Abstract
Women of childbearing age in Western societies are increasingly adopting vegetarian diets. These women are sometimes rejected as milk donors, but little about the composition of their milk is known. The present study aimed to compare the intake, nutritional status, and nutritional composition of human milk from omnivore human milk donors (Donors) and vegetarian/vegan lactating mothers (Veg). Milk, blood, and urine samples from 92 Donors and 20 Veg were used to determine their fatty acid profiles, as well as vitamins and minerals. In a representative sample of both groups, we also determined the lipid class profile as a distribution of neutral and polar lipids, the molecular species of triacylglycerols, and the relative composition of phospholipids in their milk. A dietary assessment was conducted with a five-day dietary record (while considering the intake of supplements). We highlight the following results, expressed as the mean (SE), for the Veg vs. Donors: (1) Their docosahexaenoic acid (DHA) intake was 0.11 (0.03) vs. 0.38 (0.03) g/day; the plasma DHA was 0.37 (0.07) vs. 0.83 (0.06)%; and the milk DHA was 0.15 (0.04) vs. 0.33 (0.02)%. (2) Their milk B12 levels were 545.69 (20.49) vs. 482.89 (4.11) pM; 85% of the Veg reported taking B12 supplements (mean dose: 312.1 mcg/day); and the Veg group showed no differences with Donors in terms of total daily intake or plasma B12. (3) Their milk phosphatidylcholine levels were 26.88 (0.67) vs. 30.55 (1.10)%. (4) Their milk iodine levels were 126.42 (13.37) vs. 159.22 (5.13) mcg/L. In conclusion, the Vegs’ milk was shown to be different from the Donors’ milk, mainly due to its low DHA content, which is concerning. However, raising awareness and ensuring proper supplementation could bridge this gap, as has already been achieved for cobalamin.
Keywords: breast milk, human milk bank, vegetarian, diet, nutritional status, lipid profile, vitamins, minerals, vitamin B12, docosahexaenoic acid
1. Introduction
A mother’s own milk is the gold standard for the feeding of preterm and full-term infants. The World Health Organization (WHO) recommends exclusive breastfeeding for the first 6 months of life and its continuation during the introduction of complementary foods, up to 2 years or longer [1]. For preterm infants, donor human milk (DHM) is the preferred feeding strategy when their own mother’s milk is not available [2,3,4].
Despite the importance of human milk in infant nutrition, there is a lack of robust knowledge about its nutritional composition; moreover, this is even more evident in DHM. A recent systematic review highlighted the lack of available information on the vitamin and mineral composition of DHM. Furthermore, the findings in maternal milk should not be generalized to DHM due to additional sources of variation conditioned by the processes that DHM undergoes (i.e., expression, freezing, storage, pooling, mixing, multiple container transfers, and pasteurization) [5]. Premature infants have increased nutritional needs, so studying the nutritional content of DHM and the factors that may influence it should be mandatory.
Among the many factors that influence the composition of human milk, one of the most interesting is the mother’s diet, as it is a modifiable factor that can be improved with health advice. However, the impact of the maternal diet on the nutritional profile variations in human milk between women is not yet fully understood, most likely because of the fact that there are many factors that modify the composition of the milk, not only those related to the characteristics of the breastfeeding woman, such as genetics, diet, lifestyle, etc., but also related to breastfeeding itself, such as the type and time of expression, the stage of lactation, etc. [5,6,7,8]. In addition, studies have generally focused on a few nutrients and have not assessed the full nutritional profile of milk.
In the last few years, there has been a renewed effort to better understand the relationship between the maternal diet, nutritional status, and human milk quality. This has been conducted not only in resource-poor countries, but also in resource-rich settings, since modern trends in diet and lifestyle may compromise human milk nutrient levels [9,10].
Moreover, women of childbearing age are increasingly adopting vegetarian or vegan diets in Western societies. However, despite the numerous health benefits, vegetarians without proper dietary advice are at a high risk of inadequate intake of and an inadequate status for several nutrients, mainly vitamin B12, vitamin D, iron, iodine, zinc, calcium, selenium, and docosahexaenoic acid (DHA). Additionally, the more restrictive the vegetarian diet is, the greater the risk of dietary inadequacy [11,12]. Consequently, human milk banks have been faced with the challenge of determining whether these women are suitable to be milk donors, with the aim of ensuring the safety and quality of DHM via donor selection processes [13].
Following the recommendations of the European Milk Bank Association, a vegan diet with an adequate supplementation of vitamin B12 is not an exclusion criterion for donor candidates [13]. Nevertheless, certain countries do not accept milk donations from vegans, regardless of their vitamin B12 supplementation status [12]. According to the American Dietetic Association, the breast milk of vegetarian mothers with an appropriately planned diet is similar in its composition to that of non-vegetarians, except with respect to fatty acid (FA) concentration [14].
However, only a few studies have determined the macronutrients, FAs, vitamin B12, folate, and minerals in the milk of women who follow a vegetarian/vegan diet [12,15], whereas other important nutrients have not been assessed, including fat-soluble vitamins, vitamin C, many of the B group vitamins, and other lipid compounds different from FAs.
Finally, we could not find any study focused on comparing the human milk nutritional composition of DHM with that of vegetarian women.
The objective of the present study is to compare the maternal intake, nutritional status, and nutritional composition (i.e., macronutrients, water-soluble and fat-soluble vitamins, minerals, FA profile, lipid class prolife, molecular species of triacylglycerols, and the relative composition of phospholipids) of human milk from omnivore human milk donors and vegetarian/vegan lactating mothers.
2. Materials and Methods
2.1. Study Design and Subjects
This cross-sectional, observational study was conducted at the Regional Human Milk Bank Aladina MGU (RHMB) at “12 de Octubre” University Hospital in Madrid, Spain. The subjects of study were as follows: (1) human milk donors with an omnivore diet and with full-term infants (Donors) who donated milk at least once in the last 2 months to the RHMB; and (2) healthy vegetarian/vegan lactating mothers (Veg) with a milk expression routine who were lactating 3 weeks or more postpartum. Vegetarians were defined as women who did not consume any kind of meat or fish, but did consume eggs and/or milk, including ovo-vegetarians, lacto-vegetarians, and ovo-lacto-vegetarians. Vegans were defined as those women who also excluded any animal-derived foods, such as eggs, dairy products, honey, beeswax, gelatin, and other animal-derived ingredients.
Participants were recruited between August 2017 and February 2020.
The study procedure was endorsed by the “12 de Octubre” University Hospital Clinical Research Ethics Committee (protocol code 15/269) and all participants provided written informed consent, which could be withdrawn at any time. Furthermore, the study was conducted in accordance with the Declaration of Helsinki.
2.1.1. Sample Size Calculation
The present work is part of a larger study in which 3 groups of women were studied: (1) human milk donors, (2) vegetarian/vegan lactating mothers, and (3) lactating mothers of very preterm and/or very-low-birth-weight infants—i.e., ≤32 weeks gestational age and/or ≤1500 g—who were admitted to the neonatal unit of the “12 de Octubre” University Hospital at the time of the study. One of the main aims of this work was to study the correlations between the intake, nutritional status, and human milk nutritional composition of the mothers. For this purpose, the sample size was calculated to guarantee the generalizability of the results. In this sense, considering the need for at least 10 cases per predictor and an additional 5% for possible losses, a sample of 115 participants was estimated for the models of the global study, in which a total of 10 predictors of clinical interest were included. This involved the recruitment of at least 85 milk donors, 15 healthy lactating women on a vegetarian diet, and 15 mothers of preterm infants. For this study, we selected milk donors with an omnivorous diet and a full-term infant as a group which is more comparable to that of vegetarian mothers.
2.1.2. Study Protocol
The study participants were contacted and provided with detailed information on the objectives of the study and the procedures to be carried out. Donors’ contact details were provided by the RHMB. Vegetarians/vegans were advised about the study by the pediatricians of the primary care centers in the “12 de Octubre” University Hospital’s health area, as well as through advertisements placed on online communities that were focused on breastfeeding or vegetarianism, on websites dedicated to vegetarians, or in vegetarian food shops.
An early morning meeting at the RHMB was arranged for each of the women who agreed to participate in the study. At this appointment (day 0), the participant’s blood and urine samples for biochemical studies, somatometric measurements, informed consent for the study, health and socio-demographic survey, and food frequency questionnaire (FFQ) were collected. The participants provided a sample of their first urine that morning. If they forgot, a sample was obtained at the RHMB while the women were still fasting. The urine was transferred to two 10 mL test tubes and frozen at −80 °C. A fasting blood sample was collected in four tubes of EDTA. The blood tubes were wrapped in foil to protect the light-sensitive nutrients and then refrigerated at −4 °C until they were processed within the following 2 h.
Women were weighed and measured, and their body mass index (BMI) was also calculated (using an electronic medical scale with BMI function: Model 799 with measuring rod Model 220, CE approved—Class III, brand Seca®, Hamburg, Germany).
The health and socio-demographic survey included information about mother’s age, country of birth, educational level, and work situation, as well as her details regarding food exclusion; dietary changes in the previous 2 years; consumption of iodized salt; supplementation during pregnancy and lactation; alcohol and tobacco consumption; physical activity; season during the study; diseases; medication intake; offspring; pregnancy; childbirth; lactation; milk expression; weight prior to last pregnancy; and weight gain during pregnancy. The participants also provided information regarding sex and somatometric values, as well as diseases of their breastfed infants.
The FFQ was adapted to the vegetarian diet and assessed the daily/weekly/monthly intake of the following: (1) milk; (2) other dairy products; (3) meat and meat products; (4) fish; (5) eggs; (6) fruits, e.g., fresh juices and dried fruits; (7) raw vegetables; (8) cooked vegetables; (9) legumes (including peanuts, soya beans, and derivates) and seitan; (10) bread, pasta, rice, and other cereals (including rice and oat drinks); (11) nuts and seeds; (12) sweets; and (13) fats and oils (olive, sunflower, sesame, soya, coconut, etc.). A photo atlas was used to determine the serving weight (grams) [16]. The recorded weight of food was used to calculate the daily or weekly ingested rations of each food considering the weight of each ration, as proposed by the Spanish Society of Community Nutrition [17] and adopted by the Spanish Ministry of Health [18].
At least one of the researchers reviewed all the data and assisted the participants in completing the questionnaires, provided the materials necessary for maintaining their dietary record and performing their milk collection, and explained the study procedure again. Written information as well as a telephone number was provided to the participants for those with further questions.
Within the following 15 days after their visit to the RHMB, participants were asked to choose 6 days in a row to conduct the second part of the study. During the first 5 days (days 1 to 5) they filled in a dietary record. In parallel, from days 2 to 5, they collected a milk sample of 25 mL from each expression (at least one per day) for vitamin and mineral studies. On the sixth day (day 6), they collected a complete milk sample from one of their breasts for the lipid studies. Participants were requested to bring their dietary records and milk samples to the RHMB within the following 15 days after study’s completion.
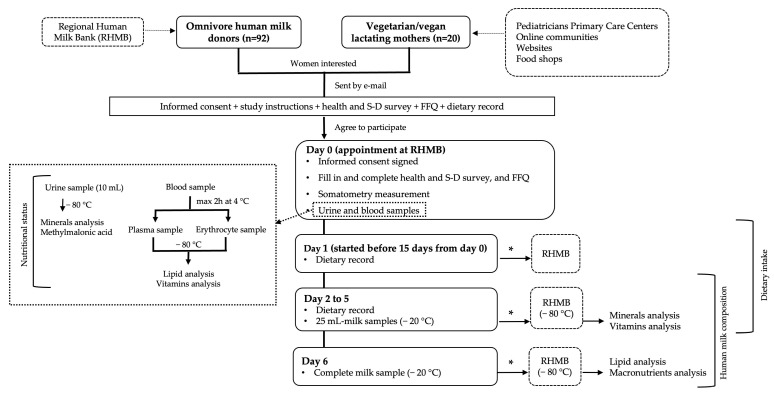
The vitamins and lipids were determined in the plasma, erythrocytes, and milk. Minerals were determined in milk and urine. Vitamins and minerals in milk were detected on each of the four consecutive days when the participants expressed milk for this purpose. Figure 1 shows the study’s protocol.
Figure 1.
Flow chart of the study’s protocol. * Delivered to the RHMB within the following 15 days. Abbreviations: S-D, socio-demographic; FFQ, food frequency questionnaire; RHMB, Regional Human Milk Bank.
2.2. Blood Samples Processing
Blood samples were processed at the Mitochondrial Diseases Laboratory of the “12 de Octubre” University Hospital within two hours of blood extraction. The tubes were protected from light by being wrapped in foil throughout the process. Plasma was obtained after centrifuging blood samples in EDTA tubes at 1200× g for 10 min at 4 °C, which were then aliquoted into 5 opaque 1 mL Eppendorf® (Hamburg, Germany) tubes for the vitamins study and 1 transparent 1 mL Eppendorf® tube for the lipid study. Erythrocytes samples were obtained following the protocol for washing red blood cells described by López Martínez et al. in the Technical Document of the Spanish Society of Clinical Biochemistry and Molecular Pathology [19], similar to that described by Klem et al. [20]. Briefly, after collecting the plasma, a 0.9% saline solution was added until the tube containing the erythrocyte pellet was filled. Then, the contents were mixed by inverting the tube several times. After centrifugation for 10 min at 1200× g, the supernatant was removed with a Pasteur pipette. This process was repeated two more times and finally the washed erythrocytes were aliquoted into 4 opaque 1 mL Eppendorf® tubes for the vitamins study and 1 transparent 1 mL Eppendorf® tube for the lipid study.
The aliquots obtained were stored and frozen at −80 °C in the RHMB.
2.3. 5-Day Dietary Record
For five consecutive days (including at least one weekend or holiday), the participants were asked to record their diet at the time that their food and beverages were consumed, including vitamin and mineral supplements, medicines, and salt (specifying whether it was iodized). Whenever possible, the food taken was weighed; household measures, such as cups or spoons, could be used if an exact measurement of weight was not possible. The participating women were also encouraged to provide ingredients and nutritional information if they consumed processed food.
DIAL® software (DIAL.EXE Version 3, February 2014, Alce Ingeniería, Madrid, Spain) was employed to calculate the energy and nutrients provided by the food [21]; this tool uses the “Food Composition Tables” published by the Department of Nutrition of the Complutense University of Madrid in 2010. The food items not included in the program were created based on the information provided by participants (i.e., food packages) or databases such as the Spanish Food Composition Database [22] or the Food Data Central of the U.S. Department of Agriculture [23]. The caloric and lipid profiles, the healthy eating index (HEI), and the daily intake values for energy and for each nutrient were obtained. The nutrients provided by the intake of vitamin or mineral supplements were taken into account in the calculation of the daily nutrient intake.
Reference values for the caloric and lipid profiles regarding the total energy of the diet were as follows: (1) 10–15% for proteins, (2) 20–35% for lipids, (3) more than 50% for carbohydrates, (4) less than 10% for saturated fatty acids (SFAs), and (5) 4–10% for polyunsaturated fatty acids (PUFAs). These values were obtained by following the nutritional objectives for the Spanish population [24]. The daily nutrient intake was compared with the dietary reference intake (DRI) of the Institute of Medicine (IOM) [25,26,27,28,29,30] and the dietary reference values of the European Food Safety Authority (EFSA) for nursing mothers [31]. The intake of PUFAs was compared with the IOM recommendations [29] and those of the Food and Agriculture Organization [32]. The diet quality evaluation of the participants was based on the HEI and was categorized as follows: (1) >80 excellent, (2) 71–80 very good, (3) 61–70 good, (4) 51–60 acceptable, and (5) 0–50 inadequate [33,34].
2.4. Milk Sample Collection and Processing
The human milk samples for vitamin and mineral assessment were collected at home over four consecutive days (at least once per day), starting one day after the first day of dietary recording. The purpose was to reproduce the conditions of their routine in terms of daily milk pumping. Therefore, participants were asked not to change their expression routine, no request was made for a time schedule, and the same transparent glass containers were used as for donation. Milk could be expressed from one or both breasts, the only requirement being a complete emptying of the breast. From each milk extraction, 25 mL was collected using a sterile syringe after gently shaking the container, and the milk was then placed in an additional sterile feeding bottle and frozen at −20 °C. For the lipid assessment, an entire sample on the sixth day via the complete expression of one breast was collected and frozen at −20 °C, without manipulation. Samples were labelled with a participant code, the date and time of expression, and the total volume expressed.
The storage time of the milk samples at the subjects’ homes averaged 10.5 days (minimum 1 day, maximum 45 days). The home milk-freezing time was slightly longer in the Donors group (mean 11.8 in the Donors group vs. 9.1 in the Veg group, p < 0.001).
Participants transported the frozen milk samples in portable coolers with cold packs and delivered them to the RHMB, where they remained frozen at −20 °C until processing. Then, every 25 mL milk bottle was thawed in a 40 °C water bath, gently shaken to homogenize the milk, and then divided into twenty 1 mL aliquots in transparent Eppendorf® tubes. If a woman had expressed more than one milk sample per day, then, after thawing the milk, the different samples from the same day were mixed, shaken gently to homogenize them, and 20 1 mL aliquots were obtained from the mixture. Milk from different days was not mixed. Each aliquot was labelled with the code of the participant and the day of expression (day 2 to 5) and then frozen at −80 °C. The complete extraction on day 6 was frozen without manipulation.
2.5. Sample Storage and Shipment
The plasma, red blood cells, urine, and milk samples were stored frozen at −80 °C in the RHMB. Samples were sent on dry ice to their respective laboratories for analyses, where they were also stored at −80 °C.
2.6. Lipid Analysis
Lipid analyses were conducted by the Food Lipid Biomarkers and Health Group at the Institute of Food Science Research (CIAL), CSIC-UAM. The FA profile was studied in the erythrocytes, plasma, and milk from all the donors and the vegetarians/vegans included in the study. The lipid class profile as distribution of neutral and polar lipids, the relative composition of phospholipids (PLs), and the molecular species of triacylglycerols (TAGs) were determined in the milk samples from 18 Veg, as well as from a randomly selected subgroup of 19 Donors.
2.6.1. Fat Extraction
The fat from the human milk samples was extracted following the method described by Löfgren et al., (2012) [35], with some modifications based on the optimization of the solvent/ratio sample as described by García-Serrano et al., (2020) [36]. The lipid extracts were filtered through 0.45 μm PVDF filters, collected in amber vials, dried under a nitrogen stream, and then stored at −35 °C until further chromatographic analysis.
2.6.2. Chromatographic Analyses
Separation and Quantification of Lipid Classes by HPLC Evaporative Light Scattering Detector (ELSD)
The separation of the lipid classes was accomplished in an HPLC system (model 1260; Agilent Technologies Inc. Palo Alto, CA, USA) coupled with an ELSD (SEDEX 85 model; Sedere SAS, Alfortville Cedex, France) while using prefiltered compressed air as the nebulizing gas at a pressure of 350 kPa at 60 °C; in addition, the gain was set at 3. Two columns in series (250 × 4.5 mm Zorbax Rx-SIL column with 5-μm particle diameter; Agilent Technologies Inc.) and a pre-column with the same packing were used. Before analysis, samples were dissolved in dichloromethane (5 mg/mL) and 50 μL was injected after column equilibration at 40 °C. The solvent gradient was conducted as detailed in Castro-Gómez et al., (2017) [37]. Both samples and standards were analyzed under the same conditions, using solvents that were freshly prepared.
Determination of Fatty Acid Methyl Esters (FAMEs) by GC-MS
FAMEs were directly prepared from the plasma and erythrocyte samples (200 μL), without prior lipid extraction. However, in the case of the milk samples, 10 mg of the lipid extracts previously obtained was used. Two independent derivatization processes were carried out for each sample following the direct acid–base methylation method, as described by Castro-Gomez et al., (2014) [38]. Prior to methylation, tritridecanoin (13:0 TAG, 75 μL; 1.0 mg/mL) was added to the samples as an internal standard.
FAMEs analysis was performed in an Agilent 6890 series gas chromatograph which was coupled to an Agilent 5973 series mass spectrometer (Agilent Technologies Inc., Palo Alto, CA, USA). A CP-Sil 88 fused-silica capillary column (100 m × 0.25 mm × 0.2 mm film thickness; Chrompack, Middelburg, The Netherlands) was employed for chromatographic separation as described by Calvo et al., (2020) [39]. The temperature program was as follows: 1 min at 100 °C; first ramp was 7 °C/min up to 170 °C; the temperature was held for 55 min; then it was increased at 10 °C/min up to 230 °C; and then it was held for 33 min. The total time for the chromatographic run was 105 min. Helium was used as a carrier gas with a column inlet pressure of 30 psi. The injection volume was 1 μL and the split ratio was 1:25. The MS detector conditions were as follows: transfer line temperature of 250 °C; ion source temperature of 230 °C; and quadrupole temperature of 150 °C. The MS was operated under an electron impact ionization at 70 eV. It was then used in total ion current (TIC) mode to scan the mass range from 40 to 500 m/z. Anhydrous milk fat (reference material BCR-164; Fedelco Inc., Madrid, Spain) was assayed to determine and calculate the response factor for FAMEs. FAMEs data analysis was presented as weight/weight percentages. In total, 15 FAMEs in erythrocytes, 14 in plasma, and 30 in milk were quantified, with a chain length of 6–22 carbon atoms. In general terms, the limit of quantitation was 1.8 ppm. Plasmalogens, as their dimethylacetal derivatives (DMA), were also determined during the GC/MS analysis.
Determination of TAG Molecular Species by GC-FID
TAG molecular species were quantified according to their number of carbon atoms (CN). Analyses were performed on a Clarus 400 GC (PerkinElmer Ltd., Beaconsfield, UK), which was equipped with an automatic split/splitless injector and a flame ionization detector. An Rtx-65TAG fused silica capillary column (30 m × 0.25 mm i.d. × 0.1-μm film thickness; Restek Corp., Bellefonte, PA, USA) was used. Experimental chromatographic conditions were the same as those published by Fontecha et al., (2006) [40]: 120 °C, held for 30 s; 10 °C/min to 220 °C and held for 30 s; and then 6 °C/min to 350 °C and held for 30 min. The injector and flame ionization detector temperatures were 355 and 370 °C, respectively. Helium was used as the carrier gas (172 kPa). Lipid extracts were dissolved in dichloromethane (20 mg/mL) and the injection volume was 0.5 μL. For the qualitative and quantitative analysis of TAGs, the response factors were calculated using an anhydrous milk fat (reference material BCR-519; EU Commission, Brussels, Belgium; purchased from Fedelco Inc., Madrid, Spain), with a known TAGs composition and glyceryl trinanoate as the internal standard (9:0 TAG, 100 μL; 1 mg/mL).
2.7. Vitamins and Minerals Analysis
The vitamins and minerals analyses were conducted by NUTREN-Nutrigenomics Group of the Department of Experimental Medicine of the University of Lleida, Spain.
2.7.1. Minerals
Minerals in breastmilk were determined following the methodology described by Huynh et al., (2015) [41]. Briefly, 1 mL of homogenized breast milk was placed into labeled 50 mL polypropylene tubes, 5 mL 8% TMAH and 0.75 mL pure water were then added to each of the tubes using the diluter, and then the tubes were recapped. Samples were mixed by shaking/vortexing at low speed and were allowed to stand overnight in a fume hood at room temperature. On the following day, samples were mixed again by shaking/vortexing and digested at 90 °C for 1 h using the heating block system. Samples were mixed by shaking/vortexing at least twice during the incubation period to ensure complete digestion. The tubes were then removed from the heating block and cooled at room temperature. Then, 2.25 mL of pure water was added to all tubes using the diluter and the volume was increased to 40 mL by the addition of 30 mL of high-purity water. The tubes were tightly recapped and shaken/vortexed until thoroughly mixed. Then 5–10 mL of each digested solution was filtered and transferred into 1.5 mL tubes, prior to ICPMS analysis. The minerals in the urine samples were directly determined after a 20-fold dilution. Briefly, 0.25 mL of urine was added to 4.75 mL of an aqueous solution containing 2% 1-butanol, 0.05% EDTA, 0.05% Triton X-100, and 1% NH4OH. Determination was then carried out using an Agilent 7500ce inductively coupled plasma mass spectrometry system, consisting of an integrated sample introduction system (ISIS) unit plus a CETAC ASX-510 auto-sampler (Agilent Technologies, Mulgrave, Australia), which was equipped with a Ceramic VeeSpray nebulizer (Glass Expansion Pty. Ltd., Melbourne, Australia).
2.7.2. Water-Soluble Vitamins and Vitamin-B12-Associated Biomarkers
All blood and milk handling in the laboratory was carried out in subdued light to minimize the effects of the light on the vitamin concentrations.
The amount of vitamin B12 in the milk was determined by an immunoassay determination in the deproteinized samples. Briefly, samples were centrifuged to separate and eliminate the fat. For 500 µL of breastmilk (without fat), 100 µL of sodium acetate (pH4, 1 M), 50 μL of 1% potassium cyanidin, and 2.2% of pepsin were added. The samples were incubated at 37 °C with an automated shaker (120 rpm) for protein digestion for 3 h. Further, the samples were heated at 90 °C to ensure a quantitative conversion of all forms of vitamin B12 to cyanocobalamin. After cooling, the samples were filtered and analyzed by a competitive immunoassay method (Ref. 33000, Acces B12 assay, Beckman Coulter, Brea, CA, USA). The blood cobalamin content was determined without previous sample preparation and with the competitive immunoassay method, as reported previously.
Plasmatic holotranscobalamin was determined by an immunoassay with an Active-B12 (Holotranscobalamin) ELISA kit (Ref. AX53101, IBL-International Gmbh, Hamburg, Germany), which was performed by following the manufacturer’s instructions. The reported range of detection was from 10 to 128 pmol/L.
The plasmatic homocysteine was determined by an enzymatic assay kit (Ref. 41057, Spinreact, Barcelona Spain), following the manufacturer’s instructions.
Methylmalonic acid was determined directly in the diluted urine samples by UPLC-MS/MS, following the procedure described by Boutin et al., (2020) [42]. In addition, a good chromatographic separation was obtained between the succinic and methylmalonic acids.
Ascorbic acid was determined by HPLC-DAD following the procedure described by Romeu-Nadal et al., (2006) [43]. Briefly, milk samples were thawed to around 22 °C in a water bath, protected from light, and then mixed. Dehydroascorbic acid was reduced to ascorbic acid with DL-dithiothreitol. Exactly 300 μL of human milk and 800 μL of DL-dithiothreitol 100 mM were added. The mixture was then shaken mechanically for 30 s and the tube was then kept in a dark place for 15 min. Next, 300 μL of meta-phosphoric acid (0.56% w/v) was added, the mixture was further shaken for 30 s, and then it was filtered with centrifuge filters at 4 °C for 30 min (Spin-X Micro Centrifuge Filter, 0.2 μm Nylon Filter). To analyze the ascorbic acid, 300 μL of breastmilk and 300 μL of meta-phosphoric acid (0.56% w/v) were mixed and filtered with centrifuge filters at 4 °C for 30 min. For chromatographic analysis, 50 μL of the filtrate was injected into an HPLC system. Isocratic chromatographic separation was carried out using a mobile phase of Milli-Q water with acetic acid (0.1% v/v) and methanol in a relative proportion of 95:5 (v/v). The effluent flowrate was 0.7 mL/min, and the column temperature was 25 °C. The analytical column used was a 5 μm Kromasil 100 Å, C18 (Tecknokroma, Barcelona, Spain). Ascorbic acid was identified by comparing the retention time of the sample peak with that of the ascorbic standard at 254 nm. Plasma samples were determined with the same methodology, with some modifications, as described by Robitaille and Hoffer (2016) [44], which were achieved mainly through the protein precipitation performed via 10% meta-phosphoric acid in 2 mM of EDTA.
The rest of the analysis for the water-soluble vitamins in human milk was carried out by UPLC tandem mass spectrometry (UPLC–MS/MS), following the method described by Hampel et al., (2012) [45]. Samples were subjected to protein precipitation and the removal of non-polar constituents by diethyl ether, prior to analysis. Quantification was performed via a ratio response to the stable-isotope-labeled internal standards. The limit of quantitation was between 0.05 and 5 ppb, depending on the vitamin. The water-soluble vitamins in the plasma and erythrocytes were determined with the same protocol that was used for its determination in human milk, with some modifications that were introduced in the sample preparation. Briefly, for 500 µL of the sample, protein precipitation was performed with the addition of 75 μL of trichloroacetic acid (500 g/L). Samples were vigorously mixed and then centrifuged at 10,500× g at 4 °C for 5 min. The supernatant was collected and mixed with 1 mL of methanol. Further samples were centrifuged at 10,500× g at 4 °C for 5 min. Moreover, the supernatant was dried under nitrogen and then finally resuspended with 500 µL of water.
The glutathione reductase activity in erythrocytes was determined by an Abcam ab83461 assay kit (Abcam, Cambridge, UK), following the manufacturer’s instructions.
2.7.3. Fat-Soluble Vitamins
The concentrations of retinol (i.e., the main form of vitamin A in milk), α-tocopherol (the main biological form of vitamin E), and γ-tocopherol in the milk and plasma were determined by HPLC with a fluorescence and UV detector, following the method described by Jiang et al., (2016) [46]. The concentration of tocopherols was determined with an excitation wavelength of 295 nm and a cut-off emission filter of 345 nm. The retinol was determined by UV detection (325 nm wavelength). The external quantification was performed based on the calibration curves for retinol and α-tocopherol prepared each day of the analysis. Briefly, samples were saponificated with a mixed solution of 0.1 g ascorbic acid, 2 mL ethanol, including 0.1% BHT, and a 0.5 mL 50% aqueous potassium hydroxide solution. Further, the retinol and tocopherols were extracted with petroleum ether and the organic fraction was dried with nitrogen and then later re-dissolved with a mixed solution of methanol and methyl-tert-butyl-ether (1:1), including 0.1% BHT.
Vitamin D metabolites in milk and plasma were determined by UPLC electrospray ionization/tandem MS, as described by Aronov et al., (2008) [47]. Briefly, the samples’ protein precipitation was performed with acetonitrile. The vitamin D metabolites were extracted with methyl-tert-butyl-ether and the organic fraction was dried under nitrogen. Further, the samples were re-dissolved with methanol and 4-phenyl-1,2,4-triazoline-3,5-dione (PTAD) for its derivatization. The internal standards of the deuterated metabolites of vitamin D were used during the whole process.
2.8. Blood Biochemistry, Hemoglobin, and Urine Creatinine
Blood biochemistry was determined by the NUTREN-Nutrigenomics Group via enzymatic kinetic colorimetric methods. The total cholesterol, HDL- and LDL-cholesterol, and triacylglycerols were determined via enzymatic assays, following the manufacturer’s instructions (Refs. 1001090, 1001096, 41023, and 1001310, respectively; Spinreact, Barcelona, Spain). Hemoglobin was determined by the Drabkin colorimetric method, following the manufacturer’s instructions from the commercial assay kit (Ref. 1001230, Spinreact, Barcelona, Spain). The urine creatinine was determined by the Jaffé colorimetric kinetic method with a commercial assay kit (Ref. 1001110, Spinreact, Barcelona, Spain).
2.9. Human Milk Macronutrients Analysis
The breast milk macronutrient analyses were carried out at the RHMB. The leftover milk samples from the lipid study at the CIAL were received frozen. The milk was then thawed in a water bath and homogenized before analyses. Total fat, protein, and lactose in human milk samples were measured by Fourier transform mid-infrared (FT-MID) spectroscopy in a milk analyzer (MilkoScan FT2, FOSS S.A., Barcelona, Spain) properly calibrated for the analysis of human milk.
2.10. Statistics
The distribution of data for normality was evaluated using the Shapiro–Wilk test.
Quantitative sociodemographic variables were presented as the mean and standard deviation (SD) when they followed a parametric (normal) distribution and as median and interquartile range (IQR) when they followed a nonparametric distribution. Qualitative variables were expressed as both absolute and relative frequencies.
The lipid, vitamin, and mineral values were expressed as the mean and standard error (SE). In the case of the vitamins and minerals in the milk, due to the wide dispersion of the values of some of the nutrients, the median and IQR were presented. For the same reason, the median and IQR for the riboflavin intake were also shown.
The inferences on the qualitative variables were analyzed using a Chi-square test. The inferences on the quantitative variables were made depending on the distribution that was obtained via the use of the Student’s t-test or the Mann–Whitney U test for comparisons between two groups. A p-value < 0.05 was considered statistically significant. All statistical analyses were performed using SAS© software (SAS Institute Inc., Cary, NC, USA), version 9.4 of the SAS System for Windows.
3. Results
3.1. Population Studied
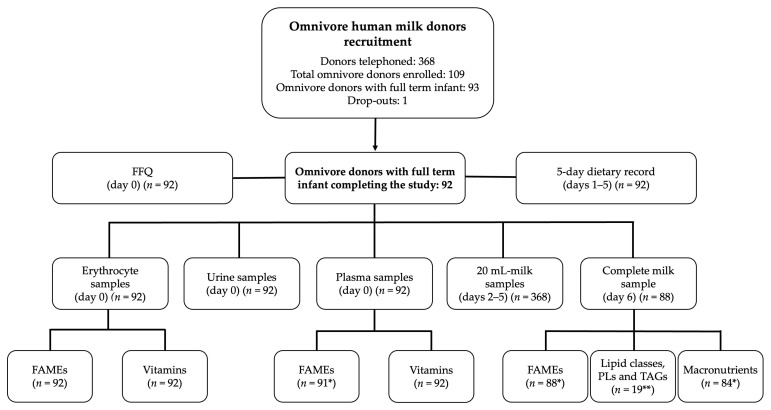
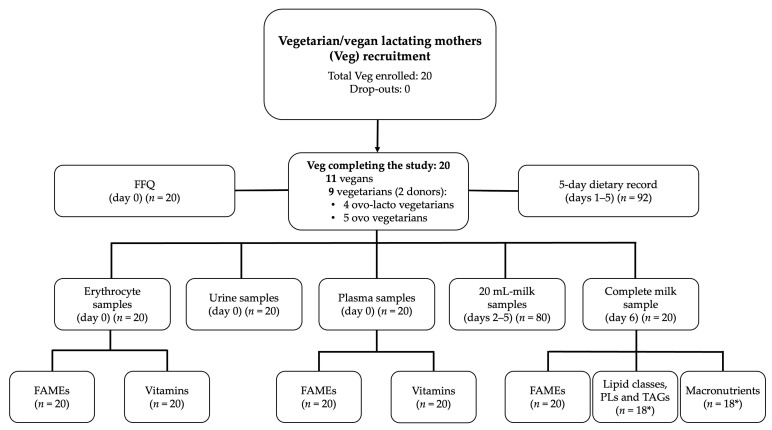
A total of 113 women were recruited: 93 were Donors and 20 Veg. A total of 112 participants completed the study: 92 Donors and 20 Veg. In the Veg group, there were 11 vegans and 9 vegetarians (5 ovo-vegetarians and 4 ovo-lacto-vegetarians). Of the 20 Veg, 10 had made some change in their diet in the last 2 years. In particular, four had switched from vegetarian to vegan, two had switched from vegan to vegetarian, and one had occasionally consumed eggs, fish, and dairy during their pregnancy. Two of the vegetarians were human milk donors. The flow chart of subject recruitment and sampling is shown in Figure 2 and Figure 3.
Figure 2.
Flow chart for omnivore human milk donors’ recruitment and sampling. * The difference between 92 and the number of samples reflects missing data. ** Corresponding to a randomly selected subgroup of 19 omnivore donors with full-term infants. Abbreviations: FFQ, food frequency questionnaire; FAMEs, fatty acid methyl esters; PLs, relative composition of phospholipids; TAGs, molecular species of triacylglycerols.
Figure 3.
Flow chart for vegetarian/vegan lactating mothers’ (Veg) recruitment and sampling. * Due to two missing data. Abbreviations: FFQ, food frequency questionnaire; FAMEs, fatty acid methyl esters; PLs, relative composition of phospholipids; TAGs, molecular species of triacylglycerols.
Table 1, Table 2 and Table 3 show the comparison of health and socio-demographic data survey results between both groups. The only differences found were a lower gestational weight gain in the Veg group by a median of 1 kg (p value = 0.024), a higher percentage of women using manual milk expression in the Veg group (25% vs. 7.6%, p value = 0.038), and a different distribution of weight percentiles for the breastfed infants at birth (p value = 0.007) and at the time of the study (p value = 0.049). There was no difference in pre-pregnancy or in current BMI. The age range of breastfed infants was 2–50 months in the Donor group and 1–36 months in the Veg group.
Table 1.
Characteristics of the omnivore human milk donors with full-term infants (Donors) and lactating vegetarian/vegan mothers (Veg).
| Characteristic | Donors (n = 92) | Veg (n = 20) | p Value |
|---|---|---|---|
| Age (years) | 36.1 (4.2); 28–47 | 33.9 (5,2); 24–42 | 0.118 |
| Weight (kg) | 60.8 (55.3, 71.0); 46–122 | 61.1 (52.8, 64.9); 48–93 | 0.365 |
| Height (cm) | 164.2 (6.3); 152–158 | 163.1 (4.4); 155–172 | 0.493 |
| Pre-pregnancy BMI (kg/m2) | 22.3 (21.0, 25.0); 18.2–44.2 | 22.2 (20.1, 23.9); 18.0–34.3 | 0.436 |
| Pre-pregnancy BMI (kg/m2) category | |||
| Underweight (<18.5) | 1 (1.1%) | 2 (10.0%) | 0.135 |
| Normal (18.5–24.9) | 69 (75.0%) | 15 (75.0%) | |
| Overweight (25–29.9) | 12 (13.0%) | 2 (10.0%) | |
| Obese (≥30) | 10 (10.9%) | 1 (5.0%) | |
| Current BMI (kg/m2) | 23.1 (21.2, 25.0); 16.7–42.8 | 22.8 (20.5, 23.7); 18.3–38.9 | 0.287 |
| Current BMI (kg/m2) category | |||
| Underweight (<18.5) | 2 (2.2%) | 1 (5.0%) | 0.624 |
| Normal (18.5–24.9) | 67 (72.8%) | 16 (80.0%) | |
| Overweight (25–29.9) | 12 (13.0%) | 1 (5.0%) | |
| Obese (≥30) | 11 (12.0%) | 2 (10.0%) | |
| Gestational weight gain (kg) | 12.0 (9.2, 15.0); 5–30 | 11.0 (8.5, 12.2); 6–13.6 | 0.024 |
| Postpartum weight retention (kg) | 0.75 (−0.8–2.2); −12–11.8 | 0.7 (−1.8–3.2); −4.2–11.2 | 0.846 |
| Number of children | |||
| 1 | 48 (52.2%) | 14 (70.0%) | 0.557 |
| 2 | 36 (39.1%) | 6 (30.0%) | |
| ≥3 | 8 (8.7%) | 0 (0.0%) | |
| Country of origin: Spain, n (%) | 84 (91.3%) | 19 (95.0%) | 0.581 |
| Education level | |||
| Secondary studies | 2 (2.2%) | 3 (15.0%) | 0.063 |
| Technical studies | 9 (9.8%) | 1 (5.0%) | |
| University studies | 81 (88.0%) | 16 (80.0%) | |
| Currently working | 43 (46.7%) | 14 (70.0%) | 0.059 |
| Physical activity | |||
| Sedentary | 21 (22.8%) | 5 (25.0%) | 0.355 |
| Low active | 49 (53.3%) | 7 (35.0%) | |
| Active/very active | 22 (23.9%) | 8 (40.0%) | |
| Tobacco consumption | |||
| Previously | 18 (19.6%) | 4 (20.0%) | 0.96 |
| Currently | |||
| Passive smoking | 18 (19.6%) | 2 (10.0%) | 0.16 |
| Active smoking | 1 (1.1%) | 1 (5.0%) | |
| Alcohol consumption | |||
| Prior to pregnancy | 47 (51.1%) | 11 (55.0%) | 0.228 |
| During pregnancy | 1 (1.1%) | 1 (5.0%) | |
| Currently | 4 (4.3%) | 3 (15.0%) | |
| Season during the study | |||
| Spring | 24 (26.1%) | 1 (5.0%) | 0.103 |
| Summer | 13 (14.1%) | 6 (30.0%) | |
| Autumn | 33 (35.9%) | 8 (40.0%) | |
| Winter | 22 (23.9%) | 5 (25.0%) |
The quantitative sociodemographic variables are presented as means (standard deviations) when they followed a parametric distribution and as medians (25th, 75th percentiles) when they followed a nonparametric distribution. The ranges are displayed after the semicolons. The qualitative variables are expressed as the absolute and relative frequencies (%). Abbreviations: BMI, body mass index.
Table 2.
Characteristics of the breastfed offspring of the omnivore human milk donors with full-term infants (Donors) and vegetarian/vegan lactating mothers (Veg).
| Characteristic | Donors (n = 92) | Veg (n = 20) | p Value |
|---|---|---|---|
| Girl | 50 (54.3%) | 7 (35%) | 0.116 |
| Boy | 42 (45.7%) | 13 (65.0%) | |
| Twin pregnancy | 0 (0.0%) | 1 (5.0%) | 0.178 |
| Gestational age (weeks) | 39+6 (39, 40+4); 37+1–42+3 | 39+4 (38+6, 40+3); 36+4–41+4 | 0.082 |
| Birth weight (grams) | 3303.4 (412.7); 2120–4640 | 3125.8 (412.6); 2240–3960 | 0.120 |
| Birth weight percentile 1 | |||
| ≤25 | 23 (25.0%) | 12 (60.0%) | 0.007 |
| 25–75 | 62 (67.4%) | 8 (40.0%) | |
| ≥75 | 7 (7.6%) | 0 (0.0%) | |
| Age of breastfed child (months) | |||
| 0–6 | 34 (37.0%) | 9 (45.0%) | 0.776 |
| 6–12 | 34 (37.0%) | 6 (30.0%) | |
| 12–50 | 24 (26.1%) | 5 (25.0%) | |
| Weight percentile of breastfed child 2 | |||
| ≤15 | 13 (14.1%) | 7 (35.0%) | 0.049 |
| 15–85 | 62 (67.4%) | 12 (60.0%) | |
| ≥85 | 17 (18.5%) | 1 (5.0%) |
The quantitative sociodemographic variables are presented as means (standard deviations) when they followed a parametric distribution and as medians (25th, 75th percentiles) when they followed a nonparametric distribution. The ranges are displayed after the semicolons. The qualitative variables are expressed as the absolute and relative frequencies (%). 1 Taking as reference the Olsen intrauterine growth curves [48]. 2 Taking as reference the World Health Organization (WHO)’s child growth standards [49].
Table 3.
Characteristics of the lactation of the omnivore human milk donors with full-term infants (Donors) and vegetarian/vegan lactating mothers (Veg).
| Characteristic | Donors (n = 92) | Veg (n = 20) | p Value |
|---|---|---|---|
| Donor previously | 19 (20.6%) | 1 (5%) | 0.118 |
| Lactation stage (months) | 7.0 (5.0, 13.5); 2–50 | 8.0 [4.5–14.0]; 1–36 | 0.942 |
| Type of lactation | |||
| Exclusive | 40 (43.5%) | 9 (45%) | 0.901 |
| Partial | 52 (56.5%) | 11 (55%) | |
| Sum of child direct breastfeeding times plus daily pumped sessions | |||
| <5 | 8 (8.7%) | 2 (10.0%) | 0.971 |
| 5–10 | 59 (64.1%) | 12 (60.0%) | |
| >10 | 24 (26.1%) | 5 (25.0%) | |
| Missing data | 1 (1.1%) | 1 (5.0%) | |
| Tandem breastfeeding | 5 (5.4%) | 1 (5.0%) | 0.937 |
| Breastfeeding twins | 0 (0%) | 0 (0%) | - |
| Type of milk expression * | |||
| Manual | 7 (7.6%) | 5 (25%) | 0.038 |
| Mechanical breast pump | 10 (10.9%) | 2 (10.0%) | 0.909 |
| Simple electric breast pump | 68 (73.9%) | 14 (70%) | 0.720 |
| Double electric breast pump | 12 (13.0%) | 2 (10%) | 0.709 |
The quantitative sociodemographic variables are presented as means (standard deviations) when they followed a parametric distribution and as medians (25th, 75th percentiles) when they followed a nonparametric distribution. The ranges are displayed after the semicolons. The qualitative variables are expressed as the absolute and relative frequencies (%). * The categories are not excluding each other.
The data reported by the participants on their consumption of pharmacological supplements during pregnancy and lactation are shown in Table 4. Most participants in both groups took supplements during gestation of vitamin B12, folic acid, and iodine. At the time of the study, a total of 17/20 (85%) Veg were taking B12 supplements, of which two were taking multi-nutrient supplements containing low doses of B12 (2.5 and 4 mcg/day), and 15 were taking high doses of B12 (1000–6000 mcg weekly). During lactation, there was a higher percentage of supplementation of vitamins A, E, C, B9, iodine, and iron in the Donors group. On the other hand, at the time of the study, higher supplementation doses of vitamins D, B7, B9, B12, and calcium were observed in the Veg group. The use of omega-3 supplements during pregnancy and lactation was low in the Veg group (30 and 35%, respectively).
Table 4.
Consumption of the pharmacological supplements during pregnancy and the lactation of the omnivore human milk donors with full-term infants (Donors) and vegetarian/vegan lactating mothers (Veg).
| Pharmacological Supplement | n (%) | p Value | Daily Dose, Mean (SE); Range | p Value | ||
|---|---|---|---|---|---|---|
| Donors (n = 92) | Veg (n = 20) | Donors (n = 92) | Veg (n = 20) | |||
| Vitamin A, mcg | ||||||
| Pregnancy | 12 (13.0) | 3 (15.0) | 0.732 | 527.6 (69.7); 23.0–700.0 | 383.3 (174.0); 100.0–700.0 | 0.389 |
| Lactation | 45 (48.9) | 3 (15.0) | 0.002 | |||
| Previously | 14 (15.2) | 2 (10.0) | 730.9 (49.2); 333.0–1000.0 | 250.0 (150.0); 100.0–400.0 | 0.022 | |
| Currently | 31 (33.7) | 1 (5.0) | 678.1 (45.2); 160.0–1000.0 | 800.0 (-); 800.0–800.0 | 0.729 | |
| Vitamin D, mcg | ||||||
| Pregnancy | 50 (54.3) | 10 (50.0) | 0.687 | 10.61 (0.8); 3.8–30.0 | 17.08 (6.2); 5.0–62.5 | 0.925 |
| Lactation | 52 (56.5) | 9 (45.0) | 0.220 | |||
| Previously | 15 (16.3) | 2 (10.0) | 5.0 (0.4); 2.5–10.0 | 6.2 (3.7); 2.5–10.0 | 0.926 | |
| Currently | 37 (40.2) | 7 (35.0) | 6.0 (0.8); 1.0–25.0 | 27.4 (9.0); 5.0–62.5 | 0.001 | |
| Vitamin E, mg | ||||||
| Pregnancy | 22 (23.9) | 7 (35.0) | 0.318 | 10.7 (0.7); 1.8–15.0 | 11. (2.0); 4.0–20.0 | 0.762 |
| Lactation | 49 (53.3) | 6 (30.0) | 0.030 | |||
| Previously | 15 (16.3) | 2 (10.0) | 11.5 (0.5); 6.0–15.0 | 13.0 (7.0); 6.0–20.0 | 0.926 | |
| Currently | 34 (37.0) | 4 (20.0) | 10.9 (0.6); 2.4–16.0 | 11.0 (0.6); 10.0–12.0 | 0.542 | |
| Vitamin C, mg | ||||||
| Pregnancy | 48 (52.2) | 10 (50.0) | 0.823 | 61.1 (4.2); 12.0–180.0 | 93.6 (30.6); 26.0–358.0 | 0.336 |
| Lactation | 51 (55.4) | 7 (35.0) | 0.050 | |||
| Previously | 16 (17.4) | 3 (15.0) | 80.0 (4.2); 40.0–110.0 | 60.0 (20.0); 40.0–100.0 | 0.260 | |
| Currently | 35 (38.0) | 4 (20.0) | 73.5 (4.2); 16.0–125.0 | 65.0 (8.7); 50.0–80.0 | 0.492 | |
| Vitamin B1, thiamine, mg | ||||||
| Pregnancy | 48 (52.2) | 9 (45.0) | 0.530 | 1.1 (0.0); 0.6–1.5 | 3.3 (1.7); 0.9–17.0 | 0.038 |
| Lactation | 50 (54.3) | 8 (40.0) | 0.149 | |||
| Previously | 16 (17.4) | 3 (15.0) | 1.0 (0.1); 0.6–1.2 | 2.8 (1.1); 0.6–4.3 | 0.221 | |
| Currently | 34 (37.0) | 5 (25.0) | 0.9 (0.1); 0.2–1.1 | 10.8 (9.8); 0.9–50.0 | 0.431 | |
| Vitamin B2, riboflavin, mg | ||||||
| Pregnancy | 48 (52.2) | 9 (45.0) | 0.530 | 1.4 (0.0); 0.7–2.5 | 2.4 (0.8); 1.0–8.5 | 0.060 |
| Lactation | 50 (54.3) | 8 (40.0) | 0.149 | |||
| Previously | 16 (17.4) | 3 (15.0) | 1.3 (0.1); 0.7–1.6 | 2.9 (1.2); 0.7–5.0 | 0.272 | |
| Currently | 34 (37.0) | 5 (25.0) | 1.3 (0.1); 0.7–1.6 | 5.0 (3.8); 1.0–20.0 | 0.750 | |
| Vitamin B3, niacin, mg | ||||||
| Pregnancy | 48 (52.2) | 8 (40.0) | 0.301 | 15.6 (0.4); 4.0–20.0 | 17.4 (2.6); 10.0–33.0 | 0.375 |
| Lactation | 50 (54.3) | 8 (40.0) | 0.149 | |||
| Previously | 16 (17.4) | 3 (15.0) | 15.0 (0.7); 8.0–16.0 | 14.0 (3.1); 8.0–18.0 | 0.718 | |
| Currently | 34 (37.0) | 5 (25.0) | 13.3 (0.7); 3.2–16.0 | 11.4 (2.1); 5.0–16.0 | 0.403 | |
| Vitamin B5, pantothenic, mg | ||||||
| Pregnancy | 48 (52.2) | 8 (40.0) | 0.301 | 5.7 (0.2); 4.0–20.0 | 8.9 (3.2); 5.0–31.0 | 0.923 |
| Lactation | 50 (54.3) | 8 (40.0) | 0.149 | |||
| Previously | 16 (17.4) | 3 (15.0) | 5.6 (0.3); 3.0–6.0 | 6.3 (2.0); 3.0–10.0 | 0.718 | |
| Currently | 34 (37.0) | 5 (25.0) | 5.1 (0.2); 2.8–6.0 | 24.4 (18.9); 5.0–100.0 | 0.488 | |
| Vitamin B6, pyridoxine, mg | ||||||
| Pregnancy | 48 (52.2) | 9 (45.0) | 0.530 | 1.4 (0.0); 0.7–2.2 | 3.6 (1.5); 0.8–2.5 | 0.533 |
| Lactation | 50 (54.3) | 8 (40.0) | 0.149 | |||
| Previously | 16 (17.4) | 3 (15.0) | 1.4 (0.1); 0.7–2.2 | 3.0 (1.4); 0.7–5.4 | 0.249 | |
| Currently | 34 (37.0) | 5 (25.0) | 1.3 (0.1); 0.3–2.0 | 3.6 (2.2); 1.3–12.5 | 0.617 | |
| Vitamin B7, biotin, mcg | ||||||
| Pregnancy | 48 (52.2) | 8 (40.0) | 0.301 | 55.3 (2.9); 25.0–150.0 | 117.1 (20.1); 50.0–187.0 | <0.001 |
| Lactation | 50 (54.3) | 7 (35.0) | 0.063 | |||
| Previously | 16 (17.4) | 2 (10.0) | 47.5 (2.3); 25.0–60.0 | 37.5 (12.5); 25.0–50−0 | 0.208 | |
| Currently | 34 (37.0) | 5 (25.0) | 42.3 (2.1); 10.0–50.0 | 116.0 (27.5); 50.0–180.0 | 0.002 | |
| Vitamin B9, folic acid, mcg | ||||||
| Pregnancy | 89 (96.7) | 20 (100.0) | 0.999 | 603.6 (93.5); 162.0–6200.0 | 636.5 (229.9); 286.0–5000.0 | 0.990 |
| Lactation | 75 (81.5) | 13 (65.0) | 0.022 | |||
| Previously | 19 (20.7) | 6 (30.0) | 272.4 (24.3); 100–400 | 416.7 (91.0); 100.0–800.0 | 0.094 | |
| Currently | 56 (60.9) | 7 (35.0) | 280.1 (16.2); 2.0–400 | 400.0 (21.8); 300.0–500.0 | 0.011 | |
| Vitamin B12, cobalamin, mcg | ||||||
| Pregnancy | 88 (95.7) | 20 (100.0) | 0.999 | 2.3 (0.1); 0.95–4.7 | 213.5 (55.8); 1.2–934.0 | 0.002 |
| Lactation | 74 (80.4) | 18 (90.0) | 0.683 | |||
| Previously | 19 (20.7) | 1 (5.0) | 2.4 (0.1); 1.3–3.5 | 2.0 (-); 2.0–2.0 | 0.213 | |
| Currently | 55 (59.8) | 17 (85.0) | 2.1 (0.1); 1.0–2.5 | 312.1 (48.0); 2.0–857.0 | <0.001 | |
| Iodine, mcg | ||||||
| Pregnancy | 89 (96.7) | 19 (95.0) | 0.452 | 199.0 (3.9); 46.5–400.0 | 177.7 (10.1); 75.0–229.4 | 0.076 |
| Lactation | 78 (84.8) | 12 (60.0) | 0.001 | |||
| Previously | 18 (19.6) | 5 (25.0) | 191.7 (6.1); 100.0–200.0 | 185.8 (22.2); 100.0–229.0 | 0.693 | |
| Currently | 60 (65.2) | 7 (35.0) | 183.2 (5.9); 46.0–300.0 | 157.1 (22.3); 75.0–200.0 | 0.101 | |
| Calcium, mg | ||||||
| Pregnancy | 3 (3.3) | 4 (20.0) | 0.019 | 62.0 (44.1); 12.0–150.0 | 391.8 (149.7); 100.0–650.0 | 0.075 |
| Lactation | 37 (40.2) | 5 (25.0) | 0.144 | |||
| Previously | 12 (13.0) | 2 (10.0) | 180.8 (17.1); 24.0–245.0 | 225.0 (125.0); 100.0–350.0 | 0.749 | |
| Currently | 25 (27.2) | 3 (15.0) | 164.0 (17.9); 40.0–500.0 | 566.7 (83.3); 400.0–650.0 | 0.004 | |
| Iron, mg | ||||||
| Pregnancy | 67 (72.8) | 15 (75.0) | 0.899 | 45.6 (3.6); 6.5–108.0 | 46.9 (6.0); 9.0–80.0 | 0.540 |
| Lactation | 66 (71.7) | 10 (50.0) | 0.021 | |||
| Previously | 26 (28.3) | 5 (25.0) | 39.9 (6.3); 7.0–105.0 | 69.0 (7.0); 47.0–80.0 | 0.061 | |
| Currently | 40 (43.5) | 5 (25.0) | 29.2 (5.3); 3.0–114.0 | 40.4 (16.2); 14.0–80.0 | 0.302 | |
| Zinc, mg | ||||||
| Pregnancy | 44 (47.8) | 8 (40.0) | 0.498 | 9.7 (0.2); 4.3–15.0 | 11.9 (2.1); 7.5–25.0 | 0.603 |
| Lactation | 48 (52.2) | 7 (35.0) | 0.097 | |||
| Previously | 16 (17.4) | 3 (15.0) | 9.2 (0.5); 5.0–10.0 | 13.3 (6.0); 5.0–25.0 | 0.718 | |
| Currently | 32 (34.8) | 4 (20.0) | 8.3 (0.5); 2.0–10.0 | 7.8 (1.0); 5.0–10.0 | 0.302 | |
| Selenium, mcg | ||||||
| Pregnancy | 42 (45.7) | 7 (35.0) | 0.363 | 52.3 (1.3); 27.0–60.0 | 40.0 (8.0); 12.5–60.0 | 0.173 |
| Lactation | 46 (50.0) | 6 (30.0) | 0.054 | |||
| Previously | 15 (16.3) | 2 (10.0) | 26.8 (4.0); 10.0–55.0 | 32.5 (22.5); 10.0–55.0 | 0.937 | |
| Currently | 31 (33.7) | 4 (20.0) | 27.1 (3.4); 4.0–55.0 | 34.0 (12.1); 13.0–55.0 | 0.579 | |
| Omega 3, g | ||||||
| Pregnancy | 48 (52.2) | 6 (30.0%) | 0.065 | 0.23 (0.02); 0.16–0.95 | 0.21 (0.04); 0.10–0.38 | 0.550 |
| Lactation | 48 (52.2) | 7 (35.0%) | 0.097 | |||
| DHA, g | ||||||
| Lactation | ||||||
| Previously | 15 (16.3) | 2 (10.0%) | 0.18 (0.01); 0.08–0.20 | 0.10 (-); 0.10–0.10 | 0.102 | |
| Currently | 33 (35.9) | 5 (25.0%) | 0.18 (0.01); 0.10–0.38 | 0.18 (0.03); 0.10–0.25 | 0.546 | |
| EPA, g | ||||||
| Lactation | ||||||
| Previously | 15 (16.3) | 2 (10.0) | 0.03 (0.00); 0.00–0.04 | 0.02 (-); 0.02–0.02 | 0.332 | |
| Currently | 33 (35.9) | 5 (25.0) | 0.05 (0.02); 0.00–0.50 | 0.15 (0.12); 0.00–0.64 | 0.727 | |
The quantitative variables are presented as means (standard error of the mean). The ranges are displayed after the semicolons. The qualitative variables are expressed as the absolute and relative frequencies (%). Women who took supplements during lactation but had stopped taking them at the time of the study were classified in the “Previously” group. Women who were still taking supplements at the time of the study were classified in the “Currently” group. Abbreviations: SE, standard error of the mean; DHA, docosahexaenoic acid; and EPA, eicosapentaenoic acid.
3.2. Diet Survey
Table 5, Table 6 and Table 7 show the results of the five-day dietary records regarding the participants’ average daily nutrient intake together with recommended daily intakes (Table 5), the prevalence of inadequate intakes of specific nutrients (Table 6), and, furthermore, the number of food servings per day, the healthy eating index (HEI), and records of supplement and iodized salt intake (Table 7). Table 8 shows the results of the FFQ.
Table 5.
Diet survey: five-day dietary record. Daily nutrients intake of the omnivore human milk donors with full-term infants (Donors) and vegetarian/vegan lactating mothers (Veg), compared with recommended daily intakes.
| Donors (n = 92) | Veg (n = 20) | p Value | Recommendations a | ||
|---|---|---|---|---|---|
| EFSA (PRI/AI *) | IOM (RDA/AI *) | ||||
| Energy (Kcal) | 2318.66 (43.49) | 2146.73 (85.06) | 0.079 | b | |
| Protein (g) | 96.36 (1.95) | 67.46 (3.12) | <0.001 | c | 71 |
| Total fat (g) | 102.66 (2.62) | 85.81 (4.55) | 0.004 | ||
| Saturated fat (g) | 33.12 (1.01) | 19.88 (1.51) | <0.001 | ALAP | ALAP |
| Polyunsaturated fat (g) | 16.29 (0.58) | 21.00 (1.42) | 0.001 | ||
| Monounsaturated fat (g) | 43.94 (1.21) | 38.06 (2.48) | 0.040 | ||
| PUFAs/SFAs | 0.54 (0.02) | 1.28 (0.11) | <0.001 | ||
| (PUFAs + MUFAs)/SFAs | 1.94 (0.05) | 3.54 (0.23) | <0.001 | ||
| Kcal from carbohydrate (%) | 43.76 (0.60) | 51.83 (0.92) | <0.001 | 45–60 ** | 45–65 ** |
| Kcal from protein (%) | 16.84 (0.26) | 12.65 (0.45) | <0.001 | 10–35 ** | |
| Kcal from fat (%) | 39.24 (0.60) | 35.29 (0.95) | 0.003 | 20–35 ** | 20–35 ** |
| Kcal from saturated fat (%) | 12.66 (0.23) | 8.18 (0.54) | <0.001 | ||
| Kcal from polyunsaturated fat (%) | 6.30 (0.19) | 8.72 (0.35) | <0.001 | ||
| Kcal from monounsaturated fat (%) | 16.81 (0.33) | 15.72 (0.67) | 0.239 | ||
| Kcal from n-3 fatty acids (%) | 0.84 (0.03) | 0.89 (0.10) | 0.858 | 0.5 | 0.6–1.2 |
| n-6 fatty acids (g) | 13.64 (0.51) | 18.27 (1.28) | <0.001 | 13 * | |
| n-3 fatty acids (g) | 2.11 (0.09) | 2.37 (0.31) | 0.846 | 1.3 * | |
| n-6/n-3 fatty acids | 7.77 (0.27) | 9.90 (0.69) | 0.002 | ||
| Myristic acid C14:0 (g) | 2.86 (0.14) | 1.28 (0.26) | <0.001 | ||
| Palmitic acid C16:0 (g) | 16.52 (0.50) | 8.40 (0.67) | <0.001 | ||
| Palmitoleic acid C16:1 n7 (g) | 1.55 (0.06) | 0.42 (0.06) | <0.001 | ||
| Stearic acid C18:0 (g) | 7.07 (0.23) | 3.53 (0.33) | <0.001 | ||
| Oleic acid C18:1n9c (g) | 40.58 (1.16) | 36.40 (2.35) | 0.106 | ||
| Linoleic acid C18:2n6c (g) | 13.45 (0.51) | 18.25 (1.28) | <0.001 | ||
| Linolenic acid C18:3n3 (g) | 1.58 (0.07) | 2.24 (0.30) | 0.016 | ||
| Eicosapentaenoic acid C20:5n3 (g) | 0.15 (0.02) | 0.04 (0.02) | <0.001 | ||
| Docosapentaenoic acid C22:5n3 (g) | 0.08 (0.03) | 0.00 (0.00) | <0.001 | ||
| Docosahexaenoic acid C22:6n3 (g) | 0.38 (0.03) | 0.11 (0.03) | <0.001 | +0.10–0.20 *d | |
| EPA + DHA (g) | 0.53 (0.04) | 0.14 (0.04) | <0.001 | 0.25 * | |
| Trans fatty acids (g) | 0.44 (0.02) | 0.13 (0.03) | <0.001 | ALAP | ALAP |
| Cholesterol (g) | 334.42 (10.56) | 58.60 (17.24) | <0.001 | ALAP | |
| Cholesterol (mg/1000 Kcal) | 144.96 (4.03) | 28.90 (8.45) | <0.001 | ||
| Thiamine (B1) (mg) | 2.02 (0.07) | 4.75 (2.46) | 0.056 | 0.1 mg/MJ | 1.4 mg |
| Riboflavin (B2) (mg) | 2.55 (0.09) 2.40 (1.70, 3.20) 1 |
2.95 (0.98) 1.70 (1.20, 2.80) 1 |
0.024 | 2.0 | 1.6 |
| Niacin (B3) (mg) | 43.21 (1.09) | 31.24 (1.75) | <0.001 | 1.6 mg/MJ e | 17 mg e |
| Pantothenic acid (B5) (mg) | 7.93 (0.31) | 12.28 (4.94) | 0.551 | 7 * | 7 * |
| Pyridoxine (B6) (mg) | 2.91 (0.10) | 3.40 (0.60) | 0.997 | 1.7 | 2 |
| Biotin (B7) (μg) | 51.60 (2.94) | 63.86 (12.69) | 0.779 | 45 * | 35 * |
| Folate food + folic acid (B9) (μg) | 473.22 (20.87) | 668.15 (46.65) | <0.001 | 500 f | 500 g |
| Cobalamin (B12) (μg) | 6.92 (0.28) | 258.40 (53.44) | 0.096 | 5 * | 2.8 |
| Vitamin C (mg) | 178.41 (8.87) | 211.86 (18.33) | 0.088 | 155 | <19y: 115 |
| ≥19y: 120 | |||||
| Vitamin A (μg) | 1430.37 (111.15) | 1357.94 (108.26) | 0.704 | 1300 h | <19y: 1200 i |
| ≥19y: 1300 i | |||||
| Vitamin D (μg) | 5.61 (0.47) | 10.78 (4.28) | 0.204 | 15 *j | 15 jk |
| Vitamin E (μg) | 17.16 (0.84) | 20.65 (1.49) | 0.029 | 11 *l | 19 |
| Iodine (μg) | 245.33 (11.76) | 259.58 (47.15) | 0.536 | 200 * | 290 |
| Calcium (mg) | 1148.32 (35.12) | 910.92 (70.48) | 0.002 | 18–24y:1000 | <19y: 1300 |
| ≥25y: 950 | ≥19y: 1000 | ||||
| Phosphorus (mg) | 1677.19 (38.38) | 1436.13 (79.71) | 0.007 | 550 * | <19y: 1250 |
| ≥19y: 700 | |||||
| Iron (mg) | 25.34 (2.17) | 31.33 (5.23) | 0.028 | 16 | <19y: 10 |
| ≥19y: 9 | |||||
| Zinc (mg) | 14.40 (0.52) | 12.07 (0.83) | 0.077 | 10.4–15.6 m | <19y: 13 |
| ≥19y: 12 | |||||
| Selenium (μg) | 118.98 (3.38) | 100.80 (6.66) | 0.010 | 85 * | 70 |
The quantitative variables are presented as means (standard error of the mean). 1 Medians and interquartile ranges for riboflavin are also shown, due to the wide dispersion of the values in the Veg group. a Recommended daily intake. Adequate intake is presented with an asterisk (*) and PRI/RDA (i.e., Population Reference Intake for the EFSA values, and Recommended Dietary Allowance for the IOM values) in ordinary type. ** Reference intake range. b Depends on age and level of physical activity. c 0.83 g/kg body weight + 19 g/day from 0–6 months postpartum or + 13 g/day if >6 months postpartum. d In addition to the combined intakes of EPA and DHA of 0.25 g/day. e As the niacin equivalents (NE) (1 mg niacin = 1 mg NE = 60 mg dietary tryptophan). f DFE: dietary folate equivalents. For the combined intakes of food folate and folic acid, DFEs can be computed as follows: μg DFE = μg food folate + (1.7 × μg folic acid). g As the dietary folate equivalents (DFE). Furthermore, 1 DFE = 1 μg of folate from food = 0.6 μg of folic acid from fortified foods or from supplements taken with food = 0.5 μg of folic acid from supplements was taken on an empty stomach. h RE: retinol equivalents, 1 μg RE equals 1 μg of retinol, 6 μg of β-carotene, and 12 μg of other provitamin A carotenoids. i As retinol activity equivalents (RAEs). 1 RAE = 1 μg of retinol, 12 μg of β-carotene, 24 μg of α-carotene, or 24 μg of β-cryptoxanthin. j Assuming minimal cutaneous synthesis. In the presence of an endogenous cutaneous synthesis of vitamin D, the dietary vitamin D requirements are lower or even zero. Further, 1 μg of vitamin D ingested = 40 International Units (IU) and 0.025 μg of vitamin D ingested = 1 IU. k As cholecalciferol. l Such as ∝-tocopherol, which includes RRR-∝-tocopherol, the only form naturally present in foods, as well as the synthetic 2R-isomeric forms of ∝-tocopherol, which are found in certain fortified foods and supplements. m Depending on the level of a phytate intake; the higher the phytate intake, the higher the zinc requirement. Abbreviations: EFSA, European Food Safety Authority; PRI, population reference intake; AI, adequate intake; IOM, Institute of Medicine; RDA, recommended dietary allowances; Kcal, kilocalories; ALAP: as low as possible, while consuming a nutritionally adequate diet; PUFAs, polyunsaturated fatty acids; SFA, saturated fatty acids; MUFAs, monounsaturated fatty acids; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; MJ: megajoule; and y, years.
Table 6.
Prevalence of inadequate intakes of specific nutrients in omnivore human milk donors with full-term infants (Donors) and in vegetarian/vegan lactating mothers (Veg) 1.
| Nutrient | H-AR * [50] | Donors (n = 92), n (%) | Veg (n = 20), n (%) | p Value |
|---|---|---|---|---|
| Thiamine (B1), mg | 1.2 | 5 (5.4%) | 0 (0.0%) | 0.286 |
| Riboflavin (B2), mg | 1.7 | 13 (14.1%) | 9 (45%) | 0.002 |
| Niacin (B3), mg | 13 | 0 (0.0%) | 0 (0.0%) | - |
| Pantothenic acid (B5), mg | 5.6 | 22 (23.9%) | 6 (30%) | 0.568 |
| Pyridoxine (B6), mg | 1.4 | 1 (1.1%) | 0 (0.0%) | 0.640 |
| Biotin (B7), μg | 36 | 34 (37.0%) | 7 (35.0%) | 0.869 |
| Folate food + folic acid (B9), μg | 380 (DFE) | 36 (39.1%) | 0 (0.0%) | <0.001 |
| Cobalamin (B12), μg | 2.4 | 0 (0.0%) | 5 (25.0%) | <0.001 |
| Vitamin C, mg | 145 | 34 (37.0%) | 4 (20.0%) | 0.147 |
| Vitamin A, μg RAE | 1020 | 32 (34.8%) | 6 (30.0%) | 0.682 |
| Vitamin D, μg | 10 | 81 (88.04) | 15 (75.00) | 0.131 |
| Vitamin E, mg | 16 | 45 (48.9%) | 5 (25.0%) | 0.051 |
| Iodine, μg | 209 | 40 (43.5%) | 8 (40.0%) | 0.776 |
| Calcium, mg | 860 (19–30 y) 750 (31–50 y) |
6 (6.5%) | 9 (45.0%) | <0.001 |
| Phosphorous, mg | 580 | 0 (0.0%) | 0 (0.0%) | - |
| Selenium, μg | 59 | 1 (1.1%) | 0 (0.0%) | 0.640 |
1 The number and percentage of women with inadequate intakes of each nutrient (below harmonized average requirements) are presented in each group. * The H-AR, the harmonized average requirement, was proposed by Allen et al., (2020) [50], after they selected values from the standards set by EFSA (for Europe) and the IOM (for the United States and Canada), giving priority to those published most recently. Abbreviations: DFE, dietary folate equivalents; RAE, retinol activity equivalents; and y, years.
Table 7.
Diet survey: five-day dietary diaries. Number of food servings per day consumed by the participants, healthy eating index (HEI), and records of supplement and iodized salt intake of the omnivore human milk donors with full-term infants (Donors) and vegetarian/vegan lactating mothers (Veg).
| Donors (n = 92) | Veg (n = 20) | p Value | Recommendations [24] a | |
|---|---|---|---|---|
| Servings per day: | ||||
| Dairy | 2.58 (0.13) | 0.58 (0.28) | <0.001 | ≥4 b |
| Grains, legumes, and nuts | 6.12 (0.20) | 8.11 (0.60) | 0.003 | ≥7 |
| Vegetables and greens | 3.40 (0.14) | 5.11 (0.33) | <0.001 | ≥4 |
| Fruits | 1.85 (0.13) | 1.87 (0.22) | 0.654 | ≥3 |
| Eggs, meat, and fish | 2.94 (0.11) | 0.28 (0.05) | <0.001 | 2–3 c |
| HEI | 63.26 (0.92) | 61.69 (1.50) | 0.478 | |
| Supplement intake: yes, n (%) | 51 (55.4%) | 17 (85%) | 0.028 | |
| Iodized salt intake: yes, n (%) | 62 (67.4%) | 14 (70.0%) | 0.359 | |
| Salt (g/day) | 1.10 (0.06) | 1.89 (0.26) | <0.001 |
The quantitative variables are presented as means (standard error of the mean). The qualitative variables are expressed as the absolute and relative frequencies (%). a Number of recommended daily servings of food for lactating women. b Preferably skimmed or semi-skimmed. c Preferably fat-free or very low-fat. Abbreviations: HEI, Health Eating Index.
Table 8.
Diet survey: food consumption frequency questionnaire (FFQ, the number of food servings per day or week that were consumed by the participants) of the omnivore human milk donors with full-term infants (Donors) and vegetarian/vegan mothers (Veg).
| Donors (n = 92) | Veg (n = 20) | p Value | Serving Size [17] a | |
|---|---|---|---|---|
| Milk (servings/day) | 1.48 (0.11) | 0.26 (0.17) | <0.001 | 200–250 mL |
| Other dairy products (servings/day) | 1.06 (0.10) | 0.21 (0.15) | <0.001 | Yogurt 200–250 g Fresh cheese 80–125 g Cured cheese 40–60 g |
| Meats and derivatives (servings/day) | 0.73 (0.06) | 0.00 (0.00) | <0.001 | 100–125 g |
| Fish (servings/week) | 2.19 (0.14) | 0.01 (0.01) | <0.001 | 125–150 g |
| Eggs (servings/week) | 2.92 (0.18) | 1.07 (0.40) | <0.001 | 60 g |
| Fruits (servings/day) | 2.22 (0.16) | 2.87 (0.44) | 0.146 | 120–200 g |
| Raw vegetables (servings/day) | 0.64 (0.06) | 1.04 (0.15) | 0.002 | 150–200 g |
| Cooked vegetables (servings/day) | 0.80 (0.05) | 1.43 (0.15) | <0.001 | 150–200 g |
| Legumes (servings/week) | 1.39 (0.09) | 5.03 (0.55) | <0.001 | 60–80 g |
| Bread (servings/day) | 1.79 (0.18) | 1.73 (0.34) | 0.959 | 40–60 g |
| Pasta, rice, other grains (servings/week) | 3.31 (0.35) | 7.28 (1.20) | <0.001 | 60–80 g |
| Nuts (servings/week) | 4.14 (0.68) | 8.32 (1.15) | 0.003 | 25 g |
| Oils and fats (servings/day) | 2.84 (0.30) | 2.27 (0.33) | 0.765 | 10 g |
| Sweets (grams/week) | 285.83 (44.06) | 124.36 (22.34) | 0.053 |
a Serving weights for each food group. Data are presented as means (standard error of the mean).
The caloric and lipid profiles of the diet of the Veg group showed to be more adequate. They consumed less saturated fat, trans FAs, and cholesterol, as well as more polyunsaturated fatty acids (PUFAs) consisting of linoleic acid (LA) and linolenic acid (ALA). However, their intake of very-long-chain n-3 FAs, DHA, and eicosapentaenoic acid (EPA) was found to be in a much lower than the recommended amount, as per the FAO/WHO (2008) [32]. Furthermore, there was a higher n-6/n-3 ratio in the Veg group. Regarding vitamins and minerals, the Donors consumed more riboflavin, niacin, calcium, phosphorus, and selenium, while Veg consumed more vitamin E, food folate + folic acid, and pyridoxine/proteins. The food folate and folic acid intake by the Donors was below the recommended amount (Table 5). However, there was no difference in the healthy eating index score between the two groups; both scores were indicative of a good diet (Table 7).
3.3. Nutritional Status
The results of the lipid, vitamin, and mineral studies in the erythrocytes, plasma, and urine are presented in Table 9 and Table 10. The contribution of the SFAs, monounsaturated fatty acids (MUFAs), and PUFAs to the total FAs content was similar in both the Donors and Veg group, with some small differences. The predominant FAs in both groups were the same: arachidonic acid (AA) followed by palmitic/stearic acids in the erythrocytes, and LA followed by palmitic/oleic acids in the plasma. Notably, Veg group had a lower proportion of SFAs in their plasma, but a significantly lower proportion of total n-3 FAs, and particularly of EPA and DHA, in both their plasma and erythrocytes, as well as a higher n-6/n-3 ratio. The levels of C16:0 DMA and C18:0 DMA and therefore the total content of plasmalogens were higher both in the plasma and erythrocytes from Donors (Table 9).
Table 9.
Erythrocytes and plasma fatty acid composition (g/100 g of total fat) of the omnivore human milk donors with full-term infants (Donors) and vegetarian/vegan lactating mothers (Veg).
| Fatty Acid (%) | Common Name | Donors (n = 92) | Veg (n = 20) | p Value |
|---|---|---|---|---|
| ERYTHROCYTES | ||||
| Saturated Fatty Acids (SFAs) | ||||
| C14:0 | Myristic | 0.12 (0.00) | 0.10 (0.01) | 0.041 |
| DMA C16:0 | Dimethylacetal C16:0 | 2.20 (0.02) | 1.64 (0.05) | <0.001 |
| C16:0 | Palmitic | 21.20 (0.21) | 19.97 (0.40) | 0.022 |
| DMA C18:0 | Dimethylacetal C18:0 | 3.46 (0.03) | 2.92 (0.13) | <0.001 |
| C18:0 | Stearic | 20.25 (0.15) | 19.40 (0.34) | 0.066 |
| C24:0 | Lignoceric | 2.40 (0.09) | 3.65 (0.22) | <0.001 |
| Monounsaturated Fatty Acids (MUFAs) | ||||
| C17:1 | Margaroleic | 0.35 (0.01) | 0.52 (0.04) | <0.001 |
| C18:1 cis-11 (n7) | Cis vaccenic | 0.24 (0.01) | 0.32 (0.03) | 0.001 |
| C18:1 cis-9 (n9) | Oleic | 12.62 (0.15) | 13.40 (0.38) | 0.007 |
| n-6 Polyunsaturated Fatty Acids (n-6 PUFAs) | ||||
| C18:2 (n6) | Linoleic | 8.12 (0.14) | 9.24 (0.31) | 0.002 |
| C20:3 (n6) | Dihomo-γ-linolenic | 0.99 (0.05) | 1.57 (0.14) | <0.001 |
| C20:4 (n6) | Arachidonic | 24.30 (0.32) | 24.91 (0.75) | 0.130 |
| n-3 Polyunsaturated Fatty Acids (n-3 PUFAs) | ||||
| C20:5 (n3) | Eicosapentaenoic | 0.12 (0.02) | 0.00 (0.00) | 0.009 |
| C22:5 (n3) | Docosapentaenoic | 0.73 (0.03) | 0.66 (0.04) | 0.151 |
| C22:6 (n3) | Docosahexaenoic | 2.90 (0.12) | 1.72 (0.32) | <0.001 |
| Fatty Acid Families | ||||
| DMAs | 5.66 (0.05) | 4.55 (0.16) | <0.001 | |
| SFAs | 43.98 (0.28) | 43.12 (0.49) | 0.347 | |
| MUFAs | 13.20 (0.15) | 14.23 (0.44) | 0.003 | |
| PUFAs | 37.09 (0.39) | 38.10 (0.65) | 0.308 | |
| MCFAs (C8-C15) | 0.12 (0.00) | 0.10 (0.01) | 0.041 | |
| LCFAs (C16-C18) | 62.78 (0.46) | 62.85 (0.73) | 0.924 | |
| VLCFAs (C20-C24) | 29.04 (0.39) | 28.85 (0.76) | 0.992 | |
| n-6 PUFAs | 33.41 (0.30) | 35.72 (0.59) | 0.007 | |
| n-3 PUFAs | 3.75 (0.16) | 2.38 (0.35) | <0.001 | |
| n-6 PUFAs/n-3 PUFAs | 10.83 (0.59) | 21.09 (2.49) | <0.001 | |
| PLASMA | ||||
| Saturated Fatty Acids (SFAs) | ||||
| C14:0 | Myristic | 0.28 (0.01) | 0.24 (0.03) | 0.062 |
| C15:0 | Pentadecylic | 0.05 (0.00) | 0.03 (0.00) | 0.003 |
| DMA C16:0 | Dimethylacetal C16:0 | 0.20 (0.01) | 0.12 (0.01) | <0.001 |
| C16:0 | Palmitic | 21.17 (0.17) | 19.10 (0.49) | <0.001 |
| DMA C18:0 | Dimethylacetal C18:0 | 0.08 (0.00) | 0.05 (0.01) | <0.001 |
| C18:0 | Stearic | 6.26 (0.07) | 5.86 (0.15) | 0.033 |
| Monounsaturated Fatty Acids (MUFAs) | ||||
| C16:1 cis-9 (n7) | Palmitoleic | 0.43 (0.02) | 0.42 (0.08) | 0.010 |
| C18:1 cis-11 (n7) | Cis vaccenic | 0.44 (0.01) | 0.55 (0.04) | 0.002 |
| C18:1 cis-9 (n9) | Oleic | 18.40 (0.26) | 19.92 (0.99) | 0.411 |
| n-6 Polyunsaturated Fatty Acids (n-6 PUFAs) | ||||
| C18:2 (n6) | Linoleic | 39.20 (0.46) | 41.28 (1.42) | 0.390 |
| C20:3 (n6) | Dihomo-γ-linolenic | 1.11 (0.06) | 1.43 (0.18) | 0.057 |
| C20:4 (n6) | Arachidonic (ARA) | 11.30 (0.33) | 10.56 (0.71) | 0.533 |
| n-3 Polyunsaturated Fatty Acids (n-3 PUFAs) | ||||
| C20:5 (n3) | Eicosapentaenoic (EPA) | 0.24 (0.03) | 0.08 (0.02) | 0.012 |
| C22:6 (n3) | Docosahexaenoic (DHA) | 0.83 (0.06) | 0.37 (0.07) | <0.001 |
| Fatty Acid Families | ||||
| DMAs | 0.29 (0.01) | 0.17 (0.01) | <0.001 | |
| SFAs | 27.76 (0.19) | 25.23 (0.48) | <0.001 | |
| MUFAs | 19.27 (0.26) | 20.89 (1.06) | 0.454 | |
| PUFAs | 52.68 (0.34) | 53.71 (1.11) | 0.309 | |
| MCFA (C8-C15) | 0.33 (0.02) | 0.27 (0.03) | 0.031 | |
| LCFA (C16-C18) | 85.91(0.40) | 87.12 (0.75) | 0.361 | |
| VLCFA (C20-C24) | 13.48 (0.39) | 12.44 (0.74) | 0.456 | |
| n-6 PUFAs | 51.61 (0.34) | 53.26 (1.10) | 0.173 | |
| n-3 PUFAs | 1.07 (0.08) | 0.45 (0.09) | <0.001 | |
| n-6 PUFAs/n-3 PUFAs | 82.35 (6.70) | 173.23 (26.35) | <0.001 | |
Data expressed as means (standard error of the mean). Abbreviations: DMA, dimethylacetal, MCFA, medium-chain fatty acids; LCFA, long-chain fatty acids; and VLCFA: very-long-chain fatty acids.
Table 10.
Erythrocytes, plasma, and urine concentrations of nutrients and biochemical determinations of the omnivore human milk donors with full-term infants (Donors) and vegetarian/vegan mothers (Veg).
| Variable 1 | Donors (n = 92) | Veg (n = 20) | p Value | Comments about the Reference Values or Studies in which the Corresponding Vitamers are Determined 3 |
||
|---|---|---|---|---|---|---|
| n | Value 2 | n | Value 2 | |||
| ERYTHROCYTES | ||||||
| Hemoglobin (Drabkin colorimetric method) | ||||||
| g/dL | 92 | 25.57 (0.38) | 20 | 23.33 (0.84) | 0.011 | |
| EGRAC (assay kit) | 74 | 7 | ||||
| 1.25 (0.03) | 1.53 (0.12) | 0.029 | ||||
| Riboflavin insufficiency/deficiency (EGRAC ≥ 1.4) [26,51,52] | 22 (29.7%) | 4 (57.1%) | 0.224 | |||
| Marginal riboflavin status (EGRAC 1.2 to <1.4) [51,52] | 20 (27.0%) | 2 (28.6%) | ||||
| Acceptable riboflavin status (EGRAC < 1.2) [51,52] | 32 (43.2%) | 1 (14.3%) | ||||
| Riboflavin, B2 (UPLC-MS/MS) | 92 | 20 |
|
|||
| ng/L | 767.92 (37.71) | 932.02 (119.42) | 0.277 | |||
| nM | 2.04 (0.10) | 2.48 (0.32) | ||||
| ng/g Hb | 3.09 (0.16) | 4.12 (0.55) | 0.115 | |||
| Riboflavin deficiency (<170 nM) [51] | 92 (100%) | 20 (100%) | - | |||
| Nicotinamide, B3 (UPLC-MS/MS) |
|
|||||
| mcg/L | 4718.23 (298.58) | 5039.97 (636.03) | 0.659 | |||
| mcM | 38.64 (2.44) | 41.27 (5.21) | ||||
| mcg/g Hb | 18.51 (1.28) | 22.41 (3.06) | 0.327 | |||
| Pantothenic acid, B5 (UPLC-MS/MS) | 92 | 20 |
|
|||
| mcg/L | 58.00 (9.32) | 111.85 (35.59) | 0.849 | |||
| nM | 264.56 (42.51) | 510.19 (162.34) | ||||
| mg/g Hb | 233.36 (42.00) | 489.65 (154.86) | 0.964 | |||
| Pyridoxamine, B6 (UPLC-MS/MS) | 92 | 20 |
|
|||
| mcg/L | 519.43 (23.59) | 612.83 (33.22) | 0.044 | |||
| mcM | 3.07 (0.14) | 3.62 (0.20) | ||||
| mcg/g Hb | 2.06 (0.11) | 2.73 (0.20) | 0.006 | |||
| PLASMA | ||||||
| Thiamin, B1 (UPLC-MS/MS) | 92 | 20 |
|
|||
| mcg/L | 0.42 (0.03) | 0.49 (0.10) | ||||
| nM | 1.24 (0.09) | 1.45 (0.30) | ||||
| Riboflavin, B2 (UPLC-MS/MS) | 92 | 20 |
|
|||
| mcg/L | 23.01 (1.86) | 14.79 (1.89) | 0.006 | |||
| nM | 61.14 (4.94) | 39.30 (5.02) | ||||
| Riboflavin < 6.7 nM [64] | 0 (0.0%) | 0 (0.0%) | ||||
| Nicotinamide, B3 (UPLC-MS/MS) | 92 | 20 |
|
|||
| mcg/L | 4.77 (0.36) | 3.46 (0.27) | 0.052 | |||
| nM | 39.06 (2.95) | 28.33 (2.21) | ||||
| Pantothenic acid, B5 (UPLC-MS/MS) | 92 | 20 | ||||
| mcg/L | 175.24 (52.12) | 170.05 (35.48) | 0.410 | |||
| nM | 799.30 (237.90) | 775.60 (161.80) | ||||
| Pyridoxine, B6 (UPLC-MS/MS) | 92 | 20 |
|
|||
| mcg/L | 139.29 (3.27) | 144.12 (7.21) | 0.548 | |||
| nM | 823.32 (19.3) | 851.87 (42.62) | ||||
| Pyridoxamine, B6 (UPLC-MS/MS) | 92 | 20 |
|
|||
| mcg/L | 264.26 (4.88) | 285.48 (7.30) | 0.006 | |||
| nM | 1571.14 (28.88) | 1691.36 (43.40) | ||||
| Folate, B9 (UPLC-MS/MS) | 91 | 20 |
|
|||
| mcg/L | 3.11 (0.45) | 5.20 (2.42) | 0.758 | |||
| nM | 7.05 (1.02) | 11.55 (5.48) | ||||
| Folate status undetermined (6.8–13.4 nM) [71] | 26 (28.6%) | 5 (25.0%) | 0.739 | |||
| Folate deficiency (<6.8 nM) [71] | 60 (65.9%) | 13 (65.0%) | ||||
| Cobalamin, B12 (Competitive immunoassay). | 92 | 20 |
|
|||
| pM | 557.30 (22.46) | 519.80 (61.17) | 0.276 | |||
| B12 depletion (148–221 pM) [72] | 1 (1.1%) | 2 (10.0%) | 0.025 | |||
| B12 deficiency (<148 pM) [72] | 0 (0.0%) | 0 (0.0%) | ||||
| Severe B12 deficiency (<75 pM) [72] | 0 (0.0%) | 0 (0.0%) | ||||
| Holotranscobalamin II (Immunoassay ELISA kit) | 92 | 20 |
|
|||
| pM | 191.52 (7.61) | 185.04 (21.41) | 0.384 | |||
| B12 depletion (Holo-TC II < 35 pM) [73] | 0 (0.0%) | 0 (0.0%) | - | |||
| Homocysteine (enzymatic assay) | 92 | 20 | ||||
| mcM | 10.37 (0.37) | 8.30 (0.78) | 0.006 | |||
| Homocysteine elevated (>13 mcM) [71,74] | 21 (22.8%) | 2 (10.0%) | 0.198 | |||
| Ascorbic acid (HPLC-DAD) | 92 | 20 |
|
|||
| mcM | 51.88 (3.23) | 62.14 (3.80) | 0.012 | |||
| <11 mcM: scurvy [27] | 1 (1.1%) | 0 (0.0%) | 0.640 | |||
| Retinol (HPLC with fluorescence and UV detector) | 91 | 20 | ||||
| mcg/dL | 51.35 (1.61) | 44.53 (3.74) | 0.056 | |||
| mcM | 1.79 (0.06) | 1.55 (0.13) | ||||
| Vit A deficiency (Retinol <20 mcg/dL, <0.7 mcM) [76,77] | 0 (0.0%) | 0 (0.0%) | - | |||
| 25(OH), D (UPLC-electrospray ionization/tandem MS) | 92 | 20 | ||||
| ng/mL | 7.37 (0.42) | 9.22 (1.29) | 0.264 | |||
| nM | 18.40 (1.05) | 23.01 (3.22) | ||||
| Risk for vit D inadequacy (25(OH)D 12- < 20 ng/mL; 30- < 50 nM) [30,78,79] | 11 (12.0%) | 4 (20.0%) | 0.044 | |||
| Risk for vitamin deficiency (25(OH)D < 12 ng/mL; <30 nM) [30,78,79] | 80 (87.0%) | 14 (70%) | ||||
| 1,25(OH)2D (UPLC-electrospray ionization/tandem MS) | 92 | 20 | ||||
| pg/mL | 146.50 (15.29) | 271.41 (35.70) | <0.001 | |||
| pM | 351.65 (36.70) | 651.49 (85.69) | ||||
| α-tocopherol (HPLC with fluorescence and UV detector) | 90 | 20 |
|
|||
| mcg/dL | 287.63 (24.42) | 311.37 (56.64) | 0.941 | |||
| mcM | 6.68 (0.57) | 7.23 (1.31) | ||||
| Vit E deficiency (<500 mcg/dL; <0.5 mg/dL; <11.6 mcM) [27] | 80 (89.9%) | 16 (80.0%) | 0.218 | |||
| Severe vit E deficiency (<5.8 mcM) [81] | 56 (62.9%) | 9 (45.0%) | 0.140 | |||
| α-tocopherol:total lipids (cholesterol+triacylglycerols) | 89 | 20 |
|
|||
| (mcmol:mmol) | 1.32 (0.12) | 1.45 (0.29) | 0.196 | |||
| Ratio < 1.6 mcmol:mmol [81] | 70 (78.7%) | 13 (65.0%) | ||||
| α-tocopherol:cholesterol (mcmol:mmol) | 89 | 1.47 (0.13) | 20 | 1.63 (0.34) | 0.457 |
|
| Ratio < 2.2 mcmol:mmol [81] | 77 (86.5%) | 16 (80.0%) | ||||
| γ-tocopherol (HPLC with fluorescence and UV detector) | 88 | 20 | ||||
| mcg/dL | 37.62 (2.16) | 52.66 (4.98) | 0.003 | |||
| Total cholesterol (enzymatic assay) | 92 | 20 | ||||
| mg/dL | 183.01 (3.51) | 182.01 (6.03) | 0.840 | |||
| mM | 4.74 (0.09) | 4.71 (0.16) | ||||
| Hypercholesterolemia (>240 mg/dL) [79] | 6 (6.5%) | 1 (5.0%) | 0.799 | |||
| Triacylglycerols (enzymatic assay) | 92 | 20 | ||||
| mg/dL | 48.93 (1.90) | 43.26 (2.43) | 0.336 | |||
| mM | 0.55 (0.02) | 0.49 (0.03) | ||||
| Hypertriglyceridemia (>200 mg/dL) [79] | 0 (0.0%) | 0 (0.0%) | - | |||
| HDL (enzymatic assay) | 92 | 20 | ||||
| mg/dL | 62.32 (1.08) | 69.49 (2.37) | 0.008 | |||
| mM | 1.61 (0.03) | 1.80 (0.06) | ||||
| Low HDL levels (<40 mg/dL) [79] | 1 (1.1%) | 0 (0.0%) | 0.640 | |||
| LDL (enzymatic assay) | 92 | 20 | ||||
| mg/dL | 103.92 (2.70) | 105.26 (6.29) | 0.790 | |||
| mM | 2.69 (0.07) | 2.73 (0.16) | ||||
| High LDL levels (>160 mg/dL) [79] | 3 (3.3%) | 0 (0.0%) | 0.413 | |||
| URINE | ||||||
| Cr (Jaffé colorimetric kinetic method) | 92 | 121.17 (5.67) | 20 | 121.22 (13.23) | 0.776 | |
| mg/dL | ||||||
| Methylmalonic acid (UPLC-MS/MS) |
91 | 20 |
|
|||
| mg/L | 6.17 (0.32) | 5.97 (0.79) | 0.618 | |||
| mcg/mg Cr | 5.28 (0.27) | 5.38 (0.81) | 0.613 | |||
| mcmol/mmol Cr | 5.06 (0.26) | 5.15 (0.78) | 0.613 |
|
||
| B12 deficiency marker (Methylmalonic acid/Cr >4 mcg/mg; >3.8 mmol/mol) [73] |
55 (61.1%) | 12 (60.0%) | 0.927 | |||
| Iodine (mcg/L) (ICP-MS) | 92 | 20 | ||||
| Mean (SE) mcg/L | 127.71 (7.57) | 112.00 (21.47) |
0.061 |
|
||
| Median (p25, p75) mcg/L | 109.64 (75.80, 159.54) * | 66.28 (50.26, 139.36) * | ||||
| mcg/mg Cr | 0.11 (0.01) | 0.10 (0.02) | 0.082 | |||
| mcg/g Cr | 110 (10) | 100 (20) |
|
|||
| Sodium (ICP-MS) | 92 | 20 | ||||
| mg/L | 3365.51 (139.32) | 3693.99 (454.86) | 0.704 | |||
| M | 0.15 (0.01) | 0.16 (0.02) | ||||
| mg/mg Cr | 3.07 (0.15) | 3.38 (0.38) | 0.528 | |||
| mmol/g Cr | 133.48 (6.52) | 146.93 (16.52) |
|
|||
| Calcium (ICP-MS) | 92 | 20 | ||||
| mg/L | 105.00 (9.28) | 72.02 (14.68) | 0.082 | |||
| mg/mg Cr | 0.09 (0.01) | 0.06 (0.01) | 0.024 |
|
||
| Phosphorus (ICP-MS) | 92 | 20 | ||||
| mg/L | 1053.17 (52.08) | 840.20 (89.73) | ||||
| mg/mg Cr | 0.88 (0.03) | 0.74 (0.06) |
|
1 The units of our results have been converted to the international system. 2 Values are presented as the means (standard error of the mean) for quantitative variables and the number of participants (%) for qualitative variables. 3 References have been sorted by year of publication. In the absence of clearly established reference values, the results of studies in which the same vitamers have been determined are presented. For the purpose of comparison, the units of some of the cited manuscripts have also been converted to the international system. * In the case of urinary iodine, the median (25th, 75th percentiles) is also shown, as it is the value used to assess the adequacy of iodine intake in a population. Abbreviations: n, number of samples; EGRAC, erythrocyte glutathione reductase activity coefficient: EGRA with an excess of flavine adenine dinucleotide (FAD)/EGRA under baseline conditions; UPLC, ultra-performance liquid chromatography; MS/MS, tandem mass spectrometry; SE, standard error; Hb, hemoglobin; SD, standard deviation; UHPLC, ultra-high-performance liquid chromatography; HPLC, high-performance liquid chromatography; LC, liquid chromatography; Holo-TCII, holotranscobalamin II; DAD, diode array detector; UV, ultraviolet; MS, mass spectrometry; Cr, creatinine; ICP, inductively coupled plasma; and M, molar.
In the assessment of the participants’ riboflavin status from the measurement of the erythrocyte glutathione reductase activity coefficient (EGRAC), a high percentage of women with a deficiency was observed in both groups (29.7% in Donors and 57.1% in Veg). Vegetarians had lower plasma levels of riboflavin, higher levels of both erythrocyte and plasma pyridoxamine, higher plasma ascorbic acid, and lower urine calcium/creatinine ratios. The low levels of plasma thiamine, erythrocyte riboflavin (albeit normal–high plasma levels), plasma niacin, plasma folate, plasma 25-OHD3, and plasma α-tocopherol were noteworthy in both groups. On the other hand, the levels of plasma ascorbic acid, retinol, cobalamin, and holotranscobalamin II were normal in both groups (Table 10).
3.4. Human Milk Composition
The results of the macronutrient, lipid, vitamin, and mineral studies in human milk are presented in Table 11, Table 12 and Table 13.
Table 11.
Macronutrient composition (g/100 mL milk), lipid classes’ profile (g/100 g fat), relative composition of phospholipids (g/100 g polar lipids) and molecular species of triacylglycerols content (g/100 g fat) according to their carbon number (CN) in the human milk of the omnivore human milk donors with full-term infants (Donors) and in the vegetarian/vegan mothers (Veg).
| Nutrient | Donors | Veg | p Value | ||
|---|---|---|---|---|---|
| n | Mean (SE) | n | Mean (SE) | ||
| Macronutrients (g/100 mL milk) | |||||
| Lipids | 84 | 3.13 (0.17) | 18 | 3.14 (0.34) | 0.958 |
| Carbohydrates | 7.73 (0.03) | 7.76 (0.06) | 0.779 | ||
| Proteins | 1.17 (0.03) | 1.16 (0.05) | 0.533 | ||
| Lipid classes (g/100 g fat) | |||||
| Triacylglycerols | 19 | 95.56 (0.55) | 18 | 95.33 (0.66) | 0.832 |
| Diacylglycerols | 3.97 (0.49) | 4.18 (0.61) | 0.808 | ||
| Monoacylglycerols | 0.04 (0.01) | 0.03 (0.01) | 0.586 | ||
| Free fatty acids + cholesterol | 0.38 (0.05) | 0.41 (0.05) | 0.543 | ||
| Polar lipids | 0.05 (0.00) | 0.06 (0.00) | 0.047 | ||
| Triacylglycerols (g/100 g fat) | |||||
| CN24 | 19 | 0.01 (0.00) | 18 | 0.01 (0.00) | 0.428 |
| CN26 | 0.10 (0.01) | 0.11 (0.01) | 0.713 | ||
| CN28 | 0.09 (0.01) | 0.06 (0.01) | 0.038 | ||
| CN30 | 0.21 (0.03) | 0.13 (0.02) | 0.024 | ||
| CN32 | 0.31 (0.05) | 0.26 (0.04) | 0.543 | ||
| CN34 | 0.36 (0.07) | 0.22 (0.03) | 0.301 | ||
| CN36 | 0.40 (0.05) | 0.38 (0.05) | 0.915 | ||
| CN38 | 1.61 (0.16) | 1.16 (0.10) | 0.022 | ||
| CN40 | 1.98 (0.12) | 2.09 (0.13) | 0.533 | ||
| CN42 | 2.65 (0.21) | 2.72 (0.26) | 0.671 | ||
| CN44 | 4.95 (0.32) | 4.47 (0.37) | 0.627 | ||
| CN46 | 7.45 (0.35) | 6.60 (0.45) | 0.346 | ||
| CN48 | 10.68 (0.34) | 10.62 (0.62) | 0.574 | ||
| CN50 | 14.83 (0.49) | 12.18 (0.52) | 0.001 | ||
| CN52 | 37.14 (1.11) | 32.37 (1.34) | 0.011 | ||
| CN54 | 17.23 (1.20) | 26.64 (1.47) | <0.001 | ||
| Phospholipids (g/100 g polar lipids) | |||||
| Phosphatidylethanolamine | 19 | 24.62 (1.86) | 18 | 30.72 (1.88) | 0.048 |
| Phosphatidylcholine | 30.55 (1.10) | 26.88 (0.67) | 0.018 | ||
| Sphingomyelin | 44.83 (2.58) | 42.40 (1.82) | 0.429 | ||
Variables are presented as means (standard error of the mean).
Table 12.
Human milk fatty acid methyl esters (FAMEs) composition (g/100 g of total fat) of the omnivore human milk donors with full-term infants (Donors) and vegetarian/vegan mothers (Veg).
| Fatty Acid (%) | Common Name | Donors (n = 88) | Veg (n = 20) | p Value | Reference Values | |
|---|---|---|---|---|---|---|
| European [88] 1 | World [89] 2 | |||||
| Saturated Fatty Acids (SFAs) | ||||||
| C6:0 | Caproic | 0.11 (0.00) | 0.10 (0.00) | 0.631 | 0.08 ± 0.02 | 0.13 ± 0.47 |
| C8:0 | Caprylic | 0.18 (0.00) | 0.17 (0.01) | 0.095 | 0.22 ± 0.06 | 0.21 ± 0.22 |
| C10:0 | Capric | 1.18 (0.03) | 1.05 (0.06) | 0.018 | 1.44 ± 0.34 | 1.37 ± 0.86 |
| C12:0 | Lauric | 5.38 (0.17) | 5.14 (0.36) | 0.719 | 5.46 ± 1.84 | 5.7 ± 2.81 |
| C14:0 | Myristic | 6.50 (0.30) | 5.45 (0.44) | 0.255 | 6.19 ± 1.93 | 6.56 ± 3.05 |
| C15:0 | 0.19 (0.01) | 0.08 (0.01) | <0.001 | |||
| C15:0 ai | C15:0 anteiso | 0.03 (0.00) | 0.02 (0.00) | <0.001 | ||
| C15:0 i | C15:0 iso | 0.04 (0.00) | 0.01 (0.00) | <0.001 | ||
| C16:0 i | C16:0 iso | 0.02 (0.00) | 0.01 (0.01) | <0.001 | ||
| C16:0 | Palmitic | 19.64 (0.27) | 15.24 (0.52) | <0.001 | 21.94 ± 2.92 | 21.5 ± 4.82 |
| C17:0 ai | C17:0 anteiso | 0.05 (0.00) | 0.01 (0.00) | <0.001 | ||
| C17:0 i | C17:0 iso | 0.27 (0.01) | 0.30 (0.02) | 0.102 | ||
| C17:0 | Margaric | 0.20 (0.01) | 0.09 (0.01 | <0.001 | 0.31 ± 0.15 | |
| C18:0 | Stearic | 5.83 (0.14) | 4.06 (0.22) | <0.001 | 6.68 ± 1.59 | 6.36 ± 2.07 |
| C20:0 | Arachidic | 0.16 (0.01) | 0.17 (0.03) | 0.733 | 0.17 ± 0.04 | 0.23 ± 0.17 |
| Monounsaturated Fatty Acids (MUFAs) | ||||||
| C14:1 cis-9 (n5) | Myristoleic | 0.08 (0.00) | 0.03 (0.01) | <0.001 | ||
| C16:1 cis-9 (n7) | Palmitoleic | 1.55 (0.05) | 1.32 (0.11) | <0.001 | 2.21 ± 0.64 | 2.3 ± 0.92 |
| C17:1 | Margaroleic | 0.07 (0.00) | 0.04 (0.00) | <0.001 | ||
| ∑ C18:1 trans | 0.27 (0.02) | 0.07 (0.03) | <0.001 | 0.66 ± 0.35 | ||
| C18:1 cis-9 (n9) | Oleic | 38.31 (0.53) | 41.38 (1.22) | 0.025 | 35.59 ± 4.17 | 32.6 ± 5.84 |
| C18:1 cis-11 (n7) | Cis vaccenic | 1.59 (0.03) | 1.66 (0.05) | 0.282 | 2.38 ± 0.53 | |
| C20:1 (n9) | Gondoic | 0.69 (0.06) | 1.42 (0.21) | <0.001 | 0.38 ± 0.12 | 0.46 ± 0.28 |
| n-6 Polyunsaturated Fatty Acids (n-6 PUFAs) | ||||||
| C18:2 (n6) | Linoleic (LA) | 15.29 (0.39) | 20.02 (1.15) | <0.001 | 14.00 ± 4.95 | 15.7 ± 7.15 |
| C20:2 (n6) | Eicosadienoic | 0.27 (0.01) | 0.32 (0.02) | 0.063 | 0.26 ± 0.07 | 0.37 ± 0.19 |
| C20:3 (n6) | Dihomo-γ-linolenic | 0.33 (0.01) | 0.36 (0.04) | 0.652 | 0.31 ± 0.09 | 0.37 ± 0.18 |
| C20:4 (n6) | Arachidonic (AA) | 0.55 (0.02) | 0.46 (0.03) | 0.012 | 0.44 ± 0.12 | 0.50 ± 0.25 |
| n-3 Polyunsaturated Fatty Acids (n-3 PUFAs) | ||||||
| C18:3 (n3) | Linolenic (ALA) | 0.52 (0.02) | 0.61 (0.05) | 0.044 | 0.94 ± 0.55 | 1.11 ± 1.05 |
| C22:5 (n3) | Docosapentaenoic (DPA) | 0.08 (0.01) | 0.04 (0.01) | <0.001 | ||
| C22:6 (n3) | Docosahexaenoic (DHA) | 0.33 (0.02) | 0.15 (0.04) | <0.001 | 0.34 ± 0.35 | 0.37 ± 0.31 |
| n-7 Polyunsaturated Fatty Acids (n-7 PUFAs) | ||||||
| C18:2 c9, t11 (n7) | Rumenic | 0.09 (0.01) | 0.04 (0.02) | |||
| Fatty Acid Families | ||||||
| Not identified | 0.21 (0.02) | 0.18 (0.02) | 0.297 | |||
| SFAs | 39.78 (0.54) | 31.90 (0.91) | <0.001 | 42.23 ± 5.29 | 42.2 ± 7.73 | |
| MUFAs | 42.55 (0.56) | 45.91 (1.17) | 0.017 | 41.34 ± 4.48 | 36.3 ± 6.46 | |
| PUFAs | 17.46 (0.39) | 22.00 (1.15) | <0.001 | 16.43 ± 5.07 | 21.2 ± 8.18 | |
| SCFAs | 0.11 (0.00) | 0.10 (0.00) | 0.654 | |||
| MCFAs (C8-C15) | 13.59 (0.47) | 11.94 (0.82) | 0.317 | |||
| LCFAs (C16-C18) | 83.60 (0.48) | 84.81 (0.93) | 0.509 | |||
| VLCFAs (C20-C24) | 2.50 (0.09) | 2.96 (0.24) | 0.071 | |||
| n-6 PUFAs | 16.44 (0.39) | 21.16 (1.16) | <0.001 | 17.8 ± 7.51 | ||
| n-3 PUFAs | 0.93 (0.04) | 0.80 (0.06) | 0.104 | 1.88 ± 2.63 | ||
| n-6 PUFAs/n-3 PUFAs | 20.90 (1.20) | 29.32 (2.49) | 0.001 | |||
| LA/ALA ratio | 33.61 (1.64) | 36.46 (3.25) | 0.272 | |||
| ARA/DHA ratio | 2.47 (0.19) | 6.68 (1.05) | <0.001 | 1.68 ± 0.89 | ||
Variables are presented as means (standard error of the mean). 1 Data are presented as the mean ± standard deviation, from 223 lactating mothers at a lactation stage of 120 ± 5 days. 2 Data of the mature milk are presented as the mean ± standard deviation. Abbreviations: SCFAs, short-chain fatty acids; MCFAs, medium-chain fatty acids; LCFAs, long-chain fatty acids; and VLCFAs, very-long-chain fatty acids.
Table 13.
Vitamins and minerals in milk of omnivore human milk donors with full-term infants (Donors) and vegetarian/vegan mothers (Veg).
| Nutrient 1 | Mature Milk Nutrient Concentration Reference |
Donors | (n = 92) | Veg | (n = 20) | p Value |
|---|---|---|---|---|---|---|
| n (o) | Mean (SE)/Median (RIQ) | n (o) | Mean (SE)/Median (RIQ) | |||
| Free thiamin, B1 (UPLC-MS/MS) mcg/L | Free thiamin 18.5 [90] Total thiamin 180 [91] |
92 (362) | 22.01 (0.92)/17.45 (0.50, 30.40) | 20 (80) | 24.75 (2.35)/22.50 (7.70, 33.95) | 0.560 |
| Free riboflavin, B2 (UPLC-MS/MS) mcg/L | Free riboflavin 11.2 [90] Total riboflavin 364 [92] |
92 (362) | 78.72 (4.93)/43.05 (22.10, 104.50) | 20 (80) | 89.49 (22.16)/15.70 (8.80, 60.20) | <0.001 |
| Nicotinamide, B3 (UPLC-MS/MS) mcg/L | Nicotinamide 275 [90] Total niacin 2100 [93] |
92 (362) | 59.43 (2.62)/44.55 (23.60, 79.30) | 20 (80) | 33.11 (2.51)/27.10 (13.80, 49.10) | <0.001 |
| Pantothenic acid, B5 (UPLC-MS/MS) mcg/L | 2500 [94] | 92 (362) | 2176.31 (25.85)/2230.70 (1763.80, 2538.30) | 20 (80) | 2305.05 (62.94)/2370.00 (1929.55, 2718.70) | 0.034 |
| Pyridoxal, B6 (UPLC-MS/MS) mcg/L | Pyridoxal 96 [90] B6 130 [95] |
92 (362) | 40.91 (1.12)/37.05 (26.40, 51.70) | 20 (80) | 48.46 (3.87)/43.35 (23.70, 59.90) | 0.233 |
| Folate, B9 (UPLC-MS/MS) mcg/L | 80 [95] | 92 (362) | 19.82 (0.40)/18.95 (14.60, 25.10) | 20 (80) | 18.79 (1.57)/15.75 (11.25, 21.30) | 0.002 |
| Cobalamin, B12 (Competitive immunoassay) | 92 (358) | 20 (79) | 0.007 | |||
| pM | 482.89 (4.11)/479.70 (435.70–527.80) | 545.69 (20.49)/510.90 (440.70–566.80) | ||||
| mcg/L | 0.5 [96] | 0.654 (0.01)/0.650 (0.591, 0.715) | 0.740 (0.03)/0.692 (0.597, 0.768) | |||
| Vitamin C * (HPLC-DAD) | 91 (358) | 20 (80) | 0.247 | |||
| mg/dL | 6.33 (0.08)/6.20 (5.45, 7.08) | 6.50 (0.16)/6.40 (5.63, 7.77) | ||||
| mg/L | 35–90 [75] | 63.30 (0.80)/62.00 (54.50, 70.80) | 65.00 (1.60)/64.00 (56.30, 77.70) | |||
| Retinol (HPLC with fluorescence and UV detector) | 91 (357) | 19 (76) | <0.001 | |||
| mcg/dL | 1130.67 (243.99)/38.80 (24.10, 68.60) | 1123.11 (400.04)/200.60 (40.60, 249.35) | ||||
| mcg/L | 530 [97] | 11306.7 (2439.9)/388.0 (241.0, 686.0) | 11231.1 (4000.4)/2000.6 (406.0, 2493.5) | |||
| Vitamin D3 (UPLC–electrospray ionization/tandem MS) | 92 (362) | 20 (80) | <0.001 | |||
| pg/mL | 3433.84 (291.29)/1141.75 (340.15, 4345.90) | 1102.01 (224.89)/336.05 (123.55, 1345.55) | ||||
| mcg/L | 0.25–2 [98] | 3.43 (0.29)/1.14 (0.34, 4.35) | 1.10 (0.22)/0.34 (0.12, 1.35) | |||
| 25(OH)D (UPLC–electrospray ionization/tandem MS) | 92 (362) | 20 (80) | 0.084 | |||
| pg/mL | 82.66 (5.57)/46.50 (23.35, 102.60) | 100.36 (11.14)/62.70 (30.70, 131.45) | ||||
| mcg/L | 0.083 (0.006)/0.047 (0.023, 0.103) | 0.104 (0.011)/0.063 (0.031, 0.131) | ||||
| α-tocopherol (HPLC with fluorescence and UV detector) | 91 (357) | 19 (76) | 0.206 | |||
| mcg/dL | 465.80 (10.85)/433.00 (309.80, 579.70) | 432.34 (23.01)/407.25 (279.35, 558.40) | ||||
| mg/L | 4.6 [99] | 4.65 (0.10)/4.33 (3.10, 5.80) | 4.32 (0.23)/4.07 (2.79, 5.58) | |||
| γ-tocopherol (HPLC with fluorescence and UV detector) | 91 (357) | 19 (76) | <0.001 | |||
| mcg/dL | 53.86 (1.36)/47.20 (35.10, 69.39) | 77.48 (4.45)/68.45 (47.10, 104.40) | ||||
| mg/L | 0.45 [90] | 0.539 (0.014)/0.472 (0.351, 0.694) | 0.775 (0.045)/0.685 (0.471, 0.104) | |||
| Vitamin E (as TE) ** mg/L | 5.2 [90] | 91 (357) | 4.77 (0.11)/4.46 (3.19, 5.94) | 19 (76) | 4.52 (0.24)/4.27 (2.99, 5.93) | 0.330 |
| Iodine (ICP-MS) ppb (mcg/L) | 50–100 [95] 100–200 [100,101] |
92 (366) | 159.22 (5.13)/135.65 (88.30, 197.50) | 20 (80) | 126.42 (13.37)/95.65 (50.25, 145.65) | <0.001 |
| Calcium (ICP-MS) ppm (mg/L) | 200–300 [102] | 92 (366) | 98.93 (2.87)/91.00 (54.80, 135.60) | 20 (80) | 83.54 (3.70)/80.00 (60.40, 102.55) | 0.093 |
| Phosphorous (ICP-MS) ppm (mg/L) | 120–140 [95,103] | 92 (366) | 131.22 (1.49)/128.85 (111.30, 145.80) | 20 (80) | 120.02 (2.25)/120.75 (107.40, 134.20) | 0.002 |
| Selenium (ICP-MS) ppb (mcg/L) | 18 [104] | 92 (366) | 11.72 (0.25)/10.80 (9.10, 13.30) | 20 (80) | 9.90 (0.37)/9.45 (7.75, 11.00) | <0.001 |
The quantitative variables are presented as the means (standard error of the mean) and as medians (interquartile range). The distribution of all variables was non-normal, except for vitamin C, which had a normal distribution. n is the total number of women in each group with data for the studied nutrient, and (o) is the total number of observations for each nutrient. The maximum for each nutrient was 368 in the Donors group and 80 in the Veg group as 4 milk samples were collected for each woman. When these values were not reached, it meant that there were losses in the milk collection or analysis. 1 For the purpose of comparison, the units of our results were converted to those used in the references for the nutrients in the human milk. * Vitamin C = ascorbic acid + dehydroascorbic acid. ** Vitamin E is calculated as the sum of each vitamer expressed as tocopherol equivalents (TE) (mg) = α-tocopherol (mg) + 0.25 × γ-tocopherol (mg) [105]. Abbreviations: SE, standard error; IQR, interquartile range; UPLC, ultra-performance liquid chromatography; MS/MS, tandem mass spectrometry; HPLC, high-performance liquid chromatography; DAD, diode array detector; UV, ultraviolet; MS, mass spectrometry; TE, tocopherol equivalents; ICP, inductively coupled plasma; ppb, parts per billion; and ppm, parts per million.
There were no differences in the distribution of macronutrients and lipid classes in the milk of the two groups, except for a slight increase in the proportion of polar lipids in the milk of the Veg group (Table 11). The TAGs profile followed a similar distribution in the milk of both groups, with a clear predominance of long-chain molecular species in the Veg group. The content of the molecular species CN54 was significantly higher in the case of milk from the Veg mothers, which correlates with the higher proportion of oleic acid and LA acids that were present in that sample. Regarding the relative proportion of the different PLs in the milk, the majority compound was sphingomyelin (SM) in both groups, which accounted for more than 40% of the total polar lipids. However, in the Veg group, there was a higher proportion of phosphatidylethanolamine (PE) and a lower proportion of phosphatidylcholine (PC) than was found in the Donor group. In the FAs distribution (Table 12), the MUFAs were predominant in both groups. In comparison, the Veg group’s milk had lower relative levels of SFAs and higher relative levels of MUFAs and PUFAs than those in the milk of the Donors group. The predominant SFA was found to be palmitic acid in both groups, followed by myristic, lauric, and stearic acids. The predominant MUFA was oleic acid, the most abundant FA in the milk, and the predominant PUFA was LA in both groups. The Veg group had a higher proportion of LA and ALA and lower proportions of AA, DHA, and docosapentaenoic acid (DPA) in their milk. EPA was undetectable in all the samples. The DHA content in the Donors group was double that of the Veg group. Notably, the proportion of n-6 PUFAs in milk was higher in the Veg group, as was the ratio of n-6 PUFAs/n-3 PUFAs. Long-chain FAs containing more than 15 carbons were the predominant FAs in both diet groups, and there was no difference in the distribution of FAs according to chain length.
In the study of the micronutrient content of human milk (Table 13), the great variability found within was striking. Furthermore, this variability was much more evident in the case of riboflavin and retinol. For this reason, we have provided the data for both, the mean (standard error) and the median (interquartile range) for a proper interpretation of the data and to allow comparisons with other published studies. It should be noted that the cobalamin levels in milk were adequate in both groups, albeit higher in the Veg group. Adequate levels of vitamin C, α-tocopherol, pantothenic acid, iodine, and phosphorous were also found in the milk of both groups, but with higher levels of pantothenic acid and lower levels of phosphorous and iodine in the Veg group, and no differences between groups in the other two micronutrients. The vitamin D3 content in the milk was lower in the Veg group, but it was within the published range. The milk of the Veg group had a higher content of gamma-tocopherol than the Donors’ milk. On the other hand, the folate, nicotinamide, selenium, and calcium contents in the milk were low in both groups; all of them, except calcium, were lower in the Veg group. Nicotinamide showed the greatest difference between the two groups, as it was almost half in the milk of the Veg group compared to that of the Donors.
4. Discussion
The present study compares the maternal intake, nutritional status, and nutritional composition of human milk from 92 omnivore milk donors with full-term infants with that of 20 vegetarian/vegan lactating mothers in inland Spain.
We determined a large number of nutrients in the human milk, comprising macronutrients, lipid classes, sixteen molecular species of TAGs, three PLs, thirty FAs, eleven vitamins, and four minerals. There are no previous studies on vegetarians/vegans in which such a quantity of nutrients has been determined in their milk or in which they have been compared with milk donors. The previously measured compounds in vegetarians’ and in vegans’ milk have been limited to macronutrients, free amino acids, FAs, minerals, trace elements, taurine, glutathione peroxidase activity, nucleotides, nucleosides, brain-derived neurotrophic factor, folic acid, and vitamin B12. It is notable that the rest of the vitamins have not yet been studied. In addition, the literature lacks a more comprehensive nutritional and dietary study, as physical measurements were the only indicators in these studies on nutritional status [12].
In our study, the most important differences in the milk of the two dietary groups were found in the distribution of PLs and the FAs’ profile, which is in accordance with a notable difference in the type and amount of fat in the vegetarian diet that has been previously described [106] and which our study corroborates. On one hand, the Veg group consumed lower total fat, one-third less saturated fat, and markedly lower amounts of cholesterol and trans FAs. According to this, the milk of the Veg group was richer in unsaturated FAs and lower in SFAs and trans FAs, which is explained by the fact that plant-based diets are rich in unsaturated FAs. Veg showed a more favorable lipid profile, and their HDL plasma levels were higher. However, this group exhibited a plasmalogens content, both in plasma and erythrocytes, significantly lower than that of Donors. Plasmalogens have been proposed to act as reservoirs of PUFAs and, based on their ability to scavenge reactive oxygen species as well as to chelate potentially harmful metal ions, they are considered natural antioxidants [107]. So, these decreased levels in the Veg group might be a marker of a certain oxidative stress. Furthermore, with respect to essential FAs, the Veg group showed significant drawbacks, due to deficient intakes of DHA and EPA, as well as a low n-3 FAs intake relative to n-6 FAs intake. Relevant to this, the DHA intake by the Veg group (110 mg/day) was less than one-half of the DHA intake by the Donors (380 mg/day), which resulted in a DHA concentration in the milk of the Veg group that was less than half that of the Donors (0.15% vs. 0.33%, respectively). This is in line with the findings of another study comparing milk DHA content from non-donor vegetarians, vegans, and omnivores [108]. It is recommended that breastfeeding women consume at least 200 mg of DHA per day [109,110,111], which should translate into a milk DHA content of 0.30% [109]. The Veg group did not reach this recommendation. DHA supply to the fetus and neonate is associated with beneficial effects on visual function and cognitive development [109,110]. The impact of a low milk DHA content is greater for premature infants, as their DHA requirements are higher than those of term infants due to the lack of placental DHA transfer in the last trimester and the very low stores in adipose tissue [112]. In addition, they must ingest preformed DHA, as their ability to obtain DHA from ALA is thought to be very limited due to the decreased activity of their enzyme systems [113]. There are greater apparent benefits provided by a higher DHA concentration in milk for preterm infants (1 vs. 0.35% of the total FAs intake) [109,114], and DHA requirements for very-low-birth-weight infants have been established in the range from 12 to 60 mg/kg/day [109,115]. Considering that the pasteurization of DHM does not affect DHA levels [116,117], the DHA content in the milk of the Donors group was sufficient (albeit at the lower end of the adequate range) and that of the Veg group was clearly insufficient to meet the DHA needs of very-low-birth-weight infants. Calculations have been formulated, taking into account the lipid concentration found in the studied milk (3.13 g/dL), as well as the 0.33% vs. 0.15% DHA contents and enteral intake of 175 mL/kg/day.
However, we do not consider this to be a reason for the exclusion of vegetarian/vegan women as milk donors. Firstly, the intakes and levels of DHA in human milk vary widely across countries. Indeed, values equal or even lower than those of our Veg group have been reported both in developed and developing countries [118,119,120,121,122]. In fact, several studies on DHM in North America found DHA values that were similar to or even lower than those of our Veg group [116,117,123]. Secondly, since DHA supplementation to lactating women has consistently been shown to increase DHA concentrations in breast milk [124,125,126,127,128,129,130], it seems an appropriate strategy to recommend DHA supplementation from algal oil to Veg women who want to become milk donors. Most studies show that vegetarians, and especially vegans, consume low to zero amounts of EPA and DHA unless they take supplements [131]. This is because good sources of EPA and DHA are typically of animal origin, mainly cold-water fish and seafood. DHA-enriched eggs and milk are an option for ovo-lacto-vegetarians, but not for vegans. Some cold-water marine algae (other than spirulina) contain long-chain n-3 fatty acids, but their high iodine content limits their regular consumption. In addition, intakes of supplements up to 1 g/day DHA have been used in clinical trials with no significant adverse effects [110]. As only 25% of women in the Veg group reported taking DHA supplements, there is an important opportunity for improvement in this area.
DHA supplementation would even be a strategy to consider in the Donors group, especially in women with a low fish intake. A study conducted in the Mother’s Milk Bank of Ohio, in which the studied omnivores donors reported low basal dietary intakes of DHA (23 mg/day), concluded that supplementation with an algal DHA product at 1 g/day improved the dietary DHA and maternal milk concentration to mimic intrauterine accretion for the preterm infant [123]. Perrin found that the use of a DHA/EPA supplement by breastfeeding women was a significant positive predictor of milk ALA, DHA, and total n-3 composition, as well as a significant negative predictor of the n-6/n-3 ratio in milk [121].
The n-6/n-3 ratio of the diet, which is used to assess the balance between essential FAs and is recommended to be less than 10:1, is another issue of great interest. In the vegetarian and vegan populations, it should be set at 2:1–4:1, because they depend on the endogenous production of DHA and EPA from ALA, and higher n-6/n-3 ratios decrease it [106,131]. In the present study, the n-6/n-3 ratio was higher in the diet of the Veg group (9.9 vs. 7.8), and also in their erythrocytes, plasma, and milk. This is due to the high LA contents of plant-based diets, making it very difficult for vegans/vegetarians to obtain ALA without also increasing the amount of LA in the diet, unless specific foods that are high in ALA are consumed, such as flaxseed/linseed oil, hemp seeds, chia, or oleate-rich, low-LA sunflower oils [131]. Increasing the consumption of these foods could be an additional recommendation for vegetarians/vegans who want to be milk donors. Nevertheless, the human conversion rate of ALA to EPA and DHA is low, so in periods of high demand, such as in lactation, experts particularly recommend considering DHA supplementation in vegans [131].
The FAs profile in breast milk may affect the status of the infant. It was observed that in the total erythrocyte lipids of vegan-breastfed infants, the LA was higher, and the DHA was lower when compared to those of omnivore-breastfed infants [108]. However, more studies are needed because of the small sample size of the study that reported these results. Providing DHA and AA supplements to breastfed and very preterm infants in the early neonatal period was associated in another study with improved recognition memory and higher problem-solving scores at 6 months [132].
Regarding the PLs content of human milk, even though they constitute only about 1% of the total lipid content of milk, dietary PLs play an important role in the digestion, absorption, and transport of TAGs [133], as well as for infants’ intestinal maturation and the establishment of the intestinal immune system [134]. SM, PE, and PC are the three major PLs that have been found in human milk in most studies [135]. In agreement with certain studies [136,137], we found SM to be the most abundant PL in human milk, accounting for more than 40% of the total polar lipids. SM is located on the outside of the lipid bilayer of cell membranes and is particularly abundant in the myelin sheaths surrounding the axons of neurons. However, there are large differences in the content and distribution of PLs between studies that have been conducted around the world [136,137,138,139,140]. Further, PE was determined to be the most prevalent PL in human milk in some of them [138,139]. We found that the Veg group showed a lower percentage of PC (26.88% vs. 30.55%) and a higher percentage of PE (30.72% vs. 24.62%) in their milk. SM and PC are good sources of choline, whose importance lies in its involvement in the structural integrity of cell membranes, as a precursor of acetylcholine and as a methyl donor for methionine synthesis [138]. The clinical impact of these findings for preterm infants is unknown. To our knowledge, this is the first study to determine the relative composition of PLs in the milk of vegetarian/vegan women. Our study suggests the potential influence of dietary pattern on the distribution of PLs in human milk in two otherwise comparable population groups, but more studies are needed to support our findings.
In terms of micronutrients, the greatest concern among vegetarians is the vitamin B12 content of their milk because vitamin B12 is scarce in plant foods, and its deficiency in the lactating mother can lead to irreversible cognitive impairment and other neurological disorders as well as hematological complications in the breastfed infant [141]. This is a main reason for the exclusion of vegan women from milk donation in certain countries. In this respect, the vitamin B12 content in the milk of our Veg group is a perfect example that the vegetarian/vegan diet can be suitable for lactating women if sufficient supplementation is provided. Our results support the approach of most worldwide milk banks, which recruit vegetarian/vegan mothers as donors on the condition that they regularly supplement vitamin B12, as well as other micronutrients if necessary [12]. In the Veg group, with a vitamin B12 supplementation rate of 85% and an average daily dose of 312 mcg/day, their plasmatic levels were similar to those of the Donors. In addition, the vitamin B12 content of milk was found to be higher than that of the Donors, and the content of cobalamin in the milk of both groups was found to be adequate. A total of 14/20 (70%) of the Veg consumed supplements with high doses of B12 that were equal to or higher than the 2000 mcg weekly amount recommended by the American Dietetic Association and other authors [142,143].
Of the remaining vitamins and minerals, the differences found in the content of certain vitamins in the milk between the Veg and Donors were probably of little clinical relevance and would not justify the exclusion of the vegetarian/vegan group studied from milk donation. Nicotinamide showed the greatest difference between the two groups, as it was almost half in the Veg group compared to the Donors. Interestingly, we found that even though the Veg group consumed more vitamin E, they did not have higher plasma or milk concentrations of α-tocopherol. However, they did have higher plasma and milk levels of γ-tocopherol, which is a vitamer of growing interest for its detoxifying, anti-inflammatory, natriuretic, and protective properties against cardiovascular disease and prostate cancer [81,144]. In general, the results of the milks’ vitamin analysis appeared to be in line with the results of the dietary record analysis, except in the case of folate and vitamin D. It is known that the folate content in milk does not depend on the mother’s intake [145], and the vitamin D status depends not only on her intake, but also on sun exposure. Except for an old study in which no significant differences in the milk’s folate content were found between lacto-vegetarians and omnivores [146], there have been no studies evaluating other vitamins (except cobalamin) in the milk of vegetarians/vegans. Additionally, there are few studies assessing the concentration of vitamins in raw DHM [5,147]. Studies assessing the mineral content in both vegetarians/vegans and milk donors are also scarce [5,12,15]. We have, therefore, compared our values in the milk with those used by the European Food Safety Authority (EFSA) [91,92,93,94,95,96,97,98,99,102,103,104] in order to establish the micronutrient adequate intakes for infants under 6 months of age. In cases in which we were not able to measure the total content of a vitamin in milk, because we determined only some of its vitamers (i.e., free thiamine, free riboflavin, nicotinamide, and pyridoxal), we compared our vitamers’ values with those of Gibson’s study [90]. The low content of calcium, folate, nicotinamide, and selenium in both the Veg and Donors groups was remarkable. In this regard, plasmatic nicotinamide was low in both groups. In addition, the folate stores were deficient in a high percentage of lactating mothers in both the Veg and Donors groups. The high percentage of women in both groups who showed biochemical values suggestive of low stores of some vitamins (B1, B2, B3, B9, and vitamins D and E) was alarming, especially regarding vitamin E; although it must be said that the values of α-tocopherol in milk were adequate. These values are difficult to interpret, mainly because there are nearly no studies assessing the biochemical nutritional status of lactating women in developed countries. As the Institute of Medicine (IOM) stated in 1991 in its document “Nutrition during lactation”, “no standards for anthropometric or biochemical indicators have been established for nutritional status among lactating women” and “it is probably inappropriate to assess the nutritional status of lactating women by comparing plasma nutrient values with reference values for a nonpregnant women” [148]. Moreover, differences in analytical techniques between studies make it even more difficult to interpret results. The incorporation of modern techniques in our study based on liquid chromatography and tandem mass spectrometry allowed us to characterize more specifically the different group B vitamers, but for some of them, there are still no established and universally accepted reference values. Another unexplored factor is how the duration of breastfeeding affects the mother’s nutrient stores. The average breastfeeding time of the women in our study was 7–8 months, whereby more than half of them were breastfeeding for more than 6 months, and some of them for up to 50 months, with 5% of them conducting tandem breastfeeding. It should be remembered that the milk donors not only breastfeed their own children, but also express milk for donation. All of this could affect their nutrient stores. We found a study conducted by Papathakis et al. in 2007 [80], which showed that 70% of lactating South African women at 24 weeks postpartum met the criteria for vitamin E deficiency (< 11.6 mcM) and the mean plasmatic α-tocopherol decreased significantly over time after their delivery. In Gibson’s study [90], in Indonesian disadvantaged mothers, most micronutrient concentrations in milk declined during lactation, independent of changes in human milk production. Furthermore, there were significant associations between maternal biomarkers and milk micronutrient concentrations at 5 months, thereby raising the need for further studies to investigate a possible link between the decline in milk micronutrients and the shifts in maternal status. Clearly, more studies are needed to assess the biochemical parameters of the nutritional status of lactating women to help establish the ranges of normality and deficiency in this population. In addition, studies that assess the behavior of maternal nutrient stores in the face of prolonged breastfeeding are required.
Regarding the mineral content of milk, we found statistically significant differences in three of the four minerals studied between the two investigated groups. Iodine, phosphorus, and selenium were lower in the milk of the Veg group. These results contrast with those of Perrin [15] and Debski [149], who found higher selenium content in the milk of women with a plant-based diet when compared to omnivore women. In our case, women in the Veg group consumed less selenium and phosphorus. The milk phosphorus content was within the reference range in both groups. However, selenium levels were low in both groups—15% lower in the Veg group compared to that of the Donors. Although there was no difference in iodine intake, the median urine iodine concentration showed that the Veg group is an iodine-deficient population, while the Donors group are iodine-sufficient. The milk iodine concentration was within the normal range in both groups, but was 20% lower in the Veg group, which may be of relevance in meeting the high requirements of preterm infants [150]. More clinically important was the low calcium content in the milk of both groups, which may mean that the Spanish women’s calcium requirements are higher than those dictated by the EFSA and IOM, according to the recommended calcium intake of 1500 mg/day for Spanish lactating women [24], which neither group reached.
Another point worth mentioning is that a lower gestational weight gain in the Veg group and lower weight percentiles of their offspring both at birth and at the time of the study were found. In this regard, vegetarian diets were associated with lower gestational weight gain in a systematic review of observational studies [151]. Furthermore, in a review on the effects of vegetarian and vegan diets during pregnancy on the health of mothers and their offspring, several studies reported an association between a vegetarian diet and lower fetal growth, lower birth weight, or a higher risk of being small for their gestational age [152].
Our study presents certain limitations. It is known that the dietary patterns and levels of the biochemical indicators regarding nutritional status can be quite different between vegetarians and vegans [121,131], but we were not able to differentiate between the vegetarian and vegan mothers because of the low sample sizes. However, this study is still one of the most complete investigations available as it not only studies the nutritional content of the milk, but also is supported by a detailed dietary study that includes a five-day dietary record, as well as biochemical determinations of the plasma of lactating women. Another strength is the multiple milk samples that were collected from each woman over four consecutive days.
Finally, we would like to emphasize that the studied milk was mature milk, which was subjected to the usual procedures in the donation process, such as extraction, freezing, and thawing. Similarly, storage was achieved by utilizing transparent glass bottles to mimic the usual donation procedure, whereby the photosensitive vitamins were not protected from light. All these processes can affect the composition of the milk to a greater or lesser extent [153,154,155]. Therefore, the results are not superimposable to those of mothers’ own milk, but they do reflect the nutritional content of raw DHM. The effect of pasteurization on milk micronutrients has also been studied previously [156,157].
5. Conclusions
In conclusion, perhaps the most important contribution of this study is the detailed and comprehensive description of micronutrients and lipids in human milk from omnivore milk donors and vegetarian/vegan women and their comparison with the reference values. The milk of vegetarians and vegans has been shown to be different from that of omnivore donors, probably according to the differences in their dietary pattern, thereby showing advantages and disadvantages that we should be aware of. Of particular concern is the lower DHA content in the milk of our vegetarians/vegans’ group. However, raising awareness and administering proper supplementation could bridge this gap, as has been the case with vitamin B12.
In view of the results of this study, in the donor selection processes going forward, it might be more appropriate to conduct an individualized dietary assessment than to simply accept or reject a woman as a potential donor depending on whether she is omnivorous or vegetarian/vegan. An additional consideration is that, due to the high variability found in the micronutrient content and lipid profile in human milk, it is recommended for milk bank pooling practices to decrease the compositional variability in DHM [116,158].
Acknowledgments
We wish to thank our donors as well as the vegetarian/vegan women who took the time to participate in this study and to kindly donate their human milk. Thank you for your generosity, which made this research possible. We also want to acknowledge the contribution of RETICS “Maternal and Child Health and Development Network” (SAMID Network).
Author Contributions
Conceptualization, N.U.-V., D.E.-V., N.R.G.-L. and C.R.P.-A.; methodology, N.U.-V., D.E.-V., J.F., M.V.C., J.M.-T. and J.C.E.S.; formal analysis, N.U.-V., C.R.F. and K.K.; investigation, N.U.-V., K.K, D.E.-V., J.F., M.V.C., J.M.-T. and J.C.E.S.; writing—original draft preparation, N.U.-V. and K.K.; writing—review and editing, N.U.-V., K.K., D.E.-V., J.F., M.V.C., J.M.-T., J.C.E.S., N.R.G.-L., C.R.F. and C.R.P.-A.; project administration, N.U.-V., N.R.G.-L. and C.R.P.-A.; funding acquisition, N.U.-V., D.E.-V., N.R.G.-L. and C.R.P.-A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Hospital 12 de Octubre’s Clinical Research Ethics Committee (protocol code 15/269, 29 September 2015).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy issues.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by grant from the Spanish Research Projects in Health funded by Instituto de Salud Carlos III (ISCIII)—the State Plan for Scientific and Technical Research and Innovation (grant FIS PI15/00995). Furthermore, this work received support from RETICS “Maternal and Child Health and Development Network” (SAMID Network) (RD16/0022/0015) and from the Spanish Ministry of Science, Innovation, and Universities (Project PDI2020-114821RB-I00).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.World Health Organization . Guideline: Protecting, Promoting and Supporting Breastfeeding in Facilities Providing Maternity and Newborn Services. World Helath Organization (WHO); Genova, Switzerland: 2017. [(accessed on 5 April 2023)]. Available online: https://www.who.int/publications/i/item/9789241550086. [PubMed] [Google Scholar]
- 2.World Health Organization . Guidelines on Optimal Feeding of Low Birth-Weight Infants in Low-and Middle-Income Countries. World Health Organization (WHO); Geneva, Switzerland: 2011. [(accessed on 5 April 2023)]. Available online: https://apps.who.int/iris/handle/10665/85670. [PubMed] [Google Scholar]
- 3.AAP Comittee on Nutrition. AAP Section on breastfeeding. AAP Comittee on Fetus and Newbornborn Donor human milk for the high- risk infant: Preparation, safety, and usage options in the United States. Pediatrics. 2017;139:e20163440. doi: 10.1542/peds.2016-3440. [DOI] [PubMed] [Google Scholar]
- 4.Moro G.E., Billeaud C., Rachel B., Calvo J., Cavallarin L., Christen L., Escuder-Vieco D., Gaya A., Lembo D., Wesolowska A., et al. Processing of Donor Human Milk: Update and Recommendations from the European Milk Bank Association (EMBA) Front. Pediatr. 2019;7:49. doi: 10.3389/fped.2019.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perrin M.T., Belfort M.B., Hagadorn J.I., McGrath J.M., Taylor S.N., Tosi L.M., Brownell E.A. The nutritional composition and energy content of donor human milk: A systematic review. Adv. Nutr. 2020;11:960–970. doi: 10.1093/advances/nmaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christian P., Smith E.R., Lee S.E., Vargas A.J., Bremer A.A., Raiten D.J. The need to study human milk as a biological system. Am. J. Clin. Nutr. 2021;113:1063–1072. doi: 10.1093/ajcn/nqab075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dror D.K., Allen L.H. Overview of nutrients in human milk. Adv. Nutr. 2018;9:278S–294S. doi: 10.1093/advances/nmy022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hampel D., Shahab-Ferdows S., Islam M.M., Peerson J.M., Allen L.H. Vitamin Concentrations in Human Milk Vary with Time within Feed, Circadian Rhythm, and Single-Dose Supplementation. J. Nutr. 2017;147:603–611. doi: 10.3945/jn.116.242941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bravi F., Wiens F., Decarli A., Dal Pont A., Agostoni C., Ferraroni M. Impact of maternal nutrition on breast-milk composition: A systematic review. Am. J. Clin. Nutr. 2016;104:646–662. doi: 10.3945/ajcn.115.120881. [DOI] [PubMed] [Google Scholar]
- 10.Keikha M., Bahreynian M., Saleki M., Kelishadi R. Macro- and Micronutrients of Human Milk Composition: Are They Related to Maternal Diet? A Comprehensive Systematic Review. Breastfeed. Med. 2017;12:517–527. doi: 10.1089/bfm.2017.0048. [DOI] [PubMed] [Google Scholar]
- 11.Elorinne A.L., Alfthan G., Erlund I., Kivimäki H., Paju A., Salminen I., Turpeinen U., Voutilainen S., Laakso J. Food and nutrient intake and nutritional status of Finnish vegans and non-vegetarians. PLoS ONE. 2016;11:e0148235. doi: 10.1371/journal.pone.0148235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karcz K., Królak-Olejnik B. Vegan or vegetarian diet and breast milk composition–a systematic review. Crit. Rev. Food Sci. Nutr. 2021;61:1081–1098. doi: 10.1080/10408398.2020.1753650. [DOI] [PubMed] [Google Scholar]
- 13.Weaver G., Bertino E., Gebauer C., Grovslien A., Mileusnic-Milenovic R., Arslanoglu S., Barnett D., Boquien C.Y., Buffin R., Gaya A., et al. Recommendations for the establishment and operation of Human Milk Banks in Europe: A consensus statement from the European Milk Bank Association (EMBA) Front. Pediatr. 2019;7:53. doi: 10.3389/fped.2019.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craig W., Mangels A., American Dietetic Association Position of the American Dietetic Association: Vegetarian diets. J. Am. Diet. Assoc. 2009;109:1266–1286. doi: 10.1016/j.jada.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 15.Perrin M.T., Pawlak R., Judd N., Cooper J., Donati G.L. Major and Trace Mineral Composition of Milk from Lactating Women Following Vegan, Vegetarian, and Omnivore Diets. Br. J. Nutr. 2022;23:1–8. doi: 10.1017/S0007114522004007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vazquez M., Witriw A. Modelos Visuales de Alimentos y Tablas de Relacion. 1st ed. Edición del Autor; Buenos Aires, Argentina: 1997. [Google Scholar]
- 17.Dapcich V., Salvador Castell G., Ribas Barba L., Pérez Rodrigo C., Aranceta-Bartrina J., Serra Majem L. Embarazo y lactancia. Necesidades especiales. In: Sociedad Española de Nutrición Comunitaria, editor. Guía de la Alimentación Saludable. Spanish Society of Community Nutrition; Madrid, Spain: 2004. [(accessed on 2 February 2023)]. p. 82. Available online: https://www.nutricioncomunitaria.org/es/otras-publicaciones. [Google Scholar]
- 18.Ministerio de Sanidad, Gobierno de España Estilos de Vida Saludable. [(accessed on 3 February 2023)]; Available online: https://estilosdevidasaludable.sanidad.gob.es/alimentacionSaludable/queSabemos/enLaPractica/tablaPlanificacion/planificaciones/home.htm.
- 19.López Martínez R., Alonso Nieva N., Serrat Orús N., Gella Tomás F., Boned Juliani B., Canalias Reverter F., Esteve Poblador S., González de la Presa B., Izquierdo Álvarez S., Macías Blanco C., et al. Procedimiento para el Estudio de la Interferencia por Hemólisis, Bilirrubina y Turbidez y Para la Verificación de los Índices de Hemólisis, Ictericia y Lipemia. Documento Técnico (2013). Documentos de la SEQC 2014(7)-junio 2014. [(accessed on 6 April 2023)]. Available online: https://publicaciones.seqc.es/recopilacion-de-documentos/33-documentos-de-la-seqc-2014-7-junio-2014.html.
- 20.Klem S., Klingler M., Demmelmair H., Koletzko B. Efficient and specific analysis of red blood cell glycerophospholipid fatty acid composition. PLoS ONE. 2012;7:e33874. doi: 10.1371/journal.pone.0033874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ortega R., López-Sobaler A., Andrés P., Requejo A., Aparicio A., Molinero L. DIAL Software for Assessing Diets and Food Calculations. Department of Nutrition (UCM) & Alceingeniería, SA; Madrid, Spain: 2014. [(accessed on 6 April 2023)]. Available online: http://www.alceingenieria.net/nutricion.htm. [Google Scholar]
- 22.Red BEDCA del Ministerio de Ciencia e Innovación Base de Datos Española de Composición de Alimentos. [(accessed on 3 February 2023)]. Available online: http://www.bedca.net/bdpub/index.php.
- 23.U.S. Department of Agriculture ARS FoodData Central. [(accessed on 1 February 2023)];2019 Available online: https://fdc.nal.usda.gov/
- 24.Ortega Anta R., Requejo Marcos A. Nutriguía. Manual de Nutrición Clínica. Editorial Médica Panamericana; Madrid, Spain: 2015. [Google Scholar]
- 25.Institute of Medicine . Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. National Academies Press; Washington, DC, USA: 1997. [PubMed] [Google Scholar]
- 26.Institute of Medicine . Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. The National Academies Press; Washington, DC, USA: 1998. [PubMed] [Google Scholar]
- 27.Institute of Medicine . Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. National Academies Press; Washington, DC, USA: 2000. [PubMed] [Google Scholar]
- 28.Institute of Medicine . Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. The National Academies Press; Washington, DC, USA: 2001. [PubMed] [Google Scholar]
- 29.Institute of Medicine . Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. The National Academies Press; Washington, DC, USA: 2005. [Google Scholar]
- 30.Institute of Medicine . In: Dietary Reference Intakes for Calcium and Vitamin D. Ross C.A., Taylor C.L., Yaktine A.L., Del Valle H.B., Institute of Medicine, editors. National Academies Press; Washington, DC, USA: 2011. [PubMed] [Google Scholar]
- 31.EFSA (European Food Safety Authority) Dietary Reference Values for nutrients. Summary report. EFSA Support. Publ. 2017;98:e15121. doi: 10.2903/sp.efsa.2017.e15121. [DOI] [Google Scholar]
- 32.Food and Agriculture Organization of the United Nations (FAO) Fats and Fatty Acids in Human Nutrition. Report of an Expert Consultation. FAO; Geneva, Switzerland: 2008. FAO Food and Nutrition Paper 91. [Google Scholar]
- 33.Basiotis P.P., Carlson A., Gerrior S.A., Juan W.Y., Lino M. In: The Healthy Eating Index: 1999–2000. Dinkins J.M., editor. U.S. Department of Agriculture, Center for Nutrition Policy and Promotion; Washington, DC, USA: 2002. [Google Scholar]
- 34.Kennedy E.T., Ohls J., Carlson S., Fleming K. The Healthy Eating Index: Design and applications. J. Am. Diet. Assoc. 1995;95:1103–1108. doi: 10.1016/S0002-8223(95)00300-2. [DOI] [PubMed] [Google Scholar]
- 35.Löfgren L., Ståhlman M., Forsberg G.-B., Saarinen S., Nilsson R., Hansson G.I. The BUME method: A novel automated chloroform-free 96-well total lipid extraction method for blood plasma. J. Lipid Res. 2012;53:1690–1700. doi: 10.1194/jlr.D023036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.García-Serrano A., Tomé-Carneiro J., Carmen Crespo M., Visitación Calvo M., Pereda-Pérez I., Baliyan S., Burgos-Ramos E., Montero O., Dávalos A., Venero C., et al. Concentrates of buttermilk and krill oil improve cognition in aged rats. Prostaglandins Leukot. Essent. Fatty Acids. 2020;155:102077. doi: 10.1016/j.plefa.2020.102077. [DOI] [PubMed] [Google Scholar]
- 37.Castro-Gómez P., Montero O., Fontecha J. In-Depth Lipidomic Analysis of Molecular Species of Triacylglycerides, Diacylglycerides, Glycerophospholipids, and Sphingolipids of Buttermilk by GC-MS/FID, HPLC-ELSD, and UPLC-QToF-MS. Int. J. Mol. Sci. 2017;18:605. doi: 10.3390/ijms18030605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castro-Gómez P., Fontecha J., Rodríguez-Alcalá L.M. A high-performance direct transmethylation method for total fatty acids assessment in biological and foodstuff samples. Talanta. 2014;128:518–523. doi: 10.1016/j.talanta.2014.05.051. [DOI] [PubMed] [Google Scholar]
- 39.Calvo M.V., Martín-Hernández M.C., García-Serrano A., Castro-Gómez M.P., Alonso-Miravalles L., García-Martín R., Megino-Tello J., Alonso L., Fontecha J. Comprehensive characterization of neutral and polar lipids of buttermilk from different sources and its milk fat globule membrane isolates. J. Food Compos. Anal. 2020;86:103386. doi: 10.1016/j.jfca.2019.103386. [DOI] [Google Scholar]
- 40.Fontecha J., Mayo I., Toledano G., Juárez M. Triacylglycerol composition of protected designation of origin cheeses during ripening. Authenticity of milk fat. J. Dairy Sci. 2006;89:882–887. doi: 10.3168/jds.S0022-0302(06)72152-X. [DOI] [PubMed] [Google Scholar]
- 41.Huynh D., Zhou S.J., Gibson R., Palmer L., Muhlhausler B. Validation of an optimized method for the determination of iodine in human breast milk by inductively coupled plasma mass spectrometry (ICPMS) after tetramethylammonium hydroxide extraction. J. Trace. Elem. Med. Biol. 2015;29:75–82. doi: 10.1016/j.jtemb.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Boutin M., Presse N., Martineau T., Perreault A., Gaudreau P., Auray-Blais C. Mass spectrometry analysis of urinary methylmalonic acid to screen for metabolic vitamin B12 deficiency in older adults. Bioanalysis. 2020;12:693–705. doi: 10.4155/bio-2020-0106. [DOI] [PubMed] [Google Scholar]
- 43.Romeu-Nadal M., Morera-Pons S., Castellote A.I., López-Sabater M.C. Rapid high-performance liquid chromatographic method for Vitamin C determination in human milk versus an enzymatic method. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2006;830:41–46. doi: 10.1016/j.jchromb.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 44.Robitaille L., Hoffer L.J. A simple method for plasma total vitamin C analysis suitable for routine clinical laboratory use. Nutr. J. 2016;15:40. doi: 10.1186/s12937-016-0158-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hampel D., York E.R., Allen L.H. Ultra-performance liquid chromatography tandem mass-spectrometry (UPLC-MS/MS) for the rapid, simultaneous analysis of thiamin, riboflavin, flavin adenine dinucleotide, nicotinamide and pyridoxal in human milk. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012;903:7–13. doi: 10.1016/j.jchromb.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 46.Jiang J., Xiao H., Wu K., Yu Z., Ren Y., Zhao Y., Li K., Li J., Li D. Retinol and α-tocopherol in human milk and their relationship with dietary intake during lactation. Food Funct. 2016;7:1985–1991. doi: 10.1039/C5FO01293G. [DOI] [PubMed] [Google Scholar]
- 47.Aronov P.A., Hall L.M., Dettmer K., Stephensen C.B., Hammock B.D. Metabolic profiling of major vitamin D metabolites using Diels-Alder derivatization and ultra-performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2008;391:1917–1930. doi: 10.1007/s00216-008-2095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olsen I.E., Groveman S.A., Lawson M.L., Clark R.H., Zemel B.S. New intrauterine growth curves based on United States data. Pediatrics. 2010;125:214–224. doi: 10.1542/peds.2009-0913. [DOI] [PubMed] [Google Scholar]
- 49.World Health Organization . WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development. World Health Organization (WHO); Geneva, Switzerland: 2006. [(accessed on 5 April 2023)]. Available online: https://www.who.int/publications/i/item/924154693X. [Google Scholar]
- 50.Allen L.H., Carriquiry A.L., Murphy S.P. Perspective: Proposed Harmonized Nutrient Reference Values for Populations. Adv. Nutr. 2020;11:469–483. doi: 10.1093/advances/nmz096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Graham J., Peerson J., Haskell M., Shrestha R., Brown K., Allen L. Erythrocyte riboflavin for the detection of riboflavin deficiency in pregnant Nepali women. Clin. Chem. 2005;51:2162–2165. doi: 10.1373/clinchem.2005.055079. [DOI] [PubMed] [Google Scholar]
- 52.Berger M.M., Shenkin A., Schweinlin A., Amrein K., Augsburger M., Biesalski H.K., Bischoff S.C., Casaer M.P., Gundogan K., Lepp H.L., et al. ESPEN micronutrient guideline. Clin. Nutr. 2022;41:1357–1424. doi: 10.1016/j.clnu.2022.02.015. [DOI] [PubMed] [Google Scholar]
- 53.Hustad S., McKinley M.C., McNulty H., Schneede J., Strain J.J., Scott J.M., Ueland P.M. Riboflavin, flavin mononucleotide, and flavin adenine dinucleotide in human plasma and erythrocytes at baseline and after low-dose riboflavin supplementation. Clin. Chem. 2002;48:1571–1577. doi: 10.1093/clinchem/48.9.1571. [DOI] [PubMed] [Google Scholar]
- 54.Andraos S., Jones B., Wall C., Thorstensen E., Kussmann M., Smith D.C., Lange K., Clifford S., Saffery R., Burgner D., et al. Plasma B vitamers: Population epidemiology and parent-child concordance in children and adults. Nutrients. 2021;13:821. doi: 10.3390/nu13030821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ehsanian R., Anderson S., Schneider B., Kennedy D., Mansourian V. Prevalence of low plasma vitamin B1 in the stroke population admitted to acute inpatient rehabilitation. Nutrients. 2020;12:1034. doi: 10.3390/nu12041034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Håglin L., Domellöf M., Bäckman L., Forsgren L. Low plasma thiamine and phosphate in male patients with Parkinson’s disease is associated with mild cognitive impairment. Clin. Nutr. ESPEN. 2020;37:93–99. doi: 10.1016/j.clnesp.2020.03.012. [DOI] [PubMed] [Google Scholar]
- 57.Khaksari M., Mazzoleni L.R., Ruan C., Kennedy R.T., Minerick A.R. Determination of water-soluble and fat-soluble vitamins in tears and blood serum of infants and parents by liquid chromatography/mass spectrometry. Exp. Eye Res. 2017;155:54–63. doi: 10.1016/j.exer.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 58.McCann A., Midttun Ø., Whitfield K.C., Kroeun H., Borath M., Sophonneary P., Ueland P.M., Green T.J. Comparable performance characteristics of plasma thiamine and erythrocyte thiamine diphosphate in response to thiamine fortification in rural cambodian women. Nutrients. 2017;9:676. doi: 10.3390/nu9070676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anwar A., Marini M., Abruzzo P.M., Bolotta A., Ghezzo A., Visconti P., Thornalley P.J., Rabbani N. Quantitation of plasma thiamine, related metabolites and plasma protein oxidative damage markers in children with autism spectrum disorder and healthy controls. Free Radic. Res. 2016;50:S85–S90. doi: 10.1080/10715762.2016.1239821. [DOI] [PubMed] [Google Scholar]
- 60.Coats D., Frank E.L., Reid J.M., Ou K., Chea M., Khin M., Preou C., Enders F.T., Fischer P.R., Topazian M. Thiamine pharmacokinetics in Cambodian mothers and their breastfed infants. Am. J. Clin. Nutr. 2013;98:839–844. doi: 10.3945/ajcn.113.062737. [DOI] [PubMed] [Google Scholar]
- 61.Gangolf M., Czerniecki J., Radermecker M., Detry O., Nisolle M., Jouan C., Martin D., Chantraine F., Lakaye B., Wins P., et al. Thiamine status in humans and content of phosphorylated thiamine derivatives in biopsies and cultured cells. PLoS ONE. 2010;5:e13616. doi: 10.1371/journal.pone.0013616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thornalley P.J., Babaei-Jadidi R., Al Ali H., Rabbani N., Antonysunil A., Larkin J., Ahmed A., Rayman G., Bodmer C.W. High prevalence of low plasma thiamine concentration in diabetes linked to a marker of vascular disease. Diabetologia. 2007;50:2164–2170. doi: 10.1007/s00125-007-0771-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guo C., Wei J., Gao W., Pu L., Tang Z., Li L. Plasma riboflavin is a useful marker for studying riboflavin requirement in Chinese male adults. Nutr. Res. 2016;36:534–540. doi: 10.1016/j.nutres.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 64.Petteys B.J., Frank E.L. Rapid determination of vitamin B2 (riboflavin) in plasma by HPLC. Clin. Chim. Acta. 2011;412:38–43. doi: 10.1016/j.cca.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 65.Powers H.J., Hill M.H., Mushtaq S., Dainty J.R., Majsak-Newman G., Williams E.A. Correcting a marginal riboflavin deficiency improves hematologic status in young women in the United Kingdom (RIBOFEM) Am. J. Clin. Nutr. 2011;93:1274–1284. doi: 10.3945/ajcn.110.008409. [DOI] [PubMed] [Google Scholar]
- 66.Ibrahim G.R., Shah I., Gariballa S., Yasin J., Barker J., Ashraf S.S. Significantly Elevated Levels of Plasma Nicotinamide, Pyridoxal, and Pyridoxamine Phosphate Levels in Obese Emirati Population: A Cross-Sectional Study Ghada. Molecules. 2020;25:3932. doi: 10.3390/molecules25173932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kouassi Nzoughet J., Chao de la Barca J.M., Guehlouz K., Leruez S., Coulbault L., Allouche S., Bocca C., Muller J., Amati-Bonneau P., Gohier P., et al. Nicotinamide deficiency in primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2019;60:2509–2514. doi: 10.1167/iovs.19-27099. [DOI] [PubMed] [Google Scholar]
- 68.Meisser Redeuil K., Longet K., Bénet S., Munari C., Campos-Giménez E. Simultaneous quantification of 21 water soluble vitamin circulating forms in human plasma by liquid chromatography-mass spectrometry. J. Chromatogr. A. 2015;1422:89–98. doi: 10.1016/j.chroma.2015.09.049. [DOI] [PubMed] [Google Scholar]
- 69.Hansen C.M., Shultz T.D., Kwak H.K., Memon H.S., Leklem J.E. Assessment of vitamin B-6 status in young women consuming a controlled diet containing four levels of vitamin B-6 provides an estimated average requirement and recommended dietary allowance. J. Nutr. 2001;131:1777–1786. doi: 10.1093/jn/131.6.1777. [DOI] [PubMed] [Google Scholar]
- 70.Driskell J.A., Chrisley B.M. Plasma B-6 vitamer and plasma and urinary 4-pyridoxic acid concentrations in young women as determined using high performance liquid chromatography. Biomed. Chromatogr. 1991;5:198–201. doi: 10.1002/bmc.1130050504. [DOI] [PubMed] [Google Scholar]
- 71.Sobczyńska-Malefora A., Harrington D.J. Laboratory assessment of folate (Vitamin B9) status. J. Clin. Pathol. 2018;71:949–956. doi: 10.1136/jclinpath-2018-205048. [DOI] [PubMed] [Google Scholar]
- 72.Allen L.H., Miller J.W., De Groot L., Rosenberg I.H., Smith A.D., Refsum H., Raiten D.J. Biomarkers of Nutrition for Development (BOND): Vitamin B-12 Review. J. Nutr. 2018;148:1995S–2027S. doi: 10.1093/jn/nxy201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pawlak R., Parrott S.J., Raj S., Cullum-Dugan D., Lucus D. How prevalent is vitamin B12 deficiency among vegetarians? Nutr. Rev. 2013;71:110–117. doi: 10.1111/nure.12001. [DOI] [PubMed] [Google Scholar]
- 74.Bailey L.B., Stover P.J., McNulty H., Fenech M.F., Gregory J.F., III, Mills J.L., Pfeiffer C.M., Fazili Z., Zhang M., Ueland P.M., et al. Biomarkers of nutrition for development-Folate review. J. Nutr. 2015;145:1636S–1680S. doi: 10.3945/jn.114.206599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.EFSA NDA Panel. Scientific Opinion on Dietary Reference Values for vitamin C. EFSA J. 2013;11:3418. doi: 10.2903/j.efsa.2013.3418. [DOI] [Google Scholar]
- 76.De Pee S., Dary O. Biochemical indicators of vitamin A deficiency: Serum retinol and serum retinol binding protein. J. Nutr. 2002;132:2895–2901. doi: 10.1093/jn/132.9.2895S. [DOI] [PubMed] [Google Scholar]
- 77.World Health Organization . Serum Retinol Concentrations for Determining the Prevalence of Vitamin A Deficiency in Populations. World Health Organization (WHO); Geneva, Switzerland: 2011. [(accessed on 5 April 2023)]. Available online: https://apps.who.int/iris/bitstream/handle/10665/85859/WHO_NMH_NHD_MNM_11.3_eng.pdf?sequence=4&isAllowed=y. [Google Scholar]
- 78.U.S. Centers for Disease Control and Prevention . Second National Report on Biochemical Indicators of Diet and Nutrition in the U.S. Population. CDC; Atlanta, GA, USA: 2012. [(accessed on 5 April 2023)]. Available online: https://www.cdc.gov/nutritionreport/pdf/nutrition_book_complete508_final.pdf. [Google Scholar]
- 79.Dietary Guidelines Advisory Committee . Scientific Report of the 2015 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Health and Human Services and the Secretary of Agriculture. Department of Agriculture, Agricultural Research Service; Washington, DC, USA: 2015. [(accessed on 5 April 2023)]. Available online: https://health.gov/sites/default/files/2019-09/Scientific-Report-of-the-2015-Dietary-Guidelines-Advisory-Committee.pdf. [Google Scholar]
- 80.Papathakis P.C., Rollins N.C., Chantry C.J., Bennish M.L., Brown K.H. Micronutrient status during lactation in HIV-infected and HIV-uninfected South African women during the first 6 mo after delivery. Am. J. Clin. Nutr. 2007;85:182–192. doi: 10.1093/ajcn/85.1.182. [DOI] [PubMed] [Google Scholar]
- 81.Dror D.K., Allen L.H. Vitamin E deficiency in developing countries. Food Nutr. Bull. 2011;32:124–143. doi: 10.1177/156482651103200206. [DOI] [PubMed] [Google Scholar]
- 82.Henjum S., Manger M., Hampel D., Brantsæter A.L., Shahab-Ferdows S., Bastani N.E., Strand T.A., Refsum H., Allen L.H. Vitamin B12 concentrations in milk from Norwegian women during the six first months of lactation. Eur. J. Clin. Nutr. 2020;74:749–756. doi: 10.1038/s41430-020-0567-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Norman E.J., Morrison J.A. Screening elderly populations for cobalamin (vitamin B12) deficiency using the urinary methylmalonic acid assay by gas chromatography mass spectrometry. Am. J. Med. 1993;94:589–594. doi: 10.1016/0002-9343(93)90209-8. [DOI] [PubMed] [Google Scholar]
- 84.World Health Organization . Vitamin and Mineral Nutrition Information System. Urinary Iodine Concentrations for Determining Iodine Status Deficiency in Populations. World Health Organization (WHO); Geneva, Switzerland: 2013. [(accessed on 8 March 2023)]. Available online: https://apps.who.int/iris/bitstream/handle/10665/85972/WHO_NMH_NHD_EPG_13.1_eng.pdf. [Google Scholar]
- 85.Ahn J., Lee J.H., Lee J., Baek J.Y., Song E., Oh H.S., Kim M., Park S., Jeon M.J., Kim T.Y., et al. Association between Urinary Sodium Levels and Iodine Status in Korea. Korean J. Intern. Med. 2020;35:392–399. doi: 10.3904/kjim.2017.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Foley K.F., Boccuzzi L. Urine calcium: Laboratory measurement and clinical utility. Lab. Med. 2010;41:683–686. doi: 10.1309/LM9SO94ZNBHEDNTM. [DOI] [Google Scholar]
- 87.Fernández-Ruiz L., Rodelo Haad C., Rodríguez-Portillo M., Santamaría-Olmo R. Variation of phosphaturia according to phosphorus intake. Actual. Med. 2020;105:18–26. doi: 10.15568/am.2020.809.or02. [DOI] [Google Scholar]
- 88.Giuffrida F., Fleith M., Goyer A., Samuel T.M., Elmelegy-Masserey I., Fontannaz P., Cruz-Hernandez C., Thakkar S.K., Monnard C., De Castro C.A., et al. Human milk fatty acid composition and its association with maternal blood and adipose tissue fatty acid content in a cohort of women from Europe. Eur. J. Nutr. 2022;61:2167–2182. doi: 10.1007/s00394-021-02788-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang Z., Wang Y., Yang X., Cheng Y., Zhang H., Xu X., Zhou J., Chen H., Su M., Yang Y., et al. Human Milk Lipid Profiles around the World: A Systematic Review and Meta-Analysis. Adv. Nutr. 2022;13:2519–2536. doi: 10.1093/advances/nmac097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gibson R.S., Rahmannia S., Diana A., Leong C., Haszard J.J., Hampel D., Reid M., Erhardt J., Suryanto A.H., Sofiah W.N., et al. Association of maternal diet, micronutrient status, and milk volume with milk micronutrient concentrations in Indonesian mothers at 2 and 5 months postpartum. Am. J. Clin. Nutr. 2020;112:1039–1050. doi: 10.1093/ajcn/nqaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.EFSA NDA Panel. Turck D., Bresson J.L., Burlingame B., Dean T., Fairweather-Tait S., Heinonen M., Hirsch-Ernst K.I., Mangelsdorf I., McArdle H.J., et al. Dietary reference values for thiamin. EFSA J. 2016;14:4653. doi: 10.2903/j.efsa.2016.4653. [DOI] [Google Scholar]
- 92.EFSA NDA Panel. Turck D., Bresson J.-L., Burlingame B., Dean T., Fairweather-Tait S., Heinonen M., Hirsch-Ernst K., Mangelsdorf I., McArdle H., et al. Dietary Reference Values for riboflavin. EFSA J. 2017;15:4919. doi: 10.2903/j.efsa.2017.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.EFSA NDA Panel Scientific Opinion on Dietary Reference Values for niacin. EFSA J. 2014;12:3759. doi: 10.2903/j.efsa.2014.3759. [DOI] [Google Scholar]
- 94.EFSA NDA Panel Scientific Opinion on Dietary Reference Values for pantothenic acid. EFSA J. 2014;12:3581. doi: 10.2903/j.efsa.2014.3581. [DOI] [Google Scholar]
- 95.EFSA NDA Panel Scientific Opinion on nutrient requirements and dietary intakes of infants and young children in the European Union. EFSA J. 2013;11:3408. doi: 10.2903/j.efsa.2013.3408. [DOI] [Google Scholar]
- 96.EFSA NDA Panel Scientific Opinion on Dietary Reference Values for cobalamin (vitamin B12) EFSA J. 2015;13:4150. doi: 10.2903/j.efsa.2015.4150. [DOI] [Google Scholar]
- 97.EFSA NDA Panel Scientific Opinion on Dietary Reference Values for vitamin A. EFSA J. 2015;13:4028. doi: 10.2903/j.efsa.2015.4028. [DOI] [Google Scholar]
- 98.EFSA NDA Panel Scientific Opinion on dietary reference values for vitamin D. EFSA J. 2016;14:4547. doi: 10.2903/j.efsa.2016.4547. [DOI] [Google Scholar]
- 99.EFSA NDA Panel Scientific Opinion on Dietary Reference Values for vitamin E as α-tocopherol. EFSA J. 2015;13:4149. doi: 10.2903/j.efsa.2015.4149. [DOI] [Google Scholar]
- 100.Semba R.D., Delange F. Iodine in human milk: Perspectives for infant health. Nutr. Rev. 2001;59:269–278. doi: 10.1111/j.1753-4887.2001.tb05512.x. [DOI] [PubMed] [Google Scholar]
- 101.Andersson M., Braegger C.P. The role of iodine for thyroid function in lactating women and infants. Endocr. Rev. 2022;43:469–506. doi: 10.1210/endrev/bnab029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.EFSA NDA Panel Scientific Opinion on Dietary Reference Values for calcium. EFSA J. 2015;13:4101. doi: 10.2903/j.efsa.2015.4101. [DOI] [Google Scholar]
- 103.EFSA NDA Panel Scientific Opinion on Dietary Reference Values for phosphorus. EFSA J. 2015;13:4185. doi: 10.2903/j.efsa.2015.4185. [DOI] [Google Scholar]
- 104.EFSA NDA Panel Scientific Opinion on Dietary Reference Values for selenium. EFSA J. 2014;12:3846. doi: 10.2903/j.efsa.2014.3846. [DOI] [Google Scholar]
- 105.LASER Analytica Comprehensive literature search and review of breast milk composition as preparatory work for the setting of dietary reference values for vitamins and minerals. EFSA Support. Publ. 2014;11:EN-629. doi: 10.2903/sp.efsa.2014.en-629. [DOI] [Google Scholar]
- 106.Davis B.C., Kris-Etherton P.M. Achieving optimal essential fatty acid status in vegetarians: Current knowledge and practical implications. Am. J. Clin. Nutr. 2003;78:640–646. doi: 10.1093/ajcn/78.3.640S. [DOI] [PubMed] [Google Scholar]
- 107.Engelmann B. Plasmalogens: Targets for oxidants and major lipophilic antioxidants. Biochem. Soc. Trans. 2004;32:147–150. doi: 10.1042/bst0320147. [DOI] [PubMed] [Google Scholar]
- 108.Sanders T., Reddy S. The influence of a vegetarian diet on the fatty acid composition of human milk and the essential fatty acid status of the infant. J. Pediatr. 1992;120:71–77. doi: 10.1016/S0022-3476(05)81239-9. [DOI] [PubMed] [Google Scholar]
- 109.Koletzko B., Boey C.C.M., Campoy C., Carlson S.E., Chang N., Guillermo-Tuazon M.A., Joshi S., Prell C., Quak S.H., Sjarif D.R., et al. Current information and asian perspectives on long-chain polyunsaturated fatty acids in pregnancy, lactation, and infancy: Systematic review and practice recommendations from an early nutrition academy workshop. Ann. Nutr. Metab. 2014;65:49–80. doi: 10.1159/000365767. [DOI] [PubMed] [Google Scholar]
- 110.Cetin I., Koletzko B. Long-chain ω-3 fatty acid supply in pregnancy and lactation. Curr. Opin. Clin. Nutr. Metab. Care. 2008;11:297–302. doi: 10.1097/MCO.0b013e3282f795e6. [DOI] [PubMed] [Google Scholar]
- 111.Koletzko B., Cetin I., Brenna J.T., Perinatal Lipid Intake Working Group. Child Health Foundation. Diabetic Pregnancy Study Group. European Association of Perinatal Medicine. European Society for Clinical Nutrition and Metabolism. European Society for Paediatric Gastroenterology. Hepatology and Nutrition et al. Dietary fat intakes for pregnant and lactating women. Br. J. Nutr. 2007;98:873–877. doi: 10.1017/S0007114507764747. [DOI] [PubMed] [Google Scholar]
- 112.Lapillonne A., Eleni Dit Trolli S., Kermorvant-Duchemin E. Postnatal docosahexaenoic acid deficiency is an inevitable consequence of current recommendations and practice in preterm infants. Neonatology. 2010;98:397–403. doi: 10.1159/000320159. [DOI] [PubMed] [Google Scholar]
- 113.Valentine C.J. Maternal Dietary DHA Supplementation to Improve Inflammatory Outcomes in the Preterm Infant. Adv. Nutr. 2012;3:370–376. doi: 10.3945/an.111.001248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Makrides M., Gibson R.A., McPhee A.J., Collins C.T., Davis P.G., Doyle L.W., Simmer K., Colditz P.B., Morris S., Smithers L.G., et al. Neurodevelopmental outcomes of preterm infants fed high-dose docosahexaenoic acid: A randomized controlled trial. JAMA. 2009;301:175–182. doi: 10.1001/jama.2008.945. [DOI] [PubMed] [Google Scholar]
- 115.Agostoni C., Buonocore G., Carnielli V.P., De Curtis M., Darmaun D., Decsi T., Domellöf M., Embleton N.D., Fusch C., Genzel-Boroviczeny O., et al. Enteral nutrient supply for preterm infants: Commentary from the european society of paediatric gastroenterology, hepatology and nutrition committee on nutrition. J. Pediatr. Gastroenterol. Nutr. 2010;50:85–91. doi: 10.1097/MPG.0b013e3181adaee0. [DOI] [PubMed] [Google Scholar]
- 116.Baack M.L., Norris A.W., Yao J., Colaizy T. Long Chain Polyunsaturated Fatty Acid Levels in U.S. Donor Human Milk: Meeting the Needs of Premature Infants? J. Perinatol. 2012;32:598–603. doi: 10.1038/jp.2011.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Valentine C.J., Morrow G., Fernandez S., Gulati P., Bartholomew D., Long D., Welty S.E., Morrow A.L., Rogers L.K. Docosahexaenoic acid and amino acid contents in pasteurized donor milk are low for preterm infants. J. Pediatr. 2010;157:906–910. doi: 10.1016/j.jpeds.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 118.Brenna J.T., Varamini B., Jensen R.G., Diersen-Schade D.A., Boettcher J.A., Arterburn L.M. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am. J. Clin. Nutr. 2007;85:1457–1464. doi: 10.1093/ajcn/85.6.1457. [DOI] [PubMed] [Google Scholar]
- 119.Forsyth S., Gautier S., Salem N.J. Global estimates of dietary intake of docosahexaenoic acid and arachidonic acid in developing and developed countries. Ann. Nutr. Metab. 2016;68:258–267. doi: 10.1159/000446855. [DOI] [PubMed] [Google Scholar]
- 120.Fu Y., Liu X., Zhou B., Jiang A.C., Chai L. An updated review of worldwide levels of docosahexaenoic and arachidonic acid in human breast milk by region. Public Health Nutr. 2016;19:2675–2687. doi: 10.1017/S1368980016000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Perrin M.T., Pawlak R., Dean L.L., Christis A., Friend L. A cross-sectional study of fatty acids and brain-derived neurotrophic factor (BDNF) in human milk from lactating women following vegan, vegetarian, and omnivore diets. Eur. J. Clin. Nutr. 2019;58:2401–2410. doi: 10.1007/s00394-018-1793-z. [DOI] [PubMed] [Google Scholar]
- 122.Barreiro R., Díaz-Bao M., Cepeda A., Regal P., Fente C.A. Fatty acid composition of breast milk in Galicia (NW Spain): A cross-country comparison. Prostaglandins Leukot. Essent. Fat. Acids. 2018;135:102–114. doi: 10.1016/j.plefa.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 123.Valentine C.J., Morrow G., Pennell M., Morrow A.L., Hodge A., Haban-Bartz A., Collins K., Rogers L.K. Randomized controlled trial of docosahexaenoic acid supplementation in midwestern U.S. human milk donors. Breastfeed. Med. 2013;8:86–91. doi: 10.1089/bfm.2011.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Makrides M., Neumann M., Gibson R. Effect of maternal docosahexaenoic (DHA) supplementation on breast milk composition. Eur. J. Clin. Nutr. 1996;50:352–357. [PubMed] [Google Scholar]
- 125.Fidler N., Sauerwald T., Pohl A., Demmelmair H., Koletzko B. Docosahexaenoic acid transfer into human milk after dietary supplementation: A randomized clinical trial. J. Lipid Res. 2000;41:1376–1383. doi: 10.1016/S0022-2275(20)33449-0. [DOI] [PubMed] [Google Scholar]
- 126.Jensen C.L., Maude M., Anderson R.E., Heird W.C. Effect of docosahexaenoic acid supplementation of lactating women on the fatty acid composition of breast milk lipids and maternal and infant plasma phospholipids. Am. J. Clin. Nutr. 2000;71:292–299. doi: 10.1093/ajcn/71.1.292S. [DOI] [PubMed] [Google Scholar]
- 127.Sherry C.L., Oliver J.S., Marriage B.J. Docosahexaenoic acid supplementation in lactating women increases breast milk and plasma docosahexaenoic acid concentrations and alters infant omega 6:3 fatty acid ratio. Prostaglandins Leukot. Essent. Fat. Acids. 2015;95:63–69. doi: 10.1016/j.plefa.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 128.Yang Y., Li G., Li F., Xu F., Hu P., Xie Z., Lu X., Ding Y., Wang Z. Impact of DHA from Algal Oil on the Breast Milk DHA Levels of Lactating Women: A Randomized Controlled Trial in China. Nutrients. 2022;14:3410. doi: 10.3390/nu14163410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Valencia-Naranjo A., Manjarres-Correa L.M., Bermúdez-Cardona J. Pilot study of the effect of EPA + DHA supplementation on the fatty acid profile of erythrocytes and breast milk of lactating women from Sonsón, Colombia. Curr. Res. Food Sci. 2022;5:789–797. doi: 10.1016/j.crfs.2022.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Smithers L.G., Markrides M., Gibson R.A. Human milk fatty acids from lactating mothers of preterm infants: A study revealing wide intra- and inter-individual variation. Prostaglandins Leukot. Essent. Fat. Acids. 2010;83:9–13. doi: 10.1016/j.plefa.2010.02.034. [DOI] [PubMed] [Google Scholar]
- 131.Burns-Whitmore B., Froyen E., Heskey C., Parker T., San Pablo G. Alpha-linolenic and linoleic fatty acids in the vegan diet: Do they require dietary reference intake/adequate intake special consideration? Nutrients. 2019;11:2365. doi: 10.3390/nu11102365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Henriksen C., Haugholt K., Lindgren M., Aurvåg A.K., Rønnestad A., Grønn M., Solberg R., Moen A., Nakstad B., Berge R.K., et al. Improved cognitive development among preterm infants attributable to early supplementation of human milk with docosahexaenoic acid and arachidonic acid. Pediatrics. 2008;121:1137–1145. doi: 10.1542/peds.2007-1511. [DOI] [PubMed] [Google Scholar]
- 133.Sæle Ø., Rød K., Quinlivan V., Li S., Farber S. A novel system to quantify intestinal lipid digestion and transport. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2018;1863:948–957. doi: 10.1016/j.bbalip.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ahmed T.B., Eggesbø M., Criswell R., Uhl O., Demmelmair H., Koletzko B. Total fatty acid and polar lipid species composition of human milk. Nutrients. 2022;14:158. doi: 10.3390/nu14010158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Demmelmair H., Koletzko B. Lipids in human milk. Best Pract. Res. Clin. Endocrinol. Metab. 2018;32:57–68. doi: 10.1016/j.beem.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 136.Benoit B., Fauquant C., Daira P., Peretti N., Guichardant M., Michalski M.-C. Phospholipid species and minor sterols in French human milks. Food Chem. 2010;120:684–691. doi: 10.1016/j.foodchem.2009.10.061. [DOI] [Google Scholar]
- 137.Sala-Vila A., Castellote A.I., Rodriguez-Palmero M., Campoy C., López-Sabater M.C. Lipid composition in human breast milk from Granada (Spain): Changes during lactation. Nutrition. 2005;21:467–473. doi: 10.1016/j.nut.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 138.Ingvordsen Lindahl I.E., Artegoitia V.M., Downey E., O’Mahony J.A., O’Shea C.A., Ryan C.A., Kelly A.L., Bertram H.C., Sundekilde U.K. Quantification of human milk phospholipids: The effect of gestational and lactational age on phospholipid composition. Nutrients. 2019;11:222. doi: 10.3390/nu11020222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang L., Shimizu Y., Kaneko S., Hanaka S., Abe T., Shimasaki H., Hisaki H., Nakajima H. Comparison of the fatty acid composition of total lipids and phospholipids in breast milk from Japanese women. Pediatr. Int. 2000;42:14–20. doi: 10.1046/j.1442-200x.2000.01169.x. [DOI] [PubMed] [Google Scholar]
- 140.Bitman J., Wood D.L., Mehta N.R., Hamosh P., Hamosh M. Comparison of the phospholipid composition of breast milk from mothers of term and preterm infants during lactation. Am. J. Clin. Nutr. 1984;40:1103–1119. doi: 10.1093/ajcn/40.5.1103. [DOI] [PubMed] [Google Scholar]
- 141.Dror D.K., Allen L.H. Effect of vitamin B12 deficiency on neurodevelopment in infants: Current knowledge and possible mechanisms. Nutr. Rev. 2008;66:250–255. doi: 10.1111/j.1753-4887.2008.00031.x. [DOI] [PubMed] [Google Scholar]
- 142.Messina V., Melina V., Mangels A.R. A new food guide for North American vegetarians. J. Am. Diet. Assoc. 2003;103:771–775. doi: 10.1053/jada.2003.50141. [DOI] [PubMed] [Google Scholar]
- 143.Rizzo G., Laganà A.S., Rapisarda A.M.C., La Ferrera G.M.G., Buscema M., Rossetti P., Nigro A., Muscia V., Valenti G., Sapia F., et al. Vitamin B12 among vegetarians: Status, assessment and supplementation. Nutrients. 2016;8:767. doi: 10.3390/nu8120767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wagner K.-H., Kamal-Eldin A., Elmadfa I. Gamma-tocopherol--an underestimated vitamin? Ann. Nutr. Metab. 2004;48:169–188. doi: 10.1159/000079555. [DOI] [PubMed] [Google Scholar]
- 145.Allen L.H. B Vitamins in Breast Milk: Relative Importance of Maternal Status and Intake, and Effects on Infant Status and Function. Adv. Nutr. Int. Rev. J. 2012;3:362–369. doi: 10.3945/an.111.001172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bijur A.M., Desai A.G. Composition of breast milk with reference to vitamin B12 and folic acid in Indian mothers. Indian J. Pediatr. 1985;52:147–150. doi: 10.1007/BF02754773. [DOI] [PubMed] [Google Scholar]
- 147.Büttner B.E., Witthöft C.M., Domellöf M., Hernell O., Öhlund I. Effect of type of heat treatment of breastmilk on folate content and pattern. Breastfeed. Med. 2014;9:86–91. doi: 10.1089/bfm.2013.0008. [DOI] [PubMed] [Google Scholar]
- 148.Institute of Medicine (US) Committee on Nutritional Status During Pregnancy and Lactation . Nutrition during Lactation. Subcommittee on Nutrition During Lactation; Washington, DC, USA: Committee on Nutritional Status During Pregnancy and Lactation; Washington, DC, USA: Food and Nutrition Board; Washington, DC, USA: Institute of Medicine; Washington, DC, USA: National Academy of Sciences; Washington, DC, USA: 1991. [Google Scholar]
- 149.Debski B., Finley D.A., Picciano M.F., Lönnerdal B., Milner J. Selenium content and glutathione peroxidase activity of milk from vegetarian and nonvegetarian women. J. Nutr. 1989;119:215–220. doi: 10.1093/jn/119.2.215. [DOI] [PubMed] [Google Scholar]
- 150.Ureta-Velasco N., Keller K., Escuder-Vieco D., Serrano J.C.E., García-Lara N.R., Pallás-Alonso C.R. Assessment of Iodine Concentration in Human Milk from Donors: Implications for Preterm Infants. Nutrients. 2022;14:4304. doi: 10.3390/nu14204304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Streuling I., Beyerlein A., Rosenfeld E., Schukat B., Von Kries R. Weight gain and dietary intake during pregnancy in industrialized countries—A systematic review of observational studies. J. Perinat. Med. 2011;39:123–129. doi: 10.1515/jpm.2010.127. [DOI] [PubMed] [Google Scholar]
- 152.Sebastiani G., Herranz Barbero A., Borrás-Novel C., Alsina Casanova M., Aldecoa-Bilbao V., Andreu-Fernández V., Pascual Tutusaus M., FerreroMartínez S., Gómez Roig M.D., García-Algar O. The effects of vegetarian and vegan diet during pregnancy on the health of mothers and offspring. Nutrients. 2019;11:557. doi: 10.3390/nu11030557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nessel I., Khashu M., Dyall S.C. The effects of storage conditions on long-chain polyunsaturated fatty acids, lipid mediators, and antioxidants in donor human milk—A review. Prostaglandins Leukot. Essent. Fat. Acids. 2019;149:8–17. doi: 10.1016/j.plefa.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 154.Wei W., Cheng J., Yang J., Chen C., Jin Q., Song J., Wang X. Phospholipid composition and fat globule structure change during low temperature storage of human milk. LWT. 2021;150:112050. doi: 10.1016/j.lwt.2021.112050. [DOI] [Google Scholar]
- 155.Leaf A., Lansdowne Z. Vitamins--conventional uses and new insights. World Rev. Nutr. Diet. 2014;110:152–166. doi: 10.1159/000358464. [DOI] [PubMed] [Google Scholar]
- 156.Peila C., Moro G.E., Bertino E., Cavallarin L., Giribaldi M., Giuliani F., Cresi F., Coscia A. The effect of holder pasteurization on nutrients and biologically-active components in donor human milk: A review. Nutrients. 2016;8:477. doi: 10.3390/nu8080477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Escuder-Vieco D., Rodríguez J.M., Espinosa-Martos I., Corzo N., Montilla A., García-Serrano A., Calvo M.V., Fontecha J., Serrano J., Fernández L., et al. High-Temperature Short-Time and Holder Pasteurization of Donor Milk: Impact on Milk Composition. Life. 2021;11:114. doi: 10.3390/life11020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Young B.E., Murphy K., Borman L.L., Heinrich R., Krebs N.F. Milk Bank Pooling Practices Impact Concentrations and Variability of Bioactive Components of Donor Human Milk. Front. Nutr. 2020;7:579115. doi: 10.3389/fnut.2020.579115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy issues.