Abstract
Since Charles Darwin and his book carnivorous plants have aroused interest and heated debate. In addition, there is growing interest in this group of plants as a source of secondary metabolites and in the application of their biological activity. The aim of this study was to trace the recent literature in search of the application of extracts obtained from families Droseraceae, Nepenthaceae, and Drosophyllaceae to show their biological potential. The data collected in the review clearly indicate that the studied Nepenthales species have great biological potential in terms of antibacterial, antifungal, antioxidant, anti-inflammatory, and anticancer use. We proposed that further investigations should include: (i) bioactivity-guided investigations of crude plant extract to connect a particular type of action with a specific compound or a group of metabolites; (ii) a search for new bioactive properties of carnivorous plants; (iii) establishment of molecular mechanisms associated with specific activity. Furthermore, further research should be extended to include less explored species, i.e., Drosophyllum lusitanicum and especially Aldrovanda vesiculosa.
Keywords: carnivorous plants, biological activity, secondary metabolites, naphthoquinones, polyphenols
1. Introduction
For centuries, plants have always been of great importance for human health and have been used to treat many diseases. Nowadays, plant-derived products or isolated compounds are also of great significance, and the exploration of their biological potential is the subject addressed by numerous research teams. New sources of biomolecules or new directions of action of known compounds are still being sought.
Since Charles Darwin and his book [1], carnivorous plants have aroused interest and heated debate [2,3,4]. Plants evolved various strategies (mycorrhiza, myrmecophily, symbiosis with nitrogen-fixing bacteria) to live in nutrient-poor habitats, and carnivory is an example of such adaptation. Macroelements are absorbed from the bodies of captured organisms; however, carnivorous species also perform photosynthesis and assimilate carbon dioxide using the C3 pathway, thus, can be considered as mixotrophic [5]. When Charles Darwin (1875) published his book about carnivorous plants, he used the word “insectivorous”. However, since then, our knowledge of this ecological group has increased significantly. Although insects are an important prey group, they capture prey ranging from protozoa and various invertebrates to even small vertebrates such tadpoles, fish fry, salamanders, geckos, or, in rare cases, mammals [6,7,8]. In some carnivorous plants, there is a change from carnivory to coprophagy. Roridula plants capture insects but have no digestive enzymes, so they use nitrogen from the feces of obligately associated, carnivorous hemipterans [9]. Some Nepenthes species developed mutualistic relationships with small mammals for nitrogen supplementation. Animal feces are a source of macroelements [10,11]. Another strategy occurs in N. ampullaria, which collects in pitchers the plant-derived materials and benefits from this material utilization [5]. This “vegetarian” trend also occurs in Utricularia and Genlisea, which capture and digest algae in addition to animal prey [12,13]. Another carnivorous plant strategy to obtain nutrients is cooperation with various organisms (arthropods, fungi, protozoa, and bacteria). Many carnivorous plants with pitcher trap type form small, relatively self-contained ecosystems in traps. Organisms in traps may act as decomposers, breaking down the proteins, fats, and carbohydrates of prey, thus may increase the availability of nutrients for the host plant. The best examples of these inquilines are known in some Nepenthes and Sarracenia species [14,15]. Similar strategies occur in Utricularia and Genlisea traps, where various species of bacteria, algae, and protozoa may live and reproduce [16,17,18,19,20].
In recent years, many carnivorous species have gained increased attention due to their multidimensional biological activity and the possibility of their application in pharmacy and medicine. The review by Miclea clearly showed the potential of carnivorous plants from Sarraceniaceae [21]. Similarly, species from the Nepenthaceae and Droseraceae families exhibit diverse biological activities. They have been used in folk medicine to treat various disorders, and recent reports confirm their effectiveness based on scientific investigations. Plants of both families are a rich source of secondary metabolites representing phenolics (flavonoids including anthocyanins, phenolic acids, and their derivatives), naphthoquinones, and volatile organic compounds [22,23,24,25,26], and the role of these components in the growth, development, and physiology of plants has previously been described in detail by Hatcher et al. [27]. The aim of our work was to summarize the current state of knowledge on the biological effectiveness of Droseraceae and Nepenthaceae, especially since both crude extracts and isolated components of these families have been intensively studied in terms of antimicrobial, anticancer, and anti-inflammatory action [28,29,30]. It should also be noted that some Dionaea, Drosera, and Nepenthes species are common in cultivation and easy to propagate and introduce into in vitro cultures. Thus, their biomass is relatively easily available, and these plants may be a valuable model for biological and phytochemical studies.
The review discusses the biological activity of plants, and the provided information can help to outline the directions for further investigation of carnivorous species. A literature survey was carried out using Scopus, PubMed, Web of Science, and google scholar databases. In the investigation following combination of terms was used: “Aldrovanda” or “Nepenthes” or “Drosera” or “Dionaea” or “Drosophyllum” and “activity” or “action”. The search covered titles, abstracts, and keywords. The lists of all retrieved articles (ca 1700 papers) were reviewed, taking into account the inclusion criteria (full-text availability, English language) and exclusion criteria (conference papers, reviews, the subject area not connected with biological science, e.g., environmental science, engineering, chemistry, physics and so on). From the selected 279 papers, further identification of potentially relevant studies was performed by abstract screening.
2. Occurrence and Taxonomy
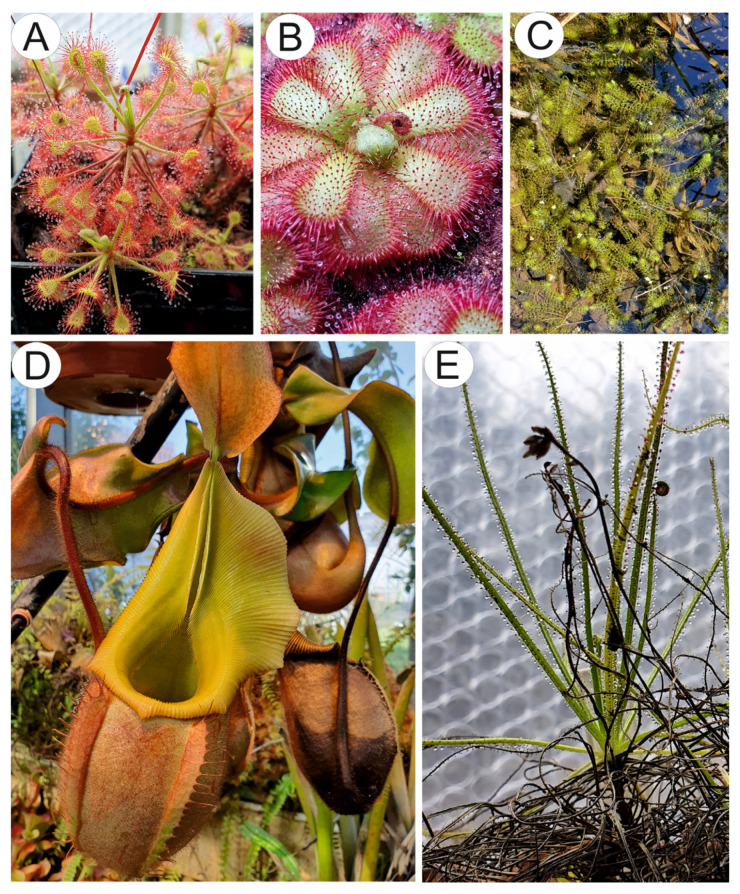
Nepenthales is an ancient (Late Cretaceous: Cenomanian and Gondwanan origin) taxonomically diverse order comprising five families: Droseraceae, Nepenthaceae, Drosophyllaceae, Dioncophyllaceae, and Ancistrocladaceae. However, the members of this monophyletic group differ from their sister core-Caryophyllales, in terms of the occurrence of acetogenic naphtho- and anthraquinones and the absence of betalains [4,31,32]. The Droseraceae family consists of three carnivorous genera: Drosera (Figure 1A,B), Dionaea, and Aldrovanda (Figure 1C). The Drosera genus is species rich about 250 species, but new species are still described (Figure 1C) [33], and is nearly cosmopolitan. Drosera are herbaceous carnivorous plants with leaves with glandular emergences [34] forming active adhesive traps [35]. The Dionaea genus is monotypic and contains one extant species of Dionaea muscipula, which is paleoendemic, with restricted distribution (to the coastal plain of North and South Carolina on the eastern seaboard of the United States) [34]. The Aldrovanda genus comprises several extinct species and one extant species, Aldrovanda vesiculosa, which used to be widespread in the Old World and in various climatic zones; however, now it is rare and endangered [36,37]. Both Dionaea and Aldrovanda have highly mobile snap traps characterized by different mechanics [38].
Figure 1.
Example of species from carnivorous Nepenthales: (A). Drosera madagascariensis DC., (B). Drosera admirabilis Debbert, (C). Aldrovanda vesiculosa L. at a replacement site near Třeboň in the Czech Republic. (D). Nepenthes veitchii Hook.f. in the carnivorous plant collection of Dr. Krzysztof Banaś (University of Gdańsk). (E). Drosophyllum lusitanicum (L.) Link.
The family Nepenthaceae is related to Droseraceae and is represented by only one genus Nepenthes (Figure 1D), which is widespread in the Australasian tropics with the highest diversity in the islands of Borneo, Sumatra, and the southern Philippines. This genus comprises more than 160 species [31,39], and still new species are being discovered [40]. Nepenthes plants produce pitchers to catch prey. However, some highly specialized species also use other nutritional sources such as mammal and bird feces or plant litter [10,11,41].
The Drosophyllaceae family comprises one species Drosophyllum lusitanicum (Figure 1E), which is a carnivorous subshrub with leaves with glandular emergences that form passive adhesive traps. It is endemic to the western Iberian Peninsula and northern Morocco, where it grows in fire-prone Mediterranean heathlands [42,43,44].
The Dioncophyllaceae family comprises one carnivorous genus, Triphyophyllum, and two non-carnivorous genera (Dioncophyllum and Habropetalum). The Triphyophyllum genus contains one species, Triphyophyllum peltatum, which occurs in tropical West Africa [31]. T. peltatum produces carnivorous leaves with emergences during its juvenile phase [45].
Figure 1 shows some examples of carnivorous species of Nepenthales.
3. Biological Activity
3.1. Antibacterial, Antiviral, and Antifungal Activity
Antibacterial and antifungal properties are the most widely explored biological activity of Droseraceae plants. In recent years, many in vitro studies have confirmed their efficacy against various pathogens that are resistant and susceptible to antibiotics. Different species have been intensively studied, and extracts have been prepared from both fresh and dried plant material using solvents with diverse polarity. The following parameters are used to describe the antibacterial effect: MIC—minimal inhibitory concentration (the lowest concentration that shows no visible growth), MBC/MFC—minimum bactericidal/fungicidal concentration (the lowest concentration that reduces viability by 99.9%), or zone of inhibition—ZOI (the radius of the circle around the discs with an antimicrobial agent, in which bacteria growth is inhibited).
Many reports show that plants from the Droseraceae and Nepenthaceae families are active against fungal pathogens and Gram-positive (G(+)) bacteria; however, their potential against Gram-negative (G(−)) bacteria is significantly lower [46,47]. This is not surprising because G(−) bacteria are, in general, more resistant to antimicrobials than G(+) bacteria due to the presence of a lipopolysaccharide-rich outer membrane, which provides additional protection against external agents [48]. For example, analyses of extracts from some Brazilian Drosera species (Dr. communis, Dr. montana var. montana, Dr. brevifolia, Dr. villosa var. graomogolensis, and Dr. villosa var. villosa) demonstrated that they were effective against Staphylococcus aureus and Candida albicans; in turn, P. aeruginosa, E. coli, and S. choleraesuis were resistant to all these extracts [46]. A similar observation was made for Dionaea muscipula extracts against food-related pathogenic and putrefactive bacteria. The inhibitory effect against G(+): B. cereus, B. subtilis, M. luteus, S. aureus, and S. faecalis was approximately two times higher than in the case of G(−): E. coli, P. aeruginosa, S. enteritidis, S. typhimurium, and S. marcescens [49]. On the other hand, no tendency was observed in the case of Dr. peltata against oral bacteria, and the activity of chloroform extract against G(−) anaerobes: Preotella oris, P. buccae, and P. intermedia was higher (or comparable) than against some Streptococcus strains [50].
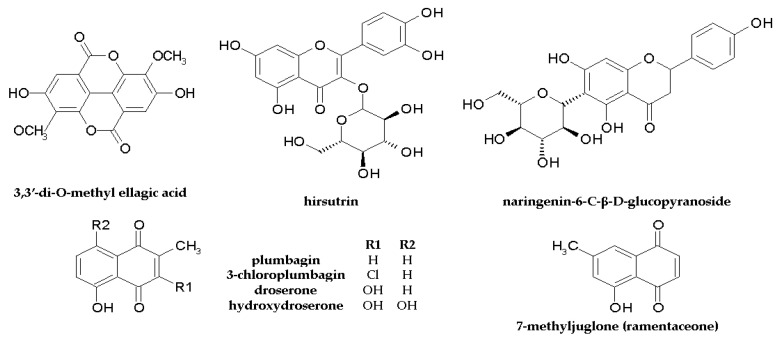
Many studies have evidenced the antimicrobial potential of Droseraceae species. Dr. rotundifolia extracts were active against various pathogens, including Bacillus thuringiensis, Clostridium perfringens, Listeria monocytogenes, E. coli, Salmonella enterica subsp. enterica, and Yersinia enterocolitica [51,52]. In addition, ethanol extracts from the field and lab-grown Dr. rotundifolia showed significant antiviral activity. They were found to be able to protect adenocarcinomic human alveolar basal epithelial cells (A549) against enteroviruses CVA9 and CVB3, responsible for many acute and chronic infections [53]. In another study, methanol extract and its fractions (petroleum ether, chloroform, ethyl acetate, n-butanol, and water residues) from Dr. peltata var. lunata were tested against Rhizopus oryzae, Aspergillus flavus, A. niger, A. oryzae, and Penicillium citrinum. The petroleum ether fraction was the most active (MIC = 5.86–46.88 µg/mL, MFC = 23.44–93.75 µg/mL), and the effect was related to plumbagin [54]. Grevenstuk et al. reported a growth-inhibiting effect of Dr. intermedia extracts and isolated plumbagin against yeasts and filamentous fungal strains responsible for food deterioration [55]. Antibacterial activity was also exhibited by some compounds from the bulbs of Dr. magna. Naringenin-6-C-β-d-glucopyranoside showed the broadest spectrum of activity and, at a concentration of 32 µg/mL, displayed a 100% inhibitory effect against methicillin-resistant S. aureus (MRSA), E. coli, K. pneumoniae, Acinetobacter baumanii, P. aeruginosa, Candida albicans, and Cryptococcus neoformans. Hirsutrin was also effective against most of the microorganisms mentioned above, except C. albicans, and was only partially active toward P. aeruginosa (79% of inhibition). In turn, hydroxydroserone and plumbagin acted against Cryptococcus neoformans and MRSA [56].
The aforementioned extracts were prepared using leaves or whole plants; however, the other parts of plants also showed an antibacterial effect. Aqueous, ethanol, and methanol extracts of thick roots, open flowers, and hair of Dr. spatulata var. bakoensis were evaluated against respiratory tract infectious microbes, including S. aureus, K. pneumoniae, S. pneumoniae, and A. niger. Among the extracts tested, ethanol was the most active, with MIC in the range of 0.35–0.50 mg/mL, 0.3–0.45 mg/mL, 0.45–0.55 mg/mL, and 0.5–0.6 mg/mL, respectively [57].
The impact on biofilm creation is another important direction of action in the context of antibacterial activity. The multilayered cellular coating formed by polysaccharides and proteins (biofilm) of bacterial strains protects against drugs and increases the resistance of pathogens to treatment [58]. The biofilm inhibitory effect of Dr. rotundifolia, Dr. intermedia, and four commercial sundew products against multidrug-resistant E. coli strains was examined by Gerschler et al. They demonstrated that plant extracts were more effective than commercial products (minimum biofilm inhibitory concentrations MBIC were 35 µg/mL and 75–140 µg/mL, respectively), and 2”-O-galloyl hyperoside was the most potent inhibitor of the isolated compounds (MBIC: 38 µg/mL). It should also be mentioned that plumbagin and 7-methyl juglone were responsible for the antibacterial effect, and the flavonoids were not active [52].
Although there are many reports on the antibacterial activity of Droseraceae species, little is known about Nepenthaceae. Only a few papers have been published on this topic. Ethanol extract from dried N. gracilis was effective against Bacillus subtilis and E. coli with an inhibition zone of 19 and 17 mm for the leaf, and 11 and 9 mm for the pitcher, respectively (control: 16 and 14 mm, respectively) [59], and hexane extract inhibited the growth of some fungi [60]. In turn, acetone extract from N. cv. Miranda showed antibacterial activity against S. aureus, P. aeruginosa, and E. coli [61] and against K. pneumoniae [62], N. mirabilis was effective against S. aureus [63], and extract from N. bicalcarata leaves was active against S. aureus, B. subtilis, B. spizizenii, and non-filamentous fungi (S. cerevisiae and C. albicans) [64]. Furthermore, an antifungal effect against human pathogens, including Candida and Aspergillus spp., was exhibited by chitin-induced pitcher liquid as well as isolated naphthoquinones from N. khasiana [65].
In turn, almost nothing is known about the activity of Dioncophyllaceae and Drosophyllaceae plants, and only one study was published. Investigations of the antibacterial/antifungal activity of Drosophyllum lusitanicum against nine bacterial and 11 yeast strains showed that it was effective against Gram (+) bacteria (S. aureus, S. epidermidis, E. faecalis, S. pyogenes), with the exception of S. pneumoniae: ZOI = 17.67–43 mm (15.6 to 250 µg/mL) and the tested yeast strains: ZOI = 23.67–42.23 mm and MIC values in the range of 31–63 µg/mL. In contrast, the growth of Gram-negative bacteria (P. aeruginosa, E. coli, E. sakazakii) was only slightly inhibited by the plant extract [66].
More details on the anti-pathogenic activities of extracts from Droseraceae, Drosophyllaceae, and Nepenthaceae species and isolated compounds, including MIC/MBC values, are given in Table 1 and Table 2. The chemical structures of the components are shown in Figure 2.
Table 1.
Antibacterial and antifungal activity of extracts from Droseraceae, Drosophyllaceae, and Nepenthaceae species.
| Species | Extract | Antibacterial/Antifungal Effect | Ref |
|---|---|---|---|
| Dr. aliciae | methanol, chloroform |
E. faecalis MBC: 125–150 mg FW/mL; S. aureus MBC: 25–75 mg FW/mL; E. coli MBC: 50–100 mg FW/mL; K. pneumoniae MBC: 125, >150 mg FW/mL; P. aeruginosa MBC:125, >150 mg FW/mL |
[47] |
| Dr. peltata var. lunata | petroleum ether fraction (PFE) from methanol extract |
R. oryzae MIC: 23.44; MFC: 93.75 µg/mL; A. flavus MIC: 11.72, MFC: 23.44 µg/mL; A. niger MIC: 23.44, MFC: 46.88 µg/mL; A. oryzae MIC: 5.86, MFC: 23.44 µg/mL; P. citrinum MIC: 46.88 MFC: 93.75 µg/mL |
[54] |
| Dr. peltata | chloroform |
S. mutans MIC: 31.25; S. sobrinus MIC: 15.62 µg/mL; S. rattus MIC: 125; S. cricetus MIC: 62.5 µg/mL; S. sanguis MIC: 125; S. milleri MIC: 125; S. mitis MIC: 250 µg/mL; S. constellatus MIC: 125; S. oralis MIC: 125 µg/mL; S. salivarius MIC: 250 Prevotella oris MIC: 125 µg/mL; P. buccae MIC: 62.5; P. intermedia MIC: 62.5 µg/mL |
[50] |
| Dr. gigantea | tetrahydrofuran | P. aeruginosa BMC 80 mg FW/mL | [67] |
| Dr. intermedia | n-hexane |
A. fumigatus, A. flavus: MIC: 15.63 μg/mL; A. niger, A. parasiticus, P. expansum MIC: 31.25 μg/mL; Z. bailii, P. membranaefaciens MIC: 7.80 μg/mL; S. cerevisiae MIC: 15.60 μg/mL; D. hansenii MIC: 3.90 μg/mL |
[55] |
| Dr. binata | chloroform | S. aureus MBC: 16 mg DW/mL | [68] |
| Dr. capensis | methanol (m), chloroform (ch) |
E. faecalis MBC: 125 (ch), 150, >150 (m) mg FW/mL; S. aureus MBC: 50 (ch), 75–100 (m) mg FW/mL; K. pneumoniae MBC: >150 (ch,m) mgFW/mL; P. aeruginosa MBC: >150 (ch, m) mgFW/mL |
[69] |
| Dr. rotundifolia | ethanol |
B. thuringiensis, C. perfringens, L. monocytogenes E. coli, S. enterica, Y. enterocolitica MIC: 25 μg DW/mL |
[51] |
| Dr. intermedia | methanol | E. coli MIC: 367–700 μg/mL | [52] |
|
Dr. spatulata var. bakoensis root (r), flower (f), hair (h). |
ethanol |
S. aureus MIC: 0.45 (r), 0.3 (f), 0.4 (h) mg dr. ex/mL; K. pneumoniae MIC: 0.55 (r), 0.45 (f, h) mg dr. ex/mL; S. pneumoniae MIC: 0.5 (r), 0.35 (f), 0.4 (h) mg dr. ex/mL; A. niger MIC: 0.6 (r), 0.5 (f), 0.65 (h) mg dr. ex/mL |
[57] |
| Di. muscipula | methanol (m), chloroform (ch) |
E. faecalis MBC:75, 100 (ch), 75 (m) mg FW/mL; S. aureus MBC: ≤25 (ch, m) mg FW/mL; K. pneumoniae MBC: 100, 125 (ch), 75 (m) mg FW/mL; P. aeruginosa MBC: >150 (ch), 100, 125 (m) mg FW/mL |
[69] |
| Di. muscipula | tetrahydrofuran | P. aeruginosa BMC: 160 mg FW/mL | [67] |
| Di. muscipula | tetrahydrofuran |
S. aureus MIC: 167, BMC: 500 μg DW/mL; E. faecalis MIC: 667, BMC: 1250 μg DW/mL; E. coli MIC: 500, BMC: 1250 μg DW/mL; P. aeruginosa MIC: 1250, BMC: 1250 μg DW/mL |
[70] |
| Drosophyllum lusitanicum | hexane | Bacteria: S. aureus MIC: 31 µg/mL S. epidermidis MIC: 15.6 µg/mL E. faecalis MIC: 250 µg/mL, S. pyogenes MIC: 125 µg/mL Fungi: C. albicans, C. catenulate, Trichosporon beigelii MIC: 31 µg/mL; C. famata, C. guilliermondi, Yarrowia lipolytica Y, T. mucoides, C. neoformans MIC: 63 µg/mL |
[66] |
| N. khasiana | chitin-induced pitcher liquid |
C. albicans MIC: 2.5, MFC: 12.5 mg DW/mL; C. krusei MIC: 7.3, MFC: 14.5 mg DW/mL C. glabrata MIC: 14.5, MFC: 14.5 mg DW/mL; A. fumigatus MIC: 3.8, MFC: 14.5 mg DW/mL; A. flavus MIC: 1.9, MFC: 1.9 mg DW/mL; A. niger MIC: 1.9, MFC: 7.3 mg DW/mL |
[65] |
| N. bicalcarata | methanol |
S. aureus, B. subtilis, B. spizizenii, C. albicans MIC: 256; S. cerevisiae MIC: 1024 (μg/mL) |
[64] |
| N. gracilis | hexane |
C. albicans, I. orientalis, T. mentagrophytes: MIC 20 µg/mL (MFC 20 µg/mL); C. parapsilosis, C. neoformans: MIC 20 µg/mL (MFC 160 µg/mL); A. brasiliensis MIC 40–80 µg/mL (MFC 160 µg/mL) |
[60] |
MIC—minimal inhibitory concentration (the lowest concentration that shows no visible growth); MBC—minimum bactericidal concentration (the lowest concentration that reduces viability by ≥99.9%); MFC—minimal fungicidal concentration; FW—fresh weight; DW—dry weight.
Table 2.
Antibacterial and antifungal activity of isolated components from the Droseraceae and Nepenthaceae families.
| Compound | Species | Antibacterial/Antifungal Effect | Ref |
|---|---|---|---|
| ramentaceone | Dr. aliciae |
E. faecalis MBC: 0.1 mg/mL; S. aureus MBC: 0.05 mg/mL; E. coli MBC: 0.08–0.165 mg/mL; K. pneumoniae MBC: >0.4 mg/mL; P. aeruginosa MBC: >0.4 mg/mL |
[47] |
| plumbagin | Dr. peltata var. lunata |
R. oryzae MIC: 5, MFC: 20 µg/mL; Aspergillus flavus MIC: 5 MFC 20 µg/mL; A. niger MIC: 5 MFC: 20 µg/mL; A. oryzae MIC: 0.625, MFC: 2.5 µg/mL; P. citrinum MIC: 0.625, MFC: 0.625 µg/mL |
[54] |
| plumbagin | Dr. intermedia |
A. fumigatus MIC: 0.08 μg/mL; A. flavus MIC: 0.98 μg/mL; A. niger, A. parasiticus, P. expansum MIC: 1.95 μg/mL; Z. bailii MIC: 2.00 μg/mL; S. cerevisiae MIC: 7.80 μg/mL; D. hansenii, P. membranaefaciens: MIC 3.90 μg/mL |
[55] |
| plumbagin 3-chloroplumbagin 3,3′-di-O-methyl ellagic acid droserone |
Dr. binata |
S. aureus MBC: 16 µg/mL; S. aureus MBC: 32 µg/mL; S. aureus MBC: >128 µg/mL; S. aureus MBC: >128 µg/mL; |
[68] |
| naringenin-6-C-β-D -glucopyranoside |
Dr. magna | S. aureus, E. coli, K. pneumoniae, A. baumanii, P. aeruginosa, C. albicans, C. neoformans: 100% inhibition at 32 µg/mL | [56] |
| hirsutrin | Dr. magna | S. aureus, E. coli, K. pneumoniae, A. baumanii, C. neoformans: 100% inhibition at 32 µg/mL; P. aeruginosa: 79% inhibition at 32 µg/mL | [56] |
| hydroxydroserone | Dr. magna |
C. albicans: 98% inhibition at 8 μg/mL; C. neoformans: 100% inhibition at 4 μg/mL, |
[56] |
| plumbagin | Dr. magna | S. aureus: 96% inhibition at <0.2 μg/mL C. neoformans: 100% inhibition at 8 μg/mL, | [56] |
| plumbagin | N. gracilis |
C. albicans, I. orientalis MIC: 2 µg/mL (MFC: 2 µg/mL); T. mentagrophytes MIC: 2 µg/mL (MFC: 4 µg/mL); C. parapsilosis MIC: 8 µg/mL (MFC: 16 µg/mL); C. neoformans MIC: 4 µg/mL (MFC: 8 µg/mL); A. brasiliensis MIC: 31 µg/mL (MFC: 63 µg/mL) |
[60] |
| 7-methyl juglone | Dr. rotundifolia | E. coli MIC: 250–333 µg/mL | [52] |
| plumbagin | Dr. rotundifolia | E. coli MIC: 104–208 µg/mL | [52] |
MIC—minimal inhibitory concentration; MBC—minimum bactericidal; MFC—minimal fungicidal concentration.
Figure 2.
Chemical structure of components with antibacterial and antifungal activity found in Nepenthales.
Different approaches were used to enhance the antibacterial activity of carnivorous plants. For example, it has been evidenced that the combination of plant extracts with silver nanoparticles (AgNPs) increased the bactericidal activity of Dr. gigantea, Di. muscipula, Dr. binata, Dr. indica, and Dr. spatulata against P. aeruginosa, S. aureus, C. albicans fungi, and plant pathogens Pectobacterium atrosepticum, P. parmentieri, and Dickeya dadantii [67,71]. It also enhanced the effectiveness of Dr. binata extract against multidrug-resistant clinical strains of S. aureus [68], as well as Dr. spatulata against E. coli [72]. Moreover, a synergistic effect with AgNPs was also observed for isolated naphthoquinones, i.e., plumbagin and 3-chloroplumbagin [67,68,73].
It has also been shown that supplementation of medium with various factors may increase the antibacterial activity of in vitro cultivated plants, as this procedure has an impact on the content of secondary metabolites from the naphthoquinone class. Such an increase was observed for Di. muscipula after elicitation with Cronobacter sakazaki lysate [74] as well as for Dr. capensis and Di. muscipula subjected to Agrobacterim rhizogenes lysate, jasmonic acid, nitrogen deficiency, L-phenylalanine, and trans-cinnamic acid [69]. Furthermore, the antibacterial potential of D. muscipula against S. aureus, E. faecalis, E. coli, and P. aeruginosa increased significantly after the genetic transformation of tissues using Rhizobium rhizogenes strains [70].
The antibacterial and antifungal activity of carnivorous plants is considered to be mainly related to the presence of naphthoquinones, such as plumbagin and its isomer ramentaceone; however, the other components of the plants act synergistically and enhance their effectiveness. It has been demonstrated that naphthoquinones are effective inhibitors of nucleic acid synthesis in pathogen cells. Moreover, plumbagin may induce apoptosis by inactivating topoisomerase II through the generation of free radicals [47,75]. In turn, flavonoids exhibit anti-biofilm activity, which has great significance in pathogen infection [52], and some flavonoids isolated from carnivorous plants have significant anti-pathogen activity as well [56]; therefore, the antimicrobial activity is a result of the synergistic action of various components.
It should also be mentioned that the proteins found in pitcher fluid also exhibit a significant antibacterial/antifungal effect. They are pathogenesis-related proteins protecting carnivorous plants during the digestion of prey [76,77,78,79].
3.2. Cytotoxic Activity
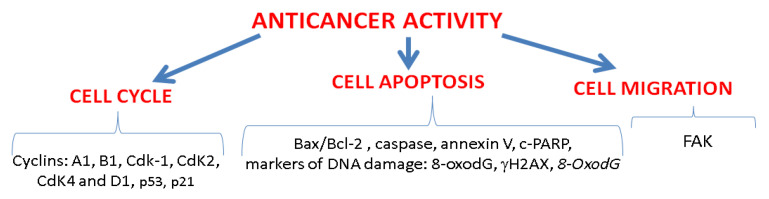
Anticancer activity is another explored direction of action of Droseraceae and Nepenthaceae plants, and some reports have been published on this topic. The investigations were based on both in vitro studies on cell lines and in vivo assays using animal models. Basic investigations included cytotoxicity assays based on cellular metabolic activity tests (MTT—mitochondrial dehydrogenase activity, LDH—lactate dehydrogenase activity) or permeability of cell membrane tests (TB—trypan blue). Additionally, some molecular mechanisms associated with anticancer activity have also been studied, focusing on (i) the cell cycle (cell cycle regulatory proteins: cyclin A1, cyclin B1, and Cdk-1), (ii) the apoptotic pathway (Bax/Bcl-2 ratio, caspase, annexin V, markers of DNA damage: 8-Hydroxy-2-Deoxyguanosine, γH2AX) and (iii) cell migration (FAK protein—focal adhesion kinase) (Figure 3). All these mechanisms are of great significance in the development of anticancer therapies [80].
Figure 3.
Main molecular mechanisms and the investigated parameters associated with anticancer activity studied in carnivorous plants from Nepenthales.
Ethanol and aqueous extracts from whole Dr. indica plants showed cytotoxicity against Dalton’s Ascitic Lymphoma (DAL) and Ehrlich Ascitic Carcinoma (EAC) cell lines and remarkably increased the percentage of dead cancer cells at 250 µg/mL (90% and 86%, respectively, in DAL cells and 89% and 80%, respectively, in EAC) [81]. Furthermore, the activity was confirmed in an in vivo study in a murine model with induced Dalton’s lymphoma ascites (DLA). After a 14-day treatment with the extracts (250 and 500 mg/kg orally), normalization of body weight, viable tumor cells, and hematological parameters were observed, which were altered in the untreated DLA group. This was accompanied by increased caspase-3 activity and decreased DNA, RNA, and protein content in the treated group, suggesting an increase in apoptosis [82]. Similar effects were exerted by Dr. burmannii extracts in an in vivo study in mice with induced EAC [83]. Furthermore, Dr. peltata and Dr. indica had a modulatory effect on the development of metabolic syndrome in tumor-bearing mice and stabilized the tumor-induced serum hormones, blood glucose, and lipid profile [84,85]. In turn, in an in vitro study, hexane, ethyl acetate, and 1-butanol fractions were obtained by fractionation of extract (10% MeOH/water) from fresh leaves of Di. muscipula and were found to be cytotoxic against P388 murine lymphocytic leukemia [86].
Dr. burmannii methanol/water (7:3) extract was cytotoxic against breast cancer (MCF-7) with IC50 120.9 µg/mL; however, it had no impact on the other cancer lines tested, i.e., lung (A549), cervical (HeLa), liver (HepG2), and brain (U87) cells (IC50 in the range of 1062–1577 µg/mL) and on the control normal fibroblast cell line (WI-38 IC50 1389 µg/mL). It downregulated the expression of cell cycle regulatory proteins (cyclin A1, B1, and cyclin-dependent kinase Cdk-1) and arrested MCF-7 cells in the G2/M phase but did not affect the G1/S regulatory proteins CdK2, CdK4, and D1. Furthermore, it activated tumor-suppressor proteins p53 and cyclin-dependent kinase inhibitor p21 [87]. p53-p21-RB signaling is an important cell cycle regulator, and p53 can induce cell cycle arrest, as it downregulates many cell cycle genes [88]. It was also evidenced that Dr. burmannii extract induced apoptosis through the disturbance of the Bax (apoptotic)/Bcl-2 (anti-apoptotic) protein ratio and the activation of the caspase cascade [87]. Bax/Bak are pore-forming proteins at the mitochondrial outer membrane, and their expression leads to an increase in the permeability of the mitochondrial membrane and the release of cytochrome c to the cytosol, where it binds to the cytosolic proteins Apaf-1 and procaspase-9 and forms an apoptosome. This is followed by the activation of caspases responsible for the proteolytic degradation of cellular structures and, thus, the induction of apoptosis [89,90]. The degradation of poly (ADP-ribose)polymerase (PARP) to its cleaved form (c-PARP) was also observed [87]. PARP is involved in various cellular processes, such as DNA repair, cell division, differentiation, and programmed cell death [91].
Further detailed insight into the mechanism of anticancer action was provided by investigation of a few Nepenthes species. In an in vitro study, it was found that an ethyl acetate fraction of methanol extract from N. thorellii x (ventricosa x maxima) exerted an anti-proliferative effect against breast cancer (MCF7 and SKBR3) [92] and oral cancer (Ca9-22, CAL 27, OECM-1, and HSC-3) [93] and exhibited antileukemic activity against three leukemia cell lines, including acute promyelocytic HL-60, chronic myelogenous K-562, and T-cell acute lymphocytic MOLT-4 (IC50 in the range of 3.68–3.85 µg/mL in a 24-h assay) [94]. It was established that the mechanism of action was associated with the induction of oxidative stress, as an increase in the ROS level and mitochondrial superoxide (MitoSOX), mitochondrial membrane depolarization, and enhanced mRNA expression of genes involved in the modulation of oxidative stress, i.e., nuclear factor erythroid 2-like 2 (NFE2L2), catalase (CAT), thioredoxin (TXN), heme oxygenase 1 (HMOX1), and NAD(P)H quinone dehydrogenase 1 (NQO1), were observed after treatment with Nepenthes extract. As a result of oxidative stress, increased apoptosis, and DNA damage were noted, which was evidenced by an increase in caspases 3/7, γH2AX, and 8-hydroxy-2-deoxyguanosine markers of DNA damage, and expression of c-PARP and cleaved caspase-3 (c-Cas 3) [92,93,94].
A similar mechanism was reported for the ethyl acetate fractions of methanol extract from N. adrianii x clipeata, N. ventricosa x maxima, and N. ventricosa x sibuyanensis. In an in vitro study, they exhibited cytotoxicity against various types of oral cancer (Ca9-22, CAL 27, OECM-1, SCC9, and HSC-3) through induction of oxidative stress-mediated apoptosis and DNA damage [95,96,97]. Furthermore, it was established that the cytotoxic effect was enhanced in a combination of the extract with ultraviolet-C (UVC) irradiation. The proliferation of Ca9-22 cells was decreased to 57% compared to the individual treatments with the extract (5 µg/mL) and a low dose of UVC (12 J/m2) (80%). No cytotoxicity against normal oral HGF-1 cells was observed [98].
Only one study was devoted to the anticancer activity of Drosophyllaceae plants. Water, methanol, and hexane leaf extracts of Drosophyllum lusitanicum were studied in terms of their anti-proliferative, cytotoxic, and apoptogenic properties against human cervical adenocarcinoma [99]. It has been shown that the hexane extract was the most active, followed by the methanol extract, and induced cell cycle arrest in the G2/M phase and apoptosis [99]. On the other hand, there are no reports on direct anticancer activity of Triphyophyllum peltatum; however, Bringmann et al. found that some dioncoquinones (which belong to naphthylisoquinoline alkaloids) occurring in stress-induced plant cell cultures showed antitumor activity against lymphoma (DOHH-2 and SU-DHL-4) and multiple myeloma (INA-6) in an in vitro study [100].
A few studies have shown that carnivorous plant extracts can enhance the activity of known anticancer compounds. N. cv. Miranda-leaf-acetone combined with 5-fluorouracil synergistically increased apoptosis of pulmonary adenocarcinoma (PC-9), carcinoma (4T1), and murine melanoma (B16F10) cells [62], and a combination of ethyl acetate extract from N. ventricosa x maxima with cisplatin can enhance the cytotoxicity against oral cancer cells (Ca9-22) [96].
It is worth mentioning that the extracts and isolated compounds were less or non-toxic against normal cell lines used as reference. Detailed information on the cytotoxic activity of Droseraceae and Nepenthaceae is summarized in Table 3.
Table 3.
Cytotoxic activity of Droseraceae and Nepenthaceae extracts.
| Species/Extract | Model/Reference Line | Effect | Ref |
|---|---|---|---|
|
Dr. indica/water (w), ethanol (e) |
Dalton’s Ascitic Lymphoma (DAL), Ehrlich Ascitic Carcinoma (EAC) | 250 µg/mL ↑ dead cells ca 90% (e), 86% (w) 250 µg/mL ↑ dead cells ca 89% (e), 80% (w) |
[81] |
| Dr. indica/water, ethanol | Mice with induced Dalton’s lymphoma ascites (DLA) | 14-day treatment (250, 500 mg/kg orally): normalization of body weight and blood parameters, ↓ viable tumor cells, ↑ 3-caspase, ↓ DNA, ↓ RNA, ↓ total protein |
[82] |
| Dr. burmannii/water, ethanol | Mice with induced Ehrlich Ascitic Carcinoma (EAC) | 14-day treatment (250, 500 mg/kg orally): normalization of body weight and blood parameters, ↓ viable tumor cells |
[83] |
| Dr. burmannii/70% methanol | breast cancer (MCF-7)/normal fibroblast cell (WI-38) | ↓ viability: IC50 120.9 µg/mL 200 μg/mL: arrested cells in the G2/M phase (↓ cyclins A1, B2, CdK1; ↑ p21, ↑p53) ↑ apoptosis (↑ annexin V, ↑ Bax/Bcl-2, ↑ caspases 9 and 3, ↓ PARP) |
[87] |
| Di. muscipula/fractions from 10% methanolic extract | murine lymphocytic leukemia (P388) | IC50: 0.01 mg/mL hexane, 6.3 mg/mL ethyl acetate, 15.8 mg/mL butanol fraction |
[86] |
| Drosophyllum lusitanicum/hexane (h), methanol (m), water (w) | human cervical adenocarcinoma (HeLa) | IC50 = 2.14 μg/mL (h); IC50 = 50.98 μg/mL (m); IC50 = 719.53 μg/mL (w) ↑ apoptosis (↑ subG1) |
[99] |
| N. cv. Miranda/acetone | human pulmonary adenocarcinoma (PC-9), 4T1 mammary carcinoma, B16F10 melanoma | 150 µg/mL: 98% death rates inhibition of cell migration |
[62] |
|
N. adrianii x clipeata/ethyl acetate fraction from methanol extract |
oral cancer (CAL 27, OECM-1, Ca9-22, HSC-3, SCC9)/normal oral cells (HGF-1) | ↓ viability: IC50: 8, 11, 12, 14, 17 μg/mL ↑ ROS: ↑ MitoSOX, depletion of MMP ↑ apoptosis (↑ annexin V, ↑ c-PARP) ↑ DNA damage (↑ γH2AX) |
[95] |
|
N. ventricosa x maxima/ethyl acetate fraction from methanol extract |
oral cancer (Ca9-22, CAL 27)/normal oral cells (HGF-1) | ↓ viability: IC50: 11, 12 μg/mL ↑ apoptosis (↑ subG1, ↑ annexin V, ↑ pancaspase) ↑ ROS: ↑MitoSOX, depletion of MMP ↑ DNA damage (↑ 8-oxodG) |
[96] |
| N. ventricosa x sibuyanensis/ethyl acetate fraction from methanol extract | oral cancer (Ca9-22, CAL 27, SCC9)/normal oral cells (HGF-1) | ↓ viability: IC50: 25, 20, 32 μg/mL ↑ apoptosis (↑ subG1, ↑ annexin V, ↑ pancaspase) ↑ ROS: ↑ MitoSOX, depletion of MMP ↑ DNA damage (↑ 8-oxodG) |
[97] |
|
N. thorellii x (ventricosa x maxima)/ethyl acetate fraction from methanol extract |
human breast cancer (MCF7 and SKBR3)/human breast normal cells (M10) | ↓ viability: IC50 10 and 15 μg/mL ↑ apoptosis (↑ subG1, ↑ annexin V) ↑ ROS: ↑ GSH, ↑ MitoSOX, depletion of MMP DNA damage (↑ γH2AX, ↑ 8-OxodG) |
[92] |
|
N. thorellii x (ventricosa x maxima)/ethyl acetate fraction from methanol extract |
oral cancer (Ca9-22, CAL 27, OECM-1, HSC-3)/normal oral cells (HGF-1) | ↓ viability: IC50 9.27, 11.05, 13.2, 24 μg/mL ↑ apoptosis (↑ subG1, ↑ annexin V, ↑ pancaspase) ↑ ROS: ↑ MitoSOX, depeletion of MMP ↑ expression of antioxidant genes DNA damage (↑ γH2AX, ↑ 8-OHdG) |
[93] |
|
N. thorellii x (ventricosa x maxima)/ethyl acetate fraction from methanol extract |
leukemia cell lines (HL-60, K-562, MOLT-4) | ↓ viability: IC50 3.85, 3.68, 3.73 μg/mL ↑ apoptosis (↑ subG1, ↑ annexin V, ↑ caspases 3/7) ↑ ROS: ↑ MitoSOX, ↑ MMP ↑ expression of antioxidant genes DNA damage (↑ γH2AX, ↑ G8-OHdG) |
[94] |
IC50—half maximal inhibitory concentration; ROS—reactive oxygen species; GSH-glutathione; MitoSOX—mitochondrial superoxide; MMP—mitochondrial membrane potential; 8-OxodG—8-Oxo-2′-deoxyguanosine; G8-OHdG—8-hydroxy-2′-deoxyguanosine; ↓—decrease; ↑—increase.
Many reports indicate that the anticancer activity of carnivorous plants is associated with naphthoquinones, whose cytotoxicity against various types of cancer lines is well documented. In particular, the anticancer potential of plumbagin has recently been extensively studied [101,102,103,104,105,106]. It was found that plumbagin affects the various signaling pathways involved in cancer cell proliferation, survival, invasion, and metastasis through suppression of some signaling molecules such as nuclear factor-kappaB (NF-κB) and signal transducer and activator of transcription 3 (Stat3) [107,108]. In vitro, investigation showed that plumbagin generates ROS and regulates the PI3K/Akt and MAPK signaling pathways to promote apoptosis and autophagy [109]. Furthermore, in silico study revealed that plumbagin can bind to cancer signaling proteins, namely PI3Kγ, AKT1/PKBα, Bcl-2, NF-κB, and Stat3, which play a key role in the pathogenesis of cancer [110]. It also can form H-bonds with PARP [111]. Some review papers have been published to summarize the latest findings on this topic [112,113,114,115,116]. Therefore, here, we focused only on naphthoquinones isolated from Droseraceae and Nepenthaceae plants.
As reported by Kawiak et al., 3-chloroplumbagin induces apoptosis as it disturbs the ratio of apoptotic (Bax, Bak) to anti-apoptotic (Bcl-2, Mcl-1) proteins [117]. Furthermore, it has been shown that the compound affects MAP kinase signaling and reduces the levels of phosphorylated MEK and ERK induced by EGF, which increases the sensitivity of cancer cells to the induction of apoptosis. These results suggest that ChPL induces apoptosis via both MAP kinase signaling and the mitochondria-mediated pathway [117].
Similarly, it was found that the cytotoxicity of ramentaceone against human promyelocytic leukemia was mediated via triggering apoptosis through the mitochondria-mediated pathway and ROS generation, and several features characteristic for induction of the process, i.e., a decrease in the mitochondrial transmembrane potential, the Bcl-2/Bax, Bak ratio, an increase in the cytochrome c level, and the activity of caspase 3, were observed [118].
The anticancer effects of plumbagin from N. alata against MCF-7 breast cancer cells were also related to an increased intracellular ROS level resulting in the induction of apoptosis via a p53-dependent pathway (↑p53 and p21). Plumbagin arrested the cell cycle in G2/M (↓cyclin B1, no effect on cyclin A). An increase in c-PARP and Bax protein and a decrease in Bcl-2 were also noted [119]. It was also found that, through ROS induction, plumbagin inhibits topoisomerase II and stabilizes the topoisomerase II−DNA cleavable complex, thus contributing to DNA damage [75]. Moreover, plumbagin turned out to be a potent inhibitor of dihydroorotase and, therefore, may decrease the biosynthesis of pyrimidines essential for the rapid growth and proliferation of cancer cells [120]. Plumbagin also exerted an inhibitory effect on allantoinase [62] and dihydropyrimidinase [121] involved in the utilization of nitrogen in purine-derived compounds. These metal-dependent enzymes belong to the cyclic amidohydrolase family and catalyze the hydrolysis of the cyclic amide bond in the metabolism of purines and pyrimidines; therefore, they are important targets for anticancer therapy.
The anticancer activity of plumbagin was confirmed in an in vivo study using an MCF-7 tumor xenograft model developed with BALB/c nude mice. Plumbagin injected at 2 mg/kg body weight for 4 weeks markedly inhibited the increase in tumor volume and decreased tumor weight [119].
Examples of the cytotoxicity of naphthoquinones isolated from Droseraceae and Nepenthaceae species against different types of cancers are shown in Table 4.
Table 4.
Cytotoxic activity of naphthoquinones isolated from Droseraceae and Nepenthaceae.
| Compound | Species | Cytotoxicity/Reference Line | Ref |
|---|---|---|---|
| plumbagin | N. thorelii x (ventricosa x maxima)/aerial parts | leukemia cells (24 h assay) acute promyelocytic (HL-60): IC50 = 0.35 µg/mL chronic myelogenous (K-562): IC50 = 0.4 µg/mL T-cell acute lymphocytic (MOLT-4): IC50 = 0.19 µg/mL |
[94] |
| plumbagin | N. alata | human breast cancer (MCF-7): IC50 = 3.5 μM (48 h assay) human epithelial ovarian cancer (SK-OV-3): IC50 = 13.1 μM (48 h assay) |
[119] |
| plumbagin | Di. muscipula | murine lymphocytic leukemia (P388): IC50 = 0.01 µg/mL human colon carcinoma (HCT116): IC50 = 0.11 µg/mL |
[86] |
| plumbagin | Di. muscipula | human breast cancer MDA-MB-468: IC50 = 0.4 μM human breast adenocarcinoma (MCF-7): IC50 = 1.8 μM/epithelial cell line (MCF-10a) |
[117] |
| plumbagin | Di. muscipula | promyelocytic leukemia (HL-60): IC50 = 2.7 μM | [75] |
| 3-hydroxymethylplumbagin | Di. muscipula | murine lymphocytic leukemia (P388): IC50 = 0.08 µg/mL | [86] |
| hydroplumbagin ß-D-glucopyranoside | Di. muscipula | murine lymphocytic leukemia (P388): IC50 = 2.0 µg/mL | [86] |
| 3-chloroplumbagin | Di. muscipula | human breast cancer (MDA-MB-468): IC50 = 0.6 μM human breast adenocarcinoma (MCF-7): IC50 = 2 μM/epithelial cell line (MCF-10a) |
[117] |
| ramentaceone | Dr. aliciae | human leukemic monocyte lymphoma (U937): IC50 = 3.2 µM human cervical cancer (HeLa): IC50 = 51 µM human breast adenocarcinoma (MCF-7): IC50 = 17 µM human colorectal carcinoma (HCT-116): IC50 = 37 µM |
[122] |
| ramentaceone | Dr. aliciae | human promyelocytic leukemia (HL-60): IC50 = 8.75 μM | [118] |
| isoshinanolone | Di. muscipula | murine lymphocytic leukemia (P388): IC50 = 0.4 µg/mL | [86] |
| diomuscinone | Di. muscipula | murine lymphocytic leukemia (P388): IC50 = 0.06 µg/mL | [86] |
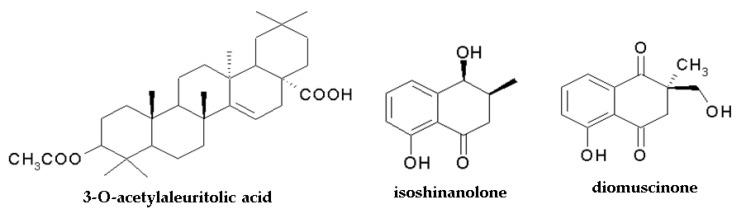
It should be mentioned that the anticancer activity of Drosera species can also be attributed to the pentacyclic triterpene 3-O-acetylaleuritolic acid (3-O-AAA), which was found in Dr. spatulata [123] and Dr. villosa [46]. The compound exerted a growth inhibitory effect on HeLa (cervical cancer), HT-29 (human colorectal adenocarcinoma), and MCF7 (human breast cancer) cell lines with IC50 2.26 μM, 3.43 μM, and 4.23 μM, respectively, after 24 h of treatment (control: non-tumorigenic breast cells IC50 = 53 μM). It moderately affected colony formation, inhibited cell migration (the scratch assay) and cell adhesion (↓FAK protein), and contributed to autophagy induction (modulation of autophagy-related proteins including mTOR and beclin1 proteins) [123].
On the other hand, the cytotoxicity of some carnivorous species may be connected with flavonoid compounds. Quercetin 3-O-(6″-n-butyl-d-glucuronide), which was one of the main active components of the fraction from N. thorellii [92], showed a cytotoxic effect against liver cancer HepG2 and breast cancer MCF7 cells [124]. Moreover, there are many reports on the anticancer properties of flavonoids [125,126,127,128], and this allows an assumption that different compounds can act synergistically.
The structures of compounds with confirmed anticancer activity found in Nepenthaceae and Droseraceae are presented in Figure 2 and Figure 4.
Figure 4.
Chemical structure of components with anticancer activity found in Nepenthales. The structure of plumbagin, 3-chloroplumbagin, and ramentaceone is shown in Figure 2.
3.3. Anti-Inflammatory Effects
Some reports describe the anti-inflammatory activity of Nepenthaceae and Droseraceae. The HET-CAM assay (hen’s eggs test–chorioallantoic membrane) revealed a strong anti-inflammatory property of ethanol and aqueous extracts from aerial parts of Dr. rotundifolia and ethanol extract from Dr. madagascariensis at the doses of 500 μg of extracts/pellet; the anti-inflammatory effect was stronger than that exerted by 50 μg of hydrocortisone/pellet (control) [129]. The anti-inflammatory activity was also noted for 70% ethanol extract from dried aerial parts of Dr. madagascariensis [130] and Dr. rotundifolia [131] based on the human neutrophil elastase activity assay, and it was found that the activity was related to flavonoids, in particular quercetin derivatives [132]. In turn, Dr. burmannii methanol/water extract (30–80 µg/mL) downregulated the NO and TNF-α level increase in RAW 264.7 (murine macrophage) cells through bacterial lipopolysaccharide (LPS)-induced inflammation by modulation of the mRNA expression of iNOS and COX-2. It also suppressed LPS-stimulated intracellular ROS production [87].
In another study, the anti-inflammatory effect of ethanol/water extract from some Drosera species on human mast cells (HMC-1) induced with T cell membrane activated by PMA (phorbol 12-myristate 13-acetate) was investigated [133]. Activation of T cell membranes (aTc-m) leads to the degranulation of mast cells and the release of inflammatory mediators, including cytokines; therefore, mast cells are involved in allergic and inflammatory reactions [134]. It was found that Dr. rotundifolia and Dr. tokaiensis at 200 µg/mL suppressed aTc-m-induced expression of TNF-α, Granzyme B (GZMB), interleukine-1 (IL1β), and intracellular adhesion molecule-1 (ICAM-1) in HMC-1 cells. In turn, no effect was observed for Dr. spatulata [133]. The analysis of the phenolic composition of the extracts revealed that hyperoside, isoquercitrin, myricetin, quercetin, and ellagic acid were the main components, with the content in the range of 2.81–4.02%, 0.40–0.71%, 0.22–0.49%, 0.41–0.53%, and 0.17–2.63%, respectively [133].
On the other hand, Thao et al., who investigated the anti-inflammatory activity of methanolic extract from N. mirabilis as well as its components in LPS-stimulated bone marrow-derived dendritic cells, found that all the isolated compounds, i.e., naphthalene derivatives, lignans, triterpenes, and flavonoids, decreased the production of pro-inflammatory cytokines IL-12p40 and IL-6; however, they had slight or no effects on TNF-α production. 2-methoxy-7-methyljuglone followed by nepenthoside B were the most potent inhibitors with IC50 0.17 (IL-12p40), 0.46 (IL-6), 8.28 (TNF-α) µM and 1.17 (IL-12 p40), 2.15 (IL-6), and 21.05 (TNF-α) µM, respectively [135].
Anti-inflammatory activity assays of Droseraceae and Nepenthaceae plants are summarized in Table 5.
Table 5.
Anti-inflammatory activity of Droseraceae and Nepenthaceae plants.
| Species/Extract | Experimental Model | Effect | Ref |
|---|---|---|---|
|
Dr. rotundifolia/water (w), 70% ethanol (e) |
human neutrophil elastase | Inhibition with IC50 5 (w), 1 (e) μg/mL | [131] |
| Dr. madagascariensis/70% ethanol | human neutrophil elastase | Inhibition with IC50 9.4 μg/mL | [130] |
| Dr. rotundifolia/water, ethanol | HET-CAM assay | 500 μg/pellet: inhibition 88% (w), 98% (e) (hydrocortisone 50 μg/pellet: 89%) | [129] |
| Dr. madagascariensis/water, ethanol | HET-CAM assay | 500 μg/pellet: inhibition 51% (w), 89% (e) (hydrocortisone 50 μg/pellet: 89%) | [129] |
| Dr. burmannii 70% methanol in water | LPS-induced murine macrophage (RAW 264.7) | 30–80 µg/mL ↓ NO, ↓ iNOS, ↓ TNF-α, ↓ COX-2 | [87] |
| Dr. rotundifolia, Dr. tokaiensis/80% ethanol in water | human mast cells (HMC-1) induced by PMA-activated T-cell membrane | 200 µg/mL suppressed morphological changes, expression of inflammatory genes, TNF-α, GZMB, IL1β, and ICAM-1 | [133] |
HET-CAM assay—hen’s eggs test—chorioallantoic membrane; LPS—lipopolysaccharide; PMA—phorbol 12-myristate 13-acetate; iNOS—inducible nitric oxide synthase; TNF-α—tumor necrosis factor-alpha, COX-2—cyclooxygenase 2; GZMB—Granzyme B; IL1β—interleukine-1; ICAM-1—intracellular adhesion molecule-1.
It is commonly believed that the anti-inflammatory effect of carnivorous plants is associated with flavonoids, which can inhibit pathological processes associated with inflammation, including proteolytic damage of tissues induced by neutrophil elastase and the release of lysosomal enzymes. They can also modulate the expression of pro-inflammatory cytokines [136,137]. For example, quercetin, isoquercitrin, and hyperoside, the main flavonoids found in Dr. rotundifolia, inhibited human neutrophil elastase with IC50 values of 0.8, 0.7, and 0.9 µg/mL, respectively, but no effect was exerted by plumbagin and junglon [131]. In turn, Fukushima et al. observed no correlation between the contents of phenolic compounds and anti-inflammatory activity [133]; therefore, further investigation is needed to clarify this issue.
3.4. Antioxidant Activity
Maintenance of the redox balance is crucial for the proper function of cells. Reactive oxygen species (ROS), normally produced as part of aerobic metabolism, play an essential role in the regulation of many physiological processes, e.g., they contribute to defense against microorganisms. However, an excessive ROS level may lead to damage to cell macromolecules, including DNA, protein, and membrane lipids, and oxidative stress is involved in aging processes and the pathogenesis of various disorders. On the other hand, increased ROS levels trigger apoptosis of cancer cells; therefore, ROS inducers have a high potential in anticancer therapy [138,139].
The antioxidant activity of Droseraceae and Nepenthaceae species was extensively investigated using in vitro (e.g., DPPH, ABTS, FRAP, ORAC, antioxidant enzyme activity) and in vivo assays. Generally, carnivorous plants contain two types of metabolites with different antioxidant effects; thus, the activity of extracts depends on the type of the extrahent used. Methanol or ethanol extracts with various water addition levels contain many polyphenolic compounds with strong antioxidant activity [53,64,74,81,140]. In turn, less polar extracts (acetone, ethyl acetate, chloroform) rich in naphthoquinones have a lower capability to scavenge ROS in in vitro assays [47,99]. Detailed analysis of the antioxidant capacity of compounds isolated from N. mirabilis (ORAC assay and reduction of Cu2+ to Cu+) showed that quercetin and kaempferol glycosides were significantly more active than naphthoquinones [141]. On the other hand, naphthoquinones stimulate ROS production and contribute to ROS-mediated cell apoptosis, which is beneficial in anticancer therapy. This was evidenced by an increased ROS level in many types of cancer cell lines (Table 2) treated with acetone or ethyl acetate extracts from N. thorelii [92,93,94], N. adrianii [95], and N. ventricosa [96,97].
It should also be noted that some extracts or plant-derived compounds exert ambiguous antioxidant effects. They exhibit activity in free radical scavenging tests, such as ABTS or DPPH; however, in assays carried out on cell line models, they strongly generate ROS. The impact of exogenous antioxidants is often concentration-dependent. They may alleviate oxidative stress at low concentrations and stimulate ROS generation at higher amounts [138].
3.5. Other Types of Activities
In addition to the most widely explored anti-pathogenic, anticancer, anti-inflammatory, and antioxidant properties mentioned above, a few reports have described other biological effects for Drosera and Nepenthes species.
3.5.1. Impact on Respiratory Tract
According to ethnomedicine, some carnivorous plants, e.g., Drosera species, are useful in the treatment of respiratory tract disorders, including chronic bronchitis, asthma, and cough. A few recent reports have verified this type of activity.
The effect of a low concentration of Dr. rotundifolia ethanolic extract containing phenolic compounds (ellagic acid, quercetin, isoquercitrin, and/or hyperoside) on the human bronchial epithelial cell line (16HBE) was evaluated. After 24 h of incubation, a ca 6.9% increase in cell viability was noted. Furthermore, the extract stimulated the cell functions by regulating the expression of dozens of genes involved in various cellular processes. For example, Dr. rotundifolia extract increased the expression of specific epidermal growth factors, which play a positive role in the regulation of cell survival, cell proliferation, and wound healing. It also enhanced the expression of proteins related to xenobiotic detoxification and cytokines; therefore, it stimulated self-repair systems impaired in respiratory tract diseases [142].
In another in vitro study, the effects on airway smooth muscle (ASM) and ciliary beat frequency (CBF) were investigated using tracheal slices of C57BL/6N mice [143]. It was found that Dr. rotundifolia extract (90% ethanol) and an aqueous fraction increased the CBF, which improved mucociliary clearance. Furthermore, the ethanol–water extract and the aqueous fraction had an antispasmodic effect against acetylcholine-induced contractions, and the water fraction abrogated potassium ions-induced contraction. Quercetin, 2″-O-galloylhyperoside, and hyperoside flavonoids were found to be responsible for an impact on smooth muscle, as they accelerated CBF, exerted an inhibitory effect on phosphodiesterases [143], enzymes involved in airway dilatation, vascular and airway smooth muscle contraction, and remodeling, and participated in the regulation of inflammation [144].
3.5.2. Anti-Osteoporotic Activity
N. mirabilis was found to be a source of polyphenols and naphthoquinones with anti-osteoporotic activity and high potential in the prevention and treatment of osteoporosis. Among the isolated compounds, pinoresiol-4-O-b-D-glucopyranoside, nepenthosides B [145], plumbagin, cis-isoshinanolone, quercetin 3-O-b-D-glucuronide, and kaempferol-3-O-a-L-rhamnoside [141] in an in vitro assay exerted the strongest inhibitory effect on the differentiation of osteoclasts, which are responsible for bone resorption and play an important role in the formation of the bone matrix. Tartrate-resistant acid phosphatase (TRAP was used) as a phenotypic marker in the study because TRAP expression is associated with the induction of osteoclast differentiation [146]. The most active compounds, namely plumbagin and kaempferol-3-O-a-L-rhamnoside, at 10.0 mM inhibited TRAP to 14.73% and 35.98%, respectively, compared to the control (RANκL-induced RAW 264.7 cells) [141].
3.5.3. Antiparasitic Activity
The antimalarial activity of chloroform extract from N. thorelii roots and isolated compounds was evaluated by Likhitwitayawuid et al. using Plasmodium falciparum [147]. The plant extract exhibited a significant inhibitory effect with IC50 10 µg/mL, and plumbagin was found to be the most active of the investigated components (IC50 = 0.27 µM); therefore, it is considered the main component responsible for the antimalarial activity of N. thorelii. Only a weak antiplasmodial effect was exhibited by the other isolated components, namely 2-methylnaphthazarin, octadecyl caffeate, isoshinanolone, and droserone (IC50 in the range of 5.79–22.06 µM).
Furthermore, hydroxydroserone isolated from Dr. magna bulbs showed anthelmintic activity against fourth-stage larvae of the blood-feeding parasitic nematode Hemonchus contortus and inhibited larval motility by 27% at 100 μM after 72 h [56].
The antimalarial activity was also reported for Triphyophyllum peltatum. Chloroform extracts from root and stem bark showed antiplasmodial activity with IC50 values of 103 and 279 ng/mL for P. berghei and 53 and 76 ng/mL for P. falciparum, respectively [148]. Presumably, the effect was related to the presence of naphthylisoquinoline alkaloids, as some of the isolated compounds, namely 5’-O-demethyldioncophylline A, dioncophylline C, dioncopeltine A, dioncopeltine A, and dioncophylline A and B, exhibited significant activity against both protozoa [148,149,150]. Interestingly, dioncophylline A also showed larvicidal activity against the mosquito Anopheles stephensi, capable of transmitting both P. falciparum and P. vivax [151] and was effective against Aedes aegypti—a known vector of several viruses, including yellow fever, dengue, chikungunya and Zika viruses [152]. On the other hand, betulinic acid, representing lupane-type triterpenes, isolated from T. peltatum also exhibited moderate to good in vitro antimalarial activity against asexual erythrocytic stages of P. falciparum [153].
It should also be mentioned that dioncoquinones found in T. peltatum showed promising antiprotozoal bioactivities against Leishmania, Trypanosoma [154], and Babesia canis [155].
3.5.4. Antiaging Activity
The antiaging effect of some Drosera species was evidenced by Tominaga et al. based on the impact of 80% MeOH extracts from aboveground parts of Dr. rotundifolia, Dr. tokaiensis, Dr. spatulata, and Dr. peltata on advanced glycation end-products (AGEs) including Nω-(carboxymethyl)arginine (CMA) and Nε-(carboxymethyl)lysine (CML). The accumulation of AGEs is correlated with aging and the development of age-related diseases, such as diabetes and arteriosclerosis. It was found that crude extracts significantly inhibited the formation of CMA and CML, and the effect was related to purified components, such as ellagic acid, 3,3′-di-O-methylellagic acid 4′-glucoside, myricitrine, and quercimelin [156].
3.5.5. Hepatoprotective Activity
70% methanolic extract from dried Dr. burmannii displayed significant hepatoprotective activity in an in vivo study in iron-overloaded Swiss albino mice, normalized the serum markers increased after hepatocellular injury, including ALAT, ASAT, ALP, and bilirubin, and enhanced the levels of liver antioxidants. The effect was a result of strong iron chelation activity [140].
3.5.6. Antiepileptic Activity
In an in vivo assay, Hema et al. found that 90% ethanolic extract from Dr. burmannii at the dose of 500 mg/kg body weight showed antiepileptic activity, delayed the onset of convulsions, and decreased the duration of seizures induced with pentylenetetrazole (PTZ) in mice administered one hour before PTZ [157].
3.5.7. Antidiabetic Effect
Methanolic leaf extract of N. bicalcarata exhibited antidiabetic activity in an in vivo study; administered orally at a dose of 300 mg/kg body weight for 6 weeks, it significantly reduced the blood glucose level in diabetic rats [64].
In turn, N. mirabilis exhibited α-glucosidase and α-amylase inhibitory activity. Both enzymes regulate blood glucose levels, and the inhibition of these enzymes can delay the digestion of carbohydrates, resulting in a decrease in the rate of glucose absorption. Therefore, the extracts have the potential for use against type 2 diabetes [158]. The effect may be associated with plumbagin because in silico study revealed that it may interact with α glucosidase, an enzyme involved in the metabolism of carbohydrates and insulin production [159].
3.5.8. Support of Celiac Disease Treatment
Proteolytic components in the N. x ventrata pitcher can support the treatment of celiac disease, i.e., a chronic autoimmune disorder triggered by gluten proteins commonly occurring in grain products. It has been shown that, due to the combined activity of prolyl endoprotease and a non-canonical aspartic protease, the fluid from pit-fall traps had a potent gluten detoxification capacity. Therefore, supplementation with enzymes derived from Nepenthes plants can help to avoid the induction of immune responses by reducing peptide size [160].
3.5.9. Antifertility Activity
An in vivo study revealed significant antifertility activity of 90% ethanol and aqueous extracts from Dr. burmannii [161]. Both extracts administered orally at a dose of 250 or 450 mg/kg body weight caused loss of implantation ability in female rats with regular estrous cycles. The alcohol extract decreased the percentage of implantation to 78.74% and 85.23%, respectively. In turn, the aqueous extract reduced the implantation to 67.22% and 83.66%. The antifertility activity may be related to the plumbagin presented in the ethanol extract [162]; however, no plumbagin was found in the water extract. Thus, further investigation is needed.
The less widely studied types of activity of carnivorous Nepenthales plants are summarized in Table 6.
Table 6.
Other types of activity of carnivorous Nepenthales plants.
| Species/Extract | Activity | Experimental Model | Effect | Ref |
|---|---|---|---|---|
| Dr. rotundifolia/ethanol | impact on respiratory tract | bronchial epithelial cell line (16HBE) | ↑ cell viability ca 6.9%, ↑ epidermal growth factors, ↑ proteins related with detoxification, ↑ cytokines |
[142] |
| Dr. rotundifolia/water (w), 70 ethanol (e) | antispasmodic | guinea-pig ileum induced with carbachol (M3 receptor) | 0.5 mg/mL depressed the response to carbachol to 86% (w) and to 64% (e) | [131] |
| Dr. madagascariensis/70% ethanol | antispasmodic | guinea-pig ileum induced with carbachol/histamine/PGF2á (M3, H1, contractile prostanoid receptors, respectively) | M3: 0.5 mg/mL depressed the response to 72%, and 1 mg/mL to 35% H1: 0.5 mg/mL depressed the response to 75% PGF2á: no effect |
[130] |
| Dr. rotundifolia/90% ethanol (e), aqueous fraction (w) | antispasmodic | tracheal slices of C57BL/6N mice | ↓ acetylcholine-induced contractions (e,w); ↓ K+ induced contraction (w) | [143] |
| Dr. rotundifolia/90% ethanol, aqueous fraction | ciliary beat frequency | tracheal slices of C57BL/6N mice | ↑ciliary beat frequency | [143] |
| Dr. rotundifolia, Dr. tokaiensis, Dr. spatulata, Dr. peltata/80% methanol | antiaging | ribose-gelatin mixture | 10,20,50 µg/mL: ↓ formation of CMA and CML | [156] |
| Dr. burmannii/90% ethanol | antiepileptic | mice with pentylenetetrazole-induced seizures | 500 mg/kg bw: delaying the onset of convulsions, shortening the duration of seizures | [157] |
| Dr. burmannii/70% methanol | hepatoprotective | iron-overloaded Swiss albino mice | ↓ ALAT, ↓ ASAT, ↓ ALP, ↓ bilirubin ↑ SOD, ↑ CAT, ↑ GST, ↑ GSH ↓ liver iron, ↓ serum ferritin ↓ lipid and protein peroxidation ↓ collagen content restoration of healthy liver |
[140] |
| Dr. burmannii/90% ethanol (e), water (w) | antifertility | female Wistar rats with normal estrus cycles | 250 and 450 mg/kg bw: ↓ implantation to 78.74% and 85.23% (e); ↓ implantation to 67.22% and 83.66% (w) |
[161] |
| N. thorelii root/chloroform | antimalarial | Plasmodium falciparum | Inhibition of growth: IC50 10 µg/mL | [147] |
| T. peltatum root (r), stem bark (s)/chloroform | antimalarial | Plasmodium falciparum | Inhibition of growth: IC50 53 ng/mL (r) IC50 76 (s) ng/mL | [148] |
| T. peltatum root (r), stem bark (s)/chloroform | antimalarial | Plasmodium berghei | Inhibition of growth: IC50 103 ng/mL (r) IC50 279 ng/mL (s) | [148] |
| N. mirabilis/methanol | anti-osteoporotic | RANκL-induced murine bone-marrow macrophages (RAW 264.7) | Inhibition of TRAP expression (↓ osteoclast differentiation) | [141] |
| N. bicalcarata/methanol | antidiabetic | diabetic rats | 300 mg/kg bw for 6 week: ↓ blood glucose level | [64] |
| N. mirabilis/ethanol (e), water (w) | antidiabetic | enzymatic activity assay |
Inhibition of α-glucosidase: IC50 = 32.7 (e), 3.3 (w) µg/mL and α –amylase: IC50 = 73.6 (e), 296.7 (w) µg/mL | [158] |
CMA—Nω-(carboxymethyl)arginine; CML—Nε-(carboxymethyl)lysine, PGF2á—prostaglandin F2á, M3—muscarinic receptors, H1—histamine receptors, TRAP—tartrate-resistant acid phosphatase; ↓ decrease; ↑ increase.
3.5.10. Activity against Plant Pathogens and Pests
Hexane extract from the leaf of N. ventricosa x maxima effectively inhibited plant pathogenic fungi, including P. capsic, R. stolonifer var. stolonifera, A. alternata, A. niger, B. oryzae, F. oxysporum, R. solani, and S. sclerotiorumand with MIC values ranging from 7.2 to 43.7 μg/mL [163].
Furthermore, pitcher and leaf tissues of N. x ventrata (hybrid of N. alata and N. ventricosa) exerted a growth inhibitory effect, and larvicidal activities against insect herbivores Spodoptera littoralis, and the action was related to naphthoquinones. It was found that the plumbagin concentration necessary for 50% growth inhibition of larvae was determined to be 226.5 µg/g diet [164]. Plumbagin isolated from Dr. muscipula leaves showed antifeedant activity against Spodoptera litura [86,165] and had significant insecticidal activity against adults of Musca domestica (LD50 20 µg/fly) and reduced their longevity, fertility, and natality [166]. Insecticidal activity against Liriomyza trifolii through contact application was also exhibited by hexane extracts from Drosophyllum lusitanicum leaves (at a concentration of 100 mg/mL, it caused 100% mortality after 1 d of treatment) [167]. Hexane and water extracts also showed toxicity against lettuce and wheat and significantly inhibited seed germination [168].
Moreover, recombinant chitinase derived from Drosera spp. showed antifungal potential and suppressed the growth of some plant pathogens, including Fusarium poae, Trichoderma viride, Alternaria solani [169] and Parastagonospora nodorum [170].
In turn, naphthylisoquinoline alkaloids, namely dioncophylline A and 5’-O-demethyl-8-O-methyl-7-epi-dioncophylline A, isolated from T. peltatum exhibited a molluscicidal effect with LD100 values of 20 and 40 ppm, respectively [171].
All these data indicate that compounds derived from carnivorous plants may be promising agents helpful in the control of plant diseases and pest management.
4. Possibility of Cultivation
Most Nepenthales plants have limited natural habitats, and some of them are strictly protected [172]. This raises the problem of obtaining plant material with sufficient biomass for phytochemical and biological investigations and for the isolation of active molecules. Therefore, alternative ways for the production of biomass are constantly being developed, e.g., field or greenhouse cultivation, in vivo propagation, or micropropagation and suspension culture [122,173,174,175,176,177,178,179,180] which may increase the possibility of large-scale applications in pharmacy and medicine.
For example, vegetative propagation was successfully applied to increase plant material of various Drosera spp. [53,122,181,182,183,184,185,186,187], Dionaea muscipula [69,188,189,190], Nepenthes spp [191,192,193] and Drosophyllum lusitanicum [177,178,194]. In this technique, explants from parent plants are cultivated in a growth medium, and 3 to 12 plants can be obtained from one explant after 6–8 weeks. In the cultivation process, various solidified and liquid media, e.g., Murashige and Skoog (MS), half-strength MS, Lindemann, Vacin and Went, Fast, etc., can be applied. The medium is usually supplemented with various growth regulators, including auxins: 2,4-dichlorophenoxyacetic acid, naphthylacetic acid, indoleacetic acid, and cytokinins: kinetin, 6-benzylaminopurine, and gibberellins. The pH of the growth medium is also an important factor affecting plant growth and development [184,185]. Obviously, there is no universal medium composition that would be optimal for in vitro propagation of different species, and optimization of experimental conditions is usually needed to obtain a high reproduction rate.
Furthermore, genetic transformation and elicitation using different growth regulators (e.g., methyl jasmonate, yeast extract, and chitosan) have been studied to increase the production of active metabolites and, in consequence, the biological potential of Droseraceae and Nepenthaceae, and it was found that carnivorous plants are susceptible to elicitation [69,74,184,193,195]. For instance, the beneficial effect on naphthoquinones content in the roots of Dr. burmanii and Dr. indica was observed for yeast extract [186,196]. Furthermore, chitosan, salicylic acid, and methyl jasmonate were found to be elicitors for plumbagin in in vitro culture of Dr. indica [196], and it was evidenced that production of plumbagin in shoots of Dr. peltata may be stimulated by 6-benzyladenine [184]. In turn, L-phenylalanine and trans-cinnamic acid enhanced the production of flavonoids (quercetin and myricetin) in Dr. capensis and Di. muscipula [69] and a combination of biotic elicitator (Cronobacter sakazakii lysate) with hydromechanical stress increased the level of polyphenolic compounds in Di. muscipula [74]. In recent years, it was also found that the color of the light source or microwave radiation used during cultivation may influence the level of metabolites in carnivorous plants [197,198,199,200].
5. Conclusions and Future Prospects
There is a growing global interest in plant-derived products containing many bioactive compounds with various biological activities and providing health benefits.
The data collected in the review clearly indicate that species from the genera Nepenthes, Drosera, and Dionaea have great biological potential, and their activity is a subject of interest for many researchers. Intensive investigation is being conducted in terms of antibacterial, antifungal, antioxidant, anti-inflammatory, and anticancer properties of extracts, isolated fractions, and pure compounds.
Among them, the greatest attention has recently been paid to cytotoxicity against different types of cancer. It has been evidenced that some Nepenthes and Drosera species show anticancer activity against lymphocytic leukemia [86,94], oral [93,95,96,97], and breast [87,92] cancer cell lines. This effect was mainly attributed to naphthoquinones, and the mechanism of action is related to increased generation of intracellular ROS resulting in apoptosis induction through MAP kinase signaling and p53-dependent and mitochondria-mediated pathways [117,118,119]. Naphthoquinones also are responsible for antibacterial, antiviral, and antifungal activities and may be of great importance in the context of the search for a new alternative to traditional antibiotics against microbial infections due to the increased prevalence of microbial resistance [54,55,56,60].
Interestingly, it has also been evidenced that the extract or isolated compounds combined with known cytostatic or antibacterial agents may enhance anticancer and bactericidal activity. Such a synergistic effect was observed, e.g., for N. cv. Miranda extract combined with 5-fluorouracil against pulmonary adenocarcinoma and murine melanoma [62], ethyl acetate extract from N. ventricosa x maxima with cisplatin against oral cancer cells [96], and 7-methyljuglone combined with antituberculous drugs against Mycobacterium tuberculosis [201,202]. In addition, the combination of Drosera spp. with silver nanoparticles (AgNPs) seems to be a promising direction for further investigation, as it increased the activity against multidrug-resistant bacterial strains, fungi, and plant pathogens [67,68,71,72]. A synergistic effect with AgNPs was also exhibited by isolated naphthoquinones, i.e., plumbagin and 3-chloroplumbagin [67,68,73].
In turn, polyphenols are related to anti-inflammatory [129,130,131,133], antiosteoporotic [141,145], antispasmodic [131,131,143], and antiaging [156] activities and to the beneficial effect on the respiratory tract [142,143].
Our paper summarizes the current state of knowledge on the biological potential of the Nepenthaceae and Droseracea families and clearly shows that detailed biochemical characterization of the species may lead to the discovery of new therapeutic agents. In addition, extracts may be an alternative to synthetic insecticides for pest and plant disease management in agriculture, as they inhibit the activity of plant pathogenic fungi and insect herbivores [163,164,165,166,169,170].
This overview can be used as a starting point for further research that should be developed and focused in the future on (i) bioactivity-guided investigations of crude plant extracts to connect the particular type of action with a specific compound or a group of metabolites, (ii) a search for new bioactive properties of carnivorous plants, and (iii) establishment of molecular mechanisms associated with the specific activity. Moreover, further investigations should be extended to include less commonly explored species. For example, still little is known of Aldrovanda and Drosophyllum, and researchers have so far focused mainly on the biogeography or morphology of these genera [7,203,204,205,206,207]. To date, only a few reports have been published on their biological activity [66] and phytochemistry [208,209,210,211,212].
Furthermore, efforts should be increased to elaborate cost-effective ways for obtaining plant material, including improvement of micropropagation protocols, genetic transformation, and in vitro cultures with different growth regulators to increase the availability of the plant material. In addition, the testing of different elicitors and cultivation conditions should be continued to enhance the production of biologically active compounds for pharmaceutical and medical purposes.
Acknowledgments
We thank the Botanical Garden of the Jagiellonian University for the opportunity to use plants from the garden collection. We would like also to thank the Center for Technology Transfer CITTRU, Jagiellonian University in Krakow for their assistance and funding for the research.
Author Contributions
Conceptualization, M.W. and B.J.P.; investigation, M.W. and B.J.P.; writing—original draft preparation, M.W., M.F., P.S. and B.J.P.; writing—review and editing, M.W., M.F. and B.J.P.; visualization, M.W. and B.J.P.; supervision, M.W. and B.J.P.; project administration, M.W. and B.J.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
In the case of B.J.P., the work was supported by the program “Excellence Initiative–Research University” at the Jagiellonian University in Kraków, Poland.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Darwin C. Insectivorous Plants. John Murray; London, UK: 1875. [Google Scholar]
- 2.Hedrich R., Fukushima K. On the Origin of Carnivory: Molecular Physiology and Evolution of Plants on an Animal Diet. Annu. Rev. Plant Biol. 2021;72:133–153. doi: 10.1146/annurev-arplant-080620-010429. [DOI] [PubMed] [Google Scholar]
- 3.Król E., Płachno B.J., Adamec L., Stolarz M., Dziubińska H., Trębacz K. Quite a Few Reasons for Calling Carnivores ‘the Most Wonderful Plants in the World’. Ann. Bot. 2012;109:47–64. doi: 10.1093/aob/mcr249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellison A.M., Adamec L., editors. Carnivorous Plants: Physiology, Ecology, and Evolution. Oxford University Press; Oxford, UK: 2018. Introduction: What Is a Carnivorous Plant. [DOI] [Google Scholar]
- 5.Pavlovič A. Photosynthesis in Carnivorous Plants: From Genes to Gas Exchange of Green Hunters. Crit. Rev. Plant Sci. 2022;41:305–320. doi: 10.1080/07352689.2022.2132710. [DOI] [Google Scholar]
- 6.Darnowski D., Bauer U., Méndez M., Horner J., Płachno B.J. Prey Selection and Specialization by Carnivorous Plants. In: Ellison A., Adamec L., editors. Carnivorous Plants: Physiology, Ecology, and Evolution. Oxford University Press; Oxford, UK: 2018. [DOI] [Google Scholar]
- 7.Poppinga S., Smaij J., Westermeier A.S., Horstmann M., Kruppert S., Tollrian R., Speck T. Prey Capture Analyses in the Carnivorous Aquatic Waterwheel Plant (Aldrovanda vesiculosa L., Droseraceae) Sci. Rep. 2019;9:18590. doi: 10.1038/s41598-019-54857-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miranda V.F.O., Silva S.R., Reut M.S., Dolsan H., Stolarczyk P., Rutishauser R., Płachno B.J. A Historical Perspective of Bladderworts (Utricularia): Traps, Carnivory and Body Architecture. Plants. 2021;10:2656. doi: 10.3390/plants10122656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson B. Adaptations to Foliar Absorption of Faeces: A Pathway in Plant Carnivory. Ann. Bot. 2005;95:757–761. doi: 10.1093/aob/mci082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke C.M., Bauer U., Lee C.C., Tuen A.A., Rembold K., Moran J.A. Tree Shrew Lavatories: A Novel Nitrogen Sequestration Strategy in a Tropical Pitcher Plant. Biol. Lett. 2009;5:632–635. doi: 10.1098/rsbl.2009.0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cross A.T., van der Ent A., Wickmann M., Skates L.M., Sumail S., Gebauer G., Robinson A. Capture of Mammal Excreta by Nepenthes Is an Effective Heterotrophic Nutrition Strategy. Ann. Bot. 2022;130:927–938. doi: 10.1093/aob/mcac134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Płachno B.J., Wołowski K., Fleischmann A., Lowrie A., Łukaszek M. Algae and Prey Associated with Traps of the Australian Carnivorous Plant Utricularia volubilis (Lentibulariaceae: Utricularia Subgenus Polypompholyx) in Natural Habitat and in Cultivation. Aust. J. Bot. 2014;62:528. doi: 10.1071/BT14176. [DOI] [Google Scholar]
- 13.Peroutka M., Adlassnig W., Volgger M., Lendl T., Url W.G., Lichtscheidl I.K. Utricularia: A Vegetarian Carnivorous Plant? Algae as Prey of Bladderwort in Oligotrophic Bogs. Plant Ecol. 2008;199:153–162. doi: 10.1007/s11258-008-9420-3. [DOI] [Google Scholar]
- 14.Adlassnig W., Peroutka M., Lendl T. Traps of Carnivorous Pitcher Plants as a Habitat: Composition of the Fluid, Biodiversity and Mutualistic Activities. Ann. Bot. 2011;107:181–194. doi: 10.1093/aob/mcq238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bittleston L.S. Commensals of Nepenthes Pitchers. In: Ellison A., Adamec L., editors. Carnivorous Plants: Physiology, Ecology, and Evolution. Oxford University Press; Oxford, UK: 2018. [DOI] [Google Scholar]
- 16.Sirová D., Borovec J., Černá B., Rejmánková E., Adamec L., Vrba J. Microbial Community Development in the Traps of Aquatic Utricularia Species. Aquat. Bot. 2009;90:129–136. doi: 10.1016/j.aquabot.2008.07.007. [DOI] [Google Scholar]
- 17.Sirová D., Borovec J., Šantrůčková H., Šantrůček J., Vrba J., Adamec L. Utricularia Carnivory Revisited: Plants Supply Photosynthetic Carbon to Traps. J. Exp. Bot. 2010;61:99–103. doi: 10.1093/jxb/erp286. [DOI] [PubMed] [Google Scholar]
- 18.Wołowski K., Płachno B.J. Algae Commensal Community in Genlisea Traps. Acta Soc. Bot. Pol. 2011;77:77–86. doi: 10.5586/asbp.2008.011. [DOI] [Google Scholar]
- 19.Płachno B.J., Łukaszek M., Wołowski K., Adamec L., Stolarczyk P. Aging of Utricularia Traps and Variability of Microorganisms Associated with That Microhabitat. Aquat. Bot. 2012;97:44–48. doi: 10.1016/j.aquabot.2011.11.003. [DOI] [Google Scholar]
- 20.Sirová D., Bárta J., Borovec J., Vrba J. The Utricularia-Associated Microbiome: Composition, Function, and Ecology. In: Ellison A., Adamec L., editors. Carnivorous Plants: Physiology, Ecology, and Evolution. Oxford University Press; Oxford, UK: 2018. [DOI] [Google Scholar]
- 21.Miclea I. Secondary Metabolites with Biomedical Applications from Plants of the Sarraceniaceae Family. Int. J. Mol. Sci. 2022;23:9877. doi: 10.3390/ijms23179877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wójciak M., Feldo M., Stolarczyk P., Płachno B.J. Carnivorous Plants from Nepenthaceae and Droseraceae as a Source of Secondary Metabolites. Molecules. 2023;28:2155. doi: 10.3390/molecules28052155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosli M.A.F., Mediani A., Azizan K.A., Baharum S.N., Goh H.H. UPLC-TOF-MS/MS-Based Metabolomics Analysis Reveals Species-Specific Metabolite Compositions in Pitchers of Nepenthes ampullaria, Nepenthes rafflesiana, and Their Hybrid Nepenthes × Hookeriana. Front. Plant Sci. 2021;12:655004. doi: 10.3389/fpls.2021.655004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egan P.A., van der Kooy F. Phytochemistry of the Carnivorous Sundew Genus Drosera (Droseraceae)—Future Perspectives and Ethnopharmacological Relevance. Chem. Biodivers. 2013;10:1774–1790. doi: 10.1002/cbdv.201200359. [DOI] [PubMed] [Google Scholar]
- 25.Hatcher C.R., Sommer U., Heaney L.M., Millett J. Metabolomic Analysis Reveals Reliance on Secondary Plant Metabolites to Facilitate Carnivory in the Cape Sundew, Drosera capensis. Ann. Bot. 2021;128:301–314. doi: 10.1093/aob/mcab065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kováčik J., Klejdus B., Repčáková K. Phenolic Metabolites in Carnivorous Plants: Inter-Specific Comparison and Physiological Studies. Plant Physiol. Biochem. 2012;52:21–27. doi: 10.1016/j.plaphy.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Hatcher C.R., Ryves D.B., Millett J. The Function of Secondary Metabolites in Plant Carnivory. Ann. Bot. 2020;125:399–411. doi: 10.1093/aob/mcz191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanusi S.B., Abu Bakar M.F., Mohamed M., Sabran S.F., Mainasara M.M. Ethnobotanical, phytochemical, and pharmacological properties of Nepenthes species: A review. Asian J. Pharm. Clin. Res. 2017;10:16. doi: 10.22159/ajpcr.2017.v10i11.20050. [DOI] [Google Scholar]
- 29.Mithöfer A. Carnivorous Pitcher Plants: Insights in an Old Topic. Phytochemistry. 2011;72:1678–1682. doi: 10.1016/j.phytochem.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 30.Gaascht F., Dicato M., Diederich M. Venus Flytrap (Dionaea muscipula Solander Ex Ellis) Contains Powerful Compounds That Prevent and Cure Cancer. Front. Oncol. 2013;3:202. doi: 10.3389/fonc.2013.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fleischmann A., Schlauer J., Smith S.A., Givnish T.J. Evolution of Carnivory in Angiosperms. Volume 1. Oxford University Press; Oxford, UK: 2018. [DOI] [Google Scholar]
- 32.Schlauer J. New Data Relating to the Evolution and Phylogeny of Some Carnivorous Plant Families. Carnivorous. Plant Newsl. 1997;26:34–38. doi: 10.55360/cpn262.js506. [DOI] [Google Scholar]
- 33.Krueger T., Robinson A., Bourke G., Fleischmann A. Small Leaves, Big Diversity: Citizen Science and Taxonomic Revision Triples Species Number in the Carnivorous Drosera microphylla Complex (D. Section Ergaleium, Droseraceae) Biology. 2023;12:141. doi: 10.3390/biology12010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fleischmann A., Cross A.T., Gibson R., Gonella P.M., Dixon K.W. Systematics and Evolution of Droseraceae. In: Ellison A., Adamec L., editors. Carnivorous Plants: Physiology, Ecology, and Evolution. Oxford University Press; Oxford, UK: 2018. pp. 45–47. [Google Scholar]
- 35.Juniper B.E., Robins R.J., Joel D.M. The Carnivorous Plants. Academic Press; London, UK: 1989. [Google Scholar]
- 36.Adamec L. Biological Flora of Central Europe: Aldrovanda vesiculosa L. Perspect. Plant Ecol. Evol. Syst. 2018;35:8–21. doi: 10.1016/j.ppees.2018.10.001. [DOI] [Google Scholar]
- 37.IUCN . Aldrovanda Vesiculosa. In: Cross A., Adamec L., editors. The IUCN Red List of Threatened Species 2020. IUCN; Fontainebleau, France: 2019. E.T162346A83998419. [DOI] [Google Scholar]
- 38.Poppinga S., Joyeux M. Different Mechanics of Snap-Trapping in the Two Closely Related Carnivorous Plants Dionaea muscipula and Aldrovanda vesiculosa. Phys. Rev. E. 2011;84:041928. doi: 10.1103/PhysRevE.84.041928. [DOI] [PubMed] [Google Scholar]
- 39.Clarke C., Schlauer J., Moran J., Robinson A. Systematics and Evolution of Nepenthes. In: Ellison A., Adamec L., editors. Carnivorous Plants: Physiology, Ecology, and Evolution. Oxford University Press; Oxford, UK: 2018. [DOI] [Google Scholar]
- 40.Dančák M., Majeský Ľ., Čermák V., Golos M.R., Płachno B.J., Tjiasmanto W. First Record of Functional Underground Traps in a Pitcher Plant: Nepenthes pudica (Nepenthaceae), a New Species from North Kalimantan, Borneo. PhytoKeys. 2022;201:77–97. doi: 10.3897/phytokeys.201.82872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clarke C., Moran J.A. Climate, Soils and Vicariance—Their Roles in Shaping the Diversity and Distribution of Nepenthes in Southeast Asia. Plant Soil. 2016;403:37–51. doi: 10.1007/s11104-015-2696-x. [DOI] [Google Scholar]
- 42.Martín-Rodríguez I., Vargas P., Ojeda F., Fernández-Mazuecos M. An Enigmatic Carnivorous Plant: Ancient Divergence of Drosophyllaceae but Recent Differentiation of Drosophyllum lusitanicum across the Strait of Gibraltar. Syst. Biodivers. 2020;18:525–537. doi: 10.1080/14772000.2020.1771467. [DOI] [Google Scholar]
- 43.Adlassnig W., Peroutka M., Eder G., Pois W., Lichtscheidl I.K. Ecophysiological Observations on Drosophyllum lusitanicum. Ecol. Res. 2006;21:255–262. doi: 10.1007/s11284-005-0116-z. [DOI] [Google Scholar]
- 44.Correia E., Freitas H. Drosophyllum lusitanicum, an Endangered West Mediterranean Endemic Carnivorous Plant: Threats and Its Ability to Control Available Resources. Bot. J. Linn. Soc. 2002;140:383–390. doi: 10.1046/j.1095-8339.2002.00108.x. [DOI] [Google Scholar]
- 45.Green S., Green T.L., Heslop-Harrison Y. Seasonal Heterophylly and Leaf Gland Features in Triphyophyllum (Dioncophyllaceae), a New Carnivorous Plant Genus. Bot. J. Linn. Soc. 1979;78:99–116. doi: 10.1111/j.1095-8339.1979.tb02188.x. [DOI] [Google Scholar]
- 46.Ferreira D.T., Andrei C.C., Saridakis H.O., de Jesus Faria T., Vinhato E., Carvalho K.E., Daniel J.F.S., Machado S.L., Saridakis D.P., Braz-Filho R. Antimicrobial Activity and Chemical Investigation of Brazilian Drosera. Mem. Inst. Oswaldo Cruz. 2004;99:753–755. doi: 10.1590/S0074-02762004000700016. [DOI] [PubMed] [Google Scholar]
- 47.Krolicka A., Szpitter A., Maciag M., Biskup E., Gilgenast E., Romanik G., Kaminski M., Wegrzyn G., Lojkowska E. Antibacterial and Antioxidant Activity of the Secondary Metabolites from in Vitro Cultures of Drosera aliciae. Biotechnol. Appl. Biochem. 2008;53:175–184. doi: 10.1042/BA20080088. [DOI] [PubMed] [Google Scholar]
- 48.Epand R.M., Walker C., Epand R.F., Magarvey N.A. Molecular Mechanisms of Membrane Targeting Antibiotics. Biochim. Biophys. Acta BBA Biomembr. 2016;1858:980–987. doi: 10.1016/j.bbamem.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 49.Ogihara H., Endou F., Furukawa S., Matsufuji H., Suzuki K., Anzai H. Antimicrobial Activity of the Carnivorous Plant Dionaea muscipula Against Food-Related Pathogenic and Putrefactive Bacteria. Biocontrol. Sci. 2013;18:151–155. doi: 10.4265/bio.18.151. [DOI] [PubMed] [Google Scholar]
- 50.Didry N., Dubreuil L., Trotin F., Pinkas M. Antimicrobial Activity of Aerial Parts of Drosera peltata Smith on Oral Bacteria. J. Ethnopharmacol. 1998;60:91–96. doi: 10.1016/S0378-8741(97)00129-3. [DOI] [PubMed] [Google Scholar]
- 51.Ďurechová D., Kačániová M., Terentjeva M., Petrová J., Hleba L., Kata I. Antibacterial activity of Drosera rotundifolia L. against gram-positive and gram-negative bacteria. J. Microbiol. Biotechnol. Food Sci. 2016;5:20–22. doi: 10.15414/jmbfs.2016.5.special1.20-22. [DOI] [Google Scholar]
- 52.Gerschler S., Guenther S., Schulze C. Antibiofilm Activity of Sundew Species against Multidrug-Resistant Escherichia coli Strains. Int. J. Mol. Sci. 2022;23:13720. doi: 10.3390/ijms232213720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tienaho J., Reshamwala D., Karonen M., Silvan N., Korpela L., Marjomäki V., Sarjala T. Field-Grown and In Vitro Propagated Round-Leaved Sundew (Drosera rotundifolia L.) Show Differences in Metabolic Profiles and Biological Activities. Molecules. 2021;26:3581. doi: 10.3390/molecules26123581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tian J., Chen Y., Ma B., He J., Tong J., Wang Y. Drosera peltata Smith var. lunata (Buch.-Ham.) C. B. Clarke as a Feasible Source of Plumbagin: Phytochemical Analysis and Antifungal Activity Assay. World J. Microbiol. Biotechnol. 2014;30:737–745. doi: 10.1007/s11274-013-1495-x. [DOI] [PubMed] [Google Scholar]
- 55.Grevenstuk T., Gonçalves S., Domingos T., Quintas C., van der Hooft J.J., Vervoort J., Romano A. Inhibitory Activity of Plumbagin Produced by Drosera intermedia on Food Spoilage Fungi. J. Sci. Food Agric. 2012;92:1638–1642. doi: 10.1002/jsfa.5522. [DOI] [PubMed] [Google Scholar]
- 56.Norman E.O., Tuohey H., Pizzi D., Saidah M., Bell R., Brkljača R., White J.M., Gasser R.B., Taki A.C., Urban S. Phytochemical Profiling and Biological Activity of the Australian Carnivorous Plant, Drosera magna. J. Nat. Prod. 2021;84:964–971. doi: 10.1021/acs.jnatprod.0c00869. [DOI] [PubMed] [Google Scholar]
- 57.Dileep K., Tulasi C.D.S.L.N., Ramakrishnaiah g. Phytochemical screening and evaluation of in-vitro antimicrobial activity of Drosera spatulata var bakoensis an indigenous carnivorous plant against respiratory tract infectious microbes. Asian J. Pharm. Clin. Res. 2016;9:274. doi: 10.22159/ajpcr.2016.v9i6.14382. [DOI] [Google Scholar]
- 58.Slobodníková L., Fialová S., Rendeková K., Kováč J., Mučaji P. Antibiofilm Activity of Plant Polyphenols. Molecules. 2016;21:1717. doi: 10.3390/molecules21121717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rodzali N.N., Mydin M.M. Antibacterial Activity of Leaves and Pitchers Extract of Nepenthes gracilis against Bacillus subtilis and Escherichia coli. J. Fundam. Appl. Sci. 2018;9((Suppl. S6)):81. doi: 10.4314/jfas.v9i6s.7. [DOI] [Google Scholar]
- 60.Gwee P.S., Khoo K.S., Ong H.C., Sit N.W. Bioactivity-Guided Isolation and Structural Characterization of the Antifungal Compound, Plumbagin, from Nepenthes gracilis. Pharm. Biol. 2014;52:1526–1531. doi: 10.3109/13880209.2014.902083. [DOI] [PubMed] [Google Scholar]
- 61.Lien Y. Pharmacological Activities of Nepenthes Miranda Extracts. FASEB J. 2019;33((Suppl. S1)):471.12. doi: 10.1096/fasebj.2019.33.1_supplement.471.12. [DOI] [Google Scholar]
- 62.Lin E.-S., Huang C.-Y. Cytotoxic Activities and the Allantoinase Inhibitory Effect of the Leaf Extract of the Carnivorous Pitcher Plant Nepenthes Miranda. Plants. 2022;11:2265. doi: 10.3390/plants11172265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wiart C., Mogana S., Khalifah S., Mahan M., Ismail S., Buckle M., Narayana A.K., Sulaiman M. Antimicrobial Screening of Plants Used for Traditional Medicine in the State of Perak, Peninsular Malaysia. Fitoterapia. 2004;75:68–73. doi: 10.1016/j.fitote.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 64.Ismail N.A., Kamariah A.S., Lim L.B.L., Ahmad N. Phytochemical and Pharmacological Evaluation of Methanolic Extracts of the Leaves of Nepenthes bicalcarata Hook. F. Int. J. Pharmacogn. Phytochem. Res. 2015;7:1127–1138. [Google Scholar]
- 65.Eilenberg H., Pnini-Cohen S., Rahamim Y., Sionov E., Segal E., Carmeli S., Zilberstein A. Induced Production of Antifungal Naphthoquinones in the Pitchers of the Carnivorous Plant Nepenthes khasiana. J. Exp. Bot. 2010;61:911–922. doi: 10.1093/jxb/erp359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gonçalves S., Quintas C., Gaspar M.N., Nogueira J.M.F., Romano A. Antimicrobial Activity of Drosophyllum lusitanicum, an Endemic Mediterranean Insectivorous Plant. Nat. Prod. Res. 2009;23:219–229. doi: 10.1080/14786410801972870. [DOI] [PubMed] [Google Scholar]
- 67.Krychowiak-Maśnicka M., Krauze-Baranowska M., Godlewska S., Kaczyński Z., Bielicka-Giełdoń A., Grzegorczyk N., Narajczyk M., Frackowiak J.E., Krolicka A. Potential of Silver Nanoparticles in Overcoming the Intrinsic Resistance of Pseudomonas Aeruginosa to Secondary Metabolites from Carnivorous Plants. Int. J. Mol. Sci. 2021;22:4849. doi: 10.3390/ijms22094849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krychowiak M., Grinholc M., Banasiuk R., Krauze-Baranowska M., Głód D., Kawiak A., Królicka A. Combination of Silver Nanoparticles and Drosera binata Extract as a Possible Alternative for Antibiotic Treatment of Burn Wound Infections Caused by Resistant Staphylococcus aureus. PLoS ONE. 2014;9:e115727. doi: 10.1371/journal.pone.0115727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krolicka A., Szpitter A., Gilgenast E., Romanik G., Kaminski M., Lojkowska E. Stimulation of Antibacterial Naphthoquinones and Flavonoids Accumulation in Carnivorous Plants Grown in Vitro by Addition of Elicitors. Enzym. Microb. Technol. 2008;42:216–221. doi: 10.1016/j.enzmictec.2007.09.011. [DOI] [Google Scholar]
- 70.Makowski W., Królicka A., Nowicka A., Zwyrtková J., Tokarz B., Pecinka A., Banasiuk R., Tokarz K.M. Transformed Tissue of Dionaea muscipula J. Ellis as a Source of Biologically Active Phenolic Compounds with Bactericidal Properties. Appl. Microbiol. Biotechnol. 2021;105:1215–1226. doi: 10.1007/s00253-021-11101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Banasiuk R., Krychowiak M., Swigon D., Tomaszewicz W., Michalak A., Chylewska A., Ziabka M., Lapinski M., Koscielska B., Narajczyk M., et al. Carnivorous Plants Used for Green Synthesis of Silver Nanoparticles with Broad-Spectrum Antimicrobial Activity. Arab. J. Chem. 2020;13:1415–1428. doi: 10.1016/j.arabjc.2017.11.013. [DOI] [Google Scholar]
- 72.Gaddam S.A., Kotakadi V.S., Subramanyam G.K., Penchalaneni J., Challagundla V.N., Dvr S.G., Pasupuleti V.R. Multifaceted Phytogenic Silver Nanoparticles by an Insectivorous Plant Drosera spatulata Labill var. bakoensis and Its Potential Therapeutic Applications. Sci. Rep. 2021;11:21969. doi: 10.1038/s41598-021-01281-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krychowiak M., Kawiak A., Narajczyk M., Borowik A., Królicka A. Silver Nanoparticles Combined with Naphthoquinones as an Effective Synergistic Strategy Against Staphylococcus aureus. Front. Pharmacol. 2018;9:816. doi: 10.3389/fphar.2018.00816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Makowski W., Tokarz K.M., Tokarz B., Banasiuk R., Witek K., Królicka A. Elicitation-Based Method for Increasing the Production of Antioxidant and Bactericidal Phenolic Compounds in Dionaea muscipula J. Ellis Tissue. Molecules. 2020;25:1794. doi: 10.3390/molecules25081794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kawiak A., Piosik J., Stasilojc G., Gwizdek-Wisniewska A., Marczak L., Stobiecki M., Bigda J., Lojkowska E. Induction of Apoptosis by Plumbagin through Reactive Oxygen Species-Mediated Inhibition of Topoisomerase II. Toxicol. Appl. Pharmacol. 2007;223:267–276. doi: 10.1016/j.taap.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 76.Buch F., Rott M., Rottloff S., Paetz C., Hilke I., Raessler M., Mithöfer A. Secreted Pitfall-Trap Fluid of Carnivorous Nepenthes Plants Is Unsuitable for Microbial Growth. Ann. Bot. 2013;111:375–383. doi: 10.1093/aob/mcs287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hatano N., Hamada T. Proteomic Analysis of Secreted Protein Induced by a Component of Prey in Pitcher Fluid of the Carnivorous Plant Nepenthes alata. J. Proteom. 2012;75:4844–4852. doi: 10.1016/j.jprot.2012.05.048. [DOI] [PubMed] [Google Scholar]
- 78.Rajninec M., Fratrikova M., Boszoradova E., Jopcik M., Bauer M., Libantova J. Basic β-1,3-Glucanase from Drosera binata Exhibits Antifungal Potential in Transgenic Tobacco Plants. Plants. 2021;10:1747. doi: 10.3390/plants10081747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sprague-Piercy M.A., Bierma J.C., Crosby M.G., Carpenter B.P., Takahashi G.R., Paulino J., Hung I., Zhang R., Kelly J.E., Kozlyuk N., et al. The Droserasin 1 PSI: A Membrane-Interacting Antimicrobial Peptide from the Carnivorous Plant Drosera capensis. Biomolecules. 2020;10:1069. doi: 10.3390/biom10071069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alimbetov D., Askarova S., Umbayev B., Davis T., Kipling D. Pharmacological Targeting of Cell Cycle, Apoptotic and Cell Adhesion Signaling Pathways Implicated in Chemoresistance of Cancer Cells. Int. J. Mol. Sci. 2018;19:1690. doi: 10.3390/ijms19061690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Raju A., Christina A.J.M., Murali A. In Vitro Antioxidant and Anticancer Activity Studies on Drosera indica L. (Droseraceae) Adv. Pharm. Bull. 2013;3:115–120. doi: 10.5681/APB.2013.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Raju A., Christina A.J.M. Anticancer Activity of Drosera indica L., on Dalton’s Lymphoma Ascites (DLA) Bearing Mice. J. Intercult. Ethnopharmacol. 2013;2:9. doi: 10.5455/jice.20120615104543. [DOI] [Google Scholar]
- 83.Raju A., Christina A.J.M., Murali A. Antitumor Activity of Ethanol and Aqueous Extracts of Drosera burmannii Vahl. in EAC Bearing Mice. Spatula DD—Peer Rev. J. Complement. Med. Drug Discov. 2012;2:83. doi: 10.5455/spatula.20120501080940. [DOI] [Google Scholar]
- 84.Raju A., Raj S.S., Christina A.J.M. Modulatory Effect of Drosera peltata J.E.Sm on Development of Metabolic Syndrome in Tumor Bearing Mice. Indones. J. Pharm. 2016;27:203. doi: 10.14499/indonesianjpharm27iss4pp203. [DOI] [Google Scholar]
- 85.Raju A., Christina A.J.M. Drosera indica L: Potential Effect on Liver Enzyme, Lipid Profile and Hormone Change in Dalton’s Lymphoma Ascites (DLA) Bearing Mice. J. Intercult. Ethnopharmacol. 2012;1:69. doi: 10.5455/jice.20120514060140. [DOI] [Google Scholar]
- 86.Tokunaga T., Dohmura A., Takada N., Ueda M. Cytotoxic Antifeedant from Dionaea muscipula Ellis: A Defensive Mechanism of Carnivorous Plants against Predators. Bull. Chem. Soc. Jpn. 2004;77:537–541. doi: 10.1246/bcsj.77.537. [DOI] [Google Scholar]
- 87.Ghate N., Das A., Chaudhuri D., Panja S., Mandal N. Sundew Plant, a Potential Source of Anti-Inflammatory Agents, Selectively Induces G2/M Arrest and Apoptosis in MCF-7 Cells through Upregulation of P53 and Bax/Bcl-2 Ratio. Cell Death Discov. 2016;2:15062. doi: 10.1038/cddiscovery.2015.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Engeland K. Cell Cycle Regulation: P53-P21-RB Signaling. Cell Death Differ. 2022;29:946–960. doi: 10.1038/s41418-022-00988-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bhadra K. A Mini Review on Molecules Inducing Caspase-Independent Cell Death: A New Route to Cancer Therapy. Molecules. 2022;27:6401. doi: 10.3390/molecules27196401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Morris J.L., Gillet G., Prudent J., Popgeorgiev N. Bcl-2 Family of Proteins in the Control of Mitochondrial Calcium Signalling: An Old Chap with New Roles. Int. J. Mol. Sci. 2021;22:3730. doi: 10.3390/ijms22073730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pazzaglia S., Pioli C. Multifaceted Role of PARP-1 in DNA Repair and Inflammation: Pathological and Therapeutic Implications in Cancer and Non-Cancer Diseases. Cells. 2019;9:41. doi: 10.3390/cells9010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ou-Yang F., Tsai I.-H., Tang J.-Y., Yen C.-Y., Cheng Y.-B., Farooqi A.A., Chen S.-R., Yu S.-Y., Kao J.-K., Chang H.-W. Antiproliferation for Breast Cancer Cells by Ethyl Acetate Extract of Nepenthes thorellii × (ventricosa x maxima) Int. J. Mol. Sci. 2019;20:3238. doi: 10.3390/ijms20133238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang K.-H., Tang J.-Y., Chen Y.-N., Chuang Y.-T., Tsai I.-H., Chiu C.-C., Li L.-J., Chien T.-M., Cheng Y.-B., Chang F.-R., et al. Nepenthes Extract Induces Selective Killing, Necrosis, and Apoptosis in Oral Cancer Cells. J. Pers. Med. 2021;11:871. doi: 10.3390/jpm11090871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu W., Lin L.-C., Wang P.-J., Chen Y.-N., Wang S.-C., Chuang Y.-T., Tsai I.-H., Yu S.-Y., Chang F.-R., Cheng Y.-B., et al. Nepenthes Ethyl Acetate Extract Provides Oxidative Stress-Dependent Anti-Leukemia Effects. Antioxidants. 2021;10:1410. doi: 10.3390/antiox10091410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tang J., Peng S., Cheng Y., Wang C., Farooqi A.A., Yu T., Hou M., Wang S., Yen C., Chan L., et al. Ethyl Acetate Extract of Nepenthes adrianii x clipeata Induces Antiproliferation, Apoptosis, and DNA Damage against Oral Cancer Cells through Oxidative Stress. Environ. Toxicol. 2019;34:891–901. doi: 10.1002/tox.22748. [DOI] [PubMed] [Google Scholar]
- 96.Tang J.-Y., Li L.-J., Ou-Yang F., Wang C.-L., Shu C.-W., Wu K.-H., Wang H.-R., Yen C.-H., Cheng Y.-B., Chang H.-W. Ethyl Acetate Extract of Nepenthes ventricosa x maxima Exerts Preferential Killing to Oral Cancer Cells. DNA Cell Biol. 2019;38:763–772. doi: 10.1089/dna.2018.4436. [DOI] [PubMed] [Google Scholar]
- 97.Tang J.-Y., Yu T.-J., Lin L.-C., Peng S.-Y., Wang C.-L., Ou-Yang F., Cheng Y.-B., Chang H.-W. Ethyl Acetate Extracts of Nepenthes ventricosa × sibuyanensis Leaves Cause Growth Inhibition against Oral Cancer Cells via Oxidative Stress. OncoTargets Ther. 2019;12:5227–5239. doi: 10.2147/OTT.S190460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peng S.-Y., Lin L.-C., Yang Z.-W., Chang F.-R., Cheng Y.-B., Tang J.-Y., Chang H.-W. Combined Treatment with Low Cytotoxic Ethyl Acetate Nepenthes Extract and Ultraviolet-C Improves Antiproliferation to Oral Cancer Cells via Oxidative Stress. Antioxidants. 2020;9:876. doi: 10.3390/antiox9090876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gonçalves S., Xavier C., Costa P., Alberício F., Romano A. Antioxidant, Cytotoxic and Apoptotic Activity of Drosophyllum lusitanicum Extracts. J. Med. Plants Res. 2010;4:1601–1608. [Google Scholar]
- 100.Bringmann G., Zhang G., Hager A., Moos M., Irmer A., Bargou R., Chatterjee M. Anti-Tumoral Activities of Dioncoquinones B and C and Related Naphthoquinones Gained from Total Synthesis or Isolation from Plants. Eur. J. Med. Chem. 2011;46:5778–5789. doi: 10.1016/j.ejmech.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 101.Jampasri S., Reabroi S., Tungmunnithum D., Parichatikanond W., Pinthong D. Plumbagin Suppresses Breast Cancer Progression by Downregulating HIF-1α Expression via a PI3K/Akt/MTOR Independent Pathway under Hypoxic Condition. Molecules. 2022;27:5716. doi: 10.3390/molecules27175716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang H., Zhang A., Gupte A.A., Hamilton D.J. Plumbagin Elicits Cell-Specific Cytotoxic Effects and Metabolic Responses in Melanoma Cells. Pharmaceutics. 2021;13:706. doi: 10.3390/pharmaceutics13050706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lin C.-L., Yu C.-I., Lee T.-H., Chuang J.M.-J., Han K.-F., Lin C.-S., Huang W.-P., Chen J.Y.-F., Chen C.-Y., Lin M.-Y., et al. Plumbagin Induces the Apoptosis of Drug-Resistant Oral Cancer in Vitro and in Vivo through ROS-Mediated Endoplasmic Reticulum Stress and Mitochondrial Dysfunction. Phytomedicine. 2023;111:154655. doi: 10.1016/j.phymed.2023.154655. [DOI] [PubMed] [Google Scholar]
- 104.Sidhu H., Capalash N. Plumbagin Downregulates UHRF1, p-Akt, MMP-2 and Suppresses Survival, Growth and Migration of Cervical Cancer CaSki Cells. Toxicol. Vitr. 2023;86:105512. doi: 10.1016/j.tiv.2022.105512. [DOI] [PubMed] [Google Scholar]
- 105.Pan S.-T., Ye F.-F., Huang G., Qiu J.-X. Plumbagin Enhances the Anticancer Effects of PF Chemotherapy via Downregulation of the PI3K/AKT/MTOR/P70S6K Pathway in Human Tongue Squamous Cell Carcinoma. J. Oncol. 2023;2023:8306514. doi: 10.1155/2023/8306514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gaascht F., Teiten M.-H., Cerella C., Dicato M., Bagrel D., Diederich M. Plumbagin Modulates Leukemia Cell Redox Status. Molecules. 2014;19:10011–10032. doi: 10.3390/molecules190710011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kuo P.-L., Hsu Y.-L., Cho C.-Y. Plumbagin Induces G2-M Arrest and Autophagy by Inhibiting the AKT/Mammalian Target of Rapamycin Pathway in Breast Cancer Cells. Mol. Cancer Ther. 2006;5:3209–3221. doi: 10.1158/1535-7163.MCT-06-0478. [DOI] [PubMed] [Google Scholar]
- 108.Sandur S.K., Ichikawa H., Sethi G., Ahn K.S., Aggarwal B.B. Plumbagin (5-Hydroxy-2-Methyl-1,4-Naphthoquinone) Suppresses NF-ΚB Activation and NF-ΚB-Regulated Gene Products Through Modulation of P65 and IκBα Kinase Activation, Leading to Potentiation of Apoptosis Induced by Cytokine and Chemotherapeutic Agents. J. Biol. Chem. 2006;281:17023–17033. doi: 10.1074/jbc.M601595200. [DOI] [PubMed] [Google Scholar]
- 109.Wei Y., Lin Y., Chen W., Liu S., Jin L., Huang D. Computational and In Vitro Analysis of Plumbagin’s Molecular Mechanism for the Treatment of Hepatocellular Carcinoma. Front. Pharmacol. 2021;12:594833. doi: 10.3389/fphar.2021.594833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jamal M.S., Parveen S., Beg M.A., Suhail M., Chaudhary A.G.A., Damanhouri G.A., Abuzenadah A.M., Rehan M. Anticancer Compound Plumbagin and Its Molecular Targets: A Structural Insight into the Inhibitory Mechanisms Using Computational Approaches. PLoS ONE. 2014;9:e87309. doi: 10.1371/journal.pone.0087309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kumar A.R., Merlin N.J., Arundhathi R.P., Dharan S.S. In-Silico Molecular Docking Evaluation of Plumbagine Derivatives for Anticancer Activity. J. Pharm. Sci. Res. 2019;11:2676–2678. [Google Scholar]
- 112.Liu Y., Cai Y., He C., Chen M., Li H. Anticancer Properties and Pharmaceutical Applications of Plumbagin: A Review. Am. J. Chin. Med. 2017;45:423–441. doi: 10.1142/S0192415X17500264. [DOI] [PubMed] [Google Scholar]
- 113.Tripathi S.K., Panda M., Biswal B.K. Emerging Role of Plumbagin: Cytotoxic Potential and Pharmaceutical Relevance towards Cancer Therapy. Food Chem. Toxicol. 2019;125:566–582. doi: 10.1016/j.fct.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 114.Yin Z., Zhang J., Chen L., Guo Q., Yang B., Zhang W., Kang W. Anticancer Effects and Mechanisms of Action of Plumbagin: Review of Research Advances. BioMed Res. Int. 2020;2020:6940953. doi: 10.1155/2020/6940953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Taneja N., Alam A., Patnaik R.S., Taneja T. Unmasking the Potential Role of Plant-based Medicine “Plumbagin” in Oral Cancer—A Novel Paradigm. Oral Sci. Int. 2022;19:3–18. doi: 10.1002/osi2.1107. [DOI] [Google Scholar]
- 116.Roy A. Plumbagin: A Potential Anti-Cancer Compound. Mini-Rev. Med. Chem. 2021;21:731–737. doi: 10.2174/1389557520666201116144421. [DOI] [PubMed] [Google Scholar]
- 117.Kawiak A., Domachowska A., Krolicka A., Smolarska M., Lojkowska E. 3-Chloroplumbagin Induces Cell Death in Breast Cancer Cells Through MAPK-Mediated Mcl-1 Inhibition. Front. Pharmacol. 2019;10:784. doi: 10.3389/fphar.2019.00784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kawiak A., Zawacka-Pankau J., Wasilewska A., Stasilojc G., Bigda J., Lojkowska E. Induction of Apoptosis in HL-60 Cells through the ROS-Mediated Mitochondrial Pathway by Ramentaceone from Drosera aliciae. J. Nat. Prod. 2012;75:9–14. doi: 10.1021/np200247g. [DOI] [PubMed] [Google Scholar]
- 119.De U., Son J.Y., Jeon Y., Ha S.-Y., Park Y.J., Yoon S., Ha K.-T., Choi W.S., Lee B.M., Kim I.S., et al. Plumbagin from a Tropical Pitcher Plant (Nepenthes alata Blanco) Induces Apoptotic Cell Death via a P53-Dependent Pathway in MCF-7 Human Breast Cancer Cells. Food Chem. Toxicol. 2019;123:492–500. doi: 10.1016/j.fct.2018.11.040. [DOI] [PubMed] [Google Scholar]
- 120.Guan H.-H., Huang Y.-H., Lin E.-S., Chen C.-J., Huang C.-Y. Plumbagin, a Natural Product with Potent Anticancer Activities, Binds to and Inhibits Dihydroorotase, a Key Enzyme in Pyrimidine Biosynthesis. Int. J. Mol. Sci. 2021;22:6861. doi: 10.3390/ijms22136861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Huang Y.-H., Lien Y., Chen J.-H., Lin E.-S., Huang C.-Y. Identification and Characterization of Dihydropyrimidinase Inhibited by Plumbagin Isolated from Nepenthes Miranda Extract. Biochimie. 2020;171–172:124–135. doi: 10.1016/j.biochi.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 122.Kawiak A., Królicka A., Łojkowska E. In Vitro Cultures of Drosera aliciae as a Source of a Cytotoxic Naphthoquinone: Ramentaceone. Biotechnol. Lett. 2011;33:2309–2316. doi: 10.1007/s10529-011-0700-y. [DOI] [PubMed] [Google Scholar]
- 123.Toton E., Kedziora I., Romaniuk-Drapala A., Konieczna N., Kaczmarek M., Lisiak N., Paszel-Jaworska A., Rybska A., Duszynska W., Budzianowski J., et al. Effect of 3-O-Acetylaleuritolic Acid from in Vitro-Cultured Drosera spatulata on Cancer Cells Survival and Migration. Pharmacol. Rep. 2020;72:166–178. doi: 10.1007/s43440-019-00008-x. [DOI] [PubMed] [Google Scholar]
- 124.Ahmed H., Moawad A., Owis A., AbouZid S., Ahmed O. Flavonoids of Calligonum polygonoides and Their Cytotoxicity. Pharm. Biol. 2016;54:2119–2126. doi: 10.3109/13880209.2016.1146778. [DOI] [PubMed] [Google Scholar]
- 125.Kopustinskiene D.M., Jakstas V., Savickas A., Bernatoniene J. Flavonoids as Anticancer Agents. Nutrients. 2020;12:457. doi: 10.3390/nu12020457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Khan A.U., Dagur H.S., Khan M., Malik N., Alam M., Mushtaque M. Therapeutic Role of Flavonoids and Flavones in Cancer Prevention: Current Trends and Future Perspectives. Eur. J. Med. Chem. Rep. 2021;3:100010. doi: 10.1016/j.ejmcr.2021.100010. [DOI] [Google Scholar]
- 127.Forni C., Rossi M., Borromeo I., Feriotto G., Platamone G., Tabolacci C., Mischiati C., Beninati S. Flavonoids: A Myth or a Reality for Cancer Therapy? Molecules. 2021;26:3583. doi: 10.3390/molecules26123583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ponte L.G.S., Pavan I.C.B., Mancini M.C.S., da Silva L.G.S., Morelli A.P., Severino M.B., Bezerra R.M.N., Simabuco F.M. The Hallmarks of Flavonoids in Cancer. Molecules. 2021;26:2029. doi: 10.3390/molecules26072029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Paper D.H., Karall E., Kremser M., Krenn L. Comparison of the Antiinflammatory Effects Of Drosera rotundifolia And Drosera madagascariensis in the HET-CAM Assay. Phytother. Res. 2005;19:323–326. doi: 10.1002/ptr.1666. [DOI] [PubMed] [Google Scholar]
- 130.Melzig M.F., Pertz H.H., Krenn L. Anti-Inflammatory and Spasmolytic Activity of Extracts from Droserae Herba. Phytomedicine. 2001;8:225–229. doi: 10.1078/0944-7113-00031. [DOI] [PubMed] [Google Scholar]
- 131.Krenn L., Beyer G., Pertz H., Karall E., Kremser M., Galambosi B., Melzig M. In Vitro Antispasmodic and Antiinflammatory Effects of Drosera rotundifolia. Arzneimittelforschung. 2004;54:402–405. doi: 10.1055/s-0031-1296991. [DOI] [PubMed] [Google Scholar]
- 132.Kolodziej H., Pertz H.H., Humke A. Main Constituents of a Commercial Drosera Fluid Extract and Their Antagonist Activity at Muscarinic M3 Receptors in Guinea-Pig Ileum. Pharm. 2002;57:201–203. doi: 10.1002/chin.200224226. [DOI] [PubMed] [Google Scholar]
- 133.Fukushima K., Nagai K., Hoshi Y., Masumoto S., Mikami I., Takahashi Y., Oike H., Kobori M. Drosera rotundifolia and Drosera tokaiensis Suppress the Activation of HMC-1 Human Mast Cells. J. Ethnopharmacol. 2009;125:90–96. doi: 10.1016/j.jep.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 134.Chen Y., Griffiths C.E.M., Bulfone-Paus S. Exploring Mast Cell–CD8 T Cell Interactions in Inflammatory Skin Diseases. Int. J. Mol. Sci. 2023;24:1564. doi: 10.3390/ijms24021564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Thao N.P., Luyen B.T.T., Koo J.E., Kim S., Koh Y.S., Thanh N.V., Cuong N.X., Kiem P.V., Minh C.V., Kim Y.H. In Vitro Anti-Inflammatory Components Isolated from the Carnivorous Plant Nepenthes mirabilis (Lour.) Rafarin. Pharm. Biol. 2016;54:588–594. doi: 10.3109/13880209.2015.1067234. [DOI] [PubMed] [Google Scholar]
- 136.Al-Khayri J.M., Sahana G.R., Nagella P., Joseph B.V., Alessa F.M., Al-Mssallem M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules. 2022;27:2901. doi: 10.3390/molecules27092901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ferraz C.R., Carvalho T.T., Manchope M.F., Artero N.A., Rasquel-Oliveira F.S., Fattori V., Casagrande R., Verri W.A. Therapeutic Potential of Flavonoids in Pain and Inflammation: Mechanisms of Action, Pre-Clinical and Clinical Data, and Pharmaceutical Development. Molecules. 2020;25:762. doi: 10.3390/molecules25030762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vassalle C., Maltinti M., Sabatino L. Targeting Oxidative Stress for Disease Prevention and Therapy: Where Do We Stand, and Where Do We Go from Here. Molecules. 2020;25:2653. doi: 10.3390/molecules25112653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Miyata Y., Mukae Y., Harada J., Matsuda T., Mitsunari K., Matsuo T., Ohba K., Sakai H. Pathological and Pharmacological Roles of Mitochondrial Reactive Oxygen Species in Malignant Neoplasms: Therapies Involving Chemical Compounds, Natural Products, and Photosensitizers. Molecules. 2020;25:5252. doi: 10.3390/molecules25225252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ghate N.B., Chaudhuri D., Das A., Panja S., Mandal N. An Antioxidant Extract of the Insectivorous Plant Drosera burmannii Vahl. Alleviates Iron-Induced Oxidative Stress and Hepatic Injury in Mice. PLoS ONE. 2015;10:e0128221. doi: 10.1371/journal.pone.0128221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Thanh N.V., Thao N.P., Huong P.T.T., Lee S.H., Jang H.D., Cuong N.X., Nam N.H., Kiem P.V., Kim Y.H., Minh C.V. Naphthoquinone and Flavonoid Constituents from the Carnivorous Plant Nepenthes mirabilis and Their Anti-Osteoporotic and Antioxidant Activities. Phytochem. Lett. 2015;11:254–259. doi: 10.1016/j.phytol.2015.01.009. [DOI] [Google Scholar]
- 142.Arruda-Silva F., Bellavite P., Marzotto M. Low-Dose Drosera rotundifolia Induces Gene Expression Changes in 16HBE Human Bronchial Epithelial Cells. Sci. Rep. 2021;11:2356. doi: 10.1038/s41598-021-81843-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hake A., Begrow F., Spiegler V., Symma N., Hensel A., Düfer M. Effects of Extracts and Flavonoids from Drosera rotundifolia L. on Ciliary Beat Frequency and Murine Airway Smooth Muscle. Molecules. 2022;27:6622. doi: 10.3390/molecules27196622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mokra D., Mokry J. Phosphodiesterase Inhibitors in Acute Lung Injury: What Are the Perspectives? Int. J. Mol. Sci. 2021;22:1929. doi: 10.3390/ijms22041929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Thanh N.V., Thao N.P., Dat L.D., Huong P.T.T., Lee S.H., Jang H.D., Cuong N.X., Nam N.H., Kiem P.V., Minh C.V., et al. Two New Naphthalene Glucosides and Other Bioactive Compounds from the Carnivorous Plant Nepenthes mirabilis. Arch. Pharm. Res. 2015;38:1774–1782. doi: 10.1007/s12272-015-0576-9. [DOI] [PubMed] [Google Scholar]
- 146.Yu M., Moreno J.L., Stains J.P., Keegan A.D. Complex Regulation of Tartrate-Resistant Acid Phosphatase (TRAP) Expression by Interleukin 4 (IL-4) J. Biol. Chem. 2009;284:32968–32979. doi: 10.1074/jbc.M109.001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Likhitwitayawuid K., Kaewamatawong R., Ruangrungsi N., Krungkrai J. Antimalarial Naphthoquinones from Nepenthes Thorelii. Planta Med. 1998;64:237–241. doi: 10.1055/s-2006-957417. [DOI] [PubMed] [Google Scholar]
- 148.François G., Bringmann G., Dochez C., Schneider C., Timperman G., Assi L.A. Activities of Extracts and Naphthylisoquinoline Alkaloids from Triphyophyllum peltatum, Ancistrocladus Aabbreviatus and Ancistrocladus barteri against Plasmodium berghei (Anka Strain) in Vitro. J. Ethnopharmacol. 1995;46:115–120. doi: 10.1016/0378-8741(95)01240-E. [DOI] [PubMed] [Google Scholar]
- 149.Bringmann G., Saeb W., God R., Schäffer M., François G., Peters K., Peters E.-M., Proksch P., Hostettmann K., Assi L.A. 5′-O-Demethyldioncophylline A, a New Antimalarial Alkaloid from Triphyophyllum peltatum. Phytochemistry. 1998;49:1667–1673. doi: 10.1016/S0031-9422(98)00231-3. [DOI] [PubMed] [Google Scholar]
- 150.François G., Timperman G., Eling W., Assi L.A., Holenz J., Bringmann G. Naphthylisoquinoline Alkaloids against Malaria: Evaluation of the Curative Potentials of Dioncophylline C and Dioncopeltine A against Plasmodium berghei in Vivo. Antimicrob. Agents Chemother. 1997;41:2533–2539. doi: 10.1128/AAC.41.11.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.François G., Van Looveren M., Timperman G., Chimanuka B., AkéAssi L., Holenz J., Bringmann G. Larvicidal Activity of the Naphthylisoquinoline Alkaloid Dioncophylline A against the Malaria Vector Anopheles stephensi. J. Ethnopharmacol. 1996;54:125–130. doi: 10.1016/S0378-8741(96)01459-6. [DOI] [PubMed] [Google Scholar]
- 152.Bringmann G., Holenz J., Saeb W., Ake Assi L., Hostettmann K. Dioncophylline A as a Larvicide against Aedes aegypti. Pharm. Pharmacol. Lett. 1999;9:24–25. [Google Scholar]
- 153.Bringmann G., Saeb W., Assi L., François G., Sankara Narayanan A., Peters K., Peters E.-M. Betulinic Acid: Isolation from Triphyophyllum peltatum and Ancistrocladus heyneanus, Antimalarial Activity, and Crystal Structure of the Benzyl Ester. Planta Med. 1997;63:255–257. doi: 10.1055/s-2006-957666. [DOI] [PubMed] [Google Scholar]
- 154.Bringmann G., Rüdenauer S., Irmer A., Bruhn T., Brun R., Heimberger T., Stühmer T., Bargou R., Chatterjee M. Antitumoral and Antileishmanial Dioncoquinones and Ancistroquinones from Cell Cultures of Triphyophyllum peltatum (Dioncophyllaceae) and Ancistrocladus abbreviatus (Ancistrocladaceae) Phytochemistry. 2008;69:2501–2509. doi: 10.1016/j.phytochem.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 155.Bringmann G., Fayez S., Shamburger W., Feineis D., Winiarczyk S., Janecki R., Adaszek Ł. Naphthylisoquinoline Alkaloids and Their Synthetic Analogs as Potent Novel Inhibitors against Babesia canis in Vitro. Vet. Parasitol. 2020;283:109177. doi: 10.1016/j.vetpar.2020.109177. [DOI] [PubMed] [Google Scholar]
- 156.Tominaga Y., Sugawa H., Hirabayashi K., Ikeda T., Hoshi Y., Nagai R. Drosera tokaiensis Extract Containing Multiple Phenolic Compounds Inhibits the Formation of Advanced Glycation End-Products. Arch. Biochem. Biophys. 2020;693:108586. doi: 10.1016/j.abb.2020.108586. [DOI] [PubMed] [Google Scholar]
- 157.Hema B., Bhupendra S., Mohamed Saleem T.S., Gauthaman K. Anticonvulsant Effect of Drosera burmannii. Int. J. Appl. Res. Nat. Prod. 2009;2:1–4. [Google Scholar]
- 158.Trinh B.T.D., Staerk D., Jäger A.K. Screening for Potential α-Glucosidase and α-Amylase Inhibitory Constituents from Selected Vietnamese Plants Used to Treat Type 2 Diabetes. J. Ethnopharmacol. 2016;186:189–195. doi: 10.1016/j.jep.2016.03.060. [DOI] [PubMed] [Google Scholar]
- 159.Tuli H.S., Bhatia G.K., Sood S., Debnath P., Aggarwal D., Upadhyay S.K. In Silico Analysis and Molecular Docking Studies of Plumbagin and Piperine Ligands as Potential Inhibitors of Alpha-Glucosidase Receptor. Biointerface Res. Appl. Chem. 2020;11:9629–9637. doi: 10.33263/BRIAC112.96299637. [DOI] [Google Scholar]
- 160.Rey M., Yang M., Lee L., Zhang Y., Sheff J.G., Sensen C.W., Mrazek H., Halada P., Man P., McCarville J.L., et al. Addressing Proteolytic Efficiency in Enzymatic Degradation Therapy for Celiac Disease. Sci. Rep. 2016;6:30980. doi: 10.1038/srep30980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Madhavan V., Hema Prem Kumar B., Murali A., Yoganarasimhan S.N. Antifertility Activity of Drosera burmannii. Pharm. Biol. 2009;47:128–131. doi: 10.1080/13880200802437149. [DOI] [Google Scholar]
- 162.Sandeep G., Dheeraj A., Sharma N.K., Jhade D., Bharti A. Effect of Plumbagin Free Alcohol Extract of Plumbago zeylanica Linn. Root on Reproductive System of Female Wistar Rats. Asian Pac. J. Trop. Med. 2011;4:978–984. doi: 10.1016/S1995-7645(11)60230-7. [DOI] [PubMed] [Google Scholar]
- 163.Shin K.-S., Lee S., Cha B.J. Suppression of Phytopathogenic Fungi by Hexane Extract of Nepenthes ventricosa × maxima Leaf. Fitoterapia. 2007;78:585–586. doi: 10.1016/j.fitote.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 164.Rahman-Soad A., Dávila-Lara A., Paetz C., Mithöfer A. Plumbagin, a Potent Naphthoquinone from Nepenthes Plants with Growth Inhibiting and Larvicidal Activities. Molecules. 2021;26:825. doi: 10.3390/molecules26040825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tokunaga T., Takada N., Ueda M. Mechanism of Antifeedant Activity of Plumbagin, a Compound Concerning the Chemical Defense in Carnivorous Plant. Tetrahedron Lett. 2004;45:7115–7119. doi: 10.1016/j.tetlet.2004.07.094. [DOI] [Google Scholar]
- 166.Pavela R. Efficacy of Naphthoquinones as Insecticides against the House Fly, Musca domestica L. Ind. Crops Prod. 2013;43:745–750. doi: 10.1016/j.indcrop.2012.08.025. [DOI] [Google Scholar]
- 167.Gonçalves S., Gonçalves M.A., Ameixa O., Nogueira J.M.F., Romano A. Insecticidal Activity of Leaf Extracts from Drosophyllum lusitanicum against Liriomyza trifolii (Burgess) (Diptera: Agromyzidae) J. Hortic. Sci. Biotechnol. 2008;83:653–657. doi: 10.1080/14620316.2008.11512438. [DOI] [Google Scholar]
- 168.Gonçalves S., Ferraz M., Romano A. Phytotoxic Properties of Drosophyllum lusitanicum Leaf Extracts and Its Main Compound Plumbagin. Sci. Hortic. 2009;122:96–101. doi: 10.1016/j.scienta.2009.03.028. [DOI] [Google Scholar]
- 169.Rajninec M., Jopcik M., Danchenko M., Libantova J. Biochemical and Antifungal Characteristics of Recombinant Class I Chitinase from Drosera rotundifolia. Int. J. Biol. Macromol. 2020;161:854–863. doi: 10.1016/j.ijbiomac.2020.06.123. [DOI] [PubMed] [Google Scholar]
- 170.Sinelnikov I.G., Siedhoff N.E., Chulkin A.M., Zorov I.N., Schwaneberg U., Davari M.D., Sinitsyna O.A., Shcherbakova L.A., Sinitsyn A.P., Rozhkova A.M. Expression and Refolding of the Plant Chitinase From Drosera capensis for Applications as a Sustainable and Integrated Pest Management. Front. Bioeng. Biotechnol. 2021;9:728501. doi: 10.3389/fbioe.2021.728501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bringmann G., Holenz J., Assi L., Zhao C., Hostettmann K. Molluscicidal Activity of Naphthylisoquinoline Alkaloids from Triphyophyllum and Ancistrocladus Species. Planta Med. 1996;62:556–557. doi: 10.1055/s-2006-957970. [DOI] [PubMed] [Google Scholar]
- 172.Cross A.T., Krueger T.A., Gonella P.M., Robinson A.S., Fleischmann A.S. Conservation of Carnivorous Plants in the Age of Extinction. Glob. Ecol. Conserv. 2020;24:e01272. doi: 10.1016/j.gecco.2020.e01272. [DOI] [Google Scholar]
- 173.Wawrosch C. An Improved 2-Step Liquid Culture System for Efficient In Vitro Shoot Proliferation of Sundew (Drosera rotundifolia L.) Sci. Pharm. 2009;77:827–836. doi: 10.3797/scipharm.0908-03. [DOI] [Google Scholar]
- 174.Kunakhonnuruk B., Kongbangkerd A., Inthima P. Improving Large-Scale Biomass and Plumbagin Production of Drosera communis A.St.-Hil. by Temporary Immersion System. Ind. Crops Prod. 2019;137:197–202. doi: 10.1016/j.indcrop.2019.05.039. [DOI] [Google Scholar]
- 175.Banasiuk R., Kawiak A., Królicka A. In Vitro Cultures of Carnivorous Plants from the Drosera and Dionaea Genus for the Production of Biologically Active Secondary Metabolites. BioTechnologia. 2012;2:87–96. doi: 10.5114/bta.2012.46572. [DOI] [Google Scholar]
- 176.Adamec L., Kondo K. Optimization of Medium for Growing the Aquatic Carnivorous Plant Aldrovanda vesiculosa In Vitro. Plant Biotechnol. 2002;19:283–286. doi: 10.5511/plantbiotechnology.19.283. [DOI] [Google Scholar]
- 177.Gonçalves S., Romano A. In Vitro Minimum Growth for Conservation of Drosophyllum lusitanicum. Biol. Plant. 2007;51:795–798. doi: 10.1007/s10535-007-0163-0. [DOI] [Google Scholar]
- 178.Gonçalves S., Romano A. Micropropagation of Drosophyllum lusitanicum (Dewy Pine), an Endangered West Mediterranean Endemic Insectivorous Plant. Biodivers. Conserv. 2005;14:1071–1081. doi: 10.1007/s10531-004-7846-z. [DOI] [Google Scholar]
- 179.Parzymies M., Pogorzelec M., Świstowska A. Optimization of Propagation of the Polish Strain of Aldrovanda vesiculosa in Tissue Culture. Biology. 2022;11:1389. doi: 10.3390/biology11101389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Blehová A., Švubová R., Lukačová Z., Moravčíková J., Matušíková I. Transformation of Sundew: Pitfalls and Promises. Plant Cell Tissue Organ Cult. PCTOC. 2015;120:681–687. doi: 10.1007/s11240-014-0635-9. [DOI] [Google Scholar]
- 181.Grevenstuk T., Coelho N., Gonçalves S., Romano A. In Vitro Propagation of Drosera intermedia in a Single Step. Biol. Plant. 2010;54:391–394. doi: 10.1007/s10535-010-0071-6. [DOI] [Google Scholar]
- 182.Yanthan J.S., Kehie M., Kumaria S., Tandon P. In Vitro Regeneration of Drosera burmannii Vahl.: A Carnivorous Plant of North-East India. 3 Biotech. 2017;7:124. doi: 10.1007/s13205-017-0777-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Taraszkiewicz A., Jafra S., Skrzypczak A., Kaminski M., Krolicka A. Antibacterial activity of secondary metabolites from in vitro culture of Drosera gigantea against the plant pathogenic bacteria Pseudomonas syringae pv. syringae and P. syringae pv. morsprunorum. J. Plant Pathol. 2012;94((Suppl. S1)):63–68. doi: 10.4454/jpp.v94i1sup.011. [DOI] [Google Scholar]
- 184.Wongsa T., Inthima P., Nakkuntod M., Premjet D., Kongbangkerd A. Effects of Cytokinin and Auxin on In Vitro Organ Development and Plumbagin Content of Drosera peltata Thunb. AGRIVITA J. Agric. Sci. 2018;40:415–424. doi: 10.17503/agrivita.v40i0.1276. [DOI] [Google Scholar]
- 185.Rejthar J., Viehmannova I., Cepkova P., Fernndez E., Milella L. In Vitro Propagation of Drosera intermedia as Influenced by Cytokinins, PH, Sucrose, and Nutrient Concentration. Emir. J. Food Agric. 2014;26:558. doi: 10.9755/ejfa.v26i6.18022. [DOI] [Google Scholar]
- 186.Putalun W., Udomsin O., Yusakul G., Juengwatanatrakul T., Sakamoto S., Tanaka H. Enhanced Plumbagin Production from in Vitro Cultures of Drosera burmanii Using Elicitation. Biotechnol. Lett. 2010;32:721–724. doi: 10.1007/s10529-010-0202-3. [DOI] [PubMed] [Google Scholar]
- 187.Wawrosch C., Vackar E., Grauwald B. Variations of Naphthoquinone Levels in Micropropaaated Drosera Species In Vitro, under Qreenhouse and Outdoor Growth Conditions. Sci. Pharm. 2005;73:251–262. doi: 10.3797/scipharm.aut-05-18. [DOI] [Google Scholar]
- 188.Hutchinson J.F. In Vitro Propagation of Dionaea muscipula Ellis (Venus Fly Trap) Sci. Hortic. 1984;22:189–194. doi: 10.1016/0304-4238(84)90100-6. [DOI] [Google Scholar]
- 189.Jang G.-W., Kim K.-S., Park R.-D. Micropropagation of Venus Fly Trap by Shoot Culture. Plant Cell Tissue Organ Cult. 2003;72:95–98. doi: 10.1023/A:1021203811457. [DOI] [Google Scholar]
- 190.Hook I.L.I. Naphthoquinone Contents of in Vitro Cultured Plants and Cell Suspensions of Dionaea muscipula and Drosera Species. Plant Cell Tissue Organ Cult. 2001;67:281–285. doi: 10.1023/A:1012708819212. [DOI] [Google Scholar]
- 191.Joshi B., Panwar G.S., Singh S.K. In Vitro Propagation of Insectivorous Plant Nepenthes khasiana Hook. F.-an Endangered Ornamental and Ethnomedicinal Species. Vegetos. 2022;35:534–539. doi: 10.1007/s42535-021-00310-1. [DOI] [Google Scholar]
- 192.Siti-Suhaila A., Norwati M. Micropropagation of Nepenthes hybrid (n. Viking × n. Miranda) using a temporary immersion bioreactor system, setistm. J. Trop. For. Sci. 2021;33:494–500. doi: 10.26525/jtfs2021.33.4.494. [DOI] [Google Scholar]
- 193.Miguel S., Michel C., Biteau F., Hehn A., Bourgaud F. In Vitro Plant Regeneration and Agrobacterium-Mediated Genetic Transformation of a Carnivorous Plant, Nepenthes mirabilis. Sci. Rep. 2020;10:17482. doi: 10.1038/s41598-020-74108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gonçalves S., Romano A. Ex vitro rooting of Drosophyllum lusitanicum micropropagated shoots improves acclimatization. Acta Hortic. 2007;748:127–131. doi: 10.17660/ActaHortic.2007.748.14. [DOI] [Google Scholar]
- 195.Pietrosiuk A., Budzianowska A., Budzianowski J., Ekiert H., Jeziorek M., Kawiak A., Kikowska M., Krauze-Baranowska M., Królicka A., Kuźma Ł., et al. Polish Achievements in Bioactive Compound Production From In Vitro Plant Cultures. Acta Soc. Bot. Pol. 2022;91:9110. doi: 10.5586/asbp.9110. [DOI] [Google Scholar]
- 196.Thaweesak J., Seiichi S., Hiroyuki T., Waraporn P. Elicitation Effect on Production of Plumbagin in in Vitro Culture of Drosera indica L. J. Med. Plants Res. 2011;5:4949–4953. [Google Scholar]
- 197.Boonsnongcheep P., Sae-foo W., Banpakoat K., Channarong S., Chitsaithan S., Uafua P., Putha W., Kerdsiri K., Putalun W. Artificial Color Light Sources and Precursor Feeding Enhance Plumbagin Production of the Carnivorous Plants Drosera burmannii and Drosera indica. J. Photochem. Photobiol. B. 2019;199:111628. doi: 10.1016/j.jphotobiol.2019.111628. [DOI] [PubMed] [Google Scholar]
- 198.Makowski W., Tokarz B., Banasiuk R., Królicka A., Dziurka M., Wojciechowska R., Tokarz K.M. Is a Blue–Red Light a Good Elicitor of Phenolic Compounds in the Family Droseraceae? A Comparative Study. J. Photochem. Photobiol. B. 2019;201:111679. doi: 10.1016/j.jphotobiol.2019.111679. [DOI] [PubMed] [Google Scholar]
- 199.López-Orenes A., Ferrer M.A., Calderón A.A. Microwave Radiation as an Inducer of Secondary Metabolite Production in Drosera rotundifolia In Vitro Plantlets. J. Nat. Prod. 2022;85:2104–2109. doi: 10.1021/acs.jnatprod.2c00031. [DOI] [PubMed] [Google Scholar]
- 200.Siatkowska K., Chraniuk M., Bollin P., Banasiuk R. Light Emitting Diodes Optimisation for Secondary Metabolites Production by Droseraceae Plants. J. Photochem. Photobiol. B. 2021;224:112308. doi: 10.1016/j.jphotobiol.2021.112308. [DOI] [PubMed] [Google Scholar]
- 201.Mahapatra A., Mativandlela S.P.N., Binneman B., Fourie P.B., Hamilton C.J., Meyer J.J.M., van der Kooy F., Houghton P., Lall N. Activity of 7-Methyljuglone Derivatives against Mycobacterium tuberculosis and as Subversive Substrates for Mycothiol Disulfide Reductase. Bioorg. Med. Chem. 2007;15:7638–7646. doi: 10.1016/j.bmc.2007.08.064. [DOI] [PubMed] [Google Scholar]
- 202.Bapela N.B., Lall N., Fourie P.B., Franzblau S.G., Van Rensburg C.E.J. Activity of 7-Methyljuglone in Combination with Antituberculous Drugs against Mycobacterium tuberculosis. Phytomedicine. 2006;13:630–635. doi: 10.1016/j.phymed.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 203.Šimura J., Spíchal L., Adamec L., Pěnčík A., Rolčík J., Novák O., Strnad M. Cytokinin, Auxin and Physiological Polarity in the Aquatic Carnivorous Plants Aldrovanda vesiculosa and Utricularia australis. Ann. Bot. 2016;117:1037–1044. doi: 10.1093/aob/mcw020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Westermeier A.S., Sachse R., Poppinga S., Vögele P., Adamec L., Speck T., Bischoff M. How the Carnivorous Waterwheel Plant (Aldrovanda vesiculosa) Snaps. Proc. R. Soc. B Biol. Sci. 2018;285:20180012. doi: 10.1098/rspb.2018.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Płachno B.J., Kapusta M., Stolarczyk P., Wójciak M., Świątek P. Immunocytochemical Analysis of Bifid Trichomes in Aldrovanda vesiculosa L. Traps. Int. J. Mol. Sci. 2023;24:3358. doi: 10.3390/ijms24043358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Płachno B.J., Kapusta M., Stolarczyk P., Świątek P., Strzemski M., Miranda V.F.O. Immunocytochemical Analysis of the Wall Ingrowths in the Digestive Gland Transfer Cells in Aldrovanda vesiculosa L. (Droseraceae) Cells. 2022;11:2218. doi: 10.3390/cells11142218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Płachno B.J., Strzemski M., Dresler S., Adamec L., Wojas-Krawczyk K., Sowa I., Danielewicz A., Miranda V.F.O. A Chemometry of Aldrovanda vesiculosa L. (Waterwheel, Droseraceae) Populations. Molecules. 2020;26:72. doi: 10.3390/molecules26010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Budzianowski J., Budzianowska A., Kromer K. Naphthalene Glucoside and Other Phenolics from the Shoot and Callus Cultures of Drosophyllum lusitanicum. Phytochemistry. 2002;61:421–425. doi: 10.1016/S0031-9422(02)00258-3. [DOI] [PubMed] [Google Scholar]
- 209.Grevenstuk T., Gonçalves S., Nogueira J.M.F., Romano A. Plumbagin Recovery from Field Specimens Of Drosophyllum lusitanicum (L.) Link. Phytochem. Anal. 2008;19:229–235. doi: 10.1002/pca.1034. [DOI] [PubMed] [Google Scholar]
- 210.Ojeda F., Carrera C., Paniw M., García-Moreno L., Barbero G.F., Palma M. Volatile and Semi-Volatile Organic Compounds May Help Reduce Pollinator-Prey Overlap in the Carnivorous Plant Drosophyllum lusitanicum (Drosophyllaceae) J. Chem. Ecol. 2021;47:73–86. doi: 10.1007/s10886-020-01235-w. [DOI] [PubMed] [Google Scholar]
- 211.Bringmann G., Irmer A., Büttner T., Schaumlöffel A., Zhang G., Seupel R., Feineis D., Fester K. Axially Chiral Dimeric Naphthalene and Naphthoquinone Metabolites, from Root Cultures of the West African Liana Triphyophyllum peltatum. J. Nat. Prod. 2016;79:2094–2103. doi: 10.1021/acs.jnatprod.6b00439. [DOI] [PubMed] [Google Scholar]
- 212.Bringmann G., Irmer A., Rüdenauer S., Mutanyatta-Comar J., Seupel R., Feineis D. 5′-O-Methyldioncophylline D, a 7,8′-Coupled Naphthylisoquinoline Alkaloid from Callus Cultures of Triphyophyllum peltatum, and Its Biosynthesis from a Late-Stage Tetrahydroisoquinoline Precursor. Tetrahedron. 2016;72:2906–2912. doi: 10.1016/j.tet.2016.04.003. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article.