Background:
Given the complexity of managing HCC, professional society guidelines advocate multidisciplinary care (MDC) for patients with HCC. However, implementation of MDC programs requires a significant investment of time and resources. We conducted a systematic review and meta-analysis to enumerate potential benefits of MDC for patients with HCC.
Methods:
We conducted a search of the PubMed/MEDLINE and EMBASE databases and national conference abstracts to identify studies published after January 2005 that reported early-stage presentation, treatment receipt, or overall survival among patients with HCC, stratified by MDC status. We calculated pooled risk ratios and HRs for clinical outcomes according to MDC receipt using the DerSimonian and Laird method for random effects models.
Results:
We identified 12 studies (n = 15,365 patients with HCC) with outcomes stratified by MDC status. MDC was associated with improved overall survival (HR = 0.63, 95% CI: 0.45–0.88); however, its association with curative treatment receipt was not statistically significant (risk ratio = 1.60, 95% CI: 0.89–2.89) and pooled estimates were limited by high heterogeneity (I 2 > 90% for both). Studies (n = 3) were discordant regarding an association between MDC and time-to-treatment initiation. MDC was associated with early-stage HCC (risk ratio = 1.60, 95% CI: 1.12–2.29), suggesting possible referral bias contributing to improved outcomes. Limitations of studies also included risk of residual confounding, loss to follow-up, and data preceding the availability of immune checkpoint inhibitors.
Conclusion:
MDC for patients with HCC is associated with improved overall survival, underscoring the likely benefit of managing patients with HCC in a multidisciplinary care setting.
INTRODUCTION
HCC is the fifth most prevalent cancer globally and claims close to 800,000 lives per year.1 It is one of the few cancers with a rising mortality rate in the US and is projected to become the third leading cause of cancer-related mortality by 2030.2 The 5-year survival of HCC approaches 70% for early-stage HCC but remains below 20% overall despite recent advances in surveillance and therapeutic options.3 This poor survival relates to failures across the cancer care continuum, including ineffective early detection strategies, diagnostic and therapeutic delays, and underuse of guideline-concordant treatment.4,5
HCC is a complex disease given the near universal presence of underlying advanced chronic liver disease as well as multiple available treatment modalities offered by various treating specialties.6 In contrast to prior treatment allocation algorithms in which one treatment was recommended for a specific tumor stage, there are now multiple treatment options to consider based on several factors including tumor burden, liver dysfunction, performance status, transplant eligibility, local expertise, and patient preferences.6 Therefore, many expert panels, including the American Association for the Study of Liver Diseases (AASLD) guidance document, advocate for multidisciplinary care (MDC) of patients with HCC.7,8
There are several models of MDC, ranging from a multidisciplinary tumor board to a fluid referral system between specialists to co-located clinics with multiple specialists being present concurrently.9 A multidisciplinary team generally comprises specialists in multiple fields including hepatology, surgical oncology, transplant surgery, interventional radiology, medical oncology, and radiation oncology. Furthermore, large MDC programs often engage other specialties, including palliative care, nutrition, social work, and nursing, as well as the patients in their own care. MDC facilitates communication between specialists to provide more individualized care with the aim of optimizing the diagnosis, treatment, and outcomes of patients with HCC. Indeed, data increasingly indicate that MDC has a beneficial association with the management and outcomes of other cancers.10–12 MDC can also decrease the number of necessary clinic visits, addressing patient-reported barriers to care including missed work and transportation.13 However, implementation of MDC programs often requires a significant investment of time and resources. Tumor boards are typically noncompensated activities, hospitals may not have access to all necessary specialists, MDC clinics can result in fewer patient encounters than would be seen otherwise, and efficient evaluation by multiple providers requires effective patient navigation services.14 Therefore, tangible evidence enumerating improvements in clinical outcomes for patients with HCC can be informative for health systems that are contemplating making this investment. To address this need, we conducted a systematic review and meta-analysis of available studies evaluating the potential benefits of MDC for patients with HCC.
METHODS
This study was conducted following Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines.15
Search strategy
Two investigators independently performed an electronic-based search of the PubMed, Ovid MEDLINE, and EMBASE databases to identify relevant articles evaluating potential benefits of MDC published between January 2005 and January 2022. The search terms included “liver ca$ OR hepatocellular ca$ OR hepatoma OR HCC” AND “multidisciplinary OR multispecialty OR multidisciplin$ OR multispecial$ OR team.” Manual searches of reference lists were also conducted to identify citations that may have been missed by the electronic-based search. A search of the AASLD, European for the Study of the Liver (EASL), Digestive Disease Week (DDW), and American College of Gastroenterology (ACG) conference abstracts for 2019–2022 was also conducted.
Study selection and inclusion/exclusion criteria
After removal of duplicate citations, screening of titles, abstracts, and full texts of the remaining citations were conducted by one investigator to generate a list of potentially relevant articles. A second investigator reviewed full texts of articles in this list and included them based on specific criteria. Disagreements and discrepancies were resolved by a third investigator. We included studies that: (1) involved patients with HCC from any etiology; (2) evaluated the clinical impact of a clearly defined MDC; and (3) compared curative treatment receipt or overall survival (OS) between patients with HCC who were under MDC and those who were not. We excluded studies that only reported outcomes among patients who received MDC without a comparator arm. Additional exclusion criteria were as follows: (1) reviews, opinion letters, case reports, or incomplete reports; (2) nonhuman data; (3) lack of original data; and (4) non-English language articles. For studies reporting data on overlapping cohorts, the publication with more complete data was included for analysis.
Data extraction and quality assessment
Articles meeting inclusion and exclusion criteria were independently reviewed by 2 investigators to extract required information using standardized forms, including cohort size and characteristics, study design, HCC staging system used [ie, Barcelona Clinic Liver Cancer (BCLC) stage, Milan criteria, and TNM staging], type of MDC model (eg, tumor board and co-located clinic), and clinical outcomes of patients with HCC. Clinical outcomes included proportion of patients with early HCC stage detection, time-to-treatment initiation, curative treatment receipt, noncurative treatment receipt, length of follow-up, and OS. Curative treatment was defined as liver transplantation, liver resection, or local ablation, whereas noncurative modalities included transarterial chemoembolization, radiation therapy, and systemic therapy. Study quality and risk of bias were assessed by 2 investigators using a modified National Institutes of Health Study Quality Assessment Tool. Discrepancies were resolved through discussion with a third investigator.
Statistical analysis
For each study, we calculated a risk ratio (RR) with the exposure being MDC and clinical outcomes being the proportion of patients with early-stage HCC and/or the proportion who underwent curative treatment. For survival, we abstracted HRs when available; if not reported, we recorded median survival for both MDC and control groups. If a HR was reported without a 95% CI, we computed it using the effect estimate and the p-value.16 We used the DerSimonian and Laird method for random effects models to calculate pooled RR estimates for early-stage HCC and curative treatment receipt and pooled HR estimates for OS.17 We used the χ2 test of heterogeneity and the inconsistency index (I 2) to quantitatively determine the extent of heterogeneity between studies. If significant heterogeneity (I 2 > 50%) was found between studies, we used sensitivity analysis to exclude outlier studies one at a time to assess if this impacted pooled effect estimates. We performed subgroup analyses to explore potential causes of heterogeneity among results. Potential publication bias was evaluated graphically using Begg’s funnel plot and statistically using Egger’s regression test and fail-safe N.18–20 All data analysis was performed using R software 4.2.1.
RESULTS
Study characteristics
Of 2484 potentially relevant titles, 189 were selected for abstract review and 53 for full-text review (Supplemental Figure 1, http://links.lww.com/HC9/A271). After full-text review, 7 articles met inclusion criteria. Searches of annual meeting abstracts yielded 5 additional relevant abstracts, resulting in the inclusion of a total of 12 studies. Egger’s test did not suggest the presence of publication bias for studies assessing early-stage HCC detection (p = 0.09), curative treatment receipt (p = 0.37), or OS (p = 0.29). Similarly, examination of the funnel plot did not suggest publication bias for studies examining the association between MDC and OS. The fail-safe N (ie, number of studies with a null effect that would render the pooled effect size statistically insignificant) was 210 for early-stage HCC detection and 388 for OS.
Characteristics of studies evaluating MDC for patients with HCC are detailed in Table 1. Seven were single-center quasi-experimental studies using a pre-post study design around the time of MDC implementation. The remainder were either single-center (n = 3) or multicenter (n = 2) retrospective analyses comparing patients who received or did not receive MDC during the study period. Most studies were conducted in the US, although there were studies from Turkey (n = 1), South Korea (n = 1), and Australia (n = 1). MDC models included a multidisciplinary conference for imaging review and discussion of management decisions across all studies; however, MDC was additionally defined by evaluation in a co-located multidisciplinary clinic in 2 studies (Yopp and colleagues and Vora and colleagues) and being seen by at least 3 disciplines in the peridiagnostic, pretreatment period in one study (Chirikov and colleagues).
TABLE 1.
Characteristics of included studies
| References | Study location | Study period | Study design | Definition of multidisciplinary care | Number of patients |
|---|---|---|---|---|---|
| Chang et al21 | San Francisco, USA | 2000–2006 | Pre vs. post, single center | Multidisciplinary management conference | 183 |
| Yopp et al22 | Dallas, USA | 2006–2011 | Pre vs. post, single center | Multidisciplinary liver tumor clinic+management conference | 355 |
| Kani et al23 a | Istanbul, Turkey | 2007–2013 | Pre vs. post, single center | Multidisciplinary management conference | 162 |
| Chirikov et al24 | SEER-Medicare | 2000–2007 | Retrospective, multicenter | Multispecialty care (3+ disciplines) between 4 wk prediagnosis to week of treatment | 2245 |
| Davison25 a | Sydney, Australia | 2003–2013 | Pre vs. post, single center | Multidisciplinary management conference | 177 |
| Vora26 a | Atlanta, USA | 2012–2015 | Retrospective, single center | Multidisciplinary liver tumor clinic | 447 |
| Agarwal et al27 | Madison, USA | 2002–2013 | Pre vs. post, single center | Multidisciplinary management conference | 655 |
| Serper et al28 | VA Health System, USA | 2008–2010 | Retrospective, multicenter | Multidisciplinary management conference | 3988 |
| Diaz et al29 a | Miami, USA | 2012–2014 | Retrospective, single center | Multidisciplinary management conference | 227 |
| Duininck et al30 | Atlanta, USA | 2009–2016 | Pre vs. post, single center | Multidisciplinary management conference | 204 |
| Sinn et al31 | Seoul, South Korea | 2005–2013 | Retrospective, single center | Multidisciplinary management conference | 6619 |
| Ehab et al32 a | Tampa, USA | 2014–2018 | Pre vs. post, single center | Multidisciplinary management conference | 259 (40% HCC) |
Conference abstract.
Quality Assessment
We used a modified National Institutes of Health Study Quality Assessment Tool to assess for risk of bias (Table 2). All studies clearly described the study objectives and patient eligibility criteria. Most studies had low risk of bias for exposure measurement; however, one study stratified patients based on count of disciplines visited as a surrogate of MDC. Most studies (n = 6) measured objective and guideline-concordant outcomes and were considered as low risk of bias. Many studies (n = 5) did not provide measures of variance, such as 95% CIs, when reporting differences in clinical outcomes between groups. A common limitation of studies (n = 6) was failure to report the length of follow-up time for outcome measurement. Many studies reporting associations between MDC and clinical outcomes did not adjust for potential confounders or were at high risk of residual confounding. For example, only 5 of 11 studies reporting survival estimates adjusted for both demographics and clinical characteristics.
TABLE 2.
Assessment for risk of bias across studies
| References | Consistent eligibility | Sample size | Time frame | Exposure measurement | Outcome measurement | Loss to follow-up | Confoundersa |
|---|---|---|---|---|---|---|---|
| Chang et al21 | Low | High | Low | Low | Medium | NR | High |
| Yopp et al22 | Low | Low | NR | Low | Low | Low | Medium |
| Kani et al23 | Low | High | NR | Low | Medium | NA | High |
| Chirikov et al24 | Low | Low | Low | High | Low | NA | Low |
| Davison25 | Low | High | NR | Low | Medium | NA | High |
| Vora26 | Low | Low | NR | Low | Medium | NA | High |
| Agarwal et al27 | Low | Low | Low | Low | Low | NA | Low |
| Serper et al28 | Low | Low | Low | Low | Low | NA | Low |
| Diaz et al29 | Low | Low | Low | Low | Medium | NA | High |
| Duininck et al30 | Low | Low | NR | Low | Low | NA | Low |
| Sinn et al31 | Low | Low | Low | Low | Low | NA | Low |
| Ehab et al32 | Low | Low | NR | Low | High | NA | High |
For studies reporting multiple outcomes, we assessed adjustment for confounders in survival analysis.
Abbreviations: NA, not applicable; NR, not reported.
Proportion of Patients With Early-stage HCC
Seven studies [n = 10,488 patients, of whom 2380 (22.7%) received MDC] reported data on tumor stage stratified by MDC status. Most studies (n = 4) used BCLC stage 0/A to define early-stage HCC, whereas 2 used the TNM staging system, and 1 used the Milan criteria (Table 3). Early-stage HCC was significantly associated with MDC, with a pooled RR of 1.60 (95% CI: 1.12–2.29) (Figure 1); however, there was significant heterogeneity (I 2 = 88%, p < 0.01). After excluding outlier studies (Chirikov and colleagues), sensitivity analysis revealed a similarly high heterogeneity (I 2 = 85%, p < 0.01) and the pooled estimate of association between early-stage presentation and MDC remained statistically significant (RR = 1.60, 95% CI: 1.15–2.23). The pooled proportions of early-stage HCC among patients who received and did not receive MDC were 47.2% (31.1%–63.8%) and 27.8% (15.1%–45.3%), respectively.
TABLE 3.
Clinical outcomes, stratified by MDC status
| References | Definition early-stage HCC | Early-stage HCC diagnosis | Curative therapy receipta | Time-to-treatment initiation | Factors adjusted in survival analysis | Overall survival |
|---|---|---|---|---|---|---|
| Chang et al21 | TNM stage I/II | MDC: 75/121 No MDC: 14/62 |
MDC: 23/121 No MDC: 4/62 |
NR | None | OR = 7.10 (95% CI: 3.46–14.5) |
| Yopp et al22 | BCLC stage A | MDC: 45/105 No MDC: 65/250 |
MDC: 22/105 No MDC: 24/250 |
Mean 2.3 vs. 5.3 (p = 0.002) | Tumor stage, HCC treatment | HR = 0.40 (95% CI: 0.33–0.49) |
| Kani et al23 | BCLC stage A | NR | Transplant: OR = 1.11, p = 0.79 Resection: OR = 1.06, p = 0.63 |
NR | None | Mean 11.1 vs. 7.4 mo (p = 0.01) |
| Chirikov et al24 | TNM stage I | MDC: 279/811 No MDC: 508/1434 |
NA | NR | Demographics, comorbidities, liver etiology, tumor stage, HCC treatment | HR = 0.86 (95% CI: 0.78–0.95)a |
| Davison25 | BCLC stage A | NR | MDC: 20.9% No MDC: 18.6% |
NR | None | Median 15.1 vs. 6.3 mo (p = 0.2) |
| Vora26 | Milan criteria | NR | Transplant: OR = 2.51, p = 0.002 | NR | None | No difference 1-y overall survival |
| Agarwal et al27 | Milan criteria | MDC: 147/306 No MDC: 90/349 |
MDC: 218/306 No MDC: 152/349 |
NR | Demographics, liver disease severity, AFP level, tumor stage, HCC treatment | HR = 0.72 (95% CI: 0.55–0.95) |
| Serper et al28 | BCLC stage A | NR | Any treatment: OR = 1.19 (95% CI: 0.98–1.46) | NR | Demographics, comorbidities, liver disease severity, tumor stage, HCC treatment, region | HR = 0.83 (95% CI: 0.77–0.90) |
| Diaz et al29 | BCLC stage A | MDC: 95/165 No MDC: 20/62 |
NR | No difference | None | Median 23.4 vs. 8.6 mo (p < 0.01) |
| Duininck et al30 | BCLC stage A | MDC: 24/134 No MDC: 4/70 |
MDC: 5/134 No MDC: 0/70 |
NR | Demographics, tumor stage, HCC treatment | HR = 0.62 (95% CI: 0.40–0.98) |
| Sinn et al31 | BCLC stage A | MDC: 523/738 No MDC: 3513/5881 |
MDC: 241/738 No MDC: 2001/5881 |
NR | Demographics, liver etiology, liver disease severity, tumor stage, AFP and DCP levels, HCC treatment | HR = 0.47 (95% CI: 0.41–0.53) |
| Ehab et al32 | Not defined | NR | NR | Median 17 vs. 24 d (p < 0.01) | NA | NR |
Calculated from p-value reported in manuscript.
Abbreviations: AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; DCP, des-gamma carboxy-prothrombin; MDC, multidisciplinary care; NA, not applicable; NR, not reported.
FIGURE 1.
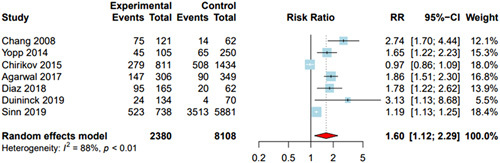
Association between multidisciplinary care and early-stage HCC. Patients under multidisciplinary care were significantly more likely to have early-stage HCC at presentation, with a pooled risk ratio (RR) of 1.60 (95% CI: 1.12–2.29). DerSimonian and Laird method was used for a random effects model.
Effect size and heterogeneity were similar in subgroup analyses restricted to studies that used BCLC stage 0/A or Milan criteria to define early-stage HCC (RR = 1.60, 95% CI: 1.15–2.23, I 2 = 85%). In this subgroup of studies, pooled proportions of early-stage HCC with and without MDC were 46.9% (24.7%–70.3%) and 27.0% (9.8%–55.6%), respectively. In additional subgroup analyses, we found that results were consistent across study location, with MDC being associated with early-stage HCC in the US (RR = 1.72, 95% CI: 1.13–2.60, I 2 = 90%). Finally, MDC was also associated with early-stage HCC among studies classified as low risk of bias (RR = 1.60, 95% CI: 1.15–2.23, I 2 = 85%) but not among those classified as higher risk of bias (RR = 1.59, 95% CI: 0.002–1158.9, I 2 = 94%).
Curative treatment receipt
Five studies [n = 8016 patients, of whom 1404 (17.5%) received MDC] reported data on curative treatment receipt stratified by MDC status (Table 3). MDC was positively associated with curative treatment; however, the pooled estimate was not statistically significant (pooled RR = 1.60; 95% CI: 0.89–2.89) and interpretation was limited by high heterogeneity (I 2 = 91%, p < 0.01) (Figure 2). Exclusion of 2 outlier studies (Agarwal and colleagues and Sinn and colleagues) led to a statistically significant association between MDC and curative treatment receipt (RR = 2.38, 95% CI: 1.34–4.23) without heterogeneity (I 2 = 0%). The pooled proportions of curative treatment receipt among patients receiving and not receiving MDC were 24.1% (5.7%–62.5%) and 10.7% (1.3%–52.5%), respectively.
FIGURE 2.
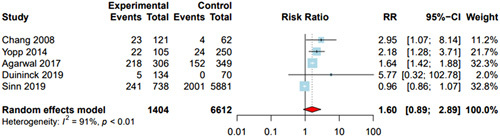
Association between multidisciplinary care and curative treatment receipt. Patients under multidisciplinary care had increased curative treatment, but the association was not statistically significant, with a pooled risk ratio (RR) of 1.60 (95% CI: 0.89–2.89). DerSimonian and Laird method was used for a random effects model.
Subgroup analysis by study location revealed a significant association between MDC and curative treatment receipt in the US (RR = 1.69; 95% CI: 1.37–2.09, I 2 = 0%). Finally, the association between MDC and curative treatment was not statistically significant among studies classified as low risk of bias (RR = 1.48, 95% CI: 0.73–3.02, I 2 = 93%) but was significant in the study at high risk of bias (RR = 2.95, 95% CI: 1.07–8.14).
Time-to-treatment initiation
Three studies (n = 685 patients) reported time-to-treatment initiation, stratified by MDC status. Shorter time-to-treatment initiation was reported by Yopp and colleagues (mean 2.3 vs. 5.3 mo, p = 0.002) and Ehab and colleagues (median 0.6 vs. 0.8 mo, p < 0.01), although both were quasi-experimental studies using a pre-post design. Diaz and colleagues compared patients with and without MDC during the same study period and found no difference in time-to-treatment initiation between the 2 groups.
OS
Eleven studies (n = 15,262 patients) assessed the association between MDC and survival. There was variability in reporting of survival data, with most studies (n = 6) reporting HRs with CIs, whereas others instead reported median or mean survival (n = 3), 1-year survival (n = 1), and OR for death (n = 1) (Table 3). Among the 4 studies that reported survival outcomes for MDC using landmark analyses, Kani and colleagues reported significantly higher mean OS (11.1 vs. 7.4 mo, p = 0.01), Diaz et al reported significantly higher median survival (23.4 vs. 8.6 mo, p < 0.01), Davison et al reported higher median OS although this did not reach statistical significance (15.1 vs. 6.3, p = 0.20), and Vora et al found no significant difference in 1-year OS. Among the 6 studies that reported HRs for death (n = 14,066 patients, of whom 3460 were under MDC), patients managed through MDC had reduced mortality, with a pooled HR of 0.63 (95% CI: 0.45–0.88); however, there was significant heterogeneity across studies (I 2 = 95%) (Figure 3). This association remained statistically significant after exclusion of an outlier study (Yopp and colleagues) in sensitivity analysis (HR = 0.69, 95% CI: 0.50–0.95); however, heterogeneity persisted (I 2 = 94%).
FIGURE 3.
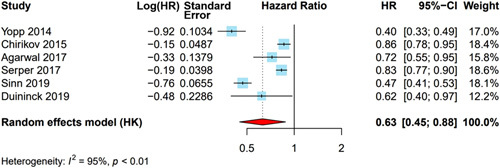
Association between multidisciplinary care and overall survival. Multidisciplinary care was significantly associated with improved survival, with a pooled HR of 0.63 (95% CI: 0.45–0.88); however, there was high heterogeneity (I 2 = 95%, p < 0.01). DerSimonian and Laird method was used for a random effects model.
MDC was associated with improved survival across subgroup analyses including studies from the US (HR = 0.67, 95% CI: 0.45–0.99, I 2 = 92%). MDC was associated with improved survival among studies classified as being at low risk of bias (HR = 0.59, 95% CI: 0.40–0.86, I 2 = 95%) and the study at high risk of bias (HR = 0.86, 95% CI: 0.78–0.95).
Four studies reported stage-stratified analyses evaluating the association between MDC and OS. Chang and colleagues found the odds of survival was greatest in patients with TNM stage II (OR = 15.5, 95% CI: 2.82–85.1) and stage IV (OR = 7.10, 95% CI: 3.46–14.5) HCC, with less benefit among those with stage I (OR = 1.44, 95% CI: 0.12–17.9) and stage III (OR = 2.19, 95% CI: 0.66–7.23) HCC. Similarly, Yopp and colleagues performed stage-stratified analyses by BCLC stage and found no significant difference in survival among patients with BCLC stage A or B HCC (p > 0.05 for both) but significantly improved survival with MDC in patients with BCLC stage C or D HCC (p < 0.001 and p = 0.01, respectively). Agarwal and colleagues reported that the benefit of MDC was observed in both patients with T1–T2 HCC (OR = 0.58, 95% CI: 0.37–0.92) and those with T3 or beyond (OR = 0.72, 95% CI: 0.55–0.93), whereas Kani and colleagues found the survival benefit of MDC was greatest in patients with BCLC stage A and stage B disease.
DISCUSSION
As HCC treatment algorithms become increasingly complex, MDC models aim to promote curative treatments and optimize OS. In this systematic review and meta-analysis, we found MDC was associated with significant improvements in OS but nonsignificant increases in curative treatment receipt. Few studies reported stage-specific analyses, although results appeared consistent across tumor stages and subgroup analyses. However, currently available data are limited by potential referral bias, residual confounding, and high inter-study heterogeneity, highlighting a need for continued research.
Multidisciplinary collaborations among health care professionals were initially implemented to amalgamate the expertise of various specialists to better treat complex diseases. HCC care has become increasingly complex, given the pathophysiologic nature of the disease itself, variation in tumor biology, expanded use of surgical resection and liver transplantation, and the continuous emergence and evolution of locoregional and systemic treatment options.30 Studies included in our systematic review all precede the use of immune checkpoint inhibitors, which is noteworthy given the growing interest in the use of these drugs for earlier tumor stages, as well as in novel combinations with surgery and locoregional therapies.33 If ongoing trials demonstrate a benefit of combination therapies, MDC may be increasingly important to facilitate effective communication between providers.9 As silos progressively break down between treating specialties, the need for MDC for patients with cancer will likely only increase in the future. Similarly, trials and other studies to define optimal care paths, including combination treatments, would also likely recruit best in an MDC setting.
Improved OS among patients with HCC managed through MDC is likely multifactorial and could be explained by enhanced treatment discussions as well as revised imaging and biopsy interpretations for both diagnosis and appropriate staging.31,34 Although the LI-RADS criteria offer objective criteria for HCC diagnosis, radiologic interpretation has imperfect interobserver reliability.35 Over time, The Liver Imaging Reporting and Data System (LI-RADS) has also improved the differentiation of LR-5 (definite HCC) from LR-M (malignancy but not definite HCC), with any questionable cases being classified as the latter category and necessitating biopsy for confirmation.36 There has also been increasing recognition that a subset of patients have combined HCC-cholangiocarcinoma, which can affect management decisions including recommended systemic therapy and eligibility for liver transplantation.37
Beyond implementation of MDC, provider adherence to MDC intervention recommendations is also important to consider.38 A retrospective cohort study among 387 patients with HCC for whom curative treatment was recommended in a multidisciplinary meeting demonstrated that provider adherence to recommendations, which occurred in 66% of patients, was associated with reduced mortality (HR = 0.39, 95% CI: 0.27–0.54).39 Results from another single-center study including 137 patients with HCC similarly showed that patients who received the recommended treatment per MDC conference were more likely to undergo liver transplantation compared with those who did not receive the recommended treatment (25.6% vs. 14.4%) and had a greater 1-year survival.38
Although MDC cannot directly contribute to early detection, as multidisciplinary conferences only manage patients already diagnosed with HCC, we found MDC implementation is associated with a higher proportion of patients with early-stage HCC. It is possible that MDC implementation increases center-level awareness of HCC and promotes surveillance and decreases time to diagnostic resolution.34 This improvement could also be related to concomitant interventions from the HCC program such as provider education or focused interventions to improve HCC surveillance use.40–42 However, given most studies used a pre-post study design, observed associations may simply represent improvements in early detection over time.43 In addition, this association may be related to referral bias, in which patients with early-stage HCC are more likely to be evaluated in MDC settings than those with advanced-stage or terminal-stage tumors. Although patients with advanced-stage or terminal-stage HCC typically are treated with systemic therapy or best supportive care, respectively, treatment decisions in patients with liver-localized disease often have multiple treatment options that must be considered. For example, patients can be bridged or downstaged with different locoregional therapies and then be considered for liver transplantation. Future studies evaluating this association and the potential for referral bias are critical to understand the magnitude of MDC benefits in patients with HCC.
Notably, MDC implementation within health care centers can face significant limitations that may challenge its applicability, particularly in resource-limited settings.44 For instance, inclusion of representatives from multiple academic disciplines into weekly team meetings requires substantial use of resources, including institutional funds and additional health care personnel time per patient.44–46 However, in light of the direct benefits of MDC on HCC management, health systems with sufficient resources should consider making this investment, particularly when it is justified by a large number of cases that would benefit from it.31,44 Furthermore, to reach optimal results, a multidisciplinary team has to surmount some of the commonly encountered barriers to effective clinical decision-making.47 These include hierarchies and lack of trust between team members as well as organizational issues such as scheduling conflicts and lack of time to prepare for meetings.47 Accordingly, effective leadership of a multidisciplinary team is crucial to promote inclusiveness, resolve logistical conflicts, and subsequently ensure more favorable outcomes.47,48
We acknowledge our study has several limitations. There was high heterogeneity across pooled estimates that persisted after sensitivity and study-level subgroup analyses. Given the lack of patient characteristics data, we were unable to generate patient-level subgroup analyses that could otherwise potentially justify heterogeneity. Furthermore, larger studies generally reported a smaller effect of MDC on clinical outcomes, suggesting that high-volume centers may derive less benefit from MDC than smaller centers with less experience.49,50 In addition, we found most studies were limited by using quasi-experimental pre-post design, so some improvements in clinical outcomes may be related to independent improvements in treatment modalities in the post-MDC period. Other limitations include inherent selection and lead-time biases that could favor clinical outcomes, poor reporting of loss to follow-up, and risk of residual confounding. Finally, there were substantial variations in the functional definition of MDC across centers; thus, we were unable to compare clinical outcomes between different types of MDC, such as the incremental value of a co-located clinic beyond a multidisciplinary tumor conference.21,51 Future studies should tackle these limitations to strengthen confidence in these results.
In summary, we found a consistent association between MDC and improved clinical outcomes for patients with HCC, including OS. However, these data must be considered in light of limitations including potential referral bias and between-study heterogeneity, highlighting a need for continued research. Current evidence as well as the evolving approach to HCC treatment suggests MDC should be considered the standard of care and implemented in most health systems, provided future studies continue to generate more high-quality evidence.
Supplementary Material
AUTHOR CONTRIBUTIONS
Amit G. Singal had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design (Amit G. Singal); acquisition, analysis, and interpretation of the data (all authors); drafting of the manuscript (Karim Seif El Dahan); critical revision of the manuscript for important intellectual content (all authors); obtained funding (Amit G. Singal); and study supervision (Amit G. Singal).
FUNDING INFORMATION
This study was conducted with support from NIH R01 CA256977, R01 MD012565, and U01 CA271888. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funding agencies had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation of the manuscript.
CONFLICTS OF INTEREST
Neehar Parikh has served as a consultant or on advisory boards for Fujifilm Medical Sciences, Exelixis, Freenome, Eisai, AstraZeneca, Genentech, and Gilead Sciences. Amit Singal has served as a consultant or on advisory boards for FujiFilm Medical Sciences, Exact Sciences, Roche, Glycotest, GRAIL, Freenome, Universal Dx, Genentech, AstraZeneca, Eisai, Bayer, Exelixis, and TARGET RWE. Ju Dong Yang has served as a consultant for AstraZeneca, Eisai, Exact Sciences, Exelixis, Fujifilm Medical Sciences, and Gilead Sciences. Hao Zhu has served as a consultant or on advisory boards for Alnylam Pharmaceuticals, Flagship Pioneering, Chroma Medicines, Ubiquitx, and AstraZeneca. Nicole Rich consults and advises AstraZeneca. Maria del Pilar Bayona Molano is on the speakers’ bureau for AstraZeneca. The remaining authors have no conflicts to report.
Footnotes
Abbreviations: AASLD, American Association for the Study of Liver Diseases; BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; HR, hazard ratio; HCC, hepatocellular carcinoma; I2, inconsistency index; LI-RADS, Liver Imaging Reporting and Data System; MDC, multidisciplinary care; OR, odds ratio; OS, overall survival; RR, risk ratio; TNM, tumor, nodes, and metastasis.
Contributor Information
Karim Seif El Dahan, Email: karim.seifeldahan@utsouthwestern.edu.
Annika Reczek, Email: annika.reczek@gmail.com.
Darine Daher, Email: Darine.Daher@UTSouthwestern.edu.
Nicole E. Rich, Email: nicole.rich@utsouthwestern.edu.
Ju Dong Yang, Email: judong.yang@cshs.org.
David Hsiehchen, Email: david.hsieh@utsouthwestern.edu.
Hao Zhu, Email: Hao.Zhu@utsouthwestern.edu.
Madhukar S. Patel, Email: madhukar.patel@utsouthwestern.edu.
Maria del Pilar Bayona Molano, Email: MariaDelPilar.BayonaMolano@UTSouthwestern.edu.
Purva Gopal, Email: purva.gopal@utsouthwestern.edu.
Neehar D. Parikh, Email: ndparikh@med.umich.edu.
Adam C. Yopp, Email: adam.yopp@utsouthwestern.edu.
Amit G. Singal, Email: amit.singal@utsouthwestern.edu.
REFERENCES
- 1. Huang DQ, Singal AG, Kono Y, Tan DJH, El-Serag HB, et al. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metab. 2022;34:969–977.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. [DOI] [PubMed] [Google Scholar]
- 3. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [DOI] [PubMed] [Google Scholar]
- 4. Rao A, Rich NE, Marrero JA, Yopp AC, Singal AG. Diagnostic and therapeutic delays in patients with hepatocellular carcinoma. J Natl Compr Canc Netw. 2021;19:1063–1071. [DOI] [PubMed] [Google Scholar]
- 5. Singal AG, Lok AS, Feng Z, Kanwal F, Parikh ND. Conceptual model for the hepatocellular carcinoma screening continuum: current status and research agenda. Clin Gastroenterol Hepatol. 2022;20:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reig M, Forner A, Rimola J, Ferrer-Fabrega J, Burrel M, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76:681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Asrani SK, Ghabril MS, Kuo A, Merriman RB, Morgan T, et al. Quality measures in HCC care by the Practice Metrics Committee of the American Association for the Study of Liver Diseases. Hepatology. 2022;75:1289–1299. [DOI] [PubMed] [Google Scholar]
- 8. Singal A, Llovet J, Yarchoan M, Mehta N, Heimbach J, et al. AASLD Guidance on Prevention, diagnosis and treatment of hepatocellular carcinoma. Hepatology. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Byrd K, Alqahtani S, Yopp AC, Singal AG. Role of multidisciplinary care in the management of hepatocellular carcinoma. Semin Liver Dis. 2021;41:1–8. [DOI] [PubMed] [Google Scholar]
- 10. Brar SS, Hong NL, Wright FC. Multidisciplinary cancer care: does it improve outcomes ? J Surg Oncol. 2014;110:494–499. [DOI] [PubMed] [Google Scholar]
- 11. Doe S, Petersen S, Buekers T, Swain M. Does a multidisciplinary approach to invasive breast cancer care improve time to treatment and patient compliance ? J Natl Med Assoc. 2020;112:268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roberts TJ, Lennes IT, Hawari S, Sequist LV, Park ER, et al. Integrated, multidisciplinary management of pulmonary nodules can streamline care and improve adherence to recommendations. Oncologist. 2020;25:431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schoenberger H, Rich NE, Jones P, Yekkaluri S, Yopp A, et al. Racial and ethnic disparities in barriers to care in patients with hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2021;21:1094–1096.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ray H, Beaumont A, Loeliger J, Martin A, Marston C, et al. Implementation of a multidisciplinary allied health optimisation clinic for cancer patients with complex needs. J Clin Med. 2020;9:2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Altman DG, Bland JM. How to obtain the confidence interval from a P value. BMJ. 2011;343:d2090. [DOI] [PubMed] [Google Scholar]
- 17. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 18. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 19. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rosenthal R. Combining results of independent studies. Psychol Bull. 1978;85:185–193. [Google Scholar]
- 21. Chang TT, Sawhney R, Monto A, Davoren JB, Kirkland JG, et al. Implementation of a multidisciplinary treatment team for hepatocellular cancer at a Veterans Affairs Medical Center improves survival. HPB (Oxford). 2008;10:405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yopp AC, Mansour JC, Beg MS, Arenas J, Trimmer C, et al. Establishment of a multidisciplinary hepatocellular carcinoma clinic is associated with improved clinical outcome. Ann Surg Oncol. 2014;21:1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kani HT, Siykhymbayev A, Demircan N, Banzragch M, Gunes Yegin E, et al. P577 Beneficial effect of multidisciplinary approach on survival in hepatocellular carcinoma patients. J Hepatol. 2014;60:S261. [Google Scholar]
- 24. Chirikov VV, Mullins CD, Hanna N, Breunig IM, Seal B, et al. Multispecialist care and mortality in hepatocellular carcinoma. Am J Clin Oncol. 2015;38:557–563. [DOI] [PubMed] [Google Scholar]
- 25. Davison S. Survival benefits of multi-disciplinary team care in hepatocellular carcinoma at Liverpool Hospital, New South Wales, 2003–2013. J Gastroenterol Hepatol. 2015;30:95–116. [Google Scholar]
- 26. Vora R. The utility of a multidisciplinary liver tumor clinic (MDTC) to treat patients with hepatocellular carcinoma (HCC) at a large volume liver transplant center. Hepatology. 2016;64:601–810. [Google Scholar]
- 27. Agarwal PD, Phillips P, Hillman L, Lucey MR, Lee F, et al. Multidisciplinary management of hepatocellular carcinoma improves access to therapy and patient survival. J Clin Gastroenterol. 2017;51:845–849. [DOI] [PubMed] [Google Scholar]
- 28. Serper M, Taddei TH, Mehta R, D’Addeo K, Dai F, et al. Association of provider specialty and multidisciplinary care with hepatocellular carcinoma treatment and mortality. Gastroenterology. 2017;152:1954–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Diaz CR, Payne D, Madrazo BL, Selvaggi G, Jones PD, et al. Tu1492—The barriers to implementation of Multidisciplinary Tumor Board Recommendations and their impact on hepatocellular carcinoma survival. Gastroenterology. 2018;154:S-1237–S-1238. [Google Scholar]
- 30. Duininck G, Lopez-Aguiar AG, Lee RM, Miller L, Dariushnia S, et al. Optimizing cancer care for hepatocellular carcinoma at a safety-net hospital: the value of a multidisciplinary disease management team. J Surg Oncol. 2019;120:1365–1370. [DOI] [PubMed] [Google Scholar]
- 31. Sinn DH, Choi GS, Park HC, Kim JM, Kim H, et al. Multidisciplinary approach is associated with improved survival of hepatocellular carcinoma patients. PLoS ONE. 2019;14:e0210730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ehab J, Powers B, Chin A, Zambrano C, Kim R, et al. Implementation of a patient-centered tumor board-based multidisciplinary program to improve quality and expedite care for hepatobiliary tumors. HPB. 2019;21:S100. [Google Scholar]
- 33. Singal AG, Kudo M, Bruix J. Breakthroughs in hepatocellular carcinoma therapies. Clin Gastroenterol Hepatol. 2023; S1542-3565(23)00106-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang J, Mavros MN, Cosgrove D, Hirose K, Herman JM, et al. Impact of a single-day multidisciplinary clinic on the management of patients with liver tumours. Curr Oncol. 2013;20:e123–e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yokoo T, Singal AG, Diaz de Leon A, Ananthakrishnan L, Fetzer DT, et al. Prevalence and clinical significance of discordant LI-RADS(®) observations on multiphase contrast-enhanced MRI in patients with cirrhosis. Abdom Radiol (NY). 2020;45:177–187. [DOI] [PubMed] [Google Scholar]
- 36. Chernyak V, Fowler KJ, Kamaya A, Kielar AZ, Elsayes KM, et al. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: imaging of hepatocellular carcinoma in at-risk patients. Radiology. 2018;289:816–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Beaufrère A, Calderaro J, Paradis V. Combined hepatocellular-cholangiocarcinoma: an update. J Hepatol. 2021;74:1212–1224. [DOI] [PubMed] [Google Scholar]
- 38. Gashin L, Tapper E, Babalola A, Lai KC, Miksad R, et al. Determinants and outcomes of adherence to recommendations from a multidisciplinary tumour conference for hepatocellular carcinoma. HPB (Oxford). 2014;16:1009–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Charriere B, Muscari F, Maulat C, Bournet B, Bonnet D, et al. Outcomes of patients with hepatocellular carcinoma are determined in multidisciplinary team meetings. J Surg Oncol. 2017;115:330–336. [DOI] [PubMed] [Google Scholar]
- 40. Wolf E, Rich NE, Marrero JA, Parikh ND, Singal AG. Use of hepatocellular carcinoma surveillance in patients with cirrhosis: a systematic review and meta-analysis. Hepatology. 2021;73:713–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mokdad A, Browning T, Mansour JC, Zhu H, Singal AG, et al. Implementation of a voice messaging system is associated with improved time-to-treatment and overall survival in patients with hepatocellular carcinoma. J Natl Compr Canc Netw. 2016;14:38–46. [DOI] [PubMed] [Google Scholar]
- 42. Singal AG, Reddy S, Radadiya Aka Patel H, Villarreal D, Khan A, et al. Multicenter randomized clinical trial of a mailed outreach strategy for hepatocellular carcinoma surveillance. Clin Gastroenterol Hepatol. 2021;20:2818–2825.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: new trends. J Hepatol. 2020;72:250–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Berardi R, Morgese F, Rinaldi S, Torniai M, Mentrasti G, et al. Benefits and limitations of a multidisciplinary approach in cancer patient management. Cancer Manag Res. 2020;12:9363–9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. De Ieso PB, Coward JI, Letsa I, Schick U, Nandhabalan M, et al. A study of the decision outcomes and financial costs of multidisciplinary team meetings (MDMs) in oncology. Br J Cancer. 2013;109:2295–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brauer DG, Strand MS, Sanford DE, Kushnir VM, Lim KH, et al. Utility of a multidisciplinary tumor board in the management of pancreatic and upper gastrointestinal diseases: an observational study. HPB (Oxford). 2017;19:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Soukup T, Lamb BW, Arora S, Darzi A, Sevdalis N, et al. Successful strategies in implementing a multidisciplinary team working in the care of patients with cancer: an overview and synthesis of the available literature. J Multidiscip Healthc. 2018;11:49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lamb BW, Brown KF, Nagpal K, Vincent C, Green JS, et al. Quality of care management decisions by multidisciplinary cancer teams: a systematic review. Ann Surg Oncol. 2011;18:2116–2125. [DOI] [PubMed] [Google Scholar]
- 49. Mokdad AA, Zhu H, Marrero JA, Mansour JC, Singal AG, et al. Hospital volume and survival after hepatocellular carcinoma diagnosis. Am J Gastroenterol. 2016;111:967–975. [DOI] [PubMed] [Google Scholar]
- 50. Mokdad AA, Murphy CC, Pruitt SL, Mansour JC, Marrero JA, et al. Effect of hospital safety net designation on treatment use and survival in hepatocellular carcinoma. Cancer. 2018;124:743–751. [DOI] [PubMed] [Google Scholar]
- 51. Patkar V, Acosta D, Davidson T, Jones A, Fox J, et al. Cancer multidisciplinary team meetings: evidence, challenges, and the role of clinical decision support technology. Int J Breast Cancer. 2011;2011:831605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


