI.0181-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liu H, Bai H, Hui E, Yang L, Evans CS, Wang Z, Kwon SE, Chapman ER, Synaptotagmin 7 functions as a Ca2+-sensor for synaptic vesicle replenishment, Elife 3 (2014) e01524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lu T, Trussell LO, Inhibitory transmission mediated by asynchronous transmitter release, Neuron 26 (3) (2000) 683–694, 10.1016/S0896-6273(00)81204-0. [DOI] [PubMed] [Google Scholar]
- [37].Luo F, Bacaj T, Sudhof TC, Synaptotagmin-7 Is Essential for Ca2+-Triggered Delayed Asynchronous Release But Not for Ca2+-Dependent Vesicle Priming in Retinal Ribbon Synapses, J. Neurosci 35 (31) (2015) 11024–11033, 10.1523/JNEUROSCI.0759-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Luo F, Sudhof TC, Synaptotagmin-7-Mediated Asynchronous Release Boosts High-Fidelity Synchronous Transmission at a Central Synapse, Neuron 94 (4) (2017) 826–839 e823, 10.1016/j.neuron.2017.04.020. [DOI] [PubMed] [Google Scholar]
- [39].Ma Y, Wang Q, Joe D, Wang M, Whim MD, Recurrent hypoglycemia inhibits the counterregulatory response by suppressing adrenal activity, J. Clin. Invest 128 (9) (2018) 3866–3871, 10.1172/JCI91921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].MacDougall DD, Lin Z, Chon NL, Jackman SL, Lin H, Knight JD, Anantharam A, The high-affinity calcium sensor synaptotagmin-7 serves multiple roles in regulated exocytosis, J. Gen. Physiol 150 (6) (2018) 783–807, 10.1085/jgp.201711944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Maximov A, Sudhof TC, Autonomous function of synaptotagmin 1 in triggering synchronous release independent of asynchronous release, Neuron 48 (4) (2005) 547–554, 10.1016/j.neuron.2005.09.006. [DOI] [PubMed] [Google Scholar]
- [42].Otsu Y, Shahrezaei V, Li B, Raymond LA, Delaney KR, Murphy TH, Competition between phasic and asynchronous release for recovered synaptic vesicles at developing hippocampal autaptic synapses, J. Neurosci 24 (2) (2004) 420–433, 10.1523/Jneurosci.4452-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Rahamimoff R, Yaari Y, Delayed release of transmitter at the frog neuromuscular junction, J. Physiol 228 (1) (1973) 241–257, 10.1113/jphysiol.1973.sp010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Schiavo G, Osborne SL, Sgouros JG, Synaptotagmins: more isoforms than functions? Biochem. Biophys. Res. Commun 248 (1) (1998) 1–8, 10.1006/bbrc.1998.8527. [DOI] [PubMed] [Google Scholar]
- [45].Sugita S, Shin OH, Han W, Lao Y, Sudhof TC, Synaptotagmins form a hierarchy of exocytotic Ca(2+) sensors with distinct Ca(2+) affinities, EMBO J. 21 (3) (2002) 270–280, 10.1093/emboj/21.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Turecek J, Jackman SL, Regehr WG, Synaptotagmin 7 confers frequency invariance onto specialized depressing synapses, Nature 551 (7681) (2017) 503–506, 10.1038/nature24474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Turecek J, Regehr WG, Synaptotagmin 7 Mediates Both Facilitation and Asynchronous Release at Granule Cell Synapses, J. Neurosci 38 (13) (2018) 3240–3251, 10.1523/JNEUROSCI.3207-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Turecek J, Regehr WG, Neuronal Regulation of Fast Synaptotagmin Isoforms Controls the Relative Contributions of Synchronous and Asynchronous Release, Neuron 101 (5) (2019) 938–949 e934, 10.1016/j.neuron.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wakade AR, Noncholinergic transmitter(s) maintains secretion of catecholamines from rat adrenal medulla for several hours of continuous stimulation of splanchnic neurons, J. Neurochem 50 (4) (1988) 1302–1308, 10.1111/j.1471-4159.1988.tb10608.x. [DOI] [PubMed] [Google Scholar]
- [50].Wang M, Wang Q, & Whim MD (2016). Fasting induces a form of autonomic synaptic plasticity that prevents hypoglycemia. Proc. Natl. Acad. Sci. U.S.A, 113 (21), E3029–3038. doi: 10.1073/pnas.1517275113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wolf K, Zarkua G, Chan S-A, Sridhar A, Smith C, Spatial and activity-dependent catecholamine release in rat adrenal medulla under native neuronal stimulation, Physiol. Rep 4 (17) (2016) e12898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zucker RS, Calcium- and activity-dependent synaptic plasticity, Curr. Opin. Neurobiol 9 (3) (1999) 305–313, 10.1016/S0959-4388(99)80045-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


