Abstract
Sphingomonas paucimobilis SYK-6 can grow on several dimeric model compounds of lignin as a carbon and energy source. It has O demethylation systems on three kinds of substrates: 5,5′-dehydrodivanillic acid (DDVA), syringate, and vanillate. We previously reported the cloning of a gene involved in the tetrahydrofolate-dependent O demethylation of syringate and vanillate. In the study reported here, we cloned the gene responsible for DDVA O demethylation. Using nitrosoguanidine mutagenesis, a mutant strain, NT-1, which could not degrade DDVA but could degrade syringate and vanillate, was isolated and was used to clone the gene responsible for the O demethylation of DDVA by complementation. Sequencing analysis showed an open reading frame (designated ligX) of 1,266 bp in this fragment. The deduced amino acid sequence of LigX had similarity to class I type oxygenases. LigX was involved in O demethylation activity on DDVA but not on vanillate and syringate. DDVA O demethylation activity in S. paucimobilis SYK-6 cell extracts was inhibited by addition of the LigX polyclonal antiserum. Thus, LigX is an essential enzyme for DDVA O demethylation in SYK-6. S. paucimobilis SYK-6 has two O demethylation systems: one is an oxygenative demethylase system, and the other is a tetrahydrofolate-dependent methyltransferase system.
Lignin is the most abundant aromatic compound in the biosphere. The degradation of lignin is a significant step in the global carbon cycle. Some bacterial strains are capable of degrading aromatic compounds. With rare exceptions, they do not open the aromatic ring unless two hydroxyl groups have been introduced in cis into the benzene nucleus. For example, the phenylmethylether bond exists in natural aromatic compounds such as lignin; its cleavage is essential to prepare the substrate of enzyme for lignin metabolism in the biosphere. Some studies have investigated the cleavage of this phenylmethylether bond by an oxygenase reaction or by the tetrahydrofolate (THF)-dependent methyltransferase reaction system (2, 5–9, 11, 17, 28, 36, 37, 39, 40). In the former reaction, monooxygenases with two- or three-component enzyme systems catalyzed methylether cleavage; these enzyme systems contained terminal enzymes such as iron-sulfur proteins and cytochrome P450-like enzymes (6–9), and they required NADH or NADPH to carry out O demethylation via electron transport. Some reports showed that in the THF-dependent methyltransferase reaction system, ATP and dl-THF are essential for O demethylation reactions (5, 17, 37).
However, successful molecular cloning of the O demethylation system genes has been described in a few reports. In the oxygenase reaction system, vanillate demethylase genes such as vanA and vanB of Pseudomonas sp. strains ATCC 19151 (7) and HR199 (36), whose products catalyzed vanillate O demethylation, have been described. vanA and vanB encode the subunits of the vanillate O demethylase (class I type oxygenase). In the THF-dependent methyltransferase reaction, we previously reported that ligH of Sphingomonas paucimobilis SYK-6 was essential to THF-dependent O demethylation of vanillate and syringate (28). And Kaufmann et al. succeeded in the molecular cloning of odmA, which is involved in the THF-dependent methyltransferase reaction of vanillate in Acetobacterium dehalogenans (18).
S. paucimobilis SYK-6, a bacterium that can grow on 5,5′-dehydrodivanillic acid (2,2′-dihydroxy-3,3′-dimethoxy-5,5′-dicarboxybiphenyl) (DDVA) as a sole carbon source, was isolated from pulp-bleaching wastewater in Japan. This bacterium can also grow on several dimeric model compounds of lignin. The metabolic pathway of DDVA and other dimeric model compounds of lignin in this bacterium has been reported previously (15, 28). We have identified several genes related to this pathway (21–25, 28, 30, 31, 34, 35). It has O demethylation systems that work on three kinds of substrates, DDVA, syringate, and vanillate, in the metabolic pathway (15, 28). The O demethylation of syringate and vanillate in SYK-6 was carried out by the THF-dependent enzyme system containing ligH, but this system had no activity on DDVA (28). These results pose important questions. How does DDVA O demethylation proceed in the metabolism of lignin model compounds by S. paucimobilis SYK-6? Is the enzyme system of DDVA O demethylation of the THF-dependent methyltransferase type or the oxygenative demethylation type?
In this study, we investigated DDVA O demethylation in SYK-6. This is the first report of an O demethylase acting on biphenyl type lignin. We first showed that two different demethylation systems exist in S. paucimobilis SYK-6: one is a DDVA-specific oxygenative O-demethylase, and the other is a syringate- and vanillate-specific O-demethylase of the THF-dependent methyltransferase type.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. The S. paucimobilis wild-type strain SYK-6 was isolated from pulp-bleaching wastewater, as described previously (15), for its ability to utilize DDVA as a carbon source. Escherichia coli MV1190 and E. coli HB101 were used as host cells. A genomic library of S. paucimobilis SYK-6 was constructed in the broad-host-range vector pKT230 (16, 29). The helper plasmid pRK2013 has been described previously (12). Plasmid pKT230MC was constructed from pKT230 as described in a previous study (3). The regulation plasmid pREP4 and the expression vector pQE32, purchased from QIAGEN Inc. (Valencia, Calif.), were used for protein expression.
TABLE 1.
Bacteria and plasmids used in this study
| Strain or plasmid | Genotype or phenotype | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| HB101 | supE44 hsdS20 recA13 proA2 lacY1 galK2 rpsL20 xyl-5 mtl-1 ara-14 leuB6 thi-1 | Takara-Shuzo Co. |
| MV1190 | Δ(lac-proAB) thi supE Δ(sri-recA) 306::Tn10(Tetr) F′[traD36 proAB+ lacIq Zd M15] | Bio-Rad Laboratories, Inc. |
| SG13009 | Lac−ara gal Mtl− F−recA+ uvr+ | Bio-Rad Laboratories, Inc. |
| S. paucimobilis | ||
| SYK-6 | Wild type; Nalr Smr | 10 |
| NT-1 | Mutant of SYK-6 deficient in DDVA O demethylation; Nalr Smr | 27 |
| NT-11 | Mutant of SYK-6 deficient in DDVA O demethylation and OH-DDVA ring cleavage; Nalr Smr | This study |
| NT-21 | Mutant of SYK-6 deficient in DDVA O demethylation and OH-DDVA ring cleavage; Nalr Smr | 33 |
| NT-23 | Mutant of SYK-6 deficient in DDVA O demethylation and OH-DDVA ring cleavage; Nalr Smr | This study |
| DC-49 | Mutant of SYK-6 deficient in vanillate and syringate O demethylation; Nalr Smr | 27 |
| Plasmids | ||
| pUC119 | Apr | 28 |
| pRK2013 | tra+ Kmr derivative of RK2 containing ColE1 replicon | 13 |
| pKT230MC | pKT230 derivative with a 200-bp EcoRI-PvuII fragment from pUC119 inserted into its EcoRI-SacI site | 27 |
| pVK100 | mob+ Kmr derivative of RK2 containing ColE1 replicon | 12 |
| pKT230 | mob+ Kmr RSF1010/pACYC177 replicon | 27 |
| pREP4 | lacI+ Kmr ColE1 replicon | QIAGEN Inc. |
| pLE6 | pKT230 derivative with a 6-kbp EcoRI fragment of S. paucimobilis SYK-6 chromosomal DNA inserted into its EcoRI site; Kmr Tcr | This study |
| pMC37 | 3.7-kbp BglII-EcoRI fragment (blunted) from pLE6 inserted into the SmaI site of pKT230MC; Kmr | This study |
| pMC19R | 1.9-kbp fragment of the 3.7-kbp BglII-EcoRI fragment of pMC37 deleted; Kmr | This study |
| pLSC17 | pLE6 derivative with a 1.7-kbp fragment of the 6-kbp EcoRI fragment deleted; Kmr | This study |
| pMC17 | 1.7-kbp SalI fragment from pLE6 inserted into the SalI site of pKT230MC; Kmr | This study |
| pMC15 | pMC17 derivative; 200-bp region from the promoter side of pKT230MC deleted; Kmr | This study |
| pUS17 | 1.7-kbp SalI fragment from pMC17 inserted into the SalI site of pUC119; Apr | This study |
| pQELigX | pQE32 derivative with a 1.3-kbp CpoI-SalI fragment (blunted) from pUS17 inserted into its SmaI site; bla+ Apr | This study |
Media and growth conditions.
E. coli and S. paucimobilis strains were routinely grown in Luria-Bertani (LB) medium at 37 and 28°C, respectively. When DDVA and other phenolic compounds were used as carbon sources, each was added to W medium (41) at a final concentration of 0.2% (wt/vol). Kanamycin and nalidixic acid were added to the selective medium at final concentrations of 25 mg/liter for S. paucimobilis strains. For E. coli, kanamycin and ampicillin were added to the selective medium at final concentrations of 50 mg/liter.
Substrates, enzymes, and reagents.
DDVA and OH-DDVA were synthesized as reported previously (15, 34). Vanillate and syringate were purchased from Tokyo Kasei Co. (Tokyo, Japan). All restriction enzymes, T4 DNA ligase, T4 DNA polymerase, E. coli Klenow fragment, and a Kilosequence kit were obtained from Takara Shuzo Co. (Kyoto, Japan). All antibiotics were purchased from Wako Pure Chemical Industries (Saitama, Japan).
Mutagenesis and screening.
Nitrosoguanidine mutagenesis of S. paucimobilis SYK-6 was performed as described by Miller (26). The final concentration of nitrosoguanidine was 50 μg/ml. The mutants were screened for a deficit in the ability to use DDVA as a carbon source. The capacity of these mutants to degrade DDVA was assessed as described in our previous study (28). Utilization of DDVA was measured by a decrease in UV absorption at 200 to 380 nm with a U-2000 spectrophotometer (Hitachi Co., Tokyo, Japan).
Cloning and nucleotide sequencing.
All of the recombinant DNA methods used to construct the plasmids or to study the cloned fragments have been described previously (20). The shotgun cloning of the genes involved in the O demethylation of DDVA proceeded as follows. The genomic library was introduced into the cells of the S. paucimobilis SYK-6 mutant NT-1 by triparental mating methods. Exconjugants were screened on LB medium containing kanamycin and nalidixic acid. Colonies growing on the plates were patched onto W medium plates containing DDVA as a carbon source. Strains that were able to grow on these plates were complemented with recombinant plasmids. The various deletion derivatives of the pLE6 plasmid were constructed with the restriction endonucleases and exonucleases of the Kilosequence kit. Subcloning was performed with plasmids pUC119 and pKT230MC. In addition, a minimum DNA fragment was determined by complementation of the degradation and assimilation abilities of mutant NT-1 for DDVA. Nucleotide sequencing was performed by the dideoxy-chain termination method with an Auto Read Sequence Kit and an ALF DNA Sequencer II obtained from Amersham Pharmacia Biotech (Uppsala, Sweden). The nucleotide sequence between the SalI restriction sites of pUS17 was determined. The nucleotide sequence and deduced amino acid sequences were analyzed with GENETIX, version 10.1, software (Software Development Co., Ltd., Tokyo, Japan), and a similarity search was carried out with the SwissProt database.
Preparation of cell extracts and enzyme assay.
S. paucimobilis SYK-6 and its mutants were cultured in LB medium containing nalidixic acid (25 mg/liter). The mutants having pVK100, pKT230MC, or their derivatives were cultured in LB medium containing nalidixic acid and kanamycin (each at 25 mg/liter). When the optical density at 550 nm reached 0.8, the cultures were centrifuged. Cells from the 200-ml cultures were washed with 100 mM Tris-HCl buffer (pH 7.5) and resuspended in 100 ml of W medium containing 0.1% DDVA and 0.1% syringate. After 24 h, the cultures were harvested, washed with 100 mM Tris-HCl buffer (pH 7.5), and resuspended in 2 ml of the same buffer. The cell suspensions were broken by a chilled French pressure cell (2,000 kg/cm2). The broken cells were centrifuged at 15,000 × g for 10 min at 4°C. The supernatants were then used as cell extracts for the enzyme reactions. The extracts were assayed with a protein assay kit (Bradford type of reagent) purchased from Bio-Rad Laboratories, Inc., Richmond, Calif.
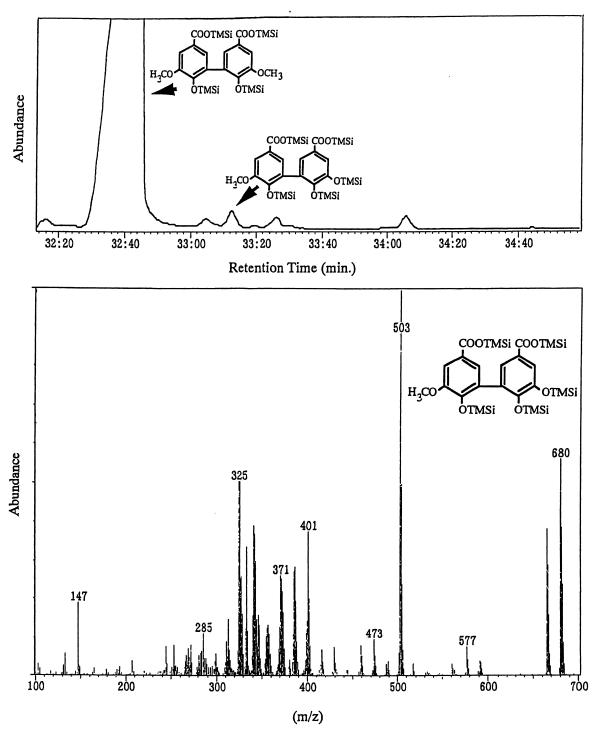
The O demethylation activities of DDVA were measured as follows. First, 1.5 ml of 100 mM Tris-HCl buffer (pH 7.5) containing 5 mg of protein from the cell extract was prepared in a 2-ml reaction cuvette (Iijima Denshi Co., Aichi, Japan) at 25°C. Then 20 μl of 0.5 M NADH or NADPH was added to the reaction mixture. After 1 min, DDVA was added, and then the substrate-dependent oxygen consumption was examined with a galvanic cell electrode purchased from Iijima Denshi Co. After incubation of the cell extracts together with NADPH and DDVA for 3 h at 28°C, the reaction mixture was acidified to pH 2 with 2 M hydrochloric acid and then extracted twice with 0.5 ml of ethyl acetate. The total organic solvent was then completely evaporated, and the residue was dissolved in 0.1 ml of pyridine. A 0.02-ml portion of the pyridine solution was mixed with the same volume of N,O-bis(trimethylsilyl)-trifluoroacetamide (Tokyo Kasei Co.). After incubation for 30 min at 60°C, OH-DDVA in the resultant reaction mixtures was subjected to gas chromatography-mass spectrometry (GC-MS) analysis with a 5890 SERIES II (Hewlett-Packard) and an Automass system II (JEOL Co.). A CP-Sil 5CB capillary column (0.32 mm by 25 m; GL Science Co.) was used. The oven temperature program was as follows: initial temperature, 100°C (for 1 min); final temperature, 280°C; rate of increase, 5°C/min. The carrier gas was He, with a flow rate of 10 ml/min.
LigX antiserum and immunoinhibition.
The ligX gene was cloned into the expression vector pQE32 (pQELigX), and the construct was used for transformation of E. coli strain SG13009 containing the repressor plasmid pREP4. At an A550 of 0.5, 2 mM isopropylthiogalactopyranoside (IPTG) was added, and the culture was grown for 5 h. Cells were harvested by centrifugation (at 6000 × g for 10 min at 4°C) and resuspended in buffer A (6 M guanidine hydrochloride, 0.1 M sodium phosphate, 0.01 M Tris [pH 8.0]) at 5 ml per g (wet weight). The extract was centrifuged at 10,000 × g for 10 min, and the supernatant was applied to a column of Ni-nitrilotriacetic acid agarose (QIAGEN). After the column was washed according to the manufacturer's instructions, the histidine-tagged protein was eluted with buffer D (8 M urea, 0.1 M sodium phosphate, 0.01 M Tris [pH 5.9]). Five milligrams of purified protein was then injected into a rabbit following the immunization procedure of Sawadi Technology (Tokyo, Japan). The final blood collection after 12 weeks was used as the LigX antiserum. LigX antiserum was stored at −20°C for subsequent studies. For immunoinhibition of LigX activity, cell extracts containing 5 mg of protein were incubated with the LigX antiserum or with the prebleed antiserum (as a control).
Nucleotide sequence accession number.
The nucleotide sequence data determined for this paper appear in the DDBJ, EMBL, and GenBank nucleotide sequence databases under accession no. AB021319.
RESULTS
Detection of DDVA O demethylation activity in S. paucimobilis SYK-6.
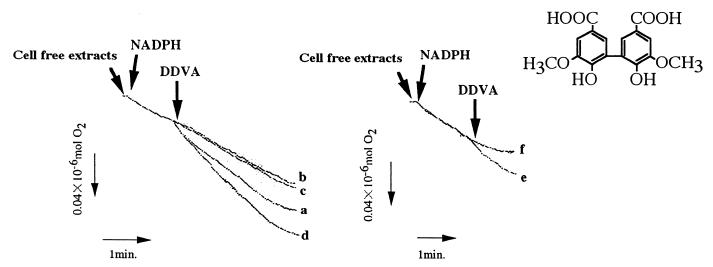
S. paucimobilis SYK-6 can O demethylate DDVA, syringate, and vanillate. In a previous study, we reported that the O demethylation reaction of syringate and vanillate depends on THF, whereas no such O demethylation activity was detected with DDVA (9). To examine the involvement of an oxygenase system such as VanA, VanB, or cytochrome P-450 in the O demethylation of DDVA, we measured substrate-dependent oxygen consumption on DDVA in the presence of SYK-6 cell extracts (oxygen uptake rate, 5.6 nmol/min/mg of protein) (Fig. 1a). Oxygen consumption activity clearly increased as a result of incubation in W medium containing 0.1% DDVA and 0.1% syringate in comparison with the activity of extracts from cells grown in LB medium (Fig. 1a and b). In this assay system, no oxygen consumption was detected on syringate and vanillate. Using the same cell extracts, DDVA was converted to OH-DDVA, which was detected by GC-MS (Fig. 2). By adding THF together with syringate and vanillate to the cell extracts, 3-O-methyl gallate and protocatechuate were detected by GC (data not shown).
FIG. 1.
Oxygen uptake in the presence of DDVA by cell extracts of SYK-6 (a), SYK-6 which was not cultured in W medium containing 0.1% DDVA and 0.1% syringate (b), the DDVA O demethylation-deficient mutant NT-1 (c), the recombinant strain NT-1/pMC15 (harboring ligX) (d), E. coli harboring ligX and ligZ plus NT-1 (e), and E. coli harboring ligX and ligZ (f). All SYK-6-derived strains were treated with octanoyl-N-methylglucamide (MEGA-8).
FIG. 2.
GC-MS analysis of DDVA oxidation by cell extracts of S. paucimobilis SYK-6.
Isolation of S. paucimobilis SYK-6 mutants deficient in DDVA O demethylation.
To further investigate DDVA O demethylation, we screened for mutants of S. paucimobilis SYK-6 deficient in DDVA O demethylation. Four mutants, NT-1, NT-11, NT-21, and NT-23, were isolated following nitrosoguanidine mutagenesis. They could not degrade DDVA but degraded syringate and vanillate. OH-DDVA dioxygenase (34) activity was detected only in cell extracts of mutant NT-1. The other mutants, NT-11, NT-21, and NT-23, did not show OH-DDVA dioxygenase activity (Table 2). Mutant NT-1 had no DDVA O demethylation activity (Fig. 1c); all mutants failed to show oxygen consumption on DDVA (data not shown). These data suggest that strain NT-1 is a mutant specifically defective in the O demethylation of DDVA (Table 2).
TABLE 2.
Enzyme activity in cell extracts of S. paucimobilis SYK-6, its mutants, and recombinant strains
| Strain | Enzyme activitya (% of activity in SYK-6)
|
|||||
|---|---|---|---|---|---|---|
| DDVA O demethylation (LigX) | OH-DDVA dioxygenase (LigZ) | O demethylation (LigH) of:
|
Protocatechuate 4,5-dioxygenase (LigAB) acting on:
|
|||
| Syringate | Vanillate | 3-O-Methylgallate | Protocatechuate | |||
| SYK-6 | 100 | 100 | 100 | 100 | 100 | 100 |
| NT-1 | ND | 95 ± 5 | 93 ± 5 | 98 ± 5 | 99 ± 2 | 98 ± 3 |
| NT-11 | ND | ND | 90 ± 2 | 92 ± 3 | 98 ± 2 | 98 ± 4 |
| NT-21 | ND | ND | 95 ± 7 | 94 ± 4 | 97 ± 2 | 96 ± 5 |
| NT-23 | ND | ND | 93 ± 5 | 98 ± 3 | 95 ± 5 | 98 ± 3 |
| DC-49 | 75 ± 6 | 90 ± 4 | ND | ND | 97 ± 5 | 99 ± 3 |
| NT-1/pLE6 | 99 ± 3 | 97 ± 5 | 94 ± 3 | 96 ± 4 | 95 ± 3 | 99 ± 3 |
| DC-49/pLE6 | 72 ± 4 | 91 ± 5 | ND | ND | 95 ± 3 | 95 ± 4 |
LigX, LigZ, and LigAB enzyme activities were detected by a galavanic cell electrode. LigH enzyme activity was detected by GC (28). ND, not detected.
Cloning and sequencing of the genes involved in DDVA O demethylation.
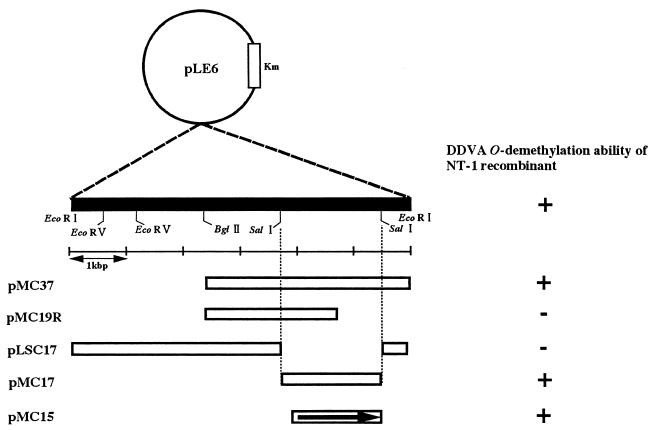
Shotgun cloning of complementary DNA for strain NT-1 was conducted as described in Materials and Methods. One exconjugant was able to grow on W medium containing DDVA as a sole carbon source. This exconjugant harbored the recombinant plasmid pLE6, which contains a 6-kbp EcoRI fragment at the EcoRI site of pKT230. Conjugation experiments with strains NT-1 and DC-49 (28) and plasmid pLE6 were performed, and the degradation abilities of transconjugants for DDVA, vanillate, and syringate were confirmed. Plasmid pLE6 complemented the O demethylation of DDVA in strain NT-1. However, strain DC-49 was not complemented for the O demethylation of syringate and vanillate by pLE6 (Table 2). Subcloning experiments revealed that the 1.5-kbp region in the 3.7-kbp BglII-EcoRI fragment of the pLE6 insert was able to complement the DDVA O demethylation of strain NT-1, as shown in Fig. 3.
FIG. 3.
Deletion analysis of ability of plasmids to complement strain NT-1 for DDVA O demethylation. Construction of plasmids is described in Table 1. The location and direction of the ligX gene are indicated by an arrow.
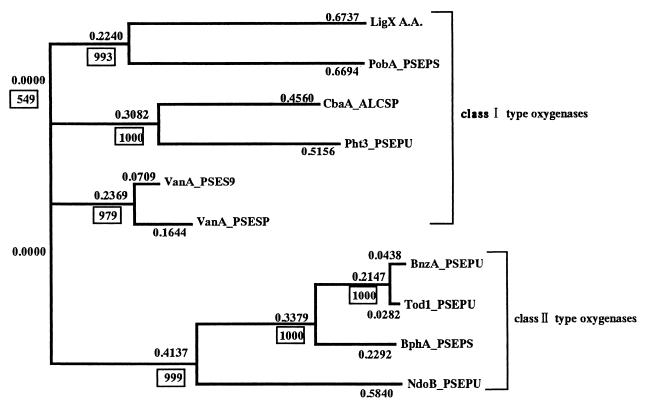
The nucleotide sequence between the SalI restriction sites of pUS17 was determined. Computer analysis of the nucleotide sequence indicated a single open reading frame (ORF). The sequence of this ORF had a G+C content of 63%. The deduced gene product of this ORF consists of 422 amino acid residues, and the molecular size was calculated to be 48,754 Da. Computer analysis also showed that 85% of the third bases of codons were G's or C's. The codon usage of this ORF was quite similar to that in order genes of S. paucimobilis SYK-6 (data not shown). The ORF, designated ligX here, thus appeared to be a functional gene in S. paucimobilis SYK-6. A similarity search indicated that the deduced amino acid sequence of LigX had similarity to those of some oxygenases (Fig. 4). LigX was designated a class I type oxygenase from the phylogenetic tree (Fig. 4). LigX was closest to the α subunit of phenoxybenzoate dioxygenase (PobA).
FIG. 4.
Phylogenetic tree of oxygenases homologous to LigX, drawn using GENETIX version 10.1 software. The numbers on some of the branches refer to the confidence estimated by bootstrap analysis (100 replications). Class I type oxygenases consist of two proteins, an oxygenase and an oxidoreductase, that act as a ferredoxin and a ferredoxin reductase. Class II type oxygenases consist of three proteins: an oxygenase, a ferredoxin, and a ferredoxin reductase. BnzA_PSEPU, benzene 1,2-dioxygenase α subunit of Pseudomonas putida (accession no. P08084) (14); BphA_PSEPS, biphenyl dioxygenase α subunit of P. pseudoalcaligenes KF707 (Q52028) (38); CbaA_ALCSP, 3-chlorobenzoate 3,4-dioxygenase of Alcaligenes sp. strain BR60 (Q44256) (27); NdoB_PSEPU, naphthalene 1,2-dioxygenase α subunit of P. putida NCIB9816 (P23094) (19); PobA_PSEPS, phenoxybenzoate dioxygenase α subunit of P. pseudoalcaligenes POB310 (Q52185) (10); Pht3_PSEPU, phthalate 4,5-dioxygenase oxygenase subunit of P. putida (Q05183) (32); Tod1_PSEPU, toluene 2,3-dioxygenase α subunit of P. putida F1 (P13450) (42); VanA_PSES9, vanillate O-demethylase oxygenase subunit of Pseudomonas sp. strain ATCC 19151 (P12609) (7); VanA_PSESP, vanillate O-demethylase oxygenase subunit of Pseudomonas sp. strain HR199 (O05616) (36).
In order to obtain positive proof that the LigX was required for the O demethylation of DDVA in SYK-6, we introduced plasmid pMC15, harboring the 1.5-kbp fragment carrying ligX, into the DDVA O demethylation-deficient mutant NT-1 and measured DDVA O demethylation activity. Oxygen consumption was measured using cell extracts of NT-1/pMC15 (oxygen uptake rate, 7.8 nmol/min/mg of protein). The oxygen uptake rate was approximately equal to the value observed for SYK-6 (Fig. 1d). The NT-1 mutant, used as a control, had no activity toward DDVA (Fig. 1c). Thus, the 1.5-kbp ligX DNA fragment was essential to DDVA O demethylation.
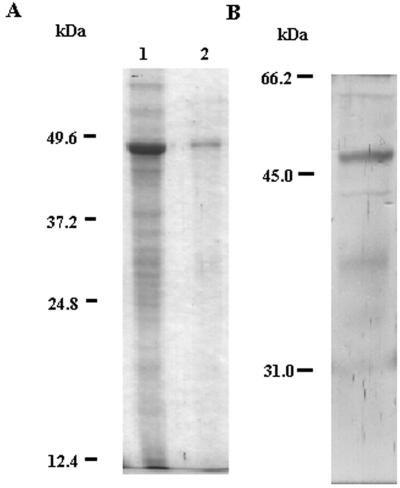
Expression of LigX and immunoinhibition of LigX activity.
The ligX gene was cloned into the expression vector pQE32 (pQELigX). Cellular proteins of the E. coli recombinant strains were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Fig. 5A). A large amount of protein with a molecular size of about 49 kDa was found in the lysate of the strain harboring pQELigX after the addition of IPTG to the culture. The molecular weight of this protein was consistent with that of the ligX gene product deduced from the nucleotide sequence. The cellular proteins of SYK-6 and E. coli harboring pQELigX were subjected to electrophoresis in an SDS-polyacrylamide gel and subsequently to Western blotting (Fig. 5B). In each cellular protein, a strong signal was detected at approximately 49 kDa with the antiserum against LigX. Using this antibody, an immunoinhibition experiment of LigX activity was performed. The LigX antiserum was preincubated for 30 min at room temperature with S. paucimobilis SYK-6 cell extracts containing 5 mg of protein before addition of the reaction mixtures of the enzyme assay. LigX activity levels were 4.575 and 1.144 nmol/min/mg of protein in the presence of 0 and 5 μg of LigX antiserum, respectively. In the presence of 10 or 20 μg of LigX antiserum, no oxygen consumption was detected on DDVA. Thus, the antibody of LigX antiserum inhibited LigX activity in cell extracts of S. paucimobilis SYK-6. When THF was added together with syringate and vanillate to the mixture (cell extracts of SYK-6 containing 10 μg of LigX antiserum), 3-O-methylgallate and protocatechuate were detected by GC (data not shown). A prebleed control did not inhibit DDVA O demethylation activity in cell extracts of S. paucimobilis SYK-6. Thus, LigX was an essential enzyme for DDVA O demethylation.
FIG. 5.
Expression and immunological detection of the ligX gene product. (A) Overexpression and purification of histidine-tagged LigX. Proteins were separated by electrophoresis on an SDS-polyacrylamide gel and stained with Coomassie brilliant blue. Lane 1, extracts of the E. coli recombinant strain SG13009(pREP4, pQELigX) after induction by IPTG; lane 2, purified histidine-tagged LigX. (B) Immunological detection of LigX in extracts. Proteins were separated by electrophoresis on an SDS-polyacrylamide gel and then transferred onto nitrocellulose membranes. Immunodetection was performed with a biotin-streptavidin-alkaline phosphatase system and LigX-specific antiserum. The electrophoresed protein was from cell extracts of S. paucimobilis SYK-6 after incubation in W medium containing 0.1% DDVA and 0.1% syringate.
Heterologous expression of ligX from S. paucimobilis SYK-6 in E. coli.
In order to obtain further positive proof that LigX was required for the O demethylation of DDVA in SYK-6, the ligX gene was ligated downstream of the lacZ promoter in pUC119 (pUS17) and introduced into E. coli. Using cell extracts of recombinant E. coli harboring pUS17, O demethylation activity against DDVA was measured. No oxygen consumption was detected. DDVA O demethylation activity was so weak that the ligZ gene was ligated downstream of the ligX gene to amplify total oxygen consumption for OH-DDVA (production for DDVA O demethylation) ring cleavage, which occurred following DDVA O demethylation. But no DDVA-dependent oxygen consumption was detected in cell extracts of recombinant E. coli harboring the ligX and ligZ genes. DDVA-dependent oxygen consumption was detected in cell extracts of E. coli carrying ligX in the presence of cell extracts of the DDVA O demethylation-deficient mutant NT-1 (oxygen uptake rate, 1.1 nmol/min/mg of protein) (Fig. 1e). These results suggested that other factors essential for DDVA O demethylation existed in SYK-6 but not in E. coli. Although the 6-kbp EcoRI fragment was sequenced to identify the other factors essential for DDVA O demethylation, such as oxidoreductase, no ORF similar to an oxidoreductase gene was found (data not shown).
DISCUSSION
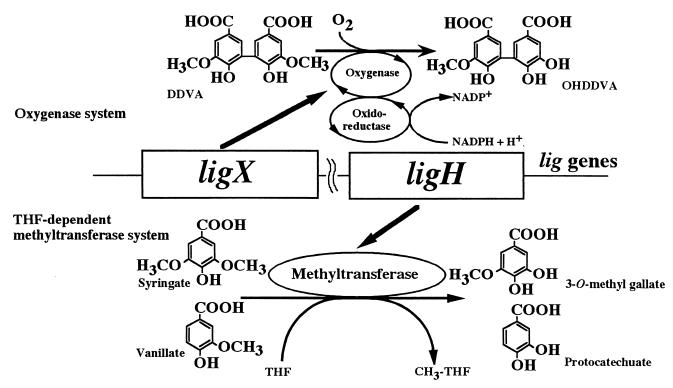
S. paucimobilis SYK-6 has an O demethylation system that acts on three kinds of substrates: DDVA, syringate, and vanillate. In a previous study, we reported that the O demethylation of syringate and vanillate was a THF-dependent methyltransferase reaction system, but this enzyme system had no O demethylation activity on DDVA (28). In this paper, we have described the investigation of the O demethylation of an intermediate in the biodegradation of lignin, DDVA. This is the first step of the metabolism of DDVA in SYK-6. DDVA O demethylation activity was detected in SYK-6 cell extracts by a substrate-dependent oxygen consumption assay using a galvanic cell electrode (Fig. 1a). It appeared that DDVA O demethylation in SYK-6 was an oxygenative demethylase system such as cytochrome P-450 in Moraxella sp. strain GU2 (9) and vanillate demethylase (encoded by vanA and vanB) in Pseudomonas sp. strains ATCC 19151 (7) and HR199 (36). Cell extracts of SYK-6 contained an O-demethylase that converted DDVA to OH-DDVA, depending on oxygen consumption and the presence of NADPH or NADH. These results suggest that two different demethylation enzyme systems exist in S. paucimobilis SYK-6: one is specific for demethylation of DDVA associated with oxygen and NADPH (or NADH), and the other is specific for demethylation of vanillate and syringate by a THF-dependent methyltransferase system. We made an interesting discovery, that two quite different O demethylation systems (Fig. 6) were functioning in the lignin metabolism of a single microorganism.
FIG. 6.
Proposed different O demethylation systems for cleavage of the methyl ether linkage in S. paucimobilis SYK-6.
To analyze lignin biphenyl-specific O-demethylase, we screened the DDVA O demethylation-deficient mutant NT-1 and cloned a gene essential to DDVA O demethylation. NT-1 had O demethylation activity toward syringate and vanillate via the THF-dependent methyltransferase system, and its activity was almost equal to that of the wild type (Table 2). Thus, it appeared that S. paucimobilis SYK-6 had at least two different O demethylation systems (Fig. 6).
A complementary DNA (ligX) of NT-1 had a coding capacity of 496 amino acids. A similarity search revealed that the deduced amino acid sequence of LigX showed similarity to the aromatic-ring-hydroxylating oxygenases PobA (accession no. Q52185) (32); CbaA (Q44256) (27); Pht3 (Q05183) (10); the α subunits of BnzA (P08084) (14), BphA (Q52028) (38), NdoB (P23094) (19), and Tod1 (P13450) (42) and was also similar to VanA (P12609 and O05616). The deduced amino acid sequence of LigX had the region containing the cysteine and histidine residues which were proposed to be the ligands of the Rieske-type iron-sulfur cluster (36). This cluster was commonly observed in these oxygenases. The enzymes that showed similarity to LigX were located at a terminal position of the electron transport system. Thus, LigX would also be a terminal enzyme that causes DDVA O demethylation by adding oxygen to DDVA.
To provide evidence that LigX was a demethylase, we tried to purify this protein. However, the purified protein had no activity against DDVA. It could be that LigX required the oxidoreductase component for its activity, so that DDVA O demethylation activity could not be measured. Purified LigX was added to cell extracts of the DDVA O demethylation-deficient mutant NT-1; however, no LigX activity was detected. LigX may have lost its activity during the purification process. Most oxygenative O-demethylases reported in the past were sensitive to oxidation and dialysis, even under a nitrogen atmosphere. Thus, normal procedures for enzyme purification (e.g., ammonium sulfate fractionation or column chromatography on ion-exchanges and gels) are unsuitable for the demethylase. It may be very difficult to purify LigX due to its instability. So we carried out immunoinhibition of LigX activity in an effort to prove directly that LigX is involved in the DDVA O demethylation reaction. In the immunoinhibition experiment, the LigX antibody inhibited LigX activity in cell extracts, whereas a prebleed antiserum did not inhibit its activity. LigX was essential to the enzyme reaction. Furthermore, LigX would have obtained its O demethylation activity during the process of evolution, although it is inherently an aromatic-ring-hydroxylating oxygenase such as PobA. Hence, we concluded that LigX is an oxygenase and DDVA O-demethylase (Fig. 6).
The phenoxybenzoate dioxygenase of Pseudomonas pseudoalcaligenes POB310 is a two-component oxygenase system comprising PobA and PobB. PobB acts as a ferredoxin and a ferredoxin reductase which transports electrons from NADH. Electron transport systems also exist in the other enzyme systems described above. So the enzyme system of DDVA O demethylation is also considered a multicomponent enzyme that consists of LigX and an electron transport component. Most of the proteins which constitute the multicomponent enzyme system are encoded in the vicinity of terminal enzymes. But no reductase component similar to PobB was observed on the 6-kbp EcoRI fragment of pLE6. DDVA O demethylation activity was not detected in cell extracts of recombinant E. coli harboring the ligX genes. But DDVA O demethylation activity was detected in cell extracts of the recombinant E. coli incubated with cell extracts of the DDVA O demethylation-deficient mutant NT-1. Although the reductase component did not exist in the vicinity of ligX, a SYK-6-specific reductase component did exist. At this time, we did not identify the reductase component, but it will exist far from the oxygenase component, as in Comamonas testosteroni B356 (4).
In this study, we succeeded for the first time in detecting enzyme activity involved in DDVA-specific O demethylation and in molecular cloning of the gene encoding that enzyme. Why do two substrate-specific enzyme systems exist in S. paucimobilis SYK-6? The O demethylation of vanillate by VanA and VanB converts vanillate to protocatechuate and formate (7, 36). LigX and the reductase component should also release formate with OH-DDVA by DDVA O demethylation. LigH was similar (60%) to the formyltetrahydrofolate synthetase (FTHS) of Clostridium thermoaceticum (EC 6.3.4.3), reported previously (28). FTHS transferred −CHO from formate to THF. LigH would transfer −CHO (from the O demethylation of DDVA) and −CH3 (from the O demethylation of syringate and vanillate) to THF. We think that SYK-6 carries out one-carbon recycling like the one-carbon metabolism shown in Saccharomyces cerevisiae (1, 18). It is thought that SYK-6 has developed two O demethylation systems in order to get energy from C1 compounds. Further study will clarify this matter for S. paucimobilis SYK-6 in detail.
ACKNOWLEDGMENT
This work was supported in part by a Grant-in Aid for Scientific Research (no. 10660159) from the Ministry of Education, Science, and Culture of Japan.
REFERENCES
- 1.Appling D R, Rabinowitz J C. Regulation of expression of the ADE3 gene for yeast C1-tetrahydrofolate synthase, a trifunctional enzyme involved in one-carbon metabolism. J Biol Chem. 1985;260:1248–1256. [PubMed] [Google Scholar]
- 2.Bache R, Pfenning N. Selective isolation of Acetobacterium woodii on methoxylated aromatic acids and determination of growth yields. Arch Microbiol. 1981;130:255–261. [Google Scholar]
- 3.Bagdasarian M, Lurz R, Rucker B, Franklin F C H, Bagdasarian M M, Frey J, Timmis K N. Specific purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981;16:237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- 4.Bergeron J, Ahmad D, Barriault D, Larose A, Sylvestre M, Powlowski J. Identification and mapping of the gene translation products involved in the first step of the Comamonas testosteroni B-356 biphenyl/chlorobiphenyl biodegradation pathway. Can J Microbiol. 1994;57:2880–2887. doi: 10.1139/m94-118. [DOI] [PubMed] [Google Scholar]
- 5.Berman M H, Frazer A C. Importance of tetrahydrofolate and ATP in the anaerobic O demethylation reaction for phenylmethylethers. Appl Environ Microbiol. 1992;58:925–931. doi: 10.1128/aem.58.3.925-931.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernhardt F H, Bill E, Trautwein A X, Twilfer H. 4-Methoxybenzoate monooxygenase from Pseudomonas putida: isolation, biochemical properties, substrate specificity, and reaction mechanisms of the enzyme components. Methods Enzymol. 1988;161:281–294. doi: 10.1016/0076-6879(88)61031-7. [DOI] [PubMed] [Google Scholar]
- 7.Brunel F, Davison J. Cloning and sequencing of Pseudomonas genes encoding vanillate demethylase. J Bacteriol. 1988;170:4924–4930. doi: 10.1128/jb.170.10.4924-4930.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buswell J A, Ribbons D W. Vanillate O-demethylase from Pseudomonas species. Methods Enzymol. 1988;161:294–301. doi: 10.1016/0076-6879(88)61032-9. [DOI] [PubMed] [Google Scholar]
- 9.Dardas A, Gal D, Barrelle M, Sauret-Ignazi G, Sterjiades R, Pelmont J. The demethylation of guaiacol by a new bacterial cytochrome P-450. Arch Biochem Biophys. 1985;236:585–592. doi: 10.1016/0003-9861(85)90662-9. [DOI] [PubMed] [Google Scholar]
- 10.Dehmel U, Engesser K H, Timmis K N, Dwyer D F. Cloning, nucleotide sequence, and expression of the gene encoding a novel dioxygenase involved in metabolism of carboxydiphenyl ethers in Pseudomonas pseudoalcaligenes POB310. Arch Microbiol. 1995;163:35–41. doi: 10.1007/BF00262201. [DOI] [PubMed] [Google Scholar]
- 11.DeWeerd K A, Saxena A, Nagle D P, Jr, Suflita J M. Metabolism of the 18O-methoxy substituent of 3-methoxybenzoic acid and other unlabeled methoxybenzoic acids by anaerobic bacteria. Appl Environ Microbiol. 1988;54:1237–1242. doi: 10.1128/aem.54.5.1237-1242.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ditta G, Stanfield S, Corbin D, Helinski D R. Broad host range DNA cloning system for Gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci USA. 1980;77:7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henikoff S. Unidirectional digestion with exonuclease creates targeted breakpoints for DNA sequencing. Gene. 1984;28:351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- 14.Irie S, Doi S, Yorifuji T, Takagi M, Yano K. Nucleotide sequencing and characterization of the genes encoding benzene oxidation enzymes of Pseudomonas putida. J Bacteriol. 1987;169:5174–5179. doi: 10.1128/jb.169.11.5174-5179.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katayama Y, Nishikawa S, Murayama A, Yamasaki M, Morohoshi N, Haraguchi T. The metabolism of biphenyl structure in lignin of the soil bacterium (Pseudomonas paucimobilis SYK-6) FEBS Lett. 1988;233:129–133. [Google Scholar]
- 16.Katayama Y, Nishikawa S, Nakamura M, Yano K, Yamasaki M, Morohoshi N, Haraguchi T. Construction of genomic libraries of lignin model compounds degradable Pseudomonas paucimobilis SYK-6 with Escherichia coli-Pseudomonas shuttle vectors. Mokuzai Gakkaishi. 1988;34:423–427. [Google Scholar]
- 17.Kaufmann F, Wohlfarth G, Diekert G. O-Methylase from Acetobacterium dehalogenans—substrate specificity and function of the participating proteins. Eur J Biochem. 1998;253:706–711. doi: 10.1046/j.1432-1327.1998.2530706.x. [DOI] [PubMed] [Google Scholar]
- 18.Kaufmann F, Wohlfarth G, Diekert G. O-Demethylase from Acetobacterium dehalogenans. Cloning, sequencing, and active expression of the gene encoding the corrinoid protein. Eur J Biochem. 1998;257:515–521. doi: 10.1046/j.1432-1327.1998.2570515.x. [DOI] [PubMed] [Google Scholar]
- 19.Kurkela S, Lehvaeslaiho H, Palva E T, Teeri T H. Cloning, nucleotide sequence and characterization of genes encoding naphthalene dioxygenase of Pseudomonas putida strain NCIB9816. Gene. 1988;73:355–362. doi: 10.1016/0378-1119(88)90500-8. [DOI] [PubMed] [Google Scholar]
- 20.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 21.Masai E, Kubota S, Katayama Y, Kawai S, Yamasaki M, Morohoshi N. Characterization of the C α-dehydrogenase gene involved in the cleavage of β-aryl ether by Pseudomonas paucimobilis. Biosci Biotechnol Biochem. 1993;57:1655–1659. doi: 10.1271/bbb.57.1655. [DOI] [PubMed] [Google Scholar]
- 22.Masai E, Shinohara S, Hara H, Nishikawa S, Katayama Y, Fukuda M. Genetic and biochemical characterization of a 2-pyrone-4,6-dicarboxylic acid hydrolase involved in the protocatechuate 4,5-cleavage pathway of Sphingomonas paucimobilis SYK-6. J Bacteriol. 1999;181:55–62. doi: 10.1128/jb.181.1.55-62.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masai E, Katayama Y, Kawai S, Nishikawa S, Yamasaki M, Morohoshi N. Cloning and sequencing of the gene for a Pseudomonas paucimobilis enzyme that cleaves β-aryl ether. J Bacteriol. 1991;173:7950–7955. doi: 10.1128/jb.173.24.7950-7955.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masai E, Katayama Y, Kubota S, Kawai S, Yamasaki M, Morohoshi N. A bacterial enzyme degrading the model lignin compound β-etherase is a member of the glutathione S-transferase superfamily. FEBS Lett. 1993;323:135–140. doi: 10.1016/0014-5793(93)81465-c. [DOI] [PubMed] [Google Scholar]
- 25.Masai E, Katayama Y, Nishikawa S, Fukuda M. Characterization of Sphingomonas paucimobilis SYK-6 genes involved in degradation of lignin-related compounds. J Ind Microbiol Biotechnol. 1999;23:364–373. doi: 10.1038/sj.jim.2900747. [DOI] [PubMed] [Google Scholar]
- 26.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. pp. 125–129. [Google Scholar]
- 27.Nakatsu C H, Straus N A, Windham R C. The nucleotide sequence of the Tn5271 3-chlorobenzoate 3,4-dioxygenase gene (cbaAB) unites the class IA oxygenases in a single lineage. Microbiology. 1995;141:485–495. doi: 10.1099/13500872-141-2-485. [DOI] [PubMed] [Google Scholar]
- 28.Nishikawa S, Sonoki T, Kasahara T, Obi T, Kubota S, Kawai S, Morohoshi N, Katayama Y. Cloning and sequencing of the Sphingomonas (Pseudomonas) paucimobilis gene essential for the O demethylation of vanillate and syringate. Appl Environ Microbiol. 1998;64:836–842. doi: 10.1128/aem.64.3.836-842.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishikawa S, Katayama Y, Yamasaki M, Morohoshi N, Haraguchi T. In vitro packaging and conjugal transfer of lignin model compounds degradable Pseudomonas paucimobilis SYK-6 chromosomal DNA. Mokuzai Gakkaishi. 1988;34:1021–1025. [Google Scholar]
- 30.Nishikawa S, Katayama Y, Yamasaki M, Morohoshi N, Haraguchi T. Proceedings of the 6th International Symposium on Wood and Pulping Chemistry. 2nd ed. 1991. Cloning of the genes involved in the protocatechuate meta-cleavage pathway; pp. 311–313. [Google Scholar]
- 31.Noda Y, Nishikawa S, Kadokura H, Nakajima H, Yoda K, Katayama Y, Morohoshi N, Yamasaki M. Molecular cloning of the protocatechuate 4,5-dioxygenase genes of Pseudomonas paucimobilis. J Bacteriol. 1990;172:2704–2709. doi: 10.1128/jb.172.5.2704-2709.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nomura Y, Nakagawa M, Ogawa N, Harashima S, Oshima Y. Genes in PHT plasmid encoding the initial degradation pathway of phthalate in Pseudomonas putida. J Ferment Bioeng. 1992;74:333–344. [Google Scholar]
- 33.Pasternack L B, Littlepage L E, Laude D A, Jr, Appling D R. 13C NMR analysis of the use of alternative donors to the tetrahydrofolate-dependent one-carbon pools in Saccharomyces cerevisiae. Arch Biochem Biophys. 1996;326:158–165. doi: 10.1006/abbi.1996.0060. [DOI] [PubMed] [Google Scholar]
- 34.Peng X, Egashira T, Hanashiro K, Masai E, Nishikawa S, Katayama Y, Kinbara K, Fukuda M. Cloning of a Sphingomonas paucimobilis SYK-6 gene encoding a novel oxygenase that cleaves lignin-related biphenyl and characterization of the enzyme. Appl Environ Microbiol. 1998;64:2520–2527. doi: 10.1128/aem.64.7.2520-2527.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng X, Masai E, Katayama Y, Fukuda M. Characterization of the meta-cleavage compound hydrolase gene involved in degradation of the lignin-related biphenyl structure by Sphingomonas paucimobilis SYK-6. Appl Environ Microbiol. 1999;65:2789–2793. doi: 10.1128/aem.65.6.2789-2793.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Priefert H, Rabenhorst J, Steinbuchel A. Molecular characterization of genes of Pseudomonas sp. strain HR199 involved in bioconversion of vanillin to protocatechuate. J Bacteriol. 1997;179:2595–2607. doi: 10.1128/jb.179.8.2595-2607.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stupperich E, Konle R. Corrinoid-dependent methyl transfer reactions are involved in methanol and 3,4-dimethoxybenzoate metabolism by Sporomusa ovata. Appl Environ Microbiol. 1993;59:3110–3116. doi: 10.1128/aem.59.9.3110-3116.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taira K, Hirose J, Hayashida S, Furukawa K. Analysis of bph operon from the polychlorinated biphenyl-degrading strain of Pseudomonas pseudoalcaligenes KF707. J Biol Chem. 1992;267:4844–4853. [PubMed] [Google Scholar]
- 39.Taylor B F. Aerobic and anaerobic catabolism of vanillic acid and some other methoxy-aromatic compounds by Pseudomonas sp. strain PN-1. Appl Environ Microbiol. 1982;46:1286–1292. doi: 10.1128/aem.46.6.1286-1292.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu Z, Daniel S L, Drake H L. Characterization of CO-dependent O-demethylating enzyme system from the acetogen Clostridium thermoaceticum. J Bacteriol. 1988;170:5747–5750. doi: 10.1128/jb.170.12.5747-5750.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yano K, Nishi T. pKJ1, a naturally occurring conjugative plasmid coding for toluene degradation and resistance to streptomycin and sulfonamides. J Bacteriol. 1980;143:552–560. doi: 10.1128/jb.143.2.552-560.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zylstra G J, Gibson D T. Toluene degradation by Pseudomonas putida F1. Nucleotide sequence of the todC1C2BADE genes and their expression in Escherichia coli. J Biol Chem. 1989;264:14940–14946. [PubMed] [Google Scholar]