Abstract
Introduction:
Up to 34% of patients with medulloblastoma develop posterior fossa syndrome (PFS) following brain tumor resection and have increased risk of long-term neurocognitive impairments. Lack of agreement in conceptualization and diagnosis of PFS calls for improvements in diagnostic methods. The current study aimed to describe psychometric properties of a new Posterior Fossa Syndrome Questionnaire (PFSQ).
Methods:
The PFSQ was informed by prior research and developed by a multidisciplinary team with subject matter expertise. Participants (N = 164; 63.4% Male; 78.7% White; Mage at diagnosis = 10.38 years, SD = 5.09, range 3 – 31 years) included patients with newly diagnosed medulloblastoma enrolled in the SJMB12 clinical trial. Forty-four patients (26.8%) were classified as having PFS based on attending physician’s post-surgical yes/no report. A PFSQ was completed by a neurologist within 2 weeks of coming to St. Jude Children’s Research Hospital for adjuvant treatment, irrespective of suspicion for PFS.
Results:
PFSQ items Ataxia (100.00%), Dysmetria (95.45%), and Speech/Language Changes (79.55%) were most sensitive. However, Ataxia (26.50%) and Dysmetria (46.61%) demonstrated low specificity. Speech/Language Changes (81.36%), Mutism (95.76%), Orofacial Apraxia (98.29%) and Irritability (96.61%) had high specificity. A principal component analysis found four components: 1) Speech/Language Changes, 2) Apraxias (including mutism), 3) Motor/Oromotor, and 4) Emotional Lability.
Conclusions:
The PFSQ is a dimensional diagnostic approach that can be used to improve diagnostic consistency across clinical and research groups to help accelerate understanding of PFS etiology, identify surgical correlates of risk, predict long-term impairments, and develop targeted interventions. Additional measure validation, including correlation with symptom resolution, is required.
Keywords: posterior fossa syndrome, cerebellar mutism syndrome, medulloblastoma, pediatric cancer, questionnaire
Introduction
Posterior fossa syndrome (PFS), also referred to as cerebellar mutism syndrome, is a condition that develops in up to 34% of patients with medulloblastoma following surgical resection of a posterior fossa tumor.1 Symptoms include delayed onset (1 – 6 days after surgery) speech and language difficulties, motor impairments, and emotional lability.1–4 Previously considered a transient condition due to significant symptom improvement over time, research has identified long-term impairments.5–9 The majority of patients with PFS continue to experience significant difficulties with ataxia, ambulation, and speech/language at greater than one year post diagnosis.1,7 Further, long-term neurobehavioral deficits have been observed, including reduced intellectual ability, processing speed, attention regulation, working memory, visual-spatial reasoning, and abnormalities on neurologic exam.1,7–8 As such, comprehensive rehabilitation, including physical and occupational therapy, speech-language therapy, and cognitive remediation, is imperative to improve quality of life following diagnosis.
Conceptualization of PFS among clinicians and researchers continues to evolve. When surveyed about the diagnostic practice of PFS, all experts conceptualized the syndrome as continuous, with symptoms that range from mild to severe, despite the typical dichotomous categorization in the research literature.10 While mutism was ranked as the most important diagnostic feature, the majority indicated that a period of mutism is not required to diagnose PFS.10 Variability in conceptualization was also evident based on years in practice, with different symptom emphasis for junior and senior experts.10 These findings challenged the representativeness of the term “cerebellar mutism syndrome” and are in-line with recent recommendations to categorize PFS into PFS1 (i.e., complete mutism) and PFS2 (i.e., diminished speech).1 Differentiating PFS1 from PFS2 allows for investigation of the importance of complete mutism with respect to diagnosis, etiology and recovery of function.
Given recent shifts and controversies in conceptualization of PFS, improvements in diagnostic methods are needed. Currently, PFS is typically diagnosed dichotomously (present/not present) by an attending physician, without evidence of inter-rater agreement among physicians and in conflict with expert consensus that PFS is a continuous condition.10 A bedside cognitive screen that includes a 10-item scale has been validated to assess Cerebellar Cognitive Affective Syndrome (CCAS)/Schmahmann Syndrome in adults with cerebellar injury.11 While PFS is sometimes conceptualized as an extreme form of CCAS,12 this measure is not specific to PFS and has not been validated for children. Although multiple groups are working on measures to aid in the diagnosis of PFS,13 to-date, only one questionnaire has been published.7 The Cerebellar Mutism Syndrome (CMS) Survey assesses time of symptom onset, and duration of mutism, ataxia, hypotonia, and irritability.7 The more delayed symptom onset and longer symptom duration, the more severe the rating.7 However, the rationale behind this diagnostic approach is unclear and survey validity has not been established. A validated dimensional diagnostic approach is needed to accelerate research investigating the etiology of PFS, prediction of long-term impairments, and development of targeted interventions.
In response to the need for enhanced diagnostic objectivity, that reflects the range of PFS presentations, a comprehensive questionnaire was developed by three of the authors (DR, RBK, HMC). The current study aims to evaluate the sensitivity and specificity of neurologist symptom ratings with respect to PFS diagnostic assignment of the attending physician. This study also aims to examine these symptom ratings to identify the core components of PFS. The overarching study goal is to develop a tool that can be used to improve diagnostic consistency across clinical and research groups.
Materials and Methods
Participants
All participants were enrolled in an ongoing St. Jude-initiated, multi-institutional clinical trial (SJMB12; NCT 01878617) for patients between the ages of 3 and 22 with newly diagnosed medulloblastoma (or less than 40 if SHH subtype). For the present study, only data from St. Jude patients with a questionnaire completed by St. Jude neurologists were included (N = 164). Data was collected from July 2013 to November 2019, and the study was approved by the Institutional Review Board. Written informed consent was obtained from the parents of all individual participants included in the study, and assent was obtained from all individual participants according to institutional age-based requirements.
All patients underwent surgical resection(s) of a posterior fossa tumor, but had no prior radiotherapy, chemotherapy, or other brain-directed therapy except corticosteroids. Following resection and initial neurological assessment, patients received low (15 CGE craniospinal radiation/51 CGE boost), standard (23.4 CGE craniospinal radiation/54 CGE boost), or high (36 CGE craniospinal radiation/54 CGE boost) radiation therapy as part of the SJMB12 protocol. Additionally, patients received 4–7 cycles of chemotherapy, with some receiving an oral targeted inhibitor as maintenance therapy based on molecular features (see Heitzer et al. 2019).14
Posterior Fossa Syndrome Questionnaire (PFSQ)
The Posterior Fossa Syndrome Questionnaire (PFSQ) was developed by a multidisciplinary team. First a neuropsychologist and author of the present study (DR) wrote items based on prior literature, input from experts, and her professional experience of following more than 50 patients with PFS from early in diagnosis through recovery. This version was shared with additional study authors (RBK [neurologist], HMC [neuropsychologist], GWR and AG [neuro-oncologists]), followed by group discussion, iterative refining of criteria, and consensus determination about items to include among DR, RBK and HMC. The final questionnaire is divided into three sections based on prior research: speech/language, motor, and emotional lability. Response options to items include “No, never,” “Yes, current,” “Yes, prior,” and “Don’t know.” Severity ratings and dates of resolutions are included whenever appropriate (see Figure 1).
Figure 1.
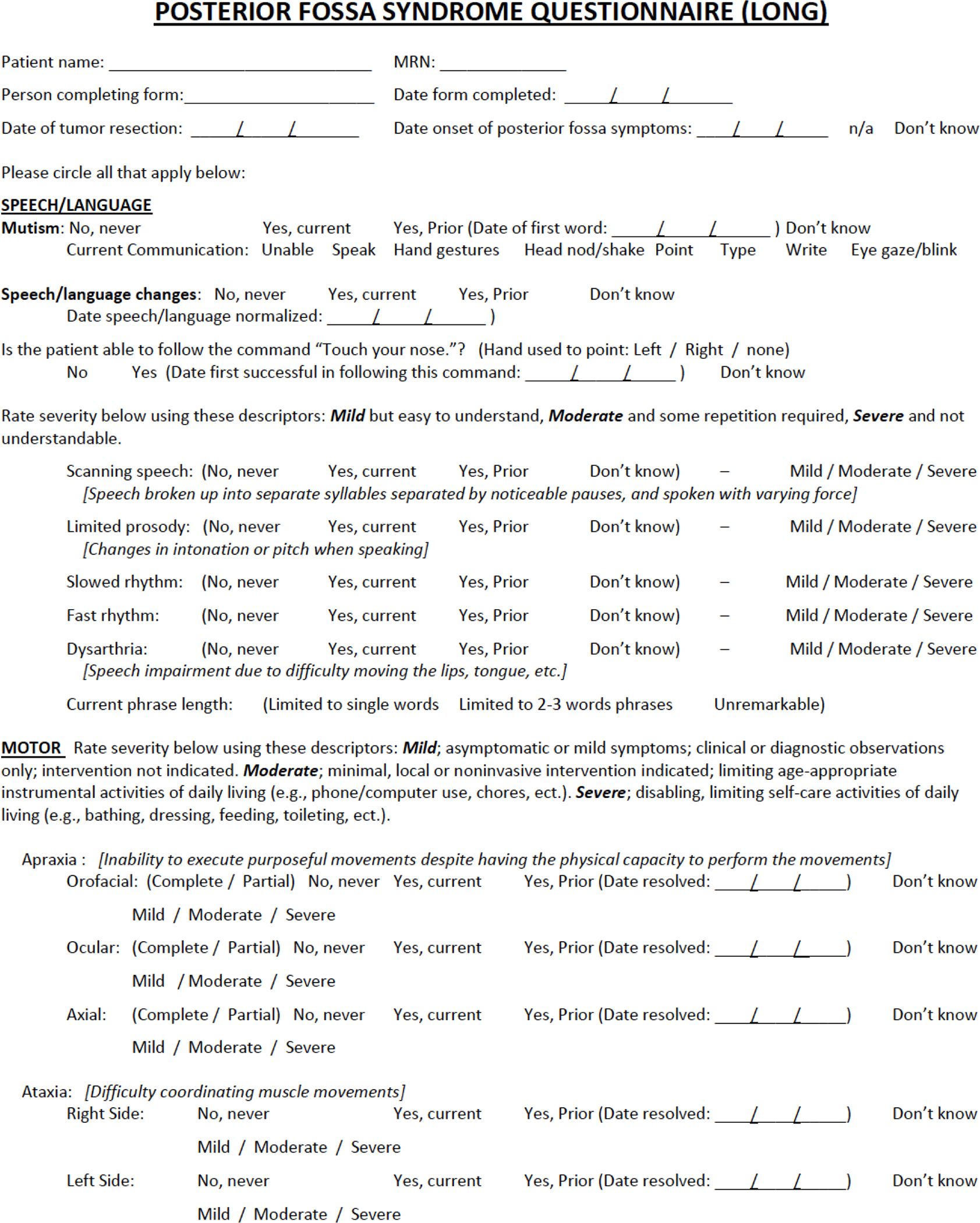

Posterior Fossa Syndrome Questionnaire (PFSQ)
Procedure
All patients in the current study were seen by neurology within the first 2 weeks of coming to St. Jude for the SJMB12 clinical trial. Referring surgical sites provided detailed documentation of patients’ symptoms in the interim between surgery and transfer of care. Almost all patients (n = 162) were seen by neurologist RBK, while two patients were seen by another St. Jude neurologist. Additional input on speech/language items was gained from a speech-language pathologist (KL) for patients when the skills were not demonstrated during neurologic examination (n = 10). As part of a neurological assessment, the PFSQ was completed once for all patients at a single time point, irrespective of suspicion of PFS. To enhance feasibility of questionnaire administration and completion, the presence/history (“Yes, current” or ”Yes, prior”) or absence (“No, never”) of main symptom-items (e.g., mutism, speech/language changes, ataxia, irritability) were assessed. Fourteen patients had a prior period of mutism documented in their transfer records that had resolved by the time of neurological exam and was coded as “Yes, prior”. Response option “Don’t know” was entered as missing. Severity ratings were not included in the present study’s analyses. Only the mutism resolution date was used in descriptive analyses, and this data was gathered from the PFSQ and from retrospective chart review. Additionally, no patients were rated as displaying fast rhythm of speech, so this item was excluded from analyses.
Statistical Analyses
All statistical analyses were conducted using SPSS version 27. Descriptive statistics of demographic and clinical variables and the PFSQ items were conducted to characterize the sample. Sensitivity and specificity analyses were used to evaluate the relationship between the ratings of the PFSQ items completed by neurology with respect to the “gold standard” yes/no PFS diagnostic assignment of the attending physician. Additionally, a principal component analysis (PCA) was conducted to investigate the core composite factors underlying the 15 PFSQ items. A PCA was used over other factor analysis techniques (e.g., principal axis factoring) because the primary aim of the present study was to reduce the symptom items into orthogonal principal components while maximizing the variance which could be accounted for. An oblique rotation (direct oblimin) was chosen to allow expected moderate correlations among symptom factors. Factor loadings with an absolute value greater than 0.40 were used, as recommended by Pituch and Stevens (2016).15
Results
At the time of data collection, most patients (n = 138; 84.1%) had only underwent one resection surgery, with a range from one to four resections. The majority were gross total resections (n = 132; 80.5%), followed by near total (n = 19; 11.6%) and subtotal (n = 13; 7.9%) resections, respectively. Le Bonheur Children’s Hospital performed 50 (30.5%) of the resections, with the remainder occurring at other institutions (see Khan et al. 2020). One-hundred-four patients (63.4%) were male, 129 (78.7%) were White, and the average age at diagnosis was 10.38 years (SD = 5.09; range 3–31 years). Based on the post-surgical attending physician’s yes/no report (following review of surgery notes and initial physical exam), 44 patients (26.8%) were classified as having PFS. There were no differences in number of resections, extent of resection or time since last resection in patients classified as having or not having PFS (p > .05). Two participants PFS statuses were classified as “Unknown” (neither “yes” or “no” PFS diagnosis) by the post-surgical attending physician and were therefore excluded from primary analyses.
Descriptive statistics of demographic and clinical variables are shown in Table 1. Of note, all patients who experienced mutism eventually had return of speech, and dates from resection until first word spoken were gathered retrospectively for all but 1 patient (n = 34). Mutism spanned a wide range (3 – 244 days), with a median of 25 days. Frequencies of PFSQ items endorsed (“Yes, current” or “Yes, prior”) among patients with and without PFS (as diagnosed by attending physician) are displayed in Table 2. Items endorsed by the rating neurologist for most patients diagnosed with PFS by the attending physician included: Ataxia (100.0%), Dysmetria (95.5%), Speech/Language Changes (93.1%), Irritability (75%), Mutism (68.2%), Hemiparesis (59.1%), and Orofacial Apraxia (53.5%). For patients without PFS, only Ataxia (73.5%) and Dysmetria (53.5%) were endorsed for the majority.
Table 1.
Demographic and Clinical Characteristics of the Sample (N = 164)
| n | (%) | |
|
| ||
| Gender | ||
| Male | 104 | 63.4 |
| Female | 60 | 36.6 |
| Race | ||
| White | 129 | 78.7 |
| Black | 12 | 7.3 |
| Asian | 9 | 5.5 |
| Pacific Islander | 1 | 0.6 |
| Multi-racial | 13 | 7.9 |
| Extent of Surgical Resection a | ||
| STR | 13 | 7.9 |
| NTR | 19 | 11.6 |
| GTR | 132 | 80.5 |
| PFS b | ||
| No | 118 | 72.0 |
| Yes | 44 | 26.8 |
| Unknownc | 2 | 1.2 |
|
| ||
| Mean ± SD | Range | |
|
| ||
| Age at Diagnosis | 10.38 ± 5.09 | 3.17 – 31.33 |
| Number of Resections d | 1.18 ± 0.46 | 1 – 4 |
| Days from resection until resolution of mutism | 36.09 ± 40.51 | 3 – 244 |
STR subtotal resection, incomplete tumor resection with gross residual disease present on neuroimaging, NTR near total resection, incomplete tumor resection with minimal residual disease present on post-operative neuroimaging, GTR gross total resection, resection of tumor without apparent gross residual disease observed by the operating neurosurgeon and confirmed on operative neuroimaging
PFS osterior fossa syndrome
Unknown classified as neither “yes” or “no” PFS diagnosis by post-surgical attending physician; participants were excluded from primary analyses
The distribution for number of resections included: 1 resection (n=138), 2 resections (n=23), 3 resections (n=2), 4 resections (n=1)
Table 2.
PSFQ Items Endorsed (“Yes, current” or “Yes, prior”)
| n (%) | ||
|
| ||
| Yes PFS | No PFS | |
|
| ||
| Speech/Language | ||
| Mutism | 30 (68.2) | 5 (4.2) |
| Speech/Language Changes | 41 (93.1) | 22 (18.7) |
| Scanning Speech | 7 (15.9) | 3 (2.5) |
| Limited Prosody | 14 (31.8) | 13 (11.0) |
| Slowed Rhythm | 16 (36.3) | 13 (11.0) |
| Dysarthria | 18 (40.9) | 11 (9.3) |
|
| ||
| Yes PFS | No PFS | |
|
| ||
| Motor | ||
| Orofacial Apraxia | 23 (53.5) | 2 (1.7) |
| Ocular Apraxia | 15 (37.5) | 3 (2.6) |
| Axial Apraxia | 20 (46.5) | 1 (0.9) |
| Ataxia | 43 (100.0) | 86 (73.5) |
| Dysmetria | 42 (95.5) | 63 (53.3) |
| Tremor | 12 (27.3) | 16 (13.5) |
| Hemiparesis | 26 (59.1) | 9 (7.8) |
|
| ||
| Yes PFS | No PFS | |
|
| ||
| Emotional Lability | ||
| Irritability | 33 (75.0) | 13 (11.0) |
| Excessive Laughter | 4 (9.1) | 4 (3.4) |
Sensitivity and specificity analyses can be found in Table 3. High sensitivity means that the PSFQ item was frequently endorsed for patients diagnosed with PFS by an attending physician. High specificity means that the PSFQ item was not frequently endorsed for patients without a PFS diagnosis. Ataxia (100.00%), Dysmetria (95.45%), and Speech/Language Changes (79.55%) were the most sensitive items (i.e., most commonly experienced by patients with PFS). However, Ataxia (26.50%) and Dysmetria (46.61%) have low specificity (i.e., a significant number of patients without PFS also experienced these symptoms). The other PSFQ items specificity ranged from 81.36% (Speech/Language Changes) to 99.15% (Axial Apraxia).
Table 3.
Sensitivity and Specificity Analyses
| Sensitivity | Specificity | |
|
| ||
| Speech/Language | ||
| Mutism | 43.18% | 95.76% |
| Speech/Language Changes | 79.55% | 81.36% |
| Scanning Speech | 35.00% | 97.37% |
| Limited Prosody | 60.00% | 88.79% |
| Slowed Rhythm | 59.09% | 88.60% |
| Dysarthria | 73.91% | 90.52% |
|
| ||
| Sensitivity | Specificity | |
|
| ||
| Motor | ||
| Orofacial Apraxia | 39.53% | 98.29% |
| Ocular Apraxia | 35.00% | 97.44% |
| Axial Apraxia | 39.53% | 99.15% |
| Ataxia | 100.00% | 26.50% |
| Dysmetria | 95.45% | 46.61% |
| Tremor | 27.27% | 86.44% |
| Hemiparesis | 56.82% | 92.24% |
|
| ||
| Sensitivity | Specificity | |
|
| ||
| Emotional Lability | ||
| Irritability | 9.09% | 96.61% |
| Excessive Laughter | 52.27% | 88.98% |
Results from the PCA (factor loadings after rotation) are displayed in Table 4. Data was excluded if any of the 15 PFSQ items were unanswered, which resulted in a sample size of 127. Missing data was a result of incomplete items on the PFSQ. There were no differences in patients included in the PCA versus those excluded on gender, race or number of surgical resections. Patients included in the PCA were older (11.05 years versus 8.10 years; p= .002). Four components were found, which largely overlapped with the conceptual domains that have been identified in the literature. The items that clustered together suggest that Component 1 represents Speech/Language Changes, accounts for 38.95% of the variance, and evidenced excellent reliability (α = .92). Component 2 represents Apraxias (including mutism), accounts for 12.88% of the variance, and demonstrated excellent reliability (α = .93). Component 3 represents Motor/Oromotor (9.47%), while Component 4 represents Emotional Lability (7.82%), both of which evidenced lower reliability (αs = .69 and .53, respectively). The Component Correlation Matrix indicated that Speech/Language Changes was correlated with Apraxias (r = −.43), Motor/Oromotor (r = .26), and Emotional Lability (r = .08); Apraxias was additionally correlated with Motor/Oromotor (r = −.14) and Emotional Lability (r = .003); and, the correlation between Motor/Oromotor and Emotional Lability was r = .14. These results confirm the use of an obliq rotation, particularly given the correlations between Speech/Language Changes, Apraxias, and Motor/Oromotor.
Table 4.
Principal Component Analysis (N = 127)
| Rotated Factor Loadings | ||||
|---|---|---|---|---|
| Item | Speech/Language Changes | Apraxia | Motor/Oromotor | Emotional Lability |
| Limited Prosody | .93 | |||
| Speech/Language Changes | .92 | |||
| Slowed Rhythm | .87 | |||
| Scanning Speech | .74 | |||
| Dysarthria | .66 | |||
| Orofacial Apraxia | −.89 | |||
| Ocular Apraxia | −.88 | |||
| Mutism | −.80 | |||
| Axial Apraxia | −.62 | |||
| Tremor | −.29 | .72 | .30 | |
| Hemiparesis | .72 | −.27 | ||
| Dysmetria | .29 | .66 | ||
| Ataxia | .59 | |||
| Excessive Laughter | .91 | |||
| Irritability | .45 | .50 | ||
| Eigenvalues | 5.84 | 1.93 | 1.42 | 1.17 |
| % of variance | 38.95 | 12.88 | 9.47 | 7.82 |
| α | .92 | .93 | .69 | .53 |
Note: Factor loadings over .40 appear in bold.
Discussion
Given the lack of agreement in conceptualization and diagnosis of PFS, the present study aimed to introduce and describe the psychometric properties of a dimensional diagnostic tool with the goal of improving diagnostic consistency across clinical and research groups. This study demonstrated the feasibility of using the PFSQ to systematically assess a large sample of prospectively followed children with recently diagnosed medulloblastoma. Findings indicated PFSQ items assessing ataxia and dysmetria are most sensitive but least specific with respect to the PFS diagnostic assignment of the attending physician. All other PFSQ items demonstrated high specificity indicating mutism, speech/language changes, apraxia, tremor, hemiparesis, irritability, and excessive laughter differentiate children with PFS from other children treated for medulloblastoma, while ataxia and dysmetria will occur frequently irrespective of PFS status.
Among speech/language changes, fast rhythm and scanning speech (i.e., speech broken into separate syllables separated by noticeable pauses and spoken with variable force) are not as commonly experienced among children diagnosed with PFS as are limited prosody, slowed rhythm and dysarthria. Mutism is highly specific but not as sensitive as we hypothesized given its historical significance,2–5,7,13 with 32% diagnosed with PFS in the present sample not having a period of complete mutism. These findings are consistent with diagnostic recommendations to categorize PFS into subtypes such as PFS1 (complete mutism) and PFS2 (diminished speech) that not only further characterize where children fall on the PFS continuum but also are of prognostic relevance with respect to recovery.1 Should other symptoms (e.g., delayed on set of PFS symptoms7 or delayed recovery of PFS symptoms1) be shown to have prognostic value with respect to log-term outcomes, they could further be incorporated into the diagnostic process.
A principal component analysis revealed four factors: 1) Speech/Language Changes, 2) Apraxias (including mutism), 3) Motor/Oromotor, and 4) Emotional Lability that largely fit with theoretical symptom categories in the literature.2–4 Interestingly apraxias separated as their own factor that included mutism, rather than mutism clustering with speech language changes. This fits with the conceptualization of apraxia as a major driver of mutism along with clinical presentations in which children cannot perform confrontational naming despite instances of reflexive speech.6,13 This finding is also consistent with demonstrated surgical injury to proximal components of the bilateral efferent cerebellar pathways resulting in disrupted cerebellar output to the supratentorial brain.6 A resultant cerebello-cerebral diaschisis has been proposed in which there is reduced perfusion of frontal brain regions that underlies speech changes.6,13 Interestingly, irritability did not load as strongly on the emotional lability factor as did excessive laughter; this finding might suggest this symptom is both a sign of emotional dysregulation as well as frustration in response to recently acquired communication and motor impairments.
Current study strengths include a large sample of prospectively followed patients, using a standardized assessment measure that was administered to patients with and without PFS. Sensitivity and specificity analyses helped elucidate the core PFS diagnostic elements from a clinician’s perspective; while, the principal components analysis provided insights into the etiology of particular PFS symptoms. Challenges in this study included conducting PFS assessments promptly after emergence of symptoms as patients transferred from outside surgical centers, and finding ways to systematically include interdisciplinary input in real-time with speech language consultation for some cases ultimately based on retrospective chart review.
Taken together, conducted analyses demonstrate the diagnostic range and homogeneity of PFS that can be used to refine diagnostic criteria. For instance, proposed diagnostic criteria for PFS include an acquired cerebellar injury with mutism or speech/language impairment, in addition to changes in mood/affect or motor dysfunction (including apraxias; see Wickenhauser et al. 2020).10 While results of the present study corroborate continued emphasis of particular speech/language impairments such as reduction of speech and slowed or gaited rhythm, scanning and ballistic features of speech might be de-emphasized while expression and melody of speech may warrant greater credence. An additional criterion that differentiates apraxias, including mutism, from general motor dysfunction may also be merited and help aid in categorization into PFS subtypes. Further, specification of behavioral indicators of mood/affect changes such as excessive laughter and/or tearfulness and flat affect may need to be distinguished from broad emotional states (e.g., irritation, agitation, anger).
Future directions include increased collaboration with speech/language pathologists to refine speech and language items including replacing scanning speech with more accurate speech descriptors, capturing dysphagia that is often seen in children with PFS, and developing items that are appropriate for young, prelinguistic children. It would also be of benefit to explore additional items as they relate to affective presentations associated with PFS including exploration of the frequency and context of flat affect in addition to emotional lability. For tools such as the PFSQ to gain broad based acceptance, it will also be critical to demonstrate ability to implement across clinical institutions and establish inter-rater reliability. The authors acknowledge many facilities may not have the resources to employ a comprehensive multi-disciplinary approach to diagnosis of PFS. Although this approach may be best clinical practice, an alternative solution that enhances scalability could be creating a shorter, single-rater questionnaire and a longer, team-based version. Future plans include further validation of the PFSQ through association of initial PFSQ ratings with resolution of PFS symptoms as this cohort of children is followed over time as well as correlation with neuroimaging findings.
Current findings indicate a dimensional diagnostic approach can be used to identify and differentiate children with a common set of PFS symptoms that reflects diagnostic practices of clinicians and is consistent with a spectrum of symptom severity. Use of a uniform diagnostic approach is needed to accelerate research discoveries related to the etiology of PFS, identify surgical correlates of risk, and predict long-term impairments. Consistent PFS diagnosis is a necessary step in reducing the incidence of this potentially debilitating postoperative condition and developing targeted interventions to mitigate long-term functional impairments.
Acknowledgements:
The authors thank the patients and families who participated in the SJMB12 clinical trial. We also thank our multidisciplinary team members for their valuable contributions to this work.
Funding:
This work was supported, in part, by the National Cancer Institute (St. Jude Cancer Center Support [CORE] Grant [P30-CA21765]) and the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Competing Interests: The authors have no relevant financial or non-financial interests to disclose.
Ethics Approval: This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of St. Jude Children’s Research Hospital.
Data Availability:
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Khan RB, Patay Z, Kilmo P, et al. Clinical features, neurologic recovery, and risk prediction of post-operative posterior fossa syndrome: A prospective study. Neuro Oncol. 2020;22:28. 10.1093/neuonc/noab030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gudrunardottir T, Morgan AT, Lux AL, et al. Consensus paper on post-operative pediatric cerebellar mutism syndrome: The Iceland Delphi results. Childs Nerv Syst. 2016;32(7):1195–1203. 10.1007/s00381-016-3093-3 [DOI] [PubMed] [Google Scholar]
- 3.De Smet HJ, Baillieux H, Wackenier P, et al. Long-term cognitive deficits following posterior fossa tumor resection: A neuropsychological and functional neuroimaging follow-up study. Neuropsychology. 2009;23(6):694–704. 10.1037/a0016106 [DOI] [PubMed] [Google Scholar]
- 4.Korah MP, Esiashvili N, Mazewski CM, et al. Incidence, risks, and sequelae of posterior fossa syndrome in pediatric medulloblastoma. Int J Radiat Oncol Biol Phys. 2010;77(1):106–112. 10.1016/j.ijrobp.2009.04.058 [DOI] [PubMed] [Google Scholar]
- 5.Gudrunardottir T, Sehested A, Juhler M, Schmiegelow K. Cerebellar mutism: Review of the literature. Childs Nerv Syst. 2011;27(3):355–363. 10.1007/s00381-010-1328-2 [DOI] [PubMed] [Google Scholar]
- 6.Patay Z Postoperative posterior fossa syndrome: Unraveling the etiology and underlying pathophysiology by using magnetic resonance imaging. Childs Nerv Syst. 2015;31(10):1853–1858. 10.1007/s00381-015-2796-1 [DOI] [PubMed] [Google Scholar]
- 7.Robertson PL, Muraszko KM, Holmes EJ, et al. Incidence and severity of postoperative cerebellar mutism syndrome in children with medulloblastoma: A prospective study by the Children’s Oncology Group. J Neurosurg. 2006;105(6 Suppl):444–451. 10.3171/ped.2006.105.6.444 [DOI] [PubMed] [Google Scholar]
- 8.Schreiber JE, Palmer SL, Conklin HM, et al. Posterior fossa syndrome and long-term neuropsychological outcomes among children treated for medulloblastoma on a multi-institutional, prospective study. Neuro Oncol. 2017;19(12):1673–1682. 10.1093/neuonc/nox135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolfe-Christensen C, Mullins L, Scott J, McNall-Knapp R. Persistent psychosocial problems in children who develop posterior fossa syndrome after medulloblastoma resection. Pediatr Blood Cancer. 2007;49(5):723–726. 10.1002/pbc.21084 [DOI] [PubMed] [Google Scholar]
- 10.Wickenhauser ME, Khan RB, Raches D, et al. Characterizing posterior fossa syndrome: A survey of experts. Pediatric neurology. 2020;104:19–22. 10.1016/j.pediatrneurol.2019.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoche F, Guell X, Vangel MG, Sherman JC, Schmahmann JD. The cerebellar cognitive affective/Schmahmann syndrome scale. Brain. 2018;141(1):248–370. 10.1093/brain/awx317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmahmann JD. Pediatric post-operative cerebellar mutism syndrome, cerebellar cognitive affective syndrome, and posterior fossa syndrome: Historical review and proposed resolution to guide future study. Childs Nerv Syst. 2020;36(6):1205–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Catsman-Berrevoets C, Patay Z. Cerebellar mutism syndrome. Hand Clin Neurol. 2018;155:273–288. 10.1007/s00381-019-04253-6 [DOI] [PubMed] [Google Scholar]
- 14.Heitzer AM, Ashford JM, Harel BT, et al. Computerized assessment of cognitive impairment among children undergoing radiation therapy for medulloblastoma. J Neurooncol. 2019;141(2):403–411. 10.1007/s11060-018-03046-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pituch KA, Stevens JP. Applied Multivariate Statistics for the Social Sciences: Analyses with SAS and IBM’s SPSS. 6th ed. London: Routledge; 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.


